PURPOSE
The FMS-related tyrosine kinase 3 (FLT3) inhibitor gilteritinib is standard therapy for relapsed/refractory FLT3-mutated (FLT3mut) acute myeloid leukemia (AML) but seldom reduces FLT3mut burden or induces sustained efficacy. Gilteritinib combines synergistically with the BCL-2 inhibitor venetoclax in preclinical models of FLT3mut AML.
METHODS
This phase Ib open-label, dose-escalation/dose-expansion study (ClinicalTrials.gov identifier: NCT03625505) enrolled patients with FLT3 wild-type and FLT3mut (escalation) or FLT3mut (expansion) relapsed/refractory AML. Patients received 400 mg oral venetoclax once daily and 80 mg or 120 mg oral gilteritinib once daily. The primary objectives were safety, identification of the recommended phase II dose, and the modified composite complete response (mCRc) rate (complete response [CR] + CR with incomplete blood count recovery + CR with incomplete platelet recovery + morphologic leukemia-free state) using ADMIRAL phase III–defined response criteria.
RESULTS
Sixty-one patients were enrolled (n = 56 FLT3mut); 64% (n = 36 of 56) of FLT3mut patients had received prior FLT3 inhibitor therapy. The recommended phase II dose was 400 mg venetoclax once daily and 120 mg gilteritinib once daily. The most common grade 3/4 adverse events were cytopenias (n = 49; 80%). Adverse events prompted venetoclax and gilteritinib dose interruptions in 51% and 48%, respectively. The mCRc rate for FLT3mut patients was 75% (CR, 18%; CR with incomplete blood count recovery, 4%; CR with incomplete platelet recovery, 18%; and morphologic leukemia-free state, 36%) and was similar among patients with or without prior FLT3 inhibitor therapy (80% v 67%, respectively). The median follow-up was 17.5 months. The median time to response was 0.9 months, and the median remission duration was 4.9 months (95% CI, 3.4 to 6.6). FLT3 molecular response (< 10−2) was achieved in 60% of evaluable mCRc patients (n = 15 of 25). The median overall survival for FLT3mut patients was 10.0 months.
CONCLUSION
The combination of venetoclax and gilteritinib was associated with high mCRc and FLT3 molecular response rates regardless of prior FLT3 inhibitor exposure. Dose interruptions were needed to mitigate myelosuppression.
INTRODUCTION
Despite advances in frontline treatment with recently approved therapies for acute myeloid leukemia (AML), most patients experience relapsed/refractory (R/R) disease.1,2 R/R AML has a median overall survival (OS) of 4-7 months with standard chemotherapy approaches,3-7 emphasizing the importance of newly approved targeted therapies and the need for additional treatment options.8-10
CONTEXT
Key Objective
The FLT3 inhibitor gilteritinib is highly active in advanced, FLT3-mutated (FLT3mut) acute myeloid leukemia (AML) but not curative. We sought to develop a tolerable combination regimen for outpatient use with improved response rate, depth, and durability relative to gilteritinib alone. The BCL‐2 inhibitor venetoclax is synergistic with gilteritinib in preclinical models of FLT3mut AML, but this combination has not been previously studied clinically.
Knowledge Generated
Through a multicenter phase Ib study in patients with relapsed/refractory FLT3mut AML, we showed that the combination of venetoclax and gilteritinib was tolerable at standard doses of each drug, generated remarkably high response rates, and markedly reduced FLT3-internal tandem duplications mutation burden. The major toxicity was myelosuppression, which was manageable with dosing modification. Early mortality was similar to gilteritinib monotherapy.
Relevance
The combination of venetoclax and gilteritinib is a highly active and tolerable oral combination regimen that potentially improves response frequency and depth over existing standards in a high-risk, mutation-defined group of patients with AML.
Activating mutations in FMS-related tyrosine kinase 3 (FLT3), including internal tandem duplications (FLT3-ITD) and tyrosine kinase domain mutations (FLT3-TKD), occur in approximately 30% of newly diagnosed AML cases.11-13 FLT3-ITD mutations are associated with higher relapse rates and reduced survival in newly diagnosed and R/R AML.14-17 FLT3 inhibition is a successful clinical strategy for treating FLT3-mutated (FLT3mut) AML, with FLT3-targeting tyrosine kinase inhibitors (TKIs) midostaurin and gilteritinib currently approved.11 Gilteritinib, a selective, potent oral FLT3 inhibitor with activity against FLT3-ITD and FLT3-TKD AML,18,19 was approved for patients with R/R FLT3mut AML on the basis of improved response and survival versus salvage chemotherapy in the phase III ADMIRAL study.8
Although single-agent gilteritinib has improved treatment of R/R FLT3mut AML, the 2-year OS rate is approximately 20%20 and few patients achieve deep and/or durable responses. Of responding patients, only 25% achieved molecular response (defined as FLT3 variant allele frequency [VAF] < 10−2), a benchmark appearing to predict better survival.21-23 Furthermore, emerging data suggest that gilteritinib alone may have reduced efficacy in patients with R/R FLT3mut AML who received prior FLT3 TKIs, which are now routinely incorporated into frontline therapy.24-26 Post hoc analysis of the ADMIRAL trial revealed numerically shorter median OS in those with previous exposure to FLT3 TKIs versus those without (6.5 v 9.6 months), despite similar composite complete response (CRc) rates (48% v 55%).24 Preclinical and clinical correlative studies of FLT3 inhibitors also indicate that a major mechanism of response is induction of terminally differentiated leukemic blasts to neutrophils; however, in many cases, this mechanism alone is not sufficient to completely eradicate FLT3mut clones without additional therapy.27-29 Improving gilteritinib efficacy likely requires combination with other antileukemic agents to avoid clonal evolution of persistent FLT3mut clones.30
Venetoclax, a selective, oral BCL-2 inhibitor, is approved and a standard treatment in combination with low-dose cytarabine or hypomethylating agents for newly diagnosed AML in patients ineligible for intensive chemotherapy.31,32 Although single-agent venetoclax has limited activity in R/R AML,33 in vitro studies have shown synthetic lethality with venetoclax combined with FLT3 inhibitors in preclinical models.34-37 We reasoned that this combination might induce earlier, deeper elimination of FLT3mut clones that drive chemotherapy-resistant disease. This study evaluated venetoclax combined with gilteritinib (VenGilt) in patients with R/R FLT3mut AML.
METHODS
Study Design and Conduct
This phase Ib, multicenter, open-label study (ClinicalTrials.gov identifier: NCT03625505; Data Supplement, online only) enrolled patients from 11 US centers. Dose escalation used a Bayesian optimal interval design to establish a maximum tolerated dose and recommended phase II dose (RP2D) that were explored in the dose-expansion portion (Data Supplement). The study protocol was approved by the Institutional Review Board or Ethics Committee at each participating institution and was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Patients
Patients age ≥ 18 years diagnosed with AML per the WHO (2016)38 who failed ≥ 1 prior line of AML therapy were eligible (no salvage limit). Patients had an Eastern Cooperative Oncology Group performance status of 0-2, adequate liver and kidney function, no history of advanced heart failure or long-QT syndrome, and white blood cell counts ≤ 25 × 109/L at study drug initiation. Hydroxyurea was permitted for cytoreduction (screening through cycle 1). Patients in dose escalation could have FLT3mut or FLT3-wild type (FLT3WT) R/R AML. Patients in dose expansion had to have documented FLT3 mutation (ITD or TKD) in the bone marrow (BM) or peripheral blood per local laboratory assay. Previous exposure to venetoclax and/or FLT3 TKIs was allowed. Prior gilteritinib exposure was only allowed for dose escalation. The Protocol (online only) lists full enrollment criteria.
