PURPOSE
Postconsolidation immunotherapy including dinutuximab, granulocyte-macrophage colony-stimulating factor, and interleukin-2 improved outcomes for patients with high-risk neuroblastoma enrolled on the randomized portion of Children's Oncology Group study ANBL0032. After random assignment ended, all patients were assigned to immunotherapy. Survival and toxicities were assessed.
PATIENTS AND METHODS
Patients with a pre-autologous stem cell transplant (ASCT) response (excluding bone marrow) of partial response or better were eligible. Demographics, stage, tumor biology, pre-ASCT response, and adverse events were summarized using descriptive statistics. Event-free survival (EFS) and overall survival (OS) from time of enrollment (up to day +200 from last ASCT) were evaluated.
RESULTS
From 2009 to 2015, 1,183 patients were treated. Five-year EFS and OS for the entire cohort were 61.1 ± 1.9% and 71.9 ± 1.7%, respectively. For patients ≥ 18 months old at diagnosis with International Neuroblastoma Staging System stage 4 disease (n = 662) 5-year EFS and OS were 57.0 ± 2.4% and 70.9 ± 2.2%, respectively. EFS was superior for patients with complete response/very good partial response pre-ASCT compared with those with PR (5-year EFS: 64.2 ± 2.2% v 55.4 ± 3.2%, P = .0133); however, OS was not significantly different. Allergic reactions, capillary leak, fever, and hypotension were more frequent during interleukin-2–containing cycles than granulocyte-macrophage colony-stimulating factor–containing cycles (P < .0001). EFS was superior in patients with higher peak dinutuximab levels during cycle 1 (P = .034) and those with a high affinity FCGR3A genotype (P = .0418). Human antichimeric antibody status did not correlate with survival.
CONCLUSION
Analysis of a cohort assigned to immunotherapy after cessation of random assignment on ANBL0032 confirmed previously described survival and toxicity outcomes. EFS was highest among patients with end-induction complete response/very good partial response. Among patients with available data, higher dinutuximab levels and FCGR3A genotype were associated with superior EFS. These may be predictive biomarkers for dinutuximab therapy.
INTRODUCTION
Despite intensive therapy, outcomes for patients with high-risk neuroblastoma remain poor.1,2 Randomized trials have shown that high-dose chemotherapy with autologous stem cell transplant (ASCT) results in improved event-free survival (EFS).3,4 To eliminate minimal residual disease and prevent relapse following ASCT, randomized trials testing postconsolidation treatments have been conducted.3,5,6 The Children's Oncology Group (COG) phase III trial ANBL0032 demonstrated that immunotherapy with dinutuximab, a chimeric antibody that targets the disialoganglioside GD2, in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and isotretinoin in the postconsolidation setting improved EFS and overall survival (OS) for patients with high-risk neuroblastoma.5 Two-year EFS for those randomly assigned to immunotherapy (n = 113) was 66 ± 5% compared with 46 ± 5% for those assigned to standard therapy (n = 113; P = .01); OS was 86 ± 4% versus 75 ± 5% (P = .02). These results led to early stopping of random assignment5 and to approval of dinutuximab for frontline therapy.7 After random assignment was halted, the immunotherapy arm remained open to refine survival estimates and obtain additional toxicity and correlative biology data.
CONTEXT
Key Objective
The seminal Children's Oncology Group phase III trial ANBL0032 demonstrated that the addition of immunotherapy with the anti-GD2 antibody dinutuximab with cytokines to isotretinoin in the postconsolidation setting improved event-free survival (EFS) and overall survival for patients with high-risk neuroblastoma. The immunotherapy arm remained open after cessation of random assignment to refine survival estimates and obtain additional toxicity and correlative biology data. This report describes the largest cohort of patients with high-risk neuroblastoma treated with dinutuximab therapy to date (n = 1,183).
Knowledge Generated
EFS and overall survival in this cohort were similar to those reported for the randomized cohort despite less stringent eligibility criteria, and toxicities were similar. Among the subset of patients with available data, higher dinutuximab levels and FCGR3A genotype were associated with superior EFS.
Relevance
The results of this study of GD2-directed postconsolidation therapy confirm the importance of immunotherapy for the frontline treatment of patients with high-risk neuroblastoma.
PATIENTS AND METHODS
Study Design and Participants
The design of ANBL0032 has been previously described.5,8 After halting of random assignment, patients were nonrandomly assigned to immunotherapy (April 30, 2009-July 31, 2015). The Protocol (online only) was approved by the institutional review boards at participating institutions. Written informed consent was obtained. The study was conducted in accordance with Good Clinical Practice principles, and the Declaration of Helsinki.
Eligibility criteria for enrollment after random assignment ended paralleled those previously reported5 with a few exceptions. Enrollment was permitted up to day +200 from last ASCT, and biopsy-proven residual disease was not required for assignment to immunotherapy. Patients had to achieve a pre-ASCT response of complete response (CR), very good partial response (VGPR), or partial response (PR) by 1993 International Neuroblastoma Response Criteria9 for primary site, soft tissue metastases and bone metastases. However, patients could have ≤ 10% tumor from a bone marrow aspirate/biopsy or newly detected marrow disease if the extent of tumor involvement was ≤ 10% (ie, patients could have an overall response < PR on the basis of protocol-specified criteria for marrow response).
Procedures
The treatment schema is summarized (Appendix Fig A1, online only). Dinutuximab was initially manufactured by the National Cancer Institute and administered intravenously over 10 hours (25 mg/m2 once per day; maximum infusion duration per dose: 20 hours) for 4 consecutive days during cycles 1-5. After January 21, 2014, dinutuximab was manufactured by United Therapeutics Corporation, and 17.5 mg/m2 once per day was administered on the same schedule. This modification reflected the change from a theoretical extinction coefficient (1.00) to a calculated extinction coefficient (1.41). Both products contained the same amount of active protein and were comparable in a phase II bioequivalence study.10 GM-CSF was administered during cycles 1, 3, and 5; IL-2 was administered during cycles 2 and 4.5 During the last 2 weeks of all cycles, patients received isotretinoin orally (80 mg/m2/dose twice daily). Cycle 6 consisted of isotretinoin alone. Disease evaluations were performed as described.5 Response was assessed using the 1993 International Neuroblastoma Response Criteria.9
Outcomes
Study end points included EFS and OS and Common Terminology Criteria for Adverse Events v4.0 grade ≥ 3 adverse events.
Statistical Analyses
Demographics, International Neuroblastoma Staging System stage, tumor biology, pre-ASCT response, ASCT number, dose modifications, days from last ASCT to enrollment, and grade ≥ 3 adverse events were summarized using descriptive statistics. Patient characteristics were compared with the cohort randomly assigned to receive immunotherapy5 using a chi-squared test or Fisher's exact test for categorical variables, and a Wilcoxon rank-sum test for continuous variables. Rates of occurrence of toxicities in GM-CSF– and IL-2–containing cycles were compared using McNemar's test for paired observations and adjusted for multiple comparisons with Bonferroni's correction. P values < .05 were considered statistically significant.
EFS was measured from time of enrollment to first occurrence of relapse, disease, progression, or death, or censored at last contact if an aforementioned event did not occur. OS was measured from time of enrollment to death or censored at last contact if death did not occur. Five-year EFS and OS estimates (estimate ± SE) and Kaplan-Meier survival curves were compared with a log-rank test.
Multivariable Cox proportional hazards (PH) models were fit for EFS and OS using the Efron method of handling tied event times and included standard risk factors (age at high-risk diagnosis, stage, MYCN status, histology, and ploidy) and pre-ASCT response, response after consolidation, number of transplants, and whether all six cycles of ANBL0032 therapy were completed. Backward selection with a P value threshold of .05 was used to arrive at the final model.
The association between the occurrence of a dose-limiting toxicity during treatment and human antichimeric antibody (HACA) positivity was tested with a chi-square test.
Correlative Biology Methods
Dinutuximab and HACA assays.
