ABSTRACT
This paper presents the consensus of the Scientific Department of Cognitive Neurology and Aging from the Brazilian Academy of Neurology on the diagnostic criteria for Alzheimer’s disease (AD) in Brazil. The authors conducted a literature review regarding clinical and research criteria for AD diagnosis and proposed protocols for use at primary, secondary, and tertiary care levels. Within this clinical scenario, the diagnostic criteria for typical and atypical AD are presented as well as clinical, cognitive, and functional assessment tools and complementary propaedeutics with laboratory and neuroimaging tests. The use of biomarkers is also discussed for both clinical diagnosis (in specific conditions) and research.
Keywords: Alzheimer Disease, Dementia, Diagnosis
RESUMO
Este artigo apresenta o consenso realizado pelo Departamento Científico de Neurologia Cognitiva e do Envelhecimento da Academia Brasileira de Neurologia sobre os critérios diagnósticos da Doença de Alzheimer (DA) no Brasil. Foi realizada uma revisão da literatura e dos critérios clínicos e de pesquisa para DA, sendo propostos protocolos para o diagnóstico de DA em níveis de atenção primária, secundária e terciária. Dentro deste cenário clínico, são apresentados os critérios diagnósticos para DA típica e atípica, além de instrumentos de avaliação clínica, cognitiva e funcional; bem como propedêutica complementar com exames laboratoriais e de neuroimagem. A utilização de biomarcadores é também apresentada, tanto para o diagnóstico clínico em situações específicas quanto para pesquisa.
Palavras-chave: Doença de Alzheimer, Demência, Diagnóstico
INTRODUCTION
Epidemiology and relevance
The continuous aging of the world population increases the prevalence and incidence of chronic and neurodegenerative diseases. Currently, dementia affects an estimated 50 million people worldwide and has 10 million new diagnoses per year, of which about 60% are due to Alzheimer’s disease (AD). Projections indicate an estimated 150 million people with dementia due to AD by 2050 1 . In Brazil, an estimated 1.7 million people have dementia, with a prevalence of approximately 1,036/100,000 inhabitants 2 .
Risk factors
Risk factors for AD can be divided into environmental and genetic. Environmental factors are more related to the sporadic form of the disease (late-onset or senile AD), whose main risk factor is aging 3 . Other risk factors include low schooling level, systemic arterial hypertension, diabetes, obesity, sedentary lifestyle, head trauma, depression, smoking, hearing loss, and social isolation 4 , which can all be prevented and modified.
From the genetic point of view, mutations responsible for the autosomal dominant forms of AD stand out. Unlike the multifactorial etiology of late-onset sporadic AD, autosomal dominant forms (which are relatively rare) have early onset, occurring before 65 years (presenile AD), and are strongly associated with mutations of the amyloid precursor protein (APP), presenilin 1, or presenilin 2 genes, which are identified in 70% of cases, and with a dominant, autosomal inheritance pattern 5 .
Though late-onset forms of AD are rarely associated with dominant inheritance, they can be related to genetic risk factors such as the ε4 allele of the apolipoprotein E (APOE) gene, which increases the risk for AD development and anticipates its onset in a few years. Homozygosis for the APOEε4 allele increases this risk in five times compared to heterozygotes 6 .
Pathophysiology
The main hypothesis in AD pathophysiology establishes that the degenerative process is triggered by a hyperproduction and/or decreased clearance and consequent accumulation of amyloid-beta peptide (Aβ) and tau protein in the affected brain tissues, accompanied by homeostatic changes that lead to a collapse of the neuronal cytoskeleton. The APP is usually cleaved by the enzyme α-secretase (ADAM10), generating soluble peptides (APPs); in AD, an alternative and sequential cleavage of APP occurs by secretases β (BACE-1) and y, generating insoluble Aβ peptides that aggregate and deposit in the extracellular space, triggering several pathological events that cause neuronal death and formation of senile or neuritic plaques (NPs). Neurofibrillary tangles (NFTs) are intracellular deposits composed of hyperphosphorylated tau protein. The tau protein maintains the integrity of intraneuronal microtubules, a function that is lost after the hyperphosphorylation process.
The initial clinical symptoms of amnestic AD are related to the increased density of NFTs in the hippocampal formation, nucleus basalis of Meynert, and paralimbic regions (fusiform gyrus and inferior and middle temporal gyri), corresponding to stages III and IV of Braak’s neuropathological staging 7 . In preclinical stages of AD (Braak stages I and II), NFTs occur almost exclusively in limbic system structures (entorhinal cortex, subiculum, and hippocampus, in addition to the amygdala, nucleus basalis of Meynert, and temporopolar cortex). In mild AD (Braak stage V), the density of NFTs increases in the limbic system and they emerge at the associative neocortical regions of the middle and superior temporal gyri (related to language symptoms) as well as at the prefrontal, retrosplenial, and posterior parietal cortices (related to executive dysfunction and spatial disorientation). NPs also deposit in these areas, progressively increasing the density of NFTs and NPs in the whole neocortex, including unimodal (visual, auditory, and somestesic) and multimodal association areas in the temporoparietal and dorsolateral frontal junction. Severe AD corresponds to Braak advanced stage VI, in which all cortical association areas and the basal ganglia are affected by NFTs and NPs, with relative sparing of the motor and sensory cortices.
Other pathophysiological mechanisms in AD include synaptic dysfunction, neurotransmitters (mainly acetylcholine) and neurotrophin depletion, mitochondrial dysfunction and deficits in insulin signaling pathways, increase in oxidative stress and inflammation, vascular changes 8 , and cholesterol metabolism 9 . Recent studies suggest that the interaction between different pathophysiological processes, such as white matter involvement associated with Aβ accumulation 10 and soluble Aβ oligomers interaction with other proteins (such as α-synuclein and tau), destabilize microtubules, mitochondrial and synaptic dysfunction, and neurodegeneration 11 ),( 12 .
CLINICAL CHARACTERIZATION
Clinical characterization
AD usually manifests in the typical amnestic presentation, with a predominant difficulty of episodic memory associated with degenerative lesions of medial temporal structures. This pattern occurs in about 85% of cases. Atypical and less frequent presentations may begin mainly with impairment in language, visual-spatial, executive, or complex motor functions. The most common atypical (usually presenile) forms are the logopenic variant of primary progressive aphasia (lvPPA) and the visual-spatial-apraxic of posterior cortical atrophy (PCA), whereas the least common forms are the corticobasal syndrome (CBS) and the behavioral and dysexecutive variant (ADbdv).
Due to these non-amnestic variants, memory impairment is no longer mandatory for AD diagnosis according to the most recent diagnostic criteria from the Brazilian Academy of Neurology 13 based on the National Institute on Aging-Alzheimer’s Association (NIA-AA) ( 14 . To help identify these initial forms of AD presentation, we will detail their clinical characteristics, evolution stages, and corresponding neuropathological substrates.
AD amnestic presentation
The typical AD presentation starts with difficulties remembering messages and recent news and repeating the same questions, comments, and narratives. Initially mild and intermittent, the symptoms first appear as subjective memory impairment (SCI) followed by mild cognitive impairment (MCI) - usually of the multiple-domain amnestic type (also impairing the language and executive functions) -, later evolving to full-blown dementia, when they begin to interfere in the activities of daily living and in the patient’s autonomy 14 .
Logopenic variant of primary progressive aphasia (lvPPA)
The first and predominant symptoms in this variant are language alterations with non-fluent speech, pauses due to word-finding difficulty, and errors (also phonological) when repeating long sentences and in spontaneous speech, but preserving semantics, syntax (grammar), comprehension of single words, and motor production of speech 15 ),( 16 .
Posterior cortical atrophy (PCA) or posterior variant
PCA is a rare form of AD which usually starts between the ages of 50 and 60 years. Its occipitotemporal variant presents with impairment of the visual identification of objects, faces, or symbols; its biparietal variant, more common, is characterized by visual-spatial dysfunction, topographical disorientation, poor hand-eye coordination, limb apraxia, visual neglect, and at clinical evaluation, elements of Balint’s syndrome (optic ataxia, oculomotor apraxia, and simultanagnosia) and/or Gerstmann syndrome (acalculia, agraphia, left-right disorientation, and finger agnosia) ( 17 ),( 18 .
In the early stages of the disease, episodic memory, language, and executive functions are still relatively preserved. PCA presents with AD neuropathology in 62 to 100% of cases 19 .
Behavioral and dysexecutive variant (ADbdv)
The AD dysexecutive variant (ADdv) affects mainly planning, working memory, and multi-tasking, with loss of inhibitory control and alternating attention, depression, anxiety and neuropsychiatric symptons. In turn, behavioural symptoms are rare.
