Abstract
Certain mammalian species are resistant to cancer, and a better understanding of how this cancer resistance arises could provide valuable insights for basic cancer research. Recent technological innovations in molecular biology have allowed the study of cancer‐resistant mammals, despite the fact that they are not the classical model animals, which are easily studied using genetic approaches. Naked mole‐rats (NMRs; Heterocephalus glaber) are the longest‐lived rodent, with a maximum lifespan of more than 37 years, and almost never show spontaneous carcinogenesis. NMRs are currently attracting much attention from aging and cancer researchers, and published studies on NMR have continued to increase over the past decade. Cancer development occurs via multiple steps and involves many biological processes. Recent research on the NMR as a model for cancer resistance suggests that they possess various unique carcinogenesis‐resistance mechanisms, including efficient DNA repair pathways, cell‐autonomous resistance to transformation, and dampened inflammatory response. Here, we summarize the molecular mechanisms of carcinogenesis resistance in NMR, which have been uncovered over the past two decades, and discuss future perspectives.
Keywords: cancer, DNA repair, inflammation, naked mole‐rat, transformation
The longest‐lived rodent, the naked mole‐rat has extraordinary resistance to carcinogenesis.

Abbreviations
- 3MC
3‐methylcholanthrene
- 53BP1
tumor protein p53‐binding protein 1
- ARF
alternative reading frame
- ASIS
ARF suppression‐induced senescence
- BMRs
blind mole‐rats
- CKI
cyclin‐dependent kinase inhibitor
- DAMPs
damage‐associated molecular patterns
- DMBA/TPA
7,12‐Dimethylbenz(a) anthracene/12‐O‐tetradecanoylphorbol‐13‐acetate
- DMRs
Damaraland mole‐rats
- DSB
double‐stranded break
- ERAS
ES cell‐expressed Ras
- HMM‐HA
high‐molecular‐mass hyaluronan
- HRASV12
oncogenic HRAS
- HSCs
hematopoietic stem cells
- iPS cells
induced pluripotent stem cells
- MHC‐I
major histocompatibility complex class I
- MLKL
mixed lineage kinase domain‐like
- NK cells
natural killer cells
- NKC
natural killer cell receptor complex
- Nkg2
natural killer group 2
- NMRs
naked mole‐rats
- NSCs
neural stem cells
- PARP1
poly ADP‐ribose polymerase 1
- RIPK3
receptor‐interacting protein kinase 3
- SIRT6
sirtuin 6
- SV40LT
simian virus 40 large T antigen
- TERT
telomerase reverse transcriptase
- γH2AX
phosphorylated histone H2AX
1. INTRODUCTION
Cancer is a disease that has long been actively studied. Basic cancer research has mainly used experimental cancer models such as laboratory mice, fruit flies, zebrafish, and tissue samples/cell lines derived from cancer patients. However, in recent years, it has become clear that some mammalian species, including elephants, bats, NMRs, and BMRs, exhibit strong resistance to carcinogenesis. Therefore, these cancer‐resistant species potentially provide unexpected insights into how humans may be able to overcome cancer. In this review, we focus on research involving the NMR, a long‐lived rodent that has strong cancer resistance and is currently attracting much attention from researchers in various research fields.
Naked mole‐rats are small rodents with a body weight similar to that of mice (~35 g; Figure 1). In the wild, NMRs live in large groups of up to 300 individuals in extensive underground nests composed of many tunnels in the savannas of eastern Africa. 1 The maximum lifespan of NMRs exceeds 37 years, an exceptionally long lifespan for their small body size, making them the longest‐lived rodent. 2 Notably, the mortality rates of NMRs do not increase with age, an essential indicator of individual aging. 3 Moreover, they rarely exhibit age‐related physiological decline, such as reduced reproductive capacity and cardiovascular function, or age‐related diseases such as cancer and Alzheimer's disease. 4 , 5 Cancer is one of the best‐known age‐related diseases, and the risk of carcinogenesis increases with age. However, NMRs exhibit extraordinary carcinogenesis resistance throughout their long lifespan. As a result, NMRs have recently received much attention as an attractive model in various research fields, especially aging and cancer research.
FIGURE 1.

Adult naked mole‐rats. Representative naked mole‐rats kept in our laboratory.
2. CARCINOGENESIS RESISTANCE IN NMR INDIVIDUALS
The conclusion that the NMR is a carcinogenesis‐resistant animal is based on several observational studies, which reported no incidences of cancer among 2000 individuals maintained in laboratories or zoos. 4 , 6 , 7 Conversely, it noteworthy that several cancer cases have been reported in necropsies performed in captive colonies in zoological collections. 8 , 9 , 10 The cancer cases reported so far comprise one case each of axillary adenocarcinoma, gastric neuroendocrine carcinoma, metastatic hepatocellular carcinoma, nephroblastoma, multicentric lymphosarcoma, cutaneous hemangioma, sacral chordoma, and presumptive esophageal adenocarcinoma. However, such cases are very rare, and it is clear that the incidence of cancer in NMRs is negligible.