Dinutuximab peak concentrations and HACA values were provided by United Therapeutics Corporation via assays performed by BioAgilytix (Durham, NC) for 262/286 patients who received National Cancer Institute–produced dinutuximab and provided blood samples. Dinutuximab peak concentrations (Cmax) were assessed before starting day 4 dinutuximab infusions during cycle 1 and cycle 4 or 5. Plasma dinutuximab levels were performed using a good laboratory practice–validated Meso Scale Discovery electrochemiluminescence immunoassay; lower limit of detection was 100 ng/mL. HACA titers were measured before cycles 1, 4, and 5 using screening, confirmatory, and titer assays. To be evaluable for HACA, patients had to have ≥ 1 evaluable sample obtained following initiation of therapy. If any sample was found to be positive for HACA, the patient was designated HACA-positive (HACA+). A master mix of biotinylated dinutuximab and ruthenium-conjugated dinutuximab in assay buffer was added to dilutions of patient sera, incubated, and added to streptavidin-coated plates. Following testing for luminescence, titers were reported as the reciprocal of the last dilution above the cutpoint. Wilcoxon rank-sum tests compared median Cmax levels and Cmax ratios in HACA+ versus HACA– patients. Cox PH models tested for association between survival and cycle 1 Cmax.
Antibody-dependent cell-mediated cytotoxicity, Fc gamma receptor genotyping, and natural killer protein 30 isoform profiling.
Antibody-dependent cell-mediated cytotoxicity.
Antibody-dependent cell-mediated cytotoxicity (ADCC) of peripheral blood mononuclear cells against neuroblastoma cell line NMB-7 was assessed at baseline and before cycle 4 using a previously described chromium51 release assay.11 Percentage lysis is expressed by lytic unit (number of effector cells required to obtain 20% target cell lysis), determined using the exponential fit equation.12 For survival analyses involving ADCC before cycle 4, EFS and OS time were measured from the start of cycle 4. Cox PH models tested for association between survival and ADCC levels.
FCGR polymorphisms.
Regions surrounding the polymorphic codon 158 of FcγRIIIA (FCGR3A, rs396991) and codon 131 of FcγRIIA (FCGR2A, rs1801274) were selectively amplified, purified, and genotyped by direct sequence analysis, as described previously.8
NCR3 (rs986475) genotyping and isoform prediction.
RNA and DNA were used to predict isoform profile of natural cytotoxicity receptors (NCRs). Isoform quantifications were performed by real-time polymerase chain reaction using natural killer protein 30 (NKp30) or beta 2 microglobulin primers,13 with expression determined using the 2−ΔΔCt algorithm. Relative isoform quantity was measured as a percentage of the total of A, B, and C isoforms. Patients harbored an immunosuppressive profile when isoform C (usually < 10% of total) was ≥ 25%.
NCR3 Single Nucleotide Polymorphism (SNP) rs986475 (correlates with isoform expression).
Genomic DNA was amplified with: NCR3-U3F, 5′-CTGAACTTTCCCTTCCACCA-3′; NCR3-U3R, 5′-GGTCCAGCCAGTAAAAACCA-3′, and sequenced with: NCR3-U3sF, 5′-TGTCCTGAGAAATGGGAAGG-3′; NCR3-U3sR, 5′-CAGTAAAAACCATGGTCCCC-3′.
RESULTS
Patient Characteristics
Patient characteristics are summarized in Table 1. After cessation of random assignment, 1,192 patients were enrolled; 1,183 eligible patients received immunotherapy (Fig 1). Most were ≥ 18 months old at diagnosis (n = 1,009; 85.3%) and had stage 4 disease (n = 765 of 921; 83.1%). Among patients with known tumor biology, 45.1% (n = 363/805) had tumors that were MYCN-amplified, 94.5% (n = 749/793) had tumors with unfavorable histology, and 54.9% (n = 397/723) had diploid tumors. Pre-ASCT response (excluding bone marrow) included CR (n = 352; 29.8%), VGPR (n = 418; 35.3%), or PR (n = 413; 34.9%). Most patients had undergone single (n = 1,042; 88.1%) rather than tandem (n = 141; 11.9%) ASCT. Because of protocol-specific bone marrow eligibility criteria, 29 had < PR pre-ASCT overall responses (progressive disease n = 3, no response n = 1, and mixed response n = 25).
TABLE 1.
Patient Characteristics
FIG 1.
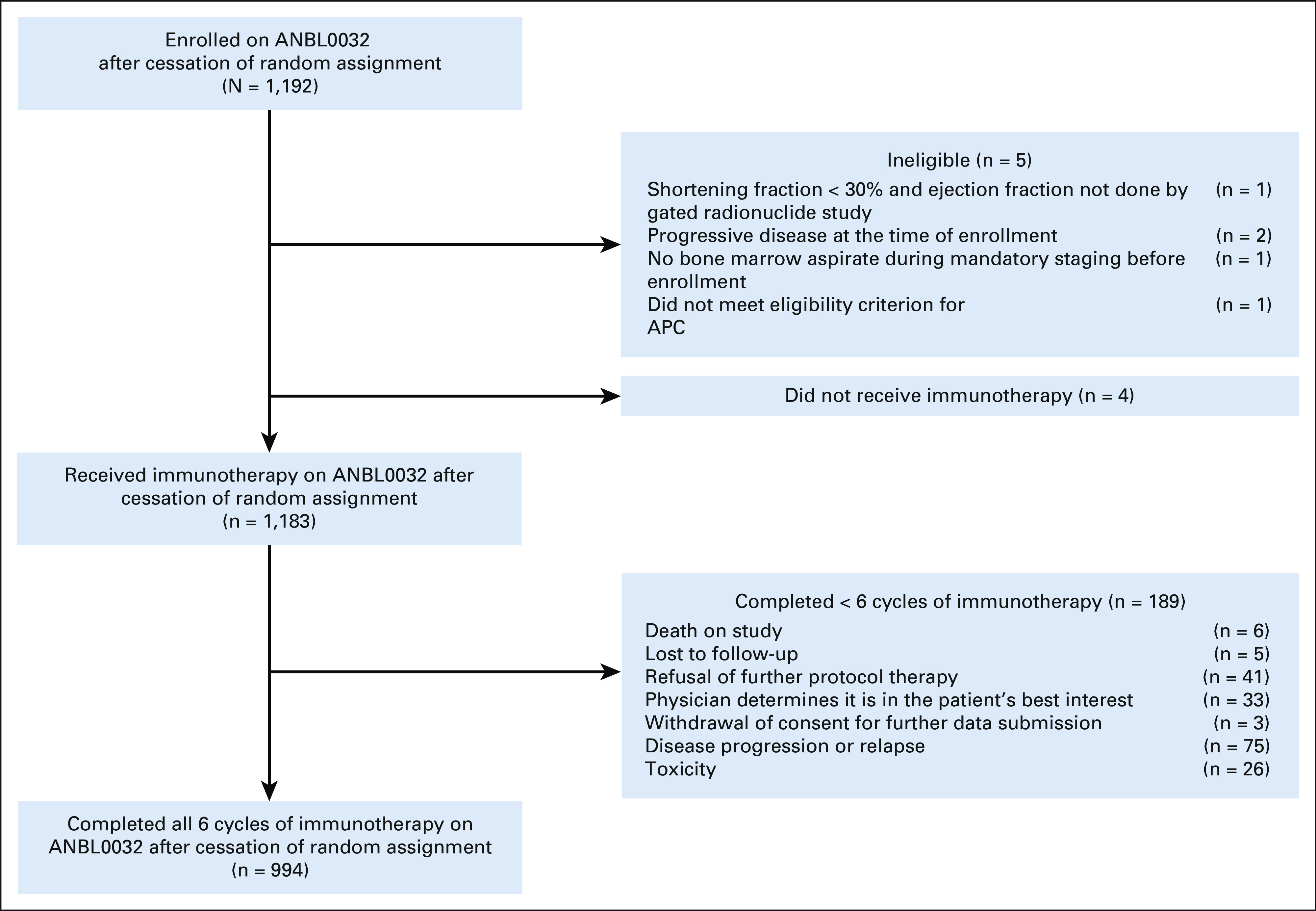
ANBL0032 immunotherapy cohort after cessation of random assignment. One thousand one hundred ninety-two patients enrolled and 1,183 patients nonrandomly received immunotherapy. Nine hundred ninety-four patients completed all six cycles of immunotherapy. APC, absolute phagocyte count.
