Abstract
Lipopolysaccharide (LPS) is one of the main virulence factors of gram-negative bacteria. The LPS from Campylobacter spp. has endotoxic properties and has been shown to play a role in adhesion. We previously cloned a gene cluster (wla) which is involved in the synthesis of the Campylobacter jejuni 81116 LPS molecule. Sequence alignment of the first gene in this cluster indicated similarity with galE genes. These genes encode a UDP-glucose 4-epimerase, which catalyzes the interconversion of UDP-galactose and UDP-glucose. A Salmonella galE mutant was transformed with the galE gene from C. jejuni. The LPS analysis of wild-type, galE, and complemented galE Salmonella strains showed that the C. jejuni galE gene could restore the smooth wild-type Salmonella LPS. A UDP-glucose 4-epimerase assay was used to demonstrate that the galE gene from C. jejuni encoded this epimerase. We constructed a C. jejuni galE mutant which expressed a lipid A-core molecule of reduced molecular weight that did not react with antiserum raised against the parental strain. These results show an essential role for the galE gene in the synthesis of C. jejuni LPS. The galE mutant also showed a reduction in its ability to adhere to and invade INT407 cells. However, it was still able to colonize chickens to the same level as the wild-type strain. The serum resistance and hemolytic activity of this mutant were not changed compared to the parent strain. The ability of the mutant to take up DNA and integrate it in its genome was reduced 20-fold. These results show that LPS of C. jejuni is an important virulence factor.
Campylobacter jejuni, an agent causing human enterocolitis, is the most common cause of bacterial diarrhea in many countries (68). Symptoms most frequently seen are acute abdominal pain and inflammatory diarrhea, often with fever (20). C. jejuni also asymptomatically colonizes the intestine of birds (12, 65).
Lipopolysaccharides (LPS) are an abundant surface component of the outer membrane of gram-negative bacteria. The LPS molecule consists of three distinct regions. Anchored in the outer membrane is the lipid A moiety, which is the endotoxic part of the LPS molecule. Attached to the lipid A is the core, which consists of an inner and outer part. Finally, the O antigen is a polysaccharide repeat and is normally attached to the outer core.
C. jejuni strains synthesize LPS molecules with or without an O-antigen-like repeat structure. The LPS molecules of Campylobacter have been shown to have endotoxic properties (17, 53). Furthermore, they have been reported to be involved in adherence (49) and may play a role in antigenic variation, as these bacteria have the ability to shift the LPS antigenic composition (50).
The sugar composition and structure of the core oligosaccharide from several C. jejuni strains, belonging to eight serotypes, have been analyzed (4–6, 8, 58). Surprising is the presence of N-acetylneuraminic acid (sialic acid), a molecule not frequently found in prokaryotes. These sialic acid residues, when attached by 2-3 linkages to β-d-galactosidase resemble gangliosides in structure (7, 8). This molecular mimicry is thought to play a role in the neuropathological autoimmune diseases Guillain-Barré syndrome and Miller-Fisher syndrome (58, 61). In Neisseria spp. and Haemophilus spp., sialylation of LPS is important in pathogenicity, by enhancing serum resistance (26, 52). The role of sialylation of the Campylobacter spp. LPS in pathogenicity has not yet been determined.
The metabolic pathways and enzymes required to synthesize the LPS molecules in C. jejuni have not yet been characterized. Rapid progress in the study of LPS synthesis in other bacteria has been made by a genetic approach in combination with knowledge on the structure of the molecules. Recently we have cloned a gene cluster (wla) which is involved in the synthesis of the C. jejuni 81116 LPS molecule (30).
Here we report the characterization of an important gene in the wla gene cluster. We show that this gene encodes a UDP-glucose 4-epimerase and that it is involved in LPS synthesis by using complementation and mutagenesis experiments. Furthermore, we show the importance of LPS in virulence of C. jejuni.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. C. jejuni 81116 was originally isolated from a human waterborne outbreak of enteritis (54). Escherichia coli DH5α (34) was used as a host for pBluescript (63) plasmid constructs, and E. coli HB101 was used as a host for pBTLPS and cosmid pBT9502. C. jejuni strains were grown under microaerophilic conditions on Skirrow's agar medium (64) or in heart infusion (HI) (Difco) broth at 42°C for 24 h; E. coli strains were grown in Luria-Bertani (LB) broth or agar for 16 h at 37°C. Salmonella enterica serovar Typhimurium strains were grown in LB broth or on MacConkey agar (Oxoid) or modified MacConkey agar (MacConkey agar without lactose) containing different concentrations of galactose.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Description | Reference |
|---|---|---|
| C. jejuni strains | ||
| 81116 | Penner 6 serotype | 54 |
| 81116galEa | 81116 transformed with KOgalEa | This study |
| 81116galEb | 81116 transformed with KOgalEb | This study |
| 81116flaB | Kanamycin resistance cassette inserted in the flaB gene | 71 |
| 81116 T1 | Tetracycline resistance cassette inserted in the flaB gene | 72 |
| E. coli strains | ||
| DH5α | F′/endA1 hsdR17(r−K m+K) subE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacZYA-argF)U169 (φ80lacZΔM15) | 34 |
| HB101 | Δ(gpt- proA)62 leuB6 thi-1 lacY1 hsdSB20 recA rpsL20 (Strr) ara-14 galK2 xyl-5 mtl-1 subE44 mcrBB | 16 |
| Salmonella serovar Typhimurium strains | ||
| SL696 | Wild type | 75 |
| SL761 | galE | 66 |
| Plasmids | ||
| pBT9502 | Cosmid vector pLA2917 containing the wla gene cluster from C. jejuni 81116 | 30 |
| pBTLPS | pBR322 containing the wla gene cluster from C. jejuni 81116 | 30 |
| pB1 | Sequencing vector pBluescript KS M13+ | 63 |
| pBR322 | Cloning vector | 15 |
| pMW2 | Kanamycin resistance cassette in pB1 | M. Wösten, unpublished |
| pBF84A | 3.2-kb BglII fragment from pBT9502 in pB1 | This study |
| pBF84B | 3.2-kb BglII fragment from pBT9502 in pB1, orientation of insert opposite that in pBF84A | This study |
| pBF84And47 | pBF84A missing 828 bp of the wlaB gene | This study |
| pBF84Bnd17 | pBF84B missing 1,300 bp of the region upstream of the galE gene | This study |
| pBF84Bnd17p | pBF84Bnd17 with a BglII site and a deletion of 481 bp in the galE gene | This study |
| KOgalEa | pBF84Bnd17p containing the kanamycin resistance gene from pMW2 in the BglII site | This study |
| KOgalEb | Like KOgalEb but with kanamycin resistance gene in opposite direction | This study |
Antibiotic concentrations used were as follows: kanamycin (Sigma Chemical), 30 μg/ml; ampicillin (Centrafarm), 100 μg/ml; and tetracycline (Sigma), 20 μg/ml.
