Abstract
The global phase 3 IMpower010 study evaluated adjuvant atezolizumab versus best supportive care (BSC) following platinum‐based chemotherapy in patients with resected stage IB–IIIA non‐small cell lung cancer (NSCLC). Here, we report a subgroup analysis in patients enrolled in Japan. Eligible patients had complete resection of histologically or cytologically confirmed stage IB (tumors ≥4 cm)–IIIA NSCLC. Upon completing 1–4 cycles of adjuvant cisplatin‐based chemotherapy, patients were randomized 1:1 to receive atezolizumab (fixed dose of 1200 mg every 21 days; 16 cycles or 1 year) or BSC. The primary endpoint of the global IMpower010 study was investigator‐assessed disease‐free survival, tested hierarchically first in patients with stage II–IIIA NSCLC whose tumors expressed programmed death‐ligand 1 (PD‐L1) on ≥1% of tumor cells, then in all randomized patients with stage II–IIIA NSCLC, and finally in the intention‐to‐treat (ITT) population (stage IB–IIIA NSCLC). Safety was evaluated in all patients who received atezolizumab or BSC. The study comprised 149 enrolled patients in three populations: ITT (n = 117; atezolizumab, n = 59; BSC, n = 58), all‐randomized stage II–IIIA (n = 113; atezolizumab, n = 56; BSC, n = 57), and PD‐L1 tumor cells ≥1% stage II–IIIA (n = 74; atezolizumab, n = 41; BSC, n = 33). At the data cutoff date (January 21, 2021), a trend toward disease‐free survival improvement with atezolizumab vs BSC was observed in the PD‐L1 tumor cells ≥1% stage II–IIIA (unstratified hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.25–1.08), all‐randomized stage II–IIIA (unstratified HR, 0.62; 95% CI, 0.35–1.11), and ITT (unstratified HR, 0.61; 95% CI, 0.34–1.10) populations. Atezolizumab‐related grade 3/4 adverse events occurred in 16% of patients; no treatment‐related grade 5 events occurred. Adjuvant atezolizumab showed disease‐free survival improvement and a tolerable toxicity profile in Japanese patients in IMpower010, consistent with the global study results.
Keywords: atezolizumab, Japanese, non‐small cell lung cancer, PD‐L1 inhibitor, PD‐L1 protein
This manuscript reports a subgroup analysis of Japanese patients in the global phase 3 IMpower010 study evaluating adjuvant atezolizumab vs best supportive care (BSC) following platinum‐based chemotherapy in resected stage IB‐IIIA non‐small cell lung cancer. Disease‐free survival (DFS) improvement with atezolizumab versus BSC was observed in the Japanese stage II‐IIIA population with PD‐L1 expression on ≥1% of tumor cells; in the Japanese all‐randomized stage II‐IIIA and ITT (stage IB‐IIIA) populations, unstratified DFS hazard ratios favored atezolizumab vs BSC. Adjuvant atezolizumab had a tolerable toxicity profile in Japanese patients in IMpower010, consistent with the global study results.
Abbreviations
- AE
adverse events
- AESI
AEs of special interest
- ALK
anaplastic lymphoma kinase
- BSC
best supportive care
- CI
confidence interval
- DFS
disease‐free survival
- EGFR
epidermal growth factor receptor
- HR
hazard ratio
- IC
tumor‐infiltrating immune cells
- ITT
intention‐to‐treat
- NR
not reached
- NSCLC
non‐small cell lung cancer
- OS
overall survival
- PD‐1
programmed death‐1
- PD‐L1
programmed death‐ligand 1
- TC
tumor cells
1. INTRODUCTION
Non‐small cell lung cancer accounts for the majority of lung cancer cases worldwide, with ~50% of these cases being classified as localized (stages I and II) or locally advanced (stage III) disease. 1 The preferred treatment for stage I–II and select cases of stage III NSCLC is curative‐intent surgery. 2 , 3 However, prognosis remains poor, particularly for patients with stage III disease, who demonstrate lower 5‐year survival rates than patients with stage I disease, 4 potentially indicating the presence of micrometastases in some patients with resected tumors.
In Japan, >85% of NSCLC cases can be categorized as stage I–IIIA, with surgical resection being the predominant treatment in this patient population. 5 , 6 Consistent with the trend observed in global populations, the 5‐year OS rates following surgical resection range from 72% to 84% for stage IA and decrease to between 30% and 38% for stage IIIA NSCLC. 5 , 6 More recent registry study data, including from patients with small cell lung cancer, show 5‐year OS rates of 84% to 89% for patients with stage IA disease and 48% to 49% for patients with stage IIIA disease following surgical resection. 7
Globally, adjuvant platinum‐based chemotherapy is a standard of care for patients with completely resected stage IB–IIIA NSCLC. 2 Although cisplatin‐based regimens have demonstrated survival benefit in several randomized clinical trials, only a modest absolute benefit of 5% in 5‐year OS is observed. 8 , 9 Cisplatin‐based adjuvant chemotherapy is also a standard treatment for patients with resected early‐stage NSCLC in Japan. However, as the Japan Intergroup Trial of Pemetrexed Adjuvant Chemotherapy for Completely Resected Nonsquamous Non‐Small Cell Lung Cancer (JIPANG) has shown, there is uncertainty as to which chemotherapy regimen offers the most convincing benefit in these settings. 10
Immune checkpoint inhibitors such as anti–PD‐L1 and anti–PD‐1 agents are standard first‐line treatments for metastatic or advanced NSCLC without oncogenic driver mutations, either as single agents or in combination with chemotherapy. 11 The humanized anti–PD‐L1 monoclonal antibody atezolizumab (Tecentriq®, F. Hoffmann‐La Roche AG, Switzerland), which inhibits the interaction between PD‐L1 and its receptors PD‐1 and B7.1, reinvigorating antitumor immunity, 12 is approved for the treatment of metastatic or recurrent NSCLC in the first‐line as well as in the second‐line and beyond. 11 , 13 , 14 In Japan, atezolizumab is approved for the treatment of unresectable, advanced, or recurrent NSCLC; untreated unresectable, advanced, or recurrent NSCLC; and untreated PD‐L1‐positive, unresectable, advanced, or recurrent NSCLC. 15 Based on the results of the interim DFS analysis of the phase 3 IMpower010 study, adjuvant atezolizumab was approved in countries including the USA and Japan for use in adult patients who received platinum‐based chemotherapy following resection of their stage II–IIIA NSCLC with PD‐L1 expression on ≥1% of tumor cells, as determined by an approved diagnostic test. 15 , 16
The global, randomized, open‐label IMpower010 study (ClinicalTrials.gov: NCT02486718) is investigating the efficacy and safety of adjuvant atezolizumab versus BSC in patients with stage IB–IIIA NSCLC who received platinum‐based chemotherapy after resection. 16 Atezolizumab (n = 507) demonstrated a statistically significant improvement in the primary efficacy endpoint of DFS versus BSC (n = 498) in patients with stage II–IIIA NSCLC who had PD‐L1 expression on TC covering ≥1% of tumor area (HR, 0.66; 95% CI, 0.50–0.88), and in all randomized patients with stage II–IIIA NSCLC (HR, 0.79; 95% CI, 0.64–0.96). The statistical significance boundary was not crossed for DFS in the ITT population comprising all randomized patients (stage IB–IIIA; HR, 0.81; 95% CI, 0.67–0.99). The toxicity profile of atezolizumab evaluated in all randomized patients who received atezolizumab or BSC was consistent with that observed for atezolizumab in previous studies of patients with NSCLC, with no new safety signals being identified. 13 , 14 , 17
Clinical response and tolerability to systemic anticancer therapy, including immunotherapy, may differ between Asian and non‐Asian patients, as demonstrated by several studies, across cancer types. 18 , 19 Some studies comparing the efficacy of PD‐L1/PD‐1 inhibitors in Asian and non‐Asian patients have shown enhanced benefit in Japanese patients in particular. 19 However, an increased risk of treatment‐related pneumonitis or hepatotoxicities has also been observed among Japanese patients receiving systemic anticancer therapy. 19 PD‐L1/PD‐1 inhibitors, which present a risk of immune‐related pneumonitis, may cause increased toxicity in Japanese patients, as was observed in the Japanese subgroup analysis of second‐line atezolizumab in patients with metastatic NSCLC in the phase 3 OAK study. 20 Therefore, it is important to explore the efficacy and safety of immunotherapies, such as atezolizumab, in specific ethnic populations, including Japanese patients, to determine the benefits in clinical response as well as identify ethnicity‐dependent toxic effects.
