Abstract
Poststroke epilepsy is a major ischaemic/haemorrhagic stroke complication. Seizure recurrence risk estimation and early therapeutic intervention are critical, given the association of poststroke epilepsy with worse functional outcomes, quality of life and greater mortality. Several studies have reported risk factors for seizure recurrence; however, in poststroke epilepsy, the role of EEG in predicting the risk of seizures remains unclear. This multicentre observational study aimed to clarify whether EEG findings constitute a risk factor for seizure recurrence in patients with poststroke epilepsy. Patients with poststroke epilepsy were recruited from the PROgnosis of POst-Stroke Epilepsy study, an observational multicentre cohort study. The enrolled patients with poststroke epilepsy were those admitted at selected hospitals between November 2014 and June 2017. All patients underwent EEG during the interictal period during admission to each hospital and were monitored for seizure recurrence over 1 year. Board-certified neurologists or epileptologists evaluated all EEG findings. We investigated the relationship between EEG findings and seizure recurrence. Among 187 patients with poststroke epilepsy (65 were women with a median age of 75 years) admitted to the lead hospital, 48 (25.7%) had interictal epileptiform discharges on EEG. During the follow-up period (median, 397 days; interquartile range, 337–450 days), interictal epileptiform discharges were positively correlated with seizure recurrence (hazard ratio, 3.82; 95% confidence interval, 2.09–6.97; P < 0.01). The correlation remained significant even after adjusting for age, sex, severity of stroke, type of stroke and generation of antiseizure medications. We detected periodic discharges in 39 patients (20.9%), and spiky/sharp periodic discharges were marginally associated with seizure recurrence (hazard ratio, 1.85; 95% confidence interval, 0.93–3.69; P = 0.08). Analysis of a validation cohort comprising 187 patients with poststroke epilepsy from seven other hospitals corroborated the association between interictal epileptiform discharges and seizure recurrence. We verified that interictal epileptiform discharges are a risk factor for seizure recurrence in patients with poststroke epilepsy. Routine EEG may facilitate the estimation of seizure recurrence risk and the development of therapeutic regimens for poststroke epilepsy.
Keywords: interictal epileptiform discharge, periodic discharge, poststroke epilepsy, seizure recurrence
Graphical Abstract
Graphical Abstract.
Abe et al. report that interictal epileptiform discharges are a risk factor for seizure recurrence in patients with poststroke epilepsy. Routine EEG may facilitate the estimation of seizure recurrence risk and the development of therapeutic regimens for poststroke epilepsy.
Introduction
Poststroke epilepsy (PSE) is a common complication of stroke. Recent studies have reported that PSE occurs in 10–12% of patients during the 5- to 10-year period after stroke onset.1,2 Although antiseizure medications (ASMs) are effective for decreasing seizure recurrence risk, patients with PSE have a higher recurrence even with ASM use. Reports indicate that the cumulative seizure recurrence rates are 42–81 and 28–84% for ischaemic and haemorrhagic stroke, respectively.3 It was previously demonstrated that 30% of patients with PSE experienced seizure recurrence during 1-year post discharge.4 Another study reported that 20% of patients with PSE were refractory to ASMs.5 The International League Against Epilepsy (ILAE) guidelines in 2014 recommended that therapeutic intervention be considered after the first unprovoked seizure >1-week post stroke unless the classical definition of epilepsy was satisfied owing to high seizure recurrence risk.6,7 Previous studies have demonstrated that the presence of PSE was associated with neurological worsening,8 changing the modified Rankin scale (mRS) from 3 to 4,9 and functional decline.10 Another study reported that health-related quality of life score was reduced in patients with PSE, and seizure frequency was an independent determinant of health-related quality of life score in patients with PSE.11 Furthermore, patients with PSE have a higher risk of short- and long-term mortality within 30-day and 20-year post seizures, respectively.12 Therefore, identifying prognostic factors that may facilitate therapeutic intervention or dosage adjustment of ASMs is essential to improve the management of patients with PSE.13,14
The risk factors for late poststroke seizure after stroke, such as stroke severity, late seizure, presence and volume of haemorrhagic stroke, younger age and presence of convulsions, remain controversial.1,4,15–19 Risk models of late seizure after stroke in patients with ischaemic stroke20 and intracerebral haemorrhage have been reported.21 EEG is an indispensable tool for diagnosing epilepsy, detecting the epileptogenic zone, identifying non-convulsive status epilepticus and predicting seizure recurrence.22 Accordingly, this approach may be useful in PSE management. Nevertheless, there is a paucity of reports investigating the relationship between EEG findings and seizure recurrence, especially in late poststroke seizure,16,23 because the detection rate of epileptiform activity is lower in patients with a first unprovoked seizure (12–50%) than in children with epilepsy (18–56%).24 This study hypothesized that epileptic discharges on EEG could predict seizure recurrence of PSE. Therefore, this study aimed to assess EEG abnormalities and seizure recurrence in patients with PSE.
