To the Editor: Remimazolam besylate is a novel, ultra-short-acting benzodiazepine with a rapid and a predictable onset and offset profile, no accumulation and extended effect, and independence of organ elimination.[1,2] There have been several reports showing the application of remimazolam besylate for sedation in short-term procedures and general anesthesia, however, no study has reported its use in the intensive care unit (ICU).[3,4] The aim of this study is to investigate the infusion doses of remimazolam besylate that provide a light to moderate level of sedation in mechanically ventilated patients after noncardiac surgeries in ICUs.
The study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (2020-0384-01), and written informed consent was obtained from all patients or their representatives according to the Declaration of Helsinki. The study was registered before enrollment at the Chinese Clinical Trial Registry (Registration number: ChiCTR2000039343).
Patients were consecutively recruited between November 2020 and March 2021 from the general ICU of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. The inclusion criteria were patients aged between 18 and 75 years old, admitted to the ICU after non-cardiac surgeries, expected to receive a postoperative sedation and mechanical ventilation for 8 to 24 h. The exclusion criteria included previous inclusion into this study or another interventional study in 3 months; a history of psychiatric disorders, acute glaucoma or myasthenia gravis; serious central nervous system disorders (acute stroke, uncontrolled seizure, or severe dementia); inability to communicate because of brain injury; liver or kidney transplant; a body mass index <18 or >30 kg/m2; unstable angina or acute myocardial infarction; left ventricular ejection fraction less than 30%; severe sinus bradycardia (<50 beats per minute); second-degree or higher atrioventricular block without a pacemaker; systolic blood pressure less than 90 mmHg despite appropriate intravenous volume replacement and vasopressors; any contraindication or allergy to benzodiazepines; acute hepatitis or serious hepatic dysfunction (Child-Pugh class C); chronic kidney disease (glomerular filtration rate < 60 ml·min−1·1.73 m−2); a history of drug or alcohol abuse; pregnancy or lactating.
Patients included were sequentially enrolled into one of the six possible treatment groups: an infusion dose of remimazolam besylate (Yichang Human well Pharmaceutical Co., Ltd., Hubei, China) 0.100, 0.125, 0.150, 0.175, 0.200, and 0.225 mg·kg−1·h−1, respectively. Up to 12 patients were enrolled into each group. On admission into our ICU, an included patient received a continuous infusion of sufentanil at 0.2 to 0.3 μg·kg−1·h−1 for analgesia. When the Richmond Agitation-Sedation Scale (RASS) score was ≥1, remimazolam besylate was given with a loading dose of 0.05 mg/kg followed by a continuous infusion according to their grouping. Propofol provided rescue sedation if required. The RASS score was measured and recorded every 2 h. The sedation goal was a RASS score of –3 to 0. If adequate sedation was not achieved, 0.2 mg/kg propofol boluses could be given. If more than three boluses were required within a 1-h period, propofol was continuously infused at 0.5 to 4.0 mg·kg−1·h−1. The infusion of remimazolam besylate could be decreased if RASS score ≤–4. Remimazolam besylate was infused until the weaning of mechanical ventilation or a maximum duration of 24 h. If a patient still required sedation after 24 h, other sedatives were given at the discretion of treating physicians.
The stop criterion of our study was at least nine patients did not need rescue propofol in both two consecutive groups. The primary outcome was to assess the infusion doses of remimazolam besylate that provide a light to moderate level of sedation in mechanically ventilated patients after noncardiac surgeries. Frequency of bolus rescue propofol was recorded along with infusion rates and duration of infusion. Heart rate and mean arterial pressure were recorded. Adverse events (AEs) included hypotension (mean arterial pressure less than 60 mmHg, requiring the infusion or increase the dose of vasopressors), bradycardia (heart rate less than 50 beats per minute), tachycardia (heart rate greater than 120 beats per minute), nausea and vomiting.
Sample size estimation was not conducted. Categorical variables were described as number (percentage) and were compared using the Chi-square test or Fisher exact test. Normally distributed continuous variables were described as mean (standard deviation) and were analyzed using one-way analysis of variance, and other continuous variables were described as medians (interquartile range [IQR]) and were analyzed using Kruskal–Wallis H test. Differences over time were explored using the paired sample t test. A P value of <0.05 was considered statistically significant. All analyses were performed using SPSS version 20.0 (IBM SPSS Statistics, Armonk, NY, USA) and GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA).
The predefined stop criterion was reached, when in both 0.125 mg·kg−1·h−1 group and 0.15 mg·kg−1·h−1 group, nine of 12 patients did not need rescue propofol. For the 36 patients included in our study, their mean age was 55.3 ± 13.6 years, 23 (63.9%) patients were male, and their mean weight was 61.5 ± 10.6 kg. Twenty-one (58.3%) patients had comorbidities, and the most common one was hypertension. The most common surgical sites were spine and extremities. The demographics of patients were similar across the three groups [Supplementary Table 1]. Results of common laboratory tests at ICU admission were presented in Supplementary Table 2.