Though the behavioral variant of AD is similar to the behavioral variant of frontotemporal dementia (bvFTD), it presents greater deficits in memory, apathy, delusional ideas, and hallucinations, with less disinhibition, compulsive or persevering behavior, affective indifference, or personality change 20 )-( 23 . This presentation of AD is rare, occurring in about 2% of large samples of AD patients and with 7-20% of patients clinically diagnosed as FTD 19 ),( 24 ),( 25 .
Corticobasal syndrome (CBS)
CBS manifests with remarkably asymmetric or unilateral signs and symptoms of stiffness, dystonia, myoclonus, bradykinesia, and tremor. It is associated with gait alteration, asymmetric apraxia, alien hand phenomenon, sensory hemineglect, and visual-spatial deficits, besides the more typical symptoms of episodic and visual-spatial memory deficits and aphasia 19 ), ( 23 . AD causes 15-50% of CBS cases, degenerating cortical structures and basal ganglia, including the substantia nigra, and manifesting clinically mainly as motor symptoms.
Clinical stages of dementia
Mild dementia
The mild dementia stage is characterized by progressive worsening of amnestic symptoms associated with other cognitive disorders, such as impaired working memory, attentional control (difficulty multitasking), language alterations (anomia), executive dysfunction (struggles with planning, problem-solving), and temporal-spatial disorientation 26 .
Neuropsychiatric symptoms occur in all stages (in up to 80% of cases) and worsen as dementia progresses, especially apathy, depression, anxiety 27 ),( 28 , and a lack of awareness regarding cognitive deficits (anosognosia), which occurs in up to 50% of patients 19 ),( 29 .
Moderate dementia
In the moderate dementia stage, patients become more dependent on others to perform instrumental activities of daily living (although still capable of self-care) and have greater difficulty remembering the name of relatives, remote events, or more significant recent events. Other cognitive symptoms may worsen, such as temporal and spatial disorientation, development of transcortical sensory aphasia, ideomotor apraxia, dyscalculia, visual agnosia, and neuropsychiatric symptoms such as delusions (typically of betrayal or theft), hallucinations, and agitation, with or without aggressiveness.
Severe dementia
In the severe dementia stage, patients are entirely dependent on a caregiver, with memory reduced to fragments of information, temporal and personal disorientation (maintaining only self-awareness), and speech restricted to a few unintelligible words. In more advanced stages, they can present urinary and fecal incontinence, parkinsonism, myoclonus, epileptic seizures (in up to 20% of cases) ( 30 ),( 31 , and gait difficulties. Later, patients have difficulty sitting and swallowing. The average survival time is five to 12 years after the onset of symptoms, but with significant variability among patients 32 .
Diagnosis
Clinical diagnosis of AD
The clinical diagnosis of AD dementia is based on a thorough evaluation, especially of the patient’s affected cognitive domains and functional impairment, as described in the diagnostic criteria and neuropsychological assesment section 13 ),( 14 . AD is a progressive pathological process with different clinical stages, and dementia occurs when pathological changes have already spread 33 .
Understanding this cognitive continuum is essential for an appropriate clinical evaluation of the patient and, with complementary tests (including biomarkers), an accurate diagnosis in atypical or early-onset cases. Biomarkers also allow identifying patients and indicating possible future specific treatments for AD 34 .
Diagnosis of dementia due to Alzheimer’s disease (modified from McKhann et al., 2011 13 and Frota et al., 2011 14
Probable Alzheimer’s disease dementia
If the patient meets the criteria for diagnosis of dementia 35 and has the following characteristics:
I. Insidious onset (months or years);
II. Clear history or observation of cognitive worsening;
III. Initial and more prominent cognitive deficits in one of the following categories:
•Amnestic presentation (there must be another affected domain).
•Non-amnestic presentation (there must be another domain affected).
•Language (memory of words).
•Visual-spatial (spatial cognition or agnosia for objects or faces, simultanagnosia, and alexia).
•Executive functions (alterations of reasoning, judgment, and problem-solving).
IV. Tomography or, preferably, magnetic resonance imaging of the brain should be performed to exclude other diagnostic possibilities or comorbidities, especially cerebrovascular disease;
V. The diagnosis of probable AD dementia should not be applied when there are:
•Evidence of significant cerebrovascular disease defined by a history of stroke temporally related to the onset or worsening of cognitive impairment; presence of multiple or extensive brain infarctions; or extensive lesions in the white matter evidenced by neuroimaging; or
•Central features of dementia with Lewy bodies (visual hallucinations, parkinsonism, REM sleep behavior disorder, and cognitive fluctuation); or
•Prominent features of the behavioral variant of FTD (hyperorality, hypersexuality, perseveration); or
•Prominent characteristics of PPA manifesting as semantic variant (with fluent speech, anomia, and semantic memory difficulties) or as non-fluent variant (with agrammatism and/or marked speech apraxia); or
•Evidence of concomitant neurological or non-neurological active disease, or medication use that may substantially affect cognition.
Anamnesis
A detailed anamnesis focused on the most common cognitive and neuropsychiatric alterations of AD allows diagnosing the disease more safely, establishing its subtype based on its initial presentation and stage, and differentiating it from other neurodegenerative diseases. Interrogation of patients and their relative/caregiver should cover (1) neuropsychiatric disorders such as depression, anxiety, apathy, delusional ideas, hallucinations, and aberrant or uninhibited motor behaviors, which are socially inappropriate; and (2) cognitive difficulties in the following domains, most affected by the disease:
Episodic memory: Does the patient forget recent facts and dates, items to purchase, appointments, or places where he/she keeps objects? Or does he/she keep repeating the same questions or comments?
Executive functions: Does the patient have difficulty staying focused, making decisions, planning activities, solving everyday problems, shopping, and dealing with small amounts of money? Do they present loss of motivation and initiative? Do they have impaired judgment?
Visual-spatial or praxic skills: Does the patient have difficulty orienting themselves spatially (outside and indoors), dressing, combing, shaving, using everyday objects, recognizing familiar faces? Have they lost dexterity in tasks in which they used to do well?
Language: Does the patient have difficulty finding words in conversations or naming objects and people? Or in understanding words or sentences, explaining situations and making themselves understood, presenting poor vocabulary and reduced speech fluency?
Neuropsychological assessment
According to specific studies on the subject 36 ),( 37 , the diagnosis of AD in its initial stage (or MCI) has greater reliability when using two tests for each of the four cognitive domains most affected by the disease and greater sensitivity when defining deficit score as >1 standard deviation (SD), and not >1.5 or >2SD), relative to normative values. Thus, in addition to conducting a global cognitive test (MMSE, Mini-Mental State Examination; or MoCA, Montreal Cognitive Assessment), the evaluation must include episodic memory, language, executive functions, and visual-spatial functions, with two subtests for each cognitive domain.
The main instruments recommended for cognitive assessment in AD in Brazil are presented below. Given the country’s socio-cultural and educational heterogeneity, it is advisable to use instruments with cutoff scores adjustable by level of education to avoid false-positive results in the diagnostic process 38 . The instruments are subdivided into cognitive screening tests, specific tests for evaluating different cognitive domains, and instruments for assessing functionality (Table 1).
Table 1. Main instruments for cognitive assessment in AD.
| Type of instrument | Main tests and normative studies |
|---|---|
| Screening tests | |
| Brief tests | Mini-Mental State Examination (MMSE) ( 39 ),( 40 , Montreal Cognitive Assessment (MoCA) ( 41 ),( 42 , Cognitive Abilities Screening Instrument - Short Version (CASI-S) ( 43 ),( 44 , Brief Cognitive Screening Battery (BBRC) ( 45 ),( 46 |
| Multi-functional batteries | Addenbrooke’s Cognitive Examination - revised version (ACE-R) ( 47 ),( 48 , Cambridge Cognitive Examination (CAMCOG) ( 49 )-( 51 , Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-COG) ( 52 ),( 53 , Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) ( 54 ),( 55 , Mattis Dementia Rating Scale (MDRS) ( 56 ),( 57 |
| Evaluation of single cognitive domains | |
| Verbal episodic memory | Rey Auditory Verbal Learning Test (RAVLT) ( 58 ),( 59 , CERAD Battery Word List Learning Subtest |
| Nonverbal memory | Subtest “figure recognition” (BBRC), Subtest “geometric figure recall” (CERAD Battery), Rey-Osterrieth Complex Figure 60 ),( 61 |
| Language | Verbal Fluency Test (phonemic and semantic) ( 62 ),( 63 , Boston Naming Test (BNT) ( 64 ),( 65 |
| Attention control and executive function | Digit Span Task in forward and inverse order 66 ),( 67 , Clock Drawing Test 68 ),( 69 , Verbal Fluency Test |
| Visual-spatial / visual-constructive abilities | CERAD / MoCA Figure Copy Subtest, Clock Drawing Test |
| Functional assessment | |
| Instrumental activities of daily living | Functional Activities Questionnaire (FAQ) ( 70 ),( 71 , Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) ( 72 ),( 73 , Direct Assessment of Functional Status-Revised (DAFS-R) ( 74 ),( 75 , Disability Assessment for Dementia (DAD) ( 76 ),( 77 , Activities of Daily Living Questionnaire (ADLQ) ( 78 ),( 79 , Bayer Activities of Daily Living Scale (B-ADL) ( 80 ),( 81 , AD8 Dementia Screening Interview 82 |
| Basic activities of daily living | Katz scale 83 ),( 84 , Functional Activities Questionnaire (FAQ) ( 70 ),( 72 |
| Dementia staging | Clinical Dementia Rating scale (CDR) ( 85 ),( 86 |
Note: The global CDR scores (CDR-GS: 0, 0.5, 1, 2, or 3) have the limitation of being based on the scores of the item Memory, considering the other items as secondary, and thus underestimating relevant information from instrumental activities that may be primarily and early affected.