PBF84And47 was made by deleting 828 bp of the wlaB gene from plasmid pBF84A by using a nested deletion kit from Pharmacia.
DNA analysis.
PC/Gene 6.70 (38) was used to analyze nucleotide and amino acid sequences, which were compared to databases available at GenomeNet using the program BLAST (3). The Macaw program (40) was used for multiple sequence alignment.
DNA techniques.
DNA isolations, restriction enzyme digestions, and DNA ligations were performed as described by Ausubel et al. (9). Restriction enzymes, alkaline phosphatase, and a nested deletion kit, obtained from Pharmacia, were used according to the manufacturer's instructions.
Transformation of Salmonella serovar Typhimurium.
The serovar Typhimurium strain SL761 was transformed with 1 μg of pBTLPS and pBF84And47 by electroporation with a Bio-Rad Gene Pulser (Biotechnologies and Experimental Research Inc., San Diego, Calif.) set at 12.5 kV/cm, 25 μF, and 200 Ω.
Preparation of polyclonal ascites antisera.
Nine-month-old BALC mice were used to raise mouse anti-C. jejuni 81116 LPS sera. Mice were administered 0.4 ml of pristane intraperitoneally. Mice were stimulated intraperitoneally after 7 days, with 100 μg of LPS and after 21 and 31 days with 75 μg of LPS. Mice were inoculated with 2 × 107 Sp/2 cells after 41 days. Sera were collected after 51 to 60 days.
LPS and protein isolation, PAGE, silver staining and immunoblotting.
Cell envelopes were isolated by the procedure of Lugtenberg et al. (45) and treated with pronase overnight at 37°C to obtain the LPS samples. The isolated LPS was resolved by Tricine polyacrylamide gel electrophoresis (PAGE) (42, 60) and analyzed by silver staining (69), zinc staining (35), and Western blotting (47). C. jejuni 81116 antiserum diluted 1:1,000 in TST (10 mM Tris-HCl [pH 7.4], 100 mM NaCl, 0.1% Tween 20) containing 5% skimmed milk was used in immunoblotting. Goat anti-mouse horseradish peroxidase-conjugated immunoglobulins (Bio-Rad) were used as the second antibody diluted 1:3,000 in TST containing 1% skimmed milk. The bound peroxidase was visualized with 4-chloro-1-naphthol. The Multimark multicolored standard (Novel Experimental Technology) was used as a molecular weight marker.
Epimerase assay.
The UDP-galactose 4-epimerase activity was measured with a colorimetric epimerase assay (51). To 10 μl of cell extract, 100 μl of 10 mM Tris-HCl (pH 8.7) and 100 μl of 5 mM UDP-galactose were added. After 10 min the reaction was stopped by addition of 25 μl of 0.1 N HCl and boiling for 5 min. To neutralize the mixture, 25 μl of 0.1 N NaOH was added. The formed glucose was assayed by addition of 2 ml of 0.1 M phosphate buffer (pH 7.0) containing 200 μg of glucose oxidase, 10 μg of peroxidase, and 600 μg of o-dianisidine. After 30 min the reaction was stopped by addition of 2.5 ml of 6 N HCl, and the color was read at 540 nm.
Construction of a galE insertional mutant.
No suitable restriction sites are present within the sequence of the galE gene of C. jejuni 81116 for insertion of a kanamycin resistance cassette. Therefore, inverse PCR was used to introduce a unique BglII restriction site and to delete part of the galE gene. Before performing inverse PCR, we deleted 1,300 bp of the insert of clone pBF84B by using a nested deletion kit from Pharmacia, resulting in plasmid pBF84Bnd17. This was done to facilitate the inverse PCR. The primers used for the inverse PCR with added BglII restriction site (shown in boldface) were galE1 (5′-GCTCAGATCTGGAGTTTGTGGTTCGCCATAAG-3′) and galE2 (5′-AATCAGATCTCTTACTTCTTGGCAGCCTA-3′, corresponding to nucleotides 1573 to 1542 and 2068 to 2096 of the complete wla cluster sequence (EMBL accession no. Y11648), respectively. The underlined nucleotides are different from the original sequence to create the BglII restriction site. The PCR conditions were 35 cycles of 1 min at 94°C, 1 min at 62°C, 9 min at 72°C, and a final extension step of 10 min at 72°C. Pfu DNA polymerase from Stratagene was used to minimize the error rate. After inverse PCR, digestion with BglII, and self-ligation, we obtained plasmid pBF84Bnd17p, which contained a unique BglII site. This site was then used for inserting a kanamycin resistance cassette (39) originating from plasmid pMW2 containing BamHI ends. The kanamycin resistance cassette was placed in the galE gene in both orientations. The resulting knockout constructs were named KOgalEa and KOgalEb. The kanamycin resistance cassette does not contain a transcriptional stop.
Natural transformation was used to introduce the constructs in C. jejuni 81116 as described previously (73).
Southern blotting.
Chromosomal DNA of kanamycin-resistant transformants was isolated and digested with restriction endonucleases HindIII and ClaI. The resulting restriction fragments were separated on an agarose gel and blotted onto Hybond-N nylon membrane. Plasmid pBF84Bnd17 was labeled with [α-32P]dATP and used as a probe. Hybridizations were performed according to Sambrook et al. (59).
Galactose sensitivity.
C. jejuni 81116, 81116galEa and 81116galEb were grown on Skirrow's agar plates and in HI broth containing 1 to 10% galactose. Growth in the broth was measured by determining the optical density at 600 nm.
Adherence and invasion assays.
Adherence and invasion assays using INT407 cells were performed as described previously (71).
Chicken colonization experiments.
A quantitative oral chick colonization assay was performed as previously described (74). Briefly, groups of specific-pathogen-free chickens (SPAFAS, Charles River, Mass.), housed in isolators, were dosed (100 μl) at 1 day of age by oral gavage with a suspension of C. jejuni 81116 or the mutant. Doses were prepared by harvesting bacteria, grown overnight on blood agar plates at 42°C, into sterile phosphate-buffered saline (PBS). At 5 days postinfection, the level of colonization was determined by plating out dilutions of cecal contents. Chick colonization levels were given as CFUs per gram of cecal contents.
Serum sensitivity assay.
A modified serum resistance assay was previously described (11, 14). Normal human serum (NHS) was collected and stored in −70°C before use. Ten percent NHS was diluted with medium 199 with Hanks balanced salt solution (HBSS). The serum sensitivity test was performed in sterile microtiter plates. C. jejuni cells (100 μl; 106 cells/ml) and 100 μl of 10% NHS were added in the microtiter. Medium 199 with HBSS but without NHS was added to the controls. After 30 and 45 min of incubation, the samples were diluted 15 times in HI broth and plated out on Skirrow's agar medium plates. Plates were incubated at 42°C for 48 h in microaerobic atmosphere, and colony counts were obtained.