Here, we describe the efficacy and safety of adjuvant atezolizumab in Japanese patients (defined as patients enrolled at sites in Japan) with resected stage IB–IIIA NSCLC from the IMpower010 study.
2. MATERIALS AND METHODS
2.1. Study design
The study design for the global IMpower010 study has been previously described. 16 Briefly, the study was conducted in two phases: enrollment and randomization. In the enrollment phase, eligible patients received one of four cisplatin‐based chemotherapy regimens for up to four 21‐day cycles, based on investigator choice: cisplatin 75 mg/m2 IV on day 1, plus either vinorelbine 30 mg/m2 IV on days 1 and 8, docetaxel 75 mg/m2 on day 1, gemcitabine 1250 mg/m2 IV on days 1 and 8, or, for patients with nonsquamous NSCLC, pemetrexed 500 mg/m2 on day 1.
Patients who completed their cisplatin‐based chemotherapy regimen without disease recurrence, and who were considered eligible for the randomization phase, were randomly assigned, 1:1, to treatment by a permuted‐block method. Patients received either atezolizumab at a fixed dose of 1200 mg IV on day 1 of a 21‐day cycle for up to 16 cycles (or 1 year) or BSC, which included observation and regular scans to detect disease recurrence. Stratification factors for randomization were sex (female vs male), tumor histology (squamous vs nonsquamous), extent of disease (stage IB vs stage II vs stage IIIA), and PD‐L1 tumor expression status (TC2/3 and any IC vs TC0/1 and IC2/3 vs TC0/1 and IC0/1) as determined by the SP142 immunohistochemistry assay (Ventana Medical Systems Inc, Tucson, AZ, USA). 21 No crossover was allowed from the BSC arm to the atezolizumab arm.
The trial was conducted in accordance with the Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board or Ethics Committee of each participating site. The safety data were periodically reviewed by an independent data monitoring committee. All patients provided written informed consent. Exploratory analysis of the Japanese subpopulation was predetermined and approved by the Institutional Review Boards in Japan.
2.2. Patients
All patients enrolled at the 24 study sites in Japan were included in this subgroup analysis. Eligible patients were aged ≥18 years, had an Eastern Cooperative Oncology Group performance status of 0 or 1, had histologically or cytologically diagnosed stage IB (tumors ≥4 cm)‐IIIA (T2–3 N0, T1–3 N1, T1–3 N2, T4 N0–1) NSCLC per the Union Internationale Contre le Cancer/American Joint Committee on Cancer staging system, 7th edition, 22 and were eligible to receive cisplatin‐based chemotherapy. As IMpower010 was based on pathological staging, all mentions of disease stage in the study refer to pathological stage. Patients with EGFR + or ALK + NSCLC tumors were also enrolled. Representative formalin‐fixed paraffin‐embedded resected tumor specimens were required to test PD‐L1 tumor expression using the SP142 immunohistochemistry assay (Ventana). The protocol was later amended to assess the primary efficacy endpoint in patients with PD‐L1 expression on TC covering ≥1% of tumor area, as determined by the SP263 IH immunohistochemistry C assay (Ventana). 16 , 23
2.3. Assessments and endpoints
The primary efficacy objective was to evaluate the efficacy of atezolizumab monotherapy compared with that of BSC, based on the investigator‐assessed DFS in the PD‐L1 TC ≥1% (SP263) stage II–IIIA, all‐randomized stage II–IIIA NSCLC, and the ITT populations, defined as all randomized patients with stage IB–IIIA NSCLC. Key secondary efficacy objectives were OS in the ITT population, 3‐year and 5‐year DFS rates in the PD‐L1 TC ≥1% (SP263) stage II–IIIA, all‐randomized stage II–IIIA NSCLC, and ITT populations. All randomized patients underwent tumor assessments by computed tomography (CT) at baseline, and every 4 months from cycle 1, day 1 in the first year and every 6 months in the second year. In patients experiencing no disease recurrence, tumor assessments were performed every 6 months by CT and X‐ray during years 3–5, and by X‐ray every year thereafter.
Safety was evaluated in patients randomized to the atezolizumab arm who received at least one dose of study drug and patients randomized to the BSC arm who had at least one post‐baseline safety assessment. All AE, serious AEs and AESI were recorded during the study and for 30 days after the last dose of study treatment for all AEs and 90 days after the last study treatment for serious AEs and AESIs.
2.4. Statistical analysis
Details of the statistical methods used in the IMpower010 study have been previously described, 16 and the same methods were followed in this exploratory analysis of the Japanese population (defined as patients enrolled at sites in Japan). The primary efficacy endpoint of DFS and the secondary efficacy endpoint of OS were hierarchically tested: first DFS in the PD‐L1 TC ≥1% (SP263) stage II–IIIA population, then DFS in the all‐randomized stage II–IIIA NSCLC population, then DFS in the ITT population, and then OS in the ITT population. The unstratified HR for DFS and OS were determined by a Cox regression model, including two‐sided 95% CIs. Kaplan–Meier methodology was used to estimate DFS and OS in both study arms, with two‐sided 95% CIs for each median survival time being determined using the Brookmeyer–Crowley method. In the Japanese subgroup analysis, demographic characteristics, such as age, sex, race/ethnicity, baseline disease characteristics, and cisplatin‐based regimen, were summarized by treatment arm. Descriptive statistics (mean, median, standard deviation, and range) are presented for continuous data; frequencies and percentages are presented for categorical data. Safety was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE version 4.0). Reported data included all‐grade AEs, grade 3–5 AEs, serious AEs, AESIs, and AEs leading to study drug discontinuation or interruption. Statistical analysis was performed using SAS (version 9.4).
