Abstract
Natural product encoding biosynthetic gene clusters (BGCs) within microbial genomes far outnumber the known natural products; chemical products from such BGCs remain cryptic. These silent BGCs hold promise not only for the elaboration of new natural products, but also for the discovery of useful biosynthetic enzymes. Here, we describe a genome mining strategy targeted towards the discovery of substrate promiscuous natural product biosynthetic enzymes. In the genome of the methanotrophic bacterium Methylovulum psychrotolerans Sph1T, we discover a transcriptionally silent natural product BGC that encoded numerous ribosomally synthesized and post-translationally modified peptide (RiPP) natural products. These cryptic RiPP natural products were accessed using heterologous expression of the substrate peptide and biosynthetic enzyme encoding genes. In line with our genome mining strategy, the RiPP biosynthetic enzymes in this BGC were found to be substrate promiscuous, which allowed us to use them in a combinatorial fashion with a similarly substrate tolerant cyanobactin biosynthetic enzyme to introduce head-to-tail macrocyclization in the proteusin family of RiPP natural products.
Graphical Abstract

Introduction
RiPP natural products possess well-established pharmaceutical utility and play important ecological roles in their native environments.1-4 Contemporary –omic technologies are enhancing the inventory and chemical diversity of RiPPs and revealing the widespread genetical potential for their production.5, 6 The emergent chemical novelty among this natural product chemical class motivates interrogating the enzymological finesse which underlies RiPP biosynthesis.7 In most cases, RiPP biosynthetic enzymes bind to the N-terminal leader sequence of the ribosomally synthesized peptide substrates to affect posttranslational modifications upon the C-terminal core region. As RiPP biosynthetic transformations are predicated upon a DNA-encoded peptide, DNA and peptide modification strategies expand the RiPP substrate chemical space.8-11 To unlock the potential to generate designer RiPPs, the substrate promiscuity of the RiPP biosynthetic enzymes needs to match the expansion of the peptide substrate pools.
How do we search for substrate promiscuous RiPP biosynthetic enzymes? Here, we posit that RiPP biosynthetic enzymes present in BGCs containing multiple substrate peptide encoding genes will inherently be substrate promiscuous as they need to modify different substrates in their native physiological context. This rationalization is supported by the discovery of a broadly substrate tolerant lanthipeptide synthetases that modify numerous different substrate peptides encoded in cyanobacterial genomes.12-15 Also reminiscent are the substrate promiscuous peptide macrocyclases that modify multiple different core regions present in a single substrate peptide for the biosynthesis of cyanobactins,16 microviridins,17 and fungal and plant macrocyclic RiPPs.18 These substrate promiscuous RiPP biosynthetic enzymes can then be used as peptide modifying biotechnology tools,19, 20 and also used combinatorically with other RiPP biosynthetic enzymes to build expansive natural product libraries.21-24
Contrary to the abovementioned instances of broad substrate tolerance, RiPP biosynthetic enzymes can also be highly selective for their substrates which constrains biosynthetic diversification. For instance, in the recently described srp RiPP BGCs, the YcaO cyclodehydratase enzyme SrpC was found to be extraordinarily selective for the SrpE substrate peptide core.25 YcaO enzymes, such as SrpC, catalyze the ATP-dependent cyclodehydration of cysteine, serine, and threonine residues to generate linear azoline-containing peptides (LAPs), macrocyclic cyanobactins and thiopeptides, among other classes of RiPP natural products.26 Typified by sequence homology to nitrile hydratase-like leader peptides (NHLPs), SrpE belongs to the proteusin subclass of RiPP substrates.27 Proteusin RiPP substrates possess unusually long leader peptides. While the srp BGC itself was found to be widespread in marine sponge microbiomes, the YcaO cyclodehydratase SrpC was found to be selective for the highly conserved SrpE core sequence (Figure S1). This, in turn, precluded any effort to use SrpC as a tool to introduce azoline heterocycles in other proteusin RiPPs.
In contrast to the substrate selective proteusin YcaO SrpC, we asked if we could devise a genome mining strategy to discover substrate promiscuous YcaO cyclodehydratases and partner them with other biosynthetic enzymes to introduce as yet unseen posttranslational modifications in proteusin RiPPs. Progressing with these motivations, we report here the discovery and biochemical characterization of a substrate promiscuous YcaO cyclodehydratase from a cryptic RiPP BGC that allowed us to construct designer head-to-tail macrocyclized proteusin RiPPs containing azol(in)e heterocycles. Typified by the previously described proteusin natural products polytheonamides and landornamides,28-31 macrolactam cyclization had not been observed for proteusin RiPPs previously.
