ABSTRACT
CoronaVac, also known as the Sinovac inactivated SARS-CoV-2 vaccine, has been widely implemented in combating the COVID-19 pandemic. We summarized the results of clinical trials and real-world studies of CoronaVac in this review. The overall efficacy for the prevention of symptomatic COVID-19 (before the emergence of variants of concern) using two doses of 3 μg CoronaVac was 67.7% (95% CI, 35.9% to 83.7%). Effectiveness in preventing hospitalizations, ICU admissions, and deaths was more prominent than that in preventing COVID-19. A third dose inherited the effectiveness against non-variants of concern and increased effectiveness against severe COVID-19 outcomes caused by omicron variants compared to two doses. Most adverse reactions were mild. Few vaccine-related serious adverse reactions have been reported. Moreover, three-dose regimen significantly increased the seroconversion levels of neutralizing antibodies against omicron as compared to two-dose regimen. This review of CoronaVac may provide a scientific basis for optimizing global immunization strategies.
KEYWORDS: COVID-19, inactivated vaccine, vaccine efficacy, safety, immunogenicity
Introduction
The biggest vaccination campaign in history is underway to provide acquired immunity against SARS-CoV-2, the virus that causes COVID-19. Globally, there have been more than 460 million confirmed cases of COVID-19, including nearly 6.06 million deaths (up to 18 March 2022) and counting. More than 10.9 billion vaccine doses have been administered and more than 5 billion persons vaccinated with at least one dose as of 17 March 2022.1 Vaccination is expected to be the ultimate weapon to end this pandemic. CoronaVac is an inactivated whole virus, aluminum-hydroxide-adjuvanted COVID-19 vaccine, created from African green monkey kidney cells (Vero cells) that have been inoculated with SARS-CoV-2 (CN02 strain).2,3 The vaccine was developed by the Chinese company Sinovac Biotech. The primary vaccination regimen for CoronaVac is two doses with an interval of 2 to 4 weeks.3 Injections should be made in the deltoid muscle 2–3 finger widths below the medial lateral process of the acromion. The raw material and final product for formulating CoronaVac can be refrigerated and transported at 2-8°C without the need to be frozen, which is importantly helpful for global distribution.4 On 1 June 2021, WHO validated the vaccine for emergency use. As of 17 March 2022, over two billion doses of CoronaVac have been administered in 52 countries and jurisdictions, most of which are low or middle-income areas.5 Therefore, CoronaVac vaccine has been enormously important in fighting the COVID-19 pandemic.
Recently, the protection offered by the vaccine has been questioned. Firstly, the emergence of five SARS-CoV-2 variants of concern since September 2020 is concerning, particularly B.1.1.529 (South Africa, Omicron). The emerging variants with multiple mutations in the spike protein or RBD are capable of escaping neutralization by vaccine-induced humoral immunity.6 Secondly, virus neutralizing antibody response, regarded as a proxy for protection from infection in humans with virus, from the two-dose regimen wanes over time,7,8 and the protection provided to the elderly and immunocompromised persons is limited. In October 2021, WHO recommended an additional dose for older adults and immunocompromised persons who have received two-dose CoronaVac to ensure sufficient protection.7,9 Hence, we reviewed the efficacy, safety, and immunogenicity of CoronaVac against SARS-CoV-2 in clinical trials and real-world studies for providing a basis for the optimization of global immunization programs.
Efficacy
Efficacy in clinical trials
Vaccine efficacy refers to the proportion of reduction of a disease among vaccinated persons during a clinical trial.10 There were three reported phase 3 clinical trials of CoronaVac. One was conducted at 24 centers in Turkey from September 2020 to January 2021, and included 10,214 adults aged 18–59 years (before the emergence of variants of concern). Individuals were randomly assigned to receive two doses of CoronaVac or placebo (vaccinations were 14 days apart).11 Another was conducted at 16 centers in Brazil from July 21 to 16 December 2020 (before the emergence of variants of concern) and included 12,396 healthcare workers aged 18–59 and 60 years or older (≥14 days apart).12 The third one was conducted in Indonesia involving 1620 healthy participants aged 18–59 years (14 days apart) from 11 August 2020 to 21 October 2020 (before the emergence of variants of concern).13,14
The efficacies for the prevention of symptomatic COVID-19 14 days or more after the second dose were 83.5% (95% CI, 65.4% to 92.1%) in Turkey,11 50.7% (95% CI, 35.9%-62.0%) in Brazil,12 65% (95% CI, 20% to 85%) in Indonesia.13,14 We estimated a pooled value using inverse variance-weighted random effect models using log-transformed effect size estimates from each phase 3 trial. The pooled efficacy for the prevention of symptomatic COVID-19 was 67.7% (95% CI, 35.9% to 83.7%) (Table 1). That is, compared to placebo recipients, two-dose CoronaVac reduced the risk of COVID-19 by 67.7%.
Table 1.
Efficacy of CoronaVac in clinical trials.
| Efficacy | Age | Symptomatic COVID-19 | Hospitalization | Requiring assistance | Moderate/severe disease |
|---|---|---|---|---|---|
| In Turkey11 | 18–59 years | 83.5(65.4–92.1) | 100.0(20.4–100.0) | NR | NR |
| In Brazil12 | ≥18 years | 50.7(35.9–62.0) | NR | 83.7(58.0–93.7) | 100.0(56.4–100.0) |
| In Indonesia13,14 | 18–59 years | 65.0(20.0–85.0) | NR | NR | NR |
| Overall | — | 67.7(35.9–83.7) | — | — | — |
Data are efficacies (%) with 95% confidence intervals for the prevention of symptomatic COVID-19 14 days or more after dose 2 before the emergence of variants of concern. NR=not reported.
The efficacy for the prevention of COVID-19-related hospitalization (in Turkey), cases requiring assistance and moderate/severe cases (in Brazil) was 100.0% (95% CI, 20.4% to 100.0%),11 83.7% (95% CI, 58.0% to 93.7%) and 100.0% (95% CI, 56.4% to 100.0%), respectively (Table 1).12 Obviously, CoronaVac are more effective in preventing severe COVID-19 outcomes than in preventing infection with SARS-CoV-2.
Effectiveness in the real world
Vaccine effectiveness refers to how well the vaccines work to protect communities as a whole in the real world, differing from the efficacy measured in a clinical trial.10
Effectiveness against non-variants of concern
In Chile, a prospective national cohort included approximately 10.2 million persons aged 16 years or older from February 2 to 1 May 2021 (lack of representative data for variant types). CoronaVac was administered using a two-dose schedule (28 days apart). The vaccine effectiveness for the prevention of COVID-19, hospitalization, ICU admission, and COVID-19-related death was 65.9% (95%CI, 65.2%-66.6%), 87.5% (95%CI, 86.7%-88.2%), 90.3% (95%CI, 89.1%-91.4%), 86.3% (95%CI, 84.5%-87.9%), respectively, at 14 days or above following the second dose. These effectiveness were significantly higher than those after the first dose (Table 2).15 The subgroup results showed that the effectiveness of two doses in preventing hospitalization and ICU admission was reduced in adults aged ≥60 years as compared to that in adults aged 16–59 years (Table 2).15
Table 2.
Effectiveness of CoronaVac in the real world studies.
