Background.
Solid-organ transplant recipients (SOTRs) have a higher risk of coronavirus disease 2019 (COVID-19) complications and death and a less powerful and lasting response to vaccines and to natural infection. In Colombia, this population was prioritized in the National Vaccination Plan against COVID-19 and received vaccines from different platforms. The aim of this study was to estimate the effectiveness of the complete vaccination schedule and of the vaccine booster for COVID-19 administered to SOTRs in Colombia.
Methods.
A nested-cohort was assembled within the population-based ESPERANZA cohort and included the subset of 16 y and older SOTRs (n = 6963); the follow-up period spanned March 11, 2021, to May 11, 2022. The vaccine effectiveness was estimated with Cox proportional-hazards models so that the overall effectiveness of the complete vaccination schedule, the vaccine booster, each used vaccine, and the homologous and heterologous schedules were estimated, adjusting by the main confounders.
Results.
The overall effectiveness of being fully vaccinated was 73.7% (95% confidence interval [CI], 68.9%-77.0%) to prevent COVID-19 infection, 83.7% (95% CI, 78.7%-87.5%) to prevent hospitalization, and 92.1% (95% CI, 88.8%-94.4%) to prevent death due to COVID-19. Similarly, the effectiveness of the vaccine booster was 76.7% (95% CI, 70.6%-81.5%), 86.9% (95% CI, 79.4%-91.6%), and 94.5% (95% CI, 89.8%-97.1%) to prevent confirmed COVID-19 infection, hospitalization, and death due to COVID-19, respectively. In both cases, there were no statistically significant differences across age groups.
Conclusions.
Findings from this work show a high protection of vaccination against infection, hospitalization, and death due to COVID-19 in SOTRs, which increases with the vaccine booster.
INTRODUCTION
Infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease 2019 (COVID-19), was declared a pandemic on March 11, 2020, by the World Health Organization.1 The first confirmed infection case in the Colombian territory was reported on March 6, 2020, which prompted the declaration of the health emergency on March 12, 2020.2
When the Colombian government began immunization for COVID-19, it acquired 5 vaccine types: ChAdOx1 nCoV-19 (AstraZeneca), CoronaVac (Sinovac), Ad26.COV2.S (Janssen), mRNA-1273 (Moderna), and BNT162b2 (Pfizer-BioNTech). Given the global and national scarcity context, the National Vaccination Plan against COVID-19 assigned the first vaccines to people who were more likely to develop complications or die because of COVID-19.
The prioritization process of the National Vaccination Plan in Colombia was determined by an ethical framework and based on the existing best epidemiological evidence at that time.3 This defined the order of equitable access to biologicals based on the population’s risk, through 5 phases. Phase 1included adults over 80 y of age and health workers in COVID-19 areas; Phase 2 included people between 60 and 79 y old and other health workers; Phase 3 included people between 50 and 59 y old, patients with selected underlying diseases, including solid-organ transplant recipients (SOTRs) and other people who, because of their occupation, presented a higher risk of infection, complication, or death from COVID-19; Phase 4 included people between 40 and 49 y old, and people who were in places with risk of outbreaks; and Phase 5 included the population between 3 and 39 y old, which was not previously prioritized.3 It is worth noting that all 5 vaccines were used in the SOTR population because no specific vaccine platform was determined for them.4
SOTRs are more prone to a SARS-CoV-2 infection because of their immunosuppression and their lower likelihood to develop an effective immune response to vaccination5-11 because their immune response to natural infection is also less powerful and lasting, rendering them more vulnerable to reinfections.12 Additionally, chronic immunosuppression might reduce the infectious dose necessary to cause COVID-19 and hinder the immune control once the infection has been established, which increases the risk of severe infection and complications.13 Furthermore, it is hypothesized that SOTRs could shed higher viral loads for longer periods than healthy hosts, which could increase their chances to spread the infection to other people.12
Similarly, previous studies have found that both humoral and cellular immune responses to vaccines and natural infection are weaker in SOTRs, whereby boosters and additional doses are required5-7,14,15 to maintain the protection against COVID-19 infection and severe disease.14 Additionally, immunosuppression has also been identified to be caused by the use of certain substances, such as antimetabolites, calcineurin inhibitors, and monoclonal antibodies, which explains the insufficient immune responses to current COVID-19 vaccination schemes in SOTRs.5,7-9,16,17
Knowing the effectiveness of vaccination in real-life conditions will allow us to evaluate the impact of prioritization in countries where SOTRs were prioritized, to wholly estimate the impact of vaccination, and to adjust the vaccination schedules in this risk group. Therefore, the aim of this study was to estimate the effectiveness of the complete vaccination schedule and of the vaccine booster for COVID-19 administered to SOTRs in Colombia.
MATERIALS AND METHODS
Design and Population Study
A nested-cohort was assembled within the ESPERANZA cohort, which is a population-based cohort made up of all Colombian residents who were eligible to receive a COVID-19 vaccine and has methodology that has been described elsewhere.18 Our nested-cohort included all SOTRs aged 16 and older that were registered in Red data—the National Health Institute (NHI) database. The follow-up period went from March 11, 2021, when the first individuals completed their vaccination schedule, to May 11, 2022, which corresponds to the latest update on the national statistics.
