PURPOSE
In patients with high-grade ovarian cancer, predictors of bevacizumab efficacy in first-line setting are needed. In the ICON-7 trial, a poor tumor intrinsic chemosensitivity (defined by unfavorable modeled cancer antigen-125 [CA-125] ELIMination rate constant K [KELIM] score) was a predictive biomarker. Only the patients with high-risk disease (suboptimally resected stage III, or stage IV) exhibiting unfavorable KELIM score < 1.0 had overall survival (OS) benefit from bevacizumab (median: 29.7 v 20.6 months; hazard ratio [HR], 0.78). An external validation study in the GOG-0218 trial was performed.
METHODS
In GOG-0218, 1,873 patients were treated with carboplatin-paclitaxel ± concurrent-maintenance bevacizumab/placebo. Patient KELIM values were calculated with CA-125 kinetics during the first 100 chemotherapy days by the Lyon University team. The association between KELIM score (favorable ≥ 1.0, or unfavorable < 1.0) and bevacizumab benefit for progression-free survival (PFS)/OS was independently assessed by NGR-GOG using univariate/multivariate analyses.
RESULTS
KELIM was assessable in 1,662 patients with ≥ 3 CA-125 available values. An unfavorable KELIM score was associated with bevacizumab benefit compared with placebo (PFS: HR, 0.70; 95% CI, 0.59 to 0.82; OS: HR, 0.87; 95% CI, 0.73 to 1.03), whereas a favorable KELIM was not (PFS: HR, 0.96; 95% CI, 0.79 to 1.17; OS: HR, 1.11; 95% CI, 0.89 to 1.39). The highest benefit was observed in patients with a high-risk disease exhibiting unfavorable KELIM, for PFS (median: 9.1 v 5.6 months; HR, 0.64; 95% CI, 0.53 to 0.78), and for OS (median: 35.1 v 29.1 months; HR, 0.79; 95% CI, 0.65 to 0.97).
CONCLUSION
This GOG-0218 trial investigation validates ICON-7 findings about the association between poor tumor chemosensitivity and benefit from concurrent-maintenance bevacizumab, suggesting that bevacizumab may mainly be effective in patients with poorly chemosensitive disease. Bevacizumab may be prioritized in patients with a high-risk and poorly chemosensitive disease to improve their PFS/OS (patient KELIM score calculator available on the Biomarker Kinetics website).
INTRODUCTION
In June 2018, the US Food and Drug Administration approved bevacizumab for patients with stage III or IV epithelial ovarian, fallopian tube, or primary peritoneal cancer in combination with carboplatin and paclitaxel, followed by single-agent bevacizumab, after initial surgical resection.1 This approval followed the outcomes of two parallel phase III trials, GOG-0218 and ICON-7, that demonstrated a benefit in progression-free survival (PFS) with the addition of bevacizumab to standard first-line chemotherapy in patients with an advanced stage III-IV ovarian cancer.2,3
CONTEXT
Key Objective
To confirm the predictive value of the tumor primary chemosensitivity (assessed by modeled cancer antigen-125 [CA-125] ELIMination rate constant K [KELIM]) regarding the efficacy of bevacizumab previously reported in the ICON-7 trial in patients with advanced ovarian carcinoma.
Knowledge Generated
This external validation analysis with GOG-0218 trial data confirmed that only the patients with poorly chemosensitive disease (unfavorable KELIM score < 1.0) experienced overall survival benefit among those with a high-risk disease. The effect of bevacizumab in patients with a low-risk disease characterized by high chemosensitivity (favorable KELIM score ≥ 1.0) is uncertain.
Relevance (K.D. Miller)
-
The results from the ICON-7 and GOG-0218 trials show that primary tumor chemosensitivity predicts the overall survival benefit of adding bevacizumab; these results informed the design of ongoing validation trials. If confirmed in those ongoing studies, bevacizumab should be prioritized in patients with a high-risk disease characterized by poor chemosensitivity (unfavorable KELIM score) during the first three cycles of neoadjuvant or adjuvant chemotherapy (patient KELIM score easily calculable on CA-125 Biomarker Kinetics in routine).*
*Relevance section written by JCO Senior Deputy Editor Kathy D. Miller, MD.
Nevertheless, identifying the patients who should be prescribed adjuvant bevacizumab treatment is still a matter of controversy.4,5 Indeed, the final survival analysis report of GOG-0218 did not find any benefit in overall survival (OS) with bevacizumab in the whole population, but suggested a potential OS gain in patients with a stage IV disease (median OS: 42.8 v 32.6 months; hazard ratio [HR], 0.75; 95% CI, 0.59 to 0.95).6 In the ICON-7 trial, an OS benefit was found in the predefined high-risk population (including patients with stage IV, and patients with stage III disease not suitable for surgery or having suboptimal debulking surgery with postoperative residual lesions > 1 cm; median OS: 39.3 v 34.5 months; HR, 0.78; 95% CI, 0.63 to 0.97).7 This discrepancy suggests that, beyond disease stage and completeness of surgery, other covariates should be identified to select the patients likely to benefit from bevacizumab.
Bevacizumab was shown to be active in patients with platinum-resistant recurrent ovarian cancer.8 Therefore, the impact of the tumor chemosensitivity on the efficacy of bevacizumab has been investigated. Several indicators of the tumor intrinsic chemosensitivity have been reported in the literature.9 Among them, the modeled cancer antigen-125 (CA-125) ELIMination rate constant K (KELIM; on the basis of the CA-125 longitudinal kinetics observed during the first 100 treatment days and calculated using mathematical modeling) was shown to be a reproducible indicator of the tumor intrinsic chemosensitivity using data from more than 12,000 patients enrolled in 12 randomized trials, the Netherlands Cancer Registry, and the Gynecologic Cancer InterGroup (GCIG) meta-analysis database.9
The relationship between KELIM and benefit from bevacizumab in the first-line setting was assessed in a post hoc analysis of the ICON-7 trial. Patients with a highly chemosensitive tumor, defined by a favorable KELIM score (≥ 1.0), had no benefit from bevacizumab, whether they had a low-risk or high-risk disease. However, a benefit in OS was found in patients with a high-risk disease exhibiting an unfavorable KELIM score < 1.0 (median OS: 29.7 v 20.6 months; absolute difference, 9.1 months; HR, 0.78; 95% CI, 0.58 to 1.04). An external validation of these data was needed.
