Background:
Little is known about environmental factors that may increase the risk of prostate cancer. We estimated associations between incident prostate cancer and environmental concentrations of five ambient volatile organic compounds (VOCs): benzene; n-decane; ethylbenzene; hexane; and 1,2,4-trimethylbenzene.
Methods:
This study is based on a population-based case-control study of incident prostate cancer (PROtEuS) in men ≤ 75 years of age living in Montreal, Canada, in 2005 to 2012. We included 1172 cases and 1177 population controls. We had personal information, lifetime residential addresses, occupational exposures, and a variety of area-wide covariables. We inferred concentrations of the five VOCs using Bayesian geostatistical models using data from a dense environmental survey conducted in Montreal in 2005 to 2006. We used different sets of adjustments to estimate odds ratios (OR) and confidence intervals.
Results:
We found nonlinear associations such that the ORs increased monotonically and then either flattened or fell off with increased exposures. The model that contained other environmental variables and contextual variables led to lower ORs and results were similar when we restricted analyses to controls recently screened or tested for prostate cancer or cases with low- or high-grade tumors. A change from the 5th to 25th percentile in mean environmental benzene levels led to an adjusted OR of 2.00 (95% confidence interval = 1.47, 2.71).
Conclusion:
We found positive associations between prostate cancer and concentrations of benzene and ethylbenzene, independently of previous testing for prostate cancer or tumor grade, suggesting that exposure to certain ambient VOCs may increase incidence.
Keywords: Prostate cancer; Volatile organic compounds; Benzene; n-Decane; Ethylbenzene; Hexane; 1,2,4-Trimethylbenzene; Case-control study; Population-based
What this study adds
This is the first study of the incidence of prostate and ambient exposures to five selected volatile organic compounds. We found nonlinear associations such that the odds ratios increased monotonically and then either flattened or fell off with increased exposures. For benzene, we observed a two-fold excess in the odds of developing prostate cancer for being exposed environmentally as compared with nonexposed men. These results suggest that some of these compounds, some of which are known carcinogens, like benzene, may increase risk even at low ambient concentrations.
Introduction
Causal risk factors for prostate cancer include age, first-degree family history and Sub-Saharan ancestry,1 and some genetic factors explain about 30% of the familial risk.2 Other possible modifiable factors include obesity, physical inactivity, alcohol, diet exposure to certain pesticides,3 monocyclic aromatic hydrocarbons, polycyclic aromatic hydrocarbons, chromium, and pesticides and possibly occupations that involve night work and firefighting.1,4–12
There is little information as to whether air pollution is associated with the risk of developing prostate cancer. In the present study population, we have found positive associations with ambient NO2 and ultrafine particles.13,14 In other studies,13–20 the role of specific ambient air pollutants in the etiology of prostate cancer has been investigated, but none included exposure to volatile organic compounds (VOCs).
Some VOCs cause cancer, such as benzene,21 and plausible mechanisms include endocrine disruption and direct action through oxidation of the metabolites of catechols of estrogens or benzene to quinones, which can cause DNA adducts and may through errors in DNA repair initiate mutations and hence malignancies.8 Many VOCs occur naturally in petroleum and crude oil products. Benzene derives from industry but also is a component of exhaust fumes of combustion engine vehicles. N-decane is often used in organic chemical synthesis, as it is an organic solvent. Ethylbenzene is emitted from burning oil, gas, and coal and from industrial applications, such as in the manufacture of styrene. Hexane is used in several industries that manufacture or use paints, adhesives, and solvents.22,23 1,2,4-trimethylbenzene (TMB) is a gasoline additive and used as a solvent, as a paint and lacquer thinner, in making dyes and in producing prescription drugs.
The objective of the present analyses was to estimate, in men under the age of 76 years living in Montreal, associations between developing prostate cancer and ambient concentrations of five VOCs (benzene, n-decane, ethylbenzene, hexane, 1,2,4-trimethylbenzene).
Methods
Study population
We conducted a population-based case-control study in Montreal that was designed to investigate the role of occupational and environmental exposures in prostate cancer (“Prostate Cancer and Environment Study,” PROtEuS).8,9,11–14,24,25 We enrolled newly diagnosed, histologically confirmed cases of prostate cancer treated in French-language Montreal hospitals in 2005 to 2009, under 76 years of age at time of diagnosis or recruitment, residing in the Montreal area, and registered on Quebec’s permanent electoral list. These hospitals covered over 80% of all prostate cancer cases diagnosed in the area.
Controls were randomly selected from the electoral list of French-speaking men, they were frequency-matched to cases on age (5-year caliper), and they had to fulfill the same criteria as cases. Controls were recruited concurrently with cases, although some controls were selected up to 2012 to increase numbers. The present study was approved by the ethics committees of all participating institutions and all participants provided written informed consent.
For the present analyses, we had estimates of VOCs only on the Island of Montreal and consequently we excluded participants living off-island (about 59% of the total study population). Response rates for the entire study were 79% for cases and 56% for controls.
Fieldwork and data collection
Using the same set of questionnaires for cases and controls, trained research assistants administered face-to-face interviews. We inquired about sociodemographic and lifetime anthropometric characteristics, medical history including previous testing for prostate cancer, diabetes, family history of cancer, physical activity at home, work and leisure, smoking, alcohol consumption, and dietary habits. Hip and waist circumferences were measured by the interviewer. The degree of aggressiveness of the tumor, defined by the Gleason score, was abstracted from pathology reports. We had their full addresses at time of diagnosis or interview and 10 years before those dates, and these were geocoded.
We included ambient NO2, ultrafine particles, and greenness because we found in the present study positive associations with the two air pollutants13,14 and a negative association with residential greenness.24
Estimating ambient concentrations of NO2 and VOCs
We conducted three dense monitoring campaigns of NO2 in Montreal in 2005 and 2006.26 Briefly, we placed over 130 samplers (December 2005, April 2006, August 2006) in areas likely to have high spatial variability of traffic-related pollution and in areas with high population density. We collocated Ogawa samplers that measured concentrations of NO2 with passive 3M 3500 Organic Vapour Monitors (3M Company, Saint Paul, MN) that measured selected VOCs. After a 2-week uninterrupted sampling period, a commercial laboratory in Mississauga, ON,27 extracted the samples with carbon disulfide and quantified the samples using gas chromatography and mass spectrometer detector (GC-MSD) using NIOSH methods 1003, 1500, and 1501 with a detection limit of 0.01 to 0.02 µg/m3 (Supplemental Table 1; http://links.lww.com/EE/A206). We corrected concentrations with three field blanks per survey. The multipoint calibration curve had an R2 > 0.999 and we have shown that co-located samplers were consistent with each other.28
We used a combination of land-use regression and geostatistical methods28 to estimate the spatial distribution for each of the five VOCs across the monitoring campaigns. We used common land-use predictors (e.g., buildings, open areas)26,29,30 and included average and total NOx and total daily traffic volume31 estimated from emission modeling system.32 Population density for 2016 was based on Canadian census data.33 Easting and northing coordinates were also included in the models. We selected variables and buffer sizes for each VOC in each campaign by using least absolute shrinkage and selection operator (LASSO).34
We fitted four regression models for each VOC, and we used Bayesian models to predict the concentrations of each VOC at all residential addresses. We modeled the natural logarithmic concentrations of each VOC at each location and campaign. The base model included an indicator variable to represent the campaign, an intercept, the land-use predictors, and the coordinates. In some of the models, we also included a latent spatial structure to accommodate for residual spatial structure after accounting for the land-use variables. We used the minimum value of the Watanabe-Akaike Information Criterion to select the final model.
We used Markov Chain Monte Carlo methods35 to obtain samples from the posterior distribution and computed the mean value at each location. After computing the posterior distribution for each campaign, we then computed mean concentrations across campaigns and these estimates were used in the present analyses.
Estimating ambient concentrations of ultrafine particles
We made use of estimated ambient concentrations of ultrafine particles from a land-use regression model using data from a mobile monitoring campaign conducted between 2011 and 2012.36
Estimating greenness
We used Landsat-5 data to estimate the daily normalized difference vegetation index (NDVI). NDVI is defined as the ratio of the difference between near-infrared (NIR) and visible radiation (R) to their sum, hence is calculated as (NIR − R)/(NIR + R) and expresses the ratio of reflected radiation to incoming radiation.37 The values of NDVI range from −1 to +1, with negative values indicating absence of vegetation.
Estimating occupational exposures
During the in-depth interview, we used methods developed originally by Gérin, Siemiatycki and colleagues38 to obtain detailed information on jobs (see Supplement; http://links.lww.com/EE/A206 for details). We used a semistructured questionnaire that elicited details regarding participants’ work histories that included all jobs held during their lifetime and an expert team translated these job histories to semiquantitative indices of occupational exposure.38–41 These methods of attributing exposure from detailed job histories using experts has been shown to be reliable.42–45
Assessment of trends in volatile organic compounds between 1995 and 2006
The Canadian National Air Pollution Surveillance Program (NAPS), operated by Environment and Climate Change Canada,46 has four monitoring sites in Montreal that use SUMMA canisters on a 6-day schedule to measure VOCs. Samples were analyzed using gas chromatography/flame ionization for C2 hydrocarbons and a combined gas chromatography/mass selective detector system for C3 to C12 hydrocarbons and chlorinated hydrocarbons.47 We abstracted from the public access files concentrations for hexane, ethylbenzene, benzene, and TMB (n-decane is not measured by NAPS) and plotted these by time.
Statistical analyses
We used unconditional logistic regression to estimate odds ratios (ORs) and associated 95% confidence intervals (CIs) of developing prostate cancer in relation to exposure to the five VOCs estimated at participants’ residences at diagnosis or interview as well as at their address 10 years prior to interview. The principal analyses are based on the addresses at time of diagnosis or interview.
We determined the shape of the response function for each continuous covariable from age-adjusted logistic models. We assessed the functional form for each continuous covariable using natural cubic spline functions of 3 and 6 degrees of freedom (df) and selected the df that provided the most plausible response curve.
