Abstract
Background
Primary healthcare, particularly Indigenous‐led services, are well placed to deliver services that reflect the needs of Indigenous children and their families. Important characteristics identified by families for primary health care include services that support families, accommodate sociocultural needs, recognise extended family child‐rearing practices, and Indigenous ways of knowing and doing business. Indigenous family‐centred care interventions have been developed and implemented within primary healthcare services to plan, implement, and support the care of children, immediate and extended family and the home environment. The delivery of family‐centred interventions can be through environmental, communication, educational, counselling, and family support approaches.
Objectives
To evaluate the benefits and harms of family‐centred interventions delivered by primary healthcare services in Canada, Australia, New Zealand, and the USA on a range of physical, psychosocial, and behavioural outcomes of Indigenous children (aged from conception to less than five years), parents, and families.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 22 September 2021.
Selection criteria
We included randomised controlled trials (RCTs), cluster RCTs, quasi‐RCTs, controlled before‐after studies, and interrupted time series of family‐centred care interventions that included Indigenous children aged less than five years from Canada, Australia, New Zealand, and the USA. Interventions were included if they met the assessment criteria for family‐centred interventions and were delivered in primary health care. Comparison interventions could include usual maternal and child health care or one form of family‐centred intervention versus another.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. overall health and well‐being, 2. psychological health and emotional behaviour of children, 3. physical health and developmental health outcomes of children, 4. family health‐enhancing lifestyle or behaviour outcomes, 5. psychological health of parent/carer. 6. adverse events or harms. Our secondary outcomes were 7. parenting knowledge and awareness, 8. family evaluation of care, 9. service access and utilisation, 10. family‐centredness of consultation processes, and 11. economic costs and outcomes associated with the interventions. We used GRADE to assess the certainty of the evidence for our primary outcomes.
Main results
We included nine RCTs and two cluster‐RCTs that investigated the effect of family‐centred care interventions delivered by primary healthcare services for Indigenous early child well‐being. There were 1270 mother–child dyads and 1924 children aged less than five years recruited. Seven studies were from the USA, two from New Zealand, one from Canada, and one delivered in both Australia and New Zealand. The focus of interventions varied and included three studies focused on early childhood caries; three on childhood obesity; two on child behavioural problems; and one each on negative parenting patterns, child acute respiratory illness, and sudden unexpected death in infancy. Family‐centred education was the most common type of intervention delivered. Three studies compared family‐centred care to usual care and seven studies provided some 'minimal' intervention to families such as education in the form of pamphlets or newsletters. One study provided a minimal intervention during the child's first 24 months and then the family‐centred care intervention for one year. No studies had low or unclear risk of bias across all domains. All studies had a high risk of bias for the blinding of participants and personnel domain.
Family‐centred care may improve overall health and well‐being of Indigenous children and their families, but the evidence was very uncertain. The pooled effect estimate from 11 studies suggests that family‐centred care improved the overall health and well‐being of Indigenous children and their families compared no family‐centred care (standardised mean difference (SMD) 0.14, 95% confidence interval (CI) 0.03 to 0.24; 2386 participants).
We are very uncertain whether family‐centred care compared to no family‐centred care improves the psychological health and emotional behaviour of children as measured by the Infant Toddler Social Emotional Assessment (ITSEA) (Competence domain) (mean difference (MD) 0.04, 95% CI −0.03 to 0.11; 2 studies, 384 participants). We assessed the evidence as being very uncertain about the effect of family‐centred care on physical health and developmental health outcomes of children. Pooled data from eight trials on physical health and developmental outcomes found there was little to no difference between the intervention and the control groups (SMD 0.13, 95% CI −0.00 to 0.26; 1961 participants). The evidence is also very unclear whether family‐centred care improved family‐enhancing lifestyle and behaviours outcomes. Nine studies measured family health‐enhancing lifestyle and behaviours and pooled analysis found there was little to no difference between groups (SMD 0.16, 95% CI −0.06 to 0.39; 1969 participants; very low‐certainty evidence). There was very low‐certainty evidence of little to no difference for the psychological health of parents and carers when they participated in family‐centred care compared to any control group (SMD 0.10, 95% CI −0.03 to 0.22; 5 studies, 975 parents/carers).
Two studies stated that there were no adverse events as a result of the intervention. No additional data were provided. No studies reported from the health service providers perspective or on outcomes for family's evaluation of care or family‐centredness of consultation processes.
Authors' conclusions
There is some evidence to suggest that family‐centred care delivered by primary healthcare services improves the overall health and well‐being of Indigenous children, parents, and families. However, due to lack of data, there was not enough evidence to determine whether specific outcomes such as child health and development improved as a result of family‐centred interventions. Seven of the 11 studies delivered family‐centred education interventions. Seven studies were from the USA and centred on two particular trials, the 'Healthy Children, Strong Families' and 'Family Spirit' trials. As the evidence is very low certainty for all outcomes, further high‐quality trials are needed to provide robust evidence for the use of family‐centred care interventions for Indigenous children aged less than five years.
Keywords: Child; Child, Preschool; Humans; Child Rearing; Health Services; Parenting; Parents; Primary Health Care
Plain language summary
Care involving families for Indigenous early childhood well‐being
Key messages
There was a small improvement on the overall health and well‐being of Indigenous children and their families when they participated in family‐centred care programmes at a primary healthcare service, but we have very low confidence in the overall evidence.
All studies used community engagement strategies, which is an important aspect of working with Indigenous communities.
Further adequately powered studies are likely to provide better estimates of the effects of family‐centred care.
What is family‐centred care?
Family‐centred care is a way of providing care that focuses on the needs of children and provides planned care around the whole family unit. It recognises that all family members are care recipients and aims to involve families in partnership with primary healthcare services.
Why is a specific focus needed on family‐centred care in Indigenous health?
Family‐centred care is important for all children, but interventions must consider sociocultural needs. Caring for children within Indigenous families often involves extended family member' roles and responsibilities, cultural child‐rearing practices, and holistic (treatment of the whole person, taking into account mental and social factors rather than just the symptoms of a disease) understandings of well‐being centred on connectedness. Engaging in family‐centred health promoting approaches through primary healthcare services could be an effective means of delivering care to children that considers the needs and functioning of the wider family.
What did we want to find out?
There has been no well‐conducted review of studies examining the effects of family‐centred health care delivered through primary healthcare services on the health and well‐being of Indigenous children and their families. One scoping review (a brief assessment of the research and evidence) completed in 2017 found 18 evaluations on family‐centred care for Indigenous children and families with three randomised controlled trials (well‐designed studies that provide the best evidence) identified. As a result, we wanted to find out if family‐centred care improved:
– the overall health and well‐being of Indigenous children and their families;
– specific aspects of care such as physical health and development of children or the psychological health of families.
We also wanted to know how delivering family‐centred care affected health service providers and the care they delivered.
What did we do?
We searched for studies that looked at family‐centred care interventions that were delivered in Canada, Australia, New Zealand, and the USA led by primary healthcare services to Indigenous children aged less than five years. We compared and summarised the results of the studies and rated our confidence in the evidence.
What did we find?
We found 11 studies that enrolled 1270 mother–child pairs and 1924 children aged less than five years. Most of the family‐centred interventions delivered to children had different foci such as childhood obesity, behavioural problems, negative parenting patterns, and acute respiratory illness. Seven studies used education as a way of delivering family‐centred care. All studies compared family‐centred care interventions to usual care or a minimal control comparison. Seven studies were from the USA, two from New Zealand, one from Canada, and one from both Australia and New Zealand.
Family‐centred care may improve overall health and well‐being of Indigenous children and their families, but the evidence was very uncertain. There was little to no difference in psychological health and emotional behaviour of children, physical health and developmental outcomes of children, family health‐enhancing lifestyle and behaviours, and psychological health of parents and carers, but the evidence was very uncertain.
What were the limitations of the evidence?
We are not confident in the evidence because people in the studies were aware of what intervention they were getting, and many people did not come back to report their results. Not all the studies reported the information we were interested in. Studies that did report on the data we were interested in were very specific to that particular study, so we had to make some assumptions about whether the data were applied to all families.
How up to date is this evidence?
The evidence is up to date to 22 September 2021.
Summary of findings
Summary of findings 1. Family‐centred care compared to any control for Indigenous children aged less than five years.
| Family‐centred care compared to any control for Indigenous children aged < 5 years | |||||
|
Patient or population: Indigenous children aged < 5 years Setting: primary health care Intervention: family‐centred care Comparison: usual care or minimal intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with any control | Risk with family‐centred care | ||||
|
Overall health and well‐being Timing: the longest time point available for each study Direction of effect: higher beneficial |
— | The mean SMD score in the intervention group was 0.14 SD higher (0.03 higher to 0.24 higher) | 2386 (11 RCTs) | ⊕⊝⊝⊝ Very lowa,b | Family‐centred care may improve the overall health and well‐being of Indigenous children and their families, but the evidence is very uncertain. |
|
Psychological health and emotional behaviour of children Timing: the longest time point available for each study Direction of effect: higher beneficial |
The mean psychological health and emotional behaviour of children ranged from 0.95 to 1.02 points | The mean MD score in the intervention group was 0.04 points higher (0.03 lower to 0.11 higher) | 384 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c | The evidence is very uncertain about the effect of family‐centred care on psychological health and emotional behaviour of children. |
|
Physical health and developmental health outcomes of children Timing: the longest time point available for each study Direction of effect: higher beneficial |
— | The mean SMD score in the intervention group was 0.13 SD higher (0.00 lower to 0.26 higher) | 1961 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b | The evidence is very uncertain about the effect of family‐centred care on physical health and developmental health outcomes of children. |
|
Family health‐enhancing lifestyle or behavioural outcomes Timing: the longest time point available for each study Direction of effect: higher beneficial |
— | The mean SMD score in the intervention group was 0.16 SD higher (0.06 lower to 0.39 higher) | 1969 (9 RCTs) | ⊕⊝⊝⊝ Very lowa,b | The evidence is very uncertain about the effect of family‐centred care on family health‐enhancing lifestyle or behavioural outcomes. |
|
Psychological health of parent/carer Timing: the longest time point available for each study Direction of effect: higher beneficial |
The mean psychological health of a parent carer was 48.71. | The mean SMD mental health scores were 0.10 SD higher (0.03 lower to 0.22 higher).e | 975 (5 RCTs) | ⊕⊝⊝⊝ Very lowa,d | The evidence is very uncertain about the effect of family‐centred care on psychological health of parent/carer. |
|
Adverse events or harms Direction of effect: lower beneficial |
2 studies reported narrative information on adverse events. 1 study measured adverse events and side effects and reported that no aspect of the intervention including the application of fluoride varnish resulted in any reported adverse events. 1 study reported on emergency department presentations and hospital admissions as adverse events. No adverse events reported were deemed to be the result of the intervention. | Unclear participants (2 RCTs) |
⊕⊝⊝⊝ Very lowa,c | The evidence is very uncertain about the effect of family‐centred care on adverse events of harm. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aCrucial biases on multiple criteria and likely to seriously alter results. This included no blinding of participants and interventionist, people delivering the intervention collected the data, high attrition, and selective outcome reporting. Downgraded two levels. bIndividual outcome measure had different constructs but we combined them to provide a broad result for the outcome. Downgraded one level. cSmall sample size. Downgraded one level. dOutcome measure had similar constructs but did not completely overlap (e.g. general mental health compared to depression). Downgraded one level. eWe converted the standardised mean difference value 0.10 to a mean difference. The mean of the control group, as measured by the Short Form Health Survey – Mental Health component (SF‐12), was used for comparison as it was the most recent study (HCSF 2 2017).
Background
Description of the condition
There are striking disparities in health between Indigenous and non‐Indigenous children in Canada, Australia, New Zealand, and the USA. Infant mortality rate ratios are 1.6 to 4 times higher among Indigenous infants than non‐Indigenous infants, and higher rates of morbidities are consistently reported (Smylie 2010). These include injuries, respiratory infections, ear infections, and increased potentially preventable hospitalisations (Barnes 2019; Falster 2016; Jervis‐Bardy 2014). Health inequalities are consistently reported across the four countries that are the focus of this review. However, there is diversity in health indicators across and within their Indigenous populations: the Aboriginal people and Torres Strait Islanders of Australia; First Nations, Metis, and Inuit peoples of Canada; the Maori of New Zealand; and American Indian, Alaskan Native, and Native Hawaiian peoples of the USA (Cunningham 2003; Welch 2015).
Indigenous populations across the included four countries have experienced colonisation by European countries as a shared and underlying determinant of Indigenous health; and harmful social policies, to varying degrees, have disrupted family relations, continuity, and functioning (Smylie 2009). Unlike many non‐Indigenous families that are typified by a nuclear family unit, Indigenous families across each of these countries commonly include childcare responsibilities for extended family members and communities, with cultural child‐rearing practices fostering physical, social, and emotional well‐being (McMahon 2017). Connectedness is central to Indigenous social and emotional well‐being (Gee 2014; Waterson 2004).
A 'functioning family' is defined as one in which members communicate, relate, maintain relationships in healthy ways, make decisions, and solve problems (Silburn 2006; Zubrick 2000). The enduring impact of colonial legacies means that some Indigenous families experience historical and transgenerational trauma and live in environments that are not conducive to good health (Atkinson 2003; Ka'apu 2019). Some families have to deal with ongoing stressors, which can impact on their contributions to work, family life, community, culture and broader society, and their ability to nurture children. Intergenerational trauma can manifest in issues that affect the health and well‐being of families (Chamberlain 2019). These include psychological distress, grief, smoking, alcohol and drug misuse, mental illnesses, and violence. In turn, families can experience issues such as lack of food security and neglect. Health‐promoting approaches (including the practices and key issues of family‐centred practice) that are effective for non‐Indigenous children might not necessarily translate effectively for Indigenous children (Health Council of Canada 2011; McCalman 2014). However, families are central to the well‐being of children, and there is a clear need to ensure that family‐centred primary healthcare interventions consider the sociocultural needs, context, and experiences Indigenous families.
Description of the intervention
Historically, child health delivered through primary healthcare services focused on the management of infants' health and development, rather than support and care for the whole family, their lives, and well‐being concerns. The concept of family‐centred care for children originated in the 1970s through the ecological theory of child development (Bronfenbrenner 1979), which stressed the importance of considering both the immediate and extended family, and home environment (Hammer 1998; Jolley 2009). The concept also draws on the theory of patient‐centred care, which advocates that healthcare delivery should focus on the patient's needs, values, and preferences (Dwamena 2012). Primary healthcare services have attempted to implement family‐centred interventions as "a way of caring for children and their families within health services which ensures that care is planned around the whole family, not just the individual child/person, and in which all the family members are recognised as care recipients" (Shields 2006; p. 1318).
Important characteristics identified by families for primary healthcare include services that support families, accommodate sociocultural needs, recognise extended family child‐rearing practices, Indigenous ways of knowing and doing business, accessibility, and delivering care responsive to holistic health (Gomersall 2017). As a result, Indigenous primary healthcare services are well‐placed to deliver family‐centred care interventions and have implemented family‐centred interventions to reflect the decision‐making processes of Indigenous families, and potentially improve early childhood outcomes. A scoping review of Indigenous family‐centred approaches targeting pregnant women and their children from birth to aged five years in Canada, Australia, New Zealand, and the USA found 18 evaluation studies of family‐centred interventions (McCalman 2017). The studies generally reported care provided to extended family members by or with Indigenous health professionals or paraprofessionals, and focused on health promotion and clinical care (McCalman 2017).
However, differing definitions of family‐centred care have prompted various approaches to the implementation of family‐centred care. For example, Homer 2012 described an urban intervention akin to a standard maternal and child healthcare approach but based on a group midwifery practice caseload model. The intervention model provided individualised care by a known midwife and Aboriginal health educator during pregnancy, labour, birth, and postnatally, with referral to child health services after discharge. In contrast, Griew 2007 proposed an intersectoral approach, linking health and childcare services, encompassing both: 1. provision of care to patients by seeing them as embedded in a family and providing services on that basis; and 2. a life course approach, which, without neglecting adult health, focused specific attention on establishing early life resilience and advantages through a focus on child development.
How the intervention might work
One literature review found that family‐centred care entailed six core principles (MacKean 2005). These were: 1. recognising the family as central to or the constant (or both) in the child's life, and the child's primary source of strength and support; 2. acknowledging the uniqueness and diversity of children and families; 3. acknowledging that parents bring expertise at both the individual caring level and the systems level; 4. recognising that family‐centred care is competency enhancing rather than weakness focused; 5. encouraging the development of true collaborative relations between families, healthcare providers, and partner organisations; and 6. facilitating family‐to‐family support and networking, and providing services that offered emotional and financial support to meet the needs of families (p. 75). Based on these principles, a checklist of the elements of family‐centred care was developed (Trivette 1993). This checklist was used to score studies that were included in one Cochrane Review of family‐centred care for hospitalised children (Shields 2012), and in a scoping review of family‐centred approaches for Indigenous children (McCalman 2017).
Considerable debate continues among health professionals, family representatives, communities, and researchers about the strategies needed for the implementation of family‐centred care. The debates centre on five key issues. First, there is debate about the necessary types of relationships between healthcare providers and families (DHS Disability Services Division 2012; Dodd 2009). Bamm 2008 described health professionals' consideration of their primary responsibility as providing education, counselling, and information. In contrast, principles most valued by families were availability, accessibility, and communication. Patients and families considered partnerships as important, yet this was not mentioned by healthcare providers (Bamm 2008). Such diversity of perspective has created tensions relating to the focus of, and strategies for, family healthcare implementation. Second is the emphasis on family choice and participation (Dodd 2009). MacKean 2005 iterated the key tension thus: "Family‐centred care is beginning to sound like something that is being defined by experts and then carried out to families, which is ironic given that the concept of family‐centred care emerged from a strong family advocacy movement" (p. 81). Third, there is a debate about the knowledge and expertise required to apply information and deliver quality family‐centred supports and services (DHS Disability Services Division 2012; Dodd 2009). Barlow 2015 suggested that well‐trained Indigenous paraprofessionals can effectively enhance parenting knowledge, parental locus of control and psychosocial outcomes; whereas D'Espaignet 2003 focused on the role of midwives supported by Aboriginal female elders (Barlow 2015; D'Espaignet 2003). Fourth, some debates centre on the optimal context/s for family‐centred care, including home visiting or clinic‐based service provision, or both (Dodd 2009). Finally, methodological issues centre on the selection of comparison interventions, defining the treatment regimen or intervention components, and identification of adverse effects (Dodd 2009).
Family‐centred care aims to improve a range of outcomes including a decrease in parental depression rates and burden in carers, and satisfaction with care and increased quality of life of the entire family (Bamm 2008). Bamm 2008 indicated that while family‐centred care required an initial investment in the education of staff and the development of new strategies, in the long term it improved the effectiveness and efficiency of health services and reduced the financial burden on the system; particularly because families are empowered to be active partners in providing care. However, the authors concluded that further research was needed to explore the direct financial benefits of a family‐centred approach.
Our scoping review of family‐centred interventions using a range of study designs found the following outcomes for Indigenous children: increased birthweight (D'Espaignet 2003); promise for obesity prevention (Harvey‐Berino 2003); and reduced behavioural problems (Barlow 2013; Barlow 2015; Turner 2007; Walkup 2009). Outcomes for primary carers included reduced maternal depression and illegal drug use (Barlow 2013; Barlow 2015); significantly better parenting knowledge, skills, attitudes, and locus of control (Barlow 2013; Barlow 2015; Harvey‐Berino 2003; Turner 2007; Walkup 2009); and improved service access and consumer satisfaction (Turner 2007). These outcomes might be expected to vary with different types of family‐centred healthcare intervention, Indigenous populations, and stages of pregnancy or child development.
Why it is important to do this review
There are no meta‐analyses of studies specifically examining the effects of family‐centred health care delivered through primary healthcare services on the health and well‐being of Indigenous children and their parents or carers. Neither has there been a review of the effects of family‐centred health care on the healthcare encounters experienced by Indigenous families, their satisfaction or healthcare behaviour, or the delivery of these services. The authors of one 2012 Cochrane Review found one randomised controlled trial (RCT) providing moderate‐quality evidence of the effects of family‐centred care for children in hospitals (Shields 2012). Based on a small sample size, the included study suggested some benefit for children's clinical care, parental satisfaction, and costs; with no evidence of harms. However, the focus of the review was on tertiary rather than primary healthcare settings, and all population groups rather than a specific focus on Indigenous populations.
With regard to Indigenous child health, one review of the health of Indigenous children (from birth to age 12 years) evaluated the quality of Indigenous child health data collection in Canada, Australia, New Zealand, and the USA (Smylie 2009). This review did not focus on family‐centred health care and is now over 10 years old. One Australian review of family‐centred primary health care for Indigenous families (Griew 2007) and another of Indigenous family functioning (Walker 2008) were completed, but these were not systematic reviews and are now over 10 years old. Other reviews were restricted to Indigenous Australian populations (Eades 2004; Herceg 2005; Jongen 2014), did not focus on family‐centred care, and were completed 15 to 20 years ago (Eades 2004; Herceg 2005).
Our previous narrative scoping review found 24 papers that described, theorised, or evaluated Indigenous family‐centred interventions. Only three of these studies (seven papers) used RCT or controlled before‐after (CBA) study designs that enabled evaluation of the effectiveness of family‐centred interventions (McCalman 2017). This Cochrane systematic review and meta‐analysis will generate data on the combined intervention effect to enable primary healthcare service providers and families to make more evidence‐informed decisions about how family‐centred approaches are likely to affect the well‐being of Indigenous children aged from conception to five years, the lifestyle and behavioural outcomes of their families, and the psychological health of their parents/carers. It will also assist services to determine whether there are likely to be any adverse events or harms from these interventions. This review may inform decisions about the likely effects of family‐centred interventions on parenting knowledge and evaluation of care, healthcare service access or utilisation, consultation processes, and economic costs and outcomes.
Objectives
To evaluate the benefits and harms of family‐centred interventions delivered by primary healthcare services in Canada, Australia, New Zealand, and the USA on a range of physical, psychosocial, and behavioural outcomes of Indigenous children (aged from conception to less than five years), parents, and families.
Methods
Criteria for considering studies for this review
Types of studies
Many family‐centred interventions are complex in nature. Therefore, this review did not limit study designs to RCTs because doing so could exclude important evidence. Additionally, there are inherent ethical considerations for researchers proposing RCTs with Indigenous populations. Some of these considerations include the distrust between predominantly non‐Indigenous researchers and Indigenous participants, and culturally inappropriate material or procedures (Bainbridge 2015; Glover 2015). People who do participate in RCTs often face barriers such as trials not addressing likely participant barriers such as telephone availability and travel costs as well as a lack of recognition in adapting and incorporating of Indigenous knowledge systems (Glover 2015). The inclusion of alternative study designs was likely to provide relevant and meaningful data. Review results are presented according to study design to facilitate meaningful comparisons and enable robust estimates of confidence.
To evaluate the effectiveness of the family‐centred interventions, we included RCTs, cluster RCTs, and quasi‐RCTs (a trial in which randomisation is attempted but subject to potential manipulation, such as allocating participants by day of the week, date of birth, or sequence of entry into trial) (CCCRG 2016). We also included CBA studies meeting the following criteria:
there were at least two intervention sites and two control sites;
the timing of the periods of study for the control and intervention groups was comparable (i.e. the pre‐ and postintervention periods of measurement for the control and intervention groups were the same); and
the intervention and control groups were comparable on key characteristics.
Interrupted time series (ITS) designs were also included if:
the intervention occurred at a clearly defined point in time specified by the researchers; and
there were at least three data points before and three data points after the intervention was introduced (CCCRG 2016).
Types of participants
We included studies in which the population included either the families who received family‐centred care or health service providers of family‐centred care, or both.
For this review, we defined 'Indigenous' as peoples who self‐identified at the individual level and were accepted by the community as a member (UN Permanent Forum on Indigenous Issues). A family was considered Indigenous if the child was identified by the family as Indigenous (one parent could be non‐Indigenous).
We defined a 'child' as a foetus, newborn infant, baby, and child up to the age of five years. Five years is a common age at which final early child health checks are carried out by primary healthcare services prior to school entry. Studies including school‐aged children were included only if the main focus of the family‐centred intervention was care for children aged under five years, or if the majority (greater than 50%) of participants were aged from conception to five years. Studies relating to family‐centred antenatal care delivered by primary healthcare services were included if they continued beyond the standard postpartum period of six weeks to at least three months.
We defined a 'family' as a basic social unit having two or more people, irrespective of age, in which each of the following conditions was present: 1. the members were related by blood, marriage, adoption, or by a contract that was either explicit or implied; 2. the members communicated with each other in terms of defined social roles such as mother, father, wife, husband, daughter, son, brother, sister, grandfather, grandmother, uncle, aunt; and 3. they adopted or created and maintained common customs and traditions (Nixon 1988). We defined 'primary healthcare providers' as those involved in providing primary health care for Indigenous children.
Types of interventions
Studies were included if they targeted Canadian, Australian, New Zealand, or USA Indigenous children aged from conception up to five years and evaluated a family‐centred intervention implemented by a primary healthcare service. The family centredness of interventions were delineated using a modified rating scale used by our scoping review (McCalman 2017). The scale was based on a validated instrument that included 13 evaluation elements that described the features of family‐centred care, clustered under three groups: 1. family as a constant, 2. culturally responsiveness, and 3. support of family individuality (Appendix 1) (Shields 2012; Trivette 1993). Pregnancy care models that did not continue beyond the standard postpartum period of six weeks to at least three months were considered as not meeting the criteria for recognition of constancy or meeting children's developmental needs. Each of the 13 elements were equally weighted and scored from 0 to 4, with 0 indicating the study included no evidence that the intervention was either implicitly or explicitly based upon the elements of family‐centred care, to 4 indicating the article included numerous instances of explicit evidence. An element of family‐centred care was considered to be implicitly addressed if it could be inferred that the author/s' descriptions or arguments were consistent with the intent of the elements of family‐centred care, whereas it was considered to explicitly address the element if the author/s clearly state and distinctly express that the element was present in health practice (Trivette 1993). The scores were added to give an overall rating of family centredness for each study. Following Shields 2012, the family‐centredness score for inclusion was 26/52 or greater than 50%; we excluded studies that did not meet criteria for family‐centredness.
Included interventions comprised a broad range of types, including the following.
Environmental interventions as evidenced by collaboration with the family or child (or both) in the design or redevelopment of the home or primary healthcare centre to provide an environment that maximised parental involvement and enhanced child health or well‐being (or both).
Communication interventions that promote parental participation in health education to plan antenatal or postnatal care, develop collaborative care pathways where both parent or child (or both) and health carer could document issues and progress, or reorganise healthcare to provide continuity of care.
Educational interventions that delivered structured educational sessions for parents, or continuing education programmes to equip staff to provide care within a family‐centred framework.
Counselling interventions such as brief interventions about family violence or other well‐being issues, home visiting and other approaches.
Family support interventions such as flexible fee charging schemes for low‐income families, referrals to other community services (such as social workers, chaplains, patient representatives, mental health professionals, home health care, rehabilitation services), or facilitation of parent‐to‐parent support.
We included studies that compared a family‐centred healthcare intervention versus usual maternal and child healthcare or one form of family‐centred intervention versus another. We disentangled complex or multifaceted interventions by noting where the intervention components were also included in the comparison arm, and hence effectively cancelled those components from the assessment. Where a component was not included in the comparison arm, we compared each component separately to no intervention/control; and with one another within the intervention arm. We then reported the effects as being attributable to the 'active' or 'unique' components of the intervention arm only.
Interventions were included only if they were implemented by a primary healthcare service. A primary healthcare service was defined as a service providing the first level of contact of individuals, families, and the community with the healthcare system (APHCRI 2009). All components of primary healthcare services that provided a service to children were included. Where studies described interventions at the interface between services (e.g. hospital discharge to primary healthcare; integrated approaches with childcare or child protection services), we included them only if the intervention was led by the primary healthcare service. We included programmes delivered within rural reservations by Indian health services or tribal health services, and early childhood centres (see Differences between protocol and review for further information).
Types of outcome measures
The focus of this review on family‐centred interventions means that study outcomes can apply to the whole family, parents/carers, children, the health practitioner, health service factors, or a combination of these. The selected outcome measures account for this potential diversity. We included an additional primary outcome called 'overall health and well‐being' with a justification and the selection of each outcome for inclusion provided in the Differences between protocol and review section.
Primary outcomes
-
Overall health and well‐being
If available, the inclusion of one primary outcome (listed below) from each included study.
-
Psychological health and emotional behaviour of children including:
level of stress, upset, crying, infant separation distress, child anxiety, insomnia, mood, fears and behavioural regression, well‐being;
self‐esteem, levels of confidence, self‐expression; and
coping, adjustment, compliance.
-
Physical health and developmental health outcomes of children including:
Clinical assessments (e.g. injury resolution);
Physiological measures (e.g. anaemia levels); and
Developmental milestones.
-
Family health‐enhancing lifestyle or behaviour outcomes including:
weight control, control over child's food intake, birth weight;
breastfeeding;
reduced substance misuse, reduced smoking, reduced alcohol consumption, reduced addictions, and other risk taking;
home safety, safe sleeping.
-
Psychological health of parent/carer including:
level of stress, anxiety, depression, mood, well‐being;
self‐esteem, levels of confidence in parenting; and
perceptions of coping, sense of control.
-
Adverse events or harms, including:
health behaviours (e.g. violence);
clinical adverse effects;
poor utilisation or access;
low quality of care; and
increased inequities.
Secondary outcomes
-
Parenting knowledge and awareness including:
knowledge about nutrition, smoking, alcohol in pregnancy, children's early years' conditions and treatment; and
awareness of home safety issues.
-
Family evaluation of care including:
family‐professional interactions' experience, relationship with healthcare practitioner, involvement in decision‐making, level of communication, flexibility and responsiveness of the intervention, cultural competency;
perceptions and ratings of care or interventions, complaints; and
family satisfaction with the information or resources (or both) provided, satisfaction with the decision/s made, satisfaction with care, sense of control.
Service access and utilisation including:
proportion of women who received antenatal care, proportion of other family members who received health education, extent to which healthcare providers gave specific advice or delivered specific interventions;
adherence to antenatal or postnatal care plans;
proportion of children who received child health checks;
linkages to other services; and
family healthcare utilisation.
Family‐centredness of consultation processes including:
practice style, level of family‐centred care, service flexibility and responsiveness, practitioner knowledge;
provision of interventions, choices offered, visiting services, home visiting, tailored literacy and language initiatives; and
service quality, adherence to recommended practice or guidelines, cultural competence.
-
Economic costs and outcomes associated with the interventions including:
costs of specific interventions (e.g. educational, clinical, immunisation);
costs of care (e.g. costs of home‐visiting care, costs of staffing requirements, time needed for the intervention); and
cost savings.
Selection of outcomes reported
Family‐centred interventions are complex and trials can report many outcomes that are measured in many ways at multiple time points. Outcomes were not used as the criteria for including studies, but selected outcomes under each of the categories listed above were identified a priori for meta‐analysis for this review. To select outcomes of inclusion of the meta‐analysis, blinded to outcomes results, two review authors (NA; CA) independently assigned the outcomes (category and timing) reported in each included study to the review's outcome categories. We resolved any differences in categorisation through consensus with a third review author (CC). This meant that more than one outcome might have been assigned to each outcome category at the review stage. Initially our plan to determine multiplicity of outcomes was to consider objective measures (e.g. anaemia levels, antenatal visit records) over subjective or self‐reported measures (e.g. self‐reported levels of parental/carer confidence or coping). In cases where one study measured the same outcome with more than one measure, we selected the most clinically important measure to avoid over‐representing these data. However, when it came to determining outcomes, the above process was deemed insufficient as some outcomes did not include any objective measures, only subjective measures. As such, we modified the process by which we chose outcomes due to multiplicity and have outlined them in the Differences between protocol and review section. Briefly, review authors (with experience working in this field) received a spreadsheet of all outcome measures with each assigned to its respective outcome category. No results were provided. Led by one review author (NS), each outcome from each study was determined by the group for inclusion in the review. Outcome measures were prioritised for inclusion based on objective versus subjective measures, author team judgement of overall relevance to family centred‐care interventions, and strengths‐based rather than deficit measures. The final outcomes included from each study in each category were based on the decision rules by the authorship team or the most frequently reported outcome, or both.
Timing of outcome assessment
Time points were determined from time since the start of the intervention grouped into short‐, medium‐, and long‐term time points with no more than one time interval for each outcome from each study selected. Short‐term outcomes were those which occur within three months, medium‐term outcomes from three to 12 months, and long‐term outcomes greater than 12 months. If multiple time points were given in each time point period, we chose the time point that was closest to the end of the time interval (Differences between protocol and review).
Main outcomes for the summary of findings table
The review's primary outcomes included in the summary of findings table were:
overall health and well‐being;
psychological health and emotional behaviour of children;
physical health and developmental health outcomes of children;
family health‐enhancing lifestyle or behaviour outcomes;
psychological health of the parent/carer;
adverse events or harms.
For each of these outcomes, we reported the typical burden of the outcome, absolute and relative magnitude of effect (where relevant), numbers of participants and studies, and the overall certainty of the body of evidence (which varied by outcome).
Search methods for identification of studies
We applied no language or date restrictions. We sought translation when necessary.
Electronic searches
We completed searches from inception to 22 September 2021 on the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 8, 2021) in the Cochrane Library;
MEDLINE OvidSP;
Embase OvidSP;
PsycINFO OvidSP;
CINAHL EBSCOhost;
Informit Indigenous Collection (Informit);
Current Contents (Ovid) (only up to 11 April 2017).
We completed searches from inception to 22 September 2021 on the following clinical trial registries:
ClinicalTrials.gov (clinicaltrials.gov);
ISRCTN registry (www.isrctn.com/); and
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (who.int/ictrp/en/).
The search strategy for all databases are in Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8.
We tailored strategies to other databases and reported them in the review.
We handsearched the reference lists of Indigenous maternal and child health reviews, reviews of family‐centred care in general populations, and any study chosen for potential inclusion in this review to identify further relevant studies. We contacted authors and inspected forward citations of included studies to determine whether there were any additional relevant studies.
Searching other resources
We searched grey literature sources for reports and conference proceedings through clearinghouses in February 2022: the Australian Indigenous Health InfoNet, Australian Institute of Family Studies, Indigenous Knowledge Network for Infants (Canada), Child and Family Health (Canada), Child Welfare Information Gateway: Working with American Indian Children and Families (USA), and New Zealand Social Policy Evaluation and Research Unit. We were unable to gain access to the Li Ka Shing database (Canada).
Data collection and analysis
Data collection and analysis followed the published protocol (McCalman 2016). Differences from the protocol are summarised in the Differences between protocol and review section.
Where review authors had published papers that might be included in the review, the review author/s in question were not involved in assessing the study for inclusion, or extracting or analysing data from that study.
Selection of studies
We downloaded results of the search into Covidence to select studies for inclusion in this review and removed duplicates within Covidence prior to screening (Covidence). Two review authors (from NS; CA; BA (non‐author acknowledged)) independently screened all titles and abstracts identified from searches to determine which met the inclusion criteria for full‐text review. We retrieved full‐text papers identified as potentially relevant by at least one review author. Two review authors (from NS; CC; SC; LS; RB; CA; JM) independently screened full‐text articles for inclusion or exclusion, with discrepancies resolved by discussion and by consulting a third review author (from NS; CC) where necessary to reach consensus. All potentially relevant papers excluded after full‐text review are listed as excluded studies, with reasons provided in the Characteristics of excluded studies table. We provided citation details and any available information about ongoing studies, and collated and reported details of duplicate publications, so that each study (rather than each report) is the unit of interest in the review. We reported the screening and selection process in an adapted PRISMA flow chart (Liberati 2009).
Data extraction and management
Two review authors (from NS; CA; CC) extracted data from the included studies. We resolved any discrepancies by discussion until reaching consensus, or through consultation with a third review author (from NS; CC) where necessary. We developed and piloted a data extraction form using the Cochrane Consumers and Communication Review Group Data Extraction Template (available at: cccrg.cochrane.org/author-resources). Data extracted included the following: study aim, study design, number and description of comparison group/s, consumer involvement, funding source, declaration of interests by authors, informed consent, ethical approval, risk of bias (including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias), overall quality rating, description of participants, geographical location, setting, methods of recruitment, participation rate, attrition, inclusion criteria, gender, Indigenous population, stage of pregnancy/age of child, exclusion of any group from study, principal health focus, study numbers, intervention name, intervention aims and rationale, type of intervention (environmental, education, communication, counselling, family support), what was done, who delivered the intervention, where it was provided, when and how often it was provided, tailoring of intervention, modification or adaptation of intervention, assessment of implementation fidelity, score on family‐centredness scale, primary and secondary outcomes (including adverse events), method of assessing outcome measures, method of follow‐up for non‐respondents, timing of outcome assessment, other information and notes (author contact details, correspondence with authors and response, translation, duplicate publication), dichotomous and continuous, and other data and results. We prepared a summary report for individual studies in the Characteristics of included studies table. One review author (NS) entered data for analysis into Review Manager Web (Review Manager Web 2020), and a second review author (CA) checked them for accuracy against the data extraction sheets.
Assessment of risk of bias in included studies
We assessed and reported on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a) and the guidelines of the Cochrane Consumers and Communication Review Group (Ryan 2013). The Cochrane Handbook for Systematic Reviews of Interventions and guidelines recommend the explicit reporting of the following individual elements for RCTs: random sequence generation; allocation sequence concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data; selective outcome reporting; and other potential sources of bias (e.g. baseline imbalance, contamination, differential diagnostic activity). We considered blinding separately for different outcomes where appropriate (e.g. blinding may have the potential to differently affect subjective versus objective outcome measures). We judged each item at high, low, or unclear risk of bias as set out in the criteria provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a), and provided a quote from the study report and a justification for our judgement for the risk of bias in within the Characteristics of included studies table.
Studies were deemed at high risk of bias if they scored at high or unclear risk of bias for either sequence generation or allocation concealment domains, or objectivity of outcome data or completeness of outcome data (intention‐to‐treat), based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2021a).
Two review authors (from NS; SC; LS; JM) independently assessed the risk of bias of included studies, with any disagreements resolved by discussion to reach consensus or consultation with a third review author (NS; CC) if needed. We contacted study authors for additional information about the included studies, or for clarification of the study methods as required. We incorporated the results of the risk of bias assessment into the review through standard tables, and systematic narrative description and commentary about each of the elements, leading to an overall assessment of the risk of bias of included studies and a judgement about the internal validity of the review's results.
For cluster‐RCTs, we assessed and reported the risk of bias associated with an additional domain: selective recruitment of cluster participants. For non‐randomised studies, we assessed and reported quasi‐RCTs as being at a high risk of bias on the random sequence generation item of the risk of bias tool.
We intended to assess CBA studies against the same criteria as RCTs and report them as being at high risk of bias on both the random sequence generation and allocation sequence concealment items. We would have excluded CBA studies that were not reasonably comparable at baseline. We also intended to assess and report ITS studies as being a high risk of bias on sequence generation. In addition, we would have assessed the following items for ITS studies: intervention independence of other changes; prespecification of the shape of the intervention effect; likelihood of intervention affecting data collection; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data; selective outcome reporting; and other sources of bias including baseline imbalance (due to lack of randomisation). However, no CBA or ITS studies were eligible for inclusion.
Where review authors had published papers that might be included in the review, the review author/s in question were not involved in assessing risk of bias for that study.
Measures of treatment effect
For dichotomous outcomes, we analysed data based on the number of events and the number of people assessed in the intervention and comparison groups. We then used these to calculate the risk ratio (RR) and 95% confidence interval (CI). For continuous measures, we analysed data based on the mean, standard deviation (SD) and number of people assessed for both the intervention and comparison groups to calculate mean difference (MD) and 95% CI. If the MD was reported without individual group data, we used this to report the study results. If more than one study measured the same outcome using different tools, we calculated the standardised mean difference (SMD) and 95% CI using the inverse variance method in Review Manager Web 2020.
For CBAs, we intended to use appropriate effect measures for dichotomous outcomes (RR, adjusted RR) and for continuous outcomes (relative % change postintervention, SMD). For ITS, we would have included the following effect measures: 1. change in level of the outcome at the first point after the introduction of the intervention, and 2. the postintervention slope minus the preintervention slope. These estimates would have been calculated from regression models adjusting for autocorrelation. It would not have been appropriate to present means and SDs of preintervention versus postintervention time points. However, no CBA or ITS studies were eligible for inclusion.
Unit of analysis issues
For cluster‐RCTs, we checked for unit‐of‐analysis errors. If errors had been found, and sufficient information was available, we would have re‐analysed the data using the appropriate unit of analysis, by taking account of the intracluster correlation (ICC). To determine estimates of ICC we would have contacted authors of included studies or, if this was not possible, imputed them using estimates from external sources. If it had not been possible to obtain sufficient information to re‐analyse the data, we would have reported effect estimates and annotated 'unit‐of‐analysis error'. However, it was not deemed necessary to re‐analyse data for the included cluster‐RCTs.
Dealing with missing data
We contacted study authors to obtain missing data (participant, outcome, or summary data). For participant data, where possible, we conducted analysis on an intention‐to‐treat basis; otherwise we analysed data as reported. We reported the levels of loss to follow‐up and assessed this as a source of potential bias. For missing outcome or summary data, we imputed missing data where possible and reported any assumptions in the Differences between protocol and review section.
Assessment of heterogeneity
Where studies were considered similar enough to allow pooling of data using meta‐analysis, we assessed the degree of heterogeneity by visual inspection of forest plots and by examining the Chi² test for heterogeneity. We reported our reasons for deciding that studies were sufficiently similar to pool statistically. We quantified heterogeneity using the I² statistic. An I² value of 50% or more represented substantial levels of heterogeneity, but this value was interpreted in light of the size and direction of effects and the strength of the evidence for heterogeneity, based on the P value from the Chi² test (Higgins 2021a). Where heterogeneity was present in pooled effect estimates, we explored possible reasons for variability by conducting subgroup analysis.
