Abstract
N6 methyladenosine (m6A) is the most abundant internal modification on mammalian messenger RNA. It is installed by a writer complex and can be reversed by erasers such as the fat mass and obesity-associated protein FTO. Despite extensive research, the primary physiological substrates of FTO in mammalian tissues and development remain elusive. Here, we show that FTO mediates m6A demethylation of long-interspersed element-1 (LINE1) RNA in mouse embryonic stem cells (mESCs), regulating LINE1 RNA abundance and the local chromatin state, which in turn modulates the transcription of LINE1-containing genes. FTO-mediated LINE1 RNA m6A demethylation also plays regulatory roles in shaping chromatin state and gene expression during mouse oocyte and embryonic development. Our results suggest broad effects of LINE1 RNA m6A demethylation by FTO in mammals.
N6-methyladenosine (m6A) is the most prevalent mammalian mRNA internal modification. It is regulated by writer and eraser proteins, thus affecting transcript fate through reader proteins (1-3). The fat mass and obesity-associated protein FTO was the first RNA demethylase shown to remove mRNA m6A (4). FTO is known to be involved in mammalian development and human diseases; for example, Fto−/− mice display severe developmental defects (5, 6). Extensive functional characterizations in human cancer cells have shown that FTO can localize to the cell cytoplasm and remove m6A from mRNA transcripts that contribute to cancer progression (7-12); however, similar activity was not apparent across mouse and human tissues, where FTO tends to exhibit nuclear localization (13). Another form of m6A, m6Am, which is enriched at the cap of a portion of mRNA and certain small nuclear RNAs, was also identified as being a substrate of FTO (14-16). However, depletion of the cap-m6Am methyltransferase PCIF1 only causes mild cellular effects (17-19) and has negligible impacts on mouse viability or fertility (20). These discordant findings indicate that the functionally relevant substrates of FTO during mammalian development remain elusive.
Unlike Pcif1 knockout (KO), Mettl3 depletion in mice causes early embryonic lethality (21). We recently found that chromatin-associated regulatory RNAs (carRNAs) can be m6A methylated by METTL3, which regulates chromatin state and transcription in mouse embryonic stem cells (mESCs) (22). Independent reports confirmed the chromatin-regulating role of carRNA m6A and further showed notable effects of m6A on the expression of endogenous retroviruses (ERVs) and heterochromatin formation (23-25). Thus, we speculated that a subset of m6A-marked carRNAs could be the physiological substrates of FTO and that FTO-mediated m6A demethylation may regulate chromatin state in mammalian tissues and during development.
Long-Interspersed Element-1 (LINE1) RNA is a major substrate of FTO in mESCs
To uncover the major substrates of FTO in these contexts, we derived Fto−/− and control wild-type (WT) mESCs (fig. S1, A to C). We quantified m6A-level changes of RNAs isolated from different subcellular fractions between Fto−/− and WT mESCs (fig. S1D). The m6A levels of RNA isolated from chromatin-associated and soluble nuclear fractions were increased (fig. S1, E to G), consistent with the nuclear localization of FTO (fig. S1H). We performed m6A methylated RNA immunoprecipitation sequencing (MeRIP-seq) to examine the chromatin-associated RNA (caRNA) methylome of WT and Fto−/− mESCs (fig. S1, I to L) and detected more hypermethylated peaks with Fto depletion (fig. S1M), accompanied by an increased overall caRNA m6A level (fig. S1N).
We annotated carRNAs as promoter-associated RNA, enhancer RNA, and RNA transcribed from transposable elements (repeat RNA) (22). Most carRNAs exhibited more hypermethylated m6A peaks (fig. S2A) and elevated m6A levels (fig. S2B) upon Fto KO. Compared with other carRNAs, Fto depletion led to more pronounced hypermethylation of repeat RNAs (Fig. 1A), more down-regulated m6A-marked repeat RNAs (fig. S2C), and greater down-regulation of hypermethylated repeat RNAs (Fig. 1B). Upon Fto KO, carRNA expression changes negatively correlated with m6A-level changes (fig. S2D), with repeat RNAs showing the strongest correlation between transcript down-regulation and m6A hypermethylation (fig. S2, E and F).
