Abstract
Novel messenger RNA (mRNA) vaccines have proven to be effective tools against coronavirus disease 2019, and they have changed the course of the pandemic. However, early reports of mRNA vaccine–induced anaphylaxis resulted in public alarm, contributing toward vaccine hesitancy. Although initial reports were concerning for an unusually high rate of anaphylaxis to the mRNA vaccines, the true incidence is likely comparable with other vaccines. These reactions occurred predominantly in young to middle-aged females, and many had a history of allergies. Although initially thought to be triggered by polyethylene glycol (PEG), lack of reproducibility of these reactions with subsequent dosing and absent PEG sensitization point away from an IgE-mediated PEG allergy in most. PEG skin testing has poor posttest probability and should be reserved for evaluating non–vaccine-related PEG allergy without influencing decisions for subsequent mRNA vaccination. Immunization stress–related response can closely mimic vaccine-induced anaphylaxis and warrants consideration as a potential etiology. Current evidence suggests that many individuals who developed anaphylaxis to the first dose of an mRNA vaccine can likely receive a subsequent dose after careful evaluation. The need to understand these reactions mechanistically remains critical because the mRNA platform is rapidly finding its way into other vaccinations and therapeutics.
Key words: COVID-19, mRNA, vaccine, anaphylaxis, immunization stress–related response, ISRR, allergy, allergic reaction, polyethylene glycol, PEG
Anaphylaxis is a potentially life-threatening allergic reaction requiring immediate medical intervention. Vaccines are a rare cause of anaphylaxis; however, initial reports were concerning when a higher rate of coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccine–induced anaphylaxis was observed compared with other vaccine platforms. In the last 18 months, we have made significant progress in how to evaluate and manage individuals who have experienced allergic reactions to the COVID-19 mRNA vaccines. In this review, we discuss the evolution of current management strategies and highlight the gaps in our understanding to emphasize areas warranting additional research (Table I).
Table I.
Knowns and unknowns of COVID-19 mRNA vaccine–induced anaphylaxis
| Current observations |
|
| Knowledge gaps |
|
HαT, Hereditary α-tryptasemia; NIAID, National Institute of Allergy and Infectious Diseases; VCD, vocal cord dysfunction.
Chronology of COVID-19 vaccines
In December 2020, at a time when deaths from COVID-19 in the United States had surpassed 0.3 million, 2 novel mRNA-based COVID-19 vaccines, Pfizer-BioNTech (Comirnaty) and Moderna (Spikevax), received emergency use authorization from the US Food and Drug Administration, having demonstrated 95% and 94% efficacy, respectively, in preventing COVID-19.1, 2, 3, 4 Subsequently, an adenovirus vector–based COVID-19 vaccine, Janssen, was granted emergency use authorization in February 2021.
The emergence of new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants with enhanced transmissibility has contributed to persistently high infection rates in the population. As of May 19, 2022, 5 variants of concern have been identified. Collectively, COVID-19 has affected more than 524 million individuals globally and resulted in the loss of 6.2 million lives.5 In the United States alone, it has infected more than 83 million individuals (ie, a quarter of the US population) and caused the demise of more than 1 million people, which is 50% more than what the flu pandemic of 1919 had caused.6,7 At the same juncture, 18 months after public vaccination for COVID-19 began, a total of 258 million individuals (77.8% of the total and 82.7% of the eligible US population, ie, age ≥ 5 years) have received at least 1 dose. Comparatively, 221 million individuals have been fully vaccinated (66.6% of the total and 70.8% of the eligible US population, ie, age ≥ 5 years), and 103 million have received their first booster (46.6% of the total and 48.4% of the eligible US population, ie, age ≥ 12 years). These numbers indicate that a significant proportion of the population is incompletely vaccinated. More than 11% of those who received the first dose of a COVID-19 mRNA vaccine have not received a second dose. Furthermore, of the individuals fully vaccinated and eligible for a booster dose, more than 22% are yet to receive their first booster dose. Of the approved COVID-19 vaccines in the United States, a total of 586 million vaccine doses have been administered, which included 346 million doses of Pfizer-BioNTech (59%), 221 million doses of Moderna (38%), and 19 million doses of Janssen (3%).7
Structure and activity of COVID-19 mRNA vaccines
Pfizer-BioNTech and Moderna vaccines used a novel mRNA-based platform, each encoding for distinctly modified SARS-CoV-2 spike protein (Table II).8,9 Lipid nanoparticles (LNPs) were used as vehicles to deliver the mRNA intracellularly. LNPs stabilize the vaccine, maintain its efficacy during storage, and potentially act as an immunologic adjuvant. LNPs comprise an external shell composed of (a) lipid-conjugated polyethylene glycol 2000 (PEG-2000) (Pfizer and Moderna have different lipid residues conjugated to PEG), (b) cholesterol residues, and (c) neutral lipid (1,2-distearoyl sn-glycero-3-phosphocholine). This shell prevents aggregation of LNPs and premature extracellular release of the mRNA. Inside the shell, LNPs contain cationic ionizable lipid (Pfizer-BioNTech: ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate); Moderna: proprietary SM-102) along with active mRNA. This ionizable lipid remains neutral at physiologic pH but assumes cationic charge intracellularly to facilitate mRNA trafficking to its destination. Although the precise mechanism has not been demonstrated in human studies, presumptively once injected, the LNP-mRNA complexes are rapidly endocytosed by nearby leukocytes (such as antigen-presenting cells) and then disintegrated in endosomes. The mRNA escapes into the cytosol through the endosomal membrane, a process facilitated by the ionizable lipid, and then traffics to the rough endoplasmic reticulum for translation. The subsequent MHC II–based antigenic loading results in maturation and proliferation of antigen-specific CD4+ T cells as well as activation of humoral immune responses (Fig 1). Preexisting antibodies to PEG may potentially enhance initial uptake of the vaccine and allow for more effective intracellular delivery.10, 11, 12
Table II.
Composition of mRNA-based COVID-19 vaccines
| Pfizer-BioNTech (Comirnaty) | Moderna (Spikevax) |
|---|---|
| Dose | |
| Primary and booster adult dose: 0.3 mL | Primary adult dose: 0.5 mL Booster adult dose: 0.25 mL |
| Ingredients | |
|
|
|
|
|
|
Note. Neither vaccine contains any preservatives. For both vaccines, the vial stoppers are not made of natural rubber latex.
Fig 1.
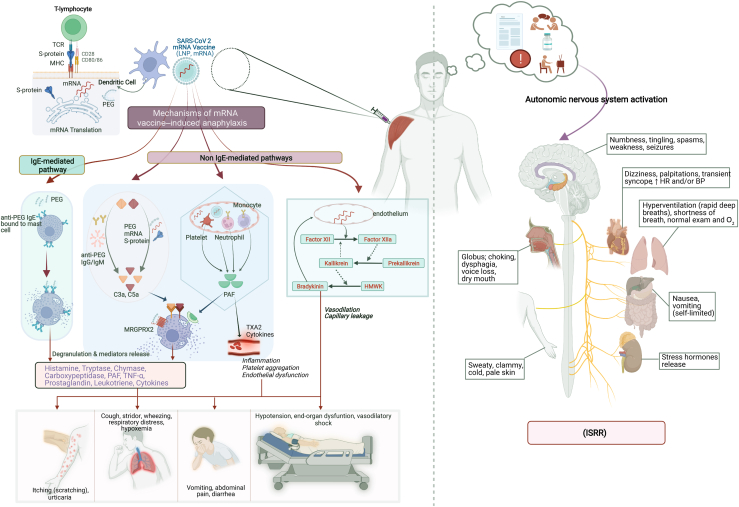
Schematic illustration of events after COVID-19 mRNA vaccination and potential mechanisms and clinical presentation of mRNA vaccine–induced anaphylaxis and Immunization Stress-Related Response (ISRR). BP, Blood pressure; HMWK, high-molecular-weight kininogen; HR, heart rate; MRGPRX2, MAS-related G-protein–coupled receptor X2; S-protein, spike protein; TCR, T-cell receptor; TXA2, thromboxane A2. Image created by Muhammad Bilal Khalid using Biorender.com.
