ABSTRACT
This study compared the immunogenicity and safety of a booster dose of HepB-CpG (HEPLISAV-B® vaccine) with HepB-Eng (Engerix-B®) and HepB-AS04 (Fendrix®) in patients receiving chronic hemodialysis. This was a multicenter, randomized, open-label, phase 3 study of adults receiving hemodialysis with antibodies to HBsAg (anti-HBs) <10 mIU/mL at study entry. The objective was to compare the seroprotection rate (SPR) induced by HepB-CpG with HepB-Eng or HepB-AS04. The SPR was defined as the percentage of patients with anti-HBs ≥10 mIU/mL post-vaccination. At 20 sites in Germany, 155 participants were randomized: HepB-CpG = 54; HepB-Eng = 50; and HepB-AS04 = 51. Of the 149 participants in the modified intention-to-treat population, 76.5% had not previously responded to at least one series of hepatitis B vaccine. Based on a post hoc analysis, the SPR in HepB-CpG recipients (52.8%; 95% confidence interval [CI]: 38.6%, 66.7%) was significantly higher than in HepB-Eng recipients (32.6%; 95% CI: 19.5%, 48.0%), and non-inferior to that in HepB-AS04 recipients (43.1%; 95% CI: 29.3%, 57.8%). Local post-injection reactions occurred in significantly fewer HepB-CpG (9.3%) than HepB-AS04 recipients (31.4%; p = .007) and at a similar rate to HepB-Eng recipients (8.2%). Systemic post-injection reactions in HepB-CpG recipients (18.5%) were similar to the HepB-AS04 group (19.6%) and higher than in the HepB-Eng group (12.2%). In this difficult-to-immunize population, a booster dose of HepB-CpG induced significantly higher levels of seroprotection than HepB-Eng with a similar safety profile. The higher levels of immunogenicity were not accompanied by higher levels of local post-injection reactions compared with HepB-AS04.
KEYWORDS: CpG 1018 adjuvant, hepatitis B virus, vaccine, hemodialysis, immunogenicity, safety
Introduction
Hepatitis B virus (HBV) infection is a major global public health problem. The World Health Organization estimates that 2 billion people have been infected worldwide and, in 2019, more than 296 million people were chronic carriers of HBV. Up to 20% of infected individuals are expected to develop cirrhosis, liver failure, or hepatocellular carcinoma. HBV infection accounted for over 820,000 deaths in 2019.1
HBV is transmitted through exposure to infected blood and body fluids. Patients with end-stage renal disease (ESRD) are at increased risk of exposure to HBV in the hemodialysis setting where frequent and prolonged vascular access is required. In an environment where multiple patients receive dialysis concurrently, repeated opportunities exist for person-to-person transmission of infectious agents, directly or indirectly via contaminated devices, equipment and supplies, environmental surfaces, or hands of personnel. In one study (2007–2009), 30% of 129 patients with new HBV infection were thought to have become infected with HBV through hemodialysis.2 At the end of 2017, an estimated 743,000 patients were on chronic dialysis in the United States with more than 124,000 patients initiating hemodialysis that year, many of whom were likely unvaccinated for hepatitis B.3 In 2015, 546,000 patients were receiving chronic dialysis in Europe, a number that has doubled since 1990.4 While the number of HBV infections reported in patients receiving hemodialysis is low, the risk of transmission remains high, and dialyzing patients with HBV infection requires special precautions, including using dedicated rooms, machines, instruments, medications, supplies, and staff to reduce the risk of infecting other patients.5
Due to a compromised immune system, patients receiving hemodialysis who become infected with HBV have a high risk of developing chronic hepatitis B infection if infected with HBV and its sequelae, including chronic active hepatitis, cirrhosis, and hepatocellular carcinoma.5 Vaccination is an important tool in preventing the transmission of HBV in the hemodialysis setting.5 Patients with chronic renal failure have defects in both humoral and cellular immunity, which lead to reduced responses to vaccination.6 The vaccine regimens for ESRD currently recommended in the US and Europe result in seroprotection rates (SPRs) of 33% to 92%, with most below 70%.7–10
In individuals with ESRD, unlike in healthy individuals, long-term protection by vaccination against HBV infection depends on persistence of a seroprotective level of antibodies against hepatitis B surface antigen (HBsAg).11 An antibody against HBsAg (anti-HBs) concentration of ≥10 mIU/mL has been shown to correlate with protection against HBV infection;12,13 long-term maintenance of an anti-HBs level of ≥10 mIU/mL in the ESRD population is necessary because breakthrough infections have occurred in persons with levels less than 10 mIU/mL.14,15 An anti-HBs concentration ≥100 mIU/mL after vaccination is desirable in patients with ESRD because the duration of seroprotection is longer than for patients with an anti-HBs concentration 10–99 mIU/mL.16
This study was conducted to evaluate the response to a booster dose of hepatitis B vaccine in patients receiving hemodialysis who had previously received hepatitis B vaccine. This study compared hepatitis B vaccines with different adjuvants: CpG 1018® adjuvant, a toll-like receptor 9 (TLR9) agonist, in HepB-CpG (referred to elsewhere as HBsAg-1018), aluminum hydroxide adjuvant in HepB-Eng (referred to elsewhere as HBsAg-Eng), and aluminum hydroxide plus monophosphoryl lipid A (MPL) adjuvant, a TLR4 agonist, in HepB-AS04 (referred to elsewhere as HBV-AS04). There are no recent reports on the response of patients on chronic hemodialysis to a booster dose of hepatitis B vaccine. This study presents data on a randomized, open-label, multicenter phase 3 trial comparing the immunogenicity and safety of a single dose of HepB-CpG with one double dose of HepB-Eng, and a single dose of HepB-AS04, in patients receiving chronic hemodialysis.
