ABSTRACT
The efficacy of hepatitis B vaccination in adults was evaluated by comparison of the positive seroprotection rates and the hepatitis B surface antibody (anti-HBs) geometric mean titers (GMTs) between intensive intervention areas and non-intensive intervention areas after 8 years post-vaccination in the Zhejiang province. Seven cities (towns) in Zhejiang province were selected as intensive intervention areas, and adults in the demonstration areas receive hepatitis B vaccine voluntarily and for free. Other areas were non-intensive intervention areas. A total of 3587 participants received the full vaccination course (three doses), and blood samples were withdrawn 8 years after the first vaccination comprised the immunized group, and 2000 participants constituted the control group. The anti-HBs positive seroprotection rates of the immunized and control groups were 65.0% and 53.0%, respectively. The anti-HBs GMT of the subjects in the immunized group was 26.30 mIU/mL compared to 9.33 mIU/mL in the control group (P < .001). Significant differences were detected in the 24–35–, 36–45-, and 46–55-year-old subgroups in the positive seroprotection rates and the anti-HBs GMTs (P < .001) between the immunized and control groups. Moreover, significant differences were found in the anti-HBs GMT in the 46–55-year-old subgroup between the two groups (P = .02), while no differences were observed in the positive seroprotection rate (P = .428). In conclusion, adults who did not receive the hepatitis B vaccine in infancy and had negative serological markers of hepatitis B, especially adults <47-years-old, need vaccination.
KEYWORDS: Hepatitis B virus, vaccine, enhanced immunization, adults
Introduction
Hepatitis B infection is a major public health concern worldwide. China is the foremost country with hepatitis B. According to the epidemiological data in recent years, the prevalence of surface antigen in the general population in China is 5–6%. Among 70 million cases of hepatitis B virus infection, 20–30 million are chronic hepatitis B patients. Vaccination is the most economical and effective measure to prevent and control hepatitis B infection. By the end of the year 2019, 96% (189/197) countries had incorporated hepatitis B vaccination in their national immunization schedule, and the coverage with three doses of hepatitis B vaccine during infancy increased to 85% in 2019.1 Hepatitis B vaccination of newborns was integrated into the national immunization program of China in 1992, and newborns’ vaccination was encouraged at the family’s expense. In 2002, all newborn vaccinations in China were free. By 2020, the coverage rate of the hepatitis B vaccine for newborns in China reached 99.58%,2 which reduced the prevalence of infection to 1.3% in children.3 However, the hepatitis B vaccine was self-financed and voluntary in adults. Yongmei et al. determined that the vaccination rate for adult hepatitis B vaccine was 36.73% in Heilongjiang and Gansu provinces,4 and the coverage rate was about 48.9% in the Zhejiang province in 2020.5
In 2016, the World Health Organization (WHO) called for the elimination of hepatitis B, a major public health threat, by 2030.6 In the past two decades, universal hepatitis B virus (HBV) vaccination at birth has significantly reduced the burden of chronic HBV infection in people <25-years-old in many highly HBV endemic countries.7 However, it was difficult to reduce the incidence of hepatitis B in adults. A large number of social activities and specific occupational exposure increased the risk of hepatitis B infection in adults. It is likely to occur in young, sexually active adults, with an increasing trend of infection in men and individuals >40-years-old. Pinto et al. analyzed the trend of hepatitis B in Brazil from 2007–2018 and found that 0–9-year-olds comprised a low proportion (2%) of the total cases of HBV infection, while a high proportion (41%) was observed among adults (20–79-years-old).8
The prevalence of hepatitis B surface antigen (HBsAg) carriers in children <15-years-old <2%, while in adults >20-years-old, the prevalence was approximately 7% in China.9 In a previous study, we showed that the prevalence of HBsAg among participants aged 15–59 years was 8.12%, confirming that hepatitis B vaccination is essential to prevent HBV infection in adults.10 The positive rate of HBsAg in 40–44– and 45–49-year-old age groups was the highest, 12.9% and 12.4% in 2012, 13.3% and 13.4%, respectively, in 2016 in Zhejiang province.11