| Effectiveness | Doses | COVID-19 | Symptomatic COVID-19 | Hospitalization | ICU admission | COVID-19-related death |
|---|---|---|---|---|---|---|
| Against non-variants of concern in adults15,31 | ≥14 days after dose 1 | |||||
| ≥16 years | 17.1(15.5–18.7) | NR | 42.9(38.9–46.1) | 44.6(38.9–49.9) | 42.0(25.6–54.8) | |
| ≥14 days after dose 2 | ||||||
| ≥16 years | 65.9(65.2–66.6) | NR | 87.5(86.7–88.2) | 90.3(89.1–91.4) | 86.3(84.5–87.9) | |
| 16–59 years | 63.5(62.4–64.6) | NR | 91.9(90.2–92.2) | 94.6(92.2–96.3) | 85.8(69.6–93.4) | |
| ≥60 years | 66.6(65.4–67.8) | NR | 85.3(84.3–86.3) | 89.2(87.6–90.6) | 86.5(84.6–88.1) | |
| At 14 days after dose 3 | ||||||
| |
Unreported age range |
70.9(65.0–75.8) |
73.6(67.5–78.5) |
80.8(72.6–86.5) |
85.1(70.4–92.5) |
NR |
| Against alpha variants in healthcare workers18 |
Mean 104 days after dose 2 Unreported age range |
39(20–64) |
NR |
NR |
NR |
NR |
| Against gamma in adults in a longitudinal study21 | ≥14 days after dose 1 | |||||
| <60 years | 31.9(29.5–34.2) | NR | 54.3(47.1–60.6) | 53.3(37.7–65.0) | 56.1(34.3–70.7) | |
| 60–69 years | 22.0(18.5–25.3) | NR | 37.5(31.5–43.0) | 38.4(28.6–46.9) | 40.4(31.1–48.5) | |
| 70–79 years | 36.8(34.1–39.4) | NR | 41.9(37.3–46.1) | 41.1(33.7–47.7) | 44.4(38.1–50.0) | |
| 80–89 years | 22.9(16.9–28.4) | NR | 28.9(20.3–36.6) | 33.6(19.4–45.4) | 30.7(20.1–39.9) | |
| ≥90 years | 17.1(2.9–29.2) | NR | 17.2(−3.8–33.9) | 11.4(−34.7–41.7) | 19.1(−6.5–38.5) | |
| ≥14 days after dose 2 | ||||||
| <60 years | 50.0(48.4–51.4) | NR | 82.8(80.0–85.1) | 84.1(78.6.0–88.2) | 84.8(77.1–89.9) | |
| 60–69 years | 59.1(57.4–60.8) | NR | 79.6(77.6–81.4) | 80.0(76.7–82.7) | 82.9(80.1–85.3) | |
| 70–79 years | 59.5(58.0–60.9) | NR | 72.7(70.8–74.4) | 73.8(70.9–76.4) | 77.5(75.3–79.5) | |
| 80–89 years | 48.8(45.5–52.0) | NR | 58.2(53.9–62.1) | 58.5(51.2–64.7) | 63.5(58.7–67.7) | |
| |
≥90 years |
36.5(27.5–44.4) |
NR |
42.4(30.2–52.4) |
36.0(8.5–55.3) |
48.6(35.0–59.3) |
| Against gamma variants in older adults in a case-control study20 | ≥14 days after dose 1 | |||||
| ≥70 years | NR | 12.5(3.7–20.6) | 16.9 (5.7–26.8) | NR | 16.9(5.7–26.8) | |
| ≥14 days after dose 2 | ||||||
| ≥70 years | NR | 46.8(38.7–53.8) | 55.5 (46.5–62.9) | NR | 61.2(48.9–70.5) | |
| 70–74 years | NR | 59.0(43.7–70.2) | 77.6(62.5–86.7) | NR | 83.9(59.2–93.7) | |
| 75–79 years | NR | 56.2(43.0–66.3) | 66.6(51.8–76.9) | NR | 78.0(58.8–88.3) | |
| |
≥80 years |
NR |
32.7(17.0–45.5) |
38.9(21.4–52.5) |
NR |
44.0(20.3–60.6) |
| Against gamma variants in healthcare workers23 | ≥14 days after dose 1 | |||||
| ≥18 years | NR | 49.6(11.3–71.4) | NR | NR | NR | |
| ≥14 days after dose 2 | ||||||
| |
≥18 years |
NR |
36.8 (−54.9–74.2) |
NR |
NR |
NR |
| Against delta variants in adults25 | 1–2 months after dose 2 | |||||
| ≥18 years | 74.5(70.6–78.0)* | NR | NR | 56.0(51.2–60.2) | 79.2(76.8–81.4) | |
| 18–39 years | 73.9(68.4–78.5)* | NR | NR | 81.9(75.1–86.8) | 88.3(81.1–92.7) | |
| 40–59 years | 70.5(64.1–75.7) | NR | NR | 71.2(66.1–75.5) | 85.5(82.1–88.3) | |
| ≥60 years | 78.6(74.4–82.2) | NR | NR | 46.1(37.1–53.8) | 76.3(72.7–79.4) | |
| 3–5 months after dose 2 | ||||||
| ≥18 years | 30.4(18.8–40.3)* | NR | NR | 28.7(12.2–42.1) | 76.2(68.8–81.9) | |
| 18–39 years | 67.3(60.1–73.3)* | NR | NR | 43.5(−5.1–69.6) | 59.5(−11.5–85.3) | |
| 40–59 years | 32.4(16.7–45.2) | NR | NR | 38.3(10.9–57.2) | 82.6(63.6–91.7) | |
| |
≥60 years |
38.6(68.4–14.1) |
NR |
NR |
30.3(7.7–47.4) |
75.4(66.7–81.9) |
| Against omicron variants28 | ≥14 days after dose 1 | |||||
| 20–59 years | NR | NR | 2.1(−53.3–37.5)a | 60.9(40.6–74.3)b | 65.4(38.6, 79.4) | |
| 60–69 years | NR | NR | NRa | 55.1(30.9, 70.9)b | 70.2(51.3, 81.7) | |
| 70–79 years | NR | NR | NRa | 33.9(8.1, 52.5)b | 48.9(28.1, 63.7) | |
| ≥80 years | NR | NR | NRa | 35.0(8.8, 53.7)b | 40.5(14.9, 58.4) | |
| ≥14 days after dose 2 | ||||||
| 20–59 years | NR | NR | 17.9(−18.0, 42.9)a | 91.7(87.8, 94.4)b | 94.0(89.6, 96.5) | |
| 60–69 years | NR | NR | NRa | 82.6(74.2, 88.2)b | 87.6(80.9, 91.9) | |
| 70–79 years | NR | NR | NRa | 80.8(72.8, 86.5)b | 84.4(77.5, 89.2) | |
| ≥80 years | NR | NR | NRa | 60.2(43.9, 71.8)b | 66.8(51.9, 77.0) | |
| ≥14 days after dose 3 | ||||||
| 20–59 years | NR | NR | 42.3(11.4, 62.4)a | 98.5(95.2, 99.5)b | NR | |
| 60–69 years | NR | NR | NRa | 98.5(95.3, 99.6)b | 98.7(94.4, 99.7) | |
| 70–79 years | NR | NR | NRa | 96.7(92.3, 98.6)b | 97.2(92.3, 99.0) | |
| ≥80 years | NR | NR | NRa | 98.6(94.3, 99.7)b | 99.2 (94.3, 99.9) |
Data are effectiveness (%) with 95% confidence intervals. Superscript letters a and b indicate the effectiveness against mild/moderate disease, severe/fatal disease, respectively. * indicates effectiveness in persons with a lower age limit of 15. Variants of concern that were not reported are not listed. NR=not reported.
