Abstract
This report describes the kinetics of T-cell responses to a panel of mycobacterial antigens (PPD-M, PPD-A, ESAT-6, Ag85, 38kD, MPB64, MPB70, MPB83, hsp16.1, hsp65, and hsp70) following experimental infection of cattle with Mycobacterium bovis. Increased antigen-specific lymphocyte proliferation, gamma interferon, and interleukin-2 responses were observed in all calves following infection. Positive lymphocyte proliferation and cytokine responses to PPD-M and ESAT-6 were observed throughout the infection period studied. In contrast, responses to all other antigens were more variable and were not constantly present, suggesting that antigen cocktails rather than individual antigens should be used for immunodiagnosis. The detection of cytokine responses in the absence of lymphocyte proliferation, particularly during the early stages of infection, suggests a role for antigen-specific cytokine readout systems in the early identification of M. bovis infection in cattle.
The control of bovine tuberculosis in Great Britain currently relies upon a test-and-slaughter strategy in which animals infected with Mycobacterium bovis are identified using the single comparative intradermal tuberculin skin test (23). However, the incidence of bovine tuberculosis is increasing in the British national herd and the urgent need for new and improved cattle vaccines and diagnostic aids has been clearly stated in a recent independent scientific review (10). For the rational development of such reagents, it is important to assess the nature of the early immune responses that occur in cattle following infection with M. bovis.
An experimental low-dose M. bovis challenge system for cattle has been previously described which results in the development of lesions that are distributed similarly to those seen in naturally occurring bovine tuberculosis (2). This model has been used to show that following infection of cattle with M. bovis, tuberculin-induced lymphocyte proliferation, gamma interferon (IFN-γ), and interleukin-2 (IL-2) responses are generated (3, 19). An intranasal-challenge model has also been used to describe the mobilization of peripheral T cells following infection with M. bovis (20), the presence of ESAT-6-driven IFN-γ responses (21), and also lymphocyte proliferation and IFN-γ responses to MPB64 and MPB70 (15) in both experimentally and naturally infected cattle.
Great variation in the cellular response, both between individuals and over time within an individual, to mycobacterial antigens purified from M. bovis culture filtrate by gel electrophoresis has been previously described in cattle infected with M. bovis (8). Such variation has important ramifications for the evaluation of potential diagnostic reagents. We therefore set out to delineate the kinetics of T-cell (proliferation, IFN-γ, and IL-2) responses to a panel of defined mycobacterial antigens in cattle experimentally infected intratracheally with an isolate of M. bovis from Great Britain. The antigens chosen for this study were selected on the basis of their ability to induce cellular immune responses in bovine or human tuberculosis (1, 8, 14–16, 21, 25–26, 31–33, 36). Antigens included were the bovine (PPD-M) and avian (PPD-A) tuberculin preparations routinely used in the diagnosis of bovine tuberculosis, a group of proteins that are either secreted by live mycobacteria or associated with the cell wall (MPB64, MPB70, MPB83, ESAT-6, Ag85, and 38kD), and three somatic stress proteins (hsp16.1, hsp65, and hsp70).
This report describes the kinetics of cellular recognition of these individual antigens by cattle experimentally infected with M. bovis from the time of infection until gross tuberculous lesions were well developed. Results generated by this report show how antigens may be differentially recognized over the time course of infection and reinforce the need for cocktails, rather than single antigens, in diagnostic tests. Our results also support the use of rapid and sensitive cytokine readout systems as a potential method for the early diagnosis of bovine tuberculosis.
MATERIALS AND METHODS
Mycobacterial antigens.
The mycobacterial antigens used in this study are described in Table 1. Bovine (PPD-M) and avian (PPD-A) tuberculin preparations and the recombinant proteins ESAT-6, MPB70, and MPB83 were produced at the Veterinary Laboratories Agency (VLA), Weybridge, United Kingdom, as described previously (32). Recombinant MPB64 was kindly provided by D. Bakker (Animal Health Science, Boxtel, The Netherlands). Native Ag85, isolated from M. bovis BCG culture filtrate as described previously (34) and comprising Ag85A, -B and -C, was donated by K. Huygen (Instituut Pasteur, Brussels, Belgium). Recombinant hsp16.1, hsp65, and hsp70 and 38kD lipoprotein were a kind gift from M. Singh (GBF, Braunschweig, Germany).