In 2012, The Chinese Preventive Medical Association and the Immunization Planning Center of the Chinese Center for Disease Control and Prevention jointly issued the Technical Guidelines for Hepatitis B Immunization Prevention in Chinese Adults.12 The guidelines recommended that adults who have not been fully vaccinated with hepatitis B vaccine and who are not fully immunized with hepatitis B vaccine should be vaccinated, and serological testing is not required before vaccination. The US Advisory Committee on Immunization Practices (ACIP) recommended that all adults who require protection from HBV infection can be vaccinated, regardless of the risk factors. In Germany, the prevalence of hepatitis B virus was 0.3%, and vaccination is not recommended in adults in high-risk groups in the general population.13 Poethko et al. estimated that the vaccination coverage was about 32.9% in the general adult population aged 18–79-years-old through checks of vaccination cards and self-reports.14
The current study was based on the adult cohort of hepatitis B vaccine immunization of the 11th Five-Year Plan Program of China. The sample was selected using a complex design involving stratification and multistage sampling. Seven cities (towns) in Zhejiang province were selected as intensive intervention areas, and adults (about 300,000 people) in these areas could voluntarily receive hepatitis B vaccine for free. A total of 11,217 participants, aged 16–59 years, were recruited in the cohort study who received the full vaccination course (three doses) were tested for HBsAg, anti-HBs, and anti-HBc before vaccination and also had a blood sample withdrawn 1 month after the first vaccination. Other areas were non-intensive intervention areas. To further evaluate the efficacy of hepatitis B vaccination in adults, we compared the positive seroprotection rates and the anti-HBs geometric mean titers (GMTs) between intensive intervention areas and non-intensive intervention areas 8 years later. This information could improve the planning and implementation strategies for hepatitis B vaccination among adults.
Materials and methods
Study procedures
This study was carried out in Zhejiang Province from May 2010 to November 2018. A total of 11,217 participants aged 16–59 years were recruited in the intensive intervention areas among those who had received the full vaccination course (three doses) and also had a blood sample withdrawn 1 month after the full vaccination. All participants were vaccinated with a hepatitis B vaccine (dose 10 μg/20 μg, made in China) and asked to complete a questionnaire on information about gender, date of birth, HBV vaccination history, telephone number, and home address. Subsequently, all the participants were tested for HBsAg, anti-HBs, and anti-HBc before vaccination, and tested for anti-HBs after 1 month and 8 years. Finally, 3587 participants comprised the immunized group whose blood sample was withdrawn 8 years after vaccination. To further evaluate the efficacy of hepatitis B vaccination in adults between the immunized and general population, 2000 adults aged 24–68 years (deadline of 31 December 2018) in the non-intensive intervention areas in Zhejiang province were selected to form the control group.
The exclusion criteria were as follows: organ transplantation, renal dialysis, participants with hepatitis C and acquired immune deficiency syndrome, vaccination contraindication. All the participants provided informed consent according to the study protocol and were willing to receive the HBV vaccine. Informed consent was signed by the guardian for participants <18-years-old. Institutional Review Board approval was obtained from the Research Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention (approval number: T-043-R).
Laboratory testing
Frozen serum samples were sent to Adicon Clinical Laboratories Inc. in Hang zhou for the quantification of HBsAg and hepatitis B core antibody (anti-HBc) and hepatitis B surface antibody (anti-HBs) by chemiluminescence microparticle immunoassay (CMIA) on Architect-i2000SR analyzer (Abbott, USA). The following signal-to-noise ratios indicated positivity: HBsAg ≥0.05 IU/mL and anti-HBc levels ≥1 S/CO. An anti-HBs antibody concentration of ≥ 10 mIU/mL measured 1 month after the administration of the last dose of the primary vaccination series was considered a reliable marker of protection against HBV infection. A low response was defined as 10 mIU/mL≤anti–HBs <100 mIU/mL, a normal response was defined as 100 mIU/mL≤anti-HBs <1000 mIU/mL, and a high response was defined as anti-HBs ≥1000 mIU/mL after the third dose of vaccine.