3. RESULTS
3.1. Patients
In total, 149 patients were recruited from 24 sites in Japan between October 2015 and September 2018 (Figure 1). Of these patients, 147 received adjuvant cisplatin‐based chemotherapy. In total, 77 patients received cisplatin plus vinorelbine, 61 patients received cisplatin plus pemetrexed, four received cisplatin plus gemcitabine, and five received cisplatin plus docetaxel. Patients received a median of 4.0, 4.0, 3.5, and 4.0 cycles of cisplatin as a part of each regimen, respectively. Patients also received a median of eight doses of vinorelbine, four cycles of pemetrexed, 6.5 doses of gemcitabine and four cycles of docetaxel, respectively. Of the patients who received cisplatin plus vinorelbine, 58 (75%) received four cycles of cisplatin and 56 (73%) received four cycles of vinorelbine. Among patients who received cisplatin plus pemetrexed, 47 (77%) received four cycles each of cisplatin and pemetrexed. Two patients (50%) in the cisplatin plus gemcitabine group received four cycles each of cisplatin and gemcitabine, and three patients (60%) in the cisplatin plus docetaxel group received four cycles each of cisplatin and docetaxel.
FIGURE 1.
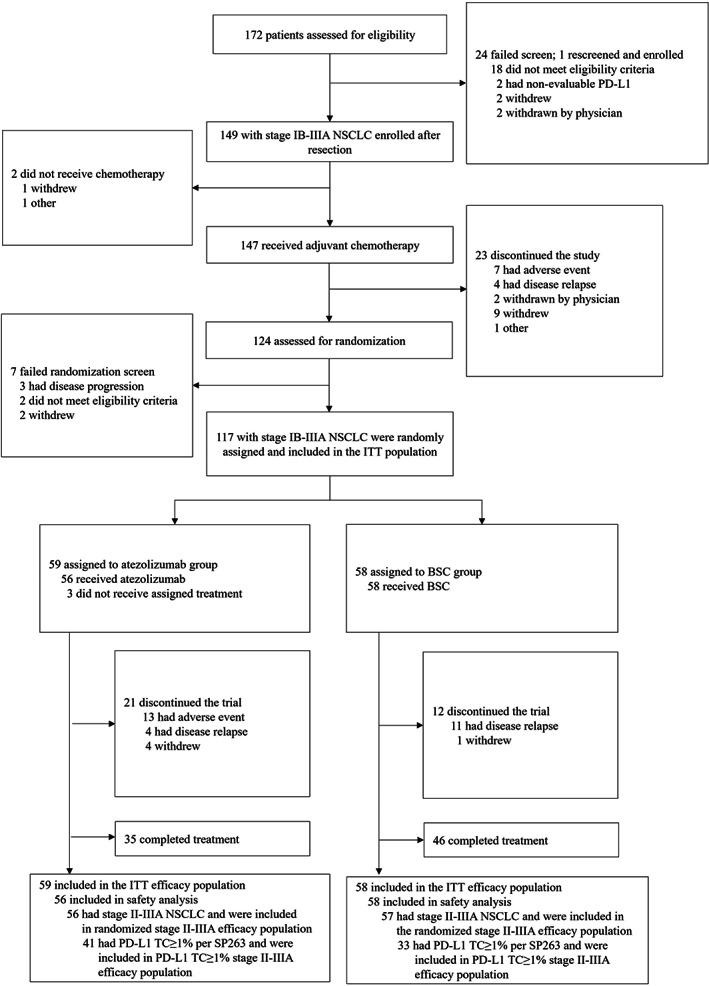
IMpower010 patient profile in the Japanese subpopulation. Screening, enrollment, and randomization of patients enrolled at Japanese sites of the global IMpower010 study. BSC, best supportive care; ITT, intention‐to‐treat; NSCLC, non‐small cell lung cancer; PD‐L1, programmed death‐ligand 1; TC, tumor cells
The ITT population comprised all randomized patients (stage IB–IIIA; n = 117) who received either atezolizumab (n = 59) or BSC (n = 58). In total, 113 patients (atezolizumab, n = 56; BSC, n = 57) had stage II–IIIA disease, of whom 74 patients (atezolizumab, n = 41; BSC, n = 33) had PD‐L1 expression on TC covering ≥1% of the tumor area, as defined by the SP263 immunohistochemistry assay, and comprised the PD‐L1 TC ≥1% stage II–IIIA population.
Baseline characteristics were generally similar between study arms across the three populations (Table 1; Table S1). In the PD‐L1 TC ≥1% stage II–IIIA population, the atezolizumab arm had fewer patients aged ≥65 years than the BSC arm (46% vs 67%), while the percentage of patients with PD‐L1 expression on TC covering ≥50% of tumor area was higher in the atezolizumab arm than the BSC arm (59% vs 49%). Similar trends in demographics and baseline characteristics were observed in the all‐randomized stage II–IIIA and ITT populations. Across populations, all patients were of Asian race.
TABLE 1.
Demographics and baseline characteristics of the Japanese PD‐L1 TC ≥1% stage II–IIIA population
| Atezolizumab (n = 41) | BSC (n = 33) | |
|---|---|---|
| Median age (range), years | 64.0 (40–75) | 68.0 (37–74) |
| Age group, n (%) | ||
| <65 years | 22 (53.7) | 11 (33.3) |
| ≥65 years | 19 (46.3) | 22 (66.7) |
| Age group, n (%) | ||
| <65 years | 22 (53.7) | 11 (33.3) |
| 65–74 years | 18 (43.9) | 22 (66.7) |
| 75–84 years | 1 (2.4) | 0 |
| Sex, n (%) | ||
| Male | 31 (75.6) | 27 (81.8) |
| Female | 10 (24.4) | 6 (18.2) |
| Race, n (%) | ||
| Asian | 41 (100) | 33 (100) |
| Ethnicity, n (%) | ||
| Not Hispanic or Latino | 40 (98) | 33 (100) |
| Not stated | 1 (2) | 0 |
| Baseline ECOG PS, n (%) | ||
| 0 | 30 (73.2) | 26 (78.8) |
| 1 | 11 (26.8) | 7 (21.2) |
| Tobacco use history, n (%) | ||
| Never | 5 (12.2) | 4 (12.1) |
| Previous | 35 (85.4) | 28 (84.8) |
| Current | 1 (2.4) | 1 (3.0) |
| Stage, n (%) | ||
| IIA | 14 (34.1) | 10 (30.3) |
| IIB | 6 (14.6) | 5 (15.2) |
| IIIA | 21 (51.2) | 18 (54.5) |
| Histology, n (%) | ||
| Squamous | 12 (29.3) | 9 (27.3) |
| Nonsquamous | 29 (70.7) | 24 (72.7) |
| EGFR mutation status, n (%) | ||
| Yes | 6 (14.6) | 6 (18.2) |
| No | 26 (63.4) | 20 (60.6) |
| Unknown | 9 (22.0) | 7 (21.2) |
| ALK mutation status, n (%) | ||
| Yes | 2 (4.9) | 2 (6.1) |
| No | 31 (75.6) | 23 (69.7) |
| Unknown | 8 (19.5) | 8 (24.2) |
| PD‐L1 status by SP263, n (%) | ||
| <50% TC | 17 (41.5) | 17 (51.5) |
| ≥50% TC | 24 (58.5) | 16 (48.5) |
Abbreviations: ALK, anaplastic lymphoma kinase; BSC, best supportive care; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; PD‐L1, programmed death‐ligand 1; TC, tumor cells.