Results and Discussion
Identification of the proteusin mpr BGC
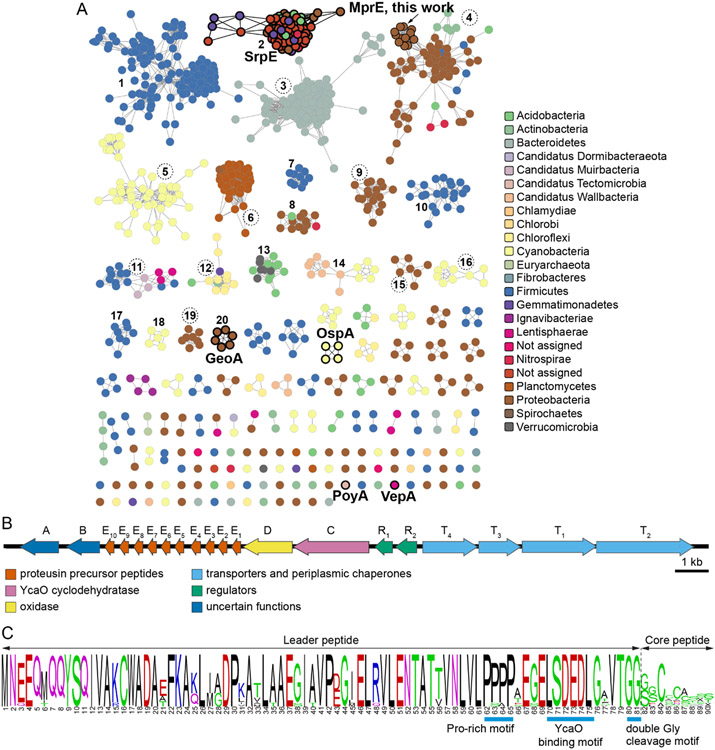
As compared to other RiPP leader sequences, proteusin RiPP substrate peptides possess unusually long leader sequences.27 Rather than mining sequence databases for RiPP biosynthetic enzymes or enzyme/substrate recognition domains, we reasoned that the long proteusin leader sequences could themselves be used as queries to mine for proteusin RiPP BGCs. Hence, we used the InterPro family IPR022513 corresponding to the NHLP domain to mine the UniProt protein sequence database using the Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST).32, 33 Using this strategy, 1290 proteusin substrate peptide sequences were identified and organized into a sequence similarity network (SSN) containing 897 nodes (multiple sequences with greater than 95% similarity were condensed into a single node). At a low alignment score threshold, all nodes coalesced into a single interconnected network (Figure S2). Within this network, we could identify nodes corresponding to the SrpE,25 PoyA,28 GeoA,29 VepA,29 and OspA30 substrate peptides that correspond to previously described proteusin RiPPs. Apart from PoyA derived polytheonamides,34 RiPP natural products for all other proteusin substrates were cryptic and accessible only via heterologous expression. A vast majority of the proteusin sequence space is as yet not connected to natural products (gray nodes, Figure S2). Upon increasing the alignment score threshold, the proteusin substrate peptide SSN split into several subnetworks. These subnetworks were congruent with source bacterium phylogeny; a majority of the subnetworks contained sequences derived from a single bacterial phylum (Figure 1A). A noteworthy exception here is the subcluster 2 which corresponds to the SrpE sequences; we have shown previously that the srp BGCs are present in multiple different bacterial phyla within marine sponge microbiomes.25
Figure 1.
Genome mining for proteusin BGCs. (A) The proteusin substrate SSN with nodes corresponding to peptides with known RiPP natural products highlighted. Clusters are arbitrarily numbered. Clusters with BGCs that contain more than one substrate peptide are labeled by numerals in dashed circles. Nodes are colored according to bacterial phyla. Nodes in cluster 4 corresponding to the Mpr substrates described in this study are labeled. (B) Organization of the mpr BGC. (C) Sequence similarity between the ten MprE1–10 substrate peptides.
Using gene neighborhood diagrams that were generated by the EFI-EST toolkit for each protein sequence, we curated clusters in Figure 1A where multiple proteusin substrate peptides were encoded within a single BGC. In the cluster 4, we identified ten proteusin substrate peptides that were encoded within the same BGC from the cold-adapted methanotrophic proteobacterium Methylovulum psychrotolerans Sph1T.35, 36 We have named this the Methylovulum psychrotolerans-derived RiPP/proteusin (mpr) BGC. The proteusin precursor peptides are encoded by ten contiguous genes, mprE1–10 (Figure 1B, Table S1). In addition to transcriptional regulators and membrane transporters, encoded in the mpr BGC were an ATP-dependent YcaO cyclodehydratase mprC, a flavin-dependent oxidase mprD, and two neighboring genes mprA and mprB. The MprE1–10 precursor peptides each bear long NHLP leader sequences characteristic of proteusin RiPPs. The highly conserved Mpr leader sequences contained a tetraproline repeat followed by a motif that directs the activity of some YcaO cyclodehydratases (Figure 1C).37, 38 The leader/core interface is demarcated by a double-glycine repeat.25, 39 In contrast to the conserved leader peptides, the MprE1–10 core sequences were highly variable which was reminiscent of the prochlorosin lanthipeptide substrates peptides.
Next, we investigated transcription of mpr by the native organism under laboratory growth conditions. We cultivated the methanotroph M. psychrotolerans Sph1T to stationary phase under methane gas and isolated total bacterial RNA. Using reverse transcription PCR (RT-PCR), we compared the transcriptional status of the mprC, mprE1, mprE7, and mprE10 genes (Figure S3, Table S2). These experiments demonstrated that mpr genes are not transcribed under the growth conditions used in this study. Azol(in)e heterocycles, conceivably installed by the cyclodehydratase MprC and oxidase MprD on the MprE substrates, are often metal chelating motifs.40-42 For example, many methanotrophs produce oxazolone-containing chalkophores known as methanobactins to uptake copper, which is required for methane oxidation by the enzyme particulate methane monooxygenase.43, 44 We therefore determined if adding the metal chelator ethylenediaminetetraacetic acid (EDTA) to stationary phase cultures would subsequently stimulate mpr transcription. However, as before, we did not observe transcription of the mpr genes (Figure S3). Collectively, these data allow us to posit that within the subset of growth conditions employed in this study, the mpr BGC is transcriptionally silent and the encoded RiPP natural products are thus cryptic.
Promiscuous activities of MprC and MprD
To gauge whether MprE1–10 peptides could be modified by YcaO cyclodehydratase MprC and flavin-dependent oxidase MprD, we coexpressed mprC and mprD with each of the mprE1–10 genes individually in Escherichia coli and queried the modifications affected upon the MprE cores by mass spectrometry. Here, azoline ring formation by cyclodehydration of cysteine, serine and threonine residues catalyzed by MprC manifests as a reduction in mass of the peptide substrate by 18 Da, and azoline to azole oxidation by MprD as a further 2 Da loss (Figure 2A). The modified MprE peptides were purified, digested with the Lys-C protease, and analyzed by mass spectrometry to reveal that all ten MprE substrates were efficiently processed by MprC and MprD for the installation of the azol(in)e heterocycles (Figure 2B-2K). Corroborating data were obtained by proteolytically separating the Mpr core peptides from the respective leader peptides by the LahT150 protease and mass spectrometric analysis (Figure S4-S17).39
Figure 2.