Statistical evaluation
Statistical analyses were performed using SPSS version 22.0 (SPSS Inc., USA). Continuous variables were expressed as means±standard deviation and categorical variables as frequencies and proportions. The positive seroprotection rates were compared by the chi-square test method, and the anti-HBs geometric mean titer (GMT) level was assessed using the t-test. The age was adjusted by logistic regression or linear regression. The comparisons of the immune response between the two groups were analyzed by rank-sum test. P–value <.05 indicated a statistically significant difference.
Results
Baseline characteristics
For 11,217 participants, the positive seroprotection rate was 43.83% before vaccination. On the other hand, the positive seroprotection rate was 87.1%, and the GMT value of anti-HBs titer was 229.09(218.78–245.47)mIU/mL at 1 month after three doses of vaccination. A total of 3587 participants who received the full vaccination course (three doses) and also had a blood sample withdrawn 8 years after the first vaccination constituted the immunized group, consisting of 1467 males (40.9%). The mean age of the cohort was 42.33 ± 8.27 years (according to the deadline of 31 December 2018). The control group consisted of 2000 participants (783 males) (39.2%), with an average age of 43.10 ± 10.4 years. Among them, 346(17.3%) had been immunized in the past, and 1236(61.8%) were not sure whether they had been immunized. The mean age difference between the two groups was statistically significant (t = 2.778,P = .005), while no difference in gender was noted (χ2 = 1.631, P = .202).
Comparison of the positive seroprotection rates and the anti-HBs GMTs between immunized and control groups
The anti-HBs positive seroprotection rate of the immunized and control groups was 65.0% and 53.0%, respectively, which differed significantly (χ2 = 77.309,P < .001). The anti-HBs GMT of the subjects in the immunized group was 26.30 mIU/mL (95% confidence interval (CI): 23.99–28.84) compared to 9.33 mIU/mL (95% CI: 7.94–10.72) in the control group, which showed significant differences (t = –13.325, P < .001).
Comparison of the positive seroprotection rates and GMTs by gender
In males, the anti-HBs-positive seroprotection rate of the immunized group was 63.4% and 50.1% in the control group, and the anti-HBs GMT of the subjects in the immunized group was 23.34 mIU/mL compared to 8.15 mIU/mL in the control group. Statistically significant differences were observed between the two groups (χ2 = 37.438, P < .001, t = –7.84, P < .001). In females, the anti-HBs-positive seroprotection rate of the immunized and control groups was 66.1% and 54.9%, respectively, and the anti-HBs GMT of the subjects in the immunized group was 28.45 mIU/mL compared to 10.0 mIU/mL in the control group. Statistically significant differences were observed between the two groups (χ2 = 41.135, P < .001; t = –9.83, P < .001; Table 1).
Table 1.
Comparison of the positive seroprotection rates and GMTs by gender.
| Gender | Group | N | n | PSR (%) | χ2 | P | GMT | 95% CI | t | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Immunized | 1467 | 930 | 63.4 | 37.438 | <.001 | 23.34 | 20.39–26.80 | −7.84 | <.001 |
| Control | 783 | 392 | 50.1 | 8.15 | 6.39–10.14 | |||||
| Female | Immunized | 2120 | 1401 | 66.1 | 41.135 | <.001 | 28.45 | 25.49–31.71 | −9.83 | <.001 |
| Control | 1217 | 668 | 54.9 | 10.00 | 8.29–11.91 |
N, total number; n1, number of positive seroprotection after 8 y; PSR, positive seroprotection rate; GMT, the geometric mean titer of anti-HBs.