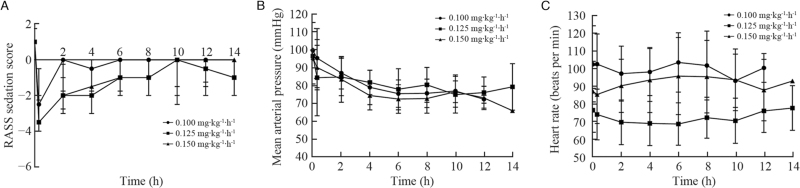
The onset of sedation was rapid, with the median RASS scores decreasing below –2 within 1 min of a loading dose [Figure 1A]. Except one patient in 0.15 mg kg−1 h−1 group experienced oversedation (four consecutive RASS scores of –4) and required a dose reduction, other patients were well-maintained at light and moderate sedation levels during the study period.
Figure 1.
(A) Median (interquartile range) RASS score for each remimazolam besylate group. (B) Mean (standard deviation) of mean arterial pressure for each remimazolam besylate group. (C) Mean (standard deviation) of heart rate for each remimazolam besylate group. RASS: Richmond Agitation-Sedation Scale.
For all the patients included, their mean duration of remimazolam besylate infusion was 11.3 ± 2.0 h, and the median length of mechanical ventilation and ICU stay was 15.0 (IQR 13.0, 19.0) h and 19.0 (IQR 16.0, 24.5) h, respectively. Requirement for rescue sedation was shown in Supplementary Table 3. Two patients in 0.100 and 0.125 mg·kg−1·h−1 group only received propofol boluses, respectively. Four patients in 0.10 mg·kg−1·h−1 group required continuous propofol infusion of 38.6 to 165.0 mg/h for 7 to 11 h, one patient in 0.125 mg·kg−1·h−1 group required 35.0 mg/h for 6 h, three patients in 0.15 mg·kg−1·h−1 group required 35.4 to 78.8 mg/h for 6 to 8 h.
After a loading dose of remimazolam besylate, mean arterial pressure dropped from 97.3 ± 15.0 mmHg to 90.0 ± 17.3 mmHg (P < 0.05), and there was a moderate decrease during the infusion [Figure 1B]. Heart rate did not change significantly between before and after a loading dose (88.7 ± 20.1 mmHg vs. 87.3 ± 19.6 mmHg, respectively, P = 0.295), as well as over the study period [Figure 1C].
AEs were identified in 13 patients [Supplementary Table 4]. The most frequent AEs were hypotension and tachycardia, which were experienced by nine and six patients, respectively. One patient developed hypotension at the loading dose of remimazolam besylate and no intervention was required, the other eight needed vasopressors and/or intravenous fluids. No deaths, serious AEs, or treatment discontinuations were identified.
As far as we know, this is the first dose-finding study of remimazolam besylate in mechanically ventilated ICU patients. Our results suggest that continuous infusion of remimazolam besylate between 0.125 and 0.15 mg·kg−1·h−1 provides a light to moderate level of sedation in mechanically ventilated patients after noncardiac surgeries, with a rapid onset and favorable safety profile and hemodynamic stability.
This study has several limitations. First, since patients of cardiac surgeries were not included in our study, the generality to these patients should be cautious. Second, the sample size was small. However, for a phase I dose-finding study, it is acceptable.[1,5] Third, comparison to other sedatives and a longer time sedation were not conducted. Both were the goals of our study in the next step, which was already registered at ClinicalTrials.gov (registration number NCT04790734).
In conclusion, continuous infusion of remimazolam besylate between 0.125 and 0.150 mg·kg−1·h−1 provides a light to moderate level of sedation in mechanically ventilated ICU patients after noncardiac surgeries. Remimazolam appeared to be an effective and safe agent for short-term postoperative sedation in ICUs. Further studies are needed to compare remimazolam with traditional sedation agents to evaluate its efficacy and safety for long-term sedation in ICU patients.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Tang Y, Yang X, Shu H, Yu Y, Xu J, Pan S, Zou X, Yuan S, Shang Y. Remimazolam besylate for sedation of postoperative patients in intensive care units: a phase I, open label, dose-finding study. Chin Med J 2022;135:2134–2136. doi: 10.1097/CM9.0000000000002243
Supplemental digital content is available for this article.
References
- 1.Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg 2012; 115:274–283. doi: 10.1213/ANE.0b013e31823f0c28. [DOI] [PubMed] [Google Scholar]
- 2.Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol 2020; 60:505–514. doi: 10.1002/jcph.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth 2020; 34:543–553. doi: 10.1007/s00540-020-02788-6. [DOI] [PubMed] [Google Scholar]
- 4.Borkett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, et al. A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg 2015; 120:771–780. doi: 10.1213/ANE.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 5.Ivy SP, Siu LL, Garrett-Mayer E, Rubinstein L. Approaches to Phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the clinical trial design task force of the National Cancer Institute Investigational Drug Steering Committee. Clin Cancer Res 2010; 16:1726–1736. doi: 10.1158/1078-0432.CCR-09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.