Abstract
The coccidian parasite Cryptosporidium parvum causes diarrhea in humans, calves, and other mammals. Neither immunization nor parasite-specific pharmaceuticals that are consistently effective against this organism are available. While polyclonal antibodies against whole C. parvum reduce infection, their efficacy and predictability are suboptimal. We hypothesized that passive immunization against cryptosporidiosis could be improved by using neutralizing monoclonal antibodies (MAbs) targeting functionally defined antigens on the infective stages. We previously reported that the apical complex and surface-exposed zoite antigens CSL, GP25-200, and P23 are critical in the infection process and are therefore rational targets. In the present study, a panel of 126 MAbs generated against affinity-purified CSL, GP25-200, and P23 was characterized to identify the most efficacious neutralizing MAb formulation targeting each antigen. To identify neutralizing MAbs, sporozoite infectivity following exposure to individual MAbs was assessed by enzyme-linked immunosorbent assay. Of 126 MAbs evaluated, 47 had neutralizing activity. These were then evaluated individually in oocyst-challenged neonatal mice, and 14 MAbs having highly significant efficacy were identified for further testing in formulations. Epitope specificity assays were performed to determine if candidate MAbs recognized the same or different epitopes. Formulations of two or three neutralizing MAbs, each recognizing distinct epitopes, were then evaluated. A formulation of MAbs 3E2 (anti-CSL [αCSL]), 3H2 (αGP25-200), and 1E10 (αP23) provided highly significant additive efficacy over that of either individual MAbs or combinations of two MAbs and reduced intestinal infection by 86 to 93%. These findings indicate that polyvalent neutralizing MAb formulations targeting epitopes on defined antigens may provide optimal passive immunization against cryptosporidiosis.
Cryptosporidium parvum is a coccidian parasite that infects intestinal epithelium and causes diarrheal disease in humans and in calves and other agriculturally important food animals worldwide (13). Cryptosporidiosis is self-limiting in hosts with normal immune systems; however, in neonates, the elderly, and hosts having congenital or acquired immunodeficiency diseases or chemotherapy-induced immunosuppression, cryptosporidial enterocolitis may become chronic and have severe consequences (13, 35). The role of C. parvum in diarrhea-related morbidity in AIDS patients and its economic impact on livestock production are now well recognized (13). No approved parasite-specific drugs, vaccines, or immunotherapies for C. parvum are presently available, although recent advances have been reported (4, 8, 15, 17, 30, 32, 35, 41, 50, 57).
Because specific immune responses prevent or terminate cryptosporidiosis, passive immunization strategies for control of the disease in neonatal and immunodeficient hosts have been investigated (reviewed in references 8 and 35). In such hosts, suboptimal active immune responses increase susceptibility to primary infection and delay or prevent termination of established infection. In previous studies, bovine colostral antibody preparations produced against whole C. parvum organisms have demonstrated specific neutralizing activity in vitro and highly significant efficacy against infection in animal models when evaluated under controlled conditions (11, 12, 33, 34, 36, 51). The efficacy of such preparations in persistently infected immunodeficient humans has been demonstrated but has been inconsistent in a limited number of studies, due in part to confounding patient and treatment variables (8, 27, 29, 35, 52, 53). While these early observations provided the rationale to investigate passive antibody-based immunization for cryptosporidiosis, possible limitations to the use of polyclonal antibodies produced against uncharacterized whole C. parvum preparations include the relatively low content of specific neutralizing antibodies in the immunoglobulin fraction, logistical restraints on production in quantity, and lot-to-lot heterogeneity in therapeutic predictability (6, 35, 59). Alternatively, the use of neutralizing monoclonal antibodies (MAbs) prepared against functionally defined C. parvum antigens may circumvent each of these factors (6, 35, 59).
We hypothesize that the efficacy of passive immunization against cryptosporidiosis can be optimized through use of a polyvalent neutralizing MAb formulation recognizing zoite antigens known to have a critical role in the infection process. We reasoned that specific and selective targeting of distinct functional epitopes would result in an additive neutralizing effect with high specific activity. The rationale for this approach is that control of C. parvum infection will likely require targeting of multiple neutralization-sensitive epitopes on the infective zoite stages (8, 35, 57). An optimal formulation of neutralizing MAbs would be expected to control infection by binding to zoites within the intestinal lumen and preventing their attachment and invasion (22, 28, 35). Alternatively, zoites bound by MAbs while extracellular, but which retain the ability to invade, might undergo MAb-mediated arrest of intracellular development (22, 24, 28, 35). Therefore, effective neutralization of the infective stages could prevent initiation of the life cycle or interrupt and terminate the cycle in an existing infection. The C. parvum antigens GP25-200 (1, 39), CSL (39, 40), and P23 (1, 23) were selected as targets for the present study. Each antigen is involved in the pathogenesis of infection, expressed on the surface of both infective zoite stages, and conserved on diverse C. parvum isolates (1, 23, 31, 39, 40). GP25-200 was originally defined by MAb C4A1 as a sporozoite apical and surface pellicle glycoprotein complex comprised of multiple 25- to 200-kDa species identified in reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (1). P23, a 23-kDa sporozoite surface pellicle protein, was originally identified by MAb C6B6 (1, 23). Passive immunization with MAbs C4A1 and C6B6 demonstrated that GP25-200 and P23 each expressed a neutralization-sensitive epitope; however, the efficacy observed was suboptimal (1, 42). Therefore, a panel of second-generation MAbs was produced against immunoaffinity-purified GP25-200 (39) and P23 (42) to determine whether the antigens contain additional neutralization-sensitive epitopes that could be targeted to enhance efficacy. We recently reported that one of the resulting MAbs produced from GP25-200-immunized mice, designated 3E2, elicited the circumsporozoite precipitate (CSP)-like reaction (36, 39). This reaction, named after the malarial CSP reaction (7), is characterized by the progressive posterior formation and release of membranous antigen-MAb complexes, after which zoites are rendered noninfective (36, 39). MAb 3E2 recognizes multiple apical complex and surface pellicle glycoproteins ranging from 46 to 770 kDa and an ∼1,300-kDa glycoprotein designated CSL (39). Of the multiple antigens recognized by 3E2, CSL was shown to be the critical surface-exposed species mechanistically bound by MAb to elicit the CSP-like reaction (39). The neutralizing activity of MAb 3E2 in vitro and its ability to passively protect against infection in vivo (19, 39) are profoundly greater than those of other MAbs we have produced against C. parvum (31, 33, 37, 38, 42). Therefore, MAb 3E2 was deemed an essential formulation candidate in the present study.
Because the large number of candidate MAbs requiring characterization added logistical complexities to the experimental design, a strategy was used to systematically determine the minimum number and specificity of MAbs required for protection. Following identification and ranking of individual MAbs having sporozoite neutralizing activity in vitro and the ability to reduce infection in vivo, epitope specificity assays were performed to select combinations of MAbs for further testing in multiple epitope-specific formulations with 3E2. The optimal formulation identified, comprised of MAbs 3E2 (anti-CSL [αCSL]), 3H2 (αGP25-200), and 1E10 (αP23), conferred highly significant additive protection over that of the individual component MAbs or combinations of two component MAbs. Further, this formulation provided complete protection in up to 40% of the treated mice, resulting in an overall 93% reduction of intestinal infection after oocyst challenge. The results indicate that polyvalent neutralizing MAb formulations selectively targeting epitopes on defined antigens may provide optimal passive immunization against cryptosporidiosis.
MATERIALS AND METHODS
Oocyst and sporozoite isolation.
The Iowa C. parvum isolate (16) used in all experiments was maintained by passage in newborn Cryptosporidium-free Holstein bull calves (37). Oocysts were isolated from calf feces by sucrose density gradient centrifugation (2), stored in 2.5% (wt/vol) KCr2O7 (4°C), and hypochlorite treated immediately prior to excystation in WRC medium (Gibco Life Technologies, Grand Island, N.Y.) containing 0.75% (wt/vol) taurocholic acid (37). Sporozoites were isolated by passage through a sterile polycarbonate filter (2.0-μm pore size) (19). For mouse challenge experiments, oocysts were used within 30 days of isolation and disinfected with 1% (vol/vol) peracetic acid instead of hypochlorite (36).
MAb production.