Processing of MprE1–10 proteusin substrates by MprC and MprD. (A) Principle for mass spectrometric detection of azol(in)e ring formation by cyclodehydration of cysteine, serine, and threonine side chains and azoline oxidation. (B–K) Modifications affected upon MprE1–10 by MprC and MprD. Primary sequences for MprE1–10 cores are illustrated with azol(in)e heterocycles denoted by pentagons. For each MprE substrate, spectral envelopes corresponding to the most highly modified peptides are labeled. In addition, several partially modified variants for each substrate peptide were also detected.
MprC and MprD modified between two (in MprE8) to six (in MprE3/E6/E9) residues, including five contiguous heterocycles installed in MprE6. Unlike the proteusin cyclodehydratase SrpC that only modified cysteine residues, MprC also modified serine and threonine residues. Heterocycles installed by MprC could be interspersed with polar, charged, or hydrophobic amino acids. Noteworthy was the observation that the C-terminal residues in MprE1–6/E9–10 were cyclodehydrated by MprC. However, azoline heterocycles at the C-termini were not oxidized by MprD (Figure S5, S9, S12, S17).
These data demonstrated that the MprC/D modification enzymes were highly promiscuous for the substrate core peptides. To assess whether the substrate promiscuity of MprC/D extended to the leader peptides, the MprE7 core sequence was appended to the PoyA,28 OspA,30 and SrpE25 proteusin leader peptides and the cyanobactin leader peptides PatE45 and TruE.22 Taking advantage of the high similarity between the MprE1–10 leader sequences, a consensus MprE leader was constructed which we term as MprEX (Supplementary Note). By coexpression in E. coli, we evaluated the ability of MprC/D to modify the MprE7 core appended to the six leaders. Each of the six leaders evaluated here, MprEX, PoyA, OspA, SrpE, PatE, and TruE, contain the recognition motif implicated in binding of the leader to the YcaO cyclodehydratases (Figure S18).37 MprC/D efficiently processed the MprEX-E7 chimeric substrate with all four possible cyclodehydrations realized (Figure S19). However, none of the other five leader sequences supported the modification of the MprE7 core, including, the proteusin PoyA and OspA leaders (Figure S19). Hence, we posit that while MprC/D are promiscuous for the core, they are highly specific for the proteusin leader sequences.
Next, we tested possible roles for MprA and MprB (Figure 1B). Upon coexpression of mprE1–10 in different combinations of mprA/mprB with mprC/mprD, we observed no modifications other than the azol(in)e heterocycles. MprA is homologous to fatty acid desaturases and has been implicated in modulating the fatty acid composition of cell membrane of M. psychrotolerans in response to decrease in growth temperatures.46 MprB bears no resemblance to any functionally characterized enzymes. As such, MprB has few sequence homologs overall. It is thus uncertain if mprB encodes a functional enzyme.
MprC activity is not unidirectional
We evaluated the directionality of cyclodehydration by MprC. The time-dependent modification of MprE7 by recombinantly produced and purified MprC and MprD was followed in vitro. The site for azol(in)e installation was discerned by proteolytic separation of the MprE7 core from the leader by the LahT150 protease,39 followed by mass spectrometric fragmentation of the thusly modified MprE7 core. Using this workflow, we could discern that the first azole heterocycle was installed by MprC/D at Cys5 (-GGCACSSK) in the MprE7 core followed by modification of the Cys3 (-GGCACSSK). The third heterocycle was installed at Ser6 (-GGCACSSK) (Figure 3A, S20). While these data establish that MprC/D do not modify their substrate in a processive unidirectional manner, they do point toward cysteine residues being modified prior to the serine residues. The preference of MprC to modify cysteines as compared to serine/threonine residues is also discernable by MS/MS analysis of incompletely modified MprE cores sequences illustrated in Figure 2. For instance, in the modified MprE6 peptide obtained upon coexpression of mprE6 with mprC/D, the first four cyclodehydrations are all affected upon the four cysteine residues in the MprE6 core (-SGCACCSCT) (Figure S21). It is after modification of all four possible cysteine residues that the Ser7 and Thr9 residues in the MprE6 core are modified by MprC.
Figure 3.
Activity of MprC. (A) Time dependent modification of MprE7 by MprC/D. The sites for azol(in)e installations were discerned using mass spectrometric fragmentation (Figure S20). (B) Chromatographic comparison to AMP and ADP standards demonstrates that ADP is generated as a product by MprC in the presence or in the absence of the MprE7 substrate. (C) Time dependent ADP formation when MprC/D were incubated with ATP/Mg+2 in the absence (blue dots) and presence (orange dots) of MprE7 substrate.
