Abstract
Immunization with urease can protect mice from challenge with Helicobacter pylori, though results vary depending on the particular vaccine, challenge strain, and method of evaluation. Unlike mice, rhesus monkeys are naturally colonized with H. pylori and so may provide a better estimate of vaccine efficacy in humans. The purpose of this study was to examine the effectiveness of H. pylori urease as a vaccine in specific-pathogen (H. pylori)-free rhesus monkeys. Monkeys raised from birth and documented to be free of H. pylori were vaccinated with orogastric (n = 4) or intramuscular (n = 5) urease. Two control monkeys were sham vaccinated. All monkeys were challenged with a rhesus monkey-derived strain of H. pylori, and the effects of vaccination were evaluated by use of quantitative cultures of gastric tissue, histology, and measurement of serum immunoglobulin G (IgG) and salivary IgA. Despite a humoral immune response, all monkeys were infected after H. pylori challenge, and there were no differences in the density of colonization. Immunization with urease therefore does not fully protect against challenge with H. pylori. An effective vaccine to prevent H. pylori infection will require different or more likely additional antigens, as well as improvements in the stimulation of the host immune response.
Helicobacter pylori infects the gastric epithelium of approximately half of the world's population and causes a histologic gastritis (9). An estimated 20% of those infected will go on to develop either peptic ulcer or gastric adenocarcinoma, which is the second-most-common cause of cancer mortality worldwide. Therapy of H. pylori requires the prolonged administration of several agents, and even under the best of circumstances it fails in 10 to 15% of cases. The growing development of antibiotic resistance further complicates the future of H. pylori management (16).
Immunization is an alternative strategy that may be useful as primary prevention and perhaps also as therapy for H. pylori infection. The early encouraging results obtained in the H. felis mouse model (4, 12, 13, 22, 27, 29) were confirmed by subsequent studies which showed that mice can be protected from H. pylori challenge by immunization with a variety of whole-cell or recombinant antigens (3, 14, 15, 26, 31). Most studies have utilized oral administration of recombinant urease, which has been shown to be effective as therapy and as primary prevention. Protection typically ranges from ca. 60 to 100%, depending on the particular adjuvant and challenge strain. More recently, parenteral administration of urease, together with a synthetic glycolipid adjuvant, has also been shown to be effective for primary prevention of H. pylori (18).
These promising results obtained in the mouse model have been only partially supported by recent studies in the rhesus monkey. Captive rhesus monkeys are naturally colonized with H. pylori that is indistinguishable from that found in humans (5–7, 33) and therefore may represent a more appropriate test of vaccine efficacy than the mouse, which is not a natural host for H. felis or H. pylori. Three studies have examined the efficacy of urease vaccine in rhesus monkeys. Lee et al. showed that immunization of naturally infected rhesus monkeys with H. pylori urease plus Escherichia coli enterotoxin (LT) was not effective therapy for H. pylori and prevented infection in only a small percentage of naturally colonized animals that were challenged following antibiotic treatment (24). However, a reduced level of colonization was seen in vaccinated monkeys compared to sham controls. A subsequent study with antibiotic-treated monkeys found that all vaccinated animals as well as controls were infected following challenge, but again the colonization level was reduced in monkeys that received oral vaccine followed by a parenteral boost (23). These studies, however, differ from the anticipated use of a vaccine in humans because the animals were not immunologically naive to H. pylori, and the effects of prior infection on vaccination are unknown. In a study of natural and presumed primary acquisition of H. pylori in very young animals, 69% of monkeys immunized with oral urease and LT developed infection, compared with 93% of controls (8). However, the results are difficult to interpret because the initial absence of infection was documented only by serology, which is not sensitive for the detection of newly acquired infection. Unlike in the challenge experiments, the vaccine produced no reduction in bacterial colonization among the animals that became infected.
We have developed a protocol for derivation of specific-pathogen (H. pylori)-free (SPF) rhesus monkeys (33). These monkeys may provide an attractive model for evaluation of vaccine efficacy because they are well characterized and have no prior immune response to H. pylori. The purpose of this experiment was to examine the effect of oral and parenteral urease vaccination on challenge with H. pylori in SPF rhesus monkeys.
MATERIALS AND METHODS
Animals.