Immunoaffinity chromatography purification of GP25-200 and CSL from C. parvum sporozoites and their use for production of a mouse MAb panel against these antigens have been previously described (39). P23 was purified from sporozoites by MAb C6B6 affinity chromatography for production of a panel of second-generation αP23 MAbs. MAb C6B6 was isolated by protein A affinity chromatography and then coupled to protein A matrix according to the protocol of the manufacturer (Bio-Rad, Hercules, Calif.). After conditions for P23 binding and elution were optimized, preparative purification was performed (4°C for all steps). Sporozoites were solubilized in binding buffer (phosphate-buffered saline [PBS] containing 5 mM EDTA, 5 mM iodoacetamide, 0.1 mM N-α-p-tosyl-l-lysine chloromethyl ketone [TLCK], 1 mM phenylmethylsulfonyl fluoride, and 1% [wt/vol] octyl glucoside), ultracentrifuged to remove insoluble material, and then bound to C6B6-coupled matrix. The matrix was washed with binding buffer (40 column volumes), after which specifically bound antigen was eluted (0.1 M glycine, pH 10), immediately neutralized (0.1 M Tris-HCl, pH 6.8), and then dialyzed (12- to 14-kDa exclusion limit) against PBS and stored at −80°C until used. The identity, purity, and reactivity of purified P23 were determined by SDS-PAGE with silver staining and Western blotting (39) using MAb C6B6. The protein concentration in the purified P23 preparation was determined by bicinchoninic acid assay (Pierce, Rockford, Ill.).
Adult female BALB/c mice (Harlan-Sprague-Dawley, Indianapolis, Ind.) were immunized by the intraperitoneal and subcutaneous routes with purified P23 (2 μg/mouse) emulsified in monophosphoryl A-trehalose dimycolate adjuvant (Ribi, Hamilton, Mont.). Mice were boosted (1 μg of P23 in adjuvant per mouse) at 7 and 11 weeks following the initial immunization. Five weeks after the last boost, mice received a final immunization by tail vein injection (1.5 μg of P23 in PBS/mouse). Three days later, spleen cells were harvested and fused with SP2/0 myeloma cells (37). Supernatants were screened for sporozoite- and merozoite-reactive antibodies by immunofluorescence assay (IFA) to identify positive hybridomas (1, 37). Commercially available kits were used to determine MAb isotypes (Isostrip; Roche Molecular Biochemicals, Indianapolis, Ind.) and concentrations (Binding Site, San Diego, Calif.).
Immunoblot analysis of antigen specificities of MAbs.
Excysted sporozoites were solubilized (4°C) in lysis buffer [50 mM Tris (pH 8.0), 5 mM EDTA, 5 mM 4-(2-aminoethyl)-benzene-sulfonylfluoride hydrochloride (AEBSF), 0.3 mM aprotinin, 10 mM E-64, 0.01 mM leupeptin, 30 mM bestatin, and 1% (wt/vol) octyl glucoside], boiled in reducing SDS-PAGE sample buffer, resolved in 4 to 20% and 2 to 12% gradient SDS-PAGE reducing gels, and Western blotted as previously described (36, 39). Blots were probed with culture supernatants containing MAbs generated against C. parvum antigens or isotype-matched control MAbs of irrelevant specificity (4 μg/ml for immunoglobulin M [IgM] and 10 μg/ml for IgG). Bound MAb was detected with affinity-purified phosphatase-conjugated goat anti-mouse IgG, IgA, and IgM (Kirkegaard and Perry, Gaithersburg, Md.) and phosphatase substrate. Selected MAbs generated against P23 were also evaluated by dot blot assay for reactivity with the recombinant P23 protein rC7 (31, 32). In brief, a nitrocellulose membrane was dotted with rC7 (15 μg/well) and probed with αP23 MAb, MAbs C6B6 and 7D10 reactive with distinct rC7 epitopes (31), or isotype-matched control MAb (11 μg/ml for IgM and 25 μg/ml for IgG). Specifically bound MAb was detected as described above.
ELISA identification of sporozoite-neutralizing MAbs.
To identify neutralizing MAbs and quantitate their specific activities, the infectivity of sporozoites following exposure to individual MAbs was assessed by enzyme-linked immunosorbent assay (ELISA). Purified sporozoites (1.2 × 105) were incubated (15 min, 37°C) with C. parvum-specific MAb or isotype-matched control MAb (11 μg/ml, final concentration) and then inoculated onto individual MDBK cell monolayers (CCL 22; American Type Culture Collection, Manassas, Va.) (10 replicates/treatment). Prior to inoculation, monolayers had been grown to ∼90% confluency in 96-well plates using fortified maintenance medium (55). Control monolayers were inoculated either with fortified maintenance medium or with sporozoites treated with (i) known neutralizing αGP25-200 MAb 4E11 or (ii) known nonneutralizing α-C. parvum CPS-500 MAb G10F5. After incubation (1.5 h, 37°C), the inoculation medium was aspirated and replaced with fortified maintenance medium. At 48 h postinoculation (p.i.), ELISA plates were centrifuged (1,310 × g, 6 min, 4°C), after which monolayers were methanol fixed, washed, blocked (Tris-buffered saline containing 3.2% [vol/vol] fish gelatin and 0.5% [wt/vol] bovine serum albumin [BSA]), and probed with MAb 3E2 (15 μg/ml in Tris-buffered saline). MAb 3E2 recognizes C. parvum stages in MDBK cells through at least 72 h p.i. (19, 42). After additional washing, plates were incubated with affinity-purified alkaline phosphatase-conjugated goat anti-mouse IgM (μ-chain specific; Cappel-Organon Teknika, Durham, N.C.), washed, and developed by addition of substrate. The optical density (OD) at 405 nm was determined with an ELISA plate reader to quantitate infection levels. Mean ODs for each treatment and control group were examined for significant differences by Student's one-tailed t test. Each experiment was performed a minimum of two times.
Evaluation of sporozoite-neutralizing MAbs for efficacy in vivo.
MAbs having in vitro neutralizing activity were produced in ascites fluid and evaluated individually for efficacy against oocyst challenge in neonatal mice (described below) to identify and rank MAbs for subsequent formulation testing. The most efficacious MAbs were then produced by growing hybridomas in bioreactors (CellMax Artificial Capillary Cell Culture System; Cellco, Germantown, Md.) using serum-free medium (Gibco Life Technologies). Bioreactor-derived MAbs were dialyzed (12- to 14-kDa exclusion limit) against PBS (4°C) and stored at −20°C until tested in mice. To assess efficacy in vivo, groups of 10 to 15 8-day-old specific-pathogen-free ICR mice (Harlan Sprague-Dawley) were administered, by gastric intubation, 3 × 104 oocysts (30 times the 50% mouse infective dose [MID50]) concurrently with individual MAbs in ascites fluid (1 mg of MAb/ml, 75 μl) or 5 × 104 oocysts (50 times the MID50) concurrently with individual or combined MAbs from bioreactor supernatants (1.5 to 4 mg of each MAb/ml, 75 μl) (37). At 3 h and every 12 h thereafter, mice received additional ascites fluid (1 mg of MAb/ml, 100 μl) or bioreactor-derived MAb (1.5 to 4 mg of each MAb/ml, 100 μl) by gastric intubation for a total of nine treatments. Cimetidine (10 mg/kg) was included with all treatments. Groups of 10 to 15 8-day-old control mice were treated identically with ascites fluid or processed bioreactor supernatant containing isotype- and concentration-matched control MAbs. After euthanasia at 92 to 94 h p.i., the jejunum, ileum, cecum, and colon were collected, coded, and examined histologically by the same investigator, without knowledge of treatment group, for C. parvum stages in mucosal epithelium. Scores of 0, 1, 2, or 3 (0, no infection; 1, < 33% of mucosa infected; 2, 33 to 66% of mucosa infected; and 3, > 66% of mucosa infected) were assigned to longitudinal sections representing the entire length of the (i) terminal jejunum, (ii) ileum, (iii) cecum, and (iv) colon and then summed to obtain an infection score (0 to 12) for each mouse (37, 39). Mean infection scores within each experiment were analyzed by Student's one-tailed t test for significant differences. Infection scores between experiments were analyzed for significant differences by one-way analysis of variance stratified by treatment group (Stata Program; Stata Corporation, College Station, Tex.). To control for computing multiple one-way analyses of variance, a Bonferonii adjusted level of significance (P ≤ 0.002) was used.
In parallel experiments, sporozoites (2 × 108/ml) were incubated (1 to 20 min, 37°C) with bioreactor-derived individual or combined MAbs (each at 1 mg of MAb/ml in PBS containing 0.5% [wt/vol] BSA) and then examined by phase-contrast microscopy to determine the effect on sporozoite morphology (36).
Epitope characterization.
To determine if neutralizing MAbs recognized the same (similar or overlapping) or different epitopes, epitope composition analyses and binding inhibition assays were performed. The ability of MAbs to bind C. parvum antigen after treatment with sodium periodate or proteinase K was determined by immunoblot assay. Solubilized sporozoite antigen was resolved in 4 to 20% and 2 to 12% gradient SDS-PAGE gels, transferred to nitrocellulose, and then treated (1 h, 21°C) with sodium periodate (2, 5, and 7.5 mM) or control buffer (58). For protease treatment, antigen was incubated (1 h, 37°C) with or without proteinase K (5 × 10−3 U per 107 excysted oocysts) and dotted onto nitrocellulose after addition of protease inhibitors (38). Replicate blots from each treatment group were then incubated with MAbs generated against GP25-200, CSL, or P23; C. parvum peptide-reactive control MAb 4D10 (19); C. parvum carbohydrate-reactive control MAb 3E2 (39); or isotype-matched control MAb and then processed as described above to detect bound MAb.