That the YcaO cyclodehydratase MprC could modify cysteine, and serine and threonine residues, and that it did not modify these substrates in a unidirectional manner is in stark contrast to the previously described activity of the proteusin RiPP cyclodehydratase SrpC.25 SrpC cyclodehydrated the three cysteine residues in the SrpE -LCCCW core in an obligate N- to C-terminal direction (Figure S1). Neither did SrpC tolerate any modification to the continuous repetition of the three cysteine residues in the SrpE core; modification of downstream cysteines was abolished if an alanine residue was introduced in between the three contiguous cysteine residues in the SrpE core sequence, nor did it modify serine or threonine residues.25 Similar strict substrate specificities have also been reported for other LAP YcaO cyclodehydratases.47, 48
YcaO cyclodehydratases employ ATP to facilitate the dehydration of the hemiorthoamide intermediate that is generated by nucleophilic addition of the serine/threonine side chain-derived alkoxide or the cysteine side chain-derived thiolate to the preceding peptide bond carbonyl carbon; ATP/Mg+2 was added in the in vitro MprE7 modification assay described above.26, 49 Dehydration is preceded by the activation of the hemiorthoamide by phosphorylation which would lead to transformation of ATP to ADP and Pi, or activation via adenylation which would lead to the formation of AMP and PPi. Both routes have been proposed to be employed by YcaO cyclodehydratases,50 though the production of AMP is also possible via ADP disproportonation.51 To discern whether MprC employs the kinase or the adenylation routes, the time-dependent formation of ADP and AMP was followed during the in vitro modification of MprE7 by MprC/D. By chromatographic separation and comparison to standards of AMP, ADP, and ATP, we could identify that MprC generated ADP as a product during the cyclodehydration reaction; AMP formation was not observed (Figure 3B). In the absence of the substrate, MprC also hydrolyzed ATP to ADP with the rate of ATP hydrolysis enhanced in the presence of the proteusin substrate (Figure 3C).
Combinatorial proteusin macrocyclization
The products of the mpr BGC resemble LAP natural products with a high density of contiguous azol(in)e heterocycles installed by the promiscuous MprC and MprD enzymes. In observance of the broad substrate tolerance of MprC, we asked if we could use it as a biotechnology tool to facilitate the introduction of entirely new modifications in proteusin RiPPs. Macrocyclization is a commonly observed biosynthetic modification in peptidic natural products. Macrocyclization affords proteolytic stability, rigidity, and by masking the charged terminal amines and carboxylates, makes the natural product membrane permeable.52 The only known instance of macrocyclization among proteusin RiPPs is the installation of (methyl)lanthionine rings in landornamides by the lanthionine synthetase OspM.30 Head-to-tail macrocyclization by amide bond formation between the terminal backbone amine and carboxyl groups has not been observed for proteusin RiPPs.
We have previously described the activity of the cyanobacterial subtilisin-like protease PatG wherein a three amino acid (-AYD) leader-like recognition sequence at the C-terminus of a peptide guides a head-to-tail macrocyclization reaction.16 To evaluate if the substrate promiscuity of MprC/D would allow their conjoined use with PatG to afford macrocyclized proteusin RiPPs, the -AYD sequence was appended to the C-termini of MprE2, MprE5, and MprE10 core sequences with the MprEX leader to afford the MprEX-E2-AYD, MprEX-E5-AYD, and MprEX-E10-AYD chimeric peptide constructs. We also generated the MprEX-PatE-AYD construct where we used one of the two physiological PatE core sequences (VTACITFC) in place of the MprE core sequences; PatE is the physiological substrate for PatG.45 All four peptide constructs were coexpressed with mprC and mprD, and upon mass spectrometric characterization of purified peptides, we observed that each construct was efficiently modified, including the PatE-AYD chimera wherein the alternating azol(in)e ring pattern was different from the MprE2/E5/E10-AYD chimeras (Figure 4A-D). These data establish that MprC/D were broadly tolerant to C-terminal extension of the MprE cores sequences.
Figure 4.
Proteusin macrocyclization. The MprEX-MprE core sequences were appended with the tripeptide -AYD at the C-termini which serves as the PatG recognition sequence (PatG RS). Installation of azol(in)e rings by MprC/D on (A) MprE2-AYD, (B) MprE5-AYD, (C) MprE10-AYD, and (D) PatE-AYD cores. Mass spectrometric characterization reveals that three (out of possible five; panel A) and two (out of possible four; panel D) azolines were oxidized to azoles by MprD for the MprE2-AYD and PatE-AYD substrates, respectively. Other detected peaks highlighted in red in panel B and in green in panel D represent partially processed peptides wherein all modifiable residues have not been cyclodehydrated by MprC. (E) Extracted ion chromatograms (EICs) for cyclic product [M+H]+ m/z 803.36 and linear substrate [M+H]+ m/z 1170.50 demonstrating in vitro cyclization of the MprC-modified PatE core in the presence of PatG. (F) EICs for cyclic product [M+Na]+ m/z 771.24 and linear substrate [M+Na]+ m/z 1139.37 demonstrating cyclization of the MprC-modified MprE5 core by PatG. (G) Mechanism for peptide macrocyclization by PatG for substrates with an azoline (top) or an azole (bottom) heterocycle preceding the AYD PatG RS. Electron delocalization (blue arrows, bottom) with the azole likely decreases the electrophilicity of the carbonyl carbon.
To mimic the 8-residue cyanobactin macrocycles generated by PatG, we performed a preparative scale isolation of the MprC modified MprE5-AYD and PatE-AYD peptides after proteolytic removal of the MprEX leaders. We did not modify these peptides using MprD. The purified peptides were delivered to PatG in vitro for macrocyclization. Here, the modified PatE-AYD peptide serves as a positive control for the native macrocyclization reaction catalyzed by PatG. Mass spectrometric characterization of the reaction extracts revealed that PatG could indeed macrocyclize the MprC modified azoline ring containing E5-AYD and PatE-AYD peptides (Figure 4E-F, S22-S23).