Hemolysis assay.
The hemolysis assay used in this study was described by Grant et al. (32). Fresh sheep erythrocytes were washed with PBS three to five times. Washed bacterial cells (1011) were spun down, resuspended in 1.5 ml of PBS, and mixed with an equal volume of 2% erythrocytes in PBS. The samples were incubated at 42°C for 3 to 40 h aerobically. Then the intact cells were spun down at 2,000 × g for 20 min, and 100 μl of supernatant was taken. Hemolysis was determined by detecting the absorbance at 550 nm. Complete hemolysis and spontaneous hemolysis were measured by adding equal volumes of distilled water and PBS, respectively.
DNA uptake assay.
Natural transformation was used to study the uptake and integration of chromosomal DNA in C. jejuni 81116 as described previously (73). A suspension of 108 CFU per ml was incubated at 37°C for 3 h under microaerophilic conditions to induce competence. C. jejuni 81116 T1 (a mutant strain containing a tetracycline resistance cassette [72]) chromosomal DNA was added, and cells were incubated for 3 h at 37°C. Cells were harvested and plated on media supplemented with tetracycline to determine the number of transformants.
RESULTS
Characteristics of the galE gene.
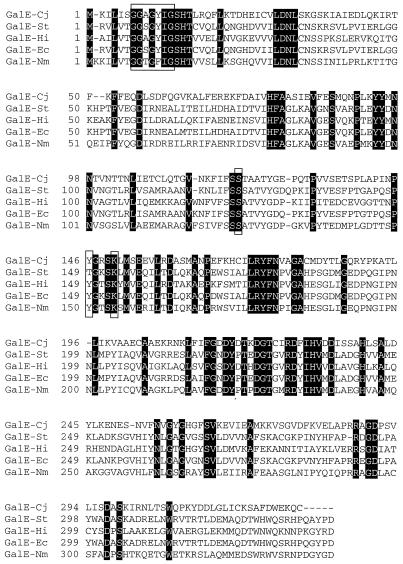
Previously we cloned and sequenced the wla gene cluster of C. jejuni 81116 (30). Here we report on the characterization of the first gene in this cluster. Sequence analysis of this gene revealed an open reading frame of 987 bp initiating with a methionine and a potential Shine-Dalgarno sequence 11 bp upstream of this start codon. No obvious putative promoter sequences were found. Comparison of the deduced amino acid sequence of this open reading frame with protein sequence databases revealed homology to proteins encoding UDP-galactose 4-epimerases (GalE [Fig. 1]) from Salmonella serovar Typhimurium (37) (33.2% identity; 56.6% similarity), Haemophilus influenzae (48) (37.7% identity; 58.0% similarity), E. coli (41) (36.3% identity; 57.5% similarity), and Neisseria meningitidis (33; 36.2% identity; 58.3% similarity). Therefore, this gene was named galE. The GalE enzyme catalyzes the interconversion between UDP-glucose and UDP-galactose. The amino acid sequence of the C. jejuni GalE contains several domains that are conserved throughout GalE proteins (24, 25) (Fig. 1). The NAD-binding domain (GxxGxxG [46]) is found between amino acids 7 and 13 (Fig. 1). Lys153 from E. coli has also been shown to bind NAD+, which is essential for enzyme activity (68). In the C. jejuni GalE, this Lys is found in position 151 (Fig. 1). Liu et al. (43) showed that serine 124 and tyrosine 149 (for C. jejuni GalE positions 122 and 147, respectively) are also important for epimerase activity.
FIG. 1.
Alignment of the deduced amino acid sequences for GalE from C. jejuni (Cj), Salmonella serovar Typhimurium (St), H. influenzae (Hi), E. coli (Ec), and N. meningitidis (Nm), performed with the program Macaw. Shaded letters indicate identical amino acids. The conserved NAD-binding domain (GxxGxxG [46]) and essential amino acids are boxed.
Functional analysis of the putative galE gene.
To confirm the function of the putative galE gene from C. jejuni, plasmids pBTLPS (carrying the complete wla cluster), pBF84And47 (carrying only the galE gene), pBR322, and pB1 were transformed into SL761 (a serovar Typhimurium galE mutant strain). To test for complementation the parent serovar Typhimurium wild-type strain, strain SL761 and the transformants were first grown on modified MacConkey agar. Serovar Typhimurium strains ferment galactose in the Leloir pathway, resulting in the production of acids. With growth on modified MacConkey agar medium, this drop in pH causes a change of color of the plates to red. However the serovar Typhimurium galE mutant strain cannot use the galactose in the medium and therefore does not cause discoloration of the plates. Growth of SL761(pBTLPS) and SL761(pBF84And47) on the modified MacConkey agar plates resulted in red plates, whereas the controls SL761(pBR322) and SL761(pB1) did not induce the color change (results not shown).
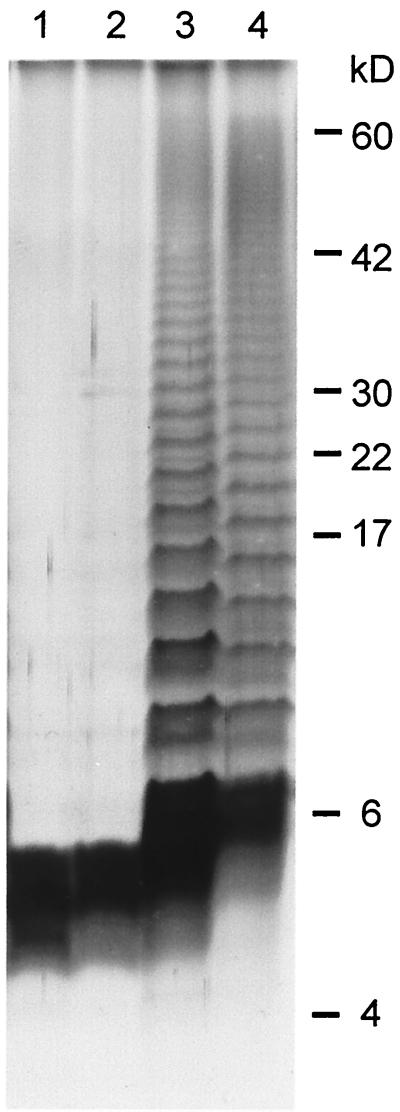
The serovar Typhimurium galE mutant transformants were also assayed for UDP-glucose epimerase activity. The UDP-glucose epimerase activity in serovar Typhimurium is induced by galactose (Table 2). Mutant strain SL761 did not show any UDP-glucose epimerase activity with or without the addition of galactose. Transformant SL761(pBF84And47) showed activity with and without galactose. This activity was twice as high as the activity measured for the serovar Typhimurium wild-type strain. Addition of isopropyl-β-d-thiogalactopyranoside to induce the lacZ promoter on pBF84And47 did not change the epimerase activity, indicating that the promoter of the C. jejuni galE gene is functional in serovar Typhimurium. The control strain SL761(pB1) showed no activity. These results show that the galE gene from C. jejuni encodes a UDP-glucose epimerase. The inactivation of the galE gene in serovar typhimurium results in the expression of a truncated LPS molecule lacking the O antigen. To investigate if the C. jejuni galE gene can restore expression of the complete LPS molecule in strain SL761, LPS was isolated from parent serovar typhimurium strain SL696, mutant strain SL761, strain SL761(pBF84And47), and strain SL761(pB1) and analyzed by Tricine sodium dodecyl sulfate (SDS) PAGE followed by silver staining. LPS isolated from SL761(pBF84And47) showed a complete O antigen as seen for LPS isolated from the wild-type strain (Fig. 2). LPS isolated from the control strain SL761(pB1) did not show the reversion to the complete LPS molecule (Fig. 2).
