To the Editor: We read with great interest “Subcutaneous panniculitis-like T-cell lymphoma after COVID-19 vaccination” by Kreher et al1 We wish to report a similar case of a lymphomatous infiltrate initially arising at the site of COVID-19 vaccination.
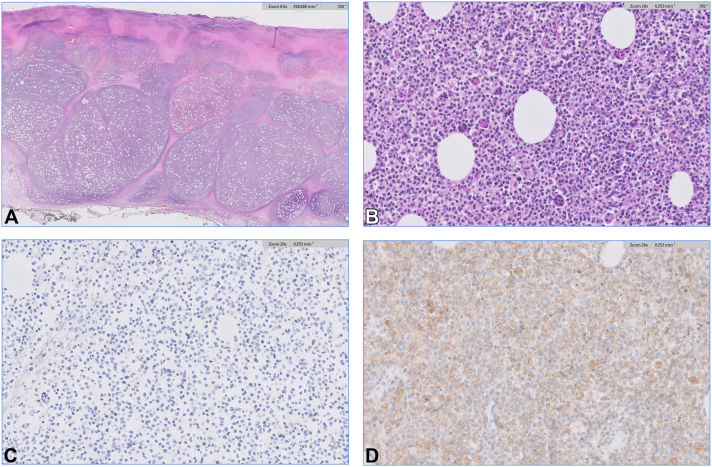
A 79-year-old male received the Moderna COVID-19 vaccine booster in the left upper arm. Three days later, he developed an ulcer at the vaccine site that progressed with surrounding erythema (Fig 1, A). He received the initial 2 doses of the vaccine 9 and 10 months prior in the same arm without complications. He was treated for cellulitis with doxycycline for 3 weeks without improvement. Due to clinical progression, he was admitted for intravenous antibiotics. Skin debridement was performed due to eschar formation. Histopathologic evaluation of the debridement specimen revealed a diffuse infiltrate involving the dermis and subcutis of atypical lymphocytes with numerous “bean-bag” cells (Fig 2). Epidermotropism and folliculotropism could not be evaluated due to extensive ulceration. Angiotropism and angiodestruction were present. Lesional cells diffusely expressed CD3 (cluster of differentiation), granzyme B, and T-cell receptor (TCR) delta. CD4, CD5, CD7, CD8, TCR BetaF1, ALK (Anaplastic lymphoma kinase), and CD30 were negative. There was weak expression by CD56 and in situ hybridization for Epstein-Barr virus was negative. TCR gene rearrangement studies were consistent with a clonal T-cell population. These findings were consistent with primary cutaneous gamma/delta T-cell lymphoma (PCGDTCL). Following debridement, the affected area on the arm improved with gradual healing and no residual lesion or masses. Clinical examination revealed no palpable lymphadenopathy. The patient reported no fever, night sweats, low appetite, or weight loss. Subsequent PET/CT (Positron emission tomographyand computed tomography) scan revealed 3 hypermetabolic subcutaneous foci in the left upper extremity near the initial ulcer without nodal hypermetabolic activity. He remained healthy without systemic symptoms or worsening skin involvement despite no additional intervention.
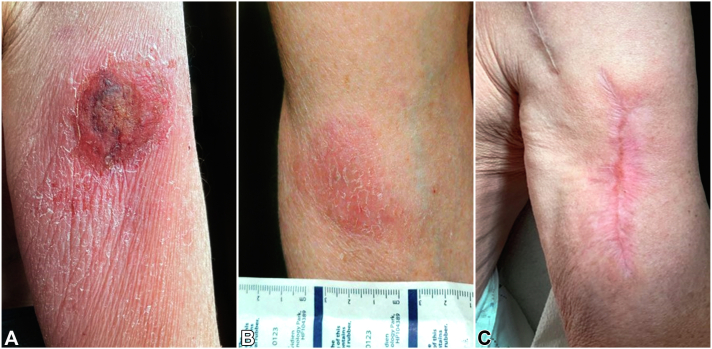
Fig 1.
A, Ulcerated plaque with surrounding erythema at the vaccine site on the left upper arm at initial presentation. B, Subtle pink patch on right upper arm 5 months following initial presentation. C, Well-healed scar on left upper arm with no palpable masses or lymphadenopathy 5 months following initial presentation.
Fig 2.