Interestingly, when the chimeric substrates were modified by both MprC and MprD, the resultant azole ring containing E5-AYD and PatE-AYD substrates were not macrocyclized by PatG. These observations can be rationalized on the basis of the proposed PatG reaction mechanism which involves the formation of an acyl intermediate with the enzyme active site serine side chain which is then resolved by nucleophilic displacement of the enzyme by the substrate terminal primary amine leading to the head-to-tail macrocyclizing amide bond formation.53, 54 We posit that electron delocalization from an azole heterocycle reduces the electrophilicity at the carbonyl carbon preceding the -AYD recognition tripeptide which could impede formation of the acyl-enzyme intermediate as well as the nucleophilic resolution of the acyl-enzyme intermediate and lactam formation (Figure 4G). No such charge delocalization is possible with an azoline ring. In light of this argument, it is instructive to observe that the macrocyclizing domain of PatG is present on the same peptide that also bears the flavin-dependent azoline oxidase domain.45 It is thus conceivable that azoline-to-azole oxidation in macrocyclic cyanobactin natural products occurs after or concomitantly with macrocyclization.
Overall, data presented herein are consistent with the assertion that RiPP biosynthetic enzymes encoded in BGCs that contain multiple substrate peptide genes display broad substrate tolerance, and that they can be used or developed as biotechnology tools. The Mpr RiPP natural products were unknown because the mpr BGC is transcriptionally silent under the laboratory growth conditions typically employed for methanotrophic bacteria. Despite access to the Mpr RiPPs via heterologous expression of the mpr genes, the physiological function of these natural products remains uncertain. Using purified Mpr RiPPs, we did not detect any antibiotic activity against bacterial test strains. While copper is required for the activity of the methane oxidizing enzyme particulate methane monooxygenase, the mpr BGC harboring methanotroph M. psychrotolerans Sph1T does not harbor genes encoding the chalkophore methanobactin biosynthetic enzymes MbnB and MbnC.55 The Mpr RiPPs did not possess any metal binding/chalkophore-like activity. Thus, it is conceivable that an alternate copper acquisition system is operable for M. psychrotolerans Sph1T.43
Prior work has revealed modifications for proteusin RiPPs that are rare or non-existent in other RiPP compound classes. These biosynthetic transformations include methylation at unactivated carbon atoms on amino acid side chains,28 irreversible epimerization around the α-carbon,29 installation of the non-proteinogenic amino acid ornithine,30 and bromination of tryptophan side chains.25 Additionally, genome mining reveals that a vast majority of BGCs encoding proteusin RiPP natural products remain unexplored, potentiating the discovery of entirely new biosynthetic transformations and substrate promiscuous biosynthetic enzymes. This then sets the stage for chemical diversity generating efforts in which enzymes from different proteusin RiPP BGCs are used together in a combinatorial fashion to generate new designer RiPP natural products. Here, our discovery of the substrate promiscuous YcaO cyclodehydratase MprC and the flavin-dependent oxidase MprD add to the toolkit of proteusin substrate peptide modification enzymes.
Methods
Generation of SSN
An SSN for NHLPs was created using EFI-EST.32, 33 Query sequences included a manually curated list of 62 NHLPs that have been described in literature. Of these 62 sequences, 58 sequences were SrpE NHLPs previously described from marine sponge microbiomes, and the other 4 sequences included three GeoA1–3 and VepA sequences.25, 29 Using this query set, 1290 NHLP sequences were retrieved to generate the SSN illustrated in Figure S1 using an alignment score of 6 (≈30% sequence identity). Applying more stringent alignment score cutoff of 23 (≈50% sequence identity) led to the creation of the SSN illustrated in Figure 1A. The SSN illustrated in Figure 1A contains 77 subclusters and 97 singletons. The ten largest subclusters contain between 16 and 198 sequences. Both SSNs were visualized in Cytoscape 3.8.2 using a 95% identity cutoff for grouping sequences into representative nodes. Node layouts were generated using the yFiles Organic algorithm.
Transcriptional status of the mpr BGC
M. psychrotolerans Sph1T (DSMZ 100602) was purchased from Leibniz-Institute DSMZ-German Collection of Microorganisms and Cell Cultures GmbH. M. psychrotolerans Sph1T was grown in 50 mL cultures in an atmosphere of 50% (v/v) methane in air in 250 mL glass serum bottles (Kimble Chase, Vineland, NJ, USA) sealed with rubber stoppers and aluminum seals (Wheaton, Millville, NJ, USA) and shaken at 200 rpm at room temperature. Cultures were grown in a modified nitrate mineral salts (NMS) medium. The NMS media contained 0.2 g/L MgSO4·7H2O, 0.2 g/L CaCl2·6H2O, 1 g/L KNO3, and 30 μM LaCl3 as well as 1X trace elements. 500X trace elements contained 1.0 g/L Na2-EDTA, 2.0 g/L FeSO4·7H2O, 0.8 g/L ZnSO4·7H2O, 0.03 g/L MnCl2·4H2O, 0.03 g/L H3BO3, 0.2 g/L CoCl2·6H2O, 0.6 g/L CuCl2·2H2O, 0.02 g/L NiCl2·6H2O, and 0.05 g/L Na2MoO4·2H2O. Immediately before use, phosphate buffer (pH 6.8), was added to a final concentration of 5.8 mM. At start of the experiment, exponentially growing cells were sub-diluted to an OD of 0.02. After 48 and 96 h, the headspace was purged with air and replenished with methane to 50% (v/v). After 96 h, EDTA was added to one culture at a final concentration 1 mM to test the effect of metal starvation on mpr BGC transcriptional status. After an additional 36 h (total growth time 132 h), a 25 mL aliquot was withdrawn, and cells pelleted by centrifugation for 15 min at 5,000×g and 4 °C. The supernatant was removed by aspiration and pellets were stored at −80 °C until further processing.