However, a cross-sectional study in an intensive care unit in Turkey found discouraging results at least 14 days after the second dose (no sampling for variant type). ICU and hospital stay, ICU and hospital mortality were similar between the vaccinated and unvaccinated groups. But the sample size in this study was only 90, and the data were from COVID-19 patients over 65 years old.16
Effectiveness against SARS-CoV-2 variants of concern
SARS-CoV-2 variants of concern have increased transmissibility or detrimental change compared to the original virus. Alpha variant (B.1.1.7) was estimated to be 1.4- to 1.9- fold more transmissible than the wild-type SARS-CoV-2.17 A retrospective cohort study was conducted among 4067 healthcare workers in Turkey between March 1 and 31 May 2021 when alpha variant was dominant. The median follow-up period was 104 days after the second dose. Two-dose CoronaVac were 39% (95% CI, 20%-64%) effective in preventing alpha variant infection (Table 2).18
Gamma variant (P.1) first discovered in Manaus in early 2021, showed 1.7- to 2.4-fold more transmissible than the ancestral virus.19 A matched, test-negative case-control real-world study was conducted in Brazil from January 17 to 29 April 2021, including 22,177 individuals aged ≥70 years across 645 cities using two-dose vaccination (28 days apart). In the setting with extensive transmission of the gamma variant, when ≥14 days following the second dose, the adjusted effectiveness for the prevention of symptomatic COVID-19, hospitalization and COVID-19-related death was 46.8% (95% CI, 38.7% to 53.8%), 55.5% (95% CI, 46.5% to 62.9%) and 61.2% (95% CI, 48.9% to 70.5%) respectively (Table 2).20 Furthermore, a retrospective longitudinal study of more than 25 million CoronaVac vaccinees in Brazil from January 18 to 24 July 2021 (P.1 dominated) showed that vaccine effectiveness 14 days and above after the second dose against hospitalization, ICU admission, and death were lower in adults ≥60 years as compared to that in adults aged <60 years (Table 2).21 In a descriptive observational study in Colombia from 24 February 2021, to 10 August 2021 among 7856 inhabitants aged 18 years and above (P.1 circulates), two-dose CoronaVac was 94.3% to prevent mild cases, and 99.9% effective in preventing moderate, severe forms and deaths. Duration after the second immunization was not provided in this study.22 However, a test-negative case-control study from 1 October 2020 to 13 April 2021 among 53,153 healthcare workers in Brazil showed less than 50% effectiveness against symptomatic gamma infection 14 days or more after receiving the second dose (vaccine effectiveness: 36.8% [95% CI, −54.9% to 74.2%]. Even, during the 0–13 days after the first vaccination, vaccinated healthcare workers were more likely to be infected than unvaccinated individuals.23
Delta (B.1.617.2) variant demonstrated 60% more transmissible than the alpha variant.24 An observational study involving 9.92 million individuals was conducted in Malaysia from 1 September 2021 to 30 September 2021, during which the delta variant was predominant. Vaccine effectiveness against COVID-19 decreased from 74.5% (95% CI, 70.6% to 78.0%) at 1–2 months after the second dose to 30.4% (95% CI, 18.8% to 40.3%) at 3–5 months after the second dose in persons aged ≥15 years. Also, the effectiveness against ICU admission decreased from 56.0% (95% CI, 51.2% to 60.2%) to 28.7% (95% CI, 12.2% to 42.1%). Effectiveness against death remained stable (Table 2).25 Similarly, a test-negative study from 18 January 2021 to 11 November 2021 involving almost 14 million people in Brazil estimated the effectiveness of CoronaVac over time. The effectiveness of CoronaVac in adults aged ≥18 years against COVID-19 (1–2 months after second dose: 51.7% [95% CI, 51.1%-52.4%]; 4–5 months after second dose: 41.8% [95% CI, 40.8% to 42.8%]; > 6 months after second dose: 34.7% [95% CI, 33.1% to 36.3%]), hospitalization or death (1–2 months: 82.6% [95%CI, 82.1% to 83.2%]; 4–5 months: 77.0% [95%CI, 76.1% to 77.8%]; > 6 months: 72.6% [95% CI, 71.0% to 74.2%]) waned over time, particularly for the elderly.26
Omicron (B.1.1.529) variant multiplied around 70-fold faster than the Delta variant in the bronchi.27 An ecological study on vaccine effectiveness was conducted from 31 December 2021 to 8 March 2022 during a omicron variant associated epidemic of COVID-19 in Hong Kong, China and 14,861 persons aged 20–69 years with confirmed SARS-CoV-2 infection were analyzed. The most critical results of this study were that CoronaVac offered protection against severe/fatal disease or mortality after infection with omicron, and even one dose was effective. Of course, two doses offered a higher level of protection against severe/fatal disease or mortality compared to one dose.28 These effectiveness of two-dose schedule for the prevention of symptomatic COVID-19, hospitalization, severe/fatal disease and deaths decreased with age (Table 2).20,28
Effectiveness after a third dose of CoronaVac
Since evidence has shown that vaccine effectiveness wanes over time,29 and as SARS-CoV-2 variants emerge,20,30 a third booster vaccination of CoronaVac was implemented to optimize immunity. As reported on 25 October 2021 in Chile, a third dose consistently prolonged the effectiveness of the second dose in general population in Chile. Effectiveness for the prevention of COVID-19, symptomatic COVID-19, hospitalization, and ICU admissions 14 days after the third dose were 70.89% (95%CI, 65.02% to 75.78%), 73.58% (95%CI, 67.50% to 78.52%), 80.77% (95%CI, 72.57% to 86.51%) and 85.10% (95%CI, 70.35% to 92.52%) respectively. The viral strains circulating at that time were not reported.31 Encouragingly, in the real world study in Hong Kong mentioned above, a third dose was not only inherited, but also improved the effectiveness against omicron of the second dose, and the effectiveness against severe/fatal or death was about 98%, whether under 60, over 60, or even over 80 years old (Table 2).28
Safety
Total adverse events/reactions
Adverse reactions, also known as side effects, are considered vaccine-related. An adverse event can be a true adverse reaction or a coincidental event that happened following vaccination.32 Values of incidence reported in different studies vary. Incidence of adverse events within 7 days after each dose in the vaccine group was higher than that in the placebo group in the phase 3 trials in Turkey (18.9% versus 16.9%, p = .01) and adverse reactions within 7 days after each injection in Brazil as well (77.1% versus 66.4%, p < .001).11,12 However, in a phase 3 trial in Indonesia, after two-dose immunization, there was no significant difference in the frequency of mild adverse events within 28 days after the second dose between the vaccine and control groups (47.9% versus 42.9%, p = .317).13 Anyway, most of the adverse reactions were mild.11–13
A phase 4 trial in Brazil included 910 patients with autoimmune rheumatic diseases (ARD) and 182 healthy controls and showed that the total adverse events within 28 days after the first dose and 6 weeks after the second dose were more frequent in patients than that in the healthy adults (50.5% versus 40.1%, p = .011).33 A cross-sectional survey reported that among 22 CoronaVac recipients with autoimmune and inflammatory rheumatic diseases (AIIRD), the incidence of adverse events within 6 months prior to study initiation was 54.5% and the vast majority were common local reactions or system response.34 Among cancer patients receiving active systemic therapy, the cumulative rate of adverse reactions after one dose and two doses was 19% and 32% respectively, and both were mild or moderate (grade 1 or 2).35 In addition, the incidence of adverse reactions within one week following the first/second immunization among 1673 medical workers in China was 15.6%/14.6%36 and the incidence of adverse events in 144 medical interns in Indonesia was 38%/35%.37 No gender differences were observed in the two surveys. However, Riad et al. surveyed 780 healthcare workers in Turkey who received either one or two doses of CoronaVac and found that women had a higher frequency of adverse reactions than men (67.9% versus 51.4%, p < .001).38 Moreover, a third booster dose after 6–8 months of primary immunization also showed a good safety profile. Adverse reactions did not increase following the third dose compared with the second dose.39,40
Other adverse events like cutaneous allergic reactions,41–43 reactive arthritis,44 subacute thyroiditis,45,46 acute thyroiditis and bilateral optic neuritis,47 transient focal neurological deficit mimicking stroke,48 and shoulder injury49 were also reported. Most, in case reports, wereconsidered self-limiting or discharged after receiving medical care.
Injection site pain was the most common adverse reaction/event (mostly greater than 10%) in clinical trials or real-world surveys.11–13–33–35–37–40,41–50 Fatigue (mostly greater than 10%) was the most frequently reported in phase 3 trial in Turkey,11 cancer patients,35 and healthcare workers.36,38,50 The most frequent systemic reactions/events in the phase 3 and phase 4 trials in Brazil were myalgia12 and headache,33 respectively. In general, the most reported systemic reaction was fatigue. Most adverse reactions/events occurred in less than 10% of CoronaVac recipients (Table 3).
Table 3.
Safety of CoronaVac in clinical trials and real world surveys.