TABLE 1.
Mycobacterial antigens used in this study
| Antigen | Characteristic(s) | Reference(s) |
|---|---|---|
| PPD-A | M. avium (environmental) tuberculin | |
| PPD-M | M. bovis tuberculin | |
| ESAT-6 | 6-kDa early secreted antigenic target | 29 |
| Ag85 | Native antigen complex, intracellular and secreted glycoprotein, fibronectin binding | 34 |
| 38 kD lipoprotein | Glycosylated lipoprotein, intracellular and secreted | 37 |
| MPB64 | Secreted protein, absent in some strains of BCG | 13 |
| MPB70 | Soluble, secreted | 17, 24 |
| MPB83 | Exported lipoprotein associated with bacterial surface | 9, 35 |
| hsp16.1 | Somatic stress protein | 30 |
| hsp65 | Somatic stress protein | 39 |
| hsp70 | Somatic stress protein | 35 |
Experimental animals.
Four female Friesian Limousin cross calves (CN1100, CN1104, CN1098, and CN1109) approximately 6 months of age were obtained from a herd free of bovine tuberculosis (i.e., with a history of negative skin test results). The calves were inoculated intratracheally in accordance with the protocol described by Buddle et al. (4) with M. bovis (AF2122/97). Two calves (CN1104 and CN1100) received 6.6 × 104 CFU, and two calves (CN1098 and CN1109) received 6.6 × 105 CFU. During the study, animals were housed in a high-security isolation unit under negative pressure and expelled air was filtered. Two weeks prior to postmortem, all animals were tested by the single comparative intradermal tuberculin skin test using avian and bovine tuberculin as stated by the European Economic Community directive (7).
Postmortem examinations and culture for M. bovis.
Euthanasia was carried out at 19 or 20 weeks postinfection by intravenous injection of sodium pentobarbitone, and a detailed postmortem was performed. Each of the following lymph nodes (or lymph node chains) was removed aseptically: right and left lateral retropharyngeal, right and left medial retropharyngeal, right and left submandibular, right and left cervical superficial, right and left bronchial, cranial mediastinal chain, caudal mediastinal chain, and representative samples of the mesenteric chain. These lymph nodes were subsequently serially sliced (approximately 2 mm) with a scalpel and inspected. Samples from individual nodes and lesions were obtained for mycobacterial culture and histological examination. The remaining superficial and visceral lymph nodes were inspected in situ. Each lung lobe was serially sliced (approximately 5 mm), and all slices were palpated and inspected. The respiratory airways were cut open as far into the lung parenchyma as possible and inspected.
Lymphocyte proliferation assay.
Peripheral blood mononuclear cells (PBMC) were separated from heparinized venous blood over Histopaque 1077 (Sigma) and resuspended in culture medium (RPMI 1640 medium with Glutamax [Gibco] supplemented with 5% CPSR [serum replacement; Gibco], nonessential amino acids [Gibco], penicillin at 100 U/ml, streptomycin at 100 μg/ml, and 5 × 10−5 M 2-mercaptoethanol [Gibco]). Cells were resuspended to 2 × 106/ml, and 0.1 ml was added per well to 96-well flat-bottom microtiter plates (Nunc). Antigens were added at 0.1 ml/well in triplicate (10-μg/ml final concentration of all antigens used in this study). The cultures were incubated for 5 days at 37°C in 5% CO2, pulsed with tritiated thymidine (37 kBq/well; Amersham, Amersham, United Kingdom), and harvested 24 h later. Incorporated radioactivity was determined as counts per minute by β-scintillation counting. Results are expressed as the change in counts per minute (mean counts per minute in the presence of antigen minus mean counts per minute in the absence of antigen) for each antigen at each time point for each animal. A positive result was taken as a mean counts per minute value of cultures stimulated with antigen that was greater than three times the mean counts per minute of unstimulated cultures and also greater than 2,000.
IFN-γ ELISA.