A hypothetical and simplified directed acyclic graph (DAG) was used to assist in defining personal, environmental, and occupational variables that should be included in the regression models (Supplemental Figure 1; http://links.lww.com/EE/A206). This DAG comprised confirmed and potential risk factors for prostate cancer as well as environmental and area-wide variables that may lead to open backdoor paths. To account for the frequency-matching by age, all models contained age, modeled as a natural cubic spline function on 3 df.
We developed a consecutive series of models that show the effects of different sets of potential confounding variables (Supplemental Table 2; http://links.lww.com/EE/A206). Including covariables not associated with exposure may decrease statistical precision48; thus, in some models we excluded covariables that were not associated with each VOC such that the covariable did not change the age-adjusted OR of the VOC by 5% or more (hereafter referred to as the “5% rule”).
We included first-degree family history, family income, marital status, smoking and alcohol consumption, diet, history of diabetes, NDVI, ambient concentrations of NO2, and ultrafine particles. We also included several area-wide (contextual) variables that provide measures of socioeconomic status in the neighborhood of the participant, using a buffer of 1 km around the centroid of the postal code area; that is, percent of the population with low education, median household income, percent with low income, percent of the population who are recent immigrants, and unemployment rate. We also included duration of probable or definite occupational exposures, lagged by 5 years, for benzene, toluene, xylenes, and styrene combined as well as chromium, pesticides, polycyclic aromatic hydrocarbons from petroleum and from other sources, diesel fumes, and unleaded gasoline fumes.
The final model included all VOCs even though including different components of the mixture, including other air pollutants, could lead to overadjustment from collider bias.49–51
We started first with the personal variables and then added in area-level variables, the two air pollutants, and finally, the selected occupational exposures, and then all the VOCs together. We show results for each VOC and we present graphs of marginal effects.52,53 We computed ORs and 95% CIs from the fitted functions for changes from the 5th to the 25th percentile, the 25th to the 50th percentile, the 50th to the 75th percentile, and the 75th to the 95th percentile using a method that we developed previously for natural cubic spline functions.54
We also conducted separate analyses for high-grade (Gleason scores >7 or [4 + 3]) and low-grade (Gleason scores <7 or [3 + 4]) tumors, retaining the full set of controls; as well, retaining all cases, we included only controls (74%) who were screened or tested for prostate cancer within the last 2 years before interview, thereby reducing the likelihood of latent, undiagnosed cancers.
Results
Characteristics of the study population
The total number of study participants was 3,987. As only participants living on the Island of Montreal were included in the present analyses, this left 1172 cases and 1177 controls (59% of the total study population). Tables 1 and 2 show the distribution of potential confounding variables. Cases were slightly younger than controls and had similar patterns of body mass index, cumulative lifetime consumption of cigarettes and alcohol, fruit and vegetable consumption, and annual family income. Almost all cases were recently screened or tested for prostate cancer, were more likely than controls to have a family history of prostate cancer, to be of Sub-Saharan ancestry, to have a lower prevalence of diabetes, and had greater physical activity levels. Cases also tended to have lower odds than controls if they completed college or university.
Table 1.
Distributions of selected continuous risk factors for incident prostate cancer and associated age-adjusted ORs and 95% CIs for an interquartile range increase, Montreal, Canada, 2005 to 2012.a
| Variable | Mean | Standard deviation | Minimum | Interquartile range | Maximum | Age-adjusted OR for an increase of the IQR | 95% CI |
|---|---|---|---|---|---|---|---|
| Ageb | 64.25 | 6.89 | 40 | 11 | 77 | 0.87 | 0.70, 1.08 |
| Maximum body mass index (kg/m2) | 28.49 | 4.72 | 12.14 | 5.61 | 68.61 | 0.92 | 0.84, 1.02 |
| Cigarette pack-years, lifetime | 23.78 | 28.52 | 0.00 | 39.50 | 225.00 | 0.97 | 0.86, 1.08 |
| Alcoholic drink-years, lifetime | 80.51 | 144.26 | 0.00 | 87.39 | 2,656.00 | 1.02 | 0.97, 1.07 |
| Area-wide variables from the census (1000 m buffer around centroid of postal code) | |||||||
| % did not complete high schoolb | 22.48 | 7.88 | 4.40 | 11.93 | 44.31 | 1.36 | 1.20, 1.54 |
| Median household income ($) | 58,211 | 15,627 | 37,153 | 12,714 | 161,047 | 0.81 | 0.76, 0.87 |
| % Low incomeb | 28.37 | 9.47 | 3.88 | 12.84 | 53.70 | 1.32 | 1.18, 1.48 |
| % Recent immigrantsb | 7.03 | 4.39 | 0.49 | 3.94 | 28.56 | 1.01 | 0.90, 1.13 |
| Unemployment rateb | 7.96 | 2.47 | 2.48 | 3.66 | 16.13 | 2.15 | 1.41, 3.30 |
| NDVI | 0.29 | 0.07 | 0.08 | 0.09 | 0.57 | 0.74 | 0.67, 0.83 |
| NO2 (ppb) | 12.15 | 2.76 | 5.59 | 4.14 | 23.16 | 1.20 | 1.06, 1.36 |
| UFP (cm−3) | 24,194 | 5,217 | 10,288 | 4,104 | 91,056 | 1.01 | 0.95, 1.08 |
| Duration of occupational exposuresb (years)c | |||||||
| Benzened | 2.15 | 7.26 | 0 | 0 | 51 | 0.99e | 0.94, 1.04 |
| MAH, not from environmental tobacco smoke | 6.36 | 12.11 | 0 | 7 | 58 | 0.91 | 0.68, 1.22 |
| Chromiumd | 1.89 | 7.39 | 0 | 0 | 58 | 0.99 | 0.95, 1.03 |
| Pesticidesd | 0.69 | 3.92 | 0 | 0 | 44 | 0.99 | 0.93, 1.05 |
| PAHs, from any sources, no ETS | 6.88 | 12.47 | 0 | 8 | 51 | 0.89 | 0.61, 1.29 |
| PAHs, from other sourcesd | 1.12 | 5.62 | 0 | 0 | 49 | 0.99 | 0.94, 1.04 |
| PAHs, from petroleum | 5.97 | 11.78 | 0 | 5 | 51 | 0.94 | 0.74, 1.19 |
| Diesel, any | 6.07 | 11.79 | 0 | 5 | 51 | 0.91 | 0.72, 1.15 |
| Unleaded gasoline | 7.08 | 11.75 | 0 | 12 | 51 | 1.21 | 0.97, 1.51 |
aFrom 1172 cases and 1177 controls.
bExposure estimated by expert assessments (see Fieldwork and data collection section of the Methods).
cModeled as natural cubic spline functions and the ORs are for an increase in the interquartile range.
dModeled as natural cubic spline functions and the ORs are for an increase of unity.
eOur estimate for ever exposed occupationally to benzene (1.30; 95% CI = 1.02, 1.68) was similar to that found in our previous article (OR = 1.24).
ETS indicates environmental tobacco smoke; MAH, monocyclic aromatic hydrocarbons; NDVI, normalized difference vegetation index; PAH, polycyclic aromatic hydrocarbons; UFP, ultrafine particles.
Table 2.
Distribution of selected categorical risk factors for incident prostate cancer and associated age-adjusted ORs and 95% CIs, Montreal, Canada, 2005 to 2012.
| Cases (N = 1,172) | Controls (N = 1,177) | |||||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Age-adjusted OR | 95% CI | |
| Annual family income | ||||||
| <$20,000 | 165 | 14.08 | 174 | 14.78 | 1 | |
| $20,000 to $29,999 | 177 | 15.10 | 164 | 13.93 | 1.16 | 0.86, 1.57 |
| $30,000 to $49,999 | 268 | 22.87 | 276 | 23.45 | 1.00 | 0.76, 1.32 |
| $50,000 to $79,999 | 224 | 19.11 | 220 | 18.69 | 1.03 | 0.78, 1.37 |
| $80,000 and more | 240 | 20.48 | 239 | 20.31 | 0.95 | 0.72, 1.27 |
| Other (prefer not to respond, do not know) | 98 | 8.36 | 104 | 8.84 | 1.00 | 0.71, 1.42 |
| Ancestry | ||||||
| European | 999 | 85.24 | 971 | 82.50 | 1 | |
| Black | 96 | 8.19 | 63 | 5.35 | 1.44 | 1.03, 2.00 |
| Asian | 13 | 1.11 | 43 | 3.65 | 0.29 | 0.16, 0.55 |
| Other | 54 | 4.61 | 91 | 7.73 | 0.56 | 0.40, 0.80 |
| Do not know | 10 | 0.85 | 9 | 0.76 | 1.05 | 0.42, 2.59 |
| Highest level of schooling | ||||||
| Elementary school | 284 | 24.23 | 249 | 21.16 | 1 | |
| High school | 359 | 30.63 | 327 | 27.78 | 0.90 | 0.72, 1.13 |
| College | 166 | 14.16 | 192 | 16.31 | 0.68 | 0.52, 0.90 |
| University | 361 | 30.80 | 408 | 34.66 | 0.70 | 0.56, 0.88 |
| Other (do not know or missing) | 2 | 0.17 | 1 | 0.08 | 1.96 | 0.18, 21.78 |
| First-degree relative with history of prostate cancer | ||||||
| No | 880 | 75.09 | 1,024 | 87.00 | 1 | |
| Yes | 252 | 21.50 | 117 | 9.94 | 2.51 | 1.98, 3.18 |
| Do not know | 40 | 3.41 | 36 | 3.06 | 1.26 | 0.80, 2.00 |
| Frequency of fruit and vegetable consumption | ||||||
| <6 | 291 | 24.83 | 305 | 25.91 | 1 | |
| >6 and <9 | 335 | 28.58 | 305 | 25.91 | 1.16 | 0.92, 1.45 |
| >9 and <12 | 273 | 23.29 | 292 | 24.81 | 0.97 | 0.77, 1.23 |
| > 12 | 265 | 22.61 | 272 | 23.11 | 1.01 | 0.80, 1.28 |
| Do not know or missing | 8 | 0.68 | 3 | 0.25 | 2.89 | 0.76, 11.04 |
| Diabetes | ||||||
| No | 1,003 | 85.58 | 950 | 80.71 | 1 | |
| Yes | 166 | 14.16 | 226 | 19.20 | 0.72 | 0.58, 0.90 |
| Do not know or missing | 3 | 0.26 | 1 | 0.08 | 2.83 | 0.29, 27.33 |
| Physical activity | ||||||
| Not very active | 285 | 24.32 | 354 | 30.08 | 1 | |
| Moderately active | 269 | 22.95 | 272 | 23.11 | 1.23 | 0.98, 1.55 |
| Very active | 613 | 52.30 | 550 | 46.73 | 1.43 | 1.18, 1.74 |
| Do not know or missing | 5 | 0.43 | 1 | 0.08 | 7.13 | 0.83, 61.45 |
| Timing of the last prostate cancer screening by prostate specific antigen or digital rectal examination | ||||||
| Within last 2 years | 1,160 | 98.97 | 871 | 74.00 | NA | |
| 2–5 years earlier | 1 | 0.09 | 98 | 8.33 | NA | |
| >5 years earlier | 0 | 0.00 | 47 | 3.99 | NA | |
| Never screened | 1 | 0.09 | 134 | 11.39 | NA | |
| Do not know | 10 | 0.85 | 27 | 2.29 | NA | |
| Gleason score | ||||||
| <6 (low grade) | 837 | 71.41 | NA | NA | ||
| >7 (high grade) | 334 | 28.50 | NA | NA | ||
| Missing | 1 | 0.09 | NA | NA |
In Table 1, the results for ultrafine particles do not quite reflect the previously reported risks and this is partly due to the use in the present analyses of data restricted to men living within Montreal. Cases and controls were similar with respect to most occupational exposures except benzene, where our estimate for ever exposed occupationally to benzene was 1.30 (95% CI = 1.02, 1.68), similar to that found in our previous paper (OR = 1.24).8
Table 3 shows the distributions of mean concentrations of the predicted posterior exposure distribution for the five VOCs, measured in µg/m3, according to the residence at time of diagnosis or interview. These estimates are comparable to the average concentrations from the four NAPS stations for 2005 to 2006, namely: hexane (2.5 µg/m3); ethylbenzene (1.4 µg/m3); benzene (2.3 µg/m3); and TMB (0.8 µg/m3). All VOCs but hexane were correlated with each other, with Spearman correlation coefficients varying from 0.5 to 0.8.