| Adverse events/reactions |
Vaccine recipients (%) |
Placebo recipients (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy adults in phase 3 in Turkeya 11 | Healthy adults in phase 3 in Brazilb 12 | Healthy adults in phase 3 in Indonesiac 13 | Healthy adults in phase 4 in Brazild 33 | ARD in phase 4 in Brazile 33 | Patients with AIIRDf 34 | Patients with cancerg 35 | Healthcare workers in Chinah 36 | Healthcare workers in Turkeyi 38 | Medical clerkship studentsj 37 | Healthy adults in following the third dosek 40 | Healthy adults in phase 3 in Turkeya 11 | Healthy adults in phase 3 in Brazilb 12 | Healthy adults in phase 3 in Indonesiac 13 | Healthy adults in following the third dosek 40 | |
| Total adverse events or reactions | 18.9 | 77.1 | 57.8 | 40.1 | 50.5 | 54.5 | 18.9 | 15.6 | 62.5 | 38.0 | 15.0 | 16.9 | 66.4 | 43.0 | 8.0 |
| Local adverse events or reactions | 2.7 | 61.5 | NR | 19.8 | 23.4 | 36.4 | 8.4 | 9.6 | 45.0 | 22.9 | NR | 1.5 | 34.6 | NR | NR |
| Pain | 2.4 | 60.3 | 33.5 | 17.0 | 19.8 | 36.3 | 4.2 | <9.6 | 41.5 | 17.4 | 12.0 | 1.1 | 32.5 | 23.7 | 0.0 |
| Erythema | 0.2 | 0.0 | 0.0 | 2.7 | 2.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
| Paraesthesia | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
| Swelling | 0.1 | 5.8 | 2.2 | 6.6 | 4.7 | 0.0 | 2.1 | <9.6 | 2.6 | 2.1 | 2.0 | 0.1 | 2.1 | 0.7 | 0.0 |
| Induration | 0.1 | 3.8 | 8.4 | 2.2 | 6.2 | 0.0 | 0.0 | <9.6 | 0.0 | 0.0 | 0.0 | 0.1 | 1.1 | 4.4 | 0.0 |
| Pruritus | 0.0 | 4.2 | 0.0 | 2.2 | 3.1 | 0.0 | 2.1 | <9.6 | 0.0 | 1.4 | 2.0 | 0.1 | 2.9 | 0.0 | 0.0 |
| Redness | 0.0 | 3.9 | 6.2 | 0.0 | 0.0 | 0.0 | 0.0 | <9.6 | 1.4 | 2.1 | 0.0 | 0.0 | 1.4 | 3.7 | 0.0 |
| Bruising | 0.0 | 0.0 | 0.0 | 3.3 | 3.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Systemic adverse events or reactions | 17.7 | 48.4 | NR | 33.5 | 43.3 | 31.8 | 10.5 | NR | 71.0 | 25.0 | NR | 16.0 | 47.6 | NR | NR |
| Fatigue | 8.2 | 0.0 | 17.3 | 7.7 | 10.9 | 13.6 | 4.2 | 8.3 | 23.6 | 5.6 | 2.0 | 7.0 | 0.0 | 8.9 | 0.0 |
| Headache | 5.9 | 34.3 | 0.0 | 11.0 | 20.2 | 13.6 | 2.1 | <6.0 | 18.7 | 1.1 | 2.0 | 5.9 | 34.8 | 0.0 | 4.0 |
| Myalgia | 4.0 | 11.7 | 25.6 | 5.5 | 8.9 | 18.1 | 2.1 | 8.1 | 11.2 | 0.0 | 0.0 | 3.0 | 10.5 | 12.6 | 0.0 |
| Chill | 2.5 | 5.0 | 0.0 | 0.0 | 0.0 | 9.0 | 0.0 | 0.0 | 2.6 | 0.7 | 0.0 | 1.8 | 5.1 | 0.0 | 0.0 |
| Fever | 1.8 | 0.2 | 2.4 | 2.7 | 2.8 | 4.5 | 2.1 | 2.9 | 3.0 | 1.1 | 2.0 | 1.5 | 0.1 | 0.0 | 4.0 |
| Diarrhoea | 1.6 | 7.9 | 0.0 | 4.9 | 6.2 | 0.0 | 0.0 | <1.6 | 0.0 | 0.0 | 2.0 | 1.7 | 8.1 | 0.0 | 0.0 |
| Cough | 0.8 | 5.5 | 0.0 | 4.4 | 6.9 | 0.0 | 0.0 | <1.2 | 0.0 | 0.0 | 0.0 | 0.7 | 5.2 | 0.0 | 0.0 |
| Arthralgia | 0.7 | 5.7 | 0.0 | 6.0 | 13.5 | 0.0 | 0.0 | 0.0 | 5.9 | 0.0 | 0.0 | 0.5 | 5.2 | 0.0 | 0.0 |
| Nausea | 0.7 | 7.9 | 0.0 | 2.2 | 6.1 | 9.0 | 0.0 | <1.4 | 5.3 | 0.0 | 0.0 | 0.2 | 8.4 | 0.0 | 0.0 |
| Vomiting | 0.3 | 1.0 | 0.0 | 0.5 | 1.5 | 0.0 | 0.0 | <1.6 | 0.0 | 0.0 | 0.0 | 0.2 | 1.0 | 0.0 | 0.0 |
| Rash | 0.1 | 0.8 | 0.0 | 1.6 | 1.0 | 0.0 | 0.0 | <1.0 | 1.5 | 0.0 | 0.0 | 0.2 | 0.7 | 0.0 | 0.0 |
| Allergic reaction | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | <1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Appetite impaired | 0.0 | 3.5 | 0.0 | 3.8 | 4.1 | 0.0 | 0.0 | <1.4 | 0.0 | 0.0 | 0.0 | 0.0 | 3.9 | 0.0 | 0.0 |
| Hypersensitivity | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 |
| Malaise | 0.0 | 0.0 | 0.0 | 4.4 | 9.5 | 0.0 | 0.0 | 0.0 | 0.0 | 13.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Somnolence | 0.0 | 0.0 | 0.0 | 10.4 | 13.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Abdominal pain | 0.0 | 0.0 | 0.0 | 3.8 | 4.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Vertigo | 0.0 | 0.0 | 0.0 | 4.9 | 7.0 | 0.0 | 0.0 | <6.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Tremor | 0.0 | 0.0 | 0.0 | 0.5 | 2.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sweating | 0.0 | 0.0 | 0.0 | 1.1 | 5.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Muscle weakness | 0.0 | 0.0 | 0.0 | 3.8 | 7.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Back pain | 0.0 | 0.0 | 0.0 | 4.9 | 9.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sneezing | 0.0 | 0.0 | 0.0 | 4.9 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Coryza | 0.0 | 0.0 | 0.0 | 7.1 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Stuffy nose | 0.0 | 0.0 | 0.0 | 4.4 | 5.7 | 0.0 | 0.0 | <0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sore throat | 0.0 | 0.0 | 0.0 | 3.8 | 7.4 | 0.0 | 0.0 | <1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Shortness of breath | 0.0 | 0.0 | 0.0 | 3.3 | 3.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Conjunctivitis | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Leg pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 9.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Dyspnea | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Lymphadenopathy | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Oropharyngeal pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Oral diseases | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 11.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Acne | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Urticaria | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | <1.0 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Dysphagia | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Serious adverse events | 0.1 | 0.5 | <0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 5.0 | 0.1 | 0.5 | <0.6 | 4.0 |
Superscript letters indicate: aadverse events after one and two doses, bsolicited adverse reactions after one and two doses, csolicited adverse events after one dose, dadverse events after one dose, eadverse events after one dose, fadverse events after vaccination, gadverse reactions after one dose, hadverse reactions after one dose, iadverse reactions after one or two doses, jadverse events after one dose, and kadverse reactions after three doses. ARD=autoimmune rheumatic diseases. AIIRD=autoimmune and inflammatory rheumatic diseases. NR=not reported.
Serious adverse events
Serious adverse events were reported in 11 (0.1%) of the 10,214 participants, including six (0.1%) of 6646 in the vaccine group and five (0.1%) of 3568 in the placebo, during a median follow-up period of 43 days after the second dose of CoronaVac in the interim results of phase 3 trial in Turkey.11 In the phase 3 trial in Brazil, during a median follow-up of two months after the second dose, 64 (0.5%) of the 12,396 participants reported serious adverse events, including 33 (0.5%) of 6195 in the vaccine group and 31 (0.5%) of 6201 in the placebo group.12 In the phase 3 trial in Indonesia, 9 (0.6%) of the 1620 subjects reported serious adverse events during a median period of 2.5 months after the second dose.13 Of the three real-world studies among healthcare workers, one showed 4 (0.5%) of 878 reported serious adverse events requiring medical care within four weeks after vaccination,38 and the other two showed none.36,50 No serious adverse events following vaccination were seen in the phase 4 trial in Brazil,33 and in real world investigations in patients with autoimmune and inflammatory rheumatic diseases,34 and patients with cancer undergoing treatment.35 After the third dose (injected 8 months after the second dose), 5 (5.0%) of 101 participants reported serious adverse events from the beginning of immunization to 28 days after the third dose (Table 3).40
There were a total of five serious adverse reactions after evaluation among the serious adverse events reported above,11,38 and the others were deemed not related to vaccination.
Immunogenicity
Immunogenicity in healthy population
Immunogenicity is the ability of a vaccine to provoke an immune response in the human body.51 In the phase 3 trial in adults aged 18–59 years in Turkey, two-dose CoronaVac induced anti-RBD antibodies in 89.7% of serum whereas 4.4% was observed in the placebo group.11 In the phase 3 trial in adults aged 18–59 years in Indonesia, neutralizing antibody seroconversion was defined as conversion from titer <1:8 to titer ≥1:8 or 4-fold increase if baseline titer ≥1:8 14 days after two doses. The seroconversion rate in the vaccine group was higher than that in the placebo group (87.15% [95% CI, 83.50–90.09] versus 0.00% [95% CI, 0.00–2.81]) with the geometric mean titer (GMT) of 15.76 (95% CI, 14.57–17.04) versus 2.02 (95% CI, 1.98–2.05) (Figure 1a).13
Figure 1.