The ability of MAbs to inhibit binding of each other to C. parvum was determined by ELISA using biotin-labeled and unlabeled MAbs (43). For biotinylation, bioreactor-derived MAb (2 mg/ml in PBS) was incubated with Sulfo-NHS-biotin (Pierce) according to the manufacturer's protocol. For use in ELISA plate preparation, sporozoites were solubilized in PBS containing 1% (wt/vol) octyl glucoside, 0.01% (wt/vol) thimerosal, and protease inhibitors (Sigma) by sonication (4°C) and freeze-thawing, ultracentrifuged to remove insoluble material, and dialyzed (3.5-kDa exclusion limit) against PBS (4°C). Immulon-4 96-well ELISA plates (Dynex, Chantilly, Va.) were coated (3 h, 37°C) with solubilized sporozoite antigen (5 × 105 sporozoites/well), washed, and blocked (in PBS containing 3.5% [vol/vol] fish gelatin and 0.5% [vol/vol] Tween 20). For binding inhibition assays, plates were incubated (2 h, 37°C) with individual unlabeled α-C. parvum MAbs (designated MAb-1; 1.5 μg in 100 μl of PBS per well) or isotype-matched control MAbs. Biotinylated α-C. parvum competitor MAb (designated MAb-2; 0.125 μg in 25 μl of PBS per well) was then added, and after incubation (2 h, 37°C) plates were washed, incubated with peroxidase-labeled streptavidin (Kirkegaard and Perry), and exposed to substrate. Mean ODs (405 nm) of six replicate wells for each treatment and control group were analyzed by Student's one-tailed t test for significant differences. Significance conclusions were verified in a replicate experiment.
RESULTS
Immunoaffinity-purified P23, GP25-200, and CSL contain multiple distinct epitopes defined by second-generation MAbs.
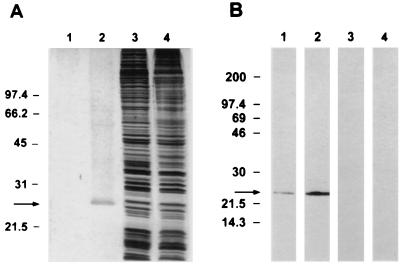
Antigen isolated by MAb C6B6 affinity chromatography comigrated with a 23-kDa antigen in sporozoites as demonstrated by SDS-PAGE and silver staining and was relatively free of contamination with other proteins (Fig. 1A). In Western blots, the purified antigen retained reactivity with MAb C6B6 and comigrated with the 23-kDa antigen in sporozoites recognized by C6B6 (Fig. 1B). Forty second-generation hybridomas were produced from P23-immunized mice in three fusions. Twenty-four resulting MAbs were selected for further study. Of these, 14 recognized a single 23-kDa sporozoite antigen in Western blots, which comigrated with that recognized by MAb C6B6 (Fig. 2, lanes 1 and 3), and 10 were unreactive in Western blots. αP23 MAb 1E10 and αP23 control MAbs C6B6 and 7D10 each reacted with rC7 in dot immunoblots, while αP23 MAbs 5B9, 4C11, and 2G6 did not (data not shown). In IFA, αP23 MAbs bound to the surface pellicle of zoites and also detected prominent antigen deposits demarcating the path traveled by motile zoites. Based on susceptibility to proteinase K and periodate, all 14 MAbs reactive with P23 in Western blots recognized peptide epitopes; 10 MAbs recognized epitopes of undetermined composition.
FIG. 1.
(A) Silver stained 10 to 20% gradient SDS-PAGE gel of solubilized sporozoites (107) before (lane 3), and after (lane 2) MAb C6B6 affinity chromatography purification of P23 (1 μg). Lane 4 contains solubilized oocysts (2.5 × 106) for comparison. Lane 1 was loaded with sample buffer to identify silver stain artifacts. Molecular mass standards (Bio-Rad) are indicated on the left (phosphorylase B, 97.4 kDa; BSA, 66.2 kDa; ovalbumin, 45 kDa; carbonic anhydrase, 31 kDa; and soybean trypsin inhibitor, 21.5 kDa). (B) Western blot recognition of affinity-purified P23 (lane 1) (1 μg) and a comigrating 23-kDa antigen in whole sporozoites (lane 2) (4 × 106) by MAb C6B6. Lane 3 (1 μg of P23) and lane 4 (4 × 106 sporozoites) were probed with isotype control MAb. Molecular mass standards (Amersham Pharmacia Biotech, Arlington Heights, Ill.) are indicated on the left (myosin, 200 kDa; β-galactosidase, 97.4 kDa; BSA, 69 kDa; carbonic anhydrase, 46 kDa; soybean trypsin inhibitor, 30 kDa; lysozyme, 21.5 kDa; and aprotinin, 14.3 kDa).
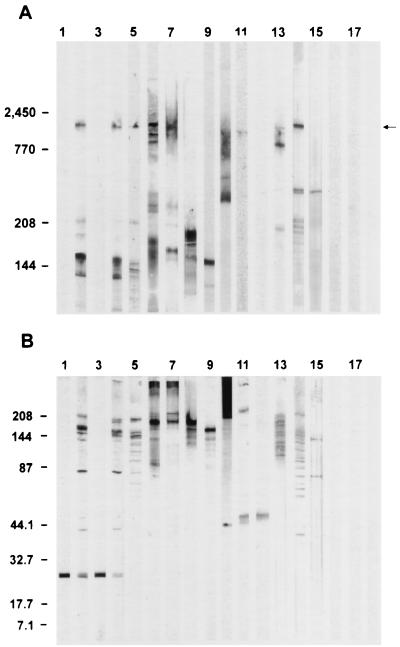
FIG. 2.
Western blot recognition of antigens from solubilized sporozoites (6 × 106/lane) resolved in 2 to 12% (A) or 4 to 20% (B) gradient SDS-PAGE by MAbs generated against immunoaffinity-purified P23 and GP25-200. Lane 1 was probed with MAb C6B6, originally used to define P23. A representative reactivity pattern of 14 second-generation αP23 MAbs is depicted in lane 3, probed with MAb 1E10. Lane 2 was probed with MAb C4A1, originally used to define GP25-200. Lanes 4 to 15 were probed with second-generation MAbs produced against GP25-200 and represent each of the 12 antigen recognition patterns into which MAbs were grouped. Depicted are reactivity patterns representative of MAbs 3H2 (lane 4; 22 MAbs), 4D10 (lane 5; 37 MAbs), 3E2 (lane 6; 6 MAbs), 1G2 (lane 7; 1 MAb), 3G7 (lane 8; 2 MAbs), 3D1 (lane 9; 4 MAbs), 4E11 (lane 10; 2 MAbs), 1C5 (lane 11; 4 MAbs), 3E8 (lane 12; 2 MAbs), 2F6 (lane 13; 19 MAbs), 4E12 (lane 14; 1 MAb), and 4F6 (lane 15; 2 MAbs). Note recognition of an ∼1,300-kDa antigen (arrow) comigrating with CSL (lane 6) by MAbs in lanes 2, 4, 5, 7, 10, 11, 13, and 14. Isotype-matched control MAbs were used to probe lanes 16 (IgM), 17 (IgG1), and 18 (IgG2a). Molecular mass standards are indicated on the left (myosin, 208 kDa; β-galactosidase, 144 kDa; BSA, 87 kDa; carbonic anhydrase, 44.1 kDa; soybean trypsin inhibitor, 32.7 kDa; lysozyme, 17.7 kDa; aprotinin, 7.1 kDa [Bio-Rad]; titin, 2,450 kDa, and nebulin, 770 kDa [obtained from K. Wang and G. Gutierrez, University of Texas, Austin]).
One hundred twelve second-generation hybridomas were produced against GP25-200 and CSL (39). One hundred two resulting MAbs which bound to the apical region and pellicle of zoites with heterogeneous IFA patterns were selected for further study. These MAbs also had heterogeneous reactivities in Western blots, represented by 12 distinct antigen recognition patterns into which MAbs could be grouped (Fig. 2). One group had a reactivity pattern indistinguishable from that of C4A1 (Fig. 2, lanes 2 and 4). Eleven additional groups recognized distinct subpopulations of antigens, most of which comprised the GP25-200 complex (Fig. 2, lanes 5 to 15). Of these, eight groups also recognized an ∼1,300-kDa antigen comigrating with CSL (Fig. 2A, lanes 4, 5, 6, 7, 10, 11, 13, and 14), and seven groups recognized antigens migrating between 200 and ∼1,300 kDa (Fig. 2A, lanes 5, 6, 7, 10, 13, 14, and 15). Five MAbs, designated αCSL, had Western blot reactivity patterns identical to that of 3E2 (Fig. 2, lane 6). All six αCSL MAbs recognized the same epitope and elicited the CSP-like reaction as previously described (39, 40). Based on susceptibility to proteinase K and periodate, 35 αGP25-200 MAbs and all 6 αCSL MAbs recognized carbohydrate or carbohydrate-dependent epitopes, 49 recognized peptide epitopes, and 12 recognized epitopes of undetermined composition.