Duplicate cultures of peripheral whole blood diluted 1:1 in culture medium (120 μl of blood–120 μl of medium per well in 96-well flat-bottom microtiter trays) were incubated in the presence or absence of antigen (10 μg/ml) for 24 h by the method described by Emery et al. (6). Neat supernatants harvested from these cultures were assessed for IFN-γ content using a commercially available antigen capture enzyme-linked immunosorbent assay (ELISA) kit (BOVIGAM; CSL Ltd., Parkville, Victoria, Australia) and following the manufacturer's instructions. The results are expressed as the change in optical density at 450 nm (ΔOD450 [mean OD450 in the presence of antigen minus mean OD450 in the absence of antigen]) for each antigen at each time point for each animal. A positive response was taken as a mean OD450 of antigen-stimulated cell supernatants that was greater than twice the mean OD450 of unstimulated cell supernatants and also greater than 0.2.
IL-2 bioassay.
IL-2 in neat peripheral blood (1:1 with antigen as for the IFN-γ ELISA described above) 24-h culture supernatants was measured by determining the ability of each supernatant to maintain the proliferation of lymphoblasts generated by stimulation with concanavalin A (ConA) by the method of Emery et al. (6). Peripheral blood culture supernatants (50 μl) were added to the ConA blasts (104/well) in duplicate. The plates were incubated for 24 h at 37°C in 5% CO2, pulsed with tritiated thymidine, and harvested 24 h later. The specificity of this bioassay for IL-2 has been previously determined by inhibition with a monoclonal antibody specific for the alpha subunit of the IL-2 receptor CD25 (11, 18). The results are expressed as the proliferation (change in counts per minute) of IL-2-dependent ConA blast cells (i.e., the mean counts per minute of cells in the presence of antigen-stimulated supernatant minus the mean counts per minute of cells in the presence of unstimulated control supernatant). A positive result was taken as a mean counts per minute value of ConA blasts in the presence of antigen-stimulated supernatants that was greater than three times the mean counts per minute of blasts in the presence of unstimulated supernatants and also greater than 2,000.
RESULTS
Pathological findings.
Gross tuberculous lesions were observed in all four infected animals, i.e., in the lateral and medial retropharyngeal, right submandibular, and bronchial lymph nodes of CN1109; in the medial retropharyngeal, bronchial, and mediastinal lymph nodes and also in the larynx, trachea, and lungs of CN1098; in the lateral and medial retropharyngeal, submandibular, bronchial, and mediastinal lymph nodes and in the nasal mucosa, trachea, bronchi, and lungs of CN1104; and in the lateral retropharyngeal, bronchial, mediastinal, and mesenteric lymph nodes and in the lungs of CN1100. The lesions were histopathologically confirmed as tuberculous granulomas, and M. bovis was cultured from these lesions.
Antigen-specific PBMC proliferation.
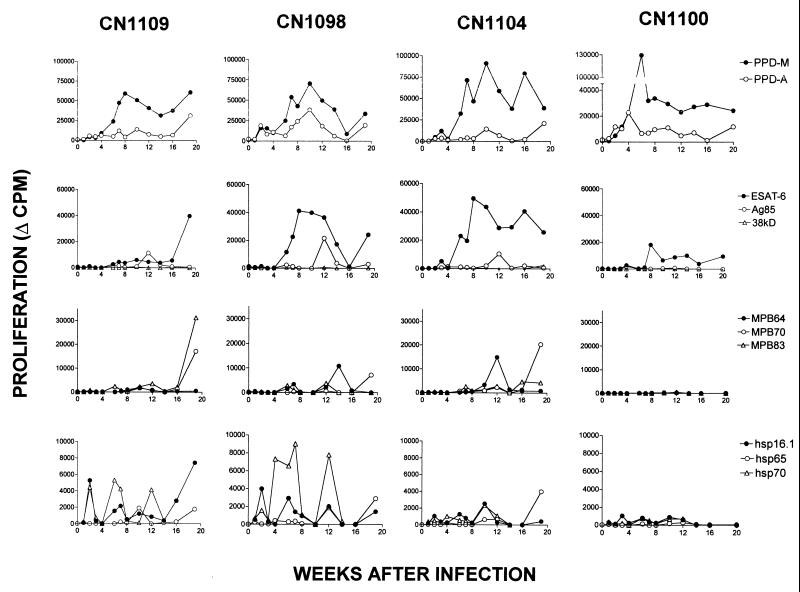
The kinetics of antigen-specific PBMC proliferation in all four animals (CN1104, CN1100, CN1098, and CN1109) is shown in Fig. 1. There were no obvious differences in the proliferative responses of these four animals that could be attributed to the dose of M. bovis received. While there were obvious differences between individual animals in their responses to the antigens, there were also similarities. First, all developed an increased response to PPD-M relative to PPD-A by 6 weeks postinfection. This M. bovis-specific response was the strongest proliferative response observed (maximum, approximately 60,000 to 130,000 cpm) and was maintained in all of the animals at higher levels relative to the avian tuberculin throughout the infection period. Second, responses to ESAT-6 developed as rapidly in two of the four animals and were, after PPD-M, the strongest responses to any of the other antigens in our panel (maximum, 20,000 to 50,000 cpm). A response to Ag85 was observed at only one time point (12 weeks postinfection), while positive responses to MPB64, MPB70, and MPB83 were observed at two or three time points (peaks of activity at 6 to 7, 12 to 14, and 20 weeks postinfection). Responses to these antigens were relatively weak compared to responses to ESAT-6 (approximate maxima: Ag85, 10,000 to 20,000 cpm; MPB64, 10,000 to 16,000 cpm; MPB70, 7,000 to 21,000 cpm; MPB83, 2,500 to 35,000 cpm).