More recently, the sum of boxes (CDR-Sum of Boxes - CDR-SB) have replaced the Clinical Dementia Rating (CDR) global scores. The first allows detecting smaller differences within and between subsequent global scores as well as within and between stages of the disease, helping differentiate MCI from initial dementia, as seen in O’Bryant et al. ( 87 , who described the following ranges of CDR-SB corresponding to CDR-GS scores: 0.5 to 4.0 for the CDR-GS of 0.5; 4.5 to 9.0 for CDR-GS of 1; 9.5 to 15.5 for CDR-GS of 2; and 16.0 to 18.0 for CDR-GS of 3.
Laboratory tests in AD clinical propaedeutics
Several clinical conditions may cause cognitive impairment, such as hypothyroidism, hypovitaminosis, and neurosyphilis. An initial medical evaluation should conduct a basic laboratory evaluation to rule out the main secondary causes of cognitive decline. It should also seek to identify systemic diseases and comorbidities that could worsen the neurological condition, such as dyslipidemia and diabetes 88 )-( 91 .
The list of recommended laboratory tests should include hematological, renal, hepatic, lipid, and metabolic profiles (serum sodium, potassium, and calcium), fasting glucose, folic acid dosage, vitamin B12, TSH, free T4, syphilis serology, and especially in atypical cases or in case of clinical suspicion, HIV testing 92 .
Structural neuroimaging
Brain evaluation by structural neuroimaging such as computed tomography (CT) or magnetic resonance imaging (MRI) is essential for properly diagnosing AD, both to rule out secondary lesions and to identify patterns of brain atrophy specific to the disease. MRI provides better anatomical resolution and different acquisition techniques that are more useful than CT for differential diagnoses with other dementias, such as those of vascular or prion pathology.
As a neurodegenerative disease, AD invariably occurs with cerebral atrophy. The most common pattern of volumetric alteration is atrophy of mesial temporal structures (MTS), in structures such as the hippocampus and entorhinal cortex, which correlates with the clinical findings of episodic memory deficit. However, atrophy can also affect different regions, especially in atypical presenile presentations, such as linguistic, dysexecutive and/or behavioral (frontal), and visual-spatial variants, among others, which will be discussed later 93 .
Computed tomography (CT) is a useful, more available, and lower-cost effective study that can be used even in primary health care. Similarly to MRI, CT can rule out structural lesions such as subdural hematoma, tumors, and hydrocephalus 94 . It can also evaluate hippocampal atrophy, especially via coronal plane reconstruction. Although MRI has a higher anatomical resolution than CT, the Medial Temporal Atrophy Scale (MTA or Scheltens scale) can also be used in CT (Figure 1) ( 95 . The MTA is sensitive to diagnose AD and specific to differentiate AD from normal older adults, although other dementias may also present hippocampal atrophy, such as vascular dementia or dementia with Lewy bodies. The Scheltens scale assesses the width of the choroidal fissure and temporal horn as well as the height of the hippocampus(Figure 2) ( 94 ),( 96 .
Figure 1. Application of the MTA scale on MRI (above) and CT (below). Under 75 years old, ≥2 is abnormal; over 75 years old, ≥3 is abnormal 95 .
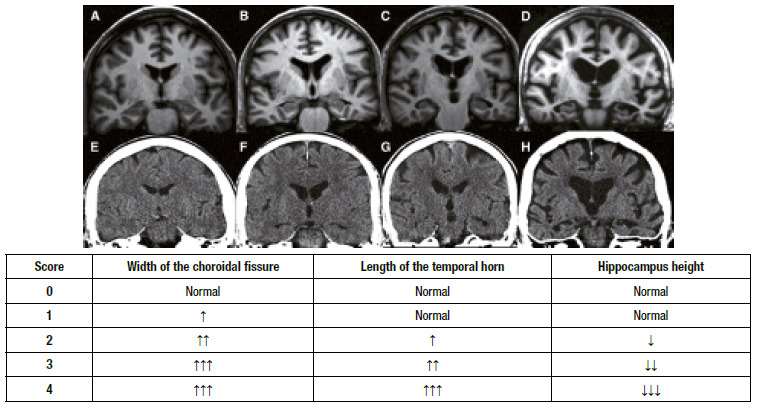
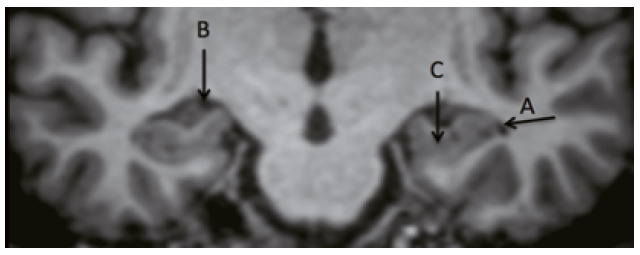
Figure 2. Structures evaluated in the MTA scale. A: temporal horn; B: choroidal fissure; C: hippocampus.
As aforementioned, MRI presents better anatomical resolution and allows performing other imaging techniques. Visual inspection of MTS using scales such as the MTA is still the most widespread and available method in the clinical diagnosis of AD, with sensitivity and specificity of 80 to 85% in differentiating individuals with AD from cognitively normal individuals 97 . Some radiological centers and software available on the Internet measure hippocampal volume by MRI, which may increase sensitivity/specificity for diagnosis. Although this information is essential, especially for follow-up, it still lacks standardization for the Brazilian population considering age and sex. Regarding MTS in AD, the subregions of the hippocampus, such as CA1 and subiculum, can be quantified, but without widespread clinical application 98 . A recent review of MCI due to AD showed low sensitivity/specificity (73 and 71%, respectively) of MTS measures to differentiate patients whose condition evolved or not to dementia 99 .
Other MRI techniques do not yet have a consolidated role in clinical practice for AD diagnosis. As an example, although the MRI proton magnetic resonance spectroscopy technique - which assesses brain metabolites such as N-acetylaspartate (Naa), creatine (Cr), and myo-inositol (mI) - shows differences between groups of patients with and without AD, it still has many limitations and technique heterogeneity as to be applied in clinical practice as a marker of the disease. The main findings of the technique are a decrease in NAA and NAA/Cr ratio and an increase in mI and mI/Cr ratio 100 .
Several other techniques, such as diffusion tensor imaging, texture analysis, MRI infusion, and functional connectivity, are still restricted for research.
Structural neuroimaging in atypical AD
A common feature of atypical presentations of AD, is the relative preservation of MTS in relation to atrophy of other brain regions. Each variant has its imaging characteristics, which generally correlate with clinical symptoms. Some neuroimaging characteristics of the three most common atypical presentations will be briefly described: visual-spatial variant (part of the PCA spectrum); linguistic variant, most commonly lvPPA; and ADbdv.
PCA: predominance of parietal and posterior temporal atrophy. The Koedam’s parietal atrophy scale ranges from 0 to 3 and assesses the integrity of the precuneus and dilatation of the posterior cingulate, parieto-occipital, and parietal lobe sulci. It may be helpful in the diagnosis of PCA, and scores ≥ 2 can be considered abnormal, according to the author’s proposal 101 ;
lvPPA: asymmetric atrophy of temporoparietal structures, predominantly in the left hemisphere (dominant for language) ( 16 ;
ADbdv: is the most heterogeneous presentation in terms of image. It presents greater atrophy in the dorsolateral prefrontal cortex compared to typical AD but may also present a pattern of temporoparietal atrophy 20 .