A subset of individual second-generation MAbs neutralizes sporozoite infectivity in vitro and passively protects against oocyst challenge in vivo.
Initial screening of MAbs was performed by ELISA. MDBK host cells were used for this assay based on their epithelial origin, permissiveness to C. parvum, and widespread use in studies to assess infectivity of C. parvum (15, 25, 50, 54, 56). The ELISA allowed rapid identification of 33 αGP25-200 MAbs (Fig. 2, lanes 4, 5, 7, 10, 11, 12, 13, and 15) and 14 αP23 MAbs (Fig. 2, lane 3) which significantly reduced infection, and quantitation of their neutralizing activity against sporozoites, the first infective stage of C. parvum (Table 1) [representative MAbs are shown]). Sporozoite-neutralizing activity of αCSL MAbs has been reported previously (19, 39, 40).
TABLE 1.
Sporozoite neutralization by individual αGP25-200 and αP23 MAbs
| MAb | Antigen specificity | ELISA OD, mean ± SD (A405)
|
% Reduction in infectiona | P | |
|---|---|---|---|---|---|
| Test MAb | Isotype control MAb | ||||
| 3H2 | GP25-200 | 1.15 ± 0.10 | 1.25 ± 0.05 | 8 | <0.05 |
| 1B3 | GP25-200 | 0.73 ± 0.03 | 1.26 ± 0.07 | 42 | <0.0001 |
| 1C5 | GP25-200 | 0.57 ± 0.06 | 0.81 ± 0.09 | 30 | <0.001 |
| 1G2 | GP25-200 | 0.81 ± 0.14 | 1.04 ± 0.16 | 22 | <0.001 |
| 2F6 | GP25-200 | 0.77 ± 0.04 | 0.93 ± 0.06 | 17 | <0.001 |
| 3D9 | GP25-200 | 0.45 ± 0.03 | 0.92 ± 0.08 | 51 | <0.0001 |
| 3D11 | GP25-200 | 0.57 ± 0.19 | 0.77 ± 0.08 | 26 | <0.01 |
| 3E8 | GP25-200 | 0.39 ± 0.02 | 0.71 ± 0.03 | 45 | <0.0001 |
| 3F7 | GP25-200 | 0.56 ± 0.19 | 0.84 ± 0.07 | 33 | <0.001 |
| 4D10 | GP25-200 | 0.76 ± 0.07 | 0.84 ± 0.06 | 10 | <0.05 |
| 4E11 | GP25-200 | 0.69 ± 0.05 | 1.04 ± 0.07 | 34 | <0.0001 |
| 4F6 | GP25-200 | 1.09 ± 0.03 | 1.19 ± 0.07 | 8 | <0.005 |
| 4H7 | GP25-200 | 1.15 ± 0.10 | 1.28 ± 0.11 | 10 | <0.01 |
| 4H9 | GP25-200 | 0.61 ± 0.06 | 0.93 ± 0.08 | 34 | <0.0001 |
| 1A9 | P23 | 0.79 ± 0.06 | 0.87 ± 0.07 | 9 | <0.025 |
| 2G6 | P23 | 0.72 ± 0.05 | 1.26 ± 0.07 | 43 | <0.0001 |
| 3C10 | P23 | 0.68 ± 0.03 | 0.84 ± 0.06 | 19 | <0.0001 |
| 3D1 | P23 | 0.61 ± 0.05 | 0.70 ± 0.04 | 13 | <0.0025 |
| 4C11 | P23 | 0.81 ± 0.06 | 0.97 ± 0.03 | 16 | <0.0025 |
| 4E4 | P23 | 0.94 ± 0.08 | 1.29 ± 0.14 | 27 | <0.0025 |
| 5B9 | P23 | 0.68 ± 0.04 | 0.78 ± 0.06 | 13 | <0.001 |
[(Isotype control MAb OD − test MAb OD) ÷ isotype control MAb OD] × 100.
Individual αGP25-200 and αP23 neutralizing MAbs were next tested in vivo to identify the most efficacious candidates for formulation. While all six αCSL neutralizing MAbs recognized the same epitope, the in vivo efficacy of each was also determined because possible differences in their affinities could significantly influence neutralizing activity (26, 33, 59). Nine of 33 αGP25-200 MAbs (Fig. 2, lanes 4, 5, 10, 11, and 13), 4 of 14 αP23 MAbs (Fig. 2, lane 3, MAb 1E10; the remaining 3 MAbs were unreactive in Western blotting), and all 6 αCSL MAbs (Fig. 2, lane 6) significantly reduced intestinal infection when administered to oocyst-challenged neonatal mice (Table 2). Of the αCSL MAbs, 3E2 had the greatest efficacy in replicate experiments, confirming its selection as a requisite formulation component.
TABLE 2.
Quantitative in vivo efficacy of individual αGP25-200, αP23, and αCSL sporozoite-neutralizing MAbs against oocyst challenge (30 times the MID50)
| MAb/isotype | Antigen specificity | Infection score (mean ± SD)
|
% Reduction in infection | Pa | |
|---|---|---|---|---|---|
| Test MAb | Isotype control MAb | ||||
| 3H2/IgM | GP25-200 | 3.1 ± 0.9 | 9.2 ± 1.5 | 66 | <0.0001 |
| 1B3/IgG1 | GP25-200 | 6.4 ± 0.8 | 10.0 ± 0.0 | 36 | <0.0001 |
| 1C5/IgM | GP25-200 | 4.7 ± 0.9 | 9.2 ± 1.5 | 49 | <0.0001 |
| 2F6/IgG1 | GP25-200 | 4.6 ± 1.1 | 7.6 ± 0.6 | 39 | <0.0001 |
| 3D9/IgG1 | GP25-200 | 6.3 ± 0.9 | 10.0 ± 0.0 | 37 | <0.0001 |
| 3D11/IgG1 | GP25-200 | 6.4 ± 1.0 | 6.0 ± 1.3 | None | NSDb |
| 3F7/IgG1 | GP25-200 | 6.3 ± 1.4 | 6.0 ± 1.3 | None | NSD |
| 4D10/IgG1 | GP25-200 | 5.5 ± 1.3 | 7.6 ± 0.6 | 28 | <0.0025 |
| 4E11/IgM | GP25-200 | 5.8 ± 1.1 | 8.1 ± 0.9 | 28 | <0.0001 |
| 4H7/IgM | GP25-200 | 4.8 ± 1.0 | 7.6 ± 0.6 | 37 | <0.0001 |
| 4H9/IgG1 | GP25-200 | 4.7 ± 1.6 | 10.0 ± 0.0 | 53 | <0.0001 |
| 1E10/IgG1 | P23 | 4.7 ± 0.8 | 6.0 ± 1.3 | 22 | <0.025 |
| 1A9/IgG3 | P23 | 7.5 ± 0.8 | 6.0 ± 1.3 | None | NSD |
| 2G6/IgMc | P23 | 5.8 ± 1.3 | 9.2 ± 1.5 | 37 | <0.001 |
| 3C10/IgM | P23 | 6.6 ± 0.9 | 6.9 ± 1.2 | 4 | NSD |
| 3D1/IgM | P23 | 7.5 ± 1.4 | 7.8 ± 1.2 | 4 | NSD |
| 4C11/IgM | P23 | 5.6 ± 0.9 | 6.9 ± 1.2 | 19 | <0.025 |
| 4E4/IgG1 | P23 | 5.8 ± 1.3 | 6.0 ± 1.3 | 3 | NSD |
| 5B9/IgM | P23 | 5.7 ± 0.8 | 6.9 ± 1.2 | 17 | <0.05 |
| 3E2/IgM | CSL | 3.4 ± 1.9 | 7.7 ± 1.4 | 56 | <0.0001 |
| 2B4/IgM | CSL | 4.1 ± 1.0 | 7.7 ± 1.4 | 47 | <0.0001 |
| 3A11/IgM | CSL | 4.8 ± 1.2 | 7.7 ± 1.4 | 38 | <0.0001 |
| 3A12/IgM | CSL | 4.3 ± 0.9 | 7.7 ± 1.4 | 44 | <0.0001 |
| 3B12/IgM | CSL | 4.9 ± 1.2 | 7.7 ± 1.4 | 36 | <0.0001 |
| 3E6/IgM | CSL | 3.7 ± 1.1 | 7.7 ± 1.4 | 52 | <0.0001 |
Significance conclusions were verified in replicate experiments.
NSD, no significant difference.
The hybridoma cell line was lost to instability.