Data Sources
All data were obtained from the Integrated Social Protection Information System (in Spanish, Sistema Integrado de Información de la Protección Social), which is the official health statistics data source in Colombia. Information from it included people who were cross-referenced in 8 Social Protection Information System data records by using an individual anonymized number that is encrypted and automatically generated by the information system to protect the person’s identity: (1) Red data include all SOTRs who reside in Colombia; (2) MIVACUNA contains sociodemographic data from vaccine candidates who were later vaccinated against COVID-19, according to the National Vaccination Plan against COVID-19; (3) PAIWEB registers people who have received any vaccine in Colombia and the basic vaccine information, such as dose, vaccine type, date, and vaccine location; (4) SEGCOVID contains information about confirmed COVID-19 cases; (5) SISMUESTRAS stores the results from polymerase chain reaction (PCR) and antigen tests conducted in Colombia; (6) Single Registry of Affiliates to the Social Protection System—Births and Deaths (Registro Único de Afiliados al Sistema de la Protección Social—Nacimientos y Defunciones, in Spanish) records death causes in Colombia; (7) the high-cost disease registry (Cuenta de Alto Costo, in Spanish) includes data about people with diseases that require a larger budget, that is, chronic kidney disease (CKD), high blood pressure (HBP), diabetes mellitus (DM), cancer, and HIV infection; and (8) unique affiliate database (Base de Datos Única de Afiliados, in Spanish) provides information regarding the affiliation regime to the health system. The listed databases are public, although they have restricted access and are currently available to the Ministry of Health and Social Protection.
Inclusion and Exclusion Criteria
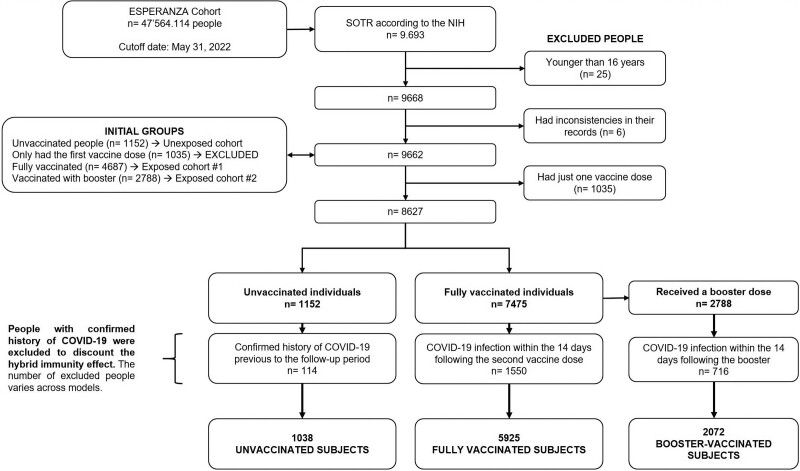
This study first included male and female SOTRs aged 16 and older residing in Colombia, regardless of their vaccination status. Subsequently, individuals were excluded if they (1) had a history of confirmed COVID-19 infection, (2) had an incomplete vaccination schedule, or (3) reported inconsistencies in their vaccination records (ie, implausible vaccine dates or doses). Definitions of a complete vaccination schedule were those originally established by the manufacturer and adopted by the HSPM.19 Figure 1 shows the complete selection process.
FIGURE 1.
Flowchart of the selection process of the analytic sample. The fully vaccinated group includes 2788 people who later received at least one booster dose. COVID-19, coronavirus disease 2019; SOTR, solid-organ transplant recipient; NHI, National Health Institute of Colombia.
Exposure Groups
Three groups were formed based on the subjects’ vaccination status: unvaccinated, fully vaccinated, and vaccinated with booster. Unvaccinated individuals were those who did not receive any vaccine during the study period. The definition of fully vaccinated people depended on the administered vaccine; hence, for AstraZeneca, Pfizer, Moderna, and Sinovac, 2 doses with a 28-d period (21 d for Pfizer) between doses was considered to be a complete schedule. It is worth noting that in the case of longer periods between doses because of any cause, people could complete their vaccination schedule without recommencing it, unless they had received a vaccine unavailable in Colombia. For the Janssen vaccine, a sole dose was deemed a complete vaccination scheme. Vaccinated with booster was defined as people who received at least 1 additional vaccine dose from the same or from a different platform; SOTRs were allowed to get a booster 1 mo after completing the vaccination schedule.20 Allocation to either the fully vaccinated or vaccinated with booster groups was done 15 d after completing the vaccination schedule or receiving the first booster, respectively. In Colombia, heterologous vaccination was used for both the booster, from September 15, 2021,20 and the initial vaccination schedule, from March 18, 2022.21
Outcomes
The study outcomes were (1) COVID-19 infection, defined as a COVID-19 diagnosis confirmed by PCR or antigen tests (these tests had to be validated by the NHI in Colombia) and registered in SISMUESTRAS; (2) hospitalization due to COVID-19, defined as having entered the general hospitalization service or the intensive care unit and having COVID-19 as one of the hospitalization causes at any moment of the hospital stay, as registered in SEGCOVID; and (3) confirmed death because of COVID-19, defined as having a confirmed COVID-19 diagnosis as the basic cause of death in the death certificate, as consulted in the Single Registry of Affiliates to the Social. Suspected deaths were not included in this study.
