Abstract
Background:
To describe characteristics of patients, providers, and clinics associated with opioid or non-opioid pain medication prescribing patterns for patients who received lower spine imaging in primary care clinics.
Methods:
In these secondary analyses of the Lumbar Imaging with Reporting of Epidemiology (LIRE) study, a randomized controlled trial conducted in 4 U.S. health systems, we evaluated characteristics associated with receipt of pain medication prescriptions. The outcomes were receipt of prescriptions for opioid or, separately, non-opioid pain medications within 90 days after imaging. Among patients who received opioid or non-opioid prescriptions, we evaluated receipt of multiple prescriptions in the year following imaging. Mixed models were used to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs).
Results:
Compared to whites, patients identified as Asian (OR: 0.53; 95% CI: 0.51–0.56), Native Hawaiian/Pacific Islander (OR: 0.73; 95% CI: 0.64–0.83), multiracial (OR: 0.84; 95% CI: 0.71–0.98) or Black (OR: 0.92; 95% CI: 0.89–0.96) had significantly reduced odds for receiving prescriptions for opioids within 90 days. Patients identified as Native American/Alaska Native had greater odds for receiving prescriptions for non-opioid pain medications within 90 days (OR: 1.12; 95% CI: 1.01–1.24). Receipt of pain prescriptions 120 days prior to imaging was strongly predictive of subsequent receipt of pain prescriptions across all categories.
Conclusions:
After adjusting for factors that could affect prescribing, the strongest differences that we observed in pain-medication prescribing were across racial categories and for patients with previous pain prescriptions. Further research is needed to understand these differences and to optimize prescribing.
Introduction
Low back pain is a complex condition with many possible etiologies.1 Treatments include medications, complementary and alternative medicine (CAM) treatments, psychological therapies, interventional therapies (e.g., injections), and spine surgeries.2 Although prescription opioid medications are generally not recommended3–6 and such use has decreased in recent years,7,8 they are still often prescribed for low back pain; in 2016, opioids were prescribed to 20% of patients after new encounters for low back pain in a large, population-based study.8 Non-opioid medications are also often prescribed for low back pain and include muscle relaxants, non-steroidal anti-inflammatory drugs (NSAIDs), gabapentinoids, tricyclic antidepressants (TCAs), and serotonin-norepinephrine re-uptake inhibitors (SNRIs).4,9
Patterns of opioid prescribing vary by patient racial/ethnic characteristics, with Blacks and Hispanic/Latinx patients less likely than whites to receive opioid prescriptions.10–13 Some evidence indicates that patients living in lower socioeconomic status (SES) neighborhoods may be more likely to receive opioid prescriptions for new episodes of back pain compared to patients in higher SES neighborhoods.14 Other patient characteristics associated with variations in opioid prescribing include age, with middle-aged patients receiving more opioid prescriptions for back pain from primary care providers (PCPs) compared to those >60 years or <30 years, and insurance type, with Medicaid or self-pay patients receiving more opioid prescriptions than those with private insurance.15 Relationships between patient sex and opioid prescribing have been examined in previous studies, with some reporting that male patients are less likely to be prescribed opioids compared to female patients16–18 and others reporting no differences by patient sex.15,19
Clinic characteristics are also likely associated with opioid prescribing. Patients who receive care in rural clinics are more likely to receive prescriptions for opioids compared to patients who receive care in urban areas.20 Opioid-related mortality rates have increased more rapidly in rural than in urban areas and prescription opioids are more commonly involved than heroin or synthetic opioids in drug-related deaths in rural areas, suggesting that opioids are more readily prescribed in rural locations.21
Little is known about provider characteristics associated with opioid prescribing. A study in two military emergency department facilities found that physician assistants were more likely than physicians to have prescribed opioids. No differences in prescribing rates between male and female providers were found.22
Increasing knowledge regarding the characteristics of patients, providers, and clinics associated with prescription of opioid or non-opioid medications for back pain may point to opportunities for optimizing care that is consistent with clinical guidelines. The purpose of this secondary analysis was to describe patient-level, provider-level, and clinic-level characteristics that were associated with pain medication prescribing for patients who received low back imaging in the primary care setting in order to generate hypotheses for future studies. Because our sample size was large, we were able to describe patterns of pain medication prescribing in under-studied patient populations such as patients in relatively small racial categories. Additionally, because we had access to a wide variety of patient, provider, and clinic characteristics, we were able to model associations adjusting for previously identified trends and report on novel associations that future work can examine.
Methods
Study Design and Data Sources
The parent study for these secondary analyses was the Lumbar Imaging with Reporting of Epidemiology (LIRE) study, which has been described previously in detail.23,24 The rationale of the LIRE study was that lumbar spine imaging often uncovers findings that are unrelated to pain and if providers were aware of how often these findings appear in patients who do not have back pain, they might be less alarmed by them and thus less likely to recommend potentially unnecessary interventions. Primary care clinics within four large healthcare systems [Mayo Clinic (Rochester, Minnesota); Henry Ford (Detroit, Michigan); Kaiser Permanente Northern California (Oakland, California); and Kaiser Permanente Washington (Seattle, Washington)] were randomly assigned to generate imaging reports containing epidemiologic benchmark text containing the prevalence of common imaging findings among patients (with or without back pain) at varying start dates.24–26 Prior to the intervention, clinics received imaging reports without the intervention text. Data were obtained from comprehensive electronic medical records (EMR) that captured healthcare utilization, including prescription medication data.
Within each health system, clinics that provided primary care for adult patients were identified. Patients ≥18 years old who received lower spine imaging from October 1, 2013 to Sept. 30, 2016, who had no spine imaging within the past year, and had not opted out of research studies were automatically enrolled in the trial. LIRE providers were defined as PCPs whose main practices were at one of these clinics and who ordered at least 1 qualifying spine image during the trial.
The LIRE study found no differences in the primary outcome, spine-related healthcare utilization in the year following the index lumbar spine image and found no differences in spine-related healthcare utilization in the year following the index spine imaging, among patients whose images did versus did not contain the intervention text. However, patients whose images contained the intervention text had a small but statistically significant decrease in the likelihood of receiving prescriptions for opioids within a year of their index images.23
For this secondary analysis, all participating institutional review boards determined that the study was minimal risk and granted waivers of consent and Health Insurance Portability and Accountability Act (HIPAA) authorization.
Prescription Drug Identification
Opioid and non-opioid pain medications were identified by a pharmacist (Supplemental Table 1) using drug information databases (IBM Micromedex® and UpToDate®). Non-opioid medication classes that we examined were skeletal muscle relaxants, NSAIDs, gabapentinoids, tricyclic antidepressants, and benzodiazepines. We also included the only SNRI that had Food and Drug Administration (FDA) approval for treatment of chronic musculoskeletal pain, duloxetine, in the non-opioid pain medication category. We included oral and topical forms and excluded pain medication prescriptions that occurred during inpatient stays and medication forms used exclusively or nearly exclusively in inpatient settings. We identified pre-index imaging pain medication prescriptions as those that occurred in the 120 days prior to the date of the spine image, and post-imaging prescriptions were those that occurred from the date the index image was finalized by the interpreting radiologist (hereafter this date is termed “index”) through 90 days later. Pre-imaging medications included outpatient prescriptions written by any provider but, because we were interested in prescribing trends among PCPs, post-imaging prescriptions were only those from LIRE providers. However, unless otherwise stated, the provider who wrote prescriptions did not have to be the same one who ordered the index image if both met the definition of LIRE providers. We analyzed ordered, not necessarily filled, prescriptions because we were interested in prescribing trends. Prescriptions written between the image date and the finalized image report were not counted as pre-imaging or post-imaging prescriptions. Non-prescription pain medications were not captured in the EMR and are not included in these analyses.