Biomarker-assisted diagnosis
Biomarkers in cerebrospinal fluid
The cerebrospinal fluid (CSF) biomarkers used for AD diagnosis are the 42-amino acid Aβ peptide (Aβ1-42) and the tau protein in its total composition and as phosphorylated residue at threonine 181 (T-tau and P-tau, respectively). The “AD pathological signature” in CSF consists of a pattern determined by reduced Aβ1-42 concentration and increased concentrations of T-tau and P-tau 102 )-( 104 .
In recent years, the use of CSF AD biomarkers for diagnosis has significantly advanced. Ideally, concentrations of Aβ1-42 should be normalized in relation to those of Aβ1-40, which do not vary significantly among dementias. The ratio Aβ1-42/Aβ1-40 can also better predict the PET measurement of amyloid load than the concentration of Aβ1-42 alone 105 )-( 107 .
There is a correspondence between the pattern of CSF biomarkers and the pathophysiological changes underlying AD. The reduction of Aβ1-42 and the increase of P-tau in CSF indicate cerebral amyloidosis and tauopathy, mechanisms that form, respectively, NPs and NFTs. The increase in T-tau signals the ongoing neurodegenerative process, usually represented by structural changes (atrophy) and regional metabolic impairment.
Molecular neuroimaging biomarkers
Pathophysiological processes related to AD can be alternatively inferred in vivo by molecular imaging methods based on positron emission tomography (PET) by injecting different radiotracers.
The progressive degenerative process of AD causes cerebral hypometabolism, which can be assessed using [18F]Fluorodeoxyglucose (FDG) as a tracer. Patients with AD present hypometabolism patterns involving the posterior cingulate, precuneus, temporoparietal, and medial temporal cortices 108 )-( 111 .
Several molecular agents can evaluate the cerebral accumulation of Aβ from peptide Aβ affinity, such as the [11C]Pittsburgh compound-B (PiB), the [18F]Flutemetamol, the [18F]Florbetaben, and [18F]Florbetapir 112 ),( 113 . PiB presents a greater limitation to clinical use since it has carbon in its molecular structure, with a half-life of only 20 minutes. Compared to the measurement of Aβ in CSF, molecular imaging by PET has the advantage of topographically identifying Aβ accumulation in the brain, involving the precuneus and bilateral fronto-temporo-parietal cortices 113 .
PET-specific radiotracers, such as [18F]Flortaucipir, can also assess the accumulation of tau protein, an essential pathological characteristic of AD 114 . Tau accumulation observed in molecular neuroimaging studies has significant clinical correlations: a greater accumulation of tau is related to the increased severity of cognitive decline 115 )-( 117 ) and, in atypical AD, the regions with higher radiotracer retention are associated with symptoms related to these regions, such as occipital lobes in PCA, and to the pattern of glycolytic hypometabolism 118 . Aβ biomarkers have good sensitivity to identify cases of incipient AD 119 whereas P-Tau markers have greater specificity to diagnose AD 120 ),( 121 .
Currently, biomarkers are used mainly in research. From the clinical point of view, biomarkers should be used to assess conditions considered atypical, either for an initial non-amnestic clinical presentation or in patients with early-onset dementia, which will be further addressed in the next section.
When to request CSF biomarkers in AD?
The indications for the CSF examination , in general, did not change since the last Brazilian Academy of Neurology consensus 92 . CSF examination is thus indicated in the investigation of presenile dementia (before 65 years) and in cases with atypical clinical presentation or course, communicating hydrocephalus, and any evidence or suspicion of inflammatory, infectious, or prion disease of the central nervous system.
If the entire diagnostic process involving anamnesis, cognitive assessment, general examination, and neuroimaging studies, the etiology of the dementia syndrome remains doubtful, CSF biomarkers may be helpful. This uncertainty commonly arises in the differential diagnosis of atypical AD presentations with other dementias, such as the behavioral variant of FTD or agrammatic and semantic PPA.
CSF biomarkers can also be used in the workup for cognitive disorders to predict dementia in oligosymptomatic cases, where the identification of the AD ‘pathological signature’ in MCI allows inferring the underlying etiology of the disease with high accuracy.
The development of potentially disease-modifying drugs, such as anti-Aβ monoclonal antibodies, will promote CSF biomarker assessment, which is essential for indicating these medications.
Biological diagnosis of AD through biomarkers
Diagnosis assisted by biomarkers allowed formulating recommendations for the biological diagnosis of AD by the A/T(N) classification 122 . According to this proposal, suspected cases of AD can be classified according to the positivity of biomarkers that allow inferring the underlying presence of one or more pathogenic processes characteristic of AD, where: “A” is the information obtained by biomarkers indicative of cerebral Aβ accumulation (i.e., Aβ1-42 reduction in the CSF or positivity in molecular imaging methods of amyloid detection); “T” is the positivity of biomarkers indicative of tau hyperphosphorylation (increased P-Tau in CSF or positivity in molecular imaging methods with tau-PET); and “N” indicates the occurrence of neurodegeneration by the increase of T-Tau levels in the CSF, hippocampal and/or temporoparietal atrophy in MRI, or loss of regional cerebral metabolic integrity, according to glucose uptake profile by [18F]FDG-PET 122 ),( 123 . According to specific topographic patterns, such measurements are further related to the presence of degenerative process at an early stage, corresponding to the degree of cerebral atrophy and cognitive decline 115 ),( 124 .
According to the 2018 NIA/AA recommendations, which included the AT(N) classification, the AD diagnostic spectrum requires evidence of accumulation of Aβ peptide and AD is defined by the combination of the positivity of this biomarker (A+) and biomarkers indicative of phosphorylated tau protein (T+). Positivity of biomarkers for cerebral amyloidosis (A+) and neurodegeneration (e.g., increase in CSF total tau) (N+) in the absence of P-tau increase (T-) markers concomitantly reflects AD pathological alteration and suspected non-AD pathological process. In short:
A -: not in the AD spectrum
A+: AD spectrum
A+/T+: AD
A+/T-/N+: AD + suspicion of another non-AD pathological process
NIA/AA authors 122 advise that these recommendations apply only to research (observational and interventional) and should not be understood as guidelines or diagnostic criteria for clinical practice, considering that: (1) they do not consider clinical symptoms, signs, and functional impairment; and (2) 40% of cognitively normal older adults may present AD biomarkers and neuropathological alterations 125 , of which about 20% will never develop dementia, even in their nineties 126 .
Pathological diagnosis of AD
The pathological diagnosis of AD is based on the presence of cortical atrophy, especially in the hippocampus and frontoparietal regions (associative areas), with marked neuronal loss and extracellular NPs and intraneuronal NFTs, which are the histopathological markers of AD that establish its definitive diagnosis. These markers are initially formed in the limbic system (hippocampus and entorhinal cortex), progressing to the association cortex, subcortical nuclei, and brainstem structures. Other neuropathological findings include neuronal loss in the pyramidal layers of the cerebral cortex and synaptic degeneration affecting associative limbic and cortical areas, starting from the hippocampus, with relative preservation of the primary areas (motor, somatosensory, and visual).
Diagnosis of AD at different levels of health care
In Brazil, economic realities and access to health services are quite heterogeneous. This requires adapting the instruments and diagnostic approaches within the levels of care established in the Guidelines of the Unified Health System (Sistema Único de Saúde - SUS) (Primary, Secondary, and Tertiary Care).
Table 2 shows the suggested protocols for the evaluation and diagnosis of AD at each level of care:
Table 2. Suggested protocol for the diagnosis of AD at each level of health care.