Where we detected substantial clinical, methodological, or statistical heterogeneity across included studies (greater than 75%) we did not report pooled results from meta‐analysis but instead used a narrative approach to data synthesis. In this event, we attempted to explore possible clinical or methodological reasons for this variation by grouping studies that were similar in terms of country, Indigenous populations, and types of family‐centred healthcare intervention to explore differences in intervention effects.
Assessment of reporting biases
We assessed reporting bias qualitatively, based on the characteristics of the included studies (e.g. if only small studies that indicated positive findings were identified for inclusion). We did not have sufficient studies (at least 10) for inclusion in the review to construct funnel plots to investigate small‐study effects, which may indicate publication bias (Higgins 2021a).
Data synthesis
We decided to meta‐analyse outcome data based on whether the interventions were sufficiently similar in terms of participants, settings, comparisons, and outcome measures to ensure meaningful conclusions from a statistically pooled result. Because of the anticipated variability in the intervention types and populations of included studies, we used a random‐effects model for meta‐analysis.
Where we were unable to pool data for a meta‐analysis, we summarised the results narratively. We explored possible clinical or methodological reasons for this variation by grouping studies that were similar in terms of the major types of intervention (i.e. environmental, communication, educational, counselling, family support) and explored differences in intervention effects. We explored the possibility of organising the data by Indigenous population and child's age; however, this was not feasible given the small number of studies. Within the data categories, we explored the main comparisons of the review:
intervention versus usual care; and
one form of family‐centred care intervention versus another.
Subgroup analysis and investigation of heterogeneity
We had planned to investigate three potential effect modifiers through subgroup analyses to determine whether they might impact the intervention effect. We were only able to report on the intervention type (i.e. environmental, communication, educational, counselling, family support), with too few studies available to report on Indigenous population (Aboriginal, Torres Strait Islander, First Nations, Metis, Inuit, American Indian, Native Alaskan, Native Hawaiian, Maori/Tangata Whenua); and age of child.
Sensitivity analysis
We intended to perform a sensitivity analysis to assess the impact on the primary outcomes of excluding studies assessed at high risk of bias and by comparing the results from fixed‐effect versus random‐effects meta‐analyses. We did not complete a sensitivity analysis for risk of bias as all studies in a meta‐analysis had a low risk for sequence generation, and it was appropriate to assess studies using a random‐effects analysis. Instead, we completed a sensitivity analysis on objective versus subjective outcomes. We excluded outcomes that were not from a validated questionnaire or tool, or were not administrative data. We reported this additional analysis in the Differences between protocol and review section.
Summary of findings and assessment of the certainty of the evidence
We prepared a summary of findings table to present the results of the meta‐analysis, based on the methods described in Chapter 15 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021). We included the major comparisons of the review, for each of the primary outcomes, including potential harms, as outlined in the Types of outcome measures section. Two review authors (NS; CA) independently assessed the certainty of evidence using the GRADE criteria. We provided a source and rationale for each assumed risk cited in the table/s, and used the GRADE system to rank the certainty of the evidence using the GRADEpro software (GRADEpro GDT). If meta‐analysis was not possible, we presented results in a narrative summary of findings table format, such as that used by Chan 2011.
Ensuring relevance to decisions in health care
The protocol received feedback from health providers and family members who receive a family‐centred intervention through Apunipima Cape York Health Service (Australia) about the meaning and relevance of family‐centred interventions for them (McCalman 2016). We intended to conduct further pre‐planned meetings using formal group methods to reach consensus decisions on key issues relating to the structure and methods of the review; however, this was not possible.
Results
Description of studies
See Characteristics of included studies table; Characteristics of excluded studies table; Characteristics of ongoing studies table; and additional features of included studies in Table 2.
1. Comparison of interventions of included studies.
| Study | Participants | Indigenous status | Country | Primary focus of intervention | Intervention | Broad category of intervention | Length of intervention | Control |
| Barlow 2006 | Pregnant adolescents aged 12–19 years at conception and at ≤ 28 weeks' gestation. | Navajo reservation (New Mexico) and White Mountain Apache reservation (Arizona) | USA | Negative parenting patterns | Home visits covering antenatal care, labour, delivery, breastfeeding, nutrition, parenting, home safety, immunisations, well‐baby care, family planning, sexually transmitted disease prevention, and maternal goal setting for personal and family development. | Education | 9 months: from 28 weeks' gestation to 6 months' postpartum; 25 home visits and 31 discrete lessons | Education: breastfeeding programme delivered in 23 home visits and 20 lessons. |
| Broughton 2013 | Pregnant women | Maori residing within the Waikato‐Tainui tribal area | New Zealand | Early childhood caries | Participants offered 4 intervention components: 1. provision of dental care during pregnancy; 2. FV application to teeth of children aged 6, 12, and 18 months; 3. MI, and 4. AG. The 3 themes covered by MI and AG are oral health knowledge, oral self‐care and oral health protection and community water fluoridation. | Counselling | 18 months: 1 intervention component delivered during pregnancy and 3 more at age 6, 12, and 18 months. | Minimal dental care: examination, x‐rays, pain relief, control of infection, scaling and prophylaxis, elimination of caries, restorations, and extractions. |
| Counselling delayed: intervention sessions were delivered after the infant was 24 months old. | ||||||||
| Family Spirit Nuture Part 1 2021 | Women aged ≥ 13 years with infants aged < 14 weeks | Navajo Nation (New Mexico) | USA | Early childhood obesity | Family Spirit Nuture curriculum lessons focus on: optimal infant feeding practices, responsive feeding, avoiding SSBs, optimal complementary feeding practices, and whole family healthy eating practices. | Education | 3 months: 6 lessons delivered from 3 to 6 months' postpartum | Education: 3 injury prevention lessons including childproofing, safe travel and outings with an infant, and strategies to avoid abuse and neglect. |
| Harrison 2010 | Women between weeks 12 and 34 of pregnancy and mothers of newborn, predentate infants | Cree Nation Eeyou Istchee (Quebec) | Canada | Early childhood caries | Mothers received MI aimed at reducing child caries. FV was offered after the infant was 1 year old. | Counselling | 24 months: 1 counselling session during pregnancy and up to 6 more sessions postnatally until their child's second birthday. | Education: mothers received 2 mailed culturally appropriate educational pamphlet describing healthy dental care practices for young children. FV was available to children in control group at local dental clinics. |
| Johnston 2010 | Infants aged 0–5 weeks | Maori and Australian Aboriginal | New Zealand and Australia | Acute respiratory illness | An 8‐week supply of free nicotine replacement therapy was available to participants and other household members. Counselling and follow‐up was provided. A fax referral to Quitline was offered, with proactive call back by Quitline. Resources were provided to support educational and behavioural coaching. | Education and counselling | 3 months: 3 home visits | Usual care |
| HCSF 1 2007 | Children aged 2–5 years | Bad River Band of Lake Superior Chippewa Indians, the Lac du Flambeau Band of Lake Superior Chippewa Indians, the Menominee Nation, and the Oneida Nation | USA | Obesity | Year 1: mentor‐led lessons with the primary carer and child, discussions and activities to help the carer and child learn about the topic, behaviour change related to the topic, and goal setting to attempt behaviour change. Families attended 3 mentor‐led group sessions. Year 2: monthly group meetings focused on topics such as basic nutrition concepts and ideas for physical activities. Monthly newsletters were disseminated for the 2 years | Education | 2 years: year 1: 12 toolkit lessons and 3 group lessons; year 2: 12 monthly group lessons | Education: year 1: same 12 lessons by mail + monthly newsletter. year 2: only monthly newsletter |
| Family Spirit Trial 2012 | Pregnant adolescents aged 12–19 years at conception and at ≤ 28 weeks' gestation. | White Mountain Apache reservation (Arizona), San Carlos Apache Reservations (Arizona), Fort Defiance communities on the Navajo Reservation (Arizona) | USA | Reduce health and behavioural risks | The Family Spirit curriculum lessons focused on 3 domains: 1. parenting skills across early childhood (0–3 years); 2. maternal drug abuse prevention; and 3. maternal life skills and positive psychosocial development. | Education | 3 years: home visits occurred weekly through the end of pregnancy, biweekly until 4 months' postpartum, monthly between 4 and 12 months' postpartum, and bimonthly between 12 and 36 months' postpartum; 45 home visits and 43 lessons | Optimised standard care: visits include 7 antenatal visits, 9 well‐baby visits during the first 3 years of life, and 4 social support visits between years 2 and 3. |
| Quissell 2014 | Head Start children aged 3–5 years and their primary carers | Navajo Nation | USA | Early childhood caries | The application of FV for children and oral health promotion activities for children and carers. | Education | 1 year: 4 times for FV; oral health promotion was 5 for children and 4 times for adults. | Usual care: usual oral health care made available by dental providers usually the Indian Health Service. FV was available at clinics. |
| Tipene‐Leach 2014 | Women booking for antenatal care | Maori | New Zealand | Sudden unexpected death in infancy | The provision of the wahakura a traditional sleeping device. | Environment | Single provision | A portable standing bassinet |
| HCSF 2 2017 | American Indian children aged 2–5 years and their primary carers | American Indian/Alaska Native | USA | Obesity | 12 monthly mailed healthy lifestyle lessons, items, and children's books addressing 6 intervention targets. Adults were supported by social media engagement via 2 weekly text messages and invitation to an optional, site‐specific Facebook group | Education | 1 year: 12 monthly mail outs; 2 weekly text messages | Education: child safety curriculum delivered in 12 mailed safety newsletters and related materials |
| Walkup 2009 | Pregnant adolescents aged 12–22 years at conception and at ≤ 28 weeks' gestation | Navajo reservation (New Mexico) and White Mountain Apache reservation (Arizona) | USA | Behavioural health problems | Home visits providing developmentally timed antenatal and infant‐care parenting lessons, as well as family planning, substance abuse prevention, and problem‐solving and coping‐skills lessons. | Education | 9 months: from 28 weeks' gestation to 6 months' postpartum; 25 home visits | Education: breastfeeding and nutrition programme delivered in 23 home visits. |
AG: anticipatory guidance; FV: fluoride varnish; MI: motivational interviewing; SSB: sugar sweetened beverage.
Results of the search
We identified 21,644 citations from electronic databases, 424 additional records from abstract searches and forward citations (Figure 1). After removal of 831 duplicates, there remained 21,237 records included for initial screening. We read the titles and abstracts and deleted 20,879 citations at this stage. We reviewed 358 full‐text articles relevant to the review and excluded 295 articles with reasons (see Characteristics of excluded studies table). The main reasons were 193 studies with an incorrect study design, 54 had the wrong intervention, 45 had the wrong study population, and we were unable to locate three articles (Figure 1). We identified eight ongoing studies (12 articles) (see Characteristics of ongoing studies table), and there were no studies awaiting classification. We included 63 articles, trials registrations, and conference proceedings that provided data on 11 studies (see Characteristics of included studies table). We contacted authors of six studies to request information about inclusion of the study or data for the meta‐analysis, or both. We received responses from three study authors (four studies; Broughton 2013; HCSF 1 2007; HCSF 2 2017; Johnston 2010).
1.
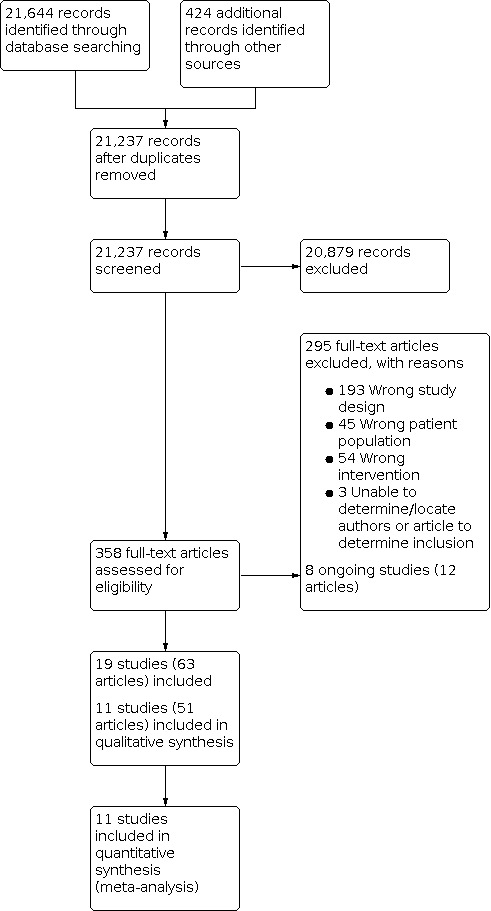
Study flow diagram.
Included studies
We included 11 studies (Barlow 2006; Broughton 2013; Family Spirit Nuture Part 1 2021; Harrison 2010; HCSF 1 2007; HCSF 2 2017; Johnston 2010; Quissell 2014; Family Spirit Trial 2012; Tipene‐Leach 2014; Walkup 2009).
Design
All included studies were published between 2006 and 2021 and were reported as RCTs. Eight RCTs were randomised at the individual level (Barlow 2006; Family Spirit Nuture Part 1 2021; HCSF 1 2007; HCSF 2 2017; Johnston 2010; Family Spirit Trial 2012; Tipene‐Leach 2014; Walkup 2009), and two studies were cluster‐RCTs (Harrison 2010; Quissell 2014). One RCT was a standard intervention comparison study design until control children were 24 months old and then received the intervention for one year. The control group then became a 'delayed intervention' (Broughton 2013). Six studies included carer–child dyads (Harrison 2010; HCSF 1 2007; HCSF 2 2017; Johnston 2010; Family Spirit Nuture Part 1 2021; Quissell 2014), and five studies randomised pregnant mothers (Barlow 2006; Broughton 2013; Family Spirit Trial 2012; Tipene‐Leach 2014; Walkup 2009). Two studies provided pilot data (Barlow 2006; Walkup 2009) for the development of the Family Spirit Program intervention (Family Spirit Trial 2012). These trials were then used to inform the Family Spirit Nuture trial (Family Spirit Nuture Part 1 2021). In addition, the Healthy Child, Strong Families programme has completed two trials, the second (HCSF 2 2017) expanding on the first (HCSF 1 2007). All other studies were independent of other included studies. All studies were funded with five studies receiving funds from government funding bodies and charities (Barlow 2006; HCSF 1 2007; Johnston 2010; Tipene‐Leach 2014; Walkup 2009), five government funded only (Broughton 2013; Harrison 2010; HCSF 2 2017; Quissell 2014; Family Spirit Trial 2012), and one was funded from multiple charities and an Indian Health Service (Family Spirit Nuture Part 1 2021).
Settings
There were seven studies in the USA (Barlow 2006; HCSF 1 2007; HCSF 2 2017; Quissell 2014; Family Spirit Trial 2012; Walkup 2009; Family Spirit Nuture Part 1 2021), two in New Zealand (Tipene‐Leach 2014; Broughton 2013), one in Canada (Harrison 2010), and one in both Australia and New Zealand (Johnston 2010). Studies delivered in a range of clinical settings including four studies in Indian Health Services (Barlow 2006; Family Spirit Trial 2012; Family Spirit Nuture Part 1 2021; Walkup 2009), two in Indian Health Services and Head Start Centres (HCSF 1 2007; HCSF 2 2017), one in Head Start Centres (Quissell 2014), one in a hospital maternity service and Aboriginal Community Controlled Organisations (Johnston 2010), one in a health clinic (Harrison 2010), and one in primary healthcare services and dental clinics (Broughton 2013). Tipene‐Leach 2014 delivered their intervention at a Maori midwifery practice, a Maori Women's Welfare League/urban marae, a Primary Health Organisation and a single weaver working with community networks in her own community. Studies provided six interventions to Indigenous families on reservation (Barlow 2006; HCSF 1 2007; Quissell 2014; Family Spirit Trial 2012; Walkup 2009; Family Spirit Nuture Part 1 2021), one in a remote area (Harrison 2010), one in a metropolitan area (Johnston 2010), one in an urban area (Tipene‐Leach 2014), one on four reservations, one urban site (HCSF 2 2017), and one in a regional area (Broughton 2013).
Participants
The 11 studies recruited 1270 mother–child dyads and 1924 children aged less than five years at baseline. There were 626 mother–child dyads and 968 children aged less than five years who were part of family‐centred care and 644 mother–child dyads and 956 children aged less than five years in the control group. Indigenous peoples varied substantially across all studies, particularly in the USA, where studies included more than one tribal nation (Table 2).
Control groups
Three studies provided usual care to the control group (Johnston 2010; Quissell 2014; Family Spirit Trial 2012). The remaining eight studies provided some form of 'minimal' intervention to the control group as it was often deemed inappropriate by participating communities for families to receive nothing (Barlow 2006; Harrison 2010; HCSF 1 2007; HCSF 2 2017; Tipene‐Leach 2014; Walkup 2009; Broughton 2013; Family Spirit Nuture Part 1 2021). One study also provided the family‐centred care intervention to the control children when they were aged 24 months for one year (Broughton 2013). Six studies providing some form of education (Barlow 2006; Harrison 2010; HCSF 1 2007; HCSF 2 2017; Walkup 2009; Family Spirit Nuture Part 1 2021), one study provided basic dental care (Broughton 2013) and one study provided an alternative sleeping device (Tipene‐Leach 2014). Education included a home visiting breastfeeding programme (Barlow 2006), a home visiting breastfeeding and nutrition programme (Walkup 2009), a home visiting child safety programme (Family Spirit Nuture Part 1 2021), monthly mailed lessons and newsletters (HCSF 1 2007), mailed culturally appropriate pamphlets and standard dental care (Harrison 2010), and monthly mailed child safety newsletters (HCSF 2 2017) (Table 2).
Intervention groups
Scores for family‐centredness ranged from 28 to 44 out of a potential score of 52 (Appendix 1). Overall, most studies met the family‐centredness criteria for sharing information with families, culturally competent health care, and parent/professional collaboration. Studies were less likely to provide financial support (e.g. supporting families to receive government supplements for parents to care for their children) and family‐to‐family support.
The focus of interventions varied and included early childhood caries (Broughton 2013; Harrison 2010; Quissell 2014), childhood obesity (Family Spirit Nuture Part 1 2021; HCSF 1 2007; HCSF 2 2017), child behavioural problems (Family Spirit Trial 2012; Walkup 2009); negative parenting patterns (Barlow 2006), child acute respiratory illness (Johnston 2010), and sudden unexpected death in infancy (Tipene‐Leach 2014) (Table 2). Based on the 'types of interventions', seven were categorised as predominantly educational (Barlow 2006; Family Spirit Nuture Part 1 2021; Family Spirit Trial 2012; HCSF 1 2007; HCSF 2 2017; Quissell 2014; Walkup 2009), two involved counselling (Broughton 2013; Harrison 2010), and one each of education and counselling (Johnston 2010) and environmental improvements (Tipene‐Leach 2014). One study also compared a family‐centred care intervention to a family‐centred care intervention through a counselling intervention received from birth compared to the same intervention (delayed) received from when the child was aged 24 months (Broughton 2013).
Nine interventions were delivered face‐to‐face either through home or group sessions except for HCSF 2 2017, which was a mailed intervention and Tipene‐Leach 2014, who provided the intervention once with written instructions. For the seven trials that had more than one face‐to‐face interaction, the duration of interventions ranged from three months to three years (Tipene‐Leach 2014).
Nearly all studies employed or supported Indigenous community members or already existing Indigenous health workers to deliver or participate in the studies, which is consistent with Indigenous research ethical guidelines (Ewen 2019). Seven studies employed local Indigenous members or respected community members to deliver the intervention (Barlow 2006; Broughton 2013; Family Spirit Nuture Part 1 2021; Family Spirit Trial 2012; HCSF 1 2007; Quissell 2014; Walkup 2009). Two studies utilised existing paraprofessional health workers to deliver their interventions (Harrison 2010; Johnston 2010). One study employed local community members to be project co‐ordinators at each site (HCSF 2 2017), and one study employed a research nurse (Tipene‐Leach 2014). Nine studies provided training to their staff. The most extensive training reported was approximately 500 hours, which also required interventionists to demonstrate mastery of the training content prior to implementing the study (Barlow 2006; Walkup 2009). This was completed to ensure fidelity to the intervention. Other studies provided training ranging from more than 80 hours' extensive training on the trial protocol, policies research ethics, and the intervention (Family Spirit Trial 2012); a two‐day training workshop in the first year, again in the second year, and follow‐up throughout the project (Harrison 2010); and two weeks of training with a refresher prior to starting, and again during the intervention (Quissell 2014). One study did not state the duration of training but gave details on what training they provided and to whom (HCSF 1 2007). Three studies provided training but did not include specific details (Family Spirit Nuture Part 1 2021; HCSF 2 2017; Johnston 2010).
Community engagement
Consumer and community involvement is a core principle for Indigenous research to help facilitate translation and sustainability of programmes. All studies included consumer and community involvement, but levels of involvement varied. Ten studies completed consultation prior to the study commencing and received input from communities on a range of research activities such as study design (Broughton 2013; Family Spirit Trial 2012; Harrison 2010), reference groups for monitoring progress of the intervention (HCSF 1 2007; HCSF 2 2017; Johnston 2010; Quissell 2014), and providing feedback on manuscripts for publications related to the study (Johnston 2010; Walkup 2009). Ten studies applied some level of cultural adaptation to resources, activities, and materials (Barlow 2006; Broughton 2013; Family Spirit Nuture Part 1 2021; Family Spirit Trial 2012; Harrison 2010; HCSF 1 2007; HCSF 2 2017; Johnston 2010; Quissell 2014; Walkup 2009). Maori community members developed the Tipene‐Leach 2014 intervention.
Outcomes
Studies reported outcomes related to overall health and well‐being, psychological health and emotional behaviour of children, physical health, developmental health outcomes of children, family health‐enhancing lifestyle or behaviour, psychological health of parent/carer, adverse events, parenting knowledge and awareness, service access and utilisation, and economic costs. There were no secondary outcomes for family evaluation of care and family‐centredness of consultation processes reported. No studies reported on the perspective of health service provider. Table 3 provides outcomes included in the review compared against intervention, control, measure, timing of outcomes, and whether the outcome was included in a meta‐analysis. We described studies with data that could not be included in a meta‐analysis narratively. The Characteristics of included studies table provides a list of outcomes and time points not used for the review.
2. Comparison of outcomes for inclusion in review.
| Study | Control group | Family‐centred care intervention category | Type of outcome | Name of outcome | Outcome measured on | Name of validated survey, questionnaire, or instrument? | Short term | Medium term | Long term | Included in a meta‐analysis |
| Primary outcomes | ||||||||||
| Overall health and well‐being | ||||||||||
| Walkup 2009 | Minimal education | Education | Continuous | Competence domain | Child | ITSEA | — | X | — | X |
| Family Spirit Trial 2012 | Usual care | Education | Continuous | Competence domain | Child | ITSEA | — | X | — | X |
| HCSF 2 2017 | Minimal education | Education | Continuous | BMI z‐score | Child | BMI; scale and measure | — | X | — | X |
| HCSF 1 2007 | Minimal education | Education | Continuous | BMI z‐score | Child | BMI; scale and measure | — | X | — | X |
| Family Spirit Nuture Part 1 2021 | Minimal education | Education | Continuous | BMI z‐score | Child | BMI; scale and measure | — | X | — | X |
| Quissell 2014 | Usual care | Education | Continuous | Caries: decayed, missing, or filled spaces | Child | NA | — | X | — | X |
| Barlow 2006 | Minimal education | Education | Continuous | Depression | Mother | CES‐D | — | X | — | X |
| Tipene‐Leach 2014 | Usual care | Environment | Binary | Fully breastfed: yes | Child | No | — | X | — | X |
| Johnston 2010 | Usual care | Education and counselling | Count | New episodes of acute respiratory illness | Child | Yes | — | X | — | X |
| Harrison 2010 | Minimal education | Counselling | Continuous | Caries: tooth level caries prevalence (d2‐4efs > 0) | Child | NA | — | — | X | X |
| Broughton 2013 | Minimal dental care | Counselling (not delayed) | Continuous | Caries: decayed, missing, or filled spaces | Child | NA | — | — | X | X |
| Psychological health and emotional behaviour of children | ||||||||||
| Walkup 2009 | Minimal education | Education | Continuous | Competence domain | Child | ITSEA | — | X | — | X |
| Family Spirit Trial 2012 | Usual care | Education | Continuous | Competence domain | Child | ITSEA | — | X | — | X |
| Harrison 2010 | Minimal education | Counselling | Binary | Parent reported 'dental caries‐related' child quality of life. Answered "yes" to > 1 quality of life question. | Child | Yes: in‐house with a previous survey involving 301 children | — | — | X | — |
| Family Spirit Trial 2012 | Usual care | Education | Continuous | Competence domain | Child | ITSEA | — | — | X | — |
| Physical health and developmental outcomes of children | ||||||||||
| Family Spirit Nuture Part 1 2021 | Minimal education | Education | Continuous | BMI z‐score | Child | BMI; scale and measure | X | — | — | — |
| Tipene‐Leach 2014 | Usual care | Environment | Binary | Fully breastfed: yes | Child | No | X | — | — | — |
| HCSF 2 2017 | Minimal education | Education | Continuous | BMI z‐score | Child | BMI; scale and measure | — | X | — | X |
| HCSF 1 2007 | Minimal education | Education | Continuous | BMI z‐score | Child | BMI; scale and measure | — | X | — | X |
| Family Spirit Nuture Part 1 2021 | Minimal education | Education | Continuous | BMI z‐score | Child | BMI; scale and measure | — | X | — | X |
| Quissell 2014 | Usual care | Education | Continuous | Caries: decayed, missing, or filled spaces | Child | NA | — | X | — | X |
| Tipene‐Leach 2014 | Usual care | Environment | Binary | Fully breastfeeding: yes | Child | No | — | X | — | X |
| Johnston 2010 | Usual care | Education and counselling | Count | New episodes of acute respiratory illness | Child | Yes | — | X | — | X |
| Harrison 2010 | Minimal education | Counselling | Continuous | Caries: tooth level caries prevalence (d2‐4efs > 0) | Child | NA | — | — | X | X |
| Broughton 2013 | Minimal dental care | Counselling (not delayed) | Continuous | Caries: decayed, missing, or filled spaces | Child | NA | — | — | X | X |
| Broughton 2013 | Counselling (delayed)a | Counselling (not delayed) | Continuous | Caries: decayed, missing, or filled spaces | Child | NA | — | — | X | — |
| Family enhancing lifestyle or behaviour outcomes | ||||||||||
| Barlow 2006 | Minimal education | Education | Continuous | Cohesion | Family | Not reported | X | — | — | — |
| Tipene‐Leach 2014 | Usual care | Environment | Binary | Maternal sleep quality: good | Mother | No | X | — | — | — |
| Tipene‐Leach 2014 | Usual care | Environment | Binary | Infants sleep position: back position | Child | No | X | — | — | — |
| HCSF 1 2007 | Minimal education | Education | Continuous | SF‐12: Physical Health component | Carer | SF‐12 | — | X | — | X |
| HCSF 2 2017 | Minimal education | Education | Continuous | SF‐12: Physical Health component | Carer | SF‐12 | — | X | — | X |
| Walkup 2009 | Minimal education | Education | Continuous | HOME | Mother | HOME | — | X | — | X |
| Barlow 2006 | Minimal education | Education | Continuous | Cohesion | Family | Not reported | — | X | — | X |
| Family Spirit Trial 2012 | Usual Care | Education | Continuous | HOME | Mother | HOME | — | X | — | X |
| Family Spirit Nuture Part 1 2021 | Minimal education | Education | Continuous | Infant SSB consumption | Child | Adapted Pre‐School Beverage Intake Questionnaire | — | X | — | X |
| Quissell 2014 | Usual care | Education | Continuous | Oral health behaviour | Carer | Not reported | — | X | — | X |
| Tipene‐Leach 2014 | Usual care | Environment | Binary | Maternal sleep quality: good | Mother | No | — | X | — | X |
| Tipene‐Leach 2014 | Usual care | Environment | Binary | Infants sleep position: back position | Child | No | — | X | — | — |
| Johnston 2010 | Usual care | Education and counselling | Binary | Full smoking ban in home | Family | No | — | X | — | X |
| Johnston 2010 | Usual care | Education and counselling | Binary | In last 7 days, infant has been around tobacco smoke | Child | No | — | X | — | — |
| Family Spirit Trial 2012 | Usual care | Education | Continuous | HOME | Mother | HOME | — | — | X | — |
| Psychological health of parent/carer | ||||||||||
| Barlow 2006 | Minimal education | Education | Continuous | Depression | Mother | CES‐D | X | — | — | — |
| Walkup 2009 | Minimal education | Education | Continuous | Depression | Mother | CES‐D | X | — | — | — |
| Family Spirit Trial 2012 | Usual care | Education | Continuous | Depression | Mother | CES‐D | X | — | — | — |
| Barlow 2006 | Minimal education | Education | Continuous | Depression | Mother | CES‐D | — | X | — | X |
| Walkup 2009 | Minimal education | Education | Continuous | Depression | Mother | CES‐D | — | X | — | X |
| HCSF 1 2007 | Minimal education | Education | Continuous | SF‐12: Mental Health component | Carer | SF‐12 | — | X | — | X |
| HCSF 2 2017 | Minimal education | Education | Continuous | SF‐12: Mental health component | Carer | SF‐12 | — | X | — | X |
| Family Spirit Trial 2012 | Usual care | Education | Continuous | Depression | Mother | CES‐D | — | X | — | X |
| Family Spirit Trial 2012 | Usual care | Education | Continuous | Depression | Mother | CES‐D | — | — | X | — |
| Adverse events | ||||||||||
| Harrison 2010 | Minimal education | Counselling | Unclear | Adverse events related to the intervention. Unclear what specific events were included | Child | NA | — | — | X | — |
| Family Spirit Nuture Part 1 2021 | Minimal education | Education | Unclear | Emergency department presentations and hospital admissions were recorded as potential adverse events | Child | NA | — | — | X | — |
| Secondary outcomes | ||||||||||
| Parenting knowledge and awareness | ||||||||||
| Barlow 2006 | Minimal education | Education | Continuous | Skills | Mother | No | X | — | — | — |
| Barlow 2006 | Minimal education | Education | Continuous | Skills | Mother | No | — | X | — | X |
| Walkup 2009 | Minimal education | Education | Continuous | Involvement | Mother | Parent Involvement Scale from the Substance Abuse and Mental Health Services Administration measure | — | X | — | — |
| Family Spirit Trial 2012 | Usual care | Education | Continuous | Home safety practices | Mother | No | — | X | — | X |
| Family Spirit Nuture Part 1 2021 | Minimal education | Education | Continuous | Maternal SSB knowledge index | Mother | No | — | X | — | — |
| Family Spirit Trial 2012 | Usual care | Education | Continuous | Knowledge | Mother | No | — | — | X | X |
| Service access and utilisation | ||||||||||
| Johnston 2010 | Usual care | Education and counselling | Count | Hospitalisation for acute respiratory illness | Child | Yes | — | X | — | — |
| Harrison 2010 | Minimal education | Counselling | Count | Number of visits to dentist for tooth pain | Child | Yes | — | — | X | — |
| Economic costs | ||||||||||
| Harrison 2010 | Minimal education | Counselling | Cost | Cost‐effectiveness | Health system | NA | — | — | X | — |
aWhen control children were aged 24 months, they received the intervention for one year and are called the delayed intervention group.
BMI: body mass index; CES‐D: Center for Epidemiologic Studies Depression Scale; HOME: Home Observation for Measurement of the Environment; ITSEA: Infant Toddler Social Emotional Assessment; NA: not applicable; SF‐12: 12‐item Short Form Survey; SSB: sugar‐sweetened beverage.
Studies included in the meta‐analysis
Where appropriate, we completed meta‐analyses grouping data by outcomes. As studies provided multiple time points for each outcome, we used the longest time point for each study. Data on available outcomes was guided by the information provided in Table 3. We included all 11 studies in at least one meta‐analysis (Barlow 2006; Broughton 2013; Family Spirit Nuture Part 1 2021; Family Spirit Trial 2012; Harrison 2010; HCSF 1 2007; HCSF 2 2017; Johnston 2010; Quissell 2014; Tipene‐Leach 2014; Walkup 2009).
Excluded studies
There were 299 studies that were considered ineligible for the review (see Characteristics of excluded studies table). Reasons for exclusion included ineligible study design, population, and intervention. Although some studies had multiple reasons for exclusion, we reported the primary reason to optimise efficiency of the screening process.
Risk of bias in included studies
Summaries of risk of bias judgements for the included studies are provided in Figure 2 and Figure 3. The authors judgements for each type of bias are presented in the Characteristics of included studies table with reasons.
2.

Risk of bias summary: review authors' judgement on each risk of bias domain across all included studies.
3.

Risk of bias graph: review authors' judgement on each risk of bias domain as percentages across all included studies.
Allocation
All 11 studies were at low risk of bias for random sequence generation through using computer or web‐based programmes, drawing lots, or providing sufficient information to deem that sequence generation had occurred at random.
Seven studies had a low risk of bias for allocation concealment with randomisation occurring after enrolment through central allocation or sealed numbered envelopes (Barlow 2006; Family Spirit Nuture Part 1 2021; Family Spirit Trial 2012; HCSF 2 2017; Johnston 2010; Tipene‐Leach 2014; Walkup 2009). The remaining four studies had an unclear risk of bias for allocation concealment with little to no information provided (Broughton 2013; Harrison 2010; HCSF 1 2007; Quissell 2014).
We assessed the two cluster‐RCTs for the domain selective cluster recruitment. Both studies recruited participants after randomisation. One study was at unclear risk of bias as the randomisation prior to enrolment of children in Head Start Centres did not appear to influence recruitment, with less than 1% of participants declining (Quissell 2014). There were similar numbers in each group; however, there were some baseline differences in participant characteristics. The other study was at high risk of bias as individuals who recruited the participants also delivered the intervention (Harrison 2010). There were also some baseline differences in recruitment including fewer intervention mothers who had already delivered their babies at the time of enrolment, had visited a dentist for toothache, and had other children with a previous tooth extraction.
Blinding
Given the nature of the interventions, it was not possible to blind participants or people delivering the interventions to group allocation. As a result, all studies were at high risk of performance bias.
Four studies reported adequate blinding of outcome assessments and were at low risk of bias on the detection bias domain (Broughton 2013; Family Spirit Trial 2012; Harrison 2010; Quissell 2014). Three studies were at unclear risk of bias for this domain due to one study reporting that only the primary outcome had a blinded assessment (Johnston 2010), one study reported that some outcomes were assessed blind but it was unclear about other outcomes (Tipene‐Leach 2014), and for one study it was unclear whether trained staff who collected data were blind to outcome assessments (Family Spirit Nuture Part 1 2021). Four studies were at high risk of bias as the same person who delivered the intervention also completed the data collection (Barlow 2006; HCSF 1 2007; HCSF 2 2017; Walkup 2009).
Incomplete outcome data
All studies completed an intention‐to‐treat or modified intention‐to‐treat analysis. Four studies were at low risk on this domain with low attrition levels during follow‐up (Family Spirit Nuture Part 1 2021; HCSF 2 2017; Johnston 2010; Tipene‐Leach 2014). The remaining seven studies were at high risk of bias due to their reported high levels of attrition (Barlow 2006; Broughton 2013; Family Spirit Trial 2012; Harrison 2010; HCSF 1 2007; Quissell 2014; Walkup 2009).
Selective reporting
Three studies were at low risk of bias with study protocols that had sufficient detail to determine that the data provided were based on definitions in the protocol and analysis completed as described (HCSF 1 2007; HCSF 2 2017; Johnston 2010). One study was at unclear risk of bias because the published protocol had insufficient detail to determine whether selective outcome reporting occurred (Harrison 2010). We were unable to locate a protocol or trial registration for one study and deemed this study at unclear risk of bias (Barlow 2006). Six studies were at high risk of bias for selective outcome reporting as they had only provided adjusted analyses, reported binary and continuous data for the same outcome, combined time points in their analysis, or did not report on all the outcomes they collected (Broughton 2013; Family Spirit Nuture Part 1 2021; Family Spirit Trial 2012; Quissell 2014; Tipene‐Leach 2014; Walkup 2009).
Other potential sources of bias
Seven studies were at low risk of bias with no other obvious sources of bias (Barlow 2006; Broughton 2013; Family Spirit Nuture Part 1 2021; HCSF 2 2017; Johnston 2010; Quissell 2014; Tipene‐Leach 2014). Three studies were at unclear risk of bias because of deviations from intended interventions and cultural appropriateness of outcome measures (Family Spirit Trial 2012; Harrison 2010; Walkup 2009). One study was at high risk of bias due to changing participant allocation after randomisation (HCSF 1 2007). No sensitivity analysis was completed to determine whether this influenced outcomes.
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
Overall health and well‐being
All 11 studies contributed data to the analysis. Pooled data included outcomes from two studies reporting psychological health and emotional behaviour of children (Family Spirit Trial 2012; Walkup 2009), eight on physical health and developmental outcomes of children (Broughton 2013; Family Spirit Nuture Part 1 2021; Harrison 2010; HCSF 1 2007; HCSF 2 2017; Johnston 2010; Quissell 2014; Tipene‐Leach 2014), and one on maternal mental health (Barlow 2006). The pooled effect estimate suggested that family‐centred care may improve the overall health and well‐being of Indigenous children and their families compared to no family‐centred care (SMD 0.14, 95% CI 0.03 to 0.24; 11 studies, 2386 participants; very low‐certainty evidence; Analysis 1.1; Table 1). There was low heterogeneity (I² = 31%). The level of confidence in the evidence is very uncertain due to high risk of bias in multiple domains for included studies and the outcomes having different underlying constructs.
1.1. Analysis.

Comparison 1: Primary outcomes, Outcome 1: Overall health and well‐being
Psychological health and emotional behaviour of children
Of the three studies that assessed psychological health and emotional behaviour of children, two could be combined for a meta‐analysis (Family Spirit Trial 2012; Walkup 2009). There was little to no difference in the competence domain of the Infant Toddler Social Emotional Assessment (ITSEA) at 12 months' postpartum between families that received the family‐centred care intervention and the control group (MD: change in ITSEA competence score 0.04, 95% CI −0.03 to 0.11; I² = 0%; 2 studies, 384 participants; very low‐certainty evidence; Analysis 1.2; Table 1).
1.2. Analysis.

Comparison 1: Primary outcomes, Outcome 2: Psychological health and emotional behaviour of children
Family Spirit Trial 2012 also measured the competence domain of ITSEA at 36 months' postpartum and found no evidence of an improvement for families who participated in the family‐centred care intervention (adjusted MD 0.04, 95% CI −0.01 to 0.09). There was no difference in dental‐related child quality of life with 19.4% (21/110) American Indian families in the family‐centred counselling intervention answering 'yes' to at least one question from the self‐reported 'Dental‐caries‐related' Child Quality of Life survey compared to 28.2% (37/131) families who participated in the control group (Harrison 2010).
Physical health and developmental health outcomes of children
Eight studies assessed 11 physical health and developmental outcomes of children. There was little to no difference between the family‐centred care and control groups (SMD 0.13, 95% CI 0.00 to 0.26; 8 studies, 1961 participants; I² = 42%; very low‐certainty evidence; Analysis 1.3; Table 1).
1.3. Analysis.

Comparison 1: Primary outcomes, Outcome 3: Physical health and developmental health outcomes of children
There were three outcomes not included in the analysis. One study reported no difference in full breastfeeding in the short‐term, with 37 infants in the family‐centred care group fully breastfeeding compared to 35 infants in the control group (intervention (95 infants) 40%; control (88 infants) 39%; P = 0.91) (Tipene‐Leach 2014). It is unclear what the definition of 'fully breastfeeding' was in this study. One study assessed body mass index (BMI) z‐score when infants were aged less than two months (Family Spirit Nuture Part 1 2021). Authors reported no difference between groups (mean: intervention −0.44 (standard error (SE) 0.13); control −0.40 (SE 0.13); adjusted MD −0.04, 95% CI −0.41 to 0.33). One study compared a family‐centred care education intervention delivered from birth (not delayed) to a delayed family‐centred care education intervention delivered at 24 months. At 36 months, the authors reported a reduction in the mean caries in the not delayed group (mean: not delayed 1.7 (SD 3.1); delayed 2.3 (SD 5.2); MD 0.73, 95% CI 0.58 to 0.93) (Broughton 2013).
Family health‐enhancing lifestyle or behaviour outcomes
Nine studies reported data on family health‐enhancing lifestyle or behaviour outcomes (Barlow 2006; Family Spirit Nuture Part 1 2021; Family Spirit Trial 2012; HCSF 1 2007; HCSF 2 2017; Johnston 2010; Quissell 2014; Tipene‐Leach 2014; Walkup 2009). There was little to no difference between receiving family‐centred care and the control group (SMD 0.16, 95% CI −0.06 to 0.39; 9 studies, 1969 participants; I² = 76%; very low‐certainty evidence; Analysis 1.4; Table 1).
1.4. Analysis.

Comparison 1: Primary outcomes, Outcome 4: Family health‐enhancing lifestyle or behavioural outcomes
The remainder of the outcomes were subjective and reported at various time points (Table 3). Two results were from studies that assessed the effect of the Family Spirit Program (Barlow 2006; Family Spirit Trial 2012). One study measured family cohesion at two months with no evidence of improvement for families who participated in family‐centred care compared to the control group (adjusted for baseline MD 0.60, 95% CI −0.3 to 1.5; 41 participants) (Barlow 2006). The second study used the Home Observation for Measurement of the Environment tool to measure the quality of a child's home environment at 36 months' postpartum and found no evidence of improvement in those that received the family‐centred care intervention (long‐term outcome; adjusted MD 0.10, 95% CI −0.54 to 0.73) (Family Spirit Trial 2012).