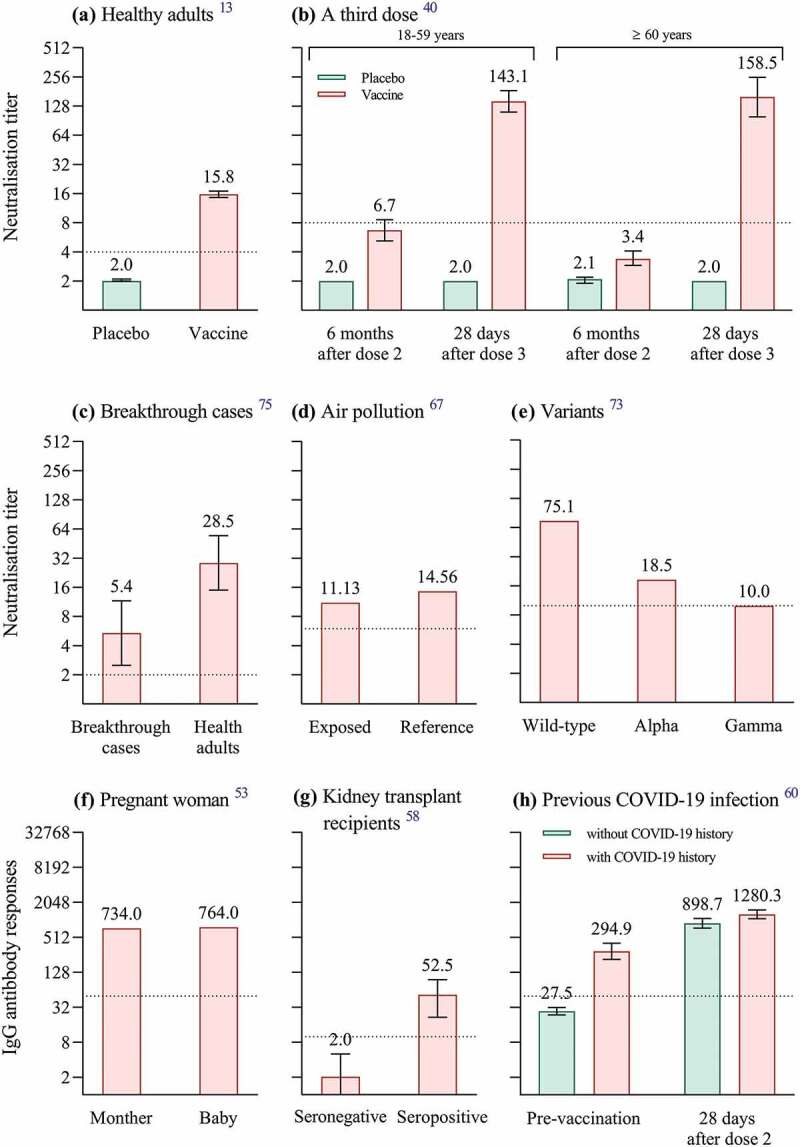
Immunogenicity of CoronaVac in different populations. We summarize immunogenicity of two-dose CoronaVac in healthy population aged 18–59 years (a) and three-dose CoronaVac in healthy population aged ≥18 years (b); immunogenicity of two-dose CoronaVac in breakthrough cases (c), in participants exposed to air pollution (d), against SARS-CoV-2 variants (e), in pregnant woman (f), in kidney transplant recipients (g) and in healthcare workers with previous COVID-19 infection (h). Numbers above the bars in panels a-h are geometric mean titers of neutralizing antibodies against SARS-CoV-2 (a-c, e), median of neutralizing antibody titers (Au/ml) (d), anti-RBD antibody levels (Au/ml) (f), median of anti-SARS-CoV-2 IgG levels (Iu/ml) (g), geometric mean titers IgG antibody of viral spike protein (h). The error bars in panels a-c, h indicate 95% confidence intervals, while those in panel g indicate interquartile ranges. All dotted line denotes the cutoff level for positivity.
Immunogenicity in special populations
Immunogenicity in patients with autoimmune rheumatic diseases (ARD)
Patients with ARD are at high risk for infectious diseases due to immune dysregulation, emphasizing the importance of the vaccine for this group of patients in reducing transmission. In the phase 4 trial in Brazil, neutralizing antibody positivity was defined as neutralization activity ≥30% and anti-SARS-CoV-2 IgG seroconversion was defined as post-vaccination serology of ≥15.0 UA/ml with a negative pre-vaccination serology at 6 weeks after two-dose schedule. Neutralizing antibody positivity rate (79.3% versus 56.3%, p < .001) and IgG seroconversion rate (95.5% versus 70.4%, p < .001) were lower in patients with ARD than those in healthy adults. Median neutralization activity (58.7% versus 64.5%, p = .013) in patients with ARD was lower compared to healthy adults, as were IgG titers (12.1 versus 29.7, p < .001).33
Immunogenicity in pregnant woman
As a risk factor for severe COVID-19, pregnancy appears to worsen the clinical course of SARS-CoV-2 infection, although it does not increase the risk of acquiring SARS-CoV-2 infection.52 The cord serum/maternal serum transfer ratio of anti-RBD antibodies was 1.04 (764/734 AU/ml) at 45 days after a mother received two doses of CoronaVac (Figure 1f).53 Moreover, human milk is the external secretion with the highest IgA concentrations, conferring protection to the newborn infants.54 Human milk samples collected from 16 healthy mothers showed elevated anti-SARS-CoV-2 specific IgA levels after two doses compared to before vaccination.55 The continued breastfeeding of infants after the mother has been vaccinated with CoronaVac, even after infection with SARS-CoV-2, should be emphasized.55
Immunogenicity in kidney transplant recipients
The COVID-19 mortality rate for kidney transplant recipients is not optimistic.56 Vaccination against COVID-19 is critical for this immunocompromised population.57 In a study of 85 kidney transplant recipients in Turkey, only 18.8% developed anti-SARS-CoV-2 IgG antibodies against nucleocapsid and spike antigens at 28 days after the second dose of CoronaVac, with median values of 52.50 and 2.04 IU/ml in the seropositive and seronegative groups, respectively (Figure 1g). The older the age, the higher the creatinine level, and the worse the antibody response.58
Immunogenicity in patients with cancer
Cancer patients are at higher risk for COVID-19 and related mortality than the healthy population.59 In a prospective observational study conducted in Turkey, seroconversion was defined as SARS-CoV-2 antibody ≥1 IU after vaccination and <1 IU before vaccination. After receiving 2 doses of CoronaVac, 30 (63.8%) of 47 cancer patients had seroconversion of SARS-CoV-2 total antibodies (including IgG and IgM). Seroconversion was 59.5% (25/42) in patients receiving at least one cytotoxic drug, and 100% (5/5) in patients receiving monoclonal antibodies or immunotherapy alone.35
Immunogenicity in healthcare workers with previous COVID-19 infection
A prospective observational study in Turkey included 148 healthcare workers (74 with previous COVID-19 infection and 74 with not). The IgG antibody titers against SARS-CoV-2 spike protein induced by two-dose CoronaVac in healthcare workers with previous SARS-CoV-2 infection were higher than those in healthcare workers without COVID-19 history (GMT: 1280.3 versus 899.7 AU/ml, p < .001) (Figure 1h).60 This result has been verified in multiple studies.61–63
Immunogenicity in participants exposed to air pollution
Exposure to air pollutants has been shown to be a critical risk factor for vaccine antibody levels64 and for COVID-19 cases.65,66 In a cross-section study conducted at 6 weeks after the second dose from China, grouped according to the combined toxic effects of air pollutants, plasma neutralizing antibody titers were lower in the high daily exposure dose group, compared with the low daily exposure group (median: 11.13 AU/mL versus 14.56 AU/mL, p < .05) (Figure 1d). Furthermore, increases in individual daily exposure to air pollutants were associated with decreases in plasma neutralizing antibody titers (rs = 0.652, p < .01).67
Immunogenicity against SARS-CoV-2 variants
More transmissible SARS-CoV-2 variants have emerged recently that may reduce vaccine effectiveness.68 The serum at 14 days after two-dose CoronaVac showed an average 1.51-, 5.27- and 3.92-fold reduction in neutralizing alpha, beta, and gamma variants compared with the activity against wild-type strains, respectively.69 At the same sampling time point in another similar study, plasma from two-dose CoronaVac recipients showed an average 2.9-, 5.5-, 4.3-, 3.4- and 12.5-fold reduction in neutralizing alpha, beta, gamma, delta and omicron variants when compared with the wild-type virus respectively.70 A study from Hong Kong, China found among two-dose CoronaVac recipients, at 56 days after the first dose of CoronaVac, 100%, 68%, 0% and 0% of serum specimens had detectable neutralizing antibody titer (≥10) against wild-type strains, delta variant, beta variant and omicron variant, respectively.71 The neutralizing ability against both P.1 isolates (P.1/28 and P.1/30) was significantly lower than that against lineage B isolate.72 Neutralizing antibody titers against alpha (GMT = 18.5) and gamma (GMT = 10.0) variants were lower than that against wild-type virus (GMT = 75.1) at 60 days after the second immunization of CoronaVac (Figure 1e).73
Immunogenicity after the third dose of CoronaVac
Although two-dose CoronaVac reduces the risk of disease, breakthrough cases may still occur defined as the detection of SARS-CoV-2 after completion of the vaccination schedule.74 In a Chilean clinical trial, 45 (1.99%) of 2263 subjects developed breakthrough infections following two-dose CoronaVac. The GMT of neutralizing antibodies against wild-type strains in the breakthrough cases was about 4-fold lower than that in CoronaVac recipients without COVID-19 (5.4 [95%CI, 2.5–11.6] versus 28.5 [95%CI, 15.0–54.6]) (Figure 1c).75
At one month after the third dose (given at approximately 5 months after 2 doses), the median titers of IgG-S (glycoprotein) and IgG-N (nucleocapsid protein) increased by 1.7- and 1.8-fold compared to one month after the second dose respectively.76 Neutralizing antibody titers against wild-type virus dropped significantly below the seropositivity cutoff value (a titer of 8) at 6 months after two doses and a third dose (given at 8 months after dose 2) recalled specific immune responses to SARS-CoV-2 in healthy adults aged 18–59 years and elderly aged 60 and over (Figure 1b).40 Compared with wild-type strains, neutralizing titers against delta and omicron were 3.3-fold and 16.5-fold lower at 28 days after three doses of CoronaVac, respectively. Three-dose CoronaVac recipients had significantly higher seroconversion rates (defined as the geometric mean half-maximal neutralizing titers >8 after vaccination) of neutralizing antibody against omicron variants, compared with the two-dose vaccine regimen (95% versus 0%).77 In addition, as shown in the meeting materials reported on 25 October 2021 by WHO, neutralizing antibody titer against wild-type virus (GMT: 50) of 6 months after the third dose was comparable to the peak of two-dose immunization (GMT: 48.4).39