The objective of the present project was to perform an external validation study to assess the prognostic and predictive value of KELIM regarding the benefit from bevacizumab in terms of PFS and OS in GOG-0218.
METHODS
Patients and Methods
This study was a retrospective investigation of the GOG-0218 trial (ClinicalTrials.gov identifier: NCT00262847). It was an international, multicenter, double-blind, placebo-controlled, phase III trial, in which 1,873 women with incompletely resected (optimal or suboptimal debulking surgery) stage III to IV disease were randomly assigned 1:1:1—to arm 1: intravenous carboplatin (area under the curve 6) and paclitaxel (175 mg/m2) once every 3 weeks (1 cycle = 3 weeks) for 6 cycles; arm 2: same chemotherapy regimen plus concurrent bevacizumab (15 mg/kg once every 3 weeks on cycles 2-6); or arm 3: chemotherapy plus concurrent and maintenance bevacizumab (15 mg/kg once every 3 weeks on cycles 2-22). The initial reports, where the other inclusion and exclusion criteria were described, demonstrated a benefit in PFS of about 4 months, but the second analysis did not find any benefit in disease-specific survival or OS.2,6
Mathematical Modeling of Longitudinal CA-125 Kinetics and Estimation of Patient Standardized KELIM by the Lyon University Team (France)
The trial design planned that CA-125 values would be locally measured at every cycle. At least three available CA-125 values during the first 100 days of treatment are required to ensure an accurate assessment of KELIM by the model. The mathematical modeling of the early CA-125 kinetics with a nonlinear mixed-effect model was described previously.10-12 To normalize the distribution of CA-125 concentrations, and to eliminate right-skewness in this distribution, CA-125 levels were log-transformed. Basic details about the semimechanistic kinetic-pharmacodynamic model adjustment, and qualification, are presented in the Data Supplement (online only).13
In this study, individual KELIM values were estimated with the model implemented in the online calculator.14 Individual KELIM values were computed using empirical Bayes estimates. As assessed in previous studies,15,16 KELIM was standardized by the prespecified optimized cutoff in an adjuvant setting (cutoff, 0.07/days; prespecified in all studies, including the initial ICON-7 trial), as a way of providing an easy reading of patient KELIM outcome, with the following equation: Standardized (std) KELIM = KELIM estimated by the model/cutoff. As a consequence, std KELIM was a continuous covariate centered on 1.0. To help the interpretation of KELIM for prognostic analyses, KELIM was dichotomized into a KELIM score: std KELIM < 1.0 was considered as unfavorable, whereas std KELIM ≥ 1.0 was considered as favorable.
The data set with patient KELIM scores (favorable or unfavorable) was sent to the NRG-GOG team to ensure an independent assessment of KELIM score prognostic and predictive value.
Assessment of the Prognostic and Predictive Values of KELIM Regarding PFS and OS
The main objective was to confirm a benefit in OS with bevacizumab addition in patients with a high-risk disease characterized by an unfavorable KELIM, as suggested in the ICON-7 trial.
The prognostic value of KELIM score for PFS and OS was assessed using univariate and multivariate tests (C-index, Kaplan-Meier method, log-rank, and Cox model). In addition to KELIM score, the other prognostic factors assessed in univariate and multivariate analyses were disease stage and quality of debulking surgery (stage III operated with optimal surgery with postoperative residual lesions ≤ 1 cm; stage III operated with suboptimal surgery with postoperative residual lesions > 1 cm, or stage IV); pathologic subtype (serous + endometrioid, v others); histologic grade (grade 1, 2, or 3); ascites (yes or no); and treatment arm (arm 1, arm 2, or arm 3). The final C-index and Cox survival models were obtained using backward selections.
Moreover, the predictive value of KELIM regarding the benefit from arm 3 (bevacizumab-concurrent-maintenance group) compared with arm 1 (placebo group) was assessed in the whole population, and then in the population of patients with a high-risk disease (stage IV, and stage III disease operated with suboptimal debulking surgery) using Kaplan-Meier method and log-rank tests.
Cox hazard-ratio regression analyses and chi-square tests were performed to assess the interactions between KELIM and treatment arms regarding PFS and OS benefit from bevacizumab in the whole population, and in patients with a high-risk disease.
All survival analyses were implemented with a landmark time point set at 100 days after the start of neoadjuvant chemotherapy or at the surgery date, whichever occurred first. As CA-125 was modeled from day 0 to 100, the patients who progressed during the first 100 days were excluded to avoid bias related to the links between early progression and CA-125 kinetics, or radiologic tumor responses.17 The median follow-up was computed using reverse Kaplan-Meier method.