There were no significant differences in stage (P = .6302), tumor MYCN status (P = .4030), or histology (P = 1.000) in this cohort compared with the randomized cohort that received immunotherapy; however, diploidy was more common in those treated after random assignment ended (54.9% v 42.4%, P = .0281). End-induction response differed between the cohorts, with a lower proportion enrolling with CR/VGPR after cessation of random assignment (65.1% v 77.2%). More patients had undergone tandem transplant in the current cohort compared with the randomized cohort, although the difference was not significant (11.9% v 6.1%, P = .0638). Time from last ASCT was longer among patients enrolled after random assignment ended (89 days [range, 40-197 days] v 73.5 days [range, 59.0-124.0 days], P < .0001).
Treatment
In total, 84.0% (n = 994) of patients completed all six cycles of therapy (Fig 1). The remaining patients completed one (n = 38; 3.2%), two (n = 47; 4.0%), three (n = 36; 3.0%), four (n = 37; 3.1%), and five (n = 31; 2.6%) cycles. Reasons for early therapy discontinuation are shown (Fig 1). Characteristics of the 101 (8.5%) patients who relapsed on therapy are shown in Appendix Table A1 (online only). IL-2 was replaced by GM-CSF in 1.9% of patients (n = 22). Data regarding 827 planned IL-2–containing cycles were available; GM-CSF replaced IL-2 in 23 cycles (2.8%).
Survival Outcomes
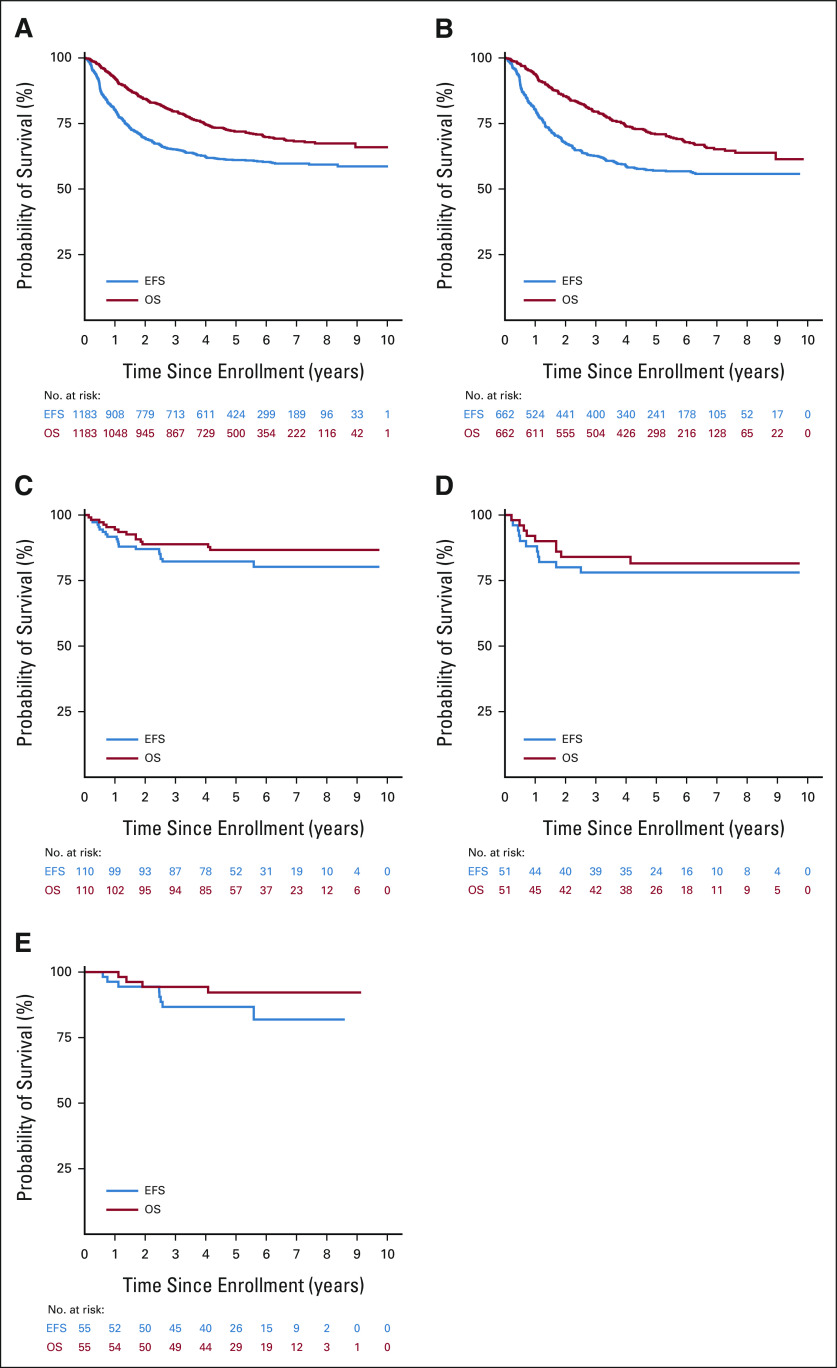
Among all patients who received immunotherapy after cessation of random assignment (n = 1,183), 2-year EFS and OS from time of enrollment were 69.4 ± 1.4% and 84.4 ± 1.1%, respectively. Five-year EFS and OS from time of enrollment were 61.1 ± 1.9% and 71.9 ± 1.7%, respectively (Fig 2A). Median follow-up time was 4.1 years (range, 0.005-10.000 years). Among stage 4 patients ≥ 18 months old at diagnosis (n = 662), 5-year EFS and OS were 57.0 ± 2.4% and 70.9 ± 2.2%, respectively (Fig 2B). For stage 3 disease (n = 110), 5-year EFS and OS were 82.3 ± 4.8% and 86.7 ± 4.2%, respectively (Fig 2C). Among stage 3 patients with MYCN-amplified (n = 51) and MYCN-nonamplified (n = 55) tumors, 5-year EFS and OS were 78.1 ± 7.5% and 81.6 ± 6.9% (Fig 2D) and 86.7 ± 6.2% and 92.2 ± 4.8% (Fig 2E), respectively.
FIG 2.
EFS and OS of the entire cohort and by patient characteristics and tumor biology: (A) entire cohort (n = 1,183), (B) patients with stage 4 disease and age ≥ 18 months at diagnosis (n = 662), (C) patients with stage 3 disease (n = 110), (D) patients with stage 3 disease and MYCN-amplified tumors (n = 51), and (E) patients with stage 3 disease and MYCN-nonamplified tumors (n = 55). EFS, event-free survival; OS, overall survival.
