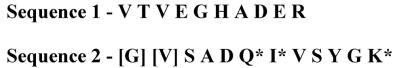Abstract
Complexes containing lipopolysaccharide (LPS) and three outer membrane proteins (OMPs) are released by gram-negative bacteria incubated in human serum and into the circulation in an experimental model of sepsis. The same OMPs are bound by immunoglobulin G (IgG) in the cross-protective antiserum raised to Escherichia coli J5 (anti-J5 IgG). This study was performed to identify the three OMPs. The 35-kDa OMP was identified as outer membrane protein A (OmpA) by immunoblotting studies using OmpA-deficient bacteria and recombinant OmpA protein. The 18-kDa OMP was identified as peptidoglycan-associated lipoprotein (PAL) based on peptide sequences from the purified protein and immunoblotting studies using PAL-deficient bacteria. The 5- to 9-kDa OMP was identified as murein lipoprotein (MLP) based on immunoblotting studies using MLP-deficient bacteria. The studies identify the OMPs released into human serum and into the circulation in an experimental model of sepsis as OmpA, PAL, and MLP.
Bacterial cell wall components released into the bloodstream are believed to be important in the pathogenesis of gram-negative sepsis. Although prior investigators have reported that bacteria release lipopolysaccharide (LPS) into serum (62, 63) and into the circulation (4, 18, 56, 66), the full composition of released bacterial products has not been established. Very little is known about release of non-LPS gram-negative outer membrane components such as outer membrane proteins (OMPs) in sepsis. Fragments containing LPS, OmpA, and another faintly staining protein, of 17 kDa, were affinity purified from filtrates of human serum incubated with Salmonella enterica serovar Abortus equi bacteria using O-chain-specific anti-LPS immunoglobulin G (IgG) (20). Similarly, we have affinity purified complexes containing LPS and at least three OMPs, with estimated molecular masses of 35, 18, and 5 to 9 kDa, from filtrates of normal human serum incubated with Escherichia coli bacteria, using O-chain-specific anti-LPS IgG (29, 30).
Previous studies indicated that passive and active immunity directed to rough mutant bacteria such as S. enterica serovar Minnesota Re595 and E. coli J5 protect in experimental and clinical gram-negative sepsis (1, 5, 11, 42, 43, 68). Protection has been attributed to antibodies directed to conserved core components of LPS (lipid A and core oligosaccharide). However, it has been difficult to prove that antisera to rough strains of bacteria contain cross-reactive anti-lipid A or anti-core oligosaccharide IgGs (15, 57), and the exact mechanism of protection remains unclear and controversial.
We have demonstrated that IgG in antiserum raised to heat-killed E. coli J5 (J5 antiserum) binds to the same three gram-negative bacterial OMPs that are released into serum in the OMP-LPS complexes described above (30). OMP-LPS complexes are also released into the bloodstream of burned rats with E. coli O18K+ sepsis (29). In addition, at least one OMP, with an estimated molecular mass of 18 kDa, is released from bacteria separately from the OMP-LPS complexes and in a form that is selectively affinity purified from human serum and septic rat plasma by IgG in J5 antiserum (29).
This study was performed to identify the 35-, 18-, and 5- to 9-kDa OMPs that are released in vitro into human serum (30) and in vivo into the circulation in experimental gram-negative sepsis (29) and are bound by IgG in J5 antiserum.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
E. coli J5 was a gift from J. C. Sadoff (Walter Reed Army Institute of Research, Washington, D.C.); E. coli O18:K1:H7 (designated E. coli O18K+), E. coli O18:K1−:G2A (a nonencapsulated derivative of O18:K1:H7, designated E. coli O18K−), E. coli O8:K45:H1, E. coli O16:K1:H6, and E. coli O25:K5:H1 were gifts from A. Cross (University of Maryland Cancer Center, Baltimore). OMP-deficient E. coli K-12 and E. coli O18 mutants and closely related OMP-containing bacteria were used for immunoblotting studies. E. coli O18 E91 (OmpA-deficient derivative of E. coli O18:K1:H7) and E69 (OmpA-restored derivative of E. coli O18:K1:H7) were generated as previously described (52). E. coli K-12 1292 (39), JC7752 (peptidoglycan-associated lipoprotein [PAL]-deficient derivative of 1292), and 7752(p417) (PAL-restored mutant of JC7752) were kindly provided by J.-C. Lazzaroni (Université Claude Bernard, Lyon, France). E. coli K-12(p400), CH202 [PAL-deficient mutant of E. coli K-12(p400)], and CH202(pRC2) (PAL-restored derivative of CH202) were kindly provided by U. Henning (Max-Planck-Institut für Biologie, Tübingen, Germany) (12). The E. coli K-12 mutant that lacks murein lipoprotein (MLP; Braun's lipoprotein) due to a deletion of the 1po gene, JE5505 (F− 1po his proA argE thi gal lac xyl mtl tsx), and its otherwise identical 1po-positive partner that contains MLP, JE5506 (F− pps his proA argE thi gal lac xyl mtl tsx), were kindly provided by H. Nikaido (University of California, Berkeley) (32).
Bacteria were cultured in Trypticase soy broth (Difco, Detroit, Mich.) from colonies stored on Trypticase soy agar (Difco). Media were supplemented with kanamycin (50 mg/ml) for E. coli K-12 CH202(pRC2) and ampicillin (100 mg/ml) for E. coli K-12 JC7752(p417) to maintain the plasmids. Bacteria were cultured at 37°C with vigorous agitation to the desired growth phase, harvested, and washed by low-speed centrifugation in sterile normal saline (5,000 to 8,000 × g, 8 to 10 min, 4°C).
Monoclonal antibodies.
Monoclonal antibodies were prepared against each of the three OMPs bound by IgG in J5 antiserum and against the O-polysaccharide of E. coli O18 LPS. For production of anti-OMP monoclonal antibodies, BALB/c mice (Charles River Laboratories, Wilmington, Mass.) were immunized with heat-killed, lyophilized E. coli J5 vaccine prepared as described elsewhere (57). Vaccine was resuspended in sterile normal saline (1 mg/ml). Increasing doses (0.1, 0.2, and 0.3 mg) were injected intraperitoneally three times per week for 3 weeks. Booster injections were given monthly for 1 to 3 months, with the final booster 3 days before the spleen was harvested. Splenocytes were harvested and fused with myeloma cells by standard laboratory protocol (27, 36). Hybridoma cell lines were cultured in Dulbecco's modification of Eagle's medium (Cellgro; Mediatech Inc., Herndon, Va.) supplemented with glucose (4.5 g/liter), l-glutamine, 20% heat-inactivated fetal calf serum (Mediatech), penicillin (100 U/ml), and streptomycin (100 mg/ml).
