PURPOSE
Sunitinib, a multitargeted tyrosine kinase inhibitor (TKI), is approved for advanced gastrointestinal stromal tumor (GIST) after imatinib failure. Ripretinib is a switch-control TKI approved for advanced GIST after prior treatment with three or more TKIs, including imatinib. We compared efficacy and safety of ripretinib versus sunitinib in patients with advanced GIST who were previously treated with imatinib (INTRIGUE, ClinicalTrials.gov identifier: NCT03673501).
PATIENTS AND METHODS
Random assignment was 1:1 to once-daily ripretinib 150 mg or once-daily sunitinib 50 mg (4 weeks on/2 weeks off) and stratified by KIT/platelet-derived growth factor α mutation and imatinib intolerance. The primary end point was progression-free survival (PFS) by independent radiologic review using modified Response Evaluation Criteria in Solid Tumors version 1.1. Secondary end points included objective response rate by independent radiologic review, safety, and patient-reported outcome measures.
RESULTS
Overall, 453 patients were randomly assigned to ripretinib (intention-to-treat [ITT], n = 226; KIT exon 11 ITT, n = 163) or sunitinib (ITT, n = 227; KIT exon 11 ITT, n = 164). Median PFS for ripretinib and sunitinib (KIT exon 11 ITT) was 8.3 and 7.0 months, respectively (hazard ratio, 0.88; 95% CI, 0.66 to 1.16; P = .36); median PFS (ITT) was 8.0 and 8.3 months, respectively (hazard ratio, 1.05; 95% CI, 0.82 to 1.33; nominal P = .72). Neither was statistically significant. Objective response rate was higher for ripretinib versus sunitinib in the KIT exon 11 ITT population (23.9% v 14.6%, nominal P = .03). Ripretinib was associated with a more favorable safety profile, fewer grade 3/4 treatment-emergent adverse events (41.3% v 65.6%, nominal P < .0001), and better scores on patient-reported outcome measures of tolerability.
CONCLUSION
Ripretinib was not superior to sunitinib in terms of PFS. However, meaningful clinical activity, fewer grade 3/4 treatment-emergent adverse events, and improved tolerability were observed with ripretinib.
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is the most common sarcoma of the gastrointestinal tract (10-15 cases/million annually) with 80%-90% being driven by activating genomic alterations in KIT or platelet-derived growth factor α (PDGFRA).1-6 First-line treatment with imatinib—a KIT and PDGFRA tyrosine kinase inhibitor (TKI)—results in initial tumor response and disease control; however, nearly all patients eventually progress.7-10
CONTEXT
Key Objective
The phase III INTRIGUE study evaluated efficacy and safety of ripretinib compared with sunitinib as a second-line therapy for patients with advanced gastrointestinal stromal tumor who were previously treated with imatinib. INTRIGUE is the largest randomized phase III trial in the second-line setting in advanced gastrointestinal stromal tumor with an active comparator arm.
Knowledge Generated
Although ripretinib was not superior to sunitinib in terms of progression-free survival (primary end point), meaningful clinical activity was observed. Patients receiving ripretinib experienced fewer grade 3/4 treatment-emergent adverse events compared with patients receiving sunitinib. Ripretinib was also associated with better scores on patient-reported outcome measures of tolerability.
Relevance
There was no significant difference in median progression-free survival between ripretinib and sunitinib. Ripretinib had a more favorable safety profile and a higher response rate compared with sunitinib. Longer follow-up is needed to assess overall survival.
Sunitinib is a multitargeted TKI with activity against the vascular endothelial growth factor receptor.11 It was approved in 2006 for second-line treatment of patients with GIST following progression on or intolerance to imatinib.12 Sunitinib is active against secondary mutations in KIT exons 13/14 (ATP-binding pocket), but less active against exons 17/18 (activation loop), yielding an overall median progression-free survival (PFS) of 5.6 months for molecularly unselected patients with advanced GIST in the historical registration trial.12-14 Given the heterogeneous nature of KIT and PDGFRA resistance mutations in GIST, there is an unmet need for an effective broad-spectrum TKI in early treatment.15
Ripretinib is a switch-control TKI with a dual mechanism of action that provides broad-spectrum inhibition of KIT or PDGFRA activity.16 It is approved for the treatment of patients with advanced GIST who received prior treatment with three or more TKIs, including imatinib, on the basis of the results of the phase III INVICTUS study.17,18 Phase I data suggest ripretinib is effective as second-line therapy in patients with advanced GIST (median PFS: 10.7 months).19 Ripretinib had higher activity against imatinib-resistant secondary KIT mutations in vitro versus sunitinib, suggesting that ripretinib may be superior in second-line GIST.16 In this phase III INTRIGUE study, we evaluate the efficacy and safety of ripretinib versus sunitinib in patients with advanced GIST previously treated with imatinib.