A, Histopathology reveals ulceration with a diffuse lymphoid infiltrate that replaces the dermis and subcutaneous fat. B, The lymphocytes are characterized by atypia and associated with numerous “bean bag” cells. C, The lymphocytes are negative for T-cell receptor (TCR) BetaF1 and positive for TCR Delta. (A, H&E original magnification × 5; B, H&E original magnification × 200; and C and D, original magnification × 200).
Five months following his initial presentation, dermatologic examination revealed a subtle pink patch without induration on the right upper arm (Fig 1, B); biopsy revealed a sparse perivascular but atypical lymphocytic infiltrate with a small population of lymphocytes expressing TCR delta. TCR gene rearrangement studies confirmed the presence of a clonal T-cell population identical to the original site. On the left upper arm, there was a well-healed scar without palpable masses or lymphadenopathy (Fig 1, C). The patient opted for conservative treatment given the unusually indolent nature of his condition and completed localized radiation therapy to both upper arms (39 Gy in 13 fractions). Subsequent PET/CT scan showed no evidence of residual disease. He remains well without evidence of recurrence 11 months after initial presentation.
PCGDTCL is typically an aggressive lymphoma with a median survival of 15 months.2 In rare cases, solitary lesions behave indolently.3, 4, 5, 6, 7 Our patient’s presentation is unusual as it arose at the site of a COVID-19 vaccination several days after vaccination. Despite a second distant site of involvement, he had a favorable disease course and continued to do well 11 months after initial presentation despite only receiving localized radiation therapy. Since this presentation and course are not typical of PCGDTCL, it is unclear if his lesions represent an indolent variant of PCGDTCL or other lymphoma with gamma/delta expression. It is unknown if the COVID-19 vaccine directly contributed to his presentation or disease course. One additional case report shows an atypical lymphoproliferative lesion with features mimicking lymphoma following the vaccine booster, monotypic proliferation of B-cells was found histologically.8
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: IRB exempt per our institutional requirements.
Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
Key words: COVID-19 vaccine; cutaneous lymphoma; gamma/delta T-cell lymphoma; general dermatology; injection site reaction; medical dermatology; oncology; pathology; viral vector vaccine.
References
- 1.Kreher M.A., Ahn J., Werbel T., Motaparthi K. Subcutaneous panniculitis-like T-cell lymphoma after COVID-19 vaccination. JAAD Case Rep. 2022;28:18–20. doi: 10.1016/j.jdcr.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guitart J., Weisenburger D.D., Subtil A., et al. Cutaneous γδ T-cell lymphomas: a spectrum of presentations with overlap with other cytotoxic lymphomas. Am J Surg Pathol. 2012;36:1656–1665. doi: 10.1097/PAS.0b013e31826a5038. [DOI] [PubMed] [Google Scholar]
- 3.Alberti-Violetti S., Maronese C.A., Benegoni L., Merlo V., Berti E. Primary cutaneous gamma-delta T cell lymphomas: a case series and overview of the literature. Dermatopathology. 2021;8:515–524. doi: 10.3390/dermatopathology8040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khallaayoune M., Grange F., Condamina M., Szablewski V., Guillot B., Dereure O. Primary cutaneous gamma-delta T-cell lymphoma: not an aggressive disease in all cases. Acta Derm Venereol. 2020;100 doi: 10.2340/00015555-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Dücker L., Fleischer M., Stutz N., et al. Primary cutaneous gamma-delta T-cell lymphoma with long-term indolent clinical course initially mimicking lupus eruthematosus profundus. Front Oncol. 2020;10:133. doi: 10.3389/fonc.2020.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galadari A., Ram-Wolff C., Al Hage J., et al. Cutaneous gamma delta T-Cell lymphoma with indolent evolution: a series of five cases. J Eur Acad Dermatol Venereol. 2022;36:e661–e740. doi: 10.1111/jdv.18204. [DOI] [PubMed] [Google Scholar]
- 7.Chen B.J., Wang R.C., Jhuang J.Y., et al. Primary cutaneous gamma/delta T-cell lymphoma in Taiwan: a series of six cases with frequent solitary presentation and relatively indolent behavior. J Cutan Pathol. 2022;49:350–357. doi: 10.1111/cup.14169. [DOI] [PubMed] [Google Scholar]
- 8.Patil A., Swerdlow S.H., Lossos I.S., Chapman J.R. Atypical follicular hyperplasia with light chain-restricted germinal centers after COVID-19 booster: a diagnostic pitfall. Virchows Arch. 2022;13:1–6. doi: 10.1007/s00428-022-03400-w. [DOI] [PMC free article] [PubMed] [Google Scholar]