Comparison of the positive seroprotection rates and GMTs by age
The positive seroprotection rate and anti-HBs GMT between the immunized and control groups in different age groups are shown in Figures 1 and 2. In the 24–35-, 36–45-, and 46–55–year–old groups, significant differences were noted in the positive seroprotection rates (χ2 = 39.397, P < .001; χ2 = 31.950, P < .001; χ2 = 12.490, P < .001) and the anti-HBs GMT GMTs (t = –7.070, P < .001; t = –8.211, P < .001; t = –5.917, P < .001) between the two groups. However, in the 46–55–year–old group, significant differences were found in the anti-HBs GMT between the two groups (t = –2.342, P = .02), while no differences were detected in the positive seroprotection rate (χ2 = 0.629, P = .428).
Figure 1.
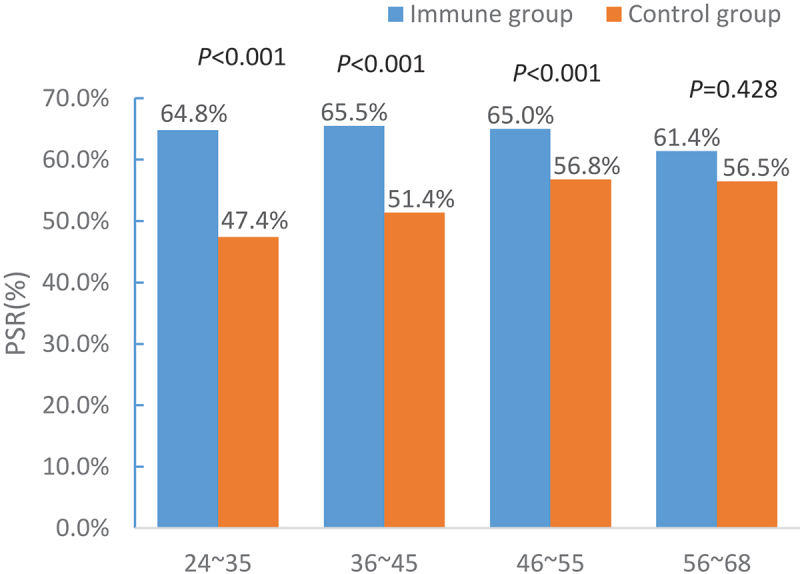
Comparison of the positive seroprotection rates by age.
Figure 2.
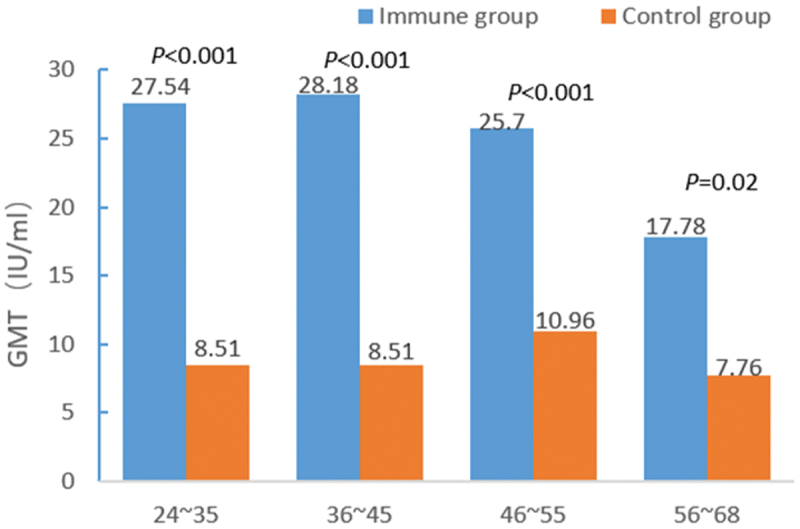
Comparison of the geometric mean titers of the hepatitis B surface antibody by age.
Comparison of the immune responses between immunized and control groups
The subgroup of participants with different immune responses, i.e., immunized and control groups, are shown in Table 2. The proportion of no-response, low-response, normal response, and high-response in the immunized group was 35.02%, 32.35%, 25.82%, and 7.92% and 47.0%, 26.60%, 20.15%, and 6.25%, in the control group, respectively.