Pathophysiology of COVID-19 mRNA vaccine-induced anaphylactic reactions (Fig 1)
The mechanisms underlying allergic reactions to the COVID-19 mRNA vaccines are poorly understood. IgE-mediated allergic reaction is a well-characterized aberrant immunologic reaction caused by allergen-induced cross-linking of specific IgE bound to the high-affinity IgE receptor on the surface of mast cells. This cross-linking results in release of preformed mediators (histamine, tryptase, chymase, carboxypeptidase, and TNF-α) and synthesis of new mediators (prostaglandins, leukotrienes, and cytokines) that are responsible for the clinical presentation of a classic allergic reaction.13 Some non-mRNA vaccine–associated allergic reactions have been attributed to inactive components, including gelatin, latex, egg protein, yeast, preservatives (thimerosal, aluminum, and phenol), and polysorbate-80.14, 15, 16 However, the mRNA vaccines do not contain any of these and instead contain another excipient, lipid-conjugated PEG-2000, which is not used in any other vaccine. PEG is ubiquitously present as an excipient in various medications, and health care and lifestyle products, and in the last 2 decades, it has been increasingly recognized as a cause of anaphylaxis.17,18 The molecular weight of PEG included in common products can range from 200 to 35,000 g/mol, and although the exact threshold for weight-based reactivity is not clearly known, generally lower-molecular-weight PEGs are well tolerated and most reactions are reported for moderate-sized PEGs such as PEG-3350 (used as laxative or bowel preparation) or PEG-5000 (pegaspargase and PEG-adenosine deaminase). Studies have shown that skin testing is effective in identifying IgE-mediated PEG hypersensitivity in non–vaccine-related PEG anaphylaxis as well as cross-reactivity with structurally similar polysorbates.19,20 Furthermore, anti-PEG IgE was found to be positive in 6 cases of PEG-induced anaphylaxis compared with controls.21
Given these findings, PEG was initially targeted as the culprit antigen responsible for reactions to the COVID-19 mRNA vaccines, and a few weeks after initial reports of anaphylaxis, an expert panel in the United States recommended skin testing with PEG- and polysorbate-containing medications in high-risk patients (those with history of IgE-mediated potential PEG allergy or an allergic reaction to the COVID-19 mRNA vaccines).22 At the time, this strategy was instituted to encourage vaccination in individuals with allergies unrelated to PEG or mRNA vaccines and to identify individuals at highest risk. A case report proposed IgE-mediated hypersensitivity to PEG as a cause of mRNA vaccine–induced anaphylaxis in a patient with previously unconfirmed medication allergies who developed anaphylaxis to the Pfizer-BioNTech vaccine. This patient had a positive skin prick test result to PEG-4000 (1% wt/vol concentration) that elicited an anaphylactic reaction requiring treatment with epinephrine. Of note, skin testing result to all other excipients including PEG-2000 and the Pfizer-BioNTech and AstraZeneca vaccines was negative.19 However, several subsequent studies have found PEG and polysorbate skin testing result to have a poor correlation and predictive value.23, 24, 25, 26 Refresh Tears eye drops, which was initially proposed to test for polysorbate-80 allergy, was noted to cause frequent irritant reactions in controls.25 In a study by Warren et al,27 all 17 patients with confirmed anaphylaxis to their first dose of a COVID-19 mRNA vaccine tested negative for PEG-2000 and polysorbate-80 by skin testing, and only 1 patient had a positive skin test result to the same mRNA vaccine that caused the initial reaction. Intriguingly, 100% (11 of 11) had a positive basophil activation test (BAT) result to the mRNA vaccine and 91% (10 of 11) had a positive BAT result to PEG-2000. None of these individuals had detectable anti-PEG IgE, but all had positive anti-PEG IgG, raising the possibility of a non-IgE pathway as the mechanism for these reactions.
Several case series have described individuals with known histories of PEG allergies to paclitaxel, docetaxel, pegaspargase, and other PEG- or polysorbate-containing medications who have tolerated the COVID-19 mRNA vaccines without reaction or increased propensity for anaphylaxis.28, 29, 30, 31, 32, 33 Furthermore, the fact that many individuals without known PEG sensitization have reacted to the first dose, the nonreproducibility of these reactions with subsequent dosing, and the minimal to no correlation between reactions and positive skin testing result and anti-PEG IgE, all suggest that IgE-mediated PEG allergy is an unlikely explanation for these reactions and reinforce that most individuals with PEG allergies can safely receive these vaccines.
The Moderna vaccine contains another excipient, tromethamine, which has been previously implicated in delayed-type reactions to gadolinium contrast magnetic resonance imaging and has occasionally been suspected to cause IgE-mediated reactions.34 Tromethamine is not found in the Pfizer vaccine. Rama et al35 reported a case of acute urticaria after Moderna vaccination in a patient who later had a positive skin test result to tromethamine-containing gadolinium contrast. This individual tolerated the Pfizer-BioNTech vaccine. Thus, although tromethamine may potentially explain some cases of anaphylaxis because of the Moderna vaccine only, more research is needed to establish a direct causal relationship.
In addition to IgE, mast cells can be activated by a wide range of chemical, physical, and other triggers that lead to signs and symptoms clinically indistinguishable from IgE-mediated anaphylaxis. PEG has also been implicated in these pseudoallergic or anaphylactoid reactions via a pathway known as complement activation–related pseudoallergy (CARPA). CARPA can occur following activation of any of the 3 complement pathways (classical, lectin, or alternative) and has been previously described following administration of pegylated liposomes, including pegylated doxorubicin. PEG-reactive IgM and IgG can trigger the classical pathway and generation of the anaphylatoxins C3a and C5a, which then induce mast cell degranulation. In the study by Warren et al,27 PEG-specific IgG antibodies were thought to be responsible for positive BAT assays in patients who experienced anaphylactic reactions to the COVID-19 mRNA vaccines. However, anti-PEG IgM and IgG antibodies have been observed in up to 40% of healthy people potentially because of prior exposure to PEG.11,12,36,37 It is unclear why this mechanism would trigger a severe allergic reaction in only a small subset of individuals who harbor these antibodies. In addition to PEG-containing LNPs, mRNA and expressed SARS-CoV-2 spike protein have been proposed to have the ability to trigger complement activation via the alternative and lectin pathways, respectively.11,38 Unlike IgE-mediated reactions, CARPA can cause anaphylactic reactions on first exposure, with lack of recurrence following subsequent doses, a pattern also observed in pegylated medication–induced hypersensitivity reactions.37
Platelet-activating factor (PAF) release has been increasingly recognized as a contributing mediator in anaphylaxis, and levels of PAF may correlate with reaction severity.39 PAF release occurs during mast cell or basophil degranulation, but can also be triggered by activated neutrophils, macrophages, or platelets, and has the ability to directly activate mast cells. In addition, direct activation of mast cells or basophils can occur independent of the IgE-FcεRI pathway via opioid receptors, MAS-related G-protein–coupled receptor X2, and Toll-like receptors. Finally, factor XII–induced activation of the kallikrein-kinin system can result in bradykinin production.12,39,40 More mechanistic studies are urgently needed to evaluate the involvement of all these pathways in COVID-19 mRNA vaccine–induced anaphylaxis.
Vaccine anaphylaxis epidemiology
The lifetime prevalence of anaphylaxis in the general US population is estimated to be 1.6%. Foods, medications, and stinging insect venoms are the most commonly identified triggers, whereas vaccines are a rare cause.41 A US-based vaccine safety datalink review estimated the incidence of all vaccine-triggered anaphylaxis cases to be 1.3/million doses. The influenza vaccine was the most commonly administered vaccine as well as the most common vaccine causing anaphylaxis at the rate of 1.35 to 1.83/million doses. The highest rates were estimated for the rabies vaccine at 86 cases/million doses, which was followed by the zoster vaccine at 9.6 cases/million doses, on the basis of 1 and 2 cases, respectively.42 Mortality rate due to any-cause anaphylaxis in the United States was estimated at less than 1/million population/year.43 A vaccine adverse event reporting system (VAERS) review of reports during 1990 to 2016 identified 8 deaths owing to possible vaccine-triggered anaphylactic reactions.44
COVID-19 vaccine anaphylaxis incidence and common traits
The phase 2/phase 3 clinical trial of Pfizer-BioNTech and the phase 3 trial of Moderna COVID-19 mRNA vaccines did not identify any cases of anaphylaxis. Of note, these studies excluded participants who had a history of an allergic reaction to any vaccine component.3,4 On the second day of public vaccine administration in the United Kingdom, 2 cases of anaphylaxis were confirmed, followed by 6 cases in the United States within the first 5 days of Pfizer-BioNTech vaccination outside of clinical trials.45,46 Although the unprecedented availability of 2 highly effective vaccines within a year of the pandemic onset was received publicly with gratification and relief, these early reports of anaphylaxis generated anxiety en masse, especially in individuals who had confirmed or presumed history of allergies. The result was an early outpouring of reports of adverse reactions with increasing allergist referrals and heightened vaccine avoidance and hesitancy. This was coupled with recommendations from national public health agencies and society guidelines advising against subsequent dosing in those with severe allergic reactions to the first dose or with a history of allergy to vaccine components and caution with longer (30 minutes instead of 15 minutes) on-site monitoring in those with a history of allergies or anaphylaxis in general and/or nonsevere allergic reactions to the first dose of the vaccine.