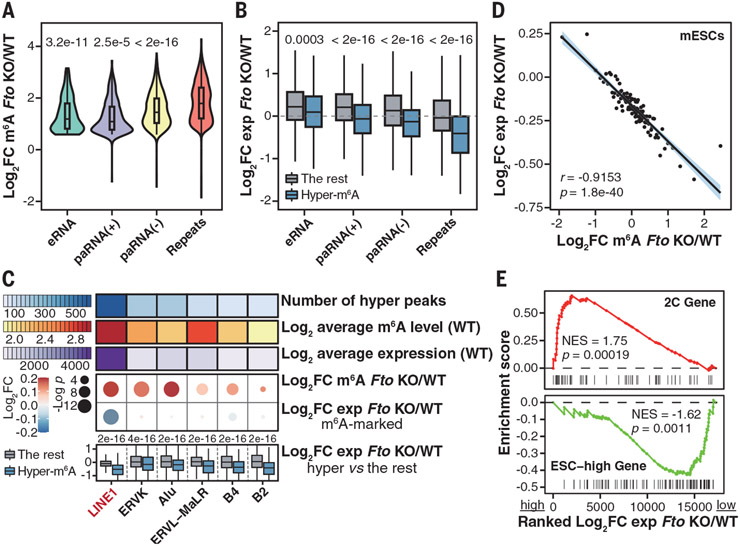
Fig. 1. m6A on LINE1 RNA is a major substrate of FTO in mESCs.
(A) Violin plots showing m6A-level fold changes of hypermethylated m6A peaks on carRNAs upon Fto KO. P values were determined using Wilcoxon rank sum tests. (B) Boxplots showing expression fold changes of hypermethylated carRNAs versus other m6A-marked carRNAs upon Fto KO. P values were determined using Wilcoxon signed-rank tests. (C) Summary of repeat RNAs on chromatin upon Fto KO. Top: number of hypermethylated peaks, average m6A level, and expression. Middle: m6A-level and expression fold changes. P values were determined using Wilcoxon signed-rank tests. Bottom: expression fold changes of hypermethylated versus non-hypermethylated repeat RNAs. P values were determined using Wilcoxon rank sum tests. (D) Scatter plot showing the negative correlation of fold changes between m6A level and expression of LINE1 RNA upon Fto KO. LINE1 RNAs were categorized into 100 bins on the basis of their ranked m6A-level fold changes upon Fto KO. r refers to Pearson’s correlation coefficient. P value was determined using t distribution. (E) Gene set enrichment analysis showing up-regulated 2C genes (top) and down-regulated ESC-high genes (bottom) from mRNA-seq upon Fto KO. NES, normalized enrichment score.
Among m6A-marked repeat RNAs, LINE1 RNA emerged as a major substrate of FTO in mESCs. It showed the highest number of hypermethylated peaks, the most increased m6A levels, the most reduced abundance (Fig. 1C), and a reduced overall expression (fig. S3, A and B) upon Fto KO. LINE1 RNA mainly associates with chromatin in mESCs (fig. S3, C and D) (26, 27). We observed colocalization of LINE1 RNA and FTO protein (fig. S3D) and validated the binding of LINE1 RNA by FTO (fig. S3E). The m6A level and expression of whole-cell LINE1 RNA exhibited changes similar to those on chromatin upon Fto KO (fig. S3, F and G), accompanied by reduced L1ORF1p expression (fig. S3H). Treating WT mESCs with an FTO inhibitor (9) recapitulated the effect of Fto KO on LINE1 RNA (fig. S3I).
Upon Fto KO, we observed that greater m6A level increases correlated with greater LINE1 RNA abundance reductions (Fig. 1D). Across published mouse and human tissue m6A methylomes (13), LINE1 RNA m6A level also negatively correlated with its expression, and high FTO expression was associated with low m6A level and high LINE1 RNA expression (fig. S4), supporting LINE1 RNA as a substrate of FTO in most tissues.