TABLE 2.
UDP-galactose epimerase activity of serovar Typhimurium strains
| Strain | UDP-galactose epimerase activity (mU/mg of protein)a
|
|
|---|---|---|
| −Galactose | +Galactose | |
| SL696 | 0 | 95 |
| SL761 | 0 | 0 |
| SL761(pBF84And47) | 214 | 219 |
| SL761(pB1) | 0 | 0 |
For strains grown on media with and without 1% galactose.
FIG. 2.
LPS analysis by tricine SDS-PAGE of LPS prepared from serovar Typhimurium strains. LPS was visualized by silver staining. Lanes: 1, SL761; 2, control SL761(pB1); 3, SL761(pBF84And47); 4, parent strain SL696.
Evidence that the C. jejuni galE gene is involved in LPS biosynthesis in C. jejuni.
To determine the function of the galE gene in C. jejuni, a mutant strain was constructed. Inverse PCR was used to introduce a unique BglII restriction site and at the same time delete part of the galE gene. To facilitate the inverse PCR, 1,300 bp of the insert of plasmid pBF84B containing the galE gene was deleted, resulting in plasmid pBF84Bnd17. The unique BglII restriction site of plasmid pBF84Bnd17p (resulting after the inverse PCR) was used to introduce a kanamycin resistance cassette. The two resulting constructs, KOgalEa and KOgalEb (containing the kanamycin resistance cassette in the same and opposite orientations relative to the galE gene, respectively) were used to transform C. jejuni strain 81116. Both plasmids KOgalEa and KOgalEb yielded kanamycin-resistant transformants, which were named 81116galEa and 81116galEb, respectively.
Introduction of the kanamycin resistance cassette in the correct position on the chromosome was verified by Southern blot analysis (Fig. 3). In all transformants, the expected homologous recombinations had occurred. The Southern blot analysis also showed that C. jejuni 81116 carries only one copy of the galE gene. Screening the genome sequence of C. jejuni 11168 (Sanger Centre website [http://www.sanger.ac.uk]) with the galE gene sequence from strain 81116 also showed the presence of only one copy.
FIG. 3.
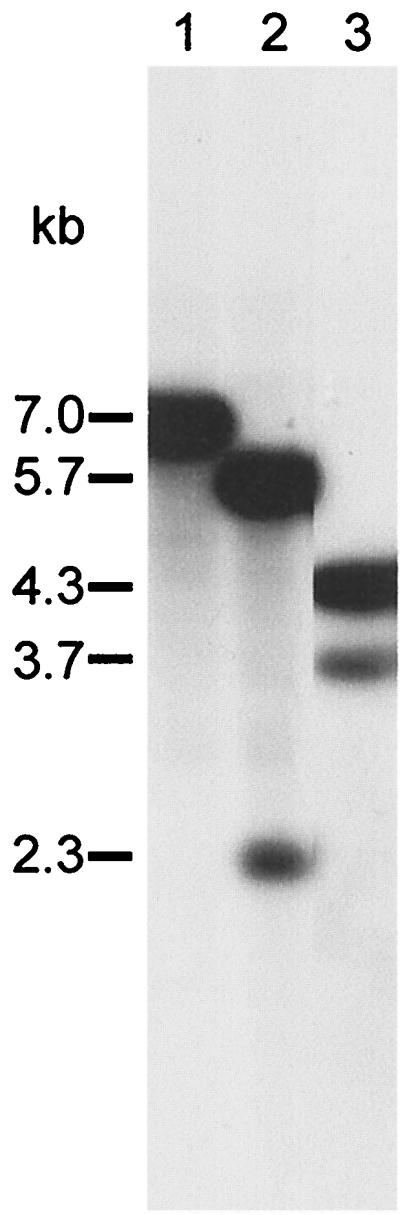
Southern blot analysis of chromosomal DNA preparations from C. jejuni wild-type and galE mutant strains. Chromosomal DNAs were digested with HindIII, resolved by agarose gel electrophoresis, and probed with plasmid pBF84Bnd17 containing the C. jejuni galE gene. Lane 1, wild-type strain 81116; lane 2, strain 81116galEa; lane 3, strain 81116galEb.
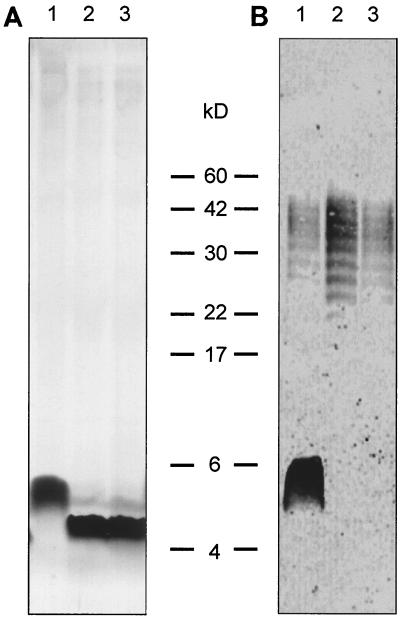
The effect of the galE mutation on LPS synthesis was investigated by isolating LPS and analyzing it by tricine PAGE followed by silver staining and Western blotting (Fig. 4). Silver staining showed that the parent strain expressed a lipid A-core molecule of around 5.5 kDa, whereas the galE mutant strain expressed a molecule of 4.5 kDa. This is consistent with the loss of galactose-containing units from the core. The Western blot showed that the lipid A-core complex, expressed by the galE mutant strain, no longer reacted with antiserum raised against C. jejuni 81116. The high-molecular-weight O-antigen-like structure could still be seen; however, it could not be stained with the LPS-specific silver staining, as reported previously (30). These results indicate that the core molecule expressed by the galE mutant strain is truncated and that this modification removes all core epitopes that react with the anti-C. jejuni 81116 antiserum.
FIG. 4.
