Abstract
Primary cutaneous B cell lymphomas (PCBCL) are rare; although data on outcomes and treatment are limited, traditionally they have been treated with radiation doses in excess of 24 Gy. We retrospectively identified and reviewed all cases of PCBCL treated at our institution from 2002–2014. Thirty-nine patients with PCBCL (42 lesions) were identified. Radiation was the only treatment for most patients. All lesions had a complete response and none had in-field failures; seven patients had out-of-field relapses, three of which were salvaged with radiation therapy. No differences in PFS or OS were found for patients given low-dose (≤ 12 Gy) versus high-dose (> 12 Gy) radiation. PCBCL is an indolent entity with a long clinical course and excellent response to radiation therapy and successful salvage of recurrent disease, even when doses are as low as 4 Gy. Given the above findings, we recommend the initial use of low-dose irradiation for PCBCL.
Keywords: B cell, follicle center, marginal zone, radiation therapy, skin lymphoma
Introduction
Primary cutaneous B-cell lymphomas (PCBCLs) are much less common than their T-cell counterparts; these two entities are currently considered in separate categories in the WHO classification [1,2]. Two subtypes of cutaneous B-cell lymphoma, primary cutaneous follicle center lymphoma (PCFCL) and primary cutaneous marginal zone lymphoma (PCMZL), share similar patterns of presentation and outcomes, and they are now considered to be different pathologic entities although originally both were frequently mistaken for each other on pathological reviews [2–4]. They present as non-painful red to violaceous papules, plaques, or nodules; treatments have varied considerably, from single- or multiple-agent chemotherapy to local radiation in doses ranging from 10–45 Gy. Complete remission is common regardless of which treatment is used [5–8]. We undertook this single-institution study to increase awareness of how to manage this rare entity and also to examine the feasibility of low-dose radiation for PCFCL and PCMZL.
Materials and methods
This retrospective review was approved by the appropriate institutional review board. After reviewing more than 1300 patients with pathologically proven cutaneous lymphoma, we identified 39 consecutive patients with primary low-grade B-cell skin lymphoma, including PCFCL, PCMZL, or other unclassified variants considered PCBCL not otherwise specified (PCBCLNOS). PCBCLNOS lesions were associated with dense dermal B-cell infiltration, lymphoid hyperplasia, and atypical lymphocytic proliferation, and all lesions expressed CD20. Medical records of these 39 patients, treated at our institution from 2002 through 2014, were reviewed retrospectively for clinical and pathologic characteristics and for details of chemotherapy and radiation treatment.
Pretreatment variables extracted from the medical records included age, sex, lesion size, and history of chemotherapy or dermatologic treatments. Staging was based on the physical examination, contrast-enhanced computed tomography (CT) of the head and neck, thorax, abdomen, and pelvis or positron emission tomography (PET/CT); magnetic resonance imaging (MRI) was also used when appropriate. Bone marrow aspiration and biopsy samples were obtained from 31 patients. In every case, the diagnosis of PCBCL had been confirmed by histologic or immunophenotypic examination of excisional or core needle biopsy specimens. All cases had been examined by flow cytometry immunophenotyping or by immunohistochemical analysis of fixed, paraffin-embedded tissue sections.
Response was assessed at the end of treatment by the treating radiation oncologist and referring dermatologist. A complete response was defined as the complete disappearance of the lesion or lesions on clinical examination; partial response was a ≥ 50% regression of clinical findings on the skin or CT or PET findings at one or more previously involved areas. Stable disease was defined as < 50% regression of the lesion based on clinical examination or imaging if obtained during follow-up. Progressive disease was defined as new or progressing abnormalities evident clinically or on PET/CT or contrast-enhanced CT, and all cases classified as a relapse were proven by biopsy. Most patients with PCMZL underwent esophagogastroduodenoscopy, colonoscopy, or both.
The primary outcomes assessed were overall survival (OS), progression-free survival (PFS), and patterns of failure. PFS was defined as time from treatment to any relapse or last available follow-up. Local control was defined as freedom from disease at the primary skin site of involvement, as evaluated by clinical examination or by CT, MRI, or PET/CT. Survival outcomes were calculated by the Kaplan-Meier method and potential prognostic factors identified with log-rank tests. Univariate analysis was done with Chi-square tests. All statistical analyses were performed using JMP version 11 (SAS Institute, Cary, NC, USA) and Stata (StataCorp, College Station, TX, USA).