In the first 10 days (December 14-23, 2020) of Pfizer-BioNTech vaccine administration in the United States, the response team of the Centers for Disease Control and Prevention and Food and Drug Administration through passive surveillance confirmed 21 cases of anaphylaxis out of 175 reports of severe allergic reactions, with an estimated anaphylaxis rate of 11.1/million doses. The median age of these individuals was 40 years (27-60 years), and 90% were females. Median onset of symptoms from time of vaccine administration was 13 minutes (2-150 minutes); 80% had a documented history of allergies and 33% had a history of anaphylaxis.47 Similarly, early surveillance of the Moderna vaccine (December 21, 2020, to January 10, 2021) identified 10 cases of anaphylaxis out of 108 suspected cases on the basis of Brighton Collaboration criteria, with an estimated anaphylaxis rate of 2.5/million doses for the Moderna vaccine. The median age of these individuals was 47 years (31-63 years), and all were females (100%). Median onset of symptoms was 7.5 minutes (1-45 minutes); 90% had a history of allergies and 50% had a history of non–vaccine-induced anaphylaxis.48 Most of these individuals had recovered at the time of reporting, and no deaths were reported to either Moderna or Pfizer-BioNTech. In both reports, nonanaphylactic mild allergic reactions had similarly rapid onset, predominantly occurring in middle-aged females, and many had a history of previous allergies (67% Pfizer-BioNTech; 60% Moderna).
An interim analysis through January 18, 2021, estimated the anaphylaxis rate to be 4.7/million doses after the Pfizer-BioNTech vaccine and 2.5/million doses after the Moderna vaccine. The clinical characteristics were similar for both mRNA vaccines—primarily middle-aged (39-41 years) females (94%-100%). More than 75% of reactions had an onset within 15 minutes of vaccine administration. Of them, 78% had a history of allergic reactions and 32% had a history of anaphylaxis (vaccines, medications, contrast, and food). Among those who experienced anaphylaxis, 27% were admitted to the intensive care unit and 10% required intubation. At the time of reporting, more than 90% had recovered and no deaths were reported.49 A single-site prospective study identified a much higher rate of severe reactions to the mRNA vaccines—16 cases of confirmed anaphylaxis in 64,900 hospital employees (a rate of 246/million).50 A meta-analysis of 26 studies (including the prospective study19), where 41 million doses of vaccines were administered, including mRNA, adenovirus vector–based, and inactivated viral vaccines, found the overall incidence of COVID-19 vaccine–induced anaphylaxis to be 7.91/million doses when adjudicated against Brighton Collaboration criteria. Among these studies, mRNA vaccines were the most frequently administered and had the highest incidence of reported anaphylaxis, ranging from 2.86 to 246/million doses (excluding the phase 2/phase 3 Pfizer-BioNTech trial, which did not observe any anaphylaxis). No fatalities related to COVID-19 vaccine–induced anaphylaxis were identified.24 Another meta-analysis estimated pooled prevalence of anaphylaxis and calculated a rate of 5.58/million doses of mRNA vaccines overall, with a slightly higher rate for the Pfizer-BioNTech vaccine (9.31/million doses) compared with the Moderna vaccine (3.42/million doses).51 A retrospective electronic health record review of participants under an insurance plan identified 2 cases of anaphylaxis after administration of 0.6 million doses of mRNA vaccines (an anaphylaxis rate of 3.29/million doses).52
Analysis of vaccine safety datalink surveillance data through June 26, 2021, estimated the anaphylaxis rate to be 4.8/million doses for the Pfizer-BioNTech vaccine and 5.1/million doses for the Moderna vaccine.53 Another meta-analysis estimated the overall risk of anaphylaxis after authorized COVID-19 vaccinations to be 107 times compared with unvaccinated individuals, with the highest risk observed for the Janssen vaccine and the lowest risk for the Moderna vaccine.54 Finally, a review of adverse event (AE) reporting public databases in the United States (VAERS) and Europe (Eudravigilance) assessed for anaphylaxis rates after administration of 837 million doses of COVID-19 vaccines through August 2021 and calculated an overall mean anaphylaxis rate of 10.67/million doses, with the highest rate for the AstraZeneca vaccine at 19.39/million doses, followed by Pfizer-BioNTech at 10.44/million doses, Moderna at 8.58/million doses, and Janssen at 7.99/million doses. These results were noted to be comparable with the anaphylaxis rates after non–COVID-19 vaccines administered during the same time period. They also identified 9 fatalities associated with COVID-19 vaccine–induced anaphylaxis in VAERS and 43 cases in Eudravigilance. However, the study was limited by lack of individual case verification to confirm a cause-effect association.55
COVID-19 vaccine–induced anaphylaxis incidence, the VAERS review
To estimate the publicly reported rates and compare them with the currently published rates of anaphylaxis for the 3 authorized vaccines in the United States (Pfizer-BioNTech, Moderna, and Janssen), we performed a VAERS database search using terms “anaphylactic reaction, anaphylactic shock, anaphylactoid reaction, and anaphylactoid shock” for events reported between December 14, 2020, and May 26, 2022.7,56
For a total of 586 million doses administered, 2320 anaphylactic events were reported in the database, with an estimated cumulative anaphylaxis rate of 4/million doses for all COVID-19 vaccines. When segregated on the basis of vaccine type, the rate was the highest for the Janssen vaccine (8/million), followed by the Moderna vaccine (4/doses) and the Pfizer-BioNTech vaccine (3.7/million). Overall, the highest number of anaphylactic events was reported for Pfizer-BioNTech (55%; n = 1280), followed by Moderna (38%; n = 887) and Janssen (7%; n = 153).
Of the 2320 anaphylactic events, only 70 events occurred in the pediatric population (<18 years), which included 68 events to Pfizer-BioNTech, 1 to Moderna, and 1 to Janssen, with a cumulative rate of 1.3 anaphylactic events/million doses for all COVID-19 vaccines. Among adults (>18 years), 1998 events (excluding 252 reports of unknown age) were reported, with a rate of 3.8 anaphylactic events/million doses. Overall, 40- to 49-year-olds had the highest rate of reactions cumulatively to the 3 vaccines and to each individual vaccine, followed by 30- to 39-yearolds.
For all 3 vaccines combined, anaphylactic events were most commonly reported in females (79%; n = 1825), followed by males (17%; n = 393) and unknown gender (4%; n = 102). For individual vaccine-related anaphylactic events, 80% (n = 1020), 79% (n = 697), and 71% (n = 108) of those affected were female for the Pfizer-BioNTech, Moderna, and Janssen vaccination, respectively.
These events comprise unverified reports aiming to estimate what has been reported in a public database; hence, the rate may be an overestimation and is expected to decrease on verification and adjudication against standardized criteria. On the contrary, there is a potential for underestimation because of missed or unreported events. Regardless, this rate is comparable with the previously reported rates and shows that anaphylaxis or anaphylaxis-like events are highly predominant in young females and reported 3 times more commonly for adults than for children. Interestingly, the reported rate of anaphylactic reactions in VAERS is twice for the Janssen vaccine compared with either mRNA vaccine.