Multiple epitope-specific neutralizing MAb combinations demonstrate additive passive protection against oocyst challenge in vivo.
Based on their quantitative in vivo efficacy when evaluated individually (Table 2), αGP25-200 and αP23 MAbs were ranked for preliminary testing in combination with αCSL MAb 3E2. Because 3E2 was considered an essential formulation component, binding inhibition assays were performed to determine whether candidate MAbs inhibited its binding to antigen. Inhibition of 3E2 binding by candidate MAbs was not observed based on comparison of test OD values to that of control 3E2 competed against itself (Table 3) (43). At the completion of preliminary testing in mice, αGP25-200 MAb 3H2, αP23 MAb 1E10, and αCSL MAb 3E2 had consistently demonstrated the greatest efficacy, individually and combined, and were selected for further study. To confirm that the three MAbs recognized distinct epitopes or did not otherwise interfere with binding of each other, additional binding inhibition assays were performed. MAbs C4A1, C6B6, and 7D10 were included for comparison. Significant inhibition was not observed when 3E2, 3H2, and 1E10 were competed against each other in all possible combinations of two based on comparison of test OD values to that of each MAb competed against itself (Table 4). These findings, together with different reactivity patterns in Western blots (Fig. 2), are consistent with recognition of a distinct epitope by each MAb. Binding inhibition data for 3E2 and 3H2 are also consistent with the observation that while both MAbs recognize a carbohydrate-dependent epitope on CSL, only 3E2 elicited the CSP-like reaction. The reaction was also observed after sporozoite incubation with all formulations of two or three MAbs containing 3E2 and was qualitatively augmented by the combination of MAbs 3E2 and 3H2. While C4A1 and 3H2 both bound to carbohydrate-dependent epitopes and were indistinguishable in Western blots, binding inhibition data indicated that the MAbs recognize distinct epitopes (Table 4). Partial inhibition of 1E10 binding by C6B6 and 7D10 was observed (Table 4). Because 1E10, C6B6, and 7D10 each recognize a peptide epitope and react with P23 and rC7 in immunoblots, binding inhibition data are consistent with the possibility that 1E10 may recognize an epitope which has similarities to or overlaps the distinct epitopes (31) recognized by 7D10 and C6B6.
TABLE 3.
Evaluation of αGP25-200 and αP23 formulation candidate MAbs for inhibition of MAb 3E2 binding
| MAb-1 | Antigen specificity | Epitope compositiona | Binding of biotinylated 3E2 (MAb-2) (A405)b |
|---|---|---|---|
| 3E2 | CSL | C | 0.07 ± 0.01 |
| 3H2 | GP25-200 | C | 1.04 ± 0.04 |
| 1B3 | GP25-200 | C | 1.01 ± 0.03 |
| 1C5 | GP25-200 | C | 1.14 ± 0.09 |
| 2F6 | GP25-200 | P | 1.03 ± 0.11 |
| 3D9 | GP25-200 | C | 1.06 ± 0.05 |
| 4D10 | GP25-200 | P | 1.19 ± 0.02 |
| 4E11 | GP25-200 | C | 1.09 ± 0.06 |
| 4H7 | GP25-200 | P | 1.08 ± 0.07 |
| 4H9 | GP25-200 | C | 1.16 ± 0.05 |
| 1E10 | P23 | P | 1.00 ± 0.02 |
| 4C11 | P23 | NDc | 0.97 ± 0.06 |
| 5B9 | P23 | ND | 1.08 ± 0.03 |
| IgM isotype control | 1.31 ± 0.04 | ||
| IgG1 isotype control | 1.11 ± 0.08 |
P, peptide; C, carbohydrate or carbohydrate-dependent (based on susceptibility to periodate and proteinase K).
Means and standard deviations from six replicates.
ND, not determined.
TABLE 4.
Recognition of distinct epitopes and lack of binding inhibition by lead formulation MAbs
| MAb-1 | Antigen specificity | Epitope compositiona | Binding of biotinylated MAb-2 (A405)b
|
||
|---|---|---|---|---|---|
| 3E2 | 1E10 | 3H2 | |||
| 3E2 | CSL | C | 0.08 ± 0.01 | 0.96 ± 0.03 | 0.63 ± 0.03 |
| 3H2 | GP25-200 | C | 0.95 ± 0.11 | 1.06 ± 0.09 | 0.21 ± 0.02 |
| 1E10 | P23 | P | 0.96 ± 0.06 | 0.31 ± 0.08 | 0.94 ± 0.05 |
| C4A1 | GP25-200 | C | 0.99 ± 0.07 | 0.96 ± 0.06 | 0.90 ± 0.10 |
| C6B6 | P23 | P | 1.05 ± 0.06 | 0.62 ± 0.03 | 1.06 ± 0.10 |
| 7D10 | P23 | P | 0.96 ± 0.06 | 0.43 ± 0.02 | 1.01 ± 0.05 |
| IgM isotype control | 1.04 ± 0.12 | 1.01 ± 0.02 | |||
| IgG1 isotype control | 1.13 ± 0.14 | ||||
P, peptide; C, carbohydrate or carbohydrate-dependent (based on susceptibility to periodate and proteinase K).
Means and standard deviations from six replicates.
After identification of lead MAbs 3E2, 3H2, and 1E10, and determination that they did not significantly inhibit binding of each other to sporozoite antigen, three experiments were performed for definitive efficacy evaluation (Table 5). Bioreactors were used to scale up production of the MAbs for these experiments and provided the means for efficiently obtaining concentrated MAb in a relatively pure form. In experiment one, MAbs were tested at a final concentration of 1.5 mg/ml each, individually and in combinations. In this experiment, an orally administered formulation containing MAbs 3E2, 3H2, and 1E10 conferred highly significant additive efficacy over that of individual component MAbs or of combinations of two component MAbs 3E2 and 3H2 or 3E2 and 1E10 (Table 5). Further, this formulation reduced intestinal infection levels by 86% in oocyst-challenged mice. While the level of protection observed was highly significant (P < 0.0001), all mice in each treatment group remained infected. Because antibody concentration influences neutralization (33), the MAbs were tested at higher concentrations in experiments two and three. In these experiments MAbs were used at final concentrations (individually or in combinations) of 4.0 mg/ml for 3E2, 3.6 mg/ml for 3H2, and 3.3 mg/ml for 1E10. Based on the analysis of variance of infection scores, there was a significant increase in efficacy of individual MAbs 3E2 (P < 0.0001) and 3H2 (P < 0.0001) and of combinations of two MAbs 3E2 and 3H2 (P < 0.0001) or 3E2 and 1E10 (P < 0.0001) when administered at higher concentrations (Table 5). Efficacy was also increased (P < 0.003) in mice receiving the combination of all three MAbs in higher concentrations in one experiment (experiment 2). In addition, complete protection against infection was observed in 5 of 15 mice receiving individual MAb 3E2 (experiment 2), 4 (experiment 2) to 6 (experiment 3) of 15 mice receiving MAbs 3E2 and 3H2 combined, 6 of 15 mice receiving MAbs 3E2 and 1E10 combined (experiment 2), and 6 of 15 mice receiving MAbs 3E2, 3H2, and 1E10 combined (experiment 2) at higher concentrations. Based on mean infection score comparisons, the additive efficacy of the higher-concentration formulation of all three MAbs over that of individual component MAb 1E10 or 3H2 (experiments 2 and 3) or combinations of two component MAbs 3H2 and 1E10 (experiments 2 and 3) or 3E2 and 1E10 (experiment 3) was similar to that observed when the MAbs were used at lower concentrations (experiment 1). However, in experiment two there was no statistically significant difference in the efficacy of the higher-concentration formulation containing all three MAbs over that of the combinations of MAbs 3H2 and 3E2 or 3E2 and 1E10, and in experiment three the efficacy of the combination of MAbs 3H2 and 3E2 was significantly greater than that of the combination of all three MAbs. Consistently, only widely scattered C. parvum stages, generally limited to the ileum and colon, were observed in mice which remained infected after treatment with higher-concentration formulations of MAbs 3E2 and 3H2 or all three MAbs (experiments 2 and 3). Similarly, only widely scattered C. parvum stages, generally limited to the ileum, cecum, and colon, were observed in mice which remained infected after treatment with the higher-concentration formulation of MAbs 3E2 and 1E10 (experiments 2 and 3). In contrast, C. parvum stages were more diffusely distributed in the jejunum, ileum, cecum, and colon in infected mice treated with higher-concentration individual MAbs or the combination of MAbs 3H2 and 1E10 (experiments 2 and 3).
TABLE 5.