Eleven newborn rhesus monkeys were taken from their dam within 24 h of birth and pair housed in temperature- and humidity-controlled incubators in the nursery. At approximately 1.5 years of age monkeys were examined for H. pylori by serology, [14C]urea breath test, and histology and culture of gastric biopsies by using methods previously described (33). All 11 animals were free of H. pylori and “H. heilmannii,” the uncultivated gastric spiral organism that commonly colonizes monkeys and other animals (6, 28, 33, 34). The [14C]urea breath test and endoscopy were repeated at approximately 2 years of age, and all 11 animals remained uninfected.
Animals were individually housed during their participation in these experiments. Ketamine (10 mg/kg of body weight) was administered intramuscularly as sedation for all procedures, which were performed following an overnight fast. Euthanasia was performed on ketamine-sedated monkeys by intravenous administration of pentobarbital (50 mg/kg of body weight). Experiments were conducted according to the Guide for the Care and Use of Laboratory Animals and were approved by the Research Advisory Committee of the California Regional Primate Research Center.
Vaccine and adjuvant preparation.
Vaccines and adjuvants were provided by OraVax (Cambridge, Mass.). Catalytically inactive but fully assembled urease apoenzyme was purified from E. coli containing either plasmid pORV214 (oral vaccine) or pORV273 (parenteral vaccine) by methods described previously (25). pORV273 is a proprietary construct identical to pORV214 except that the urease binding sites are substituted, and it was used to prepare parenteral vaccine for production rather than scientific reasons. There are no detectable antigenic differences between urease made from these two constructs. Urease for parenteral administration was purified by passing it through a Sartobind matrix (Sartorius Corp., Edgewood, N.Y.) in order to reduce contamination by E. coli endotoxin to 1.5 ng of urease or less per mg. Urease was lyophilized in 2% sucrose and stored at −20°C prior to use.
For oral immunization, 4 mg of recombinant H. pylori urease and 100 μg of E. coli LT (Berna Products Corp., Coral Gables, Fla.) were mixed and diluted to a final volume of 5 ml of lactated Ringer's solution. For parenteral immunization, 100 μg of low-endotoxin urease was mixed immediately prior to injection with 600 μg of glycolipid adjuvant (BAY R 1005; Bayer AG, Leverkusen, Germany) in lactated Ringer's solution to a final volume of 0.5 ml. The dose of oral vaccine was based on a body weight-adjusted dose found to be effective in mice (25), while the parenteral dose was chosen as the maximum likely to be used in humans.
Immunization.
Monkeys were given oral urease plus LT adjuvant (n = 4), parenteral low-endotoxin urease plus Bay adjuvant (n = 5), or sham immunization with oral lactated Ringer's solution (n = 2). Animals to receive oral vaccine were first given 2.5 ml of 8% NaHCO3 per os to neutralize gastric acidity immediately prior to immunization. Vaccine was then administered by gradually dripping the solution into the oral cavity to ensure that mucosal surfaces were exposed prior to swallowing, followed by 3 ml of phosphate-buffered saline to rinse the mouth. Parenteral vaccine was administered by intramuscular injection of half the volume (0.25 ml) into the deltoid muscle and the remaining half (0.25 ml) into the lateral thigh muscle. Each animal received four doses of vaccine or sham immunization, which were given at weeks 0, 3, 6, and 12.
Experimental challenge with H. pylori.
Two weeks after completion of vaccination (week 14), the immunized and sham control animals were orogastrically inoculated with H. pylori N246C3, a rhesus monkey-derived strain previously shown to effectively colonize rhesus monkeys (24). Low-passage stocks were subcultured once on brucella agar with 5% newborn calf serum (Gibco-BRL, Gaithersberg, Md.) supplemented with TVPA (trimethoprim, 5 mg/liter; vancomycin, 10 mg/liter; polymixin B, 2.5 IU/liter; amphotericin B, 4 mg/liter; all were from Sigma) and incubated at 37°C in 5% CO2. The subculture was then used to inoculate brucella broth (Difco Laboratories, Detroit, Mich.) with 5% newborn calf serum and TVPA, which was incubated without shaking at 37°C in 5% CO2 until the optical density at 600 nm (OD600) was approximately 0.2 (ca. 15 h). The culture was centrifuged and resuspended in brucella broth at 2 × 108 CFU/3 ml. Prior to each inoculation, the culture was examined by Gram stain, wet mount, and rapid urease assay with urea-indole medium (21). The inoculum was quantitated by plating serial dilutions. All animals were pretreated with cimetidine (10 mg/kg of body weight) 1 h prior to challenge to neutralize the gastric acid and then given 2 × 108 CFU in 3 ml delivered by orogastric tube. The challenge was repeated every other day for a total of three doses.