Patient, Provider, and Clinic Variables
We evaluated the characteristics of patients who received opioid or non-opioid pain medication prescriptions. Patient-level characteristics were obtained from the EMR and included age (categorized as 18–39; 40–60, and ≥61 years), sex, imaging modality (x-ray, computed tomography (CT), or magnetic resonance imaging (MRI)), race (identified by the patients in accordance with the Institute of Medicine report on standardization of collection of race, ethnicity, and language data;27 categories included Asian, Black/African American, Native Hawaiian/Pacific Islander, multiracial, Native American/Alaska Native, or white), ethnicity (Hispanic or not Hispanic), primary insurance at index (categorized as commercial, which included Medicare supplements; Veteran’s Affairs (VA); self-pay; Medicare; or Medicaid); Charlson comorbidity index category (0, 1, 2, and ≥3);28 receipt of prescriptions for opioids non-opioid pain medications pre-index, and the calendar time of the index image (categorized in 6-month intervals following the intervention schedule from October 1, 2013 - September 30, 2016). To determine each patient’s SES, the study sites mapped patient addresses to Federal Information Processing System (FIPS) codes using geocoding software, which were then mapped to SES indexes derived from the 2010 Census Summary File 1 and the American Community Survey 2007–2011 5-year estimate data29 and categorized into quartiles.30 Using machine learning natural language processing,31 we extracted findings on the index image from the radiology text reports. These were categorized into three mutually exclusive groups: no findings, findings that were likely clinically unimportant (e.g., disc bulge, disc space narrowing, annular fissure; hereafter termed “clinically unimportant findings”), and findings that were likely clinically important (hereafter termed “clinically important findings;” e.g., moderate-severe spinal canal stenosis, nerve root compression, disc extrusion).
Because the providers who ordered the images may not have been the same as the providers who prescribed medications, we evaluated the proportions of patients who received opioid or non-opioid pain medication prescriptions from their image-ordering providers. We also evaluated provider age, type (categorized into medical doctor [MD], doctor of osteopathic medicine [DO], or other [physician assistants and nurse practitioners; hereafter termed “PA/NP”]), sex, and specialty (family medicine, internal medicine, or other). Clinic characteristics included rural/urban, which we defined using the 2010 Rural-Urban Commuting Area (RUCA) codes,32 and clinic size (equal-size tertiles of small, medium, or large depending on the number of PCPs at the clinics).
Outcomes
To examine differences in the amount of prescribing, we subdivided patients who received any opioid or non-opioid pain prescriptions in the year following index into those with multiple time periods with prescriptions versus only a single period with a prescription. Specifically, we divided the year after index into 4 quarters of 91–92 days and counted the number of quarters in which each patient received ≥1 opioid or non-opioid pain medication prescription. We then created binary variables that were 0 if they received opioid or non-opioid pain medication prescriptions in 1 quarter and 1 if they received prescriptions in >1 quarter (hereafter termed “multiple prescriptions”). Thus, we had four binary outcome variables: 1) receipt of immediate prescriptions for opioids versus those who did not receive immediate opioid prescriptions (referent); 2) receipt of multiple prescriptions for opioids versus those who received only 1 opioid prescription (referent); 3) receipt of immediate prescriptions for non-opioid pain medications versus those who did not receive immediate non-opioid pain prescriptions (referent); and 4) receipt of multiple prescriptions for non-opioid pain prescriptions versus those who received only 1 non-opioid pain prescription (referent).
Statistical Analysis
We evaluated unadjusted relationships between the patient, provider, and clinic characteristics and those who did and did not receive prescriptions for opioid or (separately) non-opioid pain medications within 90 days after index (“immediate prescriptions”). We also examined the proportions of patients who received immediate prescriptions for 1) both opioid and non-opioid pain medication prescriptions, 2) only opioid prescriptions, 3) only non-opioid pain prescriptions, and 4) neither.
We used mixed models with random effects (which account for the fact that patients within PCPs and/or clinics would have been expected to have had prescribing patterns that were correlated) to estimate adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs). We included all the patient-level, provider-level, and clinic-level variables in the same models. All models additionally adjusted for health care system and whether the image occurred during the control (no benchmark text present on the imaging report) or intervention (benchmark text present on the imaging report) period.
To evaluate whether concordance of patient and provider sex affected pain prescriptions, we conducted secondary, unadjusted analyses that examined the proportions of immediate and multiple pain medication prescriptions, stratified by patient and provider sex. For these analyses, we only counted opioid or non-opioid pain prescriptions that were prescribed by the same provider who ordered the index images.
For patients who were missing values for race (n=28,932; 12.1% of the sample), we used the modes of the races in the zip codes where they received their index images to impute their races.33 We used the same methodology to impute SES for the n=6810 (2.9% of the population) patients missing SES. We conducted sensitivity analyses excluding the patients who were missing race or SES. A large proportion of patients were missing ethnicity data (n=163,514; 68%) but most (99%) of these patients’ data came from a health system that coded their patients’ ethnicity as either “Hispanic” or “Unknown;” therefore, everyone whose ethnicity was recorded as “Unknown” was counted as not Hispanic for these analyses. Finally, patient primary insurance status was missing for a small proportion of our sample (n=2697; 1.1%); these patients were excluded from adjusted analyses. We used SAS software (version 9.4; Cary, North Carolina) for all analyses.
Results
A total of 238,886 patients were included in our analyses (Figure 1). Of these, 34,076 (14.3%) received an immediate opioid prescription and no immediate non-opioid pain medication prescription; a total of 47,944 (20.0%) received only a non-opioid pain medication prescription and no immediate opioid prescription; and 36,119 (15.1%) received both immediate opioid and non-opioid pain medication prescriptions. Thus, approximately half of the cohort (118,139; 49.5%) received at least 1 immediate pain medication prescription. A total of 61,259 (87%) of patients who received immediate opioid prescriptions received ≥1 immediate prescription from the same provider who ordered their index images and 73,732 (88%) of patients who received immediate non-opioid pain medication prescriptions received ≥1 prescription from the image-ordering providers. Unadjusted patient-level, provider-level, and clinic-level characteristics, stratified by whether the patients received immediate prescriptions for opioids or non-opioid pain medications, are shown in Table 1.
Figure 1.