FIG. 1.
Proliferative responses of PBMC of individual cattle to each antigen (10 μg/ml) following intratracheal infection with M. bovis. Results are expressed as the change in counts per minute (Δ CPM; i.e., mean counts per minute of PBMC stimulated with antigen minus mean counts per minute of unstimulated PBMC) for each time point.
The heat shock proteins, particularly hsp16.1 and hsp70, were the first antigens to stimulate a positive proliferative response 2 to 3 weeks after infection with M. bovis. However, these responses were among the lowest overall proliferative responses observed, none of them exceeding 10,000 cpm. In addition, responses to the heat shock proteins were periodic (peaks of activity at 2, 6 to 8, 10 to 14, and 20 weeks postinfection). No positive proliferative responses were observed after stimulation with 38kD lipoprotein. One animal (CN1100) displayed a very restricted recognition of antigens compared to the other three, responding only to PPD-M and ESAT-6.
Antigen-specific cytokine production. (i) IFN-γ.
Figure 2 shows the antigen-specific IFN-γ responses of all four animals (CN1104, CN1100, CN1098, and CN1109). IFN-γ responses to bovine tuberculin were detected between 2 and 7 weeks after infection with M. bovis. Similar to proliferation, the IFN-γ response to PPD-M was thereafter maintained above that of the response to PPD-A. An IFN-γ response to ESAT-6 was observed in all of the animals almost concurrently with the IFN-γ response to PPD-M, and the strengths of these responses were very similar (maximum OD450: PPD-M, 2.4 to 3.1; ESAT-6, 1.8 to 3.1). IFN-γ responses were also observed in the absence of and before the onset of proliferation. This was particularly noticeable in animal CN1109, in which proliferative responses were relatively low or absent before 12 weeks postinfection, yet strong IFN-γ responses to all of the antigens tested were observed during this period, from as early as 4 weeks postinfection. Similarly, IFN-γ responses were observed in the absence of proliferation in CN1100 to Ag85 and hsp70; in CN1104 to Ag85, MPB70, and MPB83; and in CN1098 to Ag85 and MPB70.
FIG. 2.
IFN-γ responses of whole-blood cultures of individual cattle following intratracheal infection with M. bovis. Results are expressed as the change in OD450 (ΔO.D.450nm; i.e., mean OD450 of culture supernatants following stimulation with antigen [10 μg/ml] minus mean OD450 of unstimulated culture supernatants) for each time point.
(ii) IL-2.