To evaluate the carcinogenic resistance of the NMR, it is important not only to observe the incidence of spontaneous carcinogenesis, but also to induce in vivo experimental carcinogenesis and evaluate tissue responses. Recently, we showed that NMRs are highly resistant to chemical carcinogenesis. 11 When mice were treated with two types of chemical carcinogens, 3MC or DMBA/TPA, tumors developed in all cases within 24 or 30 weeks, respectively, whereas no NMRs treated with the same carcinogens developed tumors over a period of more than 2 years. Lewis and Buffenstein have observed that NMRs do not form tumors after 6 months of DMBA/TPA treatment (unpublished data from the Buffenstein laboratory 12 ). These observations provide strong experimental evidence confirming the exceptional resistance of NMRs to carcinogenesis.
In vivo carcinogenesis is considered to be a multistep process that starts with the generation of mutant cells in tissues as a result of DNA damage and mutations, followed by their clonal proliferation promoted by changes in the tissue microenvironment including induction of the inflammatory response around the mutant cells. 13 , 14 Recent studies have suggested that NMRs have various potential cancer resistance mechanisms to prevent different stages of this multistep process (Figure 2). In this review, we summarize the current findings in relation to NMR resistance to DNA damage, cell‐autonomous resistance to transformation, and the unique immune system that may contribute to resistance at each step in this multistage carcinogenesis process, and discuss current limitations and future perspectives.
FIGURE 2.
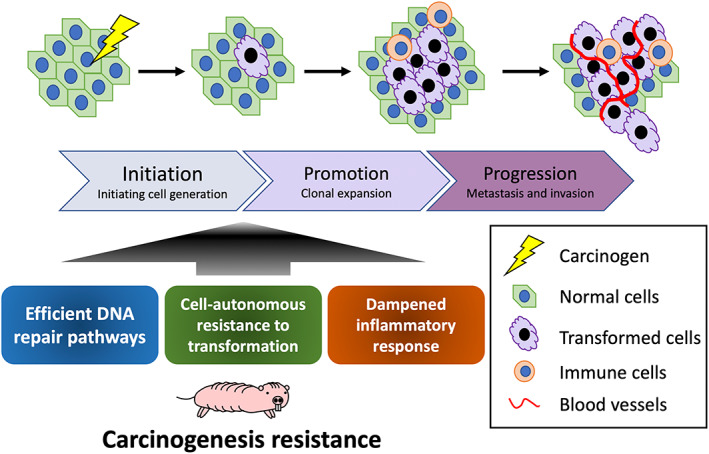
Multistep carcinogenic process and resistance mechanisms in NMRs. Many types of cancers show multistep development, with skin carcinogenesis being a typical example. NMRs possess various potential carcinogenic resistance mechanisms, which may act at different stages during this multistep process. The key mechanisms currently under consideration are efficient DNA repair pathways, cell‐autonomous resistance to transformation, and a dampened inflammatory response.
3. RESISTANCE TO DNA DAMAGE
Cancer arises from cells that have acquired many deleterious genetic alterations. Therefore, DNA mutation is a major cause of cancer. 15 In NMRs, a recent study revealed that the somatic mutation rate of individuals is unusually low for their body mass. 16 Efficient DNA repair may contribute to this exceptionally low mutation frequency. Indeed, DNA DSB repair in fibroblasts of long‐lived rodents including the NMR is much more efficient than in those of other, short‐lived rodents. 17 It is proposed that this is due to high SIRT6 activity in long‐lived rodents including the NMR (Figure 3). Similarly, NMR fibroblasts have high base excision repair efficiency as a result of high poly ADP‐ribosylation activity. 18 , 19 Poly ADP‐ribosylation occurs near the sites of DNA damage and promotes the recruitment of DNA repair factors. 20 SIRT6 can promote poly ADP‐ribosylation through PARP1. 21 Therefore, the high activity of SIRT6 may contribute to the efficient functioning of various DNA repair pathways, including DSB repair.
FIGURE 3.
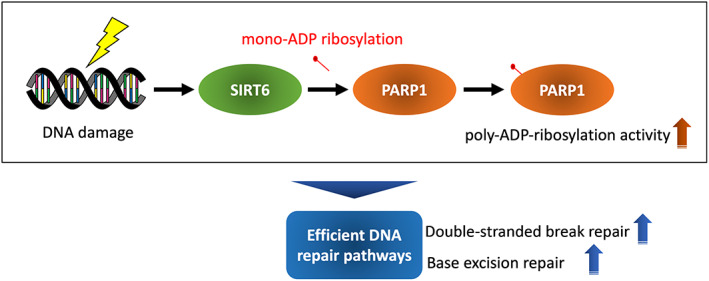
Efficient DNA repair pathways due to high activity of SIRT6. Long‐lived rodents including NMRs have higher SIRT6 activity than short‐lived rodents, which may contribute to the increased activity of PARP1. As a result, poly‐ADP‐ribosylation by PARP1, which is an important initial response for the initiation of DSB repair and base excision repair, may be activated.