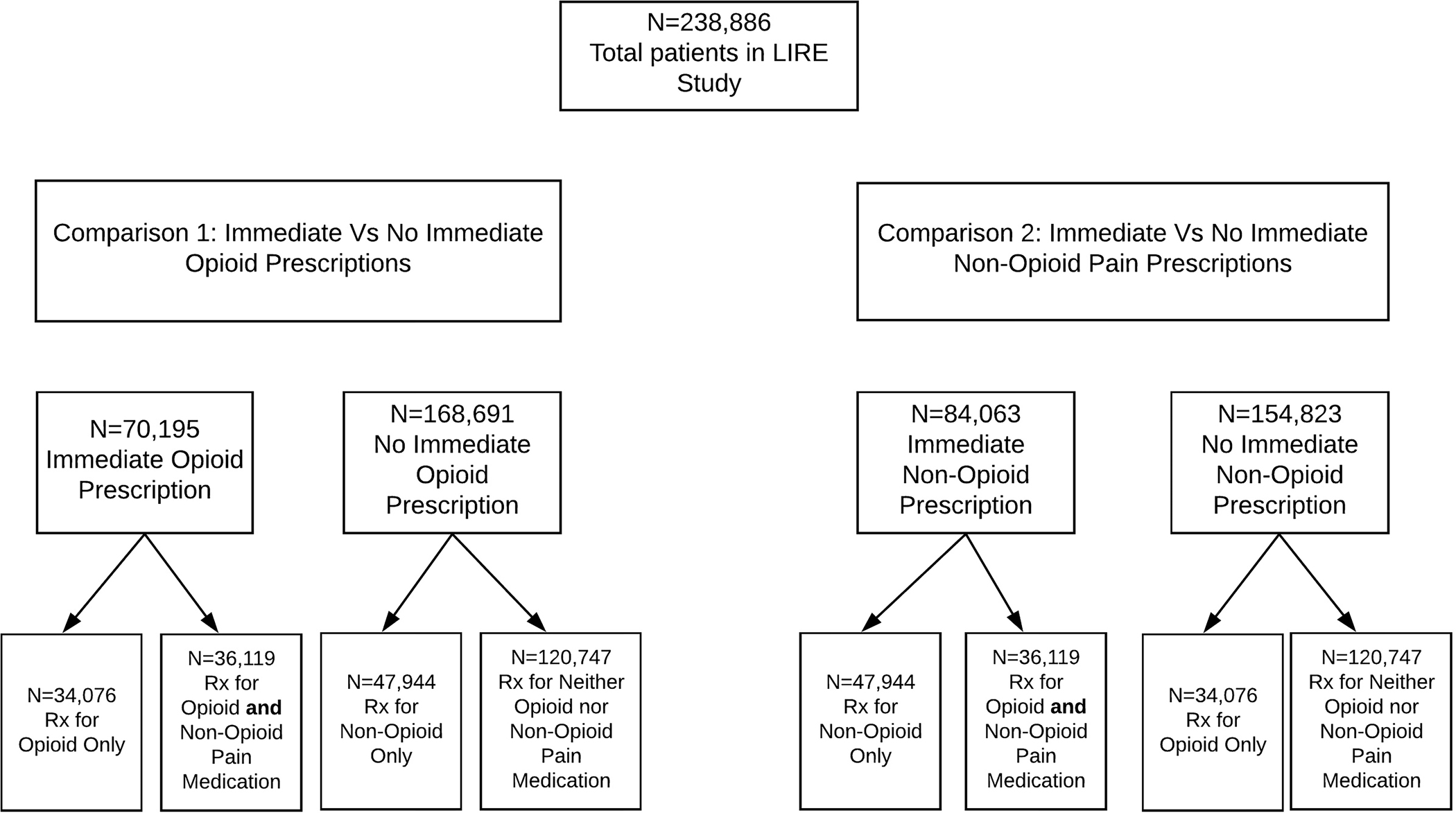
Groups of patients who received immediate prescriptions.
Table 1:
Characteristics of patients, providers, and clinics by receipt of opioid and non-opioid pain medication prescriptions within 90 days after index image.*
| All patients n (column %) n=238,886 |
Received immediate opioid prescription n (row %) n=70,195 (29%) |
Received immediate non-opioid prescription n (row %) n=84,063 (35%) |
|
|---|---|---|---|
|
| |||
| Patient-Level Characteristics | |||
|
| |||
| Age group (Years) | |||
| 18–39 | 43,342 (18%) | 9679 (22%) | 15,695 (36%) |
| 40–60 | 90,027 (38%) | 26,128 (29%) | 35,143 (39%) |
| >60 | 105,517 (44%) | 34,388 (33%) | 33,225 (31%) |
| Sex | |||
| Male | 101,499 (42%) | 28,956 (29%) | 33,365 (33%) |
| Female | 137,387 (58%) | 41,239 (30%) | 50,498 (37%) |
| Modality for index image | |||
| X-ray | 192,435 (81%) | 55,466 (29%) | 70,234 (37%) |
| CT | 943 (0.4%) | 412 (44%) | 288 (31%) |
| MRI | 45,508 (19%) | 14,317 (31%) | 13,541 (30%) |
| Race | |||
| Asian | 26,508 (11%) | 4062 (15%) | 7162 (27%) |
| Black/African-American | 23,642 (10%) | 7678 (33%) | 8416 (36%) |
| Native Hawaiian/Pacific Islanders | 1614 (0.7%) | 389 (24%) | 583 (36%) |
| Native American/Alaska Native | 1686 (0.7%) | 610 (36%) | 708 (42%) |
| Multiracial | 1005 (0.4%) | 296 (29%) | 376 (37%) |
| White | 184,431 (77%) | 57,728 (31%) | 66,818 (36%) |
| Ethnicity | |||
| Hispanic | 36,229 (15%) | 9342 (26%) | 13,702 (38%) |
| Not Hispanic | 202,657 (85%) | 60,853 (30%) | 70,361 (35%) |
| Socioeconomic status quartile | |||
| 1st | 15,824 (7%) | 5446 (34%) | 6333 (40%) |
| 2nd | 31,213 (13%) | 10,574 (34%) | 12,215 (39%) |
| 3rd | 57,564 (24%) | 18,808 (33%) | 21,879 (38%) |
| 4th | 134,285 (56%) | 35,367 (26%) | 43,636 (33%) |
| Insurance status | |||
| Medicare | 90,841 (38%) | 31,951 (35%) | 29,103 (32%) |
| Medicaid/state-subsidized | 12,056 (5%) | 4230 (35%) | 5388 (45%) |
| Commercial (including Medicare supplements) | 131,743 (55%) | 32,693 (25%) | 47,911 (36%) |
| Veterans Administration | 248 (0.1%) | 60 (24%) | 84 (34%) |
| Self-pay | 1301 (0.5%) | 490 (38%) | 596 (46%) |
| Unknown/missing | 2697 (1%) | 771 (29%) | 981 (36%) |
| Charlson comorbidity index | |||
| 0 | 153,079 (64%) | 38,227 (25%) | 53,108 (35%) |
| 1 | 41,868 (18%) | 14,449 (35%) | 15,868 (38%) |
| 2 | 23,211 (10%) | 8214 (35%) | 7828 (34%) |
| 3+ | 20,728 (9%) | 9305 (45%) | 7259 (35%) |
| Prescription for opioid in 120 days prior to index: | |||
| Yes | 61,531 (26%) | 38,581 (63%) | 35,397 (52%) |
| No | 177,355 (74%) | 31,614 (18%) | 48,666 (29%) |
| Image finding status | |||
| None | 55,546 (23%) | 13,327 (24%) | 20,627 (37%) |
| Clinically unimportant finding | 149,192 (62%) | 45,558 (31%) | 53,206 (36%) |
| Clinically important finding | 34,148 (14%) | 11,310 (33%) | 10,230 (30%) |
| Time of index image | |||
| Oct 2013 to Mar 2014 | 48,233 (20%) | 16,087 (33%) | 16,909 (35%) |
| Apr 2014 to Sept 2014 | 30,529 (13%) | 9952 (33%) | 10,833 (35%) |
| Oct 2014 to Mar 2015 | 39,424 (17%) | 11,730 (30%) | 14,204 (36%) |
| Apr 2015 to Sept 2015 | 41,796 (18%) | 11,959 (29%) | 14,816 (35%) |
| Oct 2015 to Mar 2016 | 39,177 (16%) | 10,526 (27%) | 13,726 (35%) |
| Apr 2016- Sept 2016 | 39,727 (17%) | 9941 (25%) | 13,575 (34%) |
|
| |||
| Image Ordering Provider-Level Characteristics | |||
|
| |||
| Provider mean age (IQR)† | 49.3 (43–55) | 49.6 (43–56) | 49.0 (43–55) |
| Provider type | |||
| Doctor Osteopathy | 17,288 (7%) | 5167 (30%) | 6542 (38%) |
| Medical Doctor | 213,524 (89%) | 62,250 (29%) | 74,170 (35%) |
| Physician Assistant/Nurse Practitioner | 8074 (3%) | 2778 (34%) | 3351 (42%) |
| Provider sex | |||
| Female | 125,520 (53%) | 35,315 (28%) | 44,206 (35%) |
| Male | 113,366 (47%) | 34,880 (31%) | 39,857 (35%) |
| Provider specialty | |||
| Family medicine | 117,072 (49%) | 36,042 (31%) | 44,100 (38%) |
| Internal medicine | 119,842 (50%) | 33,602 (28%) | 39,278 (33%) |
| Other | 1972 (0.8%) | 551 (28%) | 685 (35%) |
|
| |||
| Imaging Clinic-Level Characteristics | |||
|
| |||
| Rural urban commuting area type | |||
| Urban | 231,352 (97%) | 67,391 (29%) | 81,184 (35%) |
| Large rural | 4106 (1.7%) | 1436 (35%) | 1569 (38%) |
| Small rural | 3043 (1.3%) | 1247 (41%) | 1215 (40%) |
| Isolated small rural | 385 (0.2%) | 121 (31%) | 95 (25%) |
| Clinic size | |||
| Small | 48,053 (20%) | 14,044 (29%) | 15,725 (33%) |
| Medium | 74,035 (31%) | 22,315 (30%) | 25,014 (34%) |
| Large | 116,798 (49%) | 33,836 (29%) | 43,324 (37%) |
Note that 36,119 patients (15% of the total) received prescriptions for both opioid and non-opioid pain medications within 90 days and are therefore represented in multiple columns; similarly, n=120,747 received neither immediate opioid nor non-opioid pain prescriptions.