LINE elements are one of the most abundant retrotransposons in mammalian genomes, and LINE1 RNA is known to play critical roles during mammalian early development (26, 27). In mESCs, LINE1 RNA can function as a nuclear RNA scaffold for trans-regulation, with LINE1 RNA knockdown by morpholino antisense oligo (ASO) causing activated two-cell (2C) program and repressed ESC-high genes (27, 28). Fto KO largely recapitulated these transcriptomic changes (Fig. 1E), with lower 2C gene activation but greater down-regulation of ESC-high genes (fig. S5, A to E). Fto KO also caused several phenotypic changes similar to those which occurred after LINE1 ASO treatment in mESCs, including cell cycle dysregulation, self-renewal impairment, and induction of capacity to form embryoid bodies (EBs) (fig. S5, F to L). Moreover, Fto−/− mESCs exhibited a reduced ability to integrate to chimeric mice compared with WT mESCs (fig. S5, M and N).
Evolutionarily young LINE1s display higher RNA m6A levels and greater m6A increases upon Fto KO (fig. S6, A and B). Longer and less divergent LINE1 RNA also tends to exhibit a higher m6A level (fig. S6, C and D). We observed elevated m6A density near the 5′ end with Fto depletion (Fig. 2A and fig. S6E), which also responded to Mettl3 KO (fig. S6F). Our analysis suggested that several young LINE1 subfamilies were down-regulated and hypermethylated upon Fto KO (Fig. 2B and fig. S6, G to J). We used a CRISPR dCas13b system fused with WT FTO or a catalytically inactive mutant (fig. S6K) (22), and observed that delivery of dCas13b-wtFTO by guide RNA targeting LINE1 RNA reversed its m6A level and expression changes (fig. S6, L to N).
Fig. 2. FTO regulates LINE1 RNA level through m6A demethylation.
(A) m6A peak profiles on m6A-marked LINE1 RNA in WT and Fto−/− mESCs. (B) Volcano plot showing differentially expressed subfamilies of m6A-marked repeat RNAs upon Fto KO. (C) Cumulative distribution and boxplots (inset) showing nuclear LINE1 RNA lifetime in WT and Fto−/− mESCs. P value was determined using Wilcoxon signed-rank test. (D) Relative enrichment of LINE1 RNA by YTHDC1 from cross-linking and immunoprecipitation quantitative polymerase chain reaction (qPCR) in WT and Fto−/− mESCs. P values were determined using unpaired two-tailed t tests. Error bars and means ± SD are shown for n = 3 experiments. (E) Cumulative distribution and boxplots (inset) showing LINE1 RNA transcription rate in WT and Fto−/− mESCs. P value was determined using Wilcoxon signed-rank test. (F) Cumulative distribution and boxplots (inset) showing the difference in transcription rate changes between m6A-marked and -unmarked LINE1 RNA upon Fto KO. P value was determined using Wilcoxon rank sum test.
Consistent with the reported YTHDC1-mediated destabilization of m6A-marked carRNAs (22), we detected accelerated decay of LINE1 RNA upon Fto KO (Fig. 2C), accompanied by elevated binding of LINE1 RNA by YTHDC1 (Fig. 2D). Both changes were recovered by targeting LINE1 RNA with dCas13b-wtFTO in Fto−/− mESCs (fig. S7, A and B).
LINE1 transcription was markedly reduced with Fto depletion (Fig. 2E and fig. S7, C to E). Moreover, Fto KO led to greater decreases in the nascent synthesis of m6A-marked compared with unmarked LINE1 RNAs (Fig. 2F), but not ERVK or Alu transcripts (fig. S7F). We also observed reduced DNA association of LINE1 RNA and decreased R-loop formation around LINE1 loci with Fto depletion (fig. S7, G and H). These effects caused by Fto KO could all be reversed by targeting dCas13b-wtFTO to LINE1 RNA (fig. S7, I to K). Therefore, FTO appears to mediate m6A demethylation of a subset of LINE1 RNAs, maintaining their levels on chromatin.