3.2. Efficacy
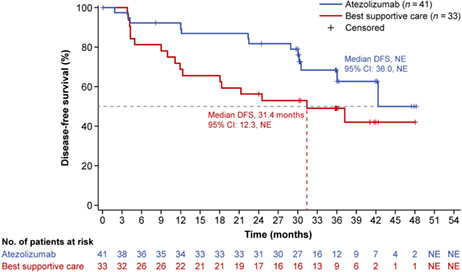
At the data cutoff date (January 21, 2021), the median survival follow‐up duration was 38.3 months (range, 0.2–52.8) in the PD‐L1 TC ≥1% stage II–IIIA population, 38.3 months (range, 0.2–52.8) in the all‐randomized stage II–IIIA population, and 38.2 months (range, 0.2–52.8) in the ITT population. Median DFS in the PD‐L1 TC ≥1% stage II–IIIA population was not reached (NR; 95% CI, 36.0‐NR) in the atezolizumab arm and 31.4 months (95% CI, 12.3 months‐NR) in the BSC arm (unstratified HR, 0.52; 95% CI, 0.25–1.08) (Figure 2). In the all‐randomized stage II–IIIA population, median DFS was 42.3 months (95% CI, 36.0‐NR) in the atezolizumab arm and 31.6 months (95% CI, 18.3‐NR) in the BSC arm (unstratified HR, 0.62; 95% CI, 0.35–1.11) (Figure S1A). Median DFS in the atezolizumab arm of the ITT population was NR (95% CI, 36.0‐NR) and 37.2 months (95% CI, 19.0‐NR) in the BSC arm (unstratified HR, 0.61; 95% CI, 0.34–1.10) (Figure S1B). DFS outcomes in key patient subgroups of the PD‐L1 TC ≥1% stage II–IIIA population are shown in Figure 3.
FIGURE 2.
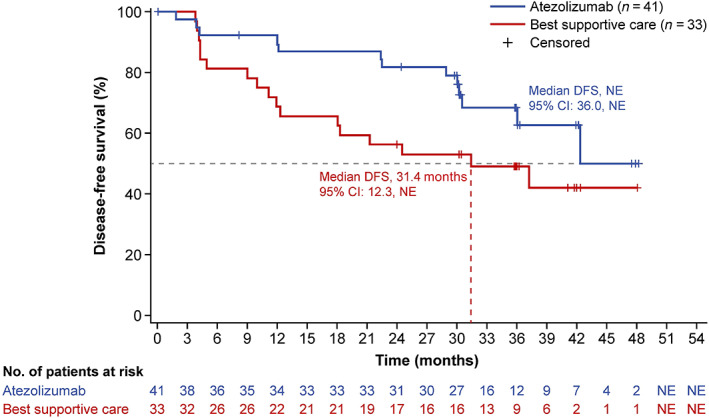
Disease‐free survival in the Japanese PD‐L1 TC ≥ 1% stage II–IIIA population. Kaplan–Meier estimates of disease‐free survival in the atezolizumab and best supportive care arms are shown for Japanese patients in the stage II–IIIA population whose tumors expressed PD‐L1 on ≥1% of tumor cells (TC). DFS, disease‐free survival; NR, not reached; NSCLC, non‐small cell lung cancer; PD‐L1, programmed death‐ligand 1; TC, tumor cells
FIGURE 3.
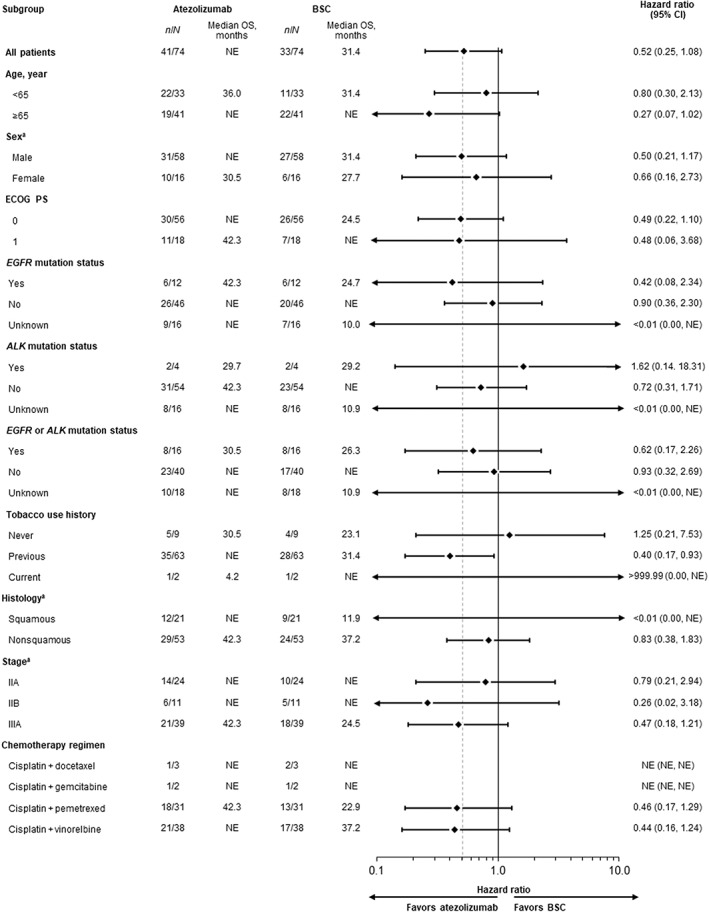
Disease‐free survival in key patient subgroups of the Japanese PD‐L1 TC ≥1% stage II–IIIA population. Forest plots of disease‐free survival in patient subgroups of the Japanese stage II–IIIA population with PD‐L1 expression on ≥1% of TC. ALK, anaplastic lymphoma kinase; BSC, best supportive care; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; NR, not reached; OS, overall survival; PD‐L1, programmed death‐ligand 1; TC, tumor cells. aPer electronic case report form
Overall survival was not mature as of the data cutoff date, with death events in the ITT population having occurred in 8.5% (n = 5) and 10.3% (n = 6) of patients in the atezolizumab and BSC arms, respectively. In the all‐randomized stage II–IIIA population, death events occurred in 8.9% of patients (n = 5) in the atezolizumab arm and 10.5% of patients (n = 6) in the BSC arm. In the PD‐L1 TC ≥1% stage II–IIIA population, death events occurred in 7.3% (n = 3) and 18.2% (n = 6) of patients, respectively. Median OS in all three study populations was NR in both arms, with an HR of 0.86 (95% CI, 0.26–2.81), 0.88 (95% CI, 0.27–2.89), and 0.41 (95% CI, 0.10–1.64) in the respective populations (Figure S2). In the PD‐L1 TC ≥1% stage II–IIIA population, 24.4% of patients (n = 10) in the atezolizumab arm and 39.4% of patients (n = 13) in the BSC arm received subsequent non‐protocol anticancer treatment (Table S2). The proportion of patients who subsequently received an antineoplastic agent was 24.4% (n = 10) in the atezolizumab arm and 39.4% of patients (n = 13) in the BSC arm.