Endoscopy and necropsy.
Four weeks after H. pylori challenge (week 18) all monkeys underwent endoscopy. Mucosal biopsies were obtained from the gastric antrum and body for quantitative culture (three from each site) and for histology (one from each site). Eleven weeks after H. pylori challenge (week 25), vaccinated and sham control monkeys were sacrificed and the stomach was opened aseptically along the greater and lesser curvature. One half of the stomach was examined by quantitative culture using 10 0.5-cm-diameter punch biopsies distributed over the gastric cardia (n = 1), body (n = 2), transition zone (n = 3), antrum (n = 3), and pylorus (n = 1). The remaining mucosa was collected by gently scraping it with a sterile microscope slide. The other half of the stomach was examined histologically using 10 1.0-cm2 tissue samples distributed over the regions of the stomach as described above.
Quantitative culture of gastric tissue.
Gastric tissue was placed in preweighed vials containing 0.9% sterile saline, weighed again, and transported immediately to the laboratory. The tissue was homogenized with a sterile ground-glass pestle and 100 μl of neat, 1:10, 1:100 and 1:1,000 dilutions were inoculated onto brucella agar containing 5% bovine calf serum (Gibco-BRL) and TVPA. The plates were incubated in an atmosphere of 5% CO2 for up to 10 days. H. pylori was identified in the conventional manner by colony morphology (pinhead-sized translucent colonies), microscopy (gram-negative curved organisms), and biochemistry (oxidase, catalase, and urease positive). The CFU per gram of gastric mucosa was calculated by enumerating colonies, adjusting for the dilution, and dividing by the tissue weight.
Histology.
Tissues were formalin fixed, paraffin embedded, and processed with hematoxylin and eosin and Warthin-Starry stains. Gastritis was determined on blinded samples by assessment of acute (polymorphonuclear leukocytes) and chronic (mononuclear cells) inflammation on a scale of 0 (none), 1 (mild), 2 (moderate), or 3 (severe) as described in the Sydney system (30).
Urease-specific H. pylori antibodies.
Blood was collected by femoral venipuncture at week 0 (prior to vaccination), week 14 (challenge), and at weeks 18 (endoscopy) and 25 (necropsy). Sera were separated by centrifugation and stored at −70°C. Saliva was collected at weeks 0, 14, and 18 by placing two cellulose wicks (Polyfiltron, Rockland, Maine) between the lower lip and gum for 5 min or more. Wicks saturated with saliva were stored at −70°C. At the time of assay, 0.5 ml of phosphate-buffered saline containing protease inhibitors (0.2 μM aminoethyl-benzene sulfonyl fluoride, 1 μg of aprotinin per ml, and 10 μM leupeptin) was added, and saliva was recovered by centrifugation.
Urease-specific antibodies were detected by enzyme-linked immunosorbent assay with native H. pylori recombinant urease as the antigen. Serum (100 μl) was added in serial dilutions (beginning at 1:100) to flat-bottom 96-well plates coated with 0.5 μg of purified H. pylori urease blocked with nonfat milk. Saliva (100 μl) was treated similarly but at a 1:5 dilution only. Goat anti-rhesus immunoglobulin G-horseradish peroxidase (IgG-HRP) conjugate or anti-rhesus IgA-HRP conjugate (Nordic Immunological Laboratories, Capistrano Beach, Calif.) were used as secondary antibodies. After incubation of plates with substrate (ABTS [N-(2-acetamido)-2-aminomethanesulfonic acid] plus H2O2 in citrate phosphate buffer) for 20 min at room temperature, the absorbance at 550 nm was determined. Results for salivary IgA were expressed as OD550; results for serum IgG were expressed as the reciprocal of the lowest titer to exceed an arbitrary threshold of 0.10 absorbance unit. All samples were run in duplicate.