Lower recurrence of anaphylaxis after second dose of COVID mRNA vaccine
Emerging data suggest that individuals who experienced an allergic reaction in general and anaphylaxis in particular to the first dose of a COVID-19 mRNA vaccine had a strikingly low recurrence rate after receiving a second dose. In a multicenter study, among 159 participants who reported immediate allergic reactions (including 19 with anaphylaxis) after their first dose of an mRNA vaccine, only 20% experienced mild allergic symptoms after their second dose (none with anaphylaxis). Another prospective study included 65 patients who had an immediate allergic reaction to their first dose, including 11 with severe allergic reactions (grade 2 according to the Ring and Messmer criteria) and 6 who received epinephrine. Of these 65 individuals, 58 received a second dose, 74% of whom tolerated it without any allergic reaction. Only 2 individuals required epinephrine because of a severe allergic reaction after the second dose. These 2 were among the 6 who had also received epinephrine after the first dose.57 In a case series, 8 patients were identified to meet validated anaphylaxis criteria after their first dose, although acute tryptase levels were not elevated in any of the 5 cases in which it was measured. PEG allergy was excluded in all by negative skin testing result and/or history of PEG tolerance. After supervised second dose administration of an mRNA vaccine, 3 had no symptoms and 5 had milder allergic symptoms than they had after their first dose.26 In addition, in a case series of 4 patients with suspected systemic allergic reactions, all 4 had negative skin prick and intradermal test result to the corresponding mRNA vaccine, and 3 of the 4 received and tolerated a second dose of an mRNA vaccine.29 In a previously described retrospective study, only 7.8% developed immediate nonanaphylactic allergic symptoms to both doses of an mRNA vaccine. Two patients who had experienced anaphylaxis after the first dose of an mRNA vaccine avoided a second dose and tolerated a subsequent Janssen vaccine dose.52 Finally, a meta-analysis of 22 studies evaluated 1366 patients (predominantly middle-aged females) who suffered from a suspected or confirmed immediate allergic reaction after their first dose of an mRNA vaccine, including 78 who had a severe allergic reaction. Of these 1366 individuals, 1360 (99.84%) tolerated the second dose administered under allergist supervision, which included 13.65% who developed mild allergic symptoms. Only 6 of the 1366 patients developed anaphylaxis (absolute risk: 0.16%). Among the 78 patients with a history of a severe allergic reaction to the first dose, only 4 (4.9%) developed recurrent anaphylaxis and 15 (9.5%) experienced mild allergic reactions after their second dose.58
The data are sparse to estimate the incidence and recurrence of allergic reactions in pediatric populations, which may partly be due to the relatively recent authorization of vaccination for 5- to 17-year-olds but may also indicate relatively higher tolerance of mRNA vaccines in children.59
What we have learned about other suspected risk factors
Patients with mastocytosis are known to be at higher risk of developing anaphylaxis with certain triggers including Hymenoptera sting and as such were initially considered to be at higher risk for receiving any COVID-19 vaccine. However, recent studies have demonstrated that these patients can tolerate the mRNA vaccines with an overall risk of anaphylaxis comparable with that of the general population. Multiple studies cumulatively evaluated 371 patients with a diagnosis of mastocytosis who received any type of COVID-19 vaccine. Of these patients, 2 (0.5%) had anaphylactic reactions, 10 (3%) had mild allergic symptoms, and the rest tolerated the vaccines without an allergic reaction. Of the 267 patients who received 1 or more doses of an mRNA vaccine, only 1 (0.3%) had an anaphylactic reaction and 4 (1%) had mild allergic symptoms. Although most patients in this and other reports received premedication in the form of oral antihistamines with or without a leukotriene receptor antagonist, those who did not receive any premedication did not appear to have an increased incidence of reactions.60, 61, 62, 63, 64, 65
The predominance of the female sex among those who report anaphylactic reactions to the mRNA vaccines is striking. Although medication-induced anaphylaxis is reported more commonly in females,66 there are no other identifiable triggers of anaphylaxis with such strong female predominance as seen in COVID-19 vaccine–related allergic reactions. Animal studies have found that estradiol exposure reduces the threshold of mast cell activation and degranulation, whereas progesterone suppresses histamine release. Both estrogen and progesterone increased allergic sensitization in mice.67,68 It remains unknown whether factors such as menstrual cycle phase, gynecological disorders, or exogenous hormonal supplementation (contraceptives or hormone replacement therapy) modify the risk of COVID-19 vaccine anaphylaxis and so additional research is clearly warranted. In some studies,69,70 higher PEG antibody titers in females have also been observed, which may contribute to a greater susceptibility for allergic reactions in women if CARPA is the predominant underlying mechanism for these anaphylactic reactions.
Immunization stress–related response—An underrecognized mimic
Shortly before the onset of the COVID-19 pandemic, the World Health Organization proposed that some AEs associated with vaccination are forms of immunization stress–related responses (ISRRs), which are reactions triggered by the process of vaccination but not caused by the components of the vaccine itself. ISRRs are often more common when an individual has negative expectations about the vaccine as a result of direct or implicit conditioning from the news, social media, their social circle and community, or their own experiences. Multiple factors may have contributed to a high risk for ISRRs during the mass vaccination campaign for COVID-19, including a high level of baseline fear and anxiety from the deadly pandemic, emotional challenges due to caring for or loss of loved ones, social isolation with minimization of support systems, high attention to COVID-19–related news reports that were pervasive and in some cases intertwined with misinformation, unprecedented social media public presence, a novel vaccine platform with lack of trust despite rigorous research-supported efficacy and safety, unconventional vaccination settings with fear of pain and adverse reactions in public with unknown medical care access, initial vaccination in health care workers who were a particularly emotionally strained subgroup during the pandemic, and finally reports of anaphylactic reactions even before public vaccination was started in the United States and many other countries. All of these factors potentially triggered a chain reaction with outcomes similar to what have been noted in other immunization experiences: vaccine hesitancy with reduced vaccination uptake on an individual as well as community level.71, 72, 73
A survey-based prospective study noted a higher incidence of incomplete vaccination in those with self-reported allergic symptoms.74 A recent systemic review and meta-analysis of placebo-controlled vaccine trials found that although the incidence of AEs was higher after vaccination (systemic: 46%-61%; local: 66%-72%), it was also quite significant following placebo injections (systemic: 31%-35%; local: 11.8%-16.2%). Overall, nocebo effect (any AE triggered by placebo) accounted for 76% and 52% of systemic AEs after first and second doses, respectively.75 Takano et al76 analyzed the incidence of ISRR after mRNA vaccines in a large cohort and reported that 2.6% of patients suffered from an ISRR after their first dose and 1.8% after their second dose, whereas only 1 case of anaphylaxis was reported for the 3929 participants who completed the survey. Similar to what has been seen with mRNA vaccine–associated anaphylaxis reports, ISRRs occurred predominantly in females (72%-91%) and a significant proportion had a history of allergies. The authors noted that participants who reported strong prevaccine anxiety were twice as likely to suffer from an ISRR.
Distinguishing the clinical phenotype of anaphylaxis and its mimics
Although immediate and delayed heterogeneous cutaneous reactions are the most commonly reported hypersensitivity reactions to the mRNA vaccines, most of these reactions are not life-threatening. Anaphylaxis, on the contrary, is a rapid-onset severe allergic reaction that develops in minutes to hours and can rapidly result in shock or death if there is delay in recognition or treatment with epinephrine. Airway, breathing, or circulatory compromises are the primary concerns; however, skin and mucosa may be involved as well, usually in combination with another organ system. Typical symptoms are outlined in Fig 1 and listed in Table III. Underlying comorbidities such as uncontrolled asthma, cardiorespiratory diseases, or recent exercise or alcohol use can result in worse outcomes.13,77,78
Table III.
Presentation of immediate reactions after COVID-19 mRNA vaccines (SVaT assessment)∗
| Anaphylaxis |
Acute stress response (ISRR) |
Vasovagal reaction (ISRR) |
Vocal cord dysfunction |
|---|---|---|---|
| Onset | |||
| Rapid (5-120 min) | Before, during, or after vaccination (very rapid, <5 min) |
Variable (can be very rapid or delayed) | |
| Symptoms | |||
| Skin | |||
| Generalized itching; feeling of warmth; hives; swelling (face, extremities, or generalized) | Pale peripheral skin color; sweaty or clammy and cold extremities | None | |
| Oral | |||
| Itching or swelling (lips, mouth, tongue) | Dry mouth | None | None |
| Ocular | |||
| Eyes itching or tearing | None | None | |
| Upper respiratory | |||
| Nasal itching; congestion; runny nose; sneezing; throat itching, swelling, or tightness; voice change (hoarseness) | Feeling of choking; “globus” sensation (mass or lump in throat); difficulty swallowing | None | Globus sensation; throat tightness or closure; voice change (hoarseness, weak) |
| Lower respiratory | |||
| Shortness of breath; chest tightness; coughing (spells) | Shortness of breath; chest tightness | None | Shortness of breath (predominant); chest tightness; cough |
| Gastrointestinal | |||
| Nausea; vomiting; diarrhea; abdominal cramps | Nausea | Nausea; vomiting (self-limited) | Dysphagia; choking sensation; reflux |
| Cardiovascular | |||
| Dizziness; chest pain; palpitations; syncope (unresponsive to positioning) | Dizziness (lightheadedness); presyncope; palpitations | Dizziness, rapidly followed by transient loss of consciousness | None |
| Neurological | |||
| Apprehension; impending doom; incontinence | Fearfulness; numbness; perioral tingling; spasms of hands and feet; weakness; seizures | ±Tonic-clonic seizure | None |
| Gynecological | |||
| Uterine cramps | None | None | None |
| Signs (observed on examination) | |||
|---|---|---|---|
|
|
|
|
| Vital signs | |||
|---|---|---|---|
| Tachycardia or dysrythmia Hypotension Hypoxemia Tachypnea |
Tachycardia Hypertension or normal blood pressure Tachypnea (hyperventilation) Normal oxygen saturation |
Bradycardia (transient) Hypotension (transient) Normal breathing Normal oxygen saturation |
Tachypnea Normal heart rate, blood pressure, and oxygen saturation |
| Biomarker | |||
|---|---|---|---|
| Acute serum tryptase∗ |
None available |
||
| Treatment | |||
|---|---|---|---|
First-line
|
|
|
|
SVaT, Symptoms, signs, Vital signs and acute Tryptase.
Obtain within 15-180 min of symptom onset.