Materials and methods
Study design and patients
This trial was conducted at 20 sites in Germany from December 2010 to April 2012; was approved by the appropriate central and local ethics committees; and was conducted according to the Declaration of Helsinki and Good Clinical Practices. Written informed consent was obtained in the patient’s native language prior to enrollment. This study was registered on ClinicalTrials.gov (NCT01195246) on September 6, 2010.
Eligible patients were aged 18 y and older who had loss of renal function, were receiving hemodialysis, and had an anti-HBs concentration of <10 mIU/mL at enrollment. Patients could have either previously responded (anti-HBs ≥10 mIU/mL) to one series of commercially available hepatitis B vaccine with or without booster(s), or failed to develop anti-HBs ≥10 mIU/mL after receiving at least one series of hepatitis B vaccine and one or more booster(s). Patients were excluded if they: were at high risk for recent exposure to HBV, hepatitis C virus, or HIV through a mode other than hemodialysis; had a known history of autoimmune or inflammatory diseases; had uncontrolled diabetes mellitus; had received a kidney transplant; had received any blood product or immunoglobulin within 3 months prior to study entry; had received any inactivated viral or bacterial vaccine 21 d prior to the vaccination or received any live viral or bacterial vaccine or systemic corticosteroids 28 d prior to the vaccination; and had an injection of DNA plasmids or oligonucleotides or an investigational hepatitis B vaccine at any time or an intradermal hepatitis B vaccine, if given as a primary vaccine series.
Eligible patients were randomly assigned 1:1:1 to receive HepB-CpG, HepB-Eng, or HepB-AS04. Randomization was stratified by site and response to the previously received hepatitis B vaccine series. Patients with prior seroprotection (hereafter referred to as “prior responders”) had an anti-HBs level of ≥10 mIU/mL after at least one series of hepatitis B vaccine, with or without booster(s). Patients without prior seroprotection (hereafter referred to as “prior non-responders”) never had an anti-HBs level of ≥10 mIU/mL after at least one series of hepatitis B vaccine and one or more booster injections of hepatitis B vaccine.
Study vaccines and administration
The test product, HepB-CpG (HEPLISAV-B® vaccine), was composed of 20 µg recombinant HBsAg, and 3000 µg of the TLR9 agonist, CpG 1018® adjuvant (lot TDG010).17 Participants in the HepB-CpG group received a single intramuscular (IM) injection (0.5 mL) in the deltoid muscle at week 0. One active comparator was HepB-Eng (Engerix-B®) (20 µg recombinant HBsAg combined with 500 µg alum adjuvant9) (lots AHBVB747AA, AHBVB988AB, and AHBVB825BE). Participants in the HepB-Eng group received a double dose of HepB-Eng in either the left or the right deltoid muscle (both injections in the same arm) at study week 0 consisting of two 1.0-mL injections of 20 µg HBsAg for a total dose of 40 µg HBsAg. The second active comparator was HepB-AS04 (Fendrix®) (20 µg recombinant HBsAg combined with 500 µg alum adjuvant and 50 µg 3-O-4ˈ-desacyl- MPL)18 (lots AFENA009AA and AFENA012AA). Participants in the HepB-AS04 group received a single IM injection (0.5 mL) into the deltoid muscle at week 0.