Treatment and Assessments
Gilteritinib was given orally, once daily beginning cycle 1/day 1 at 80 or 120 mg for dose escalation. Venetoclax was given orally, once daily starting cycle 1/day 2 with 3-day dose ramp-up (day 2, 100 mg; day 3, 200 mg; and days 4-28, 400 mg) and continued at 400 mg for cycles 2 and beyond. Protocol-specified optional higher venetoclax dose cohorts (600-800 mg) were not explored on the basis of satisfactory results from the 400 mg cohorts. Venetoclax dose was adjusted for concomitant use of moderate/strong CYP3A inhibitors per the US Food and Drug Administration label39 (strong CYP3A inducers were prohibited; Protocol). VenGilt was given in 28-day cycles and continued until disease progression, unacceptable toxicity, consent withdrawal, physician decision, or noncompliance with study procedures. Patients received prophylaxis for tumor lysis syndrome, including hydration, uric acid–reducing agents, and blood chemistry monitoring, from the day before through 24 hours after ramp-up. Growth factor support was allowed per investigator discretion after achievement of BM remission (< 5% blasts) or in the neutropenic sepsis setting. Dose-limiting toxicities (DLTs) were defined as the following events occurring within the DLT evaluation period (during cycle 1): grade ≥ 4 nonhematologic toxicity; absolute neutrophil count (ANC) < 500/μL (grade 4) or platelets < 25,000/μL (grade 4) for > 14 days off therapy without evidence of leukemia (< 5% blasts) in the BM or blood, or > 42 days from therapy initiation, whichever is longer. Disease assessments were performed using BM samples collected at screening, cycle 1/day 28, and every three cycles thereafter. Responses were evaluated on the basis of guidelines adapted from the International Working Group for AML.40 FLT3-ITD measurable residual disease was assessed using next-generation sequencing of DNA isolated from BM aspirates collected at protocol-specified time points (screening, cycle 1/day 28, and every three cycles thereafter) and ad hoc (after first study drug dose) with a detection limit of < 10−6 (Invivoscribe, San Diego, CA). Molecular response was defined as FLT3-ITD VAF < 10−2 as previously published.21,22 The Data Supplement describes peripheral blood collection for pharmacokinetic analyses, molecular data assessments, and electrocardiogram assessments.
Outcomes
Primary objectives in dose escalation were to assess safety of VenGilt, characterize DLTs, determine the RP2D, and describe pharmacokinetic parameters of venetoclax and gilteritinib. In dose expansion, the primary objective was to evaluate VenGilt efficacy using modified composite complete response (mCRc) rate among RP2D-treated patients with FLT3mut R/R AML. CRc rate was defined as the rate of complete response (CR) + CR with incomplete platelet recovery (CRp) + CR with incomplete blood count recovery (CRi); mCRc was defined to match ADMIRAL response criteria (CRc + morphologic leukemia-free state [MLFS]).8 Secondary objectives were to evaluate VenGilt safety at the RP2D and further evaluate efficacy, including CRc rate, mCRc duration of response (DOR), CR + CR with partial hematologic recovery rate, and CR + CR with partial hematologic recovery DOR. Exploratory objectives were OS, CRc DOR, and correlative biomarker evaluation.
Statistical Analyses
Planned enrollment was originally 34 patients in dose expansion on the basis of a modified Simon's Minimax 2-stage design33 using CRc as the primary end point. We assumed a historical CRc rate of 46% (on the basis of the CHRYSALIS study with single-agent gilteritinib18) and a target CRc rate of 70%, yielding a one-sided type I error rate of 2.5% and a power of 80%. After publication of the ADMIRAL study, which reported responses as CRc + MLFS, the primary end point was updated to match the same criteria of CRc + MLFS (termed mCRc in this study) to allow VenGilt data to be put into context with single-agent gilteritinib outcomes.8 At the time of this change, 51 patients were enrolled in the trial. On the basis of the primary end point change, the target mCRc rate was updated to 86.2% and planned enrollment was updated to 46 patients in dose expansion (yielding approximately 50 patients with R/R FLT3mut AML to be treated at the RP2D in escalation/expansion) to attain a precision that we defined as a Clopper-Pearson 95% CI for a mCRc of 73% to 94%.
Safety and efficacy assessments included patients who received ≥ 1 dose of study drug. Response rates were summarized with Clopper-Pearson 95% CIs. OS and DOR were assessed as medians with corresponding 95% CI using Kaplan-Meier estimation. DOR was defined as the time from achieving response until initiation of subsequent anticancer therapy or allogeneic stem-cell transplant (alloSCT), progressive disease, or death. Pharmacokinetic parameters were determined using noncompartmental methods. Adverse events (AEs) were graded on the basis of the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
RESULTS
Patients and Disposition
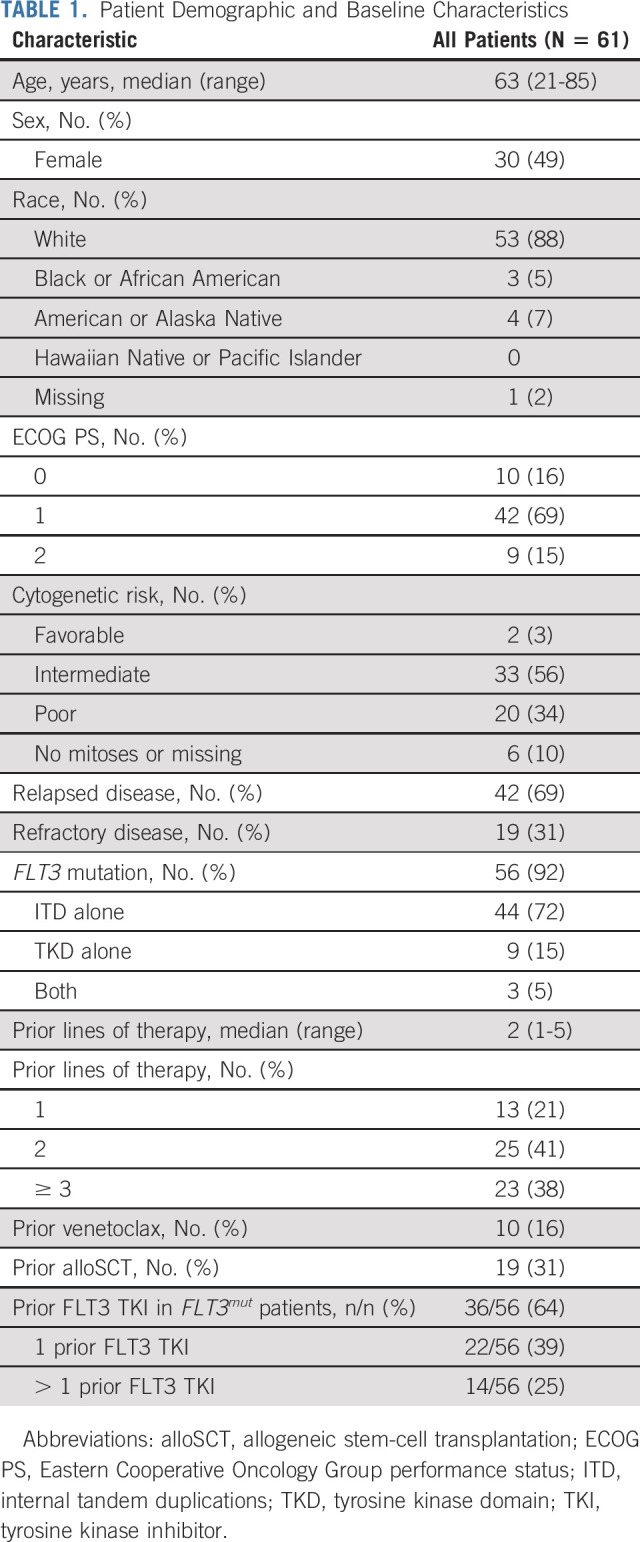
Between October 29, 2018, and December 30, 2020, 61 patients were enrolled (Fig 1). The median age was 63 (range, 21-85) years; 19 of 61 patients (31%) received prior alloSCT, 10 of 61 (16%) received prior venetoclax, and none received prior gilteritinib (Table 1). Of 56 patients with FLT3mut, 36 (64%) had prior FLT3 TKIs (14 of 56 [25%] received > 1).
