ABSTRACT
To report potential vaccine-induced inflammatory ocular adverse events following inactivated COVID-19 vaccination. Retrospective study of patients with uveitis and other ocular complications following inactivated coronavirus disease 2019 (COVID-19) vaccination at a tertiary referral center between May 2021 and August 2021. Data collection consisted of demographic and clinical data. The study included 8 eyes of 5 patients (4 females, 1 male), with a mean age of 37.2 ± 12.5 years (range 28–59 years). Mean time between vaccination and ocular complications onset was 13.2 ± 11.9 days (range 3–30 days), including two patients after the first dose of the vaccine and 3 patients after the second dose. The cases reported were three anterior uveitis, one herpetic keratitis and iridocyclitis, and one posterior uveitis. Patients received treatment with local and/or systemic steroids and all the patients had good visual outcomes. Ocular inflammatory events may occur after vaccination with possible gender preponderance. However, they are rare and manageable. Overall, the efficacy and safety of vaccination should be emphasized.
KEYWORDS: COVID-19, inactivated vaccine, ocular adverse events, vaccine-induced uveitis
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in December 2019 led to the devastating coronavirus disease 2019 (COVID-19) pandemic, which has promoted vaccine development.1–3 Currently, 3 types of COVID-19 vaccines have been used in China, including inactivated vaccines, recombinant vaccine (CHO cells), and adenovirus vector vaccine with good immunogenicity and safety. China’s Corona Vac (Sinovac Biotech Ltd., Beijing, China) and BBIBP-CorV (Sinopharm, Beijing, China) vaccines are both inactive vaccines, and are widely used in China and account for almost half of the 7.3 billion COVID-19 vaccine doses delivered globally. Since the implementation of vaccine campaigns, reports on ocular adverse effects, including anterior uveitis, multiple evanescent white dot syndrome (MEWDS), acute macular neuroretinopathy, central serous retinopathy, multifocal choroiditis, and reactivation of Vogt-Koyanagi-Harada (VKH) syndrome, scleritis, have emerged.4–11 There are also several previous reports about inactivated COVID-19 vaccine associated uveitis and other ocular complications.5,8–10,12,13 Most of them are case reports. Here, we report a case series of ocular inflammatory events associated with the administration of inactivated COVID-19 vaccination without previous ocular inflammatory history.
Patients and methods
This retrospective study included patients with uveitis and other ocular complications following COVID-19 vaccination between May 2021 and August 2021 at the Ophthalmology Department of Xinhua Hospital. Patients with previous history of uveitis, other inflammatory ocular diseases and related systemic disease were excluded.
The study and data collection were conducted in agreement with the principles of the Declaration of Helsinki and are approved by the ethics committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Informed written consent was obtained from all patients.
Data collection consisted of demographic and clinical data. The demographic data included age, sex, general medical and ocular history and medications. Clinical data included systemic and ocular symptoms post-vaccination, type of vaccine, time interval between vaccination (first and second dose) and symptom onset, laterality of eye disease, ocular findings, treatment, and outcome.
All patients underwent a complete ophthalmic examination with measurement of the best-corrected visual acuity (BCVA), slit lamp biomicroscopy, fundus examination, and ultra-widefield retina imaging, optical coherence tomography (OCT). Uveitis was graded and classified according to the Standardization of Uveitis Nomenclature (SUN) classification system.14 Laboratory tests were performed to exclude other causes of ocular inflammation. These included complete blood count, blood chemistry, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), Treponema pallidum Particle Agglutination (TPPA), interferon-gamma release assay (T-spot), serum angiotensin-converting enzyme (ACE), serum lysozyme, X ray of the chest, and magnetic resonance imaging (MRI) of the brain if necessary. In all patients, follow-up was carried out for a minimum of 3 months.