Additive in vivo efficacy of neutralizing MAb formulations against oocyst challenge (50 times the MID50)
| MAb | Expt 1a
|
Expt 2b
|
Expt 3b
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Infection score (mean ± SD) | No. infected/no. inoculated | % Reduction in infection | Infection score (mean ± SD) | No. infected/no. inoculated | % Reduction in infection | Infection score (mean ± SD) | No. infected/no. inoculated | % Reduction in infection | |
| Isotype controls | 7.6 ± 0.7 | 10/10 | 9.0 ± 0.7 | 15/15 | 9.0 ± 0.8 | 15/15 | |||
| 3E2 | 2.9 ± 0.8c | 11/11 | 62 | 0.7 ± 0.5c | 10/15 | 92 | 1.3 ± 0.9c | 15/15 | 85 |
| 3H2 | 5.6 ± 0.9c | 11/11 | 27 | 3.0 ± 1.2c | 15/15 | 66 | 2.2 ± 0.9c | 15/15 | 76 |
| 1E10 | 5.3 ± 0.7c | 11/11 | 31 | 4.5 ± 0.6c | 15/15 | 49 | 5.1 ± 0.7c | 15/15 | 43 |
| 3E2 + 3H2 | 2.0 ± 0.9cd | 11/11 | 74 | 0.8 ± 0.6cefg | 11/15 | 91 | 0.6 ± 0.5cdhi | 9/15 | 93 |
| 3E2 + 1E10 | 4.0 ± 0.6c | 11/11 | 47 | 0.7 ± 0.6cefg | 9/15 | 92 | 1.3 ± 0.6ce | 15/15 | 85 |
| 3H2 + 1E10 | NDj | ND | ND | 4.0 ± 0.9ce | 15/15 | 55 | 3.9 ± 1.1cef | 15/15 | 57 |
| 3E2 + 3H2 + 1E10 | 1.1 ± 0.3cdh | 11/11 | 86 | 0.6 ± 0.5cefg | 9/15 | 93 | 1.0 ± 0.0cefgk | 15/15 | 89 |
MAb concentrations, 1.5 mg/ml each.
MAb concentrations, 4.0 mg/ml (3E2), 3.6 mg/ml (3H2), 3.3 mg/ml (1E10), and 4.0 mg/ml each (isotype control MAbs).
P < 0.0001 compared to isotype control MAb-treated group.
P < 0.005 compared to all individual MAbs.
P < 0.0001 compared to individual MAb 1E10.
P < 0.001 compared to individual MAb 3H2.
P < 0.0001 compared to formulation of MAbs 3H2 and 1E10.
P < 0.005 compared to formulations of two MAbs.
P < 0.0025 compared to formulation of three MAbs.
ND, not determined.
P < 0.025 compared to formulation of MAbs 3E2 and 1E10.
DISCUSSION
Passive oral immunization with specific antibody has been validated as a means for protection against and treatment of a variety of enteropathogens (6, 14, 18, 46, 47, 59). In the present study, we hypothesized that the efficacy of passive antibody-mediated immunization against cryptosporidiosis could be improved by targeting distinct epitopes on defined zoite antigens known to have a role in the infection process. Surface-exposed pellicle antigens and internal apical complex antigens which are exocytosed during attachment and invasion are accessible to antibody, making such molecules viable targets for passive immunization against Apicomplexan parasites (5, 9, 24, 28). Therefore, affinity-purified CSL, GP25-200, and P23 were used to produce an expanded panel of MAbs. The rationale for this approach was to direct the immune response to biologically relevant antigens and increase the probability of obtaining neutralizing MAbs against multiple, distinct epitopes.
The heterogeneous, multiple-band reactivity patterns of MAbs generated against GP25-200 can be best explained by repetitive occurrence of a carbohydrate-dependent epitope or conserved peptide epitope on multiple glycoprotein species. While the lower-Mr antigens recognized by these MAbs could be proteolytic degradation fragments of higher-Mr antigens, this is unlikely because antigen was prepared at 4°C in the presence of broad-spectrum protease inhibitors. Alternatively, the higher-Mr antigens could be precursors of the lower-Mr antigens, or the lower-Mr antigens could be subunits of a high-Mr multimeric protein. Results of nonreducing SDS-PAGE and Western blotting suggest that the latter explanation accounts, in part, for the reactivity pattern of MAb C4A1 (42). Additional studies will be required to further define the relationship between the antigens comprising GP25-200 and CSL. Recognition of carbohydrate-dependent epitopes by many αGP25-200 MAbs was not unexpected, based on the glycosylated state reported for C. parvum sporozoite apical and surface proteins (20, 35, 48, 49) and observations that glycoconjugates are important targets of the humoral immune response against C. parvum (17, 19, 34, 35, 37, 38, 39, 49, 57). Recognition of peptide epitopes by all 14 Western blot-reactive αP23 MAbs is consistent with the reported presence of a single N-glycosylation site in P23 (31). The nonreactivity of 10 MAbs generated against P23 in Western blots suggests recognition of conformation-dependent epitopes.
Testing of the MAb panel resulted in the identification of a subset of nine αGP25-200 and four αP23 MAbs which individually protected mice from oocyst challenge (30 times the MID50). The neonatal mouse model is a more stringent assay for neutralizing activity than the ELISA (35). Therefore, the inability of some MAbs which neutralized sporozoites in vitro to reduce infection in vivo was expected. However, for MAbs which significantly reduced infection in both assays, the observation that neutralizing activity in ELISA was often lower than that in mice for a given MAb was unexpected. For example, αGP25-200 MAb 3H2 reduced infection by only 8% in the ELISA based on mean OD values. However, 3H2 reduced infection by up to 76% in neonatal mice based on mean infection scores. It is possible that exoantigens such as CSL (19, 39) and P23 (3, 10) which are released from zoites and specifically attach to host cells could be detected and be indistinguishable from intracellular stages based on OD values in ELISA. If so, the apparent in vitro neutralizing activity of MAbs which recognize such antigens could be less than the actual neutralizing activity. It is also possible that differences in neutralizing activity for a given MAb in ELISA and in mice are related to the different end points for each assay. Infection levels were evaluated at 48 h p.i. in the ELISA, prior to exponential amplification of parasite stages and completion of the life cycle that would occur in the absence of treatment (13). Infection levels were evaluated at 92 to 94 h p.i. in mice, a time point which is approximately 24 h beyond that required for completion of the life cycle (13). Therefore, the relative percent reduction of infection by neutralizing MAb might be lower at 48 h p.i. in vitro than at 92 to 94 h p.i. in vivo because infection levels are lower at 48 h p.i. in the absence of treatment. Studies to investigate these and other possible explanations are in progress.
Quantitative in vivo efficacy data for individual αGP25-200 and αP23 MAbs allowed them to be ranked for definitive testing with αCSL MAb 3E2. A formulation targeting distinct epitopes on CSL, GP25-200, and P23, and providing the greatest efficacy against a substantial oocyst challenge (50 times the MID50) was then identified. This formulation, consisting of MAbs 3E2, 3H2, and 1E10, demonstrated additive neutralizing ability when compared to individual component MAbs or combinations of two component MAbs. When the MAbs were used at higher concentrations, efficacy was significantly increased based on reduction of infection and the finding that complete protection was achieved in some mice treated with 3E2 alone and all formulations containing 3E2. These observations underscore the profound activity of 3E2 and the influence of MAb concentration on the kinetics of neutralization (33, 39). Interestingly, at higher concentrations, the combination of MAbs 3E2 and 3H2 provided protection levels which were similar (experiment 2) or significantly better (experiment 3) than that provided by the combination of MAbs 3E2, 3H2, and 1E10. Additional studies will be required to explain these unexpected observations. In any case, a compelling rationale for the use of MAb formulations targeting up to three distinct antigens rather than single MAbs is evident when considering the extended-course treatment regimens which are likely to be required for control of infection in immunodeficient hosts (8, 27, 35, 36, 51, 52, 53, 57). If antigenic variation within or between C. parvum isolates occurs, targeting multiple epitopes with therapeutic MAbs may reduce the potential for selection and emergence of variant C. parvum subpopulations. Experiments are in progress to evaluate the efficacy of 3E2, 3H2, and 1E10 in extended-course therapy against persistent infection in an adult SCID mouse model.
Knowledge of the specific mechanisms by which MAbs mediate neutralization would add to the presently limited information base on the immunobiology and molecular pathogenesis of cryptosporidiosis and might provide insight into novel control strategies (8, 35, 57). Therefore, we recently determined that MAb 3E2 neutralizes infectivity by binding to a sporozoite ligand contained in CSL, after which attachment is inhibited (19). While the mechanism of neutralization by MAb 3H2 has not yet been defined, its recognition of apical complex antigens, including CSL, and the surface pellicle of zoites suggests that it may target the attachment and/or invasion processes as well. The mechanism of neutralization by αP23 MAb 1E10 may involve interference with zoite locomotion, because P23 is deposited during gliding motility required in the invasion process (3, 10, 23). This type of sporozoan motility is characterized by attachment of zoite surface molecules to a substratum, posterior translocation, and forward locomotion (44, 45). MAb 1E10 recognized both native P23 and rC7, a recombinant polypeptide containing the 101 C-terminal amino acids of P23 (31, 32). While αP23 neutralizing MAbs C6B6 and 7D10 were identified in previous studies and each recognizes a distinct epitope on rC7 (1, 23, 31, 32), 1E10 was selected in the present study because of its greater efficacy in vivo. Interestingly, binding inhibition data suggest that 1E10 may recognize an epitope which is similar to or overlapping the epitopes recognized by C6B6 and 7D10. It will be of interest to definitively map the epitope recognized by 1E10 and determine its relationship to the epitopes defined by C6B6 and 7D10. The biological relevance of P23 and its expression of functional epitopes is further supported by the observation that immune bovine colostrum prepared against rC7 reduced oocyst shedding by 99.8% and prevented diarrhea when orally administered to oocyst-challenged calves (32).