Statistics and Computing Process
All tests were implemented using a two-sided 0.05 alpha risk. NONMEM 7.5 (ICON Development Solutions, Ellicott City, MD) software program was used to fit the semimechanistic model to CA-125 kinetic data.18 XPOSE4 program was used for graphical evaluation of model fits.19 The Kaplan-Meier analyses (LIFETEST) and Cox regression analyses (PHREG) were performed using SAS 9.4 (Cary, NC).20
RESULTS
Patient Characteristics
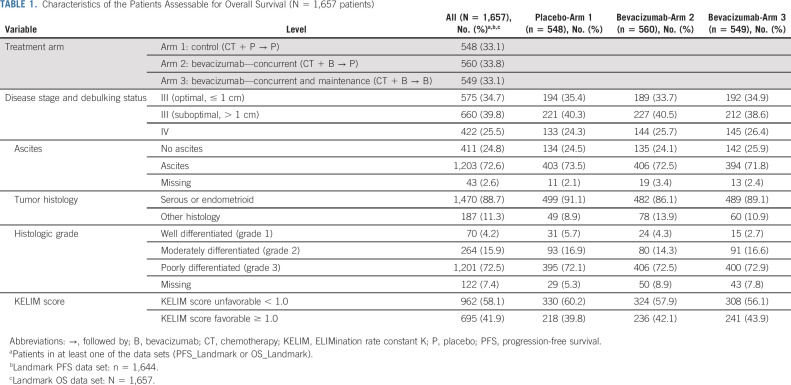
Out of 1,873 enrolled patients, 1,662 patients with ≥ 3 CA-125 available values during the first 100 days of treatment were assessable for KELIM. Among them, 1,644 and 1,657 patients were assessable for KELIM prognostic/predictive value regarding PFS and OS, respectively (87.8% and 88.4%, respectively; Data Supplement). The characteristics of the 1,657 patients assessable for OS are presented in Table 1. Among them, 575 (34.7%) had stage III disease operated with optimal debulking surgery (postoperative residual lesions ≤ 1 cm); 660 (39.8%) had stage III disease operated with suboptimal debulking surgery (postoperative residual lesions > 1 cm); and 422 patients (25.5%) had stage IV disease.
TABLE 1.
Characteristics of the Patients Assessable for Overall Survival (N = 1,657 patients)
Model Qualification
Typical parameter estimates, along with the qualification analyses from the final semimechanistic models, are presented in the Data Supplement.
The median value of KELIM was 0.063 days–1 (95% CI, 0.061 to 0.065). Std KELIM was not different across treatment arms, similar to what has been observed in previous studies (Data Supplement).
Prognostic Value of the KELIM Score Regarding PFS and OS
The median follow-up was 98.1 months for PFS (95% CI, 94.9 to 102.9), and 100.0 months for OS (95% CI, 97.5 to 103.1). The results of the univariate C-index and log-rank tests for PFS and OS are presented in the Data Supplement. In the univariate analyses for both PFS and OS, KELIM score (favorable ≥ 1.0, v unfavorable < 1.0) was a significant factor (Data Supplement), as were the disease stage and quality of debulking surgery (stage III operated with optimal surgery, v stage III operated with suboptimal surgery), ascites (yes v no), and histologic grade (grade 1, 2, or 3), in addition to treatment arm for PFS only (arm 1, arm 2, or arm 3).
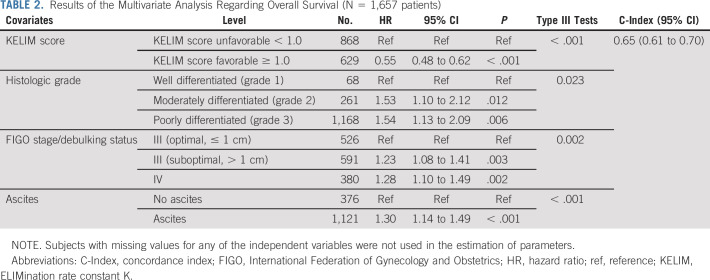
In the final multivariate models of both PFS and OS, the following covariates were significant and independent: KELIM score (favorable ≥ 1.0, v unfavorable < 1.0; PFS: HR, 0.51; 95% CI, 0.46 to 0.57; OS: HR, 0.55; 95% CI, 0.48 to 0.62), disease stage and quality of debulking surgery, histologic grade, and ascites (Table 2; Data Supplement).
TABLE 2.
Results of the Multivariate Analysis Regarding Overall Survival (N = 1,657 patients)
Benefit From Bevacizumab According to KELIM Score and Disease Risk Groups
In the whole assessable population, there was a PFS and OS improvement with bevacizumab-concurrent-maintenance arm 3 compared with the placebo arm 1 for patients with an unfavorable KELIM score (median PFS: 9.8 v 6.1 months; HR, 0.70; 95% CI, 0.59 to 0.82; median OS: 36.3 v 31.8 months; HR, 0.87; 95% CI, 0.73 to 1.03), which was not observed in patients with a favorable KELIM (median PFS: 17.6 v 13.6 months; HR, 0.96; 95% CI, 0.79 to 1.17; median OS; 55.7 v 56.7 months; HR, 1.11; 95% CI, 0.89 to 1.39; Figs 1 and 2).
FIG 1.
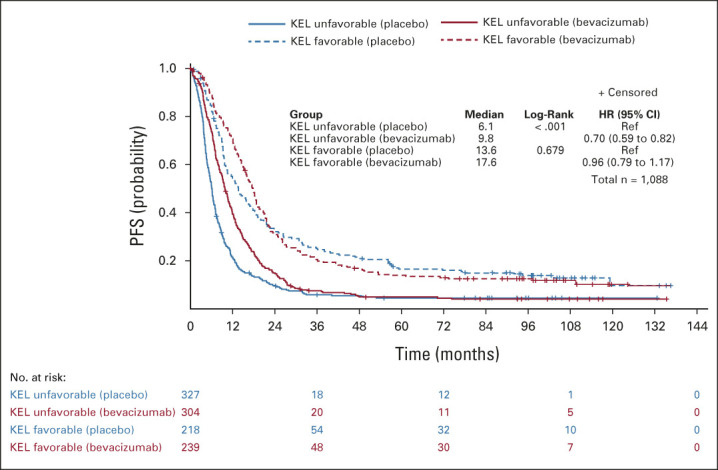
Kaplan-Meier curves of the PFS of patients according to treatment arm (arm 3 with bevacizumab concurrent-maintenance, v arm 1 with placebo) in patients with unfavorable or favorable KELIM (KEL) score, in the whole population. HR, hazard ratio; KELIM, ELIMination rate constant K; mPFS, median PFS (months); PFS, progression-free survival; Ref, reference.