Fto KO leads to closed chromatin in mESCs
LINE1 RNA and m6A on carRNAs have been shown to regulate chromatin state and transcription (22-27). Indeed, we observed decreased nascent RNA synthesis (fig. S8, A and B) accompanied by more closed chromatin (Fig. 3A) upon Fto KO. Similar effects were observed when treating WT mESCs with an FTO inhibitor (fig. S8, C and D). Additionally, LINE1 ASO treatment in WT mESCs led to more closed chromatin (fig. S8E), whereas delivering dCas13b-wtFTO to LINE1 RNA largely rescued chromatin closure observed in Fto−/− mESCs (Fig. 3B and fig. S8F). We next monitored LINE1 RNA m6A demethylation and chromatin accessibility in a time-course of FTO inhibition in WT mESCs (fig. S8, G to J). Assay for transposase-accessible chromatin sequencing (ATAC-seq) results also validated reduced chromatin accessibility upon Fto KO (fig. S9, A to C), with gained-closed regions enriching gene ontology (GO) terms relevant to development (fig. S9D). Slightly decreased H3K4me3 and H3K27ac and increased H3K9me3 and H3K27me3 were observed with Fto depletion (fig. S9E).
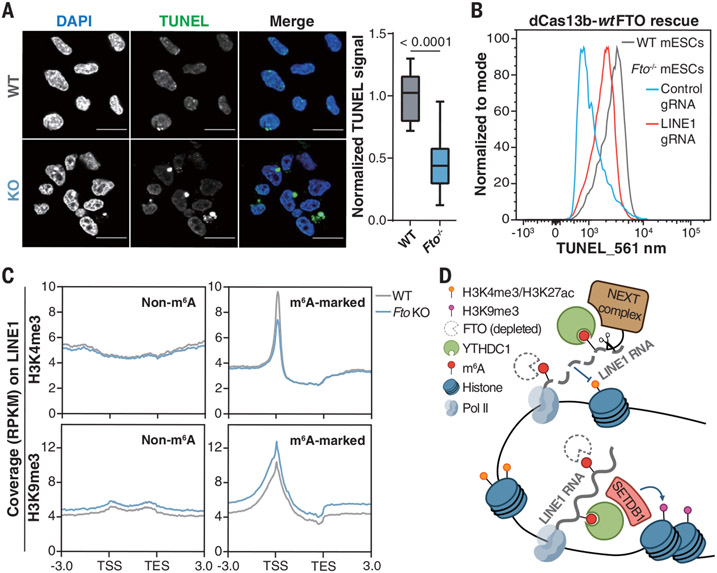
Fig. 3. FTO affects chromatin state through LINE1 RNA m6A demethylation.
(A) Left: DNase I–terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay showing more closed chromatin upon Fto KO. Scale bars, 20 μm. The nucleus was counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Representative images were selected from three independent experiments. Right: relative TUNEL intensity in WT and Fto−/− mESCs. P value was determined using an unpaired two-tailed t test. TUNEL intensity was quantified by ImageJ. (B) TUNEL signals of WT (gray) and Fto−/− mESCs rescued by dCas13b-wtFTO with guide RNA (gRNA) targeting LINE1 RNA (red) or control gRNA (blue). (C) Profiles of H3K4me3 and H3K9me3 levels on LINE1 RNA loci from 3.0 kb upstream of the transcription start site (TSS) to 3.0 kb downstream of the transcription end site (TES) in WT and Fto−/− mESCs. (D) Schematic model showing how Fto KO affects LINE1 RNA abundance and local chromatin state.
YY1 and EP300 can be recruited by caRNA to promote transcription (22, 29, 30). We found notable enrichment at gained-closed regions caused by Fto KO for H3K4me1, H3K4me3, and H3K27ac, as well as YY1, EP300, and Pol II binding, but not repressive histone marks (fig. S10A). Consistently, chromatin immunoprecipitation sequencing experiments confirmed reduced chromatin accessibility of these regions upon Fto depletion (fig. S10B). Fto KO–induced gained-closed regions were also affected by Mettl3 KO (fig. S10C) (22). In turn, chromatin closure upon Fto KO could reduce access of METTL3 to certain genomic regions, potentially explaining m6A hypomethylation within them.