Molecular fingerprinting of H. pylori.
Restriction fragment length polymorphism (RFLP) analysis was used to confirm that H. pylori recovered from vaccinated monkeys was identical to the inoculated strain and different from strains that are enzootic at the primate center. Chromosomal H. pylori DNA was prepared from bacteria grown on brucella agar with 5% bovine serum using a method previously described (2). DNA (1 μg) was digested with an excess of HaeIII (New England BioLabs, Beverly, Mass.) for 3 h at 37°C and electrophoresed in a 0.7% agarose gel (Gibco-BRL) with 1× Tris-borate buffer at 55 V for 16 h. The gel was stained with ethidium bromide and photographed under UV light.
RESULTS
Antibody response to immunization.
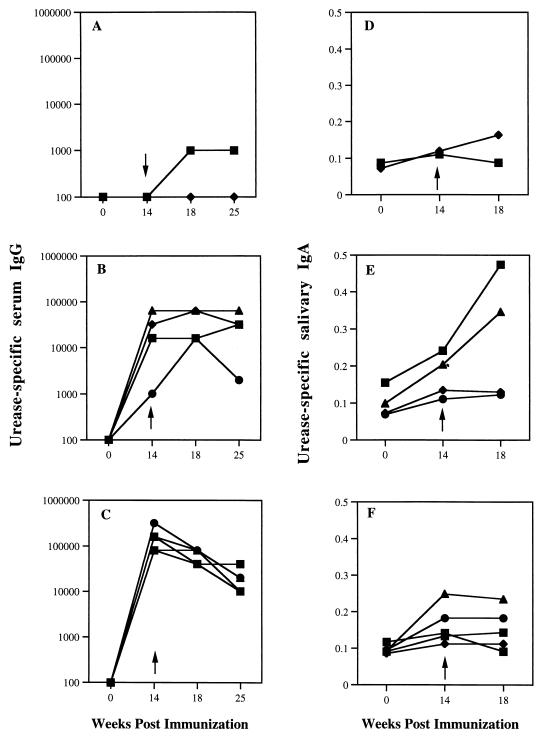
Immunization with oral and especially parenteral urease produced a marked rise in urease-specific serum IgG, which was apparent at week 14 when monkeys were challenged with H. pylori (Fig. 1). Serum IgG remained elevated at weeks 18 and 25, when monkeys underwent endoscopy and necropsy, respectively, though titers declined somewhat in monkeys that received parenteral vaccine. Immunization produced only modest elevations in urease-specific salivary IgA. As expected, control monkeys showed no immune response to sham immunization.
FIG. 1.
Urease-specific serum IgG (A, B, and C) and salivary IgA (D, E, and F) for monkeys which received sham immunization (A and D), oral immunization with urease plus E. coli LT (B and E), or intramuscular urease plus Bay (C and F). Results are shown for samples obtained before immunization (0 week), 2 weeks following immunization (14 weeks), just prior to challenge (arrow), and at 4 and 11 weeks (IgG only) after challenge (18 and 25 weeks, respectively). Serum IgG is expressed as the reciprocal of the lowest titer to exceed a threshold of 0.10 absorbance unit. Salivary IgA is expressed as A550.
Protection from H. pylori challenge.
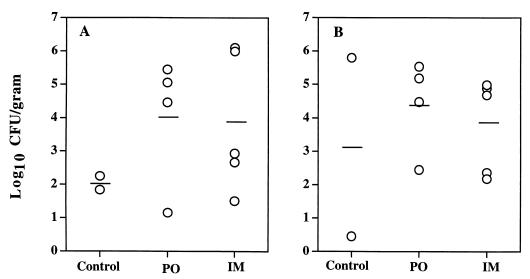
H. pylori was cultivated from gastric tissue in all control and vaccinated monkeys, both at endoscopy 4 weeks after challenge (week 18) and at necropsy 11 weeks after challenge (week 25). In order to determine if there were quantitative differences in H. pylori colonization among the three groups, the mean log10 CFU per gram of gastric tissue was calculated for each monkey. Data were averaged over the six endoscopic biopsies obtained 4 weeks after challenge (Fig. 2A) and over the 11 samples (10 punch biopsies and the gastric scraping) obtained at necropsy 11 weeks after challenge (Fig. 2B). There was considerable variability in bacterial density, which in most animals ranged between 102 and 106 CFU/g of tissue. No quantitative differences in H. pylori colony counts were apparent between the control and either vaccination group.
