Background.
High rates of nonresponse to 2 doses of mRNA severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine have been reported in transplant recipients. Several studies have investigated the efficacy of a third dose in this population. However, efficacy remains unclear, as response rates vary across studies. Therefore, we conducted a systematic review and meta-analysis to determine the efficacy of a third dose of any mRNA SARS-CoV-2 vaccine in transplant recipients.
Methods.
Preferred Reporting Items for Systematic Review and Meta-Analysis reporting guidelines (PROSPERO:CRD42021281498) were followed. Medline, Embase, and CENTRAL were searched from inception to December 2, 2021, without restrictions. All full-text studies reporting on the efficacy of a third dose of any mRNA SARS-CoV-2 vaccine in pediatric and adult transplant recipients were included. The National Institutes of Health quality assessment tool for case series and the Cochrane risk of bias tool determined study quality. Meta-analysis was performed via the DerSimonian-Laird random-effect model.
Results.
Of 84 records, 12 studies totaling 1257 patients met inclusion criteria. One study was a randomized controlled trial, whereas all other studies were observational. Across 7 studies (801 patients), humoral response after 3 doses was observed in 66.1% (95% confidence interval, 62.8%-69.4%; I2 = 0%) of transplant recipients. Triple immunosuppression, mycophenolate, antiproliferatives, and belatacept use were associated with reduced odds of humoral response in studies reporting multivariate analyses. Transplant recipients receiving a third dose displayed higher levels of neutralizing antibodies to SARS-CoV-2 variants (Alpha, Beta, and Delta) compared with placebo.
Conclusions.
A third dose SARS-CoV-2 mRNA vaccine should be strongly considered in transplant recipients. Limitations included lack of controlled studies and clinically relevant thresholds to determine response to vaccination.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for coronavirus disease 2019 (COVID-19), is a zoonotic viral pathogen that has become a global health concern. Low seroconversion rates (54%) after the second dose of mRNA SARS-CoV-2 vaccine have been reported in transplant recipients,1 and severe cases of COVID-19 have been described in transplant recipients who have received 2 doses of vaccine.2 Given the high mortality rate of COVID-19 in transplant recipients,3,4 health authorities have issued recommendations to administer a third dose in this population.1 However, the efficacy of a third dose of mRNA SARS-CoV-2 vaccine in transplant recipients varies across studies,5-7 with some studies reporting humoral response after 3 doses as low as 6.4%.5 Additionally, the relative efficacy of a third dose according to transplant type, mRNA vaccine, and factors associated with immune response remains unknown. The literature is also highly reliant on humoral response and lacks other indicators that are more suggestive of immunity, specifically cellular response and neutralizing antibody assays.8 As neutralization level is highly predictive of immune protection9 and T cells reduce viral loads and disease even when neutralizing antibody levels are low,10 these outcomes are of vital importance. Finally, these vaccines have demonstrated decreased efficacy against variants of concern (VOC),11,12 which are the dominant circulating strains in the community,13 and data on vaccine efficacy with respect to VOC in transplant populations remain unknown. Therefore, a systematic review and meta-analysis is required to determine the humoral and cellular response of transplant recipients to 3 doses of mRNA SARS-CoV-2 vaccine, according to viral strain, and factors associated with immunity in this population.
MATERIALS AND METHODS
This review was reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis14 (Tables S1 and S2, SDC, http://links.lww.com/TP/C610). Our protocol was registered on PROSPERO (CRD42021281498; September 24, 2021).
Search Strategy and Study Selection
Medline, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched from inception to December 2, 2021, without language or geographic restrictions, using a search strategy developed by a librarian (R.S.) specializing in systematic searches (Table S3, SDC, http://links.lww.com/TP/C610) according to the Peer Review of Electronic Search Strategies guidelines.15
Two independent reviewers (A.J.M.B. and H.B.M.) screened all records in Rayyan software,16 with supervising author providing consensus (D.S.A.). All studies reporting on the efficacy of a third dose of any mRNA SARS-CoV-2 vaccine in pediatric and adult transplant recipients were included. Studies reporting on nontransplant recipients (eg, Car-T-cell recipients) or studies reporting other doses (ie, first or second doses) were excluded. Studies were not required to be comparative. Randomized trials, case-control studies, cohort studies, cross-sectional studies, and single-arm studies were included, whereas commentaries, editorials, conference abstracts, reviews, gray literature, preprints, and guidelines were excluded. Manual searches through reference lists of included articles were conducted to capture any other relevant literature not captured by our search strategy.
Outcomes
Our primary outcomes consisted of the humoral and cellular response of transplant recipients of any age and transplant type to a third dose of any mRNA SARS-CoV-2 vaccine. Humoral response was considered positive if antibody titers exceeded the study’s threshold. Cellular response was considered positive if the ratio of SARS-CoV-2–specific T cells to T cells, respectively, exceeded the study threshold. If a study threshold was not provided, a threshold from another study using the same T-cell population was applied. Secondary outcomes included factors associated with immunity, adverse events, and safety.
Data Extraction
Two reviewers (A.J.M.B. and H.B.M.) independently extracted data using data extraction forms for all primary and secondary outcomes, with supervising author providing consensus (D.S.A.). Extracted data included study characteristics (authors, year, study design, patient age and sex, transplant type, time since transplant, vaccine type received, time since second dose, time of assessment of outcomes), details regarding humoral and cellular assays (antibody assay, measured antibody, threshold, cellular type, markers analyzed), and outcomes (prevalence of humoral and cellular response after 3 doses, prevalence of humoral and cellular response after 3 doses in patients without response to 2 doses, acute rejection, factors associated with humoral response, side effects, adverse events, and acute rejection episodes).
Risk of Bias Assessment
Two reviewers (A.J.M.B. and H.B.M.) independently assessed the risk of bias for all primary and secondary outcomes, with supervising author providing consensus (D.S.A.). For the synthesis of noncomparative data, such as humoral response in a single group, the National Institutes of Health quality assessment tool for case series was used (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, accessed October 2, 2021). For comparative data, version 2.0 of the Cochrane Risk of Bias Assessment Tool for randomized trials was applied (low risk, some concerns, or high risk of bias).17 All poor-quality studies or studies with a high risk of bias were excluded from the qualitative or quantitative synthesis. Sensitivity analysis was performed according to study quality. Funnel plots were used to assess for publication bias.