Resistance to DNA damage in tissue stem cells is important in carcinogenesis suppression because mutated tissue stem cells can be the origin of malignant tumors. Recently, we reported that NSCs in NMRs are resistant to DNA damage. 22 When NSCs were subjected to gamma‐irradiation, the intensity of the DSB marker, γH2AX, was significantly lower in NMR NSCs than in those of mice. Moreover, analysis of the DNA damage response marker, 53BP1, demonstrated the formation of more foci at damaged sites in NMR NSCs than in mouse NSCs. These results suggest that more DSB sites are repaired more rapidly in NMR NSCs than in mouse NSCs. The percentage of dead cells observed following treatment with the same dose of irradiation was also lower in NMR NSCs than in mouse NSCs. Therefore, we hypothesize that efficient DSB repair in NMR NSCs may reduce DNA damage and therefore cell death. Further studies are needed to determine whether other types of NMR tissue stem cells are also resistant to DNA damage.
Spontaneous mutations occur at low frequency in NMR intestinal crypts in vivo 16 ; however, the spontaneous mutation frequency in cultured NMR fibroblasts is not exceptionally lower than that of other rodent species and humans, except for short‐lived laboratory mice. 23 This disparity in results between tissues and cultured cells may result from differences in the cell types or experimental techniques, and further studies are required. Overall, the molecular mechanisms by which NMRs allow each DNA repair pathway to function so efficiently are only partially understood. Further understanding of the molecular mechanisms of high DNA repair capacity in NMRs may provide novel ways by which human DNA can be efficiently repaired.
4. CELL‐AUTONOMOUS RESISTANCE TO TRANSFORMATION INDUCTION
We and other groups have shown that some NMR cell types, including fibroblasts, iPS cells, NSCs, and HSCs, exhibit slower cell proliferation rates than the equivalent cells from laboratory mice. 22 , 24 , 25 , 26 , 27 Studies using cultured NMR fibroblasts have suggested that NMR‐specific cell proliferation suppression mechanisms, such as hypersensitivity to contact inhibition in adult fibroblasts, may contribute to their cancer resistance 28 (Figure 4). Contact inhibition is a tumor‐suppressive process whereby cell replication is arrested when cells come into contact with one another and become confluent. In general, contact inhibition is controlled by activation of the CKI p27Kip1. In addition to this p27‐mediated contact inhibition system, NMR fibroblasts induce “early contact inhibition” mediated by another CKI, p16INK4a, when cells become semiconfluent. Notably, the INK4 locus of the NMR encodes p15INK4b; ARF; p16INK4a; and an additional alternatively spliced isoform, pALT, which consists of p15INK4b exon 1 and p16INK4a exons 2 and 3. 29 This NMR‐specific pALT has a greater ability to induce cell‐cycle arrest than both p15INK4b and p16INK4a. Another major factor in contact inhibition in the NMR is the secretion of HMM‐HA by NMR fibroblasts. 30 Hyaluronan regulates cellular signaling pathways in a manner that is dependent upon its polymer length. 31 HMM‐HA secreted by NMR fibroblasts enhances sensitivity to contact inhibition via the HA‐CD44‐NF2 pathway. 30 , 32
FIGURE 4.
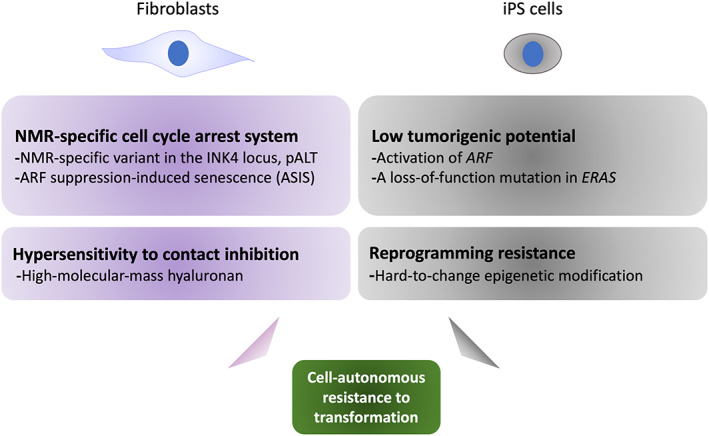
Cell‐autonomous resistance to transformation in NMRs. Several cell‐autonomous mechanisms have been proposed to contribute to the transformation‐resistance of NMRs. NMR fibroblasts exhibit unique inhibitory regulation of the cell cycle via CKIs and HMM‐HA. In NMR iPS cells, species‐specific upregulation of ARF and a loss‐of‐function mutation in ERAS are responsible for resistance to tumorigenesis. In addition, NMR fibroblasts show resistance to reprogramming induction, which may be related to the fact that they are less prone to epigenetic changes.