FIG. 2.
Mean log10 CFU/gram of tissue obtained at endoscopy 4 weeks after challenge (A) and at necropsy 11 weeks after challenge (B) with H. pylori. Results for each animal are averaged for six samples at endoscopy and for 11 samples at necropsy. Means for animals that received sham vaccination (control), oral urease (PO), and parenteral urease vaccination (IM) are shown as bars.
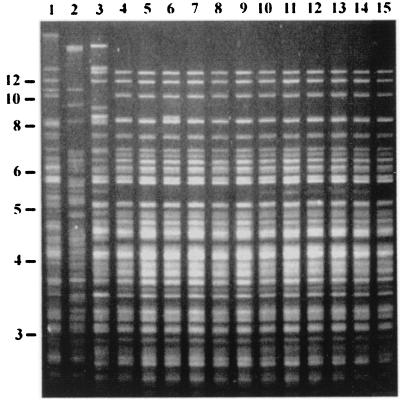
In order to confirm that the recovered H. pylori was strain N246C3 and not a strain enzootic at the primate center, we examined by RFLP a strain isolated from each of the 11 experimental animals and three representative strains isolated from naturally infected monkeys at the primate center. The fingerprint of H. pylori recovered from each monkey matched that of strain N246C3 and was easily distinguished from representative strains found in naturally infected monkeys (Fig. 3).
FIG. 3.
RFLP analysis of H. pylori DNA digested with HaeIII. Samples are from strains isolated from naturally infected monkeys at the primate center (lanes 1 to 3), strain N246C3 used as the inoculum in the present experiment (lane 4), and strains isolated from each of the 11 experimental animals (lanes 5 to 15). A size ladder in kilobases is shown at left.
Topography of H. pylori infection.
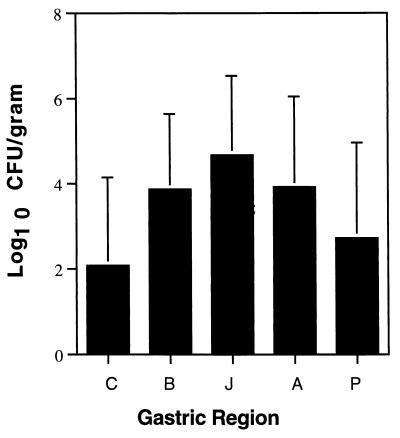
In order to examine topographical differences in H. pylori density, we calculated the log10 CFU per gram of tissue obtained at necropsy from each region of the stomach (Fig. 4). Data for the cardia and pylorus were based on a single sample, while the means of multiple samples were calculated for the body (2), transitional zone or junction (3), and antrum (3). Since animals in all groups were infected and there were no apparent topographical differences among groups, the means were computed for all 11 monkeys combined. H. pylori density was consistently lowest in the cardia and pylorus and was most often greatest in the junction (transitional zone).
FIG. 4.
Mean H. pylori log10 CFU/gram of gastric tissue in the cardia (C), body (B), junction (J), antrum (A), and pylorus (P) averaged over all 11 monkeys. The standard deviation is indicated above each bar.
Histopathology.
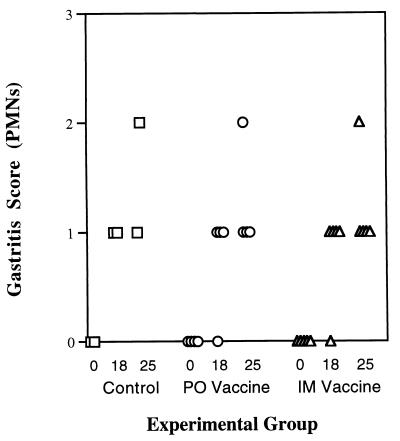
Gastritis was evaluated before immunization and at 4 and 11 weeks after H. pylori challenge. At 4 weeks after H. pylori challenge (week 18), all monkeys developed an increase in polymorphonuclear leukocytes that tended to increase at 11 weeks (week 25) after challenge (Fig. 5). Similar results were seen in other regions of the stomach and for chronic (mononuclear) inflammation (results not shown). “H. heilmannii” was not seen in any monkeys prior to vaccination but was found in two animals at 4 weeks after challenge (one oral and one parenteral immunization) and in six animals at 11 weeks after challenge (one control, two oral, and three parenteral immunizations).