3.3. Safety
The safety‐evaluable population included 56 patients in the atezolizumab arm and 58 patients in the BSC arm. The median duration of atezolizumab treatment was 10.4 months (range, 0–14 months), with a median of 16.0 (range, 1–16) atezolizumab doses received. Overall, 62.5% of patients (n = 35) completed 16 cycles of atezolizumab treatment, with 8.9% (n = 5) completing 8–15 cycles and 28.6% (n = 16) completing 0–7 cycles.
AEs of any grade occurred in 94.6% of patients (n = 53) in the atezolizumab arm and 77.6% of patients (n = 45) in the BSC arm; events were of grade 3/4 severity in 26.8% (n = 15) and 12.1% of patients (n = 7), respectively (Table 2). The most frequently occurring AEs of any grade in either study arm were pyrexia (atezolizumab arm, 33.9% [n = 19]; BSC arm, 1.7% [n = 1]), nasopharyngitis (atezolizumab arm, 16.1% [n = 9]; BSC arm, 25.9% [n = 15]), and rash (atezolizumab arm, 16.1% [n = 9]; BSC arm, 1.7% [n = 1]) (Table 3). The most frequently occurring grade 3/4 AEs in either arm were abnormal hepatic function and increased blood creatine phosphokinase, with each occurring in 3.6% of patients (n = 2) in the atezolizumab arm and no patients in the BSC arm. Death due to any cause was reported in 8.9% of patients (n = 5) in the atezolizumab arm and 10.3% of patients (n = 6) in the BSC arm, with no deaths due to AEs (Table S3). All reported deaths in the atezolizumab arm and five deaths in the BSC arm were due to disease relapse. One death in the BSC arm was a treatment‐emergent medical event that occurred beyond the AE‐reporting period.
TABLE 2.
Summary of adverse events in the safety‐evaluable population
| Japanese (N = 114) | Non‐Japanese (N = 876) | |||
|---|---|---|---|---|
| Atezolizumab (n = 56) | BSC (n = 58) | Atezolizumab (n = 439) | BSC (n = 437) | |
| Patients with ≥1 AE, n (%) | 53 (94.6) | 45 (77.6) | 406 (92.5) | 305 (69.8) |
| All events, n | 293 | 110 | 2449 | 1143 |
| Grade 3/4 | 15 (26.8) | 7 (12.1) | 93 (21.2) | 50 (11.4) |
| Grade 5 | 0 | 0 | 8 (1.8) | 3 (0.7) |
| Treatment‐related AE, n (%) | 40 (71.4) | 0 | 295 (67.2) | 0 |
| Grade 3/4 | 9 (16.1) | 0 | 44 (10.0) | 0 |
| Grade 5 | 0 | 0 | 4 (0.9) | 0 |
| Deaths, n (%) | 5 (8.9) | 6 (10.3) | 90 (20.5) | 84 (19.2) |
| Serious AE, n (%) | 10 (17.9) | 4 (6.9) | 77 (17.5) | 38 (8.7) |
| Treatment related | 7 (12.5) | 0 | 30 (6.8) | 0 |
| AE leading to dose interruption of atezolizumab, n (%) | 19 (33.9) | — | 123 (28.0) | — |
| AE leading to discontinuation of atezolizumab, n (%) | 13 (23.2) | — | 77 (17.5) | — |
| Immune‐mediated AE, n (%) | 31 (55.4) | 4 (6.9) | 225 (51.3) | 43 (9.8) |
| Grade 3/4 | 6 (10.7) a | 2 (3.4) | 33 (7.5) | 1 (0.2) |
| Grade 5 | 0 | 0 | 2 (<1) | 0 |
Abbreviations: AE, adverse event.
No grade 4 immune‐mediated AEs were reported in the Japanese safety‐evaluable population.
TABLE 3.
Adverse events of any grade occurring in ≥10% and grade 3/4 adverse events occurring in ≥2% of the Japanese safety‐evaluable population
| n (%) | Atezolizumab (n = 56) | BSC (n = 58) | ||
|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Pyrexia | 19 (33.9) | 1 (1.8) | 1 (1.7) | 0 |
| Nasopharyngitis | 9 (16.1) | 0 | 15 (25.9) | 0 |
| Rash | 9 (16.1) | 1 (1.8) | 1 (1.7) | 0 |
| Peripheral sensory neuropathy | 9 (16.1) | 0 | 1 (1.7) | 0 |
| Pruritus | 8 (14.3) | 0 | 1 (1.7) | 0 |
| Blood creatinine increased | 6 (10.7) | 0 | 0 | 0 |
| Blood creatine phosphokinase increased | 4 (7.1) | 2 (3.6) | 0 | 0 |
| Hepatic function abnormal | 3 (5.4) | 2 (3.6) | 0 | 0 |
Abbreviation: BSC, best supportive care.
Serious AEs occurred in 17.9% (n = 10) and 6.9% (n = 4) of patients in the atezolizumab and BSC arms, respectively, with the most frequently occurring serious AE in the atezolizumab arm being pyrexia, occurring in 3.6% of patients (n = 2) (Table S4).
Treatment‐related AEs of any grade were experienced by 71.4% of patients (n = 40) in the atezolizumab arm, with 16.1% (n = 9) experiencing events of grade 3/4 severity (Table 2). The most frequently occurring atezolizumab‐related AEs of any grade were pyrexia, peripheral sensory neuropathy, rash, and pruritus (Table S5). The most common atezolizumab‐related grade 3/4 AEs were abnormal hepatic function, occurring in 3.6% of patients (n = 2), and increased γ‐glutamyl transferase, neutropenia, decreased neutrophil count, inappropriate antidiuretic hormone secretion, hyponatremia, hypotension, leukopenia, rash, pyrexia, adrenal insufficiency, and drug‐induced liver injury, each occurring in 1.8% of patients (n = 1). Serious AEs deemed related to atezolizumab treatment occurred in 12.5% of patients (n = 7) (Table S4). No treatment‐related grade 5 AEs were reported. Long‐lasting AEs with a duration of ≥1 year occurred in 66% (n = 37) of patients treated with atezolizumab and 31% (n = 18) of patients treated with BSC in the Japanese population (Table S6). In the non‐Japanese population, long‐lasting AEs occurred in 58% (n = 255) of patients in the atezolizumab arm and 38% (n = 164) of patients in the BSC arm.