FIG 2.
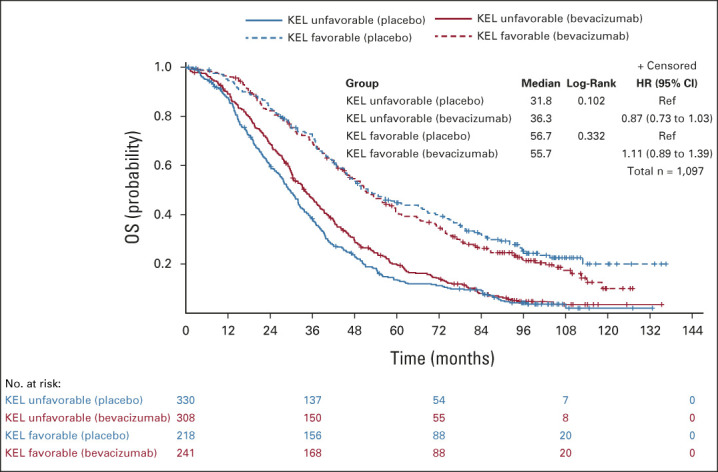
Kaplan-Meier curves of the OS of patients according to treatment arm (arm 3 with bevacizumab concurrent-maintenance, v arm 1 with placebo) in patients with unfavorable or favorable KELIM (KEL) score, in the whole population. HR, hazard ratio; KELIM, ELIMination rate constant K; mPFS, median PFS (months); PFS, progression-free survival; Ref, reference.
The maximum benefit from bevacizumab-concurrent-maintenance arm 3 compared with the placebo arm 1 was observed in patients with a high-risk disease (stage IV, or stage III operated with suboptimal surgery) exhibiting an unfavorable KELIM score (median PFS: 9.1 v 5.6 months; HR, 0.64; 95% CI, 0.53 to 0.78; median OS: 35.1 v 29.1 months; HR, 0.79; 95% CI, 0.65 to 0.97). However, as observed on Kaplan-Meier curves (Figs 3 and 4), the patients with a high-risk and highly chemosensitive disease (favorable KELIM score) did not experience benefit from bevacizumab (median PFS: 16.3 v 11.7 months; HR, 0.88; 95% CI, 0.68 to 1.13; median OS: 59.6 v 49.4 months; HR, 1.05; 95% CI, 0.79 to 1.39).
FIG 3.
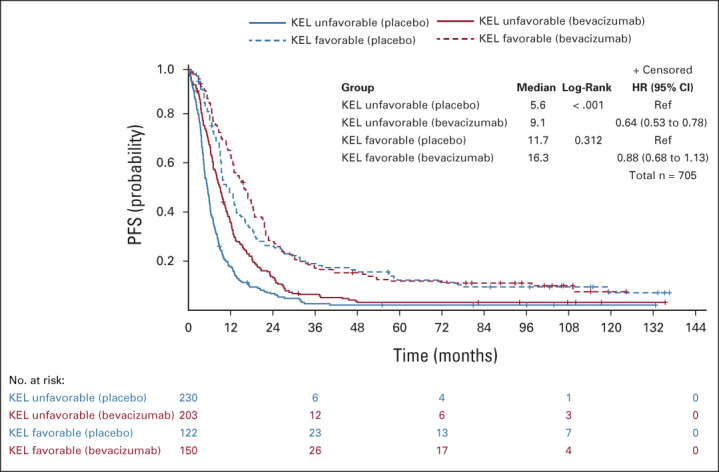
Kaplan-Meier curves of the PFS of patients according to treatment arm (arm 3 with bevacizumab concurrent-maintenance, v arm 1 with placebo) in patients with favorable or unfavorable KELIM (KEL) score, in the population of patients with a high-risk disease (stage IV + stage III operated with suboptimal surgery). HR, hazard ratio; KELIM, ELIMination rate constant K; mPFS, median PFS (months); PFS, progression-free survival; Ref, reference.
FIG 4.
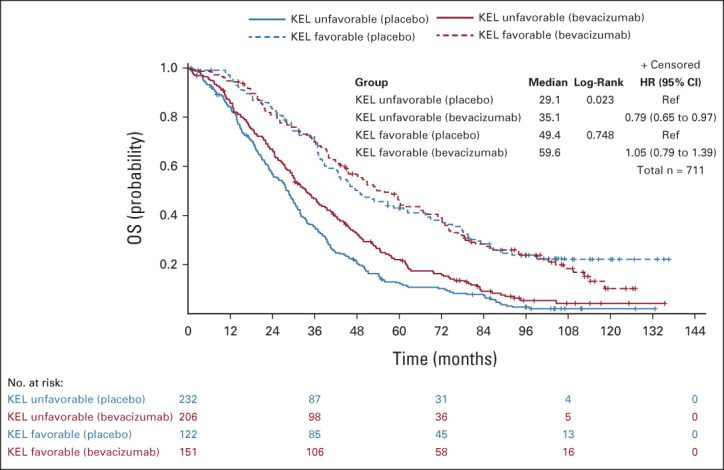
Kaplan-Meier curves of the OS of patients according to treatment arm (arm 3 with bevacizumab concurrent-maintenance, v arm 1 with placebo) in patients with favorable or unfavorable KELIM (KEL) score, in the population of patients with a high-risk disease (stage IV + stage III operated with suboptimal surgery). HR, hazard ratio; KELIM, ELIMination rate constant K; mPFS, median PFS (months); OS, overall survival; PFS, progression-free survival; Ref, reference.
By contrast, bevacizumab was associated with a potential nonsignificant deleterious effect in terms of OS in patients with a low-risk exhibiting favorable KELIM score (median OS: 51.3 v 68.6 months; HR, 1.18; 95% CI, 0.83 to 1.69; Data Supplement).