LPS separation by Tricine SDS-PAGE. (A) Silver-stained LPS samples; (B) immunoblot with C. jejuni 81116 LPS antiserum. Lanes: 1, parent strain 81116; 2, strain 81116galEa; 3, strain 81116galEb.
The effect of kanamycin on LPS synthesis was tested by growing the mutant strains on media with and without kanamycin. The effect of the kanamycin cassette insertion on LPS synthesis was tested by examining the LPS of a flagellin mutant containing the same kanamycin cassette. Neither kanamycin nor the kanamycin cassette insertion had any effect on LPS synthesis.
Addition of exogenous galactose to a galE mutant from H. influenzae has been shown to restore the phenotypic defect of the LPS (48). However, addition of galactose or UDP-galactose during growth of the C. jejuni galE mutant strain did not restore the LPS phenotype.
E. coli and serovar Typhimurium galE mutants are sensitive to galactose. Exogenous galactose is taken up and converted to UDP-galactose, which accumulates to toxic levels, resulting in lysis. The parent C. jejuni strain 81116 and its galE mutant showed no difference in growth rates (galactose sensitivity) when grown on different galactose concentrations ranging from 0 to 10%. This suggests that C. jejuni 81116 is unable to utilize exogenous galactose. Therefore, the galE mutant is not susceptible to the toxic effects associated with accumulation of UDP-galactose. This is supported by the results that the LPS phenotype as seen for the galE mutant could not be complemented by galactose or UDP-galactose.
It is unlikely that the observed phenotypic changes are due to polar effects, as the kanamycin cassette does not contain a transcription terminator. Moreover, the kanamycin cassette can be inserted in both orientations, and the gene downstream of the galE gene seems to be an essential gene (B. N. Fry, unpublished data).
The involvement of LPS in virulence.
Several virulence indicators of the galE mutant were compared to those of the parent strain. The results are given in Tables 3 and 4. The ability of the galE mutant to adhere to or invade INT407 cells was reduced 20- and 100-fold, respectively. This indicates that LPS plays a major role in these processes.
TABLE 3.
Properties of the C. jejuni 81116 galE mutant virulence indicators compared to those of the parent wild-type strain
| Strain | % Adherencea | % Invasiona | % Survivalb | Hemolysis (% of lysed sheep erythrocytes)
|
% DNA uptakec | ||
|---|---|---|---|---|---|---|---|
| 4 h | 17 h | 41 h | |||||
| Wild type | 100 | 100 | 25.6 ± 12.3 | 7.7 ± 2.0 | 34.7 ± 22.2 | 61 ± 8.9 | 100 |
| galE mutant | 4.6 ± 5.2 | 0.9 ± 1.2 | 24.1 ± 14.3 | 8.5 ± 1.3 | 46.3 ± 7.2 | 86.9 ± 26.4 | 4.5 ± 5.1 |
Adherence or invasion of INT407 cells by C. jejuni 81116 galE mutant compared to the parent strain C. jejuni 81116. The number of 81116 cells that adhered or invaded was taken as 100%.
Percentage of surviving cells after incubation for 30 min with NHS.
Transformation efficiency (calculated per microgram of DNA per 108 CFU) compared to the transformation efficiency of 81116.
TABLE 4.
Colonization of 1-day-old chicks with C. jejuni 81116 wild type and galE mutant
| Strain | Dose | No. of chicks colonized/10 infected | Colonization level (CFU/g of cecal contents) |
|---|---|---|---|
| Wild type | 2.5 × 104 | 9 | 2.5 × 104a |
| 5 × 105 | 10 | 6 × 109a | |
| galE mutant | 104 | 2 | 108 and 103 |
| 4 × 105 | 10 | 7 × 109a |
Geometric mean.
In E. coli O75:K5 (18), H. influenzae (36), and Neisseria spp. (33), LPS has been shown to play a role in resistance to serum. C. jejuni is known to be serum resistant to 10% NHS (2, 13). Therefore, we also investigated if the galE mutant of C. jejuni differed in serum resistance from the parent strain. The results show that there is no significant difference in serum resistance between the galE mutant and the parent strain. Also, the hemolytic properties of the galE mutant were unchanged compared to the parent strain.
C. jejuni strains have been shown to be able to take up DNA from the environment and integrate it into their genome without special treatment (70, 73). This natural competence for DNA uptake could be important for acquiring new advantageous features or new virulence factors. In Neisseria spp. (28) and Haemophilus spp. (23), DNA uptake involves receptors that recognize specific DNA sequences. To investigate if the changed LPS molecules had any effects on DNA uptake in C. jejuni, the ability for natural transformation of the galE mutant was studied. Chromosomal DNA from a C. jejuni mutant carrying a tetracycline cassette was used for transformation. The ability of the galE mutant to take up DNA was reduced to less than 5% of that of the parent strain. Whether LPS plays a direct role in DNA uptake as a receptor or indirect by forming complexes with receptors remains to be examined. The galE mutant was also tested for the ability to colonize the ceca of 1-day-old chicks following oral challenge. In previous work, 105 CFU of C. jejuni 81116 colonized 100% of the chicks challenged to a maximum level of about 109 CFU per g of cecal contents within 5 days (74). In these experiments, the parent strain colonized chicks to the expected levels at the doses given (Table 4). The colonization potential was slightly reduced at the lower dose level of the galE mutant but still within experimental variability. At the higher dose level the colonization potential was unaffected by the mutation, indicating that GalE was not required for optimal colonization of the avian gut.
DISCUSSION
This study shows that the first gene in a gene cluster that is involved in LPS synthesis from C. jejuni is homologous to the galE genes from several species. These genes encode for a UDP-galactose 4-epimerase, which catalyzes the interconversion of UDP-galactose and UDP-glucose. UDP-galactose is used for the synthesis of carbohydrate polymers composed of galactose, including the bacterial virulence factors LPS and exopolysaccharide. The deduced GalE protein sequence from the galE gene of C. jejuni contains a specific NAD-binding domain and amino acids that are known to be essential for epimerase activity.
The location of the galE genes on bacterial chromosomes can be classified into three groups. In E. coli (19), Salmonella serovar Typhimurium (37), Streptomyces lividans (1), and Lactobacillus casei (10), the galE, galT, and galK genes encoding proteins of the Leloir pathway are grouped together on the chromosome. In a second group of bacteria, e.g., H. influenzae (48), Rhizobium meliloti (44), Vibrio cholerae (22, 29), Neisseria spp. (33), and Erwinia stewartii (27), the galE gene is found linked to genes involved in the synthesis of polysaccharides. In the third group of bacteria (e.g., Brucella abortus [63] and Pasteurella haemolytica [56]), the galE gene is linked to neither galactose metabolism genes nor polysaccharide genes. In addition, a few bacterial species have two functional galE genes. For example, Yersinia enterocolitica has one galE gene linked to galactose utilization genes and the other linked to the LPS synthesis locus. C. jejuni has only one galE gene and belongs to the second group of bacteria, as the gene is linked with the genes involved in LPS synthesis. The galK-like gene of C. jejuni is located approximately 300 kb upstream from the galE gene in C. jejuni 11168 (Sanger Centre website).