Materials and methods
Study design and population
This study was conducted as a subanalysis of the multicentre prospective cohort PROgnosis of POst-Stroke Epilepsy (PROPOSE) study (UMIN clinical trial registry: UMIN000019940). This study enrolled consecutive patients with PSE admitted to the cerebrovascular medicine and neurology departments of the National Cerebral and Cardiovascular Center (NCVC) and seven hospitals specializing in stroke and epilepsy care in Japan between November 2014 and June 2017. Based on the new clinical definition according to ILAE,6 PSE was diagnosed in individuals aged >20 years with late seizures that occurred >1-week poststroke onset. Individuals were carefully screened to confirm PSE diagnosis using semiology, CT, MRI, single-photon emission computed tomography and EEG. This PROPOSE study excluded patients with (i) acute symptomatic seizures that occurred within 1-week poststroke onset; (ii) a history of asymptomatic stroke; (iii) a history of epilepsy pre-stroke onset; or (iv) other causes of epilepsy or potentially epileptogenic comorbidities such as intracranial tumours, traumatic brain injury, alcohol or drug abuse or other possible causes. Besides the exclusion criteria of the PROPOSE study, patients who did not undergo EEG at admission were also excluded from the present study. The final diagnosis of PSE was verified by certified neurologists and epileptologists at each hospital.
Standard protocol approvals, registrations and patient consents
Our institutional ethical committee (M26-09-7) and each participating institutional review board approved this study, which was conducted according to the relevant institutional guidelines. The need for informed consent was waived by the ethical committees in accordance with the ‘opt-out’ principle.
Acquisition of baseline clinical data
Baseline clinical data during hospitalization were systematically acquired, including age, sex, family history of epilepsy, current alcohol consumption, comorbidities (hypertension, hyperlipidaemia and diabetes mellitus), ictal/postictal symptoms, severity of stroke, stroke type, location and size of stroke lesion, history of early/late seizure and ASMs before EEG. Detailed seizure information of the ictal/postictal phase (convulsion, paresis, aphasia and consciousness alternation) from patients and eyewitnesses were collected. Stroke severity using the National Institutes of Health Stroke Scale (NIHSS) was evaluated at the latest stroke onset. Stroke subtypes were categorized as ischaemic stroke, intracerebral haemorrhage or subarachnoid haemorrhage. Haemorrhagic stroke was defined as intracerebral and subarachnoid haemorrhage. We also investigated cortical lesions and stroke size in the largest diameter (categorized as <15, 15–30 and >30 mm) using CT or MRI. The history of early seizures (i.e. occurring within 7 days post stroke) and late seizures (i.e. occurring >7-days post stroke) was assessed. At discharge, data on oral ASMs for secondary prevention into older- (valproic acid, carbamazepine, phenytoin, clonazepam and phenobarbital) or newer generations (levetiracetam, lamotrigine, lacosamide, zonisamide, gabapentin and perampanel) were collected and classified. Patients were treated with ASMs at the discretion of the attending physicians in accordance with standard clinical practice at each hospital. Functional disability at discharge using the mRS was assessed.
EEG findings
After admission, standard scalp EEG was performed for ∼20 min based on the international 10–20 system with 21 electrodes using a multichannel EEG machine (Nihon Kohden, Tokyo, Japan). The sampling rate and bandpass filter were set to 500 and 0.53–120 Hz, respectively. Board-certified epileptologists or neurologists, including two epileptologists (K.F., K.K., A.S. and M.T.) at NCVC, an epileptologist (S.M., R.M. and H.Y.) at three other hospitals and a neurologist (T.M., Y.M., M.M. and J.S.) at four hospitals, interpreted EEG results in a manner blinded to other clinical and imaging data. If necessary, EEG was repeated. The following EEG findings were assessed: interictal epileptiform discharges (IEDs), periodic discharges (PDs), rhythmic delta-wave activity (RDA) and focal irregular slow.
According to the International Federation of Clinical Neurophysiology, IEDs were defined as di- or tri-phasic waves with sharp or spiky morphology distinct to background activity, typically followed by an associated slow after wave.25 IED morphology (categorized as spike/spike and waves or sharp/sharp and wave), prevalence (categorized as ≥1 per 10 s, ≥1 per 1 min but <1 per 10 s, ≥1 per 5 min but <1 per min or <1 per 5 min) and distribution (categorized as generalized, hemispheric or focal) were also evaluated. PDs were defined as the repetition of a relatively uniform waveform (including spike, sharp, sharply contoured or blunt) with regular intervals, lasting ≤0.5 s and continuing for >6 cycles, according to the American Clinical Neurophysiology Society (ACNS) Standardized Critical Care EEG Terminology.26 Furthermore, PDs sharpness, frequency (categorized as >2.5 Hz, >1 Hz but ≤2.5 Hz or ≤1 Hz) and distribution (categorized as generalized, hemispheric or focal) were evaluated. RDA was defined as the repetition of a relatively uniform delta waveform without intervals, with a frequency of 0.5–4 Hz and continuing for >6 cycles, according to the ACNS terminology.26 Focal irregular slow activity was defined as focal irregular (excluding rhythmic waves) theta or delta activities observed at a focal area of the scalp region regardless of continuous or intermittent occurrence.
Assessment of the relationship between EEG findings and seizure recurrence
In accordance with standard clinical practice at each hospital, patients enrolled in PSE were followed up for at least 1 year after discharge. Board-certified epileptologists or neurologists performed follow-ups at 6 and 12 months after discharge at the outpatient clinic and assessed episodes of unprovoked seizure recurrences. If outpatient visits were not feasible, telephone interviews with patients, relatives or primary care physicians were conducted 6 and 12 months after discharge. The relationship between EEG findings (IEDs, PDs, RDA and focal irregular slow) and seizure recurrence was evaluated.
Validation
The outcomes of seizure recurrence were collected from the PROPOSE study.
We used data from seven hospitals other than the NCVC as a validation cohort to verify the association between IEDs and seizure recurrence. This validation cohort had the same study period and inclusion/exclusion criteria as the NCVC cohort. The investigators at each hospital confirmed the presence of IEDs based on the same definition as that of NCVC.