Study procedures
Demographic information, medical history, medication history, and smoking history were collected and physical examination including vital signs was conducted during the screening visit. In addition, laboratory testing for HBV (including HBsAg, anti-HBs, and anti–hepatitis B core antigen), hepatitis C virus, and HIV was conducted. Eligible participants were required to be serum negative for HBsAg, hepatitis C virus, and HIV. At study visits, patients underwent clinical safety evaluations and had blood drawn for measurement of anti-HBs levels.
Safety assessments
The safety and tolerability assessments included monitoring and recording of local and systemic post-injection reactions, adverse events, autoimmune adverse events, and serious adverse events.
Diary cards solicited information about the presence and severity of local post-injection reactions (redness, swelling, pain at or near the injection site), systemic post-injection reactions (malaise, headache, myalgia, fatigue), and oral temperature for 7 d after study injection. Adverse events were collected through 4 weeks after the injection, and serious adverse events and autoimmune adverse events were collected through 12 weeks after the study injection.
Immunogenicity
Anti-HBs serum concentrations were measured using the Ortho Vitros® enhanced chemiluminescence immunoassay (Ortho Clinical Diagnostics, Rochester, NY).
Statistical methods
The primary objective was to compare the immune response of HepB-CpG with HepB-Eng and HepB-AS04 as measured by the SPR defined as the percentage of patients with anti-HBs ≥10 mIU/mL at 4 weeks after the booster injection.
Secondary objectives included evaluation of the safety of HepB-CpG compared with HepB-Eng and HepB-AS04; a comparison of the immunogenicity of HepB-CpG with HepB-Eng and HepB-AS04 as measured by the percentage of patients with anti-HBs ≥100 mIU/mL following booster injection; and a comparison of the immunogenicity of HepB-CpG with HepB-Eng and HepB-AS04 as measured by anti-HBs geometric mean concentration (GMC) following booster injection.
This was a hypothesis-generating trial to compare the immune responses to a booster dose of HepB-CpG, HepB-Eng, and HepB-AS04. Because only descriptive analyses were to be performed, the sample size of approximately 50 per group was chosen. The safety population included patients who received at least one study injection and had a post-injection safety evaluation. Immunogenicity analyses used the modified intent-to-treat analysis population comprising patients who received at least one study injection and had an immunogenicity evaluation.
SPRs were calculated with associated two-sided 95% confidence intervals (CIs) using the Clopper–Pearson method.19 Adjusted GMCs were calculated with associated CIs. Analysis of variance (ANOVA), with the log transformation (log10) of the concentrations as the dependent variables, was used to calculate the least square mean log10 concentrations and CIs. Geometric means and 95% CIs of the GMCs were obtained by taking the anti-log of the least square mean and the CIs in the log10 concentrations scale.
Statistical methods and criteria for non-inferiority and superiority used in previous pivotal trials of HepB-CpG20 were applied post hoc in this hypothesis-generating trial. For the non-inferiority of the SPR, the lower limit of the 95% CI for the differences between vaccination groups (HepB-CpG minus HepB-Eng or HepB-AS04) was greater than or equal to –10%. The result was statistically significantly higher if the lower limit of the 95% CI was greater than 0%. Post hoc statistical tests of safety were performed at the two-sided 0.05 level of significance using the 2-tailed Fisher's exact test. All data analyses were performed using SAS® version 9.2.
Results
Study patients
In this trial, 155 patients were randomized (Figure 1); 117 (HepB-CpG: n = 38; HepB-Eng: n = 38; HepB-AS04: n = 41) had not responded to at least one prior hepatitis B vaccine series and 38 (HepB-CpG: n = 16; HepB-Eng: n = 12; HepB-AS04: n = 10) patients had previously responded to a hepatitis B vaccine series. Three patients died prior to having blood drawn for anti-HBs concentrations. Two patients had negative anti-HBs test results at screening but at Day 1 had anti-HBs concentrations of 13.4 mIU/mL and 12.6 mIU/mL, respectively, and were excluded from immunogenicity analyses. Overall, 96.1% of patients completed the trial. Six patients died prior to the end of the study.
Figure 1.