Three outcomes were reported from the same study with one outcome related to the mother and two for the infant (Tipene‐Leach 2014). At three months' postpartum, 93% (88/95) of mothers reported good maternal sleep quality in the intervention group compared to 90% (79/88) of mothers in the usual care group. There was no evidence of improvement between the family‐centred care intervention and the control group. For infants, the study reported whether infants slept on their back at three and six months' postpartum. The authors reported that 85% (81/95) of infants slept on their back in the intervention group at three months compared to 83% (73/88) of infants in the control group. At six months, 87% (77/89) of infants in the intervention group slept on their back compared to 85% (71/84) in the usual care group. There was also no evidence that family‐centred care improved this outcome. The third outcome was whether infants had been around tobacco smoke in the last seven days, with 18% (23/126) of infants in the intervention group being around tobacco smoke compared to 12% (15/128) in the usual care group at 12 months' postpartum (Johnston 2010).
Psychological health of parent/carer
For carer mental health, there was little to no difference between participants in family‐centred care compared to the control group (SMD 0.10, 95% CI −0.03 to 0.22; 5 studies, 975 infants; I² = 0%; very low‐certainty evidence; Analysis 1.5; Table 1).
1.5. Analysis.

Comparison 1: Primary outcomes, Outcome 5: Psychological health of parent/carer
Four outcomes were not included in the meta‐analysis. Three studies measured maternal depression using the Center for Epidemiological Studies Depression scale at two months (Barlow 2006; Family Spirit Trial 2012; Walkup 2009). Each study found little to no difference between the family‐centred care intervention and the control group (Barlow 2006: adjusted MD −3.10, 95% CI −8.8 to 2.5; 41 participants; Family Spirit Trial 2012: adjusted MD −0.34, 95% CI −1.19 to 0.51; 322 participants; Walkup 2009: adjusted MD: 0.05, 95% CI −4.0 to 4.1; 125 participants). In addition, Family Spirit Trial 2012 measured maternal depression at 36 months using repeated measures (2, 6, 12, 18, 24, 30, and 36 months). The authors found an improvement in maternal depression favouring family‐centred care compared to no family‐centred care (adjusted MD −1.17, 95% CI −2.05 to −0.28; 322 participants).
Adverse events or harms
Two studies narratively reported that there were no adverse events and side effects as a result of the intervention (Family Spirit Nuture Part 1 2021; Harrison 2010). One study reported that no aspect of the intervention including the application of fluoride varnish resulted in any adverse events (Harrison 2010). One study reported emergency department visits or hospital admissions as adverse events (Family Spirit Nuture Part 1 2021). No adverse events were deemed to be the result of the intervention. Neither study reported data to support the claim of no adverse events or side effects. As a result, we found the evidence is very uncertain about the effect of family‐centred care on adverse events of harm (Table 1).
Secondary outcomes
Parenting knowledge and awareness
There was a small improvement in parenting knowledge and awareness for families who participated in family‐centred care compared to the control group (SMD 0.20, 95% CI 0.01 to 0.38; 3 studies, 445 participants; I² = 0%; Analysis 2.1). Two studies were not included in the analysis. One study measured parenting knowledge to 36 months using repeated measures. Authors found an improvement in the family‐centred care group compared to the control group (adjusted MD 1.28, 95% CI 0.70 to 1.86) (Family Spirit Trial 2012). One study measured maternal sugar‐sweetened beverage knowledge through self‐report and only reported that more than 90% of questions were correctly answered in both groups throughout the study (Family Spirit Nuture Part 1 2021).
2.1. Analysis.

Comparison 2: Secondary outcomes, Outcome 1: Parenting knowledge and awareness
Family evaluation of care
No studies reported family evaluations of care outcomes.
Service access and utilisation
Two studies reported service access and utilisation. However, when completing a meta‐analysis there was substantial heterogeneity (I²= 81%; Analysis 2.2). One study measured the number of visits to a dentist for tooth pain at 30 (SD 3) months with 17 visits recorded for 110 children in the family‐centred care intervention group compared to 32 visits from 131 children in the usual care group (Harrison 2010). Data were provided as descriptive count data and dental pain was considered an adverse outcome (i.e. higher number of visits was a worse outcome). Johnston 2010 found no difference in the rate of hospitalisations for acute respiratory illness in Aboriginal and Maori infants in the first year of life by mothers who participated in a family‐centred care intervention compared to usual care (incidence rate ratio 1.23, 95% CI 0.70 to 2.15). Based on the data available, it appears that family‐centred care may have little to no effect on service access and utilisation for children.
2.2. Analysis.

Comparison 2: Secondary outcomes, Outcome 2: Service access and utilisation
Family‐centredness of consultation processes
No studies reported family‐centredness of consultation processes.
Economic costs and outcomes associated with the interventions
One study reported on the cost‐effectiveness of delivering a family‐centred counselling intervention compared to the control group to reduce dental caries (Harrison 2010). On a subsample of 173 children the authors investigated the cost/averted case of decayed, missing, or filled tooth surface (averted DMFTS) and the cost/averted for early childhood caries (averted ECC case). Cost‐effectiveness ratios were estimated to be USD 81/averted DMFTS and USD 3900/averted ECC case by net benefit regression using the trial data. Overall, the authors concluded that the programme is expected to improve the dental health of children; however, this would be at an increased cost in dental service provision compared to the control group.
Subgroup analysis
We explored possible clinical reasons for differences in intervention effects by subgrouping studies with similar types of intervention delivered (i.e. environmental, communication, educational, counselling, family support). Only analyses that had different types of family‐centred care interventions were included (e.g. education compared to counselling) (Table 3). We compared this to any control group (i.e. standard care, usual care, or a 'minimal' intervention group). There were no studies that compared a family‐centred care intervention to another family‐centred care intervention in the analysis.
There was little to no difference in subgroup differences reported for outcomes overall health and well‐being of children (P = 0.10; Analysis 3.1), physical health and developmental outcomes of children (P = 0.11; Analysis 3.2), or family health‐enhancing lifestyle or behaviour outcomes (P = 0.57; Analysis 3.3).
3.1. Analysis.

Comparison 3: Subgroup analysis, Outcome 1: Overall health and well‐being
3.2. Analysis.

Comparison 3: Subgroup analysis, Outcome 2: Physical health and developmental health outcomes of children
3.3. Analysis.

Comparison 3: Subgroup analysis, Outcome 3: Family health‐enhancing lifestyle or behavioural outcomes
Sensitivity analysis
We performed sensitivity analysis on primary outcomes to determine whether subjective outcomes impacted whether family‐centred care improved outcomes compared to the control group.
Overall health and well‐being outcome
Ten studies remained in the sensitivity analysis and we removed one study (Tipene‐Leach 2014). After removing the subjective outcome, there was still evidence suggesting that family‐centred care may improve the overall health and well‐being of Indigenous children and their families compared to no family‐centred care there (SMD 0.12, 95% CI 0.01 to 0.22; 10 studies, 2208 participants; I² = 34%). The results were unchanged from the original analysis.
Physical health and developmental outcomes of children
Seven studies remained in the sensitivity analysis and we removed one study (Tipene‐Leach 2014). There was no evidence that physical health and developmental outcomes of children improved when they participated in family‐centred care (SMD 0.10, 95% CI −0.02 to 0.23; 7 studies, 1783 participants; I² = 35%). The results were unchanged from the original analysis.
Family health‐enhancing lifestyle or behaviour outcomes
Four studies remained in the sensitivity analysis and we removed five studies (Barlow 2006; Johnston 2010; Quissell 2014; Tipene‐Leach 2014; Family Spirit Nuture Part 1 2021). There was no evidence of an improvement in this outcome between family‐centred care and the control group (SMD −0.00, 95% CI −0.16 to 0.15; 4 studies, 932 participants; I² = 26%). The results were unchanged from the original analysis.
Discussion
Our initial scoping study on this topic aimed to assess the certainty of the evidence and identify published literature on family‐centred care interventions for Indigenous early childhood well‐being (McCalman 2017). This scoping study identified three RCTs, which resulted in the initiation and completion of this current Cochrane Review. The evidence from this review will help primary care services base their decisions on optimal interventions to improve the health and well‐being of children and their families from Indigenous populations using the most current and reliable evidence.
Summary of main results
We included nine RCTs and two cluster‐RCTs that investigated the effect of family‐centred care delivered by primary healthcare services for Indigenous early childhood well‐being. Studies were aimed at improving various health conditions with 1270 mother–child dyads and 1924 children aged less than five years recruited. Seven studies delivered family‐centred education care, which was the most common type of intervention delivered.
Each study reported different types of measures and time points for each of our reported outcomes. As a result, we used a broad and flexible approach to determine whether family‐centred care delivered by primary healthcare services was effective in improving physical, psychosocial, and behavioural outcomes of Indigenous children and their families. We found that family‐centred care may improve the overall health and well‐being of Indigenous children and their families. However, the level of confidence in the evidence was very uncertain due to high risk of biases on multiple domains including no blinding of participants and people delivering the intervention, people delivering the intervention also collected the data, high attrition, and selective outcome reporting. In addition, we downgraded the level of certainty for indirectness as the outcomes measured had different underlying constructs.
For primary outcomes, psychological health and emotional behaviour of children, physical health and developmental health of children, family enhancing lifestyle and behaviours, and psychological health of parents and carers, there was very low‐certainty evidence of little to no improvement for families who participated in family‐centred care compared to those in the control group. Certainty of evidence was downgraded for these outcomes due to a high risk of bias for non‐blinding of participants and people delivering the intervention, people delivering the intervention also collected the data, a high attrition of participants, and selective outcome reporting. Evidence was also downgraded due to small sample size (psychological health and emotional behaviour of children outcome) and outcomes measured using different constructs (e.g. for psychological health of parent/carer we combined general mental health with specific depression scales). Two studies reported that there were no adverse events due to the intervention.
For secondary outcomes, there was a small improvement in parenting knowledge and awareness for families who participated in family‐centred care compared to the control group. Two studies reported on service utilisation and access. We were unable to combine the outcomes of these studies in a meta‐analysis; however, narratively these studies did not report improvements as a result of the family‐centred care intervention. One study reported the economic benefits of delivering family‐centred care with the authors suggesting that family‐centred care was a cost‐effective way to improve early childhood caries in Indigenous children. There were no data for family evaluation of care or family‐centredness of consultation processes. No studies reported on the perspectives of the health service providers.
We completed subgroup analyses to explore whether types of family centred‐care interventions such as environmental, communication, educational, counselling, or family support were effective in improving outcomes. We found little to no difference in subgroups for the outcomes overall health and well‐being of children, physical health and developmental outcomes of children, or family health‐enhancing lifestyle or behaviour outcomes.
Overall completeness and applicability of evidence
Overall, we do not consider that there was publication bias. Many trials did not report positive results and were still published. However, there was a dearth of evidence from Australia, Canada, and New Zealand with seven of the 11 trials from the USA. Data from six trials from the USA were also from two studies: the Family Spirit programme (four finished and two ongoing) and the two HCSF trials. All 11 trials were deemed to be culturally considered with some level of consumer and community engagement that included consultation prior to the study or reference groups for the study duration, or both.
Ten studies assessed family‐centred care as a single broad intervention type (e.g. environmental, education, counselling) and one as a multiple intervention type (e.g. education and counselling) (Table 2). Although studies delivered several types of family‐centred care interventions, there is scope for trials to consider whether combining types of family‐centred care or more complex strategies would result in improved outcomes. However, to achieve this there would need to be an increased funding commitment and workforce to deliver more complex interventions, both of which can be difficult to achieve. In addition, there were no family‐centred care interventions that included communication or family support as avenues of delivering care for children and their families. This may be the result of our search strategy not detecting some types of family‐centred care, such as communication interventions, as they are more focussed on care co‐ordination and continuity of care. We assessed studies using the family‐centred care criteria previously developed by Shields 2012; however, this assessment was based on what was provided in the publications and should be considered a minimum score for each intervention. It is likely that word limitations in peer review publishing may have prevented fuller descriptions and hence higher scores.
The control groups included in the trials were either care as usual or a minimal intervention that did not include family‐centred care. Although one study included both a minimal intervention and a delayed family‐centred care intervention delivered from 24 months of age for one year, there were no studies comparing one type of family‐centred care intervention to another. Given that 8/11 studies provided a minimal intervention to the control group, this may explain why there were only small or no effects seen. It is possible that had only usual care been delivered, there may have been more substantive effects seen for the outcomes in this review. However, we are unlikely to see many more trials that deliver usual care only as it is largely deemed inappropriate by communities for families to receive nothing.
Studies for inclusion in the review required the child to be the main focus with the delivery of family‐centred care. As such, many of our outcomes were child‐related. However, we recognise that family‐centred care, particularly in the context of Indigenous health, includes care not only for child health, antenatal care, family support, and early childhood care, but also for adults including mental health and chronic disease care. The latter aspects of care aim to improve adult health outcomes. Family‐centred care also aims to prevent, reduce, and intervene in adverse childhood events and to reduce the long‐term impacts that chronic disease, depression, and suicidal ideation have on families. Indeed, Indigenous governed health services have greater capacity to influence portfolios outside primary health care to other non‐health programmes. As such, this review does not capture this wider context of family‐centred care programmes that are delivered in communities.
Quality of the evidence
Using GRADE methodology, we assessed the certainty of evidence for all primary outcomes. Table 1 reports six outcomes comparing family‐centred care to any control group. All outcomes were at very low certainty of evidence. This was largely due to the high risk of bias in the studies, and indirectness. For the delivery of the intervention, it was not feasible to blind participants and those delivering the intervention. The location of the trials also impacted on the capacity of trials to recruit different staff to implement the trial and collect data. This occurred in four trials where the person who delivered the intervention also collected the data (Barlow 2006; HCSF 1 2007; HCSF 2 2017; Walkup 2009). Additionally, many of the outcomes reported in the studies were self‐reported, particularly in studies that were not disease specific. Lastly, long follow‐up times in some studies resulted in substantial loss to follow‐up. As a result, studies were scored at a high risk of bias particularly for blinding of participants and personnel and incomplete outcome data. We expect newer trials that overcome some of these issues will result in a change in the results presented.
Potential biases in the review process
We attempted to reduce bias in the review process. We requested clarification from three study authors whether interventions were delivered by primary healthcare services; all authors responded to our requests (four studies: Broughton 2013; HCSF 1 2007; HCSF 2 2017; Johnston 2010). We also requested data from one trial; this was supplied by the corresponding author (HCSF 2 2017). We contacted all authors of included and ongoing studies to determine whether there were any other studies for inclusion resulting in identification of one additional ongoing study (Family Spirit Nuture Part 2 2019).
We completed an intensive literature search, and included ITS and CBA study designs to ensure we captured relevant studies in this area. Compared to our scoping review (McCalman 2017), we found an additional eight RCTs and eight ongoing trials. At all stages, at least two review authors independently reviewed, selected, extracted, and completed the risk of bias on all data with discrepancies discussed with a third review author. Decisions on which outcomes to include in the review were completed by six review authors who selected outcomes of importance and relevance (as outlined in Differences between protocol and review). To minimise bias, review authors were unaware of the results of the outcomes during this process.
Agreements and disagreements with other studies or reviews
Only one Cochrane Review has investigated family‐centred care within the hospital setting for children aged from birth to 12 years and found one RCT that met the criteria (Shields 2012). The review found moderate‐quality evidence of the effects of family‐centred care for children in hospitals, which suggested some benefit for children's clinical care, parental satisfaction, and costs; there was no evidence of harms. There are several systematic and scoping reviews on non‐Indigenous populations and family‐centred care interventions. One qualitative review to support the Cochrane effectiveness review (Shields 2012) found that negotiation and perceptions by both health service providers and families influenced how family‐centred care was delivered (Shields 2006). This is supported by one scoping review examining the implementation of family‐centred care in the delivery of maternal and child health services for children aged less than five years (Ridgway 2021). Authors identified 13 qualitative studies and found four key themes regarding successful implementation: treating and maintaining respectful relationships; adapting and contextualising care; supporting autonomy and agency; and building a shared understanding through effective communication. One systematic review on the effect of family‐centred care on the health of preterm infants and parents in the neonatal intensive care unit found a significant reduction in readmission rates (Ding 2019). Similar to our review, they also found evidence of improvement in parenting skills and knowledge of families who participated in family‐centred care compared to the control group. Two additional systematic reviews on family‐centred care for children with special healthcare needs (USA population only) and preterm infants and parents in the neonatal intensive care unit described improvements for children and families who participated in the intervention (Kuhlthau 2011; Segers 2019). However, both of these studies were descriptive with no formal analysis completed. Several of these reviews are now outdated, included a range of study designs, and provided no formal data analysis to support their conclusions. Other than our scoping review, we were unable to find reviews that focused on Indigenous children or children aged from birth to five years within the primary healthcare setting (McCalman 2017).
Authors' conclusions
Implications for practice.
This review found very low‐certainty evidence that family‐centred care delivered by primary healthcare services may improve the overall health and well‐being of children and their families. There was also evidence to suggest that families who participated in family‐centred care increased their parenting knowledge and awareness to a small degree. However, for all other outcomes it is unclear whether family‐centred care improves specific child health and well‐being outcomes. We consider family‐centred care to be promising, but further research is required to establish its effectiveness.
Implications for research.
There is a need for research in several areas. High‐quality trials are needed to generate evidence to determine whether family‐centred care improves the health and well‐being of Indigenous children aged less than five years. There is a need to clarify the principles and core components of family‐centred care for Indigenous families and that is informed by Indigenous world views of family, which will guide greater consistency in outcome measurement and evaluation to determine the effect. There were no communication and family support interventions delivered with family‐centred education the most common intervention delivered. Broadening the types of family‐centred interventions to include these other styles may improve the health and well‐being of Indigenous children and their families. Furthermore, no studies reported on the quality of care and the impact of family‐centred care from the perspective of health service providers. These outcomes are important to determine the effectiveness of family‐centred care.
History
Protocol first published: Issue 12, 2016
Notes
This review is based on standard text and guidance provided by Cochrane Consumers and Communication.
Acknowledgements
We thank the editors and staff of the Cochrane Consumers and Communication Review Group, particularly the Managing Editors, Anne Jones and Bronwen Merner, for their input to the protocol. In addition, we would like to thank Editors, Rebecca Ryan and Louisa Walsh, and Information Specialist Anne Parkhill for the support and assistance during the review process. We would like to acknowledge Anne Lawson, Central Production Service, Cochrane for copy editing our review. We would like to also acknowledge our consumer peer reviewer, Victoria Sinka – Pitjantjatjara an Australian South Sea Islander parent, for her time and comments on this review.
We thank the Centre for Research Excellence (CRE) for Improving Health Services for Aboriginal and Torres Strait Islander Children for providing funding for the review, and Apunipima Cape York Health Service for initiating and supporting the review.
We thank Beatriz Ayala, in her role as research assistant, for assisting in the screening of titles and abstracts for this review. We would like to acknowledge the support of Mark Wenitong, Katrina Keith, Alan Ruben, and Komla Tsey for their support in developing the protocol for the review.
Appendices
Appendix 1. Criteria for family‐centredness
Family‐centredness rating score (13 elements)
Rating 0, 1, 2, 3, or 4
| Barlow 2006 | Broughton 2013 | Family Spirit Nuture Part 1 2021 | Family Spirit Trial 2012 | Harrison 2010 | HCSF 1 2007 | HCSF 2 2017 | Johnston 2010 | Quissell 2014 | Tipene‐Leach 2014 | Walkup 2009 | |
| C1: family as a constant | |||||||||||
| Family as a constant in child's life | 2 | 4 | 3 | 2 | 4 | 2 | 4 | 4 | 1 | 4 | 3 |
| Recognising family strengths | 2 | 4 | 2 | 2 | 3 | 2 | 4 | 2 | 1 | 4 | 2 |
| Parent/professional collaboration | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 2 | 4 | 3 | 4 |
| Needs‐based family support | 3 | 4 | 2 | 3 | 3 | 4 | 4 | 4 | 3 | 2 | 4 |
| Flexible provision of health care | 3 | 4 | 2 | 3 | 3 | 4 | 4 | 2 | 3 | 4 | 4 |
| Sharing information with families | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 4 |
| C2: culturally responsive | |||||||||||
| Culturally competent health care | 3 | 4 | 4 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| Respecting family diversity | 3 | 4 | 2 | 3 | 2 | 2 | 3 | 3 | 2 | 3 | 4 |
| Providing financial support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C3: supporting family individuality and need for different types of family support | |||||||||||
| Respecting family coping methods | 1 | 3 | 1 | 1 | 1 | 4 | 4 | 1 | 2 | 2 | 2 |
| Providing emotional support | 3 | 4 | 2 | 3 | 0 | 3 | 3 | 2 | 1 | 1 | 4 |
| Family‐to‐family support | 1 | 1 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 1 | 4 |
| Attending to the developmental needs of children and families | 3 | 4 | 2 | 3 | 3 | 2 | 3 | 0 | 3 | 3 | 4 |
| Total score | 32 (62%) | 44 (85%) | 28 (54%) | 32 (62%) | 30 (58%) | 37 (72%) | 43 (83%) | 28 (54%) | 28 (54%) | 34 (65%) | 43 (83%) |
(Exclude studies with family‐centredness score < 26 or 50%) 0: article included no evidence that the author(s) either implicitly or explicitly addressed, endorsed, or advocated adoption of adherence to the elements of family‐centred care. 1: article included a minimal amount of implicit evidence that the author(s) advanced adoption or support of the elements of family‐centred care. 2: article included numerous instances of implicit evidence that the author(s) advanced adoption or support of the elements of family‐centred care. 3: article included a minimal amount of explicit evidence that the author(s) advanced adoption or support of the elements of family‐centred care. 4: article included numerous instances of explicit evidence that the author(s) advanced adoption or support of the elements of family‐centred care Explicit evidence = an element was clearly stated and distinctly expressed. Implicit evidence = If it could be inferred that the author(s) descriptions, arguments, etc. were consistent with the intent of the elements of family‐centred care.
Appendix 2. CENTRAL search strategy
#1(aborigin* or indigen*):ti,ab,kw #2("first nation*" or "native people*"):ti,ab,kw #3"oceanic ancestry group":kw #4("first australian*" or "torres strait* islander*" or tiwi or maori* or "tangata whenua"):ti,ab,kw #5((american* or canadian) near/2 indian*):ti,ab,kw #6tribes:kw #7([mh "united states"] or [mh canada] or [mh "new zealand"] or ("united states" or america* or canad* or alaska* or "new zealand*"):ti,ab,kw) and native*:ti,ab,kw #8(amerind* or metis or navajo or ojibw* or chippewa or algonquin or cree or arikara or iroquois or cherokee or mohawk or muscogee or choctaw or seminole or zuni or lakota or sioux or hopi or pima or tohono or yaqui):ti,ab,kw #9(aleut* or eskimo* or inuit* or inupiat* or yupik* or hawai*):ti,ab,kw #10{or #1‐#9} #11(family or families or father* or mother* or husband* or wife* or paternal or maternal or grandparent* or guardian* or parent or parents or parental or parenting or "child rearing" or childrearing):ti,ab,kw #12[mh "health personnel"] #13(((general or family) next (doctor* or practitioner* or physician*)) or ((health* or medical) near/2 (service* or personnel or organi*ation*)) or nurse* or nursing or provider* or worker* or aide or aides):ti,ab,kw #14((primary near/2 (care or health*)) or "patient cent*red"):ti,ab,kw #15#10 and (#11 or #12 or #13 or #14) #16[mh pregnancy] #17pregnan*:ti,ab,kw #18[mh "maternal health services"] #19"maternal welfare":kw #20[mh infant] #21[mh "child development"] #22"child development":ti,ab,kw #23(embryo* or fetus* or foetus* or "unborn child*" or prenatal or pre‐natal or perinatal or peri‐natal or postnatal or post‐natal or postpartum or post‐partum or obstetric* or midwife* or baby or babies or newborn or neonat* or neo‐nat* or childbirth or birth or infant* or toddler* or preschool* or pre‐school* or "nursery school*" or kindergarten or "early child*"):ti,ab,kw #24[mh "pregnancy complications"] #25[mh "infant newborn diseases"] #26[mh "neurodevelopmental disorders"] #27((*developmental* or learning or "child behavio*") next (disorder* or disease* or disab*)):ti,ab,kw #28[mh "child health services"] #29(child* and (health* or care or welfare or wellbeing or "well being")) or (family next (centred or centered or based or focused or focussed)) #30"early intervention*":ti,ab,kw #31((family or families or parent* or mother* or father* or maternal or paternal or guardian*) near/5 (educat* or teach* or instruct* or train* or coach* or counsel* or advis* or advice* or inform* or support* or program* or intervention*)):ti,ab,kw #32{or #16‐#31} #33#15 and #32
Appendix 3. MEDLINE search strategy
1. (aborigin* or indigen*).ti,ab,kf.
2. (first nation* or native people*).ti,ab,kf.
3. oceanic ancestry group/
4. (first australian* or torres strait* islander* or tiwi).ti,ab,kf.
5. (maori* or tangata whenua).ti,ab,kf.
6. american native continental ancestry group/
7. indians north american/
8. ((american* or canadian) adj2 indian*).ti,ab,kf.
9. (exp united states/ or exp canada/ or exp new zealand/ or (america* or canad* or alaska* or new zealand*).ti,ab,kw.) and native*.ti,ab,kf.
10. (amerind* or metis or navajo or ojibw* or chippewa or algonquin or cree or arikara or iroquois or cherokee or mohawk or muscogee or choctaw or seminole or zuni or lakota or sioux or hopi or pima or tohono or yaqui).ti,ab,kf.
11. inuits/
12. (aleut* or eskimo* or inuit* or inupiat* or yupik* or hawai*).ti,ab,kf.
13. or/1‐12
14. (family or families).mp.
15. (father* or mother* or husband* or wife* or paternal or maternal or grandparent* or guardian*).mp.
16. parenting/
17. exp child rearing/
18. (parent or parents or parental or parenting or child rearing or childrearing).ti,ab,kf.
19. exp health personnel/
20. (((general or family) adj (doctor* or practitioner* or physician*)) or ((health* or medical) adj2 (service* or personnel or organi#ation*)) or nurse* or nursing or provider* or worker* or aide or aides).ti,ab,kf.
21. ((primary adj2 (care or health*)) or patient cent?red).mp.
22. or/14‐21
23. 13 and 22
24. exp pregnancy/
25. pregnan*.mp.
26. exp maternal health services/
27. exp "embryonic and fetal development"/
28. exp fetus/
29. exp infant/
30. child preschool/
31. exp child development/
32. (embryo* or fetus or foetus or "unborn child*" or prenatal or pre‐natal or perinatal or peri‐natal or postnatal or post‐natal or postpartum or post‐patum or baby or babies or newborn or neonat* or neo‐nat* or childbirth or birth or obstetric* or midwife* or infant* or toddler* or preschool* or pre‐school* or "nursery school*" or kindergarten or "early child*").mp.
33. exp pregnancy complications/
34. exp infant newborn diseases/
35. exp neurodevelopmental disorders/
36. ((developmental* or learning or child behavio*) adj (disorder* or disease* or disab*)).ti,ab,kf.
37. "early intervention (education)"/
38. exp child health services/
39. child health/
40. child welfare/
41. ((child* and (health* or care or welfare or wellbeing or well being)) or (family adj (cent?red or based or focus?ed))).mp.
42. ((family or families or parent* or mother* or father* or maternal or paternal or guardian*) adj5 (educat* or teach* or instruct* or train* or coach* or counsel* or advis* or advice* or inform* or support* or program* or intervention*)).ti,ab,kf.
43. or/24‐42
44. 23 and 43
45. randomized controlled trial.pt.
46. controlled clinical trial.pt.
47. random*.tw.
48. placebo*.tw.
49. trial.tw.
50. groups.ab.
51. clinical trial.pt.
52. evaluation studies.pt.
53. research design/
54. follow up studies/
55. prospective studies/
56. cross over studies/
57. comparative study.pt.
58. controlled before after studies/
59. interrupted time series analysis/
60. (experiment* or intervention*).tw.
61. (pre test or pretest or post test or posttest).tw.
62. (preintervention or postintervention).tw.
63. time series.tw.
64. (cross over or crossover or factorial* or latin square).tw.
65. (assign* or allocat* or volunteer*).tw.
66. (control* or compar* or prospectiv*).tw.
67. (impact* or effect? or chang* or evaluat*).tw.
68. or/45‐67
69. 44 and 68
Appendix 4. Embase search strategy
1. exp indigenous people/
2. (aborigin* or indigen*).ti,ab,kw.
3. (first nation* or native people*).ti,ab,kw.
4. oceanic ancestry group/
5. exp australian aborigine/
6. (first australian* or torres strait* islander* or tiwi).ti,ab,kw.
7. "maori (people)"/
8. (maori* or tangata whenua).ti,ab,kw.
9. exp amerind people/
10. ((american* or canadian) adj2 indian*).ti,ab,kw.
11. (exp united states/ or exp canada/ or exp new zealand/ or (america* or canad* or alaska* or new zealand*).ti,ab,kw.) and native*.ti,ab,kw.
12. (amerind* or metis or navajo or ojibw* or chippewa or algonquin or cree or arikara or iroquois or cherokee or mohawk or muscogee or choctaw or seminole or zuni or lakota or sioux or hopi or pima or tohono or yaqui).ti,ab,kw.
13. exp eskimo‐aleut people/
14. native hawaiian/
15. (aleut* or eskimo* or inuit* or inupiat* or yupik* or hawai*).ti,ab,kw.
16. or/1‐15
17. (family or families).mp.
18. (father* or mother* or husband* or wife* or paternal or maternal or grandparent* or guardian*).mp.
19. (parent or parents or parental or parenting or child rearing or childrearing).mp.
20. health care personnel/
21. (((general or family) adj (doctor* or practitioner* or physician*)) or ((health* or medical) adj2 (service* or personnel or organi#ation*)) or nurse* or nursing or provider* or worker* or aide or aides).ti,ab,kw.
22. ((primary adj2 (care or health*)) or patient cent?red).mp.
23. or/17‐22
24. 16 and 23
25. exp pregnancy/
26. pregnan*.mp.
27. exp prenatal development/
28. human embryo/
29. fetus/
30. exp childbirth/
31. exp infant/
32. toddler/
33. preschool child/
34. child development/
35. (embryo* or fetus or foetus or "unborn child*" or prenatal or pre‐natal or perinatal or peri‐natal or postnatal or post‐natal or postpartum or post‐patum or baby or babies or newborn or neonat* or neo‐nat* or childbirth or birth or obstetric* or midwife* or infant* or toddler* or preschool* or pre‐school* or "nursery school*" or kindergarten or "early child*").mp.
36. maternal child health care/
37. exp newborn care/
38. infant welfare/
39. exp pregnancy complication/
40. exp newborn disease/
41. exp infant disease/
42. developmental disorder/
43. exp autism/
44. exp behavior disorder/
45. exp learning disorder/
46. ((developmental* or learning or child behavio*) adj (disorder* or disease* or disab*)).ti,ab,kw.
47. child health/
48. child health care/
49. ((child* and (health* or care or welfare or wellbeing or well being)) or (family adj (cent?red or based or focus?ed))).mp.
50. early childhood intervention/
51. early intervention/
52. parent counseling/
53. ((family or families or parent* or mother* or father* or maternal or paternal or guardian*) adj5 (educat* or teach* or instruct* or train* or coach* or counsel* or advis* or advice* inform* or support* or program* or intervention*)).ti,ab,kw.
54. or/25‐53
55. 24 and 54
56. randomized controlled trial/
57. controlled clinical trial/
58. single blind procedure/ or double blind procedure/
59. crossover procedure/
60. random*.tw.
61. trial.tw.
62. placebo*.tw.
63. ((singl* or doubl*) adj (blind* or mask*)).tw.
64. (experiment* or intervention*).tw.
65. epidemiology/
66. controlled study/
67. (pre test or pretest or post test or posttest).tw.
68. (preintervention or postintervention).tw.
69. (cross over or crossover or factorial* or latin square).tw.
70. (assign* or allocat* or volunteer*).tw.
71. (control* or compar* or prospectiv*).tw.
72. (impact* or effect? or chang* or evaluat*).tw.
73. time series analysis/
74. time series.tw.
75. or/56‐74
76. 55 and 75
77. limit 76 to embase
78. limit 76 to conference abstracts
79. or/77‐78
Appendix 5. PsycINFO search strategy
1. exp indigenous populations/
2. (aborigin* or indigen*).ti,ab,id.
3. (first nation* or native people*).ti,ab,id.
4. (first australian* or torres strait* islander* or tiwi).ti,ab,id.
5. (maori* or tangata whenua).ti,ab,id.
6. ((american* or canadian) adj2 indian*).ti,ab,id.
7. tribes/
8. ((america* or canad* or alaska* or new zealand*) and native*).ti,ab,id.
9. (amerind* or metis or navajo or ojibw* or chippewa or algonquin or cree or arikara or iroquois or cherokee or mohawk or muscogee or choctaw or seminole or zuni or lakota or sioux or hopi or pima or tohono or yaqui).ti,ab,id.
10. (aleut* or eskimo* or inuit* or inupiat* or yupik*).ti,ab,id.
11. hawai*.ti,ab,hw,id.
12. or/1‐11
13. (family or families).ti,ab,hw,id.
14. (father* or mother* or husband* or wife* or paternal or maternal or grandparent* or guardian*).ti,ab,hw,id.
15. (parent or parents or parental or parenting or child rearing or childrearing).ti,ab,hw,id.
16. health personnel/
17. (((general or family) adj (doctor* or practitioner* or physician*)) or ((health* or medical) adj2 (service* or personnel or organi#ation*)) or nurse* or nursing or provider* or worker* or aide or aides).ti,ab,id.
18. ((primary adj2 (care or health*)) or patient cent?red or client cent?red).ti,ab,hw,id.
19. or/13‐18
20. 12 and 19
21. ("120" or "140" or "160").ag.
22. exp pregnancy/
23. pregnan*.ti,ab,hw,id.
24. exp prenatal development/
25. exp prenatal care/
26. exp birth/
27. exp early childhood development/
28. exp preschool students/
29. kindergarten students/
30. (embryo* or fetus* or foetus* or unborn child* or prenatal or pre‐natal or perinatal or peri‐natal or postnatal or post‐natal or postpartum or post‐patum or baby or babies or newborn or neonat* or neo‐nat* or childbirth or birth or obstetric* or midwife* or infant? or toddler* or preschool* or pre‐school* or nursery school* or kindergarten or early child*).ti,ab,hw,id.
31. exp obstetrical complications/
32. exp neonatal disorders/
33. exp developmental disabilities/
34. ((developmental* or neurodevelopmental* or learning or child behavio*) adj (disorder* or disease* or disab*)).ti,ab,hw,id.
35. early intervention/
36. ((child* and (health* or care or welfare or wellbeing or well being)) or (family adj (cent?red or based or focus?ed))).ti,ab,hw,id.
37. family life education/
38. parent training/
39. ((family or families or parent* or mother* or father* or maternal or paternal or guardian*) adj5 (educat* or teach* or instruct* or train* or coach* or counsel* or advis* or advice* or inform* or support* or program* or interven*)).ti,ab,id.
40. or/21‐39
41. 20 and 40
42. random*.ti,ab,hw,id.
43. (experiment* or intervention*).ti,ab,hw,id.
44. trial*.ti,ab,hw,id.
45. placebo*.ti,ab,hw,id.
46. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
47. treatment effectiveness evaluation/
48. mental health program evaluation/
49. (pre test or pretest or post test or posttest).ti,ab,hw,id.
50. (preintervention or postintervention).ti,ab,hw,id.
51. (cross over or crossover or factorial* or latin square).ti,ab,hw,id.
52. (assign* or allocat* or volunteer*).ti,ab,hw,id.
53. (control* or compar* or prospectiv*).ti,ab,hw,id.
54. (impact* or effect? or chang* or evaluat*).ti,ab,hw,id.
55. time series.ti,ab,hw,id.
56. exp experimental design/
57. ("0430" or "0450" or "0451" or "1800" or "2100").md.