Statistical analysis
Continuous values are presented as median [interquartile range (IQR)], whereas categorical variables are expressed as counts (percentages). Wilcoxon rank-sum test was used to analyse continuous variables and Pearson’s χ2 test or Fisher’s exact test to analyse categorical variables. We compared EEG findings and seizure recurrence using Cox proportional hazards models and calculated the hazard ratio (HR) with 95% confidence interval (CI) using univariable and multivariable regression analyses adjusted for potential risk factors for seizure recurrence (Model 1: age, sex and NIHSS; Model 2: age, sex, NIHSS, haemorrhagic stroke and newer-generation ASMs at discharge).1,4,15–19 The follow-up time was defined as the period from the discharge date from PSE to the date of death, loss or the final follow-up visit within 800 days. Seizure freedom rates between patients with and without IEDs and those with and without PDs were compared using Kaplan–Meier analysis and the log-rank test, respectively. For sensitivity analyses, the HR of IEDs for seizure recurrence in subgroups stratified by age, sex, convulsion symptoms, stroke type, location and size of stroke lesion and ASMs before EEG or at discharge was illustrated in a forest plot. The association of detailed EEG findings on IEDs and PDs with seizure recurrence was investigated using a univariate Cox proportional hazard model. In the validation analysis, the time-to-event between IEDs and no IEDs was compared using a log-rank test. Statistical significance was defined as a two-sided P-value <0.05. Statistical analyses were performed using JMP 15 software (SAS Institute Inc, Cary, NC, USA).
Results
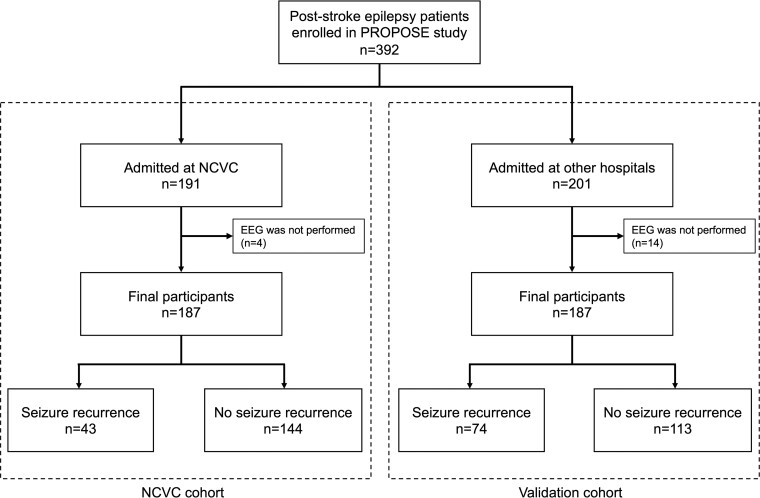
A total of 191 patients diagnosed with PSE at the NCVC were enrolled between November 2014 and September 2018 (Fig. 1). The final participants were 187 (65 women, 34.8%; median age, 75 years; IQR, 66–82 years), excluding 4 who did not undergo EEG at admission. In total, 28 patients (15.0%) dropped out within 1 year because of death (n = 24) or difficulty of contact (n = 4). During the follow-up period (median, 397 days; IQR, 337–450 days), 43 (23.0%) of 187 patients with PSE experienced seizure recurrence. Patients with seizure recurrence had higher NIHSS and higher use of newer-generation ASMs, whereas there was no difference between classifications of seizure (Supplementary Table 1).
Figure 1.
Study flow chart. A total of 392 participants were enrolled in the PROPOSE study. In the NCVC cohort, 187 participants were recruited after exclusion. In the validation cohort, 187 participants were recruited from 7 hospitals other than the NCVC.
Table 1 presents the baseline characteristics of patients with or without IEDs at NCVC. Patients with IEDs (IEDs group) were more likely to be women (29.5 versus 50.0%, P = 0.01). There were no significant differences in comorbidities or ictal/postictal symptoms. Regarding stroke characteristics, the IEDs group had higher NIHSS [11 (4–19) versus 19 (11–25.5), P = 0.02] and higher proportions of intracerebral haemorrhage (56.3%, P = 0.006) and haemorrhagic stroke (60.4%, P = 0.04). The proportions of cortical and large stroke lesions were not significantly different. Patients receiving levetiracetam monotherapy were more common in the non-IED group than in the IED group (73.0 versus 55.3%, P = 0.03). The details of ASM regimens are described in Supplementary Table 2.
Table 1.
