Abstract
The myristoylated alanine-rich C-kinase substrate (MARCKS) and the MARCKS-related protein (MARCKSL1) are ubiquitous, highly conserved membrane-associated proteins involved in the structural modulation of the actin cytoskeleton, chemotaxis, motility, cell adhesion, phagocytosis, and exocytosis. MARCKS includes an N-terminal myristoylated domain for membrane binding, a highly conserved MARCKS Homology 2 (MH2) domain, and an effector domain (which is the phosphorylation site). MARCKS can sequester phosphatidylinositol-4, 5-diphosphate (PIP2) at lipid rafts in the plasma membrane of quiescent cells, an action reversed by protein kinase C (PKC), ultimately modulating the immune function. Being expressed mostly in innate immune cells, MARCKS promotes the inflammation-driven migration and adhesion of cells and the secretion of cytokines such as tumor necrosis factor (TNF). From a clinical point of view, MARCKS is overexpressed in patients with schizophrenia and bipolar disorders, while the brain level of MARCKS phosphorylation is associated with Alzheimer’s disease. Furthermore, MARCKS is associated with the development and progression of numerous types of cancers. Data in autoimmune diseases are limited to rheumatoid arthritis models in which a connection between MARCKS and the JAK-STAT pathway is mediated by miRNAs.
We provide a comprehensive overview of the structure of MARCKS, its molecular characteristics and functions from a biological and pathogenetic standpoint, and will discuss the clinical implications of this pathway.
Keywords: Protein kinase C, Cell function, Inflammation, Immune modulation, Autoimmunity, Schizophrenia, Psychosis, Dementia, Cancer, Personalized medicine
1. The general terms of MARCKS
The myristoylated alanine-rich C-kinase substrate (MARCKS) was originally identified as Mr 87 kDa protein in the nerve terminals of rat cerebral cortex and is a member of a family (also named “MARCKS) of ubiquitous, highly conserved membrane-associated proteins. These were originally recognized as the major target of protein kinase C (PKC), and are integral to embryo development and postnatal development of the cytoskeleton, along with numerous other cell functions, including chemotaxis and motility, adhesion, migration, secretion, phagocytosis, and exocytosis. The two main members of the MARCKS family are (i) MARCKS, an acidic protein ubiquitously distributed in different tissues; (ii) MARCKSL1 (also denoted MRP, MLP, F52 MACMARCKS and MARCKS-related protein), a 20-kDa acidic protein mainly expressed in the brain, reproductive tissues, and macrophages. MARCKS and MARCKSL1 structures include a basic effector domain that binds Ca2+- calmodulin, F-actin, and acidic phospholipids and thus participates to the inhibition of fibrin formation [1] and platelet function [2]. The effector domain is also involved in the phosphorylation of PKC, which in turn phosphorylates the key serine residues within the effector domain [3]. The Ca2+ influx into the cytosol leads to phosphorylation of MARCKS [4] which in turn regulates neurological development and immunological functions. The clinical role of MARCKS has been established in neurological conditions, cancer, and inflammation, with potential therapeutic implications as shown by the effect of MARCKS inhibition in chronic obstructive pulmonary disease [5]. MARCKS is expressed at the highest levels in the brain during embryogenesis but is expressed widely throughout adulthood [6,7]. MARCKS mRNA levels are significantly upregulated in schizophrenia and bipolar disorders [8] while the phosphorylation of MARCKS, especially at Ser46, predates the aggregation of Aβ, and is sustained throughout the course of Alzheimer’s disease in human and mouse brains [9]. MARCKS might participate in microcephaly with pontine and cerebellar hypoplasia [10]. MARCKS is also involved in the development and progression of cancer and the hyperphosphorylated MARCKS affects cell proliferation, migration, drug sensitivity being associated with a poor prognosis in cancer patients. In the case of cholangiocarcinoma, phosphorylated MARCKS promotes cell migration, while unphosphorylated MARCKS promotes cell attachment thus modulating the disease prognosis [11]. In bladder carcinoma, MARCKS is hyper-phosphorylated and modulates metastasis formation [12] while in breast and lung cancer, increased phosphorylated MARCKS promotes the proliferation, invasion, and possibly resistance to chemotherapy [13–17]. Similarly, hyperphosphorylated MARCKS contributes to tumorigenesis and invasiveness of kidney [18], pancreas and skin [19–21], colon [22], and hepatocellular [23] cancers. MARCKS expression is increased during liver tumorigenesis [24] and mouse colitis associated with a worse outcome [25]. Finally, the stromal MARCKS hyperexpression contributes to activating cancer-associated fibroblasts in epithelial ovarian cancer [26].
During inflammation, MARCKS promotes the migration of inflammatory cells and the secretion of cytokines [27] mediated by PKC [28], particularly in macrophages [29]. MARCKS also modulates neutrophil migration and adhesion [30–32] by interfering with phosphorylation, actin-bundling, and cytoplasm translocation [33,34].
2. MARCKS and cell functions
The Mr “87 k” unique Ca++/phosphoprotein was first discovered in nerve terminals from rat cerebral cortex in 1982, where it modulated neurotransmission, and was later renamed MARCKS [4] based on evidence obtained from the bovine brain as [35].
MARCKS is found as a monomer or dimer, regardless of its phosphorylation status and regulates the intracellular signaling cascades via translocation and reorganization, while influencing the localization of multiple signal message molecules or complexes, thereby regulating cell polarity and domain-relative function [36].
MARCKS is an acidic, rod-shaped ubiquitous protein [37]. MARCKS and MARCKSL1 are negatively charged at neutral pH with an isoelectric point at approximately pH 4.4 while the effector domain is rich in positively charged residues (lysine), phenylalanine, and serine susceptible to phosphorylation [37] (Table 1, Fig. 1). The analysis of the chicken [38], bovine [39], mouse [40], rat [41], and human MARCKS cDNA sequences [42] demonstrated that MARCKS is abundant in amino acids alanine and glutamic acid. Conversely, MARCKS is not found in agnaths and invertebrates [43]. The human MARCKS gene (MARCKS) maps to chromosome 6q21, while the MARCKSL1 gene is located at chromosome 1p34 [44,45]. Both genes have multiple transcription factor binding sites within their promoters, lacking a TATA box in the case of MARCKS. Southern blot analysis in mice indicated that there is a single copy of the MARCKS gene per haploid genome [43]. Tumor necrosis factor alpha (TNF-α) stimulates the transcription of the human MACS gene in HL60 and U937 cells, with a 30-fold increase in mRNA levels seen after 2 h of stimulation [42]. The amino acid sequence analysis of the human MARCKS reveals the presence of a novel conserved domain in the C-terminal region compared to other species [35].
Table 1.
Amino acid sequences of the effector domains of MARCKS, MARCKS-like peptides, and the corresponding polybasic domains of potential cross-talking modular proteins. NCBI-protein (www.ncbi.nlm.nih.gov/protein) accession numbers are listed.
| Protein (residues) | NCBI/Protein ID | Effector domain sequence | Reference |
|---|---|---|---|
|
| |||
| Human MARCKS (152–176) | P29966.4 | KKKKKRFSFKKSFKLSGFSFKKNKK | [42] |
| Human MARCKSL1 (87–110) | P49006.2 | KKKKKFSFKKPFKLSGLSFKRNRK | [45] |
| Bos taurus MARCKS (151–175) | AAI15990.1 | KKKKKRFSFKKSFKLSGFSFKKNKK | [42] |
| Chicken MARCKS (117–141) | P16527.2 | KKKKKRFSFKKSFKLSGFSFKKNKK | [42] |
| Mouse MARCKS (145–169) | P26645.2 | KKKKKRFSFKKSFKLSGFSFKKSKK | [42] |
| DGK-ζ (259–273) | Q13574.4 | KKKKRASFKRKSSKK | [124–127] |
| α-Adducin (717–734) | P35611.2 | KKKKKFRTPSFLKKSKKK | [66,128,129] |
| DAKAP200 (119–141) | NP_001285753.1 | KSKSKKDKVKKKWSFRSISFGKK | [130] |
| GAP43 (30–56) | P17677.1 | KAHKAATKIQASFRGHITRKKLKGEKK | [131,132] |
| N-WASP (181–197) | BAA20128.1 | NISHTKEKKKGKAKKKR | [133,134] |
| PLD2 (553–574) | O14939.2 | RDLARHFIQRWNFTKTTKAKYK | [135] |
Abbreviations: DKG-ζ, Diacylglycerol kinase zeta; DAKAP200: Drosophila A kinase anchor protein 200; GAP43, growth-associated protein of Mr 43,000; N-WASP, neuronal Wiskott-Aldrich Syndrome protein; PLD, phosphatidylcholinespecific phospholipase D.