Covariates
Additional variables that have been deemed as relevant confounders in previous studies were also measured to include them in the analysis: age (y); sex (male versus female); affiliation regime to the health system (contributory versus subsidized); municipality of residence; comorbidities diagnosis (yes versus no), such as CKD, cancer, DM, HBP, and HIV infection; and the prevalent SARS-CoV-2 variant at the time of the COVID-19 infection (this information was taken from www.covariants.org).
Statistical Analysis
Categorical variables were described with absolute frequencies and proportions, whereas quantitative variables were described with central tendency (medians) and dispersion (range and interquartile range [IQR]) measures. Subjects’ characteristics were compared across exposure groups.
To estimate the overall vaccination effectiveness, a survival analysis was performed by using Cox proportional-hazards models to estimate the reduction in the risk of death, hospitalization, and infection in fully vaccinated individuals and in people vaccinated with booster. These models were adjusted for the confounders listed as covariates; the prevalent SARS-CoV-2 variant at the time of infection was adjusted for in the models to control the transmission risk. For those unvaccinated who did not develop any of the study outcomes, the infection risk given by a specific variant was randomly assigned proportional to its dominance during the study period. Additionally, all the time-to-event from the unvaccinated subjects during the study period was considered in the models.
Multiple types of right-censoring could occur, given by people who died by nonrelated COVID-19 causes, fully vaccinated individuals who received a booster or subjects who finished the follow-up period without developing any of the study outcomes. These censoring were considered while constructing the models. The statistical analysis was carried out by using R (4.2.0 version) and its survival (3.3.1 version) and ggplot2 (3.3.6 version) packages to perform the survival analysis and to create the graphs, respectively.
Ethics
This study used secondary data sources from public information systems. The research team did not have access to personal data from the participants at any moment and all used information was anonymized. Given that this study is classified as a research without risk according to the Colombian legislation,22 an approval from an Ethics Committee was not required.
RESULTS
The inclusion and exclusion criteria yielded a sample of 6963 SOTRs during the study period (March 11, 2021–May 11, 2022), from which 85% (n = 5925) were fully vaccinated (this figure includes 2072 individuals vaccinated with booster) and 15% (n = 1038) remained unvaccinated throughout the whole follow-up. Out of the 6963 SOTRs, 42.1% were female, and the median age was 52 y (IQR: 39–62; range: 16–97), whereas the median age of the unvaccinated group was 44 y (IQR: 33–56). Additionally, 76.7% of the participants belonged to the contributory health regime and 82.4% had at least 1 comorbidity, in which CKD (72.3%) and HBP (70.3%) were the most frequent diagnosis. Table 1 describes the main characteristics of the study individuals by exposure group.
TABLE 1.
Solid-organ transplant recipients’ sociodemographic and clinical characteristics, ESPERANZA cohort
| Variable | Unvaccinated(n = 1038)n (%) | Fully vaccinateda(n = 5925)n (%) | Vaccine booster(n = 2072)n (%) | Total(n = 6963)n (%) |
|---|---|---|---|---|
| Age (y) | ||||
| Median (IQR) | 44 (33–56) | 53 (40–63) | 56 (44–65) | 52 (39–62) |
| Range | 16–97 | 16–90 | 16–90 | 16–97 |
| 16–59 | 859 (82.8) | 3987 (67.3) | 1257 (60.7) | 4846 (69.6) |
| 60 and older | 179 (17.2) | 1938 (32.7) | 815 (39.3) | 2117 (30.4) |
| Sex | ||||
| Female | 460 (44.3) | 2469 (41.7) | 865 (41.7) | 2929 (42.1) |
| Male | 578 (55.7) | 3456 (58.3) | 1207 (58.3) | 4034 (57.9) |
| Health system affiliation regime | ||||
| Contributory | 638 (61.5) | 4704 (79.4) | 1763 (85.1) | 5342 (76.7) |
| Subsidized | 400 (38.5) | 1221 (20.6) | 309 (14.9) | 1621 (23.3) |
| Comorbidities | ||||
| None | 224 (21.6) | 999 (16.9) | 381 (18.4) | 1223 (17.6) |
| ≥1 comorbidity | 814 (78.4) | 4926 (83.1) | 1691 (81.6) | 5740 (82.4) |
| Cancer | 21 (2.0) | 183 (3.1) | 77 (3.7) | 204 (2.9) |
| Diabetes mellitus | 123 (11.8) | 1052 (17.8) | 413 (19.9) | 1175 (16.9) |
| Chronic kidney disease | 710 (68.4) | 4327 (73.0) | 1453 (70.1) | 5037 (72.3) |
| High blood pressure | 700 (67.4) | 4194 (70.8) | 1413 (68.2) | 4894 (70.3) |
| HIV infection | 5 (0.5) | 17 (0.3) | 9 (0.4) | 22 (0.3) |
| Prevalent SARS-CoV-2 variant at the time of infection | ||||
| Delta | 113 (10.9) | 960 (16.2) | 624 (30.1) | 1.073 (15.4) |
| Delta/Mu | 90 (8.7) | 312 (5.3) | 0 (0.0) | 402 (5.8) |
| Mu | 519 (50.0) | 2581 (43.6) | 0 (0.0) | 3100 (44.5) |
| Omicron | 316 (30.4) | 2072 (35) | 1448 (69.9) | 2388 (34.3) |
| Initial vaccine schedule manufacturerb | ||||
| AstraZeneca | NA | 671 (11.8) | 214 (11.4) | 671 (11.8) |
| Janssen | NA | 421 (7.4) | 68 (3.6) | 421 (7.4) |
| Moderna | NA | 234 (4.1) | 44 (2.4) | 234 (4.1) |
| Pfizer | NA | 2996 (52.7) | 1056 (56.4) | 2996 (52.7) |
| Sinovac | NA | 1362 (24) | 489 (26.1) | 1362 (24) |
This group includes people with full schedule, which is made up of 2 subgroups: 1) those who completed the schedule and did not receive any booster during the entire study period and 2) those who completed the schedule and had a booster but were included analytically in the period before receiving the booster (censored booster doses).
