Abstract
The coronavirus disease 2019 pandemic led to some of the most drastic changes in clinical care delivery ever seen in the United States. Almost overnight, providers of prenatal care adopted virtual visits and reduced visit schedules. These changes stood in stark contrast to the 12 to 14 in-person prenatal visit schedule that had been previously recommended for almost a century. As maternity care providers consider what prenatal care delivery changes we should maintain following the acute pandemic, we may gain insight from understanding the evolution of prenatal care delivery guidelines. In this paper, we start by sketching out the relatively unstructured beginnings of prenatal care in the 19th century. Most medical care fell within the domain of laypeople, and childbirth was a central feature of female domestic culture. We explore how early discoveries about “toxemia” created the groundwork for future prenatal care interventions, including screening of urine and blood pressure—which in turn created a need for routine prenatal care visits. We then discuss the organization of the medical profession, including the field of obstetrics and gynecology. In the early 20th century, new data increasingly revealed high rates of both infant and maternal mortalities, leading to a greater emphasis on prenatal care. These discoveries culminated in the first codification of a prenatal visit schedule in 1930 by the Children’s Bureau. Surprisingly, this schedule remained essentially unchanged for almost a century. Through the founding of the American College of Obstetricians and Gynecologists, significant technological advancements in laboratory testing and ultrasonography, and calls of the National Institutes of Health Task Force for changes in prenatal care delivery in 1989, prenatal care recommendations continued to be the same as they had been in 1930—monthly visits until 28 weeks’ gestation, bimonthly visits until 36 weeks’ gestation, and weekly visits until delivery. However, coronavirus disease 2019 forced us to change, to reconsider both the need for in-person visits and frequency of visits. Currently, as we transition from the acute pandemic, we should consider how to use what we have learned in this unprecedented time to shape future prenatal care. Lessons from a century of prenatal care provide valuable insights to inform the next generation of prenatal care delivery.
Key words: alloimmunization, antenatal care, blood pressure, care delivery, coronavirus disease 2019 pandemic, electronic fetal monitoring, history, preeclampsia, prenatal care, proteinuria, reduced visit schedules, telemedicine, ultrasonography
Introduction
As a chief resident working in an obstetrics clinic serving predominantly low-income women, one of the authors (A.F.P.) noticed a curious pattern in her pregnant patients’ prenatal appointment attendance. Patients would routinely present for their first appointment. They never missed their anatomy scan—the ultrasound where they could (among other things) learn the gender of the baby. They would return between 24 and 28 weeks’ gestation to confirm that their blood count was adequate and they had no signs of gestational diabetes. Subsequently, many would disappear until a few weeks before delivery.
When asked about their absence, patients described the choices they were being forced to make between recommended care and the demands of everyday life: “I couldn’t get a ride”; “I can’t miss work, I gotta put food on the table”; and “I couldn’t get my babies across town.” The reasons were always followed with the reassurance, “but I knew everything was fine.” Women would also express frustration about the frequency and brevity of the appointments (typically no more than 10 minutes) and the lack of fulfillment from their visits. As she thought more critically about the prenatal visit schedule—monthly visits until 28 weeks’ gestation, biweekly visits until 36 weeks’ gestation, and weekly visits until delivery—A.F.P started to question the status quo.
She was surprised to find that the current prenatal care schedule had first been recommended in 1930 (without supporting evidence) and had remained unchanged through the current recommendations published in the “Guidelines for perinatal practice, 8th edition,” in 2017. She learned that the United States has maintained this same one-size-fits-none prenatal care delivery guideline despite drastic changes in technology and population health, evidence to support alternative prenatal care delivery, persistently worse maternity outcomes, and deepening health disparities.1 Moreover, she wondered why.
The coronavirus disease 2019 (COVID-19) pandemic forced us to reconsider prenatal care delivery guidelines in the United States, both to reduce viral exposure during clinic visits and to conserve scarce healthcare resources. As maternity care providers consider whether we should maintain changes, such as reduced visit schedules and telemedicine, understanding prenatal care delivery guidelines over time can provide important insights. Thus, we describe the surprising evolution of prenatal care delivery guidelines over the span of 3 centuries to inform the next generation of prenatal care delivery.
Pregnancy Care in the Early Republic
During the 19th century, medical care in the United States was relatively unstructured. The absence of state licensing laws meant that anyone could claim to be a physician (and many did).2 Moreover, the predominantly rural landscape and difficulty of transportation meant that healthcare advice was often delivered by laypeople, frequently relying on widely read texts that offered advice on all sorts of medical matters, including prenatal care. If one looked, for example, to “Gunn’s domestic medicine,”3 the most important advice for pregnant women was to keep the bowels regular. Tepid baths were also recommended. Another extremely popular text, William Buchan’s “Domestic medicine,”4 suggested that bleeding—a common remedy at the time thought to correct bodily imbalances or remove inflammation through intentional blood loss—be utilized for pregnant women suffering from dependent edema or jaundice.5 Regular visits to a professional provider were neither recommended nor likely to be an option. Most prenatal care remained in the domain of other women in the family who shared an expertise through apprenticeship and lore and for whom the shared experience of pregnancy and childbirth was a central feature of domestic culture.6
By the mid-1800s, European physicians arrived at several insights about what has come to be known as preeclampsia or eclampsia (“toxemia”), which laid the groundwork for future prenatal interventions.7 , 8 It had been recognized since ancient times that pregnancy could be accompanied by headaches and convulsions, and some speculated that the seizures of pregnant women were caused by the uterus. However, it was not always easy to differentiate between convulsions owing to epilepsy and those caused by pregnancy.9 Because of the discovery of new methods for studying components of the blood, the idea that many diseases—including convulsions in pregnancy—were associated with circulating toxins led to the term “toxemia.”10 The association of convulsions in pregnant women with proteinuria was established in 1843 by John Lever,7 working at Guy’s Hospital in London. When Lever decided to examine the urine of every pregnant woman that he saw with convulsions, he found albumen in the urine of all but 1 woman. He suggested that in such cases, rapid delivery was the best course of action. Because he did not continue to find albumen in the urine of these women after delivery, he concluded that the fundamental cause of the convulsions was not an intrinsic disease of the kidney, but was related to pregnancy.11 At about the same time, some physicians noted a hard bounding pulse in pregnant women having convulsions but lacked the technology necessary to measure blood pressure. However, in 1896, the Italian physician Riva-Rocci invented a sphygmomanometer that could easily measure blood pressure, and soon after that, physicians started to assert that hypertension might be an early marker of eclampsia.8 By associating eclampsia with diagnostic findings, such as proteinuria and hypertension, physicians could start to see a rationale for routine examination of pregnant women who were asymptomatic.