Mouse fibroblasts are transformed by the introduction of HRASV12 and SV40LT and acquire tumorigenic potential. Conversely, transformation of human fibroblasts requires the transduction of TERT in addition to HRASV12 and SV40LT. Regarding NMRs, two groups have shown that introduction of HRASV12 and SV40LT is insufficient for the transformation of NMR fibroblasts. Furthermore, degradation of HMM‐HA by hyaluronidase or transduction of hTERT is reported to allow transformation of NMR cells by these two oncogenes. 30 , 33 However, another group recently reported that NMR fibroblasts derived from several tissues can be transformed by introduction of only HRASV12 and SV40LT. 34 The use of different promoters in the expression vectors used in these studies may account for this discrepancy 35 ; further studies are needed to determine how resistant NMR cells are to transformation by introduction of oncogenes.
In general, undifferentiated iPS cells have tumorigenic potential and form teratomas in vivo. 36 , 37 However, we have previously generated NMR iPS cells and found that they do not form tumors. 26 This is due to the species‐specific upregulation of the tumor‐suppressor ARF and a loss‐of‐function mutation in the oncogene, ERAS (Figure 4). In general, ARF suppression promotes cell proliferation during reprogramming and carcinogenesis. However, in NMRs, fibroblasts that suppressed ARF during reprogramming or oncogene activation entered a cellular senescence‐like state and stopped proliferating. We termed this NMR‐specific phenomenon “ASIS,” It seems probable that ASIS acts as an NMR‐specific safeguard against tumorigenesis; however, its molecular mechanisms are still unknown. Tan et al. 38 have shown that NMR fibroblasts are less prone to epigenetic changes during iPS cell induction, and that this resistance to epigenetic changes may lead to a low reprogramming efficiency. These results are significant because carcinogenesis and cellular reprogramming processes share several characteristics. In mice, induction of in vivo reprogramming by expression of Yamanaka four factors results in the development of teratomas, tumors similar to Wilms tumor, a common pediatric kidney cancer, or human germ cell tumors depending on the level and timing of transgene expression. 39 , 40 , 41 Therefore, the low reprogramming efficiency and these NMR‐specific responses to induction of reprogramming may be related to their cancer resistance.
In summary, previous studies examining the response of cultured NMR fibroblasts to induction of transformation or reprogramming have identified several NMR‐specific molecular mechanisms that may contribute to their in vivo resistance to carcinogenesis. However, it seems likely that the introduction of potent oncogenes may still result in transformation of NMR cells, as seen in mouse cells. 34 , 42 During in vivo carcinogenesis, such as the development of colon cancer, the gradual accumulation of oncogenic mutations eventually leads to carcinogenesis. 43 Therefore, further in vitro and in vivo studies are required to determine what level of oncogenic mutations NMR cells can tolerate before oncogenic transformation occurs. Moreover, it is important to evaluate the invasive and metastatic potential of the transformed NMR cells to determine the degree of malignancy they develop.
5. DAMPENED INFLAMMATORY RESPONSE AND UNIQUE IMMUNE SYSTEM
During tumorigenesis, changes in the tissue microenvironment that surrounds the mutant cells, especially persistent tissue inflammation, strongly promote carcinogenesis. 44 , 45 , 46 As described above, we recently demonstrated that NMRs are strongly resistant to induction of carcinogenesis by treatment with chemical carcinogens. 11 Notably, we found that carcinogen‐treated NMR tissues exhibit a dampened inflammatory response despite the increase in tissue damage, such as DNA damage and cell death, induced by chemical carcinogen treatment. Analysis of potential contributory factors in this attenuated inflammatory response identified NMR‐specific loss‐of‐function mutations in the master regulators of necroptosis, RIPK3, and MLKL genes. Necroptosis is a programmed necrotic cell death that causes massive release of cellular components into the intercellular space, called DAMPs. In NMRs, the lack of necroptosis‐inducing ability suppresses the release of DAMPs upon treatment with carcinogens, leading to attenuation of immune cell infiltration and suppression of cancer promotion (Figure 5). DNA damage and cell death were increased in NMR skin following exposure to chemical carcinogens. Therefore, it will be important to determine how NMR tissues clear these damaged cells and cellular components while simultaneously limiting the accumulation of immune cells.
FIGURE 5.