| Diagnosis in Primary Care | |
|---|---|
| Anamnesis | Ask the patient and their relative/caregiver about cognitive, neuropsychiatric, and behavioral symptoms. The interviews should be preferably conducted separately. |
| Clinical examination | Perform general physical examination looking for signs of systemic diseases and a complete neurological examination attentive to focal signs. |
| Laboratory tests | Perform laboratory tests to detect causes of secondary dementias and comorbidities that may contribute to the clinical picture. |
| Cognitive assessment | Applying a brief cognitive screening test, such as MoCA, BBRC, or CASI-S is suggested. BBRC stands out due to its easy application and high sensitivity, even for individuals with low schooling levels. Moreover, applying brief highly sensitive tasks, such as semantic verbal fluency (animals) and a word-learning test, which are highly accurate to detect dysfunction of the hippocampal system (amnesia), is recommended. Data on functionality can be addressed in the anamnesis or specific brief questionnaires, such as the Functional Activities Questionnaire (FAQ). |
| Structural neuroimaging | A brain CT is essential to rule out other causes of dementia (such as tumors, hydrocephalus, or cerebral infarctions) and to identify, within the limitations of the method, patterns of atrophy compatible with AD. |
| Diagnosis in Secondary Care | |
| Anamnesis | Ask the patient and their relative/caregiver about cognitive, neuropsychiatric, and behavioral symptoms. |
| Clinical examination | Perform general physical examination looking for signs of systemic diseases and a complete neurological examination attentive to focal signs. |
| Laboratory tests | Perform laboratory tests to detect causes of secondary dementias and comorbidities that may contribute to the clinical picture. If chronic meningitis is suspected, a lumbar puncture should be performed for CSF analysis. |
| Cognitive assessment | Using a brief test of cognitive screening or multifunctional battery of medium coverage is suggested, with at least one task to examine each cognitive domain; complement by applying a functional assessment tool, as described above. |
| Structural neuroimaging | Brain CT or MRI (preferably) is essential to rule out other causes of dementia and further investigate mesial structures, with visual scales or manual or automated volumetry. |
| Biomarker assessment | Using biomarkers is indicated in cases of diagnostic doubt between AD and other neurodegenerative dementias not in the amyloid spectrum, such as FTD, to correctly follow the guidelines for the use of AD approved medications, such as cholinesterase inhibitors and memantine. Other situations are referred to in biomarkers sections. Importantly, the future emergence and availability of potentially disease-modifying drugs will require evaluating all patients with mild AD as potential candidates for these treatments. Biomarkers can be requested from the secondary level of health care if they are reserved for selected cases and requested and interpreted by trained professionals. |
| Diagnosis in Tertiary Care | |
| Anamnesis | Conduct a detailed initial interview with the patient and their relative/caregiver. |
| Clinical examination | Perform a general physical examination looking for signs of systemic diseases and a complete neurological examination attentive to focal signs. |
| Laboratory tests | Perform laboratory tests to detect causes of secondary dementias and comorbidities that may contribute to the clinical picture and other tests if suspecting a relevant systemic disease. If chronic meningitis is suspected, a lumbar puncture should be performed for CSF analysis. |
| Cognitive assessment | A comprehensive neuropsychological evaluation using a cognitive screening test is suggested for applying instruments to further examine all cognitive domains and to assess functionality and neuropsychiatric disorders. |
| Structural neuroimaging | Brain MRI is essential to rule out other causes of dementia and further investigate the mesial temporal and other brain regions using visual scales or manual or automated volumetry. |
| Functional neuroimaging | FDG-PET or single-photon emission computerized tomography (SPECT) studies may show regional alterations in brain metabolism or changes in blood flow in cases of incipient and/or mild dementia, even in the absence of structural changes in neuroimaging. |
| Biomarker assessment | Using biomarkers is indicated in cases of diagnostic doubt between AD and other neurodegenerative dementias not in the amyloid spectrum, such as FTD, to correctly follow the guidelines for the use of AD approved medications, such as cholinesterase inhibitors and memantine. Other situations are referred to in biomarkers sections. Importantly, the future emergence and availability of potentially disease-modifying drugs will require evaluating all patients with mild AD as potential candidates for these treatments. |
FUTURE PROSPECTS
Diagnosis of preclinical AD
The pathogenic process of AD begins many years before the first clinical manifestations of the disease, and the analysis of biomarkers indicates the presence of asymptomatic individuals 127 . Preclinical AD is therefore a long and silent stage of the disease that precedes the first cognitive alterations which will later lead to the diagnosis of mild cognitive impairment (MCI) due to AD. It corresponds to a window of opportunity to implement interventions for delaying (or, ideally, interrupting) the pathogenic process of AD 128 . The existence of interventions modifying the pathogenesis of AD, associated with the possibility of identifying the disease in the asymptomatic phase will represent an effective way to establish the prevention of dementia.
The diagnostic criteria for preclinical AD, restricted to the research context, were proposed to identify individuals at risk of AD in the asymptomatic phase 129 )-( 131 . Three evolutionary stages inherent to preclinical AD were proposed: the first shows isolated evidence of cerebral amyloidogenesis according to the positivity of Aβ biomarkers; the second shows evidence of ongoing neurodegenerative process according to CSF and/or brain imaging biomarkers; and the third, indicates very subtle cognitive or behavioral alterations, insufficient for the diagnosis of MCI 129 .
Peripheral biomarkers of AD
Limitations for using these methods to better diagnose AD include the low availability and high cost of molecular image obtainment by PET and the need to perform lumbar puncture to obtain CSF samples. Developing new biomarkers in peripheral blood, with good diagnostic accuracy and predictive sensitivity, would thus significantly advance laboratory instrumentation in AD diagnosis. Moreover, determining plasma levels of Aβ, tau protein, and neurofilament light chain (NFL) -another neuronal cytoskeletal protein - with ultrasensitive methods could provide reliable estimates of cerebral amyloidogenesis and neurodegeneration in early stages of AD 121 ),( 132 ),( 133 . Plasma amyloid levels present a reliable correlation, measured by the relationship between peptides Aβ1-42 and Aβ1-40, and future positivity in amyloid PET 134 . Phosphorylated tau levels are present in other degenerative disorders but have been reported as elevated in plasma in individuals with AD, with the 181P-tau form showing greater specificity 133 ),( 135 . The presence of NFL in CSF indicates nonspecific neuronal damage. Recent studies have shown a positive correlation between plasma and NFL cerebrospinal fluid levels, but only regarding neurodegeneration 136 . A recent meta-analysis showed that NFL levels in both CSF and plasma have high diagnostic sensitivity for AD and other neurodegenerative dementias 137 .
Other approaches using genomics, transcriptomics, metabolomics, lipidomics, and proteomics have been applied to generate different biomarkers for AD. One study showed that altered microRNAs resulting from the failure of the synaptic function are potential plasma biomarkers for AD 138 . A Brazilian study showed decreased levels of ADAM10 PPA-secretases in platelets, decreased PSEN1 levels in platelets and leukocytes, and lower bace1 (β-secretase) levels in leukocytes 139 .
Implications of early diagnosis for disease-modifying therapies
The biomarker-based classification system proposed in 2018 indicates a broader concept of the pathological process in AD. However, clinical trials still face many challenges. Despite a growing understanding that clinical evaluation alone is limited for evaluating an intervention outcome, cognitive improvement measures are still the main outcomes in all clinical trials. Identifying the best molecular targets or a combination of them by developing better protocols to assess the results of interventions using biochemical, physiological, and neuropsychological measures as outcomes is essential to identify individuals in preclinical stages of AD and facilitate early therapeutic interventions. This is the premise of most efforts to find new therapies.
ACKNOWLEDGEMENTS
PC, LCS and RN are funded by CNPq, Brazil (bolsa de produtividade em pesquisa).