Table 3.
Distributions of mean ambient concentrations, in µg/m3, for the five VOCs, according to address at time of interview or diagnosis by case status, Montreal, Canada, 2005 to 2012.
| Ambient VOCs | Minimum | 25th percentile | Median | 75th percentile | Maximum | Mean | Interquartile range | Standard deviation |
|---|---|---|---|---|---|---|---|---|
| Mean | ||||||||
| Benzene | ||||||||
| Total | 0.56 | 0.92 | 1.14 | 1.33 | 2.59 | 1.15 | 0.41 | 0.29 |
| Cases | 0.56 | 0.99 | 1.20 | 1.35 | 2.59 | 1.19 | 0.36 | 0.28 |
| Controls | 0.56 | 0.86 | 1.06 | 1.30 | 2.18 | 1.11 | 0.44 | 0.30 |
| n-Decane | ||||||||
| Total | 1.14 | 1.56 | 1.74 | 1.94 | 2.88 | 1.77 | 0.38 | 0.29 |
| Cases | 1.20 | 1.59 | 1.77 | 1.96 | 2.88 | 1.80 | 0.37 | 0.28 |
| Controls | 1.14 | 1.53 | 1.71 | 1.93 | 2.86 | 1.75 | 0.40 | 0.30 |
| Ethylbenzene | ||||||||
| Total | 1.88 | 2.55 | 2.85 | 3.22 | 4.57 | 2.88 | 0.67 | 0.48 |
| Cases | 1.89 | 2.62 | 2.90 | 3.27 | 4.33 | 2.94 | 0.65 | 0.45 |
| Controls | 1.88 | 2.45 | 2.77 | 3.16 | 4.57 | 2.82 | 0.71 | 0.50 |
| Hexane | ||||||||
| Total | 5.36 | 6.88 | 7.30 | 7.90 | 13.19 | 7.49 | 1.02 | 0.94 |
| Cases | 5.89 | 6.90 | 7.29 | 7.87 | 12.91 | 7.47 | 0.97 | 0.88 |
| Controls | 5.36 | 6.85 | 7.31 | 7.94 | 13.19 | 7.51 | 1.08 | 0.99 |
| 1,2,4-trimethyl-benzene | ||||||||
| Total | 0.69 | 0.96 | 1.06 | 1.21 | 1.77 | 1.09 | 0.25 | 0.18 |
| Cases | 0.76 | 0.98 | 1.08 | 1.22 | 1.77 | 1.11 | 0.24 | 0.18 |
| Controls | 0.69 | 0.94 | 1.04 | 1.20 | 1.72 | 1.07 | 0.26 | 0.18 |
Using data from the NAPS with fixed-site monitors, we found that concentrations decreased monotonically from 1995 until 2006, with average percent changes of predicted values from a linear regression line by time as follows: benzene: 71%; ethylbenzene, 56%; hexane, 53%; and TMB, 81% (Supplement Figures 3; http://links.lww.com/EE/A206). However, these trends were not consistent across all monitoring stations with increases in concentrations in ethylbenzene at two stations and increases for TMB at one station starting around 2004.
Associations for exposure to VOCs at the address at time of diagnosis or interview
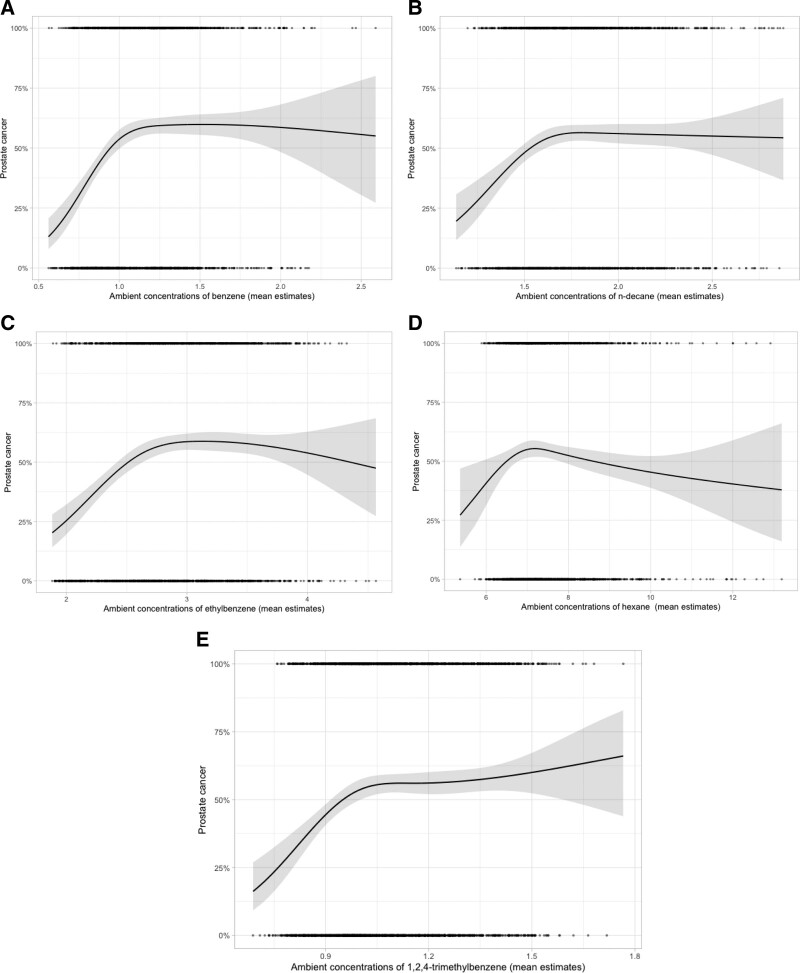
Our primary analyses were based on estimated mean concentrations of the VOCs for addresses at time of diagnosis or interview. Figure 1 show the selected marginal age-adjusted response patterns, where we found that risks increased monotonically and then either flattened or fell off with increased exposures.
Figure 1.
Marginal, age-adjusted response functions for the risk of prostate cancer for each of the five VOCs measured using mean values. The units for the exposures are in µg/m3. The solid line represents the maximum likelihood estimates using a natural cubic spline function on 3 degrees of freedom. The gray band shows the pointwise 95% confidence interval and the rug plot at the top refers to values for cases and the bottom refers to controls. A, Mean values of benzene. B, Mean values of n-decane. C, Mean values of ethylbenzene. D, Mean values of hexane. E, Mean values of 1,2,4-trimethylbenzene.
The ORs for mean estimates of the VOCs are shown in Tables 4–11 where we present estimates from the model adjusted only for age (model 1), adjusted for age and personal variables (model 2), and adjusted for all personal, environmental, and area-wide covariables, excluding covariables not associated with the VOC according to the “5% rule” (model 7). We selected these models as many of the others showed similar findings. (Supplemental Table 3; http://links.lww.com/EE/A206 show the complete results of all 10 models and Supplemental Table 4; http://links.lww.com/EE/A206 present all the models stratified by low- and high-grade tumors.) The age-adjusted analyses are presented to show the amount of confounding in the data. All the VOCs exhibited nonlinear response patterns with the incidence of prostate cancer, and we thus present ORs for changes in exposure across the 5th, 25th, 75th, and 95th percentiles.
Table 4.