The galE gene from C. jejuni can complement a Salmonella serovar Typhimurium galE mutant for the utilization of galactose, epimerase activity, and LPS synthesis. These results show that the galE gene from C. jejuni encodes for a UDP-galactose epimerase. The epimerase activity was higher in the complemented serovar Typhimurium mutant than the serovar Typhimurium wild-type strain. This can be explained by the presence of multiple copies per cell of the C. jejuni galE gene due to the use of the multicopy plasmid pBluescript to produce transformants.
The essential role that GalE plays in campylobacter LPS biosynthesis was shown by electrophoretic analysis of LPS purified from the parental and mutant strains. The lipid A-core part of the LPS molecule was reduced in size, suggesting that sugars from the outer core were missing. However, the O-antigen-like structure was still present in the galE mutant strain. Therefore, the O-antigen-like structure either is attached to the inner part of the LPS molecule or is not part of the LPS molecule. As the LPS-specific silver staining procedure does not stain the high-molecular-weight O-antigen-like structures, we can conclude that it is not part of the LPS molecule. Also, the reverse zinc staining method stained the lipid A-core molecule and not the O-antigen-like structures (results not shown), indicating that they are not related. These results support our previous suggestion that the O-antigen-like structure from C. jejuni is not linked to the LPS molecule and therefore resembles capsular polysaccharide or enterobacterial common antigen, as found in E. coli, rather than an LPS O antigen (30).
A study by McSweegan and Walker (49) suggested a role for LPS in adherence. The LPS mutant constructed in this study can now be used to study the extent of this role. The inactivation of the galE gene significantly reduced but did not completely abolish C. jejuni adherence. The presence of multiple adhesins is a common finding in pathogenic bacteria and has also been suggested for C. jejuni (55). However, so far only CheY (77) and PEB1 (55) have been shown to play a role in C. jejuni adherence. Flagella were shown to play a role in adherence in one study (76), whereas in two other studies fla mutants did not show a difference in adherence compared to the parent strain (31, 71). The galE mutant was also less invasive than the parent strain, which indicates that LPS plays an important role in epithelial cell interactions.
C. jejuni appears to have evolved to optimally colonize the avian gut. In the oral chick model, levels of colonization can be as high as 1010 CFU per g of cecal contents (21). Despite these huge numbers, there is no evidence for enteric disease in colonized chickens, suggesting that the bacterial factors required for colonization are distinct from those required for virulence. Results from previous studies using this model suggest that presumed virulence factors, like flagella, may have only a minor role in avian gut colonization (74). This probably reflects the major site of colonization, the ceca, which have restricted intestinal mucus flow, and thus colonization, once established, can be maintained without the need for bacterial motility. In contrast, a housekeeping enzyme like superoxide dismutase appears to be essential for optimal colonization (57), presumably to provide protection from some host-mediated environmental stress. Thus, the absence of the observed effect of the galE gene deletion on colonization is unsurprising and seems likely to reflect the minimal role that adherence and/or invasion apparently have in colonization of the chicken intestinal mucosa.
The reduced epithelial cell interactions together with the unchanged colonization properties of this LPS mutant make it an interesting candidate for competitive exclusion experiments. The changed LPS structure in galE mutants may also be useful in live-vaccine development. Ganglioside-like structures have been found in the outer core part of the LPS molecules from C. jejuni strains thought to be involved in the induction of Guillain-Barré syndrome. The absence of these structures in the galE mutants may enable the development of a safe, live-vaccine without the possibility of inducing an immune response to host gangliosides. In addition, the loss of galactose residues may expose the conserved inner core part of the LPS molecule and other membrane-associated structures and proteins to the immune system. Cross-reactivity of antibodies raised against the truncated galE LPS remains to be investigated.
ACKNOWLEDGMENTS
This work was funded by RMIT-University and in part by the Ministry of Agriculture, Fisheries and Foods, GB. We thank Shaun Cawthraw from the Veterinary Laboratories Agency for the chick colonization studies.
REFERENCES
- 1.Adams C W, Fornwald J A, Schmidt F J, Rosenberg M, Brawner M E. Gene organization and structure of the Streptomyces lividans gal operon. J Bacteriol. 1988;170:203–212. doi: 10.1128/jb.170.1.203-212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allos B M, Lippy F T, Carlsen A, Washburn R G, Blaser M J. Campylobacter jejuni strains from patients with Guillain-Barre syndrome. Emerg Infect Dis. 1998;4:263–268. doi: 10.3201/eid0402.980213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall G O, Lynch C M, Pang H, Shaver R T, Moran A P. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur J Biochem. 1995;231:570–578. [PubMed] [Google Scholar]
- 5.Aspinall G O, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry. 1994;33:241–249. doi: 10.1021/bi00167a032. [DOI] [PubMed] [Google Scholar]
- 6.Aspinall G O, McDonald A G, Raju T S, Pang H, Kurjanczyk L A, Penner J L, Moran A P. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharide. Eur J Biochem. 1993;213:1029–1037. doi: 10.1111/j.1432-1033.1993.tb17850.x. [DOI] [PubMed] [Google Scholar]
- 7.Aspinall G O, McDonald A G, Raju T S, Pang H, Mills S D, Kurjanczyk L A, Penner J L. Serological diversity and chemical structures of Campylobacter jejuni low-molecular-weight lipopolysaccharides. J Bacteriol. 1992;174:1324–1332. doi: 10.1128/jb.174.4.1324-1332.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aspinall G O, McDonald A G, Raju T S, Pang H, Moran A P, Penner J L. Chemical structures of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur J Biochem. 1993;216:880. [PubMed] [Google Scholar]
- 9.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 10.Bettenbrock K, Alpert C A. The gal genes for the Leloir pathway of Lactobacillus casei 64H. Appl Environ Microbiol. 1998;64:2013–2013. doi: 10.1128/aem.64.6.2013-2019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaser M J, Perez G P, Smith P F, Patton C, Tenover F C, Lastovica A J, Wang W I. Extraintestinal Campylobacter jejuni and Campylobacter coli infections: host factors and strain characteristics. J Infect Dis. 1986;153:552–559. doi: 10.1093/infdis/153.3.552. [DOI] [PubMed] [Google Scholar]