Referências
- 1.Gauthier S, Rosa-Neto P, Morais JA, Webster C. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London: Alzheimer's Disease International (UK); 2021. 313 p [Google Scholar]
- 2.Melo SC, Champs APS, Goulart RF, Malta DC, Passos VMA. Dementias in Brazil: increasing burden in the 2000-2016 period. Estimates from the Global Burden of Disease Study 2016. Arq Neuropsiquiatr. 2020;78(12):762–771. doi: 10.1590/0004-282X20200059. [DOI] [PubMed] [Google Scholar]
- 3.Robinson M, Lee BY, Hane FT. Recent progress in Alzheimer's disease research, part 2: genetics and epidemiology. J Alzheimers Dis. 2017;57(2):317–330. doi: 10.3233/JAD-161149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JH. Genetics of Alzheimer's disease. Dement Neurocogn Disord. 2018;17(4):131–136. doi: 10.12779/dnd.2018.17.4.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkowitz CL, Mosconi L, Rahman A, Scheyer O, Hristov H, Isaacson RS. Clinical application of APOE in Alzheimer's prevention: a precision medicine approach. J Prev Alzheimers Dis. 2018;5(4):245–252. doi: 10.14283/jpad.2018.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braak H, Zetterberg H, Del Tredici K, Blennow K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but Amyloid-ß changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol. 2013;126(5):631–641. doi: 10.1007/s00401-013-1139-0. [DOI] [PubMed] [Google Scholar]
- 8.Brundel M, Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32(3):425–436. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sáiz-Vazquez O, Puente-Martínez A, Ubillos-Landa S, Pacheco-Bonrostro J, Santabárbara J. Cholesterol and Alzheimer's disease risk: a meta-meta- analysis. Brain Sci. 2020;10(6):386–386. doi: 10.3390/brainsci10060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schilling LP, Pascoal TA, Zimmer ER, Mathotaarachchi S, Shin M, Rieder CRM. Regional Amyloid-ß load and white matter abnormalities contribute to hypometabolism in Alzheimer's dementia. Mol Neurobiol. 2019;56(7):4916–4924. doi: 10.1007/s12035-018-1405-1. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero-Muñoz MJ, Gerson J, Castillo-Carranza DL. Tau oligomers: the toxic player at synapses in Alzheimer's disease. Front Cell Neurosci. 2015;9:464–464. doi: 10.3389/fncel.2015.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengupta P, Satpute-Krishnan P, Seo AY, Burnette DT, Patterson GH, Lippincott-Schwartz J. ER trapping reveals Golgi enzymes continually revisit the ER through a recycling pathway that controls Golgi organization. Proc Natl Acad Sci U S A. 2015;112(49):E6752–E6E61. doi: 10.1073/pnas.1520957112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frota NAF, Nitrini R, Damasceno BP, Forlenza OV, Dias-Tosta E, Silva AB. Criteria for the diagnosis of Alzheimer's disease. Recommendations os the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Dement Neuropsychol. 2011;5(3):146–152. doi: 10.1590/S1980-57642011DN05030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montembeault M, Brambati SM, Gorno-Tempini ML, Migliaccio R. Clinical, anatomical, and pathological features in the three variants of primary progressive aphasia: a review. Front Neurol. 2018;9:692–692. doi: 10.3389/fneur.2018.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS. van der Flier WM Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13(8):870–884. doi: 10.1016/j.jalz.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fumagalli GG, Basilico P, Arighi A, Mercurio M, Scarioni M, Carandini T. Parieto-occipital sulcus widening differentiates posterior cortical atrophy from typical Alzheimer disease. Neuroimage Clin. 2020;28:102453–102453. doi: 10.1016/j.nicl.2020.102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130(10):2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 20.Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, Vogel JW. The behavioural/dysexecutive variant of Alzheimer's disease clinical, neuroimaging and pathological features. Brain. 2015;138(9):2732–2749. doi: 10.1093/brain/awv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ossenkoppele R, Singleton EH, Groot C, Dijkstra AA, Eikelboom WS, Seeley WW. Research criteria for the behavioral variant of Alzheimer disease: a systematic review and meta-analysis. JAMA Neurol. 2022;79(1):48–60. doi: 10.1001/jamaneurol.2021.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townley RA, Graff-Radford J, Mantyh WG, Botha H, Polsinelli AJ, Przybelski SA. Progressive dysexecutive syndrome due to Alzheimer's disease: a description of 55 cases and comparison to other phenotypes. Brain Commun. 2020;2(1):1–19. doi: 10.1093/braincomms/fcaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graff-Radford J, Yong KXX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222–234. doi: 10.1016/S1474-4422(20)30440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snowden JS, Stopford CL, Julien CL, Thompson JC, Davidson Y, Gibbons L. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex. 2007;43(7):835–845. doi: 10.1016/s0010-9452(08)70683-x. [DOI] [PubMed] [Google Scholar]
- 25.Mendez MF, Joshi A, Tassniyom K, Teng E, Shapira JS. Clinicopathologic differences among patients with behavioral variant frontotemporal dementia. Neurology. 2013;80(6):561–568. doi: 10.1212/WNL.0b013e3182815547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisberg B, Ferris SH, Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 27.Tatsch MF, Bottino CMC, Azevedo D, Hototian SR, Moscoso MA, Folquitto JC. Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: prevalence and relationship with dementia severity. Am J Geriatr Psychiatry. 2006;14(5):438–445. doi: 10.1097/01.JGP.0000218218.47279.db. [DOI] [PubMed] [Google Scholar]
- 28.Gauthier S, Cummings J, Ballard C, Brodaty H, Grossberg G, Robert P. Management of behavioral problems in Alzheimer's disease. Int Psychogeriatr. 2010;22(3):346–372. doi: 10.1017/S1041610209991505. [DOI] [PubMed] [Google Scholar]
- 29.Lacerda IB, Santos RL, Belfort T, Neto JPS, Dourado MCN. Patterns of discrepancies in different objects of awareness in mild and moderate Alzheimer's disease. Aging Ment Health. 2020;24(5):789–796. doi: 10.1080/13607863.2018.1544219. [DOI] [PubMed] [Google Scholar]
- 30.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer's disease. Arch Neurol. 2009;66(4):435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, Lavrencic L, Radford K, Booth A, Yoshimura S, Anstey KJ. Systematic review of coexistent epileptic seizures and Alzheimer's disease: incidence and prevalence. J Am Geriatr Soc. 2021;69(7):2011–2020. doi: 10.1111/jgs.17101. [DOI] [PubMed] [Google Scholar]
- 32.Vermunt L, Sikkes SAM. van den Hout A.Handels R.Bos I.van der Flier WM Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15(7):888–898. doi: 10.1016/j.jalz.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M. The antibody aducanumab reduces Aß plaques in Alzheimer's disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 35.Smid J, César-Freitas KG, Dourado MCN, Kochhann R, Studart-Neto A, Barbosa BJAPB, et al. Subjective cognitive decline, mild cognitive impairment, and dementia - syndromic approach. Dement Neuropsychol. 2022;16(S1) doi: 10.1590/1980-5764-DN-2022-S101PT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42(1):275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega LFV, Aprahamian I, Borges MK, Cação JC, Yassuda MS. Screening for Alzheimer's disease in low-educated or illiterate older adults in Brazil: a systematic review. Arq Neuropsiquiatr. 2019;77(4):279–288. doi: 10.1590/0004-282X20190024. [DOI] [PubMed] [Google Scholar]
- 39.Folstein MF, Folstein SE, Folstein , McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 40.Brucki SM, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH. Suggestions for utilization of the mini-mental state examination in Brazil. Arq Neuropsiquiatr. 2003;61(3B):777–781. doi: 10.1590/s0004-282x2003000500014. [DOI] [PubMed] [Google Scholar]
- 41.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 42.Memória CM, Yassuda MS, Nakano EY, Forlenza OV. Brief screening for mild cognitive impairment: validation of the Brazilian version of the Montreal cognitive assessment. Int J Geriatr Psychiatry. 2013;28(1):34–40. doi: 10.1002/gps.3787. [DOI] [PubMed] [Google Scholar]
- 43.Teng EL, Larson EB, Lin KN, Graves AB, Liu HC. Screening for dementia: the Cognitive Abilities Screening Instrument - short version (CASI Short); 106Annual Convention of the American Psychological Association; 1998 Aug 14-18; San Francisco, US. Washington, DC: American Psychological Association; 1998. [Google Scholar]
- 44.Damasceno A, Delicio AM, Mazo DFC, Zullo JFD, Scherer P, Ng RTY. Validation of the Brazilian version of mini-test CASI-S. Arq Neuropsiquiatr. 2005;63(2b):416–421. doi: 10.1590/S0004-282X2005000300010. [DOI] [PubMed] [Google Scholar]
- 45.Nitrini R, Lefèvre BH, Mathias SC, Caramelli P, Carrilho PE, Sauaia N. Neuropsychological tests of simple application for diagnosing dementia. Arq Neuropsiquiatr. 1994;52(4):457–465. doi: 10.1590/s0004-282x1994000400001. [DOI] [PubMed] [Google Scholar]