Discussion
Our review showed that a two-dose regimen of CoronaVac conferred 67.7% protection against symptomatic COVID-19. Effectiveness of two doses waned with age and time after vaccination and was better than a single dose. A third dose inherited the effectiveness against non-variants of concern and increased effectiveness against severe COVID-19 outcomes caused by omicron variants compared to two doses. Effectiveness in preventing hospitalizations, ICU admissions, and deaths was more prominent than that in preventing COVID-19. Most of the adverse reactions following CoronaVac were mild. A two-dose regimen demonstrated a better immunogenicity profile in healthy populations compared to that in patients with autoimmune rheumatic diseases, kidney transplant recipients, patients with cancer, or individuals exposed to air pollution. A third dose enhanced immune responses to omicron variants as compared to two-dose CoronaVac.
Although two-dose CoronaVac met the 50% or more efficacy requirements approved for emergency use by the WHO,10 and the probability of true vaccine efficacy greater than 30% exceeded the minimum FDA criteria for authorization,78 CoronaVac was less effective in preventing infection with SARS-CoV-2 than in preventing severe COVID-19 outcomes. Therefore, the efficacy of CoronaVac to prevent SARS-CoV-2 virus into the human body needs to be improved. This also suggested that non-vaccine interventions are still needed for people vaccinated with CoronaVac.
Of note, humoral immunity is integral but probably not unique to the prevention of COVID-19. On the one hand, the poor performance of neutralizing antibodies in breakthrough cases relative to vaccine-protected individuals suggested that neutralizing antibodies may be indispensable in the prevention of COVID-19. On the other hand, vaccination with CronaVac could reduce the risk of severe illness or death after infection with Omicron, and even one dose was effective. However, none of the serum specimens after two-dose regimen showed neutralizing antibody seroconversion against omicron, implying the possibility that humoral immunity may represent not all protection against severe COVID-19 outcomes. It is likely that there are other potential factors worth exploring that can resist the invasion of the Omicron variant in the human body.
Surprisingly, Hitchings et al found in Brazil that vaccinated healthcare workers were more susceptible to gamma virus infection than unvaccinated individuals shortly after the first dose of CoronaVac.23 However, this conclusion was only for one dose and less than 14 days after vaccination, after all, it took time for neutralizing antibodies to develop after vaccination. Importantly, in their study, healthcare workers who prioritize vaccinations had a slightly higher rate of previous positive RT-PCR or antigen tests, meaning that vaccinated individuals were at higher risk of exposure than unvaccinated individuals. There was also a reason that previous natural infections in Brazil may have provided protection for unvaccinated individuals.23
Adverse reactions/events varied across studies due to differences in study protocols including different sample sizes. There was approximately a four-fold difference in total adverse reactions/events between the Turkish and Brazilian phase 3 trials. Uniform standards are needed for the collection of safety data before clinical trials. Also, few vaccine-related serious adverse reactions were reported in studies with large sample sizes, suggesting that surveillance for serious adverse reactions needs to be intensified during the trial process. Some cases, such as cutaneous allergic reactions, reactive arthritis, and thyroiditis, although rarely reported in clinical trials or real-world studies, need to be vigilant.
Findings of immunogenicity in special populations indicated that disease status, environmental pollution and previous COVID-19 infection that need to be considered in future CoronaVac strategies. Moreover, the durability of the antibody response induced by the third dose requires further observation. Germline origin, evolution and decay of dominant neutralizing antibodies following CoronaVac vaccination needs further study.
Currently, it is not clear what level of antibody titer can ensure protection from SARS-CoV-2. The weakening of antibody responses over time is a natural process of humoral immunity. In the future, for individuals who have been vaccinated with two doses of CoronaVac as primary immunization, the third booster immunization is recommended. For individuals with weakened immunity, new immunization schemes, such as heterologous prime-boost immunization, can be considered. Additionally, continuous monitoring for SARS-CoV-2 variants is warranted.
There are some limitations in this review. Firstly, we did not review heterologous prime-boost studies, although we included the effectiveness of a homologous prime-boost with CoronVac. Secondly, we summarized the effectiveness of CoronaVac in the adult and geriatric populations, but not in the pediatric population, which was rarely reported. Thirdly, we did not directly compare the results of humoral immune responses in different studies due to different test kits used. Finally, we summarized the efficacy, safety, and immunogenicity of CoronaVac without comparing it to other vaccine candidates. However, this review of CoronaVac may provide a scientific basis for optimizing global immunization strategies.
Funding Statement
This work was supported by the key project of science and technology plan of Jiangsu Province, China [grant number: BE2021738].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.WHO . WHO’s COVID-19 dashboard. [accessed 2022 Mar 18]. https://covid19.who.int/.
- 2.Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):1–13. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184–1191. doi: 10.1080/21645515.2015.1017694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strategic Advisory Group of Experts on Immunization - SAGE (WHO) . Evidence assessment: sinovac/CoronaVac COVID-19 vaccine. [Reported 2021 Apr 29. https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf.
- 6.Afkhami S, D’Agostino MR, Zhang A, Stacey HD, Marzok A, Kang A, Singh R, Bavananthasivam J, Ye G, Luo X, et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185(5):896–915.e19. doi: 10.1016/j.cell.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallapaty S. China’s COVID vaccines have been crucial - now immunity is waning. Nature. 2021;598(7881):398–399. doi: 10.1038/d41586-021-02796-w. [DOI] [PubMed] [Google Scholar]
- 8.Jantarabenjakul W, Chantasrisawad N, Puthanakit T, Wacharapluesadee S, Hirankarn N, Ruenjaiman V, Paitoonpong L, Suwanpimolkul G, Torvorapanit P, Pradit R, et al. Short-term immune response after inactivated SARS-CoV-2 (CoronaVac®, Sinovac) and ChAdox1 nCov-19 (Vaxzevria®, Oxford-AstraZeneca) vaccinations in health care workers. Asian Pac J Allergy Immunol. 2021. doi: 10.12932/AP-250721-1197. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.WHO . Interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac. [Reported 2021 Oct 21]. https://apps.who.int/iris/handle/10665/346788.
- 10.WHO . Vaccine efficacy, effectiveness and protection. [Reported 2021 July 14]. https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection.
- 11.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palacios R, Batista AP, Albuquerque CSN, Patiño EG, Santos JP, Conde MTRP, Piorelli RO, Júnior LCP, Raboni SM, Ramos F, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: the PROFISCOV study. [Posted 2021 Apr 11]. SSRN. https://ssrn.com/abstract=3822780.