Certainty of the Evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE)18 was used to assess the certainty of the evidence for all outcomes. Although there is no formal guidance for GRADE in systematic reviews of prevalence,19 a recommended framework was applied.20 Two reviewers (A.J.M.B. and W.C.) independently assessed certainty of the evidence, with supervising author providing consensus (D.S.A.).
Statistical Analysis
Meta-analysis of the prevalence of humoral and cellular response of transplant recipients after 3 doses of mRNA SARS-CoV-2 vaccine was performed in OpenMetaAnalyst21 using the DerSimonian-Laird random-effect model.22 I2 >50% represented substantial heterogeneity,23 and heterogeneity was evaluated using the Cochrane Q-statistics with a significance level of P < 0.10.24 P <0.05 was considered as significant for all other analyses. Prespecified subgroup analyses were performed according to transplant (ie, heart, lung, kidney) and vaccine type (ie, mRNA-1273 versus BNT162b2). Subgroup analyses were also performed according to study threshold and according to correlates of protection according to SARS-CoV-2 variant (wild type, alpha, delta).25 The presence of publication bias was only assessed if >10 studies were available for each outcome.24 Solid organ transplant recipients were analyzed separately from hematopoietic stem cell transplant (HSCT) recipients.
For analysis of associations, the odds ratios (ORs) of predictive factors for humoral response could not be pooled as the multivariable logistic regression performed across studies accounted/adjusted for different covariates. Thus, associations were synthesized qualitatively.
All data are presented with 95% confidence intervals (CIs). All tests were 2-sided and statistical significance was based on the 95% CIs excluding the null.
RESULTS
Study Selection
Of 84 records, 12 studies5-7,25-33 were included encompassing 1257 patients (Figure S1, SDC, http://links.lww.com/TP/C610).34 Along with editorials and commentaries, 3 studies were excluded in the full-text phase. One study reported prevalence of humoral response in a single cohort of transplant recipients receiving 2 or 3 doses,35 another study did not adequately characterize its study populations (ie, various data such as time since transplant and immunosuppressive regimens were not reported) and did not meet threshold for quality,36 and another study applied inconsistent thresholds in their data reporting.37
Study Characteristics
Study and patient characteristics are summarized in Table 1. All patients were aged >18 y, and the range of medians/means was 54.8 to 66.6 y. In most (8/10) studies, the third dose was administered 2 mo after the second dose (range of medians/means, 21–168 d), with outcome measurement occurring 1 mo afterward (range of medians/means, 14–52 d). All studies used defined thresholds to determine humoral and cellular response, although assays and thresholds varied across studies (Table 2). Transplant types included 878 kidney (69.8%), 147 heart (11.7%), 101 liver (8.0%), 42 allogenic HSCT (aHSCT; 3.3%), 30 lung (2.4%), and 59 other transplants (4.7%), such as pancreas and combined transplants.
TABLE 1.
Characteristics of included studies
| Author (location) | Study design | Age, y (%M) | Transplant | Time since transplant (y) | Vaccine | Time since second dose | Time of assessment | Humoral assay (threshold) |
|---|---|---|---|---|---|---|---|---|
| Hall et al7(Canada) and Kumar et al27 (Canada) | RCT | 66.6 [63.3–71.4] (74%) | 29 Kidney29 Lung20 Liver18 Heart24 Other | 3.16 [1.71–6.12] | mRNA-1273 | 60 d | 30 d | Antispike-RBD IgG(≥100 U/mL) |
| Kamar et al28(France) | Single arm | 57.6 ± 17.2 (69.7%) | 76 Kidney12 Liver7 Other | 8.1 ± 1.0 | BNT162b2 | 61 ± 1 d | 30 d | Anti–SARS-CoV-2(S/Co>1.1) |
| Del Bello et al29(France) | Single arm | 54.8 ± 12.8 (65.4%) | 277 Kidney69 Liver33 Heart17 Other | 6.8 ± 8.1 | BNT162b2 | 59 d [47–67] | 30 d | Anti–SARS-CoV-2(S/Co>1.1) |
| Chavarot et al5 (France) | Single arm | 63.5 [51–72] (58%) | 62 Kidney | 4.0 [2.1–6.6] | BNT162b2 | 69.5 d [40–84] | 28 [28–33] | SARS-CoV-2 spike protein(≥50 AU/mL) |
| Peled et al30(Israel) | Single arm | 61.0 [49.8–68.0] (71%) | 96 Heart | 6.3 [3.5–13.6] | BNT162b2 | 168 ± 18 d | 18 d | Antispike-RBD IgG(>12.6 geometric titer) |
| Westhoff et al31 (Germany) | Single arm | 59.5 ± 11.6 (80%) | 10 Kidney | 5.2 ± 4.7 | mRNA-1273 | 64 ± 15 d | 14 d | SARS-CoV-2 spike and nucleocapsid protein(≥0.75 U/mL) |
| Benotmane et al6(France) | Single arm | 57.6 [49.6–66.1] (61.6%) | 159 Kidney | 5.3 [1.9–11.1] | mRNA-1273 | 51 d [48–59] | 28 d [28–32] | Antispike-RBD IgG(>50 AU/mL) |
| Redjoul et al32 (France) | Single arm | 59 [50–64] (65%) | 42 Allogenic HSCT | <1 y (22)>1 y (20) | BNT162b2 | 51 ± 22 d | 26 ± 6 d | Antispike-RBD IgG(≥4160 AU/mL) |
| Masset et al33(France) | Single arm | 63.7 ± 11.7 (63.2%) | 124 Kidney12 Other | 9.5 ± 8.1 | BNT162b2 | 50 d ± NR | 28 d [27–33] | Antispike-RBD IgG(>250 UI/L) |
| Bertrand et al34 (France) | Single arm | 63.6 ± 15.7 (60%) | 80 Kidney | 7.3 [3.4–14.1] | BNT162b2 | 67.5 d[57–70] | At least 28 d | Antispike-RBD IgG(>50 AU/mL) |
| Massa et al26 (France) | Single arm | 58 [47.1–66.1] (72.1%) | 61 Kidney | 4.5 [1.8–11.3] | BNT162b2 | 21 d | 28 d | Antispike-RBD IgG(>50 AU/mL) |
Interquartile range is presented in square brackets.
AU, Arbitrary units; HSCT, hematopoietic stem cell transplantation; IgG, immunoglobulin G; IU, international units; %M, % male; NR, not reported; RBD, receptor-binding domain; RCT, randomized controlled trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; S/Co, signal to cutoff; U, units.