Results
Patients and therapies
Patient and treatment characteristics are summarized in Table I. The median follow-up time for the 39 patients (42 lesions) was 29 months and the average follow-up 49 months. The median follow-up in our cohort of lesions which had been treated with 12 Gy or less, was 19 months (n = 13), and for lesions treated with 4 Gy (n = 8) was 14 months. Most patients (80%) were older than 40 years, and the most common site of involvement was the scalp (31% of lesions). Twenty-seven cases (64%) presented with a single lesion and were staged as IAE per the Ann Arbor staging classification (correlating to stage T1N0M0 based on ISCL/EORTC classification); another 15 cases (36%) were classified as stage IV because of multiple skin lesions (correlating to T2N0M0 based on ISCL/EORTC classification). About half of the lesions measured at 2 cm or less on clinical examination.
Table I.
Baseline patient and treatment characteristics.
| Characteristics | Value or No. (%) |
|---|---|
| Sex | |
| Male | 23 (59) |
| Female | 16 (41) |
| Age at diagnosis, years, median [range]) | 54 (24–83) |
| < 40 | 8 (20) |
| ≥ 40 | 31 (80) |
| Pathologic diagnosis (n = 42 lesions) | |
| PCMZL | 21 (50) |
| PCFCL | 16 (38) |
| PCBCLNOS | 5 (12) |
| Lesion size (median, [range]) | 2 (0.6–12) |
| ≤ 2 cm | 22 (52) |
| > 2 cm | 20 (48) |
| Lesion location | |
| Scalp | 13 (31) |
| All other sites | 29 (69) |
| Dermatological treatment | |
| Imiquimod | 2 (5%) |
| Intralesional steroids | 4 (10%) |
| None | 36 (85%) |
| Chemotherapy | |
| No chemo | 33 (79) |
| Rituximab | 4 (10) |
| R-CHOP | 2 (4) |
| Other | 3 (7) |
| Radiation modality | |
| Electrons | 39 (93) |
| Photons | 3 (7) |
| Radiation dose, Gy, median, (range) | 30 (4–39.6) |
| ≤ 12 | 13 (31) |
| > 12 | 29 (69) |
| Number of lesions | |
| 1 | 27 (65) |
| > 1 | 15 (35) |
| Relapse | |
| Yes | 10 (24) |
| No | 32 (76) |
| Stage | |
| I | 27 (64) |
| IV | 15 (36) |
PCMZL, primary cutaneous marginal zone lymphoma; PCFC, primary cutaneous follicle center lymphoma; PCBCLNOS, PCBCL not otherwise specified; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
Most patients (93%) were treated with electron-beam radiation, and most (79%) did not receive chemotherapy before or after the radiation. The most common chemotherapy regimen was single-agent rituximab. Radiation-related toxicity was minimal, with 13 patients experiencing acute grade 1 erythema of the skin, one experiencing grade 2-skin erythema, and the remainder with no acute toxicity. None of the patients experienced any long-term radiation-related side-effects.
Response to therapy
All treated lesions displayed a complete response after therapy. Of the 42 treated lesions, 10 relapses occurred in seven patients with a median time to relapse of 10 months, and all relapses occurred outside the radiation field. Among those with relapse, three lesions in two patients were salvaged successfully with 4 Gy in two fractions, with no evidence of disease at last follow-up. One relapse was treated with surgical excision, two were observed without further progression, three were treated with systemic chemotherapy, and one with intralesional steroids; and all had no evidence of, or stable disease at last follow-up. Two of the other relapses were originally PCMZL, but one recurred as PCFCL and the other as PCBCLNOS. Only two patients in the group had died, and neither death was related to PCBCL.
We found no differences in PFS or OS according to radiation dose (low-dose being ≤ 12 Gy and high-dose > 12 Gy) (Figure 1). On univariate analysis, only pathologic subtype was associated with risk of relapse (Table II), with PMZL being associated with a higher risk of relapse than PCFCL.
Figure 1.