We observed distinct profiles of H3K4me3 and H3K9me3 between loci with m6A-marked and -unmarked LINE1 RNA (Fig. 3C). At m6A-marked LINE1 RNA loci, we observed decreased H3K4me3 and H3K27ac levels and Pol II binding upon Fto KO, accompanied by increased H3K9me3 levels (Fig. 3C and fig. S11, A to C). Similar patterns were observed for FTO-targeted LINE1 subfamilies but not L1M2b, an unmarked LINE1 subfamily, or IAPEz-int, an ERVK subfamily regulated by METTL3 (23-25) (fig. S11D). Targeting LINE1 RNA with dCas13b-wtFTO reversed the dys-regulated histone marks (fig. S11E). Moreover, Fto KO led to decreased chromatin association and LINE1 RNA binding of YY1 and EP300 (fig. S11, F to H).
Altogether, our data reveal an interplay between LINE1 RNA m6A demethylation by FTO and chromatin state. After Fto depletion, increased m6A on LINE1 RNA could promote YTHDC1 binding, which could recruit nuclear decay machinery (22) and histone modifiers that add repressive histone marks (23, 24), resulting in reduced LINE1 RNA abundance and more closed chromatin (Fig. 3D).
FTO regulates gene expression mostly through intragenic LINE1 RNA
We observed that Fto and LINE1 RNA abundance increased after EB differentiation induction, but LINE1 RNA increase was diminished upon Fto KO (fig. S12A). Targeting dCas13b-wtFTO to LINE1 RNA in Fto−/− mESCs partially rescued aberrantly expressed differentiation markers associated with induced EB differentiation and impaired self-renewal (fig. S12, B to G).
Therefore, we investigated the mechanism underlying the impact of Fto KO on mESC differentiation. We found that genes containing LINE1 RNA were down-regulated upon Fto KO compared with genes beyond 10 kb from LINE1 RNA (Fig. 4A and fig. S13A) (26). The expression of LINE1-containing genes and intragenic LINE1 RNA tended to decrease with Fto depletion, accompanied by increased intragenic LINE RNA m6A level (Fig. 4B and fig. S13B). LINE1-containing genes also displayed greater transcription rate reductions compared with other genes upon Fto KO (fig. S13, C to E).
Fig. 4. Fto KO deactivates LINE1-containing genes by repressing intragenic LINE1 RNA.
(A) Boxplots showing gene expression fold changes upon Fto KO from caRNA sequencing. Genes were categorized according to their genomic distance to the nearest LINE1 RNA with at least 10 reads. P value was determined using Wilcoxon rank sum test. (B) Scatter plot showing the negative correlation of fold changes between expression of LINE1-containing genes and m6A level of corresponding intragenic LINE1 RNA upon Fto KO. Intragenic LINE1 RNAs were categorized into 100 bins on the basis of their ranked expression fold changes upon Fto KO. r refers to Pearson’s correlation coefficient. P value was calculated based on t distribution. (C) Boxplots showing fold changes of gene expression from caRNA sequencing (left) and gene transcription rate (right) upon Fto KO. Genes were categorized into three groups: genes containing down-regulated LINE1 RNA, genes near (<1 Mb) down-regulated LINE1 RNA, and genes containing other LINE1 RNA. P values were determined using Wilcoxon rank sum tests. (D) Profiles of H3K4me3, H3K9me3, H3K27ac, and Pol II levels on loci of genes containing down-regulated LINE RNA from 3.0 kb upstream of the TSS and 3.0 kb downstream of the TES in WT and Fto−/− mESCs.