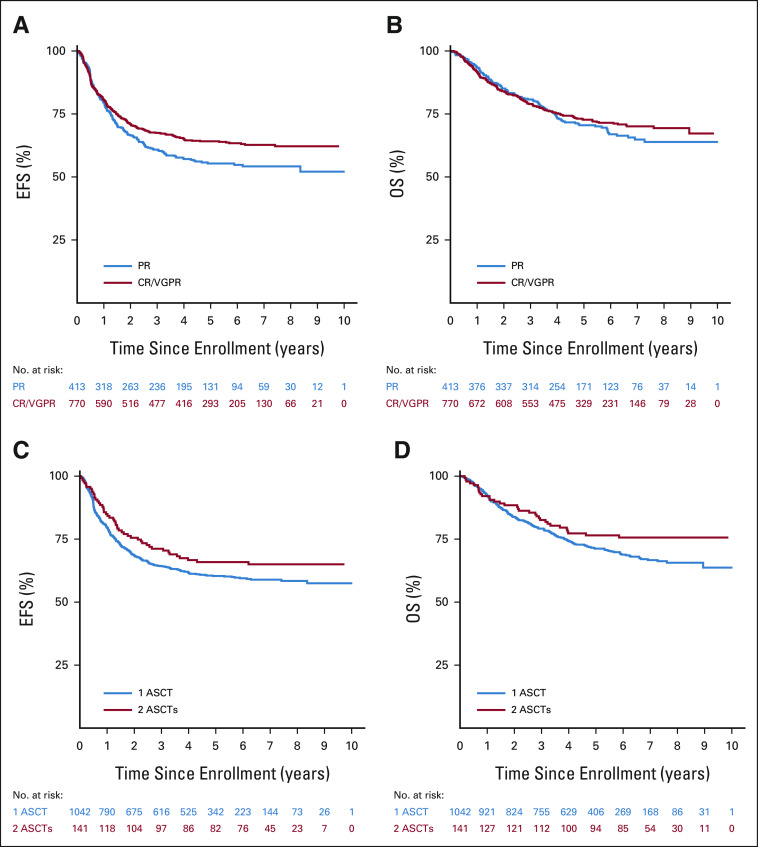
EFS and OS by prognostic factors, pre-ASCT response, response after consolidation, number of transplants, and completion of therapy are summarized (Table 2). Stage 4 patients had inferior survival compared with non–stage 4 patients (5-year EFS: 58.4 ± 2.3% v 79.3 ± 4.3%, P = .0001; OS: 70.9 ± 2.1% v 84.5 ± 3.8%, P = .0005). EFS, but not OS, was significantly higher for patients with favorable versus unfavorable histology (5-year EFS: 82.8 ± 7.3% v 61.1 ± 2.3%, P = .0116; OS: 85.5 ± 6.8% v 72.8 ± 2.1%, P = .1241) and for patients with a pre-ASCT response (excluding marrow) of CR/VGPR versus PR (5-year EFS: 64.2 ± 2.2% v 55.4 ± 3.2%, P = .0133, Fig 3A; OS: 72.7 ± 2.1% v 70.5 ± 2.9%, P = .3811, Fig 3B). EFS for patients ≥ 18 months old at diagnosis was inferior to those < 18 months old, although the difference was not significant (5-year EFS: 59.7 ± 2.0% v 69.8 ± 4.9%, P = .0831). Although EFS did not differ on the basis of number of ASCTs (Fig 3C), there was a trend toward improved OS for tandem patients (5-year OS: 76.5 ± 3.8% v 71.2 ± 1.9%, P = .0704, Fig 3D). There was no difference in EFS on the basis of MYCN status. However, there was a trend toward improved OS for those with MYCN-nonamplified versus MYCN-amplified tumors (5-year OS, 76.4 ± 2.6% v 69.2 ± 3.1%, P = .0631). OS, but not EFS, was significantly worse among those who did not complete protocol therapy compared with those who did (5-year EFS: 56.2 ± 6.9% v 66.4 ± 1.9%, P = .0827; 5-year OS: 64.6 ± 6.6% v 78.0 ± 1.7%, P = .0056).
TABLE 2.
EFS and OS by Patient Characteristics
FIG 3.
Survival by pre-ASCT INRC response (CR/VGPR v PR) and number of ASCTs: (A) EFS by pre-ASCT INRC response (P = .0133), (B) OS by pre-ASCT INRC response (P = .3811), (C) EFS by number of ASCTs (P = .1282), and (D) OS by number of ASCTs (P = .0704). ASCT, autologous stem cell transplant; CR, complete response; EFS, event-free survival; INRC, International Neuroblastoma Response Criteria; OS, overall survival; PR, partial response; VGPR, very good partial response.
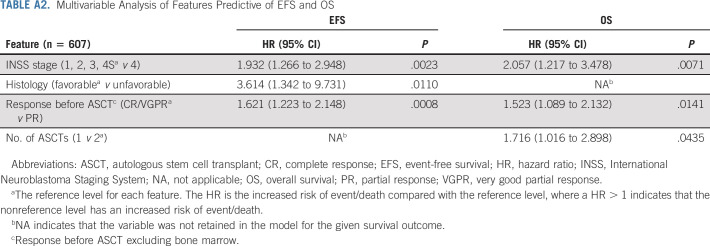
In the multivariable Cox model for EFS (n = 607 with complete data; Appendix Table A2, online only), patients with stage 4 (hazard ratio [HR], 1.93, P = .0023), unfavorable histology (HR, 3.61, P = .011), and PR pre-ASCT (HR, 1.62, P = .0008) had statistically significantly higher risk of event. For OS, patients with stage 4 (HR, 2.06, P = .0071), PR pre-ASCT (HR, 1.52, P = .0141), and one ASCT (HR, 1.72, P = .0435) had statistically significantly higher risk of death.
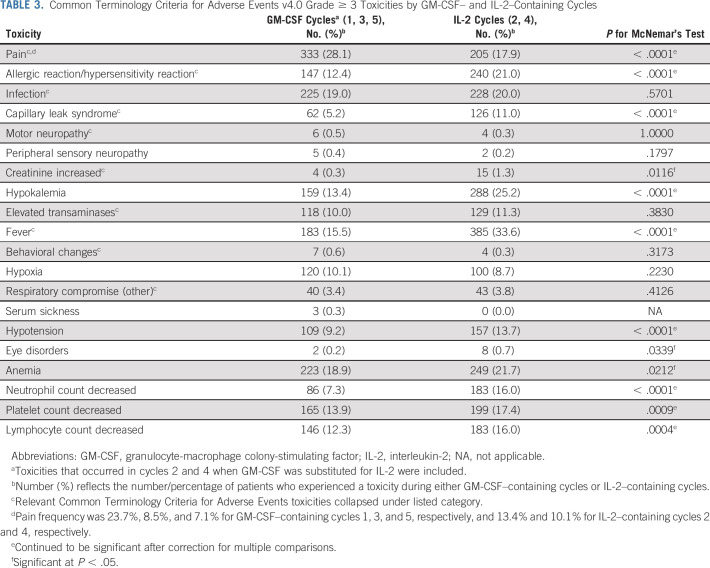
Toxicities
Adverse events monitored during the trial are summarized (Table 3; grade ≥ 3). Pain was most frequent during cycle 1 (23.7%). In cycles 2-5, grade ≥ 3 pain was reported in a higher percentage of patients during IL-2–containing cycles than GM-CSF–containing cycles (17.9% v 11.7%; P < .0001). Other toxicities reported more commonly during IL-2–containing cycles included allergic reaction (21.0% v 12.4%), capillary leak (11.0% v 5.2%), fever (33.6% v 15.5%), and hypotension (13.7% v 9.2%; P < .0001). Respiratory compromise and elevated creatinine were rare (< 5%); however, elevated creatinine was more frequent during IL-2–containing cycles (1.3% v 0.3%, P = .0116). Hematologic toxicities were more frequent during IL-2–containing cycles. No treatment-related deaths were reported.
TABLE 3.
Common Terminology Criteria for Adverse Events v4.0 Grade ≥ 3 Toxicities by GM-CSF– and IL-2–Containing Cycles
Correlative Biology Studies
HACA and dinutuximab levels.