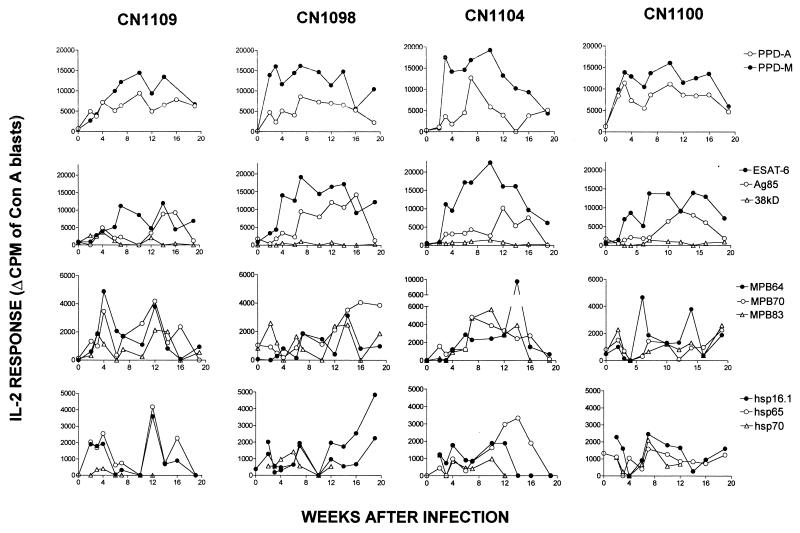
Figure 3 shows the antigen-specific IL-2 responses of all four animals (CN1104, CN1100, CN1098, and CN1109). PPD-M, PPD-A, and ESAT-6 elicited positive IL-2 responses between 2 and 6 weeks postinfection. These antigens, together with Ag85, induced the strongest IL-2 responses observed (maximum, 9,000 to 20,000 cpm). The IL-2 responses to all of the other antigens tested were relatively low (most were less than 5,000 cpm). Similar to the IFN-γ responses described above, IL-2 responses were also observed in the absence of and before the onset of proliferation, e.g., in CN1109 to Ag85, MPB64, MPB70, and hsp65; in CN1098 to Ag85 and MPB83; in CN1104 to Ag85, MPB83, and MPB70; and in CN1100 to Ag85 and MPB64.
FIG. 3.
IL-2 responses of whole-blood cultures of individual cattle following intratracheal infection with M. bovis. Results are expressed at each time point as the proliferation of IL-2-dependent ConA blast cells following their incubation with whole-blood supernatants (change in counts per minute [Δ CPM] equals mean counts per minute of ConA blasts in the presence of antigen [10 μg/ml]-stimulated supernatant minus mean counts per minute of ConA blasts in the presence of control unstimulated supernatant).
DISCUSSION
This report describes the specificities of T-cell responses in cattle experimentally infected with M. bovis and follows the kinetics of these responses from infection to the development of tuberculous lesions. Antigen-specific T-cell proliferation and IFN-γ and IL-2 responses to a panel of mycobacterial antigens were assessed at regular intervals throughout the infection period.
All four calves responded in broadly similar manners in that positive responses generated to bovine tuberculin and ESAT-6 were largely sustained throughout the infection period, while responses to all of the other antigens tested displayed marked periodicity within each individual animal, and positive responses to these latter antigens were not always detectable. These results are in full agreement with those of Fifis et al. (8), who showed that the cellular response of M. bovis-infected cattle to individual antigens not only varied between animals but also changed over the time course of infection. Whether this periodic nature of antigen recognition in cattle is related to the turnover of mycobacterial growth in vivo (data suggest intervals of 5 to 7 weeks between peaks of cellular activity) or due to the sequestration of reactive clones, as suggested for human tuberculosis (27), or some other form of suppression is unknown. Presumably, clones reactive to immunodominant antigens such as ESAT-6 may be present in the circulation at a higher frequency and therefore would not be so noticeably affected by sequestration.
Our experimental bovine tuberculosis data confirm ESAT-6 as a good candidate antigen for diagnosis (22). However, a recent study of naturally infected field reactors showed that ESAT-6 elicited positive proliferative responses in just 67% of the animals tested (32). Therefore, cocktails of different antigens, rather than reliance upon a single antigen, are required for confidence in the diagnosis of large numbers of cattle from diverse genetic backgrounds. This is further illustrated by the use of protein (5) and peptide (32) cocktails of several mycobacterial antigens, including ESAT-6, MPB64, MPB70, and MPB83, to differentiate between BCG-vaccinated and M. bovis-infected cattle. The use of cocktails also avoids the possibility of selecting for deletion mutants of M. bovis, i.e., those lacking that particular diagnostic antigen, such as described for strains of M. tuberculosis which do not express the 19-kDa lipoprotein (12).