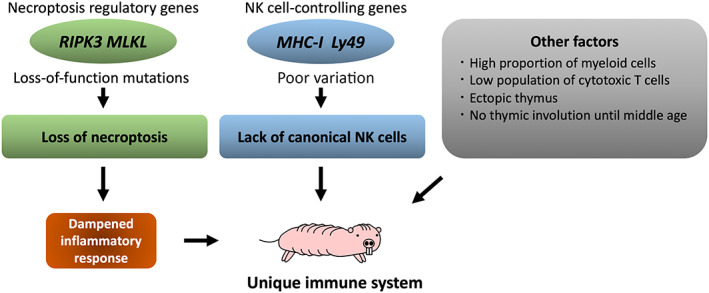
Unique immunological features of NMRs. NMRs lack key regulators of necroptosis, which may contribute to the attenuation of the tissue inflammatory response in these animals. Furthermore, gene family expansion of MHC‐I and Ly49 genes, the major NK cell receptors, is significantly lower in NMRs than in other species, suggesting a loss of the control mechanisms of canonical NK cells that may be relevant to the absence of these cells in NMRs. NMRs also have some unique immune characteristics, such as a high myeloid‐to‐lymphoid cell ratio, ectopic thymi, and no thymic involution for more than 10 years.
Recent studies have also suggested that NMRs possess a unique immune system 47 (Figure 5). An interspecies comparative analysis of single‐cell RNA‐sequencing data between NMRs and mice revealed that while myeloid cells were prominent in NMR spleens, mouse spleens were dominated by lymphoid cells, which includes B cells, T cells, and natural killer (NK) cells. A similar differentiation bias was also observed in NMR HSCs. 27 Intriguingly, lymphoid‐derived NK cells were entirely absent from NMRs. In rodents, NK cell function is controlled by genes encoding their MHC‐I ligands and NKC. 48 , 49 There are 22 MHC‐I genes in the mouse genome, but only two in that of the NMR. 47 The Ly49 gene, a major NKC gene, expands in the mouse genome to form a gene family, 49 but only encodes a single protein in the NMR genome. 47 Therefore, it seems that NMRs lack the control mechanisms of canonical NK cells at the genomic level. Notably, we previously demonstrated that NMR macrophages reacted to the anti‐NK1.1 antibody, 50 commonly used as an NK cell marker in mouse immunology research. 51 It will therefore be interesting to investigate whether NMR macrophages partly shoulder the function of NK cells. Another study revealed that the frequency of cytotoxic T cells was lower in the NMR compared with their murine counterpart. 52 , 53 Notably, NMRs have the ectopic thymus and show no thymic involution up to 11 years of age. 53 Although immunological research on NMRs has only recently begun, the unique regulation of their immune system is gradually becoming clear. Further studies on how the NMR immune system contributes to their carcinogenesis resistance are expected.
6. CONCLUSION
In this review, we have summarized recent progress in understanding of NMR cancer resistance in terms of resistance to DNA damage, cell‐autonomous resistance to transformation, and the unique NMR immune system. To further our understanding of cancer resistance in NMRs, it will be important to consider these processes from an evolutionary perspective by comparison with other cancer‐resistant species as well as more closely related mole‐rat species. We and another group previously demonstrated that NMR NSCs and fibroblasts are less prone than those of mice to cell death following gamma‐irradiation‐induced DNA damage. 22 , 54 However, cancer‐resistant elephants have a different strategy to protect against DNA damage. Elephant lymphocytes are more prone to cell death in response to DNA damage than human lymphocytes. 55 This high susceptibility of damaged cells to cell death is thought to be due to the presence of more than 20 copies of the p53 gene in elephant genome, and may contribute to the elimination of highly damaged cells and therefore to cancer resistance in elephants. As described above, we have shown that NMRs have strong resistance to carcinogenesis induced using 3MC or DMBA/TPA. When BMRs, another cancer‐resistant rodent that is phylogenetically distinct from the NMR, 56 was subjected to carcinogenesis induction by treatment with these same chemical carcinogens, the incidence of carcinogenesis following 3MC treatment was low (~9%), and tumor formation was not observed with DMBA/TPA treatment. Notably, the skin of BMRs showed a robust inflammatory and necrotic response upon carcinogenic insult. Consistent with this observation, BMR fibroblasts possess a unique necrotic cell death induction mechanism, which acts via the activation of retrotransposons and the innate immune response through the cGAS–STING pathway. 57 , 58 The strong inflammatory response to chemical carcinogens exhibited by BMRs contrasts with the dampened inflammatory response seen in NMRs. Therefore, mechanisms of cancer resistance appear diverse between cancer‐resistant species.
In NMR studies, cell and tissue responses of NMRs are often compared with those of laboratory mice, because the mouse is the best‐studied rodent and has a similar body mass to the NMR. However, the observed differences may sometimes not be “NMR‐specific.” Therefore, in addition to mice, comparisons with more closely related, noncancer‐resistant rodents, such as guinea pigs, would be useful. Simultaneous comparisons in multiple species, or with more closely related species, would provide more robust results. DMRs, which are closely related to NMRs and have a relatively long lifespan (~20 years), remain poorly studied. In vivo and in vitro studies using DMRs may reveal the evolution of cancer resistance mechanisms in these mole‐rat species.