Results
Demographics and clinical data
The study included 8 eyes of 5 patients (4 females, 1 male), with a mean age of 37.2 ± 12.5 years (range 28–59 years). Mean time between vaccination and ocular complications onset was 13.2 ± 11.9 days (range 3–30 days). Two patients reported ocular complications at 3 and 14 days after the first dose of the vaccination. The other three patients reported at 7, 12, and 30 days after the second dose of the vaccination. None of the patients had previous history of uveitis, other inflammatory ocular diseases or related systemic disease. Patients’ demographic and clinical data is shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients having ocular adverse events following COVID-19 vaccination.
| PatientNo | Gender | Age (years) | Laterality | Diagnosis | VaccineReceived | Doses | Days AfterVaccination | PositiveSerologicTest | TreatmentReceived | Presenting BCVA | Final BCVA | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 36 | OS | Anterior uveitis | Corona Vac | 1 | 3 | Topical steroidoral steroid | 20/100 | 20/25 | 1 mild recurrent episode and resolved | |
| 2 | F | 32 | OD | Anterior uveitis | Corona Vac | 2 | 30 | Topical steroid | 20/30 | 20/20 | Resolved | |
| 3 | F | 31 | OU | Anterior uveitis | 1. Corona Vac2. BBIBP-CorV | 2 | 7 | HLAB27(+) | Topical steroid | 20/20, 20/20 | 20/20, 20/20 | Resolved |
| 4 | M | 59 | OS | Herpetic keratitisiridocyclitis | BBIBP-CorV | 2 | 12 | Topical steroidanti-viral treatment | 20/30 | 20/25 | Resolved | |
| 5 | F | 28 | OU | Posterior uveitis | Corona Vac | 1 | 14 | IVT dexamethasone | 20/30, 20/40 | 20/20, 20/20 | Resolved |
COVID-19 vaccination = coronavirus disease 2019, BCVA = best-corrected visual acuity, M = male, F = Female, OD = right eye, OS = left eye, OU = both eye, HLA-B27 = human leukocyte antigen B27, IVT = intravitreal injection.
Inflammatory ocular adverse events
The study included three anterior uveitis, one herpetic keratitis and iridocyclitis, and one posterior uveitis.
Patient 1–3 had anterior uveitis. Patients 1 and 2 were unilateral anterior uveitis and HLA-B27 negative, while Patient 3 was bilateral and HLA-B27 positive. In addition to the topical application of prednisolone acetate, Patient 1 received a fast systemic tapering dose of glucocorticoids due to the severity of the symptom. She had a recurrence of anterior uveitis 8 months after the first episode and was controlled well with a short time period of topical steroid (Figure 1). The BCVA improved from 20/100 to 20/25 at the last follow-up. The other two patients were controlled well with topical steroid and the BCVA of the affected eyes returned to 20/20 at the last follow-up.
Figure 1.
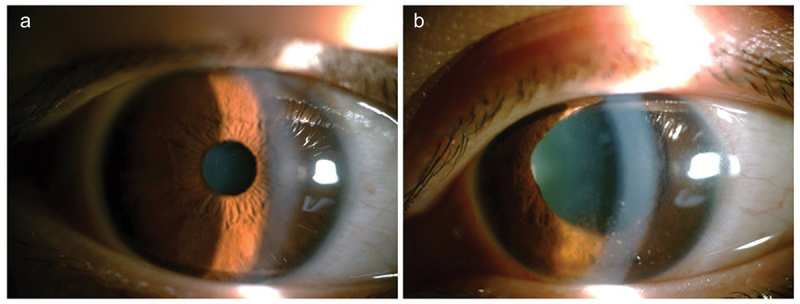
Patient 1 was diagnosed as anterior uveitis of left eye. She received both topical and a fast systemic tapering dose of glucocorticoids due to the severe symptom. (a) Showed normal anterior segment of right eye. (b) Showed anterior inflammation and irregular pupil of left eye.
Patient 4 developed herpetic keratitis and iridocyclitis 12 days after the second dose. He had no previous history of herpetic disease before vaccination (Figure S1). He recovered after topical and systemic anti-viral therapy (oral Acyclovir) and topical steroid. His BCVA recovered from 20/30 to 20/25 at the last follow-up.
Patient 5 was a 28-year-old female who complained of bilateral blurred vision 14 days after the first dose of inactivated COVID vaccine. He experienced headache and fatigue right after the vaccination. The patient denied any previous ocular or systemic medical history. On examination, the best visual acuity was, 20/40(OD), 20/60(OS), respectively. Anterior segment examination was unremarkable. There was 1+ vitreous cell and serous retinal detachments in both eyes. The left eye was more severe with macular involved (Figure 2). She was diagnosed as bilateral posterior uveitis which was similar to Harada disease. Intravitreal dexamethasone implantation was given. Intraocular pressure (IOP) was controlled well by using anti-glaucoma medications (Brinzolamide and Timolol Maleate Eye Drops). The visual acuities increased to 20/20 for both eyes during the follow-up without recurrence. The patient did not opt for a second dose of the vaccination.