Refers to the initial schedule’s manufacturer. The additional dose is not considered for the group that received a booster, because this information is described in detail in Table 2.
IQR, interquartile range; NA, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In the fully vaccinated group, the most frequently used vaccines were Pfizer (52.7%), Sinovac (24.0%), AstraZeneca (11.8%), Janssen (7.4%), and Moderna (4.1%). Furthermore, most subjects received a homologous schedule (n = 3425) in this group, with mainly Pfizer being administered (n = 1720), followed by Sinovac (n = 785), AstraZeneca (n = 407), Jansen (n = 349), and Moderna (n = 164). Regarding the vaccinated with booster group who had a homologous schedule (ie, 3 doses from the same manufacturer), Pfizer (61.7%), Sinovac (20.2%), and AstraZeneca (11.4%) were the most used. In the case of heterologous schedule in those who had a booster, the main combinations were Pfizer/Moderna (29.1%), Pfizer/AstraZeneca (21.8%), and Sinovac/Pfizer (22.8%). The complete description of all combinations is shown in Table 2.
TABLE 2.
Complete vaccination schedule and booster in solid-organ transplant recipients in Colombia, by manufacturer, ESPERANZA cohort
| Vaccination schedule | Homologous(n = 947) | |
|---|---|---|
| n | % | |
| Pfizer | 584 | 61.7 |
| AstraZeneca (AZ) | 108 | 11.4 |
| Moderna | 11 | 1.2 |
| Sinovac | 192 | 20.2 |
| Janssen | 52 | 5.5 |
| Heterologous (n = 990) | ||
| n | % | |
| 2 AZ + Pfizer | 67 | 6.8 |
| 2 AZ + Moderna | 37 | 3.7 |
| 2 AZ + Janssen | 4 | 0.4 |
| 2 Pfizer + AZ | 216 | 21.8 |
| 2 Pfizer + Moderna/Pfizer | 289 | 29.1 |
| 2 Pfizer + Janssen | 9 | 1.0 |
| 2 Moderna + AZ | 5 | 0.5 |
| 2 Moderna + Pfizer/Moderna | 27 | 2.7 |
| 2 Moderna + Janssen | 1 | 0.1 |
| 2 Sinovac + AZ | 73 | 7.4 |
| 2 Sinovac + Pfizer/Moderna | 226 | 22.8 |
| 2 Sinovac + Janssen | 7 | 0.7 |
| Other combinations | 29 | 3.0 |
Records that did not specify the manufacturer of the vaccination schedule were excluded from this table
As to the outcomes occurrence in the exposure groups, the risk of COVID-19 infection, hospitalization, and death due to COVID-19 was 26.4%, 9.6%, and 7.5%, respectively, in the unvaccinated group. On the other hand, figures were significantly lower in the fully vaccinated group, so that the risk of COVID-19 infection was 11.5%; risk of hospitalization, 2.7%; and risk of death due to COVID-19, 1.2%. Moreover, these risks were even lower in the subset that received a booster: 7.8% for COVID-19 infection; 2.7% for hospitalization; and 0.7% for death due to COVID-19. The listed differences between groups were statistically significant (P < 0.01). Table 3 lists the outcomes’ occurrence and their time-to-event in detail, according to the exposure group.
TABLE 3.
Study outcomes in unvaccinated and fully vaccinated solid-organ transplant recipients in Colombia, ESPERANZA cohort
| Outcome | Unvaccinated(n = 1038)n (%) | Fully vaccinateda(n = 5925)n (%) | Vaccine booster(n = 2072)n (%) | Total(n = 6963)n (%) |
|---|---|---|---|---|
| Confirmed COVID-19 infection | 272 (26.4) | 679 (11.5) | 153 (7.8) | 951 (13.7) |
| COVID-19 hospitalization | 99 (9.6) | 160 (2.7) | 33 (1.7) | 259 (3.7) |
| Death due to COVID-19 | 77 (7.5) | 70 (1.2) | 14 (0.7) | 147 (2.1) |
| Time-to-outcome (d)b | ||||
| Time-to-infection; median (IQR) | 411 (255–411) | 292 (222–309) | 103 (62–147) | 294 (223–316) |
| Time-to-hospitalization; median (IQR) | 411 (411–411) | 298 (264–313) | 111 (71–153) | 300 (268–338) |
| Time-to-death; median (IQR) | 411 (411–411) | 298 (267–315) | 112 (72–153) | 301 (272–344) |
This group includes people with full schedule, which is made up of 2 subgroups: 1) those who completed the schedule and did not receive any booster during the entire study period and 2) those who completed the schedule and had a booster but were included analytically in the period before receiving the booster (censored booster doses).