While progress has been made in our understanding of cancer resistance in NMRs since cancer research in NMRs began in the 2000s, many unanswered questions remain; in particular, there is a dearth of in vivo experimental studies. To conduct more sophisticated in vivo studies, it will be essential to establish developmental engineering techniques to facilitate generation of genetically engineered NMR individuals, which can be studied using various recent technologies such as multiomics and tissue imaging. Furthermore, other NMR features, such as tolerance to hypoxia, longevity, and sociality, which may have been acquired during adaptation to their underground habitat, may be linked to their cancer resistance. Therefore, further investigation of the various properties that are unique to NMRs would help to elucidate their cancer resistance mechanism and its evolutionary process from a new perspective.
FUNDING INFORMATION
This work was supported in part by JSPS KAKENHI Grant Numbers JP22K15024 (to K.O.), JP22K06069 (to Y.K.), JP21H05143, JP21H02392 and JP22K18355 (to K.M.); and JST FOREST Program Grant Number JPMJFR216C (to K.M.). Y.Y. is a Research Fellow of the Japanese Society for the Promotion of Science.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
ACKNOWLEDGMENTS
We thank all members of the K.M. laboratories for scientific discussion.
Yamamura Y, Kawamura Y, Oka K, Miura K. Carcinogenesis resistance in the longest‐lived rodent, the naked mole‐rat. Cancer Sci. 2022;113:4030‐4036. doi: 10.1111/cas.15570
REFERENCES
- 1. Jarvis JUM. Eusociality in a mammal: cooperative breeding in naked mole‐rat colonies. Science. 1981;212(4494):571‐573. doi: 10.1126/science.7209555 [DOI] [PubMed] [Google Scholar]
- 2. Lee BP, Smith M, Buffenstein R, Harries LW. Negligible senescence in naked mole rats may be a consequence of well‐maintained splicing regulation. Geroscience. 2020;42(2):633‐651. doi: 10.1007/s11357-019-00150-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruby JG, Smith M, Buffenstein R. Naked Mole‐Rat mortality rates defy gompertzian laws by not increasing with age. Elife. 2018;7:e31157. doi: 10.7554/eLife.31157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buffenstein R. Negligible senescence in the longest living rodent, the naked mole‐rat: insights from a successfully aging species. J Comp Physiol B. 2008;178(4):439‐445. doi: 10.1007/s00360-007-0237-5 [DOI] [PubMed] [Google Scholar]
- 5. Edrey YH, Medina DX, Gaczynska M, et al. Amyloid beta and the longest‐lived rodent: the naked mole‐rat as a model for natural protection from Alzheimer's disease. Neurobiol Aging. 2013;34(10):2352‐2360. doi: 10.1016/j.neurobiolaging.2013.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grimes KM, Lindsey ML, Gelfond JAL, Buffenstein R. Getting to the heart of the matter: age‐related changes in diastolic heart function in the longest‐lived rodent, the naked mole rat. J Gerontol A Biol Sci Med Sci. 2012;67(4):384‐394. doi: 10.1093/gerona/glr222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delaney MA, Nagy L, Kinsel MJ, Treuting PM. Spontaneous histologic lesions of the adult naked mole rat (Heterocephalus glaber): a retrospective survey of lesions in a zoo population. Vet Pathol. 2013;50(4):607‐621. doi: 10.1177/0300985812471543 [DOI] [PubMed] [Google Scholar]
- 8. Delaney MA, Ward JM, Walsh TF, et al. Initial case reports of cancer in naked mole‐rats (Heterocephalus glaber). Vet Pathol. 2016;53(3):691‐696. doi: 10.1177/0300985816630796 [DOI] [PubMed] [Google Scholar]
- 9. Taylor KR, Milone NA, Rodriguez CE. Four cases of spontaneous neoplasia in the naked mole‐rat (Heterocephalus glaber), a putative cancer‐resistant species. J Gerontol A Biol Sci Med Sci. 2017;72(1):38‐43. doi: 10.1093/gerona/glw047 [DOI] [PubMed] [Google Scholar]
- 10. Cole JE, Steeil JC, Sarro SJ, Kerns KL, Cartoceti A. Chordoma of the sacrum of an adult naked mole‐rat. J Vet Diagn Invest. 2020;32(1):132‐135. doi: 10.1177/1040638719894985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oka K, Fujioka S, Kawamura Y, et al. Resistance to chemical carcinogenesis induction via a dampened inflammatory response in naked mole‐rats. Commun Biol. 2022;5(1):287. doi: 10.1038/s42003-022-03241-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hadi F, Smith ESJ, Khaled WT. Naked mole‐rats: resistant to developing cancer or good at avoiding it? Adv Exp Med Biol. 2021;1319:341‐352. doi: 10.1007/978-3-030-65943-1_14 [DOI] [PubMed] [Google Scholar]
- 13. DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54(1):63‐128. doi: 10.1016/0163-7258(92)90051-z [DOI] [PubMed] [Google Scholar]