- 12.Blaser M J, Reller L B. Campylobacter enteritis. N Engl J Med. 1981;305:1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- 13.Blaser M J, Smith P F, Kohler P F. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis. 1985;151:227–235. doi: 10.1093/infdis/151.2.227. [DOI] [PubMed] [Google Scholar]
- 14.Blaser M J, Smith P F, Repine J E, Joiner K A. Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Investig. 1988;81:1434–1444. doi: 10.1172/JCI113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 16.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 17.Branquinho M R, Alviano C S, Ricciardi I D. Chemical composition and biological action of lipopolysaccharide (LPS) of Campylobacter fetus ss. jejuni. Rev Microbiol. 1983;14:90–96. [Google Scholar]
- 18.Burns S M, Hull S I. Comparison of loss of serum resistance by defined lipopolysaccharide mutants and an acapsular mutant of uropathogenic Escherichia coli O75:K5. Infect Immun. 1998;66:4244–4253. doi: 10.1128/iai.66.9.4244-4253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busby S, Dreyfus M. Segment-specific mutagenesis of the regulatory region in the Escherichia coli galactose operon: isolation of mutations reducing the initiation of transcription and translation. Gene. 1983;21:121–131. doi: 10.1016/0378-1119(83)90154-3. [DOI] [PubMed] [Google Scholar]
- 20.Butzler J P, Skirrow M B. Campylobacter enteritis. Clin Gastroenterol. 1979;8:737–765. [PubMed] [Google Scholar]
- 21.Cawthraw S A, Wassenaar T M, Ayling R, Newell D G. Increased colonization potential of Campylobacter jejuni strain 81116 after passage through chickens and its implication on the rate of transmission within flocks. Epidemiol Infect. 1996;117:213–215. doi: 10.1017/s0950268800001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comstock L E, Johnson J A, Michalski J M, Morris J G, Jr, Kaper J B. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol. 1996;19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x. [DOI] [PubMed] [Google Scholar]
- 23.Danner D B, Deich R A, Sisco K L, Smith H O. An eleven-base-pair sequence determines the specificity of DNA uptake in Haemophilus transformation. Gene. 1980;11:311–318. doi: 10.1016/0378-1119(80)90071-2. [DOI] [PubMed] [Google Scholar]
- 24.Darrow R A, Rodstrom R. Purification and properties of uridine diphosphate galactose 4-epimerase from yeast. Biochemistry. 1968;7:1645–1654. doi: 10.1021/bi00845a005. [DOI] [PubMed] [Google Scholar]
- 25.Daude N, Gallaher T K, Zeschnigk M, Starzinski-Powitz A, Petry K G, Haworth I S, Reichardt J K. Molecular cloning, characterization, and mapping of a full-length cDNA encoding human UDP-galactose 4′-epimerase. Biochem Mol Med. 1995;56:1–7. doi: 10.1006/bmme.1995.1048. [DOI] [PubMed] [Google Scholar]
- 26.Demarco de Hormaeche R, Macpherson A, Bowe F, Hormaeche C E. Alterations of the LPS determine virulence of Neisseria gonorrhoeae in guinea-pig subcutaneous chambers. Microb Pathog. 1991;11:159–170. doi: 10.1016/0882-4010(91)90046-d. [DOI] [PubMed] [Google Scholar]
- 27.Dolph P J, Majerczak D R, Coplin D L. Characterization of a gene cluster for exopolysaccharide biosynthesis and virulence in Erwinia stewartii. J Bacteriol. 1988;170:865–871. doi: 10.1128/jb.170.2.865-871.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elkins C, Thomas C E, Seifert H S, Sparling P F. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J Bacteriol. 1991;173:3911–3913. doi: 10.1128/jb.173.12.3911-3913.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fallarino A, Mavrangelos C, Stroeher U H, Manning P A. Identification of additional genes required for O-antigen biosynthesis in Vibrio cholerae O1. J Bacteriol. 1997;179:2147–2153. doi: 10.1128/jb.179.7.2147-2153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fry B N, Korolik V, ten Brinke J A, Pennings M T, Zalm R, Teunis B J, Coloe P J, van der Zeijst B A. The lipopolysaccharide biosynthesis locus of Campylobacter jejuni 81116. Microbiology. 1998;144:2049–2061. doi: 10.1099/00221287-144-8-2049. [DOI] [PubMed] [Google Scholar]
- 31.Grant C C, Konkel M E, Cieplak W, Jr, Tompkins L S. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993;61:1764–1771. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant K A, Belandia I U, Dekker N, Richardson P T, Park S F. Molecular characterization of pldA, the structural gene for a phospholipase A from Campylobacter coli, and its contribution to cell-associated hemolysis. Infect Immun. 1997;65:1172–1180. doi: 10.1128/iai.65.4.1172-1180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammerschmidt S, Birkholz C, Zahringer U, Robertson B D, van Putten J, Ebeling O, Frosch M. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol Microbiol. 1994;11:885–896. doi: 10.1111/j.1365-2958.1994.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 35.Hardy E, Pupo E, Castellanos-Serra L, Reyes J, Fernandez-Patron C. Sensitive reverse staining of bacterial lipopolysaccharides on polyacrylamide gels by using zinc and imidazole salts. Anal Biochem. 1997;244:28–32. doi: 10.1006/abio.1996.9719. [DOI] [PubMed] [Google Scholar]
- 36.Hood D W, Makepeace K, Deadman M E, Rest R F, Thibault P, Martin A, Richards J C, Moxon E R. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol Microbiol. 1999;33:679–692. doi: 10.1046/j.1365-2958.1999.01509.x. [DOI] [PubMed] [Google Scholar]
- 37.Houng H S, Kopecko D J, Baron L S. Molecular cloning and physical and functional characterization of the Salmonella typhimurium and Salmonella typhi galactose utilization operons. J Bacteriol. 1990;172:4392–4398. doi: 10.1128/jb.172.8.4392-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korn L J, Queen C. Analysis of biological sequences on small computers. DNA. 1984;3:421–436. doi: 10.1089/dna.1.1984.3.421. [DOI] [PubMed] [Google Scholar]
- 39.Labigne-Roussel A, Courcoux P, Tompkins L. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence C E, Altschul S F, Boguski M S, Liu J S, Neuwald A F, Wootton J C. Detecting subtle sequence signals: a Gibbs sampling strategy for multiple alignment. Science. 1993;262:208–214. doi: 10.1126/science.8211139. [DOI] [PubMed] [Google Scholar]
- 41.Lemaire H G, Muller-Hill B. Nucleotide sequences of the gal E gene and the gal T gene of E. coli. Nucleic Acids Res. 1986;14:7705–7711. doi: 10.1093/nar/14.19.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lesse A J, Campagnari A A, Bittner W E, Apicella M A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990;126:109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Thoden J B, Kim J, Berger E, Gulick A M, Ruzicka F J, Holden H M, Frey P A. Mechanistic roles of tyrosine 149 and serine 124 in UDP-galactose 4-epimerase from Escherichia coli. Biochemistry. 1997;36:10675–10684. doi: 10.1021/bi970430a. [DOI] [PubMed] [Google Scholar]