TABLE 2.
Detailed description of humoral and cellular assays across studies
| Study | Cellular assay(s) | Threshold (s) | Humoral assay (s) | Antibody measuring | Threshold (s) positive |
|---|---|---|---|---|---|
| Del Bello et al29 | Not reported | Not reported | Viral Ab titer: Wantai microplate ELISA | Anti–SARS-CoV-2 antibodies | S/Co>1.1 |
| Hall et al7 andKumar et al27 | Intracellularcytokine stainingMarkers analyzed: IFN-gamma/IL-2Cells analyzed: CD4+, CD8+ cells | <0.01(absolute values were reported)a | Viral Ab titer: anti–SARS-CoV-2 spike enzyme immunoassay (Roche Elecsys) | Antispike-RBD IgG | ≥100 U/mL |
| Neutralizing antibody test:(i) SARS-CoV-2 surrogate virus neutralization test(ii) SARS-CoV-2 pseudovirus neutralization assay (Kumar et al27 only) | (i) Antispike-RBD IgG(ii) N/A | (i) ≥30%(ii) Absence of 50% neutralization with undiluted serum | |||
| Kamar et al28 | Not performed | Not reported | Viral Ab titer: Wantai microplate ELISA | Anti–SARS-Cov-2 antibodies | S/Co>1.1 |
| Redjoul et al32 | Not performed | Not reported | Viral Ab titer:Abbott Architect SARS-CoV-2 IgG Quant II assay (Abbott, Sligo, Ireland) | Antispike-RBD IgG | ≥4160 AU/mL |
| Westhoff et al31 | Intracellularcytokine stainingMarkers analyzed: IL-2/IL-4/IFN-γ/TNF-α/GrzBCell analyzed: CD4+CD154+CD127+and CD8+ CD137+ | >0% | Viral Ab titer: measure using Elecsys Anti–SARS-CoV-2-S (Roche, Mannheim, Germany) | SARS-CoV-2 spike and nucleocapsid protein | ≥0.75 U/mL |
| Neutralizing antibody test: VSVG (FLuc) pseudovirus system bearing the SARS-CoV-2 spike protein | SARS-CoV-2 spike protein | ≥0 AU | |||
| Benotmane et al6 | Not reported | Not reported | Viral Ab titer: ARCHITECT IgG IIQuant test (Abbott) | Antispike-RBD IgG | >50 AU/mL |
| Chavarot et al5 | Not reported | Not reported | Viral Ab titer: SARS-CoV-2 IgG II Quant antibody test (Abbott, USA) | SARS-CoV-2 spike protein | ≥50 AU/mL |
| Masset et al33 | Not performed | Not reported | Viral Ab titer:(i) Chemiluminescent microparticle immunoassay (Abbott Architect)(ii) Chemiluminescence immunoassay (Siemens Atellica)(iii) Electrochemiluminescence immunoassay (Roche Elecsys) | Antispike-RBD IgG | >250 IU/L |
| Peled et al30 | T lymphocytes(T-cell markers are not described) | Not reported | Viral Ab titer: in-house ELISA | Antispike-RBD IgG | >12.6 geometric titer |
| Neutralizing antibody test: GFP reporter-based VSVG backbone coated with the SARS-CoV-2 spike protein | SARS-CoV-2 spike protein | >1024 geometric titer | |||
| Bertrand et al34 | Intracellularcytokine stainingMarkers analyzed: IFN-gammaCells analyzed: CD3+ T cells | S1 > 25 SFC/106 CD3+ T cellsS2 > 40 SFC/106CD3+ T cells | Viral Ab titer: ARCHITECT IgG II Quant test (Abbott) | Antispike-RBD IgG | >50 AU/mL |
| Massa et al26 | ELISpot Path Human IFN-g (SARS-CoV-2, S1 scan+S2N+SNMO) ALP assay (MABTECH) measured IFN-gamma–secreting SARS-CoV-2–specific T cells per 106 PBMCs | None | Viral Ab titer: Abbott SARS-CoV-2 IgG assay | Antispike-RBD IgG | >50 AU/mL |
| Neutralizing antibody test: V-PLEX! SARS-CoV-2 Panel 7 (ACE2) Kit (MSD) was used to measure binding of the SARS-CoV-2 full-length Spike protein to soluble ACE2 receptor | Anti–full-length SARS-CoV-2 spike protein antibodies | Noneb |
Threshold from Westhoff et al31 was applied in meta-analysis.
Threshold from Kumar et al27 was applied in meta-analysis.
Ab, antibody; ACE2, angiotensin-converting enzyme 2; ALP, Alkaline phosphatase; AU, Arbitrary units; ELISA, enzyme-linked immunosorbent assay; GFP, Green fluorescent protein; IFN, interferon; IgG, immunoglobulin G; IL, interleukin; IU, international units; MSD, Meso scale diagnostics; N/A, not available; PBMC, peripheral blood mononuclear cells; RBD, receptor-binding domain; S1, 15-mer peptide pools spanning the sequence of SARS-CoV-2 spike protein S spanning the N-terminal; S2, 15-mer peptide pools spanning the sequence of SARS-CoV-2 spike protein S spanning the C-terminal; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFC, spot-forming cells; S/Co, signal to cutoff; TNF, tumor necrosis factor; U, units; VSVG, vesicular stomatitis virus glycoprotein.