Table 2.
Comparison of the different immune responses between immunized and control groups.
| Group | N | No response |
Low response |
Normal response |
High response |
χ2 | P |
|---|---|---|---|---|---|---|---|
| Immunized | 3587 | 1256 (35.02) | 1121 (32.25) | 926 (25.82) | 284 (7.92) | 77.309 | <.001 |
| Control | 2000 | 940 (47.00) | 532 (26.60) | 403 (20.15) | 125 (6.25) |
Discussion
The current findings indicated that adults are adequately protected even 8 years after receiving the hepatitis B vaccine. Some studies suggested that vaccine-induced immunity might persist for 15–20 years or even longer after a complete course.15–17 The anti–HBs–positive seroprotection rate of the immunized and controls groups was 65.4% and 53.0%, respectively, and the anti-HBs GMT of the subjects in the immunized group was 26.30 mIU/mL compared to 9.33 mIU/mL in the control group. In Hunan, 44.75% of freshmen University students were anti-HBs-positive.18 Wang et al. suggested that the prevalence of anti-HBs decreased with advancing age from 84.8% (age <1 year) to 49.0% (age 50–59 years) in Changchun City, Jilin province.19 Thus, hepatitis B vaccination seems beneficial to increasing the positive rate of anti-HBs in adults. Another study by Li et al.20 showed that the positive rate of surface antibody in adults 12 years after hepatitis B vaccination was 63%, which remained above the protective level.
Strategies for HBV control were initially focused on vaccination of high-risk groups in adults. However, ≥30% of people with acute hepatitis B infection did not have identifiable risk factors and therefore, were missed by a high-risk group approach.1 Hence, decision–makers and health professionals worldwide discussed a strategy of universal hepatitis B immunization for a specific age cohort, even in low–endemic countries. According to the Center for Disease Control and Prevention (CDC), an estimated 68% of individuals with chronic hepatitis B are unaware of the infection,21 and many remain asymptomatic until the onset of cirrhosis or end-stage liver disease.22
International guidelines recommend HBV screening for people born in regions with ≥2% disease prevalence.1,23 Universal screening has not yet been implemented in most HBV-endemic countries because of concerns related to high costs and insufficient infrastructure for implementation. Prabhu et al. conducted a cost–effectiveness analysis among pregnant women in the USA and found that universal HBV immunity screening and vaccination of susceptible individuals is cost–effective compared to no screening and vaccinating routinely.24 Although most countries have screened for high-risk groups, the diagnosis rates remain low. In order to resolve this issue, MehlikaUniversal HBsAg screening of adults for chronic hepatitis B(CHB) in the general population of the USA was carried out and found to be cost–effective and potentially cost-saving compared to the current CHB screening recommendations.25 However, universal screening has not been proposed or implemented for chronic HBV infection in China. Nonetheless, Lei et al. used a Markov cohort model and input parameters based on data from previous studies and public databases to assess the cost-effectiveness of the four HBV serological screening strategies in China. The study suggested that universal HBV screening is cost-effective and can be applied to every screening strategy if implemented early. The most cost-effective strategy was the serum HBsAg/HBsAb/hepatitis B e antibody (HBeAg)/HBeAb/HBcAb (five-test) screening strategy in people aged 18–70 years.26
Stratified by age, significant differences were detected in the positive rate and titer of surface antibodies between the immunized and the general populations, except for the 56–68–year-old age group. These results indicated that hepatitis B vaccination has a significant effect on adults, especially those <47–years–old. This effect could be attributed to the reason at the older adults (>40–years-old) are less likely to achieve a seroprotective response to hepatitis B vaccination.1 China introduced free hepatitis B vaccination in 2002 and hence, almost all children and adolescents are vaccinated before the age of 20 years, while adults, especially those <47–years-old are recommended immunization.