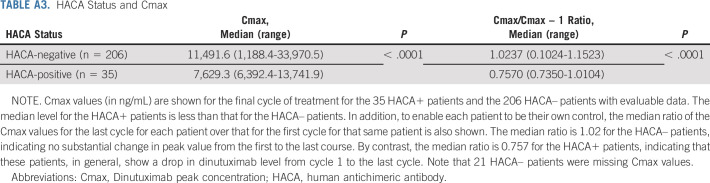
HACA data were available from BioAgilytix for 262 patients (HACA+: n = 53; HACA–: n = 209). Characteristics of these patients were similar to those for whom HACA data were unavailable (n = 921), except that a higher proportion of the latter had a pre-ASCT response of CR/VGPR rather than PR and underwent single rather than tandem ASCT. The BioAgilytix system is more sensitive than the enzyme-linked immunosorbent assay used previously.8 To avoid focusing on low HACA values unlikely to be clinically meaningful, previous studies called specimens positive only if levels were sufficiently above background detected in pretreatment specimens.8 Eighteen patients considered weakly HACA+ by BioAgilytix were considered negative in this analysis (HACA+: n = 35; HACA–: n = 227). As noted in other trials,14 fewer dose-limiting toxicities were seen in HACA+ than in HACA– patients (17.1% v 37.9%, P = .0167); however, EFS and OS did not differ (P = .6327 and .8531, respectively). HACA– patients showed no change in median peak dinutuximab levels from first to final cycles, whereas 34/35 HACA+ patients had detectible drops in peak dinutuximab levels (P < .0001), indicating an in vivo effect (Appendix Table A3, online only). Higher cycle 1 peak dinutuximab levels were associated with improved EFS (P = .0341) and a trend toward improved OS (P = .0721). A 5,000 ng/mL increase in cycle 1 Cmax was associated with a 33% lower risk of event.
Fc gamma receptor genotype, NKp30 isotype, and ADCC.
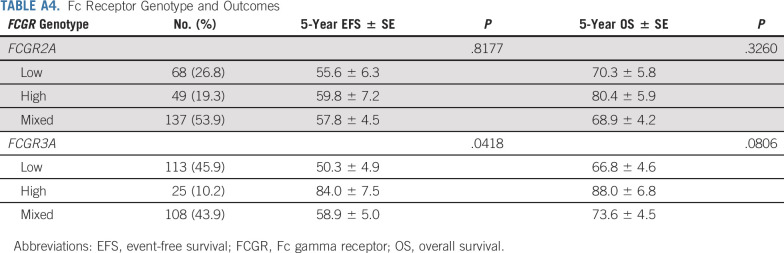
In patients with genotyping data available (n = 262), frequencies of polymorphisms of FCGR2A codon 131 and FCGR3A codon 158 (Appendix Table A4, online only) are similar to those reported previously.8,15 Among patients with high- (n = 49), low- (n = 68), or mixed- (n = 137) affinity FCGR2A genotypes, no differences in EFS or OS were identified. Although the number of patients with available samples was small, EFS was significantly better for patients with high-affinity FCGR3A genotype (n = 25) than those with low-/mixed-affinity genotypes (n = 221; P = .0418; Appendix Table A4, Appendix Fig A2A, online only). The difference in OS on the basis of FCGR3A genotype did not reach statistical significance (P = .0806; Appendix Fig A2B).
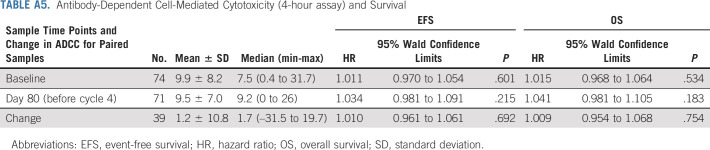
ADCC activities of peripheral blood mononuclear cells were evaluated at baseline (n = 74) and before starting cycle 4 IL-2 (n = 71). No relationship was found between ADCC and EFS/OS (Appendix Table A5, online only).
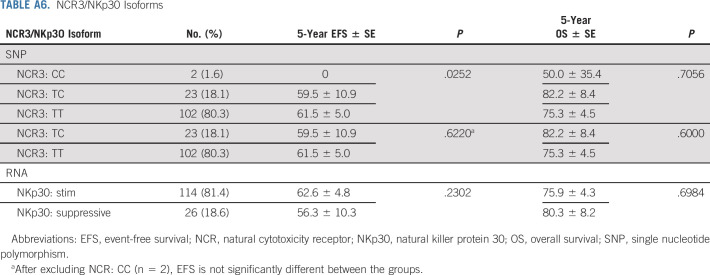
NKp30 genotype and transcript data were concordant in 37/39 (95%) patients for whom data were available. Most had an immunostimulatory NCR/NKp30 isoform (TT in SNP rs986475; Appendix Table A6, online only). Twenty-six patients had the immunosuppressive isoform on the basis of RNA isoform expression and/or by SNP (TC or CC). Only two harbored the homozygous immunosuppressive isoform (CC); these patients relapsed at 1.06 and 1.26 years after enrollment. NKp30 isoform was not correlated with EFS/OS.
DISCUSSION
This report describes the largest cohort of patients with high-risk neuroblastoma treated with anti-GD2 antibody therapy to date. Two- and 5-year EFS and OS were consistent with those of the randomized portion of ANBL00325,8 despite the less stringent eligibility criteria for the current cohort. Superior EFS and OS were observed for those with non–stage 4 disease, and superior EFS was observed for those with pre-ASCT responses of CR/VGPR compared with PR, consistent with prior results.5,8 There was a trend toward improved OS among patients treated with tandem versus single ASCT; however, only a small proportion of patients underwent tandem ASCT. The results of COG ANBL0532 (ClinicalTrials.gov identifier: NCT00567567)16 demonstrating superior survival in tandem ASCT were released in May 2015; thus, single ASCT was still considered standard for much of the study duration. Nevertheless, in the multivariable Cox model, there was an increased risk of death among those who underwent single transplant.
Most patients completed therapy despite treatment-related toxicities. As GD2 is expressed on nerve fibers,17 pain is expected. More patients experienced severe pain during cycle 1 compared with subsequent cycles, as noted previously.5 Allergic reactions and capillary leak were more frequently observed during IL-2–containing cycles, similar to prior observations5 and consistent with established IL-2–associated toxicities.18 GM-CSF and IL-2 were administered with dinutuximab to enhance ADCC.19-22 However, a randomized trial conducted by the International Society of Paediatric Oncology European Neuroblastoma found added toxicity without survival benefit with addition of subcutaneous IL-2 to dinutuximab beta.6 Long-term continuous infusion dinutuximab beta with subcutaneous IL-2 has been shown to result in elevated levels of regulatory T cells, correlating with inferior progression-free survival.23 Together, these findings led COG to remove IL-2 from postconsolidation therapy. Strategies to potentially mitigate toxicities (including extended duration schedules and optimization of supportive care) and incorporation of patient-reported outcomes as part of future trials may improve the patient experience. Home administration may also be of interest. Development of next-generation anti-GD2 antibodies may help decrease treatment-associated pain.24,25
The rate of HACA positivity in this cohort was similar to that reported in the randomized cohort. The development of HACA was associated with lower dinutuximab levels and decreased toxicity, as reported previously.14,24,26,27 However, HACA status was not associated with survival in this study. Higher cycle 1 dinutuximab levels were associated with improved EFS, consistent with the findings of two chemoimmunotherapy trials.28,29 This suggests that greater in vivo exposure to anti-GD2 antibody could be associated with improved outcome. If alternative dosing schedules are used, the association between dinutuximab levels and survival should be evaluated.
Identification of biomarkers associated with outcome in the context of immunotherapy for neuroblastoma has long been a high priority in the field, particularly in light of the toxicity that accompanies GD2-directed therapy. The association of FCGR3A high-affinity genotype with better outcome is consistent with the trend reported in patients treated with dinutuximab beta in a European study.30 However, no correlation between outcome and FCGR2A/FCGR3A status was detected in the randomized cohort from ANBL0032. The association of Fc gamma receptor genotype and EFS requires validation in an independent cohort.
Because natural killer (NK) cells are important for ADCC, NKp30 isoforms have been evaluated in several dinutuximab trials. The lack of association between NKp30 isoform and survival suggests that NK cell number and/or expression of other NK-activating receptors might overshadow the role of NKp30. Complete or partial loss of GD2 expression can occur during therapy or at the time of neuroblastoma recurrence,31 which may affect response to anti-GD2 therapy. Improved understanding of the tumor microenvironment and immune profiles of patients may also provide insight into mechanisms of resistance to GD2-directed immunotherapy.32-36 Elucidating these mechanisms could help identify additional biomarkers to guide treatment and improve outcomes for children with high-risk neuroblastoma.
ACKNOWLEDGMENT
The authors express their gratitude to United Therapeutics Corporation for supplying dinutuximab.