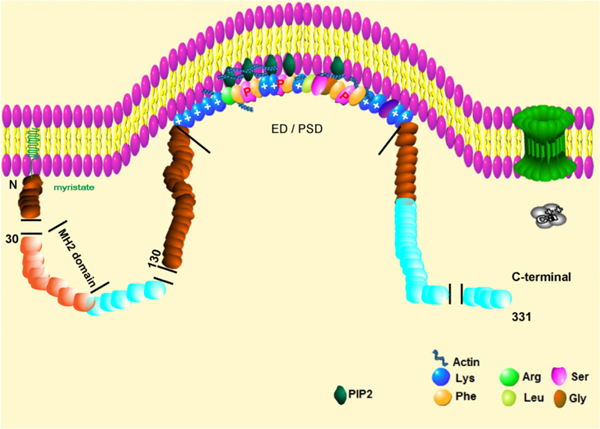
Fig. 1.
The MARCKS protein includes three highly conserved domains: i. the N-Terminal domain, which can be myristoylated (Myr); ii. the MH2 domain (MH2D), and iii. The effector domain, highly positively charged with 4 potential phosphorylation sites.
The MARCKS and MARCKSL1 mRNAs are present in the brain and spinal cord during the early embryonic development while mice heterozygous for MARCKS exhibit normal phenotype [6], with spatial learning being the only impaired function [46]. On the other hand, studies in genetic depletion murine models have revealed that MARCKS and MARCKSL1 are both necessary for embryogenesis [6,45,47–49].
The highly conserved effector domain is the phosphorylation site which can interact with both PKC and or Ca2+/calmodulin [50] (Fig. 1) and contains four potential phosphorylation sites [50,51] and an actin-filament-binding site [52] (Table 1, Fig. 2). Characterization of the four MARCKS phosphorylation sites as the prominent cellular substrate for chicken and bovine PKC revealed that all harbor a highly conserved domain with 25 identical amino acid residues [53]. MARCKS phosphorylation has been demonstrated in oligodendroglial progenitors [54], HIR 3.5 cells [55], and Swiss 3 T3 fibroblasts [56].
Fig. 2.
In the quiescent status and in the absence of calcium/calmodulin binding, MARCKS is tethered to the membrane by the N-terminal and effector domains.
MARCKS modulates the actin plasticity interaction with the plasma membrane thus participating in cytoskeleton regulation [57], and the anchoring of MARCKS to actin and calmodulin is finely modulated. The binding of MARCKS to actin filaments is disrupted by either phosphorylation, causing its translocation from the plasma membrane to the cytoplasm, or binding to calcium/calmodulin (Fig. 2). It has been hypothesized that MARCKS modulates the pathways involving downstream effector molecule, actin [57], and the activation of this pathway leads to the unbinding of actin from MARCKS; the resulting increased plasticity with softening actin cytoskeleton, promotes actin-related cell bioactivities, such as chemotaxis and motility, cell adhesion, migration, secretion, phagocytosis, exocytosis.
During the quiescent status, MARCKS is anchored to the plasma membrane via a electrostatic interaction of the N-terminal domain [58]. MARCKS is highly expressed in the nervous tissue and spleen, moderately in the heart and kidney and, less in the liver [40]. Several mitogenic agents may reduce MARCKS expression, indicating that its downregulation might be essential for the cell cycle [59]. In macrophages, activated PKC phosphorylates the membrane MARCKS to support the cytoskeleton [60] while in response to inflammatory stimuli, such as bacterial lipopolysaccharide (LPS) and TNF-α, MARCKS influences neutrophil migration [61]. Murine macrophage express MARCKS which is regulated by LPS [40] while MARCKS negatively modulates the LPS signaling [28]. MARCKS is distributed primarily in the substrate-adherent surface of pseudopodia and filopodia in quiescent macrophage [60] where its phosphorylation by PKC prevents MARCKS from proteolytic degradation [62]. Membrane-bound or overexpressed MARCKS significantly impact the lateral mobility of B cell receptors (BCR) while the formed reduces mouse primary splenic B cells in vitro and in vivo [63] ultimately regulating BCR signaling [64]. Conversely, our group could not detect MARCKS expression in T cells (unpublished data).
A growing amount of evidence supports the role of the effector domain of MARCKS [65], the human MARCKS gene [42], and MARCKSL1 adducin [66]. MARCKS is phosphorylated by calmodulin [51], and its subcellular distribution is demonstrated in transformed 3 T3 cells [67] as well as in models of neurotransmitter release modulation [68].
MARCKSL1 is attached to the vesicular phospholipid membrane [69] while MARCKS modulates endothelial cell proliferation [70], influences dual-directional protein translocation [71] and there is a computational model for the electrostatic sequestration of PI(4,5)P2 by membrane- tethered MARCKS [72]. In the case of the liver and the biliary tract, choleretic and cholestatic agents activate the intracellular signaling kinases, including phosphoinositide-3-kinase (PI3K) and PKC which then regulate MARCKS [73] and secondary bile acids promote MARCKS translocation [74], while MARCKS phosphorylation modulates taurolithocholate (TLC)-induced cholestasis [75] and its hyperexpression is observed in the hepatic ischemia/reperfusion injury [76].
3. MARCKS domains
The comparison of the MARCKS proteins from different species (including bovine, mouse, chicken, and human) reveals three highly conserved domains. These three domains include an N-terminal myristoylated domain that binds to the membranes, a highly conserved Homology 2 (MH2) domain, and an effector domain acting as a phosphorylation site [57]. The N-terminal domain includes 14 amino acids with a consensus sequence of GAQFSKTA recognized by N-myristoyl transferase at the glycine residue [77], mediating the interaction of MARCKS with the plasma membrane through the insertion of the N-terminal myristoyl moiety into the hydrophobic lipid bilayer [78]. N-terminal myristoylation takes place not only during translation but also at post-translation [79], with the myristoylation site undergoing a reversible co-translational attachment of myristic acid to its N-terminal glycine residue [80]. The MH2 domain can interact with actin or contains a potential dimerization motif [81].
Next to the N-terminal and the MH2 domains, the previously mentioned MARCKS effector domain (KKKKKRFSFKKSFKLSGF SFKKNKK) [42] has an overall charge of +13 where lysine residues are concentrated at both ends and likely contribute to an extended effector domain conformation via electrostatic repulsion. The multiple serine residues are susceptible to phosphorylation by protein kinase C (PKC), or other protein kinases, such as Rho kinase (ROCK) [82–86], and the Ser 166 is the most bioactive [87]. The MARCKS effector domain is also unusually enriched with 5 phenylalanine (F) residues and three out of 4 serines (positions 152, 156, and 163 in the mouse) known to be phosphorylated by classical and novel isoforms of PKC [87–89]. The highly positively charged effector domain is less than 10% α-helical and does not form any secondary structure, regardless of extensive phosphorylation [88]. The MARCKSL1 effector domain is very similar but contains a significant replacement of Ser-97 with a proline residue, which has important implications both for the conformation of the effector domain and its net charge upon phosphorylation. Besides PKC and Ca2+/calmodulin, the highly conserved effector domain also interacts with the PI3K-AKT signaling pathway (Fig. 3), BCR, and TLR signaling pathways. Moreover, the MARCKS effector domain could also induce polymerization of G-actin, via the N-terminus five continuous ranked lysine of the effector domain and could be reversed by calmodulin binding or PKC phosphorylation [90] (Fig. 4).