58. or/42‐57
59. 41 and 58
Appendix 6. CINAHL search strategy
S26 s25 Limiters ‐ Exclude MEDLINE records
S25 s9 and s24
S24 s10 or s11 or s12 or s13 or s14 or s15 or s16 or s17 or s18 or s19 or s20 or s21 or s22 or s23
S23 "early intervention*" or ((family or families or parent* or mother* or father* or maternal or paternal or guardian*) N5 (educat* or teach* or instruct* or train* or coach* or counsel* or advis* or advice* or inform* or support* or program* or intervention*))
S22 (child* and (health* or care or welfare or wellbeing or "well being")) or (family N1 (cent#red or based or focus#ed))
S21 MH child health services+
S20 (developmental* or learning or "child behavio*") N1 (disorder* or diasease* or disab*)
S19 MH mental disorders diagnosed in childhood+
S18 MH infant, newborn, diseases+
S17 MH pregnancy complications+
S16 embryo* or fetus* or foetus* or "unborn child*" or prenatal or pre‐natal or perinatal or peri‐natal or postnatal or post‐natal or postpartum or post‐patum or baby or babies or newborn or neonat* or neo‐nat* or childbirth or birth or obstetric* or midwife* or infant* or toddler* or preschool* or pre‐school* or "nursery school*" or kindergarten or "early child*"
S15 "child development*"
S14 MH infant+
S13 MH (maternal health services+ or maternal‐child care+ or maternal‐child welfare+)
S12 MH fetus+
S11 MH embryo+
S10 (MH pregnancy+) or pregnan*
S9 s7 and s8
S8 family or families or father* or mother* or husband* or wife* or paternal or maternal or grandparent* or guardian* or parent or parents or parental or parenting or "child rearing" or childrearing or ((general or family) N1 (doctor* or practitioner* or physician*)) or ((health* or medical) N2 (service* or personnel or organi?ation*)) or nurse* or nursing or provider* or worker* or aide or aides or (primary N2 (care or health*)) or "patient centred" or "patient centered" or MH health personnel+
S7 s1 or s2 or s3 or s4 or s5 or s6
S6 aleut* or eskimo* or inuit* or inupiat* or yupik* or hawai*
S5 amerind* or metis or navajo or ojibw* or chippewa or algonquin or cree or arikara or iroquois or cherokee or mohawk or muscogee or choctaw or seminole or zuni or lakota or sioux or hopi or pima or tohono or yaqui
S4 ((MH (united states+ or canada+)) or ("united states" or america* or canad* or alaska* or "new zealand*")) and native*
S3 ((american* or canadian*) N2 indian*) or tribes
S2 "first australian*" or "torres strait* islander*" or tiwi or maori* or "tangata whenua"
S1 aborigin* or indigen* or "first nation*" or "native people*"
Appendix 7. Informit search strategy
Indigenous Collection 14 databases: A+Education, AGIS Plus Text, Asia Collection, Australian Public Affairs (APAFT), Business Collection, EduTV, Engineering Collection, Families & Society Collection, Health Collection, Humanities & Social Sciences Collection, Indigenous Collection, Literature & Culture Collection, New Zealand Collection, TVNews
(family OR families OR father* OR mother* OR husband* OR wife* OR paternal OR maternal OR grandparent* OR guardian* OR parent*)
AND
(pregnan* OR embryo* OR fetus* OR foetus* OR "unborn child*" OR prenatal OR "pre‐natal" OR perinatal OR "peri‐natal" OR postnatal OR "post‐natal" OR postpartum OR "post‐partum" OR obstetric* OR midwife* OR baby OR babies OR newborn OR neonat* OR "neo‐nat*" OR childbirth OR birth OR infant* OR toddler* OR preschool* OR "pre‐school*" OR "nursery school*" OR kindergarten OR "early child*" OR "child develoment" OR "newborn disease*" OR "infant disease*" OR "early childhood disease*" OR developmental OR "learning disorder*" OR "child behavio*" OR "early intervention*" OR (child* AND (health* OR care OR welfare OR wellbeing OR "well being")))
AND
(random* OR control* OR trial OR assign* OR allocat* OR volunteer* OR experiment* OR intervention* OR compar* OR prospectiv* OR assess* OR impact* OR effect* OR chang* OR evaluat* OR blind* OR "cross over" OR crossover OR factorial OR "latin square" OR "time series")
Appendix 8. CENTRAL search strategy (scoping)
#1(aborigin* or indigen*):ti,ab,kw (Word variations have been searched) #2(first nation* or native people*):ti,ab,kw (Word variations have been searched) #3MeSH descriptor: [Oceanic Ancestry Group] explode all trees #4(first australian* or torres strait* islander*):ti,ab,kw (Word variations have been searched) #5maori*:ti,ab,kw (Word variations have been searched) #6MeSH descriptor: [American Native Continental Ancestry Group] explode all trees #7MeSH descriptor: [Indians, North American] explode all trees #8((american* or canadian) near/1 indian*) #9MeSH descriptor: [United States] explode all trees #10MeSH descriptor: [Canada] explode all trees #11MeSH descriptor: [New Zealand] explode all trees #12(america* or canad* or alaska* or new zealand*):ti,ab,kw (Word variations have been searched) #13native*:ti,ab,kw (Word variations have been searched) #14(#9 or #10 or #11 or #12) and #13 #15(metis or cherokee* or chippewa* or choctaw* or navajo* or sioux):ti,ab,kw (Word variations have been searched) #16MeSH descriptor: [Inuits] explode all trees #17(inuit* or eskimo* or inupiat* or yupik*):ti,ab,kw (Word variations have been searched) #18hawai*:ti,ab,kw (Word variations have been searched) #19#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #14 or #15 or #16 or #17 or #18 #20(family or families):ti,ab,kw (Word variations have been searched) #21(father* or mother* or husband* or wife* or paternal or maternal or grandparent* or guardian*):ti,ab,kw (Word variations have been searched) #22MeSH descriptor: [Parenting] explode all trees #23(parent or parents or parental or parenting):ti,ab,kw (Word variations have been searched) #24MeSH descriptor: [Maternal Health Services] explode all trees #25(prenatal or perinatal or postnatal or postpartum):ti,ab,kw (Word variations have been searched) #26#20 or #21 or #22 or #23 or #24 or #25 #27#19 and #26 #28MeSH descriptor: [Pregnancy] explode all trees #29pregnan*:ti,ab,kw (Word variations have been searched) #30MeSH descriptor: [Embryonic and Fetal Development] explode all trees #31MeSH descriptor: [Fetus] explode all trees #32MeSH descriptor: [Infant] explode all trees #33MeSH descriptor: [Child, Preschool] explode all trees #34(fetus* or foetus* or unborn child* or baby or babies or newborn or neonat* or infant? or toddler* or preschool* or preschool* or kindergarten or early child*):ti,ab,kw (Word variations have been searched) #35MeSH descriptor: [Pregnancy Complications] explode all trees #36MeSH descriptor: [Infant, Newborn, Diseases] explode all trees #37MeSH descriptor: [Neurodevelopmental Disorders] explode all trees #38MeSH descriptor: [Early Intervention (Education)] explode all trees #39#28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 #40#27 and #39
Data and analyses
Comparison 1. Primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall health and well‐being | 11 | 2386 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [0.03, 0.24] |
| 1.1.1 Body mass index (BMI) z‐score | 3 | 668 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.08, 0.25] |
| 1.1.2 Caries | 3 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.02, 0.37] |
| 1.1.3 Fully breastfed | 1 | 178 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.05, 0.99] |
| 1.1.4 New episode of acute respiratory illness | 1 | 289 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 1.1.5 Infant‐Toddler Social and Emotional Assessment (ITSEA) | 2 | 384 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.08, 0.32] |
| 1.1.6 Maternal mental health | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.09, 1.17] |
| 1.2 Psychological health and emotional behaviour of children | 2 | 384 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.03, 0.11] |
| 1.2.1 ITSEA | 2 | 384 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.03, 0.11] |
| 1.3 Physical health and developmental health outcomes of children | 8 | 1961 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.00, 0.26] |
| 1.3.1 BMI z‐score | 3 | 668 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.08, 0.25] |
| 1.3.2 Caries | 3 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.02, 0.37] |
| 1.3.3 Fully breastfeed | 1 | 178 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.05, 0.99] |
| 1.3.4 New episode of acute respiratory illness | 1 | 289 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 1.4 Family health‐enhancing lifestyle or behavioural outcomes | 9 | 1969 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.06, 0.39] |
| 1.4.1 Home environment | 2 | 404 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.13, 0.27] |
| 1.4.2 Parent/carer general physical health | 2 | 528 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.33, 0.27] |
| 1.4.3 Family cohesion | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.77, 0.48] |
| 1.4.4 Maternal sleep quality | 1 | 169 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.24, 0.93] |
| 1.4.5 Oral health behaviour | 1 | 451 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.02, 0.45] |
| 1.4.6 Full smoking ban in the home | 1 | 254 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.62, 0.47] |
| 1.4.7 Sugar‐sweetened beverages consumption | 1 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 1.02 [0.64, 1.40] |
| 1.5 Psychological health of parent/carer | 5 | 975 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.03, 0.22] |
| 1.5.1 12‐item Short Form Survey (SF‐12) Mental Health | 2 | 530 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.16, 0.19] |
| 1.5.2 Center for Epidemiologic Studies Depression Scale (CES‐D) | 3 | 445 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.01, 0.39] |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Parenting knowledge and awareness | 3 | 445 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.01, 0.38] |
| 2.1.1 Skills | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.38, 0.85] |
| 2.1.2 Involvement | 1 | 82 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.29, 0.59] |
| 2.1.3 Home safety practices | 1 | 322 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.02, 0.42] |
| 2.2 Service access and utilisation | 2 | 530 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.54, 0.30] |
Comparison 3. Subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Overall health and well‐being | 11 | 2386 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [0.03, 0.24] |
| 3.1.1 Family‐centred care (FCC) education | 7 | 1541 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [0.02, 0.23] |
| 3.1.2 FCC counselling | 2 | 378 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.15, 0.53] |
| 3.1.3 FCC environment | 1 | 178 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.05, 0.99] |
| 3.1.4 FCC education and counselling | 1 | 289 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 3.2 Physical health and developmental health outcomes of children | 8 | 1961 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.00, 0.26] |
| 3.2.1 FCC education | 4 | 1116 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.01, 0.24] |
| 3.2.2 FCC counselling | 2 | 378 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.15, 0.53] |
| 3.2.3 FCC environment | 1 | 178 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.05, 0.99] |
| 3.2.4 FCC education and counselling | 1 | 289 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 3.3 Family health‐enhancing lifestyle or behavioural outcomes | 9 | 1969 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.06, 0.39] |
| 3.3.1 FCC education | 7 | 1546 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.09, 0.43] |
| 3.3.2 FCC environment | 1 | 169 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.24, 0.93] |
| 3.3.3 FCC education and counselling | 1 | 254 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.62, 0.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barlow 2006.
| Study characteristics | ||
| Methods |
Study design: RCT Study recruitment: July 2001–February 2002; follow‐up 6 months' postpartum Published protocol/trial: none found |
|
| Participants |
Description: pregnant American Indian adolescents aged 12–19 years at conception and at ≤ 28 weeks' gestation (intervention n = 28, comparison n = 25) Exclusion criteria: serious medical, legal, or social problems that would preclude their ability to fully participate in the intervention and assessments Indigenous population: Navajo and White Mountain Apache reservations in New Mexico and Arizona Setting: USA, reservation Place of delivery: Indian Health Service Principle health condition: negative parenting patterns Age of mother: intervention < 18 years: 16 (57%), comparison < 18 years: 17 (68%) Gender of child: not reported First child in family: intervention 21 (75%), comparison 19 (76%) Family unit: living with parents: intervention 20 (71%), comparison 16 (64%) Socioeconomic status: not reported Employment of mother: currently employed: intervention 4 (14%), comparison 3 (12%) Education of mother: still in school: intervention 16 (57%), comparison 15 (60%) |
|
| Interventions |
Intervention Intervention name: Family Strengthening Intervention Intervention aim: to promote childcare knowledge, skills, and involvement among pregnant American Indian adolescents. Theory used to develop intervention: intervention was modelled on "Healthy Families America" a national programme founded on 12 research‐based principles to ensure quality of home‐visiting interventions for at‐risk families. The content was derived from extensive community input on what teen parents needed to learn and was based on the American Academy of Pediatrics guide to baby care: Caring for Your Baby and Young Child: Birth to Age 5. Consumer and community involvement: a community‐based participatory process was used to culturally adapt the programme including style, graphics, delivery, and content. Overall grouping: education Fees, reimbursement, or incentives: none reported Procedures: lessons covered antenatal care, labour, delivery, breastfeeding, nutrition, parenting, home safety, immunisations, well‐baby care, family planning, sexually transmitted disease prevention, and maternal goal setting for personal and family development. The curricular content was scheduled chronologically to provide key instruction at developmentally appropriate times for participants' children. The protocol included 25 home visits and 41 discrete lessons taught from 28 weeks' gestation to 6 months' postpartum (about 9 months total) by the educators using tabletop flip charts. Home visits were scheduled to last approximately 1.5 hours. Cultural adaptations, including style, graphics, delivery, and content, were achieved through a community‐based participatory process (Barlow 2006, p. 1102). Incentives, reimbursements, fees paid to individuals or organisations: none reported Materials: not reported Mode of delivery: individual home visits When and how often was the intervention delivered? intervention delivered from 28 weeks' gestation to 6 months' postpartum (about 9 months in total). 25 home visits and 41 discrete lessons could be provided. Who delivered the intervention? educators: American Indian women, bilingual, job history in health and human services, passed a background screening test, been a teen mother, or had a special interest in this group. Was there any training provided to the people who delivered the intervention? educators participated in > 500 hours of training and were tested to ensure they had mastered lesson content and delivery strategies prior to study implementation. Educators received daily supervision at the site and weekly supervision through cross‐site conference calls. Ongoing training occurred bimonthly throughout the study. Every 3 months, supervisors observed educators with participants and rated educators' professionalism, rapport, interpersonal skills, and adherence to the home‐visitation protocol. Was the study modified or adapted? none reported Was the fidelity assessed? fidelity to dose of intervention was completed. Intervention group completed 82% of 41 lessons and 85% of 25 expected home visits. The control group completed 86% of 20 lessons and 63% of 23 expected home visits. Comparison Breastfeeding Education Programme This was an education programme that was developed in 1996–1997 by Johns Hopkins Center for American Indian Health and the participating communities. Participants assigned to this arm received 23 home visits covering 20 breastfeeding lessons. Expected visit duration was 1–1.5 hours. Fees, reimbursement, or incentives: none reported |
|
| Outcomes | Overall health and well‐being: depression Family enhancing lifestyle or behaviour outcomes: family cohesion Parent/carer psychological health: depression Parenting knowledge and awareness: skills Time points: 2 and 6 months |
|
| Funding source and conflicts of interest |
Funding: Substance Abuse Mental Health Services Administration, the Ford Foundation, the Annie E. Casey Foundation, and the C. S. Mott Foundation Conflict of interest: none reported |
|
| Notes |
Other outcomes collected but not used within the review Mother: knowledge, involvement, family conflict, locus of control, self‐esteem, drug use, social support |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using web program. Quote: "Randomization stratified by site was determined by the Randomization.com Website prior to enrolling any study participants." |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred after enrolment; central allocation was used. Quote: "The randomization sequence for each site was stored in Baltimore, Md, by our data manager and was concealed from the key investigators and on‐site educators at all times. After each participant signed consent/assent forms and completed the baseline assessment, the educators faxed these materials to the data manager in Baltimore. The data manager checked that all assessments were properly completed, confirmed that the teen met inclusion criteria and no exclusion criteria, and then informed the educator of the participant's group assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to the intervention. Quote: "The participants and evaluators were not blind to intervention assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Evaluators both delivered the intervention and completed the data collection for all mothers. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis completed. There was a higher dropout in the intervention group compared to the control group. The intervention group were more likely to be living with their parents, still in school, and be recruited earlier in their pregnancies. The authors acknowledged this limitation. |
| Selective reporting (reporting bias) | Unclear risk | Unable to find a protocol or trial registration. |
| Other bias | Low risk | No other obvious sources of bias. |
Broughton 2013.
| Study characteristics | ||
| Methods |
Study design: RCT Study recruitment: July 2011–December 2012; follow‐up 36 months' postpartum Published protocol/trial: protocol published and trial registered; ACTRN12611000111976 and ACTRN12610000422022 |
|
| Participants |
Description: pregnant Maori women residing within the Waikato‐Tainui tribal area (intervention n = 126, control n =133) Exclusion criteria: none reported Indigenous population: Maori Setting: New Zealand, tribal area Place of delivery: primary healthcare services and dental clinics Principle health condition: early childhood caries Age of mother (years), mean: intervention 27.1 (SD 6.1), control 26.6 (SD 7.0) Gender of child: not reported First child in family: intervention 39 (32%), control 52 (40%) Family unit: none reported Socioeconomic status: held a community services card: intervention 83 (69%), control 81 (63%) Employment of mother: employed only: intervention 37 (34%), control 33 (29%); employed and benefits intervention: 11 (10%), control 10 (9%); benefits only: intervention: 51 (46%), control 58 (52%) Education of mother: up to secondary school: intervention 42 (38%), control 43 (38%); trade/polytechnic intervention: 45 (41%), control 39 (35%); university intervention: 45 (41%), control 28 (25%) |
|
| Interventions |
Intervention Intervention 1 name: not delayed intervention Intervention aim: to reduce dental disease burden and oral health inequalities among Maori children living in the Waikato‐Tainui tribal area of Aotearoa/New Zealand. Theory used to develop intervention: intervention was modelled on the Te Whare Tapa Whā model of health and well‐being which compares health to the 4 walls of a house and employs a set of values, principles, philosophy, and practice that is iwi‐derived and grounded in Waikato‐Tainui maatauranga (knowledge). Consumer and community involvement: study conducted within the North Island Tribal area of Waikato‐Tainui and was the responsibility of 2 tribally derived organisations: Raukura Hauora O Tainui and the Waikato‐Tainui College for Research and Development. Both organisations worked in partnership with a research team from the University of Otago. Overall grouping: counselling Fees, reimbursement, or incentives: none reported Procedures: participants were offered 4 intervention components: 1. provision of dental care during pregnancy; 2. FV application to the teeth of children aged 6, 12, and 18 months; 3. MI; and 4. AG. The themes covered by each MI/AG phase include: 1. oral health knowledge, including teeth eruption and teething, causes and prevention of childhood dental disease, and foods, beverages, and behaviours that are harmful to oral tissue; 2. oral self‐care – including ways to look after children's teeth, use of toothbrush, toothpaste and disclosing solution; and use of oral health services; 3. oral health protection and community water fluoridation. Materials: participants were provided with free basic dental care and support was provided for participants to access the dental clinic. Oral health packs, including toothbrushes and toothpaste, were provided to be consistent with the AG, Maori‐focused oral health promotion materials, and the New Zealand oral healthcare system (fluoridation and oral health aids). Mode of delivery: individual, face‐to‐face sessions When and how often was the intervention delivered? dental care was arranged during pregnancy or when the participant could attend, FV was provided at 6, 12 and 18 months for children, and the MI/AG sessions were implied to be delivered at the same interval as the FV. Who delivered the intervention? oral health professional: delivered the dental care and FV component; motivational interviewer: delivered the MI/AG component. Was there any training provided to the people who delivered the intervention? none reported Was the study modified or adapted? none reported Was the fidelity assessed? none reported Intervention 2 name: delayed intervention Intervention 2 description: after 24 months the control group received the intervention described above and was named the 'Delayed intervention' group. Comparison Minimal dental care Basic dental care was provided that included examination, x‐rays, pain relief, control of infection, scaling and prophylaxis, elimination of caries, restorations, and extractions. Fees, reimbursement, or incentives: none reported |
|
| Outcomes | Overall health and well‐being: early childhood caries Child physical health and development: early childhood caries Time points: 24 and 36 months |
|
| Funding source and conflicts of interest |
Funding: Te Kaunihera Rangahau Hauora O Aotearoa, the New Zealand Health Research Council (HRC ICIHRP Grant application ID 09/644) Conflict of interest: none reported |
|
| Notes |
Other outcomes recorded but not used within the review Child physical health and development: other measures of early childhood caries Time points: 24 and 36 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using envelopes. Quote: "Once the mother had agreed to participate in the study they were randomly allocated to either the intervention group or the delayed intervention group by choosing an envelope which contained the name of the group." |
| Allocation concealment (selection bias) | Unclear risk | There was little information provided about allocation concealment other than the drawing of envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | People collecting data were blinded to the intervention. Quote: "The therapist did not know which intervention group the child had been allocated to." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition. Outcome data were available for less than half of the intervention group and there were differences in self‐reported health status and education level between those who completed the study and those who withdrew. Quote: "At the follow‐up when the child was 24 months old, under half of the mothers in the intervention group were assessed." |
| Selective reporting (reporting bias) | High risk | A protocol was available; however, there was insufficient information to determine how the data were to be analysed. Multiple outcome measures were reported. |
| Other bias | Low risk | No other obvious sources of bias. |
Family Spirit Nuture Part 1 2021.
| Study characteristics | ||
| Methods |
Study design: RCT Study recruitment: March 2017–May 2018; follow‐up 12 months' postpartum Published protocol/trial: trial registered NCT03101943 |
|
| Participants |
Description: Navajo mothers aged ≥ 13 years with infants aged < 14 weeks living within 50 miles of the Northern Navajo Medical Center (intervention n = 68, control n = 66) Exclusion criteria: unable to fully participate or unwilling to undergo randomisation Indigenous population: Navajo Nation (New Mexico) Setting: USA, reservation Place of delivery: Indian Health Service: Northern Navajo Medical Center Principle health condition: childhood obesity Age of mother (years), mean: intervention: 27.4 (SD 6.4), control 27.5 (SD 6.1) Gender of child born: intervention 35 (52%) girls, control 35 (53%) girls Number of children in family: intervention 2.4 (SD 1.6), control 2.4 (SD 1.4) Family unit: married intervention 10 (15%), control: 15 (23%) Socioeconomic status: 1 financial hardship: intervention 13 (19%), control 19 (29%); 2–5 financial hardships: intervention 22 (32%), control 20 (30%); no financial hardship: intervention 33 (48%), control 27 (41%) Employment of mother: not employed: intervention 54 (79%), control 59 (89%); full‐time: intervention 8 (12%), control 4 (6%); part‐time: intervention 6 (9%), control 3 (5%) Education of mother: less than high school: intervention 8 (12%), control 14 (21%); still attending or completed high school or general equivalency diploma: intervention 27 (40%), control 19 (29%); completed more than high school: intervention 33 (49%), control 33 (50%) |
|
| Interventions |
Intervention Intervention name: FSN Intervention aim: to address specific parent feeding practices in infancy associated risk for early childhood obesity, including SSB consumption, responsive parenting and infant feeding practices, and optimal growth through 12 months' postpartum. Theory used to develop intervention: FSN model was built on the previously published, evidence‐based Family Spirit home‐visiting early childhood intervention. Consumer and community involvement: the brief FSN early childhood home‐visiting intervention was designed in partnership with tribal communities. Overall grouping: education Fees, reimbursement, or incentives: participants received USD 20 in gift cards and 2 gift packages worth USD 25 for the completion of assessment at baseline, 4, and 12 months. Procedures: home visit lessons were delivered between 3‐ and 6‐months' postpartum and covered the following: 1. whole family healthy eating practices, 2. optimal infant feeding practices, 3. responsive feeding, 4. avoiding SSBs, 5. complementary feeding practices, and 6. parental healthy eating role modelling. Materials: 45‐minute lessons included a warm‐up, lesson content and activities, a question‐and‐answer period, referrals as needed and summary handouts. Mode of delivery: individual home visits When and how often was the intervention delivered? 6 lessons were delivered at specific infant age intervals at 3, 3.5, 4, 4.5, 5, and 6 months' postpartum. Who delivered the intervention? Navajo paraprofessionals Was there any training provided to the people who delivered the intervention? yes; however, the type of training was unspecified. Was the study modified or adapted? none reported Was the fidelity assessed? none reported Comparison Minimal education Participated in 3 injury prevention lessons at 3, 4, and 5 months' postpartum drawn from the original Family Spirit intervention but avoided confounding FSN content and outcomes. Content included childproofing, safe travel practices, and strategies to avoid abuse and neglect. Control sessions followed the same format as the FSN lessons. Fees, reimbursement, or incentives: participants received USD 20 in gift cards and 2 gift packages worth USD 25 for the completion of assessment at baseline, 4, and 12 months. |
|
| Outcomes | Overall health and well‐being: infant growth Child physical health and development: infant growth Family‐enhancing lifestyles or behavioural outcomes: SSB consumption Adverse events: emergency department presentations or hospital admissions Parenting knowledge and awareness: maternal SSB consumption index Time points: < 2 months and 12 months |
|
| Funding source and conflicts of interest |
Funding: Navajo Area Indian Health Service, the Osprey Foundation (grant# 132271), the McCune Charitable Foundation, and a private donor Conflict of interest: none reported |
|
| Notes |
Other outcomes recorded but not used within the review Child: infant growth, age at complementary food introduction, % introduced complementary food at ≥ 6 months' postpartum, SSB consumption Mother: responsive feeding Time points not used: 4, 6, and 9 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists were created using statistical software. Quote: "Two randomization lists will be created prior to study initiation using STATA statistical software." |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred after enrolment. Quote: "Participants who completed a baseline assessment were randomized 1:1 to the FSN intervention or the IPE control. Randomization status was delivered by text message or phone call to staff who enrolled the participant." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and staff were not blinded to the intervention. Quote: "Participants and study staff were not blind to randomization status." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear who collect the study data. The protocol discussed trained staff; however, it is not clear who this was in the study and whether they were blinded to outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a low level of attrition which was balanced between groups. Quote: "Follow‐up assessments were completed by 92% (123 of 134) of participants at 12 months post partum." |
| Selective reporting (reporting bias) | High risk | Protocol available. Data were provided as per primary outcome; however, it is unclear how these data were defined (i.e. binary or continuous) both are provided. Adjusted effect sizes are only reported. |
| Other bias | Low risk | No other obvious sources of bias. |
Family Spirit Trial 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Study period: May 2006–September 2011; follow‐up 36 months' postpartum Published protocol/trial: protocol published and trial registered; NCT00373750 |
|
| Participants |
Description: all pregnant American Indian adolescents aged 12–19 years at conception and at ≤ 28 weeks' gestation (intervention n = 159, comparison n = 163) Exclusion criteria: currently participating in other mental or behavioural research or if life circumstances precluded full participation in the intervention protocol, such as severe mental illness or legal status that required high‐intensity residential care. Indigenous population: White Mountain Apache, San Carlos Apache Reservations, and the Tuba City and Fort Defiance communities on the Navajo Reservation in northern Arizona Setting: USA, reservations Place of delivery: Indian Health Service Principle health condition: reduce health and behavioural risks Age of mother (years), mean: intervention 18.2 (SD 1.4), control 18.1 (SD 1.6) Gender of infant: none reported First child in family: intervention 122 (76.7%), control 125 (76.7%) Family unit: lives with parents: intervention 96 (60.8%), control 95 (58.6%) Socioeconomic status: none reported Employment of mother: currently employed: 12 (7.5%), control 11 (6.8%) Education of mother: currently in school: intervention 63 (39.6%), control 68 (41.7%) |
|
| Interventions |
Intervention Intervention name: Family Spirit Intervention + optimised standard care Intervention aim: to promote family‐based protective factors and reduce behavioural health disparities among American Indian teen parents and their children. Theory used to develop intervention: the theoretical model underpinning the Family Spirit intervention was based on G.R. Patterson's developmental model, which posits parenting as the critical link between parents' personal characteristics and environmental context and their children's individual risks and ultimate outcomes. Based on this framework, the Theory of Planned Behaviour was used to inform the intervention development. Consumer and community involvement: community‐based participatory research was used in the design, prioritising topic areas, and recommendations to use local paraprofessionals. Overall grouping: education Fees, reimbursement, or incentives: incentives through gift cards were given for assessments and increased with duration of participation in the study. For example, they started at USD 10 for initial assessment and increase by USD 5 per time point for maximum of USD 50 for final assessment. Procedures: the Family Spirit curriculum lessons focused on 3 domains: 1. parenting skills across early childhood (0–3 years); 2. maternal drug abuse prevention; and 3. maternal life skills and positive psychosocial development. Each visit was delivered using a flip chart. Lessons are structured to include building rapport, review of previous lesson, and past referrals, teaching of lessons and related activities, question/answer period and distribution of lesson summary hand‐outs. The home visitor is trained to use the lesson outline to create a comfortable teaching dialogue, rather than reading points by rote. Materials: lesson summary hand‐outs Mode of delivery: individual home visits or other private location When and how often was the intervention delivered? each visit lasted approximately 1 hour with 43 lessons delivered over 45 visits from. Home visits occurred weekly through to the end of pregnancy, biweekly until 4 months' postpartum, monthly between 4 and 12 months' postpartum, and bimonthly between 12 and 36 months' postpartum. Who delivered the intervention? Family Health Educator: local female American Indian paraprofessionals, bilingual, minimum of high school diploma, ≥ 2 years of job‐related education or work experience. Was there any training provided to the people who delivered the intervention? staff received extensive training (> 80 hours) in trial protocol and policies, protection of human research subjects, and intervention delivery. Family Health Educators had to demonstrate mastery of the Family Spirit curriculum through written and oral examinations. During the first year of employment, supervisors observed educators conducting home visits on a quarterly basis and rated them on professionalism, rapport, interpersonal skills, and protocol adherence. Was the study modified or adapted? none reported Was the fidelity assessed? both Family Health Educators and Evaluators audiotaped each participant visit and a random 20% of tapes are reviewed by study co‐ordinators for protocol adherence. The proportion of intervention lessons completed was recorded. Comparison Usual care Usual care included transportation assistance to regularly scheduled, clinic‐based antenatal and well‐baby visits. There were 7 antenatal visits, 9 well‐baby visits during the first 3 years of life (at 1 and 2 weeks, and 2, 4, 6, 9, 12, 24, and 36 months' postpartum), and 4 social support visits between years 2 and 3. Fees, reimbursement, or incentives: incentives through gift cards given for assessments and increased with duration of participation in study (e.g. started at USD 10 for initial assessment and increased by USD 5 per time point for maximum of USD 50 for final assessment). |
|
| Outcomes | Overall health and well‐being: ITSEA – competence domain Child psychological health and emotional behaviour: ITSEA – competence domain Family enhancing lifestyle or behaviour outcomes: Home Observation for Measurement of the Environment Parent/carer psychological health: depression Parenting knowledge and awareness: home safety practices, knowledge Time points: 12 and 36 months |
|
| Funding source and conflicts of interest |
Funding: National Institute on Drug Abuse grant R01 DA‐019042 Conflict of interest: Dr Compton has served as a consultant for Shire Pharmaceuticals, as a principal investigator on a study for Shire Pharmaceuticals, and as an associate editor for the Journal of Consulting and Clinical Psychology and the Journal of Child and Adolescent Psychopharmacology. Dr Carter receives royalties for the Infant‐Toddler Social and Emotional Assessment. Dr Walkup has served as a consultant for Shire Pharmaceuticals and has received research support from, served on the advisory board of, and received travel support and honoraria from the Tourette Syndrome Association; he has received free medication and placebo for NIH‐funded studies from Eli Lilly and from Pfizer; and he receives royalties from Guilford Press and Oxford University Press. |
|
| Notes |
Other outcomes recorded but not used within the review Child: internalising, externalising, and dysregulation behaviour Mother: substance use, parenting knowledge, home safety attitudes, parenting‐related stress, self‐efficacy, internalising problems, externalising problems, total behavioural and social problems, alcohol and substance abuse Time points not used: 6 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer program. Quote: "The data manager created the randomization sequence using Stata 9.0, and the study coordinator delivered the randomization status of each individual over the telephone to the unblinded field staff member who had enrolled the participant." |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred after enrolment; central allocation used. Quote: "The data manager created the randomization sequence using Stata 9.0, and the study coordinator delivered the randomization status of each individual over the telephone to the unblinded field staff member who had enrolled the participant." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent evaluators blinded to randomisation collected data. Quote: "Independent Evaluators complete all participants' observational and interview assessments and medical chart reviews and are masked to participants' randomization assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis completed. High level of attrition at end of study with a higher attrition rate in intervention than control group. Quote: "Attrition was higher in the intervention group than in the control group. Attrition in both conditions primarily occurred before 12 months, followed by relatively stable participation through 36 months. Analyses of differential attrition showed significant effects for two variables. Women in the intervention group who reported no substance use during pregnancy were more likely to drop out, and participants in the control group who reported never using cigarettes were more likely to remain in the study. Attrition did not favor the intervention group." |
| Selective reporting (reporting bias) | High risk | There was a protocol but no data analysis provided. Data for time points at 36 months were combined and only mean adjusted scores were given. |
| Other bias | Unclear risk | There was some discussion on the cultural appropriateness of the outcomes in particular the Home Observation Measurement of the Environment. No other obvious sources of bias. |
Harrison 2010.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT Study recruitment: January 2005–October 2007; follow‐up 30 (SD 3) months Published protocol/trial: protocol published and trial registered; ISRCTN41467632 |
|
| Participants |
Description: women at 12–34 weeks of pregnancy and mothers of newborn, predentate infants from 9 communities (intervention n = 131 in 5 communities, control n = 141 in 4 communities) Exclusion criteria: any woman knowing of an impending, permanent move out of her community. Indigenous population: Quebec Cree Nation Eeyou Istchee Setting: Canada, remote Place of delivery: health clinic Principle health condition: early childhood caries Age of mother (years), mean: intervention 25.5 (SD 6.4), control 25.6 (SD 5.8) Gender of infant: none reported Number of children in family: has other children: intervention 83 (64.3%), control 92 (65.7%) Family unit: none reported Socioeconomic status: none reported Employment of primary carer: none reported Education of primary carer: none reported |
|
| Interventions |
Intervention Intervention name: MI Intervention aim: to control caries in Indigenous children Theory used to develop intervention: follows the principles of MI, a client‐centred but directive counselling style. Consumer and community involvement: 2 years of community consultation was completed. This was mainly around study design and the use of RCT. The decision to use an RCT was decided based on no health promotion programme for oral health previously being completed. Community Health Representatives who delivered the intervention modified the intervention protocol and resources (menus) to fit better with the "way of being" of the Cree. The menus were customised for various stages of infant and toddler development and were printed on flip charts that included images of local children and families. Overall grouping: counselling Fees, reimbursement, or incentives: none reported Procedures: at the counselling sessions, mothers were given the resources to enable them to adopt their selected behaviours, e.g. infant toothbrushes and fluoride toothpaste, and xylitol gum for the mother. Menus were developed at specific age ranges to reflect changes over time in each child's feeding and snacking habits, and dental development. FV was provided as a choice of care after the child's first birthday. Mothers also received a 'Privilege Card' to expedite dental care. Materials: mothers received resources at each MI visit to enable them to implement selected behaviours, e.g. infant toothbrushes, toothpaste, and sippy cups. Mothers also received a pamphlet explaining children's dental care practices. Mode of delivery: well‐child visit provided individually to each family face‐to‐face with home visits as an option. When and how often was the intervention delivered? mothers were counselled once during pregnancy and up to 6 times postnatally to correspond with the 2‐, 4‐, 6‐, 12‐, 18‐, and 24‐month well‐child visits at local clinics. Who delivered the intervention? Community Health Representatives: existing health workers and, where not available, local women were employed. Was there any training provided to the people who delivered the intervention? a 2‐day training workshop for Community Health Representatives was held in the first year of the project in a community adjacent to Eeyou Istchee that was accessible by air or ground transport. An MI consultant delivered the training. The consultant presented theory and principles of MI. Explanatory notes were developed for the Community Health Representatives to guide their counselling sessions. The MI consultant followed up months later with an MI coaching conference call to problem‐solve MI with and support the Community Health Representatives. A second 2‐day workshop for all intervention Community Health Representatives was held in year 2 in an intervention community. Following this workshop, the Project Manager regularly visited each of the communities individually to problem‐solve issues with recruiting and delivery of the counselling interventions. The Programme Manager also maintained regular telephone contact with the project's staff in each community. Was the study modified or adapted? recruitment and delivery of the intervention changed between sites. In 2/5 intervention communities and 2/4 control communities, recruitment and, for the intervention communities, delivery of the intervention was eventually completed by the Project Manager. Local women who were not Community Health Representatives completed recruitment in the remaining 2 control communities. Was the fidelity assessed? no Comparison Pamphlet group This was an education programme. The mothers received a culturally appropriate educational pamphlet describing healthy dental care practices for young children. Pamphlets were mailed to mothers when their child was aged 6 and 18 months. FV was available to control children at local dental clinics. Fees, reimbursement, or incentives: none reported |
|
| Outcomes | Overall health and well‐being: tooth level caries Child psychological health and emotional behaviour: parent answered 'yes' to > 1 quality of life question Child physical health and development: tooth level caries Service access and utilisation: number of visits to the dentist for tooth pain Adverse events: whether there were adverse events Economic costs: cost‐effectiveness Time points: 30 (SD 3) months of age |
|
| Funding source and conflicts of interest |
Funding: Canadian Institute of Health Research (grant #FRN 67817) Conflict of interest: none reported |
|
| Notes |
Other outcomes recorded but not used within the review Child: data on dental caries including enamel caries, dentinal caries, pulpal caries, restorations, and absence due to caries Mother: dental health knowledge and home care behaviours |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communities were alphabetically ordered to receive their intervention, which was drawn from lots. Quote: "Communities were randomized in each round by alphabetical ordering of the communities' names. For example, for each round, the first name on the alphabetical list of communities was announced, followed by the drawing of an envelope from the basket; the next name was announced, followed by another draw until all envelopes were allocated." |
| Allocation concealment (selection bias) | Unclear risk | There was little information provided about allocation concealment other than the drawing of envelopes. Quote: "Randomization was done over community radio with two "rounds" of a constrained randomization process. Two baskets contained envelopes marked "test" or "control": one basket for large communities (2 envelopes: 1 test, 1 control) and another for smaller communities (7 envelopes: 4 test, 3 control)." |
| Selective cluster recruitment | High risk | Communities knew of their allocation prior to enrolment. Individuals who recruited women also delivered the intervention. There was some baseline differences in recruitment including fewer intervention mothers had already delivered at time of enrolment, had visited a dentist for toothache, and had other children with a previous tooth extraction. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to the intervention. Quote: "Mothers and interveners were aware of their community's allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | People collecting data were blinded to the intervention. Quote: "The examiners were blinded to allocation and were unfamiliar with the intervention." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis completed. Greater loss to follow‐up in control group compared to intervention group (83% vs 93%). |
| Selective reporting (reporting bias) | Unclear risk | Protocol available. Data provided as per definition; however, caries reported at tooth and child level and this was not distinguished in the protocol and was the primary outcome. |
| Other bias | Unclear risk | Deviation from intended intervention: intervention mothers were given "Privilege Cards" to allow for priority access to dental services. However, because of the turnover of dental staff, not all clinics honoured the cards. About one‐third recalled using the cards; however, it is unclear how many mothers were turned away. No other obvious sources of bias. |
HCSF 1 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Study recruitment: not reported, follow‐up 2 years Published protocol/trial: protocol published and trial not registered |
|
| Participants |
Description: American Indian children aged 2–5 years (interventions n = 67, control n = 83) Exclusion criteria: children with presence of major physical or behavioural conditions that would preclude participation. Indigenous population: Bad River Band of Lake Superior Chippewa Indians, the Lac du Flambeau Band of Lake Superior Chippewa Indians, the Menominee Nation, and the Oneida Nation Setting: USA, reservation Place of delivery: Indian Health Service and Head Start sites Principle health condition: obesity Age of child (years), mean: intervention 4.0 (SD 0.9), control 4.0 (SD 0.9) Gender of child: intervention 34 (50.7%) girls, control 36 (43.4%) girls First child in family: none reported Family unit: none reported Primary carer of child: mother: intervention 58 (86.6%), control 70 (84.3%); father: intervention 2 (3.0%), control 1 (1.2%); grandparent/other: intervention 7 (6.0%), control 12 (14.5%) Socioeconomic status: none reported Employment of primary carer: none reported Education of primary carer: high school or less: intervention 15 (19.7%), control 16 (19.3%); some college: intervention 24 (35.8%), control 30 (36.1%); completed college and beyond: intervention 16 (23.9%), control 22 (26.5%); unknown: intervention 12 (17.9%), control 15 (18.1%) |
|
| Interventions |
Intervention Intervention name: Mentored Health Child, Strong Families (Mentored HCSF) Intervention aim: HCSF aims to change behaviours through increased knowledge of healthy lifestyles, enhanced parenting and increased self‐efficacy. Theory used to develop intervention: HCSF is based on social cognitive and family systems theories and seeks to change behaviours at the family level. Consumer and community involvement: tool kit lessons and activities were developed by the University of Wisconsin–Madison and Great Lakes Inter‐Tribal Council research team and University of Wisconsin Extension specialists. Community members and tribal leaders were integral throughout the conceptualisation and planning of the intervention. HCSF's Supportive Communities component, which worked with 3 tribal communities to develop community advisory boards was aimed at assessing and eliminating environmental barriers to health. Overall grouping: education Fees, reimbursement, or incentives: gift cards to local merchants and lesson‐specific incentives (non‐monetary) were provided. Individual amounts varied but averaged USD 175/person. Procedures: year 1: initial contact with the family was made by telephone, and mentors were encouraged to share information to create a friendly and supportive relationship. The first in‐person lesson was designed to create dialogue between the mentor and the family and to begin building a supportive rapport. During each visit, mentors reviewed the lesson with the primary carer and child, led discussions and activities to help the carer and child learn about the topic, considered behaviour change related to the topic, and assisted the family in setting goals to attempt behaviour change. Ideally, mentor‐led discussions assisted the primary carer and child in progressing along the continuum of motivation towards actual behaviour change, while helping the primary carer build skills and confidence in his or her own ability to adopt healthier lifestyle choices. During year 1, intervention families were also invited with their extended family to 3 mentor‐led group sessions. Year 2: intervention families participated in monthly group meetings and continued to receive a monthly newsletter with parenting tips/recipes/local programme notices to help in sustaining behaviour changes implemented in year 1. Monthly group meetings focused on topics such as basic nutrition concepts (sugar, fats, appropriate serving sizes, food choice variety) and ideas for physical activities. Monthly newsletters were disseminated for the 2 years Materials: lesson‐specific incentives included an HCSF calendar to track goals and progress, cooking utensils, and physical activity items such as balls, frisbees, pedometers, exercise videos, etc. Mode of delivery: year 1: individual face‐to‐face home visits, year 2 group session When and how often was the intervention delivered? year 1: 12 toolkit lessons, 3 group lessons; year 2: 12 monthly group meetings Who delivered the intervention? mentors: tribal members or other people connected to the tribe. This included parents, grandparents, and respected community members who were able to deliver the intervention. Was there any training provided to the people who delivered the intervention? mentors were trained extensively by the University of Wisconsin Extension staff, tribal well‐ness staff (including nurses, diabetes educators, and dieticians), knowledgeable tribal elders, and HCSF research staff. Additional training was provided on child development, nutrition, and physical activity. Mentors received a full protocol manual, yearly training, and refresher sessions. The University of Wisconsin–Madison and Great Lakes Inter‐Tribal Council project co‐ordinators worked with mentors, discussing issues with in‐home visiting and families' lack of progress, and assessed mentor progress. Was the study modified or adapted? if at any time the home visits were unable to be scheduled or completed for participants, the intervention materials were provided by mail. This was not outlined in the protocol. Was the fidelity assessed? not reported Comparison Mailed group This was an education intervention. In year 1, the control families received the same 12 lessons by mail + the monthly newsletter. In year 2, the control families received only the monthly newsletter. Fees, reimbursement, or incentives: gift cards to local merchants and lesson‐specific incentives (non‐monetary) were provided. Individual amounts varied but averaged USD 175/person. |