The three OMPs are exposed on the surface of bacteria after incubation in human serum (30). Accordingly, monoclonal antibodies were initially screened by bacterial enzyme-linked immunosorbent assay (ELISA), using serum-exposed smooth E. coli isolates (E. coli O8:K45:H1, O16:K1:H6, and O25:K5:H1) as coating antigen and hybridoma culture supernatants as primary antibody. Antibodies that bound to serum-exposed bacteria were then tested by immunoblotting using E. coli O25:K5:H1 bacterial lysates as antigen and hybridoma culture supernatants as primary antibody. A MilliBlot-MP membrane processor (Millipore Corporation, Bedford, Mass.) was used for application of primary antibody. Blots were developed as described below. Following initial screening, hybridomas of interest were subcloned by limiting dilution to one cell in every fourth well to derive subclones with strong growth characteristics and high production of the antibodies with the binding characteristics described below. Polyclonal mouse anti-J5 IgG was used as a positive control, and preimmune serum served as the negative control. Antibodies directed against the OMPs were selected based on binding to bands in bacterial lysates that were the same molecular weights as the three OMPs bound by anti-J5 IgG (30). To confirm that the monoclonal antibodies were binding OMPs, immunoblotting was performed with outer membranes that were incubated with or without proteinase K prior to electrophoresis as previously described (30).
Two methods were used to prepare large amounts of the monoclonal IgGs from the hybridoma cell lines isolated as described above. Monoclonal antibodies directed to each of the three OMPs and to the O polysaccharide of E. coli O18 LPS (monoclonal anti-O18 IgG) were produced in ascites fluid of BALB/c mice by mouse hybridoma cell lines. The hybridoma cell line producing anti-O IgG was the kind gift of A. Cross (34). Ten days after intraperitoneal instillation of 0.5 ml of pristane (Sigma, St. Louis, Mo.), 5 × 106 to 10 × 106 hybridoma cells were collected, washed twice in Hanks' balanced salt solution (Mediatech), and injected intraperitoneally. Ascites fluid was collected by aspiration every 2 to 3 days three times (27). Monoclonal antibody against the 18-kDa OMP was also produced in an artificial capillary cell culture system (Cellmax; Cellco, Laguna Hills, Calif.). The cartridge (Cellmax 011 module) was inoculated with 2.5 × 107 viable cells. Culture medium was Dulbecco's modification of Eagle's medium (Mediatech) supplemented with glucose (4.5 g/liter), l-glutamine, 2.5 to 10% heat-inactivated fetal calf serum (Mediatech), penicillin (100 U/ml), and streptomycin (100 mg/ml). The concentration of IgG produced in the artificial capillary cell culture was 0.3 to 1.0 mg/ml as determined by ELISA. Anti-OMP antibodies showed no cross-reactivity with LPS or with proteins in human serum by immunoblotting. The monoclonal anti-O18 IgG does not cross-react with LPS from other organisms, with the OMPs, or with proteins in human serum by immunoblotting.
Sera, antisera, IgG, and immunobeads.
Sera and antisera were prepared from the blood of 2- to 3-kg New Zealand White rabbits (ARI Breeding Laboratories, East Bridgewater, Mass.) and BALB/c mice. Antiserum to a vaccine of heat-killed E. coli J5 (J5 antiserum) was prepared from pooled blood from 10 rabbits as previously described (30, 57). Murine antiserum against E. coli J5 vaccine was collected from mice immunized as described above. A vaccine of E. coli O18 O polysaccharide consisting of the purified O-polysaccharide conjugated to toxin A of Pseudomonas aeruginosa was a gift from A. Cross (17). Rabbit antiserum to the O polysaccharide of E. coli O18 LPS (polyclonal anti-O18 IgG) was prepared using 10 mg of vaccine per inoculation as previously described (30). All sera were immediately prepared after venipuncture, aliquoted, and frozen (−80°C) until use. IgG in polyclonal rabbit antisera to heat-killed E. coli J5 does not cross-react with components of normal human serum by immunoblotting. Polyclonal anti-O18 IgG does not cross-react with LPS from heterologous bacteria, OMPs, or components of normal human serum by ELISA and/or immunoblotting.
Sera from rabbits and healthy human volunteers were prepared from blood collected into sterile polypropylene tubes containing sterile glass beads to initiate clotting. After 2 h at room temperature, the clot was removed by centrifugation (1,000 × g, 10 min, 4°C). Sera were either used immediately or frozen (−80°C) and thawed immediately prior to use.
IgG was purified from hyperimmune serum, from artificial capillary cell culture supernatants, and from ammonium sulfate-precipitated ascites fluid by passage over a protein G-Sepharose 4 fast-flow column (Pharmacia, Piscataway, N.J.) as described elsewhere (27, 64). Bound IgG was eluted from the column with 0.1 M glycine (pH 2.7) and was immediately neutralized with 1 M Tris buffer (pH 9.0). Purified IgG was dialyzed against phosphate-buffered saline (PBS; pH 7.2) and stored at −80°C. Protein concentration was determined by ELISA (69) and by UV absorption (A280).
IgGs were covalently conjugated to magnetic beads (BioMag Amine Terminated 8-4100, PerSeptive Diagnostics, Cambridge, Mass.) according to the manufacturer's instructions and as previously described (30). Monoclonal IgG directed against the 18-kDa OMP was covalently conjugated to cyanogen bromide-activated Sepharose 4B beads (Pharmacia) according to the manufacturer's instructions.
Immunoblotting.
Immunoblotting was used to detect binding of antisera and monoclonal antibodies to lysates of bacteria (106/well), samples collected during the purification of the 18-kDa OMP, and bacterial antigens that were affinity purified from filtrates of human serum incubated with bacteria. All samples were prepared in sample buffer (2.5% sodium dodecyl sulfate [SDS], 22% glycerol, 0.5% β-mercaptoethanol, and trace bromophenol blue in Tris base), electrophoresed on SDS–16% polyacrylamide gels, and transferred to nitrocellulose (Bio-Rad Laboratories, Hercules, Calif.) by applying 200 mA of constant current at 4°C for 1 h (Hoefer Scientific Instruments, San Francisco, Calif.). The nitrocellulose was blocked with 1% powdered skim milk in TTBS (150 mM NaCl, 50 mM Tris, 0.1% Tween 20 [pH 7.5]), washed with TTBS, incubated with primary antibodies in TTBS, and washed. Primary antibodies included rabbit or mouse J5 antiserum (diluted 500- and 4,000-fold respectively), murine monoclonal antibodies to each of the three OMPs (at a concentration of 1 mg/ml), or polyclonal anti-O18 IgG (diluted 500-fold). Blots were then incubated for 30 min with biotin-conjugated anti-rabbit or anti-mouse IgG antibody (Vectastain; Vector Laboratories, Burlingame, Calif.) diluted 1:240 in TTBS, washed, and then incubated for 30 min in a mixture of avidin and biotinylated horseradish peroxidase complex, as specified by the manufacturer (Vectastain). After a final wash with PBS, peroxidase substrate (2 ml of 4-chloro-1-naphthol, [3 mg/ml], 8 ml of PBS, 10 μl of 30% H2O2) was added. The reaction was stopped after 20 to 30 min.