Nevertheless, the present study had some limitations. (1) The high loss to follow-up caused by the floating population may influence the reliability and the sample size of this study; (2) Because it was a retrospective investigation, a lot of people couldn’t give an accurate answer of vaccination history; (3) HBsAg was not monitored in the immunized population for 8 years after immunization. However, the positive rate of HBsAg in the non-intensive intervention areas was 14.3%. Compared to 8.12% in the 15–59–year–old population in Zhejiang province, the effect of the hepatitis B vaccine is distinct in adults.
In conclusion, adults who did not receive the hepatitis B vaccine in infancy and had negative serological markers of hepatitis B, especially adults <47-years-old should be vaccinated. Also, additional economic studies are needed to determine whether universal hepatitis B screening or vaccination is necessary.
Acknowledgments
The authors thank the NanXun, TongLu, ShaoXing, SanMen, TongXiang, WuXing, AnJi and ChangXing Disease Control and Prevention Centers and other relevant personnel for their contributions to this study.
Funding Statement
This study was supported by the National Scientific and Technological Major Project of China [2018ZX10715014, 2017ZX10105001, 2014ZX10004008, 2013ZX10004-904, 2011ZX10004-901].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Pattyn J, Hendrickx G, Vorsters A, Van Damme P.. Hepatitis B vaccines. J Infect Dis. 2021;224(12 Suppl 2):1–5. doi: 10.1093/infdis/jiaa668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jing W, Liu J, Liu M. Eliminating mother-to-child transmission of HBV: progress and challenges in China. Front Med. 2020;14(1):21–29. doi: 10.1007/s11684-020-0744-2. [DOI] [PubMed] [Google Scholar]
- 3.Wan Y, Fan C, Liu Y, Xu J, Zhao T, Qiu J, et al. Coverage and influencing factors of hepatitis B vaccination among adults in Heilongjiang and Gansu provinces. Chin J Vaccines Immunization. 2020;26(2):207–212. [Google Scholar]
- 4.Xu Y, Wu Q, Xu S, Xu J, Huang Y. Analysis on hepatitis B vaccination and its influencing factors among adults in Zhejiang Province. Chin J Health Educ. 2020;36(3):259–61+284. [Google Scholar]
- 5.World Health Organization . Hepatitis B [OL]. 2019. July 18 [accessed 2022 Apr 5]. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- 6.Cox AL, El-Sayed MH, Kao JH, Lazarus JV, Lemoine M, Lok AS, Zoulim F. Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol. 2020;17(9):533–542. doi: 10.1038/s41575-020-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block TM, Chang KM, Guo JT. Prospects for the Global Elimination of Hepatitis B. Annu Rev Virol. 2021;8(1):437–458. doi: 10.1146/annurev-virology-091919-062728. [DOI] [PubMed] [Google Scholar]
- 8.Pinto CS, Costa GB, Allaman IB, Gadelha SR. Clinical, epidemiological aspects, and trends of Hepatitis B in Brazil from 2007 to 2018. Sci Rep. 2021;11(1):13986. doi: 10.1038/s41598-021-93434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Men P, Xiao Y, Gao P, Lv M, Yuan Q, Chen W, Bai S, Wu J. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(1):811. doi: 10.1186/s12879-019-4428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren W, Ren J, Wu Z, Shen L, Shan H, Dai X, Li J, Liu Y, Qiu Y, Yao J, et al. Long-term persistence of anti-HBs after hepatitis B vaccination among adults: 8-year results. Hum Vaccin Immunother. 2020;16(3):687–692. doi: 10.1080/21645515.2019.1666612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Z, Deng M, Peng C, Song X, Chen Y, Zhang X, Liu Q, Li Y, Jiang H, Xu X, et al. A system dynamics modelling simulation based on a cohort of hepatitis B epidemic research in east China community. Epidemiol Infect. 2019;147:e86. doi: 10.1017/S0950268819000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang X, Cui F. Technical guide for adult Hepatitis B immunization in China. Chin J Gastroenterol Hepatol. 2012;21:197–201. [Google Scholar]