|
| Outcomes | Overall health and well‐being: BMI z‐score Child physical health and development: BMI z‐score Family enhancing lifestyle or behaviour outcomes: 12‐item Short Form Survey (SF‐12) Physical Health Parent/carer psychological health: SF‐12 Mental Health Time points: 12 months |
|
| Funding source and conflicts of interest |
Funding: Wisconsin Partnership Program Community–Academic Partnership Fund and the NIH (grant number U01 HL 087381). Author EJT was supported through an NIH T32 training grant to the University of Wisconsin Department of Nutritional Sciences (grant number 5T32DK007665). The funders had no role in the design, analysis, or writing of the article. Conflict of interest: none reported |
|
| Notes |
Other outcomes recorded but not used within the review Child: waist circumference; fruit and vegetable servings/day; soda/sweetened drink and candy servings/day; hours physical activity/day; hours television viewing time/day; accelerometry Mother: waist circumference; height and weight; BMI; glucose tolerance; blood lipid profile; C‐reactive protein level; urine microalbumin level; creatinine level; nutrition and physical activity behaviours; fruit and vegetable servings/day; soda/sweetened drink and sweets (candy) servings/day; hours of physical activity/day; hours television viewing time/day; accelerometry; health behavioural efficacy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is unclear how sequence generation was completed; however, there appeared to be a sufficient process of randomisation used. Quote: "Within each stratum, half of the families were randomly assigned to the intervention condition and half to the control condition. Furthermore, within each stratum, a blocked randomization strategy was used to ensure that there was an equal number of families in the intervention and control groups." |
| Allocation concealment (selection bias) | Unclear risk | Randomisation occurred after enrolment; no information about allocation concealment. Quote: "Randomization at the family level was done after obtaining consent from and completing baseline measurements on participating families." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The same people who delivered the intervention also collected the data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis completed. High level of missing data with more data missing in the intervention (83%) than in the control (67%) group; intention‐to‐treat analysis. Missing imputation was completed but did not account for the high attrition rate in the intervention group. |
| Selective reporting (reporting bias) | Low risk | Protocol available. Data provided as per definition. |
| Other bias | High risk |
Changes in participant allocation after randomisation Participants were moved after randomisation. This could have influenced outcomes in favour of the intervention. No sensitivity analysis was completed. No other obvious sources of bias. Quote: "After randomization, participants who were unable to be scheduled for their initial mentoring visit within two months were moved to the mailed toolkit group, resulting in a higher number of participants in this group (eight families were transferred before any intervention was administered, resulting in eighty‐three in the mailed only group instead of the seventy‐five expected after randomization)." |
HCSF 2 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Study recruitment: January 2013–April 2015; follow‐up 1 year Published protocol/trial: protocol published and trial registered; NCT01776255 |
|
| Participants |
Description: American Indian children aged 2–5 years and their primary carers (intervention n = 225, control n = 225) Exclusion criteria: minimal exclusion criteria applied due to community's value for inclusion in community projects. Indigenous population: American Indian/Alaska Native Setting: USA, 4 reservations and 1 urban site Place of delivery: Indian Health services, Head Start centres, and social service centres Principle health condition: obesity Age of child (months), mean: intervention 45.9 (SD 12.8), control 44.1 (SD 13.2) Gender of child: intervention 115 (51.1%), control 111 (49.3%) Number of children in family: none reported Family unit: none reported Primary carer of child: none reported Socioeconomic status: < USD 5000: intervention 69 (31.7%), control 63 (28.4%); USD 5000–19,999: intervention 61 (28.0%), control 63 (28.4%); USD 20,000–34,999: intervention 45 (20.6%), control 49 (22.1%); ≥ USD 35,000: intervention 43 (19.7%), control 47 (21.2%) Employment of primary carer: none reported Education of primary carer: high school or less: intervention 83 (36.9%), control 86 (38.2%); some college: intervention 120 (53.3%), control 115 (51.1%); completed degree or postgraduate: intervention 22 (9.8%), control 24 (10.7%) |
|
| Interventions |
Intervention Intervention name: Wellness Journey Intervention aim: none reported Theory used to develop intervention: none reported Consumer and community involvement: each selected community vetted the final study design and provided input. Community partners (including tribal wellness staff and community advisory boards) also developed additional lessons targeting stress and sleep that were not part of the original programme (HCSF 1: see Adams 2011 under HCSF 1 2007). Overall grouping: education Fees, reimbursement, or incentives: all families received as USD 50 gift care after completing baseline, 12‐month and 24‐month testing. Families who were randomly chosen to complete dietary recalls also received an additional USD 25 gift card at each time point. Lesson‐specific incentives, such as cooking utensils, balls, books, games, and pedometers were also provided. Procedures: each monthly toolkit included 1. printed educational lessons with information and suggestions for activities, 2. supportive items (e.g. measuring cups, recipes, pedometers, games), and 3. a children's book relating to 1 of the intervention targets to foster family interaction. There were 6 intervention targets, which included increasing fruit and vegetable consumption, decreasing sugar consumption, increasing physical activity, decreasing screen time, improving sleep habits, and decreasing stress (adult only). Wellness Journey adults were supported by social media engagement and invited invitation to an optional, site‐specific Facebook group where intervention targets were discussed. Materials: each Wellness Journey lesson included a children's book related to the topic and items to support behaviour change (e.g. pedometers, apple corers, measuring cups, exercise DVDs) Mode of delivery: mailed to individuals When and how often was the intervention delivered? 12 monthly mailed lessons; twice weekly text messages and an invitation to optional site‐specific Facebook group. Who delivered the intervention? local community members were employed as the project co‐ordinator at each site. Was there any training provided to the people who delivered the intervention? local co‐ordinators were trained in‐person by the central study co‐ordinator on all research protocols. Was the study modified or adapted? none reported Was the fidelity assessed? none reported Comparison Safety Journey This was a child safety curriculum intervention. 12 mailed safety newsletters and related materials including safety reflectors for biking and cabinet safety locks were sent to participating families. Fees, reimbursement, or incentives: all families received as USD 50 gift care after completing baseline, 12‐month and 24‐month testing. Families who were randomly chosen to complete dietary recalls also received an additional USD 25 gift card at each time point. Safety‐focused incentives included bike reflectors, outlet covers, and books. |
|
| Outcomes | Overall health and well‐being: BMI z‐score Child physical health and development: BMI z‐score Family enhancing lifestyle or behaviour outcomes: SF‐12 Physical Health Parent/carer psychological health: SF‐12 Mental Health Time points: 12 months |
|
| Funding source and conflicts of interest |
Funding: National Institutes of Health, National Heart, Lung, and Blood Institute (grant number 1R01HL114912). Authors, EJT and VMG were supported through NIH T32 training grants to the University of Wisconsin Department of Nutritional Sciences (5T32DK007665) and the Department of Family Medicine and Community Health (T32HP10010), respectively, at the time of the work. Conflict of interest: none reported |
|
| Notes |
Other outcomes recorded but not used within the review Child: waist circumference; diet screener; physical activity; 24‐hour diet recall; screen time survey; physical activity, weekday sleep (hours) Mother/carer: BMI; waist circumference; diet screener; physical activity; 24‐hour diet recall; screen time survey; physical activity; sleep survey; self‐efficacy; safety survey, family nutrition and physical activity total score, adult perceived stress Follow‐up 2 years; however, in year 2, participants crossed over and completed the alternative intervention/control. We used results from year 1 as the effectiveness trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer program. Quote: "Randomization was conducted by the REDCap data management system (Research Electronic Data Capture data management system) using a permuted block strategy prepared by the study biostatistician." |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred after enrolment; central allocation was used. Quote: "Randomization was conducted by a centralized study coordinator after baseline enrolment data were collected by local site coordinators at each study site." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were collected by the people delivering the intervention. People completing data entry and analyses were blinded by group assignment. Quote: "Site coordinators were not blinded to study arm for the post intervention/Year 1 data collection due to in‐person delivery of intervention Lesson 1 and administration of the Wellness Journey Facebook group. Data input and analysis were conducted by study personnel who were blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis completed. Approximately 9% of data were missing from each group. |
| Selective reporting (reporting bias) | Low risk | Protocol available. Data provided as per definition. |
| Other bias | Low risk | No other obvious sources of bias. |
Johnston 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Study recruitment: December 2009–January 2012; follow‐up 12 months Published protocol/trial: protocol published and trial registered; ACTRN12609000937213 |
|
| Participants |
Description: singleton or firstborn in multiple birth infants aged 0–5 weeks whose mother was aged > 16 years, Indigenous, permanent resident of the location, and currently smoked or the infant lived in a household where there was ≥ 1 other person who smoked (intervention n = 145, usual care n = 148) Exclusion criteria: infants excluded if they had serious neonatal respiratory complications, other serious neonatal complications, or had major organ abnormalities. Mothers/carers excluded in they had been previously recruited in the research study, or they lived in the same household as a mother/carer who had previously been recruited into study. Indigenous population: Maori and Aboriginal Setting: New Zealand and Australia, urban areas, Place of delivery: hospital maternity health services Manukau City (New Zealand) and Aboriginal Community Controlled Health Organisation Darwin (Australia) Principle health condition: acute respiratory illness Age of child (weeks), mean: intervention 6.3 (SD 2.7), usual care 6.0 (SD 2.7) Gender of child: intervention 40% girls, usual care 46% girls Number of children in family, mean: children in house aged < 5 years: intervention 1.9 (SD 1.0), usual care 1.9 (SD 1.0) Family unit: married/de facto/living with partner: intervention 72 (50%), usual care 91 (62%) Primary carer of child: mother: intervention 145 (100%), usual care 148 (100%) Socioeconomic status: none reported Employment of mothers: none reported Education of mothers: highest level of education – technical and further education/polytechnic/university: intervention 41 (28%), usual care 34 (23%) |
|
| Interventions |
Intervention Intervention name: none Intervention aim: to provide information and education about the health effects of environmental tobacco smoke exposure and use behavioural 'coaching' techniques to help mothers/carers and family members implement strategies to reduce the infant's environmental tobacco smoke exposure, and identify the smokers among other household members and deliver culturally appropriate smoking cessation advice, counselling, and treatment options as requested. Theory used to develop intervention: programme about environmental tobacco smoke was framed around an Indigenous model of health promotion. This considered the psychological, physical, spiritual, and cultural well‐being of the individual and the family/community, as it related to the project. In New Zealand, Te Whare Tapa Wha was used and was applied to understanding Maori smoking cessation behaviour and for guiding the development of culturally appropriate smoking cessation programmes and strategies. In Australia, the model drew on similar concepts as the New Zealand health promotion model. Consumer and community involvement: in New Zealand, the research advisory group gave support and input into the study and provided guidance as the study progressed. In Australia, the reference group monitored the study's progress and authorised publication and dissemination of findings. Overall grouping: education and counselling Fees, reimbursement, or incentives: none reported Procedures: an 8‐week supply of free nicotine replacement therapy patches or gum was available to participants and other household members. Indigenous Health Workers provided nicotine replacement therapy with appropriate counselling and follow‐up. Those who were interested also received a fax referral to Quitline with proactive call back by Quitline. Culturally appropriate resources were obtained from relevant health groups in each country who hold a repository of such resources. These were used to assist in both educational and behavioural 'coaching'. Materials: 8‐week supply of free nicotine replacement therapy (patches or gum) was available to participants and other household members. Culturally appropriate resources for example flip charts, 'No Smoking' stickers, posters were also provided. Mode of delivery: individual face‐to‐face sessions When and how often was the intervention delivered? 3 face‐to‐face home visits of approximately 45–60 minutes' duration were completed over the first 3 months of the infant's life. Who delivered the intervention? Indigenous Health Workers (paraprofessional health workers) Was there any training provided to the people who delivered the intervention? Indigenous Health Workers delivered the programme after appropriate training. They completed standardised progress reports after each programme session, which was used at weekly team meetings with the health workers and study personnel for discussion and ongoing training. Was the study modified or adapted? none reported Was the fidelity assessed? a mix of quantitative and qualitative measures to assess how well the intervention programme was implemented according to protocol, e.g. number of 'coaching' activities completed, obstacles and successes in delivering programme, parent satisfaction with the programme were taken. Comparison Usual care Usual care through their community health provider that included routine visits for maternal and child health checks in the first 12 months of an infant's life. Fees, reimbursement, or incentives: none reported |
|
| Outcomes | Overall health and well‐being: new episodes of acute respiratory disease Child physical health and development: new episodes of acute respiratory disease Family enhancing lifestyles or behavioural outcomes: in the last 7 days, infant had been around tobacco smoke; full smoking ban in the home Service access and utilisation: child rate of hospitalisations for acute respiratory infections Time points: 12 months |
|
| Funding source and conflicts of interest |
Funding: National Health and Medical Research Council of Australia (545203); the Health Research Council of NZ (09/626); Cure Kids NZ (3525) and the James Russell Lewis Trust, New Zealand (13787/15734). Conflict of interest: CB has previously undertaken research on behalf of NicoNovum, but prior to the purchase of the company by RJ Reynolds. NW has provided consultancy to the manufacturers of smoking cessation medications, received honoraria for speaking at a research meeting, and received benefits in kind and travel support from a manufacturer of smoking cessation medications. MG has provided consultancy to the manufacturers of smoking cessation medications. NW, CB, MG, and VP have also undertaken two trials of very low nicotine content cigarettes, which were purchased from two different tobacco companies. The companies concerned had no role in development of the study design, data collection, data analysis, data interpretation, or writing of the trial publications. |
|
| Notes |
Other outcomes recorded but not used within the review Child: urinary cotinine, infant was breastfed Mother/carer: self‐report of smoking cessation; number quit attempts Time points not used: 4 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer program. Quote: "Participants will be randomized by computer with stratification using permuted blocks by country (Australia, NZ) and infant age (0‐5 weeks, >5‐10 weeks)." |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred after enrolment; central allocation used. Quote: "All participants (i.e. the infants) will be assigned a unique registration number allocated by a central computer following the submission of their details on a web‐based form." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Staff assessing the primary outcome were blinded to who received the intervention; however, other measures were not. The trial statistician was blinded to group assignment. Quote: "Research assistants, who will be responsible for collecting the minor outcome measures will accompany the health workers to the participants' homes for all visits and thus cannot be blinded. The primary outcome will however be a double‐blinded measure." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified intention‐to‐treat analysis completed. There was a low level of attrition that was balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Protocol available. Data provided as per definition. |
| Other bias | Low risk | No other obvious sources of bias. |
Quissell 2014.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT Study recruitment: cohort 1 2011–2012, cohort 2 2012–2013; follow‐up 1 year Published protocol/trial: protocol published and trial registered; NCT01116739 |
|
| Participants |
Description: Head Start children aged 3–5 years and their primary parents or carers (intervention n = 463 in 20 sites, control n = 434 in 19 sites) Exclusion criteria: children aged < 3 years at time of Head Start enrolment, children without a consenting legal guardian, adults unable to understand English well enough to consent or to complete the computerised survey in English. In the intervention classrooms, children were excluded if they presented with an allergy to any components of the FV or if they were home‐based rather than enrolled in the Head Start classroom. Indigenous population: Navajo Nation Setting: USA, reservation Place of delivery: Head Start Centres Principle health condition: early childhood caries Age of parents (years), mean: total participating parents 32.4 (SD 9.6) Age of child (years), mean: intervention 3.7 (SE 0.03), control 3.7 (SE 0.04) Gender of child: intervention 236 (51.0%) girls, control 213 (49.1%) girls Number of children in family, mean: intervention 3.0 (SD 0.1), control 2.9 (SD 0.1) Family unit: none reported Primary carer of child: mother: intervention 359 (77.5%), control 332 (76.5%) Socioeconomic status, mean: intervention 4.0 (SD 0.1), control 4.0 (SD 0.2); score of 4 = an income of USD 10,000–14,999/year Employment of primary carer: none reported Education of primary carer (years), mean: intervention 13.7 (SD 0.2), control 13.5 (SD 0.1) |
|
| Interventions |
Intervention Intervention name: combined oral health promotion–FV intervention Intervention aim: to reduce early childhood caries among Navajo preschool‐age children Theory used to develop intervention: none reported Consumer and community involvement: representative of the tribe provided input into planning the research project, introducing and explaining the study to community members and tribal leaders. A Community Advisory Group reviewed all intervention activities and materials and provide ongoing community oversight, advice, and encouragement on the project. Overall grouping: education Fees, reimbursement, or incentives: a fruit basket raffle for enrolled carers who attended parent events. Procedures: the intervention included the application of FV for the children and oral health promotion activities for children and carers. Community Oral Health Specialists applied FV to children's teeth in the Head Start classroom. The Community Oral Health Specialists provided oral health promotion activities. Oral health promotion activities began with a kick‐off event for carers and children that introduced the project. The first event was a kick‐off to provide an opportunity for carers to learn about the project. The 3 remaining parent events included 1. an overview of the importance of primary teeth, prevention of tooth decay, consequences of tooth decay, and carers' roles in prevention; 2. 2 small‐group oral health promotion activities; 3. a simple goal‐setting activity; and 4. a fruit basket raffle for enrolled carers who attended. The remaining 4 child events incorporated brief, highly interactive activities into a Head Start classroom session. Topics included teeth, tooth brushing, nutrition (avoidance of sticky foods), visiting the dentist, and fluoride. Materials: all families received toothbrushes and toothpaste for all family members at enrolment; intervention children and participating carers received additional supplies throughout the study period. Mode of delivery: face‐to‐face in groups When and how often was the intervention delivered? 4 times each school year, the Community Oral Health Specialists applied FV to children's teeth. The Community Oral Health Specialists provided oral health promotion activities to children 5 times per year and to carers 4 times per year. Who delivered the intervention? American Indian community members who were hired and trained to be Community Oral Health Specialists. Was there any training provided to the people who delivered the intervention? training included an initial week of orientation to the project: instruction in oral disease and health, introduction to relevant behavioural and educational foundations, preparation for enrolment, and acquisition of required research training and credentials. The university study personnel then provided a second week of hands‐on intervention and FV application training in the field, with an additional intervention training session just before initiating the programme in the field and subsequent periodic refresher sessions. The field staff dental hygienist trained the Community Oral Health Specialists to understand the function and application of FV and provided hands‐on experience. Was the study modified or adapted? none reported Was the fidelity assessed? the number of adherers to the intervention was recorded. Those who received ≥ 3 child oral health promotion events, ≥ 1 carer oral health promotion event, and ≥ 3 FVs were considered to adhered (n = 247, 53.3%). Comparison Usual care Usual oral health care was made available by dental providers, usually by the Indian health service. Participants in the usual care arm were not prevented from having FV from other sources but did not receive it through the intervention. The usual care group also received toothbrushes and toothpaste at enrolment and data collection events. Fees, reimbursement, or incentives: none reported |
|
| Outcomes | Overall health and well‐being: dental caries Child physical health and development: dental caries Family enhancing lifestyle or behaviour outcomes: oral health behaviour Time points: 12 months |
|
| Funding source and conflicts of interest |
Funding: National Institute of Dental and Craniofacial Research of the National Institutes of Health under Award Number U54DE019259‐01. Conflict of interest: none reported |
|
| Notes |
Other outcomes recorded but not used within the review Mother: oral health knowledge Time points not used: 24 and 36 months Follow‐up was for 3 years; however, we only collected data for year 1 as cohort 1 had an increased dose effect during the second year. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer program Quote: "The unit of randomization for this study is the Headstart Center (HSC), which may contain one or multiple classrooms. The HSCs were stratified by agency and by one vs. multiple classrooms. Within these strata, the HSCs were randomized into the intervention or the usual‐care groups using a random number generator." |
| Allocation concealment (selection bias) | Unclear risk | No information is provided about allocation concealment. |
| Selective cluster recruitment | Unclear risk | Centres were randomised prior to enrolment; however, this did not appear to have influenced recruitment with < 1% of participants declining to participate from Head Start Centres. There were similar numbers in each group; however, there were some baseline differences. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | People collecting data were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Modified intention‐to‐treat analysis completed. Large attrition after 1 year. It is likely this would have affected outcomes. |
| Selective reporting (reporting bias) | High risk | Protocol has some information; however, not very detailed. Caries reported as binary and continuous and this was not distinguished in the protocol. Adjusted analysis only provided from some outcomes. |
| Other bias | Low risk | No other obvious sources of bias. |
Tipene‐Leach 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Study recruitment: June 2011–April 2013; follow‐up 6 months' postpartum Published protocol/trial: protocol published and trial registered; ACTRN12610000993099 |
|
| Participants |
Description: all women booking for antenatal care from 2 midwifery practices working with mainly Maori women from low socioeconomic areas in the Hawke's Bay region (intervention n = 101, control n = 96) Exclusion criteria: babies born < 36 weeks' gestation, < 2500 g birth weight, admitted to the neonatal intensive care unit for > 3 days, severe congenital anomalies. Mothers if they had a previous unexplained sudden infant death, severe mental health problems, involved in a methadone maintenance programme. Indigenous population, n (%): Maori intervention 75 (74.3%), control 64 (66.7%) Setting: New Zealand, Urban area Place of delivery: Maori midwifery services Principle health condition: sudden unexpected death in infancy Age of mother (years), mean: intervention 25.9 (SD 6.2), control 25.6 (SD 6.3) Gender of infant: intervention 50 (49.5%) girls, control 48 (50.0%) girls Number of children in family: first child: intervention 35 (34.7%), control 32 (33.3%) Family unit: single: intervention 30 (29.7%), control 28 (29.2%); separated/divorced: intervention 0 (0.0%), control 5 (5.2%); married/civil union/defacto relationship: intervention 71 (70.3%), control 63 (65.6%) Socioeconomic status: none reported Employment of primary carer: none reported Education of primary carer: completed primary school to year 11: intervention 46 (45.5%), control 47 (49.0%); completed year 12 (required level): intervention 19 (18.8%), control 7 (7.3%) |
|
| Interventions |
Intervention Intervention name: wahakura group Intervention aim: none reported Theory used to develop intervention: none reported Consumer and community involvement: the wahakura is developed by Maori community. The wahakura is a woven flax bassinet with a thin, firm mattress. It is specifically designed to create a separate sleeping surface in the shared sleeping space. Overall grouping: environment Fees, reimbursement, or incentives: participants were given USD 50 grocery voucher gift after the 1‐month sleep study, and USD 25 voucher on completion of each of the 3‐ and 6‐month interviews. Procedures: devices were provided to mothers during pregnancy with evidence‐based safe sleep instructions. Mothers were recommended to always use the assigned device for their baby, regardless of location. Materials: the wahakura and education brochures Mode of delivery: individually face‐to‐face to set up the project When and how often was the intervention delivered? mothers were recommended to use the wahakura regardless of location. Who delivered the intervention? research nurse Was there any training provided to the people who delivered the intervention? none reported Was the study modified or adapted? none reported Was the fidelity assessed? when the infant was 1 month old they were videoed overnight to see how many people were using their sleeping device. Comparison Bassinet group Used a portable standing bassinet, custom designed in New Zealand for distribution to infants at high risk. Bassinet could easily be moved and transported in a car. The base contained an identical 20 mm foam sponge mattress as used in the wahakura. Families also received an education brochure. Fees, reimbursement, or incentives: participants were given USD 50 grocery voucher gift after the 1‐month sleep study, and USD 25 voucher on completion of each of the 3‐ and 6‐month interviews. |
|
| Outcomes | Overall health and well‐being: fully breastfed Child physical and development: fully breastfed Family enhancing lifestyle or behaviour: maternal sleep quality: good; infant sleep position: back Time points: 3 and 6 months |
|
| Funding source and conflicts of interest |
Funding: Health Research Council of New Zealand, and a University of Otago Research Grant Conflict of interest: none reported |
|
| Notes |
Other outcomes recorded but not used within the review Child: infant health, physiological sleep study variables, dummy use, infant sleep behaviour Mother: frequency and duration bed sharing Time point not used: 1 month |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation appeared sufficient to ensure random assignment. Quote: "The randomized order was generated by the statistician by using random length blocks." |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred after enrolment; allocation through numbered envelopes. Quote: "Allocation will be concealed and performed, following application of inclusion/exclusion criteria and consent to participate in the study, by opening a sealed envelope opened in numbered sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to the intervention. Quote: "Neither researchers nor participants could be blinded to the group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is clear some outcomes were analysed blind such as video and audio recording. However, it unclear whether the interviewers at 1, 3, and 6 months were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis completed. There was a low level of attrition and was balanced between groups. For binary outcomes, the events were much higher than the missing data. |
| Selective reporting (reporting bias) | High risk | There was a very brief description in the protocol on the analysis. Data were collected such as full, exclusive, or partial breastfeeding and only full breastfeeding data were reported. |
| Other bias | Low risk | No other obvious sources of bias. |
Walkup 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Study recruitment: May 2002–May 2004; follow‐up 12 months' postpartum Published protocol/trial: protocol not published and trial registered; NCT00356551 |
|
| Participants |
Description: pregnant American Indian adolescents aged 12–22 years at conception and at ≤ 28 weeks' gestation (intervention n = 81, control n = 86). Exclusion criteria: mothers were ineligible if they had extreme medical, legal, or social problems that precluded their ability to participate in visits or assessments; mothers who were at acute risk for self or others at the time of consent Indigenous population: Navajo and White Mountain Apache reservations in New Mexico and Arizona. Setting: USA, reservation Place of delivery: Indian Health Service Principle health condition: child behavioural health problems Age of mother: aged < 18 years: intervention 36 (44%), control 43 (50%) Gender of children born: not reported First child in family: intervention 73 (90%), control 78 (91%) Family unit: living with parents: intervention 63 (78%), control 58 (67%); married: intervention 9 (11%), control 5 (6%) Socioeconomic status: not reported Employment of mother: currently employed intervention: 9 (11%), control 11 (13%) Education of mother: high school/general equivalency diploma/some college: intervention 31 (38%), control 35 (41%) |
|
| Interventions |
Intervention Intervention name: Family Spirit Intervention Intervention aim: to address antenatal and newborn care and maternal life skills Theory used to develop intervention: curricular content for the Family Spirit intervention was based on recommendations and standards documented in the American Academy of Pediatrics' Caring for Your Baby and Child: Birth to Age 5. This theoretical model hypothesises that parenting is the critical link between parent domains and child domains and mediates children's outcomes. Consumer and community involvement: the Navajo and White Mountain Apache leaders and community stakeholders contributed to the design of the intervention, research protocol, and reviewed the article. Overall grouping: education Fees, reimbursement, or incentives: incentives in the form of gift cards to a local grocery store were provided to participants on completion of study assessments. Procedures: curriculum included developmentally timed antenatal and infant‐care parenting lessons, as well as family planning, substance abuse prevention, and problem solving and coping‐skills lessons. The Family Spirit reflects local native practices but not community‐specific traditions or spiritual beliefs. Materials: none reported Mode of delivery: individual home visit or other private location When and how often was the intervention delivered? intervention was delivered from 28 weeks' gestation until 6 months' postpartum. 25 home visits were available each lasting approximately 1 hour. Who delivered the intervention? interventionists: local American Indian women from the community, bilingual, at least a high school degree and had work experience in health and human services Was there any training provided to the people who delivered the intervention? interventionists delivered both the intervention and control interventions to mothers. They received approximately 500 hours of training in home‐visiting methods and curricular content and demonstrating mastery and fidelity to the study protocol on oral and written examinations. Interventionists also served as evaluators and were specifically trained to administer self‐report and observational assessments with objectivity. Was the study modified or adapted? none reported Was the fidelity assessed? dose of intervention and control groups were collected. Treatment group mothers completed a median of 20/25 (80%) expected home visits. Control group mothers completed a median of 21/23 (91%) expected home visits (p. 598). Comparison Breastfeeding Nutrition Group Education programme. Control group's curricular content included a previously developed breastfeeding/nutrition education programme that included 23 home visits, each lasting approximately 1 hour. Fees, reimbursement, or incentives: gift cards to a local grocery store on completion of study assessments. |
|
| Outcomes | Overall health and well‐being: ITSEA – Competence domain Child psychological health and emotional behaviour: ITSEA – Competence domain Family enhancing lifestyle or behaviour outcomes: Home Observation for Measurement of the Environment Parent carer psychological health: depression Parenting knowledge and awareness: involvement Time points: 12 months' postpartum |
|
| Funding source and conflicts of interest |
Funding: Substance Abuse Mental Health Services Administration (SAMHSA I: Grant No. UD1SP08860, SAMHSA II: Grant No. UD1SP09588), and the Ford Foundation, the Annie E. Casey Foundation, and the C.S. Mott Foundation. Conflict of interest: Dr Walkup has received research grant support from Eli Lilly, Pfizer, and Abbott. He has been a consultant to GlaxoSmithKline, Eli Lilly, and the Cliff 's Communities. He has received speaker's honoraria from the Tourette Syndrome Association. The other authors report no conflicts of interest. |
|
| Notes |
Other outcomes collected but not used within the review Maternal outcomes: parenting knowledge, parenting stress, substance use, social support Time points not used: 6 months' postpartum |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using web program Quote: "The randomization sequence, generated by the Website http://randomization.com was stored confidentially by the data manager in Baltimore, MD." |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred after enrolment; central allocation was used. Quote: "Randomization was revealed to participants after the baseline assessment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and people delivering the intervention were not blinded to the intervention. Quote: "Neither the participants nor the interventionists were blind to study group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | People who delivered the intervention also collected data. Quote: "Interventionists also served as evaluators and were specifically trained to administer self‐report and observational assessments with objectivity." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis completed. There was a high number of missing data by the end of the trial. In addition, the intervention group consistently had increased proportions of attrition at 2 and 6 months. |
| Selective reporting (reporting bias) | High risk | Trial was registered with no information provided on analysis. Only reported adjusted results and it was unclear how many confounders were included in the analysis. |
| Other bias | Unclear risk | There is some discussion on the cultural appropriateness of the outcomes in particular the Home Observation Measurement of the Environment outcome. |
AG: anticipatory guidance; BMI: body mass index; FSN: Family Spirit Nurture; FV: fluoride varnish; HCSF: Healthy Child, Strong Families; ITSEA: Infant‐Toddler Social and Emotional Assessment; MI: motivational interviewing; n: number; RCT: randomised controlled trial; SD: standard deviation; SE: standard error; SF‐12: 12‐item Short Form Survey; SSB: sugar‐sweetened beverage; USD: US dollars.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abad 2007 | Wrong study design |
| ACTRN12608000073303 | Wrong intervention |
| ACTRN12617000210370 | Wrong intervention |
| ACTRN12618001079235 | Wrong intervention |
| Adirim 2013 | Wrong population |
| Affonso 1993 | Wrong study design |
| Affonso 1995 | Wrong study design |
| Ah 2016 | Wrong study design |
| Ahmat 2012 | Wrong study design |
| Albright 2009 | Wrong population |
| Albright 2012 | Wrong population |
| Albright 2014 | Wrong population |
| Albright 2015 | Wrong population |
| Alicata 2016 | Wrong study design |
| Alto 1994 | Wrong study population |
| Anand 2007 | Wrong study population |
| Anderson 2015 | Wrong study design |
| Anderson 2019 | Wrong population |
| ANTaR 2007 | Wrong study design |
| Araujo 2016 | Wrong study design |
| Arney 2010 | Wrong study design |
| Atkinson 2010 | Wrong study design |
| Azur 2007 | Wrong study design |
| Bagshaw 2006 | Wrong study design |
| Bair‐Merritt 2010 | Wrong population |
| Baldwin 2001 | Wrong study design |
| Barclay 2014 | Wrong study design |
| Barlett 1988 | Wrong study design |
| Bar‐Zeev 2015 | Wrong population |
| Batliner 2014 | Wrong intervention |
| Batliner 2018 | Wrong intervention |
| Benzies 2011a | Wrong study design |
| Benzies 2011b | Wrong study design |
| Berns 2017 | Wrong intervention |
| Bernstein 2005 | Wrong study design |
| Bertilone 2015 | Wrong study design |
| Bertilone 2017 | Wrong study design |
| Best 2011 | Wrong study design |
| Best 2013 | Wrong study design |
| BigFoot 2009 | Wrong study design |
| BigFoot 2011 | Wrong study design |
| Billard 2014 | Wrong study design |
| Black 2013a | Wrong study design |
| Black 2013b | Wrong study design |
| Black 2014 | Wrong study design |
| Blinkhorn 2012 | Wrong study design |
| Blue 2020 | Wrong intervention |
| Boffa 2007 | Wrong study design |
| Bond 2009a | Wrong study design |
| Bond 2009b | Wrong study design |
| Bonnici 2008 | Wrong study design |
| Booth‐LaForce 2020 | Wrong intervention |
| Bovill 2017 | Wrong population |
| Bowes 2014 | Wrong study design |
| Boychuk 1984 | Wrong study design |
| Bradley 1994 | Wrong study design |
| Brega 2020 | Wrong intervention |
| Breslin 2009 | Wrong study design |
| Brewin 2002 | Wrong study design |
| Brewin 2004 | Wrong study design |
| Brown 2015 | Wrong study design |
| Brown 2016 | Wrong study design |
| Bruerd 1989 | Wrong study design |
| Bucharski 1999 | Wrong study design |
| Buckskin 2013 | Wrong study design |
| Bulterys 1990 | Wrong study design |
| Burd 2007 | Wrong study design |
| Burrows 2014 | Wrong study design |
| Bussey 2013 | Wrong study design |
| Byrnes 2020 | Wrong population |
| Calabria 2012 | Wrong study design |
| Carlisle 2020 | Wrong study design |
| Chaffin 2012 | Wrong intervention |
| Chamberlain 1998 | Wrong study design |
| Chamberlain 2017 | Wrong study design |
| Chambliss 2000 | Wrong population |
| Chang 2010 | Wrong study design |
| Chartier 2020 | Wrong study design |
| Chi 2013 | Wrong study design |
| Cidro 2015 | Wrong study design |
| CIRCA 2014 | Wrong study design |
| Cleary 2006 | Wrong study design |
| Cresp 2016 | Wrong study design |
| Cruz 2016a | Wrong population |
| Cruz 2016b | Wrong population |
| D'Espaignet 2003 | Wrong population |
| Daro 1998 | Wrong population |
| Davis 1999 | Wrong study design |
| Davis 2001 | Wrong study design |
| Davis 2013 | Wrong population |
| dela Cruz 2010 | Wrong study design |
| Department of Family and Community Services 2004 | Wrong study design |
| Department of Family and Community Services 2005 | Wrong study design |
| Dew 2014 | Wrong study design |
| Dietrich 1986 | Wrong study design |
| Dinges 1974 | Wrong study design |
| Dionne 2009 | Wrong population |
| Dobson 2017 | Wrong study design |
| Douglas 2013 | Wrong study design |
| Duffy 1994 | Wrong study design |
| Duggan 1999 | Wrong population |
| Duggan 2000 | Wrong population |
| Duggan 2004a | Wrong population |
| Duggan 2004b | Wrong population |
| Eades 2004 | Wrong study design |
| Eades 2007 | Wrong study design |
| Eades 2012 | Wrong population |
| El‐Kamary 2004 | Wrong population |
| Emerson 2015 | Wrong study design |
| Engeler 1997 | Wrong study design |
| Eni 2011 | Wrong study design |
| Enns 2019 | Wrong study design |
| Esquivel 2016 | Wrong population |
| Fejo 1994 | Wrong study design |
| Fernald 2017 | Wrong population |
| Fialkowski 2014 | Wrong study design |
| Fisher 2002 | Wrong study design |
| Floden 1989 | Wrong study design |
| Flynn 1999 | Wrong study design |
| Frances 2011 | Wrong study design |
| Franklin 1995 | Wrong study design |
| Frenza 1993 | Wrong study design |
| Frow 2010 | Wrong study design |
| Fuddy 2002 | Wrong study design |
| Gao 2014 | Wrong study design |
| George 2007 | Wrong study design |
| Gerlach 2009 | Wrong study design |
| Ginsburg 2012 | Wrong study population |
| Glor 1987 | Wrong study design |
| Glover 1996 | Wrong intervention |
| Glover 2000 | Wrong intervention |
| Glover 2009 | Wrong intervention |
| Glover 2015a | Wrong study design |
| Glover 2015b | Wrong intervention |
| Glover 2016 | Wrong study design |
| Gomby 2007 | Wrong study design |
| Gould 2013 | Wrong study design |
| Grace 2016 | Wrong study design |
| Gray‐Donald 2000 | Wrong population |
| Gregory 2008 | Wrong study design |
| Guyer 2000 | Wrong population |
| Haag 2019 | Wrong intervention |
| Hamerton 2014 | Wrong study design |
| Harnett 1998 | Wrong study design |
| Harrison 2006 | Wrong study design |
| Harvey‐Berino 2003 | Wrong intervention |
| Haswell‐Elkins 2009 | Wrong study design |
| Hewer 2006 | Wrong study design |
| Hilferty 2010 | Wrong study design |
| Holdaway Smith 2021 | Wrong population |
| Homel 2006a | Wrong study design |
| Homel 2006b | Wrong study design |
| Hucul 2015 | Wrong study design |
| Hunter 2014 | Wrong population |
| Iglesias 2010 | Wrong study design |
| ISRCTN41467632 | Wrong intervention |
| Jamieson 2016 | Wrong intervention |
| Jamieson 2018 | Wrong intervention |
| Jamieson 2019a | Duplicate |
| Jamieson 2019b | Wrong intervention |
| Jan 2004 | Wrong study design |
| Jersky 2016 | Wrong study design |
| Johnson 1994 | Wrong study design |
| Johnson 2006 | Wrong study design |
| Johnson 2011 | Wrong study design |
| Johnston 2011 | Wrong study design |
| Jones 2020 | Wrong study design |
| Jongen 2014 | Wrong study design |
| Karanja 2010 | Wrong study design |
| Karanja 2012 | We attempted to contact multiple authors of the publication and received no reply. We decided to exclude. |
| Karol 2016 | Wrong study design |
| Kegler 2003 | Wrong study design |
| Kegler 2004 | Wrong study design |
| Kemp 2010 | Wrong population |
| Kemp 2018 | Wrong study design |
| Keown 2018 | Wrong intervention |
| Kildea 2016 | Wrong study design |
| Kildea 2017 | Wrong study design |
| King‐Hooper 1996 | Wrong study design |
| Kira 2016 | Wrong population |
| Koniak‐Griffin 1999 | Wrong study design |
| Lawrence 2004 | Wrong study design |
| Lawrence 2008 | Wrong intervention |
| Lee 2010 | Wrong study design |
| Lees 2014 | Wrong intervention |
| Leijten 2016 | Wrong population |
| Letourneau 2008 | Wrong study design |
| Long 1995 | Wrong study design |
| Lucero 2012 | Wrong study design |
| Lucero 2015 | Wrong study design |
| Macedo 2020 | Wrong intervention |
| Mackerras 1998 | Wrong study design |
| Madden 1984 | Wrong population |
| Makin 2001 | Wrong study design |
| Mares 2012 | Wrong study design |
| Martens 2002 | Wrong study design |
| Mathu‐Muju 2016 | Wrong study design |
| Matthews 2013 | Wrong study design |
| Maupome 2010 | Wrong study design |
| May 1989 | Wrong study design |
| May 2008 | Wrong study design |
| Mayberry 1999 | Wrong study design |
| Mayfield 1984 | Wrong study design |
| Mayfield 1985 | Wrong study design |
| Mayfield 1986 | Wrong study design |
| McIntosh 2018 | Wrong intervention |
| McKay 2015 | Wrong study design |
| McKenzie 1995 | Wrong study design |
| McShane 2008 | Wrong study design |
| Middleton 2017 | Wrong study design |
| Mondy 2004 | Wrong study design |
| Montag 2015 | Wrong intervention |
| Mraz Esposito 2014 | Wrong study design |
| Munns 2010 | Wrong study design |
| Munns 2015 | Wrong study design |
| Munro 2011 | Wrong study design |
| Murphy 2012 | Wrong study design |
| Mylant 2021 | Wrong study design |
| Nations 2004 | Unable to determine study design, contacted author but received no reply. |
| NCT00428805 | Wrong population |
| NCT00435500 | Wrong intervention |
| NCT01116726 | Wrong intervention |
| NCT02091804 | Wrong intervention |
| NCT03142009 | Wrong population |
| Nguyen 2015 | Wrong study design |
| Novotny 2013 | Wrong population |
| Novotny 2017 | Wrong study design |
| NSW Centre for Parenting and Research 2005 | Wrong study design |
| Nutting 1979 | Wrong study design |
| Oxford 2020 | Wrong intervention |
| Patten 2012a | Wrong population |
| Patten 2012b | Wrong population |
| Phillips 2014 | Wrong intervention |
| Poland 1991 | Wrong study design |
| Pollack 2011 | Wrong study design |
| Poppe 1992 | Wrong study design |
| Prater 2002 | Wrong study design |
| Pullon 2003 | Wrong population |
| Ratima 1999 | Wrong study design |
| Ratnaike 1994 | Wrong study design |
| Reeve 2014 | Wrong study design |
| Reeve 2016 | Wrong study design |
| Richardson 2008 | Wrong study design |
| Richer 2018 | Wrong study design |
| Ricks 2015 | Wrong study design |
| Riley 2010 | Wrong study design |
| Ring 2007 | Wrong study design |
| Roberts 1993 | Wrong study design |
| Roberts‐Thomson 2010 | Wrong intervention |
| Robinson 2008 | Wrong study design |
| Robinson 2009 | Wrong study design |
| Robinson 2012 | Wrong study design |
| Robinson 2013 | Wrong study design |
| Robinson 2017 | Wrong study design |
| Rogers 2003 | Wrong study design |
| Ryan 2001 | Wrong study design |
| Saggers 2009 | Wrong study design |
| Sanghavi 2005 | Wrong study design |
| Santos 1999 | Wrong study design |
| Sawchuk 1998 | Wrong study design |
| Sawyer 2014 | Wrong study design |
| Shan 2014 | Wrong study design |
| Simmons 2008 | Wrong population |
| Simonet 2009 | Wrong population |
| Sivak 2008 | Wrong study design |
| Smith 1993 | Wrong study design |
| Smith 2000 | Wrong study design |
| Smith 2018 | Wrong study design |
| Smithers 2017 | Wrong intervention |
| Smithers 2021 | Wrong intervention |
| Soares 2021a | Wrong intervention |
| Soares 2021b | Wrong intervention |
| Soltzberg 1997 | Wrong study design |
| Sparrow 2011 | Wrong study design |
| TAIHS 2003 | Wrong study design |
| Thompson 2006 | Wrong study design |
| Tipa 2015 | Wrong study design |
| Trenholme 2016 | Wrong intervention |
| Tsey 2007 | Wrong study design |
| Turner 2007 | Wrong population |
| Turner 2017 | Wrong intervention |
| Valery 2007 | Wrong population |
| Valery 2008 | Wrong population |
| Valery 2009 | Wrong population |
| Valery 2010 | Wrong population |
| Vicary 2001 | Wrong study design |
| Volpe 2014 | Wrong study design |
| Wen 2012 | Wrong population |
| Wilken 2013 | Wrong intervention |
| Williams 2017 | Wrong study design |
| Wilson 2013 | Wrong population |
| Wilson 2018 | Wrong intervention |
| Wright 1997 | Wrong study design |
| Yoshimoto 2014 | Wrong study design |
| Young 2016 | Wrong study design |
Characteristics of ongoing studies [ordered by study ID]
Baby Teeth Talk Study (BTT).
| Study name | Preventing early childhood caries in Indigenous children: the Baby Teeth Talk study (BTT) |
| Methods | RCT |
| Participants | Pregnant women who identify as Aboriginal Peoples in Canada (First Nations, Metis, Inuit) and live in urban and on‐reserve communities in Ontario and Manitoba. |
| Interventions | Baby Teeth Talk. MI and AG provided pre‐ and postnatally to mothers by community‐based researchers, concerning how to care for children's teeth. Delivered at the time of tooth eruption (6–10 months), 12, 18, and 24 months. Dental care and fluoride varnish applied at time of tooth eruption, 12, 18, and 24 months. Control group received a delayed dental care programme; MI and AG, dental care, and fluoride varnish provided at 24, 30, and 36 months. |
| Outcomes | Outcomes assessed from preconception to 3 years' postpartum. Child dental caries as incidence and increment over 2 years; maternal/carer oral health‐related knowledge, self‐care, self‐efficacy, and literacy; maternal/carer dental health service utilisation. |
| Starting date | June 2011 |
| Contact information | Herenia P Lawrence |
| Notes |
Back to Basics.
| Study name | Back to Basics: addressing childhood obesity through traditional foods in Alaska |
| Methods | Randomised mixed‐methods intervention trial |
| Participants | Alaskan Native residents from rural communities in Yukon‐Kuskokwim region: children aged 0–5 years and enrolled in the RurAL CAP, Head Start, Early Head Start, or Parents as Teachers programmes. |
| Interventions | Back to Basics. A physical activity programme; 9‐month traditional food menu programmes within Head Start programmes; home‐based nutrition programme; and documentation mechanism to record traditional food important to each community. Programmes repeated annually. Control group received standard education and menu programmes. |
| Outcomes | Outcomes assessed annually over 4 years of intervention. BMI; traditional food content in diet. |
| Starting date | 14 May 2018 |
| Contact information | Timothy K Thomas |
| Notes |
Family Spirit Nuture Part 2 2019.