ISRR, a biopsychosocial event emanating from what may very well be a nocebo effect, likely represents a critically underrecognized proportion of the SARS-CoV-2 vaccine–induced reactions75,76 because of its ability to closely mimic symptoms of anaphylaxis and its potential for being mislabeled and mismanaged as an allergic reaction. Stress-triggered autonomic nervous system activation in ISRRs can manifest before, during, or immediately after vaccination as an acute stress response (sympathetic overdrive; fight or flight) or vasovagal syncope (parasympathetic overdrive), and both can closely mimic a severe allergic reaction. However, close monitoring can distinguish the 2 on the basis of a Symptoms and signs, Vitals and acute Tryptase assessment (see Table III and Fig 2). Factors that increase the probability of an ISRR versus anaphylaxis include high blood pressure, globus sensation, hyperventilation in the absence of other respiratory signs, numbness, tingling, other neurological symptoms such as headache or brain fog, transient dizziness with stable vital signs, isolated palpitations, pale clammy or sweaty hands and feet, absence of urticaria and angioedema (although ISRR can occur concurrently with isolated cutaneous symptoms, making the distinction difficult), and lack of classic anaphylaxis signs such as stridor, wheezing, coughing spells, vomiting, abdominal cramps, diarrhea, and hypotension. Timing of onset and lack of tryptase elevation in the presence of severe symptoms may further aid in distinguishing these reactions. Alternatively, ISRR may present with predominantly neurological symptoms, a reaction called dissociative neurological symptom reaction, which presents acutely after vaccination and is typically short-lived, with motor weakness, impaired sensations, abnormal movements, limb posturing, loss of speech or abnormal speech, and nonepileptic (psychogenic) seizures.73,79 Other conditions that can mimic an immediate allergic reaction are listed in Table IV.
Fig 2.
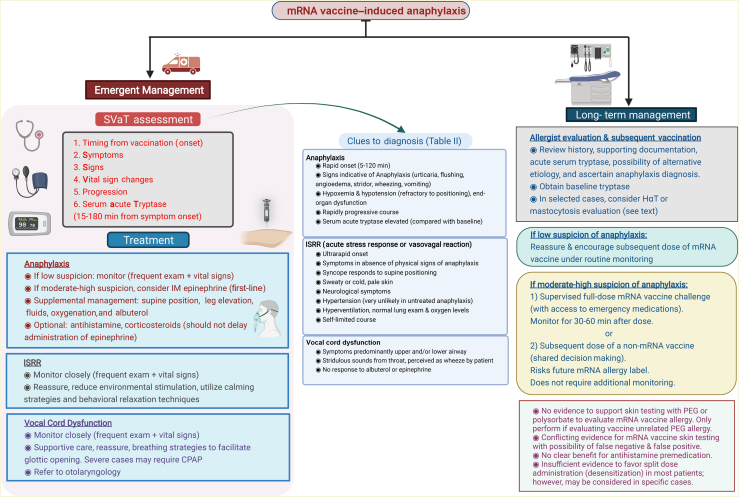
Acute evaluation and management of suspected SARS-CoV-2 mRNA vaccine–induced anaphylaxis and its mimics, and subsequent evaluation and strategies for vaccination on the basis of current evidence. CPAP, Continuous positive airway pressure ventilation; HαT, hereditary α-tryptasemia; IM, intramuscular; SVaT, Symptoms, signs, Vital signs, and acute Tryptase. Image created by Muhammad Bilal Khalid using Biorender.com.
Table IV.
Differential diagnosis of mRNA vaccine–induced allergic reactions and considerations
| Reaction | Consideration |
|---|---|
| ISRR |
Acute stress response (sympathetic overdrive) Symptomatically mimics anaphylaxis; clinical signs characteristically absent; heart rate and blood pressure may be elevated or normal Vasovagal syncope (parasympathetic overdrive) Rapid onset and recovery when placed in supine position Dissociative neurological symptom reaction Nonepileptic (psychogenic) seizures, motor weakness, impaired sensations, abnormal movements, limb posturing, loss of speech or abnormal speech |
| Vocal cord dysfunction (inducible laryngeal obstruction) | Precipitants: emotional stress, exercise, inhaled irritants, gastroesophageal reflux; because of upper and lower respiratory tract symptoms, it can closely mimic anaphylaxis or asthma exacerbation If suspected, refer to otolaryngologist |
| Asthma exacerbation | Consider if recent history indicates uncontrolled asthma or increased use of rescue inhaler |
| Solitary cutaneous reaction (acute urticaria or angioedema) | Co-occurrence with ISRR may mimic anaphylaxis, resulting in an indiscernible clinical presentation |
| Allergic reaction to an alternative trigger | Consider alternative explanations (eg, food, medication, other) if plausible alternative etiology or symptom onset from time of vaccination is >4 h |
| Chronic idiopathic urticaria with or without angioedema; hereditary angioedema | Consider breakthrough episode as a possibility, especially if unclear past diagnosis of urticaria or angioedema |
| Cardiac dysrhythmia | Consider if predominant history of palpitations during the reaction; may warrant further cardiac evaluation including EKG and cardiology referral |
| Flush syndromes (perimenopause, carcinoid syndrome) | Flushing and abdominal symptoms predominate |
EKG, Electrocardiogram.
A careful assessment by on-site or emergency staff can not only guide acute management but also assist with planning subsequent vaccination. In addition to further research, there is a need to educate and improve awareness regarding ISRRs in the health care staff involved in managing acute reactions and specialists involved directly or indirectly in vaccine administration or consultation. Furthermore, a national approach is required to counteract a potentially vicious cycle of ISRRs.
Acute management of COVID-19 vaccine–induced anaphylaxis (Fig 2 and Table III)
Premedication with antihistamine or corticosteroids
Several studies have reported prophylactic use of antihistamines before vaccination; however, the evidence has not shown a clear benefit in preventing or reducing the severity of anaphylactic reactions.23, 24, 25, 26,60, 61, 62, 63, 64,80, 81, 82, 83 Because corticosteroids can result in suppression of both innate and adaptive immune response, these should be avoided before vaccine administration, if possible.
Acute assessment, management, and documentation
Use of a focused assessment approach (Symptoms and signs, Vital signs, and acute Tryptase) in evaluating these reactions and clear documentation can not only guide appropriate management but also allow correct diagnosis of these overlapping reactions and assist with long-term guidance regarding how to advise patients with an mRNA vaccine “allergy.” The following are particularly important for providers involved in early care of these patients, such as vaccine site staff and emergency care personnel.
-
•
Observing symptom onset from time of vaccination (before, during, or within 5 minutes after vaccine administration should strongly prompt consideration of an ISRR [acute stress response or vasovagal syncope], whereas 5-120 minutes is more typical of anaphylaxis72,76,77);
-
•
Identifying symptoms more likely associated with different types of reactions;
-
•
Targeted examination to identify signs associated with allergic reactions;
-
•
Obtaining vital signs frequently, especially if symptoms are ongoing, can help with distinguishing the underlying cause; for example, hypertension is typical in acute stress responses, bradycardia, or transient hypotension, with rapid recovery on lying supine or leg elevation in vasovagal syncope, and significant hypotension refractory to position change in anaphylaxis. Similarly, hypoxemia will be seen only with anaphylaxis, and isolated tachypnea more likely in ISRRs; and
-
•
Obtaining acute serum tryptase within 15 minutes to 3 hours of symptom onset (usually peaks around 90 minutes).8 Tryptase has an excellent positive predictive value (>90%) but poor negative predictive value (<20%).84 Because of a delayed turnaround, the result will not impact acute management but, if elevated, will provide greater confidence for the diagnosis of anaphylaxis. Complement levels should not be obtained in clinical settings because these require rigorous and rapid processing, and faulty processing can lead to falsely elevated levels. BAT has unknown validity for mRNA vaccine anaphylaxis and is currently used primarily in research studies.12
Treatment should be based on the aforementioned evaluation and suspected diagnosis. The patient should be immediately placed in a supine position if hypotensive. If hypotension improves, vasovagal syncope is the likely diagnosis and requires supportive treatment. If anaphylaxis is suspected, epinephrine should be administered promptly as first-line treatment. No other treatment should delay epinephrine because delay can lead to worse outcomes. If hypotension persists, epinephrine can be repeated every 5 to 15 minutes and intravenous fluid resuscitation can be used as an adjunctive therapy. Oxygen and airway support should be provided in case of hypoxemia, and albuterol can be used as an adjunctive therapy in case of respiratory distress. Because of delayed onset, antihistamines and corticosteroids have minimal role in the management of anaphylaxis and may help only in the treatment of cutaneous symptoms or as an adjunct treatment of respiratory symptoms; however, these agents should not be used as first-line treatment for anaphylaxis.13,77,78,85 Treatment with oral antihistamines is appropriate if a nonanaphylactic cutaneous reaction such as urticaria or angioedema occurs. If suspicion is high for an acute stress response, close monitoring until symptoms improve with effective reassurance and behavioral relaxation techniques may promote a quick recovery.72,73 Vocal cord dysfunction should be suspected in a patient whose reaction manifests as an asthma exacerbation but does not improve despite albuterol inhalation. In these cases, referral to an otolaryngologist for further evaluation may be warranted.86
Chronic evaluation and management of COVID-19 vaccine–induced anaphylaxis (Fig 2)
When a patient with a possible reaction is referred to an allergist, a careful history is essential to make a correct diagnosis. Supporting documentation and an acute serum tryptase level provide helpful additional information. If suspicion for anaphylaxis is high, one should consider obtaining baseline tryptase because 5% of the White population carries a recently discovered genetic trait called hereditary α-tryptasemia, which is associated with baseline elevated tryptase levels. Additional history of severe anaphylaxis due to insect venom or drugs should prompt further evaluation for a possible mast cell disorder or hereditary α-tryptasemia.