- 46.Nitrini R, Brucki SMD, Yassuda MS, Fichman HC, Caramelli P. The Figure Memory Test: diagnosis of memory impairment in populations with heterogeneous educational background. Dement. Neuropsychol. 2021;15(2):173–185. doi: 10.1590/1980-57642021dn15-020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21(11):1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- 48.Carvalho VA, Caramelli P. Brazilian adaptation of the Addenbrooke's Cognitive Examination-Revised (ACE-R) Dement Neuropsychol. 2007;1(2):212–216. doi: 10.1590/S1980-57642008DN10200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 50.Nunes PV, Diniz BS, Radanovic M, Abreu ID, Borelli DT, Yassuda MS. CAMcog as a screening tool for diagnosis of mild cognitive impairment and dementia in a Brazilian clinical sample of moderate to high education. Int J Geriatr Psychiatry. 2008;23(11):1127–1133. doi: 10.1002/gps.2038. [DOI] [PubMed] [Google Scholar]
- 51.Aprahamian I, Martinelli JE, Cecato J, Izbicki R, Yassuda MS. Can the CAMCOG be a good cognitive test for patients with Alzheimer's disease with low levels of education? Int Psychogeriatr. 2011;23(1):96–101. doi: 10.1017/S104161021000116X. [DOI] [PubMed] [Google Scholar]
- 52.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 53.Schultz RR, Siviero MO, Bertolucci PH. The cognitive subscale of the "Alzheimer's Disease Assessment Scale" in a Brazilian sample. Braz J Med Biol Res. 2001;34(10):1295–1302. doi: 10.1590/s0100-879x2001001000009. [DOI] [PubMed] [Google Scholar]
- 54.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 55.Bertolucci PHF, Okamoto IH, Brucki SMD, Siviero MO, Toniolo J, Neto, Ramos LR. Applicability of the CERAD neuropsychological battery to Brazilian elderly. Arq Neuropsiquiatr. 2001;59(3A):532–536. doi: 10.1590/S0004-282X2001000400009. [DOI] [PubMed] [Google Scholar]
- 56.Vitaliano PP, Breen AR, Russo J, Albert M, Vitiello MV, Prinz PN. The clinical utility of the dementia rating scale for assessing Alzheimer patients. J Chronic Dis. 1984;37(9-10):743–753. doi: 10.1016/0021-9681(84)90043-2. [DOI] [PubMed] [Google Scholar]
- 57.Porto CS, Fichman HC, Caramelli P, Bahia VS, Nitrini R. Brazilian version of the Mattis dementia rating scale: diagnosis of mild dementia in Alzheimer's disease. Arq Neuropsiquiatr. 2003;61(2B):339–345. doi: 10.1590/s0004-282x2003000300004. [DOI] [PubMed] [Google Scholar]
- 58.Rey A. L'examen clinique en psychologie [The clinical examination in psychology] Paris (France): Presses Universitaires de France; 1964. [Google Scholar]
- 59.Malloy-Diniz LF, Lasmar VA, Gazinelli LS, Fuentes D, Salgado JV. The Rey Auditory-Verbal Learning Test: applicability for the Brazilian elderly population. Braz J Psiquiatry. 2007;29(4):324–329. doi: 10.1590/S1516-44462006005000053. [DOI] [PubMed] [Google Scholar]
- 60.Osterrieth PA. Le test de copie d'une figure complexe; contribution à l'étude de la perception et de la mémoire [Test of copying a complex figure; contribution to the study of perception and memory] Arch Psychol. 1944;30:206–356. [Google Scholar]
- 61.Foss MP, Bastos-Formigheri MS, Speciali JG. Rey's complex figures for the elderly. Aval Psicol. 2010;9(1):53–61. [Google Scholar]
- 62.Hazin I, Leite G, Oliveira RM, Alencar JC, Fichman HC, Marques PN. Brazilian Normative Data on Letter and Category Fluency Tasks: effects of gender, age, and geopolitical region. Front Psychol. 2016;7:684–684. doi: 10.3389/fpsyg.2016.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caramelli P, Carthery-Goulart MT, Porto CS, Fichman HC, Nitrini R. Category fluency as a screening test for Alzheimer disease in illiterate and literate patients. Alzheimer Dis Assoc Disord. 2007;21(1):65–67. doi: 10.1097/WAD.0b013e31802f244f. [DOI] [PubMed] [Google Scholar]
- 64.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 65.Mansur LL, Radanovic M, Araújo GC, Taquemori LY, Greco LL. Boston Naming Test performance of Brazilian population from São Paulo. Pro Fono. 2006;18(1):13–20. doi: 10.1590/S0104-56872006000100003. [DOI] [PubMed] [Google Scholar]
- 66.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 67.Zimmermann N, Cardoso CO, Trentini CM, Grassi-Oliveira R, Fonseca RP. Brazilian preliminary norms and investigation of age and education effects on the Modified Wisconsin Card Sorting Test, Stroop Color and Word test and Digit Span test in adults. Dement Neuropsychol. 2015;9(2):120–127. doi: 10.1590/1980-57642015DN92000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shulman KI, Shedletsky R, Silver IL. The challenge of time: clock-drawing and cognitive function in the elderly. Int J Geriatr Psychiatry. 1986;1(2):135–140. doi: 10.1002/gps.930010209. [DOI] [Google Scholar]
- 69.Fuzikawa C, Lima-Costa MF, Uchoa E, Barreto SM, Shulman K. Bambuí Health and Ageing Study A population based study on the intra and inter-rater reliability of the clock drawing test in Brazil: the Bambuí Health and Ageing Study. Int J Geriatr Psychiatry. 2003;18(5):450–456. doi: 10.1002/gps.863. [DOI] [PubMed] [Google Scholar]
- 70.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 71.Herrera E, Jr, Caramelli P, Silveira AS, Nitrini R. Epidemiologic survey of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2002;16(2):103–108. doi: 10.1097/00002093-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19(4):1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez MA, Lawrence RA. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): adaptação transcultural para uso no Brasil. Cad Saude Publica. 2009;25(7):1455–1465. doi: 10.1590/S0102-311X2009000700003. [DOI] [PubMed] [Google Scholar]
- 74.Loewnstein DA, Bates CB. The Direct Assessment of Functional Status-Revised (DAFS-R). Manual for administration and scoring. Miami: Mount Sinai Medical Center; 2006. [Google Scholar]
- 75.Pereira FS, Oliveira AM, Diniz BS, Forlenza OV, Yassuda MS. Cross-cultural adaptation, reliability and validity of the DAFS-R in a sample of Brazilian older adults. Arch Clin Neuropsychol. 2010;25(4):335–343. doi: 10.1093/arclin/acq029. [DOI] [PubMed] [Google Scholar]
- 76.Gauthier L, Gélinas I, McIntyre M, Gauthier S, Laberge H, Dauphinee SW. Disability Assessment for Dementia (DAD) user's guide. 1994. [Google Scholar]
- 77.Bahia VS, Carthery-Goulart MT, Novelli MM, Kato-Narita EM, Areza-Fegyveres R, Caramelli P. Functional disability in Alzheimer disease: a validation study of the Brazilian version of Disability Assessment for Dementia (DAD-Br) Alzheimer Dis Assoc Disord. 2010;24(3):291–295. doi: 10.1097/WAD.0b013e3181cfc878. [DOI] [PubMed] [Google Scholar]
- 78.Johnson N, Barion A, Rademaker A, Rehkemper G, Weintraub S. The Activities of Daily Living Questionnaire: a validation study in patients with dementia. Alzheimer Dis Assoc Disord. 2004;18(4):223–230. [PubMed] [Google Scholar]
- 79.Medeiros ME, Guerra RO. Translation, cultural adaptation and psychometric analysis of the Activities of Daily Living Questionnaire (ADLQ) for functional assessment of patients with Alzeimer's disease. Braz J Phys Ther. 2009;13(3):257–266. doi: 10.1590/S1413-35552009005000027. [DOI] [Google Scholar]
- 80.Hindmarch I, Lehfeld H, Jongh P, Erzigkeit H. The Bayer Activities of Daily Living Scale (B-ADL) Dement Geriatr Cogn Disord. 1998;9(Suppl 2):20–26. doi: 10.1159/000051195. [DOI] [PubMed] [Google Scholar]
- 81.Folquitto JC, Bustamante SEZ, Barros SB, Azevedo D, Lopes MA, Hototian SR. The Bayer: Activities of Daily Living Scale (B-ADL) in the differentiation between mild to moderate dementia and normal aging. Braz J Psychiatry. 2007;29(4):350–353. doi: 10.1590/S1516-44462006005000037. [DOI] [PubMed] [Google Scholar]
- 82.Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 83.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 84.Lino VTS, Pereira SRM, Camacho LAB, Ribeiro ST, Filho, Buksman S. Cross-cultural adaptation of the Independence in Activities of Daily Living Index (Katz Index) Cad Saude Publica. 2008;24(1):103–112. doi: 10.1590/s0102-311x2008000100010. [DOI] [PubMed] [Google Scholar]
- 85.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 86.Chaves ML, Camozzato AL, Godinho C, Kochhann R, Schuh A, Almeida VL. Validity of the clinical dementia rating scale for the detection and staging of dementia in Brazilian patients. Alzheimer Dis Assoc Disord. 2007;21(3):210–217. doi: 10.1097/WAD.0b013e31811ff2b4. [DOI] [PubMed] [Google Scholar]
- 87.O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol. 2008;65(8):1091–1095. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N. Practice parameter: diagnosis of dementia (an evidence- based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 89.Nitrini R, Caramelli P, Bottino CM, Damasceno BP, Brucki SM, Anghinah R. Diagnosis of Alzheimer's disease in Brazil: cognitive and functional evaluation. Recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Arq Neuropsiquiatr. 2005;63(3A):720–727. doi: 10.1590/s0004-282x2005000400034. [DOI] [PubMed] [Google Scholar]
- 90.van der Flier WM.Scheltens P Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 5):v2–v7. doi: 10.1136/jnnp.2005.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hort J. O'Brien JT.Gainotti G.Pirttila T.Popescu BO.Rektorova I EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010;17(10):1236–1248. doi: 10.1111/j.1468-1331.2010.03040.x. [DOI] [PubMed] [Google Scholar]