APPENDIX
FIG A1.
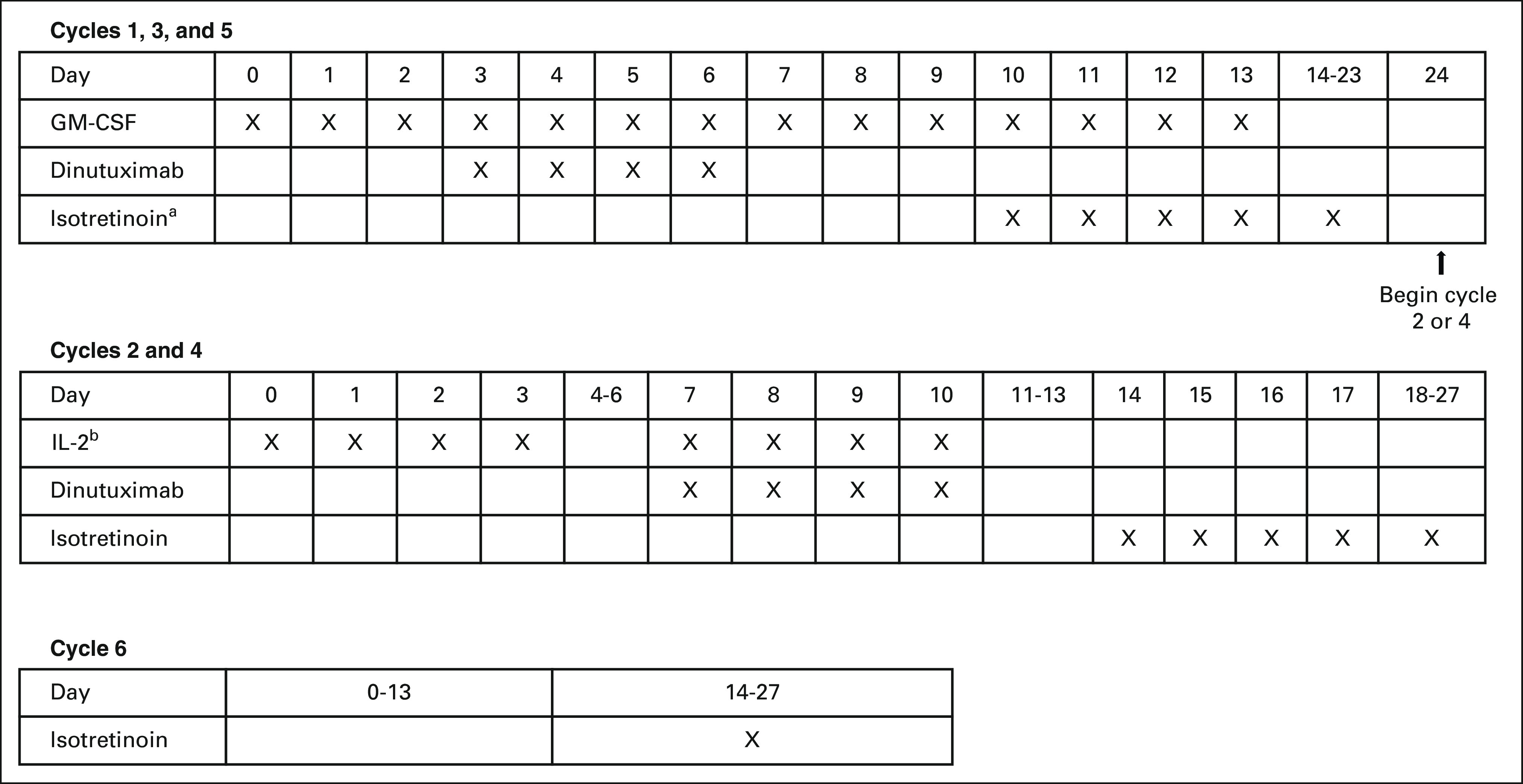
ANBL0032 immunotherapy treatment regimen. aIsotretinoin is given on days 11-24 during cycle 1. bIL-2, days 0-3: 3 MIU/m2 daily (continuous infusion); days 7-10: 4.5 MIU/m2 daily (continuous infusion). GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2, interleukin-2.
FIG A2.
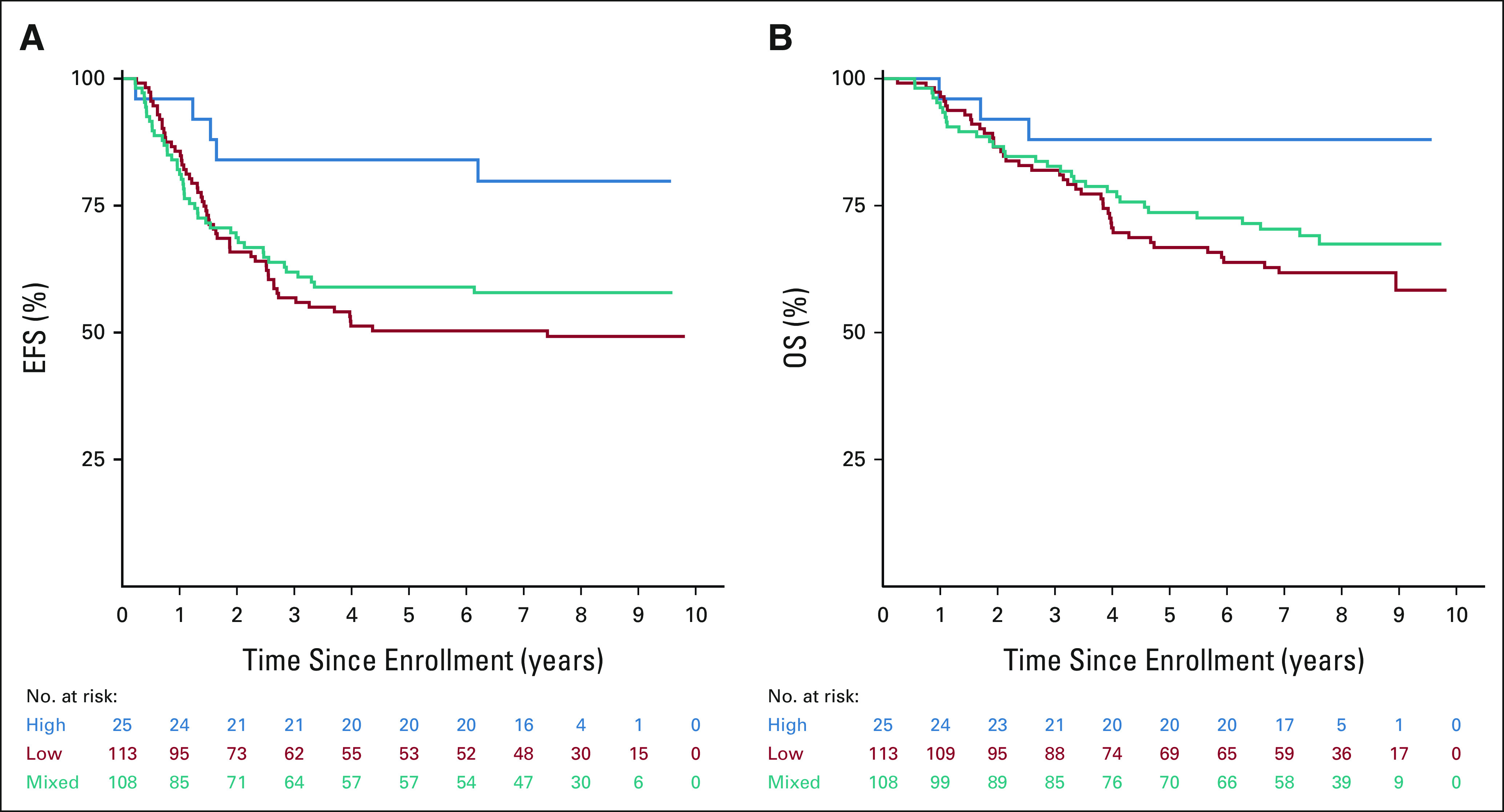
Survival by FCGR3A affinity: (A) EFS (P = .0418) and (B) OS (P = .0806). EFS, event-free survival; OS, overall survival.