The follow-up time of unvaccinated individuals was longer for all the assessed outcomes. Given that this group was made up of individuals who remained unvaccinated throughout the whole study period, they contributed a larger time-to-event than vaccinated people, whose time-to-event only counted after receiving the vaccine.
COVID-19, coronavirus disease 2019; IQR, interquartile range.
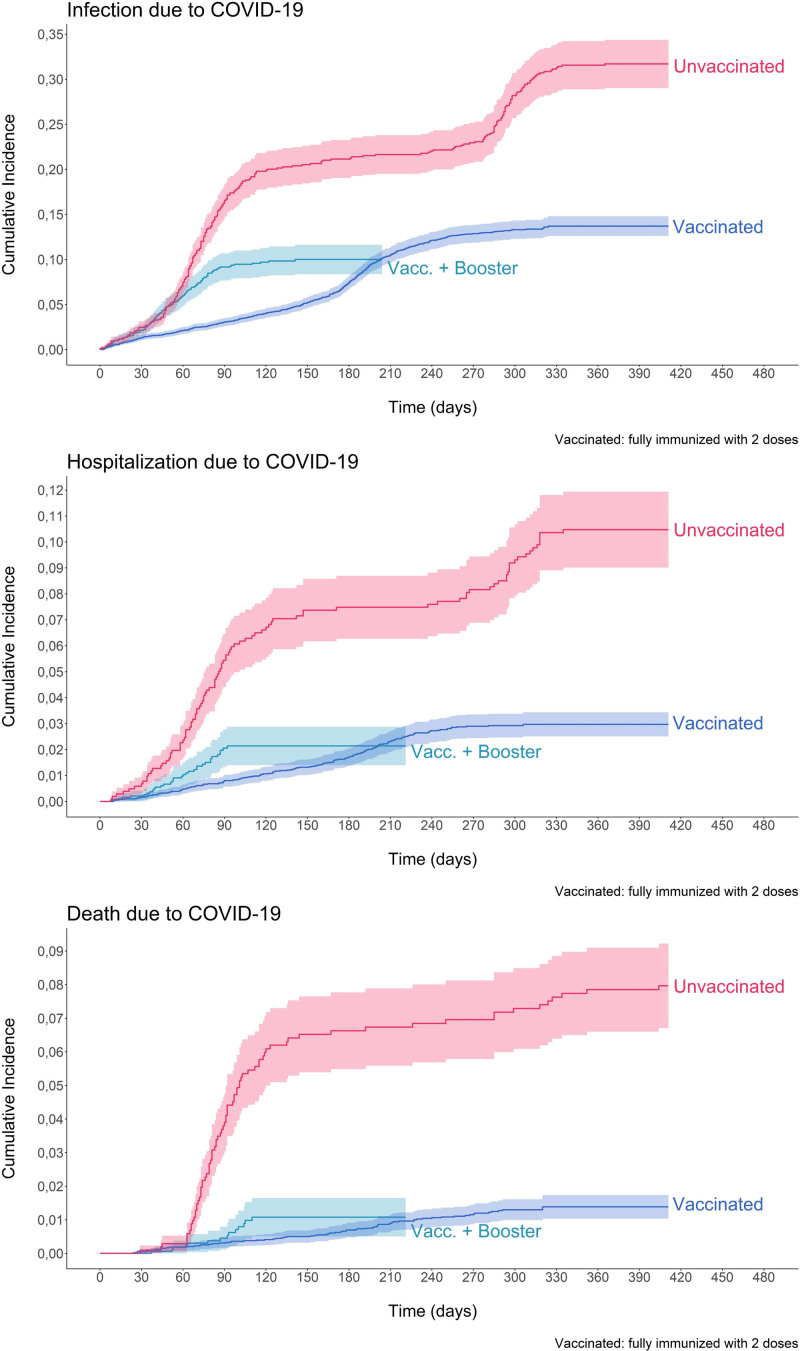
In relation to the survival analysis, a lower survival was found in those unvaccinated compared with fully vaccinated individuals. Furthermore, the difference in the survival time when comparing unvaccinated subjects to those who received a booster was not as large as that found when the comparison was made between the unvaccinated and the fully vaccinated groups; and this trend persisted over time. The unadjusted survival analysis curves are depicted in Figure 2.
FIGURE 2.
Kaplan-Meier survival curves for COVID-19 infection, hospitalization, and death, according to vaccination status in solid-organ transplant recipients in Colombia. ESPERANZA cohort. COVID-19, coronavirus disease 2019.
On the other hand, the overall effectiveness of the complete vaccination schedule was 73.3% (95% CI, 68.9%-77.0%) to prevent COVID-19 infection; 83.7% (95% CI, 78.7%-87.5%) to prevent hospitalization; and 92.1% (95% CI, 88.8%-94.4%) to prevent death due to COVID-19. Table 4 shows the effectiveness estimates of being fully vaccinated according to age groups.
TABLE 4.