Humoral Response
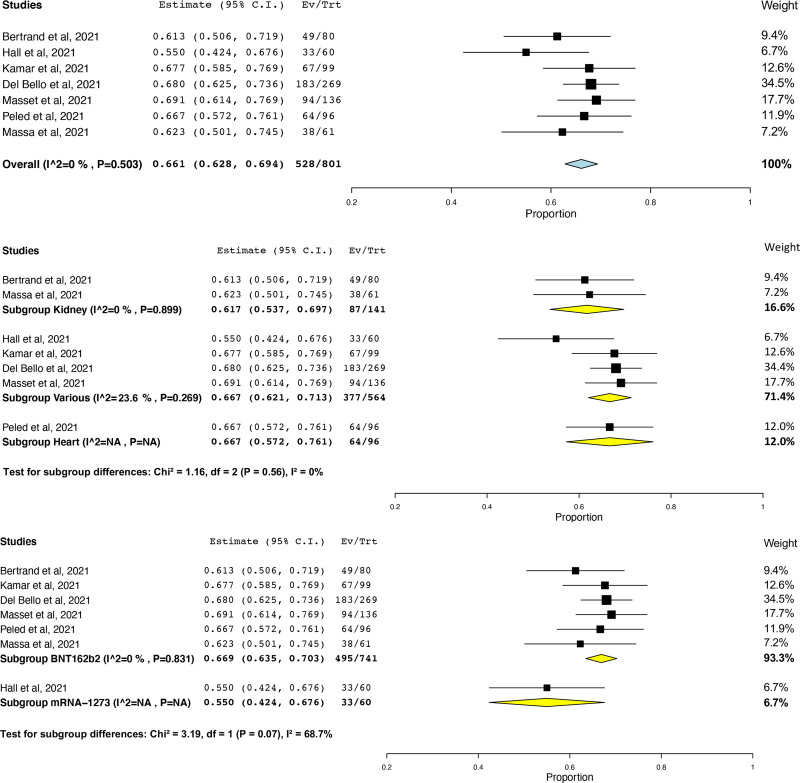
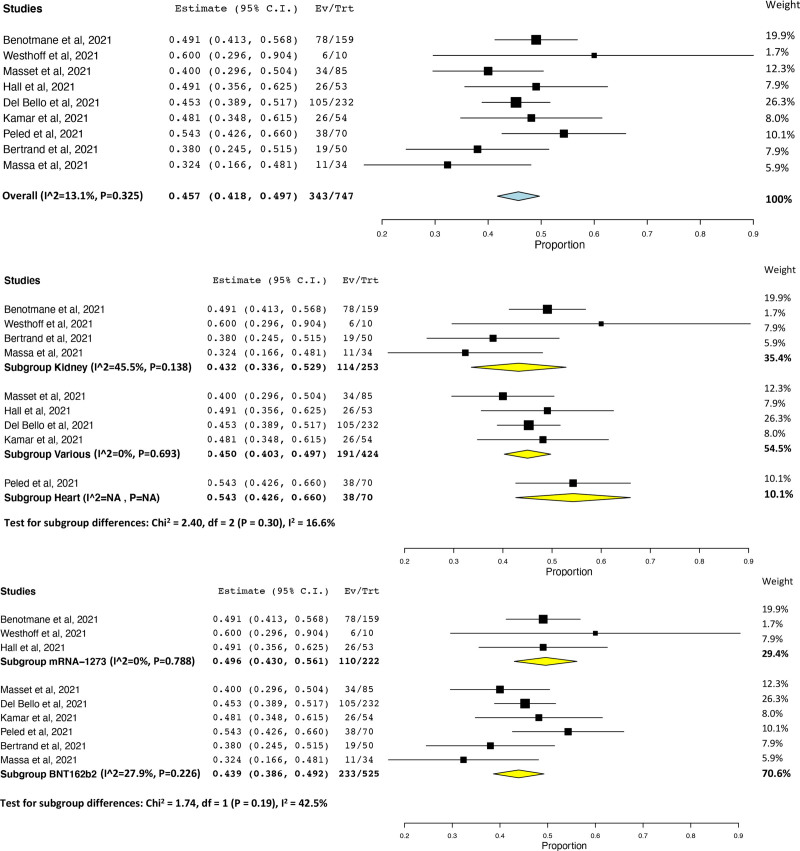
Although all studies measured response against SARS-CoV-2 spike protein, assays differed across studies—depending on the manufacturer and the kit sensitivity, the threshold cutoff varied. When the prevalence of humoral response after 3 doses was pooled, high heterogeneity (I2 = 97.6%) was observed (Figure S2, SDC, http://links.lww.com/TP/C610). This heterogeneity was found to be because of one study,5 which reported 6.4% response in a cohort of kidney transplant recipients (KTRs) receiving belatacept, and was removed as an outlier. Across 7 studies7,26,28-30,33,34 (801 patients), the prevalence of humoral response after 3 doses was 66.1% (95% CI, 62.8%-69.4%, I2 = 0%; Figure 1A), with similar rates found across studies of kidney (61.7%; 95% CI, 53.7%-69.7%; I2 = 0%)26,34 and heart (66.7%; 95% CI, 57.2%-76.1%)30 transplant recipients (test for subgroup differences, P = 0.56; Figure 1B). Prevalence of humoral response according to mRNA vaccine was higher in transplant recipients who received 3 doses of BNT162b2 (66.9%; 95% CI, 63.5%-70.3%; I2 = 0%)26,28-30,33,34 versus 3 doses of mRNA-1273 (55.0%; 95% CI, 42.4%-67.6%; I2 = N/A)7 (test for subgroup differences, P = 0.07; Figure 1C). Across 9 studies6,7,26,28-31,33,34 (747 patients), humoral response to a third dose after humoral nonresponse to 2 doses was observed in 45.7% of patients (95% CI, 41.8%-49.7%; I2 = 13.1%; Figure 2A), with similar rates found across studies of kidney (43.2%; 95% CI, 33.6%-52.9%; I2 = 45.5%)6,26,31,34 and heart (54.3%; 95% CI, 42.6%-66.0%)30 (test for subgroup differences, P = 0.30; Figure 2B). In aHSCT recipients, prevalence was 47.6% (95% CI, 32.5%-62.7%).32 In one randomized controlled trial (RCT),7 a greater number of patients in the mRNA-1273 group (55%) demonstrated humoral response compared with the placebo group (18%; risk ratio = 3.1; 95% CI, 1.7-5.8; P < 0.001). No differences in humoral response after humoral nonresponse to 2 doses were observed according to mRNA vaccine (mRNA-12736,7,31 49.6%; 95% CI, 43.0%-56.1%; I2 = 0% versus BNT162b226,28-30,32-34 43.9%; 95% CI, 38.6%-49.2%; I2 = 15.6%; test for subgroup differences, P = 0.19; Figure 2C). Notably, humoral response after 3 doses was observed in 52 of 64 patients (81.2%; 95% CI, 71.7%-90.8%) who had a weak humoral response and 26 of 95 patients (27.4%; 95% CI, 18.4%-36.3%) who had no humoral response to 2 doses.6 Prevalence of humoral response after 3 doses of any mRNA SARS-CoV-2 vaccine did not vary according to study threshold (test for subgroup differences, P = 0.43; Figure S3, SDC, http://links.lww.com/TP/C610). Prevalence of humoral response after 3 doses also did not vary according to correlates of protection for the wild type, alpha variant, and delta variant (test for subgroup difference P = 0.17; Figure S4, SDC, http://links.lww.com/TP/C610).