Purification of the 18-kDa OMP.
The final purification procedure for the 18-kDa OMP consisted of (i) preparation of total bacterial membranes, (ii) Triton X-100 extraction of bacterial membranes, (iii) affinity chromatography using Sepharose beads conjugated with 6D7 (the anti-18 kDa OMP monoclonal antibody), and (iv) reverse-phase high-pressure liquid chromatography (HPLC) separation. Details of the purification steps are described below.
Total bacterial membranes were prepared from mid-late-log-phase cultures of E. coli O18K− essentially as described previously (30, 49). Unless otherwise indicated, all steps were performed at 4 to 6°C. Two-liter cultures of bacteria were harvested by centrifugation, and the resultant pellets were resuspended in a total of 60 ml of prechilled 10 mM HEPES buffer (pH 7.4) with 25% sucrose (wt/vol) and 0.2 mM dithiothreitol (Fisher Biochemicals, Fair Lawn, N.J.). RNase and DNase (Sigma) were each added to a final concentration of 4 μg/ml. Cells were disrupted by sonicating the suspension on ice (microtip, 30- to 60-s bursts separated by 60 to 90 s, total sonication time of 4 min). Unbroken bacteria and other debris were removed by centrifugation (10,000 × g, 40 min), and the supernatant was collected (volume of 60 ml); 15 ml of HEPES buffer (pH 7.4) containing EDTA (25 mM), and dithiothreitol (0.2 mM) was added to the 60 ml to adjust the concentration of sucrose to 20% (wt/vol) and the concentration of EDTA to 5 mM. Samples were layered onto a 60% (wt/vol) sucrose cushion (7.5 ml of sample per 4.5 ml of cushion), and ultracentrifuged (100,000 × g, 3 h, 6°C). Bacterial membranes present in the hazy white/yellow band at the interface were collected by puncturing the side of the tube with a 20-gauge needle and aspirating with a 1-ml syringe (approximately 0.5 ml/tube, final volume, 5 ml). Total membranes were dialyzed against 6 liters of Tris-HCl (20 mM, pH 8). The final volume of dialyzed material was approximately 15 ml per 2 liters of the starting bacterial culture.
Sixty milliliters of dialyzed total membranes representing 8 liters of the starting bacterial culture was concentrated to 36 ml using a nitrogen pressurized system and a Diaflo ultrafiltration membrane, YM30 filter (Millipore Company, Danvers, Mass.), and extracted with Triton X-100. Twelve milliliters of 10% Triton X-100 in Tris-HCl (20 mM, pH 8.4) with the protease inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride (Sigma) and EDTA was added to the membranes [final concentrations; 2.5% Triton X-100, 0.5 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 5 mM EDTA]. The sample was incubated at room temperature for 30 min and then ultracentrifuged (TH641 swinging-bucket rotor, 100,000 × g, 2 h, 6°C). The resultant supernatant (48 ml) was circulated overnight at 9 to 10 ml/h through a 5.5-ml column of mouse monoclonal IgG directed against the 18-kDa OMP covalently conjugated to cyanogen bromide-activated Sepharose 4B beads (4°C). The column was washed (36 ml, 2.5% Triton X-100 in 200 mM sodium phosphate, 0.5 M NaCl [pH 6.8]), and then bound antigen was eluted in 0.5 and 1% SDS (in 200 mM phosphate, 0.5 M NaCl [pH 6.8]). Eluted material was concentrated to 4 ml by centrifugation in a Centricon Plus-20 centrifugal filter device (10-kDa cutoff; Biomax-8 series; Millipore).
Three milliliters of the concentrated affinity-purified sample was applied to an analytical C4 reverse-phase HPLC column (Vydac, Hesperia, Calif.) and eluted using a linear gradient of 5 to 95% acetonitrile–0.1% trifluoroacetic acid–H2O at a flow rate of 1 ml/min. Fractions were collected at 1-min intervals into 20 μl of twofold-concentrated SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer (5% SDS and 44% glycerol in Tris base) and lyophilized. Lyophilized samples were resuspended in 40 μl of water with β-mercaptoethanol (0.5%) and trace bromophenol blue and heated (100°C, 5 to 10 min). Fractions were electrophoresed and analyzed for the 18-kDa OMP by immunoblotting using anti-J5 IgG or 6D7 (the monoclonal anti-18-kDa OMP IgG) as the primary antibody.
Sequencing of the purified 18-kDa OMP.
The peak fraction from the C4 HPLC separation was electrophoresed on an SDS–16% polyacrylamide gel and stained with Coomassie brilliant blue. The faintly staining 18-kDa band was then cut from the gel, washed twice (50% acetonitrile, 0.5 ml, 3 min), and frozen. Sequence analysis of two peptides of a trypsin digestion of the protein in the gel was performed at the Harvard Microchemistry Facility by tandem mass spectrometry on a Finnigan LCQ quadrupole ion trap mass spectrometer.
Recombinant OmpA.
The coding region of the 325-amino-acid mature OmpA protein, excluding the 21-amino-acid signal sequence (GenBank accession no. V00307), was generated by PCR amplification of DNA from an extract of E. coli O18:K1:H7. OmpA-specific PCR primers (OmpABac1 [5′GACGACGACAAGGCTCCGAAAGATAACACCTG3′] and OmpABac2 [5′GAGGAGAAGCCCGGTTAAGCCTGCGGCTGAGTTAC3′]) contained 5′ extensions for cloning into the transfer plasmid pBACgus-2cp (Novagen, Madison, Wis.). The transfer plasmid containing the OmpA coding sequence, OmpA/pBACgus-2cp, was then transfected into the BacVector-2000 Triple Cut Baculovirus DNA in Sf9 cells as instructed by the manufacturer (Novagen, Madison, Wis.). Positive recombinants were expanded, and high-titer virus was produced, to give multiplicities of infection in the range of 10 to 20 for maximal protein expression in Sf9 cells. The final baculovirus construct contained the OmpA coding sequence, with an in-frame amino-terminal extension (fusion sequences were encoded by the pBACgus-2cp transfer plasmid) containing an enterokinase recognition sequence, an S-protein binding site, and a polyhistidine tail. The 36.5-kDa (calculated molecular mass) OmpA fusion protein was purified from baculovirus-infected Sf9 cell lysates by polyhistidine affinity chromatography over a Talon cobalt metal affinity resin as specified by the manufacturer (Clontech, Palo Alto, Calif.).