| Study name | Family Spirit Nurture |
| Methods | RCT |
| Participants | Pregnant Native American women living in 2 Navajo communities and the Fort Apache Indian Reservation aged 14–24 who are having their first or second baby. |
| Interventions | Family Spirit Nurture + optimised standard care. The Family Spirit Nurture home‐visiting module consists of 36, 60‐minute lessons delivered by trained local Family Health Coaches, from 28 weeks' gestation to 18 months' postpartum. Optimised standard care consists of transportation assistance to antenatal and well‐baby clinic visits. These checks are recommended by the Indian Health Service and American Academy of Pediatrics. Control group will receive optimised standard care only. |
| Outcomes | Outcomes collected between 2 and 24 months old. Breastfeeding, complementary feeding practices, infant feeding style, toddler feeding style, consumption of fruit and vegetable intake, child physical activity levels, screen time and sedentary behaviour, BMI z‐scores, maternal stress, maternal depression, maternal alcohol and drug use, infant metabolic health |
| Starting date | 25 September 2017 |
| Contact information | Allison Barlow |
| Notes | ClinicalTrials.gov Identifier: NCT03334266 |
Great Beginnings for Healthy Native Smiles.
| Study name | Great Beginnings for Healthy Native Smiles: an early childhood caries prevention project |
| Methods | RCT |
| Participants | Native American women aged > 18 years, living within 100 miles of the Hopi or Crow nations who are currently 3–7 months pregnant. |
| Interventions | Early Childhood Caries Prevention. Face‐to‐face educational sessions provided twice before childbirth then at 6, 12, 18, and 24 months provided by Community Health Workers. Children received up to 4 fluoride varnish applications during the study and MI with mothers. Control group received standard antenatal/postnatal healthy lifestyle intervention designed to improve maternal/child health knowledge. |
| Outcomes | Outcomes assessed through study completion at 30–36 months for the early enrolment cohort, and 24 months for the late cohort. Number of decayed, missing, or filled primary tooth surfaces or teeth (or both); maternal/carer oral health knowledge, behaviour, and attitudes. |
| Starting date | 29 March 2021 |
| Contact information | Julie A Baldwin, Kristan Elwell |
| Notes |
Infant Care Practices Study (ICP).
| Study name | Infant Care Practices Study (ICP) |
| Methods | RCT |
| Participants | Pregnant American Indian women aged > 14 years, from Western South Dakota who are < 20 weeks' gestation. |
| Interventions | Protecting Babies While they Sleep Curriculum. 3 antenatal contacts at study site offices involving engagement with the curriculum and activities aimed to ascertain the role of carer knowledge, beliefs, and access to resources in implementation of infant safe sleep practices, led by trained study staff. Control group attend antenatal contacts and receive educational material available from local healthcare facilities. |
| Outcomes | Outcomes assessed at 3, 6, and 12 months postnatal. Implementation of safe sleeping practices; maternal/carer changes in Safe Sleep knowledge, beliefs, and practice. |
| Starting date | 28 March 2018 |
| Contact information | Amy Elliott |
| Notes |
LEAP‐CP.
| Study name | LEAP‐CP: Learning through Everyday Activities with Parents for Indigenous Australian infants at high risk of cerebral palsy and neurodevelopmental disabilities |
| Methods | RCT |
| Participants | Infants, aged 3 months to 2 years, assessed as a high risk of cerebral palsy/neurodevelopmental disabilities or a confirmed diagnosis of cerebral palsy with 1 or both parents identifying as Aboriginal or Torres Strait Islander in the study geographical area. |
| Interventions | LEAP‐CP. 30 weekly 2‐hour visits over 7–10 months to deliver multidisciplinary family‐centred intervention, delivered through face‐to‐face home visits by an Indigenous Allied Health Worker. The structure of the visits followed: feedback and troubleshooting, therapeutic modules, and education modules. Carers are provided with written and pictographic programme and study‐specific information to facilitate the strategies provided during the sessions. Control group received care as usual from primary and allied health programmes provided in the community. |
| Outcomes | Outcomes assessed at 7–10 months postintervention commencement. Parent‐perceived changes in child's performance; carer depression, anxiety, and stress; infant/toddler motor skills outcomes; infant/toddler cognitive and communication skills; parent‐infant emotional availability; quality and quantity of parent and home infant/toddler stimulation; infant/toddler quality of life; infant/toddler social emotional assessment; infant/toddler near vision detection; nutritional status; and infant/toddler self‐care, mobility and social function. |
| Starting date | 1 September 2019 |
| Contact information | Katherine Benfer |
| Notes |
Precision Family Spirit.
| Study name | Precision Family Spirit: a pilot randomized implementation trial of a precision home visiting approach with families in Michigan |
| Methods | RCT |
| Participants | Native American women aged ≥ 14 years who are either pregnant or have an infant aged ≤ 2 months living in both the Lower and Upper Peninsula communities in Michigan |
| Interventions | Precision Family Spirit. Face‐to‐face home‐visiting module consisting of a minimum of 25 lessons from pregnancy to 12‐months' postpartum delivered by culturally matched paraprofessionals. Additional lessons, up to 41 lessons in total during the duration of enrolment, will be provided based on emergent needs assessed during home visit lessons. Control group will receive care as usual through the Michigan Family Spirit programme. |
| Outcomes | Outcomes are collected at 2, 6, and 12 months' postpartum. Quality of the relationship between the home visitor and the client, including satisfaction, retention, and adherence; infant feeding practices, including the introduction of SSBs; child development delays, maternal substance abuse (tobacco, alcohol, drugs); maternal depression and stress; maternal parenting knowledge, self‐efficacy, and feeding practices. |
| Starting date | 24 June 2019 |
| Contact information | Allison Ingalls |
| Notes |
Wakȟáŋyeža (Little Holy One).
| Study name | Wakȟáŋyeža (Little Holy One) – an intergenerational intervention for Native American parents and children: a protocol for a randomized controlled trial with embedded single‐case experimental design |
| Methods | RCT |
| Participants | Parent or carer aged > 18 years of children aged 3–5 years enrolled in Head Start class and is a member or descendent of Fort Peck Tribes, with an exposure to ≥ 1 adverse childhood event or historical trauma. |
| Interventions | Wakȟáŋyeža. 12 weekly 1‐hour lessons provided to carers/parents. Curriculum includes lessons on cultural connection and traditions; parenting adapted from Family Spirit intervention; and stress and trauma. Control group received 6 × 1‐hour lessons on nutrition over 16 weeks. |
| Outcomes | Outcomes were assessed at 6 and 12 weeks, 6, 12, 18, and 24 months. Change in carer trauma symptoms; change in parenting stress symptoms; change in carer depression; parent baseline assessment of stressful life events, positive childhood experiences, adverse childhood experiences, historical loss experiences, experience relating to historical trauma; changes to parental practices, control, substance use, family routines, communal mastery, tribal identity, social networks information, and suicide risk; child Head Start school attendance; child's externalisation and internalisation of symptoms. |
| Starting date | 18 November 2019 |
| Contact information | Teresa Brockie |
| Notes |
AG: anticipatory guidance; BMI: body mass index; MI: motivational interviewing; RCT: randomised controlled trial.
Differences between protocol and review
Types of participants
If the population had both Indigenous and non‐Indigenous children, we included studies that had more than 50% Indigenous children. If studies included children aged five years or older, we included studies that had more than 50% of children aged less than five years old or where the mean of the children's age was five years or less.
All studies were required to be implemented or led from primary healthcare services. In the USA, child wellness promotion and healthcare services delivered in rural reservations are often run in collaboration with Indian Health services or tribal health services, and early childhood centres. As a result, we decided to include trials from rural reservations in the USA where primary healthcare services were not the sole source of implementing or leading the family‐centred care but did so in collaboration with other services.
Types of outcome measures
Primary outcome
We included an additional primary outcome named 'overall health and well‐being'. Although we built in specific outcomes, we did not consider a broader outcome that could answer the aim of whether family‐centred care interventions were effective in improving the health and well‐being of Indigenous children and their families. We developed this outcome based on the guidance provided in Chapter 3 of the Cochrane Handbook for Systematic Reviews of Interventions (McKenzie 2021). To determine which outcomes would be included we used a stepwise approach. The first step was the inclusion of a study outcome by order of priority of the second to fifth primary outcomes as listed under Primary outcomes. The outcomes were included if they were measured using administrative data followed by a validated question and where neither were available, a subjective outcome. The longest time point was used in the analysis and if data were not available, then the next longest data point until a primary outcome was chosen for each study.
Selection of outcomes reported
As anticipated, there was more than one outcome assigned to each outcome category. Review authors JM, RB, SC, LS, and CC were provided with a spreadsheet of all outcomes with each outcome assigned to its respective outcome category and no results. Led by review author NS, each outcome was determined by the group for inclusion into the review. Outcomes were included based on objective versus subjective measures, their overall relevance to family centred‐care interventions and from a strengths‐based rather than deficit perspective. After outcomes were determined for inclusion, they were cross‐checked within each category to determine if there were any outcomes that were similar across studies. The final outcomes included from each paper in each category were based on decision rules by the review team or the most frequently reported outcome, or both.
Timing of outcome assessment
We grouped timing of assessment into short‐, medium‐, and long‐term time points with no more than one time interval for each outcome from each study selected. If there were multiple time points in each time point period, we chose the time point that was closest to the end of the time interval. For example, for medium‐term outcomes from greater than three to 12 months when trials gave outcomes at six and 12 months, we used the 12‐month time point. This was determined by the review team before any results were seen.
Main outcomes for summary of findings table
We decided to include all primary outcomes in the summary of findings table. This included the addition of the overall health and well‐being and the physical health and developmental health of Indigenous children outcomes.
Measures of treatment effect
When analysing continuous data, we multiplied data by −1 for scales that were in the opposite direction. For outcomes that had both continuous and dichotomous data, we combined both outcomes in the meta‐analysis and calculated the standardised mean difference (SMD) and 95% confidence interval (CI) using the general inverse variance method (Deeks 2021). For count data, we considered the outcomes to be common and, therefore, calculated these data as continuous (i.e. means and standard deviations). We then converted these data into SMDs using methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b). We included the longest time point for each outcome in the analysis.
Dealing with missing data
For missing data, we used the methods outlined in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b). We used these methods to determine standard deviations for continuous data. For cluster‐RCTs, we converted the individual means and standard deviations to SMDs using the methods outlined in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021c; White 2005).
Sensitivity analysis
Where available, we included an additional sensitivity analysis by excluding outcomes that were subjective and not from validated questionnaires or tools, or were not administrative data (i.e. hospital records).
Contributions of authors
NS: assisted with drafting the protocol, completed first screening, full‐text screening and data extracting, assessed levels of family‐centredness, carried out the analysis, interpreted the analysis, completed risk of bias, drafted and revised the final manuscript. NS will take responsibility of updating the review.
CC: assisted with drafting the protocol, assisted with full‐text screening and data extraction, assessed levels of family‐centredness, provided systematic review expertise, drafted and revised the final manuscript.
SC: assisted with drafting the protocol, completed full‐text screening, assessed levels of family‐centredness, interpreted the analysis, completed risk of bias, revised the final manuscript.
LS: assisted with drafting the protocol, completed full‐text screening, assessed levels and advised on family‐centredness, interpreted the analysis, completed risk of bias, revised the final manuscript.
RB: assisted with drafting the protocol, completed full‐text screening, assessed levels of family‐centredness, interpreted the analysis, revised the final manuscript.
CA: completed first screening and data extracting, revised and edited the final manuscript.
KE: provided advice about the clinical relevance of outcomes and revise the final manuscript.
RM: provided advice about the clinical relevance of outcomes and revise the final manuscript.
JM: completed the protocol, contributed to full‐text screening and assessed levels of family centredness, interpreted the analysis, completed risk of bias, revised the final manuscript.
Sources of support
Internal sources
-
No sources of support provided, Other
No sources of support provided
External sources
-
NHMRC Centre of Research Excellence for Improving Health Services for Aboriginal and Torres Strait Islander Children (ISAC), Australia
ISAC provided a grant of USD 21,107 to Apunipima Cape York Health Services to produce the Cochrane protocol and a review of family‐centred interventions for Indigenous early childhood health. Apunipima contracted James Cook University researchers to complete the protocol and a review.
-
Poche Centre for Indigenous Health at the University of Western Australia, Australia
Provided funds for evidence synthesis activities to support the completion of the review.
-
Catherine Chamberlain, Australia
Catherine Chamberlain is supported by a National Health and Medical Research Council Early Career Fellowship (1161065).
-
Roxanne Bainbridge, Australia
Roxanne Bainbridge was supported by a National Health and Medical Research Council Career Development Fellowship (GNT1104795).
-
Janya McCalman, Australia
Janya McCalman was supported by a National Health and Medical Research Council Early Career Fellowship (GNT1113392).
Declarations of interest
Where review authors had published papers that might be included in the review, the review author/s in question were not involved in assessing the study for inclusion, or extracting or analysing data from that study.
NS: none.
CC: I am a recipient of an Australian National Health and Medical Research Council Early Career Development Fellowship, which has a focus on Indigenous maternal public health.
SC: none.
LS: none.
RB: none.
CA: none.
MW: none.
KE: none.
RM: none.
JM: none.
New
References
References to studies included in this review
Barlow 2006 {published data only}
- Barlow A, Varipatis-Baker E, Speakman K, Ginsburg G, Friberg I, Goklish N, et al. Home-visiting intervention to improve child care among American Indian adolescent mothers: a randomized trial. Archives of Pediatrics & Adolescent Medicine 2006;160(11):1101-7. [DOI] [PubMed] [Google Scholar]
Broughton 2013 {published data only}
- ACTRN12611000111976. Reducing disease burden and health inequalities arising from chronic dental disease among Indigenous children: an early childhood caries intervention. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336353 (first received 20 December 2010).
- Broughton JR, Lawrence HP, Jamieson L. Tikanga Māori (Māori Customary Practices) in oral health research. Journal of Health Care for the Poor and Underserved 2016;27:101-9. [DOI] [PubMed] [Google Scholar]
- Broughton JR, Maipi JT, Person M, Thomson WM, Morgaine KC, Tiakiwai SJ, et al. Reducing disease burden and health inequalities arising from chronic disease among Indigenous children: an early childhood caries intervention in Aotearoa/New Zealand. BMC Public Health 2013;13:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton JR, Person M, Maipi JT, Cooper-Te KR, Smith-Wilkinson A, Tiakiwai S, et al. Ukaipo niho: the place of nurturing for oral health. New Zealand Dental Journal 2014;110(1):18-23. [PubMed] [Google Scholar]
- Broughton JR, Thomson WM, Maipi JT, Person M, Morgaine KC, Jamieson L, et al. Mokopuna Maori me oranga niho (Maori infants and oral health) an early childhood caries randomised control trial among New Zealand Maori children. New Zealand Dental Journal 2021;117:129-36. [Google Scholar]
Family Spirit Nuture Part 1 2021 {published data only}
- NCT03101943. Preventing early childhood obesity, part 1: Family Spirit Nurture, 3–9 months. clinicaltrials.gov/ct2/show/NCT03101943 (first received 5 April 2017).
- Rosenstock S, Ingalls A, Foy Cuddy R, Neault N, Littlepage S, Cohoe L, et al. Effect of a home-visiting intervention to reduce early childhood obesity among Native American children: a randomized clinical trial. JAMA Pediatrics 2021;175(2):133-42. [PMID: 10.1001/jamapediatrics.2020.3557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Family Spirit Trial 2012 {published data only}
- Barlow A, Mullany B, Neault N, Compton S, Carter A, Hastings R, et al. Effect of a paraprofessional home-visiting intervention on American Indian teen mothers' and infants' behavioral risks: a randomized controlled trial. American Journal of Psychiatry 2013;170(1):83-93. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A, Mullany B, Neault N, Goklish N, Billy T, Hastings R, et al. Paraprofessional-delivered home-visiting intervention for American Indian teen mothers and children: 3-year outcomes from a randomized controlled trial. American Journal of Psychiatry 2015;172(2):154-62. [DOI: 10.1176/appi.ajp.2014.14030332] [DOI] [PubMed] [Google Scholar]
- Haroz EE, Ingalls A, Kee C, Goklish N, Neault N, Begay M, et al. Informing precision home visiting: identifying meaningful subgroups of families who benefit most from Family Spirit. Prevention Science 2019;20(8):1244-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany B, Barlow A, Neault N, Billy T, Jones T, Tortice I, et al. The Family Spirit trial for American Indian teen mothers and their children: CBPR rationale, design, methods and baseline characteristics. Prevention Science 2012;13(5):504-18. [DOI] [PubMed] [Google Scholar]
- NCT00373750. Cradling our future through family strengthening study [In-home prevention of SA risks for Native teen families]. clinicaltrials.gov/ct2/show/NCT00373750 (first received 8 September 2006).
Harrison 2010 {published data only}
- Harrison R, Veronneau J, Leroux B. Design and implementation of a dental caries prevention trial in remote Canadian Aboriginal communities. Trials 2010;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RL, Veronneau J, Leroux B. 1a: a clinical trial of the effectiveness of a dental caries prevention program for Cree mothers and their infants. www.creehealth.org/sites/default/files/CreeC_Final%20Report%20to%20Funders_31Jan11.pdf (accessed prior to 21 November 2022).
- Harrison RL, Veronneau J, Leroux B. Effectiveness of maternal counseling in reducing caries in Cree children. Journal of Dental Research 2012;91(11):1032-7. [DOI: 10.1177/0022034512459758] [DOI] [PubMed] [Google Scholar]
- ISRCTN41467632. Dental caries prevention program for Cree mothers and infants [A clinical trial of the effectiveness of a dental caries prevention program for Cree mothers and their infants]. www.isrctn.com/ISRCTN41467632 (first received 29 June 2004).
- NCT00175318. Testing a tooth decay prevention program with Cree mothers and infants. clinicaltrials.gov/ct2/show/NCT00175318 (first received 15 September 2005).
HCSF 1 2007 {published data only}
- Adams A, LaRowe T, Cronin KA, Prince RJ, Jobe JB. Healthy children, strong families: results of a randomized trial of obesity prevention for preschool American Indian children and their families. Obesity 2011;19:S110. [DOI: ] [Google Scholar]
- Adams AK, LaRowe TL, Cronin KA, Prince RJ, Wubben DP, Parker T, et al. The Healthy Children, Strong Families intervention: design and community participation. Journal of Primary Prevention 2012;33(4):175-85. [DOI: 10.1007/s10935-012-0275-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AK, Scott JR, Prince R, Williamson A. Using community advisory boards to reduce environmental barriers to health in American Indian communities, Wisconsin, 2007–2012. Preventing Chronic Disease 2014;11:E160. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe TL, Wubben DP, Cronin KA, Vannatter SM, Adams AK. Development of a culturally appropriate, home-based nutrition and physical activity curriculum for Wisconsin American Indian families. Preventing Chronic Disease 2007;4(4):A109. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Tomayko EJ, Prince RJ, Cronin KA, Adams AK. The Healthy Children, Strong Families intervention promotes improvements in nutrition, activity and body weight in American Indian families with young children. Public Health Nutrition 2016;19(15):2850-9. [DOI: 10.1017/S1368980016001014] [DOI] [PMC free article] [PubMed] [Google Scholar]
HCSF 2 2017 {published data only}
- Grant VM, Tomayko EJ, Prince RJ, Cronin K, Adams, A. Understanding correlates of physical activity in American Indian families: the Healthy Children Strong Families-2 Study. Journal of Physical Activity & Health 2018;15(11):866-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01776255. Healthy Children, Strong Families: American Indian communities preventing obesity (HCSF2). clinicaltrials.gov/ct2/show/NCT01776255 (first received 28 January 2013).
- Tomayko EJ, Prince RJ, Cronin KA, Kim K, Parker T, Adams AK. The Healthy Children, Strong Families 2 (HCSF2) randomized controlled trial improved healthy behaviors in American Indian families with young children. Current Developments in Nutrition 2019;3(Suppl 3):53-62. [DOI: 10.1093/cdn/nzy087] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomayko EJ, Prince RJ, Cronin KA, Parker T, Kim K, Grant VM, et al. Healthy Children, Strong Families 2: a randomized controlled trial of a healthy lifestyle intervention for American Indian families designed using community-based approaches. Clinical Trials 2017;14(2):152-61. [DOI: 10.1177/1740774516685699] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomayko EJ, Webber EJ, Cronin KA, Prince RJ, Adams AK. Use of text messaging and Facebook groups to support the Healthy Children, Strong Families 2 healthy lifestyle intervention for American Indian families. Current Developments in Nutrition 2021;5(Suppl 4):32-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2010 {published data only}
- ACTRN12609000937213. A family tobacco control program to reduce respiratory illness in Indigenous infants [A randomised controlled trial of a family-centred tobacco control program about environmental tobacco smoke (ETS) to reduce respiratory illness in Indigenous infants]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=320761 (first received 30 October 2009).
- Johnston V, Walker N, Thomas DP, Glover M, Chang AB, Bullen C, et al. The study protocol for a randomized controlled trial of a family-centred tobacco control program about environmental tobacco smoke (ETS) to reduce respiratory illness in Indigenous infants. BMC Public Health 2010;10:114-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N, Johnston V, Glover M, Bullen C, Trenholme A, Chang A, et al. Effect of a family-centered, secondhand smoke intervention to reduce respiratory illness in indigenous infants in Australia and New Zealand: a randomized controlled trial. Nicotine & Tobacco Research 2015;17(1):48-57. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quissell 2014 {published data only}
- Braun PA, Quissell DO, Henderson WG, Bryant LL, Gregorich SE, George C, et al. A cluster-randomized, community-based, tribally delivered oral health promotion trial in Navajo Head Start children. Journal of Dental Research 2016;95(11):1237-44. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brega AG, Henderson WG, Harper MM, Thomas, JF, Manson SM, Batliner TS, et al. Association of ethnic identity with oral health knowledge, attitudes, behavior, and outcomes on the Navajo Nation. Journal of Health Care for the Poor and Underserved 2019;30(1):143-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brega AG, Thomas JF, Henderson WG, Batliner TS, Quissell DO, Braun PA, et al. Association of parental health literacy with oral health of Navajo Nation preschoolers. Health Education Research 2016;31(1):70-81. [DOI: 10.1093/her/cyv055] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant LL, Quissell DO, Braun PA, Henderson WG, Johs N, George C, et al. A community-based oral health intervention in Navajo Nation Head Start: participation factors and contextual challenges. Journal of Community Health 2016;41(2):340-53. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01116739. Preventing caries in preschoolers: testing a unique service delivery model in American Indian Head Start programs. clinicaltrials.gov/ct2/show/NCT01116739 (first received 5 May 2010).
- Quissell DO, Bryant LL, Braun PA, Cudeii D, Johs N, Smith VL, et al. Preventing caries in preschoolers: successful initiation of an innovative community-based clinical trial in Navajo Nation Head Start. Contemporary Clinical Trials 2014;37(2):242-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AR, Brega AG, Campagna EJ, Braun PA, Henderson WG, Bryant LL, et al. Validation and impact of caregivers' oral health knowledge and behavior on children's oral health status. Pediatric Dentistry 2016;38(1):47-54. [PMC free article] [PubMed] [Google Scholar]
- Wilson AR, Brega AG, Thomas JF, Henderson WG, Lind KE, Braun PA, et al. Validity of measures assessing oral health beliefs of American Indian parents. Journal of Racial and Ethnic Health Disparities 2018;5(6):1254-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tipene‐Leach 2014 {published data only}
- ACTRN12610000993099. The Kahungunu infant safe sleep study: a randomised controlled trial of the use of the 'wahakura', a flax woven basket versus portable bassinet for the sleep of infants at risk of sudden unexpected infant death. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336229 (first received 12 November 2010).
- Baddock S, Tipene-Leach D, Tangiora A, Jones R, Williams S, Taylor B. The technical and cultural issues of home monitoring to evaluate a Maori infant sleep device. Sleep and Biological Rhythms 2012;10(Suppl 1):52. [DOI: ] [Google Scholar]
- Baddock S, Tipene-Leach D, Williams S, Tangiora A, Jones R, Iosua E, et al. Physiological measures from a randomised controlled trial to investigate infant sleep risks and benefits when using a culturally-derived sleep device (Wahakura) compared to a standard bassinet. Sleep and Biological Rhythms 2015;13:8. [Google Scholar]
- Baddock S, Tipene-Leach D, Williams S, Tangiora A, Jones R, Taylor B. Infant sleep and breastfeeding in a culturally derived sleep device (wahakura) compared with a standard bassinet-A randomised controlled trial. Maternal & Child Nutrition 2018;14(S2):e12587. [DOI: ] [Google Scholar]
- Baddock S, Williams S, Tipene-Leach D, Tangiora A, Jones R, Taylor B. A randomised controlled trial using overnight video in the home to compare a culturally derived sleep device (wahakura) with a standard bassinet. Sleep and Biological Rhythms 2014;12:38. [DOI: ] [Google Scholar]
- Baddock SA, Tipene-Leach D, Williams SM, Tangiora A, Jones R, Iosua E, et al. Wahakura versus bassinet for safe infant sleep: a randomized trial. Pediatrics 2017;139(2):e20160162. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Baddock SA, Tipene-Leach D, Williams SM, Tangiora A, Jones R, Macznik AK, et al. Physiological stability in an indigenous sleep device: a randomised controlled trial. Archives of Disease in Childhood 2018;103(4):377-82. [DOI: 10.1136/archdischild-2017-313512] [DOI] [PubMed] [Google Scholar]
- Taylor B, Tipene-Leach D, Baddock S, Macleod E, Williams S, Tangiora A, et al. A randomized controlled trial to investigate infant sleep risks and benefits when using a culturally-appropriate sleep device compared to a standard bassinet. Sleep and Biological Rhythms 2014;12:52. [DOI: ] [Google Scholar]
- Tipene-Leach D, Abel S. Innovation to prevent sudden infant death: the wahakura as an Indigenous vision for a safe sleep environment. Australian Journal of Primary Health 2019;25(5):406-9. [DOI] [PubMed] [Google Scholar]
- Tipene-Leach D, Abel S. SUDI prevention: a review of Maori safe sleep innovations for infants. New Zealand Medical Journal 2013;126(1379):86-94. [PMID: ] [PubMed] [Google Scholar]
- Tipene-Leach D, Baddock S, Williams S, Jones R, Tangiora A, Abel S, et al. Methodology and recruitment for a randomised controlled trial to evaluate the safety of wahakura for infant bedsharing. BMC Pediatrics 2014;14:1-10. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walkup 2009 {published data only}
- NCT00356551. Family Spirit Study [Family strengthening in Native communities project]. clinicaltrials.gov/ct2/show/NCT00356551 (first received 26 July 2006).
- Walkup JT, Barlow A, Mullany BC, Pan W, Goklish N, Hasting R, et al. Randomized controlled trial of a paraprofessional-delivered in-home intervention for young reservation-based American Indian mothers. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(6):591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abad 2007 {published data only}
- Abad V, Williams KE. Early intervention music therapy: reporting on a 3-year project to address needs with at-risk families. Music Therapy Perspectives 2007;25(1):52-8. [Google Scholar]
ACTRN12608000073303 {published data only}
- ACTRN12608000073303. Study of environment on Aboriginal resilience and child health [Randomised controlled trial of a health broker intervention to improve adherence to the primary healthcare practitioner's care plan for otitis media in Aboriginal children]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000073303 (first received 8 February 2008).
ACTRN12617000210370 {published data only}
- ACTRN12617000210370. A randomised controlled trial of Te Whanau Pou Toru: Maori adaptation of primary care Triple P Discussion Groups for Maori parents of young children with behavioural difficulties [A randomised controlled trial of an adaptation of Triple P Positive Parenting Program Discussion Groups for Maori parents of young children with behavioural difficulties.]. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617000210370 (first received 8 February 2017).
ACTRN12618001079235 {published data only}
- ACTRN12618001079235. HARTI HAUORA TAMARIKI a randomised controlled trial of an opportunistic, holistic and family centred approach to improving outcomes for hospitalised children and their families [HARTI HAUORA TAMARIKI a randomised controlled trial of an opportunistic, holistic and family centred approach to reducing risk of readmission and other health risks for hospitalised children and their families]. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12618001079235 (first received 28 June 2018).
Adirim 2013 {published data only}
- Adirim T, Supplee L. Overview of the Federal Home Visiting Program. Pediatrics 2013;132(Suppl 2):S59-64. [DOI] [PubMed] [Google Scholar]
Affonso 1993 {published data only}
- Affonso DD, Mayberry LJ, Graham K, Shibuya J, Kunimoto J. Prenatal and postpartum care in Hawaii: a community-based approach. Journal of Obstetric, Gynecologic, & Neonatal Nursing 1993;22(4):320-5. [DOI] [PubMed] [Google Scholar]
Affonso 1995 {published data only}
- Affonso DD, Mayberry LJ, Inaba A, Robinson E, Matsuno R. Neighborhood Women's Health Watch: partners in community care. Advanced Practice Nursing Quarterly 1995;1(3):34-40. [PubMed] [Google Scholar]
Ah 2016 {published data only}
- Ah Chee D, Boffa JD, Tilton E. Towards an integrated model for child and family services in central Australia. Medical Journal of Australia 2016;205(1):8. [DOI] [PubMed] [Google Scholar]
Ahmat 2012 {published data only}
- Ahmat D, Huxley N, Youl P. Stop the smokes for a healthy bub: addressing smoking in pregnant Aboriginal and Torres Strait Islander women. Asia-Pacific Journal of Clinical Oncology 2012;8:332. [Google Scholar]
Albright 2009 {published data only}
- Albright CL, Maddock JE, Nigg CR. Increasing physical activity in postpartum multiethnic women in Hawaii: results from a pilot study. BMC Women's Health 2009;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albright 2012 {published data only}
- Albright CL, Steffen AD, Novotny R, Nigg CR, Wilkens LR, Saiki K, et al. Baseline results from Hawaii's Nā Mikimiki Project: a physical activity intervention tailored to multiethnic postpartum women. Women & Health 2012;52(3):265-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albright 2014 {published data only}
- Albright CL, Steffen AD, Wilkens LR, White KK, Novotny R, Nigg CR, et al. Effectiveness of a 12-month randomized clinical trial to increase physical activity in multiethnic postpartum women: results from Hawaii's N Mikimiki Project. Preventive Medicine 2014;69:214-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albright 2015 {published data only}
- Albright CL, Saiki K, Steffen AD, Woekel E. What barriers thwart postpartum women's physical activity goals during a 12-month intervention? A process evaluation of the Nā Mikimiki project. Women & Health 2015;55(1):1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alicata 2016 {published data only}
- Alicata D, Schroepfer A, Unten T, Agoha R, Helm S, Fukuda M, et al. Telemental health training, team building, and workforce development in cultural context: the Hawaii experience. Journal of Child & Adolescent Psychopharmacology 2016;26(3):260-5. [DOI] [PubMed] [Google Scholar]
Alto 1994 {published data only}
- Alto WA, Fury D, Condo A, Doran M, Aduddell M. Improving the immunization coverage of children less than 7 years old in a family practice residency. Journal of the American Board of Family Practice 1994;7(6):472-7. [PubMed]
Anand 2007 {published data only}
- Anand SS, Davis AD, Ahmed R, Jacobs R, Xie C, Hill A, et al. A family-based intervention to promote healthy lifestyles in an aboriginal community in Canada. Canadian Journal of Public Health 2007;98(6):447-52. [DOI] [PMC free article] [PubMed]
Anderson 2015 {published data only}
- Anderson YC, Taylor GM, Grant CC, Fulton RB, Hofman PL. The Green Prescription Active Families programme in Taranaki, New Zealand 2007–2009: did it reach children in need? Journal of Primary Health Care 2015;7(3):192-7. [PubMed] [Google Scholar]
Anderson 2019 {published data only}
- Anderson YC, Wynter LE, Cave TL, Wild CE, Grant CC, Derraik JG, et al. Whānau Pakari: a multi-disciplinary assessment and intervention programme for children and adolescents with obesity. Journal of Paediatrics and Child Health 2019;55:7-10. [Google Scholar]
ANTaR 2007 {published data only}
- Australians for Native Title and Reconciliation. Success Stories in Indigenous Health: a Showcase of Successful Aboriginal and Torres Strait Islander Health Projects. Sydney (NSW): Australians for Native Title and Reconciliation, 2007. [Google Scholar]
Araujo 2016 {published data only}
- Araujo M, Moraga C, Chapman E, Barreto J, Illanes E. Interventions to improve access to health services by indigenous peoples in the Americas. Pan American Journal of Public Health 2016;40(5):371-81. [PubMed] [Google Scholar]
Arney 2010 {published data only}
- Arney F, Bowering K, Chong A, Healy V, Volkmer B. Sustained nurse home visiting with families of Aboriginal children. In: Arney F, Scott D, editors(s). Working with Vulnerable Families: a Partnership Approach. Cambridge (UK): Cambridge University Press, 2010:109-34. [DOI: ] [Google Scholar]
Atkinson 2010 {published data only}
- Atkinson G. Strong in culture and community: an Aboriginal and Torres Strait Islander approach to caring for children and families. International Journal of Equity and Innovation in Early Childhood 2010;8(1):1-11. [Google Scholar]
Azur 2007 {published data only}
- Azur MJ. The Effects of Maternal Depression on Child Health and Development [Doctoral dissertation]. Baltimore (MD): The Johns Hopkins University, 2007. [Google Scholar]
Bagshaw 2006 {published data only}
- Bagshaw DM, Quinn K, Schmidt B. Children and families in transition: towards a child-centred integrated model of practice. apo.org.au/node/4934 (accessed prior to 24 October 2022).
Bair‐Merritt 2010 {published data only}
- Bair-Merritt MH, Jennings JM, Chen R, Burrell L, McFarlane E, Fuddy L, et al. Reducing maternal intimate partner violence after the birth of a child: a randomized controlled trial of the Hawaii Healthy Start Home Visitation Program. Archives of Pediatrics & Adolescent Medicine 2010;164(1):16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldwin 2001 {published data only}
- Baldwin M, Stevenson Y. A model for providing prenatal health care to indigenous women living in remote areas. International Journal of Circumpolar Health 2001;60(4):623-31. [PubMed] [Google Scholar]
Barclay 2014 {published data only}
- Barclay L, Kruske S, Bar-Zeev S, Steenkamp M, Josif C, Narjic CW, et al. Improving Aboriginal maternal and infant health services in the 'Top End' of Australia; synthesis of the findings of a health services research program aimed at engaging stakeholders, developing research capacity and embedding change. BMC Health Services Research 2014;14:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlett 1988 {published data only}
- Barlett M, Hecker R, Jan S, Delaney S, Capon A, Conaty S. Evaluation of the Daruk Antenatal Program: quantitative findings. In: Report to the National Health and Medical Research Council. 1998.
Bar‐Zeev 2015 {published data only}
- Bar-Zeev Y, Bovill M, Bonevski B, Gould G. Indigenous Counselling And Nicotine (ICAN) quit in pregnancy – developing an evidence-based intervention for smoking cessation for indigenous pregnant women. Asia-Pacific Journal of Clinical Oncology 2015;11:8-9. [Google Scholar]
Batliner 2014 {published data only}
- Batliner T, Fehringer KA, Tiwari T, Henderson WG, Wilson A, Brega AG, et al. Motivational interviewing with American Indian mothers to prevent early childhood caries: study design and methodology of a randomized control trial. Trials 2014;15:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batliner 2018 {published data only}
- Batliner TS, Tiwari T, Henderson WG, Wilson AR, Gregorich SE, Fehringer KA, et al. Randomized trial of motivational interviewing to prevent early childhood caries in American Indian children. JDR Clinical and Translational Research 2018;3(4):366-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benzies 2011a {published data only}
- Benzies K, Edwards N, Tough S, Nagan K, Mychasiuk R, Keown L, et al. Effects of a two-generation preschool programme on receptive language skill in low-income Canadian children. Early Child Development and Care 2011;181(3):397-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benzies 2011b {published data only}
- Benzies K, Tough S, Edwards N, Mychasiuk R, Donnelly C. Aboriginal children and their caregivers living with low income: outcomes from a two-generation preschool program. Journal of Child & Family Studies 2011;20(3):311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berns 2017 {published data only}
- Berns RM, Tomayko EJ, Cronin KA, Prince RJ, Parker T, Adams AK. Development of a culturally informed child safety curriculum for American Indian families. Journal of Primary Prevention 2017;38(1-2):195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernstein 2005 {published data only}
- Bernstein VJ, Harris EJ, Long CW, Iida E, Hans SL. Issues in the multi-cultural assessment of parent-child interaction: an exploratory study from the starting early starting smart collaboration. Journal of Applied Developmental Psychology 2005;26(3):241-75. [Google Scholar]
Bertilone 2015 {published data only}
- Bertilone C, McEvoy S. Success in closing the gap: favourable neonatal outcomes in a metropolitan Aboriginal maternity group practice program. Medical Journal of Australia 2015;203(6):262.e1-7. [DOI] [PubMed] [Google Scholar]
Bertilone 2017 {published data only}
- Bertilone CM, McEvoy SP, Gower D, Naylor N, Doyle J, Swift-Otero V. Elements of cultural competence in an Australian Aboriginal maternity program. Women & Birth 2017;30(2):121-8. [DOI] [PubMed] [Google Scholar]
Best 2011 {published data only}
- Best E. Closing the gap through innovative maternity care (the Aboriginal Maternal and Infant Health Service). Women & Birth 2011;24:S16. [Google Scholar]
Best 2013 {published data only}
- Best E, Raymond J. An innovative model for delivering culturally safe Mental Health Drug and Alcohol Services in Aboriginal Maternal and Infant Health Services in NSW. Women & Birth 2013;26:S15-6. [Google Scholar]
BigFoot 2009 {published data only}
- BigFoot DS, Schmidt SR. Science-to-practice: adapting an evidence based child trauma treatment for American Indian and Alaska native populations. International Journal of Child Health and Human Development 2009;2(1):33-44. [Google Scholar]
BigFoot 2011 {published data only}
- BigFoot DS. The process and dissemination of cultural adaptations of evidence-based practices for American Indian and Alaska Native children and their families. In: Sarche MC, Spicer P, Farrell P, Fitzgerald HE, editors(s). American Indian and Alaska Native Children and Mental Health: Development, Context, Prevention, and Treatment. Santa Barbara (CA): Praeger, 2011:285-307. [Google Scholar]
Billard 2014 {published data only}
- Billard I. The Hearing and Otitis Program: a model of community based ear and hearing care services for Inuit of Nunavik. Canadian Journal of Speech-Language Pathology & Audiology 2014;38(2):206-17. [Google Scholar]
Black 2013a {published data only}
- Black AP, Vally H, Morris P, Daniel M, Esterman A, Karschimkus CS, et al. Nutritional impacts of a fruit and vegetable subsidy programme for disadvantaged Australian Aboriginal children. British Journal of Nutrition 2013;110(12):2309-17. [DOI] [PubMed] [Google Scholar]
Black 2013b {published data only}
- Black AP, Vally H, Morris PS, Daniel M, Esterman AJ, Smith FE, et al. Health outcomes of a subsidised fruit and vegetable program for Aboriginal children in northern New South Wales. Medical Journal of Australia 2013;199(1):46-50. [DOI] [PubMed] [Google Scholar]
Black 2014 {published data only}
- Black AP, Vally H, Morris P, Daniel M, Esterman A, Smith F, et al. High folate levels in Aboriginal children after subsidised fruit and vegetables and mandatory folic acid fortification. Australian & New Zealand Journal of Public Health 2014;38(3):241-6. [DOI] [PubMed] [Google Scholar]
Blinkhorn 2012 {published data only}
- Blinkhorn F, Brown N, Freeman R, Humphris G, Martin A, Blinkhorn A. A phase II clinical trial of a dental health education program delivered by Aboriginal health workers to prevent early childhood caries. BMC Public Health 2012;12:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blue 2020 {published data only}
- Blue CM, Arnett MC, Ephrem H, Lunos S, Ruoqiong C, Jones R. Using motivational interviewing to reduce parental risk related behaviors for early childhood caries: a pilot study. BMC Oral Health 2020;20(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boffa 2007 {published data only}
- Boffa JD, Bell AI, Davies TE, Paterson J, Cooper DE. The Aboriginal medical services alliance Northern Territory: engaging with the intervention to improve primary health care. Medical Journal of Australia 2007;187(11-12):617-8. [DOI] [PubMed] [Google Scholar]
Bond 2009a {published data only}
- Bond C. Starting at strengths … an Indigenous early years intervention. Medical Journal of Australia 2009;191(3):175-7. [DOI] [PubMed] [Google Scholar]
Bond 2009b {published data only}
- Bond M, Wyatt K, Lloyd J, Welch K, Taylor R. Systematic review of the effectiveness and cost-effectiveness of weight management schemes for the under fives: a short report. Health Technology Assessment 2009;13(61):1-75, iii. [DOI] [PubMed] [Google Scholar]
Bonnici 2008 {published data only}
- Bonnici R. Promoting awareness of nutritional risk factors contributing to chronic disease in Indigenous communities: an outreach model. Developing Practice: the Child, Youth and Family Work Journal 2008;21:53-62. [Google Scholar]
Booth‐LaForce 2020 {published data only}
- Booth-LaForce C, Oxford M, Barbosa-Leiker C, Burduli E, Buchwald D. Randomized controlled trial of the Promoting First Relationships® preventive intervention for primary caregivers and toddlers in an American Indian community. Prevention Science 2020;21(1):98-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bovill 2017 {published data only}
- Bovill M, Bar-Zeev Y, Gruppetta M, O'Mara P, Cowling B, Gould GS. Collective and negotiated design for a clinical trial addressing smoking cessation supports for Aboriginal and Torres Strait Islander mothers in NSW, SA and Qld? Developing a pilot study. Australian Journal of Primary Health 2017;31:31. [DOI] [PubMed] [Google Scholar]
Bowes 2014 {published data only}
- Bowes J, Grace R. Review of early childhood parenting, education and health intervention programs for Indigenous children and families in Australia. Canberra (ACT): Closing the Gap Clearinghouse, Australian Institute of Health and Welfare; 2014 February. Report No.: IHW 116.