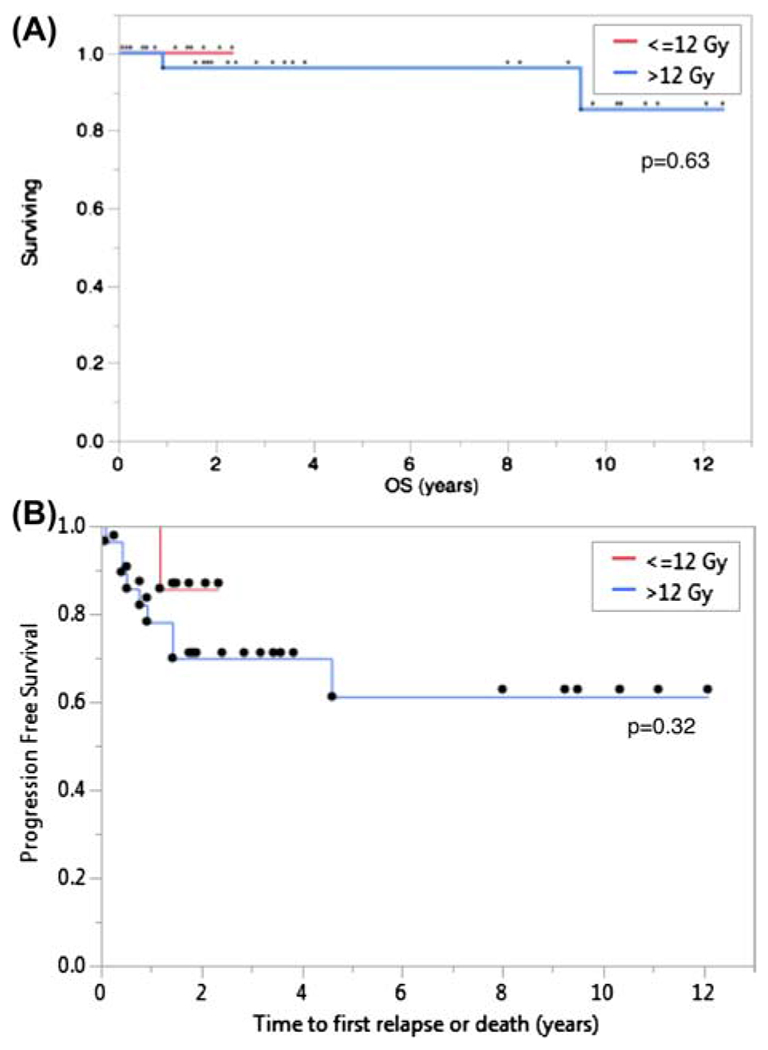
(A) Overall survival for patients treated with ≤ 12 Gy (red line) and > 12 Gy (blue line); p = 0.63. (B) Progression-free survival for lesions treated with ≤ 12 Gy (red line) and > 12 Gy (blue line); p = 0.32.
Table II.
Lesion characteristics and risk of relapse.
| Characteristics | Relapses (% of total lesions treated) | p-value |
|---|---|---|
| Lesion size | 0.36 | |
| < 2 cm | 4 (18) | |
| > 2 cm | 6 (30) | |
| Number of lesions | 0.28 | |
| One lesion | 5 (33) | |
| More than one lesion | 5 (23) | |
| Dose | 0.1 | |
| ≤ 12 Gy | 1 (8) | |
| > 12 Gy | 9 (31) | |
| Pathology | 0.009 | |
| MALT | 9 (43) | |
| PCFCL | 0 | |
| PCBCLNOS | 1 (20) |
PCMZL, primary cutaneous marginal zone lymphoma; PCFC, primary cutaneous follicle center lymphoma; PCBCLNOS, PCBCL not otherwise specified.
A total of eight lesions (seven patients) were treated with the very-low-dose radiation of 4 Gy in two fractions. All lesions showed complete response with no in-field failures after a median follow-up of 14 months (Figure 2). Only one lesion recurred outside the radiation field, and that recurrence was salvaged with another course of 4 Gy in two fractions.
Figure 2.