In summary, neutralizing MAbs against distinct epitopes on the target antigens CSL, GP25-200, and P23 were produced and characterized in the present study. Formulations containing the most efficacious MAbs provided highly significant protection and validated the concept of additive neutralization through targeting distinct epitopes expressed by both infective stages. Further, the results confirmed that MAb-mediated neutralization of C. parvum can occur in the gastrointestinal lumen during the brief period of time (13, 21) that zoites are extracellular. It is difficult to accurately compare the efficacy data reported here to those in previous studies for other α-C. parvum MAbs (1, 10, 33, 49) or polyclonal antibodies (11, 12, 33) in oocyst-inoculated neonatal mice because of differences in experimental designs. However, some comparisons can be made with similarly designed studies using a lower challenge dose of oocysts (104; 10 times the MID50) to evaluate efficacy of the MAbs originally used to define P23 and GP25-200 (31, 42). In these studies, infection levels in ileum, cecum, and colon at 92 to 94 h p.i. were reduced 29 to 36% by MAb C6B6 (31), 18 to 33% by MAb 7D10 (31), 27 to 44% by the combination of MAbs C6B6 and 7D10 (31), and 25% by the combination of MAbs C6B6 and C4A1 (42). While each treatment significantly reduced infection, all mice in each group remained infected. Therefore, in the present study, complete protection in 40% of the treated mice and an overall 93% reduction in intestinal infection by the combinations of MAbs 3E2 and 3H2 or 3E2, 3H2, and 1E10 after challenge with 5 × 104 oocysts (50 times the MID50) indicate highly substantial efficacy. The results support the hypothesis that polyvalent formulations of high-activity neutralizing MAbs targeting functionally defined C. parvum antigens may provide optimal passive immunization against cryptosporidiosis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI 30223 from the National Institutes of Health, Bethesda, Md.; United States Department of Agriculture NRICGP grant 37204-0496; and funds from the Agricultural Experiment Station, University of Arizona. Deborah A. Schaefer was supported in part by funds from the Microbiology and Immunology Graduate Program, University of Arizona.
We thank Phaedra A. Yount, Alice L. Stone, Kathryn Huey Tubman, and Rebecca C. Langer for excellent technical assistance; Erin Siegel (Arizona Prevention Center, Biostatistics Consulting, Tucson) for assistance with statistical analyses; Lance E. Perryman (North Carolina State University, Raleigh) for providing rC7; and Charles R. Sterling (University of Arizona, Tucson) for providing MAbs C6B6 and C4A1.
REFERENCES
- 1.Arrowood M J, Mead J R, Mahrt J L, Sterling C R. Effects of immune colostrum and orally administered antisporozoite monoclonal antibodies on the outcome of Cryptosporidium parvum infections in neonatal mice. Infect Immun. 1989;57:2283–2288. doi: 10.1128/iai.57.8.2283-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 3.Arrowood M J, Sterling C R, Healey M C. Immunofluorescent microscopic visualization of trails left by gliding Cryptosporidium parvum sporozoites. J Parasitol. 1991;77:315–317. [PubMed] [Google Scholar]
- 4.Blagburn B L, Soave R. Prophylaxis and chemotherapy: human and animal. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 111–128. [Google Scholar]
- 5.Carruthers V B, Sibley L D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 6.Casadevall A. Passive antibody therapies: progress and continuing challenges. Clin Immunol. 1999;93:5–15. doi: 10.1006/clim.1999.4768. [DOI] [PubMed] [Google Scholar]
- 7.Cochrane A, Aikawa H M, Jeng M, Nussenzweig R S. Antibody-induced ultrastructural changes of malarial sporozoites. J Immunol. 1976;116:859–867. [PubMed] [Google Scholar]
- 8.Crabb J H. Antibody-based immunotherapy of cryptosporidiosis. Adv Parasitol. 1998;40:121–149. doi: 10.1016/s0065-308x(08)60119-0. [DOI] [PubMed] [Google Scholar]
- 9.Dubremetz J F. Apical organelles (rhoptries, micronemes, dense granules) and host cell invasion by Coccidia: what do we know now? In: Barta J R, Fernando M A, editors. Proceedings of the VIth International Coccidiosis Conference. Vol. 6. Guelph, Ontario, Canada: Moffit Print Craft, Ltd.; 1993. pp. 3–9. [Google Scholar]
- 10.Enriquez F J, Riggs M W. Role of immunoglobulin A monoclonal antibodies against P23 in controlling murine Cryptosporidium parvum infection. Infect Immun. 1998;66:4469–4473. doi: 10.1128/iai.66.9.4469-4473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fayer R, Guidry A, Blagburn B L. Immunotherapeutic efficacy of bovine colostral immunoglobulins from a hyperimmunized cow against cryptosporidiosis in neonatal mice. Infect Immun. 1990;58:2962–2965. doi: 10.1128/iai.58.9.2962-2965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fayer R, Perryman L E, Riggs M W. Hyperimmune bovine colostrum neutralizes Cryptosporidium sporozoites and protects mice against oocyst challenge. J Parasitol. 1989;75:151–153. [PubMed] [Google Scholar]
- 13.Fayer R, Speer C A, Dubey J P. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 1–42. [Google Scholar]
- 14.Freedman D J, Tacket C O, Delehanty A, Maneval D R, Nataro J, Crabb J H. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J Infect Dis. 1998;177:662–667. doi: 10.1086/514227. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths J K, Balakrishnan R, Widmer G, Tzipori S. Paromomycin and geneticin inhibit intracellular Cryptospordium parvum without trafficking through the host cell cytoplasm: implications for drug delivery. Infect Immun. 1998;66:3874–3883. doi: 10.1128/iai.66.8.3874-3883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heine J, Pohlenz J F L, Moon H W, Woode G N. Enteric lesions and diarrhea in gnotobiotic calves monoinfected with Cryptosporidium species. J Infect Dis. 1984;150:768–775. doi: 10.1093/infdis/150.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins M C, O'Brien C, Trout J, Guidry A, Fayer R. Hyperimmune bovine colostrum specific for recombinant Cryptosporidium parvum antigen confers partial protection against cryptosporidiosis in immunosuppressed adult mice. Vaccine. 1999;17:2453–2460. doi: 10.1016/s0264-410x(98)00369-7. [DOI] [PubMed] [Google Scholar]
- 18.Lamm M E. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 19.Langer R C, Riggs M W. Cryptosporidium parvum apical complex glycoprotein CSL contains a sporozoite ligand for intestinal epithelial cells. Infect Immun. 1999;67:5282–5291. doi: 10.1128/iai.67.10.5282-5291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luft B J, Payne D, Woodmansee D, Kim C W. Characterization of the Cryptosporidium antigens from sporulated oocysts of Cryptosporidium parvum. Infect Immun. 1987;55:2436–2441. doi: 10.1128/iai.55.10.2436-2441.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumb R, Smith K, O'Donoghue P J, Lanser J A. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue culture cells. Parasitol Res. 1988;74:531–536. doi: 10.1007/BF00531630. [DOI] [PubMed] [Google Scholar]
- 22.Mazier D, Mellouk S, Beaudoin R L, Texier B, Druilhe P, Hockmeyer W, Trosper J, Paul C, Charoenvit Y, Young J, Miltgen F, Chedid L, Chigot J P, Galley B, Brandicourt O, Gentilini M. Effect of antibodies to recombinant and synthetic peptides on P. falciparum sporozoites in vitro. Science. 1986;231:156–159. doi: 10.1126/science.3510455. [DOI] [PubMed] [Google Scholar]
- 23.Mead J R, Arrowood M J, Sterling C R. Antigens of Cryptosporidium sporozoites recognized by immune sera of infected animals and humans. J Parasitol. 1988;74:135–143. [PubMed] [Google Scholar]
- 24.Mineo J R, Kahn I A, Kasper L H. Toxoplasma gondii: a monoclonal antibody that inhibits intracellular replication. Exp Parasitol. 1994;79:351–361. doi: 10.1006/expr.1994.1097. [DOI] [PubMed] [Google Scholar]