FIGURE 1.
Prevalence of humoral response after 3 doses of any mRNA SARS-CoV-2 vaccine in transplant recipients (A, top panel), also according to transplant type (B, middle panel), and mRNA vaccine (C, bottom panel). CI, confidence interval; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
FIGURE 2.
Prevalence of humoral response after 3 doses of any mRNA SARS-CoV-2 vaccine in transplant recipients that did not display a humoral response to 2 doses of an mRNA SARS-CoV-2 vaccine (A, top panel), also according to transplant type (B, middle panel), and mRNA vaccine (C, bottom panel). CI, confidence interval; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Cellular Response
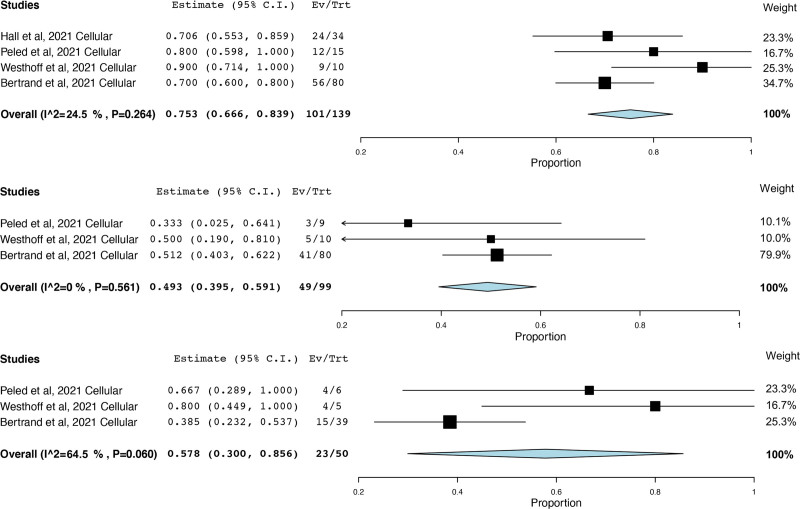
Five studies reported cellular response.7,26,30,31,34 Intracellular cytokine staining was used as a marker to measure cellular response, and markers analyzed within the studies largely varied. One study26 was not able to be included in the meta-analysis because it reported the number of SARS-CoV-2–specific T cells per peripheral blood mononuclear cells. The percentage of patients achieving cellular response after 3 doses (75.3%; 95% CI, 66.6%-83.9%; I2 = 24.5%)7,30,31,34 (Figure 3A) was significantly higher (P = 0.0001) compared with after 2 doses (49.3%; 95% CI, 39.5%-59.1%; I2 = 0%)30,31,34 (Figure 3B). Among patients with cellular nonresponse to 2 doses, 57.8% (95% CI, 30.0%-85.6%; I2 = 64.5%)30,31,34 displayed cellular response after 3 doses (Figure 3C).30,31,34 In one RCT,7 median SARS-CoV-2–specific T-cell counts after 3 doses of mRNA-1273 were greater than placebo (432 versus 67 cells per 106 CD4+ T cells, 95% CI for the between-group difference, 46–986).
FIGURE 3.
Prevalence of cellular response after 3 doses of any mRNA SARS-CoV-2 vaccine in transplant recipients (A, top panel), after 2 doses of any mRNA SARS-CoV-2 vaccine in transplant recipients (B, middle panel), and after 3 doses of any mRNA SARS-CoV-2 vaccine in transplant recipients that did not display a cellular response to 2 doses (C, bottom panel). CI, confidence interval; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Neutralizing Antibody Response
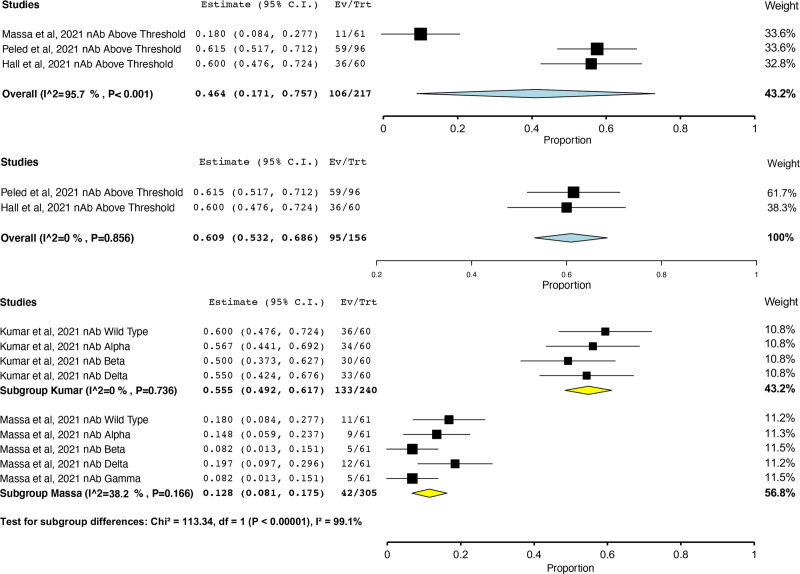
Four studies reported neutralizing antibodies.7,26,30,31 Although all studies measured response against SARS-CoV-2 spike protein, detected spike protein variants varied on the basis of the manufacturer. Furthermore, although 1 study27 used a standard enzyme-linked immunosorbent assay strategy to measure neutralizing antibody response, 2 studies30,31 used vesicular stomatitis virus glycoprotein pseudovirus expressed luciferase or Green fluorescent protein to detect neutralizing antibody responses. One study26 used a high throughput alternative to the traditional neutralization assay that identified over 8 variants of receptor-binding domain antigens as supposed to the traditional 3 variants. When the percentage of patients demonstrating a neutralizing antibody response above study threshold after 3 doses was pooled, high heterogeneity (I2 = 95.7%) was observed (Figure 4A). This heterogeneity was found to be because of one study,26 which used an alternative to the traditional neutralization assay, and was removed as an outlier. With this study removed, the percentage of patients demonstrating a neutralizing antibody response above study threshold after 3 doses was 60.9% (95% CI, 53.2%-68.6%; I2 = 0%; Figure 4B). In one RCT,7 neutralizing antibody positivity was observed in 60% of patients after 3 doses of mRNA-1273 versus 25% receiving placebo, respectively (risk ratio = 2.4; 95% CI, 1.5-4.0).