FIG. 5.
Gastritis score for acute (polymorphonuclear leukocytes) inflammation on a scale of 0 (none), 1 (mild), 2 (moderate), or 3 (severe) as described in the Sydney System (30). Data are shown for each animal in the control, oral vaccine (PO), and parenteral vaccine (IM) groups before immunization (0 week), 4 weeks after challenge (18 weeks), and 11 weeks after challenge (25 weeks).
DISCUSSION
Development of the rhesus monkey model of H. pylori provides an opportunity to study vaccine efficacy in a naturally infected host that is closely related to humans. Using procedures we developed to derive SPF rhesus monkeys, we examined for the first time the effectiveness of an H. pylori vaccine in monkeys that were documented to have no prior H. pylori infection. The results showed that immunization with recombinant urease, formulated with E. coli LT for oral delivery or a synthetic glycolipid for intramuscular delivery, does not prevent infection after experimental challenge with rhesus monkey-derived H. pylori. These results are consistent with two previous studies in rhesus monkeys which showed that immunization with four different urease and adjuvant preparations, including oral urease plus LT (24) and intramuscular urease plus Bay (23), prevented infection in only 1 of 19 animals challenged with H. pylori.
Although previous studies in rhesus monkeys did not demonstrate complete protection, they suggested that oral urease-LT alone or followed by a urease parenteral boost could produce 1- to 2-log reductions in H. pylori colony counts compared with sham-vaccinated controls (23, 24). Recent studies in gnotobiotic piglets (10) and in mice (20) have also found that when careful quantitative cultures are performed, the effect of vaccination may be quantitative rather than complete protection. We failed to find quantitative differences in H. pylori colonization between vaccinated and control animals, perhaps because of small sample size and variability in quantitative cultures. However, in unpublished studies we found that in five treated monkeys experimentally infected with H. pylori N246C3, the mean (range) log10 CFU/g at 2 and 10 weeks postinoculation (similar time intervals to those in the present study) were 4.3 (0 to 6.1) and 3.6 (0 to 7.5), respectively (one of five animals was uninfected). These results are similar to those we found in vaccinated animals (Fig. 2). Others have also failed to demonstrate quantitative differences in natural H. pylori colonization of rhesus monkeys after urease vaccination (8). Future studies will be required to determine whether immunization can consistently reduce H. pylori density and whether such a reduction is clinically important in the reduction of clinical endpoints such as peptic ulcer disease and gastric cancer. Studies on the latter point have produced mixed results (1, 17, 19). However, since the clinical sequelae of H. pylori infection are presumed to result from chronic inflammation, a quantitative reduction in H. pylori density might be expected to be clinically significant.
A recent study examined the effects of oral vaccination with urease plus LT on naturally acquired H. pylori infection in 9-month-old rhesus monkeys (8). The results showed that 69% of vaccinated animals were H. pylori infected 10 months after vaccination compared to 93% of controls. Strengths of this study were that the follow-up was prolonged and that natural infection may provide a more realistic estimate of vaccine efficacy than experimental challenge with a large inoculum. But the absence of H. pylori prior to vaccination was determined only by serology, which may be falsely negative during the period of rapid seroconversion which occurs in young rhesus monkeys during the first few years of life (7, 33). Therefore, the authors used seroprevalence data to estimate the actual prevalence of infection among vaccinated and control animals at the time of vaccination and then used these data to calculate that the vaccine was 69% effective. However, since the animals did not undergo endoscopy prior to immunization, the actual effect of the vaccine could not be determined.
Infection with H. pylori developed in all vaccinated animals despite a significant induction of humoral immunity. Immunization induced a marked increase in urease-specific serum IgG, particularly when administered parenterally, but only a modest increase in salivary IgA (Fig. 1). Similar results have been reported previously in rhesus monkeys vaccinated with H. pylori urease (8, 24). These results indicate that although the vaccine preparations were immunogenic, they were not sufficient to elicit protection. Recent results in B-cell knockout mice suggest that in the mouse model, protection from H. pylori challenge can occur in the absence of an antibody response (11).