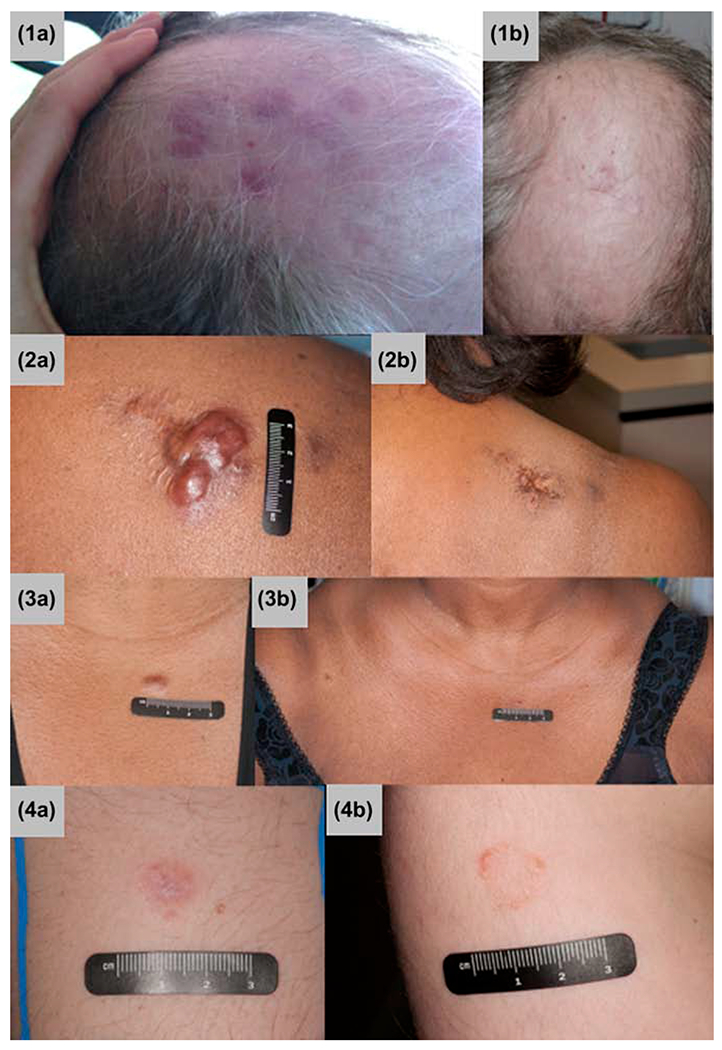
Representative photographs of lesions before treatment (a, left) and after treatment with 4 Gy (b, right). Row 1: primary cutaneous follicle center lymphoma; Row 2: primary cutaneous marginal zone lymphoma; Row 3: recurrence in patient 2, PCBCLNOS; Row 4: primary cutaneous marginal zone lymphoma.
Discussion
We found that patients with cutaneous primary BCL, including the PCMZL and PCFCL subtypes, had excellent outcomes; local control was easily achieved, mostly with local radiation, in some cases with doses as low as 4 Gy. We also showed that patients with PCBCL were at very low risk of systemic involvement, even for those with more than one lesion (stage IV disease). The overall disease course was benign, and at a median follow-up interval of 29 months, only two patients had died, both of causes unrelated to lymphoma. Multiple recurrences do not seem to affect outcomes, as recurrences were easily salvageable with further radiation therapy, chemotherapy, or intralesional injection. Lesion size (> 2 cm), the presence of multiple nearby lesions, and radiation dose less than 12 Gy did not correlate with increased risk of relapse. However, PCMZL was more likely to recur compared with PCFCL or PCBCLNOS. Interestingly, none of the patients with PCFCL had relapses, again confirming the relatively benign pathology and good outcome of this disease.
Our institutional experience is similar to others in terms of local control, for which local radiation is the main and most effective therapy. Santucci et al. [8] described the nonaggressive clinical behavior, the substantial tendency to remain localized to limited areas of the skin, and the good response to nonaggressive treatment, suggesting a benign overall prognosis on long-term follow-up. These authors also emphasized the need for recognition and proper diagnosis of this entity to avoid overtreatment. The recommended curative dose in the literature has varied from 20–45 Gy, with 24–40 Gy being most common [5,9–13]. The European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma recommends radiation to 20–36 Gy for PCMZL and > 30 Gy for PCFCL [9], The National Comprehensive Cancer Network recommends 24–30 Gy for PCMZL and 24–40 Gy for PCFCL (www.nccn.org/about/nhl.pdf). We initially started to dose de-escalate treatment of patients with PCBCLs to 12 Gy based on our own institutional experience and other reports in treatment of other indolent hematologic conditions, for instance Langerhans cell histiocytosis [14]. This was further supported by Lowry et al.’s randomized trial, which showed that doses as low as 12 Gy can be adequate in controlling non-Hodgkin lymphomas [15]. Along with the emerging evidence that doses as low as 4 Gy can be used for palliation of PCBCLs based on a complete response of 72% seen by Neelis et al. [12], or even result in cure as seen in orbital lymphomas based on the Stanford series [16], we further decreased our initial radiation dose in treatment of PCBCLs to 4 Gy.
We have now added to this growing international experience by showing that for patients receiving definitive therapy for PCBCL who have had a complete staging work-up and no evidence of systemic disease, there was no difference in outcome for those receiving < 12 Gy versus > 12 Gy. Moreover, we found that even doses as low as 4 Gy can control this disease in both the primary and relapsed setting, as evidenced by the eight lesions treated in our series. Based on our findings and the indolent nature of this disease, we believe that after considering the overall clinical picture of a patient with primary or relapsed PCBCL, strong consideration should be given to initial treatment with low-dose of 4 Gy with close monitoring post-treatment for response, progression, or relapse of disease. If a complete response is not seen or the patient relapses in the same site or even a different location, they can easily be salvaged with further radiation therapy. This recommendation is also supported by the 100% complete response rate, overall high survival rates, and multiple salvage options including re-irradiation with 4 Gy.