FIGURE 4.
Prevalence of neutralizing antibody response above study threshold after 3 doses of any mRNA SARS-CoV-2 vaccine in transplant recipients with outlier study (A, top panel) and without outlier study (B, middle panel) and prevalence of neutralizing antibody response above study threshold after 3 doses of any mRNA SARS-CoV-2 vaccine in transplant recipients according to SARS-CoV-2 variant and study (C, bottom panel). CI, confidence interval; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Two studies26,27 quantified the percentage of transplant recipients mounting a neutralizing antibody response to SARS-CoV-2 VOC, such as the Alpha,26,27 Beta,26,27 Delta,26,27 and Gamma26 variants. Although the prevalence across studies varied widely (I2 = 99.1%), the percentage of patients mounting a neutralizing antibody response above study threshold according to SARS-CoV-2 variant was similar within each study (Figure 4C). One RCT27 found a smaller percentage of transplant recipients displayed a neutralizing antibody response above threshold after 2 doses of mRNA-1273 to all 3 variants versus wild-type virus (differences in proportions: Alpha, 19% [95% CI, 8%-31%]; Beta, 21% [95% CI, 10%-32%]; and Delta, 16% [95% CI, 4% to 28%]). After 3 doses of mRNA-1273 vaccine, the number of transplant recipients displaying a neutralizing antibody response above threshold was greater for each variant compared with placebo (differences in proportions: Alpha variant 45% [95% CI, 28%-61%], Beta variant 38% [95% CI, 21%-55%], and Delta variant 37% (95% CI, 20%-55%] via surrogate virus neutralization test and wild-type virus 35% [95% CI, 17%-51%], Alpha variant 36% [95% CI, 17%-51%], Beta variant 31% [95% CI, 15%-46%], and Delta variant 37% [95% CI, 19%-53%] via pseudovirus neutralization assay, with P < 0.001 for all comparisons).
Factors Associated With Humoral Response
Five studies investigated factors associated with humoral response.5,29,30,32,33 These studies could not be pooled as the multivariable logistic regression performed across these studies accounted and adjusted for different covariates. In 2 multivariable analyses,29,30 mycophenolate use was associated with reduced odds of response across a cohort of kidney, liver, heart, and lung transplant recipients (OR = 0.28; 95% CI, 0.14-0.54; P < 0.001)29 and in heart transplant recipients alone (OR = 0.1; 95% CI, 0.01-0.49; P = 0.01).30 Additionally, in another multivariable analysis,29 belatacept was associated with reduced odds of humoral response across a cohort of kidney, liver, heart, and lung transplant recipients (OR = 0.14; 95% CI, 0.04-0.46; P = 0.001), and, in one study5 of 62 belatacept-treated KTRs, only 4 patients (6.4%) developed anti–SARS-CoV-2 immunoglobulin G with low antibody titers (median 209, interquartile range 20–409 AU/mL).
Across multivariable analyses, lymphocyte count <1500 mm3 (OR = 0.31; 95% CI, 0.10-0.63; P = 0.004)33 and lymphocyte count <1000 mm3 (OR = 0.20; 95% CI, 0.04-0.65; P = 0.02),26 antiproliferatives (OR = 0.06; 95% CI, 0.01-0.41; P = 0.01),26 triple immunosuppression (OR = 0.42; 95% CI, 0.21–0.86; P = 0.02),29 and higher CRP (OR = 0.88; 95% CI, 0.79-0.96; P = 0.02)30 were associated with nonhumoral response, whereas allograft function (OR = 1.03; 95% CI, 1.01-1.06; P = 0.02)33 and higher eGFR (OR = 1.03; 95% CI, 1.01-1.06; P = 0.04)30 were associated with an increased likelihood of achieving a positive antibody response in solid organ transplant recipients. Conflicting results were found with respect to age29,30 and sex.30,33 Finally, in a multivariable analysis of aHSCT recipients,32 only a B-cell count >0.25 g/L was associated with a humoral response (OR = 7.1; 95% CI, 1.5-34.1; P < 0.001).
Adverse Events
No serious (grade 3–4) adverse events or acute rejection episodes were reported across studies. Most common side effects included pain at injection site (62.4%), fatigue (18.5%), and headache (9.6%) with BNT162b226,30 and pain at injection site (76.7%), fatigue (43.3%), and myalgias (28.3%) with mRNA-1273 vaccine.7 One RCT7 found local and systematic events were more common after mRNA-1273 versus placebo.
Risk of Bias
All included noncomparative data, such as humoral response in a single group, were of good quality5-7,25-33 (Table S4, SDC, http://links.lww.com/TP/C610), and all comparative data were of low risk of bias7,27 (Table S5, SDC, http://links.lww.com/TP/C610). Funnel plot asymmetry was not detected (Figure S5, SDC, http://links.lww.com/TP/C610). When poor-quality studies36 were included in the analysis, significant heterogeneity was detected (Figure S6, SDC, http://links.lww.com/TP/C610).5-7,26-34
GRADE Assessments
Table S6 (SDC, http://links.lww.com/TP/C610) summarizes the certainty levels of all outcomes. Most outcomes had a low level (67%) or moderate level of certainty (17%). Most common reasons for decreasing certainty of the estimate included varying thresholds across studies and imprecision of the estimate.
DISCUSSION
Across solid organ and hematopoietic transplant recipients, this meta-analysis confirms 66.1% of transplant recipients overall and 45.9% of recipients without humoral response to 2 doses display a humoral response after 3 doses of mRNA SARS-CoV-2 vaccine, which did not significantly vary across transplant types. As humoral response has been shown to correlate with neutralizing antibody titers38 and a relationship between neutralization level after SARS-CoV-2 vaccination and protection against COVID-19 has been demonstrated,9 this suggests humoral response to vaccination may be clinically relevant. Given approximately half of transplant recipients do not display humoral response after 2 doses,1 a third dose of mRNA SARS-CoV-2 vaccine should be strongly considered. However, despite this, roughly a third of transplant recipients do not display humoral response after 3 doses, which suggests that this patient population remains at risk for infection.