- 44.Long S, Reed J W, Himawan J, Walker G C. Genetic analysis of a cluster of genes required for synthesis of the calcofluor-binding exopolysaccharide of Rhizobium meliloti. J Bacteriol. 1988;170:4239–4248. doi: 10.1128/jb.170.9.4239-4248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lugtenberg B, Meijers J, Peters R, van der Hoek P, van Alphen L. Electrophoretic resolution of the “major outer membrane protein” of Escherichia coli K12 into four bands. FEBS Lett. 1975;58:254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- 46.Macpherson D F, Manning P A, Morona R. Characterization of the dTDP-rhamnose biosynthetic genes encoded in the rfb locus of Shigella flexneri. Mol Microbiol. 1994;11:281–292. doi: 10.1111/j.1365-2958.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 47.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 48.Maskell D J, Szabo M J, Butler P D, Williams A E, Moxon E R. Molecular analysis of a complex locus from Haemophilus influenzae involved in phase-variable lipopolysaccharide biosynthesis. Mol Microbiol. 1991;5:1013–1022. doi: 10.1111/j.1365-2958.1991.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 49.McSweegan E, Walker R I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986;53:141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mills S D, Kuzniar B, Shames B, Kurjanczyk L A, Penner J L. Variation of the O antigen of Campylobacter jejuni in vivo. J Med Microbiol. 1992;36:215–219. doi: 10.1099/00222615-36-3-215. [DOI] [PubMed] [Google Scholar]
- 51.Moreno F, Rodicio R, Herrero P. A new colorimetric assay for UDP-glucose 4-epimerase activity. Cell Mol Biol. 1981;27:589–592. [PubMed] [Google Scholar]
- 52.Moxon R E, Maskell D. Haemophilus influenzae lipopolysaccharide: the biochemistry and biology of a virulence factor. Symp Soc Gen Biol. 1992;49:75–96. [Google Scholar]
- 53.Naess V, Hofstad T. Chemical composition and biological activity of lipopolysaccharides prepared from type strains of Campylobacter jejuni and Campylobacter coli. Acta Pathol Microbiol Immunol Scand. 1984;92:217–222. doi: 10.1111/j.1699-0463.1984.tb02824.x. [DOI] [PubMed] [Google Scholar]
- 54.Palmer S R, Gully P R, White J M, Pearson A D, Suckling W G, Jones D M, Rawes J C, Penner J L. Water-borne outbreak of campylobacter gastroenteritis. Lancet. 1983;1:287–290. doi: 10.1016/s0140-6736(83)91698-7. [DOI] [PubMed] [Google Scholar]
- 55.Pei Z, Burucoa C, Grignon B, Baqar S, Huang X Z, Kopecko D J, Bourgeois A L, Fauchere J L, Blaser M J. Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect Immun. 1998;66:938–943. doi: 10.1128/iai.66.3.938-943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potter M D, Lo R Y. Cloning and characterization of a gene from Pasteurella haemolytica A1 involved in lipopolysaccharide biosynthesis. FEMS Microbiol Lett. 1995;129:75–81. doi: 10.1016/0378-1097(95)00140-Z. [DOI] [PubMed] [Google Scholar]
- 57.Purdy D, Cawthraw S, Dickinson J H, Newell D G, Park S F. Generation of a superoxide dismutase (SOD)-deficient mutant of Campylobacter coli: evidence for the significance of SOD in Campylobacter survival and colonization. Appl Environ Microbiol. 1999;65:2540–2546. doi: 10.1128/aem.65.6.2540-2546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salloway S, Mermel L A, Seamans M, Aspinall G O, Nam Shin J E, Kurjanczyk L A, Penner J L. Miller-Fisher syndrome associated with Campylobacter jejuni bearing lipopolysaccharide molecules that mimic human ganglioside GD3. Infect Immun. 1996;64:2945–2949. doi: 10.1128/iai.64.8.2945-2949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 60.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 61.Schwerer B, Neisser A, Polt R J, Bernheimer H, Moran A P. Antibody cross-reactivities between gangliosides and lipopolysaccharides of Campylobacter jejuni serotypes associated with Guillain-Barré syndrome. J Endotox Res. 1995;2:395–403. [Google Scholar]
- 62.Scupham A J, Triplett E W. Isolation and characterization of the UDP-glucose 4′-epimerase-encoding gene, galE, from Brucella abortus 2308. Gene. 1997;202:53–59. doi: 10.1016/s0378-1119(97)00453-8. [DOI] [PubMed] [Google Scholar]
- 63.Short J M, Fernandez J M, Sorge J A, Huse W D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988;16:7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skirrow M B. Campylobacter enteritis: a “new” disease. Br Med J. 1977;2:9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stern N J, Bailey J S, Blankenship L C, Cox N A, McHan F. Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 1988;32:330–334. [PubMed] [Google Scholar]
- 66.Sugiyama T, Kido N, Arakawa Y, Mori M, Naito S, Ohta M, Kato N. Rapid small-scale preparation method of cell surface polysaccharides. Microbiol Immunol. 1990;34:635–641. doi: 10.1111/j.1348-0421.1990.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 67.Swanson B A, Frey P A. Identification of lysine 153 as a functionally important residue in UDP-galactose 4-epimerase from Escherichia coli. Biochemistry. 1993;32:13231–13236. doi: 10.1021/bi00211a035. [DOI] [PubMed] [Google Scholar]
- 68.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 69.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Taylor D E. Natural transformation in Campylobacter species. J Bacteriol. 1990;172:949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wassenaar T M, Bleumink-Pluym N M C, Van der Zeijst B A M. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wassenaar T M, Fry B N, van der Zeijst B A. Variation of the flagellin gene locus of Campylobacter jejuni by recombination and horizontal gene transfer. Microbiology. 1995;141:95–101. doi: 10.1099/00221287-141-1-95. [DOI] [PubMed] [Google Scholar]
- 73.Wassenaar T M, Fry B N, van der Zeijst B A M. Genetic manipulation of Campylobacter: evaluation of natural transformation and electro-transformation. Gene. 1993;132:131–135. doi: 10.1016/0378-1119(93)90525-8. [DOI] [PubMed] [Google Scholar]
- 74.Wassenaar T M, van der Zeijst B A, Ayling R, Newell D G. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J Gen Microbiol. 1993;139:1171–1175. doi: 10.1099/00221287-139-6-1171. [DOI] [PubMed] [Google Scholar]
- 75.Wilkinson R G, Gemski P, Jr, Stocker B A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972;70:527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- 76.Yao R, Burr D H, Doig P, Trust T J, Niu H, Guerry P. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol Microbiol. 1994;14:883–893. doi: 10.1111/j.1365-2958.1994.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 77.Yao R, Burr D H, Guerry P. CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol. 1997;23:1021–1031. doi: 10.1046/j.1365-2958.1997.2861650.x. [DOI] [PubMed] [Google Scholar]