Patient characteristics at baseline
| Non-IEDs | IEDs | P-value | |
|---|---|---|---|
| (n = 139) | (n = 48) | ||
| Age, years, median (IQR) | 72.5 (64–82.3) | 75 (68–82) | 0.20 |
| Female (%) | 41 (29.5) | 24 (50.0) | 0.01 |
| Family history of epilepsy | 2 (1.4) | 1 (2.1) | >0.99 |
| Current alcohol consumption (%) | 26 (18.7) | 4 (8.3) | 0.11 |
| Duration between seizure and EEG findings, days, median (IQR) | 1 (1–2.8) | 1 (1–2) | 0.32 |
| Comorbidities | |||
| Hypertension (%) | 117 (84.2) | 40 (83.3) | 0.89 |
| Hyperlipidaemia (%) | 83 (59.7) | 24 (50.0) | 0.24 |
| Diabetes mellitus (%) | 32 (23.0) | 8 (16.7) | 0.35 |
| Classification of seizures | 0.12 | ||
| Focal aware seizure | 25 (18.0) | 2 (4.2) | |
| Focal impaired aware seizure | 50 (36.0) | 18 (37.5) | |
| Focal-to-bilateral tonic-clonic seizure | 62 (44.6) | 27 (56.3) | |
| Others | 2 (1.4) | 1 (2.1) | |
| Ictal/postictal symptom | |||
| Convulsion (%) | 92 (66.2) | 34 (70.8) | 0.55 |
| Ictal/postictal paresis (%)a | 30 (21.9) | 7 (14.9) | 0.30 |
| Aphasia (%)a | 19 (13.9) | 11 (23.4) | 0.13 |
| Consciousness alternation (%)a | 56 (40.9) | 25 (53.2) | 0.14 |
| Clinical/imaging findings of stroke | |||
| NIHSS at the latest stroke onset (IQR) | 11 (4–19) | 19 (11.5–25.5) | 0.02 |
| Stroke type | |||
| Ischaemic stroke (%) | 84 (60.4) | 22 (45.8) | 0.08 |
| Haemorrhagic strokeb (%) | 60 (43.2) | 29 (60.4) | 0.04 |
| Intracerebral haemorrhage (%) | 47 (33.8) | 27 (56.3) | 0.006 |
| Subarachnoid haemorrhage (%) | 13 (9.4) | 2 (4.2) | 0.36 |
| Cortical involvement | 116 (83.5) | 38 (79.2) | 0.50 |
| Stroke lesion size (n = 183) | 0.89 | ||
| <1.5 mm (%) | 13 (9.6) | 4 (8.3) | |
| 15–30 mm (%) | 27 (20.0) | 11 (22.9) | |
| >30 mm (%) | 95 (70.4) | 33 (68.8) | |
| History of early seizure (%)c | 8 (7.4) | 0 (0) | 0.11 |
| History of late seizure (%)c | 6 (5.6) | 2 (5.4) | >0.99 |
| ASMs | |||
| ASMs before EEG (%) | 32 (23.0) | 5 (10.4) | 0.06 |
| ASMs at discharge (%) | 126 (90.7) | 47 (97.9) | 0.12 |
| Newer-generation ASMsd | 119 (94.4) | 41 (87.2) | 0.11 |
| Levetiracetam monotherapy | 92 (73.0) | 26 (55.3) | 0.03 |
| Only older-generation ASMs | 7 (5.6) | 6 (2.8) | 0.11 |
| mRS at discharge, median (IQR) | 3 (2.3–4) | 3 (1–4) | 0.10 |
Data are presented as n (%) or median (interquartile range). ASMs, antiseizure medications; IEDs, interictal epileptiform discharges; IQR, interquartile range; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale.
There were missing data in 3 cases, and we analysed 184 cases.
Haemorrhagic stroke includes cerebral haemorrhage and subarachnoid haemorrhage.
There were missing data in 32 cases, and we analysed 155 cases.
Newer-generation ASMs include newer-generation ASMs and combination therapy of newer-generation and older-generation ASMs.
Among 187 patients who underwent 1 (100%) or 2 (38.0%) EEGs, IEDs, PDs, RDA and focal irregular slow were detected in 48 (25.7%), 39 (20.9%), 12 (6.4%) and 159 (85.0%) patients, respectively. The univariate Cox proportional hazards analysis showed that the IEDs group had a higher seizure recurrence risk [HR 3.82 (2.09–6.97), Table 2]. All models in the multivariable analysis demonstrated that IEDs were an independent risk factor for seizure recurrence [Model 1: HR, 3.22 (1.11–9.33); Model 2: HR, 3.36 (1.06–10.6)]. PDs, RDA and focal irregular slow were not significantly associated with seizure recurrence. Kaplan–Meier analysis indicated that IEDs were a significant risk factor for seizure recurrence, whereas the presence of PDs was not (Fig. 2).
Table 2.
Relationship between seizure recurrence and EEG findings
| Non-seizure recurrence (n = 144) | Seizure recurrence (n = 43) | Unadjusted | Model 1a | Model 2b | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| IEDs (n = 48) | 26 (18.1) | 22 (51.2) | 3.82 (2.09–6.97) | <0.01 | 3.22 (1.11–9.33) | 0.03 | 3.36 (1.06–10.6) | 0.04 |
| PDs (n = 39) | 26 (18.1) | 13 (30.2) | 1.67 (0.87–3.21) | 0.12 | 2.51 (0.82–7.67) | 0.11 | 2.51 (0.68–9.25) | 0.17 |
| RDA (n = 12) | 9 (6.2) | 3 (7.0) | 1.08 (0.33–3.48) | 0.90 | 0 (0) | >0.99 | 0 (0) | >0.99 |
| Focal irregular slow (n = 159) | 121 (84.0) | 38 (88.4) | 1.41 (0.56–3.60) | 0.47 | 1.27 (0.29–5.66) | 0.75 | 1.06 (0.22–5.11) | 0.94 |
Data are presented as n (%) or HR (95% CI).
ASMs, antiseizure medications; CI, confidence interval; HR, hazard ratio; IEDs, interictal epileptiform discharges; NIHSS, National Institutes of Health Stroke Scale; PDs, periodic discharges; RDA, rhythmic delta-wave activity.
Model 1: age, sex and NIHSS.
Model 2: Age, sex, NIHSS, haemorrhagic stroke and new-generation ASMs at discharge.
Figure 2.
Kaplan–Meier curves of each EEG finding for seizure recurrence. Time to seizure recurrence according to the presence of IEDs (A) and PDs (B) in the NCVC cohort.