FIG 1.
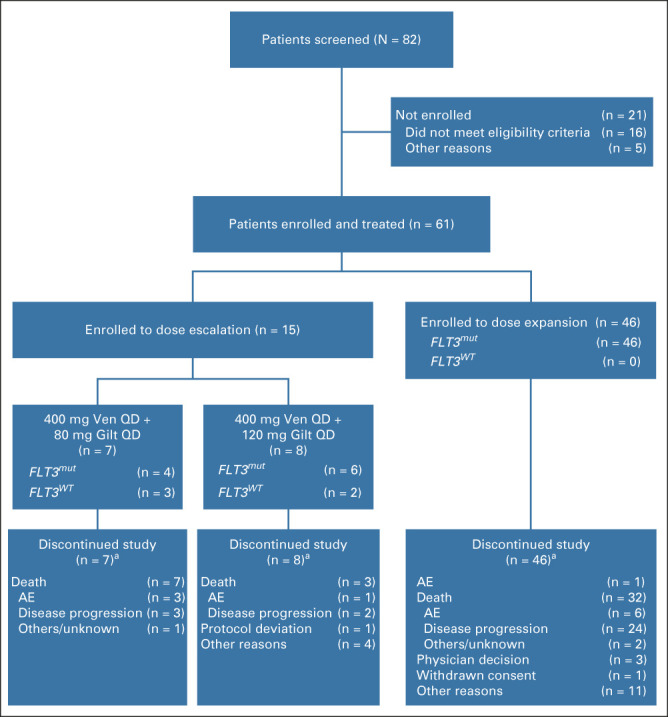
Patient enrollment and disposition. Data cutoff, November 10, 2021. aSome patients had multiple reasons given for study discontinuation. AE, adverse event; Gilt, gilteritinib; QD, once daily; Ven, venetoclax.
TABLE 1.
Patient Demographic and Baseline Characteristics
The median duration of exposure was 2.6 (range, 0.07-16.8) months for venetoclax (Data Supplement) and 2.6 (range, 0.1-17.2) months for gilteritinib (Data Supplement). All patients have discontinued the study as of the data cutoff of November 10, 2021 (Fig 1).
Dose Escalation and Pharmacokinetics
Fifteen patients were enrolled to dose escalation (Fig 1 and Data Supplement). Five patients had FLT3WT AML. One patient, who received 80 mg gilteritinib once daily, experienced a DLT of prolonged myelosuppression with hypocellular BM. Both drugs were held until counts recovered after treatment with granulocyte colony-stimulating factor (G-CSF).
Venetoclax exposures did not appear to vary with increasing doses of gilteritinib (Data Supplement). Exposures of venetoclax and gilteritinib when coadministered were similar to those described for each drug alone,41,42 indicating no apparent drug-drug interaction. Although the protocol allowed for higher venetoclax dose cohorts, 400 mg venetoclax once daily plus 120 mg gilteritinib once daily was chosen as the RP2D because of achievement of sufficient response rates and concerns of worsening myelosuppression at higher doses of venetoclax.
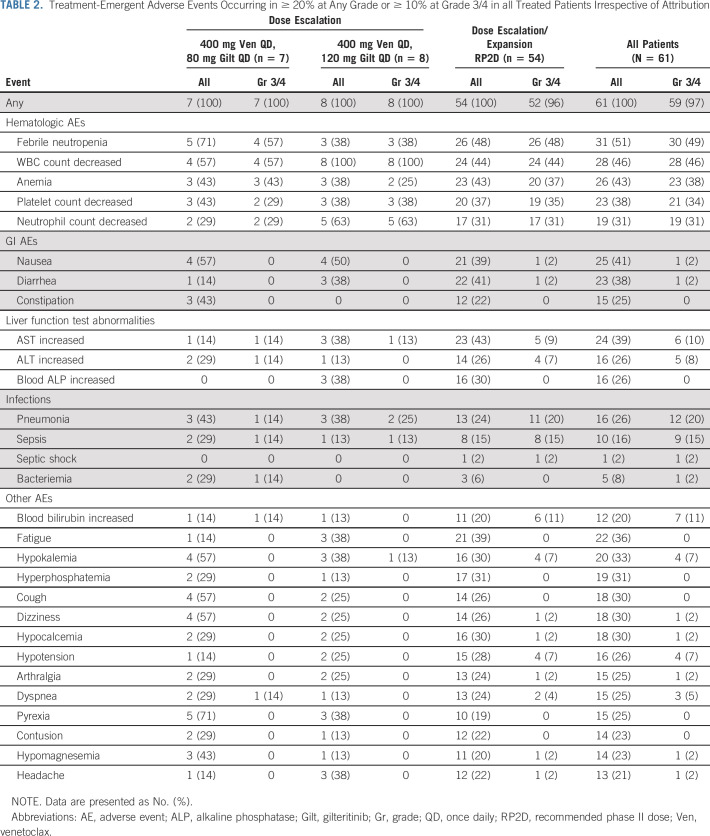
Safety
Fifty-nine of 61 patients (97%) experienced a grade 3/4 AE irrespective of attribution (Table 2). The Data Supplement summarizes AEs of special interest. No cases of posterior reversible encephalopathy syndrome or differentiation syndrome occurred. Forty-six patients (75%) experienced a serious AE (Data Supplement), most commonly (≥ 10%) febrile neutropenia (27 of 61, 44%) and pneumonia (8 of 61, 13%). The most common (≥ 25%) grade 3/4 AEs related to venetoclax and gilteritinib, respectively (Data Supplement) were white blood cell count decreased (36%; 33%), platelet count decreased (25%; 20%), and anemia (25%; 20%). AEs led to venetoclax and gilteritinib interruptions of any length in 31 of 61 (51%; Data Supplement) and 29 of 61 patients (48%; Data Supplement), respectively. Nine of 61 patients (15%) discontinued venetoclax, and 8 of 61 patients (13%) discontinued gilteritinib because of AEs (Data Supplement). The Data Supplement shows dose adjustments for each individual patient throughout treatment.
TABLE 2.
Treatment-Emergent Adverse Events Occurring in ≥ 20% at Any Grade or ≥ 10% at Grade 3/4 in all Treated Patients Irrespective of Attribution
Forty-nine patients (80%) experienced a grade 3/4 cytopenia, leading to venetoclax and gilteritinib dose interruptions ≥ 7 days in 8 of 61 (13%) and 5 of 61 (8%) patients, respectively (Data Supplement). Twenty-five of 42 patients in mCRc (60%) experienced grade 3/4 cytopenias while in mCRc, leading to venetoclax and gilteritinib dose interruptions ≥ 7 days in six and five patients, respectively.
The 30-day and 60-day mortality rates were 0 and 13% (8 of 61), respectively, in all patients (0 and 13% [7 of 56] in FLT3mut). Of 42 deaths on study, 29 were due to disease progression. Ten patients died of AEs (Data Supplement). Five of these 10 deaths occurred in patients while in mCRc (complicated fungal infection, aspergillus pneumonia, multiorgan failure, respiratory failure, and typhlitis), with 4 deaths occurring ≤ 30 days of stopping treatment and 1 death (complicated fungal infection) occurring > 30 days after treatment discontinuation. The other 5 of 10 AE-related deaths occurred in patients not in mCRc (sepsis, multiorgan failure, pseudomonas bacteremia, subdural hematoma, and death of unknown cause).