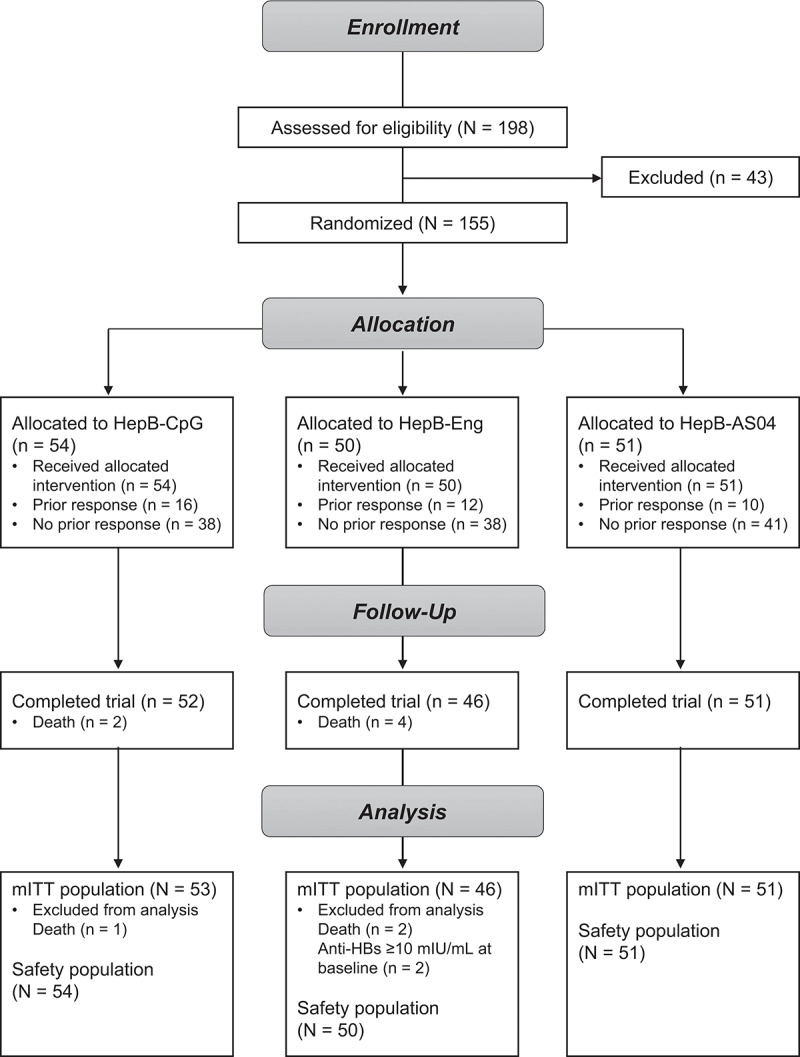
Patient disposition.
Demographic and baseline characteristics were balanced among vaccination groups, except a higher proportion of HepB-Eng recipients were aged ≥70 y compared with patients who received HepB-CpG or HepB-AS04, and a lower proportion of HepB-Eng recipients were smokers compared with patients who received HepB-CpG or HepB-AS04 (Table 1). Participants had received hemodialysis for a median of 3.8 y.
Table 1.
Demographics and baseline characteristics (safety population).
| Characteristic | HepB-CpG (N = 54) | HepB-Eng (N = 50) | HepB-AS04 (N = 51) | Total (N = 155) |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 68.0 | 72.0 | 71.0 | 70.0 |
| Range | 26-88 | 36-91 | 23-89 | 23-91 |
| Age by category, n (%) | ||||
| 18 to <50 | 4 (7.4) | 4 (8.0) | 3 (5.9) | 11 (7.1) |
| 50 to <60 | 6 (11.1) | 2 (4.0) | 7 (13.7) | 15 (9.7) |
| 60 to <70 | 19 (35.2) | 12 (24.0) | 13 (25.5) | 44 (28.4) |
| ≥70 | 25 (46.3) | 32 (64.0) | 28 (54.9) | 85 (54.8) |
| Sex, n (%) | ||||
| Male | 35 (64.8) | 30 (60.0) | 33 (64.7) | 98 (63.2) |
| Female | 19 (35.2) | 20 (40.0) | 18 (35.3) | 57 (36.8) |
| Race, n (%) | ||||
| White | 54 (100.0) | 50 (100.0) | 51 (100.0) | 155 (100.0) |
| BMI (kg/m2) | ||||
| n | 53 | 50 | 51 | 154 |
| Median | 27.2 | 26.6 | 26.5 | 26.7 |
| Range | 17.4-51.7 | 17.3-46.5 | 17.3-40.2 | 17.3-51.7 |
| Smoking status,a n (%) | ||||
| Yes | 11 (20.4) | 5 (10.0) | 14 (27.5) | 30 (19.4) |
| History of diabetes mellitus, n (%) | ||||
| Yes | 25 (46.3) | 22 (44.0) | 25 (49.0) | 72 (46.5) |
| Duration of hemodialysis (y) | ||||
| Median | 4.2 | 3.9 | 3.6 | 3.8 |
| Range | 0.9-17.4 | 0.5-16.7 | 0.6-10.2 | 0.5-17.4 |
| Response to prior hepatitis B vaccination, n (%) | ||||
| Responded | 16 (29.6) | 12 (24.0) | 10 (19.6) | 38 (24.5) |
| Did not respond | 38 (70.4) | 38 (76.0) | 41 (80.4) | 117 (75.5) |
BMI, body mass index.
aSubject smoked within the past year.