During this time, the US medical profession was becoming more organized. States passed licensing laws. The American Medical Association (AMA) was founded in 1846, followed by the formation of specialty groups, including the obstetrical societies in New York and Philadelphia.12 The American Gynecologic Society and American Association of Obstetricians and Gynecologists were founded in 1876 and 1888, respectively, with similar goals of promoting high-quality practice, education, and research.13 Although the societies’ names suggested a national presence, these organizations actually admitted relatively few members—most of whom lived on the East Coast, thus limiting their influence on obstetrical practice.14 However, the formation of these societies created a foundation for further specialization of the field of obstetrics, which would become the dominant platform for prenatal care delivery.
The Early 1900s
Around the turn of the 20th century, some physicians started to advocate for routine prenatal care as a method to reduce maternal and infant mortalities. In 1901, the Scottish practitioner John William Ballantyne pleaded for “promaternity wards”—not only to provide care for women with complications but also to study maternal and neonatal diseases in pregnancy. Later that year, he received funding from the Edinburgh Obstetric Society for the first antenatal bed in the Royal Maternity Hospital; eventually, this promaternity ward grew to over 23 beds.15 , 16 Ballantyne studied pregnancy using the latest technology of the day—in this case, X-rays and pulse measurement. He also emphasized the value of having patients seen by specialized practitioners who were skilled in obstetrical practice rather than general practitioners. However, this single hospital ward could obviously help only a limited number of women.
Ballantyne’s message was amplified in the United States through the American Journal of Obstetrics in 1901.17, 18, 19 Prominent leaders, such as Johns Hopkins obstetrician John Whitridge Williams, recognized the precariousness of health in pregnancy, stating “it is apparent that the border-line between health and disease is less clearly marked during gestation, and derangements… may readily give risk to pathological conditions which seriously threaten the life of mother, child, or both.”20
One of the first broad-based attempts at intervention came in 1901 in Boston, when public health nurses from the District Nursing Association began trying to reduce infant mortality by conducting home prenatal care visits with the Boston Lying-in Hospital for childbirth.21 , 22 New York City public health nurses followed in 1907.23 National attention became increasingly focused on high infant mortality rates. In 1909, the White House held a Conference on the Care of Dependent Children.24 Hoping that a new federal agency could improve children’s health throughout the country, US President William Howard Taft formed the Federal Children’s Bureau in 1912.25
A year later, in 1913, the Children’s Bureau released a slim booklet offering advice on prenatal care. It provided information on common symptoms and complications of pregnancy, preparation for childbirth, and hints for a smooth postpartum recovery. Of note, women were encouraged to consult with the doctor from the beginning of pregnancy, although the booklet noted that “he [sic] may have very little to do beyond giving advice and making the routine examinations of the urine [for protein].”26 The booklet did not offer advice on how often pregnant women needed to see their physician.
Infant deaths increasingly came to the attention of the medical profession. In his 1914 presidential address to the American Association for Study and Prevention of Infant Mortality, John Whitridge Williams presented a massive study of 10,000 consecutive admissions of pregnant women, with 705 fetal deaths. He concluded that 40% of infant deaths could be prevented with prenatal care. Williams outlined the ideal prenatal care plan: all women would present for an early prenatal visit and receive a full physical examination and Wassermann test (for syphilis). He suggested that a nurse visit every woman in her home to assess her “social situation” and that women return 1 month before delivery to assess for proper delivery location (home vs hospital). Of note, the author stressed the much worse outcomes for African American mothers.27 Williams was one of the most influential obstetricians of his time. He was the founding author of the dominant reference text, “Williams obstetrics,” which went through 17 editions from 1903 to 1985. Williams’ work not only raised concerns about prenatal deaths but also offered a systematic approach to improving outcomes through prenatal care.28
To systematically keep track of births throughout the country, the Census created the national birth-registration area in 1915, which provided national data to study the connection between prenatal care and infant and maternal deaths.29 If such data could demonstrate a connection between increasing care and better outcomes, it offered a means to improve health and an opportunity for physicians to strengthen their own position in the marketplace: “As the knowledge grows that the attendance of pregnancy and the guarding of young infant life are a great and important scientific function, the market will be created for good obstetric care.”30 Thus, prenatal care became not only a preventive service but also a reason for routine physician services.31
Women’s groups were becoming a political force in the Progressive Era. They worked to pass the 19th Amendment to the US Constitution in 1919, which enfranchised women. They went on to push for passage of the Sheppard-Towner Bill in 1921, which provided federal funding for 2987 prenatal care centers and public health nurses and community distribution of educational materials.32 However, funding was discontinued in 1929 following lobbying by the AMA that this was a “step toward socialized medicine.”22
Perhaps in response to the growing awareness of prenatal care’s ability to influence both infant and maternal outcomes, the Children’s Bureau published a new booklet on prenatal care in 1930. Unlike earlier publications, this guideline detailed a specific schedule for prenatal physician visits: monthly visits until 28 weeks’ gestation, biweekly visits until 36 weeks’ gestation, and weekly visits until delivery. In other words, depending on precisely how early a pregnancy was diagnosed, this was a recommendation for 12 to 14 visits during pregnancy. The booklet did not reference any evidence supporting the recommended visit schedule, nor did it specify how or if the schedule should be modified for patients with additional risk factors. The number of recommended visits remained remarkably unchanged over the years.