The interaction tests between KELIM and treatment arms were significant for PFS (in the whole population, P = .01; high-risk disease group, P = .03) and OS in the whole population (P = .08; P = .04 by KELIM strates). However, it was not significant for OS in the high-risk disease group (P = .1), potentially as the consequence of the limited statistical power related to the smaller number of patients, and the more modest effects of bevacizumab on OS compared with PFS.
DISCUSSION
Many studies meant to find reproducible predictors of bevacizumab activity in patients with ovarian carcinoma treated in first-line setting have been reported in the literature.4,5 These attempts highlight the need for biomarkers to identify which patients will benefit from this drug.4,5
After the publication by Oza et al7 showing an OS gain in patients with a high-risk cancer (suboptimally resected stage III, and stage IV disease) in the ICON-7 trial, and those by Tewari et al6 suggesting an OS benefit in patients with a stage IV disease in GOG-0218, the most widely adopted parameter for bevacizumab prescription in routine has been high disease bulk. However, the inconsistency between the two studies suggested additional biomarkers of bevacizumab efficacy were needed for improving the selection of patients.
The AURELIA trial demonstrated that bevacizumab was active in women with platinum-resistant recurrent ovarian cancer,8 and supported the concept of an association between bevacizumab efficacy and tumor chemosensitivity. The investigation of the predictive value of KELIM in the ICON-7 trial suggested that only the patients with poorly chemosensitive disease, characterized by an unfavorable KELIM score, experienced a survival benefit (9 months) from bevacizumab among those with a high-risk disease.21 This finding needs to be verified.
The current external validation study on the GOG-2018 trial data was performed in collaboration with the US NRG-GOG group. The statistical analyses, independently performed by the NGR-GOG statistician team, confirm the initial ICON-7 trial findings. The tumor-intrinsic chemosensitivity assessed by the KELIM score exhibits predictive value for benefit from the addition of bevacizumab in the first-line setting. An unfavorable KELIM score < 1.0 is associated with benefit from bevacizumab, whereas a favorable KELIM score ≥ 1.0 is related to a lack of benefit from bevacizumab.
Consistent with the ICON-7 trial results, the maximum benefit from bevacizumab was observed in patients with a high-risk disease associated with unfavorable KELIM score. The superimposability of the OS curves according to treatment arm and KELIM scores in ICON-7 and GOG-0218 reconciles the data from these two large front-line phase III trials of bevacizumab, and strongly suggests that KELIM optimizes the identification of patients benefiting from this drug (Data Supplement). Bevacizumab should be prioritized in patients with a high-risk and poorly chemosensitive disease. By contrast, it may be avoided in patients with a low-risk disease and favorable KELIM score, as the effect on OS is unclear (Data Supplement).
These data are also consistent with a recent investigation of the predictive value of KELIM score in the ICON-8 trial, suggesting that the patients belonging to a poor prognostic group (characterized by an incomplete debulking surgery and an unfavorable KELIM score) were those who had the maximum benefit from the weekly dose-dense chemotherapy compared with the standard three-weekly regimen.22 Several preclinical studies have suggested that metronomic taxane administration is associated with improved drug delivery and antiangiogenic effects.23,24
The present analysis has several limitations. First, this is a post hoc analysis of the GOG-0218 trial, and the predictive value of KELIM was not prospectively assessed in this trial. The prespecified KELIM score identified 58% of patients with an unfavorable KELIM score and 42% of patients with a favorable KELIM score, which is slightly different from the distribution of the ICON-7 trial (42% and 58%, respectively). In both trials, the percentages of patients with unfavorable KELIM score were higher among those with high-risk disease (63% of patients in the GOG-0218 trial, and 53% of patients in the ICON-7 trial). As per inclusion criteria, a higher proportion of patients with a high-risk disease were enrolled in the GOG-0218 trial compared with the ICON-7 trial, which may explain this difference. The proportionality of hazard assumption was confirmed for Figure 4 (OS in patients with a high-risk disease), but failed for Figures 1-3. To address this issue, the interaction term was computed using the parameter estimates and the standard errors from the models stratified by KELIM. Since the hazard-ratio results and the P values for the interactions (between treatment and KELIM score) between the initial model and the stratified models were significant, the results about the initial model were considered reliable. The nonsignificativity of the interaction tests between KELIM score and treatment arms regarding OS in the patients with high-risk disease might not be related to a lack of discriminative power of KELIM score, but rather to the reduced number of patients, and the lower impact of bevacizumab on OS compared with PFS. Moreover, GOG-0218 was conducted before the emergence of poly(ADP-ribose) polymerase (PARP) inhibitors, which have changed the prognosis and the standard management of patients with ovarian cancer. As a consequence, these results may not be applicable to the current patients who are frequently treated with PARP inhibitors, especially when bevacizumab is given concurrently with olaparib as done in the PAOLA-1 trial.4,25,26 The prognostic value of BRCA mutational status was not assessed in this study, but this covariate was already investigated by Tewari et al, and was not found to be associated with the benefit from bevacizumab.6,27 Integrating this parameter in this study would have led to very small subgroups with limited statistical power. However, a post hoc analysis of the VELIA trial, where patients were treated with carboplatin-paclitaxel ± concurrent and maintenance veliparib, suggested that KELIM could help select the patients with highly chemosensitive disease (favorable KELIM score), who would have maximum benefit from this PARP inhibitor.28
The objective of the present validation study on the GOG-0218 trial was to externally confirm the predictive value of the KELIM score regarding the benefit from bevacizumab in the first-line setting, as initially reported in the ICON-7 trial. Bevacizumab should be prioritized in patients with a high-risk and poorly chemosensitive disease to improve their PFS and OS. Since patient KELIM score can easily be calculated on the basis of a minimum of three CA-125 values observed during the first three cycles of chemotherapy using the online calculator (Biomarker Kinetics29 for patients treated with neoadjuvant chemotherapy, and Biomarker Kinetics14 for patients treated with adjuvant chemotherapy), this parameter could be used routinely for adjusting the disease management.