Skin testing to excipients
After the initial reports of anaphylaxis to the mRNA vaccines with most attention focused on PEG as the culprit, Banerji et al22 recommended skin testing with medications containing PEG-3350, polysorbate-80, and polysorbate-20 in high-risk patients because of low vaccine availability. However, as more evidence accumulated, an expert panel then advised against routine excipient or mRNA vaccine skin testing because of poor sensitivity.24 Over time, multiple studies have shown that skin testing with these excipients has poor predictability. Although false positives were reported especially because of polysorbate-80–containing Refresh Tears eye drops,25 result of testing with PEG of different molecular weights, including PEG-3350 and PEG-2000, has been frequently negative in patients with a history of an mRNA vaccine allergy or positive in those who tolerated an mRNA vaccine.23,25, 26, 27,81,87,88 In some situations in which PEG testing result was positive, subsequent PEG challenges confirmed a true PEG allergy instead and these patients either avoided or were able to tolerate an mRNA vaccine. The current consensus is that PEG and polysorbate have minimal role, if any, in evaluation of mRNA vaccine allergy and should primarily be used to assess for PEG or polysorbate allergy in those who have suspicious histories for allergic reactions to other PEG-containing products such as medications or laxatives.89 It is not clear at this point whether PEG may be activating an alternative pathway, such as complement activation, or whether it has any role at all in mediating allergic reactions to the mRNA vaccines.
Skin testing to vaccines
Skin testing has been recommended in the past for evaluation of allergy to non–COVID-19 vaccines.90 However, many studies have found that although skin testing to mRNA vaccines is safe and nonirritating,91 its predictability is variable with both false-negative and false-positive results.23,27,81,82,92,93
Subsequent dose administration if history of anaphylaxis to previous dose is confirmed
Current guidelines of the Centers for Disease Control and Prevention contraindicate subsequent doses of the mRNA vaccine in patients with a severe allergic reaction or a nonsevere immediate allergic reaction to a prior dose of mRNA vaccine or those with a known history of allergy to the components of the vaccine and recommend vaccination with alternative platforms.94 However, growing evidence suggests that recurrence of reactions is much lower compared with first-dose reactions, and after appropriate evaluation, most individuals who had allergic reactions to the mRNA vaccines may be able to receive subsequent doses following shared decision making.
One of the current options include administering the full dose of an mRNA vaccine in a supervised setting in the presence of trained staff and access to emergency medications including epinephrine. Although studies have tried several different types of split dosing or desensitization (2-5 steps), it is unclear whether this approach offers any additional benefit because patients who develop subjective symptoms after partial dosing may risk incomplete vaccination.83,92,93 Split dosing can be time- and resource-consuming. It has unknown efficacy with the mRNA vaccines and may be associated with increased anticipatory anxiety without a clear superiority. Single full-dose challenge also offers diagnostic advantage, which may help with future needs for mRNA vaccines. Another option is to use an alternative vaccine platform such as the Janssen vaccine, although notably the risk of thrombosis with thrombocytopenia syndrome with this vaccine is most common in the age group that has the highest rate of allergic reactions to the mRNA vaccines.95 The Novavax protein-based vaccine was recently approved in July 2022 and may offer another reasonable alternative.96
Conclusions
mRNA vaccines have come out as powerful tools against COVID-19. Anaphylaxis to these vaccines has been reported at least 3 times more commonly compared with the cumulative risk of vaccine anaphylaxis or other commonly administered vaccines, such as the influenza vaccine. These reactions have occurred predominantly in young to middle-aged females with a history of drug allergies or anaphylaxis. This demographic was also most at risk for ISRRs, which may represent a significant proportion of “allergic” reactions to the vaccines. Indeed, a recent meta-analysis noted that nocebo effects accounted for more systemic adverse effects than the vaccine in controlled trials. Currently, the mechanism of these anaphylactic reactions is unclear, although PEG appears to have much less of a role than previously thought. Furthermore, there is no significant evidence to stratify at-risk patients, which highlights the need for additional research. Currently, a multisite study is underway that may shed more light on who may be at risk for these reactions (NCT04761822).97 Another study is aiming to assess the safety of subsequent mRNA vaccination in patients with a history of systemic allergic reaction to the first dose of an mRNA vaccine (NCT04977479).98 Hopefully, these studies will also offer more insight into the pathophysiology underlying these reactions. Reassuringly and paradoxically, preliminary studies suggest that recurrence of these reactions and incidence with subsequent dosing is significantly lower compared with the first dose.
The completion of a vaccination series in these individuals is important because waning of the immune response is known to occur with time. This can best be achieved by identifying those with nonsevere allergic reactions or nonallergic reactions including ISRR through careful evaluation, performing risk assessment, and finally proceeding with subsequent dosing with the most appropriate strategy using shared decision making. Mislabeling patients with an allergy to mRNA vaccines may not only render these patients at high risk for COVID-19 but also lead to future challenges because this platform is being used for other infectious diseases (influenza, HIV, respiratory syncytial virus, herpes, cytomegalovirus, and Zika are already undergoing early trials) as well as cancer (studies ongoing in treatment of solid tumors and melanoma in conjunction with immunotherapy). Allergists are poised to play a central role in promoting greater awareness of the potential causes for these reactions, elucidating the underlying pathophysiological mechanisms, and reducing vaccine hesitancy.
Footnotes
This study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Piret J., Boivin G. Pandemics throughout history. Front Microbiol. 2020;11:631736. doi: 10.3389/fmicb.2020.631736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC museum COVID-19 timeline. Atlanta, GA: Centers for Disease Control and Prevention; 2022. https://www.cdc.gov/museum/timeline/covid19 Available at:
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO coronavirus (COVID-19) dashboard. Geneva, Switzerland: World Health Organization; 2020. Available at: https://covid19.who.int/. Accessed May 26, 2022.
- 6.The deadliest flu: the complete story of the discovery and reconstruction of the 1918 pandemic virus. Atlanta, GA: Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/flu/pandemic-resources/reconstruction-1918-virus Available at:
- 7.COVID data tracker. Atlanta, GA: Centers for Disease Control and Prevention; 2020. https://covid.cdc.gov/covid-data-tracker/ Available at:
- 8.DailyMed. Moderna COVID-19 vaccine (cx-024414) injection, suspension. Nih.gov. Last Updated: October 17, 2022. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo. Accessed May 26, 2022.
- 9.DailyMed - PFIZER-BIONTECH COVID-19 VACCINE- bnt162b2 injection, suspension. Last Updated: December 12, 2022. Nih.gov. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo. Accessed May 26, 2022.