Affinity purification of OMPs from sterile filtrates of serum-exposed bacteria.
E. coli O18K+ was grown to mid-log phase, harvested, and washed. The resultant bacterial pellet was resuspended in an equal volume of normal human serum (108 bacteria/ml) with ampicillin (200 μg/ml) and incubated for 2 h at 37°C on a rotating drum. The serum was filtered through a 0.45-μm-pore-size filter to remove intact bacteria. The serum filtrate was then incubated with antibody-conjugated magnetic beads. Antibodies used for these affinity purification studies included polyclonal anti-O18 IgG, IgG from J5 antiserum, and IgG from normal rabbit serum. Two hundred microliters of each sample was incubated with IgG-conjugated beads that had previously been washed and resuspended in 800 μl of PBS. The final concentration of IgG was 100 μg/ml. The final concentration of filtered serum was 20%. Reaction mixtures were incubated for 16 to 20 h at 4°C, with end-over-end mixing. The antibody-conjugated beads with attached antigens were then separated from the 20% filtered serum by placing the tubes in a strong magnetic field, and the beads were washed three times with PBS. Antigen was eluted by heating the beads (5 min, 100°C) in 100 μl of SDS-PAGE sample buffer (2.5% SDS and 22% glycerol in Tris base). Supernatants were carefully separated from the beads, and β-mercaptoethanol (0.5%) and trace bromophenol blue were added. Twenty microliters of each sample was then electrophoresed on lanes of 16% gels and transferred to nitrocellulose. Blots were stained with mouse anti-J5 IgG, monoclonal anti-O18 IgG, and mouse monoclonal antibodies directed against each of the OMPs. Blots were developed as described above, using biotinylated horse anti-mouse IgG as secondary antibody.
RESULTS
The 35-kDa OMP is OmpA.
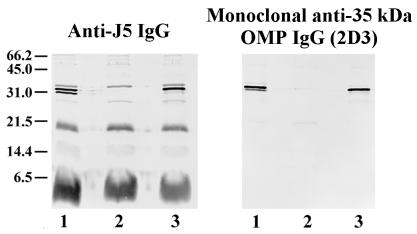
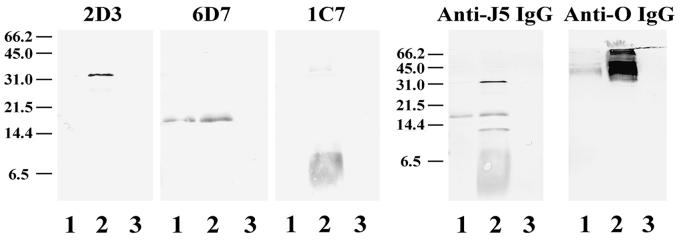
We hypothesized that the 35-kDa protein was OmpA based on the apparent molecular weight and the fact that the electrophoretic mobility of the band was altered by boiling (data not shown) (31). Immunoblotting studies were performed to identify this protein. Isolates of E. coli O18 bacteria in which the OmpA gene had been deleted and then reinserted into the strain (52) and recombinant OmpA were electrophoresed on SDS–16% polyacrylamide gels, transferred to nitrocellulose, and used as antigen. Staining antibodies included anti-J5 IgG and our monoclonal IgG that is directed against the 35-kDa OMP (2D3). Anti-J5 IgG and 2D3 did not react with the 35-kDa band in lysates of bacteria in which the OmpA gene was deleted but did react with a 35-kDa band in the wild-type strain and the strain in which the gene was reinserted (Fig. 1). Recombinant OmpA was stained by anti-J5 IgG and 2D3 (Fig. 2). Recombinant OmpA ran at a slightly higher molecular weight, presumably because of the polyhistidine tag that is present on the recombinant protein. These results indicate that the 35-kDa OMP is OmpA and that 2D3 is a monoclonal anti-OmpA IgG.
FIG. 1.
Immunoblot of OmpA-deficient bacteria. Bacteria were grown to mid-log phase and then boiled in SDS-PAGE sample buffer. Bacterial lysates were then electrophoresed on SDS–16% polyacrylamide gels and transferred to nitrocellulose. Staining antibodies included polyclonal rabbit anti-J5 IgG (left) and a monoclonal antibody directed to the 35-kDa OMP (2D3; right). Bacterial strains: wild-type OmpA+ E. coli O18:K1:H7 (lane 1); E91, an OmpA-deleted mutant of E. coli O18:K1:H7 (lane 2); E69, an OmpA-restored mutant of E. coli O18:K1:H7 (lane 3). Positions of molecular weight markers (in kilodaltons) are shown at the left.
FIG. 2.
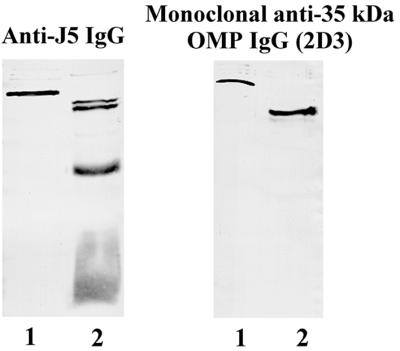
Immunoblot of recombinant OmpA. Recombinant OmpA (lane 1 of each panel) and lysates of E. coli O18:K1:H7 bacteria (lane 2 of each panel) were electrophoresed on an SDS–16% polyacrylamide gel and transferred to nitrocellulose. Primary antibodies included polyclonal mouse anti-J5 IgG (left) and the monoclonal antibody directed against the 35-kDa OMP (2D3; right).
The 18-kDa OMP is PAL.
The 18-kDa OMP was identified by sequencing purified protein. Total bacterial membranes were extracted with Triton X-100 detergent containing EDTA, and the 18-kDa OMP was affinity purified from the extracted material by using our monoclonal IgG that binds to the 18-kDa OMP (6D7). The protein was further purified to homogeneity by passage over a reverse-phase C4 HPLC column. Immunoblots of the fractions revealed that the 18-kDa OMP eluted from the column at 95% acetonitrile. The fraction containing the peak of the 18-kDa OMP was subjected to SDS-PAGE, the gel was stained with Coomassie blue, and the single lightly stained band was cut from the gel and sequenced by tandem mass spectrometry as indicated in Materials and Methods (Harvard Microchemistry, Cambridge, Mass.). Two peptide sequences (10 and 14 amino acids) that each mapped with 100% homology to the PAL were obtained (Fig. 3).