IQR=Interquartile range
Patient Race and Ethnicity
Results of adjusted analyses are shown in Figures 2–5. Compared to whites, patients identified as Asian had reduced odds of receiving immediate opioid (OR (95% CI): 0.53 (0.51–0.56)) or non-opioid (0.75 (0.73–0.77)) pain medication prescriptions and of receiving multiple prescriptions (opioids: 0.60 (0.56–0.64); non-opioids: 0.69 (0.66–0.73)). Patients identified as Black (0.92 (0.89–0.96)), Native Hawaiian/Pacific Islander (0.73 (0.64–0.83)), or multiracial (0.84 (0.71–0.98) also had reduced odds of receiving immediate opioid prescriptions, but we did not observe statistically significant associations with the receipt of multiple opioid prescriptions or immediate non-opioid pain prescriptions. Patients identified as Black (0.87 (0.83–0.91)) or Native Hawaiian (0.83 (0.71–0.97)), however, had reduced odds relative to whites for receiving multiple prescriptions for non-opioid pain medications. Patients identified as Native America/Alaskan had greater odds of receiving immediate non-opioid pain medications compared to whites (1.12 (1.01–1.24)). Patients identified as Hispanic had reduced odds of receiving immediate prescriptions for opioids (0.79 (0.77–0.82)) and of receiving multiple opioid (0.80 (0.77–0.84) or non-opioid pain medications (0.86 (0.83–0.89)) within a year, but they had greater odds of receiving immediate non-opioid pain medications prescriptions (1.02 (1.00–1.05)).
Figure 2.
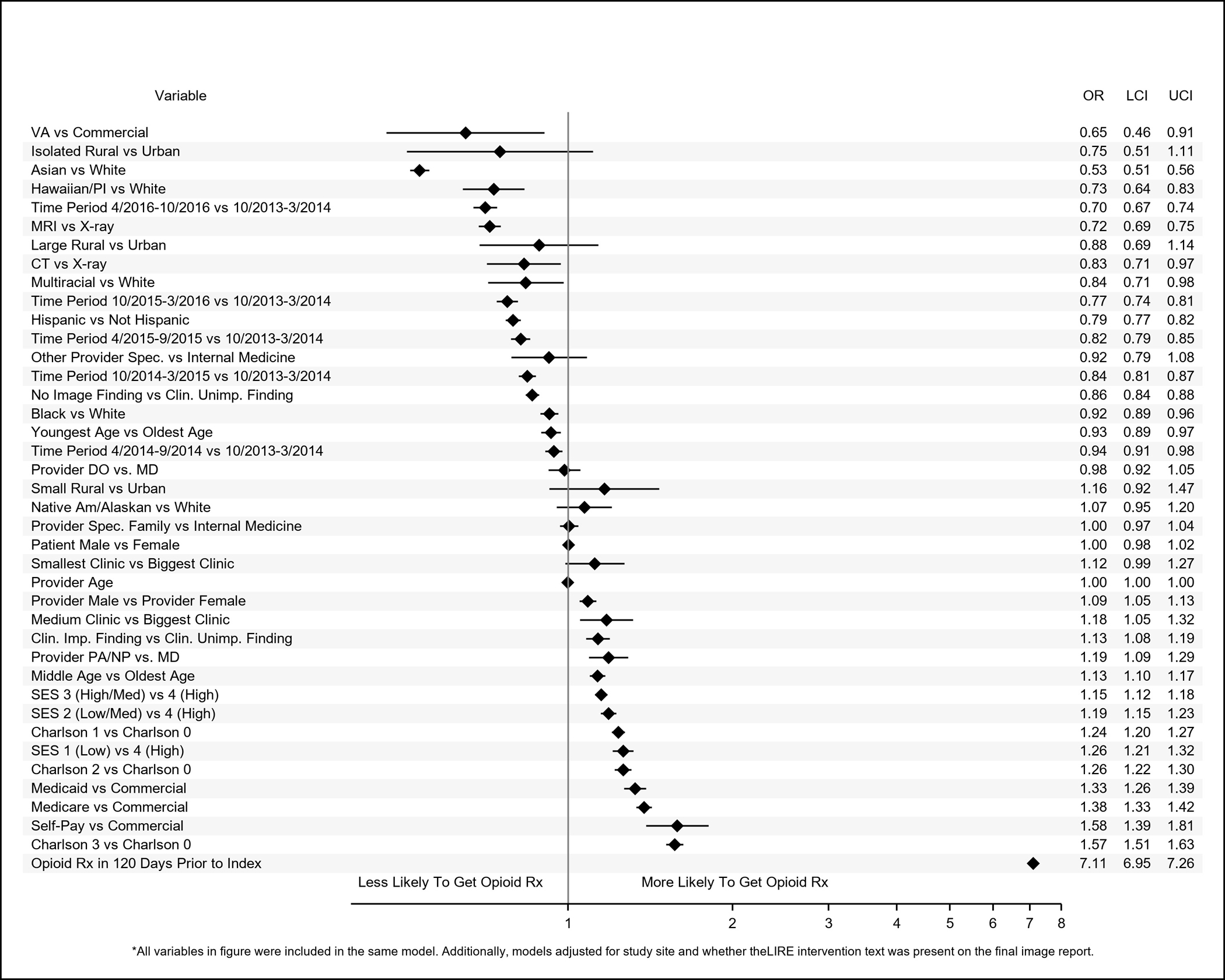
Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) of patient-level, provider-level, and clinic-level variables associated with receipt of immediate prescriptions for opioids within 90 days of receipt of lower back imaging.
Figure 5.