Cell pellets were resuspended in 1 mL TRIzol (ThermoFisher) before cells were lysed by bead beating with 0.1 mm zirconia-silica beads for 4 min. Cells were kept cold and were placed on ice halfway through the bead beating process. Chloroform (200 μL) was added and the mixture transferred to phasemaker tubes (ThermoFisher) for separation by centrifugation. Then, 1.5 volumes of 100% ethanol were added to the aqueous phase of the extract before sample cleanup with the Invitrogen RNA PureLink mini kit (Invitrogen) according to the manufacturer’s instructions. The resulting RNA sample was treated with DNase I (Ambion) according to the manufacturer’s instructions and was subsequently extracted with 3 volumes of acid-phenol chloroform (Ambion) in a phasemaker tube (ThermoFisher) by centrifugation. Lithium chloride was added to the extracted RNA to a final concentration of 2.5 M before the sample was chilled at −20 °C for 30 min. RNA was pelleted by centrifugation at 4 °C for 15 min, washed with 200 μL ice cold 70% ethanol, and resuspended in 100 μL nuclease-free water. Purified RNA was checked for DNA contamination using the primers listed in Table S2.
Using the thusly isolated RNA, cDNA was prepared for RT-PCR by reverse transcribing 500 ng RNA using iScript Reverse Transcription Supermix (BioRad). PCR was performed with OneTaq Quick-Load 2X Master Mix (NEB) containing 400 nM primers and a normalized concentration of cDNA in a total reaction volume of 10 μL. PCR reactions were performed on a BioRad C1000 Touch thermal cycler. The M. psychrotolerans Sph1T genomic DNA (gDNA) was prepared using the GeneJET Genomic DNA Purification Kit (ThemoFisher) and included as a positive control.
Enzymatic assays
Cyclodehydration and oxidation assay:
Time-course reactions were performed in 200 μL volume at 25 °C. The reaction mixture contained 10 mM HEPES-Na (pH 7.5), 1 mM TCEP, 100 μM substrate peptide (MprE7), 17.5 μM YcaO cyclodehydratase (MprC), 5 μM oxidase (MprD), 50 μM FMN, 5 mM ATP, and 5 mM MgCl2. The reactions were quenched by heating to 40 °C and then centrifuged to remove precipitates. The supernatant was digested by LysC or LahT150 and analyzed by mass spectrometry.
For ADP/AMP analysis, reaction mixtures (20 μL) were quenched sequentially by 1 μL of 10 N NaOH and 3 μL of 3 N HCl before dilution to 240 μL. A portion of the mixture (3 μL) was analyzed by an ion paired chromatography using Luna® 5 μm C18 reversed phase HPLC column (100 × 4.6 mm) at a flow rate of 0.5 mL/min of an isocratic mobile phase: 0.2 M NaH2PO4, 25 mM tetrabutyl ammonium bromide, 19.5% MeOH. UV absorbance was monitored at 260 nm.
In vitro peptidase assay:
LahT150 cleavage reaction was performed at 30 °C in 2 h for assay analysis or overnight for modified core peptide purification. The reaction mixture contained 50 mM HEPES-Na (pH 7.5), 5 mM DTT, 20 μM LahT150 and up to 200 μM purified peptides. LahT150 assays were quenched by addition of 50 μL 5% v/v trichloroacetic acid (TCA) in MeOH and centrifugated to remove precipitates. The supernatants were analyzed by mass spectrometry. Peptide digestion with LysC was performed by adding 1 μL 0.2 mg/mL LysC protease to 200 μL of purified peptides and incubated at 30 °C for 90 min. The reaction was then desalted and analyzed by mass spectrometry.
Macrocyclization assay:
Reaction mixtures (20 μL) contained 50 mM Tris-HCl (pH 7.5), 200 μM modified core peptide, 20 μM PatG,56 and 5 mM MgCl2. After incubation at 37 °C for 20 h, reactions were quenched by heating at 100 °C for 5 min. Diluted reaction in MeOH were analyzed by mass spectrometry.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (GM142882 to V.A.; GM122521 to E.W.S.; GM118762 to A.W.P.). The authors thank C. J. Fahrni for insightful discussions, S. G. Moore at the Georgia Institute of Technology’s Systems Mass Spectrometry Core facility for mass spectrometry data acquisition, and J. Deutsch for assistance with mass spectrometry data deposition.
Footnotes
Supplementary Information
The accompanying Supplementary Information (SI) document contains Supplementary Materials and Methods, sequence of the MprEX leader, annotation of the roles of the mpr genes, and mass spectrometry data detailing the characterization of the MprE peptides.