Associations from selected models between incident prostate cancer and exposure to ambient benzene evaluated from address at time of interview, Montreal, Canada, 2005 to 2012.
| Model 1 (age-adjusted) | Model 2 (adjusted for personal variablesa) | Model 7—(adjusted for all personal, environmental and area-wide covariablesb excluding covariables not associated with the VOC according to the 5% rulec) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Mean Benzene | |||
| From 5% to 25% percentile (0.73 to 0.92) | 2.35 (1.82, 3.03) | 2.28 (1.76, 2.95) | 2.00 (1.47, 2.71) |
| Mean benzene—change from 25% to 75% percentile (0.92 to 1.33) | 1.60 (1.33, 1.93) | 1.65 (1.35, 2.01) | 1.42 (1.16, 1.74) |
| Mean benzene—change from 75% to 95% percentile (1.33 to 1.64) | 1.01 (0.88, 1.15) | 1.01 (0.88, 1.16) | 0.95 (0.82, 1.10) |
| Including only recently screened or testedd controls | |||
| Mean benzene—change from 5% to 25% percentile (0.73 to 0.93) | 2.45 (1.87, 3.21) | 2.29 (1.74, 3.02) | 1.93 (1.39, 2.69) |
| Mean benzene—change from 25% to 75% percentile (0.93 to 1.33) | 1.69 (1.38, 2.08) | 1.68 (1.36, 2.09) | 1.42 (1.13, 1.77) |
| Mean benzene—change from 75% to 95% percentile (1.33 to 1.64) | 1.02 (0.88, 1.18) | 1.03 (0.88, 1.20) | 0.95 (0.81, 1.13) |
| Low-gradee | |||
| Mean benzene—change from 5% to 25% percentile (0.73 to 0.91) | 2.17 (1.64, 2.87) | 2.12 (1.59, 2.82) | 2.43 (1.79, 3.31) |
| Mean benzene—change from 25% to 75% percentile (0.91 to 1.33) | 1.64 (1.34, 2.02) | 1.75 (1.41, 2.18) | 1.64 (1.32, 2.02) |
| Mean benzene—change from 75% to 95% percentile (1.33 to 1.64) | 1.02 (0.87, 1.18) | 1.01 (0.86, 1.18) | 0.95 (0.81, 1.12) |
| High-gradee | |||
| Mean benzene—change from 5% to 25% percentile (0.72 to 0.89) | 2.83 (1.82, 4.40) | 2.65 (1.69, 4.14) | 2.15 (1.28, 3.60) |
| Mean benzene—change from 25% to 75% percentile (0.89 to 1.32) | 1.78 (1.34, 2.37) | 1.69 (1.25, 2.28) | 1.55 (1.12, 2.15) |
| Mean benzene—change from 75% to 95% percentile (1.32 to 1.64) | 0.99 (0.80, 1.21) | 0.97 (0.78, 1.20) | 0.98 (0.78, 1.24) |
aThis model included age, ancestry, first-degree family history, family income, marital status, body mass index, pack-years of smoking, alcohol drink-years, frequency of fruit and vegetable consumption, and history of diabetes.
bThe environmental variables included NDVI using a 1-km buffer from the centroid of the 6-character postal code and concentrations of NO2 and ultrafine particles inferred from land-use regression models at the address of participants. The census variables included percentages not complete high school, low income, and recent immigrants, unemployment rate, and median household income evaluated for the 6-character postal code. The occupational exposures considered were monocyclic aromatic hydrocarbons, chromium, pesticides, polycyclic aromatic hydrocarbons, diesel fumes, and unleaded gasoline fumes.
cThe 5% rule refers to excluding a variable that in the age-adjusted models for each VOC did not change the estimate of effect by more than 5%. For VOCs modeled as cubic splines, we applied this rule to any of the regression coefficients on the cubic spline function.
dScreened or tested controls refer to those who were screened or tested medically for prostate cancer within the last two years before interview.
eHigh-grade defined as Gleason scores ≥7 or [4 + 3] and low-grade were Gleason scores <7 or [3 + 4] tumors, retaining the full set of controls.
Table 11.
Selected results including all five VOCs together in the model, concentrations evaluated from addresses at time of interview for incident prostate cancer, Montreal, 2005 to 2012.
| Benzene | n-Decane | Ethylbenzene | Hexane | TMB | |
|---|---|---|---|---|---|
| Model 1 – age-adjusted + all VOCs | |||||
| VOC—change from 5% to 25% percentile | 1.80 (1.29, 2.52) | 0.95 (0.71, 1.25) | 1.49 (0.95, 2.36) | 1.04 (0.85, 1.28) | 1.13 (0.81, 1.57) |
| VOC—change from 25% to 75% percentile | 1.52 (1.20, 1.93) | 0.91 (0.73, 1.15) | 1.15 (0.84, 1.57) | 0.94 (0.80, 1.11) | 0.94 (0.70, 1.26) |
| VOC—change from 75% to 95% percentile | 1.02 (0.88, 1.17) | 0.95 (0.78, 1.15) | 0.87 (0.69, 1.12) | 0.91 (0.77, 1.07) | 1.08 (0.82, 1.42) |
| Model 2a + all VOCs | |||||
| VOC—change from 5% to 25% percentile | 1.81 (1.29, 2.55) | 0.94 (0.70, 1.25) | 1.47 (0.92, 2.35) | 1.02 (0.82, 1.25) | 1.11 (0.79, 1.56) |
| VOC—change from 25% to 75% percentile | 1.56 (1.22, 2.00) | 0.92 (0.73, 1.17) | 1.12 (0.81, 1.54) | 0.96 (0.81, 1.14) | 0.96 (0.71, 1.31) |
| VOC—change from 75% to 95% percentile | 1.02 (0.88, 1.18) | 0.99 (0.81, 1.21) | 0.85 (0.66, 1.09) | 0.94 (0.80, 1.11) | 1.14 (0.86, 1.51) |
| Model 7b + all VOCs | |||||
| VOC—change from 5% to 25% percentile | 1.77 (1.25, 2.52) | 0.94 (0.70, 1.26) | 1.29 (0.79, 2.11) | 1.05 (0.85, 1.29) | 1.04 (0.74, 1.47) |
| VOC—change from 25% to 75% percentile | 1.47 (1.14, 1.91) | 0.93 (0.73, 1.20) | 1.06 (0.76, 1.48) | 0.92 (0.76, 1.10) | 0.87 (0.62, 1.21) |
| VOC—change from 75% to 95% percentile | 0.96 (0.82, 1.13) | 1.08 (0.88, 1.32) | 0.83 (0.64, 1.06) | 0.90 (0.75, 1.07) | 1.00 (0.74, 1.35) |
aAdjusted for age, ancestry, first-degree relative with history of prostate cancer, income, marital status, BMI continuous, cigarette pack-years lifetime continuous, drink-years lifetime continuous, frequency of fruit and vegetable consumption and diabetes.
bMean VOC model adjusted for age, first-degree relative with history of prostate cancer, NDVI, % not complete high school, median household income, % low income and % recent immigrants.
Model 7 generally showed lower ORs and, in some instances, this model may have included some area-wide variables that may not be true confounding variables, thereby attenuating the ORs because of overadjustments arising from spatial correlations. We demonstrate in the section on n-decane how the ORs attenuate by adding contextual covariables.
Benzene
Benzene exhibited a nonlinear pattern (Figure 1A) with the ORs increasing and then flattening at about 1 µg/m3. The flattening of the curve is not due to influential data points, as the rug plots are quite dense in that region. We did not find dramatic changes in the estimates when we added in various covariables (Table 4). For model 7, the OR for mean benzene in the lower part of the curve (5th to 25th percentile) was 2.00 (95% CI = 1.47, 2.71), and from 25th to the 75th percentile, it was 1.42 (95% CI = 1.16, 1.74), a decrease from the age-adjusted model of 15% and 11%, respectively. When we only included controls who were screened for prostate cancer 2 years before the index date and restricted to either low- or high-grade tumors, the patterns in response approximated those found among all participants.
We estimated the total OR for being exposed to ambient and occupational exposures to benzene. Extending model 7 to include exposure to occupational benzene we found no interaction between occupational and environmental exposures (results not shown). The OR for ever exposed occupationally was 1.30 (95% CI = 1.02, 1.68) and for a 10-year increase in the duration of occupational exposure the OR was 1.1 (95% CI = 0.98, 1.23). As these models are multiplicative, exposure to benzene in both environments is computed by multiplying the environmental ORs by 1.3 and 1.1, respectively, and Table 5 shows the total increase in OR for exposure to benzene in both environments.
Table 5.
Total odds ratio for ever exposed to occupational benzene and to different levels of the mean estimates of environmental benzene.a
| Categories of mean concentrations of environmental benzene | OR for environmental exposure to benzene for an increase across percentiles | Total OR for ever exposed to occupational benzene and to environmental benzene | Total OR for every 10 years of duration of exposure to occupational benzene and to environmental benzene |
|---|---|---|---|
| 5th to 25th percentile | 2.00 | 2.60 | 2.20 |
| 25th to 75th percentile | 1.45 | 1.89 | 1.60 |
| 75th to 95th percentile | 0.95 | 1.23 | 1.04 |
aFrom multiplicative models using the same covariables as in model 7 and including occupational exposures to benzene, in separate models, for ever exposed and duration of exposure.
n-Decane
Similar to the response function for benzene, the ORs increased and then flattened at about 1.6 µg/m3. For model 7 (Table 6), the OR for mean n-decane for an increase from the 5th to the 25th percentile was 1.26 (95% CI = 0.98, 1.61), and from 25th to the 75th percentile, it was 1.09 (95% CI = 0.88, 1.33), and from the 75th to the 95th percentile, it was 0.97 (95% CI = 0.82, 1.16). Results in the subgroup analyses were similar to the main ones. We did not measure occupational exposure to n-decane nor to any of the remaining VOCs.
Table 6.