Subsequent editions of the booklet did reflect changing ideas and knowledge. For example, in 1942, updated booklets added recommendations for a public health or private nurse to help patients achieve recommended care.33 In 1949, the revised booklet acknowledged the role of the father in the pregnancy and birth process and the importance of social and emotional health.34 By the 1962 revision, mothers were admonished to seek a doctor with training and experience in delivering prenatal care, such as a specialist obstetrician. However, despite the many changes occurring in medical practice, new editions of the booklet continued to recommend the same schedule of 12 to 14 prenatal visits.35
During this period, prenatal care was not the only obstetrical service increasingly delivered by physicians. Birth moved from the home to the hospital as physicians continued to campaign for the medicalization of childbirth. In addition, the increasingly popular method of “twilight sleep delivery” (use of anesthetics during delivery to allow women to experience a pain-free childbirth) required physician supervision in a hospital setting, further medicalizing birth and prenatal care.36, 37, 38 By 1938, only about half of all births remained in women’s homes.39
Midcentury
Systematizing birth within the hospital supported the argument that births ought to be attended by specialists in obstetrical care—an argument consistent with a general trend toward the importance of specialization within medicine. Specialization came to be marked by certificates that were provided by private organizations (specialty boards), and in 1930, the incorporation of the American Board of Obstetricians and Gynecologists provided a formal mechanism by which physicians could legitimately claim particular expertise in caring for pregnant women.14 , 40
In 1951, the American Academy of Obstetrics and Gynecology (AAOG) was formed to serve the “average obstetrician gynecologist” by promoting high standards of practice, education, and research; promoting positive relationships with the public; and contributing to the scientific literature. In 1957, the name was changed to the American College of Obstetricians and Gynecologists (ACOG).12 Around the same time, Certified Nurse Midwifery became increasingly organized with the founding of the American College of Nurse Midwives in 1955.39
In 1959, the ACOG released their first “Manual of standards in obstetric-gynecologic practice” intended for a wide audience.41 The authors stressed that clinical practice was rapidly developing and that changes in their recommendations were to be expected. They upheld many of the recommendations of the previous Children’s Bureau pamphlets. A section went over fees and suggested a single bill that would include any needed operative procedures. The section on lay education did not contemplate any parental pairing other than the traditional husband and wife. In a nod to how care may have changed since previous generations, a separate section on discussion with the patient’s “mother and mother in law” suggested that the physician point out “differences in modern practice.” However, most significant for this paper, the ACOG saw no reason to reconsider the same 12 to 14 visit schedules that had first been articulated some 3 decades ago or to provide additional specifications for patients with varying levels of medical or social risk.
Just as earlier technological discoveries, such as the X-ray machine and the sphygmomanometer, had been used to improve prenatal care, the next few decades saw the introduction of several more technological innovations. The 1959 guidelines emphasized Rh testing, and the first clinical trial documenting the efficacy of Rh immunoglobulin for preventing alloimmunization was published in 1968.42 In the 1970s, radioimmunoassay detection of human chorionic growth hormone laid the foundation for earlier discovery of pregnancy and home pregnancy tests,43 whereas use of ultrasound and electronic fetal heart monitoring became routine in the late 1970s.44, 45, 46, 47 Genetic screening through amniocentesis and alpha fetal protein was introduced in the 1970s, with widespread adoption by the 1980s—predominantly for high-risk populations, including women of advanced maternal age—giving pregnant patients access to earlier diagnosis of genetic disorders and congenital anomalies.48 , 49 Additional changes included the first use of the Kessner Index (a composite measure of the timing of prenatal care initiation and total visit number completed) to assess the adequacy of prenatal care in 1970.50 Simultaneously, increasing ability of digital access to data enabled a detailed analysis of the impact of low birthweight as one of many racial disparities in the United States.51, 52, 53
In 1980, the US Surgeon General declared that a major national health objective was reduction of low birthweight infants.54 In 1982, the Institute of Medicine convened the Committee to Study the Prevention of Low Birthweight to investigate the most promising strategies for improving infant outcomes. Findings were published in 1985. The committee concluded that evidence supported the causal relationship between prenatal care and reduction of infants with low birthweight,53 , 55 estimating that $3.38 could be saved for every preventive dollar spent on prenatal care. Following the conference, several federal and state initiatives attempted to improve prenatal care access—particularly for low-income women—through Medicaid expansion and increased funding for prenatal care programs.56
The committee also called for a revision of prenatal care to “encourage the provision of improved, more flexible prenatal care services,” including use of medical and social assessments to determine appropriate care.53 Therefore, the Department of Health and Human Services commissioned the Public Health Service Expert Panel on the Content of Prenatal Care in 1989 to review the “effectiveness and efficiency of current prenatal care.”57 , 58 As they reviewed existing evidence, it became clear, as the panel’s chair concluded, that “the amazing and humbling message... was how little we knew.” Although data were insufficient to guide recommendations for a specific frequency of prenatal appointments, the committee felt comfortable recommending a flexible schedule of prenatal visits based on patients’ medical and social risk factors. Their proposed schedule included 7 visits for low-risk multiparous patients and 9 visits for low-risk nulliparous patients, with additional visits added as needed for high-risk patients based on medical and social risk factors. Interestingly, they suggested a phone visit for multiparous patients at 10 weeks’ gestation, perhaps a first step toward what we now see as telemedicine for prenatal care. In addition, the document advocated for preconception care, postpartum care extending through the first year after delivery, and a variety of social and mental health services designed to support the pregnant patient. The director of the National Institute of Child Health and Human Development, Dr Duane Alexander, anticipated controversy surrounding the new guidelines for the number of visits for low-risk patients, foreshadowing to 1 reporter “these changes will be fought by a lot of people.”59
As Alexander anticipated, this high-profile advice to cut down on prenatal visits attracted quick attention from the national media, including a front page article in the New York Times. It also drew attention from leading obstetrics and gynecology physicians.59 In 1990, the ACOG Executive Committee discussed the new recommendations. Even though ACOG members had been involved in the panel, the committee found the rationale for some changes to be unconvincing, reporting the panel’s “objectives were very broad and not always supported by data.” Perhaps unsurprisingly, they focused on the new visit schedule. Although existing historic data do not allow a detailed analysis of the committee’s discussions, they did note that “the data recommends reducing the number of prenatal visits for low-risk women on the assumption that this will produce more resources for those at risk of delivering prematurely. However, the organization of healthcare delivery services does not make such a direct transfer of resources possible” (October 1990 Executive Committee minutes, retrieved from the ACOG archives). Thus, the committee doubted (and was probably correct) whether saving money on fewer visits for low-risk patients would lead to more money for high-risk patients. To match prenatal services to patients’ needs, the new recommendations were apparently rejected for insufficient evidence. Of note, rejecting these recommendations implied maintaining an existing visit structure that was also not evidence based. In a 1991 commentary, 3 prominent ACOG members publicly questioned the new advice on visit timing, noting concern that lay press coverage might lead pregnant women to make fewer visits to their obstetrician.58 However, for low-risk women, that was, of course, precisely the point.