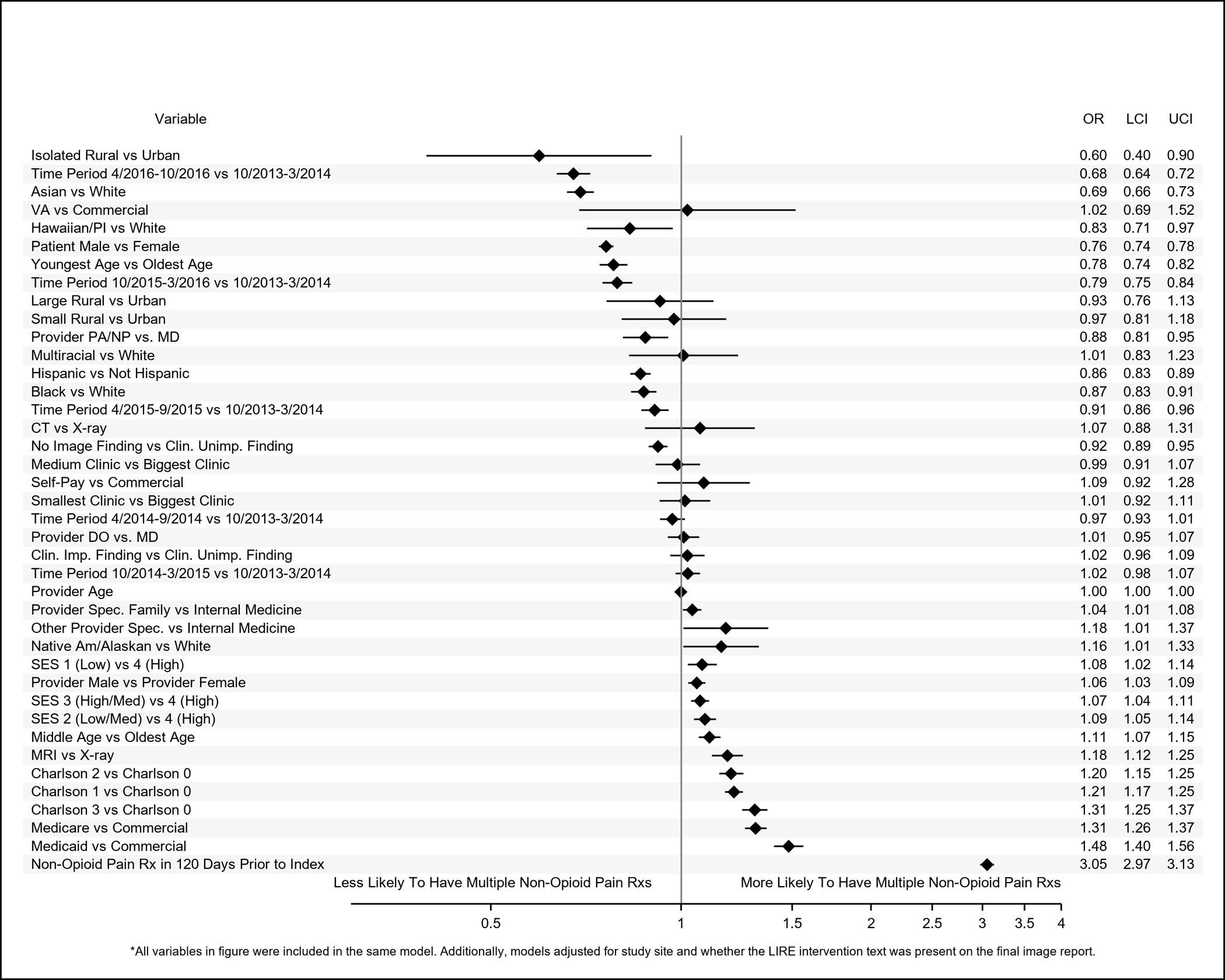
Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) of patient-level, provider-level, and clinic-level variables associated with having 1 quarter (referent category) versus multiple quarters with at least 1 non-opioid pain medication prescription within 1 year of index low back image.
Index Imaging
Compared to patients with clinically unimportant findings on their index images, patients with no findings had reduced odds of receiving immediate opioid (0.86 (0.84–0.88)) or non-opioid (0.96 (0.94–0.99)) prescriptions or of receiving multiple prescriptions (opioids: 0.87 (0.84–0.91); non-opioids: 0.92 (0.89–0.95)). Patients with clinically important findings had reduced odds of receiving multiple opioid prescriptions compared to patients with clinically unimportant findings (0.91 (0.84–0.98)).
Other Patient-Level Factors
Patients who were insured by the VA were less likely to have received prescriptions for immediate opioid (0.65 (0.46–0.91) or non-opioid pain medications (0.73 (0.55–0.98)) compared to those with commercial insurance. Patients who received pre-index prescriptions for opioid non-opioid pain medications had increased odds of receiving immediate opioid or non-opioid pain prescriptions and of receiving multiple prescriptions after index; these ORs ranged from 2.75 (95% CI: 2.70–2.80) for an immediate non-opioid pain prescription to 7.11 (6.95–7.26) for immediate opioid prescriptions; ORs for all other characteristics did not exceed 1.60. Patients in the youngest age category had reduced odds of receiving immediate opioid prescriptions (0.93 (0.89–0.97)) and multiple prescriptions for non-opioids (0.78 (0.74–0.82)) but increased odds (1.13 (1.09–1.17)) of receiving immediate non-opioid pain prescriptions compared to patients in the oldest age group. Patients whose index images occurred later in calendar time had reduced odds of receiving prescription pain medications by almost all metrics compared to patients who received their images earlier.
Patient and Provider Sex
Relative to female patients, males had reduced odds of receiving immediate prescriptions for non-opioid pain medications (0.90 (0.89, 0.92)) or multiple prescriptions for opioids (0.86 (0.84–0.89)) or non-opioids (0.76 (0.74–0.78)). However, patients whose index-image ordering providers were male had increased odds of receiving immediate (1.09 (1.05–1.13)) and multiple prescriptions for opioids (1.05 (1.02–1.09)) or non-opioids (1.06 (1.03–1.09)). Though male and female patients of female providers received opioid and non-opioid pain medication prescriptions equally, 29% of female patients of male providers received immediate opioid prescriptions compared to only 26% of male patients of male providers (Supplemental Table 2). Similarly, 33% of female patients of male providers received immediate prescriptions for non-opioid pain medications in contrast to 29% of male patients of male providers. Higher proportions of female patients of both male and female providers received multiple prescriptions for opioids and non-opioid pain medications compared to male patients.
Clinic-Level Factors
Patients who received their index images at medium-sized relative to large clinics had increased odds of receiving immediate opioid prescriptions (1.18(1.05–1.32)). Patients whose index images took place at the smallest clinics had reduced odds of receiving immediate non-opioid pain prescriptions (0.83 (0.72–0.96)). Patients whose index images occurred at isolated rural relative to urban clinics had reduced odds of receiving immediate non-opioid pain prescriptions (0.60 (0.39–0.91)) and of receiving multiple opioid (0.58 (0.37–0.91)) or non-opioid (0.60 (0.40–0.90)) pain prescriptions.
Sensitivity analyses that excluded patients who were missing race or SES revealed very similar results to those described above (Supplemental Figures 1–4). For example, in the main analysis in which race was imputed, patients identified as Black had 8% reduced odds (95% CI: 4%-11%) compared to whites of receiving immediate prescriptions for opioids; in the sensitivity analysis in which those with missing race and SES were excluded, Blacks had 10% reduced odds (95% CI: 6%-13%) of receiving immediate opioid prescriptions compared with whites.
Discussion
We report patient-level, provider-level, and clinic-level characteristics associated with pain medication prescribing patterns that have not been well-described in past literature. Notably, we found that Asian patients had lower odds of receiving opioid or non-opioid pain medication prescriptions compared to white patients across all measures of those prescriptions. We also found that, compared with older patients, middle-aged patients had higher adjusted odds of receiving pain medication prescriptions in all outcome measures. Patients who had prescriptions prior to index for opioid or non-opioid pain medications also had greater odds of receiving prescriptions for pain medications post-index. Additionally, consistent with prior research, we found that patients identified as Black/African American,12,34 identified as Hispanic,12,34 of higher SES,14 with no comorbidities,35 or who received low back imaging in more recent years7,8 had reduced odds of receiving immediate prescriptions for opioids. It is likely that the reduction in opioid prescriptions in the later years of our study was influenced both by the increasing proportion of patients whose images contained the benchmark text for the LIRE study, which was associated with small but significant decreases in the likelihood of subsequent opioid prescriptions, 23 as well as by increasingly broad societal concern about the harms associated with opioid prescriptions. These concerns were summarized in the 2016 Centers for Disease Control and Prevention (CDC) opioid prescribing guidelines,9 which called attention to the lack of evidence supporting the efficacy of long-term opioid therapy and its potential harms.