FIG. 3.
Peptide sequences of purified 18-kDa protein. Protein was purified as indicated in Materials and Methods. The lightly staining band on an SDS-polyacrylamide gel was excised and sequenced by mass tandem spectrometry. After trypsin digestion, two peptide sequences were obtained. Brackets indicate that the amino acid has been identified with reasonable confidence; an asterisk indicates that the amino acid is isobaric and cannot be unambiguously differentiated by mass spectrometric sequencing. All other amino acids are assigned with the highest confidence.
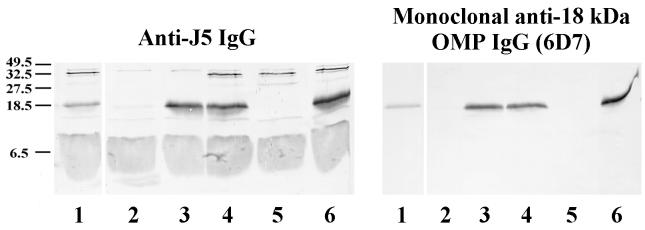
The identity of PAL was confirmed by immunoblotting studies. Lysates of E. coli K-12 in which the PAL gene (excC) was deleted, or was deleted and then replaced, were immunoblotted with anti-J5 IgG or 6D7 as primary antibody. Anti-J5 IgG and 6D7 did not react with the 18-kDa band in lysates of PAL-deficient bacteria but did react with an 18-kDa band in the wild-type strain and the strain with the gene reinserted (Fig. 4). These results indicate that the 18-kDa OMP is PAL and that 6D7 is a monoclonal anti-PAL antibody.
FIG. 4.
Immunoblot of PAL-deficient bacteria. Overnight cultures of bacteria were boiled in SDS-PAGE sample buffer. Bacterial lysates were then electrophoresed on SDS–16% polyacrylamide gels and transferred to nitrocellulose. Staining antibodies included polyclonal rabbit anti-J5 IgG (left) and a monoclonal antibody directed against the 18-kDa OMP (6D7, right). Bacterial strains: E. coli K-12 p400 containing PAL (lane 1); CH202, a PAL-deficient mutant of E. coli K-12(p400) (lane 2); CH202(pRC2), a PAL-restored mutant of CH202 (lane 3); E. coli K-12 1292 containing PAL (lane 4); JC7752, a PAL-deficient mutant of 1292 (lane 5); and JC7752(p417), a PAL-restored mutant of JC7752 (lane 6). Positions of molecular weight markers (in kilodaltons) are on the left.
The 5- to 9-kDa OMP is MLP.
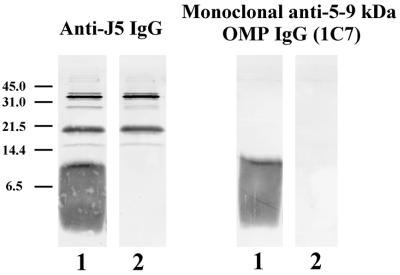
We hypothesized that the 5- to 9-kDa OMP was MLP based on its low molecular weight and size heterogeneity (26). Accordingly, a mutant of E. coli K-12 lacking MLP (JE5505) and its MLP-positive, otherwise identical partner (JE5506) were used as antigens on immunoblots (Fig. 5). One blot was developed with anti-J5 IgG, and the other blot was developed with our monoclonal antibody, 1C7. Anti-J5 IgG and 1C7 IgG did not react with the 5- to 9-kDa band in bacterial lysates of the MLP-deficient strain. These results indicate that the lower cross-reactive OMP is MLP and that 1C7 is an anti-MLP IgG.
FIG. 5.
Immunoblot of MLP-deficient bacteria. Bacteria were grown to mid-log phase and boiled in SDS-PAGE sample buffer, and the resultant bacterial lysates were used as antigen. Staining antibodies included polyclonal rabbit anti-J5 IgG (left) and the monoclonal antibody directed against the 5- to 9-kDa OMP (1C7; right). Bacterial strains: E. coli K-12 JE5505, an MLP-deficient mutant of E. coli K-12 (lane 2); E. coli K-12 JE5506, the MLP-positive otherwise identical partner of JE5505 (lane 1). Positions of molecular weight markers (in kilodaltons) are on the left.
Identification of the OMPs released by bacteria incubated in human serum.
Our prior studies (30) and work by others (20) have demonstrated that E. coli and Salmonella bacteria incubated in human serum release complexes of OMPs and LPS that can be affinity purified using O-chain specific anti-LPS IgG. To test the hypothesis that OmpA, PAL, and MLP are present in OMP-LPS complexes released by bacteria into human serum, polyclonal anti-O18 IgG was used to affinity purify LPS from sterile filtrates of human serum incubated with E. coli O18K+, a strain that is resistant to the bactericidal activity of human serum (16), as described in Materials and Methods. To further study the interaction of anti-J5 IgG with released OmpA, PAL, and MLP, filtrates were also incubated with magnetic beads that were previously conjugated with rabbit anti-J5 IgG. Captured antigens were immunoblotted with the murine monoclonal IgGs against OmpA (2D3), MLP (1C7), and PAL (6D7), murine monoclonal anti-LPS (O-chain specific) IgG, and murine polyclonal anti-J5 IgG. OmpA, PAL, and MLP were all detected in samples that were affinity purified using O-chain-specific anti-LPS IgG, indicating that bacteria release complexes containing these OMPs and LPS (Fig. 6). PAL, but not OmpA or MLP, was also detected in samples that were affinity purified using anti-J5 IgG (Fig. 6). No OMPs were detected in immunoblots of control samples that were affinity purified using IgG from normal rabbit serum. Only slight staining of the OMPs was present in control samples prepared from sterile filtrates of bacteria incubated with ampicillin in saline in the absence of normal human serum, indicating that serum factors are important in release of the OMPs (29).
FIG. 6.
Immunoblot of OMP-containing samples released into human serum. E. coli O18:K1:H7 was grown to mid-log phase, incubated for 2 h at 37°C in 100% human serum with ampicillin (200 mg/ml), and then passed through 0.45-μm-pore-size filters to remove intact bacteria. Filtrates were incubated with polyclonal rabbit anti-J5 IgG, polyclonal rabbit O-chain-specific anti-LPS IgG, and normal rabbit IgG (control) that were previously conjugated covalently to beads. Captured antigens were eluted from the beads and stained with murine monoclonal IgGs directed against OmpA (2D3), PAL (6D7), MLP (1C7), polyclonal mouse anti-J5 IgG, or a murine monoclonal IgG directed to the O-polysaccharide chain of E. coli O18 LPS, as indicated above the blots. Samples were affinity purified with rabbit anti-J5 IgG (lane 1), rabbit O-chain-specific anti-LPS IgG (lane 2), and normal rabbit IgG (lane 3). Positions of molecular weight markers (in kilodaltons) are indicated to the left of each panel.