Efficacy
In FLT3mut patients treated at any dose (n = 56), the mCRc rate (CR + CRi + CRp + MLFS, per ADMIRAL criteria8) was 75% (42 of 56; CR, 18%; CRi, 4%; CRp, 18%; MLFS, 36%) with a DOR of 4.9 months (95% CI, 3.4 to 6.6; Fig 2 and Data Supplement) after a median follow-up of 17.5 (range, 0.8-27.5) months. The median time to first mCRc was 0.9 (range, 0.7-3.5) months. The CRc rate (CR + CRi + CRp) was 39% (22 of 56) with a median DOR of 4.9 months (95% CI, 2.6 to not reached [NR]). The median time to first CRc was 2.1 (range, 0.7-4.6) months. The mCRc rate was 82% (36 of 44; CR, 20%; CRi, 5%; CRp, 18%; and MLFS, 39%) in those with FLT3-ITD and 56% (5 of 9; CR, 11%; CRi, 0%; CRp, 22%; and MLFS, 22%) in those with FLT3-TKD mutations. Five patients had FLT3WT AML; one achieved a response (MLFS) lasting 1.7 months. The median OS was 10.0 months (95% CI, 6.3 to 12.3) for all FLT3mut patients (Fig 3A). The Data Supplement shows median OS by response. Efficacy in RP2D-treated patients is reported in the Data Supplement.
FIG 2.
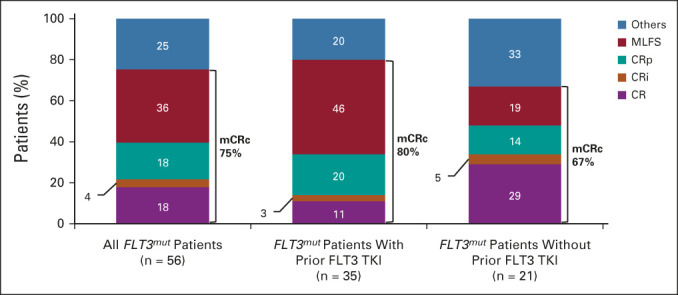
Response rates in all FLT3mut patients treated at any dose (n = 56) and in those who did (n = 35) or did not (n = 21) receive prior treatment with a FLT3 TKI. mCRc was defined as CR + CRi + CRp per criteria used in the ADMIRAL study. CR, complete response; CRi, complete response with incomplete blood count recovery; CRp, complete response with incomplete platelet recovery; mCRc, modified composite complete response; MLFS, morphologic leukemia-free state; TKI, tyrosine kinase inhibitor.
FIG 3.
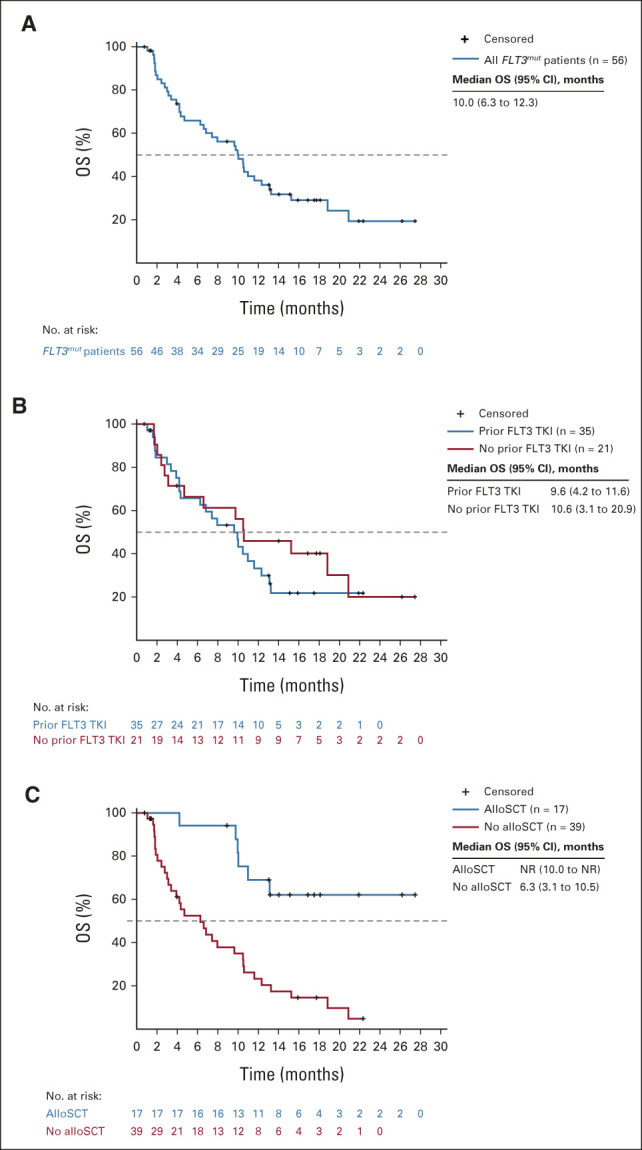
(A) OS in all FLT3mut patients treated at any dose (n = 56). (B) OS in FLT3mut patients who did (n = 35) or did not (n = 21) receive prior treatment with a FLT3 TKI. (C) OS in FLT3mut patients who did (n = 17) or did not (n = 39) receive alloSCT after VenGilt. alloSCT, allogeneic stem-cell transplantation; Gilt, gilteritinib; NR, not reached; OS, overall survival; TKI, tyrosine kinase inhibitor; Ven, venetoclax.
Among FLT3mut patients treated at any dose, the mCRc rate was 67% (14 of 21; CR, 29%; CRi, 5%; CRp, 14%; and MLFS, 19%) in 21 patients without prior FLT3 TKI exposure and 80% (28 of 35; CR, 11%; CRi, 3%; CRp, 20%; and MLFS, 46%) in 35 patients with prior FLT3 TKI exposure (Fig 2). The median OS was 10.6 months (95% CI, 3.1 to 20.9) and 9.6 months (95% CI, 4.2 to 11.6) in patients without and with prior FLT3 TKI exposure, respectively (Fig 3B). Among FLT3mut patients with prior venetoclax exposure (n = 10), the mCRc rate was 60% (four CR, two MLFS) and the median OS was 6.7 months (95% CI, 1.7 to 10.6).
The median OS was NR in 17 of 56 (30%) FLT3mut patients who received alloSCT after VenGilt and 6.3 months (95% CI, 3.1 to 10.5) for 39 of 56 (70%) patients who did not receive alloSCT (Fig 3C). In 18 FLT3mut patients who had received prior alloSCT, 67% achieved mCRc (12 of 18; CR, 1 of 18; CRi, 0 of 18; CRp, 3 of 18; and MLFS, 8 of 18) with a response duration of 4.9 months (95% CI, 1.1 to NR) and a median OS of 8.8 months (95% CI, 1.9 to 18.8; Data Supplement).
Molecular Response
Twenty-eight RP2D-treated patients with FLT3-ITD mutations were available for assessment of longitudinal allelic burden, and 25 of 28 (89%) achieved mCRc. Of those, 15 (60%) achieved molecular response (FLT3-ITD VAF < 10−2),22 and 11 (44%) and 5 (20%) achieved molecular clearance < 10−3 and < 10−4, respectively (Fig 4A and Data Supplement). The median OS in mCRc patients achieving molecular response (n = 15; < 10−2) was 11.6 months (95% CI, 7.43 to NR) and 8.2 months (95% CI, 1.05 to NR) in those who did not (n = 10; Fig 4B).
FIG 4.