Our sample was notable for having substantial numbers of patients in racial categories for whom trends in pain medication prescribing have not been well-described, including patients identified as Asian, Native Hawaiian, Native American, or multiracial. Rates of opioid prescribing tend to be dramatically lower in Asian countries than in the US, likely due to variances in cultural norms and legal regulations.36,37 It is possible that reduced expectations of pain prescriptions were also present in the populations of Asian patients in this study, which resulted in their receiving fewer pain prescriptions than whites. We did not have data on patient preferences or attitudes to assess this hypothesis, but future work should examine this in greater detail.
We did not observe significant differences in the opioid outcomes among patients identified as Native American/Alaskan Native versus whites, but they had greater odds of receiving immediate prescriptions for non-opioids. A recent study found that fatal opioid-involved overdoses (which included both prescription and illegal drugs) in American Indian/Alaska Natives had increased from a rate similar to that of whites in 1999–2001 to 4.1 times greater than that in whites by 2013–2015.38 Given this trend, it is possible that providers were prescribing non-opioid pain medications in this population to reduce their exposure to opioids, but we could not address this hypothesis with our data.
Our finding that patients who received pre-index prescriptions for pain medications had much higher odds of receiving subsequent pain prescriptions may reflect several factors. Providers may be subject to “prescribing inertia” and continue patients on medications they are already on, regardless of their efficacy.39 Additionally, patients who received previous prescriptions for pain medications may have been more likely to request pain prescriptions following their low back imaging, particularly if they were experiencing acute episodes of low back pain. We were unable to evaluate the appropriateness of the pain medication prescriptions.
Although some prior research has reported that male patients are less likely to be prescribed opioids compared to female patients,16–18 we found no associations between patient sex and receipt of immediate opioid prescriptions, but females had greater odds of having multiple pain prescriptions. We found that male image-ordering providers were more likely to write immediate opioid prescriptions and to write multiple prescriptions for opioids or non-opioid pain medications relative to female providers. This is the opposite of the findings of an analysis of PCPs in the United Kingdom (not specific to patients with low back pain), which found that female providers were more likely to prescribe opioids.40 In unadjusted analyses, we also found that male providers were slightly more likely to prescribe pain medications to female patients than to males, and higher proportions of female patients received multiple prescriptions from both female and male providers. Concordance of patient-provider sex and pain medication prescribing has not been widely researched, but a study of prescribing patterns in an emergency department found that male physicians were more likely to prescribe opioids to male patients and female physicians were more likely to prescribe to female patients.41 Further investigation into these patterns that can account for potentially confounding factors is necessary to replicate and to understand the reasons for these findings.
Limitations
Our study included a large and diverse cohort of patients, but it has some limitations. First, although all patients had received low back imaging, we cannot be certain that the index image was ordered because of low back pain. We also lacked information on the duration of pain, if any, and thus could not examine prescribing differences for patients with acute versus chronic pain. We could not be sure that prescriptions were for low back pain rather than some other indication, including opioid use disorder. Second, we lacked data for over-the-counter medications. Third, patient race and ethnicity may have been misclassified. Although the policy at each of the health systems in our study was to have patients self-identify, we could not be certain that race and ethnicity in the EMR were identified by patients themselves or by staff, although some genomics literature suggests that observer-assigned race is concordant with ancestry.42,43 Imputing missing race by using the most common races in the zip code where the imaging took place may have misclassified some patients into incorrect racial categories and although we found similar results when we conducted sensitivity analyses that excluded patients with missing data, our results may have been subject to bias. Fourth, data were not available on confounders that may have affected opioid prescribing, including education, employment status, smoking status, anxiety and depression, pain severity, and use of health-related services (e.g., acupuncture) that were not available in the EMR. While ICD-9-CM and ICD-10-CM diagnosis codes for opioid use disorder (OUD) exist, they have been shown to be under-utilized in EMR44 and we found very few instances of patients with these codes in our data, so we were unable to adjust for OUD. Similarly, we lacked data on provider characteristics that might affect pain medication prescribing, such as race, cultural and pain management competencies, and language capabilities. Finally, given the number of characteristics that we analyzed, some of the associations that we found could have been due to chance.
Conclusion
We highlighted several novel patient-level characteristics associated with the receipt of prescription pain medications, including fewer immediate pain medication prescriptions among patients identified as Asian, Black, Native Hawaiian/Pacific Islander, or multiracial and greater immediate prescribing of non-opioid pain medications for patients identified as Native American/Alaska Native. Further large, population-based studies are needed to replicate and better understand these findings. Patients who had prior prescriptions for opioid or non-opioid pain medications were more likely to receive subsequent prescriptions for pain medications. These findings could be useful in future studies to investigate how healthcare systems could identify opportunities to optimize pain medication management for individual patients.
Supplementary Material
Figure 3.
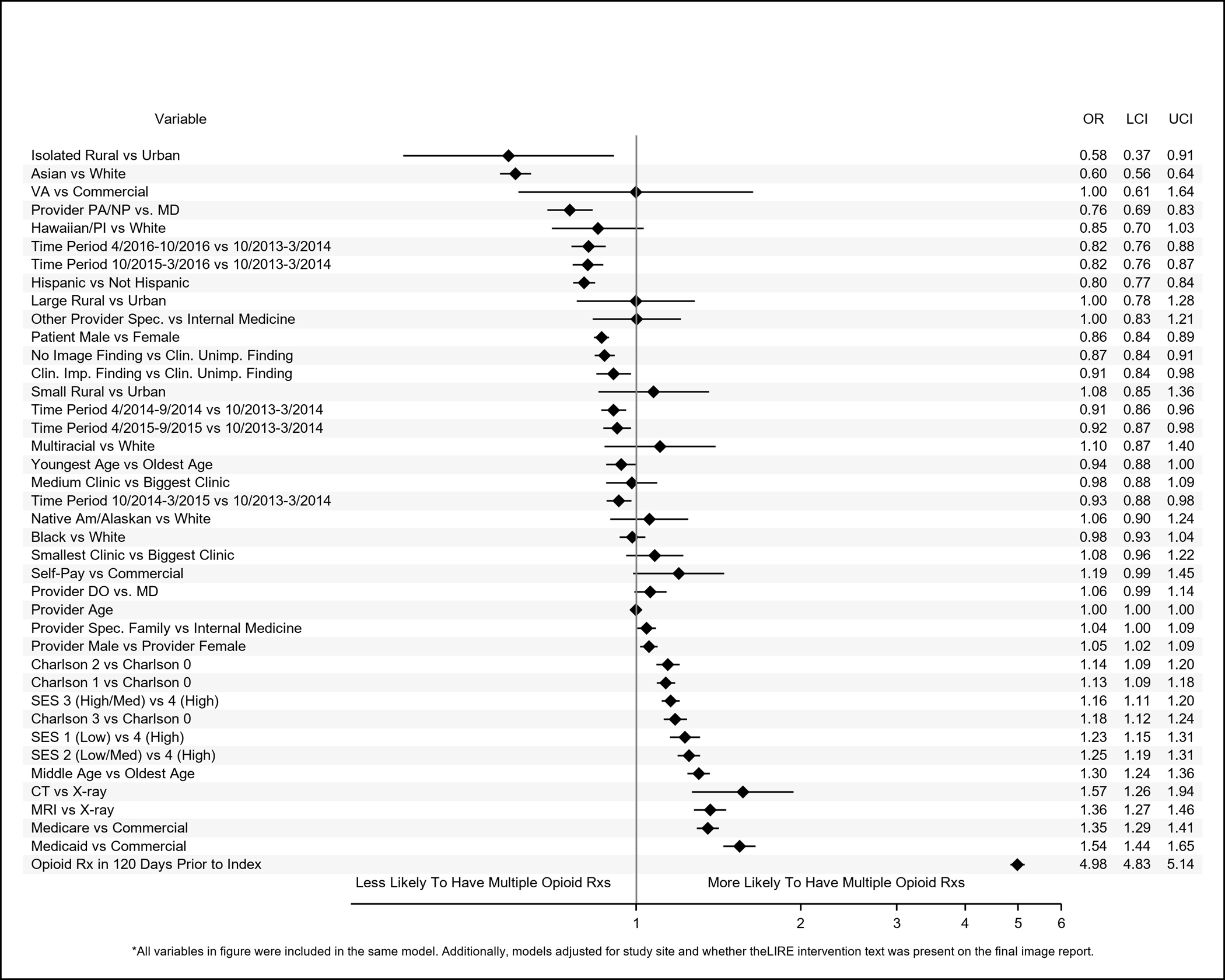
Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) of patient-level, provider-level, and clinic-level variables associated with having 1 quarter (referent category) versus multiple quarters with at least 1 opioid prescription within a year of index low back image.