DISCUSSION
This study identifies three outer membrane proteins that are released by E. coli bacteria into human serum in vitro (30) and in an animal model of gram-negative sepsis (29) as OmpA, PAL, and MLP. OmpA, PAL, and MLP are structural outer membrane proteins (3, 10, 39, 45, 54, 58, 61) that are highly conserved among different enteric gram-negative bacteria (2, 8, 33, 47). Proteins similar to each exist in nonenteric gram-negative bacteria (46, 48, 59). MLP and PAL are lipoproteins with covalently attached lipids. The OMPs are tightly associated with LPS (37, 53). OmpA, PAL, and MLP have not been studied extensively in the context of gram-negative sepsis, although it has been known for several decades that many proteins associated with LPS are biologically active (14, 19, 21, 22, 24, 25, 28, 38, 40, 41, 44, 51, 67).
OmpA, initially described by Henning and colleagues (23, 31), has 325 amino acid residues (13) and exhibits heat-modifiable electrophoretic mobility on SDS-PAGE (13, 50). The N-terminal domain of OmpA is comprised of 177 amino acids and is believed to traverse the outer membrane eight times (35). The C-terminal domain is believed to protrude into the periplasmic space. OmpA is involved in maintaining the shape of bacteria (58), serves as a phage receptor and a receptor for F-mediated conjugation, and may have pore-forming properties (60). OmpA enhances uptake of LPS into macrophages (38) and has been reported to be involved in E. coli invasion of the central nervous system (52). An OmpA-deficient mutant of the virulent strain E. coli O18K1 was shown to be less virulent than its OmpA+ parent strain in neonatal rat and embryonated chick egg models of sepsis (65).
PAL was initially described and characterized by Mizuno (47). It has 173 amino acid residues and is closely, but not covalently, associated with the peptidoglycan layer (39, 46, 47). PAL has a hydrophobic region of 22 amino acids at the N-terminal domain that interacts with the outer membrane (39). The C-terminal domain is involved in interactions with the peptidoglycan layer (39).
MLP, first described and characterized by Braun and colleagues (7, 10, 26), is the most abundant outer membrane protein (10). MLP has 58 amino acid residues and exists in two forms, a free form and a form that is covalently linked to peptidoglycan by the C-terminal domain (6, 7). Recently Zhang reported that MLP induces lethal shock in a strain of mouse (C3H/HeJ) that is genetically hyporesponsive to LPS (67). Furthermore, they found that MLP was synergistic with LPS for lethal toxicity.
We have previously shown that epitopes of all three OMPs are exposed on the surface of bacteria that have been incubated in human serum and that antiserum raised to a rough mutant vaccine of E. coli J5 results in high titers of antibodies that bind to the same three OMPs on the bacterial surface (30). The identity of two of these proteins as PAL and MLP is surprising, as both proteins are situated in the deep periplasmic space and only short N-terminal segments are believed to interact with the outer membrane (9, 39). Therefore, the increased clearance of heterologous smooth bacterial strains by infusion of antiserum to E. coli J5 (55) may be mediated through binding of immunoglobulin in this antiserum to epitopes of OmpA, PAL, and MLP on the bacterial surface.
Previous investigators have focused on LPS as the primary bacterial toxin released in gram-negative sepsis. Release of bacterial membrane components other than LPS, either alone or in conjunction with LPS, has not been studied extensively. Our studies indicate that bacteria incubated in human serum release at least three OMPs in addition to LPS. Although the present studies focused on the three predominant proteins that we have demonstrated are bound by anti-J5 IgG, it seems likely that other bacterial components, including additional OMPs, may also be present in the OMP-containing fragments that are released into serum.
More recent studies suggest that complexes containing these three OMPs and LPS are released by E. coli bacteria into human serum (30) and into septic rat blood (29). To our knowledge, release of OmpA, PAL, or MLP into the bloodstream in sepsis has not been previously described. A pathogenic role for at least one of these OMPs is suggested by studies indicating that MLP causes lethal shock in C3H/HeJ mice (67). More study will be needed to test the hypothesis that these three OMPs may contribute to the pathogenesis of gram-negative infection.
ACKNOWLEDGMENTS
This work was supported by Shriners Hospital for Crippled Children grant 8510, U.S. Navy grant N0014-94-C-0021, and NIH grant AI39617-02 and by the Charles H. Hood Foundation, Boston, Mass.
REFERENCES
- 1.Baumgartner J, McCutchan J A, Van Melle G, Vogt M, Luethy R, Glauser M P, Ziegler E J, Klauber M R, Muehlen E, Chiolero R, Geroulanos S. Prevention of gram-negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet. 1985;ii:59–63. doi: 10.1016/s0140-6736(85)90176-x. [DOI] [PubMed] [Google Scholar]
- 2.Beher M B, Schnaitman C A, Pugsley A P. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the OmpA protein of Escherichia coli. J Bacteriol. 1980;143:906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouveret E, Derouiche R, Rigal A, Lloubes R, Lazdunski C, Benedetti H. Peptidoglycan-associated lipoprotein-TolB interaction. A possible key to explaining the formation of contact sites between the inner and outer membranes of Escherichia coli. J Biol Chem. 1995;270:11071–11077. doi: 10.1074/jbc.270.19.11071. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Oktedalen O, Kierulf P, Opstad P K. Elevated VIP and endotoxin plasma levels in human gram-negative septic shock. Regul Pept. 1989;24:37–44. doi: 10.1016/0167-0115(89)90209-7. [DOI] [PubMed] [Google Scholar]
- 5.Braude A I, Douglas H, Davis C E. Treatment and prevention of intravascular coagulation with antiserum to endotoxin. J Infect Dis. 1973;128:S157–S164. doi: 10.1093/infdis/128.supplement_1.s157. [DOI] [PubMed] [Google Scholar]
- 6.Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975;415:335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- 7.Braun V, Bosch V. Sequence of the murein lipoprotein and the attachment site of the lipid. Eur J Biochem. 1972;28:51–69. doi: 10.1111/j.1432-1033.1972.tb01883.x. [DOI] [PubMed] [Google Scholar]
- 8.Braun V, Bosch V, Klumpp E R, Nef I, Mayer H, Schlecht S. Antigenic determinants of murein lipoprotein and its exposure at the surface of enterobacteriaceae. Eur J Biochem. 1976;62:555–566. doi: 10.1111/j.1432-1033.1976.tb10190.x. [DOI] [PubMed] [Google Scholar]