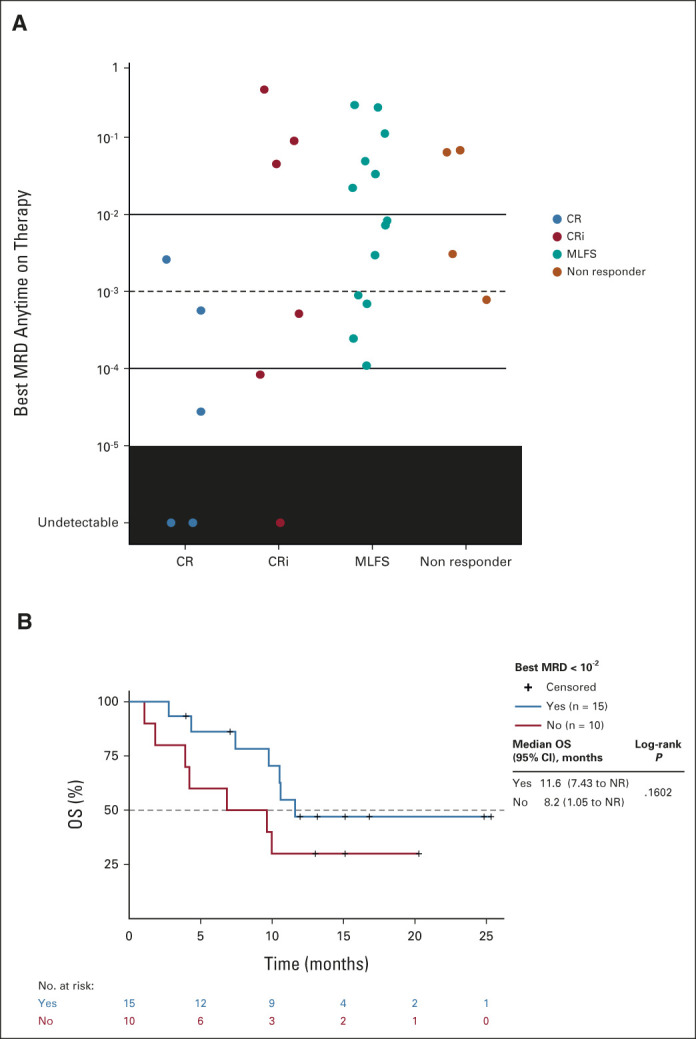
(A) FLT3-ITD MRD was assessed using next-generation sequencing of DNA isolated from bone marrow aspirates collected before and after the first dose of study drug with a sensitivity threshold of 10−6 (lowest level of FLT3-ITD clones achieved). The best reported FLT3-ITD MRD value is shown for patients divided by best clinical response achieved. (B) OS in FLT3mut patients treated at the recommended phase II dose who achieved a modified composite complete response and molecular response (FLT3-ITD variant allele frequency < 10−2). CR, complete response; CRi, complete response with incomplete blood count recovery; ITD, internal tandem duplications; MLFS, morphologic leukemia-free state; MRD, minimal residual disease; NR, not reached; OS, overall survival.
Of 31 patients with FLT3mut and baseline next-generation sequencing data, 17 had concomitant mutations in DNMT3A, 13 in NPM1, nine in both DNMT3A and NPM1, and eight in IDH1/2; mCRc rates were generally consistent regardless of the presence or absence of comutations (Data Supplement).
DISCUSSION
VenGilt yielded high mCRc and molecular response rates in patients with R/R FLT3mut AML, including those who failed multiple prior lines of therapy and those who were exposed to ≥ 1 prior FLT3 TKIs, which is representative of the current real-world population after integration of frontline midostaurin with induction therapy and the common use of sorafenib maintenance post-transplant.43 Most patients in remission achieved molecular response (FLT3-ITD VAF < 10−2); response occurred rapidly (median < 1 month), indicating that this combination could induce deep FLT3 clonal responses. Similar to single-agent gilteritinib data, reduction in FLT3-ITD mutation burden during VenGilt therapy was potentially associated with longer median survival.
Grade 3/4 cytopenias were frequent with VenGilt, consistent with the known safety profile of venetoclax-based therapies in AML. However, few patients in mCRc required venetoclax or gilteritinib dose interruptions for ≥ 7 days (13% and 8%, respectively), suggesting that blood counts generally recovered once BM leukemia burden was reduced. Despite this, several patients experienced prolonged cytopenias during response, suggesting that the shorter duration of venetoclax treatment, lower gilteritinib dose, and/or earlier G-CSF use in those with persistent cytopenias after achieving BM remission should be considered. This may be especially important for older/unfit patients with R/R FLT3mut AML who may be ineligible for alloSCT and require long-term ongoing therapy. On the basis of the authors' experiences with this and other venetoclax-based combinations, recommendations for managing myelosuppression include delaying initiation of subsequent cycles until achieving ANC > 500/μL and platelets > 50,000/μL; reducing the venetoclax duration to 21 days, or subsequently to 14 days, for patients with prolonged ANC and/or platelet recovery time (> 42 days) after achieving at least BM remission (< 5% blasts); using G-CSF for patients with confirmed BM remission and ANC < 500/μL lasting > 42 days to boost ANC before starting the next cycle; allowing longer cycles (4-6 weeks), if needed, for count recovery after achieving BM remission; and using azole antifungals to reduce fungal infections with appropriate venetoclax dose reductions. We generally avoided gilteritinib interruption to maintain FLT3-targeting suppression; however, in patients experiencing prolonged myelosuppression despite the above measures, we first recommend considering reducing gilteritinib to 80 mg once daily and subsequently a short gilteritinib interruption, if needed. Nonetheless, myelosuppression with VenGilt remained manageable with appropriate dose modifications. Although infections were common, 5 of 42 patients achieving mCRc (12%) died because of infections while in mCRc (four died ≤ 30 days and one died > 30 days after stopping treatment). Still, the 30-day and 60-day mortality rates were consistent with single-agent gilteritinib in R/R AML,8 and study discontinuation from myelosuppression in patients in remission was uncommon.
The mCRc rate with VenGilt in R/R FLT3mut patients (75%) was defined using the same response criteria as the CRc rate with single-agent gilteritinib in the ADMIRAL study (reported as 54%).8 Although survival reported here is similar to that reported in ADMIRAL, VenGilt response rates and OS are encouraging, as our study included all salvage patients (38% received ≥ 3 prior lines of therapy), whereas ADMIRAL included only first relapse/primary refractory FLT3mut patients. Furthermore, this study included a substantially higher proportion of patients who received ≥ 1 prior FLT3 TKI compared with ADMIRAL (59% v 13%).8 mCRc rates with VenGilt were 80% versus 67% for those with versus without prior FLT3 TKI exposure, which compared favorably with findings from retrospective analyses of CHRYSALIS (42% v 43%) or ADMIRAL (48% v 55%).24,44
VenGilt induced deep molecular responses, with 60% of evaluable responding patients achieving FLT3-ITD clearance (< 10−2) and 12% reaching undetectable levels (< 10−6). This compares favorably with a recent CHRYSALIS subgroup analysis in which 25% of responding R/R patients achieved molecular response (< 10−2) with single-agent gilteritinib.22 More data from ongoing studies are needed to conclusively determine the impact of FLT3-ITD clearance on outcomes.
Survival of patients receiving alloSCT after VenGilt salvage treatment was particularly encouraging (median OS, NR v 6.3 months in nontransplanted) although interpretation is limited by a small number of transplants and lack of a dedicated survival analysis for patients who would be transplant-eligible upon response to VenGilt. With a median follow-up of 17.5 months, approximately 60% of patients who received alloSCT post-VenGilt were alive, suggesting that VenGilt could be an effective bridge to transplant in young/fit patients with relapsed FLT3mut AML. In this setting, achieving marrow clearance and FLT3 allelic reduction in a few cycles before alloSCT could mitigate concerns of prolonged myelosuppression from ongoing treatment; however, a dedicated prospective study evaluating outcomes with alloSCT in VenGilt-treated patients is needed to make definitive conclusions. VenGilt also appeared to be effective in post-transplant patients, with an mCRc rate of 67% and a median OS of 8.8 months (v 10 months in all patients). VenGilt should be evaluated further in this population.