Histologic examination showed that colonization with H. pylori in all monkeys was often greatest at the junction or the transitional zone between the gastric antrum and body and least at the cardia and pylorus (Fig. 4). Similar results have been obtained in rhesus monkeys (24) and in mice (11). This finding emphasizes the recent proposal that the transitional zone is a unique environment in the stomach where local acid production may determine whether H. pylori is restricted distally to the antrum, where it is more likely to cause duodenal ulcer, or moves proximally into the body where it may lead to atrophy, metaplasia, and adenocarcinoma (32). It was surprising that “H. heilmannii” was observed in some monkeys following H. pylori challenge, despite the fact that two prior endoscopic examinations failed to show it. Since “H. heilmannii” was seen much more frequently at necropsy than at endoscopy, this may mean that low-grade infections were present in some animals despite the fact that they were isolated within 24 h of birth.
In conclusion, we have examined the effect of immunization with oral and parenteral urease against challenge with H. pylori in SPF rhesus monkeys. The results show that although the vaccine is immunogenic, it does not prevent infection. While we did not observe a reduction in colonization density in vaccinated animals compared to controls, the possibility that vaccination produces a reduction in the level of colonization cannot be excluded due to the small sample size. An effective vaccine to prevent H. pylori infection will probably require different or more likely additional antigens, as well as improvements in stimulation of the host immune response.
ACKNOWLEDGMENTS
This work was supported by the Pasteur Merieux Connaught-OraVax joint venture on H. pylori vaccine development, venture funds from the California Regional Primate Research Center (NIH RR00169), and Public Health Service grant AI-42081 from the National Institute of Allergy and Infectious Diseases.
We thank Cynthia Lee, Tom Monath, and Richard Weltzin for assistance with study design and methodology.
REFERENCES
- 1.Atherton J C, Tham K T, Peek R M, Jr, Cover T L, Blaser M J. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J Infect Dis. 1996;174:552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1990. [Google Scholar]
- 3.Corthésy-Theulaz I E, Hopkins S, Bachmann D, Saldinger P F, Porta N, Haas R, Zheng-Xin Y, Meyer T, Bouzourène H, Blum A L, Kraehenbuhl J P. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun. 1998;66:581–586. doi: 10.1128/iai.66.2.581-586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czinn S J, Cai A, Nedrud J G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 5.Drazek E S, Dubois A, Holmes R K. Characterization and presumptive identification of Helicobacter pylori isolates from rhesus monkeys. J Clin Microbiol. 1994;32:1799–1804. doi: 10.1128/jcm.32.7.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois A, Fiala N, Heman-Ackah L M, Drazek E S, Tarnawski A, Fishbein W N, Perez-Perez G I, Blaser M J. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology. 1994;106:1405–1417. doi: 10.1016/0016-5085(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 7.Dubois A, Fiala N, Weichbrod R H, Ward G S, Nix M, Mehlman P, Taub D M, Perez-Perez G I, Blaser M J. Seroepizootiology of Helicobacter pylori gastric infection in nonhuman primates housed in social environments. J Clin Microbiol. 1995;33:1492–1495. doi: 10.1128/jcm.33.6.1492-1495.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois A, Lee C K, Fiala N, Kleanthous H, Mehlman P T, Monath T. Immunization against natural Helicobacter pylori infection in nonhuman primates. Infect Immun. 1998;66:4340–4346. doi: 10.1128/iai.66.9.4340-4346.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton K A, Ringler S S, Krakowka S. Vaccination of gnotobiotic piglets against Helicobacter pylori. J Infect Dis. 1998;178:1399–1405. doi: 10.1086/314463. [DOI] [PubMed] [Google Scholar]
- 11.Ermak T H, Giannasca P J, Nichols R, Myers G A, Nedrud J, Weltzin R, Lee C K, Kleanthous H, Monath T P. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrero R L, Thiberge J-M, Huerre M, Labigne A. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrero R L, Thiberge J-M, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gómez-Duarte O G, Lucas B, Yan Z X, Panthel K, Haas R, Meyer T F. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine. 1998;16:460–471. doi: 10.1016/s0264-410x(97)00247-8. [DOI] [PubMed] [Google Scholar]