Associations from selected models between incident prostate cancer and exposure to ambient n-decane evaluated from addresses at time of interview, Montreal, Canada, 2005 to 2012.
| Model 1 (age-adjusted) | Model 2 (adjusted for personal variablesa) | Model 7 – (adjusted for all personal and environmental and area-wide covariablesb excluding covariables not associated with the VOC according to the 5% rulec) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Mean n-decane | |||
| Mean n-decane—change from 5% to 25% percentile (1.37 to 1.56) | 1.72 (1.38, 2.13) | 1.65 (1.32, 2.06) | 1.26 (0.98, 1.61) |
| Mean n-decane—change from 25% to 75% percentile (1.56 to 1.94) | 1.20 (0.99, 1.46) | 1.21 (0.99, 1.48) | 1.09 (0.88, 1.33) |
| Mean n-decane—change from 75% to 95% percentile (1.94 to 2.30) | 0.97 (0.82, 1.15) | 1.01 (0.85, 1.21) | 0.97 (0.82, 1.16) |
| Including only recently screened or tested controlsd | |||
| Mean n-decane—change from 5% to 25% percentile (1.37 to 1.55) | 1.83 (1.46, 2.28) | 1.73 (1.38, 2.18) | 1.30 (1.00, 1.67) |
| Mean n-decane—change from 25% to 75% percentile (1.55 to 1.94) | 1.29 (1.05, 1.60) | 1.28 (1.03, 1.60) | 1.17 (0.94, 1.46) |
| Mean n-decane—change from 75% to 95% percentile (1.94 to 2.31) | 0.96 (0.79, 1.16) | 1.00 (0.82, 1.21) | 0.92 (0.76, 1.12) |
| Low-gradee | |||
| Mean n-decane—change from 5% to 25% percentile (1.36 to 1.55) | 1.62 (1.27, 2.07) | 1.57 (1.22, 2.02) | 1.20 (0.91, 1.59) |
| Mean n-decane—change from 25% to 75% percentile (1.55 to 1.93) | 1.15 (0.93, 1.41) | 1.15 (0.93, 1.43) | 1.04 (0.84, 1.30) |
| Mean n-decane—change from 75% to 95% percentile (1.93 to 2.30) | 0.96 (0.80, 1.17) | 1.01 (0.83, 1.23) | 0.94 (0.78, 1.14) |
| High-gradee | |||
| Mean n-decane—change from 5% to 25% percentile (1.36 to 1.55) | 2.25 (1.48, 3.42) | 2.11 (1.38, 3.25) | 1.55 (0.97, 2.48) |
| Mean n-decane—change from 25% to 75% percentile (1.55 to 1.94) | 1.43 (1.07, 1.91) | 1.41 (1.04, 1.91) | 1.30 (0.96, 1.77) |
| Mean n-decane—change from 75% to 95% percentile (1.94 to 2.29) | 0.98 (0.77, 1.24) | 1.00 (0.78, 1.28) | 0.95 (0.74, 1.22) |
Screened controls refer to those who were screened for prostate cancer within the last two years before interview. High-grade defined as Gleason scores >7 or [4 + 3] and low-grade were Gleason scores <7 or [3 + 4] tumors, retaining the full set of controls.
aThis model included age, ancestry, first-degree family history, family income, marital status, body mass index, pack-years of smoking, alcohol drink-years, frequency of fruit and vegetable consumption, and history of diabetes.
bThe environmental variables included NDVI using a 1-km buffer from the centroid of the 6-character postal code and concentrations of NO2 and ultrafine particles inferred from land-use regression models at the address of participants. The census variables included percentages not complete high school, low income, and recent immigrants, unemployment rate, and median household income evaluated for the 6-character postal code. The occupational exposures considered were monocyclic aromatic hydrocarbons, chromium, pesticides, polycyclic aromatic hydrocarbons, diesel fumes, and unleaded gasoline fumes.
cThe 5% rule refers to excluding a variable that in the age-adjusted models for each VOC did not change the estimate of effect by more than 5%. For VOCs modeled as cubic splines, we applied this rule to any of the regression coefficients on the cubic spline function.
dScreened or tested controls refer to those who were screened or tested medically for prostate cancer within the last 2 years before interview.
eHigh-grade defined as Gleason scores ≥7 or [4 + 3] and low-grade were Gleason scores <7 or [3 + 4] tumors, retaining the full set of controls.
To show possible spurious attenuation of ORs that may be due to incorrectly including contextual variables in the model, Table 7 shows that adding NDVI and three contextual variables attenuated the slope for n-decane in the part of the curve from the 5th to the 25th percentile.
Table 7.
Effect of adding different area-wide variables to model 7 for exposure to ambient n-decane evaluated from address at time of interview, Montreal, Canada, 2005 to 2012.
| Mean n-decane—change from 5% to 25% percentile (1.37 to 1.56) | Mean n-decane—change from 25% to 75% percentile (1.56 to 1.94) | Mean n-decane—change from 75% to 95% percentile (1.94 to 2.30) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| M7.1: Age only | 1.72 | 1.38, 2.13 | 1.21 | 0.99, 1.46 | 0.97 | 0.82, 1.15 |
| M7.2: M7.1 + First-degree relative with history of prostate cancer | 1.71 | 1.38, 2.13 | 1.19 | 0.98, 1.44 | 1.00 | 0.84, 1.18 |
| M7.3: M7.2 + NDVI | 1.56 | 1.24, 1.95 | 1.16 | 0.95, 1.41 | 0.97 | 0.82, 1.15 |
| M7.4: M7.3 + % not completed high school | 1.48 | 1.17, 1.86 | 1.16 | 0.95, 1.42 | 0.96 | 0.81, 1.14 |
| M7.5: M7.4 + Median household income | 1.45 | 1.15, 1.83 | 1.15 | 0.95, 1.41 | 0.97 | 0.81, 1.15 |
| M7.6: M7.5 + % Low income | 1.26 | 0.98, 1.61 | 1.09 | 0.88, 1.33 | 0.97 | 0.82, 1.16 |
Ethylbenzene
A similar pattern to that of n-decane was observed, but there was more of a tendency for decreased ORs at higher exposures rather than a flattening of the curve, although CIs were quite broad. Table 8 shows that the corresponding ORs from model 7 for the same set of percentiles of mean ethylbenzene were 1.82 (95% CI = 1.26, 2.62), 1.34 (95% CI = 1.07, 1.68), and 0.89 (95% CI = 0.73, 1.08), respectively. Results were similar when excluding unscreened controls or restricting to low- or high-grade tumors.
Table 8.
Associations from selected models between incident prostate cancer and exposure to ambient ethylbenzene evaluated from address at time of interview, Montreal, Canada, 2005 to 2012.
| Model 1 (age-adjusted) | Model 2 (adjusted for personal variablesa) | Model 7—(adjusted for all personal and environmental and area-wide covariablesb excluding covariables not associated with the VOC according to the 5% rulec) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Mean ethylbenzene | |||
| Mean ethylbenzene—change from 5% to 25% percentile (2.08 to 2.55) | 2.55 (1.91, 3.40) | 2.47 (1.84, 3.33) | 1.82 (1.26, 2.62) |
| Mean ethylbenzene—change from 25% to 75% percentile (2.55 to 3.22) | 1.35 (1.10, 1.66) | 1.37 (1.10, 1.71) | 1.34 (1.07, 1.68) |
| Mean ethylbenzene—change from 75% to 95% percentile (3.22 to 3.70) | 0.91 (0.75, 1.11) | 0.93 (0.76, 1.13) | 0.89 (0.73, 1.08) |
| Including only recently screened or tested controls4 | |||
| Mean ethylbenzene—change from 5% to 25% percentile (2.08 to 2.55) | 2.30 (1.88, 2.82) | 2.19 (1.77, 2.70) | 1.73 (1.35, 2.23) |
| Mean ethylbenzene—change from 25% to 75% percentile (2.55 to 3.22) | 1.67 (1.46, 1.90) | 1.63 (1.42, 1.87) | 1.39 (1.18, 1.65) |
| Mean ethylbenzene—change from 75% to 95% percentile (3.22 to 3.69) | 0.85 (0.71, 1.01) | 0.86 (0.72, 1.04) | 0.89 (0.74, 1.08) |
| Low-grade5 | |||
| Mean ethylbenzene—change from 5% to 25% percentile (2.07 to 2.54) | 2.04 (1.65, 2.52) | 2.08 (1.67, 2.60) | 1.86 (1.44, 2.40) |
| Mean ethylbenzene—change from 25% to 75% percentile (2.54 to 3.22) | 1.59 (1.39, 1.82) | 1.64 (1.42, 1.90) | 1.51 (1.28, 1.79) |
| Mean ethylbenzene—change from 75% to 95% percentile (3.22 to 3.70) | 0.89 (0.74, 1.07) | 0.91 (0.75, 1.10) | 0.92 (0.76, 1.10) |
| High-grade5 | |||
| Mean ethylbenzene—change from 5% to 25% percentile (2.05 to 2.50) | 2.23 (1.64, 3.02) | 2.09 (1.53, 2.87) | 1.54 (1.05, 2.28) |
| Mean ethylbenzene—change from 25% to 75% percentile (2.50 to 3.17) | 1.60 (1.31, 1.96) | 1.51 (1.22, 1.87) | 1.23 (0.94, 1.62) |
| Mean ethylbenzene—change from 75% to 95% percentile (3.17 to 3.66) | 0.76 (0.59, 1.00) | 0.76 (0.57, 1.00) | 0.80 (0.61, 1.05) |
Screened controls refer to those who were screened for prostate cancer within the last two years before interview. High-grade defined as Gleason scores >7 or [4 + 3] and low-grade were Gleason scores <7 or [3 + 4] tumors, retaining the full set of controls.
aThis model included age, ancestry, first-degree family history, family income, marital status, body mass index, pack-years of smoking, alcohol drink-years, frequency of fruit and vegetable consumption, and history of diabetes.
bThe environmental variables included NDVI using a 1-km buffer from the centroid of the 6-character postal code and concentrations of NO2 and ultrafine particles inferred from land-use regression models at the address of participants. The census variables included percentages not complete high school, low income and recent immigrants, unemployment rate, and median household income evaluated for the 6-character postal code. The occupational exposures considered were monocyclic aromatic hydrocarbons, chromium, pesticides, polycyclic aromatic hydrocarbons, diesel fumes, and unleaded gasoline fumes.
cThe 5% rule refers to excluding a variable that in the age-adjusted models for each VOC did not change the estimate of effect by more than 5%. For VOCs modeled as cubic splines, we applied this rule to any of the regression coefficients on the cubic spline function.
dScreened or tested controls refer to those who were screened or tested medically for prostate cancer within the last 2 years before interview.
eHigh-grade defined as Gleason scores ≥7 or [4 + 3] and low-grade were Gleason scores <7 or [3 + 4] tumors, retaining the full set of controls.