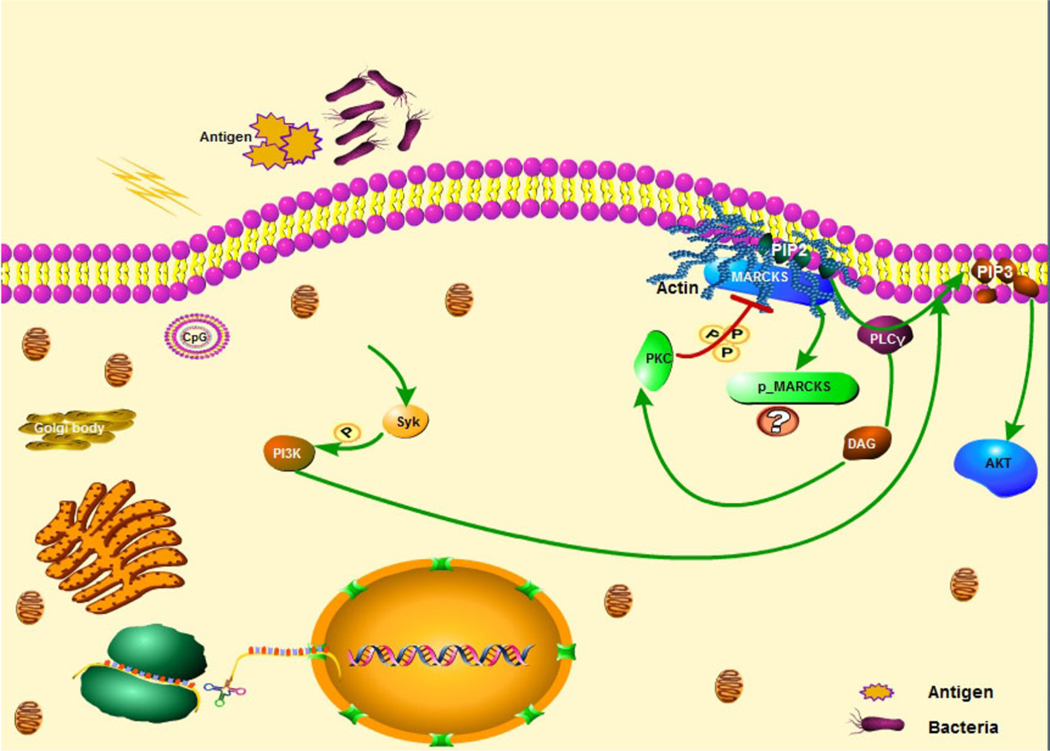
Fig. 3.
MARCKS and PIP2 colocalize in the nucleus indicating that the MARCKS effector domain is pivotal for regulation of PIP2 traffic to the nucleus and for downstream gene expression. The PI3K-AKT signaling and PIP2 mediated actin skeleton pathways of MARCK are illustrated, along with the positive feedback loop of PKC-MARCKS-PIP2-PLCγ-DAG.
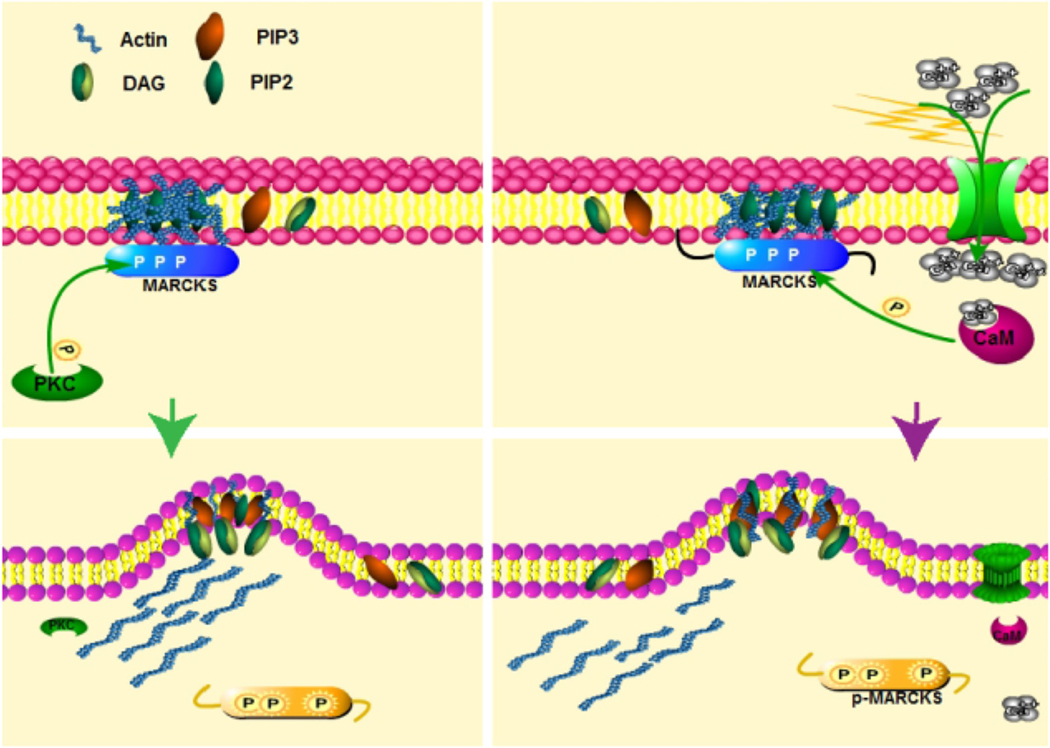
Fig. 4.
A proposed model for the modulated phosphorylation of MARCKS via activated PKC and/or Ca2+-calmodulin (CaM). In this time-dependent mode, PKC activity predominates before Ca2+ reaches its peak (Left panel) while once the Ca2+ peak is reached, Ca2+-CaM interacts with MARCKS and prevents its PKC-mediated phosphorylation (Right panel); in both cases leading to the release of PIP2 and actin.
4. MARCKS membrane binding and functions
MARCKS has been identified as an essential modulator of plasma membrane phosphoinositide, nuclear localization regulator, and cytoplasm, such as phagosomes [91], and filamentous actin cytoskeleton [92]. Phosphatidylinositol 4,5-bisphosphate (PI (4,5) P2, also named PIP2), is a vital lipid second messenger, which include the diacylglycerol (DAG) and IP3 (which activate protein kinase C, promote intracellular calcium releasing, respectively), and other signaling poly phosphoinositides, like PI(3,4,5)P3 [93,94]. PIP2 acts as a docking site and as a direct activator of numerous membrane proteins. MARCKS proteins binding to membrane require both the hydrophobic insertion into the bilayer of the N-terminal myristate, and electrostatic interaction with lipids by the effector domain (Fig. 2). Immunofluorescence studies demonstrated that MARCKS and PIP2 colocalize in the nucleus indicating that the MARCKS effector domain is pivotal for regulation of PIP2 traffic to the nucleus and for downstream gene expression [95]. The MARCKS effector domain and the accumulating actins at PIP2- containing rafts are both essential for actin cytoskeleton construction. The overexpression of wild-type MARCKS (WT) and deletion of effector domain MARCKS in a glioblastoma multiforme (GBM) cell line demonstrate that MARCKS-WT localizes to the nucleus, while in the absence of the effector domain MARCKS remains in the cytoplasm. Intriguingly, the over-expression of MARCKS-WT is associated with an increase of total cellular PIP2, implying MARCKS modulates PIP2.
Both myristoylation of the N-terminus and phosphorylation of the phosphorylation site domain by PKC induces a change in affinity for Ca2+/calmodulin, indicating that it may play a role in calcium-dependent signaling by sequestering calmodulin in the plasma membrane until activation by PKC induces exocytosis of the secondary messenger (Fig. 2). The N-terminus of calmodulin interacts with the C-terminal lobe of the effector domain, and the binding of MARCKS to the plasma membrane locally sequesters the phosphatidylinositol-4,5-diphosphate (PIP2) pool in quiescent cells [96], MARCKS sequesters (PIP2) at lipid rafts in the plasma membrane, as proven by the electrostatic interactions between phosphorylation site domain of MARCKS and PIP2 [97]. The electrostatic sequestration of PIP2 could be reversed either by binding to the effector domain mediated with calcium/ calmodulin or by phosphorylation of the effector domain via PKC, which both decrease the positive electrostatic potential [98]. Bundling of MARCKS with PIP2 prevents phospholipase C (PLC)-δ or PLC-β hydrolyzing PIP2, likely via interaction with the catalytic site [99,100]. Subsequently, the release of sequestered PIP2 locating at the lipid rafts promotes cell motility [101,102], cell migration mediated by accumulated MARCKS. It has been hypothesized that the abundant membrane bundled bound MARCKS would enrich more PIP2 sequestered at plasma membrane while the PKC-dependent phosphorylation displaces membrane-attached MARCKS, and dephosphorylation leads to its relocalization to the membrane [52]. Nuclear magnetic resonance (NMR) and circular dichroism have elaborated that calmodulin bundling does not induce a secondary conformation in the effector domain [103,104]. A specific MARCKS peptide (N-KKKKKRFSFKKSFKLSGFSFKKNKK-C) at 10–100 nM can inhibit the hydrolysis of PIP2 mediated by PLC-β or -δ through strong binding of the peptide to PIP2 [100].
Several other proteins contain shared MARCKS homology region and could potentially interact with PIP2; these include MARCKSL1, diglyceride kinase-ζ (DKG-zeta), adducin, neuromodulin (GAP43), Drosophila A kinase anchor protein 200 (DAKAP200), phospholipase D (PLD), gelsolin, N-WASP, and the N-methyl-D-aspartate receptor [105]. The translocation of diacylglycerol kinase (DGK) - ζ (zeta) to the nucleus relies on the phosphorylation of the effector domain of MARCKS.