With respect to the number of seizures after stroke, presence of convulsions, stroke type and lesion size, IEDs exhibited similar effect sizes for seizure recurrence (Fig. 3). Table 3 presents the relationship between EEG findings and seizure recurrence. Regardless of morphology and prevalence, IEDs were significantly associated with seizure recurrence. For PDs, the sharpness of spiky/sharp PDs was marginally associated with seizure recurrence [HR, 1.85 (0.93–3.69), P = 0.08].
Figure 3.
Forest plot of HR of IEDs for seizure recurrence in the different subgroups. The hazard risks with 95% CIs for seizure recurrence in each subgroup were calculated using a Cox proportional hazard model. *First-ever seizure refers to patients without a history of late seizures before admission. **Recurrent seizure refers to patients with a history of late seizure before admission.
Table 3.
Relationship between seizure recurrence and EEG findings of IEDs and PDs
| Non-seizure recurrence (n = 144) | Seizure recurrence (n = 43) | HR (95% CI) | P-value | |
|---|---|---|---|---|
| IEDs (n = 48) | 26 (18.1) | 22 (25.7) | 3.82 (2.09–6.97) | <0.0001 |
| Morphology | ||||
| Spike/spike and wave | 8 (5.6) | 7 (16.3) | 2.58 (1.14–5.81) | 0.02 |
| Sharp/sharp and wave | 18 (12.5) | 15 (34.9) | 3.14 (1.67–5.89) | 0.0004 |
| Prevalence | ||||
| ≥1 per 10 s, but not periodic | 6 (4.2) | 6 (14.0) | 2.72 (1.15–6.48) | 0.02 |
| ≥1 per 1 min but <1 per 10 s | 13 (9.0) | 12 (27.9) | 2.87 (1.48–5.62) | 0.002 |
| ≥1 per 5 min but <1 per min | 7 (4.9) | 5 (11.6) | 2.43 (0.95–6.17) | 0.06 |
| <1 per 5 min | 8 (5.6) | 8 (18.6) | 2.98 (1.38–6.45) | 0.006 |
| Distribution | ||||
| Generalized | 0 (0) | 1 (2.3) | – | 0.002 |
| Hemispheric | 3 (2.1) | 2 (4.7) | 2.70 (0.65–11.2) | 0.17 |
| Focal | 25 (17.4) | 22 (51.2) | 3.96 (2.17–7.21) | <0.0001 |
| PDs (n = 39) | 26 (18.1) | 13 (30.2) | 1.67 (0.87–3.21) | 0.12 |
| Sharpness | ||||
| Spiky/sharp | 18 (12.5) | 11 (25.6) | 1.85 (0.93–3.69) | 0.08 |
| Sharply contoured/blunt | 8 (5.6) | 2 (4.7) | 0.93 (0.23–3.87) | 0.93 |
| Frequency | ||||
| ≤1 Hz | 18 (12.5) | 6 (14.0) | 1.00 (0.42–2.38) | >0.99 |
| >1 Hz, but ≤2.5 Hz | 4 (2.8) | 3 (7.0) | 2.82 (0.87–9.19) | 0.08 |
| >2.5 Hz | 4 (2.8) | 4 (9.3) | 2.39 (0.85–6.71) | 0.10 |
| Distribution | ||||
| Generalized | 0 (0) | 0 (0) | – | – |
| Hemispheric | 11 (7.7) | 6 (14.0) | 1.81 (0.76–4.29) | 0.18 |
| Focal | 18 (12.5) | 10 (23.3) | 1.76 (0.87–3.58) | 0.12 |
CI, confidence interval; HR, hazard ratio; IEDs, interictal epileptiform discharges; PDs, periodic discharges.
In the validation cohort, 201 patients were admitted at 7 hospitals other than NCVC and 14 were excluded owing to lack of EEG data; thus, 187 patients (median age, 72 years; IQR, 64–80 years; 68 women, 36.4%) were finally enrolled (Fig. 1). Figure 4 presents Kaplan–Meier curves of IEDs for seizure recurrence in the validation cohort. IEDs were detected in 62 patients (33.2%), and seizure recurrence was observed in 31 patients (50.0%) with IEDs and 43 (34.4%) without IEDs. The seizure recurrence rate was higher in patients with IEDs (P = 0.048).
Figure 4.
Kaplan–Meier curves of IEDs for seizure recurrence in the validation cohort. The seizure freedom rate at 1 year of follow-up was lower in patients with IEDs compared with non-IEDs (62.9 versus 49.9%).
Discussion
The present study demonstrated that EEG findings of IEDs indicate seizure recurrence risk in patients with PSE, regardless of prior ASMs and risk factors. The findings of the study highlight the association between seizure recurrence and EEG findings in patients with PSE. Although PDs were not statistically significant, spiky/sharp PDs were marginally associated with seizure recurrence of PSE. Furthermore, external validation analysis consistently demonstrated that IEDs were an indicator of seizure recurrence risk in patients with PSE.