PATIENTS AND METHODS
Study Design
INTRIGUE (ClinicalTrials.gov identifier: NCT03673501) is a randomized, open-label, international, multicenter phase III study comparing efficacy and safety of ripretinib versus sunitinib in patients with advanced GIST who progressed on or were intolerant to first-line treatment with imatinib. This study used a biomarker-positive/overall group design in which end points were assessed in two populations.20,21 INTRIGUE was active at 122 sites in 22 countries. Patients were stratified by mutational status (KIT exon 11, KIT exon 9, KIT/PDGFRA wild-type [WT], and other KIT [other than exon 9 or exon 11]/PDGFRA mutations) and imatinib intolerance and subsequently randomly assigned (1:1) to receive once-daily ripretinib 150 mg (continuous dosing) or once-daily sunitinib 50 mg, 4 weeks on/2 weeks off (4/2) in 6-week cycles. Crossover was not allowed.
Patients requiring dose interruptions > 28 consecutive days were discontinued from treatment. With ripretinib, first and second dose reductions were once-daily 100 mg and once-daily 50 mg, respectively. Dose modifications for sunitinib were per approved prescribing information or institutional guidelines (Data Supplement, online only).
This study was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation Guidelines for Good Clinical Practice. The Protocol (online only), protocol amendments, and informed consent documents were approved by an institutional review board or ethics committee at each site and by appropriate regulatory authorities. Patients provided written informed consent.
Eligibility Criteria
Eligible patients were age ≥ 18 years, had histologically confirmed GIST with ≥ 1 measurable lesion by modified Response Evaluation Criteria in Solid Tumors version 1.1 (mRECIST v1.1; modifications followed those described by Demetri et al22) within 21 days before administration of study drug, provided an archival tissue sample and pathology report containing KIT/PDGFRA mutation status using a tissue-based PCR or other DNA sequencing assay, had disease progression on or documented intolerance to imatinib, had imatinib treatment discontinued 10 days before first dose of study drug, and had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2 with adequate organ function and bone marrow reserve. Complete inclusion/exclusion criteria are provided in the Data Supplement.
Outcomes
The primary end point was PFS by independent radiologic review (IRR) using mRECIST v1.1 and was tested in the two intention-to-treat (ITT) populations of KIT exon 11 participants and all patients. PFS was defined as time between random assignment and first disease progression or death because of any cause, whichever occurred first. PFS was censored on the date of the last adequate disease assessment for patients with no event. Key secondary end points were objective response rate (ORR) by IRR using mRECIST v1.1 and overall survival (OS), and were also analyzed for both the KIT exon 11 ITT and overall ITT populations. Other secondary end points included safety, PFS by investigator, and quality of life (QoL). The ITT population, defined as all randomly assigned patients who provided informed consent, was used for efficacy analyses. The safety population was defined as all patients who received ≥ 1 dose of study drug. End point definitions are available in the Data Supplement.
Assessments
Tumor assessments (CT or MRI) were completed at screening, Day (D) 1 of Cycles (C) 2-7, every other cycle thereafter, and end-of-treatment visit. Following C7 D1, an initial indication of a partial response or CR on the basis of investigator assessment must also be confirmed ≥ 4 weeks following the initial response. After treatment discontinuation, patients and families were contacted by phone every 3 months for survival data.
The safety profile was based on physical examinations, clinical laboratory tests, ECOG PS, changes from baseline in vital signs, electrocardiograms, left ventricular ejection fraction, dermatologic examinations, and adverse event (AE) reporting. Safety evaluations included treatment-emergent AEs (TEAEs), serious AEs (SAEs), treatment-related TEAEs, dose interruptions, dose reductions, and study drug discontinuations. AE severity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. AEs were monitored from informed consent to safety follow-up (30 days after last dose); AEs were considered treatment emergent if they occurred after administration of first dose of study drug and through 30 days after last dose of study drug or the day before start of subsequent new anticancer drug, whichever occurred first. Drug-related AEs reported after 30 days after the last dose of study drug were also considered TEAEs.
QoL was assessed with patient-reported outcome (PRO) measures (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for Cancer-30 and Dermatology Life Quality Index).23,24
Statistical Analyses
Approximately 426 patients and 262 PFS events in the ITT population were calculated to provide 90% power to detect a 50% improvement in PFS (median PFS ripretinib: 9 months; sunitinib: 6 months; hazard ratio [HR], 0.667) with a two-sided alpha of .05. In the KIT exon 11 ITT population, 151 PFS events were estimated to provide 95% power to detect an 80% PFS improvement (median PFS ripretinib: 9 months; sunitinib: 5 months; HR, 0.556) with a two-sided alpha of .05.
To control familywise type I error at a two-sided 0.05 level, hypothesis testing followed a hierarchical sequence: primary end point PFS, then key secondary end points ORR followed by OS. Within each end point, the KIT exon 11 ITT population was tested before the overall ITT population. If a testing in the sequence failed to meet statistical significance, subsequent P values were considered nominal.25 Therefore, outcomes for the KIT exon 11 ITT population are presented first followed by the overall ITT population.