All‐grade immune‐mediated AEs were reported in 55.4% of patients (n = 31) in the atezolizumab arm and 6.9% of patients (n = 4) in the BSC arm, with grade 3 immune‐mediated AEs occurring in 10.7% (n = 6) and 3.4% (n = 2) of patients in the respective arms (Table 2). No grade 4 or 5 immune‐mediated AEs were reported in either the atezolizumab or BSC arm. The most common immune‐mediated AE was rash, occurring in 33.9% of patients (n = 19) receiving atezolizumab and 1.7% of patients (n = 1) receiving BSC (Table 4).
TABLE 4.
Immune‐mediated adverse events occurring in the safety‐evaluable population
| n (%) | Japanese (N = 114) | Non‐Japanese (N = 876) | ||||||
|---|---|---|---|---|---|---|---|---|
| Atezolizumab (n = 56) | BSC (n = 58) | Atezolizumab (n = 439) | BSC (n = 437) | |||||
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Rash | 19 (33.9) | 1 (1.8) | 1 (1.7) | 0 | 72 (16.4) | 6 (1.4) | 10 (2.3) | 0 |
| Hepatitis (diagnosis and laboratory abnormalities) | 10 (17.9) | 4 (7.1) | 1 (1.7) | 0 | 76 (17.3) | 16 (3.6) | 21 (4.8) | 1 (<1) |
| Hepatitis (laboratory abnormalities) | 9 (16.1) | 3 (5.4) | 1 (1.7) | 0 | 72 (16.4) | 13 (3.0) | 20 (4.6) | 1 (<1) |
| Pneumonitis | 6 (10.7) | 0 | 0 | 0 | 13 a (3.0) | 4 (<1) | 3 (<1) | 0 |
| Hyperthyroidism | 2 (3.6) | 0 | 1 (1.7) | 0 | 30 (6.8) | 2 (<1) | 3 (<1) | 0 |
| Hypothyroidism | 3 (5.4) | 0 | 0 | 0 | 83 (18.9) | 0 | 3 (<1) | 0 |
| Ocular inflammatory toxicity | 1 (1.8) | 0 | 1 (1.7) | 1 (1.7) | 0 | 0 | 0 | 0 |
| Adrenal insufficiency | 1 (1.8) | 1 (1.8) | 0 | 0 | 5 (1.1) | 1 (<1) | 0 | 0 |
| Hepatitis (diagnosis) | 1 (1.8) | 1 (1.8) | 0 | 0 | 6 (1.4) | 3 (<1) | 1 (<1) | 0 |
| Meningitis | 1 (1.8) | 0 | 0 | 0 | 1 (<1) | 1 (<1) | 0 | 0 |
| Meningoencephalitis | 1 (1.8) | 0 | 0 | 0 | 3 (<1) | 3 (<1) | 0 | 0 |
| Myositis | 1 (1.8) | 0 | 0 | 0 | 3 (<1) | 0 | 1 (<1) | 0 |
| Myositis (myositis and rhabdomyolysis) | 1 (1.8) | 0 | 0 | 0 | 3 (<1) | 0 | 1 (<1) | 0 |
| Pancreatitis | 0 | 0 | 1 (1.7) | 1 (1.7) | 2 (<1) | 1 (<1) | 0 | 0 |
| Severe cutaneous reactions | 1 (1.8) | 0 | 0 | 0 | 1 (<1) | 0 | 0 | 0 |
| Infusion‐related reactions | 0 | 0 | 0 | 0 | 7 (1.6) | 1 (<1) | 0 | 0 |
| Colitis | 0 | 0 | 0 | 0 | 4 (<1) | 2 (<1) | 1 (<1) | 0 |
| Diabetes mellitus | 0 | 0 | 0 | 0 | 4 (<1) | 0 | 1 (<1) | 0 |
| Autoimmune hemolytic anemia | 0 | 0 | 0 | 0 | 2 (<1) | 0 | 0 | 0 |
| Encephalitis | 0 | 0 | 0 | 0 | 2 (<1) | 2 (<1) | 0 | 0 |
| Myocarditis | 0 | 0 | 0 | 0 | 2 b (<1) | 0 | 0 | 0 |
| Guillain–Barré syndrome | 0 | 0 | 0 | 0 | 1 (<1) | 1 (<1) | 0 | 0 |
| Hypophysitis | 0 | 0 | 0 | 0 | 1 (<1) | 0 | 0 | 0 |
| Nephritis | 0 | 0 | 0 | 0 | 1 (<1) | 0 | 0 | 0 |
| Vasculitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<1) | 0 |
Abbreviation: BSC, best supportive care.
One patient had grade 5 pneumonitis.
One patient had grade 5 myocarditis.
Atezolizumab was discontinued in 23.2% of patients (n = 13) due to AEs. The most common AEs leading to atezolizumab discontinuation were pneumonitis, occurring in 5.4% of patients (n = 3), and abnormal hepatic function and peripheral sensory neuropathy, each occurring in 3.6% of patients (n = 2). All AEs that led to atezolizumab discontinuation were considered treatment related.
4. DISCUSSION
Several PD‐L1/PD‐1 inhibitors are approved in Japan for first‐ or second‐line treatment of advanced or metastatic NSCLC 15 , 24 , 25 as part of clinical practice. However, treatment options for Japanese patients with completely resected early‐stage NSCLC remain limited to platinum‐based adjuvant chemotherapy. Immune checkpoint inhibition has now emerged as a treatment strategy for early‐stage NSCLC following surgery and adjuvant chemotherapy, as demonstrated by the pivotal global IMpower010 study. Although previous studies have shown that immunotherapies may have an enhanced benefit in Japanese patients, these agents have also been associated with a relatively higher risk of toxicity in Asian populations. 19 , 26 Here, we performed a subgroup analysis of Japanese patients from IMpower010 to assess the benefit–risk profile of atezolizumab in the Japanese population and found that the results were consistent with those seen in the primary analysis of the global IMpower010 study population. 16 Atezolizumab demonstrated a trend toward improvement in DFS vs BSC in the Japanese PD‐L1 TC ≥1% stage II–IIIA population and an acceptable overall safety profile in the Japanese population, with no new safety signals identified. Unstratified DFS HRs in the stage II–IIIA population and the ITT population favored atezolizumab vs BSC in the Japanese subpopulation.
Analysis of DFS in key patient subgroups of the Japanese PD‐L1 TC ≥1% stage II–IIIA population demonstrated a trend toward improvement with atezolizumab vs BSC in most groups. Interestingly, results in patients whose tumor harbored EGFR or ALK mutations were similar to those whose tumors did not have these alterations. However, these results must be interpreted with caution given the small number of EGFR‐ or ALK‐positive patients (n = 15) and that the mechanism driving results in this population is unclear. Treatment decisions should be made with due consideration of available alternative targeted treatment options in this population.