Prior to participating in this study, patients were vaccinated with HBVAXPRO (HBVAXPRO or HBVAXPRO 40 µg [MSD Vaccines]), HepB-Eng (Engerix, Engerix-B, Engerix-B Erwachsene [GlaxoSmithKline]), or Gen-H-B Vax (Aventis Pasteur). Prior non-responders had received an average of 5.3 hepatitis B vaccinations prior to this trial, and responders had received an average of 7.0 hepatitis B vaccinations prior to this trial.
Immunogenicity
The SPR was 52.8% in HepB-CpG recipients (42.1% in prior non-responders); the SPR was 32.6% in HepB-Eng recipients (18.9% in prior non-responders); and the SPR was 43.1% in HepB-AS04 recipients (29.3% in prior non-responders). The SPR in the HepB-CpG group was statistically significantly higher than in the HepB-Eng group and non-inferior to the SPR in the HepB-AS04 group (Table 2). The percentage of patients with anti-HBs concentrations of ≥100 mIU/mL was 26.4% in the HepB-CpG group, was 13.0% in the HepB-Eng group, and was 25.5% in the HepB-AS04 group.
Table 2.
Seroprotection rate at week 4 (mITT population).
| HepB-CpG |
HepB-Eng |
HepB-AS04 |
Difference in SPRs (HepB-CpG – HepB-Eng) (95% CI)a | Difference in SPRs (HepB-CpG – HepB-AS04) (95% CI)a | Difference in SPRs (HepB-Eng – HepB-AS04) (95% CI)a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Visit | Number (n/N) of patients | SPR (95% CI)a | Number (n/N) of patients | SPR (95% CI)a | Number (n/N) of patients | SPR (95% CI)a | |||
| Week 4 | 28/53 | 52.8% (38.6, 66.7) | 15/46 | 32.6% (19.5, 48.0) | 22/51 | 43.1% (29.3, 57.8) | 20.2%b (0.5, 38.3) | 9.7%c (–9.5, 28.2) | –10.5% (–29.1, 8.9) |
anti-HBs, antibody to hepatitis B surface antigen; CI, confidence interval; mITT, modified intent-to-treat population; SPR, seroprotection rate.
aExact binomial 95% CI (2-sided) for the percentage of patients.
bStatistically significant difference because lower bound of the 95% CI is >0%.
cNon-inferiority met because lower bound of the 95% CI is greater than –10%.
The baseline anti-HBs concentration was similar among the three groups (Table 3). The median GMC after vaccination in the HepB-CpG group was 9.64 mIU/mL (3.8 mIU/mL in prior non-responders), in the HepB-Eng group was 2.57 mIU/mL (1.2 mIU/mL in prior non-responders), and in the HepB-AS04 group was 4.98 mIU/mL (1.9 mIU/mL in prior non-responders) (Table 3). The GMC in the HepB-CpG group was statistically significantly higher than in the HepB-Eng group.
Table 3.
Anti-HBs geometric mean concentrations at week 4 (mITT population).
| Visit | Adjusted GMC (mIU/mL) (95% CI)a,b |
Ratio of GMCs | Ratio of GMCs | Ratio of GMCs | ||
|---|---|---|---|---|---|---|
| HepB-CpG (Total N = 53) | HepB-Eng (Total N = 46) | HepB-AS04 (Total N = 51) | HepB-CpG/HepB-Eng (95% CI) | HepB-CpG/HepB-AS04 (95% CI) | HepB-Eng/HepB-AS04 (95% CI) | |
| Day 1 | 0.32 (0.2, 0.4) | 0.31 (0.2, 0.4) | 0.33 (0.2, 0.5) | NC | NC | NC |
| Week 4 | 9.64 (4.2, 22.1) | 2.57 (1.1, 6.0) | 4.98 (1.9, 13.0) | 3.74c (1.2, 12.2) | 1.94 (0.6, 6.8) | 0.52 (0.1, 1.9) |
ANOVA, analysis of variance; anti-HBs, antibody to hepatitis B surface antigen; CI, confidence interval; GMC, geometric mean concentration; mITT, modified intent-to-treat population; NC, not calculated.
aEstimates were obtained through ANOVA with vaccine as the only factor in the model.
bTo estimate GMCs for each stratum, an ANOVA model with log10 anti-HBs as a dependent variable and vaccine as a factor was used. Adjusted GMC and the corresponding 95% CI were obtained by taking the anti-log of the least squares mean of log10 concentrations and the corresponding 95% CI. Since there is only one factor in the ANOVA model, the adjusted GMCs are identical to the unadjusted GMCs.
cStatistically significant difference because lower bound of the 95% CI is >1.0.