A prospective validation of KELIM score utility is warranted. The predictive value of KELIM score is being prospectively assessed in the ongoing large international phase III trial NIRVANA-1, which compares the efficacy of niraparib with/without bevacizumab in patients who have undergone complete primary debulking surgery (ClinicalTrials.gov identifier: NCT05183984). Moreover, on the basis of the consistent outcomes from ICON-7, ICON-8, and GOG-0218 trials, a prospective trial will be conducted soon. It will assess the efficacy of a salvage weekly chemotherapy combined with bevacizumab and an innovative chemoresistance reverser in patients with unfavorable KELIM score during neoadjuvant chemotherapy and incomplete interim debulking surgery.
ACKNOWLEDGMENT
The authors thank all the patients and their families, the investigators, study nurses, pharmacists, pathologists, and all study teams.
The following NRG Oncology/Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Oklahoma Health Sciences Center, Washington University School of Medicine, Abington Memorial Hospital—Asplundh Cancer Pavilion, University of Alabama at Birmingham, Women's Cancer Center of Nevada, Memorial Sloan-Kettering Cancer Center, University of Kentucky, Sudarshan K Sharma MD Limited—Gynecologic Oncology, Yale University, Metro-Minnesota CCOP, Roswell Park Comprehensive Cancer Center, Women and Infants Hospital, Mount Sinai School of Medicine, Northwestern University, Morristown Medical Center, Abramson Cancer Center of the University of Pennsylvania, Duke University Medical Center, University of Hawaii, Center of Hope at Renown Medical Center, Washington University School of Medicine, Fox Chase Cancer Center, The Hospital of Central Connecticut, Ohio State University Comprehensive Cancer Center, University of California Medical Center At Irvine-Orange Campus, Mayo Clinic, Walter Reed National Military Medical Center, Saitama Medical University International Medical Center, Gynecologic Oncology Network/Brody School of Medicine, Rush University Medical Center, University of Iowa Hospitals and Clinics, Fred Hutchinson Cancer Research Center, University of Chicago, Cleveland Clinic Foundation, University of Mississippi Medical Center, University of North Carolina at Chapel Hill, Cooper Hospital University Medical Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Seoul National University Hospital, University of Colorado Cancer Center - Anschutz Cancer Pavilion, University of California at Los Angeles Health System, Wake Forest University Health Sciences, University of New Mexico, Stony Brook University Medical Center, University of Virginia, Case Western Reserve University, Fletcher Allen Health Care, Georgia Center for Oncology Research and Education (CORE), Cancer Research for the Ozarks NCORP, Wayne State University/Karmanos Cancer Institute, University of Minnesota Medical Center-Fairview, Northern Indiana Cancer Research Consortium, Tufts-New England Medical Center, University of Pittsburgh Cancer Institute (UPCI), State University of New York Downstate Medical Center, MD Anderson Cancer Center, Moffitt Cancer Center and Research Institute, University of Wisconsin Hospital and Clinics, University of Texas—Galveston, Gynecologic Oncology of West Michigan PLLC, Carle Cancer Center, Cancer Research Consortium of West Michigan NCORP, Central Illinois CCOP, Virginia Commonwealth University, Saint Vincent Hospital, Penn State Milton S Hershey Medical Center, New York University Medical Center, Michigan Cancer Research Consortium Community Clinical Oncology Program, Northern New Jersey CCOP, University of Cincinnati, Memorial Sloan Kettering Cancer Center, University of Massachusetts Memorial Health Care, Aurora Women's Pavilion of Aurora West Allis Medical Center, Kansas City CCOP, Wisconsin NCI Community Oncology Research Program, Missouri Valley Cancer Consortium CCOP, Delaware/Christiana Care CCOP, William Beaumont Hospital, Saint Louis-Cape Girardeau CCOP, and Wichita CCOP.
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by Université Claude Bernard Lyon 1 (France), the employer of Olivier Colomban. Additionally, this project was supported by the following grants received from the National Cancer Institute: U10CA180822 (NRG Oncology SDMC) and U10CA180868 (NRG Oncology Operations).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.01207.