Hexane
Hexane exhibited a similar response function to ethylbenzene, as it increased until a value of about 7 µg/m3 and then decreased in a linear fashion. Table 9 shows that the corresponding ORs for model 7 for the same set of percentiles of mean hexane were 1.28 (95% CI = 1.06, 1.56), 0.94 (95% CI = 0.81, 1.10), and 0.82 (95% CI = 0.71, 0.95), respectively. After excluding unscreened controls and referring to the range from the 5th to the 25th percentiles, we found that the OR was attenuated (OR = 1.00 vs. 1.28). We also found attenuations for this exposure range amongst cases with low-grade tumors (OR = 0.97 vs. 1.28) but similar ORs for those with high-grade tumors.
Table 9.
Associations from selected models between incident prostate cancer and exposure to ambient hexane evaluated from addresses at time of interview, Montreal, Canada, 2005 to 2012.
| Model 1 (age-adjusted) | Model 2 (adjusted for personal variablesa) | Model 7—(adjusted for all personal and environmental and area-wide covariablesb excluding covariables not associated with the VOC according to the 5% rulec) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Mean hexane | |||
| Mean hexane—change from 5% to 25% percentile (6.38 to 6.88) | 1.28 (1.06, 1.56) | 1.24 (1.02, 1.52) | 1.28 (1.06, 1.56) |
| Mean hexane—change from 25% to 75% percentile (6.88 to 7.90) | 0.94 (0.81, 1.10) | 0.95 (0.81, 1.11) | 0.94 (0.81, 1.10) |
| Mean hexane—change from 75% to 95% percentile (7.90 to 9.19) | 0.82 (0.71, 0.95) | 0.84 (0.73, 0.98) | 0.82 (0.71, 0.95) |
| Including only recently screened or tested controlsd | |||
| Excluding unscreened controls | |||
| Mean hexane—change from 5% to 25% percentile (6.37 to 6.87) | 1.11 (0.99, 1.23) | 1.10 (0.98, 1.22) | 1.00 (0.90, 1.12) |
| Mean hexane—change from 25% to 75% percentile (6.87 to 7.90) | 1.09 (0.95, 1.25) | 1.09 (0.94, 1.25) | 0.93 (0.80, 1.07) |
| Mean hexane—change from 75% to 95% percentile (7.90 to 9.14) | 0.90 (0.78, 1.03) | 0.91 (0.79, 1.04) | 0.79 (0.69, 0.91) |
| Low-gradee | |||
| Mean hexane—change from 5% to 25% percentile (6.38 to 6.87) | 1.05 (0.95, 1.16) | 1.03 (0.93, 1.15) | 0.97 (0.87, 1.09) |
| Mean hexane—change from 25% to 75% percentile (6.87 to 7.91) | 1.04 (0.90, 1.19) | 1.02 (0.88, 1.18) | 0.91 (0.79, 1.06) |
| Mean hexane—change from 75% to 95% percentile (7.91 to 9.24) | 0.91 (0.78, 1.05) | 0.93 (0.79, 1.08) | 0.83 (0.71, 0.96) |
| High-gradee | |||
| Mean hexane—change from 5% to 25% percentile (6.35 to 6.86) | 1.39 (1.01, 1.92) | 1.32 (0.95, 1.85) | 1.28 (0.92, 1.77) |
| Mean hexane—change from 25% to 75% percentile (6.86 to 7.92) | 0.94 (0.73, 1.20) | 0.93 (0.72, 1.20) | 0.76 (0.59, 0.99) |
| Mean hexane—change from 75% to 95% percentile (7.92 to 9.20) | 0.71 (0.55, 0.92) | 0.74 (0.57, 0.95) | 0.61 (0.47, 0.80) |
Screened controls refer to those who were screened for prostate cancer within the last two years before interview. High-grade defined as Gleason scores >7 or [4 + 3] and low-grade were Gleason scores <7 or [3 + 4] tumors, retaining the full set of controls.
aThis model included age, ancestry, 1st degree family history, family income, marital status, body mass index, pack-years of smoking, alcohol drink-years, frequency of fruit and vegetable consumption, and history of diabetes.
bThe environmental variables included NDVI using a 1-km buffer from the centroid of the 6-character postal code and concentrations of NO2 and ultrafine particles inferred from land-use regression models at the address of participants. The census variables included percentages not complete high school, low income and recent immigrants, unemployment rate, and median household income evaluated for the 6-character postal code. The occupational exposures considered were monocyclic aromatic hydrocarbons, chromium, pesticides, polycyclic aromatic hydrocarbons, diesel fumes, and unleaded gasoline fumes.
cThe 5% rule refers to excluding a variable that in the age-adjusted models for each VOC did not change the estimate of effect by more than 5%. For VOCs modeled as cubic splines, we applied this rule to any of the regression coefficients on the cubic spline function.
dScreened or tested controls refer to those who were screened or tested medically for prostate cancer within the last 2 years before interview.
eHigh-grade defined as Gleason scores ≥7 or [4 + 3] and low-grade were Gleason scores <7 or [3 + 4] tumors, retaining the full set of controls.
1,2,4-Trimethylbenzene
This VOC showed a monotonically, nonlinear trend that slightly increased at higher concentrations but with very wide CIs. The largest slope was again found in the lowest exposure range and thereafter the slopes were constant (Table 10); viz., for the same set of percentiles, we found ORs for model 7 of 1.33 (95% CI = 0.98, 1.79), 1.03 (95% CI = 0.80, 1.32), and 1.03 (95% CI = 0.81, 1.32), respectively. Accounting for the wider CIs at higher exposures in the analyses of the three subgroups, we concluded that the patterns were similar to that of all participants.
Table 10.
Associations from selected models between incident prostate cancer and exposure to ambient TMB evaluated from addresses at time of interview, Montreal, Canada, 2005 to 2012.
| Model 1 (age-adjusted) | Model 2 (adjusted for personal variablesa) | Model 7—(adjusted for all personal and environmental and area-wide covariablesb excluding covariables not associated with the VOC according to the 5% rulec) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Mean TMB | |||
| Mean TMB—change from 5% to 25% percentile (0.83 to 0.96) | 1.94 (1.51, 2.49) | 1.87 (1.45, 2.41) | 1.33 (0.98, 1.79) |
| Mean TMB—change from 25% to 75% percentile (0.96 to 1.21) | 1.24 (1.00, 1.53) | 1.26 (1.01, 1.57) | 1.03 (0.80, 1.32) |
| Mean TMB—change from 75% to 95% percentile (1.21 to 1.43) | 1.11 (0.89, 1.39) | 1.15 (0.91, 1.45) | 1.03 (0.81, 1.32) |
| Including only recently screened or tested controlsd | |||
| Mean TMB—change from 5% to 25% percentile (0.83 to 0.96) | 2.12 (1.63, 2.75) | 2.03 (1.55, 2.65) | 1.35 (0.99, 1.83) |
| Mean TMB—change from 25% to 75% percentile (0.96 to 1.21) | 1.28 (1.02, 1.62) | 1.24 (0.98, 1.58) | 1.17 (0.89, 1.52) |
| Mean TMB—change from 75% to 95% percentile (1.21 to 1.43) | 1.12 (0.87, 1.44) | 1.17 (0.90, 1.52) | 1.14 (0.87, 1.48) |
| Low-gradee | |||
| Mean TMB—change from 5% to 25% percentile (0.83 to 0.96) | 1.92 (1.45, 2.53) | 1.90 (1.42, 2.53) | 1.54 (1.11, 2.12) |
| Mean TMB—change from 25% to 75% percentile (0.96 to 1.21) | 1.23 (0.98, 1.54) | 1.29 (1.01, 1.64) | 1.15 (0.86, 1.53) |
| Mean TMB—change from 75% to 95% percentile (1.21 to 1.43) | 1.20 (0.94, 1.52) | 1.23 (0.96, 1.58) | 1.19 (0.91, 1.55) |
| High-gradee | |||
| Mean TMB—change from 5% to 25% percentile (0.82 to 0.95) | 2.08 (1.34, 3.22) | 1.94 (1.24, 3.04) | 1.10 (0.67, 1.82) |
| Mean TMB—change from 25% to 75% percentile (0.95 to 1.20) | 1.32 (0.96, 1.80) | 1.26 (0.90, 1.74) | 1.01 (0.70, 1.47) |
| Mean TMB—change from 75% to 95% percentile (1.20 to 1.42) | 0.91 (0.65, 1.27) | 0.90 (0.64, 1.27) | 0.89 (0.62, 1.27) |
Screened controls refer to those who were screened for prostate cancer within the last two years before interview. High-grade defined as Gleason scores >7 or [4 + 3] and low-grade were Gleason scores <7 or [3 + 4] tumors, retaining the full set of controls.
aThis model included age, ancestry, 1st degree family history, family income, marital status, body mass index, pack-years of smoking, alcohol drink-years, frequency of fruit and vegetable consumption, and history of diabetes.
bThe environmental variables included NDVI using a 1-km buffer from the centroid of the 6-character postal code and concentrations of NO2 and ultrafine particles inferred from land-use regression models at the address of participants. The census variables included percentages not complete high school, low income and recent immigrants, unemployment rate, and median household income evaluated for the 6-character postal code. The occupational exposures considered were monocyclic aromatic hydrocarbons, chromium, pesticides, polycyclic aromatic hydrocarbons, diesel fumes, and unleaded gasoline fumes.
cThe 5% rule refers to excluding a variable that in the age-adjusted models for each VOC did not change the estimate of effect by more than 5%. For VOCs modeled as cubic splines, we applied this rule to any of the regression coefficients on the cubic spline function.
dScreened or tested controls refer to those who were screened or tested medically for prostate cancer within the last 2 years before interview.
eHigh-grade defined as Gleason scores ≥7 or [4 + 3] and low-grade were Gleason scores <7 or [3 + 4] tumors, retaining the full set of controls.