Safety
Local post-injection reactions occurred in a similar proportion of HepB-CpG recipients (9.3%) to HepB-Eng recipients (8.2%) and a significantly lower proportion of patients than HepB-AS04 recipients (31.4%; p = .007) (Table 4). All post-injection reactions in HepB-CpG and HepB-Eng recipients were of mild severity. Four patients who received HepB-AS04 reported moderate injection-site pain. No local post-injection reaction was reported as severe in any of the vaccination groups. Most post-injection reactions peaked in frequency between 1 and 3 d after the injection and were infrequent by 7 d after the injection.
Table 4.
Safety events by vaccination group (safety population).
| HepB-CpG (N = 54) | HepB-Eng (N = 50) | HepB-AS04 (N = 51) | |
|---|---|---|---|
| N | 54 | 49 | 51 |
| Any post-injection reaction a | 13 (24.1) | 8 (16.3) | 20 (39.2) |
| Local reactions | |||
| Total, n (%) | 5 (9.3) | 4 (8.2) | 16 (31.4) |
| Severe | 0 | 0 | 0 |
| Injection site redness | |||
| Total, n (%) | 0 | 0 | 1 (2.0) |
| Severe (>100 mm) | 0 | 0 | 0 |
| Injection site swelling | |||
| Total, n (%) | 0 | 0 | 0 |
| Severe (>100 mm) | 0 | 0 | 0 |
| Injection site pain | |||
| Total, n (%) | 5 (9.3) | 4 (8.2) | 15 (29.4) |
| Severe | 0 | 0 | 0 |
| Systemic reactions | |||
| Total, n (%) | 10 (18.5) | 6 (12.2) | 10 (19.6) |
| Severe | 1 (1.9) | 1 (2.0) | 1 (2.0) |
| Fever (elevated body temperature), N | 54 | 49 | 51 |
| Total, n (%) | 1 (1.9) | 2 (4.1) | 0 (0.0) |
| Severe (39°C or higher) | 1 (1.9) | 1 (2.0) | 0 (0.0) |
| Malaise, N | 54 | 48 | 51 |
| Total, n (%) | 5 (9.3) | 2 (4.2) | 1 (2.0) |
| Severe | 0 | 0 | 0 |
| Headache, N | 54 | 48 | 51 |
| Total, n (%) | 5 (9.3) | 1 (2.1) | 1 (2.0) |
| Severe | 0 | 0 | 0 |
| Myalgia, N | 54 | 49 | 51 |
| Total, n (%) | 4 (7.4) | 2 (4.1) | 4 (7.8) |
| Severe | 0 | 0 | 0 |
| Fatigue, N | 54 | 48 | 51 |
| Total, n (%) | 4 (7.4) | 0 | 5 (9.8) |
| Severe | 0 | 0 | 1 (2.0) |
| Any AE, n (%) | 24 (44.4) | 22 (44.0) | 22 (43.1) |
| Any related AE, n (%) | 2 (3.7) | 2 (4.0) | 2 (3.9) |
| Any new-onset autoimmune AE, n (%) | 0 | 0 | 0 |
| Any SAE, n (%) | 10 (18.5) | 9 (18.0) | 7 (13.7) |
| Any related SAE, n (%) | 0 | 0 | 0 |
| Any AE leading to withdrawal, n (%) | 0 | 0 | 0 |
| Death, n (%) | 2 (3.7) | 4 (8.0) | 0 |
AE, adverse event; SAE, serious adverse event.
aPercentages are based on the number of patients (N) providing data for each category. Two patients in each vaccination group did not provide oral temperature data.
Local reactions include redness ≥25 mm, swelling ≥25 mm, and pain. Local pain, malaise, headache, myalgia, and fatigue were graded as severe if they were significant and prevented daily activity.
Systemic post-injection reactions occurred in a lower percentage of patients in the HepB-Eng group compared with the HepB-CpG and HepB-AS04 groups. Malaise, headache, fatigue, and myalgia were most common in the HepB-CpG group. Myalgia and fatigue were the most common in the HepB-AS04 group. One patient in each group reported severe systemic post-injection reactions.
Rates of adverse events were comparable across all vaccination groups, and medically important events were infrequent. No new-onset autoimmune events were reported. The frequency of serious adverse events was similar across the three vaccine groups, and no serious adverse event was considered by the investigator as possibly or probably related to study vaccine.