Time-to-event data were summarized using the Kaplan-Meier method with associated two-sided 95% CIs. HRs and P values were obtained from stratified Cox regression model and two-sided stratified log-rank tests, respectively. The associated 95% CI for PFS was obtained using the Wald method. ORR was analyzed by Cochran-Mantel-Haenszel chi-square test for the association between treatment and ORR with 95% CI of the ORR difference calculated using Newcombe method. Descriptive statistics were used to summarize safety data; P values reported for safety were not prespecified. An independent data monitoring committee reviewed safety and efficacy data periodically throughout the study. Statistical analyses were done with SAS (version 9.4; Cary, NC) and did not deviate from the statistical analysis plan.
RESULTS
Patients
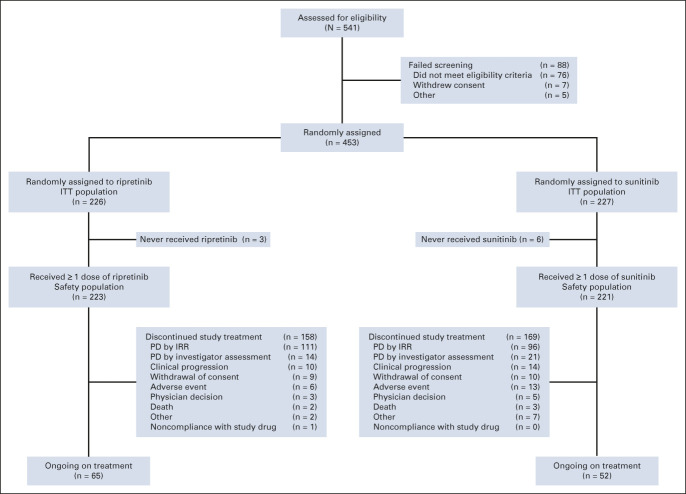
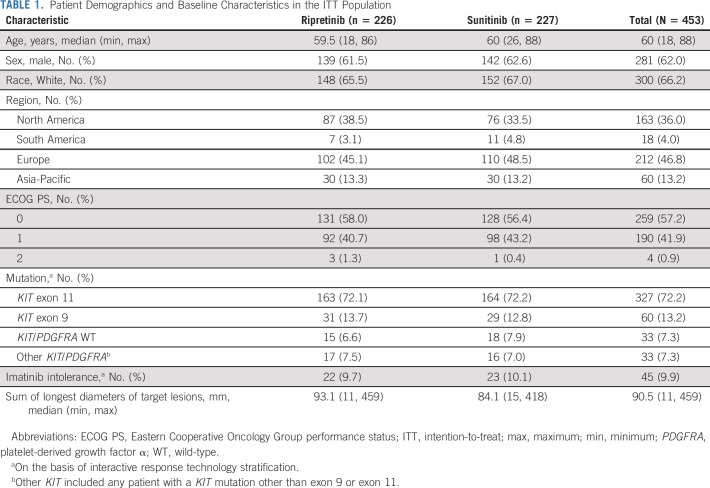
Overall, 453 patients were randomly assigned to once-daily ripretinib 150 mg (n = 226) or once-daily sunitinib 50 mg (4/2, n = 227; Fig 1). Median age was 60 (range, 18-88) years, 62.0% were male, 66.2% were White, and 46.8% were from Europe (ITT; Table 1). Most patients had a baseline ECOG PS score ≤ 1 (99.1%). A total of 327 patients (72.2%) had a primary KIT exon 11 mutation (ripretinib, n = 163; sunitinib, n = 164; KIT exon 11 ITT), 60 (13.2%) had a primary KIT exon 9 mutation, 33 (7.3%) were KIT/PDGFRA WT, and 33 (7.3%) had a primary mutation in another KIT exon (other than 9 or 11) or a PDGFRA mutation. Overall, 9.9% had reported imatinib intolerance. Demographic and clinical characteristics were well balanced between treatment arms (Table 1).
FIG 1.
CONSORT diagram for the phase III INTRIGUE study. IRR, independent radiologic review; ITT, intention-to-treat; PD, progressive disease.
TABLE 1.