Boychuk 1984 {published data only}
- Boychuk RB, Nelson JC. Improving perinatal knowledge and care. A self-instruction program. Hawaii Medical Journal 1984;43(4):100-6. [PubMed] [Google Scholar]
Bradley 1994 {published data only}
- Bradley PJ, Martin J. The impact of home visits on enrollment patterns in pregnancy-related services among low-income women. Public Health Nursing 1994;11(6):392-8. [DOI] [PubMed] [Google Scholar]
Brega 2020 {published data only}
- Brega AG, Jiang L, Johnson RL, Wilson AR, Schmiege SJ, Albino J. Health literacy and parental oral health knowledge, beliefs, behavior, and status among parents of American Indian newborns. Journal of Racial and Ethnic Health Disparities 2020;7:598-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breslin 2009 {published data only}
- Breslin L. Supporting our relative and kinship carers. Developing Practice: the Child, Youth and Family Work Journal 2009;24:23-35. [Google Scholar]
Brewin 2002 {published data only}
- Brewin M, Coggan C. Evaluation of a New Zealand indigenous community injury prevention project. Injury Control & Safety Promotion 2002;9(2):83-8. [DOI] [PubMed] [Google Scholar]
Brewin 2004 {published data only}
- Brewin M, Coggan C. Evaluation of the Ngati Porou Community Injury Prevention Project. Ethnicity & Health 2004;9(1):5-15. [DOI] [PubMed] [Google Scholar]
Brown 2015 {published data only}
- Brown SJ, Weetra D, Glover K, Buckskin M, Ah Kit J, Leane C, et al. Improving Aboriginal women's experiences of antenatal care: findings from the Aboriginal families study in South Australia. Birth 2015;42(1):27-37. [DOI] [PubMed] [Google Scholar]
Brown 2016 {published data only}
- Brown S, Glover K, Weetra D, Ah Kit J, Stuart-Butler D, Leane C, et al. Improving access to antenatal care for Aboriginal women in South Australia: evidence from a population-based study. Birth: Issues in Perinatal Care 2016;43(2):134-43. [DOI] [PubMed] [Google Scholar]
Bruerd 1989 {published data only}
- Bruerd B, Kinney MB, Bothwell E. Preventing baby bottle tooth decay in American Indian and Alaska native communities: a model for planning. Public Health Reports 1989;104(6):631-40. [PMC free article] [PubMed] [Google Scholar]
Bucharski 1999 {published data only}
- Bucharski D, Brockman L, Lambert D. Developing culturally appropriate prenatal care models for aboriginal women. Canadian Journal of Human Sexuality 1999;8(2):151-4. [Google Scholar]
Buckskin 2013 {published data only}
- Buckskin M, Ah Kit J, Glover K, Mitchell A, Miller R, Weetra D, et al. Aboriginal Families Study: a population-based study keeping community and policy goals in mind right from the start. International Journal for Equity in Health 2013;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bulterys 1990 {published data only}
- Bulterys M, Morgenstern H, Welty TK, Kraus JF. The expected impact of a smoking cessation program for pregnant women on infant mortality among Native Americans. American Journal of Preventive Medicine 1990;6(5):267-73. [PubMed] [Google Scholar]
Burd 2007 {published data only}
- Burd L, Peterson M, Face GC, Face FC, Shervold D, Klug MG. Efficacy of a SIDS risk factor education methodology at a native American and Caucasian site. Maternal & Child Health Journal 2007;11(4):365-71. [DOI] [PubMed] [Google Scholar]
Burrows 2014 {published data only}
- Burrows A, Allen B, Gorton S. Evaluation of the Bumps to Babes and Beyond program. Melbourne (VIC): The Queen Elizabeth Centre and Mallee District Aboriginal Services; 2014 December.
Bussey 2013 {published data only}
- Bussey M, Lucero NM. Re-examining child welfare's response to ICWA: collaborating with community-based agencies to reduce disparities for American Indian/Alaska Native children. Children and Youth Services Review 2013;35(3):394-401. [Google Scholar]
Byrnes 2020 {published data only}
- Byrnes CA, Trenholme A, Lawrence S, Aish H, Higham JA, Hoare K, et al. Prospective community programme versus parent-driven care to prevent respiratory morbidity in children following hospitalisation with severe bronchiolitis or pneumonia. Thorax 2020;75(4):298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calabria 2012 {published data only}
- Calabria B, Clifford A, Shakeshaft AP, Doran CM. A systematic review of family-based interventions targeting alcohol misuse and their potential to reduce alcohol-related harm in indigenous communities. Journal of Studies on Alcohol & Drugs 2012;73(3):477-88. [DOI] [PubMed] [Google Scholar]
Carlisle 2020 {published data only}
- Carlisle K, Felton-Busch C, Cadet-James Y, Taylor J, Bailie R, Farmer J, et al. WOmen's Action for Mums and Bubs (WOMB) trial protocol: a non-randomized stepped wedge implementation trial of participatory women's groups to improve the health of Aboriginal and Torres Strait Islander mothers and children in Australia. Frontiers in Public Health 2020;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaffin 2012 {published data only}
- Chaffin M, Bard D, Bigfoot DS, Maher EJ. Is a structured, manualized, evidence-based treatment protocol culturally competent and equivalently effective among American Indian parents in child welfare? Child Maltreatment 2012;17(3):242-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 1998 {published data only}
- Chamberlain M, Nair R, Nimrod C, Moyer A, England J. Evaluation of a midwifery birthing center in the Canadian north. International Journal of Circumpolar Health 1998;57(Suppl 1):116-20. [PubMed] [Google Scholar]
Chamberlain 2017 {published data only}
- Chamberlain C, O'Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD001055. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chambliss 2000 {published data only}
- Chambliss JW. An Experimental Trial of a Home Visiting Program to Prevent Child Maltreatment [Doctoral dissertation]. Ann Arbor (MI): Georgia State University, 2000. [Google Scholar]
Chang 2010 {published data only}
- Chang AB, Taylor B, Masters IB, Laifoo Y, Brown AD. Indigenous healthcare worker involvement for Indigenous adults and children with asthma. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD006344. [DOI: 10.1002/14651858.CD006344.pub3] [DOI] [PubMed] [Google Scholar]
Chartier 2020 {published data only}
- Chartier M, Enns JE, Nickel NC, Campbell R, Phillips-Beck W, Sarkar J, et al. The association of a paraprofessional home visiting intervention with lower child maltreatment rates in First Nation families in Canada: a population-based retrospective cohort study. Children and Youth Services Review 2020;108:e104675. [Google Scholar]
Chi 2013 {published data only}
- Chi DL. Reducing Alaska Native paediatric oral health disparities: a systematic review of oral health interventions and a case study on multilevel strategies to reduce sugar-sweetened beverage intake. International Journal of Circumpolar Health 2013;72:21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cidro 2015 {published data only}
- Cidro J, Zahayko L, Lawrence HP, Folster S, McGregor M, McKay K. Breast feeding practices as cultural interventions for early childhood caries in Cree communities. BMC Oral Health 2015;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
CIRCA 2014 {published data only}
- Cultural and Indigenous Research Centre Australia (CIRCA). Evaluation of NSW Aboriginal child and family centres: final report. Sydney (NSW): NSW Department of Family and Community Services; 2014 December. Report No.: ISBN 978-0-9924253-3-3.
Cleary 2006 {published data only}
- Cleary E, Ludwig S, Riese N, Grant L. Educational strategies to improve screening for gestational diabetes mellitus in Aboriginal women in a remote northern community. Canadian Journal of Diabetes 2006;30(3):264-8. [Google Scholar]
Cresp 2016 {published data only}
- Cresp R, Clarke K, McAuley KE, McAullay D, Moylan CA, Peter S, et al. Effectiveness of the Koorliny Moort out-of-hospital health care program for Aboriginal and Torres Strait Islander children in Western Australia. Medical Journal of Australia 2016;204(5):1971e-7. [DOI] [PubMed] [Google Scholar]
Cruz 2016a {published data only}
- Cruz TH, Davis SM, Myers OB, O'Donald ER, Sanders SG, Sheche JN. Effects of an obesity prevention intervention on physical activity among preschool children: the CHILE study. Health Promotion Practice 2016;17(5):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruz 2016b {published data only}
- Cruz TH, Davis SM, FitzGerald CA, Canaca GF, Keane PC. Engagement, recruitment, and retention in a trans-community, randomized controlled trial for the prevention of obesity in rural American Indian and Hispanic children. Journal of Primary Prevention 2016;35(3):135-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Espaignet 2003 {published data only}
- D'Espaignet ET, Measey ML, Carnegie MA, Mackerras D. Monitoring the 'Strong Women, Strong Babies, Strong Culture Program': the first eight years. Journal of Paediatrics & Child Health 2003;39(9):668-72. [DOI] [PubMed] [Google Scholar]
Daro 1998 {published data only}
- Daro D, McCurdy K, Harding K. The role of home visitation in preventing child abuse: an evaluation of the Hawaii Healthy Start Project. Chicago (IL): National Committee to Prevent Child Abuse, 1998.
Davis 1999 {published data only}
- Davis S. American Indian communities seek to improve their children's health. Chronic Disease Notes & Reports 1999;12(1):10-3. [Google Scholar]
Davis 2001 {published data only}
- Davis CL, Prater SL. A perinatal intervention program for urban American Indians part 1: design, implementation, and outcomes. Journal of Perinatal Education 2001;10(3):9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2013 {published data only}
- Davis SM, Sanders SG, FitzGerald CA, Keane PC, Canaca GF, Volker-Rector R. CHILE: an evidence-based preschool intervention for obesity prevention in Head Start. Journal of School Health 2013;83(3):223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
dela Cruz 2010 {published data only}
- dela Cruz AM, McCarthy P. Alberta Aboriginal Head Start in urban and northern communities: longitudinal study pilot phase. Chronic Diseases in Canada 2010;30(2):40-5. [PubMed] [Google Scholar]
Department of Family and Community Services 2004 {published data only}
- Department of Family and Community Services. Review of the Early Intervention Parenting Program and Good Beginnings Prototypes. Canberra (ACT): Department of Family and Community Services, Commonwealth Government; 2004.
Department of Family and Community Services 2005 {published data only}
- Department of Family and Community Services. Updating Stronger Families and Communities Strategy projects. Stronger Families Learning Exchange 2005;(7):32-47.
Dew 2014 {published data only}
- Dew B, Breakey G. An evaluation of Hawaii's Healthy Start Program using child abuse hospitalization data. Journal of Family Violence 2014;29(8):893-900. [Google Scholar]
Dietrich 1986 {published data only}
- Dietrich AJ, Olson AL. Model system of ongoing care for native Americans – a 5-year followup. Public Health Reports 1986;101(2):184-6. [PMC free article] [PubMed] [Google Scholar]
Dinges 1974 {published data only}
- Dinges NG, Yazzie ML, Tollefson GD. Developmental intervention for Navajo family mental health. Personnel and Guidance Journal 1974;52(6):390-5. [Google Scholar]
Dionne 2009 {published data only}
- Dionne R, Davis B, Sheeber L, Madrigal L. Initial evaluation of a cultural approach to implementation of evidence-based parenting interventions in American Indian communities. Journal of Community Psychology 2009;37(7):911-21. [Google Scholar]
Dobson 2017 {published data only}
- Dobson R, Whittaker R, Bartley H, Connor A, Chen R, Ross M, et al. Development of a culturally tailored text message maternal health program: TextMATCH. JMIR mHealth and uHealth 2017;5(4):e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Douglas 2013 {published data only}
- Douglas ML, McGhan SL, Tougas D, Fenton N, Sarin C, Latycheva O, et al. Asthma education program for First Nations children: an exemplar of the knowledge-to-action framework. Canadian Respiratory Journal 2013;20(4):295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 1994 {published data only}
- Duffy SA, Bonino K, Gallup L, Pontseele R. Community baby shower as a transcultural nursing intervention. Journal of Transcultural Nursing 1994;5(2):38-41. [DOI] [PubMed] [Google Scholar]
Duggan 1999 {published data only}
- Duggan AK, McFarlane EC, Windham AM, Rohde CA, Salkever DS, Fuddy L, et al. Evaluation of Hawaii's Healthy Start Program. The Future of Children 1999;9(1):66-90; discussion 177-8. [PubMed] [Google Scholar]
Duggan 2000 {published data only}
- Duggan A, Windham A, McFarlane E, Fuddy L, Rohde C, Buchbinder S, et al. Hawaii's Healthy Start Program of home visiting for at-risk families: evaluation of family identification, family engagement, and service delivery. Pediatrics 2000;105(1):250-9. [PubMed] [Google Scholar]
Duggan 2004a {published data only}
- Duggan A, McFarlane E, Fuddy L, Burrell L, Higman SM, Windham A, et al. Randomized trial of a statewide home visiting program: impact in preventing child abuse and neglect. Child Abuse & Neglect 2004;28(6):597-622. [DOI] [PubMed] [Google Scholar]
Duggan 2004b {published data only}
- Duggan A, Fuddy L, Burrell L, Higman SM, McFarlane E, Windham A, et al. Randomized trial of a statewide home visiting program to prevent child abuse: impact in reducing parental risk factors. Child Abuse & Neglect 2004;28(6):623-43. [DOI] [PubMed] [Google Scholar]
Eades 2004 {published data only}
- Eades S. Maternal and child health care services: actions in the primary health care setting to improve the health of Aboriginal and Torres Strait Islander women of childbearing age, infants and young children. Canberra (ACT): Office for Aboriginal and Torres Strait Islander Health, Department of Health and Ageing; 2004. Report No.: 6.
Eades 2007 {published data only}
- Eades S, Lea T, Peltola C, Robert Griew Consulting. Family centred primary health care: review of evidence and models. Sydney (NSW): Office of Aboriginal and Torres Strait Islander Health, Department of Health and Ageing; 2007 November.
Eades 2012 {published data only}
- Eades SJ, Sanson-Fisher RW, Wenitong M, Panaretto K, D'Este C, Gilligan C, et al. An intensive smoking intervention for pregnant Aboriginal and Torres Strait Islander women: a randomised controlled trial. Medical Journal of Australia 2012;197(1):42-6. [DOI] [PubMed] [Google Scholar]
El‐Kamary 2004 {published data only}
- El-Kamary SS, Higman SM, Fuddy L, McFarlane E, Sia C, Duggan AK. Hawaii's healthy start home visiting program: determinants and impact of rapid repeat birth. Pediatrics 2004;114(3):e317-26. [DOI] [PubMed] [Google Scholar]
Emerson 2015 {published data only}
- Emerson L, Fox S, Smith C. Good beginnings: getting it right in the early years: review of the evidence on the importance of a healthy start to life and on interventions to promote good beginnings. Melbourne (VIC): Lowitja Institute; 2015 July. Report No.: 978-1-921889-44-8.
Engeler 1997 {published data only}
- Engeler T, McDonald MA, Miller ME, Groos A, Black ME, Leonard D. Review of current interventions and identification of best practice currently used by community based Aboriginal and Torres Strait Islander health service providers in promoting and supporting breastfeeding and appropriate infant nutrition. Canberra (ACT): Commonwealth of Australia, Commonwealth Department of Health and Family Services; 1997 November. Report No.: 0 642 36732 9.
Eni 2011 {published data only}
- Eni R, Phillips-Beck W. Transcending jurisdictions: developing partnerships for health in Manitoba First Nation communities. International Journal of Circumpolar Health 2011;70(4):434-9. [DOI] [PubMed] [Google Scholar]
Enns 2019 {published data only}
- Enns JE, Chartier M, Nickel N, Chateau D, Campbell R, Phillips-Beck W, et al. Association between participation in the Families First Home Visiting programme and First Nations families' public health outcomes in Manitoba, Canada: a retrospective cohort study using linked administrative data. BMJ Open 2019;9:e030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esquivel 2016 {published data only}
- Esquivel MK, Nigg CR, Fialkowski MK, Braun KL, Li F, Novotny R. Influence of teachers' personal health behaviors on operationalizing obesity prevention policy in head start preschools: a project of the children's healthy living program (CHL). Journal of Nutrition Education & Behavior 2016;48(5):318-25.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fejo 1994 {published data only}
- Fejo L. The Strong Women, Strong Babies, Strong Culture Program. Aboriginal and Islander Health Worker Journal 1994;18(6):16. [Google Scholar]
Fernald 2017 {published data only}
- Fernald LC, Kagawa RM, Knauer HA, Schnaas L, Guerra AG, Neufeld LM. Promoting child development through group-based parent support within a cash transfer program: experimental effects on children's outcomes. Developmental Psychology 2017;53(2):222-36. [DOI] [PubMed] [Google Scholar]
Fialkowski 2014 {published data only}
- Fialkowski MK, DeBaryshe B, Bersamin A, Nigg C, Leon Guerrero R, Rojas G, et al. A community engagement process identifies environmental priorities to prevent early childhood obesity: the Children's Healthy Living (CHL) program for remote underserved populations in the US Affiliated Pacific Islands, Hawaii and Alaska. Maternal and Child Health Journal 2014;18(10):2261-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2002 {published data only}
- Fisher PA, Ball TJ. The Indian Family Wellness project: an application of the tribal participatory research model. Prevention Science 2002;3(3):235-40. [DOI] [PubMed] [Google Scholar]
Floden 1989 {published data only}
- Floden A. Tiospaye Teca: working with young Native American families in the Dakotas. Children Today 1989;18(5):28-32. [Google Scholar]
Flynn 1999 {published data only}
- Flynn L. The adolescent parenting program: improving outcomes through mentorship. Public Health Nursing 1999;16(3):182-9. [DOI] [PubMed] [Google Scholar]
Frances 2011 {published data only}
- Frances K, Saggers S. Evaluation of Palmerston Association's 'Yarning and Parenting Program' for parents and children experiencing drug and alcohol problems. Perth (WA): National Drug Research Institute, Curtin University; 2011 April. Report No.: ISBN 978-0-9807054-4-7.
Franklin 1995 {published data only}
- Franklin C, Waukechon J, Larney PS. Culturally relevant school programs for American Indian children and families. Social Work in Education 1995;17(3):183-93. [Google Scholar]
Frenza 1993 {published data only}
- Frenza L. An early intervention approach to ending child abuse and neglect: Hana Like home visitor program. Journal of Psychohistory 1993;21(1):29-36. [Google Scholar]
Frow 2010 {published data only}
- Frow L. A W.I.S.H. that brings hope for young Aboriginal mothers. Developing Practice: the Child, Youth and Family Work Journal 2010;27:52-8. [Google Scholar]
Fuddy 2002 {published data only}
- Fuddy LJ, Thompson RA. Healthy Start: a statewide system for the prevention of child abuse and neglect – and more. In: Melton GB, Thompson RA, Small MA, editors(s). Toward a Child-Centered, Neighborhood-Based Child Protection System: A Report of the Consortium on Children, Families, and the Law. Westport (CT): Praeger Publishers/Greenwood Publishing Group, 2002:214-32. [Google Scholar]
Gao 2014 {published data only}
- Gao Y, Gold L, Josif C, Bar-Zeev S, Steenkamp M, Barclay L, et al. A cost-consequences analysis of a midwifery group practice for Aboriginal mothers and infants in the top end of the Northern Territory, Australia. Midwifery 2014;30(4):447-55. [DOI] [PubMed] [Google Scholar]
George 2007 {published data only}
- George MA, Masotti P, MacLeod S, Bibber M, Loock C, Fleming M, et al. Bridging the research gap: aboriginal and academic collaboration in FASD prevention. The Healthy Communities, Mothers and Children Project. Alaska Medicine 2007;49(2 Suppl):139-41. [PubMed] [Google Scholar]
Gerlach 2009 {published data only}
- Gerlach A. My journey towards 'Steps in the right direction: connecting & collaborating in early intervention therapy with Aboriginal children & families in British Columbia'. Occupational Therapy Now 2009;11(3):22-3. [Google Scholar]
Ginsburg 2012 {published data only}
- Ginsburg GS, Barlow A, Goklish N, Hastings R, Baker EV, Mullany B, et al. Postpartum depression prevention for reservation-based American Indians: results from a pilot randomized controlled trial. Child & Youth Care Forum 2012;41(3):229-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glor 1987 {published data only}
- Glor ED. Impacts of a prenatal program for native women. Canadian Journal of Public Health 1987;78(4):249-54. [PubMed] [Google Scholar]
Glover 1996 {published data only}
- Glover GJ. Filial Therapy with Native Americans on the Flathead Reservation [Doctoral dissertation]. Ann Arbor (MI): University of North Texas, 1996. [Google Scholar]
Glover 2000 {published data only}
- Glover GJ, Landreth GL. Filial therapy with Native Americans on the Flathead Reservation. International Journal of Play Therapy 2000;9(2):57-80. [Google Scholar]
Glover 2009 {published data only}
- Glover GJ, Landreth GL. "Filial therapy with Native Americans on the Flathead Reservation": Correction to Glover and Landreth (2000). International Journal of Play Therapy 2009;18(1):68. [Google Scholar]
Glover 2015a {published data only}
- Glover M, Kira A, Johnston V, Walker N, Brown N, Thomas D. Australian and New Zealand Indigenous mothers' report respect for smoking bans in homes. Women & Birth 2015;28(1):1-7. [DOI] [PubMed] [Google Scholar]
Glover 2015b {published data only}
- Glover M, Kira A, Walker N, Bauld L. Using incentives to encourage smoking abstinence among pregnant indigenous women? A feasibility study. Maternal and Child Health Journal 2015;19(6):1393-9. [DOI] [PubMed] [Google Scholar]
Glover 2016 {published data only}
- Glover M, Kira A, Smith C. Enlisting "Aunties" to support Indigenous pregnant women to stop smoking: feasibility study results. Nicotine & Tobacco Research 2016;18(5):1110-5. [DOI] [PubMed] [Google Scholar]
Gomby 2007 {published data only}
- Gomby DS. The promise and limitations of home visiting: implementing effective programs. Child Abuse & Neglect 2007;31(8):793-9. [DOI] [PubMed] [Google Scholar]
Gould 2013 {published data only}
- Gould GS, McEwen A. An intensive smoking intervention for pregnant Aboriginal and Torres Strait Islander women: a randomised controlled trial. Medical Journal of Australia 2013;198(1):23. [DOI] [PubMed] [Google Scholar]
Grace 2016 {published data only}
- Grace R, Bowes J, McKay-Tempest J, Burnstein J, Tregeagle S. Early parenting education to strengthen Aboriginal parents in a remote area: the development and piloting of a group programme. Children Australia 2016;41(4):249-57. [Google Scholar]
Gray‐Donald 2000 {published data only}
- Gray-Donald K, Robinson E, Collier A, David K, Renaud L, Rodrigues S. Intervening to reduce weight gain in pregnancy and gestational diabetes mellitus in Cree communities: an evaluation. CMAJ: Canadian Medical Association Journal 2000;163(10):1247-51. [PMC free article] [PubMed] [Google Scholar]
Gregory 2008 {published data only}
- Gregory D. He Waka Tapu: working together for the well-being of family. In: Carrillo R, Tello J, editors(s). Family Violence and Men of Color: Healing the Wounded Male Spirit. 2nd edition. New York (NY): Springer, 2008:163-79. [Google Scholar]
Guyer 2000 {published data only}
- Guyer B, Hughart N, Strobino D, Jones A, Scharfstein D. Assessing the impact of pediatric-based development services on infants, families, and clinicians: challenges to evaluating the Health Steps Program. Pediatrics 2000;105(3):E33. [DOI] [PubMed] [Google Scholar]
Haag 2019 {published data only}
- Haag DG, Jamieson LM, Hedges J, Smithers LG. Is there an association between breastfeeding and dental caries among three-year-old Australian Aboriginal children? Nutrients 2019;11(11):2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamerton 2014 {published data only}
- Hamerton H, Mercer C, Riini D, McPherson B, Morrison L. Evaluating Maori community initiatives to promote healthy eating, healthy action. Health Promotion International 2014;29(1):60-9. [DOI] [PubMed] [Google Scholar]
Harnett 1998 {published data only}
- Harnett P, Clarke C, Shochet I. Promoting family and community resilience in Indigenous communities: cultural adaptation of the Resourceful Adolescent Parent Program. Auseinetter 1998;7:1-3. [Google Scholar]
Harrison 2006 {published data only}
- Harrison RL, MacNab AJ, Duffy DJ, Benton DH. Brighter Smiles: service learning, inter-professional collaboration and health promotion in a First Nations community. Canadian Journal of Public Health 2006;97(3):237-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harvey‐Berino 2003 {published data only}
- Harvey-Berino J, Rourke J. Obesity prevention in preschool Native-American children: a pilot study using home visiting. Obesity Research 2003;11(5):606-11. [DOI] [PubMed] [Google Scholar]
Haswell‐Elkins 2009 {published data only}
- Haswell-Elkins M, Reilly L, Fagan R, Ypinazar V, Hunter E, Tsey K, et al. Listening, sharing understanding and facilitating consumer, family and community empowerment through a priority driven partnership in Far North Queensland. Australasian Psychiatry 2009;17(Suppl 1):S54-8. [DOI] [PubMed] [Google Scholar]
Hewer 2006 {published data only}
- Hewer LA, Whyatt D. Improving the implementation of an early literacy program by child health nurses through addressing local training and cultural needs. Contemporary Nurse 2006;23(1):111-9. [DOI] [PubMed] [Google Scholar]
Hilferty 2010 {published data only}
- Hilferty F, Mullan K, Gool K, Chan S, Eastman C, Reeve R, et al. The evaluation of Brighter Futures, NSW Community Services' early intervention program: final report. Sydney (NSW): Social Policy Research Centre, University of New South Wales; 2010 December. Report No.: 13/10.
Holdaway Smith 2021 {published data only}
- Holdaway Smith N, Lutz T, Maupomé G, Lapidus J, Jimenez C, Janis M, et al. Long-term effects of a toddler-focused caries prevention programme among Northwestern US tribal children: the TOTS-to-Tweens study. Community Dentistry & Oral Epidemiology 2021;49(3):284-90. [DOI] [PubMed] [Google Scholar]
Homel 2006a {published data only}
- Homel R, Freiberg K, Lamb C, Leech M, Batchelor S, Carr A, et al. The pathways to prevention project: doing developmental prevention in a disadvantaged community. Trends and Issues in Crime and Criminal Justice 2006;323:1-6. [Google Scholar]
Homel 2006b {published data only}
- Homel R, Freiberg K, Lamb C. Working with the Indigenous community in the pathways to prevention project. Family Matters 2006;75:18-23. [Google Scholar]
Hucul 2015 {published data only}
- Hucul M, Anand A. Healthy together program: an innovative family education model for children in care (0–18y) and their families. The Bridge Youth & Family Services, Kelowna BC. Canadian Journal of Diabetes 2015;39:S33. [Google Scholar]
Hunter 2014 {published data only}
- Hunter K, Keay L, Clapham K, Lyford M, Brown J, Bilston L, et al. Buckle up safely (shoalhaven): a process and impact evaluation of a pragmatic, multifaceted preschool-based pilot program to increase correct use of age-appropriate child restraints. Traffic Injury Prevention 2014;15(5):483-90. [DOI] [PubMed] [Google Scholar]
Iglesias 2010 {published data only}
- Iglesias A, Iglesias S, Arnold D. Birth in Bella Bella: emergence and demise of a rural family medicine birthing service. Canadian Family Physician 2010;56(6):e233-40. [PMC free article] [PubMed] [Google Scholar]
ISRCTN41467632 {published data only}
- ISRCTN41467632. A clinical trial of the effectiveness of a dental caries prevention program for Cree mothers and their infants. www.isrctn.com/ISRCTN41467632 (first received 1 April 2004).
Jamieson 2016 {published data only}
- Jamieson L, Bradshaw J, Lawrence H, Broughton J, Venner K. Fidelity of motivational interviewing in an early childhood caries intervention involving Indigenous Australian mothers. Journal of Health Care for the Poor and Underserved 2016;27(1A):125-38. [DOI] [PubMed] [Google Scholar]
Jamieson 2018 {published data only}
- Jamieson L, Smithers L, Hedges J, Parker E, Mills H, Kapellas K, et al. Dental disease outcomes following a 2-year oral health promotion program for Australian Aboriginal children and their families: a 2-arm parallel, single-blind, randomised controlled trial. eClinicalMedicine 2018;1:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamieson 2019a {published data only}
- Jamieson L, Smithers L, Hedges J, Mills H, Kapellas K, Ha D, et al. Follow-up of intervention to prevent dental caries among Indigenous children in Australia: a secondary analysis of a randomized clinical trial. JAMA Network Open 2019;2(11):e1915611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamieson 2019b {published data only}
- Jamieson LM, Smithers LG, Hedges J, Aldis J, Mills H, Kapellas K, et al. Follow-up of an intervention to reduce dental caries in Indigenous Australian children: a secondary analysis of a randomized clinical trial. JAMA Network Open 2019;2(3):e190648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jan 2004 {published data only}
- Jan S, Conaty S, Hecker R, Bartlett M, Delaney S, Capon T. An holistic economic evaluation of an Aboriginal community-controlled midwifery programme in Western Sydney. Journal of Health Services & Research Policy 2004;9(1):14-21. [DOI] [PubMed] [Google Scholar]
Jersky 2016 {published data only}
- Jersky M, Titmuss A, Haswell M, Freeman N, Osborne P, Callaghan L, et al. Improving health service access and wellbeing of young Aboriginal parents in an urban setting: mixed methods evaluation of an arts-based program. Australian & New Zealand Journal of Public Health 2016;40:S115-21. [DOI] [PubMed] [Google Scholar]
Johnson 1994 {published data only}
- Johnson JL, Yuen J, Nishimoto P, Johnson RC, Johnson RL. Family-centered care: thriving in Hawaii under Part H. Clinics in Communication Disorders 1994;4(4):254-65. [PubMed] [Google Scholar]
Johnson 2006 {published data only}
- Johnson CL. An innovative healing model: empowering urban Native Americans. In: Witko TM, editors(s). Mental Health Care for Urban Indians: Clinical Insights from Native Practitioners. Washington (DC): American Psychological Association, 2006:189-204. [Google Scholar]
Johnson 2011 {published data only}
- Johnson JL, Brown S, Chang C, Nelson D, Mrazek S. The cost of serving infants and toddlers under Part C. Infants & Young Children 2011;24(1):101-13. [Google Scholar]
Johnston 2011 {published data only}
- Johnston V, Thomas DP, McDonnell J, Andrews RM. Maternal smoking and smoking in the household during pregnancy and postpartum: findings from an Indigenous cohort in the Northern Territory. Medical Journal of Australia 2011;194(10):556-9. [DOI] [PubMed] [Google Scholar]
Jones 2020 {published data only}
- Jones LJ, Wassenhove-Paetzold J, Thomas K, Bancroft C, Ziatyk EQ, Kim LS, et al. Impact of a fruit and vegetable prescription program on health outcomes and behaviors in young Navajo children. Current Developments in Nutrition 2020;4(8):nzaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jongen 2014 {published data only}
- Jongen C, McCalman J, Bainbridge R, Tsey K. Aboriginal and Torres Strait Islander maternal and child health and wellbeing: a systematic search of programs and services in Australian primary health care settings. BMC Pregnancy & Childbirth 2014;14:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karanja 2010 {published data only}
- Karanja N, Lutz T, Ritenbaugh C, Maupome G, Jones J, Becker T, et al. The TOTS community intervention to prevent overweight in American Indian toddlers beginning at birth: a feasibility and efficacy study. Journal of Community Health 2010;35(6):667-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karanja 2012 {published data only}
- Karanja N, Aickin M, Lutz T, Mist S, Jobe JB, Maupomé G, et al. A community-based intervention to prevent obesity beginning at birth among American Indian children: study design and rationale for the PTOTS study. Journal of Primary Prevention 2012;33(4):161-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karol 2016 {published data only}
- Karol S, Tah T, Kenon C, Meyer J, Yazzie J, Stephens C, et al. Bringing baby-friendly to the Indian Health Service. Journal of Human Lactation 2016;32(2):369-72. [DOI] [PubMed] [Google Scholar]
Kegler 2003 {published data only}
- Kegler MC, Stern R, Whitecrow-Ollis S, Malcoe LH. Assessing lay health advisor activity in an intervention to prevent lead poisoning in Native American children. Health Promotion Practice 2003;4(2):189-96. [DOI] [PubMed] [Google Scholar]
Kegler 2004 {published data only}
- Kegler MC, Malcoe LH. Results from a lay health advisor intervention to prevent lead poisoning among rural Native American children. American Journal of Public Health 2004;94(10):1730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemp 2010 {published data only}
- Kemp L, Harris E, McMahon C, Matthey S, Vimpani G, Anderson T, et al. Sustained nurse home visiting improves outcomes for vulnerable children in disadvantaged communities. Journal of Paediatrics and Child Health 2010;46:8-9. [Google Scholar]
Kemp 2018 {published data only}
- Kemp L, Grace R, Comino E, Jackson Pulver L, McMahon C, Harris E, et al. The effectiveness of a sustained nurse home visiting intervention for Aboriginal infants compared with non-Aboriginal infants and with Aboriginal infants receiving usual child health care: a quasi-experimental trial – the Bulundidi Gudaga study. BMC Health Services Research 2018;18(1):599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keown 2018 {published data only}
- Keown LJ, Sanders MR, Franke N, Shepherd M. Te Whānau Pou Toru: a randomized controlled trial (RCT) of a culturally adapted low-intensity variant of the Triple P-Positive Parenting Program for Indigenous Māori families in New Zealand. Prevention Science 2018;19(7):954-65. [DOI] [PubMed] [Google Scholar]
Kildea 2016 {published data only}
- Kildea S, Gao Y, Rolfe M, Josif CM, Bar-Zeev SJ, Steenkamp M, et al. Remote links: redesigning maternity care for Aboriginal women from remote communities in Northern Australia – a comparative cohort study. Midwifery 2016;34:47-57. [DOI] [PubMed] [Google Scholar]
Kildea 2017 {published data only}
- Kildea S, Hickey S, Nelson C, Currie J, Carson A, Reynolds M, et al. Birthing on Country (in Our Community): a case study of engaging stakeholders and developing a best-practice Indigenous maternity service in an urban setting. Australian Health Review 2017;42(2):230-8. [DOI] [PubMed] [Google Scholar]
King‐Hooper 1996 {published data only}
- King-Hooper B, Schulz C, Watts J. A First Nations community-based diabetes education program: the Nuu-chah-nulth experience. Canadian Journal of Diabetes Care 1996;20(2):20-5. [Google Scholar]
Kira 2016 {published data only}
- Kira A, Glover M, Walker N, Bauld L. Recruiting pregnant Indigenous women who smoke into a high contact incentivized cessation trial: a feasibility study. Nicotine & Tobacco Research 2016;18(10):2036-40. [DOI] [PubMed] [Google Scholar]
Koniak‐Griffin 1999 {published data only}
- Koniak-Griffin D, Mathenge C, Anderson NL, Verzemnieks I. An early intervention program for adolescent mothers: a nursing demonstration project. Journal of Obstetric, Gynecologic, & Neonatal Nursing 1999;28(1):51-9. [DOI] [PubMed] [Google Scholar]
Lawrence 2004 {published data only}
- Lawrence HP, Romanetz M, Rutherford L, Cappel L, Binguis D, Rogers JB. Effects of a community-based prenatal nutrition program on the oral health of Aboriginal preschool children in northern Ontario. Probe 2004;38(4):172-88. [Google Scholar]
Lawrence 2008 {published data only}
- Lawrence HP, Binguis D, Douglas J, McKeown L, Switzer B, Figueiredo R, et al. A 2-year community-randomized controlled trial of fluoride varnish to prevent early childhood caries in Aboriginal children. Community Dentistry and Oral Epidemiology 2008;36(6):503-16. [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee L, Griffiths C, Glossop P, Eapen V. The Boomerangs Parenting Program for Aboriginal parents and their young children. Australasian Psychiatry 2010;18(6):527-33. [DOI] [PubMed] [Google Scholar]
Lees 2014 {published data only}
- Lees DG, Fergusson DM, Frampton CM, Merry SN. A pilot study to evaluate the efficacy of adding a structured home visiting intervention to improve outcomes for high-risk families attending the Incredible Years Parent Programme: study protocol for a randomised controlled trial. Trials 2014;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leijten 2016 {published data only}
- Leijten P, Shaw DS, Gardner F, Wilson MN, Matthys W, Dishion TJ. The family check-up and service use in high-risk families of young children: a prevention strategy with a bridge to community-based treatment. Prevention Science 2016;16(3):397-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Letourneau 2008 {published data only}
- Letourneau RJ, Crump CE, Bowling J, Kuklinski DM, Allen CW. Ride Safe: a child passenger safety program for American Indian/Alaska native children. Maternal & Child Health Journal 2008;12(Suppl 1):S55-63. [DOI] [PubMed] [Google Scholar]
Long 1995 {published data only}
- Long DG, Funk-Archuleta MA, Geiger CJ, Mozar AJ, Heins JN. Peer counselor program increases breastfeeding rates in Utah Native American WIC population. Journal of Human Lactation 1995;11(4):279-84. [DOI] [PubMed] [Google Scholar]
Lucero 2012 {published data only}
- Lucero NM, Bussey M. A collaborative and trauma-informed practice model for urban Indian child welfare. Child Welfare 2012;91(3):89-112. [PubMed] [Google Scholar]
Lucero 2015 {published data only}
- Lucero NM, Bussey M. Practice-informed approaches to addressing substance abuse and trauma exposure in urban native families involved with child welfare. Child Welfare 2015;94(4):97-117. [PubMed] [Google Scholar]
Macedo 2020 {published data only}
- Macedo DM, Smithers LG, Roberts RM, Jamieson LM. Racism, stress, and sense of personal control among Aboriginal Australian pregnant women. Australian Psychologist 2020;55(4):336-48. [Google Scholar]
Mackerras 1998 {published data only}
- Mackerras D. Evaluation of the Strong Women, Strong Babies, Strong Culture Program: Results for the Period 1990–1996 in the Three Pilot Communities. Darwin (Australia): Menzies School of Health Research, 1998. [Google Scholar]
Madden 1984 {published data only}
- Madden J, O'Hara J, Levenstein P. Home again: effects of the Mother-Child Home Program on mother and child. Child Development 1984;55(2):636-47. [PubMed] [Google Scholar]
Makin 2001 {published data only}
- Makin L, Spedding S. Support at Home for Early Language and Literacies (SHELLS): collaboration and challenge. Early Child Development and Care 2001;170:45-56. [Google Scholar]
Mares 2012 {published data only}
- Mares S, Robinson G. Culture, context and therapeutic processes: delivering a parent-child intervention in a remote Aboriginal community. Australasian Psychiatry 2012;20(2):102-7. [DOI] [PubMed] [Google Scholar]
Martens 2002 {published data only}
- Martens PJ. Increasing breastfeeding initiation and duration at a community level: an evaluation of Sagkeeng First Nation's community health nurse and peer counselor programs. Journal of Human Lactation 2002;18(3):236-46. [DOI] [PubMed] [Google Scholar]
Mathu‐Muju 2016 {published data only}
- Mathu-Muju KR, McLeod J, Walker ML, Chartier M, Harrison RL. The Children's Oral Health Initiative: an intervention to address the challenges of dental caries in early childhood in Canada's First Nation and Inuit communities. Canadian Journal of Public Health 2016;107(2):e188-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 2013 {published data only}
- Matthews G, Burton J. Promising practice in intensive family support for Aboriginal and Torres Strait Islander families. Developing Practice: the Child, Youth and Family Work Journal 2013;34:56-66. [Google Scholar]
Maupome 2010 {published data only}
- Maupome G, Karanja N, Ritenbaugh C, Lutz T, Aickin M, Becker T. Dental caries in American Indian toddlers after a community-based beverage intervention. Ethnicity & Disease 2010;20(4):444-50. [PMC free article] [PubMed] [Google Scholar]
May 1989 {published data only}
- May PA, Hymbaugh KJ. A macro-level fetal alcohol syndrome prevention program for Native Americans and Alaska Natives: description and evaluation. Journal of Studies on Alcohol 1989;50(6):508-18. [DOI] [PubMed] [Google Scholar]
May 2008 {published data only}
- May PA, Miller JH, Goodhart KA, Maestas OR, Buckley D, Trujillo PM, et al. Enhanced case management to prevent fetal alcohol spectrum disorders in Northern Plains communities. Maternal & Child Health 2008;12(6):747-59. [DOI] [PubMed] [Google Scholar]
Mayberry 1999 {published data only}
- Mayberry LJ, Affonso DD, Shibuya J, Clemmens D. Integrating cultural values, beliefs, and customs into pregnancy and postpartum care: lessons learned from a Hawaiian public health nursing project. Journal of Perinatal & Neonatal Nursing 1999;13(1):15-26. [DOI] [PubMed] [Google Scholar]
Mayfield 1984 {published data only}
- Mayfield MI, Davies G. An early intervention program for native Indian infants and their families. Canadian Journal of Public Health 1984;75(6):450-3. [PubMed] [Google Scholar]
Mayfield 1985 {published data only}
- Mayfield MI. Parents, children and reading: helping Canadian Native Indian parents of preschoolers. Reading Teacher 1985;39(3):301-5. [Google Scholar]
Mayfield 1986 {published data only}
- Mayfield MI. Policy and planning implications of home-based infant stimulation programs for native Indians in North America. Early Child Development and Care 1986;24(3-4):181-95. [Google Scholar]
McIntosh 2018 {published data only}
- McIntosh C, Trenholme A, Stewart J, Vogel A. Evaluation of a sudden unexpected death in infancy intervention programme aimed at improving parental awareness of risk factors and protective infant care practices. Journal of Paediatrics and Child Health 2018;54(4):377-82. [DOI] [PubMed] [Google Scholar]
McKay 2015 {published data only}
- McKay CC, Chang AB, Versteegh LA, McCallum GB. Culturally appropriate flipcharts improve the knowledge of common respiratory conditions among Northern Territory Indigenous families. Health Promotion Journal of Australia 2015;26(2):150-3. [DOI] [PubMed] [Google Scholar]
McKenzie 1995 {published data only}
- McKenzie B, Seidl E, Bone N. Child and family services standards in First Nations: an action research project. Child Welfare 1995;74(3):633-53. [Google Scholar]
McShane 2008 {published data only}
- McShane K. Family Health and Parenting in an Urban Inuit Community [PhD Thesis]. Montreal (Canada): Concordia University, 2008. [Google Scholar]
Middleton 2017 {published data only}
- Middleton P, Bubner T, Glover K, Rumbold A, Weetra D, Scheil W, et al. 'Partnerships are crucial': an evaluation of the Aboriginal Family Birthing Program in South Australia. Australian & New Zealand Journal of Public Health 2017;41(1):21-6. [DOI] [PubMed] [Google Scholar]
Mondy 2004 {published data only}
- Mondy L, Mondy S. Engaging the community in child protection programmes: the experience of NEWPIN in Australia. Child Abuse Review 2004;13(6):433-40. [Google Scholar]
Montag 2015 {published data only}
- Montag AC, Brodine SK, Alcaraz JE, Clapp JD, Allison MA, Calac DJ, et al. Preventing alcohol-exposed pregnancy among an American Indian/Alaska Native population: effect of a screening, brief intervention, and referral to treatment intervention. Alcoholism: Clinical and Experimental Research 2015;39(1):126-35. [DOI] [PubMed] [Google Scholar]
Mraz Esposito 2014 {published data only}
- Mraz Esposito A, Del Grosso P, Kleinman R, Sama-Miller E, Paulsell D. Assessing the evidence of effectiveness of home visiting program models implemented in tribal communities: final report. Washington (DC): Office of Planning, Research and Evaluation, Administration for Children and Families, U.S. Department of Health and Human Services; 2014 September. Report No.: 2014-57.