5. MARCKS in cancer
The ability of MARCKS to induce cellular migration underlies its effects on regeneration and cancer [106]. The hyperexpression of MARCKS facilitates angiogenesis and growth in vivo in a renal cell carcinoma (RCC) xenograft carcinoma while a reduced expression, both in RNA and protein levels, is observed in high-grade RCC cell lines ex vivo causing alleviation of proliferation and migration. The abrogation of MARCKS via MPS (MARCKS effect domain) peptide modulates the effects of regorafenib, an oral multikinase inhibitor, and influences the survival of kidney cancer cells through mitigating bioactivation of AKT and mTOR [18]. MARCKS promotes cell proliferation via the ErbB2- mediated signal pathway and cell polarity proteins [36,107]. ErbB2 overexpression induces astrocytes to dedifferentiate and revert to the progenitor status [108].
Data from translational research show the role of MARCKS in numerous cancers and it is potentially of prognostic value. In primary liver cancer, the transcription factor Zic family member 2 (ZIC2) is highly expressed in liver tumor-initiating cells and proves essential for the disease perpetuation. The long noncoding RNA (lncRNA) lncZic2 locates in proximity to the ZIC2 locus and is upregulated in liver cancer and liver tumor-initiating cells, and promotes the production of MARCKS and (MARCKSL1. The LncZic2 then drives BRM/SWI2-related gene 1 (BRG1) transcriptional regulator to the promoters of the MARCKS and MARCKSL1 gene. Depletion of lncZic2 and BRG1 down regulates MARCKS and MARCKSL1. lncZic2 is essential for the self-renewal of liver T tumor-initiating cell ICs through up-regulating MARCKS and MARCKSL1 gene via BRG1 [24]. In the case of lung cancer, the phosphorylation status of the plasma membrane-anchored protein MARCKS is vital in the response to radiation; the unphosphorylated status of the effector domain is associated with a better response to ionizing radiation therapy. A549, H1792, and H1975 lung cancer cell lines treated with MARCKS effector domain peptide manifest reduced phosphorylation of MARCKS and Akt serine/threonine kinase 1, and MPS treatment could inhibit proliferation of lung cancer cell and increase radiation sensitivity. MARCKS effector domain peptide regulates MARCKS phosphorylation and modulates its function [109].
When comparing MARCKS protein expression between inflammatory and non-inflammatory breast cancer samples, inflammatory samples show an 18% rate of MARCKS-positivity (i.e. stained cells ≥1%), and MARCKS expression is also upregulated (36% vs. 11% in non- inflammatory samples). In inflammatory breast cancer, MARCKS over-expression is also associated with poor metastasis-free survival and prognosis [14]. In prostate cancer, MARCKS is upregulated in biochemically recurring cases (i.e. patients with at least a 0.2 mg/dl increase in prostate specific antigen levels), compared with non-recurrent ones; silencing of MARCKS attenuates the migration and invasion ability of prostate cancer cells, by downregulating the MMP9 mRNA expression, and cell spreading in contrast with the increased cell-cell adhesion [20]. Comparing MARCKS expression in normal versus epithelial ovarian cancer samples through immunohistochemistry, 75% of normal samples are positive for epithelial MARCKS staining versus 50% of tumor samples. Intriguingly, stromal MARCKS expression is upregulated in tumor samples (77%) compared to normal samples (22%), being correlated with shorter overall survival [26]. Studies on the expression of MARCKS in other types of cancer showed the association of glioma with the protein reduced phosphorylation inhibiting the PI3K/AKT pathway [110] or its phosphorylation associated with reduced cell proliferation in choroidal melanoma [111].
The impact of MARCKS in cancer is further supported by miRNA studies. In osteosarcoma, MARCKS is upregulated and inversely correlates with miR-34c-3p while being a direct target of miR-34c-3p, and when overexpressed, partly reversing the effects of this miRNA [112].
6. MARCKS and immune-mediated diseases
As previously discussed, MARCKS is highly expressed in macrophages and its inhibition blocks the LPS-induced expression of TNF-α while also affecting the macrophage transmigration [33,34]. The treatment of macrophages with phosphorylation site domain peptides, which prevent MARCKS phosphorylation at the N-terminus sequence, abolishes LPS-induced expression of TNF-α through inhibition of p38 and JNK MAPKs and NF-κB. The same peptides in mice decrease the TNF-α and IL-6 serum levels and are associated with a 40% improved survival after a lethal dose of LPS, likely based on the decreased cytokine production in macrophages [32]. In the case of neutrophils, MARCKS influences the migration and adhesion [31] as well as the production of inflammatory cytokines [113,114]. Decreased binding of MARCKS to the plasma membrane leads to increased cell proliferation in both B-cell tumor and mouse primary splenic B cells in vitro, as also observed by transfer experiments in vivo. The importance of MARCKS in the modulation of the immune function is supported by both clinical and basic research in B-cell chronic lymphocytic leukemia (B-CLL) [115].
Similar to what has been discussed for cancer, there is a direct correlation between MARCKS immune effects and miRNA changes. During an acute infection, miR-21 regulates phagocytosis while increased MARCKS and the Rho–B, as in in miR-21(− /− ) bone marrow-derived macrophages, correlate with augmented uptake of Listeria. The intra- peritoneal injection of Listeria monocytogenes causes higher bacterial invasiveness of the liver tissue in miR-21(− /− ) mice compared to WT mice [116]. In the case of ulcerative colitis, dextran sulfate sodium DSS- induced colitis model compared to normal colon tissues points at the downregulation of miR-429. Among the 41 genes influenced by miR- 429, MARCKS is a direct target gene, being downregulated both in mRNA and protein levels. The same miR-429 regulates the expression of MARCKS and MARCKS-associated mucin secretion both in DSS-induced colitis and colorectal cells, and targeting miR-429 upregulates MARCKS expression in colorectal cell lines. Further, miR-429 regulates mucin secretion in human colorectal cells and mouse colitis tissues through up- regulating MARCKS expression [25].
Data on the role of MARCKS in autoimmune diseases are limited to rheumatoid arthritis. Data from the collagen-induced arthritis model demonstrated that histone deacetylase 1 (HDAC1)is overexpressed and miR-124 and MARCKS poorly expressed in synovial tissues while the silencing of HDAC1 reduces synovial hyperplasia and inflammation by elevating MARCKS and miR-124 both in vitro and in vivo in a fashion with is hypothesized to be mediated by the JAK/STAT signaling pathway [117], a therapeutic target in rheumatoid arthritis [118]. As previously noted, the translocation of DGK-ζ to the nucleus relies on the phosphorylation of the effector domain of MARCKS and this pathway has also been proposed as a therapeutic target in numerous human diseases, including autoimmunity [119].
7. MARCKS and neurological disorders
MARCKS is highly expressed in neurological tissues, especially during embryogenesis and subsequent developmental phases as MARCKS is central to hemisphere fusion, neurulation, forebrain commissure formation, cortical development, and retinal laminations commissure formation [6,47]. The disruption of the MARCKS gene in mice leads to numerous neural tube defects [45] and perinatal death is invariably observed in MARCKS-deficient pups which also exhibit a characteristic lamination disorder of the cortex and retina. On the other hand, the MARCKSL1 gene deletion results in the suppression of the cranial neural tube closure with anencephaly [48,49].
In adult neurological diseases, phosphorylation of MARCKS is associated with neuron degeneration at the early stage of Alzheimer’s disease, while extracellular Aβ aggregates are observed at histology. MARCKS is also a cross-talking modulator between lipid raft and endocytosis in the process of amyloid-β peptide production, which may represent a new therapeutic target for Alzheimer’s disease treatment [120]. The expression of MARCKS is altered in dementia with Lewy bodies and Parkinson’s disease both in mouse models and human tissues. The increase in the level of pSer46-MARCKS is observed before α-synuclein aggregate formation, while human α-Syn-BAC-Tg/GBA- hetero-KO mice demonstrate no abnormalities during aging, consistent with the pattern in human post mortem brains. Pre-aggregation neurite degeneration is present in Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease [121]. Patients with microcephaly with pontine and cerebellar hypoplasia were studied by mutational search of CASK and screening of candidate genes via SNP array, targeted resequencing, and whole-exome sequencing [10]. Of note, MARCKS is one of the genes has been associated with microcephaly with pontine and cerebellar hypoplasia. MARCKS-related increase in free PIP2 is also reported to be involved in the endocytosis signaling, and mediates endocytosis through cross-talking with Neural Wiskott-Aldrich Syndrome Protein (N-WASP) [122].