- 9.Braun V, Rotering H, Ohms J-P, Hagenmaier H. Conformational studies on murein-lipoprotein from the outer membrane of Escherichia coli. Eur J Biochem. 1976;70:601–610. doi: 10.1111/j.1432-1033.1976.tb11051.x. [DOI] [PubMed] [Google Scholar]
- 10.Braun V, Wolff H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem. 1970;14:387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 11.Chedid L, Parant M, Boyer F. A proposed mechanism for natural immunity to enterobacterial pathogens. J Immunol. 1968;100:292–301. [PubMed] [Google Scholar]
- 12.Chen R, Henning U. Nucleotide sequence of the gene for the peptidoglycan-associated lipoprotein of Escherichia coli K12. Eur J Biochem. 1987;163:73–77. doi: 10.1111/j.1432-1033.1987.tb10738.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Schmidmayr W, Kramer C, Chen-Schmeisser U, Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K12. Proc Natl Acad Sci USA. 1980;77:4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Hancock R, Mishell R. Mitogenic effects of purified outer membrane proteins from Pseudomonas aeruginosa. Infect Immun. 1980;28:178–184. doi: 10.1128/iai.28.1.178-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross A S. Antiendotoxin antibodies: a dead end? Ann Intern Med. 1994;121:58–59. doi: 10.7326/0003-4819-121-1-199407010-00011. [DOI] [PubMed] [Google Scholar]
- 16.Cross A S, Kim K S, Wright C, Sadoff J C, Gemski P. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J Infect Dis. 1986;154:497–503. doi: 10.1093/infdis/154.3.497. [DOI] [PubMed] [Google Scholar]
- 17.Cryz S J, Jr, Cross A S, Sadoff J C, Furer E. Synthesis and characterization of Escherichia coli O18 O-polysaccharide conjugate vaccines. Infect Immun. 1990;58:373–377. doi: 10.1128/iai.58.2.373-377.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danner R L, Elin R J, Hosseini J M, Wesley R A, Reilly J M, Parillo J E. Endotoxemia in human septic shock. Chest. 1991;99:169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- 19.Doe W F, Yang S T, Morrison D C, Betz S J, Henson P M. Macrophage stimulation by bacterial lipopolysaccharides. II. Evidence for differentiation signals delivered by lipid A and by a protein rich fraction of lipopolysaccharides. J Exp Med. 1978;148:557–568. doi: 10.1084/jem.148.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freudenberg M A, Meier-Dieter U, Staehelin T, Galanos C. Analysis of LPS released from Salmonella abortus equi in human serum. Microb Pathog. 1992;10:93–104. doi: 10.1016/0882-4010(91)90070-q. [DOI] [PubMed] [Google Scholar]
- 21.Galdiero F, Cipollaro de L'Ero G, Benedetto N, Galdiero M, Tufano M A. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun. 1993;61:155–161. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galdiero F, Tufano M A, Sommese L, Folgore A, Tedesco F. Activation of complement system by porins extracted from Salmonella typhimurium. Infect Immun. 1984;46:559–563. doi: 10.1128/iai.46.2.559-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garten W, Hindennach I, Henning U. The major proteins of the Escherichia outer cell envelope membrane. Characterization of proteins II and III, comparison of all proteins. Eur J Biochem. 1975;59:215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 24.Giambartolomei G H, Dennis V A, Lasater B L, Philipp M T. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman G W, Sultzer B M. Characterization of the chemical and physical properties of a novel B-lymphocyte activator, endotoxin protein. Infect Immun. 1979;24:685–696. doi: 10.1128/iai.24.3.685-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hantke K, Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N terminal end of the murein lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973;34:284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 27.Harlow E, Lane D, editors. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 28.Hauschildt S, Hoffmann P, Beuscher H U, Dufhues G, Heinrich P, Wiesmuller K-H, Jung G, Bessler W G. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur J Immunol. 1990;20:63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- 29.Hellman, J., P. M. Loiselle, E. M. Zanzot, J. E. Allaire, M. M. Tehan, L. A. Boyle, J. T. Kurnick, and H. S. Warren. Release of Gram-negative outer membrane proteins into human serum and septic rat blood and their interactions with immunoglobulins in antiserum to Escherichia coli J5. J. Infect. Dis., in press. [DOI] [PubMed]
- 30.Hellman J, Zanzot E M, Loiselle P M, Amato S F, Black K M, Ge Y, Kurnick J T, Warren H S. Antiserum against Escherichia coli J5 contains antibodies reactive with outer membrane proteins of heterologous Gram-negative bacteria. J Infect Dis. 1997;176:1260–1268. doi: 10.1086/514121. [DOI] [PubMed] [Google Scholar]
- 31.Hindennach I, Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975;59:207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- 32.Hirota Y, Suzuki H, Nishimura Y, Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci USA. 1977;74:1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofstra H, Dankert J. Major outer membrane proteins: common antigens in Enterobacteriaceae species. J Gen Microbiol. 1980;119:123–131. doi: 10.1099/00221287-119-1-123. [DOI] [PubMed] [Google Scholar]
- 34.Kim K S, Kang J H, Cross A S, Kaufman B, Zollinger W, Sadoff J. Functional activities of monoclonal antibodies to the O side chain of Escherichia coli lipopolysaccharides in vitro and in vivo. J Infect Dis. 1988;157:47–53. doi: 10.1093/infdis/157.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Klose M, Störiko A, Stierhof Y-D, Hindennach I, Mutschler B, Henning U. Membrane assembly of the outer membrane protein OmpA of Escherichia coli. J Biol Chem. 1993;268:25664–25670. [PubMed] [Google Scholar]
- 36.Kohler G, Howe S C, Milstein C. Fusion between immunoglobulin-secreting and non-secreting myeloma cell lines. Eur J Immunol. 1976;6:292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- 37.Korhonen T K, Dawes E A, Makela P H, editors. Enterobacterial surface antigens: methods for molecular characterization. FEMS Symposia. Vol. 25. Amsterdam, The Netherlands: Elsevier Science Publishers B. V.; 1985. [Google Scholar]