Modeling all of the volatile organic compounds together
Table 11 shows the results when including all the VOCs in the age-adjusted model, in model 2 and model 7. For models 2 and 7, we selected all covariables that appeared in any of the analyses of the individual VOCs.
The results across all three models were similar but these estimates were attenuated compared with the analyses presented above (Tables 6, 8, 10–11). In the age-adjusted models for a change from the 5th to the 25th percentiles, the ORs from the main analyses were all highly attenuated, with reductions of: benzene, 23%; n-decane, 45%; ethylbenzene, 42%; hexane, 19%; and TMB, 42%. Only benzene and ethylbenzene showed positive associations for this range of exposures whereas the response patterns for n-decane, hexane, and TMB were flat.
Modeling the volatile organic compounds using past residences
We also had address information in 1996, about 10 years before interview, available for 1625 of 2349 participants. The Spearman correlation coefficients for mean estimates between current and past addresses varied between 0.77 and 0.90. Although we had fewer participants in these analyses, we found similar results to the main findings (Supplement Table 5; http://links.lww.com/EE/A206), although the slopes for all but benzene were attenuated.
Discussion
In this large population-based case-control study, we found nonlinear associations for all five VOCs such that the ORs increased monotonically and then either flattened or fell off with increased exposures. For benzene, we estimated the total OR from occupational and environmental exposures. The model (model 7) that contained other environmental variables and contextual variables led to lower ORs, and they were attenuated when including all VOCs in the model. We did not find important differences in sub-analyses in which we only included controls recently screened or tested for prostate cancer, or those with low- or high-grade tumors. The ORs using both sets of addresses were similar.
Estimating past exposure
In some of our previous studies, we made use of backcasting methods55 that rescaled by time the spatial estimates from the land-use models developed from our dense monitoring programs, thus producing spatial-temporal estimates. These methods, which made use of the network of fixed-site monitors, would not perform adequately in the present study as there are only four sampling sites.
Our analyses of these fixed-site monitors showed secular decreases in concentrations, although increases were found in a few VOCs starting around 2004. The percent reductions in concentrations varied between 53% and 81% from 1995 to 2006 and these declines parallel those of other criteria air pollutants, such as NO2.56 Assuming no spatial variability in secular trends that would differentially affect values assigned to participants, these decreases should not change the shape of the distributions and thus should not alter the ORs computed between percentiles.
Modeling covariables
Our intent with using a complex series of models that made various assumptions about causal processes was to provide a plausible range of estimates of relative risk. We had information on multiple suspected potential confounders for prostate cancer, such as socioeconomic and lifestyle factors, and these were included in our models even though they may not be causal. In general, the shape of response curves and the estimates derived from these did not vary dramatically by model, except for the co-pollutant model of the five VOCs, and based on our current knowledge and what we measured we are confident that we captured the important covariables.
The ORs decreased when we added in contextual variables defined from the census (model 7). This model may not be correct because the addition of contextual variables may lead to overadjustment arising solely from spatial correlations rather than causal associations.49–51 Contextual covariables or random effects models are sometimes used because of concerns with spatial autocorrelation that may be induced because of similarity in adjacent areas. As participants were drawn from across the city, we would not expect a high degree of clustering. On the other hand, these contextual variables may capture indirectly unknown or unmeasured causal variables that vary spatially, but it our view that these models likely underestimate the true relative risk.
The ORs were attenuated when we modeled the VOCs together, although our conclusion for benzene did not change. We caution that these latter results may not be valid as there may be overadjustment because of the correlations between the VOCs, collider bias, unmeasured confounding, including unmeasured components of the mixture, and measurement errors.49–51 As Goldberg et al. suggested “Given the intrinsic difficulties of assessing effects with co-pollutants, we would recommend conducting sensitivity analyses adjusting for different sets of potential confounding factors,”51 which is the strategy we followed here. In sum, it is likely that inclusion of contextual variables and modeling the VOCs together likely underestimated the ORs, but we would expect the “true” ORs are somewhere in between models 2 and 7.
It is unclear why we found nonlinear patterns in the ORs, such that they decreased or remained flat after certain concentrations, other than the obvious one that more controls were exposed at higher concentrations. These may reflect true exposure-response patterns, such as due to people being exposed to complex mixtures where there maybe synergies between pollutants, and possibly from measurement error as most pollutants in the mixture were not measured.
On the other hand, it is possible that the lower response rates in the control series (56%) could be responsible for these nonlinearities. (Participation rates in our study were comparable or better to other studies entailing extensive in-person data collection.57) Differential response rates could have influenced our results if participation was associated with socioeconomic characteristics that were also associated with ambient exposure to the VOCs. However, according to census tract data, the rates for recent immigration, unemployment, low educational level, and low household income were similar in areas of participants and nonparticipants, both among cases and controls, suggesting that the potential for selection bias may be limited.13,14
Other methodological considerations
We fitted Bayesian spatial regression models using data from three dense sampling campaigns in Montreal28 in which over 130 sites were used. A strength of our spatial model was that both the spatial- and campaign-specific variability was accounted for, instead of averaging the data across campaigns, and this provided comparisons of concentrations across campaigns and VOCs. We used 3M passive monitors because they were easy to install on fixed city poles at about 3 m, they did not require electricity or pumps, and if stolen they would not be costly to replace. The passive samplers have been shown to be reliable in measuring VOCs over extended periods of time,58 and an analysis of the dosimeters from outdoor sampling with a duration of 72 hours was comparable to automated continuous gas chromatography measurements.59 In our exposure survey, we found that co-located samplers were reliable and that mean concentrations of four of the VOCs for participants were similar to that from the four fixed-site stations.
Other methods could have been used that could lead to more accurate estimates, such as Summa canisters and flame and photoionization detectors, but they are not suitable for remote sites without electricity, their operation is difficult in cold weather, they require knowledge of the proportions of concentrations of the different VOCs, and these methods are expensive.
Misclassification of exposure is inevitable, as we estimated concentrations near participants’ residences over the course of a year, we did not have complete exposure information on all previous addresses, and thus we could not estimate accurately historical exposures which would be more relevant given the expected long latency for most solid tumors. One may expect that these errors are nondifferential and should lead to an attenuation of relative risk.
A strength of the study is the large sample size and the availability of information on disease aggressiveness, as well as screening and previous medical testing for prostate cancer. The study was set in a population with free and universal access to healthcare and this population was regularly screened for prostate cancer at the time of subjects’ ascertainment, thereby reducing the potential for disease misclassification due to under-detection of prostate cancer amongst controls. Moreover, we had the ability to restrict analyses to recently screened men, which yielded results like those in the main analyses.
This is the first study of associations between exposure to these environmental VOCs and prostate cancer. The positive associations with ambient benzene are consistent with our own evaluations of occupational benzene,8 strengthening the case that exposure to benzene may increase the risk of developing prostate cancer. On the other hand, occupational exposures to benzene are associated with other solvents, notably toluene and xylenes, underscoring the difficulty of uniquely identifying an etiological agent in complex mixtures.49–51 We know of no other studies of other VOCs, so it is premature to make any causal statements.
Conclusions
Our findings provide evidence for an increased risk of prostate cancer among men exposed to five ambient VOCs.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
Supplementary Material
Footnotes
Published online 9 November 2022
The case-control study was supported financially through grants from the Canadian Cancer Society (grants no. 13149, 19500, 19864, 19865), the Cancer Research Society, the Fonds de Recherche du Québec-Santé (FRQS), the Ministère du Développement économique, de l’Innovation et de l’Exportation du Québec, and the Canadian Institutes of Health Research (grant no. 159704). M.-E.P. received career awards from the FRQS. The analyses reported herein were funded through a contract with Health Canada under the program “Addressing Air Pollution Horizontal Initiative.”
The data from the case-control study cannot be made available publicly as the consent form signed by participants did not have such a stipulation. For the ambient exposure assessments of the five volatile organic compounds, the data and the code used to fit the different models as well as the maps are available at https://github.com/SaraZM/VOCs.