8. Concluding remarks
Numerous unanswered questions remain from the available evidence on the MARCKS family, especially based on the limited understanding of the mechanisms by which MARCKS alterations produce specific phenotypes. As an example, we have only a partial understanding of the mechanisms by which knocking out the MARCKS genes results in perinatal and not intrauterine death. In the case of immunity, we observe that MARCKS expression is found primarily in macrophages and neutrophils while being virtually absent or found only marginally in T and B cells, two cell populations which are crucial for embryo development and aging. While some lines of evidence have been proposed linking MARCKS with autoimmune diseases, particularly rheumatoid arthritis, we may hypothesize that modulating this intracellular pathway may be beneficial to treat chronic inflammation, particularly in conditions where JAK inhibition is a viable therapeutic option [123]. We also envision that the role of MARCKS in cancer may be better defined, particularly by resolving the conflicting evidence from expression studies in specific cancers and elucidating the MARCKS signaling networks. In the neurological area, MARCKS influences endo- and exocytosis, neurite outgrowth, and synaptic plasticity with numerous clinical implications which should be better characterized mechanistically.
In conclusion, the current understanding of MARCKS well illustrates the common ground among complex areas such as immunology, oncology, and neurology and highlights the need for a multidisciplinary approach with the aim to translate basic science into clinical and therapeutic implications.
Acknowledgments
Thanks for the scholarship program launched by China Scholarship Council, China (No. 201906210461). Compliance with ethical standards. This work was supported by the Pinnacle Research Award in Liver Disease from American Association for the Study of Liver Diseases (AASLD) to W.Z; and NIH R01DK123262 to M.E.G.
Footnotes
Conflicts of interest
The authors declare that they have no conflict of interests.
References
- [1].Kastelowitz N, Tamura R, Onasoga A, Stalker TJ, White OR, Brown PN, et al. Peptides derived from MARCKS block coagulation complex assembly on phosphatidylserine. Sci Rep 2017;7:4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Elzagallaai A, Rose SD, Trifaro JM. Platelet secretion induced by phorbol esters stimulation is mediated through phosphorylation of MARCKS: a MARCKS-derived peptide blocks MARCKS phosphorylation and serotonin release without affecting pleckstrin phosphorylation. Blood 2000;95:894–902. [PubMed] [Google Scholar]
- [3].Brudvig JJ, Weimer JM. X MARCKS the spot: myristoylated alanine-rich C kinase substrate in neuronal function and disease. Front Cell Neurosci 2015;9:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu WC, Walaas SI, Nairn AC, Greengard P. Calcium/phospholipid regulates phosphorylation of a Mr “87k” substrate protein in brain synaptosomes. Proc Natl Acad Sci U S A 1982;79:5249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ha EV, Rogers DF. Novel therapies to inhibit mucus synthesis and secretion in airway hypersecretory diseases. Pharmacology 2016;97:84–100. [DOI] [PubMed] [Google Scholar]
- [6].Stumpo DJ, Bock CB, Tuttle JS, Blackshear PJ. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc Natl Acad Sci U S A 1995;92:944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014;34:11929–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Konopaske GT, Subburaju S, Coyle JT, Benes FM. Altered prefrontal cortical MARCKS and PPP1R9A mRNA expression in schizophrenia and bipolar disorder. Schizophr Res 2015;164:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fujita K, Motoki K, Tagawa K, Chen X, Hama H, Nakajima K, et al. HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease. Sci Rep 2016;6:31895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hayashi S, Uehara DT, Tanimoto K, Mizuno S, Chinen Y, Fukumura S, et al. Comprehensive investigation of CASK mutations and other genetic etiologies in 41 patients with intellectual disability and microcephaly with pontine and cerebellar hypoplasia (MICPCH). PLoS One 2017;12:e0181791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Techasen A, Loilome W, Namwat N, Takahashi E, Sugihara E, Puapairoj A, et al. Myristoylated alanine-rich C kinase substrate phosphorylation promotes cholangiocarcinoma cell migration and metastasis via the protein kinase C-dependent pathway. Cancer Sci 2010;101:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yokoyama Y, Ito T, Hanson V, Schwartz GK, Aderem AA, Holland JF, et al. PMA-induced reduction in invasiveness is associated with hyperphosphorylation of MARCKS and Talin in invasive bladder cancer cells. Int J Cancer 1998;75:774–9. [DOI] [PubMed] [Google Scholar]
- [13].Chen CH, Cheng CT, Yuan Y, Zhai J, Arif M, Fong LW, et al. Elevated MARCKS phosphorylation contributes to unresponsiveness of breast cancer to paclitaxel treatment. Oncotarget 2015;6:15194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Manai M, Thomassin-Piana J, Gamoudi A, Finetti P, Lopez M, Eghozzi R, et al. MARCKS protein overexpression in inflammatory breast cancer. Oncotarget 2017; 8:6246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen CH, Chiu CL, Adler KB, Wu R. A novel predictor of cancer malignancy: up-regulation of myristoylated alanine-rich C kinase substrate phosphorylation in lung cancer. Am J Respir Crit Care Med 2014;189:1002–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen CH, Statt S, Chiu CL, Thai P, Arif M, Adler KB, et al. Targeting myristoylated alanine-rich C kinase substrate phosphorylation site domain in lung cancer. Mechanisms and therapeutic implications. Am J Respir Crit Care Med 2014;190: 1127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hanada S, Kakehashi A, Nishiyama N, Wei M, Yamano S, Chung K, et al. Myristoylated alanine-rich C-kinase substrate as a prognostic biomarker in human primary lung squamous cell carcinoma. Cancer Biomark 2013;13:289–98. [DOI] [PubMed] [Google Scholar]
- [18].Chen CH, Fong LWR, Yu E, Wu R, Trott JF, Weiss RH. Upregulation of MARCKS in kidney cancer and its potential as a therapeutic target. Oncogene 2017;36: 3588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brandi J, Dalla Pozza E, Dando I, Biondani G, Robotti E, Jenkins R, et al. Secretome protein signature of human pancreatic cancer stem-like cells. J Proteomics 2016;136:1–12. [DOI] [PubMed] [Google Scholar]
- [20].Dorris E, O’Neill A, Hanrahan K, Treacy A, Watson RW. MARCKS promotes invasion and is associated with biochemical recurrence in prostate cancer. Oncotarget 2017;8:72021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen X, Rotenberg SA. PhosphoMARCKS drives motility of mouse melanoma cells. Cell Signal 2010;22:1097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rombouts K, Carloni V, Mello T, Omenetti S, Galastri S, Madiai S, et al. Myristoylated Alanine-Rich protein Kinase C Substrate (MARCKS) expression modulates the metastatic phenotype in human and murine colon carcinoma in vitro and in vivo. Cancer Lett 2013;333:244–52. [DOI] [PubMed] [Google Scholar]
- [23].Naboulsi W, Megger DA, Bracht T, Kohl M, Turewicz M, Eisenacher M, et al. Quantitative tissue proteomics analysis reveals versican as potential biomarker for early-stage hepatocellular carcinoma. J Proteome Res 2016;15:38–47. [DOI] [PubMed] [Google Scholar]
- [24].Chen Z, Liu Y, Yao L, Guo S, Gao Y, Zhu P. The long noncoding RNA lncZic2 drives the self-renewal of liver tumor-initiating cells via the protein kinase C substrates MARCKS and MARCKSL1. J Biol Chem 2018;293:7982–92. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [25].Mo JS, Alam KJ, Kim HS, Lee YM, Yun KJ, Chae SC. MicroRNA 429 regulates mucin gene expression and secretion in murine model of colitis. J Crohns Colitis 2016;10:837–49. [DOI] [PubMed] [Google Scholar]
- [26].Doghri R, Manai M, Finetti P, Driss M, Agavnian E, Lopez M, et al. Stromal expression of MARCKS protein in ovarian carcinomas has unfavorable prognostic value. Int J Mol Sci 2017;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hamilton TA, Adams DO. Molecular mechanisms of signal transduction in macrophages. Immunol Today 1987;8:151–8. [DOI] [PubMed] [Google Scholar]