Patient Demographics and Baseline Characteristics in the ITT Population
Efficacy
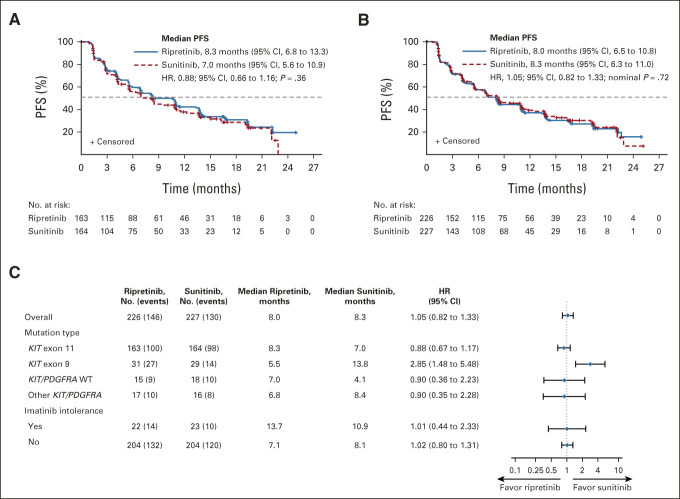
Ripretinib did not demonstrate statistically significant improvement over sunitinib in PFS by IRR in the KIT exon 11 ITT (HR, 0.88; 95% CI, 0.66 to 1.16; P = .36; median 8.3 v 7.0 months; Fig 2A) or ITT population (HR, 1.05; 95% CI, 0.82 to 1.33; nominal P = .72; median 8.0 v 8.3 months; Fig 2B) at the data cutoff (September 1, 2021). Similar results were observed in sensitivity analyses. In the KIT exon 11 population, median PFS by investigator was 13.3 months for ripretinib and 10.8 months for sunitinib (HR, 0.92; 95% CI, 0.68 to 1.24; nominal P = .57); median PFS by investigator in the ITT population for ripretinib and sunitinib was 10.6 and 10.3 months, respectively (HR, 1.03; 95% CI, 0.80 to 1.34; nominal P = .81).
FIG 2.
Kaplan-Meier analysis of PFS for patients treated with ripretinib or sunitinib in the (A) KIT exon 11 ITT population and (B) the ITT population; (C) forest plot of PFS by stratification factors for patients treated with ripretinib or sunitinib. HRs were obtained from stratified Cox regression model and P values were from two-sided stratified log-rank tests. HR, hazard ratio; ITT, intention-to-treat; PDGFRA, platelet-derived growth factor receptor alpha; PFS, progression-free survival; WT, wild-type.
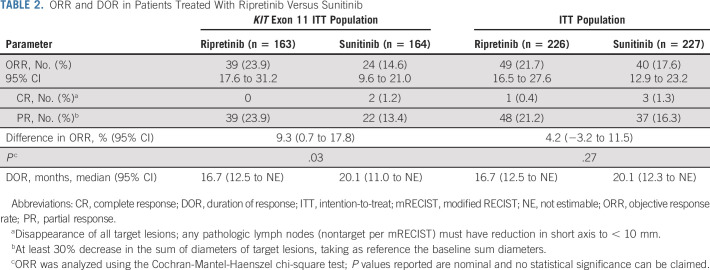
ORR in the KIT exon 11 ITT population was 23.9% with ripretinib and 14.6% with sunitinib (nominal P = .03; Table 2); ORR in the ITT population was 21.7% with ripretinib versus 17.6% with sunitinib (nominal P = .27; Table 2). Median duration of response for ripretinib and sunitinib in both populations was 16.7 and 20.1 months, respectively (Table 2).
TABLE 2.
ORR and DOR in Patients Treated With Ripretinib Versus Sunitinib
OS data were highly immature with event rates of 21.1% and 22.3% in the KIT exon 11 ITT and overall ITT populations, respectively. The median OS was not reached for either arm. Mature OS data will be presented in a subsequent publication.
PFS subgroup analyses on the basis of stratification factors revealed that patients with primary KIT exon 9 mutations (n = 60) were the only subgroup in which PFS benefit favored treatment with sunitinib versus ripretinib (HR, 2.85; 95% CI, 1.48 to 5.48; median 13.8 v 5.5 months; Fig 2C, Data Supplement).
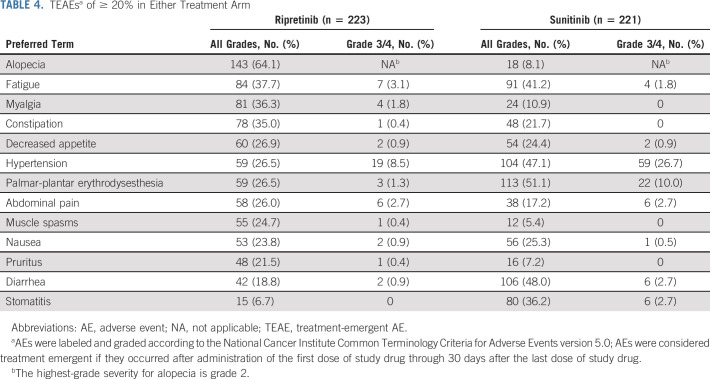
Safety
Ripretinib was generally well tolerated and its safety profile was consistent with existing prescribing information (Table 3).18 In the safety population (ripretinib, n = 223; sunitinib, n = 221), the most common TEAEs with ripretinib were alopecia (64.1%), fatigue (37.7%), and myalgia (36.3%). Palmar-plantar erythrodysesthesia syndrome (PPES; 51.1%), diarrhea (48.0%), and hypertension (47.1%) were the most common with sunitinib (Table 4).