Two deaths occurred in the HepB-CpG group, four in the HepB-Eng group, and none in the HepB-AS04 group. The cause of death was typical for this population of patients with ESRD, including cardiovascular causes and sepsis. No death was considered by the investigator to be related to study vaccine.
Discussion
Long-term protection against HBV in individuals with ESRD depends on the persistence of seroprotective levels of antibodies to HBsAg.12,21 Patients with ESRD not only have lower peak antibody levels in response to primary vaccination but also have a more rapid decline of anti-HBs levels compared with healthy patients.22 To maintain protection against HBV, booster doses of vaccine need to be administered to patients receiving hemodialysis when anti-HBs levels decline to <10 mIU/mL.23
This was a randomized, phase 3 study of hepatitis B vaccines using different adjuvants in patients who had been on hemodialysis for a median of nearly 4 y, ~75% of whom had never been seroprotected by 1 or more previous series of hepatitis B vaccine. In this population, a single dose of HepB-CpG induced a statistically significantly higher SPR than a double dose of HepB-Eng and non-inferior SPR to a single dose of HepB-AS04. HepB-CpG induced seroprotection in more than 40% of patients who had never responded to a previous series of hepatitis B vaccine, compared with 19% for HepB-Eng and 29% for HepB-AS04. HepB-CpG also induced a significantly higher anti-HBs GMC than HepB-Eng and a similar anti-HBs GMC to HepB-AS04.
HepB-CpG induced similar levels of seroprotection as HepB-AS04 while inducing a significantly lower frequency of local injection-site reactions than HepB-AS04. Moderate injection site pain was reported only by recipients of HepB-AS04, suggesting that the targeted stimulation of TLR9 was better tolerated than the broad immune stimulation induced by alum with the addition MPL, a TLR4 agonist. Safety was similar across the three vaccines, with similar frequencies of adverse events and serious adverse events. While there were six deaths in the study, none were considered related to vaccine. No new-onset autoimmune events were reported.
A limitation of this study was that a small number of patients receiving hemodialysis who had previously responded to hepatitis B vaccines were enrolled, precluding meaningful subpopulation analyses. Nonetheless, these data offer information to a clinician with a patient receiving hemodialysis who needs a booster dose of hepatitis B vaccine regardless of the patient’s response to previous hepatitis B vaccines. Another limitation of this study was an imbalance in the demographics between groups according to age and smoking status, both of which are factors known to affect immune response to hepatitis B vaccines.24,25
In conclusion, in this very difficult to immunize population of patients, a single booster dose of HepB-CpG induced statistically significantly higher levels of seroprotection and antibody levels than HepB-Eng with a similar safety profile. Of note, the higher levels of immune response to HepB-CpG were not accompanied by higher levels of local reactions and pain as induced by HepB-AS04. In this population, a booster dose of HepB-CpG may provide protection in a high proportion of patients with a well-tolerated safety profile.
Acknowledgments
The authors wish to thank the patients for their participation in this study and the staff and investigators for conducting the study. We also thank Fang Xie and Randall Hyer for their review and comments on the manuscript.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This study was funded by Dynavax Technologies Corporation.
Disclosure statement
Matthias Girndt reports participating on advisory boards/speaker boards for Amgen, Astellas, Bayer Vital, Daiichi Sankyo, Hexal, Novartis, Novo Nordisk, Pfizer, Sanofi, and Vifor Fresenius. Oliver Witzke has received research grants for clinical studies, speaker’s fees, honoraria, and travel expenses from Amgen, Astellas, Bristol-Myers Squibb, Chiesi, Hexal, Janssen-Cilag, MSD, Novartis, Roche, Pfizer, and Sanofi. Oliver Witzke is supported by an unrestricted grant of the Rudolf-Ackermann-Stiftung (Stiftung für Klinische Infektiologie). Anne K. Michelsen has been a speaker for Berlin Chemie and Lilly. Robert Janssen is an employee of Dynavax Technologies Corporation.
References
- 1.World Health Organization . Global progress report on HIV, viral hepatitis and sexually transmitted infections. 2021. Jul 15 [accessed 2022 Aug 31]. https://www.who.int/publications/i/item/9789240027077.