- 92.Caramelli P, Teixeira AL, Buchpiguel CA, Lee HW, Livramento JA, Fernandez LL. Diagnosis of Alzheimer's disease in Brazil: supplementary exams. Dement Neuropsychol. 2011;5(3):167–177. doi: 10.1590/S1980-57642011DN05030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sintini I, Graff-Radford J, Senjem ML, Schwarz CG, Machulda MM, Martin PR. Longitudinal neuroimaging biomarkers differ across Alzheimer's disease phenotypes. Brain. 2020;143(7):2281–2294. doi: 10.1093/brain/awaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barkhof F, Fox NC, Bastos-Leite AJ, Scheltens P. Neuroimaging in dementia. Berlin: Springer; 2011. [Google Scholar]
- 95.Amato ACS, Filho, Balthazar MLF. Neurologia cognitiva e do envelhecimento: do conhecimento básico à abordagem clínica. São Paulo: Omnifarma; 2016. Neuroimagem nas demências: como ela pode nos ajudar? [Google Scholar]
- 96.Wattjes MP, Henneman WJ. van der Flier WM.de Vries O.Träber F.Geurts JJ Diagnostic imaging of patients in a memory clinic: comparison of MR imaging and 64-detector row CT. Radiology. 2009;253(1):174–183. doi: 10.1148/radiol.2531082262. [DOI] [PubMed] [Google Scholar]
- 97.Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer's disease. Nat Rev Neurol. 2010;6(2):67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leocadi M, Canu E, Calderaro D, Corbetta D, Filippi M, Agosta F. An update on magnetic resonance imaging markers in AD. Ther Adv Neurol Disord. 2020;13:1–17. doi: 10.1177/1756286420947986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lombardi G, Crescioli G, Cavedo E, Lucenteforte E, Casazza G, Bellatorre AG. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment. Cochrane Database Syst Rev. 2020;3(3):CD009628–CD009628. doi: 10.1002/14651858.CD009628.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maul S, Giegling I, Rujescu D. Proton magnetic resonance spectroscopy in common dementias - current status and perspectives. Front Psychiatry. 2020;11:769–769. doi: 10.3389/fpsyt.2020.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koedam EL, Lehmann M. van der Flier WM.Scheltens P.Pijnenburg YA.Fox N Visual assessment of posterior atrophy development of an MRI rating scale. Eur Radiol. 2011;21(12):2618–2625. doi: 10.1007/s00330-011-2205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 103.Diniz BS, Pinto JA, Jr, Forlenza OV. Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer's disease? A systematic review and meta- analysis of the literature. World J Biol Psychiatry. 2008;9(3):172–182. doi: 10.1080/15622970701535502. [DOI] [PubMed] [Google Scholar]
- 104.Forlenza OV, Radanovic M, Talib LL, Aprahamian I, Diniz BS, Zetterberg H. Cerebrospinal fluid biomarkers in Alzheimer's disease: diagnostic accuracy and prediction of dementia. Alzheimers Dement (Amst) 2015;1(4):455–463. doi: 10.1016/j.dadm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dorey A, Perret-Liaudet A, Tholance Y, Fourier A, Quadrio I. Cerebrospinal fluid Aß40 improves the interpretation of Aß42 concentration for diagnosing Alzheimer's disease. Front Neurol. 2015;6:247–247. doi: 10.3389/fneur.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Racine AM, Koscik RL, Nicholas CR, Clark LR, Okonkwo OC, Oh JM. Cerebrospinal fluid ratios with Aß42 predict preclinical brain ß-amyloid accumulation. Alzheimers Dement (Amst) 2016;2:27–38. doi: 10.1016/j.dadm.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid ß (Aß) 42/40 ratio in the diagnosis of Alzheimer's disease. Alzheimers Res Ther. 2019;11(1):34–34. doi: 10.1186/s13195-019-0485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silverman DH, Small GW, Phelps ME. Clinical value of neuroimaging in the diagnosis of dementia. Sensitivity and specificity of regional cerebral metabolic and other parameters for early identification of Alzheimer's disease. Clin Positron Imaging. 1999;2(3):119–130. doi: 10.1016/s1095-0397(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 109.Silverman DH, Small GW, Chang CY, Lu CS, De Aburto MAK, Chen W. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286(17):2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 110.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32(4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 111.Schöll M, Damián A, Engler H. Fluorodeoxyglucose PET in neurology and psychiatry. PET Clin. 2014;9(4):371–390. doi: 10.1016/j.cpet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 113.Schilling LP, Zimmer ER, Shin M, Leuzy A, Pascoal TA, Benedet AL. Imaging Alzheimer's disease pathophysiology with PET. Dement Neuropsychol. 2016;10(2):79–90. doi: 10.1590/S1980-5764-2016DN1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fleisher AS, Pontecorvo MJ, Devous MD, Lu M, Arora AK, Truocchio SP. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020;77(7):829–839. doi: 10.1001/jamaneurol.2020.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM. Tau PET in Alzheimer's disease and mild cognitive impairment. Neurology. 2016;87(4):375–383. doi: 10.1212/WNL.0000000000002892. [DOI] [PubMed] [Google Scholar]
- 116.Counts SE, Ikonomovic MD, Mercado N, Vega IE, Mufson EJ. Biomarkers for the early detection and progression of Alzheimer's disease. Neurotherapeutics. 2017;14(1):35–53. doi: 10.1007/s13311-016-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsuda H, Shigemoto Y, Sato N. Neuroimaging of Alzheimer's disease: focus on amyloid and tau PET. Jpn J Radiol. 2019;37(11):735–749. doi: 10.1007/s11604-019-00867-7. [DOI] [PubMed] [Google Scholar]
- 118.Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139(5):1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimers Dement (N Y) 2019;5(1):272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blennow K. A review of fluid biomarkers for Alzheimer's disease: moving from CSF to blood. Neurol Ther. 2017;6(Suppl 1):15–24. doi: 10.1007/s40120-017-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J. Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9(7):560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 124.Wang L, Benzinger TL, Su Y, Christensen J, Friedrichsen K, Aldea P. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between ß-Amyloid and tauopathy. JAMA Neurol. 2016;73(9):1070–1077. doi: 10.1001/jamaneurol.2016.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer's disease. Neurobiol Aging. 2009;30(7):1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Josefsson M, Luna X, Pudas S, Nilsson LG, Nyberg L. Genetic and lifestyle predictors of 15-year longitudinal change in episodic memory. J Am Geriatr Soc. 2012;60(12):2308–2312. doi: 10.1111/jgs.12000. [DOI] [PubMed] [Google Scholar]
- 127.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mormino EC, Sperling RA, Holmes AJ, Buckner RL, De Jager PL, Smoller JW. Polygenic risk of Alzheimer's disease is associated with early- and late-life processes. Neurology. 2016;87(5):481–488. doi: 10.1212/WNL.0000000000002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bondi MW, Edmonds EC, Salmon DP. Alzheimer's disease: past, present, and future. J Int Neuropsychol Soc. 2017;23(9-10):818–831. doi: 10.1017/S135561771700100X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henriksen K. O'Bryant SE.Hampel H.Trojanowski JQ.Montine TJ.Jeromin A The future of blood-based biomarkers for Alzheimer's disease. Alzheimers Dement. 2014;10(1):115–131. doi: 10.1016/j.jalz.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bateman RJ, Blennow K, Doody R, Hendrix S, Lovestone S, Salloway S. Plasma biomarkers of AD emerging as essential tools for drug development: an EU/US CTAD task force report. J Prev Alzheimers Dis. 2019;6(3):169–173. doi: 10.14283/jpad.2019.21. [DOI] [PubMed] [Google Scholar]
- 134.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T. Amyloid ß concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841–849. doi: 10.1016/j.jalz.2017.06.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J. Plasma tau levels in Alzheimer's disease. Alzheimers Res Ther. 2013;5(2):9–9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mattsson N, Andreasson U, Zetterberg H, Blennow K. Alzheimer's Disease Neuroimaging Initiative Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74(5):557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao Y, Xin Y, Meng S, He Z, Hu W. Neurofilament light chain protein in neurodegenerative dementia: a systematic review and network meta-analysis. Neurosci Biobehav Rev. 2019;102:123–138. doi: 10.1016/j.neubiorev.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 138.Siedlecki-Wullich D, Català-Solsona J, Fábregas C, Hernández I, Clarimon J, Lleó A. Altered microRNAs related to synaptic function as potential plasma biomarkers for Alzheimer's disease. Alzheimers Res Ther. 2019;11(1):46–46. doi: 10.1186/s13195-019-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bram JMF, Talib LL, Joaquim HPG, Sarno TA, Gattaz WF, Forlenza OV. Protein levels of ADAM10, BACE1, and PSEN1 in platelets and leukocytes of Alzheimer's disease patients. Eur Arch Psychiatry Clin Neurosci. 2019;269(8):963–972. doi: 10.1007/s00406-018-0905-3. [DOI] [PubMed] [Google Scholar]