- 14. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 15. Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458(7239):719‐724. doi: 10.1038/nature07943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cagan A, Baez‐Ortega A, Brzozowska N, et al. Somatic mutation rates scale with lifespan across mammals. Nature. 2022;604(7906):517‐524. doi: 10.1038/s41586-022-04618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian X, Firsanov D, Zhang Z, et al. SIRT6 is responsible for more efficient DNA double‐strand break repair in long‐lived species. Cell. 2019;177(3):622‐638.e22. doi: 10.1016/j.cell.2019.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evdokimov A, Kutuzov M, Petruseva I, et al. Naked mole rat cells display more efficient excision repair than mouse cells. Aging (Albany NY). 2018;10(6):1454‐1473. doi: 10.18632/aging.101482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kosova AA, Kutuzov MM, Evdokimov AN, et al. Poly(ADP‐ribosyl)ation and DNA repair synthesis in the extracts of naked mole rat, mouse, and human cells. Aging (Albany NY). 2019;11(9):2852‐2873. doi: 10.18632/aging.101959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu C, Vyas A, Kassab MA, Singh AK, Yu X. The role of poly ADP‐ribosylation in the first wave of DNA damage response. Nucleic Acids Res. 2017;45(14):8129‐8141. doi: 10.1093/nar/gkx565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mao Z, Hine C, Tian X, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332(6036):1443‐1446. doi: 10.1126/science.1202723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamamura Y, Kawamura Y, Oiwa Y, et al. Isolation and characterization of neural stem/progenitor cells in the subventricular zone of the naked mole‐rat brain. Inflamm Regen. 2021;41(1):31. doi: 10.1186/s41232-021-00182-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Dong X, Tian X, et al. Maintenance of genome sequence integrity in long‐ and short‐lived rodent species. Sci Adv. 2021;7(44):eabj3284. doi: 10.1126/sciadv.abj3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seluanov A, Hine C, Bozzella M, et al. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell. 2008;7(6):813‐823. doi: 10.1111/j.1474-9726.2008.00431.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee SG, Mikhalchenko AE, Yim SH, et al. Naked mole rat induced pluripotent stem cells and their contribution to interspecific Chimera. Stem Cell Reports. 2017;9(5):1706‐1720. doi: 10.1016/j.stemcr.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyawaki S, Kawamura Y, Oiwa Y, et al. Tumour resistance in induced pluripotent stem cells derived from naked mole‐rats. Nat Commun. 2016;7:11471. doi: 10.1038/ncomms11471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Emmrich S, Trapp A, Zakusilo FT, et al. Characterization of naked mole‐rat hematopoiesis reveals unique stem and progenitor cell patterns and neotenic traits. EMBO J. 2022;41:e109694. doi: 10.15252/embj.2021109694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seluanov A, Hine C, Azpurua J, et al. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole‐rat. Proc Natl Acad Sci USA. 2009;106(46):19352‐19357. doi: 10.1073/pnas.0905252106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tian X, Azpurua J, Ke Z, et al. INK4 locus of the tumor‐resistant rodent, the naked mole rat, expresses a functional p15/p16 hybrid isoform. Proc Natl Acad Sci USA. 2015;112(4):1053‐1058. doi: 10.1073/pnas.1418203112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian X, Azpurua J, Hine C, et al. High‐molecular‐mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499(7458):346‐349. doi: 10.1038/nature12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015;2015:563818. doi: 10.1155/2015/563818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morrison H, Sherman LS, Legg J, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15(8):968‐980. doi: 10.1101/gad.189601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang S, Mele J, Wu Y, Buffenstein R, Hornsby PJ. Resistance to experimental tumorigenesis in cells of a long‐lived mammal, the naked mole‐rat (Heterocephalus glaber). Aging Cell. 2010;9(4):626‐635. doi: 10.1111/j.1474-9726.2010.00588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hadi F, Kulaberoglu Y, Lazarus KA, et al. Transformation of naked mole‐rat cells. Nature. 2020;583(7814):E1‐E7. doi: 10.1038/s41586-020-2410-x [DOI] [PubMed] [Google Scholar]
- 35. Zhao J, Tian X, Zhu Y, et al. Reply to: Transformation of naked mole‐rat cells. Nature. 2020;583(7814):E8‐E13. doi: 10.1038/s41586-020-2411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okita K, Ichisaka T, Yamanaka S. Generation of germline‐competent induced pluripotent stem cells. Nature. 2007;448(7151):313‐317. doi: 10.1038/nature05934 [DOI] [PubMed] [Google Scholar]
- 37. Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27(8):743‐745. doi: 10.1038/nbt.1554 [DOI] [PubMed] [Google Scholar]