The findings of this phase Ib study are limited by smaller sample size. Nonetheless, they provide the first evidence supporting venetoclax combined with FLT3 inhibitors for FLT3mut AML. Although this all-oral regimen is designed for outpatient administration, attention to myelosuppression11 and the potential for serious infections are warranted. VenGilt is a highly active, tolerable salvage regimen that retains clinical activity among FLT3mut patients exposed to prior lines of therapy from either class of agents.26 To definitively establish VenGilt as a standard of care in FLT3mut AML, further trials are required, including randomized controlled trials of VenGilt versus gilteritinib in R/R FLT3-TKD and/or FLT3-ITD–mutated AML with an end point of mCRc or OS. Furthermore, as a group of experienced investigators assessed VenGilt here in a multicenter setting and generated detailed efficacy, safety, and dose optimization data from dose escalation/expansion, these findings provide a strong foundation for evaluating VenGilt combinations in earlier disease. Our results will play an important role in guiding design, dose optimization, and myelosuppression mitigation strategies of ongoing and future trials evaluating frontline VenGilt with azacitidine in older/unfit patients with FLT3mut AML,45,46 an area of significant unmet need. Thus, experience from this first data set of VenGilt could play a major role in efforts to redefine both R/R and frontline FLT3mut AML treatment.
ACKNOWLEDGMENT
AbbVie and the authors thank all the trial investigators and the patients who participated in this clinical trial. Medical writing assistance was provided by Allison Cherry, PhD, of Bio Connections LLC and funded by AbbVie Inc.
Naval Daver
Consulting or Advisory Role: Celgene, Agios, Jazz Pharmaceuticals, Pfizer, AbbVie, Astellas Pharma, Daiichi Sankyo, Novartis, Bristol Myers Squibb, Amgen, Immunogen, Genentech, Servier, Syndax, Trillium Therapeutics, Gilead Sciences, Arog, Shattuck Labs
Research Funding: Bristol Myers Squibb, Pfizer, Immunogen, Genentech, AbbVie, Astellas Pharma, Servier, Daiichi Sankyo, Gilead Sciences, Amgen, Trillium Therapeutics, Hanmi, Trovagene, FATE Therapeutics, Novimmune, GlycoMimetics
Alexander E. Perl
Honoraria: Astellas Pharma, Daiichi Sankyo
Consulting or Advisory Role: Astellas Pharma, Actinium Pharmaceuticals, Daiichi Sankyo, AbbVie, FORMA Therapeutics, Sumitomo Dainippon, Celgene/Bristol Myers Squibb, Syndax, Genentech, BerGenBio, Immunogen
Research Funding: Astellas Pharma (Inst), Bayer (Inst), Daiichi Sankyo (Inst), Fujifilm (Inst), AbbVie (Inst), Syndax (Inst)
Mark Levis
Consulting or Advisory Role: Daiichi Sankyo, Amgen, Fujifilm, Astellas Pharma, Menarini, Bristol Myers Squibb, AbbVie/Genentech, GlaxoSmithKline, Jazz Pharmaceuticals
Research Funding: Astellas Pharma (Inst), Fujifilm (Inst)
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Astellas Pharma
Ellen Ritchie
Consulting or Advisory Role: Incyte, Celgene, Pfizer, Novartis, Bristol Myers Squibb
Speakers' Bureau: Celgene, Incyte
Research Funding: Astellas Pharma (Inst), Novartis (Inst), Pfizer (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Pfizer
Mark Litzow
Consulting or Advisory Role: Omeros, Jazz Pharmaceuticals
Research Funding: Amgen, Astellas Pharma, Actinium Pharmaceuticals, Pluristem Therapeutics, AbbVie/Genentech, Tolero Pharmaceuticals, AbbVie
Other Relationship: BioSight
James McCloskey
Honoraria: BluPrint Oncology, Bristol Myers Squibb/Pfizer
Consulting or Advisory Role: Bristol Myers Squibb/Pfizer, Blueprint Medicines, Novartis
Speakers' Bureau: Jazz Pharmaceuticals, Incyte, Bristol Myers Squibb/Pfizer, Stemline Therapeutics, Takeda, Amgen, Blueprint
Catherine C. Smith
Consulting or Advisory Role: Astellas Pharma, AbbVie/Genentech
Research Funding: AbbVie, Revolution Medicines, FUJIFILM Pharmaceuticals (Inst), Celgene/Bristol Myers Squibb (Inst)
Gary Schiller
Stock and Other Ownership Interests: Bristol Myers Squibb, Amgen, Johnson & Johnson
Consulting or Advisory Role: Ono Pharmaceutical, Agios, Celgene, Incyte, Jazz Pharmaceuticals, Novartis, AbbVie, Astellas Pharma
Speakers' Bureau: Astellas Pharma, Kite, a Gilead Company, Jazz Pharmaceuticals, Stemline Therapeutics, Bristol Myers Squibb, Sanofi, Karyopharm Therapeutics, Incyte, AbbVie
Research Funding: AbbVie, Actinium Pharmaceuticals, Actuate Therapeutics, Arog, Astellas Pharma, Bristol Myers Squibb/Celgene, Celator, Constellation Pharmaceuticals, Daiichi Sankyo, Deciphera, Delta-Fly Pharma, FORMA Therapeutics, Fujifilm, Gamida Cell, Genentech/Roche, Geron, Incyte, Karyopharm Therapeutics, Kite, a Gilead Company, Mateon Therapeutics, Onconova Therapeutics, Pfizer, Precog, REGiMMUNE, Samus Therapeutics, Sangamo Bioscience, SELLAS Life Sciences, Stemline Therapeutics, Takeda, Tolero Pharmaceuticals, Trovagene, Agios, Amgen, Jazz Pharmaceuticals, ElevateBio, Ono Pharmaceutical, Novartis, Sanofi, AVM Biotechnology, Syros Pharmaceuticals
Terrence Bradley
Consulting or Advisory Role: AbbVie, Novartis
Speakers' Bureau: Novartis, AbbVie
Ramon V. Tiu
Employment: Takeda, Astellas Pharma
Stock and Other Ownership Interests: Lilly, Takeda
Patents, Royalties, Other Intellectual Property: Methods for predicting prognosis of a subject with a myeloid malignancy, Publication no.: 20130316014 (Inst), Combination of ERK1/2 inhibitor compound with gemcitabine or with gemcitabine and NAB-paclitaxel for use in treatment of pancreatic cancer, Publication no.: 20200306254 (Inst)
Kiran Naqvi
Employment: Genentech/Roche
Leadership: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Consulting or Advisory Role: Novartis
Research Funding: Genentech/Roche
Travel, Accommodations, Expenses: Genentech/Roche
Monique Dail
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche/Genentech
Deanna Brackman
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Satya Siddani
Stock and Other Ownership Interests: AbbVie
Jing Wang
Employment: AbbVie, Novartis
Stock and Other Ownership Interests: AbbVie, Novartis
Brenda Chyla
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Paul Lee
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jessica K. Altman
Consulting or Advisory Role: GlycoMimetics, Kura Oncology, AbbVie, Astellas Pharma, Syros Pharmaceuticals, BioSight, Bluebird Bio, Stemline Therapeutics, Curio Science
Research Funding: Astellas Pharma (Inst), Pfizer (Inst), Agios (Inst), Bristol Myers Squibb (Inst), Cyclacel (Inst), Celgene (Inst), Boehringer Ingelheim (Inst), BioSight (Inst), Kura Oncology (Inst), AbbVie (Inst), Amgen (Inst), Aprea AB (Inst), Amphivena (Inst), Fujifilm (Inst), Kartos Therapeutics (Inst), Aptose Biosciences (Inst), ALX Oncology (Inst), Immunogen (Inst), Kura Oncology (Inst), Loxo (Inst), Telios (Inst)
Travel, Accommodations, Expenses: BioSight, Astellas Pharma, Daiichi Sankyo
Other Relationship: NCI, Oncology Learning Network
No other potential conflicts of interest were reported.