Figure 4.
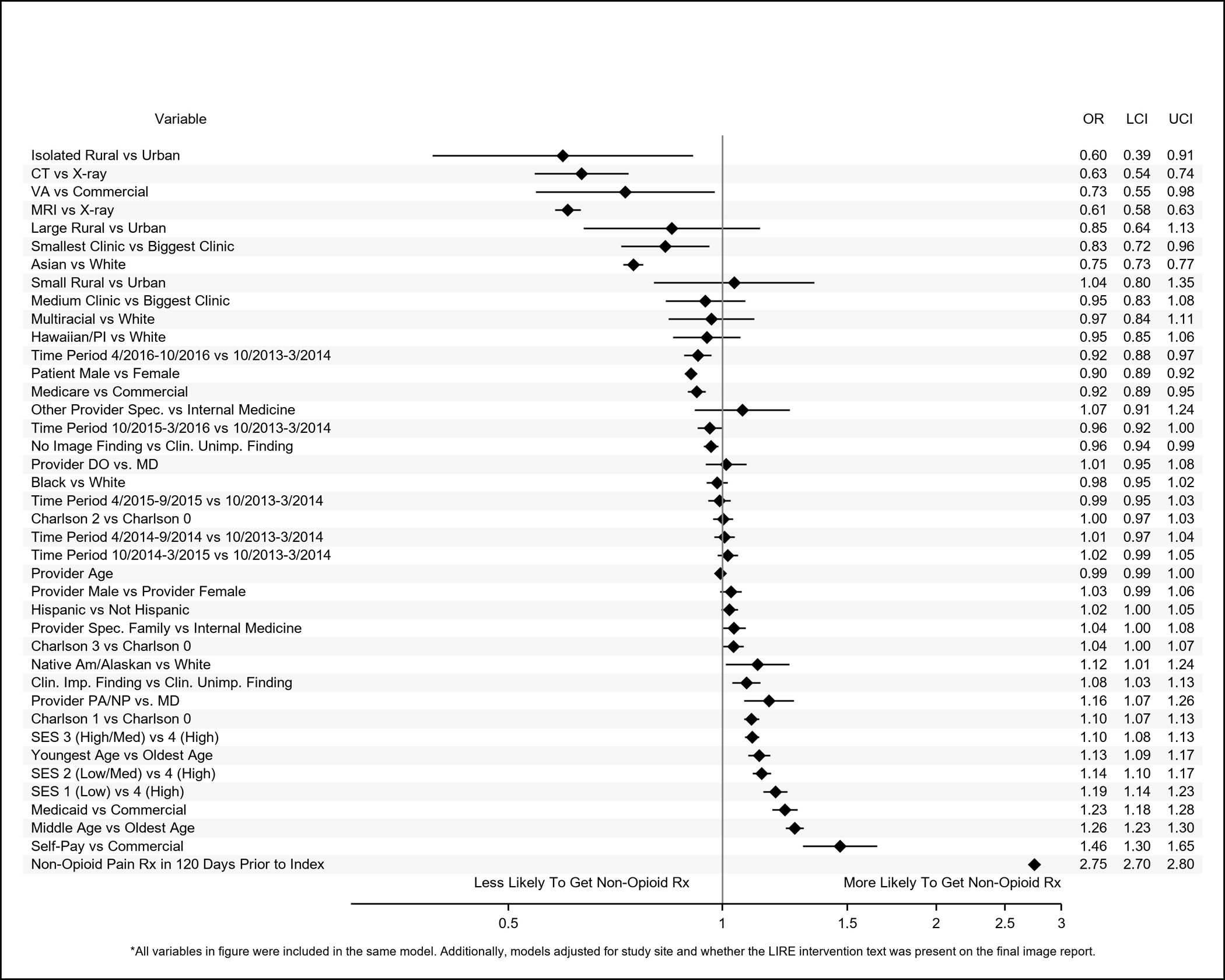
Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) of patient-level, provider-level, and clinic-level variables associated with receipt of immediate prescriptions for non-opioid pain medications within 90 days of receipt of lower back imaging.
Funding Statement:
This work was supported within the National Institutes of Health (NIH) Health Care Systems Research Collaboratory by the NIH Common Fund through cooperative agreement U24AT009676 from the Office of Strategic Coordination within the Office of the NIH Director and cooperative agreements UH2AT007766 and UH3AR066795 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). Research was completed by investigators at the University of Washington Clinical Learning, Evidence And Research (CLEAR) Center for Musculoskeletal Disorders and supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health under Award Number P30AR072572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work is registered at ClinicalTrials.gov identifier: NCT02015455.
Footnotes
Conflicting and Competing Interests: In the spirit of full disclosure, we have several potential conflicts of interest to report. Dr. Judith A Turner reports receiving royalty payments from Psychological Assessment Resources, Inc (PAR). Dr. David F. Kallmes holds patients related to spine augmentation and has licensed intellectual property related to spine augmentation from the Mayo Clinic. Dr. Richard A Deyo reports receiving royalties from Wolters Kluwer/UpToDate for authoring topics on low back pain and consulting fees from Kaiser Permanente Washington Health Research Institute, as well as partial salary support from an endowment from Kaiser Permanente. Dr. Jeffrey G Jarvik reports royalties as a book co-editor from Springer Publishing and travel reimbursement for Faculty Board of Review from GE-Association of University Radiologists Radiology Research Academic Fellowship (GERRAF) and royalties as a chapter author from Wolters Kluwer/UpToDate.
NOTE FOR NIH, TO PROVIDE LINK TO PUBLISHED VERSION: The final published version of this manuscript is available for free at: www.jabfm.org/content/34/5/950
References
- 1.Urits I, Burshtein A, Sharma M, et al. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr Pain Headache Rep. 2019;23(3):23. [DOI] [PubMed] [Google Scholar]
- 2.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478–491. [DOI] [PubMed] [Google Scholar]
- 3.North American Spine Society. Choosing Wisely. https://www.choosingwisely.org/clinician-lists/nass-opioids-for-acute-or-chronic-low-back-pain/. Published 2019. Accessed April 27, 2020.
- 4.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of P. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(7):514–530. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health Care and Excellence (NICE). Low Back Pain and Sciatica in Over 16s: Assessment and Management. In: Low Back Pain and Sciatica in Over 16s: Assessment and Management. London: 2016. [Google Scholar]
- 6.van Tulder M, Becker A, Bekkering T, et al. Chapter 3. European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15 Suppl 2:S169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoots BE, Xu L, Kariisa M, et al. 2018 Annual surveillance report of drug-related risks and outcomes--United States. 2018.