In our study, proliferation generally coincided with cytokine (IFN-γ and IL-2) production; however, there were some noticeable discrepancies. For example, modest proliferation responses to Ag85 were observed only at week 12 whereas this antigen appeared to be a strong stimulus for the production of both IFN-γ and IL-2 at a number of time points both before and after week 12. Similarly, we observed cytokine responses to a number of other antigens, including hsp65, hsp70, MPB64, MPB70, and MPB83, both in the absence of proliferation and, in some cases, before the onset of proliferative responses following infection. In particular, one animal (CN1100) that had a very limited antigen-specific proliferation response (tuberculin and ESAT-6 only) nevertheless showed positive cytokine responses to other antigens (e.g., IFN-γ and IL-2 responses to Ag85 and IL-2 response to MPB64).
These results all support the measurement of antigen-specific cytokine responses as a method of early, sensitive detection of infection. How cytokine responses compare with detection by the tuberculin skin test is not known. However, the fact that cytokine tests may detect infected animals as early as 2 to 3 weeks after infection, together with the convenience of a single farm visit and test result availability within 48 h, has obvious attractions. We are aware that the final measurements in this study, of T-cell proliferation and cytokine production, were made 2 weeks after the tuberculin skin test, which may have had some boosting effect upon the cellular responses. This was most noticeable in our study in the PBMC proliferative responses of two of the four (high-dose) animals. The boosting effect of skin testing upon PPD-specific IFN-γ production in whole-blood cultures from M. bovis-infected cattle has been previously documented (28). However, while we believe that skin testing may have contributed to the responses seen immediately prior to necropsy, boosting of all responses was not apparent at this time point in all of the animals, and therefore, measurements taken at this time point may also constitute part of the natural fluctuations observed in individual-antigen recognition.
In summary, this report describes in detail the lymphocyte proliferation, IFN-γ, and IL-2 responses of M. bovis-infected cattle to a panel of defined mycobacterial antigens, from infection to the development of tuberculous lesions. This study confirms that ESAT-6 is a dominant antigen during the early stages of M. bovis infection in cattle. However, our results support the rationale for using cocktails of antigens for diagnostic assays, rather than relying on individual antigens in order to counter the variability observed in antigen recognition throughout the infection process. This study also confirms that the detection of antigen-specific cytokine production would be a useful tool for the early diagnosis and identification of M. bovis-infected cattle.
ACKNOWLEDGMENTS
We thank veterinary surgeons Derek Clifford and Neil Palmer (VLA) and also Mark Chambers and Paul Cockle (VLA) for carrying out the infection and postmortem procedures. Thanks also go to K. Huygen (Instituut Pasteur, Brussels, Belgium) and D. Bakker (Animal Health Science, Boxtel, The Netherlands) for supplying the antigens Ag85 and MPB64, respectively.
This work was funded by the Ministry of Agriculture, Fisheries and Food, United Kingdom.
REFERENCES
- 1.Boom W H, Wallis R S, Chervenak K A. Human Mycobacterium tuberculosis-reactive CD4+ T cell clones: heterogeneity in antigen recognition, cytokine production and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991;59:2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buddle B M, Aldwell F E, Pfeffer A, de Lisle G W, Corner L A. Experimental Mycobacterium bovis infection of cattle: effect of dose of M. bovis and pregnancy on immune responses and distribution of lesions. N Z Vet J. 1994;42:167–172. doi: 10.1080/00480169.1994.35814. [DOI] [PubMed] [Google Scholar]
- 3.Buddle B M, de Lisle G W, Pfeffer A, Aldwell F E. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine. 1995;13:1123–1130. doi: 10.1016/0264-410x(94)00055-r. [DOI] [PubMed] [Google Scholar]