TABLE 3.
Exposure and AE Overview in the Safety Population
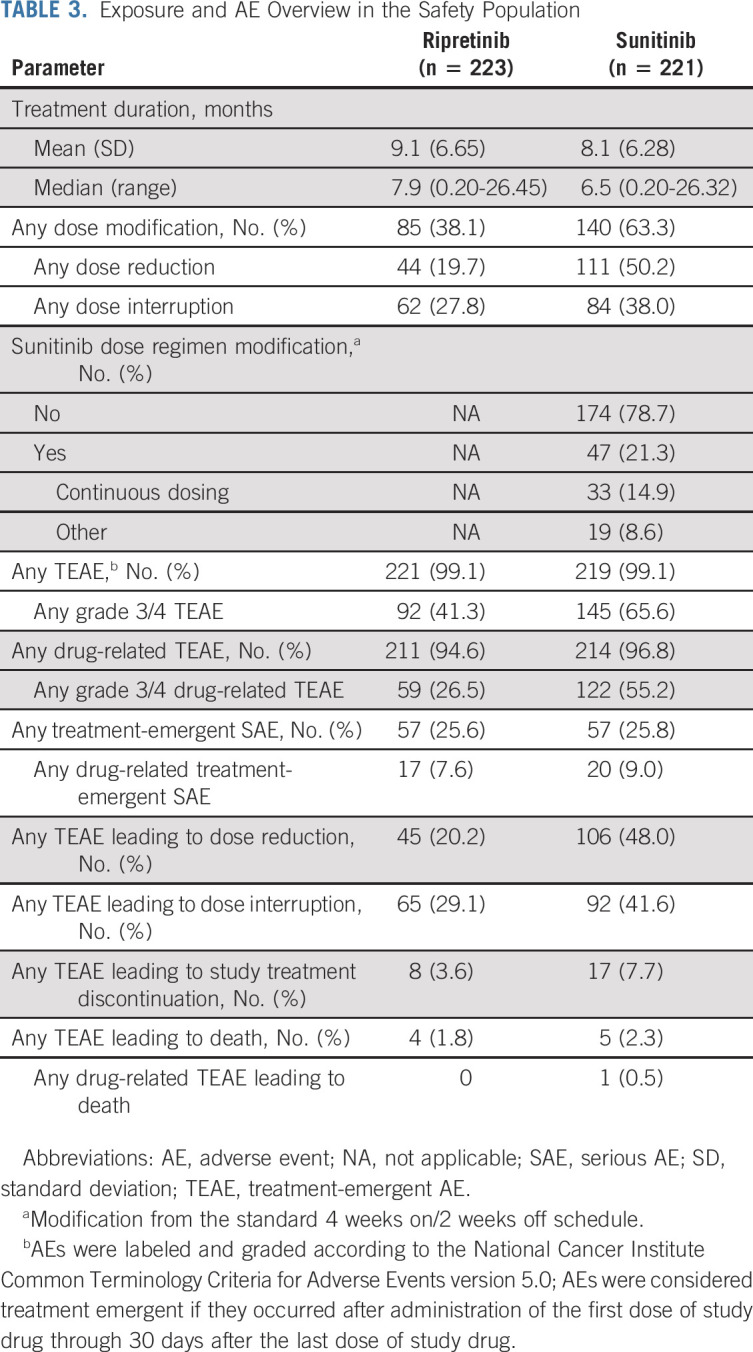
TABLE 4.
TEAEsa of ≥ 20% in Either Treatment Arm
Fewer patients had grade 3/4 TEAEs with ripretinib (n = 92, 41.3%) compared with sunitinib (n = 145, 65.6%; nominal P < .0001; Table 3, Fig 3). Similarly, there were fewer patients with grade 3/4 drug-related TEAEs with ripretinib (n = 59, 26.5%) compared with sunitinib (n = 122, 55.2%; Table 3). Grade 3/4 TEAEs (≥ 2% of patients in either arm) were mostly lower with ripretinib versus sunitinib and included hypertension (8.5% v 26.7%), neutropenia or neutrophil count decreased (0% v 13.1%), PPES (1.3% v 10%), diarrhea (0.9% v 2.7%), hypertriglyceridemia (0.4% v 3.2%), lymphocyte count decreased (0.4% v 2.3%), and stomatitis (0% v 2.7%; Fig 3). Hypertension was the single most common grade 3/4 drug-related TEAE in either arm; however, patients receiving sunitinib were nearly four times more likely to develop drug-related grade 3/4 hypertension (22.6%) compared with ripretinib (5.8%). There were no drug-related TEAEs leading to death with ripretinib and one with sunitinib (intracranial hemorrhage; Table 3).
FIG 3.