- 15.Goto T, Nishizono A, Fujioka T, Ikewaki J, Mifune K, Nasu M. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect Immun. 1999;67:2531–2539. doi: 10.1128/iai.67.5.2531-2539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham D Y, de Boer W A, Tytgat G N J. Choosing the best anti-Helicobacter pylori therapy: effect of antimicrobial resistance. Am J Gastroenterol. 1996;91:1072–1076. [PubMed] [Google Scholar]
- 17.Gunn M C, Stephens J C, Stewart J A, Rathbone B J, West K P. The significance of cagA and vacA subtypes of Helicobacter pylori in the pathogenesis of inflammation and peptic ulceration. J Clin Pathol. 1998;51:761–764. doi: 10.1136/jcp.51.10.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy B, Hessler C, Fourage S, Haensler J, Vialon-Lafay E, Rokbi B, Millet M J. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850–856. doi: 10.1016/s0264-410x(97)00258-2. [DOI] [PubMed] [Google Scholar]
- 19.Khulusi S, Mendall M A, Patel P, Levy J, Badve S, Northfield T C. Helicobacter pylori infection density and gastric inflammation in duodenal ulcer and non-ulcer subjects. Gut. 1995;37:319–324. doi: 10.1136/gut.37.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleanthous H, Myers G A, Georgakopoulos K M, Tibbitts T J, Ingrassia J W, Gray H L, Ding R, Zhang Z Z, Lei W, Nichols R, Lee C K, Ermak T H, Monath T P. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect Immun. 1998;66:2879–2886. doi: 10.1128/iai.66.6.2879-2886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991;173:1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C K, Soike K, Giannasca P, Hill J, Weltzin R, Kleanthous H, Blanchard J, Monath T P. Immunization of rhesus monkeys with a mucosal prime, parenteral boost strategy protects against infection with Helicobacter pylori. Vaccine. 1999;17:3072–3082. doi: 10.1016/s0264-410x(99)00144-9. [DOI] [PubMed] [Google Scholar]
- 24.Lee C K, Soike K, Hill J, Georgakopoulos K, Tibbitts T, Ingrassia J, Gray H, Boden J, Kleanthous H, Giannasca P, Ermak T, Weltzin R, Blanchard J, Monath T P. Immunization with recombinant Helicobacter pylori urease decreases colonization levels following experimental infection of rhesus monkeys. Vaccine. 1999;17:1493–1505. doi: 10.1016/s0264-410x(98)00365-x. [DOI] [PubMed] [Google Scholar]
- 25.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 27.Michetti P, Corthésy-Theulaz I, Davin C, Haas R, Vaney A C, Heitz M, Bille J, Kraehenbuhl J P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 28.Norris C R, Marks S L, Eaton K A, Torabian S Z, Munn R J, Solnick J V. Healthy cats are commonly colonized with “Helicobacter heilmannii” that is associated with minimal gastritis. J. Clin. Microbiol. . 1999;37:189–194. doi: 10.1128/jcm.37.1.189-194.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappo J, Thomas W D, Jr, Kabok Z, Taylor N S, Murphy J C, Fox J G. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246–1252. doi: 10.1128/iai.63.4.1246-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price A B. The Sydney system: histological division. J Gastroenterol Hepatol. 1991;6:209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 31.Radcliff F J, Hazell S L, Kolesnikow T, Doidge C, Lee A. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect Immun. 1997;65:4668–4674. doi: 10.1128/iai.65.11.4668-4674.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sander J O, Veldhuyzen van Zanten S J, Dixon M F, Lee A. The gastric transitional zones: neglected links between gastroduodenal pathology and Helicobacter ecology. Gastroenterology. 1999;116:1217–1229. doi: 10.1016/s0016-5085(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 33.Solnick J V, Canfield D R, Yang S, Parsonnet J. The rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific pathogen free monkeys. Lab Anim Sci. 1999;49:197–201. [PubMed] [Google Scholar]
- 34.Solnick J V, O'Rourke J, Lee A, Paster B, Dewhirst F E, Tompkins L S. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993;168:379–385. doi: 10.1093/infdis/168.2.379. [DOI] [PubMed] [Google Scholar]