- 4.Buddle B M, Keen D, Thomson A, Jowett G, Heslop J, deLisle G W, Stanford J L. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res Vet Sci. 1995;59:10–16. doi: 10.1016/0034-5288(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 5.Buddle B M, Parlane N A, Keen D L, Aldwell F E, Pollock J M, Lightbody K, Andersen P. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin Lab Diagn Immunol. 1999;6:1–5. doi: 10.1128/cdli.6.1.1-5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery D L, Duffy F H, Wood P R. An analysis of cellular proliferation and synthesis of lymphokines and specific antibody in vitro by leucocytes from immunized cattle. Vet Immunol Immunopathol. 1988;18:67–80. doi: 10.1016/0165-2427(88)90037-2. [DOI] [PubMed] [Google Scholar]
- 7.European Economic Community. EEC directive 80/219 EEC, ammending directive 64/432 annexe B. Off. J. 1980. L047:25–32. [Google Scholar]
- 8.Fifis T, Corner L A, Rothel J S, Wood P R. Cellular and humoral immune responses of cattle to purified Mycobacterium bovis antigens. Scand J Immunol. 1994;39:267–274. doi: 10.1111/j.1365-3083.1994.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 9.Hewinson R G, Michell S L, Russell W P, McAdam R A, Jacobs W R., Jr Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand J Immunol. 1996;43:490–499. doi: 10.1046/j.1365-3083.1996.d01-78.x. [DOI] [PubMed] [Google Scholar]
- 10.Krebs J R, Anderson R M, Clutton-Brock T, Morrison W I, Young D, Donnely C. Bovine tuberculosis in cattle and badgers. Report to the Rt. Hon. Dr. Jack Cunningham M.P. London, United Kingdom: MAFF Publications; 1997. [Google Scholar]
- 11.Kuhnle G, Collins R A, Scott J E, Keil G M. Bovine interleukins 2 and 4 expressed in recombinant bovine herpesvirus 1 are biologically active secreted glycoproteins. J Gen Virol. 1996;77:2231–2240. doi: 10.1099/0022-1317-77-9-2231. [DOI] [PubMed] [Google Scholar]
- 12.Lathigra R, Zhang Y, Hill M, Garcia M J, Jackett P S, Ivanyi J. Lack of production of the 19-kDa glycolipoprotein in certain strains of Mycobacterium tuberculosis. Res Microbiol. 1996;147:237–249. doi: 10.1016/0923-2508(96)81384-2. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Ulstrup J C, Jonassen T O, Melby K, Nagai S, Harboe M. Evidence for the absence of the MBP64 gene in some substrains of Mycobacterium bovis BCG. Infect Immun. 1993;61:1730–1734. doi: 10.1128/iai.61.5.1730-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lightbody K A, Girvin R M, Pollock D A, Mackie D P, Neill S D, Pollock J M. Recognition of a common mycobacterial T cell epitope in MPB59 of Mycobacterium bovis. Immunology. 1998;93:314–322. doi: 10.1046/j.1365-2567.1998.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lightbody K A, Girvin R M, Mackie D P, Neil S D, Pollock J M. T-cell recognition of mycobacterial proteins MPB70 and MPB64 in cattle immunised with antigen and infected with Mycobacterium bovis. Scand J Immunol. 1998;48:44–51. doi: 10.1046/j.1365-3083.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- 16.Lyashchenko K P, Pollock J M, Colangeli R, Gennaro M L. Diversity of antigen recognition by serum antibodies in experimental bovine tuberculosis. Infect Immun. 1998;66:5344–5349. doi: 10.1128/iai.66.11.5344-5349.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muira K, Nagai S, Kinomoto M, Haga S, Tokunaga T. Comparative studies with various substrains of Mycobacterium bovis BCG on the production of an antigenic protein. Infect Immun. 1983;39:540–545. doi: 10.1128/iai.39.2.540-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naessens G, Selighem M, MacHugh N, Park Y H, Davis W C, Toye P. Selection of BoCD25 monoclonal antibodies by screening mouse L cells transfected with the bovine P55-interleukin 2 (IL-2) receptor gene. Immunology. 1992;76:305–309. [PMC free article] [PubMed] [Google Scholar]
- 19.Ng K H, Aldwell F E, Wedlock N, Watson J D, Buddle B M. Antigen-induced interferon-γ and interleukin-2 responses of cattle inoculated with Mycobacterium bovis. Vet Immunol Immunopathol. 1997;57:59–68. doi: 10.1016/s0165-2427(96)05760-1. [DOI] [PubMed] [Google Scholar]