References
- 1.Dang T; Süssmuth RD, Bioactive peptide natural products as lead structures for medicinal use. Acc Chem Res 2017, 50 (7), 1566–1576. [DOI] [PubMed] [Google Scholar]
- 2.Li Y; Rebuffat S, The manifold roles of microbial ribosomal peptide–based natural products in physiology and ecology. J Biol Chem 2020, 295 (1), 34–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muttenthaler M; King GF; Adams DJ; Alewood PF, Trends in peptide drug discovery. Nat Rev Drug Discov 2021, 20 (4), 309–325. [DOI] [PubMed] [Google Scholar]
- 4.Cao L; Do T; Link AJ, Mechanisms of action of ribosomally synthesized and posttranslationally modified peptides (RiPPs). J Ind Microbiol Biotechnol 2021, 48 (3-4), kuab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell AH; Truman AW, Genome mining strategies for ribosomally synthesised and post-translationally modified peptides. Comp Struc Biotechnol 2020, 18, 1838–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloosterman AM; Medema MH; van Wezel GP, Omics-based strategies to discover novel classes of RiPP natural products. Curr Opin Biotechnol 2021, 69, 60–67. [DOI] [PubMed] [Google Scholar]
- 7.Montalbán-López M; Scott TA; Ramesh S; Rahman IR; van Heel AJ; Viel JH; Bandarian V; Dittmann E; Genilloud O; Goto Y; et al. , New developments in RiPP discovery, enzymology and engineering. Nat Prod Rep 2021, 38 (1), 130–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu W; Schmidt EW, Three principles of diversity-generating biosynthesis. Acc Chem Res 2017, 50 (10), 2569–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto Y; Suga H, Engineering of RiPP pathways for the production of artificial peptides bearing various non-proteinogenic structures. Curr Opin Chem Biol 2018, 46, 82–90. [DOI] [PubMed] [Google Scholar]
- 10.Wu C; van der Donk WA, Engineering of new-to-nature ribosomally synthesized and post-translationally modified peptide natural products. Curr Opin Biotechnol 2021, 69, 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkhart BJ; Kakkar N; Hudson GA; van der Donk WA; Mitchell DA, Chimeric leader peptides for the generation of non-natural hybrid RiPP products. ACS Cent Sci 2017, 3 (6), 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B; Sher D; Kelly L; Shi Y; Huang K; Knerr PJ; Joewono I; Rusch D; Chisholm SW; van der Donk WA, Catalytic promiscuity in the biosynthesis of cyclic peptide secondary metabolites in planktonic marine cyanobacteria. Proc Natl Acad Sci U S A 2010, 107 (23), 10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee S; van der Donk WA, Mechanistic studies on the substrate-tolerant lanthipeptide synthetase ProcM. J Am Chem Soc 2014, 136 (29), 10450–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cubillos-Ruiz A; Berta-Thompson JW; Becker JW; van der Donk WA; Chisholm SW, Evolutionary radiation of lanthipeptides in marine cyanobacteria. Proc Natl Acad Sci U S A 2017, 114 (27), E5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arias-Orozco P; Inklaar M; Lanooij J; Cebrián R; Kuipers OP, Functional expression and characterization of the highly promiscuous lanthipeptide synthetase SyncM, enabling the production of lanthipeptides with a broad range of ring topologies. ACS Synth Biol 2021, 10 (10), 2579–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIntosh JA; Robertson CR; Agarwal V; Nair SK; Bulaj GW; Schmidt EW, Circular logic: nonribosomal peptide-like macrocyclization with a ribosomal peptide catalyst. J Am Chem Soc 2010, 132 (44), 15499–15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y; Li K; Yang G; McBride JL; Bruner SD; Ding Y, A distributive peptide cyclase processes multiple microviridin core peptides within a single polypeptide substrate. Nat Commun 2018, 9 (1), 1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin GM; Ding Y, Recent advances in the biosynthesis of RiPPs from multicore-containing precursor peptides. J Ind Microbiol Biotechnol 2020, 47 (9-10), 659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czekster CM; Ludewig H; McMahon SA; Naismith JH, Characterization of a dual function macrocyclase enables design and use of efficient macrocyclization substrates. Nat Commun 2017, 8 (1), 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohanty I; Nguyen NA; Moore SG; Biggs JS; Gaul DA; Garg N; Agarwal V, Enzymatic synthesis assisted discovery of proline-rich macrocyclic peptides in marine sponges. Chembiochem 2021, 22 (16), 2614–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sardar D; Lin Z; Schmidt Eric W., Modularity of RiPP enzymes enables designed synthesis of decorated peptides. Chem Biol 2015, 22 (7), 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruffner DE; Schmidt EW; Heemstra JR, Assessing the combinatorial potential of the RiPP cyanobactin tru pathway. ACS Synth Biol 2015, 4 (4), 482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sardar D; Schmidt EW, Combinatorial biosynthesis of RiPPs: docking with marine life. Curr Opin Chem Biol 2016, 31, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le T; van der Donk WA, Mechanisms and evolution of diversity-generating RiPP biosynthesis. Trends Chem 2021, 3 (4), 266–278. [Google Scholar]
- 25.Nguyen NA; Lin Z; Mohanty I; Garg N; Schmidt EW; Agarwal V, An obligate peptidyl brominase underlies the discovery of highly distributed biosynthetic gene clusters in marine sponge microbiomes. J Am Chem Soc 2021, 143 (27), 10221–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkhart BJ; Schwalen CJ; Mann G; Naismith JH; Mitchell DA, YcaO-dependent posttranslational amide activation: biosynthesis, structure, and function. Chem Rev 2017, 117 (8), 5389–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haft DH; Basu MK; Mitchell DA, Expansion of ribosomally produced natural products: a nitrile hydratase- and Nif11-related precursor family. BMC Biol 2010, 8 (1), 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman MF; Gurgui C; Helf MJ; Morinaka BI; Uria AR; Oldham NJ; Sahl H-G; Matsunaga S; Piel J, Metagenome mining reveals polytheonamides as posttranslationally modified ribosomal peptides. Science 2012, 338 (6105), 387. [DOI] [PubMed] [Google Scholar]
- 29.Bhushan A; Egli PJ; Peters EE; Freeman MF; Piel J, Genome mining- and synthetic biology-enabled production of hypermodified peptides. Nat Chem 2019, 11 (10), 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bösch NM; Borsa M; Greczmiel U; Morinaka BI; Gugger M; Oxenius A; Vagstad AL; Piel J, Landornamides: antiviral ornithine-containing ribosomal peptides discovered through genome mining. Angew Chem Int Ed Engl 2020, 59 (29), 11763–11768. [DOI] [PubMed] [Google Scholar]