- 2.Ozer A, Yakupogullari Y, Beytur A, Beytur L, Koroglu M, Salman F, Aydogan F.. Risk factors for hepatitis B virus infection in Turkey: a population-based, case-control study. Hepat Mon. 2011;11:1–7. [PMC free article] [PubMed] [Google Scholar]
- 3.United States Renal Data System . 2019 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD); 2018. [Google Scholar]
- 4.Kramer A, Pippias M, Noordzij M, Stel VS, Afentakis N, Ambühl PM, Andrusev AM, Fuster EA, Arribas Monzón FE, Åsberg A, et al. The European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2015: a summary. Clin Kidney J. 2018;11(1):108–22. doi: 10.1093/ckj/sfx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50(RR–5):1–43. [PubMed] [Google Scholar]
- 6.Johnson DW, Fleming SJ. The use of vaccines in renal failure. Clin Pharmacokinet. 1992;22(6):434–46. doi: 10.2165/00003088-199222060-00003. [DOI] [PubMed] [Google Scholar]
- 7.Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. 2003;63:1021–51. doi: 10.2165/00003495-200363100-00006. [DOI] [PubMed] [Google Scholar]
- 8.Engerix-B® (hepatitis B vaccine [recombinant]). GlaxoSmithKline. Prescribing Information; 2019. [Google Scholar]
- 9.Tong NKC, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, Vilella A, de Juanes JR, Arrazola P, Calbo-Torrecillas FC, et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 2005;68(5):2298–303. doi: 10.1111/j.1523-1755.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 10.Merck Sharp and Dohme Limited . HBVAXPRO 40 mcg. Summary of Product Characteristics. 2019. London, UK: Merck Sharp & Dohme UK Ltd. [Google Scholar]
- 11.Girndt M, Litjens NH. Unmet needs and new promises in hepatitis B vaccination for chronic kidney disease patients. Eur Nephrol. 2010;4:14–18. [Google Scholar]
- 12.Centers for Disease Control and Prevention . Protection against viral hepatitis. Recommendations of the immunization practices advisory committee (ACIP). MMWR Morbid Mortal Wkly Rep. 1990;39(RR–2):1–26. [PubMed] [Google Scholar]
- 13.Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179(2):489–92. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 14.Buti M, Viladomiu L, Jardi R, Olmos A, Rodriguez JA, Bartolome J, Esteban R, Guardia J. Long-term immunogenicity and efficacy of hepatitis B vaccine in hemodialysis patients. Am J Nephrol. 1992;12(3):144–47. doi: 10.1159/000168436. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney FJ, Kane MA. Hepatitis B vaccine. In: S P, Orenstein W, editors. Vaccines. Philadelphia: W.B. Sauders Company; 1999. p. 158–82. [Google Scholar]
- 16.Chaves SS, Daniels D, Cooper BW, Malo-Schlegel S, Macarthur S, Robbins KC, Kobetitsch JF, McDaniel A, D’Avella JF, Alter MJ. Immunogenicity of hepatitis B vaccine among hemodialysis patients: effect of revaccination of non-responders and duration of protection. Vaccine. 2011;29(52):9618–23. doi: 10.1016/j.vaccine.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 17.HEPLISAV-B (hepatitis B vaccine [recombinant], adjuvanted). Dynavax Technologies Corporation. Prescribing Information; 2017. [Google Scholar]
- 18.Fendrix® Summary of product characteristics. GlaxoSmithKline; 2018. [Google Scholar]
- 19.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–13. doi: 10.1093/biomet/26.4.404. [DOI] [Google Scholar]
- 20.Janssen RS, Mangoo-Karim R, Pergola PE, Girndt M, Namini H, Rahman S, Bennett SR, Heyward WL, Martin JT. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in patients with chronic kidney disease. Vaccine. 2013;31(46):5306–13. doi: 10.1016/j.vaccine.2013.05.067. [DOI] [PubMed] [Google Scholar]
- 21.Litjens NH, Huisman M, van den Dorpel M, Betjes MG. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J Am Soc Nephrol. 2008;19:1483–90. doi: 10.1681/ASN.2007090971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janus N, Vacher LV, Karie S, Ledneva E, Deray G. Vaccination and chronic kidney disease. Nephrol Dial Transplant. 2008;23(3):800–07. doi: 10.1093/ndt/gfm851. [DOI] [PubMed] [Google Scholar]
- 23.Miller ER, Alter MJ, Tokars JI. Protective effect of hepatitis B vaccine in chronic hemodialysis patients. Am J Kidney Dis. 1999;33(2):356–60. doi: 10.1016/S0272-6386(99)70312-4. [DOI] [PubMed] [Google Scholar]
- 24.Fisman DN, Agrawal D, Leder K. Effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis. 2002;35(11):1368–75. doi: 10.1086/344271. [DOI] [PubMed] [Google Scholar]
- 25.Winter AP, Follett EAC, McIntyre J, Stewart J, Symington IS. Influence of smoking on immunological responses to hepatitis B vaccine. Vaccine. 1994;12(9):771–72. doi: 10.1016/0264-410X(94)90283-6. [DOI] [PubMed] [Google Scholar]