- 20.Pollock J M, Pollock D A, Campbell D G, Girvin R M, Crockard A D. Dynamic changes in circulating and antigen-responsive T-cell sub-populations post-Mycobacterium bovis infection in cattle. Immunology. 1996;87:236–241. doi: 10.1046/j.1365-2567.1996.457538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollock J M, Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J Infect Dis. 1997;175:1251–1254. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 23.Pritchard D G. A century of bovine tuberculosis 1888–1988: conquest and controversy. J Comp Pathol. 1988;15:651–663. doi: 10.1016/0021-9975(88)90058-8. [DOI] [PubMed] [Google Scholar]
- 24.Radford A J, Wood P R, Billman-Jacobe H, Geysen H M, Mason T J, Tribbick G. Epitope mapping of the Mycobacterium bovis secretory protein MPB70 using overlapping peptide analysis. J Gen Microbiol. 1990;136:265–272. doi: 10.1099/00221287-136-2-265. [DOI] [PubMed] [Google Scholar]
- 25.Roche P W, Peake P W, Billman-Jocobe H, Doran T, Britton W J. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319–5326. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roche P W, Feng C G, Britton W J. Human T cell epitopes on the Mycobacterium tuberculosis secreted protein MPT64. Scand J Immunol. 1996;43:662–670. doi: 10.1046/j.1365-3083.1996.d01-260.x. [DOI] [PubMed] [Google Scholar]
- 27.Rook G A, Carswell J W, Stanford J L. Preliminary evidence for the trapping of antigen-specific lymphocytes in the lymphoid tissue of “anergic” tuberculosis patients. Clin Exp Immunol. 1976;26:129–132. [PMC free article] [PubMed] [Google Scholar]
- 28.Rothel J S, Jones S L, Corner L A, Cox J C, Wood P R. The gamma-interferon assay for diagnosis of bovine tuberculosis in cattle: conditions affecting the production of gamma-interferon in whole blood culture. Aust Vet J. 1992;69:1–4. doi: 10.1111/j.1751-0813.1992.tb09848.x. [DOI] [PubMed] [Google Scholar]
- 29.Sørensen A L, Nagai S, Houen G, Andersen P, Andersen Å B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbon A, Hartskeerl R A, Schuitema A, Kolk A H J, Young D B, Lathigra R. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992;174:1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vordermeier H M, Harris D P, Frisca G, Roman E, Surcel H-M, Moreno C, Pasvol G, Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38kDa antigen in patients with active disease. Eur J Immunol. 1992;22:2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- 32.Vordermeier H M, Cockle P C, Whelan A O, Rhodes S, Palmer N, Bakker D, Hewinson R G. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin Diagn Lab Immunol. 1999;6:675–682. doi: 10.1128/cdli.6.5.675-682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiker H G, Lyashchenko K P, Aksoy A M, Lightbody K A, Pollock J M, Komissarenko S V, Bobrovnik S O, Kolesnikova I N, Mykhalsky L O, Gennaro M L, Harboe M. Immunochemical characterisation of the MPB70/80 and MPB83 proteins of Mycobacterium bovis. Infect Immun. 1998;66:1445–1452. doi: 10.1128/iai.66.4.1445-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiker H G, Sletten K, Nagai S, Harboe M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 complex. Infect Immun. 1990;58:272–274. doi: 10.1128/iai.58.1.272-274.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiker H G, Nagai S, Hewinson R G, Russell W P, Harboe M. Heterogenous expression of the related MPB70 and MPB83 proteins distinguish various substrains of Mycobacterium bovis BCG and Mycobacterium tuberculosis H37Rv. Scand J Immunol. 1996;43:374–380. doi: 10.1046/j.1365-3083.1996.d01-61.x. [DOI] [PubMed] [Google Scholar]
- 36.Wood P R, Corner L A, Rothel J S, Ripper J L, Fifis T, McCormic B S, Francis B, Melville L, Small K, DeWhittle K, Tolston J, Ryan T J, deLisle G W, Cox J C, Jones S L. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet Microbiol. 1992;31:71–79. doi: 10.1016/0378-1135(92)90142-g. [DOI] [PubMed] [Google Scholar]
- 37.Young D, Kent L, Rees A, Lamb J, Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986;54:177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young D, Lathigra R, Hendrix R, Sweetser D, Young R A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci USA. 1988;85:4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young R A, Bloom B R, Grossinsky C M, Ivanyi J, Thomas D, Davis R W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci USA. 1985;82:2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]