AUTHOR CONTRIBUTIONS
Conception and design: Benoit You, Larry J. Copeland, Elizabeth M. Swisher, Robert Coleman, Bradley J. Monk, Joan L. Walker, Fabio Cappuccini, Olivier Colomban
Administrative support: David Mutch
Provision of study materials or patients: Larry J. Copeland, Michael A. Bookman, Leslie M. Randall, Bradley J. Monk, Robert S. Mannel, Joan L. Walker, David Cohn, David Mutch
Collection and assembly of data: Benoit You, Larry J. Copeland, Robert Coleman, Leslie M. Randall, Krishnansu S. Tewari, Bradley J. Monk, Robert S. Mannel, Joan L. Walker, Mahvish Muzaffar, David Mutch, Andrea Wahner-Hendrickson, Lainie Martin
Data analysis and interpretation: Benoit You, Christopher Purdy, Elizabeth M. Swisher, Michael A. Bookman, Gini Fleming, Robert Coleman, Leslie M. Randall, Krishnansu S. Tewari, Bradley J. Monk, David Cohn, David Mutch, Lainie Martin, Olivier Colomban, Robert A. Burger
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Identification of Patients With Ovarian Cancer Experiencing the Highest Benefit From Bevacizumab in the First-Line Setting on the Basis of Their Tumor-Intrinsic Chemosensitivity (KELIM): The GOG-0218 Validation Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Benoit You
Consulting or Advisory Role: `Roche/Genentech, AstraZeneca, Novartis, LEK, TESARO, Bayer, Amgen, Clovis Oncology, GlaxoSmithKline, ECS PROGASTRIN, Immunomedics, Daiichi Sankyo Europe GmbH, Myriad Genetics, MSD Oncology, Seattle Genetics
Research Funding: Merck Serono (Inst), Roche/Genentech (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, AstraZeneca, BMS, MSD Oncology, Bayer
Larry J. Copeland
Consulting or Advisory Role: Myriad Genetics, GlaxoSmithKline, Elevar Therapeutics, Toray Industries, Rubius Therapeutics, Sorrento Therapeutics, Celsion, Corcept Therapeutics, VBL Therapeutics, Onconova Therapeutics, InxMed, Immunogen
Elizabeth M. Swisher
Leadership: IDEAYA Biosciences
Michael A. Bookman
Employment: The Permanente Medical Group
Consulting or Advisory Role: AstraZeneca, AbbVie, Immunogen, Merck Sharp & Dohme, Genentech/Roche, Seattle Genetics, Aravive
Gini Fleming
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Curio Science, Physicans' Education Resource
Research Funding: Corcept Therapeutics (Inst), AbbVie (Inst), Iovance Biotherapeutics (Inst), Syros Pharmaceuticals (Inst), Sermonix Pharmaceuticals (Inst), Compugen (Inst), Plexxikon (Inst), Roche (Inst), GlaxoSmithKline (Inst), Celldex (Inst), AstraZeneca (Inst), Molecular Templates (Inst), CytomX Therapeutics (Inst), Astellas Pharma (Inst), K-Group Beta (Inst)
Other Relationship: DSI (Inst), Merck (Inst), Caris Life Sciences (Inst), Eisai (Inst), AstraZeneca (Inst)
Uncompensated Relationships: AbbVie
Robert Coleman
Employment: US Oncology
Leadership: Onxeo
Stock and Other Ownership Interests: McKesson
Consulting or Advisory Role: Clovis Oncology, Genentech/Roche, AstraZeneca/MedImmune, Genmab, Tesaro, OncoMed, Sotio, Oncolytics, AbbVie/Stemcentrx, Immunogen, AbbVie, Agenus, Novocure, Merck, OncXerna Therapeutics, Alkermes, Gradalis, Regeneron
Research Funding: AstraZeneca/MedImmune, Esperance Pharmaceuticals, Array BioPharma, Clovis Oncology, Johnson & Johnson, Merck, Roche/Genentech, Abbott/AbbVie, Immunogen (Inst), Mirati Therapeutics (Inst), Amgen (Inst), Pfizer (Inst), Lilly (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Merck, AstraZeneca/MedImmune, Array BioPharma, Clovis Oncology, Roche/Genentech, Research to Practice, GOG Foundation, Sotio, Vaniam Group
Leslie M. Randall
Honoraria: BluPrint Oncology, PER, Curio Science, Projects in Knowledge, Projects in Knowledge
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, GOG Foundation, Merck, Mersana, Agenus, Rubius Therapeutics, Myriad Genetics, EMD Serono, Genentech/Roche, Seattle Genetics, Novartis, Eisai
Speakers' Bureau: AstraZeneca, Tesaro, Merck
Research Funding: Genentech/Roche (Inst), On Target Laboratories (Inst), Pfizer (Inst), Aivita Biomedical (Inst), Tesaro (Inst), AstraZeneca (Inst), Merck (Inst), Akeso Biopharma (Inst), GEICO (Inst)
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology, Merck, Eisai, AstraZeneca, Genmab
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology, Eisai, Genmab
Research Funding: AbbVie (Inst), Genentech/Roche (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Bradley J. Monk
Leadership: US Oncology
Honoraria: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, GOG Foundation, Elevar Therapeutics, Novocure, Gradalis, Karyopharm Therapeutics, Bayer, EMD Serono/Merck, Macrogenics, Sorrento Therapeutics, US Oncology, Myriad Pharmaceuticals, Novartis, OncoC4, Pieris Pharmaceuticals
Consulting or Advisory Role: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Myriad Pharmaceuticals, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure, Bayer, Elevar Therapeutics, EMD Serono/Merck, Gradalis, US Oncology, Novartis, Pieris Pharmaceuticals, OncoC4
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, TESARO/GSK, Merck
Research Funding: Novartis (Inst), Amgen (Inst), Genentech (Inst), Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), Immunogen (Inst), Regeneron (Inst), Nucana (Inst)
Robert S. Mannel
Stock and Other Ownership Interests: Edwards Lifesciences, Stryker, Danaher
Joan L. Walker
Research Funding: us biotest, Genentech, Pieces Tech (Inst)
Fabio Cappuccini
Honoraria: GlaxoSmithKline, AstraZeneca
Speakers' Bureau: GlaxoSmithKline, AstraZeneca
Patents, Royalties, Other Intellectual Property: sponsored programs
David Cohn
Research Funding: NRG Oncology (Inst), Advaxis (Inst), Agenus (Inst), Ajinomoto (Inst), Array BioPharma (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), Ergomed (Inst), Exelixis (Inst), Genentech (Inst), GlaxoSmithKline (Inst), Gynecologic Oncology Group (Inst), ImmunoGen (Inst), INC Research (Inst), inVentiv Health (Inst), Janssen Research & Development (Inst), Ludwig Institute for Cancer Research (Inst), PRA International (Inst), EMD Serono (Inst), Stemcentrx (Inst), Tesaro (Inst), AbbVie (Inst), Henry Jackson Foundation (Inst), PharmaMar (Inst), Sanofi (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Regeneron (Inst), Tricon Pharmaceuticals (Inst)
Other Relationship: Elsevier, UpToDate
David Mutch
Consulting or Advisory Role: lilly
Andrea Wahner-Hendrickson
Consulting or Advisory Role: Oxcia
Research Funding: ProLynx (Inst), Amgen (Inst)
Lainie Martin
Consulting or Advisory Role: Sutro Biopharma, Elucida Oncology, GlaxoSmithKline