Figure 2.
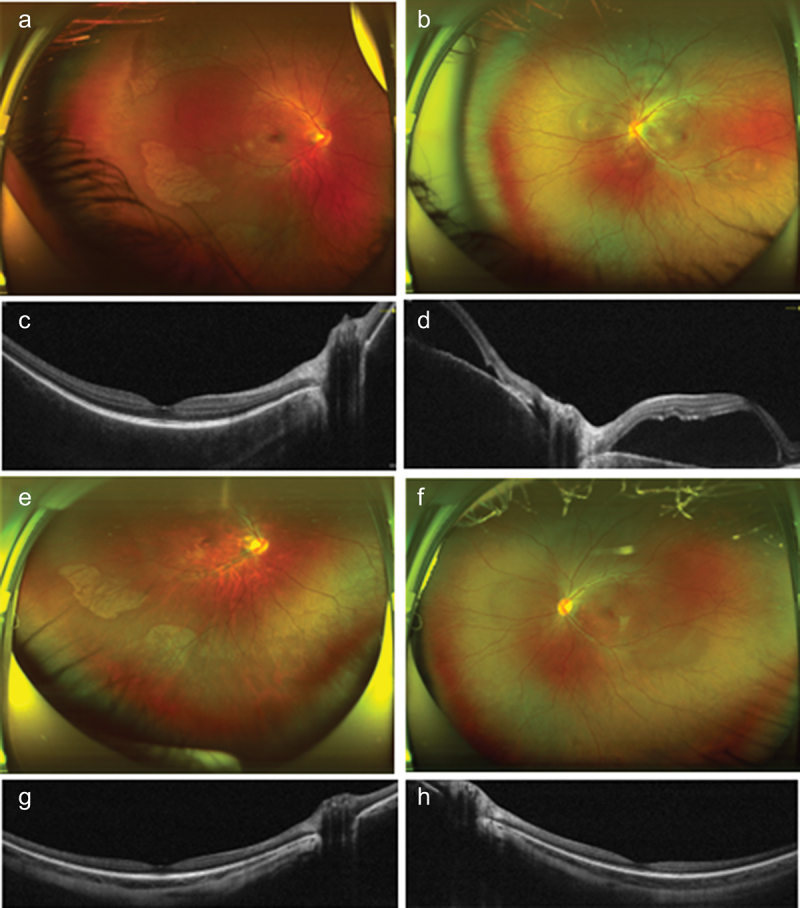
Patient 5 was diagnosed as bilateral posterior uveitis resembling Harada disease 14 days after first dose of inactivated COVID vaccine. (a and b) Fundus photos of right and left eye at the first presentation, which showed serous retinal detachment in both eyes. The left eye was more severe than the right eye. (c and d) optical coherence tomography (OCT) of right and left eye. Macula was involved in the left eye. Intravitreal dexamethasone implantations were performed and the bilateral episode completely resolved without recurrence. (e and f) Fundus photos after treatment. (g and h) OCT of right eye and left eye respectively after treatment.
Discussion
Many vaccines have been implicated in causing ocular adverse events based on the temporal association of the exposure and the putative complication.15,16 This is also true for COVID-19 vaccines.4–6,10,12,13 Three types of COVID-19 vaccines exist: the mRNA vaccine, the inactivated virus vaccine and the virus-vectored vaccine. According to the population-based pharmacovigilance surveillance systems, the incidence of uveitis after COVID-19 vaccination was 0.02–0.6/1,000,000. There were several case reports on inactivated COVID-19 vaccines BBIBP-CorV or Corona Vac-associated uveitis and other ocular complications.5,8–10,12,13 However, it is not known whether the three types of COVID-19 vaccines have significant difference in safety, particularly in relation to ocular health.