- 25.Mitschler R R, Welti R, Upton S J. A comparative study of lipid compositions of Cryptosporidium parvum (Apicomplexa) and Madin-Darby bovine kidney cells. J Eukaryot Microbiol. 1994;41:8–12. doi: 10.1111/j.1550-7408.1994.tb05927.x. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee J, Kozel T R, Casadevall A. Monoclonal antibodies reveal additional epitopes of serotype D Cryptococcus neoformans capsular glucuronoxylomannan that elicit protective antibodies. J Immunol. 1998;161:3557–3568. [PubMed] [Google Scholar]
- 27.Nord J, Ma P, DiJohn D, Tzipori S, Tacket C O. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. AIDS. 1990;4:581–584. doi: 10.1097/00002030-199006000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Nudelman S, Renia L, Charoenvit Y, Yuan L, Miltgen F, Beaudoin R L, Mazier D. Dual action of anti-sporozoite antibodies in vitro. J Immunol. 1989;143:996–1000. [PubMed] [Google Scholar]
- 29.Okhuysen P C, Chappell C L, Crabb J, Valdez L M, Douglass E T, DuPont H L. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin Infect Dis. 1998;26:1324–1329. doi: 10.1086/516374. [DOI] [PubMed] [Google Scholar]
- 30.Perkins M E, Riojas Y A, Wu T W, Le Blancq S M. CpABC, a Cryptosporidium parvum ATP-binding cassette protein at the host-parasite boundary in intracellular stages. Proc Natl Acad Sci USA. 1999;96:5734–5739. doi: 10.1073/pnas.96.10.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perryman L E, Jasmer D P, Riggs M W, Bohnet S G, McGuire T C, Arrowood M J. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol Biochem Parasitol. 1996;80:137–147. doi: 10.1016/0166-6851(96)02681-3. [DOI] [PubMed] [Google Scholar]
- 32.Perryman L E, Kapil S J, Jones M L, Hunt E L. Protection of calves against cryptosporidiosis with immune bovine colostrum induced by a Cryptosporidium parvum recombinant protein. Vaccine. 1999;17:2142–2149. doi: 10.1016/s0264-410x(98)00477-0. [DOI] [PubMed] [Google Scholar]
- 33.Perryman L E, Riggs M W, Mason P H, Fayer R H. Kinetics of Cryptosporidium parvum sporozoite neutralization by monoclonal antibodies, immune bovine serum, and immune bovine colostrum. Infect Immun. 1990;58:257–259. doi: 10.1128/iai.58.1.257-259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen C, Gut J, Doyle P S, Crabb J H, Nelson R G, Leech J H. Characterization of a >900,000-MrCryptosporidium parvum sporozoite glycoprotein recognized by protective hyperimmune bovine colostral immunoglobulin. Infect Immun. 1992;60:5132–5138. doi: 10.1128/iai.60.12.5132-5138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riggs M W. Immunology: host response and development of passive immunotherapy and vaccines. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 129–161. [Google Scholar]
- 36.Riggs M W, Cama V A, Leary H L, Jr, Sterling C R. Bovine antibody against Cryptosporidium parvum elicits a circumsporozoite precipitate-like reaction and has immunotherapeutic effect against persistent cryptosporidiosis in SCID mice. Infect Immun. 1994;62:1927–1939. doi: 10.1128/iai.62.5.1927-1939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riggs M W, McGuire T C, Mason P H, Perryman L E. Neutralization-sensitive epitopes are exposed on the surface of infectious Cryptosporidium parvum sporozoites. J Immunol. 1989;143:1340–1345. [PubMed] [Google Scholar]
- 38.Riggs M W, McNeil M R, Perryman L E, Stone A L, Scherman M S, O'Connor R M. Cryptosporidium parvum sporozoite pellicle antigen recognized by a neutralizing monoclonal antibody is a β-mannosylated glycolipid. Infect Immun. 1999;67:1317–1322. doi: 10.1128/iai.67.3.1317-1322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riggs M W, Stone A L, Yount P A, Langer R C, Arrowood M J, Bentley D L. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J Immunol. 1997;158:1787–1795. [PubMed] [Google Scholar]
- 40.Riggs M W, Yount P A, Stone A L, Langer R C. Protective monoclonal antibodies define a distinct, conserved epitope on an apical complex exoantigen of Cryptosporidium parvum sporozoites. J Eukaryot Microbiol. 1996;43:74S–75S. doi: 10.1111/j.1550-7408.1996.tb05005.x. [DOI] [PubMed] [Google Scholar]
- 41.Sagodira S, Buzoni-Gatel D, Iochmann S, Naciri M, Bout D. Protection of kids against Cryptosporidium parvum infection after immunization of dams with CP15-DNA. Vaccine. 1999;17:2346–2355. doi: 10.1016/s0264-410x(99)00041-9. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer D A. M.S. thesis. Tucson: University of Arizona; 1999. [Google Scholar]
- 43.Stahli C, Miggiano V, Stocker J, Staehelin T, Haring P, Takacs B. Distinction of epitopes by monoclonal antibodies. Methods Enzymol. 1983;92:242–253. doi: 10.1016/0076-6879(83)92023-2. [DOI] [PubMed] [Google Scholar]
- 44.Stewart M J, Vanderberg J P. Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J Protozool. 1988;35:389–393. doi: 10.1111/j.1550-7408.1988.tb04115.x. [DOI] [PubMed] [Google Scholar]
- 45.Stewart M J, Vanderberg J P. Malaria sporozoites release circumsporozoite protein from their apical end and translocate it along their surface. J Protozool. 1991;38:411–421. doi: 10.1111/j.1550-7408.1991.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 46.Tacket C O, Binion S B, Bostwick E, Losonsky G, Roy M J, Edelman R. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg. 1992;47:276–283. doi: 10.4269/ajtmh.1992.47.276. [DOI] [PubMed] [Google Scholar]
- 47.Tacket C O, Losonsky G, Link H, Hoang Y, Guesry P, Hilpert H, Levine M M. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. New Engl J Med. 1988;318:1240–1243. doi: 10.1056/NEJM198805123181904. [DOI] [PubMed] [Google Scholar]
- 48.Tilley M, Eggleston M T, Upton S J. Multiple oral inoculations with Cryptosporidium parvum as a means of immunization for production of monoclonal antibodies. FEMS Microbiol Lett. 1993;113:235–240. doi: 10.1111/j.1574-6968.1993.tb06520.x. [DOI] [PubMed] [Google Scholar]
- 49.Tilley M, Upton S J, Fayer R, Barta J R, Chrisp C E, Freed P S, Blagburn B L, Anderson B C, Barnard S M. Identification of a 15-kilodalton surface glycoprotein on sporozoites of Cryptosporidium parvum. Infect Immun. 1991;59:1002–1007. doi: 10.1128/iai.59.3.1002-1007.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzipori S. Cryptosporidiosis: laboratory investigations and chemotherapy. Adv Parasitol. 1998;40:186–221. doi: 10.1016/s0065-308x(08)60121-9. [DOI] [PubMed] [Google Scholar]
- 51.Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diagn Lab Immunol. 1994;1:450–463. doi: 10.1128/cdli.1.4.450-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzipori S, Roberton D, Cooper D A, White L. Chronic cryptosporidial diarrhoea and hyperimmune cow colostrum. Lancet. 1987;ii:344–345. doi: 10.1016/s0140-6736(87)90944-5. [DOI] [PubMed] [Google Scholar]
- 53.Ungar B L P, Ward D J, Fayer R, Quinn C A. Cessation of Cryptosporidium-associated diarrhea in an acquired immunodeficiency syndrome patient after treatment with hyperimmune bovine colostrum. Gastroenterology. 1990;98:486–489. doi: 10.1016/0016-5085(90)90842-o. [DOI] [PubMed] [Google Scholar]
- 54.Upton S J, Tilley M, Brillhart D B. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol Lett. 1994;118:233–236. doi: 10.1111/j.1574-6968.1994.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 55.Upton S J, Tilley M, Brillhart D B. Effects of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J Clin Microbiol. 1995;33:371–375. doi: 10.1128/jcm.33.2.371-375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villacorta I, deGraaf D, Charlier G, Peeters J E. Complete development of Cryptosporidium parvum in MDBK cells. FEMS Microbiol Lett. 1996;142:129–132. doi: 10.1111/j.1574-6968.1996.tb08419.x. [DOI] [PubMed] [Google Scholar]
- 57.Ward H, Cevallos A M. Cryptosporidium: molecular basis of host-parasite interaction. Adv Parasitol. 1998;40:151–185. doi: 10.1016/s0065-308x(08)60120-7. [DOI] [PubMed] [Google Scholar]
- 58.Woodward M P, Young W W, Jr, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- 59.Zeitlin L, Cone R A, Whaley K J. Using monoclonal antibodies to prevent mucosal transmission of epidemic infectious diseases. Emerg Infect Dis. 1999;5:54–64. doi: 10.3201/eid0501.990107. [DOI] [PMC free article] [PubMed] [Google Scholar]