COVID-19 complete schedule effectiveness for infection, hospitalization, and death in solid-organ transplant recipients in Colombia, by age group, ESPERANZA cohort
| COVID-19 complete schedule effectiveness | ||||||
|---|---|---|---|---|---|---|
| Age group | Infection | Hospitalization | Death | |||
| %(95% CI) | P | %(95% CI) | P | %(95% CI) | P | |
| All age groups | 73.3 (68.9-77.0) | <0.001 | 83.7 (78.7-87.5) | <0.001 | 92.1 (88.8-94.4) | <0.001 |
| 16–59 | 73.1 (67.8-77.5) | <0.001 | 83.3 (76.6-88.1) | <0.001 | 93.6 (89.5-96.1) | <0.001 |
| 60 and older | 72.6 (63.6-79.3) | <0.001 | 82.5 (72.8-88.8) | <0.001 | 91.0 (84.8-94.7) | <0.001 |
Estimates adjusted for sex, affiliation regime, municipality of residence, presence of comorbidities (CKD, cancer, DM, HBP, and HIV infection), and prevalent variant at the time of infection. Unvaccinated individuals were the reference group in all models.
CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HBP, high blood pressure.
Likewise, the effectiveness of the vaccine booster to prevent the study outcomes was higher for death due to COVID-19 (94.5%; 95% CI, 89.8%-97.1%), followed by hospitalization (86.9%; 95% CI, 79.4%-91.6%) and COVID-19 infection (76.7%; 95% CI, 70.6%-81.5%). Similar results were obtained in both age groups (16–59 y and 60 y and older), with only a minimum difference between age groups by outcome. The complete estimates of the vaccine booster effectiveness according to age groups are presented in Table 5.
TABLE 5.
COVID-19 booster effectiveness for infection, hospitalization, and death in solid-organ transplant recipients in Colombia, by age group, ESPERANZA cohort
| COVID-19 booster effectiveness | ||||||
|---|---|---|---|---|---|---|
| Age group | Infection | Hospitalization | Death | |||
| %(95% CI) | P | %(95% CI) | P | %(95% CI) | P | |
| All age groups | 76.7 (70.6-81.5) | <0.001 | 86.9 (79.4-91.6) | <0.001 | 94.5 (89.8-97.1) | <0.001 |
| 16–59 | 75.9 (67.1-82.3) | <0.001 | 84.2 (69.6-91.8) | <0.001 | 95.8 (86.2-98.8) | <0.001 |
| 60 and older | 70.2 (56.5-79.6) | <0.001 | 86.4 (74.2-92.8) | <0.001 | 91.1 (80.9-95.9) | <0.001 |
Estimates adjusted for sex, affiliation regime, municipality of residence, presence of comorbidities (CKD, cancer, DM, HBP, and HIV infection), and prevalent variant at the time of infection. Unvaccinated individuals were the reference group in all models.
CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HBP, high blood pressure.
Finally, the effectiveness of all the complete vaccination and booster schedules are presented in Tables S1 and S2 (SDC, http://links.lww.com/TP/C615). A high effectiveness of the analyzed vaccines was observed with all the homologous and heterologous combinations analyzed. However, some estimates were very imprecise with wide confidence intervals given the small sample size for some of the studied groups. Finally, it is important to highlight that in all cases the effectiveness in preventing hospitalization and death due to COVID-19 was greater than in preventing the occurrence of confirmed infection.
DISCUSSION
This research found a high effectiveness of the complete vaccination schedule and the vaccination with booster in SOTRs, which represents a significant impact of vaccination in immunized SOTRs when compared with unvaccinated individuals. It is important to highlight that our findings are a measure of the infection and complications risk reduction within the SOTR population; hence, they cannot be directly extrapolated to other populations, nor are they comparable to effectiveness estimates from immunocompetent people, who are known to have a better response to vaccines.23 To make comparisons against other groups, impact measures (absolute estimates) would be required, which is beyond the scope of the present investigation.
The obtained results also that suggest the protection granted by immunization in SOTRs begins with a complete vaccination schedule, initially defined by the manufacturers, and that a booster could strengthen such protection in time. On the other hand, this research included one of the biggest SOTR samples evaluated to this day, which not only was assessed in real-life conditions but also allowed the estimation of the vaccination effectiveness by using national epidemiologic data, whereas most of the published research in the SOTR population regarding this topic are immunogenicity studies.8,14,15,24-26 Furthermore, this investigation also estimated the effectiveness of several heterologous schedules, including combinations of inactivated, adenovirus vector-based and mRNA-based vaccines and adjusted for prevalent SARS-CoV-2 variants in Colombia, evidencing a high effectiveness of the COVID-19 vaccines in the SOTR population.
The need to vaccinate immunosuppressed individuals was also underlined because our findings showed immunization significantly reduced mortality and morbidity relative to not receiving any vaccine in this group. This strengthens the public policy of vaccination aimed at preventing disease and the risk prioritization as a main component of public health, primary healthcare systems, and national vaccination programs. Accordingly, our findings also suggest the prioritization of SOTRs in Colombia might have significantly impacted the reduction of the morbimortality of this population subset.