Notably, humoral response after 3 doses was markedly attenuated with immunosuppression, as patients receiving triple immunosuppression, antiproliferatives, or belatacept had significantly reduced odds of developing a humoral response. Specifically, with belatacept use, only 6.4% of KTRs developed immunity. Thus, these patients seem to be at particular risk. Similarly, low lymphocyte counts were associated with markedly reduced response. Given the increased risk for progression to severe COVID-19 in high-risk patients who are vaccinated but are not expected to mount an adequate immune response, current guidelines recommend measures in addition to vaccination.39 These measures include primary prevention, such as masking and social distancing, and secondary prevention, such as consideration of the administration of anti–SARS-CoV-2 monoclonal antibodies upon confirmation of SARS-CoV-2 infection.39 Furthermore, in nonresponders and weak responders to 3 doses of vaccine, a fourth dose of SARS-CoV-2 vaccine may lead to an improved serological response vaccine.40
Prevalence of humoral response was found to be lower in transplant recipients after 3 doses of mRNA-1273 versus BNT162b2. However, analysis of 1647 healthcare workers demonstrated higher antibody titers in participants vaccinated with 2 doses of mRNA-1273 compared with those vaccinated with BNT162b2.41 Given only 1 study was included in the mRNA-1273 group in our analysis and other analyses did not demonstrate a difference in efficacy between the 2 vaccines, it is likely that this result will be altered by future investigation.
Prevalence of humoral response did not significantly vary according to study thresholds. Given the wide variety of thresholds reported across studies, this result may change because more investigations are completed using similar thresholds to those already present in the literature. It is important to note that correlate of protection likely exists on a spectrum42 and that higher antibody levels are needed to achieve the same level of protection after vaccination compared with unvaccinated individuals with prior infection.43 Thus, correlates of protection must be taken from vaccinated populations. Unfortunately, none of the studies in this review used thresholds that were based on clinical correlates of protection in vaccinated populations.43 Individual studies7 used thresholds that conferred 50% protective neutralization in previous clinical studies of SARS-CoV-2–infected individuals. Other studies6,32 used thresholds that correlated with in vitro neutralization of SARS-CoV-2. Therefore, the use of common, established thresholds that are proven to correlate with clinical protection in vaccinated individuals are required in future investigations of SARS-CoV-2 vaccine efficacy in transplant populations.
Prevalence of humoral response after 3 doses did not vary according to published correlates of protection for the wild type, alpha variant, and delta variant. This result suggests that mRNA SARS-CoV-2 vaccines provided transplant recipients protection from VOC, which is significant considering the higher risk of severe disease and hospitalization with certain variants, such as the Delta variant.44 Future research is needed to establish correlates for protection for other VOC to guide vaccine administration and policy.42
A third dose was associated with increased cellular and neutralizing antibody response. Specifically, the percentage of transplant recipients displaying a cellular response above study threshold after 3 doses was significantly higher compared with after 2 doses, with higher SARS-CoV-2–specific T-cell counts compared with placebo. Neutralizing antibody levels were also significantly increased after 3 doses of mRNA SARS-CoV-2 vaccine. As neutralization level is highly predictive of immune protection9 and T cells reduce viral loads and disease even when neutralizing antibody levels are low,10 these data suggest that a third dose may provide at least partial protection and reduce the likelihood of severe COVID-19 and infection in transplant recipients. Notably, the proportion of transplant recipients demonstrating a neutralizing antibody response above threshold to VOCs was higher after 3 doses compared with 2 doses, with a greater proportion of patients demonstrating a neutralizing antibody response to VOCs compared with placebo. Given neutralizing antibody positivity after 2 doses of mRNA-1273 vaccine was low for all 3 SARS-CoV-2 VOCs (Alpha, Beta, and Delta), these data suggest that a third dose may at least provide partial protection of transplant recipients to VOCs.
Across select cellular and neutralizing antibody outcomes, heterogeneity was observed. With respect to cellular response, this may be because of the use of different assays across studies. For example, the study34 reporting the lowest prevalence measured a single cytokine whereas other studies30,31 measured multiple cytokines in their cellular assays. In addition, heterogeneity was also observed with neutralizing antibody response to 3 doses. This heterogeneity was found to be because of one study,26 which only allowed for 21 d between second and third dose administration versus >60 d for the other studies.7,30 Given previous investigations have demonstrated that neutralizing antibody titers peak around 1 mo after infection45,46 and decrease significantly thereafter,45,47 high neutralizing antibody titers at the time of third dose administration (because of a short interval between second and third doses) may explain this study’s reduced neutralizing antibody titers after 3 doses. Additionally, this study22 used a commercially available high throughput alternative to the traditional neutralization assay that identified over 8 variants of receptor-binding domain antigens as supposed to the traditional 3 variants.23 These differences could largely contribute to the heterogeneity in the results observed. Thus, standardization of cellular response assays, particularly with respect to measured cytokines, and neutralizing antibody assays could be considered. Future investigation of the cellular and neutralizing antibody response is required.
Interestingly, no consensus in relation to the tests used for cellular assays, neutralizing assays, or humoral assays was observed among the studies included in our analysis. Considering the assays differed according to measured cytokines (cellular assays) and had variable cutoffs (humoral and neutralizing assays), emphasis should be made toward standardizing the tools used to measure cellular and humoral responses. Alternatively, information should be provided on how the results from 1 assay correlate with other standard assays used. Such an effort would enable direct comparison and contrast of results across studies, which would minimize heterogeneity and allow for more robust recommendations.
A paucity of literature exists regarding humoral and cellular response in other populations after 3 doses of mRNA SARS-CoV-2 vaccine. Nonetheless, when compared with existing literature on other patient populations, transplant recipients seem to have a lower prevalence of humoral response. For example, in a cohort of patients on maintenance hemodialysis,48 92.4% of patients displayed humoral response after 3 doses compared with 66.1% of transplant recipients. Additionally, similar to transplant recipients with absent or minimal humoral response to 2 doses, small case series have demonstrated that a third dose of vaccine augments humoral response in patients with rheumatoid arthritis with absent or minimal humoral response to 2 doses.49 Finally, meta-analyses50,51 on the efficacy of 2 doses in patients with immune-mediated inflammatory diseases have demonstrated an association between attenuation of humoral response and anti-CD20 (rituximab) and anticytotoxic T lymphocyte–associated antigen therapies. This attenuation of humoral response has also been noted in transplant recipients receiving antimetabolites after 2 doses of vaccine.1 As seen in our review, some immunosuppressive therapies were associated with reduced humoral response after 3 doses. Future investigation is required to determine interventions that can promote serological response in these populations, with some investigations already planned.52
Limitations of this review include (1) the lack of literature reporting on cellular response and neutralizing antibodies and (2) the various assays and thresholds used across studies. Further studies are needed for standardization of SARS-CoV-2 serology and to correlate clinical protection with humoral and cellular response in this population.53 Additionally, the inclusion of the poor-quality study resulted in significant heterogeneity. This study was judged to be of poor quality as, among other factors, this study did not report important data such as time since transplant and immunosuppressive regimens. As seen from our analyses, immunosuppressive regimens seem to greatly influence humoral response after 3 doses of vaccine. Thus, unreported factors, such as immunosuppressive regimen, may lead to the reduced humoral response reported in this poor-quality study. Finally, in this review, the duration of response and the effect of previous immunization, such as viral vector, were not able to be assessed.