Although not mentioned in the brief comments recorded in the ACOG Committee minutes, payment incentives may have played a role in the deliberations. Most births in the 1980s and early 1990s were covered by commercial insurance60 and largely financed through a fee-for-service structure, which meant higher physician reimbursement for more prenatal visits.60, 61, 62 Although states implemented global fee structures for physician services within Medicaid as early as 1983, over 40% of private physicians refused to take patients with Medicaid.63 , 64 Therefore, at the time of the task force’s recommendations, physicians may have had significant financial motivation to maintain more intensive visit schedules. Although private payers may have had financial incentives to advocate for the new guidelines, they may have not pushed for changes because (1) prenatal care is relatively inexpensive, (2) reduced visit schedules were not widely supported by providers or specialty leadership, and (3) they wished to avoid covering other expanded services that the panel recommended, such as education and nutrition (Milton Kotelchuck, PhD, MPH, e-mail communication, September 27, 2020). It was not until managed care became more common later in the 1990s that global provider payments became ubiquitous, removing one of the incentives for more prenatal visits.65 It is also possible that patient and provider preferences drove resistance to the new visit schedule. Morton Lebow, an ACOG spokesperson, reflected that prenatal care was “based on experience, and that experience has been very good.”59
Some elements of the Public Health Service Expert Panel’s work were adopted by the ACOG in their guidelines, such as the emphasis on preconception visits, care tailoring, and psychosocial support.66 Simultaneously, the question as to how many visits were needed was studied more intensively. During the 1990s and early 2000s, clinical trials studied reduced visits for low-risk women and more intensive services—often known as “enhanced prenatal care”—for women at higher risk of preterm birth and low birthweight.67 , 68 A meta-analysis of more than 5000 patients from the United States and other high-income countries demonstrated equivalent maternal and neonatal outcomes when antenatal visits were reduced from 12 to 14 visits to 9 visits for low-risk patients.69 The World Health Organization has recommended an 8-visit schedule, with the use of women-held case notes, community-based interventions, and task-shifting components of prenatal care to community-based health workers to improve access and patient experience, particularly in low-resource settings.70 Although peer countries adopted reduced visit schedules for low-risk patients with no clear harmful effect, most major US maternity care organizations maintained the same visit schedule originally proposed in 1930.71 Attempting to reduce rates of preterm birth and low birthweight, public health researchers studied numerous other models of enhanced prenatal care, including increased case management, prenatal education, and better integration of social services.72 Most trials showed equivocal results, with large investments in prenatal care delivery not yielding significant changes in outcomes.73 , 74
Over the past decades, the United States has seen the introduction of still more innovative prenatal care delivery models. Group prenatal care, which includes enhanced education and relationship building, started in the 1990s and has recently enjoyed greater popularity.75 Some studies have documented improved patient outcomes, particularly for medically and socially complex patients.76 , 77 Starting in 2014, the University of Utah and the Mayo Clinic introduced new approaches to prenatal care, including virtual visits and leveraging nurse care managers. Preliminary evidence from these new telemedicine models demonstrates equivalent maternal and neonatal outcomes, high patient satisfaction, and even lower healthcare costs.78 , 79 However, further data are needed as results are from highly controlled trial settings; include largely homogenous, high-income patient populations; and are focused on low-risk patients. In recent years, significant innovation has been driven by the private sector, with startups, such as Babyscripts and Maven, offering consumers new, flexible ways to engage in prenatal care, through home monitoring, digital educational platforms, and telemedicine visits.80
However, despite all the new technologies that has been developed over the past century and despite all the new sciences and innovative ideas and techniques, the same 12 to 14 in-person prenatal visit schedule first advocated in 1930 has remained stubbornly and firmly in place until the COVID-19 pandemic.
The Coronavirus Disease 2019 Pandemic and Beyond
In March 2020, the world changed. Patients and providers became increasingly concerned about viral exposure in healthcare settings. In-person prenatal visits no longer seemed so benign.81 Practices across the country rapidly adopted reduced prenatal visit schedules, telemedicine, or hybrid care models. The ACOG endorsed the use of virtual care. In addition, for the first time since 1930, ACOG also endorsed reducing the number of prenatal visits.82 Early data on the feasibility and acceptability of both reduced visit schedules and telemedicine during this time are promising; however, data on outcomes, patient experience, and equity across diverse settings are still pending.81 , 83, 84, 85 The Table summarizes the key events that have shaped prenatal care delivery from the 1800s to today.
Table.