See accompanying editorial on page 4033
DISCLAIMER
Venetoclax is being developed in collaboration between AbbVie and Genentech. AbbVie, Genentech, and Astellas sponsored the study and participated in the design, study conduct, analysis, collection, and interpretation of the data, as well as the writing, review, and approval of the publication. All the authors had access to the full study data and approved the decision to submit the manuscript. The corresponding author had final responsibility for the decision to submit.
PRIOR PRESENTATION
Presented in part at the European Hematology Association Virtual Meeting, June 9-17, 2021, and the American Society of Hematology Annual Meeting, December 11-14, 2021, Atlanta, GA.
CLINICAL TRIAL INFORMATION
N.D. and A.E.P. contributed equally to this work as cofirst authors.
DATA SHARING STATEMENT
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
AUTHOR CONTRIBUTIONS
Conception and design: Naval Daver, Alexander E. Perl, Mark Levis, James McCloskey, Gary Schiller, Ramon V. Tiu, Kiran Naqvi, Monique Dail, Jing Wang, Paul Lee, Jessica K. Altman
Financial support: Gary Schiller
Provision of study materials or patients: Naval Daver, Alexander E. Perl, Mark Levis, Ellen Ritchie, Mark Litzow, Catherine C. Smith, Gary Schiller, Jessica K. Altman
Collection and assembly of data: Naval Daver, Alexander E. Perl, Joseph Maly, Mark Levis, Ellen Ritchie, Mark Litzow, James McCloskey, Catherine C. Smith, Gary Schiller, Kiran Naqvi, Satya Siddani, Jing Wang, Brenda Chyla, Paul Lee, Jessica K. Altman
Data analysis and interpretation: Naval Daver, Alexander E. Perl, Joseph Maly, Mark Levis, Mark Litzow, James McCloskey, Catherine C. Smith, Gary Schiller, Terrence Bradley, Ramon V. Tiu, Monique Dail, Deanna Brackman, Satya Siddani, Jing Wang, Brenda Chyla, Paul Lee, Jessica K. Altman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Venetoclax Plus Gilteritinib for FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Naval Daver
Consulting or Advisory Role: Celgene, Agios, Jazz Pharmaceuticals, Pfizer, AbbVie, Astellas Pharma, Daiichi Sankyo, Novartis, Bristol Myers Squibb, Amgen, Immunogen, Genentech, Servier, Syndax, Trillium Therapeutics, Gilead Sciences, Arog, Shattuck Labs
Research Funding: Bristol Myers Squibb, Pfizer, Immunogen, Genentech, AbbVie, Astellas Pharma, Servier, Daiichi Sankyo, Gilead Sciences, Amgen, Trillium Therapeutics, Hanmi, Trovagene, FATE Therapeutics, Novimmune, GlycoMimetics
Alexander E. Perl
Honoraria: Astellas Pharma, Daiichi Sankyo
Consulting or Advisory Role: Astellas Pharma, Actinium Pharmaceuticals, Daiichi Sankyo, AbbVie, FORMA Therapeutics, Sumitomo Dainippon, Celgene/Bristol Myers Squibb, Syndax, Genentech, BerGenBio, Immunogen
Research Funding: Astellas Pharma (Inst), Bayer (Inst), Daiichi Sankyo (Inst), Fujifilm (Inst), AbbVie (Inst), Syndax (Inst)
Mark Levis
Consulting or Advisory Role: Daiichi Sankyo, Amgen, Fujifilm, Astellas Pharma, Menarini, Bristol Myers Squibb, AbbVie/Genentech, GlaxoSmithKline, Jazz Pharmaceuticals
Research Funding: Astellas Pharma (Inst), Fujifilm (Inst)
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Astellas Pharma
Ellen Ritchie
Consulting or Advisory Role: Incyte, Celgene, Pfizer, Novartis, Bristol Myers Squibb
Speakers' Bureau: Celgene, Incyte
Research Funding: Astellas Pharma (Inst), Novartis (Inst), Pfizer (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Pfizer
Mark Litzow
Consulting or Advisory Role: Omeros, Jazz Pharmaceuticals
Research Funding: Amgen, Astellas Pharma, Actinium Pharmaceuticals, Pluristem Therapeutics, AbbVie/Genentech, Tolero Pharmaceuticals, AbbVie
Other Relationship: BioSight
James McCloskey
Honoraria: BluPrint Oncology, Bristol Myers Squibb/Pfizer
Consulting or Advisory Role: Bristol Myers Squibb/Pfizer, Blueprint Medicines, Novartis
Speakers' Bureau: Jazz Pharmaceuticals, Incyte, Bristol Myers Squibb/Pfizer, Stemline Therapeutics, Takeda, Amgen, Blueprint
Catherine C. Smith
Consulting or Advisory Role: Astellas Pharma, AbbVie/Genentech
Research Funding: AbbVie, Revolution Medicines, FUJIFILM Pharmaceuticals (Inst), Celgene/Bristol Myers Squibb (Inst)
Gary Schiller
Stock and Other Ownership Interests: Bristol Myers Squibb, Amgen, Johnson & Johnson
Consulting or Advisory Role: Ono Pharmaceutical, Agios, Celgene, Incyte, Jazz Pharmaceuticals, Novartis, AbbVie, Astellas Pharma
Speakers' Bureau: Astellas Pharma, Kite, a Gilead Company, Jazz Pharmaceuticals, Stemline Therapeutics, Bristol Myers Squibb, Sanofi, Karyopharm Therapeutics, Incyte, AbbVie
Research Funding: AbbVie, Actinium Pharmaceuticals, Actuate Therapeutics, Arog, Astellas Pharma, Bristol Myers Squibb/Celgene, Celator, Constellation Pharmaceuticals, Daiichi Sankyo, Deciphera, Delta-Fly Pharma, FORMA Therapeutics, Fujifilm, Gamida Cell, Genentech/Roche, Geron, Incyte, Karyopharm Therapeutics, Kite, a Gilead Company, Mateon Therapeutics, Onconova Therapeutics, Pfizer, Precog, REGiMMUNE, Samus Therapeutics, Sangamo Bioscience, SELLAS Life Sciences, Stemline Therapeutics, Takeda, Tolero Pharmaceuticals, Trovagene, Agios, Amgen, Jazz Pharmaceuticals, ElevateBio, Ono Pharmaceutical, Novartis, Sanofi, AVM Biotechnology, Syros Pharmaceuticals
Terrence Bradley
Consulting or Advisory Role: AbbVie, Novartis
Speakers' Bureau: Novartis, AbbVie
Ramon V. Tiu
Employment: Takeda, Astellas Pharma
Stock and Other Ownership Interests: Lilly, Takeda
Patents, Royalties, Other Intellectual Property: Methods for predicting prognosis of a subject with a myeloid malignancy, Publication no.: 20130316014 (Inst), Combination of ERK1/2 inhibitor compound with gemcitabine or with gemcitabine and NAB-paclitaxel for use in treatment of pancreatic cancer, Publication no.: 20200306254 (Inst)
Kiran Naqvi
Employment: Genentech/Roche
Leadership: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Consulting or Advisory Role: Novartis
Research Funding: Genentech/Roche
Travel, Accommodations, Expenses: Genentech/Roche
Monique Dail
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche/Genentech
Deanna Brackman
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Satya Siddani
Stock and Other Ownership Interests: AbbVie
Jing Wang
Employment: AbbVie, Novartis
Stock and Other Ownership Interests: AbbVie, Novartis
Brenda Chyla
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Paul Lee
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jessica K. Altman
Consulting or Advisory Role: GlycoMimetics, Kura Oncology, AbbVie, Astellas Pharma, Syros Pharmaceuticals, BioSight, Bluebird Bio, Stemline Therapeutics, Curio Science
Research Funding: Astellas Pharma (Inst), Pfizer (Inst), Agios (Inst), Bristol Myers Squibb (Inst), Cyclacel (Inst), Celgene (Inst), Boehringer Ingelheim (Inst), BioSight (Inst), Kura Oncology (Inst), AbbVie (Inst), Amgen (Inst), Aprea AB (Inst), Amphivena (Inst), Fujifilm (Inst), Kartos Therapeutics (Inst), Aptose Biosciences (Inst), ALX Oncology (Inst), Immunogen (Inst), Kura Oncology (Inst), Loxo (Inst), Telios (Inst)
Travel, Accommodations, Expenses: BioSight, Astellas Pharma, Daiichi Sankyo
Other Relationship: NCI, Oncology Learning Network
No other potential conflicts of interest were reported.