It is always challenging to determine the causality between vaccination and the adverse response. The temporal relationship is probably the most important fact. Previous studies showed the median number of days between vaccination and onset of uveitis was 16 days (range: 1 day to 6 years; SD = 362 days).17 In our study, mean time between vaccination and ocular complications onset was 13.2 ± 11.9 days (range 3–30 days). This is well within the Bradford-Hill criteria and World Health Organization Causality Assessment Guide of Suspected Adverse Reactions.18 COVID-19 vaccine-associated uveitis has been reported with onset ranging from 1 to 30 days following vaccine administration.4,6–8,11,19 None of the patients had previous history of ocular or systematic conditions related to the symptoms in our study. Although the coincidental occurrence of uveitis or ocular inflammatory diseases could not be completely excluded, we believe that the relationship between COVID-19 vaccination and ocular inflammatory diseases is possible.18,20 The potential mechanism underlying the ocular inflammatory response following COVID-19 vaccination is not known. Commonly proposed mechanisms include molecular mimicry secondary to a close resemblance between vaccine peptide fragments and uveal self-peptides, delayed-type hypersensitivity with deposition of immune complexes, and an immune reaction to vaccination adjuvants.17,21,22
There was a greater preponderance of female patients in vaccine-associated uveitis. In a previous study of 289 vaccine-associated uveitis cases reported between 1984 and 2014, a female preponderance was noticed: excluding 13 cases of missing gender, there were 199 females and 77 males.17 In particular to COVID-19 vaccination, another study of 70 patients reported 35 (56.5%) females and 27 (43.5%) males.23 However, it should be noted that there is a gender difference with a greater female preponderance for noninfectious uveitis. While the cause for this is not completely understood, recent studies suggested that sex hormones may play a role in modulating autoimmune responses: estrogen stimulates the response whereas androgens suppress it. Women primarily respond to infection and vaccination with classical Th2 mechanisms (leading to increased antibody production), whilst men develop a stronger Th1 response.24,25 This may explain the increased prevalence of Th2-mediated autoimmune disorders in women. Increased awareness of the possible gender differences after vaccination should allow for early diagnosis and timely treatment and may aid patient management.
Most of the events were mild and had a good visual outcome. No one had recurrence except one patient of anterior uveitis (Patient 1), who had a recurrence of anterior uveitis 8 months after the first episode and was controlled well with a short time period of topical steroid. Despite these rare ocular adverse events, vaccination is necessary in the management of the COVID-19 pandemic. The physician must be aware of this potential reaction and pay attention to such events.
Study limitations included its retrospective nature and limited data. Despite the fact that we discuss mechanisms, the possibility that some adverse events are due to random chance could not be completely excluded only based on a temporal association and good prognosis. Additionally, there is a potential sample bias due to the form of data collection, and the data collected might not be representative of all population.
Conclusion
Vaccination is a vital public health tool in the management of the COVID-19 pandemic. While ophthalmologists should be aware of potential inflammatory reactions associated with vaccination, especially in female patients, the rare and manageable occurrence of reported events further supported the general safety of the vaccines.
Supplementary Material
Acknowledgments
We sincerely thank all the patients for their participation.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This research was supported by National Natural Science Foundation Project of China (81770963).