Previous studies have reported that EEG abnormalities, including epileptiform abnormality and focal slowing of a background rhythm, are risk factors for seizure recurrence.27,28 A meta-analysis of 15 studies and 1799 participants with genetic, idiopathic, structural seizure reported that the sensitivity and specificity of IEDs in routine EEG after a single unprovoked seizure for seizure recurrence was 17.3 and 94.7%, respectively.29 In patients with juvenile myoclonic epilepsy, the maximum duration of epileptic discharge sequences is considered an indicator of seizure recurrence.30 In patients with ischaemic stroke, sharp waves/spikes on follow-up EEG after acute symptomatic seizures positively correlate with seizure recurrence risk.23 This research identified IEDs as a risk indicator of seizure recurrence in two PSE cohorts (both n = 187). A previous study reported that EEG findings did not correlate with seizure recurrence in 71 patients with PSE. However, the study differed from ours since it was a small, retrospective, single-centre study without information about ASMs regimens or detailed criteria for EEG abnormalities. Moreover, the detection rate of epileptic abnormalities was lower than that in our study (17.2 versus 25.7%).16 The current study may have an advantage over the previous study in that abnormal EEG findings were evaluated more extensively by board-certified epileptologists in a larger number of patients.
Additionally, spiky/sharp PDs were weakly associated with seizure recurrence. In the presence of PDs, potential spike/sharp waves can be masked by continuous periodic waves. Although few studies investigated whether the relationship between PDs and seizure recurrence exists, Bentes et al.31 reported that the presence of PDs was an independent predictor of IEDs, suggesting that spiky/sharp PDs coexist with IEDs in patients with PSE. In this regard, long-term or frequent EEG monitoring should be considered in patients with PDs.
In the subgroup analysis of the forest plot, there was no heterogeneity within each factor. Of note, the risk was similar regardless of ASMs type at discharge. There is a high degree of epileptogenesis associated with IEDs, and that can overwhelm the type of ASMs that determine seizure recurrence in PSE.32 A systematic review of 7082 patients among the whole epilepsy population reported that epileptiform abnormality while on ASMs was an independent predictor of seizure recurrence after ASMs withdrawal (adjusted concordance statistics, 0.65; 95% CI, 0.65–0.66).33 These findings suggest that the finding of EEG is an indicator of seizure recurrence, although the systematic review did not focus on PSE.
The present study had several limitations. First, there was an issue with the diagnosis of epilepsy. In particular, in the non-IEDs group, PSE was determined based solely on semiology. In this regard, 20–30% of patients diagnosed with epilepsy are misdiagnosed at epilepsy centres.34,35 However, in the present study, board-certified epileptologists and neurologists carefully diagnosed PSE and evaluated EEG in detail. Moreover, forest plot analysis revealed that IEDs were associated with seizure recurrence in patients with convulsions and those with two or more episodes of seizure recurrence, which strengthens the diagnostic accuracy of PSE. Second, ASMs may affect EEG findings. A systematic review including randomized controlled trials on genetically generalized epilepsy reported that ASMs reduced the density, frequency, cumulative duration and burst duration of epileptic discharge.36,37 In this study, the risk of IEDs for seizure recurrence was consistent regardless of the type of ASMs used before EEG. Another study limitation was the EEG recording time. Participants were examined with a routine EEG for ∼20 min, which may have been insufficient to detect low-frequency IEDs. In patients for whom diagnosis with routine EEG is difficult, long-term EEG monitoring can be useful to confirm a diagnosis.24 Reports indicate that prolonged EEG following a routine EEG increases additional findings by 30%.38 Recent studies on prolonged EEG, such as video and ambulatory EEG, reported the utility of these approaches for seizure recurrence.39,40 To increase the detection power of abnormal EEG findings, future studies should consider a combination of various EEGs, including long-term EEG, video monitoring and ambulatory EEG.
The strengths of this study include the following: first, the study design, which is a prospective cohort study, is less susceptible to selection and recall bias. Second, it was a multicentre study, and we were able to confirm the results with external validation. Third, several board-certified epileptologists and neurologists evaluated the EEG in detail, which may have clarified the relationship between IEDs and seizure recurrence. In this study, we also prospectively followed up on ASMs and evaluated the relationship between ASMs and EEG findings.
Conclusion
Our findings suggest that the presence of IEDs on EEG among patients with PSE is a risk factor for seizure recurrence. Evaluating recurrence risk and treatment effectiveness using EEG may facilitate earlier intervention and thus contribute to improved prognosis.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge all the participants and investigators for our study. They thank Ms Mariko Yasui for her data management work. They are also grateful to Mrs Yoko Ohashi and Sayaka Wada for their contributions.
Abbreviations
- ACNS =
American Clinical Neurophysiology Society
- ASMs =
antiseizure medications
- HR =
hazard ratio
- IEDs =
interictal epileptiform discharges
- ILAE =
International League Against Epilepsy
- IQR =
interquartile range
- mRS =
modified Rankin scale
- NCVC =
National Cerebral and Cardiovascular Center
- NIHSS =
National Institutes of Health Stroke Scale
- PDs =
periodic discharges
- PROPOSE =
PROgnosis of POst-Stroke Epilepsy
- PSE =
poststroke epilepsy
- RDA =
rhythmic delta-wave activity
Appendix
The names of PROPOSE investigators except co-authors: Kazuyuki Nagatsuka, Fumiaki Nakamura, Shinya Tomari, Yoshitaka Yamaguchi, Takashi Nakamura, Naoki Makita, Yuki Nakamura, Yoshiaki Okuno, Satoshi Hosoki, Ryo Fujii and Takuro Arimizu.
Contributor Information
Soichiro Abe, Department of Neurology, National Cerebral and Cardiovascular Center, Osaka 5648565, Japan.
Tomotaka Tanaka, Department of Neurology, National Cerebral and Cardiovascular Center, Osaka 5648565, Japan.
Kazuki Fukuma, Department of Neurology, National Cerebral and Cardiovascular Center, Osaka 5648565, Japan.
Soichiro Matsubara, Department of Neurology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto 8608556, Japan.