Key events in the evolution of prenatal care delivery guidelines
| Period | Key events in prenatal care delivery |
|---|---|
| Early 1800s | Prenatal care relatively unstructured and delivered by laypeople. |
| Mid-1800s | Recognition of association among blood pressure, proteinuria, and preeclampsia or eclampsia. |
| Late 1800s | Increasing organization of the medical profession. |
| 1901 | John Ballantyne (Edinburgh General Hospital) introduced “promaternity wards”; first home prenatal visits conducted by the Boston Lying-in Hospital. |
| 1909 | White House Conference on the Care of Dependent Children. |
| 1912 | The Children’s Bureau was formed. |
| 1913 | The Children’s Bureau released the first prenatal care booklet, recommending consultation with a physician early in pregnancy. |
| 1914 | John Whitridge Williams (Johns Hopkins Hospital) presented data suggesting that prenatal care can reduce infant mortality. |
| 1915 | The national birth-registration area was formed, providing national data on maternal and infant deaths. |
| 1921 | The Sheppard-Towner Bill was passed, providing federal funding for prenatal care. |
| 1930 | The Children’s Bureau released a second prenatal care booklet, with specific recommendations for physician visit schedule; the American Board of Obstetricians and Gynecologists first provides specialty certification. |
| 1951 | AAOG was formed. |
| 1955 | The ACNM was founded. |
| 1957 | The AAOG changed its name to the ACOG. |
| 1959 | The ACOG released the first “Manual of standards in obstetric-gynecologic practice,” which maintains the original prenatal visit schedule. |
| 1970 | The Kessner Index was introduced to assess the adequacy of prenatal care. |
| 1985 | Findings from the Institute of Medicine Committee to Study the Prevention of Low Birthweight were released, supporting the causal relationship between prenatal care and reduction of low birthweight infants. |
| 1989 | NIH Public Health Service Expert Panel on the Content of Prenatal Care that recommended a schedule of prenatal visits based on medical and social risk factors; Medicaid expansion occurred to improve prenatal care access. |
| 1990s | Clinical trials demonstrated the safety of reduced visit schedules for low-risk patients; group prenatal care was first introduced. |
| 2019 | The first RCT of the prenatal care model integrating telemedicine was published. |
| 2020 | Outbreak of COVID-19, which resulted in a pandemic. |
AAOG, American Academy of Obstetrics and Gynecology; ACNM, American College of Nurse Midwives; ACOG, American College of Obstetricians and Gynecologists; COVID-19, coronavirus disease 2019; NIH, National Institutes of Health; RCT, randomized clinical trial.
Peahl. Evolution of prenatal care guidelines. Am J Obstet Gynecol 2021.
What comes next? As we transition out of the acute pandemic into our “new normal,” what can be learned from a century of prenatal care history? First, we should continue to be humbled by how little we know about appropriate prenatal care delivery. Although we now know more about what services are important for improving maternal and neonatal outcomes, we still lack key information on how to deliver them, and how often. The right visit number, frequency, and modality—in person, telemedicine, group care, etc.—remain elusive. Similarly, we continue to struggle with how best to tailor services to patients’ medical and social needs. However, after a century, we seem to be ready to seriously reconsider the prenatal visit schedule originally proposed in 1930.
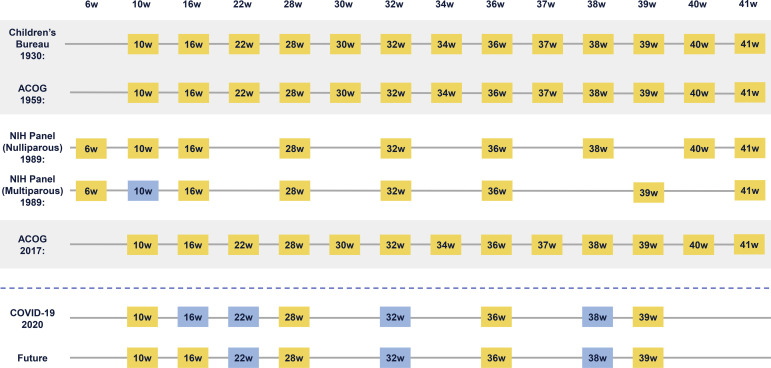
Studying the history of prenatal care delivery guidelines reveals a recurrent flaw in our design of prenatal care delivery—namely, that we have ignored it. Therefore, we have treated visit frequency and modality as fixed boxes, into which we must fit an ever-changing set of care recommendations. The 1989 National Institutes of Health panel reconsidered this idea, recommending a prenatal visit schedule anchored around the delivery of key services that could be individualized to patients’ medical and social risk factors. More recently, at our institution, we have redesigned prenatal care based on 2 fundamental principles: designing care delivery around essential services and creating flexible services to address the needs of specific patients.81 , 86 It is important to note that this does not mean a universal reduction in the number of visits; medically high-risk patients may benefit from additional healthcare contacts, as would low-risk patients with psychosocial risk factors (eg, intimate partner violence, low support). Some of these additional services may be better delivered outside of routine in-person prenatal visits with physicians, through programs, such as home visiting programs,87 peer support,88 nutritional interventions,89 and numerous others. We do not have data to support a specific prenatal visit schedule, recommended number of telemedicine visits, or specifications of additional services, and we never have. However, one thing is clear: we are long overdue for new prenatal care delivery guidelines in the United States. The Figure provides an overview of how prenatal visit schedules have changed over time and what they may look like in the future.
Figure.
Low-risk prenatal care guidelines from 1930 to 2020
ACOG, American College of Obstetricians and Gynecologists; COVID-19, coronavirus disease 2019; NIH, National Institutes of Health.
Peahl. Evolution of prenatal care guidelines. Am J Obstet Gynecol 2021.
Over 100 years after Ballantyne proposed “promaternity care,” his optimism for the future of prenatal care still rings true. Thinking back to those who called progress in prenatal care to be “fantastic, imaginary, and impossible,” he asked “who shall dare, in full remembrance of what has been accomplished in the past century, to set limits to the progress to be achieved in the present.”16 The COVID-19 pandemic has provided an opportunity for us to reflect on over a century of prenatal care delivery, incorporate what evidence has been gained, and strive to generate new knowledge to inform the next century of care for pregnant patients.
Acknowledgments
The authors would like to thank Dr Milton Kotelchuck for his historic insights and contribution to the manuscript and Mary Hyde, former senior director of the American College of Obstetricians and Gynecologists Resource Center, for her contributions to the historic research for this project. They would also like to thank Sarah Block for her assistance with the preparation of this manuscript. Ms Block is employed by the University of Michigan. Dr Kotelchuck, Ms Hyde, and Ms Block did not receive compensation for their contributions.