- 38.Korn A, Rajabi Z, Wassum B, Ruiner W, Nixdorff K. Enhancement of uptake of lipopolysaccharide in macrophages by the major outer membrane protein OmpA of gram-negative bacteria. Infect Immun. 1995;63:2697–2705. doi: 10.1128/iai.63.7.2697-2705.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazzaroni J-C, Portalier R. The excC gene of Escherichia coli K-12 required for cell envelope integrity encodes the peptidoglycan-associated lipoprotein (PAL) Mol Microbiol. 1992;6:735–742. doi: 10.1111/j.1365-2958.1992.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 40.Mangan D F, Wahl S M, Sultzer B M, Mergenhagen S E. Stimulation of human monocytes by endotoxin-associated protein: inhibition of programmed cell death (apoptosis) and potential significance in adjuvanticity. Infect Immun. 1992;60:1684–1686. doi: 10.1128/iai.60.4.1684-1686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manthey C L, Perera P-Y, Henricson B E, Hamilton T A, Qureshi N, Vogel S N. Endotoxin-induced early gene expression in C3H/HeJ (Lpsd) macrophages. J Immunol. 1994;153:2653–2663. [PubMed] [Google Scholar]
- 42.McCabe W R, Bruins S C, Craven D E, Johns M. Cross-reactive antigens: their potential for immunization-induced immunity to gram-negative bacteria. J Infect Dis. 1977;136:S161–S166. doi: 10.1093/infdis/136.supplement.s161. [DOI] [PubMed] [Google Scholar]
- 43.McCabe W R, DeMaria A, Berberich H, Johns M A. Immunization with rough mutants of Salmonella minnesota: protective activity of IgM and IgG antibody to the R595 (Re chemotype) mutant. J Infect Dis. 1988;158:291–300. doi: 10.1093/infdis/158.2.291. [DOI] [PubMed] [Google Scholar]
- 44.Melchers F, Braun V, Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975;142:473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuno T. Structure of peptidoglycan-associated lipoprotein (PAL) of the Proteus mirabilis outer membrane. I. Isolation and characterization of fatty acid-containing peptides from PAL. J Biochem. 1981;89:1051–1058. [PubMed] [Google Scholar]
- 46.Mizuno T. A novel peptidoglycan-associated lipoprotein found in the cell envelope of Pseudomonas aeruginosa and Escherichia coli. J Biochem. 1979;86:991–1000. doi: 10.1093/oxfordjournals.jbchem.a132631. [DOI] [PubMed] [Google Scholar]
- 47.Mizuno T. A novel peptidoglycan-associated lipoprotein (PAL) found in the outer membrane of Proteus Mirabilis and other gram-negative bacteria. J Biochem. 1981;89:1039–1049. [PubMed] [Google Scholar]
- 48.Mizuno T, Kageyama M. Isolation and characterization of a major outer membrane protein of Pseudomonas aeruginosa. J Biochem. 1979;85:115–122. doi: 10.1093/oxfordjournals.jbchem.a132300. [DOI] [PubMed] [Google Scholar]
- 49.Munford R S, Hall C L, Rick P D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980;144:630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura K, Mizushima S. Effects of heating is dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976;80:1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- 51.Porat R, Yanoov M, Johns M A, Shibolet S, Michalevicz R. Effects of endotoxin-associated protein on hematopoiesis. Infect Immun. 1992;60:1756–1760. doi: 10.1128/iai.60.5.1756-1760.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S-H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puohiniemi R, Muotiala A, Helander I M, Sarvas M. Conformation of Escherichia coli outer membrane protein OmpA produced in Bacillus subtilis: influence of lipopolysaccharide. FEMS Microbiol Lett. 1993;106:105–110. doi: 10.1111/j.1574-6968.1993.tb05942.x. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Herva J, Ramos-Gonzalez M, Ramos J. The Pseudomonas putida peptidoglycan-associated outer membrane lipoprotein is involved in maintenance of the integrity of the cell envelope. J Bacteriol. 1996;178:1699–1706. doi: 10.1128/jb.178.6.1699-1706.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakulramrung R, Dominigue G J. Cross-reactive immunoprotective antibodies to Escherichia coli O111 rough mutant J5. J Infect Dis. 1985;151:995–1004. doi: 10.1093/infdis/151.6.995. [DOI] [PubMed] [Google Scholar]
- 56.Shenep J L, Flynn P M, Barrett F F, Stidham G L, Westenkirchner D F. Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J Infect Dis. 1988;157:565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- 57.Siber G R, Kania S A, Warren H S. Cross-reactivity of rabbit antibodies to lipopolysaccharides of Escherichia coli J5 and other gram-negative bacteria. J Infect Dis. 1985;152:954–964. doi: 10.1093/infdis/152.5.954. [DOI] [PubMed] [Google Scholar]
- 58.Sonntag I, Schwarz H, Hirota Y, Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978;136:280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spinola S M, Griffiths G E, Shanks K L, Blake M S. The major outer membrane protein of Haemophilus ducreyi is a member of the OmpA family of proteins. Infect Immun. 1993;61:1346–1351. doi: 10.1128/iai.61.4.1346-1351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugawara E, Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992;267:2507–2511. [PubMed] [Google Scholar]
- 61.Suzuki H, Nishimura Y, Yasuda S, Nishimura A, Yamada M, Hirota Y. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol Gen Genet. 1978;167:1–9. doi: 10.1007/BF00270315. [DOI] [PubMed] [Google Scholar]
- 62.Tesh V L, Duncan R L, Jr, Morrison D C. The interaction of Escherichia coli with normal human serum: the kinetics of serum-mediated lipopolysaccharide release and its dissociation from bacterial killing. J Immunol. 1986;137:1329–1335. [PubMed] [Google Scholar]
- 63.Tesh V L, Morrison D C. The physical-chemical characterization and biologic activity of serum released lipopolysaccharides. J Immunol. 1988;141:3523–3531. [PubMed] [Google Scholar]
- 64.Warren H S, Glennon M, de Deckker F A, Tello D. Role of normal serum in the binding of lipopolysaccharide to IgG fractions from rabbit antisera to Escherichia coli J5 and other gram-negative bacteria. J Infect Dis. 1991;163:1256–1266. doi: 10.1093/infdis/163.6.1256. [DOI] [PubMed] [Google Scholar]
- 65.Weiser J N, Gotschlich E C. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun. 1991;59:2252–2258. doi: 10.1128/iai.59.7.2252-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winchurch R A, Thupari J N, Munster A M. Endotoxemia in burn patients: levels of circulating endotoxins are related to burn size. Surgery. 1987;102:808–812. [PubMed] [Google Scholar]
- 67.Zhang H, Peterson J W, Niesel D W, Klimpel G R. Bacterial lipoprotein and lipopolysaccharide act synergistically to induce lethal shock and proinflammatory cytokine production. J Immunol. 1997;159:4868–4878. [PubMed] [Google Scholar]
- 68.Ziegler E J, McCutchan J A, Fierer J, Glauser M P, Sadoff J C, Douglas H, Braude A I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982;307:1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- 69.Zollinger W D, Boslego J W. A general approach to standardization of the solid-phase radioimmunoassay for quantitation of class-specific antibodies. J Immunol Meth. 1981;46:129–140. doi: 10.1016/0022-1759(81)90130-7. [DOI] [PubMed] [Google Scholar]