This study did have shortcomings, in particular its retrospective nature and the relatively short follow-up time for those who received low-dose radiation therapy, the latter reflecting the recent adoption of less-than-12-Gy regimens at our institution. However, our median follow-up for patients treated with 12 Gy or less (19 months) was longer than the median time to relapse (10 months). Additionally, the rarity of this disease precludes its being studied in prospective trials.
Our results add to the body of the literature regarding PCBCL, with this report of one of the largest number of patients treated with definitive intent with low doses of radiation therapy to date. It is important to continue to increase awareness regarding PCBCL, particularly its indolent clinical course and excellent outcomes, to reduce the risk of over-treating this disease with high doses of radiation therapy or systemic chemotherapy.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- [1].Jaffe ES, Harris NL, Stein H, et al. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood 2008;112:4384–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768–3785. [DOI] [PubMed] [Google Scholar]
- [3].Schreuder MI, Hoefnagel JJ, Jansen PM, et al. FISH analysis of MALT lymphoma-specific translocations and aneuploidy in primary cutaneous marginal zone lymphoma. J Pathol 2005;205:302–310. [DOI] [PubMed] [Google Scholar]
- [4].Triscott JA, Ritter JH, Swanson PE, et al. Immunoreactivity for bcl-2 protein in cutaneous lymphomas and lymphoid hyperplasias. J Cutan Pathol 1995;22:2–10. [DOI] [PubMed] [Google Scholar]
- [5].Senff NJ, Kluin-Nelemans HC, Willemze R. Results of bone marrow examination in 275 patients with histological features that suggest an indolent type of cutaneous B-cell lymphoma. Br J Haematol 2008;142:52–56. [DOI] [PubMed] [Google Scholar]
- [6].Senff NJ, Noordijk EM, Kim YH, Bagot M, Berti E, Cerroni L, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood 2008;112:1600–1609. [DOI] [PubMed] [Google Scholar]
- [7].Cerroni L, Arzberger E, Putz B, et al. Primary cutaneous follicle center cell lymphoma with follicular growth pattern. Blood 2000;95:3922–3928. [PubMed] [Google Scholar]
- [8].Santucci M, Pimpinelli N, Arganini L. Primary cutaneous B-cell lymphoma: a unique type of low-grade lymphoma. Clinicopathologic and immunologic study of 83 cases. Cancer 1991;67:2311–2326. [DOI] [PubMed] [Google Scholar]
- [9].Eich HT, Eich D, Micke O, et al. Long-term efficacy, curative potential, and prognostic factors of radiotherapy in primary cutaneous B-cell lymphoma. Int J Radiat Oncol Biol Phys 2003;55:899–906. [DOI] [PubMed] [Google Scholar]
- [10].Hoefnagel JJ, Vermeer MH, Jansen PM, et al. Primary cutaneous marginal zone B-cell lymphoma: clinical and therapeutic features in 50 cases. Arch Dermatol 2005;141:1139–1145. [DOI] [PubMed] [Google Scholar]
- [11].Kirova YM, Piedbois Y, Le Bourgeois JP. Radiotherapy in the management of cutaneous B-cell lymphoma. Our experience in 25 cases. Radiother Oncol 1999;52:15–18. [DOI] [PubMed] [Google Scholar]
- [12].Neelis KJ, Schimmel EC, Vermeer MH, et al. Low-dose palliative radiotherapy for cutaneous B- and T-cell lymphomas. Int J Radiat Oncol Biol Phys 2009;74:154–158. [DOI] [PubMed] [Google Scholar]
- [13].Smith BD, Glusac EJ, McNiff JM, et al. Primary cutaneous B-cell lymphoma treated with radiotherapy: a comparison of the European Organization for Research and Treatment of Cancer and the WHO classification systems. J Clin Oncol 2004;22:634–639. [DOI] [PubMed] [Google Scholar]
- [14].Jahraus CD, Russo S, Peñagarícano J, et al. Radiotherapy dose fractionation in pediatric Langerhans cell histiocytosis. Southern Med J 2004;97:1268–1269. [DOI] [PubMed] [Google Scholar]
- [15].Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomized phase III trial. Radiother Oncol 2011;100:86–92. [DOI] [PubMed] [Google Scholar]
- [16].Fasola CE, Jones JC, Huang DD, et al. Low-dose radiation therapy (2 Gy × 2) in the treatment of orbital lymphoma. Int J Radiat Oncol Biol Phys 2013;86:930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]