- 8.Raad M, Pakpoor J, Harris AB, et al. Opioid Prescriptions for New Low Back Pain: Trends and Variability by State. J Am Board Fam Med. 2020;33(1):138–142. [DOI] [PubMed] [Google Scholar]
- 9.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee P, Le Saux M, Siegel R, et al. Racial and ethnic disparities in the management of acute pain in US emergency departments: Meta-analysis and systematic review. Am J Emerg Med. 2019;37(9):1770–1777. [DOI] [PubMed] [Google Scholar]
- 11.Milani CJ, Rundell SD, Jarvik JG, et al. Associations of Race and Ethnicity With Patient-Reported Outcomes and Health Care Utilization Among Older Adults Initiating a New Episode of Care for Back Pain. Spine. 2018;43(14):1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13(2):150–174. [DOI] [PubMed] [Google Scholar]
- 13.Harrison JM, Lagisetty P, Sites BD, Guo C, Davis MA. Trends in Prescription Pain Medication Use by Race/Ethnicity Among US Adults With Noncancer Pain, 2000–2015. Am J Public Health. 2018;108(6):788–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebauer S, Salas J, Scherrer JF. Neighborhood Socioeconomic Status and Receipt of Opioid Medication for New Back Pain Diagnosis. J Am Board Fam Med. 2017;30(6):775–783. [DOI] [PubMed] [Google Scholar]
- 15.Jeffrey Kao MC, Minh LC, Huang GY, Mitra R, Smuck M. Trends in ambulatory physician opioid prescription in the United States, 1997–2009. PM R. 2014;6(7):575–582 e574. [DOI] [PubMed] [Google Scholar]
- 16.Smith JA, Fuino RL, Pesis-Katz I, et al. Differences in opioid prescribing in low back pain patients with and without depression: a cross-sectional study of a national sample from the United States. Pain Rep. 2017;2(4):e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serdarevic M, Striley CW, Cottler LB. Sex differences in prescription opioid use. Curr Opin Psychiatry. 2017;30(4):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: findings from the National Survey on Drug Use and Health. Addict Behav. 2010;35(11):1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agnoli A, Jerant A, Franks P. Prescription Opioids and Patient Sex: A National Cross-Sectional Study. J Womens Health (Larchmt). 2020. [DOI] [PubMed] [Google Scholar]
- 20.Luu H, Slavova S, Freeman PR, Lofwall M, Browning S, Bush H. Trends and Patterns of Opioid Analgesic Prescribing: Regional and Rural-Urban Variations in Kentucky From 2012 to 2015. J Rural Health. 2019;35(1):97–107. [DOI] [PubMed] [Google Scholar]
- 21.Rigg KK, Monnat SM, Chavez MN. Opioid-related mortality in rural America: Geographic heterogeneity and intervention strategies. Int J Drug Policy. 2018;57:119–129. [DOI] [PubMed] [Google Scholar]
- 22.Ganem VJ, Mora AG, Varney SM, Bebarta VS. Emergency Department Opioid Prescribing Practices for Chronic Pain: a 3-Year Analysis. J Med Toxicol. 2015;11(3):288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvik JG, Meier EN, James KT, et al. The Effect of Including Benchmark Prevalence Data of Common Imaging Findings in Spine Image Reports on Health Care Utilization Among Adults Undergoing Spine Imaging: A Stepped-Wedge Randomized Clinical Trial. JAMA Network Open. 2020;3(9):e2015713–e2015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvik JG, Comstock BA, James KT, et al. Lumbar Imaging With Reporting Of Epidemiology (LIRE)--Protocol for a pragmatic cluster randomized trial. Contemp Clin Trials. 2015;45(Pt B):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roland M, van Tulder M. Should radiologists change the way they report plain radiography of the spine? Lancet. 1998;352(9123):229–230. [DOI] [PubMed] [Google Scholar]
- 27.Nerenz DR, McFadden B, Ulmer C. Race, ethnicity, and language data: standardization for health care quality improvement. 2009. [PubMed]
- 28.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. American journal of epidemiology. 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 29.Anderson MLP, S. Linking Demographic and Socioeconomic Data to the Electronic Health Record. Living Textbook of Pragmatic Clinical Trials Web site. https://rethinkingclinicaltrials.org/resources/linking-electronic-health-record-data-to-socioeconomic-status-methods-and-documentation/. Published 2014. Accessed August 7, 2020. [Google Scholar]
- 30.Agency for Healthcare Research and Quality. Chapter 3: Creation of New Race-Ethnicity Codes and SES Indicators for Medicare Beneficiaries. https://archive.ahrq.gov/research/findings/final-reports/medicareindicators/medicareindicators3.html. Published 2008. Updated 2008. Accessed May 23, 2018.
- 31.Tan WK, Hassanpour S, Heagerty PJ, et al. Comparison of Natural Language Processing Rules-based and Machine-learning Systems to Identify Lumbar Spine Imaging Findings Related to Low Back Pain. Acad Radiol. 2018;25(11):1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.United States Department of Agriculture. Documentation 2010 Rural-Urban Commuting Area (RUCA) Codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/. Published 2019. Updated Oct 24. Accessed March 11, 2020.
- 33.Xu X, Chong W, Li S, Arabo A, Xiao J. MIAEC: Missing data imputation based on the evidence chain. IEEE Access. 2018;6:12983–12992. [Google Scholar]
- 34.Ly DP. Racial and Ethnic Disparities in the Evaluation and Management of Pain in the Outpatient Setting, 2006–2015. Pain Med. 2019;20(2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobscha SK, Morasco BJ, Duckart JP, Macey T, Deyo RA. Correlates of prescription opioid initiation and long-term opioid use in veterans with persistent pain. Clin J Pain. 2013;29(2):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onishi E, Kobayashi T, Dexter E, Marino M, Maeno T, Deyo RA. Comparison of Opioid Prescribing Patterns in the United States and Japan: Primary Care Physicians’ Attitudes and Perceptions. J Am Board Fam Med. 2017;30(2):248–254. [DOI] [PubMed] [Google Scholar]
- 37.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi S, Weiser T, Warren-Mears V. Drug, Opioid-Involved, and Heroin-Involved Overdose Deaths Among American Indians and Alaska Natives - Washington, 1999–2015. MMWR Morb Mortal Wkly Rep. 2018;67(50):1384–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinman MA, Landefeld CS. Overcoming Inertia to Improve Medication Use and Deprescribing. JAMA. 2018;320(18):1867–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen TC, Chen LC, Kerry M, Knaggs RD. Prescription opioids: Regional variation and socioeconomic status - evidence from primary care in England. Int J Drug Policy. 2019;64:87–94. [DOI] [PubMed] [Google Scholar]
- 41.Safdar B, Heins A, Homel P, et al. Impact of physician and patient gender on pain management in the emergency department--a multicenter study. Pain Med. 2009;10(2):364–372. [DOI] [PubMed] [Google Scholar]
- 42.Dumitrescu L, Ritchie MD, Brown-Gentry K, et al. Assessing the accuracy of observer-reported ancestry in a biorepository linked to electronic medical records. Genet Med. 2010;12(10):648–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall JB, Dumitrescu L, Dilks HH, Crawford DC, Bush WS. Accuracy of administratively-assigned ancestry for diverse populations in an electronic medical record-linked biobank. PLoS One. 2014;9(6):e99161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palumbo SA, Adamson KM, Krishnamurthy S, et al. Assessment of Probable Opioid Use Disorder Using Electronic Health Record Documentation. JAMA Netw Open. 2020;3(9):e2015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