- 13.Steffen G, Sperle I, Harder T, Sarma N, Beermann S, Thamm R, Bremer V, Zimmermann R, Dudareva S. Hepatitis B vaccination coverage in Germany: systematic review. BMC Infect Dis. 2021;21(1):817. doi: 10.1186/s12879-021-06400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poethko-Müller C, Zimmermann R, Hamouda O, Faber M, Stark K, Ross RS, Thamm M. Epidemiology of hepatitis A, B, and C among adults in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56(5–6):707–715. doi: 10.1007/s00103-013-1673-x. [DOI] [PubMed] [Google Scholar]
- 15.Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53(1):68–75. doi: 10.1093/cid/cir270. [DOI] [PubMed] [Google Scholar]
- 16.Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017;35(46):6302–6307. doi: 10.1016/j.vaccine.2017.09.076. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi FP, Gallone MS, Gallone MF, Larocca AMV, Vimercati L, Quarto M, Tafuri S. HBV seroprevalence after 25 years of universal mass vaccination and management of non-responders to the anti-Hepatitis B vaccine: an Italian study among medical students. J Viral Hepat. 2019;26(1):136–144. doi: 10.1111/jvh.13001. [DOI] [PubMed] [Google Scholar]
- 18.Xie M, Quan H, Zeng Y, Yuan S, Liu Y, Yang Y. Sero-epidemiology study of hepatitis B virus surface antibodies from 2017 to 2019 among Chinese young adults in Hunan Province: a three-year retrospective study. Medicine (Baltimore). 2021;100(29):e26665. doi: 10.1097/MD.0000000000026665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Tao Y, Tao Y, Jiang J, Yan L, Wang C, Ding Y, Yu J, Zhao D, Chi X, et al. Epidemiological study of hepatitis B and hepatitis C infections in Northeastern China and the beneficial effect of the vaccination strategy for hepatitis B: a cross-sectional study. BMC Public Health. 2018;18(1):1088. doi: 10.1186/s12889-018-5984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Yan B, Lu J, Liu J, Kong Q, Wu W, et al. Persistence of immune memory and its related factors at 12 years after hepatitis B vaccination among adults. Chin J Prev Med. 2019;05:497–502. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services . Hepatitis B basic information. Updated 2020. Aug 31 [accessed 2020 Nov 18]. https://www.hhs.gov/hepatitis/learnabout-viral-hepatitis/hepatitis-b-basics/index.html.
- 22.U.S. Preventive Services Task Force . Screening for Hepatitis B virus infection in adolescents and adults: recommendation statement. Am Fam Physician. 2021;103(8):495–501. [PubMed] [Google Scholar]
- 23.Abara WE, Qaseem A, Schillie S, McMahon BJ, Harris AM, Abraham GM, et al. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167(11):794–804. doi: 10.7326/M17-1106. [DOI] [PubMed] [Google Scholar]
- 24.Prabhu M, Susich MK, Packer CH, Hersch AR, Riley LE, Caughey AB. Universal hepatitis B antibody screening and vaccination in pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2022;139(3):357–367. doi: 10.1097/AOG.0000000000004652. [DOI] [PubMed] [Google Scholar]
- 25.Toy M, Hutton D, Harris AM, Nelson N, Salomon JA, So S. Cost-effectiveness of 1-time universal screening for chronic Hepatitis B infection in adults in the United States. Clin Infect Dis. 2022;74(2):210–217. doi: 10.1093/cid/ciab405. [DOI] [PubMed] [Google Scholar]
- 26.Su S, Wong WC, Zou Z, Cheng DD, Ong JJ, Chan P, Ji F, Yuen M-F, Zhuang G, Seto W-K, et al. Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob Health. 2022;10(2):e278–278e287. doi: 10.1016/S2214-109X(21)00517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