Footnotes
The authors report no conflict of interest.
References
- 1.Severe maternal morbidity in the United States. Centers for Disease Control and Prevention. 2017. www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html Available at:
- 2.Shryock R.H. Johns Hopkins University Press; Baltimore, MD: 1967. Medical licensing in America, 1650-1965. [Google Scholar]
- 3.Gunn J.C. a physician of Knoxville; 1830. Gunn’s domestic medicine. Knoxville, TN: printed under the immediate supervision of the author. [Google Scholar]
- 4.Buchan W. Otis Broaders, and Company; Boston, MA: 1848. Domestic medicine. [Google Scholar]
- 5.Davis A.B., Appel T.A. Smithsonian Institution Press; Washington, DC: 1979. Bloodletting instruments in the National Museum of History and Technology. [Google Scholar]
- 6.Merkatz I.R., Thompson J.E. Elsevier; New York, NY: 1990. New perspectives on prenatal care. [Google Scholar]
- 7.Lever J.C.W. Cases of puerperal convulsions, with remarks. Guy’s Hosp Rep. 1843;1:495–517. [Google Scholar]
- 8.Cook H.W., Briggs J.B. Clinical observations on blood pressure. Johns Hopkins Hosp Rep. 1903;11:451–534. [Google Scholar]
- 9.Temkin O. 2nd ed. Johns Hopkins University Press; Baltimore, MD: 1971. The falling sickness: a history of epilepsy from the Greeks to the beginnings of modern neurology. [Google Scholar]
- 10.Bell M.J. A historical overview of preeclampsia-eclampsia. J Obstet Gynecol Neonatal Nurs. 2010;39:510–518. doi: 10.1111/j.1552-6909.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purkerson M.L., Vekerdy L. A history of eclampsia, toxemia and the kidney in pregnancy. Am J Nephrol. 1999;19:313–319. doi: 10.1159/000013467. [DOI] [PubMed] [Google Scholar]
- 12.Mengert W.F. American College of Obstetricians and Gynecologists; Washington, DC: 1971. History of the American College of Obstetricians and Gynecologists 1950-1970. [Google Scholar]
- 13.About the American Association of Obstetricians and Gynecologists Foundation. The American Association of Obstetricians and Gynecologists Foundation; 2020. https://www.aaogf.org/about.asp Available at: [Google Scholar]
- 14.American College of Obstetricians and Gynecologists . American College of Obstetricians and Gynecologists; Washington, DC: 2019. History of the American College of Obstetricians and Gynecologists: 1951-2017. [Google Scholar]
- 15.Ballantyne J.W. A plea for a pro-maternity hospital. Br Med J. 1901;1:813–814. doi: 10.1136/bmj.1.2101.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballantyne J.W. William Green & Sons; Edinburgh, Scotland: 1902. Manual of antenatal pathology and hygiene. [Google Scholar]
- 17.Ballantyne J.W. Visits to the wards of the pro-maternity hospital, a vision of the twentieth century. Am J Obstet Dis Women Child. 1901;43:596. [Google Scholar]
- 18.Alexander G.R., Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep. 2001;116:306–316. doi: 10.1016/S0033-3549(04)50052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 20.Williams J.W., editor. Obstetrics: a text-book for the use of students and practitioners. 2nd ed. D. Appleton and Company; New York, NY: 1910. The management of normal pregnancy; pp. 199–204. [Google Scholar]
- 21.Worcester A. The Boston Lying-in Hospital fifty years ago. N Engl J Med. 1933;209:1109–1112. [Google Scholar]
- 22.Thompson J.E., Walsh L.V., Merkatz I.R. In: New perspectives on parental care. Merkatz I.R., Thompson J.E., editors. Elsevier; New York, NY: 1990. The history of prenatal care: cultural, social, and medical contexts; pp. 9–30. [Google Scholar]
- 23.Heaton C. Fifty years of progress in obstetrics and gynecology. N Y State J Med. 1951;51:83–85. [PubMed] [Google Scholar]
- 24.Yarrow A.L. History of U.S. Children’s Policy 1900-present. 2009. https://firstfocus.org/wp-content/uploads/2014/06/Childrens-Policy-History.pdf Available at:
- 25.Centennial series: the creation of the Children’s Bureau. Children’s Bureau Express. 2012. https://cbexpress.acf.hhs.gov/index.cfm?event=website.viewArticles&issueid=134§ionid=24&articleid=3463 Available at:
- 26.Prenatal care. United States Department of Labor, Children’s Bureau. 1930. https://www.mchlibrary.org/history/chbu/2265-1930.PDF Available at:
- 27.Williams J.W. The limitations and possibilities of prenatal care: based on the study of 705 fetal deaths occurring in 10,000 consecutive admissions to the obstetrical department of the Johns Hopkins Hospital. J Am Med Assoc. 1915;64:95–101. [Google Scholar]
- 28.Williams J.W. D. Appleton and Company; New York, NY: 1905. ed. Obstetrics: a text-book for the use of students and practitioners. 1st ed. [Google Scholar]
- 29.Shapiro S. Development of birth registration and birth statistics in the United States. Popul Stud (Camb) 1950;4:86–111. [Google Scholar]
- 30.Hill I.L. Extending the care of pregnancy. JAMA. 1918;71:885–887. [Google Scholar]
- 31.Hsiao F. Controlling pregnancy: Fred Lyman Adair and the influence of eugenics on the development of prenatal care. 2019. https://elischolar.library.yale.edu/ymtdl/3504 Available at: Accessed May 10, 2020.