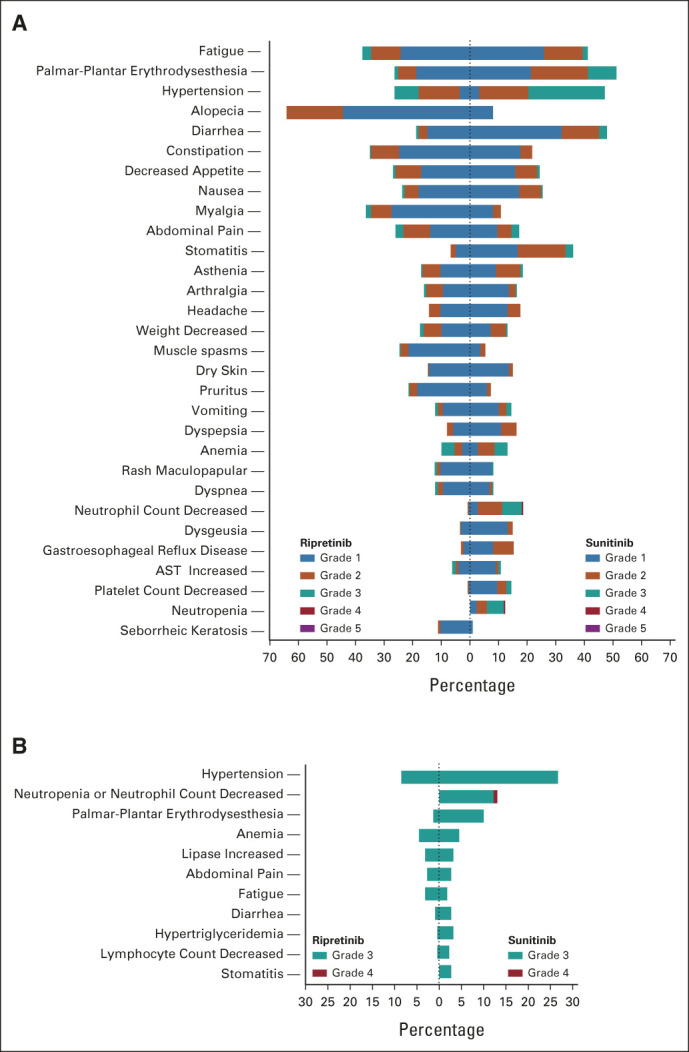
Butterfly plots of TEAEs (≥ 10% in either arm) of (A) all grades and (B) grade 3/4 TEAEs (≥ 2% in either arm). AEs were labeled and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0; AEs were considered treatment emergent if they occurred after administration of the first dose of study drug through 30 days after the last dose of study drug. AE, adverse event; TEAE, treatment-emergent AE.
Fewer patients who received ripretinib needed dose modifications (38.1%) versus sunitinib (63.3%; Table 3). Dose interruptions, dose reductions, and treatment discontinuations because of TEAEs were lower with ripretinib (29.1%, 20.2%, and 3.6%) versus sunitinib (41.6%, 48.0%, and 7.7%; Table 3). The incidence of treatment-emergent SAEs was similar between ripretinib (25.6%) and sunitinib (25.8%). Overall, 7.6% and 9.0% of patients experienced drug-related treatment-emergent SAEs with ripretinib and sunitinib, respectively (Table 3).
Quality of Life
Because of high incidence of dermatologic AEs reported with sunitinib, patients used the Dermatology Life Quality Index to assess impact of skin issues on QoL. Impact on QoL was less frequently reported with ripretinib versus sunitinib across treatment cycles (C7 D29: 14.3% v 26.0%; Data Supplement). Patients receiving sunitinib also experienced greater deterioration in European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for Cancer-30 role functioning (ability to work or engage in leisure activities) across all treatment cycles (mean change from baseline at C7 D29: −8.7 v −22.7 for ripretinib v sunitinib). In both measures, patients receiving sunitinib reported less impact/deterioration on D1 of each cycle immediately following the 2-week off period compared with D29, whereas ripretinib scores did not demonstrate cyclical variation (Data Supplement). Detailed analysis of QoL data will be presented in a separate publication.
DISCUSSION
To our knowledge, INTRIGUE is the largest randomized phase III trial in second-line GIST with an active comparator arm. Ripretinib did not meet the primary end point of superior PFS versus sunitinib. However, PFS observed with ripretinib was comparable to PFS with sunitinib in the KIT exon 11 (8.3 v 7.0 months) and ITT populations (8.0 v 8.3 months), demonstrating clinical activity of ripretinib in second-line GIST. ORR with ripretinib was higher than with sunitinib in the KIT exon 11 population. Additionally, the safety profile observed with ripretinib in INTRIGUE was consistent with existing prescribing information.18 Patients receiving ripretinib experienced fewer grade 3/4 TEAEs and reported better role functioning and less skin toxicity compared with patients receiving sunitinib.