Munns 2010 {published data only}
- Munns A. Yanan Ngurra-ngu Walalja Halls Creek community families programme. Neonatal, Paediatric & Child Health Nursing 2010;13(1):18-21. [Google Scholar]
Munns 2015 {published data only}
- Munns A, Walker R. The Halls Creek Community Families Program: elements of the role of the child health nurse in development of a remote Aboriginal home visiting peer support program for families in the early years. Australian Journal of Rural Health 2015;23(6):322-6. [DOI] [PubMed] [Google Scholar]
Munro 2011 {published data only}
- Munro A, Allan J. Can family-focussed interventions improve problematic substance use in Aboriginal communities? A role for social work. Australian Social Work 2011;64(2):169-82. [Google Scholar]
Murphy 2012 {published data only}
- Murphy E, Best E. The Aboriginal Maternal and Infant Health Service: a decade of achievement in the health of women and babies in NSW. New South Wales Public Health Bulletin 2012;23(3-4):68-72. [DOI] [PubMed] [Google Scholar]
Mylant 2021 {published data only}
- Mylant M, Isaacson MJ, Heil J. The feasibility of the strengthening family and an adapted American Indian nutrition and physical activity program: a pilot study. South Dakota Medicine: the Journal of the South Dakota State Medical Association 2021;74(4):172-80. [PubMed] [Google Scholar]
Nations 2004 {published data only}
- Nations V. Six-Year Follow-up Evaluation of a Home Visitation Program: the Prevention of Child Abuse/Neglect in an Alaska Native Population [PhD thesis]. San Francisco (CA): Faculty of Saybrook Graduate School and Research Center, 2004. [Google Scholar]
NCT00428805 {published data only}
- NCT00428805. Child Health Initiative for Lifelong Eating and Exercise (CHILE). clinicaltrials.gov/show/NCT00428805 (first received 30 January 2007).
NCT00435500 {published data only}
- NCT00435500. Fluoride varnish in the prevention of dental caries in Aboriginal and Non-Aboriginal children. clinicaltrials.gov/show/NCT00435500 (first received 15 February 2007).
NCT01116726 {published data only}
- NCT01116726. Promoting behavioral change for oral health in American Indian mothers and children. clinicaltrials.gov/show/NCT01116726 (first received 5 May 2010).
NCT02091804 {published data only}
- NCT02091804. Promoting first relationships: a strengths-based primary prevention project in a native community. clinicaltrials.gov/ct2/show/NCT02091804 (first received 19 March 2014).
NCT03142009 {published data only}
- NCT03142009. Family listening program: multi-tribal implementation and evaluation. clinicaltrials.gov/show/NCT03142009 (first received 5 May 2017).
Nguyen 2015 {published data only}
- Nguyen KH, Smith AC, Armfield NR, Bensink M, Scuffham PA. Cost-effectiveness analysis of a mobile ear screening and surveillance service versus an outreach screening, surveillance and surgical service for Indigenous children in Australia. PLOS One 2015;10(9):e0138369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Novotny 2013 {published data only}
- Novotny R, Nigg C, Li F, Renda G, Wilkens L. Pacific kids DASH for health (PACDASH) tailored multilevel intervention for prevention of obesity and elevated blood pressure among multiethnic children. Annals of Nutrition and Metabolism 2013;63:401-2. [Google Scholar]
Novotny 2017 {published data only}
- Novotny R. Multilevel multicomponent intervention strategies for childhood obesity prevention. Nutrients 2017;9(3):25. [Google Scholar]
NSW Centre for Parenting and Research 2005 {published data only}
- NSW Centre for Parenting and Research. Conclusions from a literature review on effectiveness of early intervention programs and strategies. Developing Practice 2005;(12):39-41.
Nutting 1979 {published data only}
- Nutting PA, Barrick JE, Logue SC. The impact of a maternal and child health care program on the quality of prenatal care: an analysis by risk group. Journal of Community Health 1979;4(4):267-79. [DOI] [PubMed] [Google Scholar]
Oxford 2020 {published data only}
- Oxford M, Booth-LaForce C, Echo-Hawk A, Madesclaire O, Parrish L, Widner M, et al, the CATCH Project Team. Promoting First Relationships®: implementing a home visiting research program in two American Indian communities. Canadian Journal of Nursing Research 2020;52(2):149-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patten 2012a {published data only}
- Patten CA. Tobacco cessation intervention during pregnancy among Alaska Native women. Journal of Cancer Education 2012;27(1 Suppl):S86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patten 2012b {published data only}
- Patten CA, Windsor RA, Renner CC, Enoch C, Hochreiter A, Nevak C, et al. Feasibility of a tobacco cessation intervention for pregnant Alaska Native women. Nicotine & Tobacco Research 2012;12(2):79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2014 {published data only}
- Phillips JH, Wigger C, Beissbarth J, McCallum GB, Leach A, Morris PS. Can mobile phone multimedia messages and text messages improve clinic attendance for Aboriginal children with chronic otitis media? A randomised controlled trial. Journal of Paediatrics and Child Health 2014;50(5):362-7. [DOI] [PubMed] [Google Scholar]
Poland 1991 {published data only}
- Poland ML, Giblin PT, Waller JB Jr, Bayer IS. Development of a paraprofessional home visiting program for low-income mothers and infants. American Journal of Preventive Medicine 1991;7(4):204-7. [PubMed] [Google Scholar]
Pollack 2011 {published data only}
- Pollack J, Yoong T, Eastwood J. A collaborative model of a sustained nurse home visiting programme and paediatric screening for urban aboriginal children. Journal of Paediatrics and Child Health 2011;47:25. [Google Scholar]
Poppe 1992 {published data only}
- Poppe P, Aller Atucha LM. It reaches remote areas. Integration 1992;33:65-9. [PubMed] [Google Scholar]
Prater 2002 {published data only}
- Prater SL, Davis CL. A perinatal intervention program for urban American Indians part 2: the story of a program and its implications for practice. Journal of Perinatal Education 2002;11(2):23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pullon 2003 {published data only}
- Pullon S, McLeod D, Benn C, Viccars A, White S, Cookson T, et al. Smoking cessation in New Zealand: education and resources for use by midwives for women who smoke during pregnancy. Health Promotion International 2003;18(4):315-25. [DOI] [PubMed] [Google Scholar]
Ratima 1999 {published data only}
- Ratima MM, Fox C, Fox B, Te Karu H, Gemmell T, Slater T, et al. Long-term benefits for Maori of an asthma self-management program in a Maori community which takes a partnership approach. Australian and New Zealand Journal of Public Health 1999;23(6):601-5. [DOI] [PubMed] [Google Scholar]
Ratnaike 1994 {published data only}
- Ratnaike RN, Chinner TL. The importance of health education in programs to control diarrhea: experiences in an Australian Aboriginal community. Journal of Health Education 1994;25(5):283-8. [Google Scholar]
Reeve 2014 {published data only}
- Reeve C, Thomas A, Mossenson A, Reeve D, Davis S. Evaluation of an ear health pathway in remote communities: improvements in ear health access. Australian Journal of Rural Health 2014;22(3):127-32. [DOI] [PubMed] [Google Scholar]
Reeve 2016 {published data only}
- Reeve C, Banfield S, Thomas A, Reeve D, Davis S. Community outreach midwifery-led model improves antenatal access in a disadvantaged population. Australian Journal of Rural Health 2016;24(3):200-6. [DOI] [PubMed] [Google Scholar]
Richardson 2008 {published data only}
- Richardson B. Comparative analysis of two community-based efforts designed to impact disproportionality. Child Welfare 2008;87(2):297-317. [PubMed] [Google Scholar]
Richer 2018 {published data only}
- Richer F, Robert E, Boileau-Falardeau M, Gauthier AM. Supporting Indigenous families in the Cree territory: lessons from the  Mashkûpímâtsît Awash initiative. Canadian Journal of Public Health 2018;109(5-6):710-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ricks 2015 {published data only}
- Ricks TL, Phipps KR, Bruerd B. The Indian Health Service Early Childhood Caries Collaborative: a five-year summary. Pediatric Dentistry 2015;37(3):275-80. [PubMed] [Google Scholar]
Riley 2010 {published data only}
- Riley C, Crawford R. Reducing health disparities for low decile children and families: a nurse-led response. Journal of Primary Health Care 2010;2(3):243-8. [PubMed] [Google Scholar]
Ring 2007 {published data only}
- Ring IT, Wenitong M. Interventions to halt child abuse in Aboriginal communities. Medical Journal of Australia 2007;187(4):204-5. [DOI] [PubMed] [Google Scholar]
Roberts 1993 {published data only}
- Roberts RN. Early education as community intervention: assisting an ethnic minority to be ready for school. American Journal of Community Psychology 1993;21(4):521-35. [DOI] [PubMed] [Google Scholar]
Roberts‐Thomson 2010 {published data only}
- Roberts-Thomson KF, Slade GD, Bailie RS, Endean C, Simmons B, Leach AJ, et al. A comprehensive approach to health promotion for the reduction of dental caries in remote Indigenous Australian children: a clustered randomised controlled trial. International Dental Journal 2010;60(3 Suppl 2):245-9. [PubMed] [Google Scholar]
Robinson 2008 {published data only}
- Robinson G, Tyler W. Ngaripirliga'ajirri: the implementation of Exploring Together on the Tiwi Islands. Australian e-Journal for the Advancement of Mental Health 2008;7(1):1-11. [Google Scholar]
Robinson 2009 {published data only}
- Robinson G, Zubrick S, Silburn S, Tyler WB, Jones Y, D'Aprano A, et al. Let's Start: Exploring Together. An early intervention program for Northern Territory children and families. Final evaluation report. Darwin (NT): School for Social and Policy Research, Institute of Advanced Studies, Charles Darwin University; 2009 January. Report No.: ISBN 978-0-9806800-1-0.
Robinson 2012 {published data only}
- Robinson G, Tyler W, Jones Y, Silburn S, Zubrick SR. Context, diversity and engagement: early intervention with Australian Aboriginal families in urban and remote contexts. Children & Society 2012;26(5):343-55. [Google Scholar]
Robinson 2013 {published data only}
- Robinson G, Tyler W, Silburn S, Zubrick SR. Gender, culture and intervention: exploring differences between Aboriginal and non-Aboriginal children's responses to an early intervention programme. Children & Society 2013;27(6):459-70. [Google Scholar]
Robinson 2017 {published data only}
- Robinson G, Mares S, Arney F. Continuity, engagement and integration: early intervention in remote Australian Aboriginal communities. Australian Social Work 2017;70(1):116-24. [Google Scholar]
Rogers 2003 {published data only}
- Rogers R. The Indigenous Early Childhood Project (2003). In: National Indigenous Child Welfare and Development Seminar , editors(s). Our future generations. National Indigenous Child Welfare and Development Seminar; 2003 July 22-24; Melbourne (VIC). Northcote (VIC): Secretariat of National Aboriginal and Islander Child Care, 2003:175.
Ryan 2001 {published data only}
- Ryan S, Wallstrom T. Program evaluation and implications of a rural early intervention associate preparation program. Infant-Toddler Intervention 2001;11(1):27-47. [Google Scholar]
Saggers 2009 {published data only}
- Saggers S, Frances K, Edith Cowan University Social Justice Research Centre, Save the Children Australia. Local evaluation of East Kimberley Communities for Children initiative for children and their families: final report. Perth (WA): Social Justice Research Centre, Edith Cowan University; 2009.
Sanghavi 2005 {published data only}
- Sanghavi DM. Taking well-child care into the 21st century: a novel, effective method for improving parent knowledge using computerized tutorials. Archives of Pediatrics & Adolescent Medicine 2005;159(5):482-5. [DOI] [PubMed] [Google Scholar]
Santos 1999 {published data only}
- Santos EL. A Mental Health Service Delivery Model for Urban Native Americans: an Evaluation of Utilization Rates and Mental Health Treatment Factors in an Urban Setting [Doctoral dissertation]. Ann Arbor (MI): Pacific Graduate School of Psychology, 1999. [Google Scholar]
Sawchuk 1998 {published data only}
- Sawchuk P, Rauliuk M, Kotaska A, Townsend S, Wilson E, Starr M. Infant nutrition program effectively prevents iron-deficiency anemia in a First Nations community. International Journal of Circumpolar Health 1998;57(Suppl 1):189-93. [PubMed] [Google Scholar]
Sawyer 2014 {published data only}
- Sawyer MG, Pfeiffer S, Sawyer A, Bowering K, Jeffs D, Lynch J. Effectiveness of nurse home visiting for families in rural South Australia. Journal of Paediatrics and Child Health 2014;50(12):1013-22. [DOI] [PubMed] [Google Scholar]
Shan 2014 {published data only}
- Shan H, Muhajarine N, Loptson K, Jeffery B. Building social capital as a pathway to success: community development practices of an early childhood intervention program in Canada. Health Promotion International 2014;29(2):244-55. [DOI] [PubMed] [Google Scholar]
Simmons 2008 {published data only}
- Simmons D, Rush E, Crook N. Development and piloting of a community health worker-based intervention for the prevention of diabetes among New Zealand Maori in Te Wai o Rona: Diabetes Prevention Strategy. Public Health Nutrition 2008;11(12):1318-25. [DOI] [PubMed] [Google Scholar]
Simonet 2009 {published data only}
- Simonet F, Wilkins R, Labranche E, Smylie J, Heaman M, Martens P, et al. Primary birthing attendants and birth outcomes in remote Inuit communities – a natural "experiment" in Nunavik, Canada. Journal of Epidemiology & Community Health 2009;63(7):546-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sivak 2008 {published data only}
- Sivak L, Arney F, Lewig K. A pilot exploration of a family home visiting program for families of Aboriginal and Torres Strait Islander children: report and recommendations: perspectives of parents of Aboriginal children and organisational considerations. Adelaide (SA): Australian Centre for Child Protection, University of South Australia; 2008 February.
Smith 1993 {published data only}
- Smith PB, Weinman M, Reeves GC, Wait RB, Hinkley CM. Educational efforts in preventing preterm delivery among inner city adolescents. Patient Education and Counseling 1993;21(1-2):71-5. [DOI] [PubMed] [Google Scholar]
Smith 2000 {published data only}
- Smith RM, Smith PA, McKinnon M, Gracey M. Birthweights and growth of infants in five Aboriginal communities. Australian and New Zealand Journal of Public Health 2000;24(2):124-35. [DOI] [PubMed] [Google Scholar]
Smith 2018 {published data only}
- Smith L, Blinkhorn F, Moir R, Blinkhorn A. Results of a two year dental health education program to reduce dental caries in young Aboriginal children in New South Wales, Australia. Community Dental Health 2018;35(4):211-6. [DOI] [PubMed] [Google Scholar]
Smithers 2017 {published data only}
- Smithers LG, Lynch J, Hedges J, Jamieson LM. Diet and anthropometry at 2 years of age following an oral health promotion programme for Australian Aboriginal children and their carers: a randomised controlled trial. British Journal of Nutrition 2017;118(12):1061-9. [DOI] [PubMed] [Google Scholar]
Smithers 2021 {published data only}
- Smithers LG, Hedges J, Ribeiro Santiago PH, Jamieson LM. Dietary intake and anthropometric measurement at age 36 months among Aboriginal and/or Torres Strait Islander children in Australia: a secondary analysis of the Baby Teeth Talk randomized clinical trial. JAMA Network Open 2021;4(7):e2114348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soares 2021a {published data only}
- Soares GH, Ribeiro Santiago PH, Biazevic MG, Michel-Crosato E, Jamieson L. Dynamics in oral health-related factors of Indigenous Australian children: a network analysis of a randomized controlled trial. Community Dentistry and Oral Epidemiology 2021;50(4):251-9. [DOI] [PubMed] [Google Scholar]
Soares 2021b {published data only}
- Soares GH, Santiago PH, Biazevic MG, Jamieson L, Michel-Crosato E. Do network centrality measures predict dental outcomes of Indigenous children over time? A network analysis of a randomised controlled trial. International Journal of Paediatric Dentistry 2021;31(5):634-46. [DOI] [PubMed] [Google Scholar]
Soltzberg 1997 {published data only}
- Soltzberg B, Raube K, Merrell K. The impact of a community-based intervention on children's utilization of early and periodic screening, diagnostic and treatment services [abstract]. Association for Health Services Research 1997;14:313-4.
Sparrow 2011 {published data only}
- Sparrow J, Armstrong MI, Bird C, Grant E, Hilleboe S, Olson-Bird B, et al. Community-based interventions for depression in parents and other caregivers on a Northern Plains Native American reservation. In: Sarche MC, Spicer P, Farrel P, Fitzgerald E, editors(s). American Indian and Alaska Native Children and Mental Health: Development, Context, Prevention, and Treatment. Denver (CO): Praeger, 2011:205-31. [Google Scholar]
TAIHS 2003 {published data only}
- Townsville Aboriginal and Islanders Health Services. The Mums and Babies Project. Townsville Aboriginal and Islanders Health Services Ltd 2003;3:10-1.
Thompson 2006 {published data only}
- Thompson NL, Hare RD. Reaching native children and families: early education for American Indian and Alaska Native children in rural America. Zero to Three Journal 2006;26(4):43-5. [Google Scholar]
Tipa 2015 {published data only}
- Tipa Z, Wilson D. Cultural responsiveness and the family partnership model. Nursing Praxis in New Zealand 2015;31(2):35-49. [Google Scholar]
Trenholme 2016 {published data only}
- Trenholme A, Stewart J, Aish H, Hoare K, Higham JA, McIntosh C, et al. The Healthy Lungs Study: respiratory outcomes after 2 years of intensive primary care for high risk Maori and Pacific infants. European Respiratory Journal 2016;48(Suppl 60):PA1315. [Google Scholar]
Tsey 2007 {published data only}
- Tsey K, Wilson A, Haswell-Elkins M, Whiteside M, McCalman J, Cadet-James Y, et al. Empowerment-based research methods: a 10-year approach to enhancing Indigenous social and emotional wellbeing. Australasian Psychiatry 2007;15(Suppl 1):S34-8. [DOI] [PubMed] [Google Scholar]
Turner 2007 {published data only}
- Turner KM, Richards M, Sanders MR. Randomised clinical trial of a group parent education programme for Australian Indigenous families. Journal of Paediatrics and Child Health 2007;43(6):429-37. [DOI] [PubMed] [Google Scholar]
Turner 2017 {published data only}
- Turner NM, Charania NA, Chong A, Stewart J, Taylor L. The challenges and opportunities of translating best practice immunisation strategies among low performing general practices to reduce equity gaps in childhood immunisation coverage in New Zealand. BMC Nursing 2017;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valery 2007 {published data only}
- Valery PC, Masters IB, Clements V, Taylor B, Laifoo Y, Chang AB. A randomised controlled study on education intervention for childhood asthma by the Aboriginal and Torres Strait Islander health workers in Torres Strait region. Respirology 2007;12(Suppl 4):A193. [Google Scholar]
Valery 2008 {published data only}
- Valery PC, Masters IB, Taylor B, O'Rourke P, Laifoo Y, Chang AB. A randomised controlled study on education intervention for childhood asthma by Indigenous health workers in the Torres Strait. Respirology 2008;13(Suppl 2):A67. [Google Scholar]
Valery 2009 {published data only}
- Valery PC, Masters IB, Taylor B, O'Rourke, Laifoo Y, Chang AB. Education intervention for childhood asthma by Indigenous health workers in the Torres Strait, Australia. Respirology 2009;14(Suppl 1):A41. [DOI] [PubMed] [Google Scholar]
Valery 2010 {published data only}
- Valery PC, Masters IB, Taylor B, Laifoo Y, O'Rourke PK, Chang AB. An education intervention for childhood asthma by Aboriginal and Torres Strait Islander health workers: a randomised controlled trial. Medical Journal of Australia 2010;192(10):574-9. [DOI] [PubMed] [Google Scholar]
Vicary 2001 {published data only}
- Vicary D, Andrews H. A model of therapeutic intervention with Indigenous Australians. Australian & New Zealand Journal of Public Health 2001;25(4):349-51. [DOI] [PubMed] [Google Scholar]
Volpe 2014 {published data only}
- Volpe T, Boydell KM, Pignatiello A. Mental health services for Nunavut children and youth: evaluating a telepsychiatry pilot project. Rural & Remote Health 2014;14(2):2673. [PubMed] [Google Scholar]
Wen 2012 {published data only}
- Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children's BMI at age 2: randomised controlled trial. BMJ (Clinical Research Ed.) 2012;344:e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilken 2013 {published data only}
- Wilken LR, Novotny R, Fialkowski MK, Boushey CJ, Nigg C, Paulino Y, et al. Children's Healthy Living (CHL) Program for remote underserved minority populations in the Pacific region: rationale and design of a community randomized trial to prevent early childhood obesity. BMC Public Health 2013;13:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2017 {published data only}
- Williams K, Berthelsen D, Viviani M, Nicholson J. Participation of Australian Aboriginal and Torres Strait Islander families in a parent support programme: longitudinal associations between playgroup attendance and child, parent and community outcomes. Child: Care, Health and Development 2017;43(3):441-50. [DOI] [PubMed] [Google Scholar]
Wilson 2013 {published data only}
- Wilson LB, Debaryshe B, Singh M, Taba S. Evaluating two oral health video interventions with early head start families. International Journal of Dentistry 2013;2013:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2018 {published data only}
- Wilson AR, Fehringer KA, Henderson WG, Venner K, Thomas J, Harper MM, et al. Fidelity of motivational interviewing in an American Indian oral health intervention. Community Dentistry and Oral Epidemiology 2018;46(3):310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1997 {published data only}
- Wright L, Naylor A, Wester R, Bauer M, Sutcliffe E. Using cultural knowledge in health promotion: breastfeeding among the Navajo. Health Education & Behavior 1997;24(5):625-39. [DOI] [PubMed] [Google Scholar]
Yoshimoto 2014 {published data only}
- Yoshimoto DK, Robertson NT, Hayes DK. Insights in public health: the Hawai'i Home Visiting Network: evidence-based home visiting services in Hawai'i. Hawai'i Journal of Medicine & Public Health: a Journal of Asia Pacific Medicine & Public Health 2014;73(5):155-60. [PMC free article] [PubMed] [Google Scholar]
Young 2016 {published data only}
- Young C, Gunasekera H, Kong K, Purcell A, Muthayya S, Vincent F, et al. A case study of enhanced clinical care enabled by Aboriginal health research: the Hearing, EAr health and Language Services (HEALS) project. Australian & New Zealand Journal of Public Health 2016;40(6):523-8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Baby Teeth Talk Study (BTT) {published data only}
- Cidro J, Maar M, Peressini S, Schroth RJ, Broughton J, Jamieson L, et al. Strategies for meaningful engagement between community-based health researchers and First Nations participants. Frontiers in Public Health 2017;5:138. [DOI: 10.3389/fpubh.2017.00138] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02151916. Preventing early childhood caries in Indigenous children: the Baby Teeth Talk Study (BTT). clinicaltrials.gov/ct2/show/NCT02151916 (first received 2 June 2014).
Back to Basics {published data only}
- NCT03601299. Back to Basics: addressing childhood obesity through traditional foods in Alaska. clinicaltrials.gov/ct2/show/NCT03601299 (first received 26 July 2018).
Family Spirit Nuture Part 2 2019 {published data only}
- Ingalls A, Rosenstock S, Cuddy RF, Neault N, Yessilth S, Goklish N, et al. Family Spirit Nurture (FSN) – a randomized controlled trial to prevent early childhood obesity in American Indian populations: trial rationale and study protocol. BMC Obesity 2019;6:18. [DOI: 10.1186/s40608-019-0233-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03334266. Preventing early childhood obesity, part 2: Family Spirit Nurture, prenatal – 18 months. clinicaltrials.gov/ct2/show/NCT03334266 (first received 7 November 2017).
Great Beginnings for Healthy Native Smiles {published data only}
- NCT04556175. Great beginnings for healthy native smiles: an early childhood caries prevention project. clinicaltrials.gov/ct2/show/NCT04556175 (first received 21 September 2020).
Infant Care Practices Study (ICP) {published data only}
- NCT03494621. Infant Care Practices Study (ICP) [A CBPR initiative for reducing infant mortality in American Indian communities]. clinicaltrials.gov/ct2/show/NCT03494621 (first received 11 April 2018).
LEAP‐CP {published data only}
- ACTRN12619000969167. LEAP-CP: Learning through Everyday Activities with Parents for Indigenous Australian infants at high risk of cerebral palsy and neurodevelopmental disabilities [Peer delivered early intervention for infants at high risk of cerebral palsy and neurodevelopmental disabilities in Indigenous Australia]. trialsearch.who.int/?TrialID=ACTRN12619000969167 (first received 9 July 2019).
Precision Family Spirit {published data only}
- Ingalls A, Barlow A, Kushman E, Leonard A, Martin L, West AL, et al. Precision Family Spirit: a pilot randomized implementation trial of a precision home visiting approach with families in Michigan – trial rationale and study protocol. Pilot and Feasibility Studies 2021;7(8):1-11. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03975530. Piloting a precision approach to home visiting. clinicaltrials.gov/ct2/show/NCT03975530 (first received 5 June 2019).
Wakȟáŋyeža (Little Holy One) {published data only}
- Brockie T, Haroz EE, Nelson KE, Cwik M, Decker E, Ricker A, et al. Wakȟáŋyeža (Little Holy One) – an intergenerational intervention for Native American parents and children: a protocol for a randomized controlled trial with embedded single-case experimental design. BMC Public Health 2021;21(1):2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04201184. Wakȟáŋyeža (Little Holy One). clinicaltrials.gov/ct2/show/NCT04201184 (first received 17 December 2019).
Additional references
APHCRI 2009
- Australian Primary Health Care Research Institute. Primary health care reform in Australia: report to support Australia's first national primary health care strategy, September 2009. www.phcris.org.au/guides/about_phc.php (accessed prior to 30 September 2016).
Atkinson 2003
- Atkinson J. Trauma Trails: Recreating Song Lines. Melbourne (VIC): Spinifex Press, 2003. [Google Scholar]
Bainbridge 2015
- Bainbridge R, Tsey K, McCalman J, Kinchin I, Saunders V, Watkin Lui F, et al. No one's discussing the elephant in the room: contemplating questions of research impact and benefit in Aboriginal and Torres Strait Islander Australian health research. BMC Public Health 2015;15(696):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bamm 2008
- Bamm EL, Rosenbaum PL. Family-centred theory: origins, development, barriers, and supports to implementation in rehabilitation medicine. Archives of Physical Medicine and Rehabilitation 2008;89(8):1618-24. [DOI] [PubMed] [Google Scholar]
Barlow 2013
- Barlow A, Mullany B, Neault N. Effect of a paraprofessional home-visiting intervention on American Indian teen mothers' and infants' behavioral risks: a randomized controlled trial. American Journal of Psychiatry 2013;170(1):83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlow 2015
- Barlow A, Mullany B, Neault N. Paraprofessional delivered, home-visiting intervention for American Indian teen mothers and children: three-year outcomes from a randomized controlled trial. American Journal of Psychiatry 2015;172(2):154-62. [DOI] [PubMed] [Google Scholar]
Barnes 2019
- Barnes R, Blythe CC, Klerl N, Lee WH, Borland ML, Richmond R, et al. Geographical disparities in emergency department presentations for acute respiratory infections and risk factors for presenting: a population-based cohort study of Western Australian children. BMJ Open 2019;9(2):e025360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bronfenbrenner 1979
- Bronfenbrenner U. The Ecology of Human Development. Boston (MA): Harvard University Press, 1979. [Google Scholar]
CCCRG 2016
- Cochrane Consumers and Communication Review Group. Standard Protocol Text and Additional Guidance for Review Authors. Available at cccrg.cochrane.org (accessed prior to 25 October 2022).
Chamberlain 2019
- Chamberlain C, Gee G, Harfield S, Campbell S, Brennan S, Clark Y, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PLOS One 2019;14(3):e0213460. [DOI: 10.1371/journal.pone.0213460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2011
- Chan RJ, Webster J, Marquart L. Information interventions for orienting patients and their carers to cancer care facilities. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD008273. [DOI: 10.1002/14651858.CD008273.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 7 June 2017. Available at covidence.org.
Cunningham 2003
- Cunningham C, Stanley F. Indigenous by definition, experience, or world view. BMJ 2003;327(7412):403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Ding 2019
- Ding X, Zhu L, Zhang R, Wang L, Wang T-T, Latour JM. Effects of family-centred care interventions on preterm infants and parents in neonatal intensive care units: a systematic review and meta-analysis of randomised controlled trials. Australian Critical Care 2019;32(1):63-75. [DOI: 10.1016/j.aucc.2018.10.007] [DOI] [PubMed] [Google Scholar]
Dodd 2009
- Dodd J, Saggers S, Wildy H. Constructing the 'ideal' family for family-centred practice: challenges for delivery. Disability & Society 2009;24(2):173-86. [DOI: 10.1080/09687590802652447] [DOI] [Google Scholar]
Dwamena 2012
- Dwamena F, Holmes-Rovner M, Gaulden CM, Jorgenson S, Sadigh G, Sikorskii A, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD003267. [DOI: 10.1002/14651858.CD003267.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ewen 2019
- Ewen SC, Ryan T, Platania-Phung C. Capacity building of the Australian Aboriginal and Torres Strait Islander health researcher workforce: a narrative review. Human Resources for Health 2019;17:10. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falster 2016
- Falster K, Banks E, Lujic S, Falster M, Lynch J, Zwi K, et al. Inequalities in pediatric avoidable hospitalizations between Aboriginal and non-Aboriginal children in Australia: a population data linkage study. BMC Pediatrics 2016;16(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gee 2014
- Gee G, Dudgeon P, Schultz C, Hart A, Kelly K. Chapter 4: Aboriginal and Torres Strait Islander social and emotional wellbeing. In: Dudgeon P, Milroy H, Walker R, editors(s). Working Together: Aboriginal and Torres Strait Islander Mental Health and Wellbeing Principles and Practice. Canberra (ACT): Commonwealth of Australia, 2014:55-68. [Google Scholar]
Glover 2015
- Glover M, Kira A, Johnston V, Walker N, Thomas D, Chang AB, et al. A systematic review of barriers and facilitators to participation in randomized controlled trials by Indigenous people from New Zealand, Australia, Canada and the United States. Global Health Promotion 2015;22(1):21-31. [DOI: 10.1177/1757975914528961] [DOI] [PubMed] [Google Scholar]
Gomersall 2017
- Gomersall JS, Gibson O, Dwyer J, O'Donnell K, Stephenson M, Carter D, et al. What Indigenous Australian clients value about primary health care: a systematic review of qualitative evidence. Australian and New Zealand Journal of Public Health 2017;41:417-23. [DOI: 10.1111/1753-6405.12687] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 8 June 2021. Available at gradepro.org.
Griew 2007
- Griew R, Tilton E, Stewart J. Family Centred Primary Health Care: Review of Evidence and Models. Canberra (ACT): Department of Health and Ageing, 2007. [Google Scholar]
Hammer 1998
- Hammer C. Toward a "thick description" of families: using ethnography to overcome the obstacles to providing family-centered early intervention services. American Journal of Speech-Language Pathology 1998;7:5-22. [Google Scholar]
Health Council of Canada 2011
- Health Council of Canada. Understanding and improving Aboriginal maternal and child health in Canada: conversations about promising practices across Canada. publications.gc.ca/collections/collection_2011/ccs-hcc/H174-23-2011-eng.pdf (accessed 29 August 2016).
Herceg 2005
- Herceg A. Improving Health in Aboriginal and Torres Strait Islander Mothers, Babies and Young Children: a Literature Review. Canberra (ACT): Department of Health and Ageing, 2005. [Google Scholar]
Higgins 2021a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021b
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021c
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Available from training.cochrane.org/handbook/archive/v6.2.
Homer 2012
- Homer CS, Foureur MJ, Allende T, Pekin F, Caplice S, Catling-Paull C. It's more than just having a baby' women's experiences of a maternity service for Australian Aboriginal and Torres Strait Islander families. Midwifery 2012;28(4):449-55. [DOI] [PubMed] [Google Scholar]
Jervis‐Bardy 2014
- Jervis-Bardy J, Sanchez L, Carney AS. Otitis media in Indigenous Australian children: review of epidemiology and risk factors. Journal of Laryngology & Otology 2014;128:S16-27. [DOI] [PubMed] [Google Scholar]
Jolley 2009
- Jolley J, Shields L. The evolution of family centered care. Journal of Pediatric Nursing 2009;24(2):164-70. [DOI: 10.1016/j.pedn.2008.03.010] [DOI] [PubMed] [Google Scholar]
Jongen 2014
- Jongen C, McCalman J, Bainbridge R, Tsey K. Aboriginal and Torres Strait Islander maternal and infant health and wellbeing: a systematic review of programs and services in Australian primary health care settings. BMC Pregnancy and Childbirth 2014;14(251):1-20. [DOI: 10.1186/1471-2393-14-251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ka'apu 2019
- Ka'apu K, Burnette CE. A culturally informed systematic review of mental health disparities among adult indigenous men and women of the USA: what is known? British Journal of Social Work 2019;49(4):880-98. [DOI: 10.1093/bjsw/bcz009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuhlthau 2011
- Kuhlthau KA, Bloom S, Cleave J, Knapp AA, Romm D, Klatka K, et al. Evidence for family-centered care for children with special health care needs: a systematic review. Academic Pediatrics 2011;11(2):136-43. [DOI: 10.1016/j.acap.2010.12.014] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care Interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1-34. [DOI] [PubMed] [Google Scholar]
MacKean 2005
- MacKean GL, Thurston WE, Scott CM. Bridging the divide between families and health professionals' perspectives on family-centred care. Health Expectations 2005;8(1):74-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCalman 2014
- McCalman J, Tsey K, Bainbridge R, Rowley K, Percival N, O'Donoghue L, et al. The characteristics, implementation and effects of Aboriginal and Torres Strait Islander health promotion tools: a systematic literature search. BMC Public Health 2014;14(712):1-12. [DOI: 10.1186/1471-2458-14-712] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCalman 2017
- McCalman J, Heyeres M, Campbell S, Bainbridge R, Chamberlain C, Strobel N, et al. Family-centred interventions by primary healthcare services for Indigenous early childhood wellbeing in Australia, Canada, New Zealand and the United States: a systematic scoping review. BMC Pregnancy Childbirth 2017;17(71):1247-71. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2021
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, Thomas J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Available from training.cochrane.org/handbook/archive/v6.2.
McMahon 2017
- McMahon, M. Lotjpa-nhanuk: Indigenous Australian Child-Rearing Discourses [Doctoral thesis]. Melbourne (VIC): La Trobe University, 2017. [Google Scholar]
Nixon 1988
- Nixon JW. Family Cohesion in Families with an Impaired Child [Doctoral thesis]. Brisbane (QLD): University of Queensland, 1989. [Google Scholar]
Review Manager Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. Copenhagen: The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Ridgway 2021
- Ridgway L, Hackworth N, Nicholson JM, McKenna L. Working with families: a systematic scoping review of family-centred care in universal, community-based maternal, child, and family health services. Journal of Child Health Care 2021;25(2):268-89. [DOI: 10.1177/1367493520930172] [DOI] [PubMed] [Google Scholar]
Ryan 2013
- Ryan R, Hill S, Prictor M, McKenzie J. Cochrane Consumers and Communication group Study quality Guide. opal.latrobe.edu.au/articles/journal_contribution/Study_Quality_guide/6818903. La Trobe University, Melbourne (accessed 16 August 2016).
Schünemann 2021
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Segers 2019
- Segers E, Ockhuijsen H, Baarendse P, Eerden I, den Hoogen A. The impact of family centred care interventions in a neonatal or paediatric intensive care unit on parents' satisfaction and length of stay: a systematic review. Intensive Critical Care Nursing 2019;50:63-70. [DOI: 10.1016/j.iccn.2018.08.008] [DOI] [PubMed] [Google Scholar]
Shields 2006
- Shields L, Pratt J, Hunter J. Family centred care: a review of qualitative studies. Journal of Clinical Nursing 2006;15(10):1317-23. [DOI] [PubMed] [Google Scholar]
Shields 2012
- Shields L, Zhou H, Pratt J, Taylor M, Hunter J, Pascoe E. Family-centred care for hospitalised children aged 0–12 years. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD004811. [DOI: 10.1002/14651858.CD004811.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silburn 2006
- Silburn S, Zubrick S, De Maio J, Shepherd C, Griffin J, Mitrou FG, et al. The Western Australian Aboriginal Child Health Survey: Strengthening the Capacity of Aboriginal Children, Families and Communities. Perth (WA): Curtin University of Technology and Telethon Institute for Child Health Research, 2006. [Google Scholar]
Smylie 2009
- Smylie J, Adomako P. Indigenous children's health report: health assessment in action. Toronto (ON): Keenan Research Centre, Centre for Research on Inner City Health; 2009.
Smylie 2010
- Smylie J, Crengle S, Freemantle J, Taualii M. Indigenous birth outcomes in Australia, Canada, New Zealand and the United States – an overview. Open Women's Health Journal 2010;4:7-17. [DOI: 10.2174/1874291201004010007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trivette 1993
- Trivette CM, Dunst CJ, Allen S, Wall L. Family-centredness of the Children's Health Care Journal. Children's Health Care 1993;22(4):241-56. [DOI] [PubMed] [Google Scholar]
UN Permanent Forum on Indigenous Issues
- UN Permanent Forum on Indigenous Issues. Who are Indigenous peoples? www.un.org/esa/socdev/unpfii/documents/5session_factsheet1.pdf (accessed 16 August 2016).
Walker 2008
- Walker R, Shepherd C. Strengthening Aboriginal family functioning: what works and why? Melbourne (VIC): Australian Institute of Family Studies; 2008. Report No.: 7.
Walkup 2009
- Walkup JT, Barlow A, Mullany BC. Randomized controlled trial of a paraprofessional-delivered in-home intervention for young reservation-based American Indian mothers. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(6):591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waterson 2004
- Waterston T, Alperstein G, Stewart Brown S. Social capital: a key factor in child health inequalities. Archives of Disease in Childhood 2004;89(5):456-9. [DOI: 10.1136/adc.2002.024422] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2015
- Welch V, Boyer Y, Chamberlain C. Can Cochrane Reviews inform decisions to improve Indigenous people's health? [editorial]. Cochrane Database of Systematic Reviews 2015;8. [DOI: 10.1002/14651858.ED000103] [DOI] [PMC free article] [PubMed]
White 2005
- White IR, Thomas J. Standardized mean differences in individually-randomized and cluster-randomized trials, with applications to meta-analysis. Clinical Trials 2005;2:141-51. [DOI] [PubMed] [Google Scholar]
Zubrick 2000
- Zubrick S, Williams A, Silburn S, Vimpani G. Indicators of social and family functioning. www.dss.gov.au/sites/default/files/documents/indicators_of_social_and_family_functioning_full_report.pdf (accessed prior to 26 October 2022).
References to other published versions of this review
McCalman 2016
- McCalman J, Campbell SK, Chamberlain C, Strobel NA, Bainbridge RG, Wenitong M, Ruben A, Edmond KM, Marriott R, Tsey K, Keith K, Shields L. Family-centred interventions for Indigenous early childhood well-being by primary healthcare services. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD012463. [DOI: 10.1002/14651858.CD012463] [DOI] [PMC free article] [PubMed] [Google Scholar]