- 32.Kobrin F.E. The American midwife controversy: a crisis of professsionalization. Bull Hist Med. 1966;40:350–363. [PubMed] [Google Scholar]
- 33.Prenatal care United States Department of Labor, Children’s Bureau. 1942. https://www.mchlibrary.org/history/chbu/2265-1942.PDF Available at:
- 34.Prenatal care Federal Security Agency, Social Security Administration, Children’s Bureau. 1949. https://www.mchlibrary.org/history/chbu/2265-1949.PDF Available at:
- 35.Prenatal care United States Department of Health, Education, and Welfare, Office of Child Development, Children’s Bureau. 1962. https://www.mchlibrary.org/history/chbu/2265-1962.PDF Available at:
- 36.Litoff J.B. Greenwood Press; Westport, CT: 1978. American midwives: 1860 to the present. [Google Scholar]
- 37.Wertz R.W., Wertz D.C. Yale University Press; New Haven, CT: 1989. Lying-in: a history of childbirth in America. [Google Scholar]
- 38.McCool W.F., Simeone S.A. Birth in the United States: an overview of trends past and present. Nurs Clin North Am. 2002;37:735–746. doi: 10.1016/s0029-6465(02)00020-8. [DOI] [PubMed] [Google Scholar]
- 39.Leavitt J.W. Oxford University Press; New York, NY: 1986. Brought to bed: childbearing in America, 1750-1950. [Google Scholar]
- 40.Everett H.S., Taylor E.S. The history of the American Gynecological Society and the scientific contributions of its Fellows. Am J Obstet Gynecol. 1976;126:908–919. doi: 10.1016/0002-9378(76)90678-5. [DOI] [PubMed] [Google Scholar]
- 41.American College of Obstetricians and Gynecologists . American College of Obstetricians and Gynecologists; Chicago, IL: 1959. Manual of standards in obstetric-gynecologic practice. [Google Scholar]
- 42.Pollack W., Gorman J.G., Freda V.J., Ascari W.Q., Allen A.E., Baker W.J. Results of clinical trials of RhoGAM in women. Transfusion. 1968;8:151–153. doi: 10.1111/j.1537-2995.1968.tb04895.x. [DOI] [PubMed] [Google Scholar]
- 43.Vaitukaitis J.L., Braunstein G.D., Ross G.T. A radioimmunoassay which specifically measures human chorionic gonadotropin in the presence of human luteinizing hormone. Am J Obstet Gynecol. 1972;113:751–758. doi: 10.1016/0002-9378(72)90553-4. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg B.B., Isard H.J., Gershon-Cohen J., Ostrum B.J. Ultrasonic fetal cephalometry. Radiology. 1966;87 doi: 10.1148/87.2.328. 328–32 passim. [DOI] [PubMed] [Google Scholar]
- 45.McLeary R.D. The radiologist’s role in obstetric ultrasound. Radiology. 1980;137:565–566. doi: 10.1148/radiology.137.2.7433694. [DOI] [PubMed] [Google Scholar]
- 46.Kubli F.W., Hon E.H., Khazin A.F., Takemura H. Observations on heart rate and pH in the human fetus during labor. Am J Obstet Gynecol. 1969;104:1190–1206. doi: 10.1016/s0002-9378(16)34294-6. [DOI] [PubMed] [Google Scholar]
- 47.Myers R.E., Mueller-Heubach E., Adamsons K. Predictability of the state of fetal oxygenation from a quantitative analysis of the components of late deceleration. Am J Obstet Gynecol. 1973;115:1083–1094. doi: 10.1016/0002-9378(73)90557-7. [DOI] [PubMed] [Google Scholar]
- 48.Burke W., Tarini B., Press N.A., Evans J.P. Genetic screening. Epidemiol Rev. 2011;33:148–164. doi: 10.1093/epirev/mxr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prenatal care United States Department of Health and Human Services. 1983. https://www.mchlibrary.org/history/chbu/2265-1983.PDF Available at: [DOI] [PubMed]
- 50.Institute of Medicine. Infant Death: An Analysis by Maternal Risk and Health Care. Kessner DM, ed. Vol. 1. Washington, DC: National Academy of Sciences; 1973.
- 51.Eisner V., Brazie J.V., Pratt M.W., Hexter A.C. The risk of low birthweight. Am J Public Health. 1979;69:887–893. doi: 10.2105/ajph.69.9.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.United Nations, Department of Economic and Social Affairs, Statistics Division . United Nations; New York, NY: 1983. Demographic and Social Statistics Branch. United Nations demographic yearbook, 1981. [Google Scholar]
- 53.Committee to Study the Prevention of Low Birthweight . National Academies Press; Washington, DC: 1985. Division of Health Promotion and Disease Prevention, Institute of Medicine. Preventing low birthweight. [PubMed] [Google Scholar]
- 54.Brown S.S. Can low birth weight be prevented? Fam Plann Perspect. 1985;17:112–118. [PubMed] [Google Scholar]
- 55.Krans E.E., Davis M.M. Preventing low birthweight: 25 years, prenatal risk, and the failure to reinvent prenatal care. Am J Obstet Gynecol. 2012;206:398–403. doi: 10.1016/j.ajog.2011.06.082. [DOI] [PubMed] [Google Scholar]
- 56.Omnibus Budget Reconciliation Act of 1989, HR 3299, 101st Cong, Pub L No. 101–239 (1989-1990). Congress.gov. https://www.congress.gov/bill/101st-congress/house-bill/3299 Available at:
- 57.Caring for our future: the content of prenatal care A report of the Public Health Service Expert Panel on the Content of Prenatal Care. National Institutes of Health. 1989. https://eric.ed.gov/?id=ED334018 Available at:
- 58.Rosen M.G., Merkatz I.R., Hill J.G. Caring for our future: a report by the expert panel on the content of prenatal care. Obstet Gynecol. 1991;77:782–787. [PubMed] [Google Scholar]
- 59.Kolata G. Less prenatal care urged for most healthy women. New York Times. October 4, 1989:A1. https://www.nytimes.com/1989/10/04/us/less-prenatal-care-urged-for-most-healthy-women.html Available at: Accessed Oct. 12, 2020.