- [28].Mancek-Keber M, Bencina M, Japelj B, Panter G, Andra J, Brandenburg K, et al. MARCKS as a negative regulator of lipopolysaccharide signaling. J Immunol 2012;188:3893–902. [DOI] [PubMed] [Google Scholar]
- [29].Damera G, Jester WF, Jiang M, Zhao H, Fogle HW, Mittelman M, et al. Inhibition of myristoylated alanine-rich C kinase substrate (MARCKS) protein inhibits ozone-induced airway neutrophilia and inflammation. Exp Lung Res 2010;36: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Takashi S, Park J, Fang S, Koyama S, Parikh I, Adler KB. A peptide against the N-terminus of myristoylated alanine-rich C kinase substrate inhibits degranulation of human leukocytes in vitro. Am J Respir Cell Mol Biol 2006;34:647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eckert RE, Neuder LE, Park J, Adler KB, Jones SL. Myristoylated alanine-rich C- kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am J Respir Cell Mol Biol 2010;42:586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee SM, Suk K, Lee WH. Myristoylated alanine-rich C kinase substrate (MARCKS) regulates the expression of proinflammatory cytokines in macrophages through activation of p38/JNK MAPK and NF-kappaB. Cell Immunol 2015;296:115–21. [DOI] [PubMed] [Google Scholar]
- [33].Chun KR, Bae EM, Kim JK, Suk K, Lee WH. Suppression of the lipopolysaccharide-induced expression of MARCKS-related protein (MRP) affects transmigration in activated RAW264.7 cells. Cell Immunol 2009;256:92–8. [DOI] [PubMed] [Google Scholar]
- [34].Green TD, Park J, Yin Q, Fang S, Crews AL, Jones SL, et al. Directed migration of mouse macrophages in vitro involves myristoylated alanine-rich C-kinase substrate (MARCKS) protein. J Leukoc Biol 2012;92:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yamauchi E, Kiyonami R, Kanai M, Taniguchi H. Presence of conserved domains in the C-terminus of MARCKS, a major in vivo substrate of protein kinase C: application of ion trap mass spectrometry to the elucidation of protein structures. J Biochem 1998;123:760–5. [DOI] [PubMed] [Google Scholar]
- [36].Weimer JM, Yokota Y, Stanco A, Stumpo DJ, Blackshear PJ, Anton ES. MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex. Development 2009;136:2965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ramsden JJ. MARCKS: a case of molecular exaptation? Int J Biochem Cell Biol 2000;32:475–9. [DOI] [PubMed] [Google Scholar]
- [38].Graff JM, Stumpo DJ, Blackshear PJ. Molecular cloning, sequence, and expression of a cDNA encoding the chicken myristoylated alanine-rich C kinase substrate (MARCKS). Molecular Endocrinology (Baltimore, Md) 1989;3:1903–6. [DOI] [PubMed] [Google Scholar]
- [39].Stumpo DJ, Graff JM, Albert KA, Greengard P, Blackshear PJ. Molecular cloning, characterization, and expression of a cDNA encoding the “80- to 87-kDa” myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A 1989;86:4012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Seykora JT, Ravetch JV, Aderem A. Cloning and molecular characterization of the murine macrophage “68-kDa” protein kinase C substrate and its regulation by bacterial lipopolysaccharide. Proc Natl Acad Sci U S A 1991;88:2505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Erusalimsky JD, Brooks SF, Herget T, Morris C, Rozengurt E. Molecular cloning and characterization of the acidic 80-kDa protein kinase C substrate from rat brain. Identification as a glycoprotein. J Biol Chem 1991;266:7073–80. [PubMed] [Google Scholar]
- [42].Harlan DM, Graff JM, Stumpo DJ, Eddy RL Jr, Shows TB, Boyle JM, et al. The human myristoylated alanine-rich C kinase substrate (MARCKS) gene (MACS). Analysis of its gene product, promoter, and chromosomal localization. J Biol Chem 1991;266:14399–405. [PubMed] [Google Scholar]
- [43].Prieto D, Zolessi FR. Functional diversification of the four MARCKS family members in zebrafish neural development. J Exp Zool B Mol Dev Evol 2017;328: 119–38. [DOI] [PubMed] [Google Scholar]
- [44].Blackshear PJ, Tuttle JS, Oakey RJ, Seldin MF, Chery M, Philippe C, et al. Chromosomal mapping of the human (MACS) and mouse (Macs) genes encoding the MARCKS protein. Genomics 1992;14:168–74. [DOI] [PubMed] [Google Scholar]
- [45].Stumpo DJ, Eddy RL Jr, Haley LL, Sait S, Shows TB, Lai WS, et al. Promoter sequence, expression, and fine chromosomal mapping of the human gene (MLP) encoding the MARCKS-like protein: identification of neighboring and linked polymorphic loci for MLP and MACS and use in the evaluation of human neural tube defects. Genomics 1998;49:253–64. [DOI] [PubMed] [Google Scholar]
- [46].McNamara RK, Stumpo DJ, Morel LM, Lewis MH, Wakeland EK, Blackshear PJ, et al. Effect of reduced myristoylated alanine-rich C kinase substrate expression on hippocampal mossy fiber development and spatial learning in mutant mice: transgenic rescue and interactions with gene background. Proc Natl Acad Sci U S A 1998;95:14517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Blackshear PJ, Lai WS, Tuttle JS, Stumpo DJ, Kennington E, Nairn AC, et al. Developmental expression of MARCKS and protein kinase C in mice in relation to the exencephaly resulting from MARCKS deficiency. Brain Res Dev Brain Res 1996;96:62–75. [DOI] [PubMed] [Google Scholar]
- [48].Chen J, Chang S, Duncan SA, Okano HJ, Fishell G, Aderem A. Disruption of the MacMARCKS gene prevents cranial neural tube closure and results in anencephaly. Proc Natl Acad Sci U S A 1996;93:6275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu M, Chen DF, Sasaoka T, Tonegawa S. Neural tube defects and abnormal brain development in F52-deficient mice. Proc Natl Acad Sci U S A 1996;93:2110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Graff JM, Young TN, Johnson JD, Blackshear PJ. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem 1989;264:21818–23. [PubMed] [Google Scholar]
- [51].McIlroy BK, Walters JD, Blackshear PJ, Johnson JD. Phosphorylation-dependent binding of a synthetic MARCKS peptide to calmodulin. J Biol Chem 1991;266: 4959–64. [PubMed] [Google Scholar]
- [52].Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature 1991;351:320–2. [DOI] [PubMed] [Google Scholar]
- [53].Graff JM, Stumpo DJ, Blackshear PJ. Characterization of the phosphorylation sites in the chicken and bovine myristoylated alanine-rich C kinase substrate protein, a prominent cellular substrate for protein kinase C. J Biol Chem 1989; 264:11912–9. [PubMed] [Google Scholar]
- [54].Bhat NR. Phosphorylation of MARCKS (80-kDa) protein, a major substrate for protein kinase C in oligodendroglial progenitors. J Neurosci Res 1991;30:447–54. [DOI] [PubMed] [Google Scholar]
- [55].Blackshear PJ, Haupt DM, Stumpo DJ. Insulin activation of protein kinase C: a reassessment. J Biol Chem 1991;266:10946–52. [PubMed] [Google Scholar]
- [56].Brooks SF, Herget T, Erusalimsky JD, Rozengurt E. Protein kinase C activation potently down-regulates the expression of its major substrate, 80K, in Swiss 3T3 cells. EMBO J 1991;10:2497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell 1992;71:713–6. [DOI] [PubMed] [Google Scholar]
- [58].Graff JM, Gordon JI, Blackshear PJ. Myristoylated and nonmyristoylated forms of a protein are phosphorylated by protein kinase C. Science (New York, NY) 1989; 246:503–6. [DOI] [PubMed] [Google Scholar]
- [59].Brooks SF, Herget T, Broad S, Rozengurt E. The expression of 80K/MARCKS, a major substrate of protein kinase C (PKC), is down-regulated through both PKC-dependent and -independent pathways. Effects of bombesin, platelet-derived growth factor, and cAMP. J Biol Chem 1992;267:14212–8. [PubMed] [Google Scholar]
- [60].Rosen A, Keenan KF, Thelen M, Nairn AC, Aderem A. Activation of protein kinase C results in the displacement of its myristoylated, alanine-rich substrate from punctate structures in macrophage filopodia. J Exp Med 1990;172:1211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Thelen M, Rosen A, Nairn AC, Aderem A. Tumor necrosis factor alpha modifies agonist-dependent responses in human neutrophils by inducing the synthesis and myristoylation of a specific protein kinase C substrate. Proc Natl Acad Sci U S A 1990;87:5603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Spizz G, Blackshear PJ. Protein kinase C-mediated phosphorylation of the myristoylated alanine-rich C-kinase substrate protects it from specific proteolytic cleavage. J Biol Chem 1996;271:553–62. [DOI] [PubMed] [Google Scholar]