Research Funding: Agenus (Inst), AstraZeneca (Inst), Sutro bioPharma (Inst), Immunogen (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Robert A. Burger
Employment: Genentech/Roche, Mersana
Stock and Other Ownership Interests: Genentech/Roche, Mersana
Consulting or Advisory Role: Myriad Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.US Food and Drug Administration: FDA approves bevacizumab in combination with chemotherapy for ovarian cancer. 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-bevacizumab-combination-chemotherapy-ovarian-cancer
- 2.Burger RA, Brady MF, Bookman MA, et al. : Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Perren TJ, Swart AM, Pfisterer J, et al. : A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484-2496, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Colombo N, Sessa C, Bois AD, et al. : ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer 30:672-705, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Collinson F, Hutchinson M, Craven RA, et al. : Predicting response to bevacizumab in ovarian cancer: A panel of potential biomarkers informing treatment selection. Clin Cancer Res 19:5227-5239, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tewari KS, Burger RA, Enserro D, et al. : Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol 37:2317-2328, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oza AM, Cook AD, Pfisterer J, et al. : Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol 16:928-936, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pujade-Lauraine E, Hilpert F, Weber B, et al. : Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 32:1302-1308, 2014 [DOI] [PubMed] [Google Scholar]
- 9.You B, Freyer G, Gonzalez-Martin A, et al. : The role of the tumor primary chemosensitivity relative to the success of the medical-surgical management in patients with advanced ovarian carcinomas. Cancer Treat Rev 100:102294, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Colomban O, Tod M, Leary A, et al. : Early modeled longitudinal CA-125 kinetics and survival of ovarian cancer patients: A GINECO AGO MRC CTU study. Clin Cancer Res 25:5342-5350, 2019 [DOI] [PubMed] [Google Scholar]
- 11.You B, Colomban O, Heywood M, et al. : The strong prognostic value of KELIM, a model-based parameter from CA 125 kinetics in ovarian cancer: Data from CALYPSO trial (a GINECO-GCIG study). Gynecol Oncol 130:289-294, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Lauby A, Colomban O, Corbaux P, et al. : The increasing prognostic and predictive roles of the tumor primary chemosensitivity assessed by CA-125 elimination rate constant K (KELIM) in ovarian cancer: A narrative review. Cancers (Basel) 14:98, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dartois C, Brendel K, Comets E, et al. : Overview of model-building strategies in population PK/PD analyses: 2002-2004 literature survey. Br J Clin Pharmacol 64:603-612, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biomarker Kinetics: Modeled CA-125 KELIM™ in patients with stage III-IV high grade serous ovarian carcinomas treated with first line adjuvant chemotherapy. https://www.biomarker-kinetics.org/CA-125
- 15.Van Wagensveld L, Colomban O, Van der AA MA, et al. : The prognostic value of chemosensitivity, estimated by the modeled CA-125 KELIM, in ovarian cancer patients treated with neo-adjuvant chemotherapy in the Netherlands. Ann Oncol 31, 2020. (suppl; abstr 847P) [Google Scholar]
- 16.You B, Robelin P, Tod M, et al. : CA-125 ELIMination rate constant K (KELIM) is A marker of chemosensitivity in patients with ovarian cancer: Results from the phase II CHIVA trial. Clin Cancer Res 26:4625-4632, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Heller G, McCormack R, Kheoh T, et al. : Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: A comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol 36:572-580, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beal SL, Boeckmann A, Bauer R, et al. : NONMEM User’s Guides (1989–2009). Ellicott City, MD, Icon, 2009 [Google Scholar]
- 19.Jonsson EN, Karlsson MO: Xpose—An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51-64, 1999 [DOI] [PubMed] [Google Scholar]
- 20.van Driel WJ, Koole SN, Sikorska K, et al. : Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 378:230-240, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Colomban O, Tod M, Peron J, et al. : Bevacizumab for newly diagnosed ovarian cancers: Best candidates among high-risk disease patients (ICON-7). JNCI Cancer Spectr 4:pkaa026, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You B, Clamp A, Cook A, et al. : Differential benefit from fractionated dose-dense first-line chemotherapy for epithelial ovarian cancer (EOC) according to KELIM-evaluated tumor primary chemosensitivity: Exploratory analyses of ICON-8 trial. J Clin Oncol 39, 2021. (suppl 15; abstr 5530) [Google Scholar]
- 23.Clamp AR, James EC, McNeish IA, et al. : Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): Primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 394:2084-2095, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Printezi MI, Kilgallen AB, Bond MJG, et al. : Toxicity and efficacy of chronomodulated chemotherapy: A systematic review. Lancet Oncol 23:e129-e143, 2022 [DOI] [PubMed] [Google Scholar]
- 25.Wright AA, Bohlke K, Armstrong DK, et al. : Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 34:3460-3473, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tew WP, Lacchetti C, Ellis A, et al. : PARP inhibitors in the management of ovarian cancer: ASCO Guideline. J Clin Oncol 38:3468-3493, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norquist BM, Brady MF, Harrell MI, et al. : Mutations in homologous recombination Genes and outcomes in ovarian carcinoma patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group study. Clin Cancer Res 24:777-783, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You B, Sehgal V, Hosmane B, et al. : The CA-125 KELIM as a potential complementary tool for predicting veliparib benefit: An exploratory analysis from the VELIA/GOG-3005 Study. J Clin Oncol 10.1200/JCO.22.00430 [epub ahead of print on July 22, 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biomarker Kinetics: Modeled CA-125 KELIM™ in patients with stage III-IV high grade serous ovarian carcinomas treated with first line neo-adjuvant chemotherapy. https://www.biomarker-kinetics.org/CA-125-neo
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.01207.