- 13.Fadlyana E, Rusmil K, Tarigan R, Rahmadi AR, Prodjosoewojo S, Sofiatin Y, Khrisna CV, Sari RM, Setyaningsih L, Surachman F, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: an interim analysis in Indonesia. Vaccine. 2021;39(44):6520–6528. doi: 10.1016/j.vaccine.2021.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strategic Advisory Group of Experts on Immunization-SAGE (WHO) . Working group on COVID-19 vaccines. Evidence assessment: Sinovac/CoronaVac COVID-19 vaccine. [Reported 2021 Apr 29]. https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf.
- 15.Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevinc SA, Metin S, Basi NB, Ling J, Cinar AS, Oba S. Effectiveness of inactivated SARS-CoV-2 vaccine (CoronaVac) on intensive care unit survival. Epidemiol Infect. 2022;150:e35. doi: 10.1017/S0950268822000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, Pearson CAB, Russell TW, Tully DC, Washburne AD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538):eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Can G, Acar HC, Aydin SN, Balkan II, Karaali R, Budak B, Saltoglu N. Waning effectiveness of CoronaVac in real life: a retrospective cohort study in health care workers. Vaccine. 2022;40(18):2574–2579. doi: 10.1016/j.vaccine.2022.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DDS, Mishra S, Crispim MAE, Sales FCS, Hawryluk I, McCrone JT, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372(6544):815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranzani OT, Hitchings MDT, Dorion M, D’-Agostini TL, de Paula RC, de Paula OFP, Villela EFM, Torres MSS, de Oliveira SB, Schulz W, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374(2015). doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerqueira-Silva T, Oliveira VA, Boaventura VS, Pescarini JM, Júnior JB, Machado TM, Flores-Ortiz R, Penna GO, Ichihara MY, de Barros JV, et al. Influence of age on the effectiveness and duration of protection of Vaxzevria and CoronaVac vaccines: a population-based study. Lancet Reg Health Am. 2022;6:100154. doi: 10.1016/j.lana.2021.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serrano-Coll H, Miller H, Guzmán C, Rivero R, Gastelbondo B, Miranda J, Galeano K, Montaña-Restrepo J, Mattar S. Effectiveness of the CoronaVac® vaccine in a region of the Colombian Amazon, was herd immunity achieved? Trop Dis Travel Med Vaccines. 2022;8(1):2. doi: 10.1186/s40794-021-00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hitchings MDT, Ranzani OT, Torres MSS, de Oliveira SB, Almiron M, Said R, Borg R, Schulz WL, de Oliveira RD, da Silva PV, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Health Am. 2021;1:100025. doi: 10.1016/j.lana.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the delta variant B.1.617.2 COVID-19. Clin Pract. 2021;11(4):778–784. doi: 10.3390/clinpract11040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suah JL, Husin M, Keng PS, Hwa B, Thevananthan T, Low EV, Appannan MR, Zin FM, Zin SM, Yahaya H, et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study. Int J Infect Dis. 2022;S1201-9712(22):00167–9. doi: 10.1016/j.ijid.2022.03.028. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerqueira-Silva T, Katikireddi SV, de Araujo Oliveira V, Flores-Ortiz R, Júnior JB, Paixão ES, Robertson C, Penna GO, Werneck GL, Barreto ML, et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med. 2022;28(4):838–843. doi: 10.1038/s41591-022-01701-w. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvard Medical School . As coronavirus continues to spread, many questions and answers. [Reported 2022 Jan 6]. https://web.archive.org/web/20220111054542/https://www.health.harvard.edu/diseases-and-conditions/coronavirus-resource-center.
- 28.McMenamin ME, Nealon J, Lin Y, Wong YJ, Cheung JK, Lau EHY, Wu P, Leung GM, Cowling BJ. Vaccine effectiveness of two and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong. Medrxiv. 2022. preprint. doi: 10.1101/2022.03.22.22272769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, Al Khatib HA, Coyle P, Ayoub HH, Al Kanaani Z, et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med. 2021;385(24):e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Iketani S, Guo Y, Chan JF, Wang M, Liu L, Luo Y, Chu H, Huang Y, Nair MS, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 31.WHO . Covid-19 vaccine effectiveness assessment in Chile. [Reported 2021 Oct 25]. https://cdn.who.int/media/docs/default-source/blue-print/chile_rafael-araos_who-vr-call_25oct2021.pdf?sfvrsn=7a7ca72a_7.
- 32.CDC . Understanding adverse events and side effects. [Reported 2021 Mar 30]. https://www.cdc.gov/vaccinesafety/ensuringsafety/sideeffects/index.html.
- 33.Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, Yuki EFN, Pedrosa T, Fusco SRG, Rojo PT, Pereira RMR, Shinjo SK, Andrade DCO, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27(10):1744–1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 34.Esquivel-Valerio JA, Skinner-Taylor CM, Moreno-Arquieta IA, Cardenas-de la Garza JA, Garcia-Arellano G, Gonzalez-Garcia PL, Almaraz-Juarez FDR, Galarza-Delgado DA. Adverse events of six COVID-19 vaccines in patients with autoimmune rheumatic diseases: a cross-sectional study. Rheumatol Int. 2021;41(12):2105–2108. doi: 10.1007/s00296-021-05017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karacin C, Eren T, Zeynelgil E, Imamoglu GI, Altinbas M, Karadag I, Basal FB, Bilgetekin I, Sutcuoglu O, Yazici O, et al. Immunogenicity and safety of the CoronaVac vaccine in patients with cancer receiving active systemic therapy. Future Oncol. 2021;17(33):4447–4456. doi: 10.2217/fon-2021-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, Chen HX. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021;20(7):891–898. doi: 10.1080/14760584.2021.1925112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Supangat SE, Nugraha MY, Qodar TS, Mulyono BW, Tohari AI. COVID-19 vaccines programs: adverse events following immunization (AEFI) among medical clerkship student in Jember, Indonesia. BMC Pharmacol Toxicol. 2021;22(1):58. doi: 10.1186/s40360-021-00528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, Klugar M. Prevalence and risk factors of CoronaVac side effects: an independent cross-sectional study among healthcare workers in Turkey. J Clin Med. 2021;10(12):2629. doi: 10.3390/jcm10122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO . Updated safety & immunogenicity of 3rd dose CoronaVac® (Sinovac). [Reported 2021 Oct 25]. https://cdn.who.int/media/docs/default-source/blue-print/developers_sinovac_who-vr-call_25oct2021.pdf?sfvrsn=1aabe8d8_7.