- 31.Rust M; Helfrich EJN; Freeman MF; Nanudorn P; Field CM; Rückert C; Kündig T; Page MJ; Webb VL; Kalinowski J; et al. , A multiproducer microbiome generates chemical diversity in the marine sponge Mycale hentscheli. Proc Natl Acad Sci U S A 2020, 117 (17), 9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zallot R; Oberg N; Gerlt JA, Discovery of new enzymatic functions and metabolic pathways using genomic enzymology web tools. Curr Opin Biotechnol 2021, 69, 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zallot R; Oberg N; Gerlt JA, The EFI web resource for genomic enzymology tools: leveraging protein, genome, and metagenome databases to discover novel enzymes and metabolic pathways. Biochemistry 2019, 58 (41), 4169–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamada T; Matsunaga S; Yano G; Fusetani N, Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J Am Chem Soc 2005, 127 (1), 110–118. [DOI] [PubMed] [Google Scholar]
- 35.Oshkin IY; Belova SE; Danilova OV; Miroshnikov KK; Rijpstra WIC; Sinninghe Damsté JS; Liesack W; Dedysh SN, Methylovulum psychrotolerans sp. nov., a cold-adapted methanotroph from low-temperature terrestrial environments, and emended description of the genus Methylovulum. Int J Syst Evol Microbiol 2016, 66 (6), 2417–2423. [DOI] [PubMed] [Google Scholar]
- 36.Oshkin IY; Miroshnikov KK; Belova SE; Korzhenkov AA; Toshchakov SV; Dedysh SN, Draft genome sequence of Methylovulum psychrotolerans Sph1T, an obligate methanotroph from low-temperature environments. Genome Announc 2018, 6 (11), e01488–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koehnke J; Mann G; Bent AF; Ludewig H; Shirran S; Botting C; Lebl T; Houssen WE; Jaspars M; Naismith JH, Structural analysis of leader peptide binding enables leader-free cyanobactin processing. Nat Chem Biol 2015, 11 (8), 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sardar D; Pierce E; McIntosh JA; Schmidt EW, Recognition sequences and substrate evolution in cyanobactin biosynthesis. ACS Synth Biol 2015, 4 (2), 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobeica SC; Dong S-H; Huo L; Mazo N; McLaughlin MI; Jiménez-Osés G; Nair SK; van der Donk WA, Insights into AMS/PCAT transporters from biochemical and structural characterization of a double Glycine motif protease. eLife 2019, 8, e42305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertram A; Pattenden G, Marine metabolites: metal binding and metal complexes of azole-based cyclic peptides of marine origin. Nat Prod Rep 2007, 24 (1), 18–30. [DOI] [PubMed] [Google Scholar]
- 41.Comba P; Dovalil N; Gahan LR; Haberhauer G; Hanson GR; Noble CJ; Seibold B; Vadivelu P, Cu(II) coordination chemistry of patellamide derivatives: possible biological functions of cyclic pseudopeptides. Chemistry 2012, 18 (9), 2578–90. [DOI] [PubMed] [Google Scholar]
- 42.Miller BW; Schmidt EW; Concepcion GP; Haygood MG, Halogenated metal-binding compounds from shipworm symbionts. J Nat Prod 2022, 85 (3), 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semrau JD; DiSpirito AA; Yoon S, Methanotrophs and copper. FEMS Microbiol Rev 2010, 34 (4), 496–531. [DOI] [PubMed] [Google Scholar]
- 44.Kenney GE; Rosenzweig AC, Chalkophores. Ann Rev Biochem 2018, 87 (1), 645–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt EW; Nelson JT; Rasko DA; Sudek S; Eisen JA; Haygood MG; Ravel J, Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc Natl Acad Sci U S A 2005, 102 (20), 7315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bale NJ; Rijpstra WIC; Sahonero-Canavesi DX; Oshkin IY; Belova SE; Dedysh SN; Sinninghe Damsté JS, Fatty acid and hopanoid adaption to cold in the methanotroph Methylovulum psychrotolerans. Front Microbiol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deane CD; Melby JO; Molohon KJ; Susarrey AR; Mitchell DA, Engineering unnatural variants of plantazolicin through codon reprogramming. ACS Chem Biol 2013, 8 (9), 1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deane CD; Burkhart BJ; Blair PM; Tietz JI; Lin A; Mitchell DA, In vitro biosynthesis and substrate tolerance of the plantazolicin family of natural products. ACS Chem Biol 2016, 11 (8), 2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunbar KL; Melby JO; Mitchell DA, YcaO domains use ATP to activate amide backbones during peptide cyclodehydrations. Nat Chem Biol 2012, 8 (6), 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koehnke J; Bent AF; Zollman D; Smith K; Houssen WE; Zhu X; Mann G; Lebl T; Scharff R; Shirran S; et al. , The cyanobactin heterocyclase enzyme: a processive adenylase that operates with a defined order of reaction. Angew Chem Int Ed Engl 2013, 52 (52), 13991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge Y; Czekster CM; Miller OK; Botting CH; Schwarz-Linek U; Naismith JH, Insights into the mechanism of the cyanobactin heterocyclase enzyme. Biochemistry 2019, 58 (16), 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shinbara K; Liu W; van Neer RHP; Katoh T; Suga H, Methodologies for backbone macrocyclic peptide synthesis compatible with screening technologies. Front Chem 2020, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal V; Pierce E; McIntosh J; Schmidt Eric W.; Nair Satish K., Structures of cyanobactin maturation enzymes define a family of transamidating proteases. Chem Biol 2012, 19 (11), 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koehnke J; Bent A; Houssen WE; Zollman D; Morawitz F; Shirran S; Vendome J; Nneoyiegbe AF; Trembleau L; Botting CH; Smith MCM; Jaspars M; Naismith JH, The mechanism of patellamide macrocyclization revealed by the characterization of the PatG macrocyclase domain. Nat Struct Mol Biol 2012, 19 (8), 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kenney Grace E; Dassama Laura MK; Pandelia M-E; Gizzi Anthony S; Martinie Ryan J; Gao P; DeHart Caroline J; Schachner Luis F; Skinner Owen S; Ro Soo Y; et al. , The biosynthesis of methanobactin. Science 2018, 359 (6382), 1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarkar S; Gu W; Schmidt EW, Expanding the Chemical Space of Synthetic Cyclic Peptides Using a Promiscuous Macrocyclase from Prenylagaramide Biosynthesis. ACS Catal 2020, 10 (13), 7146–7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.