In conclusion, a third dose SARS-CoV-2 mRNA vaccine should be strongly considered in transplant recipients. Future study and establishment of standardized assays and clinically relevant thresholds for humoral and cellular response are required. As this population seems to remain at risk for infection post 3 doses, investigations regarding the efficacy of future doses should be considered, and patients and healthcare providers should remain vigilant regarding exposure to infection.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
All authors met the International Committee of Journal Editors criteria for authorship. A.J.M.B., H.B.M., W.C., and D.S.A. gave substantial contributions to the conception or design of the work, the acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. R.S. and C.A.B. gave substantial contributions to the conception or design of the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This study met the definition of Institutional Review Board exempt research.
All additional data can be obtained by contacting the corresponding author.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadei HM, Gonwa TA, Leoni JC, et al. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021;21:3496–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljungman P, de la Camara R, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35:2885–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muñoz Serrano A, Arias A, Moreno-Torres V, et al. Coronavirus disease 2019 (COVID-19) in solid organ transplant recipients: a case-control study. Ann Transplant. 2021;26:e933152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavarot N, Morel A, Leruez-Ville M, et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am J Transplant. 2021;21:4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326:1063–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nixon DF, Ndhlovu LC. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;385:e7. [DOI] [PubMed] [Google Scholar]
- 9.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. [DOI] [PubMed] [Google Scholar]
- 10.Shroff RT, Chalasani P, Wei R, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27:2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenforde MW, Self WH, Naioti EA, et al. ; IVY Network Investigators; IVY Network. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults—United States, March-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callaway E. Delta coronavirus variant: scientists brace for impact. Nature. 2021;595:17–18. [DOI] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. [DOI] [PubMed] [Google Scholar]
- 16.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 19.Borges Migliavaca C, Stein C, Colpani V, et al. ; Prevalence Estimates Reviews – Systematic Review Methodology Group (PERSyst). How are systematic reviews of prevalence conducted? A methodological study. BMC Med Res Methodol. 2020;20:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. [DOI] [PubMed] [Google Scholar]
- 21.Wallace B, Dahabreh I, Trikalinos T, et al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49:1–15. [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. Cochrane; 2019. [Google Scholar]
- 25.Goldblatt D, Fiore-Gartland A, Johnson M, et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine. 2022;40:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massa F, Cremoni M, Gérard A, et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine. 2021;73:103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar D, Ferreira VH, Hall VG, et al. Neutralization of SARS-CoV-2 variants in transplant recipients after two and three doses of mRNA-1273 vaccine: secondary analysis of a randomized trial. Ann Intern Med. 2022;175:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Bello A, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 2022;22:322–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peled Y, Ram E, Lavee J, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2022;41:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westhoff TH, Seibert FS, Anft M, et al. A third vaccine dose substantially improves humoral and cellular SARS-CoV-2 immunity in renal transplant recipients with primary humoral nonresponse. Kidney Int. 2021;100:1135–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redjoul R, Le Bouter A, Parinet V, et al. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8:e681–e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masset C, Kerleau C, Garandeau C, et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021;100:1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stumpf J, Tonnus W, Paliege A, et al. Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation. 2021;105:e267–e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marlet J, Gatault P, Maakaroun Z, et al. Antibody responses after a third dose of covid-19 vaccine in kidney transplant recipients and patients treated for chronic lymphocytic leukemia. Vaccines (Basel). 2021;9:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-COV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubio-Acero R, Castelletti N, Fingerle V, et al. ; KoCo19 study team. In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized oligo-/asymptomatic patients. Infect Dis Ther. 2021;10:1505–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institutes of Health. Anti-SARS-CoV-2 monoclonal antibodies. 2022. Available at https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies. Accessed June 1, 2022.
- 40.Midtvedt K, Vaage JT, Heldal K, et al. Fourth dose of the SARS-CoV-2 vaccine in kidney transplant recipients with previously impaired humoral antibody response. Am J Transplant. 2022;22:2704–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steensels D, Pierlet N, Penders J, et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry J, Osman S, Wright J, et al. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS One. 2022;17:e0266852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei J, Pouwels KB, Stoesser N, et al. ; COVID-19 Infection Survey team. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022;28:1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Twohig KA, Nyberg T, Zaidi A, et al. ; COVID-19 Genomics UK (COG-UK) consortium. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang K, Long QX, Deng HJ, et al. Longitudinal dynamics of the neutralizing antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Clin Infect Dis. 2021;73:e531–e539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karuna S, Li SS, Grant S, et al. ; HVTN 405/HPTN 1901 Study Team. Neutralizing antibody responses over time in demographically and clinically diverse individuals recovered from SARS-CoV-2 infection in the United States and Peru: a cohort study. PLOS Med. 2021;18:e1003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crawford KHD, Dingens AS, Eguia R, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;223:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dekervel M, Henry N, Torreggiani M, et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin Kidney J. 2021;14:2349–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmiedeberg K, Vuilleumier N, Pagano S, et al. Efficacy and tolerability of a third dose of an mRNA anti-SARS-CoV-2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses. Lancet Rheumatol. 2022;4:e11–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jena A, Mishra S, Deepak P, et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev. 2022;21:102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakuraba A, Luna A, Micic D. Serologic response to coronavirus disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis. Gastroenterology. 2022;162:88–108.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yahav D, Rozen-Zvi B, Mashraki T, et al. Immunosuppression reduction when administering a booster dose of the BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant recipients without adequate humoral response following two vaccine doses: protocol for a randomised controlled trial (BECAME study). BMJ Open. 2021;11:e055611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perkmann T, Perkmann-Nagele N, Koller T, et al. Anti-spike protein assays to determine sars-cov-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr. 2021;9:e0024721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.