Our work also allows recognition of vaccination as a potential cost-effective strategy in terms of the burden of disease caused by COVID-19 and years of potential life lost because of mortality or disability in a young population that has several comorbidities.27-29 There are also implications for clinical practice because receiving immunosuppressive drugs is a direct risk factor for COVID-19 death,30 although vaccination could be a preventive intervention for it. It also has to be considered that both the ability to prevent infection by activating the immune system and the risk of COVID-19 infection are related to the person’s immunosuppression status and to its competence to mount an immune response; therefore, the greater the immunodeficiency, the higher chance of an inadequate response to the biologic or the vaccine-induced immunization. Risk factors for an insufficient immune response comprise several individual aspects, such as age and receiving immunosuppressive therapy, which, in the case of SOTRs, needs to be considered along the underlying disease that caused the organ transplant in the first place (eg, kidney or liver failure). Thus, an adequate immune response cannot be assumed in all cases despite vaccination confers benefits to immunosuppressed individuals; therefore, the relevance of booster doses.31-34 Unfortunately, this study could not collect information about the transplanted organ or the therapy the participants were using, which impeded the analysis of their role in the effectiveness of vaccines.
Previous investigations have also found vaccination to be a good strategy in SOTRs to prevent COVID-19. For example, a research found a reduction in the incidence of symptomatic COVID-19 in vaccinated SOTRs (0.065 per 1000 person-days; 95% CI, 0.024-0.17) compared with unvaccinated subjects (0.34 per 1000 person-days; 95% CI, 0.26-0.44), which evidences a high effectiveness in this risk group.35 Other examples include an investigation carried out in Israel, where a cohort was immunized with mRNA-based vaccines and the effectiveness for symptomatic COVID-19 infection was found to be 71% (95% CI, 37%-87%) in immunosuppressed patients, whereas it was 94% (95% CI, 88%-97%) in general population36; and retrospective studies that have suggested a lower vaccination effectiveness to prevent COVID-19-related hospitalization in immunocompromised patients, as shown in a population with an immunosuppression prevalence of 44%.37
With regard to the booster dose, a study also found that only two-thirds of the included SOTRs generated anti-SARS-CoV-2 antibodies.38 This correlates to our findings, in which the survival time between the unvaccinated and vaccinated with boosted individuals was lower than when the comparison was made between the unvaccinated and the fully vaccinated subjects. Additionally, previous publications have also reported a poor response to COVID-19 vaccines in SOTRs, as indicated in a meta-analysis that estimated seroconversion was 16 times less likely to occur after vaccination in SOTRs.39
Results on this subject are heterogeneous and show that differences in control groups influence the conclusions. It is important to clarify that information related to the effectiveness of booster vaccines in SOTR was not found because most published studies assessed the vaccine-induced immune response, with a focus on the humoral response, but did not estimate its impact on the protection against SARS-CoV-2, which does not allow a direct comparison with our results.8,11,40
The limitations of this work comprise of high effectiveness estimates of the COVID-19 vaccines because the comparison was made against unvaccinated SOTRs instead of nontransplanted individuals. Moreover, these estimates might be affected by the lack of inclusion of certain covariates that could act as potential confounders, such as other diseases, the type of the administered immunosuppressive drugs, the educational level or the transplanted organ (although kidney transplants are the most frequent in Colombia). Lastly, vaccination effectiveness throughout time was not estimated, which is one of the main questions to be answered, considering the insufficient immune response seen in SOTRs. New studies are required that aim to not only respond this query but also address the impact of the hybrid immunity and compare the vaccines, effectiveness over time against other immunocompromised individuals and the general population.
In conclusion, our research evidences the relevant and coherent measures taken by the Colombian government when implementing the COVID-19 vaccination, which focused on prioritizing the most vulnerable groups, intervening the possible virus-related mortality causes, and decreasing the health inequities potentially caused by the COVID-19 syndemic. This study also serves as input to keep the recommendation to prioritize SOTR vaccination worldwide and to guarantee the timely access to booster doses.
Supplementary Material
Footnotes
This study was conducted using financial and economic resources provided by the Ministry of Health and Social Protection of Colombia (Ministerio de Salud y Protección Social).
M.P.A., J.F.N., L.A.C., and M.R.B. did design and statistical analysis conceptualization. J.F.N., L.A.C., and A.F.P. did data acquisition. M.P.A., J.F.N., L.A.C., M.R.B., A.F.P., and M.G.P. did data verification. M.P.A. and A.F.P. did dataset construction. M.P.A., J.F.N., and A.F.P. did statistical analysis. M.P.A., J.F.N., L.A.C., and M.R.B. drafted of the article. M.P.A., J.F.N., L.A.C., M.R.B., A.F.P., M.G.P., and F.R.G. did critical revision and discussion of the article.
J.F.N., L.A.C., and F.R.G. were members of the Colombian COVID-19 vaccine advisory committee. All other authors declare no competing interests.
This manuscript has been previously submitted to Social Science Research Network as a preprint; https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4166380.
Supplemental Visual Abstract; http://links.lww.com/TP/C616.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.World Health Organization. WHO Target Product Profiles for COVID-19 Vaccines. 2022. Available at https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines.