TABLE A1.
Characteristics of Patients With On-Therapy Relapse or Disease Progression
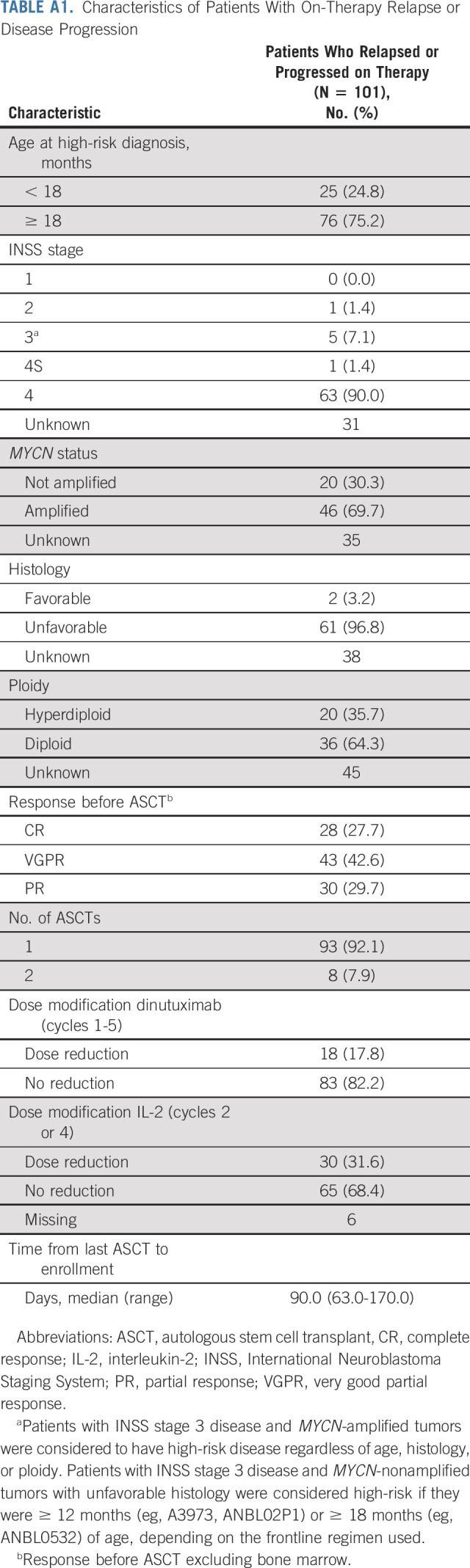
TABLE A2.
Multivariable Analysis of Features Predictive of EFS and OS
TABLE A3.
HACA Status and Cmax
TABLE A4.
Fc Receptor Genotype and Outcomes
TABLE A5.
Antibody-Dependent Cell-Mediated Cytotoxicity (4-hour assay) and Survival
TABLE A6.
NCR3/NKp30 Isoforms
Ami V. Desai
Stock and Other Ownership Interests: Pfizer, Viatris
Consulting or Advisory Role: Ology Medical Education, Ymabs Therapeutics Inc
Research Funding: Merck (Inst), Roche (Inst), Jubilant DraxImage (Inst), Ymabs Therapeutics Inc (Inst), Lilly (Inst), GlaxoSmithKline (Inst), Actuate Therapeutics (Inst)
Arlene Naranjo
Consulting or Advisory Role: Novartis
Wendy London
Consulting or Advisory Role: Jubilant Radiopharma, Merck
Research Funding: Agios, Bristol Myers Squibb, Novartis, Aileron Therapeutics, Bluebird Bio
Barry L. Shulkin
Consulting or Advisory Role: Navidea
Katherine K. Matthay
Consulting or Advisory Role: Resonance, Innervate RAdiopharmaceuticals
Susan L. Cohn
Stock and Other Ownership Interests: Merck, Amgen (I), Pfizer (I), AbbVie, Lilly, Sanofi, Accelerated Medical Diagnostics, Novo Nordisk, Gilead Sciences, United Health Group, Teva, AstraZeneca/MedImmune, Johnson & Johnson/Janssen, Novartis, Teva
Honoraria: Y-mAbs Therapeutics
Research Funding: United Therapeutics (Inst), Merck (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/46569/summary
John M. Maris
Stock and Other Ownership Interests: Tantigen Bio
Consulting or Advisory Role: Auron Therapeutics, Illumina Radiopharmaceuticals, Jubilant DraxImage
Patents, Royalties, Other Intellectual Property: GPC2 binders and CARs (Inst), Neuroblastoma antigens (Inst)
Rochelle Bagatell
Uncompensated Relationships: Ymabs Therapeutics Inc
Paul Sondel
Research Funding: Invenra (Inst)
Patents, Royalties, Other Intellectual Property: I have partial interest in patents related to my work at the University of Wisconsin-Madison WI, which are held by and managed by the University of Wisconsin Foundation; I am an unpaid medical advisor to Invenra Inc, a monoclonal antibody biotech firm in Madison WI. My UW laboratory is collaborating with Invenra and receiving research reagents from them for mutual research (Inst)
Uncompensated Relationships: Invenra
Alice L. Yu
Leadership: OPKO Health
Stock and Other Ownership Interests: OPKO Health/GeneDx
Honoraria: EUSA Pharma
Consulting or Advisory Role: OBI Pharma
Speakers' Bureau: EUSA Pharma
Research Funding: United Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Globo H-Diphtheria toxoid vaccine for cancer therapy, NKT stimulatory phenyl-glycolipids for cancer therapy and vaccine adjuvant, cancer-targeting peptides, methods for suppressing cancer by inhibition of TMCC3
Travel, Accommodations, Expenses: EUSA Pharma
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented in part as abstract form at the 2020 ASCO annual meeting, virtual, May 29-31, 2020; and the 2021 Advances in Neuroblastoma Research Meeting, virtual, January 25-27, 2021.