Contributors
P.F. and F.Z. wrote the manuscript. P.F., F.Z., M.L., J. Luo and H.Y. contributed to providing cases and interpreting the results to the manuscript. J. Li contributed to analyzing the results. P.F. and P.Z. supervised and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Ethics approval
This study was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Approval No. XHEC-D-2022-106). All investigations followed the tenets of the Declaration of Helsinki.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2138051
References
- 1.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ (Clinical Research Ed). 2021;373(1088). doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsang HF, Chan LWC, Cho WCS, Yu AC, Yim AK, Chan AK, Ng LP, Wong YK, Pei XM, Li MJ, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021;19(7):1–6. doi: 10.1080/14787210.2021.1863146. [DOI] [PubMed] [Google Scholar]
- 3.Vitiello A, Ferrara F, Troiano V, La Porta R.. COVID-19 vaccines and decreased transmission of SARS-CoV-2. Inflammopharmacology. 2021;29(5):1357–60. doi: 10.1007/s10787-021-00847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolletta E, Iannetta D, Mastrofilippo V, De Simone L, Gozzi F, Croci S, Bonacini M, Belloni L, Zerbini A, Adani C, et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med. 2021;10(24):5960. doi: 10.3390/jcm10245960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Wang B, Li X. Acute-onset Vogt-Koyanagi-Harada like uveitis following Covid-19 inactivated virus vaccination. Am J Ophthalmol Case Rep. 2022;26:101404. doi: 10.1016/j.ajoc.2022.101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ElSheikh RH, Haseeb A, Eleiwa TK, Elhusseiny AM. Acute uveitis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29(6):1207–09. doi: 10.1080/09273948.2021.1962917. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Murthy SI, Annum S. Bilateral multifocal choroiditis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29(4):753–57. doi: 10.1080/09273948.2021.1957123. [DOI] [PubMed] [Google Scholar]
- 8.Mudie LI, Zick JD, Dacey MS, Palestine AG. Panuveitis following vaccination for COVID-19. Ocul Immunol Inflamm. 2021;29(4):741–42. doi: 10.1080/09273948.2021.1949478. [DOI] [PubMed] [Google Scholar]
- 9.Pan L, Zhang Y, Cui Y, Wu X. Bilateral uveitis after inoculation with COVID-19 vaccine: a case report. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis. 2021;113:116–18. doi: 10.1016/j.ijid.2021.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang K, Pan L, Guo H, Wu X. Case report: associated ocular adverse reactions with inactivated COVID-19 vaccine in China. Front Med. 2021;8:823346. doi: 10.3389/fmed.2021.823346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinovitch T, Ben-Arie-Weintrob Y, Hareuveni-Blum T, Shaer B, Vishnevskia-Dai V, Shulman S, Newman H, Biadsy M, Masarwa D, Fischer N, et al. Uveitis After ThE BNT162b2 mRNA vaccination against SARS-CoV-2 Infection: a possible association. Retina (Philadelphia, Pa). 2021;41(12):2462–71. doi: 10.1097/IAE.0000000000003277. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Li X, Li H, Li M, Gong S. Ocular adverse events after inactivated COVID-19 vaccination in Xiamen. Vaccines. 2022;10(3):482. doi: 10.3390/vaccines10030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139(10):1131–35. doi: 10.1001/jamaophthalmol.2021.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabs DA, Nussenblatt RB, Rosenbaum JT . Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang MTM, Niederer RL, McGhee CNJ, Danesh-Meyer HV. COVID-19 vaccination and the eye. Am J Ophthalmol. 2022;240:79–98. doi: 10.1016/j.ajo.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng JY, Margo CE. Ocular adverse events following vaccination: overview and update. Surv Ophthalmol. 2022;67(2):293–306. doi: 10.1016/j.survophthal.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Benage M, Fraunfelder FW. Vaccine-associated uveitis. Mo Med. 2016;113:48–52. [PMC free article] [PubMed] [Google Scholar]
- 18.World Health O . Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification 2nd ed edn. Geneva/Switzerland: World Health Organization; 2018. [Google Scholar]
- 19.Furer V, Eviatar T, Zisman D, Peleg H, Braun-Moscovici Y, Balbir-Gurman A, Paran D, Levartovsky D, Zisapel M, Elalouf O, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–38. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 20.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escott S, Tarabishy AB, Davidorf FH. Multifocal choroiditis following simultaneous hepatitis A, typhoid, and yellow fever vaccination. Clin Ophthalmol (Auckland, NZ). 2013;7:363–65. doi: 10.2147/OPTH.S37443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraunfelder FW, Suhler EB, Fraunfelder FT. Hepatitis B vaccine and uveitis: an emerging hypothesis suggested by review of 32 case reports. Cutan Ocul Toxicol. 2010;29(1):26–29. doi: 10.3109/15569520903427717. [DOI] [PubMed] [Google Scholar]
- 23.Testi I, Brandão-de-Resende C, Agrawal R, Pavesio C. Ocular inflammatory events following COVID-19 vaccination: a multinational case series. J Ophthalmic Inflamm Infect. 2022;12(1):4. doi: 10.1186/s12348-021-00275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhary MM, Hajj-Ali RA, Lowder CY. Gender and ocular manifestations of connective tissue diseases and systemic vasculitides. J Ophthalmol. 2014;2014:403042. doi: 10.1155/2014/403042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173(3):600–09. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