- 2.Ministerio de Salud y de la Protección Social. Resolución 385. Bogotá D.C., Colombia; 2020. [Google Scholar]
- 3.Departamento Administrativo De La Presidencia De La República (DAPRE). Decreto 109 de 2021: Por El Cual Se Adopta El Plan Nacional de Vacunación Contra El COVID-19 y Se Dictan Otras Disposiciones. Bogotá D.C., Colombia; 2021. [Google Scholar]
- 4.Ministerio de Salud y de la Protección Social, Departamento Nacional de Planeación, Ministerio de Hacienda y Crédito Público, Instituto de Evaluación Tecnológica en Salud. Plan Nacional de Vacunación Contra El COVID-19. Bogotá D.C., Colombia; 2021. Available at https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/pnv-contra-covid-19.pdf. [Google Scholar]
- 5.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients—a prospective cohort study. Eur J Heart Fail. 2021;23:1555–1559. [DOI] [PubMed] [Google Scholar]
- 7.Prendecki M, Thomson T, Clarke CL, et al. Comparison of humoral and cellular responses in kidney transplant recipients receiving BNT162b2 and ChAdOx1 SARS-CoV-2 vaccines. medRxiv. [Preprint. July 14, 2021]. doi:10.1101/2021.07.09.21260192. [Google Scholar]
- 8.Kamar N, Abravanel F, Marion O, et al. Three doses of an mrna covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruminhent J, Boongird S, Assanatham M, et al. Anti–SARS-CoV-2 spike protein S1 receptor-binding domain antibody after vaccination with inactivated whole-virus SARS-CoV-2 in end-stage kidney disease patients: an initial report. 2021;100:1136–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limaye A, Hardinger K. COVID-19: issues related to solid organ transplantation. 2022. Available at https://www.uptodate.com/contents/covid-19-issues-related-to-solid-organ-transplantation. Accessed July 16, 2022.
- 13.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. [DOI] [PubMed] [Google Scholar]
- 14.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar D, Ferreira VH, Hall VG, et al. Neutralization of SARS-CoV-2 variants in transplant recipients after two and three doses of mRNA-1273 vaccine: secondary analysis of a randomized trial. Ann Intern Med. 2022;175:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holden IK, Bistrup C, Nilsson AC, et al. Immunogenicity of SARS-CoV-2 mRNA vaccine in solid organ transplant recipients. J Intern Med. 2021;290:1264–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanathan M, Murugesan K, Yang LM, et al. Cell-mediated and humoral immune response to 2-dose SARS-CoV2 mRNA vaccination in immunocompromised patient population. medRxiv. [Preprint. July 23, 2021]. doi:10.1101/2021.07.21.21260921. [Google Scholar]
- 18.Arregocés-Castillo L, Fernández-Niño J, Rojas-Botero M, et al. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022;3:e242–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministerio de Salud y de la Protección Social. Resolución 1151. Colombia; 2021. [Google Scholar]
- 20.Ministerio de Salud y de la Protección Social. Resolución 1426. Bogotá D.C., Colombia; 2021. [Google Scholar]
- 21.Ministerio de Salud y de la Protección Social. Resolución 419. Bogotá D.C., Colombia; 2022. [Google Scholar]
- 22.Ministerio de Salud de la República de Colombia. Resolución 8430 de 1993. Bogotá D.C., Colombia; 1993. [Google Scholar]
- 23.Ssentongo P, Ssentongo AE, Voleti N, et al. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2022;22:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali H, Alterki A, Sindhu S, et al. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Front Immunol. 2021;12:752233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidov Y, Tsaraf K, Cohen-Ezra O, et al. Immunogenicity and adverse effects of the 2-dose BNT162b2 messenger RNA vaccine among liver transplantation recipients. Liver Transpl. 2022;28:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurdi A Al, Gassen RB, Borges TJ, et al. Diminished antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. medRxiv. [Preprint. January 6, 2022]. doi:10.1101/2022.01.03.22268649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaezi A, Meysamie A. COVID-19 vaccines cost-effectiveness analysis: a scenario for Iran. Vaccines (Basel). 2021;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson CAB, Bozzani F, Procter SR, et al. COVID-19 vaccination in Sindh Province, Pakistan: a modelling study of health impact and cost-effectiveness. PLOS Med. 2021;18:e1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy KP, Fitzmaurice KP, Scott JA, et al. Clinical outcomes and cost-effectiveness of COVID-19 vaccination in South Africa. Nat Commun. 2021;12:6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim AHJ, Sparks JA. Immunosuppression and SARS-CoV-2 breakthrough infections. Lancet Rheumatol. 2022;4:e379–e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gangappa S, Kokko KE, Carlson LM, et al. Immune responsiveness and protective immunity after transplantation. Transpl Int. 2008;21:293–303. [DOI] [PubMed] [Google Scholar]
- 32.Eckerle I, Rosenberger KD, Zwahlen M, et al. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8:e56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong PP, Avery RK. A Comprehensive review of immunization practices in solid organ transplant and hematopoietic stem cell transplant recipients. Clin Ther. 2017;39:1581–1598. [DOI] [PubMed] [Google Scholar]
- 34.Kotton CN, Hibberd PL. Immunizations in solid organ transplant candidates and recipients. 2022. Available at https://www.uptodate.com/contents/immunizations-in-solid-organ-transplant-candidates-and-recipients. Accessed July 16, 2022.
- 35.Aslam S, Adler E, Mekeel K, et al. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021;23:e13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chodick G, Tene L, Rotem RS, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2022;74:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenforde MW, Patel MM, Ginde AA, et al. ; Influenza and Other Viruses in the Acutely Ill (IVY) Network. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis. 2022;74:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamar N, Abravanel F, Marion O, et al. Anti-SARS-CoV-2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2 vaccine in a large cohort of solid organ transplant patients. Am J Transplant. 2022;22:1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of Covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326:1063–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.