SUPPORT
Supported by COG NCTN Operations Center Grant No. U10CA180886, COG NCTN Statistics & Data Center Grant No. U10CA180899, St Baldrick's Foundation: NCI Grant No. R35 CA220500 (J.M.M.).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Ami V. Desai, Andrew L. Gilman, Mehmet Fevzi Ozkaynak, Wendy B. London, Jacquelyn A. Hank, Malcolm Smith, Katherine K. Matthay, Susan L. Cohn, John M. Maris, Paul M. Sondel, Julie R. Park, Alice L. Yu
Administrative support: Jeffrey A. Moscow
Provision of study materials or patients: Mehmet Fevzi Ozkaynak, Jacquelyn A. Hank, Katherine K. Matthay, Susan L. Cohn, Paul M. Sondel
Collection and assembly of data: Ami V. Desai, Mehmet Fevzi Ozkaynak, Sheena C. Tenney, Mitchell Diccianni, Jacquelyn A. Hank, Julie R. Park, Alice L. Yu
Data analysis and interpretation: Ami V. Desai, Andrew L. Gilman, Arlene Naranjo, Sheena C. Tenney, Mitchell Diccianni, Jacquelyn A. Hank, Marguerite T. Parisi, Barry L. Shulkin, Jeffrey A. Moscow, Hiroyuki Shimada, Susan L. Cohn, John M. Maris, Rochelle Bagatell, Paul M. Sondel, Julie R. Park, Alice L. Yu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcomes Following GD2-Directed Postconsolidation Therapy for Neuroblastoma After Cessation of Random Assignment on ANBL0032: A Report From the Children's Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ami V. Desai
Stock and Other Ownership Interests: Pfizer, Viatris
Consulting or Advisory Role: Ology Medical Education, Ymabs Therapeutics Inc
Research Funding: Merck (Inst), Roche (Inst), Jubilant DraxImage (Inst), Ymabs Therapeutics Inc (Inst), Lilly (Inst), GlaxoSmithKline (Inst), Actuate Therapeutics (Inst)
Arlene Naranjo
Consulting or Advisory Role: Novartis
Wendy London
Consulting or Advisory Role: Jubilant Radiopharma, Merck
Research Funding: Agios, Bristol Myers Squibb, Novartis, Aileron Therapeutics, Bluebird Bio
Barry L. Shulkin
Consulting or Advisory Role: Navidea
Katherine K. Matthay
Consulting or Advisory Role: Resonance, Innervate RAdiopharmaceuticals
Susan L. Cohn
Stock and Other Ownership Interests: Merck, Amgen (I), Pfizer (I), AbbVie, Lilly, Sanofi, Accelerated Medical Diagnostics, Novo Nordisk, Gilead Sciences, United Health Group, Teva, AstraZeneca/MedImmune, Johnson & Johnson/Janssen, Novartis, Teva
Honoraria: Y-mAbs Therapeutics
Research Funding: United Therapeutics (Inst), Merck (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/46569/summary
John M. Maris
Stock and Other Ownership Interests: Tantigen Bio
Consulting or Advisory Role: Auron Therapeutics, Illumina Radiopharmaceuticals, Jubilant DraxImage
Patents, Royalties, Other Intellectual Property: GPC2 binders and CARs (Inst), Neuroblastoma antigens (Inst)
Rochelle Bagatell
Uncompensated Relationships: Ymabs Therapeutics Inc
Paul Sondel
Research Funding: Invenra (Inst)
Patents, Royalties, Other Intellectual Property: I have partial interest in patents related to my work at the University of Wisconsin-Madison WI, which are held by and managed by the University of Wisconsin Foundation; I am an unpaid medical advisor to Invenra Inc, a monoclonal antibody biotech firm in Madison WI. My UW laboratory is collaborating with Invenra and receiving research reagents from them for mutual research (Inst)
Uncompensated Relationships: Invenra
Alice L. Yu
Leadership: OPKO Health
Stock and Other Ownership Interests: OPKO Health/GeneDx
Honoraria: EUSA Pharma
Consulting or Advisory Role: OBI Pharma
Speakers' Bureau: EUSA Pharma
Research Funding: United Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Globo H-Diphtheria toxoid vaccine for cancer therapy, NKT stimulatory phenyl-glycolipids for cancer therapy and vaccine adjuvant, cancer-targeting peptides, methods for suppressing cancer by inhibition of TMCC3
Travel, Accommodations, Expenses: EUSA Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pinto NR, Applebaum MA, Volchenboum SL, et al. : Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol 33:3008-3017, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay KK, Maris JM, Schleiermacher G, et al. : Neuroblastoma. Nat Rev Dis Primers 2:16078, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, et al. : Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med 341:1165-1173, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Matthay KK, Reynolds CP, Seeger RC, et al. : Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A Children's Oncology Group study. J Clin Oncol 27:1007-1013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu AL, Gilman AL, Ozkaynak MF, et al. : Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363:1324-1334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladenstein R, Potschger U, Valteau-Couanet D, et al. : Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol 19:1617-1629, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Dhillon S: Dinutuximab: First global approval. Drugs 75:923-927, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Yu AL, Gilman AL, Ozkaynak MF, et al. : Long-term follow-up of a phase III study of ch14.18 (dinutuximab) + cytokine immunotherapy in children with high-risk neuroblastoma: COG study ANBL0032. Clin Cancer Res 27:2179-2189, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodeur GM, Pritchard J, Berthold F, et al. : Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11:1466-1477, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Marachelian A, Desai A, Balis F, et al. : Comparative pharmacokinetics, safety, and tolerability of two sources of ch14.18 in pediatric patients with high-risk neuroblastoma following myeloablative therapy. Cancer Chemother Pharmacol 77:405-412, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batova A, Kamps A, Gillies SD, et al. : The Ch14.18-GM-CSF fusion protein is effective at mediating antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity in vitro. Clin Cancer Res 5:4259-4263, 1999 [PubMed] [Google Scholar]
- 12.Pross HF, Baines MG, Rubin P, et al. : Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol 1:51-63, 1981 [DOI] [PubMed] [Google Scholar]
- 13.Delahaye NF, Rusakiewicz S, Martins I, et al. : Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 17:700-707, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Siebert N, Troschke-Meurer S, Marx M, et al. : Impact of HACA on immunomodulation and treatment toxicity following ch14.18/CHO long-term infusion with interleukin-2: Results from a SIOPEN phase 2 trial. Cancers (Basel) 10:387, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Gordon M, Schultheis AM, et al. : FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 25:3712-3718, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Park JR, Kreissman SG, London WB, et al. : Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: A randomized clinical trial. JAMA 322:746-755, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svennerholm L, Bostrom K, Fredman P, et al. : Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim Biophys Acta 1214:115-123, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Siegel JP, Puri RK: Interleukin-2 toxicity. J Clin Oncol 9:694-704, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Barker E, Reisfeld RA: A mechanism for neutrophil-mediated lysis of human neuroblastoma cells. Cancer Res 53:362-367, 1993 [PubMed] [Google Scholar]
- 20.Albertini MR, Hank JA, Schiller JH, et al. : Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res 3:1277-1288, 1997 [PubMed] [Google Scholar]
- 21.Kendra K, Malkovska V, Allen M, et al. : In vivo binding and antitumor activity of Ch14.18. J Immunother 22:423-430, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Hank JA, Robinson RR, Surfus J, et al. : Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res 50:5234-5239, 1990 [PubMed] [Google Scholar]
- 23.Troschke-Meurer S, Siebert N, Marx M, et al. : Low CD4(+)/CD25(+)/CD127(-) regulatory T cell- and high INF-gamma levels are associated with improved survival of neuroblastoma patients treated with long-term infusion of ch14.18/CHO combined with interleukin-2. Oncoimmunology 8:1661194, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navid F, Sondel PM, Barfield R, et al. : Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol 32:1445-1452, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erbe AK, Hernandez R, Gerhardt D, et al. : Improving specific targeting of tumors through bispecific SNIPER antibodies. J Immunol 204:91.2, 2020 [Google Scholar]
- 26.Hank JA, Gan J, Ryu H, et al. : Immunogenicity of the hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin Cancer Res 15:5923-5930, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bishop MW, Hutson PR, Hank JA, et al. : A Phase 1 and pharmacokinetic study evaluating daily or weekly schedules of the humanized anti-GD2 antibody hu14.18K322A in recurrent/refractory solid tumors. MAbs 12:1773751, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mody R, Yu AL, Naranjo A, et al. : Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: A report from the Children's Oncology Group. J Clin Oncol 38:2160-2169, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furman WL, Federico SM, McCarville MB, et al. : A phase II trial of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma. Clin Cancer Res 25:6320-6328, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siebert N, Jensen C, Troschke-Meurer S, et al. : Neuroblastoma patients with high-affinity FCGR2A, -3A and stimulatory KIR 2DS2 treated by long-term infusion of anti-GD2 antibody ch14.18/CHO show higher ADCC levels and improved event-free survival. Oncoimmunology 5:e1235108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumacher-Kuckelkorn R, Volland R, Gradehandt A, et al. : Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr Blood Cancer 64:46-56, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Nassin ML, Nicolaou E, Gurbuxani S, et al. : Immune reconstitution following autologous stem cell transplantation in patients with high-risk neuroblastoma at the time of immunotherapy. Biol Blood Marrow Transplant 24:452-459, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Das RK, Vernau L, Grupp SA, et al. : Naive T-cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov 9:492-499, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asgharzadeh S, Salo JA, Ji L, et al. : Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol 30:3525-3532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei JS, Kuznetsov IB, Zhang S, et al. : Clinically relevant cytotoxic immune cell signatures and clonal expansion of T-cell receptors in high-risk MYCN-not-amplified human neuroblastoma. Clin Cancer Res 24:5673-5684, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao R, Spranger S, Hernandez K, et al. : Immunogenomic determinants of tumor microenvironment correlate with superior survival in high-risk neuroblastoma. J Immunother Cancer 9:e002417, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]