- 10.Ndeupen S., Qin Z., Jacobsen S., Bouteau A., Estanbouli H., Igyarto B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24:103479. doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szebeni J., Storm G., Ljubimova J.Y., Castells M., Phillips E.J., Turjeman K., et al. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat Nanotechnol. 2022;17:337–346. doi: 10.1038/s41565-022-01071-x. [DOI] [PubMed] [Google Scholar]
- 12.Risma K.A., Edwards K.M., Hummell D.S., Little F.F., Norton A.E., Stallings A., et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147:2075–2082.e2. doi: 10.1016/j.jaci.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberman P. Mechanisms of anaphylaxis beyond classically mediated antigen- and IgE-induced events. Ann Allergy Asthma Immunol. 2017;118:246–248. doi: 10.1016/j.anai.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 14.McNeil M.M., DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141:463–472. doi: 10.1016/j.jaci.2017.12.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone C.A., Jr., Rukasin C.R.F., Beachkofsky T.M., Phillips E.J. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85:2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badiu I., Geuna M., Heffler E., Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenande E., Garvey L.H. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 18.Stone C.A., Jr., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9:670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Bruusgaard-Mouritsen M.A., Jensen B.M., Poulsen L.K., Duus Johansen J., Garvey L.H. Optimizing investigation of suspected allergy to polyethylene glycols. J Allergy Clin Immunol. 2022;149:168–175.e4. doi: 10.1016/j.jaci.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z.H., Stone C.A., Jr., Jakubovic B., Phillips E.J., Sussman G., Park J., et al. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021;9:1731–1733.e3. doi: 10.1016/j.jaip.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitlick M.M., Sitek A.N., D’Netto M.E., Dages K.N., Chiarella S.E., Gonzalez-Estrada A., et al. Utility and futility of skin testing to address concerns surrounding messenger RNA coronavirus disease 2019 vaccine reactions. Ann Allergy Asthma Immunol. 2022;128:153–160. doi: 10.1016/j.anai.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenhawt M., Abrams E.M., Shaker M., Chu D.K., Khan D., Akin C., et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9:3546–3567. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfson A.R., Robinson L.B., Li L., McMahon A.E., Cogan A.S., Fu X., et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9:3308–3320.e3. doi: 10.1016/j.jaip.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krantz M.S., Bruusgaard-Mouritsen M.A., Koo G., Phillips E.J., Stone C.A., Jr., Garvey L.H. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don’t give up on the second dose! Allergy. 2021;76:2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren C.M., Snow T.T., Lee A.S., Shah M.M., Heider A., Blomkalns A., et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. 2021;4:e2125524. doi: 10.1001/jamanetworkopen.2021.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerji A., Wolfson A.R., Robinson L.B., McMahon A.E., Cogan A.S., Saff R.R., et al. COVID-19 vaccines tolerated in patients with paclitaxel and docetaxel allergy. Allergy. 2022;77:1048–1051. doi: 10.1111/all.15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo G., Anvari S., Friedman D.L., Zarnegar-Lumley S., Szafron V., Kahwash B.M., et al. mRNA COVID-19 vaccine safety in patients with previous immediate hypersensitivity to pegaspargase. J Allergy Clin Immunol Pract. 2022;10:322–325. doi: 10.1016/j.jaip.2021.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mark C., Gupta S., Punnett A., Upton J., Orkin J., Atkinson A., et al. Safety of administration of BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine in youths and young adults with a history of acute lymphoblastic leukemia and allergy to PEG-asparaginase. Pediatr Blood Cancer. 2021;68:e29295. doi: 10.1002/pbc.29295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otani I.M., Tsao L.R., Tang M. COVID-19 vaccine administration in patients with reported reactions to polyethylene glycol- and polysorbate-containing therapeutics. Ann Allergy Asthma Immunol. 2022;29:88–94.e1. doi: 10.1016/j.anai.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picard M., Drolet J.P., Masse M.S., Filion C.A., AlMuhizi F., Fein M., et al. Safety of COVID-19 vaccination in patients with polyethylene glycol allergy: a case series. J Allergy Clin Immunol Pract. 2022;10:620–625.e1. doi: 10.1016/j.jaip.2021.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brockow K., Mathes S., Fischer J., Volc S., Darsow U., Eberlein B., et al. Experience with polyethylene glycol allergy-guided risk management for COVID-19 vaccine anaphylaxis. Allergy. 2022;77:2200–2210. doi: 10.1111/all.15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukawska J., Mandaliya D., Chan A.W.E., Foggitt A., Bidder T., Harvey J., et al. Anaphylaxis to trometamol excipient in gadolinium-based contrast agents for clinical imaging. J Allergy Clin Immunol Pract. 2019;7:1086–1087. doi: 10.1016/j.jaip.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 35.Rama T.A., Coutinho R.M., Mota D., Moreira A., Cernada J. Hypersensitivity to the Moderna COVID-19 vaccine caused by tromethamine: PEG is not always the culprit excipient. J Investig Allergol Clin Immunol. 2022;32:414–415. doi: 10.18176/jiaci.0773. [DOI] [PubMed] [Google Scholar]
- 36.Kozma G.T., Shimizu T., Ishida T., Szebeni J. Anti-PEG antibodies: properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. 2020;154-155:163–175. doi: 10.1016/j.addr.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. 2014;61:163–173. doi: 10.1016/j.molimm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 38.Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruhns P., Chollet-Martin S. Mechanisms of human drug-induced anaphylaxis. J Allergy Clin Immunol. 2021;147:1133–1142. doi: 10.1016/j.jaci.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Vadas P., Gold M., Perelman B., Liss G.M., Lack G., Blyth T., et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 41.Wood R.A., Camargo C.A., Jr., Lieberman P., Sampson H.A., Schwartz L.B., Zitt M., et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:461–467. doi: 10.1016/j.jaci.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 42.McNeil M.M., Weintraub E.S., Duffy J., Sukumaran L., Jacobsen S.J., Klein N.P., et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma L., Danoff T.M., Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:1075–1083. doi: 10.1016/j.jaci.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su J.R., Moro P.L., Ng C.S., Lewis P.W., Said M.A., Cano M.V. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990-2016. J Allergy Clin Immunol. 2019;143:1465–1473. doi: 10.1016/j.jaci.2018.12.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahase E. Covid-19: people with history of significant allergic reactions should not receive Pfizer vaccine, says regulator. BMJ. 2020;371:m4780. doi: 10.1136/bmj.m4780. [DOI] [PubMed] [Google Scholar]
- 46.Clark T. Anaphylaxis following m-RNA COVID-19 vaccine receipt. In: Presentation to ACIP COVID-19 Vaccines Work Group (December 19, 2020, meeting). Atlanta, GA: Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-19/05-COVID-CLARK-508.pdf Available at:
- 47.CDC COVID-19 Response Team, Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.CDC COVID-19 Response Team, Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B., et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alhumaid S., Al Mutair A., Al Alawi Z., Rabaan A.A., Tirupathi R., Alomari M.A., et al. Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2021;17:109. doi: 10.1186/s13223-021-00613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macy E., Pandya S., Sheikh J., Burnette A., Shi J.M., Chung J., et al. Population-based incidence, severity, and risk factors associated with treated acute-onset COVID-19 mRNA vaccination-associated hypersensitivity reactions. J Allergy Clin Immunol Pract. 2022;10:827–836. doi: 10.1016/j.jaip.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sobczak M., Pawliczak R. The risk of anaphylaxis behind authorized COVID-19 vaccines: a meta-analysis. Clin Mol Allergy. 2022;20:1. doi: 10.1186/s12948-022-00167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maltezou H.C., Anastassopoulou C., Hatziantoniou S., Poland G.A., Tsakris A. Anaphylaxis rates associated with COVID-19 vaccines are comparable to those of other vaccines. Vaccine. 2022;40:183–186. doi: 10.1016/j.vaccine.2021.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hhs.gov. Vaccine adverse event reporting system (VAERS). Last reviewed December 12, 2022. Available at: https://vaers.hhs.gov/. Accessed May 26, 2022.