- 60.Gold R.B., Kenney A.M., Singh S. Paying for maternity care in the United States. Fam Plann Perspect. 1987;19:190–206. [PubMed] [Google Scholar]
- 61.Brown S.S., editor. Prenatal care: reaching mothers, reaching infants. National Academy Press; Washington, DC: 1988. Barriers to the use of prenatal care; pp. 54–87. [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC) Adequacy of prenatal-care utilization--California, 1989-1994. MMWR Morb Mortal Wkly Rep. 1996;45:653–656. [PubMed] [Google Scholar]
- 63.Howell E.M., Brown G.A. Prenatal, delivery, and infant care under Medicaid in three states. Health Care Financ Rev. 1989;10:1–15. [PMC free article] [PubMed] [Google Scholar]
- 64.Orr M.T., Forrest J.D. The availability of reproductive health services from U.S. private physicians. Fam Plann Perspect. 1985;17:63–69. [PubMed] [Google Scholar]
- 65.Schwalberg R., Mathis S.A., Giffen M., Mohamadi L., Zimmerman B., Sines E. Medicaid coverage of perinatal services: results of a national survey. The Henry J. Kaiser Family Foundation. 2013. https://www.kff.org/wp-content/uploads/2013/01/medicaid-coverage-of-perinatal-services-results-of-a-national-survey-report.pdf Available at:
- 66.American College of Obstetricians and Gynecologists . 7th ed. American College of Obstetricians and Gynecologists; Washington, DC: 1989. Standards for obstetric gynecologic services. [Google Scholar]
- 67.Medicaid prenatal care: states improve access and enhance services, but face new challenges. United States General Accounting Office. 1994. https://www.gao.gov/assets/80/78856.pdf Available at:
- 68.Alexander G.R., Korenbrot C.C. The role of prenatal care in preventing low birth weight. Future Child. 1995;5:103–120. [PubMed] [Google Scholar]
- 69.Dowswell T., Carroli G., Duley L., et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2015;7:CD000934. doi: 10.1002/14651858.CD000934.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WHO recommendations on antenatal care for a positive pregnancy experience. World Health Organization. 2016. https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/anc-positive-pregnancy-experience/en/ Available at: [PubMed]
- 71.Friedman Peahl A., Heisler M., Essenmacher L.K., et al. A comparison of international prenatal care guidelines for low-risk women to inform high-value care. Am J Obstet Gynecol. 2020;222:505–507. doi: 10.1016/j.ajog.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 72.Hill I., Dubay L., Courtot B., et al. Strong start for mothers and newborns evaluation: year 5 project synthesis. Urban Institute. 2018. https://downloads.cms.gov/files/cmmi/strongstart-prenatal-finalevalrpt-v1.pdf Available at:
- 73.Anum E.A., Retchin S.M., Strauss J.F., 3rd Medicaid and preterm birth and low birth weight: the last two decades. J Womens Health (Larchmt) 2010;19:443–451. doi: 10.1089/jwh.2009.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krans E.E., Davis M.M. Strong start for mothers and newborns: implications for prenatal care delivery. Curr Opin Obstet Gynecol. 2014;26:511–515. doi: 10.1097/GCO.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rising S.S. Centering pregnancy. An interdisciplinary model of empowerment. J Nurse Midwif. 1998;43:46–54. doi: 10.1016/s0091-2182(97)00117-1. [DOI] [PubMed] [Google Scholar]
- 76.Byerley B.M., Haas D.M. A systematic overview of the literature regarding group prenatal care for high-risk pregnant women. BMC Pregnancy Childbirth. 2017;17:329. doi: 10.1186/s12884-017-1522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carter E.B., Temming L.A., Akin J., et al. Group prenatal care compared with traditional prenatal care: a systematic review and meta-analysis. Obstet Gynecol. 2016;128:551–561. doi: 10.1097/AOG.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Butler Tobah Y.S., LeBlanc A., Branda M.E., et al. Randomized comparison of a reduced-visit prenatal care model enhanced with remote monitoring. Am J Obstet Gynecol. 2019;221:638.e1–638.e8. doi: 10.1016/j.ajog.2019.06.034. [DOI] [PubMed] [Google Scholar]
- 79.Virtual prenatal care. University of Utah Health. 2020. https://healthcare.utah.edu/virtual-care/virtual-prenatal-care/ Available at:
- 80.Marko K.I., Krapf J.M., Meltzer A.C., et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200. doi: 10.2196/resprot.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peahl A.F., Smith R.D., Moniz M.H. Prenatal care redesign: creating flexible maternity care models through virtual care. Am J Obstet Gynecol. 2020;223:389.e1–389.e10. doi: 10.1016/j.ajog.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.COVID-19 FAQs for obstetrician-gynecologists, obstetrics. American College of Obstetricians and Gynecologists. 2020. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics Available at:
- 83.Peahl A.F., Powell A., Berlin H., et al. Patient and provider perspectives of a new prenatal care model introduced in response to the coronavirus disease 2019 pandemic. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.10.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fryer K., Delgado A., Foti T., Reid C.N., Marshall J. Implementation of obstetric telehealth during COVID-19 and beyond. Matern Child Health J. 2020;24:1104–1110. doi: 10.1007/s10995-020-02967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aziz A., Zork N., Aubey J.J., et al. Telehealth for high-risk pregnancies in the setting of the COVID-19 pandemic. Am J Perinatol. 2020;37:800–808. doi: 10.1055/s-0040-1712121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peahl A.F., Gourevitch R.A., Luo E.M., et al. Right-sizing prenatal care to meet patients’ needs and improve maternity care value. Obstet Gynecol. 2020;135:1027–1037. doi: 10.1097/AOG.0000000000003820. [DOI] [PubMed] [Google Scholar]
- 87.Issel L.M., Forrestal S.G., Slaughter J., Wiencrot A., Handler A. A review of prenatal home-visiting effectiveness for improving birth outcomes. J Obstet Gynecol Neonatal Nurs. 2011;40:157–165. doi: 10.1111/j.1552-6909.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- 88.Dennis C.L., Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev. 2013;2:CD001134. doi: 10.1002/14651858.CD001134.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soneji S., Beltrán-Sánchez H. Association of special supplemental nutrition program for women, infants, and children with preterm birth and infant mortality. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.16722. [DOI] [PMC free article] [PubMed] [Google Scholar]