- [63].Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J 1997;16: 3078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Xu C, Fang Y, Yang Z, Jing Y, Zhang Y, Liu C, et al. MARCKS regulates tonic and chronic active B cell receptor signaling. Leukemia 2019;33:710–29. [DOI] [PubMed] [Google Scholar]
- [65].Graff JM, Rajan RR, Randall RR, Nairn AC, Blackshear PJ. Protein kinase C substrate and inhibitor characteristics of peptides derived from the myristoylated alanine-rich C kinase substrate (MARCKS) protein phosphorylation site domain.J Biol Chem 1991;266:14390–8. [PubMed] [Google Scholar]
- [66].Joshi R, Gilligan DM, Otto E, McLaughlin T, Bennett V. Primary structure and domain organization of human alpha and beta adducin. J Cell Biol 1991;115: 665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Reed JC, Rapp U, Cuddy MP. Transformed 3T3 cells have reduced levels and altered subcellular distribution of the major PKC substrate protein MARCKS. Cell Signal 1991;3:569–76. [DOI] [PubMed] [Google Scholar]
- [68].Robinson PJ. The role of protein kinase C and its neuronal substrates dephosphin, B-50, and MARCKS in neurotransmitter release. Mol Neurobiol 1991;5:87–130. [DOI] [PubMed] [Google Scholar]
- [69].Vergères G, Ramsden JJ. Binding of MARCKS (myristoylated alanine-rich C kinase substrate)-related protein (MRP) to vesicular phospholipid membranes. Biochem J 1998;330(Pt 1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhao Y, Neltner BS, Davis HW. Role of MARCKS in regulating endothelial cell proliferation. Am J Physiol Cell Physiol 2000;279:C1611–20. [DOI] [PubMed] [Google Scholar]
- [71].Disatnik MH, Boutet SC, Pacio W, Chan AY, Ross LB, Lee CH, et al. The bi-directional translocation of MARCKS between membrane and cytosol regulates integrin-mediated muscle cell spreading. J Cell Sci 2004;117:4469–79. [DOI] [PubMed] [Google Scholar]
- [72].Wang J, Gambhir A, McLaughlin S, Murray D. A computational model for the electrostatic sequestration of PI(4,5)P2 by membrane-adsorbed basic peptides. Biophys J 2004;86:1969–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Anwer MS. Role of protein kinase C isoforms in bile formation and cholestasis. Hepatology (Baltimore, Md) 2014;60:1090–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lau BW, Colella M, Ruder WC, Ranieri M, Curci S, Hofer AM. Deoxycholic acid activates protein kinase C and phospholipase C via increased Ca2+ entry at plasma membrane. Gastroenterology 2005;128:695–707. [DOI] [PubMed] [Google Scholar]
- [75].Park SW, Webster CRL, Anwer MS. Mechanism of inhibition of taurolithocholate-induced retrieval of plasma membrane MRP2 by cyclic AMP and tauroursodeoxycholate. Physiol Rep 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Li Y, Gao M, Xu LN, Yin LH, Qi Y, Peng JY. MicroRNA-142–3p attenuates hepatic ischemia/reperfusion injury via targeting of myristoylated alanine-rich C-kinase substrate. Pharmacol Res 2020;156:104783. [DOI] [PubMed] [Google Scholar]
- [77].Aderem A. The role of myristoylated protein kinase C substrates in intracellular signaling pathways in macrophages. Curr Top Microbiol Immunol 1992;181: 189–207. [DOI] [PubMed] [Google Scholar]
- [78].Vergéres G, Manenti S, Weber T, Stürzinger C. The myristoyl moiety of myristoylated alanine-rich C kinase substrate (MARCKS) and MARCKS-related protein is embedded in the membrane. J Biol Chem 1995;270:19879–87. [DOI] [PubMed] [Google Scholar]
- [79].McIlhinney RA, McGlone K. Evidence for a non-myristoylated pool of the 80 kDa protein kinase C substrate of rat brain. Biochem J 1990;271:681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Manenti S, Sorokine O, Van Dorsselaer A, Taniguchi H. Demyristoylation of the major substrate of protein kinase C (MARCKS) by the cytoplasmic fraction of brain synaptosomes. J Biol Chem 1994;269:8309–13. [PubMed] [Google Scholar]
- [81].Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium- calmodulin. Nature 1992;356:618–22. [DOI] [PubMed] [Google Scholar]
- [82].Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-( +)-2-methyl-1-[(4-methyl-5-isoquinoline)sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol Ther 2002;93:225–32. [DOI] [PubMed] [Google Scholar]
- [83].Li J, O’Connor KL, Greeley GH Jr, Blackshear PJ, Townsend CM Jr, Evers BM. Myristoylated alanine-rich C kinase substrate-mediated neurotensin release via protein kinase C-delta downstream of the Rho/ROK pathway. J Biol Chem 2005; 280:8351–7. [DOI] [PubMed] [Google Scholar]
- [84].Tatsumi S, Mabuchi T, Katano T, Matsumura S, Abe T, Hidaka H, et al. Involvement of Rho-kinase in inflammatory and neuropathic pain through phosphorylation of myristoylated alanine-rich C-kinase substrate [MARCKS]. Neuroscience 2005;131:491–8. [DOI] [PubMed] [Google Scholar]
- [85].Tanabe A, Kamisuki Y, Hidaka H, Suzuki M, Negishi M, Takuwa Y. PKC phosphorylates MARCKS Ser159 not only directly but also through RhoA/ROCK. Biochem Biophys Res Commun 2006;345:156–61. [DOI] [PubMed] [Google Scholar]
- [86].Ohsawa M, Ishikura K, Mutoh J, Hisa H. Involvement of inhibition of RhoA/Rho kinase signaling in simvastatin-induced amelioration of neuropathic pain. Neuroscience 2016;333:204–13. [DOI] [PubMed] [Google Scholar]
- [87].Blackshear PJ. The MARCKS family of cellular protein kinase C substrates. J Biol Chem 1993;268:1501–4. [PubMed] [Google Scholar]
- [88].Manenti S, Sorokine O, Van Dorsselaer A, Taniguchi H. Affinity purification and characterization of myristoylated alanine-rich protein kinase C substrate (MARCKS) from bovine brain. Comparison of the cytoplasmic and the membrane-bound forms. J Biol Chem 1992;267:22310–5. [PubMed] [Google Scholar]
- [89].Herget T, Oehrlein SA, Pappin DJ, Rozengurt E, Parker PJ. The myristoylated alanine-rich C-kinase substrate (MARCKS) is sequentially phosphorylated by conventional, novel and atypical isotypes of protein kinase C. Eur J Biochem 1995;233:448–57. [DOI] [PubMed] [Google Scholar]
- [90].Wohnsland F, Schmitz AA, Steinmetz MO, Aebi U, Vergéres G. Interaction between actin and the effector peptide of MARCKS-related protein. Identification of functional amino acid segments. J Biol Chem 2000;275:20873–9. [DOI] [PubMed] [Google Scholar]
- [91].Allen LH, Aderem A. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J Exp Med 1995;182: 829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Eustace NJ, Anderson JC, Langford CP, Trummell HQ, Hicks PH, Jarboe JS, et al. Myristoylated alanine-rich C-kinase substrate effector domain phosphorylation regulates the growth and radiation sensitization of glioblastoma. Int J Oncol 2019;54:2039–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell 2000;100:603–6. [DOI] [PubMed] [Google Scholar]
- [94].Insall RH, Weiner OD. PIP3, PIP2, and cell movement–similar messages, different meanings? Dev Cell 2001;1:743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rohrbach TD, Shah N, Jackson WP, Feeney EV, Scanlon S, Gish R, et al. The effector domain of MARCKS is a nuclear localization signal that regulates cellular PIP2 levels and nuclear PIP2 localization. PLoS One 2015;10:e0140870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ulrich A, Schmitz AA, Braun T, Yuan T, Vogel HJ, Vergères G. Mapping the interface between calmodulin and MARCKS-related protein by fluorescence spectroscopy. Proc Natl Acad Sci U S A 2000;97:5191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tinsley JH, Teasdale NR, Yuan SY. Involvement of PKCdelta and PKD in pulmonary microvascular endothelial cell hyperpermeability. Am J Physiol Cell Physiol 2004;286:C105–11. [DOI] [PubMed] [Google Scholar]
- [98].Murray D, Arbuzova A, Honig B, McLaughlint S. The role of electrostatic and nonpolar interactions in the association of peripheral proteins with membranes. In: Current topics in membranes. Academic Press; 2002. p. 277–307. [Google Scholar]
- [99].Glaser M, Wanaski S, Buser CA, Boguslavsky V, Rashidzada W, Morris A, et al. Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J Biol Chem 1996;271:26187–93. [DOI] [PubMed] [Google Scholar]