- 40.Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, Wu Z, Jiang D, Deng X, Chu K, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022;22(4):483–495. doi: 10.1016/S1473-3099(21)00681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durmaz K, Aykut Temiz S, Metin Z, Dursun R, Abdelmaksoud A. Allergic and cutaneous reactions following inactivated SARS-CoV-2 vaccine (CoronaVac®) in healthcare workers. Clin Exp Dermatol. 2022;47(1):171–173. doi: 10.1111/ced.14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akdaş E, İ̇lter N, Öğüt B, Erdem Ö. Pityriasis rosea following CoronaVac COVID-19 vaccination: a case report. J Eur Acad Dermatol Venereol. 2021;35(8):e491–e493. doi: 10.1111/jdv.17316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akdaş E, Öğüt B, Erdem Ö, Öztaş MO, İ̇lter N. Cutaneous reactions following CoronaVac COVID-19 vaccination: a case series of six healthcare workers from a single centre. J Eur Acad Dermatol Venereol. 2021;35(12):e861–e864. doi: 10.1111/jdv.17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An QJ, Qin DA, Pei JX. Reactive arthritis after COVID-19 vaccination. Hum Vaccin Immunother. 2021;17(9):2954–2956. doi: 10.1080/21645515.2021.1920274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.İ̇remli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106(9):2600–2605. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saygılı ES, Karakilic E. Subacute thyroiditis after inactive SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14(10):e244711. doi: 10.1136/bcr-2021-244711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leber HM, Sant’Ana L, Konichi da Silva NR, Raio MC, Tjmm M, Endo CM, Nascimento H, de Souza CE. Acute thyroiditis and bilateral optic neuritis following SARS-CoV-2 vaccination with CoronaVac: a case report. Ocul Immunol Inflamm. 2021;29(6):1200–1206. doi: 10.1080/09273948.2021.1961815. [DOI] [PubMed] [Google Scholar]
- 48.Rattanawong W, Akaratanawat W, Tepmongkol S, Chutinet A, Tantivatana J, Suwanwela NC. Acute prolonged motor aura resembling ischemic stroke after COVID -19 vaccination (CoronaVac): the first case report. J Headache Pain. 2021;22(1):93. doi: 10.1186/s10194-021-01311-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chuaychoosakoon C, Parinyakhup W, Tanutit P, Maliwankul K, Klabklay P. Shoulder injury related to Sinovac COVID-19 vaccine: a case report. Ann Med Surg (Lond). 2021;68:102622. doi: 10.1016/j.amsu.2021.102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soysal A, Gönüllü E, Karabayır N, Alan S, Atıcı S, Yıldız İ, Engin H, Çivilibal M, Karaböcüoğlu M. Comparison of immunogenicity and reactogenicity of inactivated SARS-CoV-2 vaccine (CoronaVac) in previously SARS-CoV-2 infected and uninfected health care workers. Hum Vaccin Immunother. 2021;17(11):3876–3880. doi: 10.1080/21645515.2021.1953344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banaszkiewicz A, Radzikowski A. Efficacy, effectiveness, immunogenicity–are not the same in vaccinology. World J Gastroenterol. 2013;19(41):7217–7218. doi: 10.3748/wjg.v19.i41.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, Woodworth KR, Nahabedian JF, 3rd, Azziz-Baumgartner E, Gilboa SM, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soysal A, Bilazer C, Gönüllü E, Barın E, Çivilibal M. Cord blood antibody following maternal SARS-CoV-2 inactive vaccine (CoronaVac) administration during the pregnancy. Hum Vaccin Immunother. 2021;17(10):3484–3486. doi: 10.1080/21645515.2021.1947099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maertens K, De Schutter S, Braeckman T, Baerts L, Van Damme P, De Meester I, Leuridan E. Breastfeeding after maternal immunisation during pregnancy: providing immunological protection to the newborn: a review. Vaccine. 2014;32(16):1786–1792. doi: 10.1016/j.vaccine.2014.01.083. [DOI] [PubMed] [Google Scholar]
- 55.Calil VMLT, Palmeira P, Zheng Y, Krebs VLJ, Carvalho WB, Carneiro-Sampaio M. CoronaVac can induce the production of anti-SARS-CoV-2 IgA antibodies in human milk. Clinics (Sao Paulo). 2021;76:e3185. doi: 10.6061/clinics/2021/e3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hilbrands LB, Duivenvoorden R, Vart P, Franssen CFM, Hemmelder MH, Jager KJ, Kieneker LM, Noordzij M, Pena MJ, Vries H, et al. COVID-19-Related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Combe C, Kirsch AH, Alfano G, Luyckx VA, Shroff R, Kanbay M, van der Sande F, Basile C, EUDIAL Working Group of the ERA-EDTA . At least 156 reasons to prioritize COVID-19 vaccination in patients receiving in-centre haemodialysis. Nephrol Dial Transplant. 2021;36(4):571–574. doi: 10.1093/ndt/gfab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eren Sadioğlu R, Demir E, Evren E, Aktar M, Şafak S, Artan AS, Meşe S, Ağaçfidan A, Çınar G, Önel M, et al. Antibody response to two doses of inactivated SARS-CoV-2 vaccine (CoronaVac) in kidney transplant recipients. Transpl Infect Dis. 2021;23(6):e13740. doi: 10.1111/tid.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garassino MC, Vyas M, de Vries EGE, Kanesvaran R, Giuliani R, Peters S. European society for medical oncology. The ESMO call to action on COVID-19 vaccinations and patients with cancer: vaccinate. Monitor Educate Ann Oncol. 2021;32(5):579–581. doi: 10.1016/j.annonc.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yalçın TY, Topçu DI, Doğan Ö, Aydın S, Sarı N, Erol Ç, Kuloğlu ZE, Azap ÖK, Can F, Arslan H. Immunogenicity after two doses of inactivated virus vaccine in healthcare workers with and without previous COVID-19 infection: prospective observational study. J Med Virol. 2022;94(1):279–286. doi: 10.1002/jmv.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayram A, Demirbakan H, Günel Karadeniz P, Erdoğan M, Koçer I. Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in healthcare workers. J Med Virol. 2021;93(9):5560–5567. doi: 10.1002/jmv.27098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ulhaq ZS, Soraya GV, Indriana K, Devitasari R, Pradiptha IPY, Zulfikar DB, Uxiana V, Zulkarnain, Rachma LN, Arisanti D. The level of Ig anti-RBD SARS-CoV-2 after two doses of CoronaVac vaccine. J Med Virol. 2021. doi: 10.1002/jmv.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Şenol Akar Ş, Akçalı S, Özkaya Y, Gezginci FM, Cengiz Özyurt B, Deniz G, Karadağ Yalçın F, Özer D, Dündar Erbay P, Eser E, et al. Factors affecting side effects, seroconversion rates and antibody response after inactivated SARS-CoV-2 Vaccination in healthcare workers. Mikrobiyol Bul. 2021;55(4):519–538. Turkish. doi: 10.5578/mb.20219705. [DOI] [PubMed] [Google Scholar]
- 64.Lin X, Xu X, Zeng X, Xu L, Zeng Z, Huo X. Decreased vaccine antibody titers following exposure to multiple metals and metalloids in e-waste-exposed preschool children. Environ Pollut. 2017;220(Pt A):354–363. doi: 10.1016/j.envpol.2016.09.071. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Y, Xie J, Huang F, Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Travaglio M, Yu Y, Popovic R, Selley L, Leal NS, Martins LM. Links between air pollution and COVID-19 in England. Environ Pollut. 2021;268(Pt A):115859. doi: 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang S, Chen S, Xiao G, Zhao M, Li J, Dong W, Hu J, Yuan T, Li Y, Liu L. The associations between air pollutant exposure and neutralizing antibody titers of an inactivated SARS-CoV-2 vaccine. Environ Sci Pollut Res Int. 2022;29(9):13720–13728. doi: 10.1007/s11356-021-16786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mistry P, Barmania F, Mellet J, Peta K, Strydom A, Viljoen IM, James W, Gordon S, Pepper MS. SARS-CoV-2 variants, vaccines, and host immunity. Front Immunol. 2022;12:809244. doi: 10.3389/fimmu.2021.809244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Shen H, Huang R, Tong X, Wu C. Serum neutralising activity against SARS-CoV-2 variants elicited by CoronaVac. Lancet Infect Dis. 2021;21(8):1071–1072. doi: 10.1016/S1473-3099(21)00287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Ma Y, Xu Y, Liu J, Li X, Chen Y, Chen Y, Xie J, Xiao L, Xiang Z, et al. Resistance of SARS-CoV-2 Omicron variant to convalescent and CoronaVac vaccine plasma. Emerg Microbes Infect. 2022;11(1):424–427. doi: 10.1080/22221751.2022.2027219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu L, Mok BW, Chen LL, Chan JM, Tsang OT, Lam BH, Chuang VW, Chu AW, Chan WM, Ip JD, et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin Infect Dis. 2021;ciab1041. doi: 10.1093/cid/ciab1041. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Souza WM, Amorim MR, Sesti-Costa R, Coimbra LD, Brunetti NS, Toledo-Teixeira DA, de Souza GF, Muraro SP, Parise PL, Barbosa PP, et al. Neutralisation of SARS-CoV-2 lineage P.1 by antibodies elicited through natural SARS-CoV-2 infection or vaccination with an inactivated SARS-CoV-2 vaccine: an immunological study. Lancet Microbe. 2021;2(10):e527–e535. doi: 10.1016/S2666-5247(21)00129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernández J, Bruneau N, Fasce R, Martín HS, Balanda M, Bustos P, Ulloa S, Mora J, Ramírez E. Neutralization of alpha, gamma, and D614G SARS-CoV-2 variants by CoronaVac vaccine-induced antibodies. J Med Virol. 2022;94(1):399–403. doi: 10.1002/jmv.27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, Schaefer-Babajew DJ, DaSilva J, Muecksch F, Gaebler C, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384(23):2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duarte LF, Gálvez NMS, Iturriaga C, Melo-González F, Soto JA, Schultz BM, Urzúa M, González LA, Vázquez Y, Ríos M, et al. Immune profile and clinical outcome of breakthrough cases after vaccination with an inactivated SARS-CoV-2 vaccine. Front Immunol. 2021;12:742914. doi: 10.3389/fimmu.2021.742914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keskin AU, Bolukcu S, Ciragil P, Topkaya AE. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J Med Virol. 2022;94(1):39–41. doi: 10.1002/jmv.27350. [DOI] [PubMed] [Google Scholar]
- 77.Wang K, Jia Z, Bao L, Wang L, Cao L, Chi H, Hu Y, Li Q, Zhou Y, Jiang Y, et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature. 2022;603(7903):919–925. doi: 10.1038/s41586-022-04466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Food and Drug Administration . Guidance for industry: emergency use authorization for vaccines to prevent COVID-19. [Reported 2021 May 25]. https://www.fda.gov/media/142749/download.