Both intragenic and intergenic LINE1 RNAs showed suppressed expression upon Fto KO, but intragenic RNAs were generally more responsive (fig. S13, F and G). We further explored the cis-regulatory role of intragenic LINE1 RNAs suppressed by Fto KO and observed significantly decreased expression and reduced transcription rate for genes containing down-regulated LINE1 RNA compared with other genes (Fig. 4C). Accordingly, reduced H3K4me3 and H3K27ac levels and Pol II binding, accompanied by elevated H3K9me3 levels, were observed for these gene loci (Fig. 4D) and those containing FTO-targeted LINE1 subfamilies (fig. S13H).
Most genes containing down-regulated LINE1 RNA were also down-regulated upon Fto KO (fig. S14A), and these genes enriched pathways involved in differentiation and development (fig. S14B). We examined several key genes in ESC pluripotency and early development (Esrrb, Nek5, Phf3, and Zfp982) and found that all of them contained intragenic LINE1 RNA with increased m6A level and decreased expression upon Fto KO, associated with more closed local chromatin and reduced gene transcription rate (fig. S14, C to G), which could all be reversed by targeting LINE1 RNA with dCas13b-wtFTO (fig. S15, A to I).
Certain 2C genes, such as Dub1 and Zscan4, do not contain LINE1 RNA These genes and MERVL RNA, a 2C-specific transposon, could be repressed by LINE1 RNA through trans-regulation (27). For these transcripts, we observed reduced expression when applying dCas13b-wtFTO to LINE1 RNA in Fto−/− mESCs (fig. S15J).
The FTO-LINE1 RNA axis is functionally relevant in mouse development
We examined the mouse cerebellum, hippocampus, and adult neural stem cells because Fto is highly expressed in mouse brain tissues (31). We observed increased LINE1 RNA m6A levels, decreased LINE1 RNA expressions, and more closed chromatin from samples derived from Fto−/− mice compared with WT controls (fig. S16, A to J). Similar trends of Fto and LINE1 RNA abundance during mESC differentiation were also observed for adult neural stem cells (fig. S16, K and L).
We next investigated the effects of FTO during early development. Fto−/− pups were born at a lower rate than the expected Mendelian ratio (fig. S17A). Fto−/− female mice showed ovarian defects and impaired fertility (fig. S17, B and C). Previous studies revealed that LINE1 is activated before the sex determination of primordial germ cells and the meiotic entry of oocytes (32, 33). Indeed, both Fto and LINE1 RNA expression decreased from primordial germ cell to the metaphase II (MII) stage (fig. S17D). The number of germinal vesicle (GV) oocytes and the ratio of surrounded nucleolus GV oocytes from Fto−/− mice were lower compared with WT controls (Fig. 5, A and B). Fto depletion led to significantly reduced LINE1 RNA expression in GV and MII oocytes (Fig. 5C and fig. S17E), with down-regulated LINE1 subfamilies largely resembling those observed in mESCs (fig. S17F). We observed more closed chromatin for GV oocytes from Fto−/− mice compared with WT controls (Fig. 5D). Fto−/− GV oocytes could mature to the MII stage (fig. S17G), but Fto−/− MII oocytes showed increased chromosome misalignment and spindle collapse (fig. S17H). RNA-sequencing (RNA-seq) results revealed greater expression decreases of genes containing down-regulated LINE1 RNA compared with other genes in Fto−/− GV and MII oocytes, respectively (fig. S17, I and J). GO analysis suggested that Fto KO caused the observed defects in oocyte development, likely through LINE1 RNA, which regulates LINE1-containing genes (fig. S17K).
Fig. 5. The FTO-LINE1 RNA axis plays critical roles during early development.