- [100].Wang J, Arbuzova A, Hangyas-Mí halý ne G, McLaughlin S. The effector domain of´ myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J Biol Chem 2001;276:5012–9. [DOI] [PubMed] [Google Scholar]
- [101].Sheats MK, Sung EJ, Adler KB, Jones SL. In vitro neutrophil migration requires protein kinase C-Delta (δ-PKC)-mediated myristoylated alanine-rich C-kinase substrate (MARCKS) phosphorylation. Inflammation 2015;38:1126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Yu D, Makkar G, Strickland DK, Blanpied TA, Stumpo DJ, Blackshear PJ, et al. Myristoylated alanine-rich protein kinase substrate (MARCKS) regulates small GTPase Rac1 and Cdc42 activity and is a critical mediator of vascular smooth muscle cell migration in intimal hyperplasia formation. J Am Heart Assoc 2015;4: e002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Porumb T, Crivici A, Blackshear PJ, Ikura M. Calcium binding and conformational properties of calmodulin complexed with peptides derived from myristoylated alanine-rich C kinase substrate (MARCKS) and MARCKS-related protein (MRP). Eur Biophys J 1997;25:239–47. [DOI] [PubMed] [Google Scholar]
- [104].Matsubara M, Yamauchi E, Hayashi N, Taniguchi H. MARCKS, a major protein kinase C substrate, assumes non-helical conformations both in solution and in complex with Ca2+− calmodulin. FEBS Lett 1998;421:203–7. [DOI] [PubMed] [Google Scholar]
- [105].McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct 2002;31: 151–75. [DOI] [PubMed] [Google Scholar]
- [106].Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–21. [DOI] [PubMed] [Google Scholar]
- [107].Jin Cho S, La M, Ahn JK, Meadows GG, Joe CO. Tob-mediated cross-talk between MARCKS phosphorylation and ErbB-2 activation. Biochem Biophys Res Commun 2001;283:273–7. [DOI] [PubMed] [Google Scholar]
- [108].Ghashghaei HT, Weimer JM, Schmid RS, Yokota Y, McCarthy KD, Popko B, et al. Reinduction of ErbB2 in astrocytes promotes radial glial progenitor identity in adult cerebral cortex. Genes Dev 2007;21:3258–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rohrbach TD, Jones RB, Hicks PH, Weaver AN, Cooper TS, Eustace NJ, et al. MARCKS phosphorylation is modulated by a peptide mimetic of MARCKS effector domain leading to increased radiation sensitivity in lung cancer cell lines. Oncol Lett 2017;13:1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jarboe JS, Anderson JC, Duarte CW, Mehta T, Nowsheen S, Hicks PH, et al. MARCKS regulates growth and radiation sensitivity and is a novel prognostic factor for glioma. Clin Cancer Res 2012;18:3030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Manenti S, Malecaze F, Chap H, Darbon JM. Overexpression of the myristoylated alanine-rich C kinase substrate in human choroidal melanoma cells affects cell proliferation. Cancer Res 1998;58:1429–34. [PubMed] [Google Scholar]
- [112].Liu H, Su P, Zhi L, Zhao K. miR-34c-3p acts as a tumor suppressor gene in osteosarcoma by targeting MARCKS. Mol Med Rep 2017;15:1204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wang CN, Lin YC, Chang BC, Chen CH, Wu R, Lee CC. Targeting the phosphorylation site of myristoylated alanine-rich C kinase substrate alleviates symptoms in a murine model of steroid-resistant asthma. Br J Pharmacol 2019; 176:1122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Li J, D’Annibale-Tolhurst MA, Adler KB, Fang S, Yin Q, Birkenheuer AJ, et al. A myristoylated alanine-rich C kinase substrate-related peptide suppresses cytokine mRNA and protein expression in LPS-activated canine neutrophils. Am J Respir Cell Mol Biol 2013;48:314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Carballo E, Colomer D, Vives-Corrons JL, Blackshear PJ, Gil J. Characterization and purification of a protein kinase C substrate in human B cells. Identification as lymphocyte-specific protein 1 (LSP1). J Immunol 1996;156:1709–13. [PubMed] [Google Scholar]
- [116].Johnston DGW, Kearney J, Zasłona Z, Williams MA, O’Neill LAJ, Corr SC. MicroRNA-21 limits uptake of Listeria monocytogenes by macrophages to reduce the intracellular niche and control infection. Front Cell Infect Microbiol 2017;7: 201. 10.3389/fcimb.2017.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Meng Q, Pan B, Sheng P. Histone deacetylase 1 is increased in rheumatoid arthritis synovium and promotes synovial cell hyperplasia and synovial inflammation in the collagen-induced arthritis mouse model via the microRNA- 124-dependent MARCKS-JAK/STAT axis. Clin Exp Rheumatol 2020. [DOI] [PubMed] [Google Scholar]
- [118].Isailovic N, Ceribelli A, Cincinelli G, Vecellio M, Guidelli G, Caprioli M, et al. Lymphocyte modulation by tofacitinib in patients with rheumatoid arthritis. Clin Exp Immunol 2021;205:142–9. 10.1111/cei.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sakane F, Imai S, Kai M, Yasuda S, Kanoh H. Diacylglycerol kinases as emerging potential drug targets for a variety of diseases. Curr Drug Targets 2008;9:626–40. [DOI] [PubMed] [Google Scholar]
- [120].Su R, Han ZY, Fan JP, Zhang YL. A possible role of myristoylated alanine-rich C kinase substrate in endocytic pathway of Alzheimer’s disease. Neurosci Bull 2010; 26:338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Fujita K, Homma H, Kondo K, Ikuno M, Yamakado H, Tagawa K, et al. Ser46-phosphorylated MARCKS is a marker of neurite degeneration at the pre- aggregation stage in PD/DLB pathology. eNeuro 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].van Rheenen J, Achame EM, Janssen H, Calafat J, Jalink K. PIP2 signaling in lipid domains: a critical re-evaluation. EMBO J 2005;24:1664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].McInnes IB, Byers NL, Higgs RE, Lee J, Macias WL, Na S, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther 2019;21:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bunting M, Tang W, Zimmerman GA, McIntyre TM, Prescott SM. Molecular cloning and characterization of a novel human diacylglycerol kinase zeta. J Biol Chem 1996;271:10230–6. [PubMed] [Google Scholar]
- [125].Goto K, Kondo H. A 104-kDa diacylglycerol kinase containing ankyrin-like repeats localizes in the cell nucleus. Proc Natl Acad Sci U S A 1996;93:11196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ding L, McIntyre TM, Zimmerman GA, Prescott SM. The cloning and developmental regulation of murine diacylglycerol kinase zeta. FEBS Lett 1998; 429:109–14. [DOI] [PubMed] [Google Scholar]
- [127].Rincón E, Gharbi SI, Santos-Mendoza T, Mérida I. Diacylglycerol kinase ζ : at the crossroads of lipid signaling and protein complex organization. Prog Lipid Res 2012;51:1–10. [DOI] [PubMed] [Google Scholar]
- [128].Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol 1998;142:485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Matsuoka Y, Hughes CA, Bennett V. Adducin regulation. Definition of the calmodulin-binding domain and sites of phosphorylation by protein kinases A and C. J Biol Chem 1996;271:25157–66. [DOI] [PubMed] [Google Scholar]
- [130].Rossi EA, Li Z, Feng H, Rubin CS. Characterization of the targeting, binding, and phosphorylation site domains of an A kinase anchor protein and a myristoylated alanine-rich C kinase substrate-like analog that are encoded by a single gene. J Biol Chem 1999;274:27201–10. [DOI] [PubMed] [Google Scholar]
- [131].Skene JHP. Axonal growth-associated proteins. Annu Rev Neurosci 1989;12: 127–56. [DOI] [PubMed] [Google Scholar]
- [132].Kosik KS, Orecchio LD, Bruns GA, Benowitz LI, MacDonald GP, Cox DR, et al. Human GAP-43: its deduced amino acid sequence and chromosomal localization in mouse and human. Neuron 1988;1:127–32. [DOI] [PubMed] [Google Scholar]
- [133].Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J 1996;15:5326–35. [PMC free article] [PubMed] [Google Scholar]
- [134].Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science (New York, NY) 2000;290:801–6. [DOI] [PubMed] [Google Scholar]
- [135].Sciorra VA, Rudge SA, Prestwich GD, Frohman MA, Engebrecht J, Morris AJ. Identification of a phosphoinositide binding motif that mediates activation of mammalian and yeast phospholipase D isoenzymes. EMBO J 1999;18:5911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]