- 38. Tan L, Ke Z, Tombline G, et al. Naked mole rat cells have a stable epigenome that resists iPSC reprogramming. Stem Cell Reports. 2017;9(5):1721‐1734. doi: 10.1016/j.stemcr.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abad M, Mosteiro L, Pantoja C, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013;502(7471):340‐345. doi: 10.1038/nature12586 [DOI] [PubMed] [Google Scholar]
- 40. Ohnishi K, Semi K, Yamamoto T, et al. Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell. 2014;156(4):663‐677. doi: 10.1016/j.cell.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 41. Taguchi J, Shibata H, Kabata K, et al. DMRT1‐mediated reprogramming drives development of cancer resembling human germ cell tumors with features of totipotency. Nat Commun. 2021;12(1):5041. doi: 10.1038/s41467-021-25249-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deuker MM, Lewis KN, Ingaramo M, Kimmel J, Buffenstein R, Settleman J. Unprovoked stabilization and nuclear accumulation of the naked mole‐rat p53 protein. Sci Rep. 2020;10(1):6966. doi: 10.1038/s41598-020-64009-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159‐170. doi: 10.1016/s0092-8674(00)81333-1 [DOI] [PubMed] [Google Scholar]
- 44. Hoste E, Arwert EN, Lal R, et al. Innate sensing of microbial products promotes wound‐induced skin cancer. Nat Commun. 2015;6:5932. doi: 10.1038/ncomms6932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bald T, Quast T, Landsberg J, et al. Ultraviolet‐radiation‐induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507(7490):109‐113. doi: 10.1038/nature13111 [DOI] [PubMed] [Google Scholar]
- 46. Mittal D, Saccheri F, Vénéreau E, Pusterla T, Bianchi ME, Rescigno M. TLR4‐mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010;29(13):2242‐2252. doi: 10.1038/emboj.2010.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hilton HG, Rubinstein ND, Janki P, et al. Single‐cell transcriptomics of the naked mole‐rat reveals unexpected features of mammalian immunity. PLoS Biol. 2019;17(11):e3000528. doi: 10.1371/journal.pbio.3000528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guethlein LA, Norman PJ, Hilton HG, Parham P. Co‐evolution of MHC class I and variable NK cell receptors in placental mammals. Immunol Rev. 2015;267(1):259‐282. doi: 10.1111/imr.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kelley J, Walter L, Trowsdale J. Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet. 2005;1(2):129‐139. doi: 10.1371/journal.pgen.0010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wada H, Shibata Y, Abe Y, et al. Flow cytometric identification and cell‐line establishment of macrophages in naked mole‐rats. Sci Rep. 2019;9(1):17981. doi: 10.1038/s41598-019-54442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bartel Y, Bauer B, Steinle A. Modulation of NK cell function by genetically coupled C‐type lectin‐like receptor/ligand pairs encoded in the human natural killer gene complex. Front Immunol. 2013;4:362. doi: 10.3389/fimmu.2013.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shebzukhov Y, Holtze S, Hirseland H, et al. Identification of cross‐reactive antibodies for the detection of lymphocytes, myeloid cells and haematopoietic precursors in the naked mole rat. Eur J Immunol. 2019;49(11):2103‐2110. doi: 10.1002/eji.201948124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Emmrich S, Zakusilo FT, Trapp A, et al. Ectopic cervical thymi and no thymic involution until midlife in naked mole rats. Aging Cell. 2021;20(10):e13477. doi: 10.1111/acel.13477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao Y, Tyshkovskiy A, Muñoz‐Espín D, et al. Naked mole rats can undergo developmental, oncogene‐induced and DNA damage‐induced cellular senescence. Proc Natl Acad Sci USA. 2018;115(8):1801‐1806. doi: 10.1073/pnas.1721160115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abegglen LM, Caulin AF, Chan A, et al. Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. JAMA. 2015;314(17):1850‐1860. doi: 10.1001/jama.2015.13134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Manov I, Hirsh M, Iancu TC, et al. Pronounced cancer resistance in a subterranean rodent, the blind mole‐rat, Spalax: in vivo and in vitro evdence. BMC Biol. 2013;11:91. doi: 10.1186/1741-7007-11-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gorbunova V, Hine C, Tian X, et al. Cancer resistance in the blind mole rat is mediated by concerted necrotic cell death mechanism. Proc Natl Acad Sci USA. 2012;109(47):19392‐19396. doi: 10.1073/pnas.1217211109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao Y, Oreskovic E, Zhang Q, et al. Transposon‐triggered innate immune response confers cancer resistance to the blind mole rat. Nat Immunol. 2021;22(10):1219‐1230. doi: 10.1038/s41590-021-01027-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