Among Japanese (n = 56) and non‐Japanese (n = 439) patients in the atezolizumab arm, similar incidences of any‐grade AEs (95% vs 93%) and any‐grade treatment‐related AEs (71% vs 67%) were observed. Slightly higher incidences of any‐cause grade 3/4 AEs (27% vs 21%), grade 3/4 treatment‐related AEs (16% vs 10%) and any‐grade long‐lasting AEs (66% vs 58%) were seen in Japanese patients compared with non‐Japanese patients. However, these data should be interpreted with caution due to the smaller sample size of the Japanese population. No AEs led to death in the Japanese safety‐evaluable population. The incidence of immune‐mediated AEs was similar between the Japanese (55%) and non‐Japanese (51%) patients; grade 3/4 immune‐mediated AEs occurred at comparable rates in the two groups (11% vs 8%), with no grade 4 immune‐mediated AEs reported in the Japanese population. Atezolizumab treatment discontinuations due to AEs were reported in 23% of Japanese patients compared with 18% of non‐Japanese patients. The most frequent AE leading to atezolizumab discontinuation was pneumonitis, occurring in 5.4% of patients. While Japanese patients receiving anticancer therapy typically present a higher risk of pneumonitis than non‐Japanese populations, 19 , 20 pneumonitis was reported as one of the most frequent reasons for atezolizumab discontinuation in the global IMpower010 population as well. 16 No new long‐lasting risks with atezolizumab monotherapy were identified.
Atezolizumab treatment regimens, including monotherapy and combinations with chemotherapy, have been specifically investigated in Asian populations, including Japanese patients in several studies, and have shown consistent clinical benefit and safety in global study populations and Japanese subpopulations. 20 , 27 , 28 , 29 The results of this subgroup analysis of Japanese patients from IMpower010 expand upon these findings and suggest a positive benefit–risk profile of atezolizumab for this group of patients with resected stage II–IIIA NSCLC. Limitations of this subgroup analysis include the open‐label nature of the trial, small sample size, lack of power for efficacy comparisons, and assessment of DFS by investigator rather than a central review committee. The use of DFS, rather than OS, as the primary endpoint may be considered a limitation of this study, given that OS is widely accepted as the most clinically relevant endpoint in NSCLC trials. 30 However, DFS is increasingly gaining acceptance as a primary endpoint in the adjuvant setting, 31 with multiple phase 3 trials of adjuvant therapies for NSCLC evaluating DFS or progression‐free survival as a primary endpoint, including the ADUARA 32 and PEARLS/KEYNOTE‐091 33 trials. Given the extended follow‐up times required to measure OS, the use of DFS as a surrogate endpoint can allow for the timely approval of new agents. 30
In conclusion, consistent with findings in the global IMpower010 study, there was a trend toward DFS improvement with adjuvant atezolizumab following cisplatin‐based chemotherapy compared with BSC in Japanese patients with completely resected stage II–IIIA NSCLC, including those with PD‐L1 TC ≥1% tumors. Longer median DFS in the atezolizumab arm versus the BSC arm was observed in the stage IB–IIIA ITT population. Adjuvant atezolizumab was tolerable in this Japanese patient population, and the observed toxicity profile was similar to that reported in the safety‐evaluable population of the global IMpower010 study. No new safety signals were identified.
FUNDING INFORMATION
F. Hoffmann‐La Roche Ltd/Genentech Inc sponsored this study. All authors disclose medical writing support funded by Chugai Pharmaceutical Co., Ltd.
DISCLOSURE
Hirotsugu Kenmotsu received research funding (Chugai Pharmaceutical Co., Ltd., Novartis Pharma K.K., Daiichi‐Sankyo Co., Ltd., AstraZeneca K.K., and Loxo Oncology). Shunichi Sugawara received honoraria (Chugai Pharmaceutical Co., Ltd); Haruhiro Saito received fees for pamphlet development (Ono Pharmaceutical Co., Ltd) and received research funding (AstraZeneca K.K. and Chugai Pharmaceutical Co., Ltd.). Morihito Okada received honoraria (Johnson & Johnson K.K., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., CSL Behring K.K., and Bristol‐Myers Squibb K.K.) and received research funding (Chugai Pharmaceutical Co., Ltd., Bristol‐Myers Squibb K.K, and AstraZeneca K.K). Yuichiro Ohe received honoraria (AstraZeneca K.K. and Chugai Pharmaceutical Co., Ltd.), research funding (AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly K.K, Ono Pharmaceutical Co., Ltd., Bristol‐Myers Squib K.K., Kyorin Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Pfizer Inc., Taiho Pharmaceutical Co., Ltd., Novartis Pharma K.K., Takeda Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Daiichi‐Sankyo Co., Ltd., Janssen Pharmaceutical K.K., and Loxo Oncology), and is an editorial board member (Cancer Science). Shizuka Nakagawa and Haruka Nagao are employees of Chugai Pharmaceutical Co., Ltd. Yasutaka Watanabe, Toyofumi Fengshi Chen‐Yoshikawa, and Wataru Nishio have no conflicts of interest.
STUDY CONDUCT
F. Hoffmann‐La Roche Ltd/Genentech Inc sponsored the study, provided study drugs, was involved in the study design, data collection, data analysis, and data interpretation for the IMpower010 analysis, and gave approval to submit for this publication. Chugai Pharmaceutical Co Ltd was involved in the data collection for the IMpower010 analysis, data analysis, data interpretation, and writing of the report for the Japanese subgroup analysis of IMpower010 and gave approval to submit for publication. The study was funded by F. Hoffmann‐La Roche Ltd. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
ETHICS STATEMENT
Approval of research protocol: The trial was conducted in accordance with the Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board or Ethics Committee of each participating site. The safety data were periodically reviewed by an independent data monitoring committee.
Informed consent: All patients provided written informed consent.
Clinical trial registration: ClinicalTrials.gov, NCT02486718.
Animal studies: N/A.
Supporting information
Data S1
ACKNOWLEDGMENTS
We would like to thank the patients and their families, participating study investigators and clinical sites. Medical writing assistance was provided by Madhubrata Ghosh, PhD, and Akshaya Srinivasan, PhD, of MediTech Media and funded by Chugai Pharmaceutical Co., Ltd.
Kenmotsu H, Sugawara S, Watanabe Y, et al. Adjuvant atezolizumab in Japanese patients with resected stage IB‐IIIA non‐small cell lung cancer (IMpower010). Cancer Sci. 2022;113:4327‐4338. doi: 10.1111/cas.15564
The Japanese investigators of the IMpower010 study are listed in the Supplementary Appendix.
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to individual patient‐level data through the clinical study data request platform at (https://www.clinicalstudydatarequest.com/Default.aspx). For further details on Chugai's Data Sharing Policy and how to request access to related clinical study documents, see here (www.chugai‐pharm.co.jp/english/profile/rd/ctds_request.html).