(A) Number of GV oocytes from 4-week-old WT and Fto−/− mice. Error bars and means ± SEM are shown for n = 4 WT mice and n = 6 Fto−/− mice. (B) Ratio of surrounded nucleolus (SN)/nonsurrounded nucleolus (NSN) oocytes from 4-week-old WT mice (n = 5) and Fto−/− mice (n = 4). (C) Relative LINE1 RNA expression measured by reverse transcription qPCR in WT and Fto−/− oocytes. (D) Left: DNase I-TUNEL assay showing more closed chromatin in oocytes upon Fto KO. Scale bars, 50 μm. The nucleus was counterstained by DAPI. Representative images were selected from three independent experiments. Right: relative TUNEL intensity in WT and Fto−/− oocytes (n = 12 each). pSN, partly surrounded nucleolus. (E) Implantation rate (left) and E7.5 embryo rate (right) of FtoP+/M+, FtoP+/M−, FtoP−/M+, and FtoP−/M− zygotes. (F) Relative LINE1 RNA expression measured by reverse transcription qPCR in FtoP+/M+ and FtoP−/M− morulae. For (A), (C), (D), and (F), P values were determined using unpaired two-tailed t tests; error bars and means ± SD are shown for n = 3 experiments in (C) and (F). For (B) and (E), P values were determined using two-tailed z tests.
We further generated WT (FtoP+/M+), maternal Fto-deficient (FtoP+/M−, paternal Fto-deficient (FtoP−/M+), and homozygous KO (FtoP−/M−) embryos (fig. S18, A and B). Embryos from all four groups could reach the blastocyst stage [embryonic day 3.5 (E3.5)] and hatch out of the zona pellucida at E4.5, but Fto-deficient embryos showed a slightly weakened ability to do so (fig. S18C). Moreover, maternal loss of Fto severely impeded decidua formation and the generation of E7.5 embryos, with no E7.5 embryos produced upon homozygous Fto depletion (Fig. 5E and fig. S18D). We examined the transcriptome differences between FtoP−/M− and FtoP+/M+ morulae, in which we detected repressed LINE1 RNA and down-regulated LINE1 subfamilies similar to those in mESCs (Fig. 5F and fig. S18, E and F). We again observed induced expression of Zscan4 and MERVL RNA (fig. S18, G and H) and greater expression decreases of genes containing down-regulated LINE1 RNA, including regulators essential during early embryonic development, such as Lin28b, Tet2, and Gsk3b (fig. S18, I to L).
Finally, we applied dCas13b-wtFTO in Fto−/− MII oocytes and fertilized them with Fto−/− sperm. Embryos were developed in vitro to the morula stage for subsequent analyses (fig. S18, M and N). Induced LINE1 RNA associated with more open chromatin was observed in FtoP−/M− morulae by targeting dCas13b-wtFTO to LINE1 RNA in Fto−/− MII oocytes (fig. S18, O and P). The expression of Zscan4, MERVL RNA, and selected genes containing down-regulated LINE1 RNA were also reversed in the rescued embryos (fig. S18, Q and R).
Discussion
In this work, we have shown functionally relevant substrates and proposed mechanisms of RNA m6A demethylation through FTO in mammalian development. Our findings support LINE1 RNA as a major substrate of FTO in mESCs, although FTO may additionally mediate m6A demethylation of other carRNAs to affect gene expression (supplementary text and figs. S19 to S21). In contrast to certain cancer cells in which FTO can be hijacked to mediate mRNA m6A demethylation (7-12), which may dominate chromatin state regulation (34) (supplementary text and fig. S22), we found that FTO-mediated m6A demethylation maintained LINE1 RNA abundance in mESCs, which contributes to promoting local chromatin openness and activating LINE1-containing genes. We further showed that the FTO-LINE1 RNA axis is functionally relevant in mouse oocyte and embryonic development. How FTO achieves target selectivity in different cellular contexts still needs future investigation. In addition to m6A, RNA 5-methylcytosine oxidation is also known to affect transcription of ERVL and ERVL-associated genes in mESCs (35), suggesting a potential widespread presence of regulation through retrotransposon RNA modifications (36).