Rie Motoyama, Department of Neurology, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Tokyo 1730015, Japan.
Masahiro Mizobuchi, Department of Neurology, Nakamura Memorial Hospital, Sapporo 0608570, Japan; Clinic of Minami-ichijyo Neurology, Sapporo 0600061, Japan.
Hajime Yoshimura, Department of Neurology, Kobe City Medical Center General Hospital, Kobe 6500047, Japan.
Takayuki Matsuki, Department of Neurology, St Mary’s Hospital, Fukuoka 8300047, Japan.
Yasuhiro Manabe, Department of Neurology, National Hospital Organization Okayama Medical Center, Okayama 7011192, Japan.
Junichiro Suzuki, Department of Neurology, Toyota Memorial Hospital, Toyota 4718513, Japan.
Hiroyuki Ishiyama, Department of Neurology, National Cerebral and Cardiovascular Center, Osaka 5648565, Japan.
Maya Tojima, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 6068507, Japan.
Katsuya Kobayashi, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 6068507, Japan.
Akihiro Shimotake, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 6068507, Japan.
Kunihiro Nishimura, Department of Preventive Medicine and Epidemiology, National Cerebral and Cardiovascular Center, Osaka 5648565, Japan.
Masatoshi Koga, Department of Cerebrovascular Medicine, National Cerebral and Cardiovascular Center, Osaka 5648565, Japan.
Kazunori Toyoda, Department of Cerebrovascular Medicine, National Cerebral and Cardiovascular Center, Osaka 5648565, Japan.
Shigeo Murayama, Department of Neurology, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Tokyo 1730015, Japan.
Riki Matsumoto, Division of Neurology, Kobe University Graduate School of Medicine, Kobe 6500017, Japan.
Ryosuke Takahashi, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto 6068507, Japan.
Akio Ikeda, Department of Epilepsy, Movement Disorders and Physiology, Kyoto University Graduate School of Medicine, Kyoto 6068507, Japan.
Masafumi Ihara, Department of Neurology, National Cerebral and Cardiovascular Center, Osaka 5648565, Japan.
PROPOSE Study Investigators:
Kazuyuki Nagatsuka, Fumiaki Nakamura, Shinya Tomari, Yoshitaka Yamaguchi, Takashi Nakamura, Naoki Makita, Yuki Nakamura, Yoshiaki Okuno, Satoshi Hosoki, Ryo Fujii, and Takuro Arimizu
Funding
This research was funded by the Practical Research Project for Lifestyle-related Diseases including Cardiovascular Diseases and Diabetes Mellitus from the Japan Agency for Medical Research and Development (AMED#JP18ek0210057) and partially supported by the Japan Society for the Promotion of Science (19K16888). Role of the Funder/Sponsor: AMED had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests
A.I. belongs to the Department of Epilepsy, Movement Disorders and Physiology, Kyoto University Graduate School of Medicine, an Industry-Academia Collaboration Course, and was supported by a grant from Eisai Corporation, Nihon Kohden Corporation, Otsuka Pharmaceutical Co. and UCB Japan Co., Ltd. All other authors declared no conflicts of interest.
Supplementary material
Supplementary material is available at Brain Communications online.
Data availability
All de-identified participant data generated for this work are available upon reasonable request from any qualified investigator.
References
- 1. Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: A prospective multicenter study. Arch Neurol. 2000;57(11):1617–1622. [DOI] [PubMed] [Google Scholar]
- 2. Roivainen R, Haapaniemi E, Putaala J, Kaste M, Tatlisumak T. Young adult ischaemic stroke related acute symptomatic and late seizures: Risk factors. Eur J Neurol. 2013;20(9):1247–1255. [DOI] [PubMed] [Google Scholar]
- 3. Zelano J. Recurrence risk after a first remote symptomatic seizure in adults: Epilepsy or not? Epilepsia Open. 2021;6(4):634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanaka T, Yamagami H, Ihara M, et al. Seizure outcomes and predictors of recurrent post-stroke seizure: A retrospective observational cohort study. PLoS One. 2015;10(8):e0136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lattanzi S, Rinaldi C, Cagnetti C, et al. Predictors of pharmaco-resistance in patients with post-stroke epilepsy. Brain Sci. 2021;11(4):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. [DOI] [PubMed] [Google Scholar]
- 7. Hesdorffer DC, Benn EK, Cascino GD, Hauser WA. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia. 2009;50(5):1102–1108. [DOI] [PubMed] [Google Scholar]
- 8. Bryndziar T, Sedova P, Kramer NM, et al. Seizures following ischemic stroke: Frequency of occurrence and impact on outcome in a long-term population-based study. J Stroke Cerebrovasc Dis. 2016;25(1):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Reuck J, Claeys I, Martens S, et al. Computed tomographic changes of the brain and clinical outcome of patients with seizures and epilepsy after an ischaemic hemispheric stroke. Eur J Neurol. 2006;13(4):402–407. [DOI] [PubMed] [Google Scholar]
- 10. Yoshimura H, Tanaka T, Fukuma K, et al. Impact of seizure recurrence on one-year functional outcome and mortality in patients with post-stroke epilepsy. Neurology. 2022;99(4):e376–e384. [DOI] [PubMed] [Google Scholar]
- 11. Winter Y, Daneshkhah N, Galland N, Kotulla I, Kruger A, Groppa S. Health-related quality of life in patients with poststroke epilepsy. Epilepsy Behav. 2018;80:303–306. [DOI] [PubMed] [Google Scholar]