- 57.Kelso J.M. Misdiagnosis of systemic allergic reactions to mRNA COVID-19 vaccines. Ann Allergy Asthma Immunol. 2021;127:133–134. doi: 10.1016/j.anai.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu D.K., Abrams E.M., Golden D.B.K., Blumenthal K.G., Wolfson A.R., Stone C.A., Jr., et al. Risk of second allergic reaction to SARS-CoV-2 vaccines: a systematic review and meta-analysis. JAMA Intern Med. 2022;182:376–385. doi: 10.1001/jamainternmed.2021.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosa Duque J.S., Leung D., Au E.Y.L., Lau Y.L. Second dose of COVID-19 vaccination in immediate reactions to the first BNT162b2. Pediatr Allergy Immunol. 2022;33:e13683. doi: 10.1111/pai.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rama T.A., Moreira A., Castells M. mRNA COVID-19 vaccine is well tolerated in patients with cutaneous and systemic mastocytosis with mast cell activation symptoms and anaphylaxis. J Allergy Clin Immunol. 2021;147:877–878. doi: 10.1016/j.jaci.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaakati R., Khokhar D., Akin C. Safety of COVID-19 vaccination in patients with mastocytosis and monoclonal mast cell activation syndrome. J Allergy Clin Immunol Pract. 2021;9:3198–3199. doi: 10.1016/j.jaip.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazarinis N., Bossios A., Gulen T. COVID-19 vaccination in the setting of mastocytosis-Pfizer-BioNTech mRNA vaccine is safe and well tolerated. J Allergy Clin Immunol Pract. 2022;10:1377–1379. doi: 10.1016/j.jaip.2022.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruano-Zaragoza M., Carpio-Escalona L.V., Diaz-Beya M., Piris-Villaespesa M., Castano-Diez S., Munoz-Cano R., et al. Safety of COVID-19 vaccination in patients with clonal mast cell disorders. J Allergy Clin Immunol Pract. 2022;10:1374–1376.e3. doi: 10.1016/j.jaip.2022.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sriskandarajah P., Hobart J., Radia D.H., Whyte A.F. A UK survey examining the experience of adults with mastocytosis receiving COVID-19 vaccination. Hemasphere. 2021;5:e650. doi: 10.1097/HS9.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rama T.A., Miranda J., Silva D., Amaral L., Castro E., Coimbra A., et al. COVID-19 vaccination is safe among mast cell disorder patients, under adequate premedication. Vaccines (Basel) 2022;10:718. doi: 10.3390/vaccines10050718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eaddy Norton A., Broyles A.D. Drug allergy in children and adults: is it the double X chromosome? Ann Allergy Asthma Immunol. 2019;122:148–155. doi: 10.1016/j.anai.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Chen W., Mempel M., Schober W., Behrendt H., Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy. 2008;63:1418–1427. doi: 10.1111/j.1398-9995.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 68.Somiya M., Mine S., Yasukawa K., Ikeda S. Sex differences in the incidence of anaphylaxis to LNP-mRNA COVID-19 vaccines. Vaccine. 2021;39:3313–3314. doi: 10.1016/j.vaccine.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen B.M., Su Y.C., Chang C.J., Burnouf P.A., Chuang K.H., Chen C.H., et al. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal Chem. 2016;88:10661–10666. doi: 10.1021/acs.analchem.6b03109. [DOI] [PubMed] [Google Scholar]
- 70.Neun B.W., Barenholz Y., Szebeni J., Dobrovolskaia M.A. Understanding the role of anti-PEG antibodies in the complement activation by Doxil in vitro. Molecules. 2018;23:1700. doi: 10.3390/molecules23071700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loharikar A., Suragh T.A., MacDonald N.E., Balakrishnan M.R., Benes O., Lamprianou S., et al. Anxiety-related adverse events following immunization (AEFI): a systematic review of published clusters of illness. Vaccine. 2018;36:299–305. doi: 10.1016/j.vaccine.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gold M.S., MacDonald N.E., McMurtry C.M., Balakrishnan M.R., Heininger U., Menning L., et al. Immunization stress-related response—redefining immunization anxiety-related reaction as an adverse event following immunization. Vaccine. 2020;38:3015–3020. doi: 10.1016/j.vaccine.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 73.Immunization stress-related response: a manual for program managers and health professionals to prevent, identify and respond to stress-related responses following immunization. Geneva, Switzerland: World Health Organization; 2019. https://www.who.int/publications/i/item/9789241515948 Available at:
- 74.Robinson L.B., Landman A.B., Shenoy E.S., Hashimoto D., Fu X., Camargo C.A., Jr., et al. Allergic symptoms after mRNA COVID-19 vaccination and risk of incomplete vaccination. J Allergy Clin Immunol Pract. 2021;9:3200–3202.e1. doi: 10.1016/j.jaip.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haas J.W., Bender F.L., Ballou S., Kelley J.M., Wilhelm M., Miller F.G., et al. Frequency of adverse events in the placebo arms of COVID-19 vaccine trials: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2143955. doi: 10.1001/jamanetworkopen.2021.43955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takano T., Hirose M., Yamasaki Y., Hara M., Okada T., Kunishima H. Investigation of the incidence of immunisation stress-related response following COVID-19 vaccination in healthcare workers. J Infect Chemother. 2022;28:735–740. doi: 10.1016/j.jiac.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cardona V., Ansotegui I.J., Ebisawa M., El-Gamal Y., Fernandez Rivas M., Fineman S., et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13:100472. doi: 10.1016/j.waojou.2020.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaker M.S., Wallace D.V., Golden D.B.K., Oppenheimer J., Bernstein J.A., Campbell R.L., et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145:1082–1123. doi: 10.1016/j.jaci.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 79.Marchetti R.L., Gallucci-Neto J., Kurcgant D., Proenca I., Valiengo L., Fiore L.A., et al. Immunization stress-related responses presenting as psychogenic non-epileptic seizures following HPV vaccination in Rio Branco, Brazil. Vaccine. 2020;38:6714–6720. doi: 10.1016/j.vaccine.2020.08.044. [DOI] [PubMed] [Google Scholar]
- 80.Krantz M.S., Kwah J.H., Stone C.A., Jr., Phillips E.J., Ortega G., Banerji A., et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181:1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kohli-Pamnani A., Zapata K., Gibson T., Kwittken P.L. Coronavirus disease 2019 vaccine hypersensitivity evaluated with vaccine and excipient allergy skin testing. Ann Allergy Asthma Immunol. 2022;128:97–98. doi: 10.1016/j.anai.2021.08.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tuong L.C., Capucilli P., Staicu M., Ramsey A., Walsh E.E., Shahzad Mustafa S. Graded administration of second dose of Moderna and Pfizer-BioNTech COVID-19 mRNA vaccines in patients with hypersensitivity to first dose. Open Forum Infect Dis. 2021;8:ofab507. doi: 10.1093/ofid/ofab507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.AlMuhizi F., Ton-Leclerc S., Fein M., Tsoukas C., Garvey L.H., Lee D., et al. Successful desensitization to mRNA COVID-19 vaccine in a case series of patients with a history of anaphylaxis to the first vaccine dose. Front Allergy. 2022;3:825164. doi: 10.3389/falgy.2022.825164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buka R.J., Knibb R.C., Crossman R.J., Melchior C.L., Huissoon A.P., Hackett S., et al. Anaphylaxis and clinical utility of real-world measurement of acute serum tryptase in UK emergency departments. J Allergy Clin Immunol Pract. 2017;5:1280–1287.e2. doi: 10.1016/j.jaip.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 85.Lieberman P., Nicklas R.A., Randolph C., Oppenheimer J., Bernstein D., Bernstein J., et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015;115:341–384. doi: 10.1016/j.anai.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 86.Leong P., Al-Harrasi M., Carr B., Leahy E., Bardin P.G., Barnes S. Vocal cord dysfunction/inducible laryngeal obstruction(s) mimicking anaphylaxis during SARS-CoV-2 (COVID-19) vaccination. J Allergy Clin Immunol Pract. 2022;10:1380–1381. doi: 10.1016/j.jaip.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carpenter T., Konig J., Hochfelder J., Siegel S., Gans M. Polyethylene glycol and polysorbate testing in 12 patients before or after coronavirus disease 2019 vaccine administration. Ann Allergy Asthma Immunol. 2022;128:99–101. doi: 10.1016/j.anai.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaplan B., Farzan S., Coscia G., Rosenthal D.W., McInerney A., Jongco A.M., et al. Allergic reactions to coronavirus disease 2019 vaccines and addressing vaccine hesitancy: Northwell Health experience. Ann Allergy Asthma Immunol. 2022;128:161–168.e1. doi: 10.1016/j.anai.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Banerji A., Norton A.E., Blumenthal K.G., Stone C.A., Jr., Phillips E. Rapid progress in our understanding of Covid-19 vaccine allergy: a cause for optimism, not hesitancy. J Allergy Clin Immunol. 2022;150:12–16. doi: 10.1016/j.jaci.2022.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kelso J.M., Greenhawt M.J., Li J.T., Nicklas R.A., Bernstein D.I., Blessing-Moore J., et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 91.Marcelino J., Farinha S., Silva R., Didenko I., Proenca M., Tomaz E. Nonirritant concentrations for skin testing with SARS-CoV-2 mRNA vaccine. J Allergy Clin Immunol Pract. 2021;9:2476–2477. doi: 10.1016/j.jaip.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pienkowski M.M., Pienkowski S.M. Evaluation of anaphylaxis risk by skin testing with coronavirus disease 2019 messenger RNA vaccines on patients with anaphylaxis. Ann Allergy Asthma Immunol. 2022;128:101–103. doi: 10.1016/j.anai.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romantowski J., Kruszewski J., Solarski O., Bant A., Chcialowski A., Pietrzyk I., et al. Protocol of safe vaccination against COVID-19 in patients with high risk of allergic reactions. Clin Transl Allergy. 2022;12:e12152. doi: 10.1002/clt2.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.COVID-19 vaccines for people with allergies. Atlanta, GA: Centers for Disease Control and Prevention; 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/specific-groups/allergies.html Available at:
- 95.See I., Su J.R., Lale A., Woo E.J., Guh A.Y., Shimabukuro T.T., et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dunkle L.M., Kotloff K.L., Gay C.L., Anez G., Adelglass J.M., Barrat Hernandez A.Q., et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clinicaltrials.gov. COVID19 SARS vaccinations: systemic allergic reactions to SARS-CoV-2 vaccinations. Last updated: September 21, 2022. Available at: https://clinicaltrials.gov/ct2/show/study/NCT04761822. Accessed May 26, 2022.
- 98.Clinicaltrials.gov. The safety of administering a second dose of a COVID-19 mRNA vaccine in individuals who experienced a systemic allergic reaction to an initial dose. Last Updated: November 14, 2022. Available at: https://clinicaltrials.gov/ct2/show/study/NCT04977479. Accessed May 26, 2022.