REFERENCES
- 1. Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943‐953. doi: 10.2147/CMAR.S187317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non‐small‐cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28(suppl_4):iv1‐iv21. doi: 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 3. Remon J, Soria JC, Peters S, ESMO Guideline Committee . Early and locally advanced non‐small‐cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32(12):1637‐1642. doi: 10.1016/j.annonc.2021.08.1994 [DOI] [PubMed] [Google Scholar]
- 4. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39‐51. doi: 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 5. Asamura H, Goya T, Koshiishi Y, et al. A Japanese lung cancer registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3(1):46‐52. doi: 10.1097/JTO.0b013e31815e8577 [DOI] [PubMed] [Google Scholar]
- 6. Tsuchiya R. Surgery for stage I NSCLC in Japan. J Thorac Oncol. 2007;2(7 suppl 3):S113‐S114. doi: 10.1097/JTO.0b013e318074e522 [DOI] [PubMed] [Google Scholar]
- 7. Okami J, Shintani Y, Okumura M, et al. Demographics, safety and quality, and prognostic information in both the seventh and eighth editions of the TNM classification in 18,973 surgical cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol. 2019;14(2):212‐222. doi: 10.1016/j.jtho.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 8. Arriagada R, Dunant A, Pignon JP, et al. Long‐term results of the international adjuvant lung cancer trial evaluating adjuvant cisplatin‐based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28(1):35‐42. doi: 10.1200/JCO.2009.23.2272 [DOI] [PubMed] [Google Scholar]
- 9. Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26(21):3552‐3559. doi: 10.1200/JCO.2007.13.9030I [DOI] [PubMed] [Google Scholar]
- 10. Kenmotsu H, Yamamoto N, Yamanaka T, et al. Randomized phase III study of pemetrexed plus cisplatin versus vinorelbine plus cisplatin for completely resected stage II to IIIA nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2020;38(19):2187‐2196. doi: 10.1200/JCO.19.02674 [DOI] [PubMed] [Google Scholar]
- 11. Planchard D, Popat S, Kerr K, et al. Metastatic non‐small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29(Suppl 4):iv192‐iv237. doi: 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 12. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563‐567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first‐line treatment of PD‐L1‐selected patients with NSCLC. N Engl J Med. 2020;383(14):1328‐1339. doi: 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 14. Mazieres J, Rittmeyer A, Gadgeel S, et al. Atezolizumab versus docetaxel in pretreated patients with NSCLC: final results from the randomized phase 2 POPLAR and phase 3 OAK clinical trials. J Thorac Oncol. 2021;16(1):140‐150. doi: 10.1016/j.jtho.2020.09.022 [DOI] [PubMed] [Google Scholar]
- 15. Pharmaceuticals and Medical Devices Agency . Review Report (Tecentriq). Accessed November 9, 2021. https://www.pmda.go.jp/files/000235287.pdf
- 16. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non‐small‐cell lung cancer (IMpower010): a randomised, multicentre, open‐label, phase 3 trial. Lancet. 2021;398(10308):1344‐1357. doi: 10.1016/S0140-6736(21)02098-5 [DOI] [PubMed] [Google Scholar]
- 17. Ardizzoni A, Azevedo S, Rubio‐Viqueira B, et al. Primary results from TAIL: a global single‐arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non‐small cell lung cancer. J Immunother Cancer. 2021;9(3):e001865. doi: 10.1136/jitc-2020-001865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng L, Qin BD, Xiao K, et al. A meta‐analysis comparing responses of Asian versus non‐Asian cancer patients to PD‐1 and PD‐L1 inhibitor‐based therapy. Onco Targets Ther. 2020;9(1):1781333. doi: 10.1080/2162402X.2020.1781333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng L, Wu YL. Immunotherapy in the Asiatic population: any differences from Caucasian population? J Thorac Dis. 2018;10(Suppl 13):S1482‐S1493. doi: 10.21037/jtd.2018.05.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hida T, Kaji R, Satouchi M, et al. Atezolizumab in Japanese patients with previously treated advanced non‐small‐cell lung cancer: a subgroup analysis of the phase 3 OAK study. Clin Lung Cancer. 2018;19(4):e405‐e415. doi: 10.1016/j.cllc.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 21. Vennapusa B, Baker B, Kowanetz M, et al. Development of a PD‐L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl Immunohistochem Mol Morphol. 2019;27(2):92‐100. doi: 10.1097/PAI.0000000000000594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136(1):260‐271. doi: 10.1378/chest.08-0978 [DOI] [PubMed] [Google Scholar]
- 23. Rebelatto MC, Midha A, Mistry A, et al. Development of a programmed cell death ligand‐1 immunohistochemical assay validated for analysis of non‐small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. 2016;11(1):95. doi: 10.1186/s13000-016-0545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pharmaceuticals and Medical Devices Agency . Review Report (Keytruda). Accessed February 23, 2022. https://www.pmda.go.jp/files/000231921.pdf
- 25. Pharmaceuticals and Medical Devices Agency . Review Report (Opdivo). Accessed November 9, 2021. https://www.pmda.go.jp/files/000241165.pdf
- 26. Soo RA, Kawaguchi T, Loh M, et al. Differences in outcome and toxicity between Asian and caucasian patients with lung cancer treated with systemic therapy. Future Oncol. 2012;8(4):451‐462. doi: 10.2217/fon.12.25 [DOI] [PubMed] [Google Scholar]
- 27. Iwata H, Inoue K, Kaneko K, et al. Subgroup analysis of Japanese patients in a phase 3 study of atezolizumab in advanced triple‐negative breast cancer (IMpassion130). Jpn J Clin Oncol. 2019;49(12):1083‐1091. doi: 10.1093/jjco/hyz135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishio M, Saito H, Goto K, et al. IMpower132: atezolizumab plus platinum‐based chemotherapy vs chemotherapy for advanced NSCLC in Japanese patients. Cancer Sci. 2021;112(4):1534‐1544. doi: 10.1111/cas.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishio M, Sugawara S, Atagi S, et al. Subgroup analysis of Japanese patients in a phase III study of atezolizumab in extensive‐stage small‐cell lung cancer (IMpower133). Clin Lung Cancer. 2019;20(6):469‐476 e1. doi: 10.1016/j.cllc.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 30. Cheema PK, Burkes RL. Overall survival should be the primary endpoint in clinical trials for advanced non‐small‐cell lung cancer. Curr Oncol. 2013;20(2):e150‐e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mauguen A, Pignon J‐P, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re‐analysis of meta‐analyses of individual patients' data. Lancet Oncol. 2013;14(7):619‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu Y‐L, Tsuboi M, He J, et al. Osimertinib in resected EGFR‐mutated non–small‐cell lung cancer. N Engl J Med. 2020;383(18):1711‐1723. [DOI] [PubMed] [Google Scholar]
- 33. Paz‐Ares L, O'Brien MER, Mauer M, et al. VP3‐2022: pembrolizumab (pembro) versus placebo for early‐stage non‐small cell lung cancer (NSCLC) following complete resection and adjuvant chemotherapy (chemo) when indicated: randomized, triple‐blind, phase III EORTC‐1416‐LCG/ETOP 8‐15 – PEARLS/KEYNOTE‐091 study. Ann Oncol. 2022;33(4):451‐453. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
Qualified researchers may request access to individual patient‐level data through the clinical study data request platform at (https://www.clinicalstudydatarequest.com/Default.aspx). For further details on Chugai's Data Sharing Policy and how to request access to related clinical study documents, see here (www.chugai‐pharm.co.jp/english/profile/rd/ctds_request.html).