- 12. Arntz RM, Rutten-Jacobs LC, Maaijwee NA, et al. Poststroke epilepsy is associated with a high mortality after a stroke at young age: Follow-up of transient ischemic attack and stroke patients and unelucidated risk factor evaluation study. Stroke. 2015;46(8):2309–2311. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka T, Ihara M. Post-stroke epilepsy. Neurochem Int. 2017;107:219–228. [DOI] [PubMed] [Google Scholar]
- 14. Debicki DB. Electroencephalography after a single unprovoked seizure. Seizure. 2017;49:69–73. [DOI] [PubMed] [Google Scholar]
- 15. Arntz R, Rutten-Jacobs L, Maaijwee N, et al. Post-stroke epilepsy in young adults: A long-term follow-up study. PLoS One. 2013;8(2):e55498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim HJ, Park KD, Choi KG, Lee HW. Clinical predictors of seizure recurrence after the first post-ischemic stroke seizure. BMC Neurol. 2016;16(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neshige S, Kuriyama M, Yoshimoto T, et al. Seizures after intracerebral hemorrhage; risk factor, recurrence, efficacy of antiepileptic drug. J Neurol Sci. 2015;359(1-2):318–322. [DOI] [PubMed] [Google Scholar]
- 18. Abraira L, Toledo M, Guzman L, et al. Long-term epilepsy after early post-stroke status epilepticus. Seizure. 2019;69:193–197. [DOI] [PubMed] [Google Scholar]
- 19. Lin R, Yu Y, Wang Y, et al. Risk of post-stroke epilepsy following stroke-associated acute symptomatic seizures. Front Aging Neurosci. 2021;13:707732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galovic M, Döhler N, Erdélyi-Canavese B, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): A multivariable prediction model development and validation study. Lancet Neurol. 2018;17(2):143–152. [DOI] [PubMed] [Google Scholar]
- 21. Haapaniemi E, Strbian D, Rossi C, et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke. 2014;45(7):1971–1976. [DOI] [PubMed] [Google Scholar]
- 22. Smith SJ. EEG in the diagnosis, classification, and management of patients with epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(Suppl. 2):ii2–ii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. George P, Punia V, Natteru PA, Hantus S, Newey C. Predictors of seizure recurrence after acute symptomatic seizures in ischemic stroke patients. Neurosci J. 2019;2019:8183921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wirrell EC. Prognostic significance of interictal epileptiform discharges in newly diagnosed seizure disorders. J Clin Neurophysiol. 2010;27(4):239–248. [DOI] [PubMed] [Google Scholar]
- 25. Kane N, Acharya J, Benickzy S, et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract. 2017;2:170–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirsch LJ, Fong MWK, Leitinger M, et al. American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol. 2021;38(1):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: A quantitative review. Neurology. 1991;41(7):965–972. [DOI] [PubMed] [Google Scholar]
- 28. Pohlmann-Eden B, Newton M. First seizure: EEG and neuroimaging following an epileptic seizure. Epilepsia. 2008;49(Suppl 1):19–25. [DOI] [PubMed] [Google Scholar]
- 29. Bouma HK, Labos C, Gore GC, Wolfson C, Keezer MR. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23(3):455–463. [DOI] [PubMed] [Google Scholar]
- 30. Turco F, Bonanni E, Milano C, et al. Prolonged epileptic discharges predict seizure recurrence in JME: Insights from prolonged ambulatory EEG. Epilepsia. 2021;62(5):1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bentes C, Martins H, Peralta AR, et al. Early EEG predicts poststroke epilepsy. Epilepsia Open. 2018;3(2):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanaka T, Fukuma K, Abe S, et al. Antiseizure medications for post-stroke epilepsy: A real-world prospective cohort study. Brain Behav. 2021;11(9):e2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamberink HJ, Otte WM, Geerts AT, et al. Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: A systematic review and individual participant data meta-analysis. Lancet Neurol. 2017;16(7):523–531. [DOI] [PubMed] [Google Scholar]
- 34. Chadwick D, Smith D. The misdiagnosis of epilepsy. BMJ. 2002;324(7336):495–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith D, Defalla BA, Chadwick DW. The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. QJM. 1999;92(1):15–23. [DOI] [PubMed] [Google Scholar]
- 36. Gunawan C, Seneviratne U, D’Souza W. The effect of antiepileptic drugs on epileptiform discharges in genetic generalized epilepsy: A systematic review. Epilepsy Behav. 2019;96:175–182. [DOI] [PubMed] [Google Scholar]
- 37. Fattore C, Boniver C, Capovilla G, et al. A multicenter, randomized, placebo-controlled trial of levetiracetam in children and adolescents with newly diagnosed absence epilepsy. Epilepsia. 2011;52(4):802–809. [DOI] [PubMed] [Google Scholar]
- 38. Fisch L, Lascano AM, Vernaz Hegi N, et al. Early specialized care after a first unprovoked epileptic seizure. J Neurol. 2016;263(12):2386–2394. [DOI] [PubMed] [Google Scholar]
- 39. Chen T, Si Y, Chen D, et al. The value of 24-hour video-EEG in evaluating recurrence risk following a first unprovoked seizure: A prospective study. Seizure. 2016;40:46–51. [DOI] [PubMed] [Google Scholar]
- 40. Michel V, Mazzola L, Lemesle M, Vercueil L. Long-term EEG in adults: Sleep-deprived EEG (SDE), ambulatory EEG (Amb-EEG) and long-term video-EEG recording (LTVER). Neurophysiol Clin. 2015;45(1):47–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All de-identified participant data generated for this work are available upon reasonable request from any qualified investigator.