All authors were involved in the design, analysis, interpretation, and preparation of the manuscript. All authors read and approved the final version of the manuscript.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Giannandrea F. Malignant tumours of the male reproductive system. In: Anttila A, Boffeta P, eds. Occupational cancers. Second ed. Springer Nature Switzerland AG, 2020;455–465. [Google Scholar]
- 2.Eeles R, Goh C, Castro E, et al. The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol. 2014;11:18–31. [DOI] [PubMed] [Google Scholar]
- 3.Alavanja MCR, Ross MK, Bonner MR. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA Cancer J Clin. 2013;63:120–142. [DOI] [PubMed] [Google Scholar]
- 4.Gerin M, Siemiatycki J, Desy M, Krewski D. Associations between several sites of cancer and occupational exposure to benzene, toluene, xylene, and styrene: results of a case-control study in Montreal. Am J Ind Med. 1998;34:144–156. [DOI] [PubMed] [Google Scholar]
- 5.Piscator M. Role of cadmium in carcinogenesis with special reference to cancer of the prostate. Environ Health Perspect. 1981;40:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva JF, Mattos IE, Luz LL, Carmo CN, Aydos RD. Exposure to pesticides and prostate cancer: systematic review of the literature. Rev Environ Health. 2016;31:311–327. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484–e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanc-Lapierre A, Sauve JF, Parent ME. Occupational exposure to benzene, toluene, xylene and styrene and risk of prostate cancer in a population-based study. Occup Environ Med. 2018;75:562–572. [DOI] [PubMed] [Google Scholar]
- 9.Demoury C, Karakiewicz P, Parent ME. Association between lifetime alcohol consumption and prostate cancer risk: A case-control study in Montreal, Canada. Cancer Epidemiol. 2016;45:11–17. [DOI] [PubMed] [Google Scholar]
- 10.Sritharan J, Pahwa M, Demers PA, Harris SA, Cole DC, Parent M-E. Prostate cancer in firefighting and police work: a systematic review and meta-analysis of epidemiologic studies. Environ Health. 2017;16:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barul C, Richard H, Parent ME. Night-shift work and risk of prostate cancer: results from a canadian case-control study, the prostate cancer and environment study. Am J Epidemiol. 2019;188:1801–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barul C, Parent ME. Occupational exposure to polycyclic aromatic hydrocarbons and risk of prostate cancer. Environ Health. 2021;20:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parent M-E, Goldberg MS, Crouse DL, et al. Traffic-related air pollution and prostate cancer risk: a case–control study in Montreal, Canada. Occup Environ Med. 2013;70:511–518. [DOI] [PubMed] [Google Scholar]
- 14.Weichenthal S, Lavigne E, Valois MF, et al. Spatial variations in ambient ultrafine particle concentrations and the risk of incident prostate cancer: a case-control study. Environ Res. 2017;156:374–380. [DOI] [PubMed] [Google Scholar]
- 15.Winkelstein W, Jr., Kantor S. Prostatic cancer: relationship to suspended particulate air pollution. Am J Publ Health Nation’s Health. 1969;59:1134–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raaschou-Nielsen O, Andersen ZJ, Hvidberg M, et al. Air pollution from traffic and cancer incidence: a Danish cohort study. Environ Health. 2011;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datzmann T, Markevych I, Trautmann F, et al. Outdoor air pollution, green space, and cancer incidence in Saxony: a semi-individual cohort study. BMC Public Health. 2018;18:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman NC, Burnett RT, Ezzati M, et al. Fine particulate matter exposure and cancer incidence: analysis of SEER cancer registry data from 1992-2016. Environ Health Perspect. 2020;128:107004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayakumar V, Abern MR, Jagai JS, Kajdacsy-Balla A. Observational study of the association between air cadmium exposure and prostate cancer aggressiveness at diagnosis among a nationwide retrospective cohort of 230,540 patients in the United States. Int J Environ Res Public Health. 2021;18:8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin M, Kim OJ, Yang S, Choe SA, Kim SY. Different mortality risks of long-term exposure to particulate matter across different cancer sites. Int J Environ Res Public Health. 2022;19:3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer. Benzene/IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 120. International Agency for Research on Cancer; 2017. [Google Scholar]
- 22.U.S. Environmental Protection Agency. Integrated risk information system. 2022. Available at: https://www.epa.gov/iris. Accessed August 2, 2022.
- 23.Agency for Toxic Substances and Disease Registry. Toxicological profiles. 2022. Available at: https://www.atsdr.cdc.gov/toxprofiledocs/. Accessed August 2, 2022. [DOI] [PubMed]
- 24.Demoury C, Thierry B, Richard H, et al. Residential greenness and risk of prostate cancer: A case-control study in Montreal, Canada. Environ Int. 2016;98:129–136. [DOI] [PubMed] [Google Scholar]
- 25.Sauve JF, Lavoue J, Parent ME. Occupation, industry, and the risk of prostate cancer: a case-control study in Montreal, Canada. Environ Health. 2016;15:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crouse DL, Goldberg MS, Ross NA. A prediction-based approach to modelling temporal and spatial variability of traffic-related air pollution in Montreal, Canada. Atmos Environ. 2009;43:5075–5084. [Google Scholar]
- 27.Airzone. Airzone. Available at: https://www.airzoneone.com/lab-analysis/. Accessed August 18, 2021.
- 28.Zapata-Marin S, Schmidt AM, Crouse D, et al. Spatial modeling of ambient concentrations of volatile organic compounds in Montreal, Canada. Environ Epidemiol. 2022;6:e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie X, Semanjski I, Gautama S, et al. A review of urban air pollution monitoring and exposure assessment methods. ISPRS Int J Geo-Inf. 2017;6:389. [Google Scholar]
- 30.Ramos Y, Requia WJ, St-Onge B, Blanchet J-P, Kestens Y, Smargiassi A. Spatial modeling of daily concentrations of ground-level ozone in Montreal, Canada: a comparison of geostatistical approaches. Environ Res. 2018;166:487–496. [DOI] [PubMed] [Google Scholar]
- 31.PTV Vissim. Traffic simulation software. Available at: https://www.ptvgroup.com/en/solutions/products/ptv-vissim/. Accessed August 19, 2021.
- 32.United States Environmental Protection Agency. MOVES and other mobile source emissions models. Available at: http://www.epa.gov/moves. Accessed August 19, 2021.
- 33.Statistics Canada. Census profile, 2016 census. Available at: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=CMACA&Code1=462&Geo2=PR&Code2=01&Data=Count&SearchText=Montreal&SearchType=Begins&SearchPR=01&TABID=1&B1=All. Accessed July 30, 2021.
- 34.James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning: With Applications in R. Springer, 2013. [Google Scholar]
- 35.Peters G. Markov Chain Monte Carlo: stochastic simulation for Bayesian inference (2nd edn). Dani Gamerman and Hedibert F. Lopes, Chapman & Hall/CRC, Boca Raton, FL, 2006. No. of pages: xvii +323. Price: $69.95. ISBN10: 1-58488-587-4, ISBN13: 978-1-58488-587-0. Stat Med. 2008;27:3213–3214. [Google Scholar]
- 36.Weichenthal S, Ryswyk KV, Goldstein A, Bagg S, Shekkarizfard M, Hatzopoulou M. A land use regression model for ambient ultrafine particles in Montreal, Canada: a comparison of linear regression and a machine learning approach. Environ Res. 2016;146:65–72. [DOI] [PubMed] [Google Scholar]
- 37.Pettorelli N. The Normalized Difference Vegetation Index. Oxford University Press; 2013. [Google Scholar]
- 38.Gerin M, Siemiatycki J, Kemper H, Begin D. Obtaining occupational exposure histories in epidemiologic case-control studies. J Occup Med. 1985;27:420–426. [PubMed] [Google Scholar]
- 39.Gerin M, Siemiatycki JS, Laroche LM. Translating job histories into histories of occupational exposure for epidemiological purposes. Job Exposure Matrices, Proceedings of a Conference. 1983:78-82-78-82. [Google Scholar]
- 40.Stewart PA, Stewart WF, Siemiatycki J, Heineman EF, Dosemeci M. Questionnaires for collecting detailed occupational information for community-based case control studies. Am Ind Hyg Assoc J. 1998;59:39–44. [DOI] [PubMed] [Google Scholar]
- 41.Sauve JF, Lavoue J, Nadon L, et al. A hybrid expert approach for retrospective assessment of occupational exposures in a population-based case-control study of cancer. Environ Health. 2019;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg MS, Siemiatycki J, Gerin M. Inter-rater agreement in assessing occupational exposure in a case-control study. Br J Ind Med. 1986;43:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fritschi L, Nadon L, Benke G, et al. Validation of expert assessment of occupational exposures. Am J Ind Med. 2003;43:519–522. [DOI] [PubMed] [Google Scholar]
- 44.Florath I, Glass DC, Rhazi MS, Parent ME, Fritschi L. Inter-rater agreement between exposure assessment using automatic algorithms and using experts. Ann Work Expo Health. 2019;63:45–53. [DOI] [PubMed] [Google Scholar]
- 45.Batisse E, Labrèche F, Goldberg MS, et al. Inter-rater reliability of occupational exposure assessment in a case-control study of female breast cancer. J Occup Environ Hyg. 2021;18:522–531. [DOI] [PubMed] [Google Scholar]
- 46.Government of Canada. The Canadian National Air Pollution Surveillance (NAPS) program. Available at: https://www.canada.ca/en/environment-climate-change/services/air-pollution/monitoring-networks-data/national-air-pollution-program.html. Accessed 1, October 2022.
- 47.Canadian Council of Ministers of the Environment. Ambient Air Monitoring and Quality Assurance/Quality Control Guidelines. National Air Pollution Surveillance Program. Canadian Council of Ministers of the Environment; 2019. [Google Scholar]
- 48.Day NE, Byar DP, Green SB. Overadjustment in case-control studies. Am J Epidemiol. 1980;112:696–706. [DOI] [PubMed] [Google Scholar]
- 49.Weisskopf MG, Webster TF. Trade-offs of personal vs. more proxy exposure measures in environmental epidemiology. Epidemiology. 2017;28:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisskopf MG, Seals RM, Webster TF. Bias amplification in epidemiologic analysis of exposure to mixtures. Environ Health Perspect. 2018;126:047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldberg MS, Baumgartner J, Chevrier J. Statistical adjustments of environmental pollutants arising from multiple sources in epidemiologic studies: the role of markers of complex mixtures. Atmos Environ. 2022;270:118788. [Google Scholar]
- 52.Lüdecke D. Tidy data frames of marginal effects from regression models. J Open Source Softw. 2018;3:772. [Google Scholar]
- 53.Lüdecke D. sjPlot: Data Visualization for Statistics in Social Science. R package version 2.8.8. Comprehensive R Archive Network; 2021. [Google Scholar]
- 54.Cao J, Valois MF, Goldberg MS. An S-Plus function to calculate relative risks and adjusted means for regression models using natural splines. Comput Methods Programs Biomed. 2006;84:58–62. [DOI] [PubMed] [Google Scholar]
- 55.Chen H, Goldberg MS, Crouse DL, et al. Back-extrapolation of estimates of exposure from current land-use regression models. Atmos Environ. 2010;44:4346–4354. [Google Scholar]
- 56.Canadian Council of Ministers of the Environment. Canada’s air. Available at: https://ccme.ca/en/air-quality-report. Accessed August 12, 2022.
- 57.Xu M, Richardson L, Campbell S, Pintos J, Siemiatycki J. Response rates in case-control studies of cancer by era of fieldwork and by characteristics of study design. Ann Epidemiol. 2018;28:385–391. [DOI] [PubMed] [Google Scholar]
- 58.Shields HC, Weschler CJ. Analysis of ambient concentrations of organic vapors with a passive sampler. JAPCA. 1987;37:1039–1045. [Google Scholar]
- 59.Stock TH, Morandi MT, Afshar M, Chung KC. Evaluation of the use of diffusive air samplers for determining temporal and spatial variation of volatile organic compounds in the ambient air of urban communities. J Air Waste Management Assoc. 2008;58:1303–1310. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.