REFERENCES
- 1.Orlowski RJ, Mangan JK, Luger SM: Approach to patients with primary refractory acute myeloid leukemia. Curr Opin Hematol 22:97-107, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Thol F, Ganser A: Treatment of relapsed acute myeloid leukemia. Curr Treat Options Oncol 21:66, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandwein JM, Saini L, Geddes MN, et al. : Outcomes of patients with relapsed or refractory acute myeloid leukemia: A population-based real-world study. Am J Blood Res 10:124-133, 2020 [PMC free article] [PubMed] [Google Scholar]
- 4.Stahl M, DeVeaux M, Montesinos P, et al. : Hypomethylating agents in relapsed and refractory AML: Outcomes and their predictors in a large international patient cohort. Blood Adv 2:923-932, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roboz GJ, Rosenblat T, Arellano M, et al. : International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol 32:1919-1926, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Ravandi F, Ritchie EK, Sayar H, et al. : Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): A randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol 16:1025-1036, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Short NJ, Konopleva M, Kadia TM, et al. : Advances in the treatment of acute myeloid leukemia: New drugs and new challenges. Cancer Discov 10:506-525, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Perl AE, Martinelli G, Cortes JE, et al. : Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 381:1728-1740, 2019 [DOI] [PubMed] [Google Scholar]
- 9.DiNardo CD, Stein EM, de Botton S, et al. : Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 378:2386-2398, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Stein EM, DiNardo CD, Pollyea DA, et al. : Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130:722-731, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daver N, Venugopal S, Ravandi F: FLT3 mutated acute myeloid leukemia: 2021 treatment algorithm. Blood Cancer J 11:104, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakao M, Yokota S, Iwai T, et al. : Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 10:1911-1918, 1996 [PubMed] [Google Scholar]
- 13.Yamamoto Y, Kiyoi H, Nakano Y, et al. : Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 97:2434-2439, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Tao S, Wang C, Chen Y, et al. : Prognosis and outcome of patients with acute myeloid leukemia based on FLT3-ITD mutation with or without additional abnormal cytogenetics. Oncol Lett 18:6766-6774, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chevallier P, Labopin M, Turlure P, et al. : A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: A GOELAMS study. Leukemia 25:939-944, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Kottaridis PD, Gale RE, Frew ME, et al. : The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 98:1752-1759, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Wattad M, Weber D, Dohner K, et al. : Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia 31:1306-1313, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Perl AE, Altman JK, Cortes J, et al. : Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: A multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol 18:1061-1075, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarver TC, Hill JE, Rahmat L, et al. : Gilteritinib is a clinically active FLT3 inhibitor with broad activity against FLT3 kinase domain mutations. Blood Adv 4:514-524, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perl AE, Martinelli G, Neubauer A, et al. : Long-term survivors and gilteritinib safety beyond one year in FLT3-mutated R/R AML: ADMIRAL trial follow-up. J Clin Oncol 38, 2020. (abstr 7514) [Google Scholar]
- 21.Altman JK, Perl AE, Hill JE, et al. : The impact of FLT3 mutation clearance and treatment response after gilteritinib therapy on overall survival in patients with FLT3 mutation-positive relapsed/refractory acute myeloid leukemia. Cancer Med 10:797-805, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levis MJ, Perl AE, Altman JK, et al. : A next-generation sequencing-based assay for minimal residual disease assessment in AML patients with FLT3-ITD mutations. Blood Adv 2:825-831, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hourigan CS, Dillon LW, Gui G, et al. : Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol 38:1273-1283, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perl AE, Altman JK, Hosono N, et al. : Clinical outcomes in patients with relapsed/refractory acute myeloid leukemia treated with gilteritinib who received prior midostaurin or sorafenib. Blood 136:22-23, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone RM, Mandrekar SJ, Sanford BL, et al. : Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377:454-464, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yilmaz M, Alfayez M, DiNardo CD, et al. : Outcomes with sequential FLT3-inhibitor-based therapies in patients with AML. J Hematol Oncol 13:132, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon CM, Canaani J, Rea B, et al. : Gilteritinib induces differentiation in relapsed and refractory FLT3-mutated acute myeloid leukemia. Blood Adv 3:1581-1585, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sexauer A, Perl A, Yang X, et al. : Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood 120:4205-4214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng R, Friedman AD, Levis M, et al. : Internal tandem duplication mutation of FLT3 blocks myeloid differentiation through suppression of C/EBPalpha expression. Blood 103:1883-1890, 2004 [DOI] [PubMed] [Google Scholar]
- 30.McMahon CM, Ferng T, Canaani J, et al. : Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov 9:1050-1063, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiNardo CD, Jonas BA, Pullarkat V, et al. : Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383:617-629, 2020 [DOI] [PubMed] [Google Scholar]
- 32.Wei AH, Montesinos P, Ivanov V, et al. : Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 135:2137-2145, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konopleva M, Pollyea DA, Potluri J, et al. : Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov 6:1106-1117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh Mali R, Zhang Q, DeFilippis RA, et al. : Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models. Haematologica 106:1034-1046, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinton LT, Zhang P, Williams K, et al. : Synergistic effect of BCL2 and FLT3 co-inhibition in acute myeloid leukemia. J Hematol Oncol 13:139, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma J, Zhao S, Qiao X, et al. : Inhibition of Bcl-2 synergistically enhances the antileukemic activity of midostaurin and gilteritinib in preclinical models of FLT3-mutated acute myeloid leukemia. Clin Cancer Res 25:6815-6826, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu R, Li L, Nguyen B, et al. : FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation. Signal Transduct Target Ther 6:186, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arber DA, Orazi A, Hasserjian R, et al. : The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391-2405, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Venetoclax [package insert]. 2021. https://www.rxabbvie.com/pdf/venclexta.pdf [Google Scholar]
- 40.Cheson BD, Bennett JM, Kopecky KJ, et al. : Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21:4642-4649, 2003 [DOI] [PubMed] [Google Scholar]
- 41.James AJ, Smith CC, Litzow M, et al. : Pharmacokinetic profile of gilteritinib: A novel FLT-3 tyrosine kinase inhibitor. Clin Pharmacokinet 59:1273-1290, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salem AH, Agarwal SK, Dunbar M, et al. : Pharmacokinetics of venetoclax, a novel BCL-2 inhibitor, in patients with relapsed or refractory chronic lymphocytic leukemia or non-Hodgkin lymphoma. J Clin Pharmacol 57:484-492, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Burchert A, Bug G, Fritz LV, et al. : Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol 38:2993-3002, 2020 [DOI] [PubMed] [Google Scholar]
- 44.Numan Y, Abdel Rahman Z, Grenet J, et al. : Gilteritinib clinical activity in relapsed/refractory FLT3 mutated acute myeloid leukemia previously treated with FLT3 inhibitors. Am J Hematol 97:322-328, 2022 [DOI] [PubMed] [Google Scholar]
- 45.Maiti A, DiNardo CD, Daver NG, et al. : Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood Cancer J 11:25, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Short NJ, DiNardo CD, Daver N, et al. : A triplet combination of azacitidine, venetoclax and gilteritinib for patients with FLT3-mutated acute myeloid leukemia: Results from a phase I/II study. Presented at the American Society of Hematology annual meeting, Atlanta, GA, December 11-14, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.