The design assumption that median PFS would be 6 months for sunitinib may have affected the outcome. The PFS for sunitinib observed in this trial was higher (8.3 months, ITT) than the PFS observed in the original phase III sunitinib trial (5.6 months).13 In that trial, efficacy end points were evaluated using RECIST, not mRECIST v1.1.13 Although it may be a function of different versions of RECIST, median baseline tumor burden was notably higher in the original sunitinib trial (233 mm [range, 26-722 mm]) than the current study (sunitinib arm, 84.1 mm [range, 15-418 mm]).13 Similarly, the original study had fewer patients in the sunitinib arm with imatinib intolerance (4.3%) compared with the current study (10.1%), which likely contributed to higher PFS.13 Furthermore, the baseline sum of longest diameters of target lesions was larger for ripretinib compared with sunitinib in this study. Taken together, these differences may have contributed to the current efficacy outcomes.
In the current study, sunitinib demonstrated greater PFS benefit in patients harboring a KIT exon 9 mutation (13.8 months) compared with an exon 11 mutation (7.0 months). This finding is in line with previous studies.14,26-28 Conversely, patients who received ripretinib in the current study fared better if they had a primary KIT exon 11 mutation (8.3 months) versus an exon 9 mutation (5.5 months). In contrast to INVICTUS trial data for ripretinib versus placebo in fourth-line treatment where primary mutation did not predict ripretinib activity, in this study, the primary KIT mutation appeared to predict ripretinib activity in second-line treatment.29 Ripretinib demonstrated good tolerability across all mutation types; although not tested in this study, similar to imatinib, a higher dose of ripretinib may benefit some patients (particularly those with KIT exon 9 mutations) and would be interesting to investigate.30-32
Similar to INVICTUS, alopecia was the most common TEAE of any grade observed with ripretinib in INTRIGUE; however, most patients who experienced alopecia were of grade 1 severity (< 50% hair loss).17,33 Patients who received ripretinib were less likely to experience grade 3/4 TEAEs compared with sunitinib. Additionally, patients receiving sunitinib were more likely to experience TEAEs that are considered clinically impactful and distressing such as stomatitis and diarrhea.34-36 Even lower-severity diarrhea (moderate or grade 2) can be associated with psychologic distress and limitations in daily living activities, and patients report pain and discomfort with stomatitis/oral mucositis.33,35,37
Nearly all grade 3/4 TEAEs (≥ 2% in either arm) were more common with sunitinib (Fig 3B). Patients receiving sunitinib were three times more likely to develop grade 3 hypertension compared with patients receiving ripretinib, increasing the need for closer monitoring to avoid potential exacerbation of prior cardiovascular disease.33,38 Similarly, patients receiving sunitinib were seven times more likely to develop grade 3 PPES—the highest grade of severity for PPES, characterized by severe skin changes, pain, and limitations in activities of daily living—versus patients receiving ripretinib.33 PRO measures were also improved with ripretinib versus sunitinib. These results suggest the safety profile of ripretinib is substantially more favorable than that of sunitinib.
Although once-daily 50 mg (4/2) is the recommended starting dose/schedule for sunitinib for patients with GIST, once-daily 37.5-mg continuous dosing is often implemented in clinical practice. This may be due to promising efficacy demonstrated in a small phase II trial (N = 60, median PFS: 34 weeks); however, 23% of patients in this phase II study still required a dose reduction.39 In a treatment-use study, patients receiving sunitinib who altered their dose or schedule had similar rates of common grade 3/4 TEAEs compared with patients who remained on the once-daily 50-mg (4/2) dose.40 Without more robust clinical evidence that reduced doses provide at least comparable tolerability and efficacy as the label-recommended dose, patients in INTRIGUE were required to start at once-daily 50 mg (4/2).
Limitations for this study include small sample sizes of additional mutational subgroups (KIT exon 9, KIT/PDGFRA WT, and other KIT/PDGFRA). The study was powered to investigate the KIT exon 11 population, and limited information was available for the other subgroups. Additionally, it is challenging to control familywise type I error for an excessive number of hypothesis tests.
In conclusion, ripretinib was not superior to sunitinib for PFS in patients with advanced GIST previously treated with imatinib. However, median PFS observed with ripretinib was comparable with median PFS observed with sunitinib, suggesting that ripretinib is active as second-line therapy for GIST. Additionally, ORR for patients receiving ripretinib in the KIT exon 11 population was higher compared with patients receiving sunitinib. Ripretinib also demonstrated a more favorable safety profile and better responses on PRO measures than sunitinib. Further analysis is ongoing to assess mature OS, pharmacokinetic exposure, circulating tumor DNA, and additional PRO measures.