Materials and methods summary
Fto−/− and control WT mESCs were derived from the inner cell mass of E3.5 blastocysts. m6A immunoprecipitation was performed for non-ribosomal RNA isolated from the chromatin-associated fraction or from whole cell, as indicated, using the EpiMark N6-Methyladenosine Enrichment Kit (New England Biolabs). All RNA-seq libraries were prepared using SMARTer Stranded Total RNA-Seq Kit version 2, Pico Input Mammalian (TaKaRa). For most samples, libraries were sequenced on an Illumina NovaSeq 6000 in a 100-base-pair paired-end mode. For RNA-seq data, trimmomatic trimmed reads were aligned to the mm10 reference genome using HISAT2. Read counts were calculated by featureCounts, and differential expression was analyzed using DESeq2. For the generation of preimplantation embryos, MII oocytes were subjected to intracytoplasmic sperm injection and embryo culture. Detailed materials and methods are available in the supplementary materials.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. Zhang for Fto−/− mice and S. Liu, H.-L. Sun, and H. Shi for discussions.
Funding:
This work was supported by the National Institutes of Health (grants R01 ES030546 and RM1 HG008935 to C.H.); the National Key R&D Program of China (grants 2020YFA0113200 and 2018YFA0108900 to Y.G.); and the National Natural Science Foundation of China (grants 31922022, 31721003, 31820103009, and 82071720 to Y.G., S.G., and B.H.). C.S.-P. is supported by Medical Scientist Training Program grant T32GM007281 and National Cancer Institute fellowship F30 CA253987. C.H. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Data and materials availability: Sequencing data are available at the Gene Expression Omnibus (GEO accession nos. GSE151704 and GSE151780). All other data are available in the manuscript or the supplementary materials.
Competing interests: C.H. is a scientific founder and a scientific advisory board member of Accent Therapeutics, Inc., Inferna Green, Inc., and AccuaDX, Inc. The remaining authors declare no competing interests.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Roundtree IA, Evans ME, Pan T, He C, Cell 169, 1187–1200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frye M, Harada BT, Behm M, He C, Science 361, 1346–1349 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi H, Wei J, He C, Mol. Cell 74, 640–650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia G et al. , Nat. Chem. Biol 7, 885–887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Hoeven F et al. , Development 120, 2601–2607 (1994). [DOI] [PubMed] [Google Scholar]
- 6.Fischer J et al. , Nature 458, 894–898 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Li Z et al. , Cancer Cell 31, 127–141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su R et al. , Cell 172, 90–105.e23 (2018).29249359 [Google Scholar]
- 9.Huang Y et al. , Cancer Cell 35, 677–691.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su R et al. , Cancer Cell 38, 79–96.e11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qing Y et al. , Mol. Cell 81, 922–939.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y et al. , Cell Metab. 33, 1221–1233.e11 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Liu J et al. , Mol. Cell 77, 426–440.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Mauer J et al. , Nature 541, 371–375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauer J et al. , Nat. Chem. Biol 15, 340–347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei J et al. , Mol. Cell 71, 973–985.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulias K et al. , Mol. Cell 75, 631–643.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sendinc E et al. , Mol. Cell 75, 620–630.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akichika S et al. , Science 363, eaav0080 (2019).30467178 [Google Scholar]
- 20.Pandey RR et al. , Cell Rep. 32, 108038 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Batista PJ et al. , Cell Stem Cell 15, 707–719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J et al. , Science 367, 580–586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J et al. , Nature 591, 322–326 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Xu W et al. , Nature 591, 317–321 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Chelmicki T et al. , Nature 591, 312–316 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Jachowicz JW et al. , Nat. Genet 49, 1502–1510 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Percharde M et al. , Cell 174, 391–405.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu JY et al. , Cell Rep. 30, 3296–3311.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigova AA et al. , Science 350, 978–981 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bose DA et al. , Cell 168, 135–149.e22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerken T et al. , Science 318, 1469–1472 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malki S, van der Heijden GW, O’Donnell KA, Martin SL, Bortvin A, Dev. Cell 29, 521–533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seisenberger S et al. , Mol. Cell 48, 849–862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y et al. , Nat. Neurosci 21, 195–206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guallar D et al. , Nat. Genet 50, 443–451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei J, He C, Curr. Opin. Cell Biol 70, 109–115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.