ACKNOWLEDGMENT
The INTRIGUE study was funded by Deciphera Pharmaceuticals, LLC. The authors thank the patients and their families and caregivers, the investigators, and the investigational site staff of the INTRIGUE study. Medical writing support was provided by Lauren Hanlon, PhD, of AlphaBioCom, LLC, King of Prussia, PA, and was funded by Deciphera Pharmaceuticals, LLC, in compliance with international good publication practice guidelines. MCH received partial salary support from the following sources: a research grant from the Jonathan David Foundation, a VA Merit Review Grant (I01BX005358), and an NCI R21 grant (R21CA263400). INTRIGUE study investigators are listed in Appendix Table A1 (online only).
APPENDIX
TABLE A1.
Study Investigators and Investigational Sites
Sebastian Bauer
Honoraria: Novartis, Pfizer, Bayer, Pharmamar, GlaxoSmithKline, Deciphera
Consulting or Advisory Role: Blueprint Medicines, Bayer, Lilly, Deciphera, Nanobiotix, Daiichi Sankyo, Exelixis, Janssen-Cilag, ADC Therapeutics, Mundipharma, GlaxoSmithKline, Adcendo, Boehringer Ingelheim
Research Funding: Blueprint Medicines, Novartis, Incyte (Inst)
Travel, Accommodations, Expenses: Pharmamar
Yoon-Koo Kang
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol Myers Squibb, Zymeworks, ALX Oncology, Amgen, Novartis, Macrogenics, Surface Oncology, Blueprint Medicines
Tao Wang
Employment: Deciphera
Stock and Other Ownership Interests: Deciphera, Pfizer
Robin L. Jones
Consulting or Advisory Role: Lilly, Immune Design, Merck Serono, Adaptimmune, Daiichi Sankyo, Eisai, Morphotek, TRACON Pharma, Immodulon Therapeutics, Deciphera, PharmaMar, Blueprint Medicines, Clinigen Group, Epizyme, Boehringer Ingelheim, Bayer, Karma Oncology, UpToDate, Athenex, Immunicum, Mundipharma, SpringWorks Therapeutics, SynOX
Research Funding: GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: PharmaMar
Albiruni Abdul Razak
Consulting or Advisory Role: Adaptimmune, Bayer, GlaxoSmithKline, Medison, Inhibrx
Research Funding: Deciphera, Karyopharm Therapeutics, Pfizer, Roche/Genentech, Bristol Myers Squibb, MedImmune, Amgen, GlaxoSmithKline, Blueprint Medicines, Merck, AbbVie, Adaptimmune, Iterion Therapeutics, Neoleukin Therapeutics, Daiichi Sankyo, Symphogen, Rain Therapeutics
Expert Testimony: Medison
Suzanne George
Stock and Other Ownership Interests: Abbott Laboratories
Honoraria: CStone Pharmaceuticals
Consulting or Advisory Role: Blueprint Medicines, Deciphera, Bayer, Lilly, UpToDate, Research to Practice, MORE Health, Daiichi, Kayothera, Immunicum
Research Funding: Blueprint Medicines (Inst), Deciphera (Inst), Daiichi Sankyo RD Novare (Inst), Merck (Inst), Eisai (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst), Theseus Pharmaceuticals (Inst), BioAtla
Patents, Royalties, Other Intellectual Property: UptoDate
Expert Testimony: Bayer
Other Relationship: Research to Practice, WCG
Axel Le Cesne
Honoraria: Bayer, PharmaMar, Deciphera
Michael C. Heinrich
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Blueprint Medicines, Deciphera, Theseus Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Patent on treatment of GIST-licensed to Novartis (Inst)
Patrick Schöffski
Honoraria: Blueprint Medicines
Consulting or Advisory Role: Deciphera, Ellipses Pharma, Blueprint Medicines, Transgene, Exelixis, Boehringer Ingelheim, Studiecentrum voor Kernenergie (SCK CEN), SQZ Biotechnology, Adcendo, PharmaMar, Merck, Medpace
Research Funding: CoBioRes NV (Inst), Eisai (Inst), G1 Therapeutics (Inst), PharmaMar (Inst), Genmab (Inst), Merck (Inst), Sartar Therapeutics (Inst), ONA Therapeutics (Inst)
Ying Su
Employment: Codiak Biosciences, Deciphera
Stock and Other Ownership Interests: Deciphera, Codiak Biosciences
Margaret von Mehren
Consulting or Advisory Role: Deciphera, GlaxoSmithKline
Research Funding: Novartis (Inst), Deciphera (Inst), Gradalis (Inst), Genmab (Inst), ASCO (Inst), Solaris Health (Inst)
Patents, Royalties, Other Intellectual Property: Cell line
Other Relationship: NCCN
Hans Gelderblom
Research Funding: Novartis (Inst), Ipsen (Inst), Deciphera (Inst), Daiichi (Inst)
Steven Attia
Research Funding: AB Science (Inst), TRACON Pharma (Inst), Bayer (Inst), Novartis (Inst), Lilly (Inst), Immune Design (Inst), Karyopharm Therapeutics (Inst), Epizyme (Inst), Blueprint Medicines (Inst), Genmab (Inst), CBA Pharma (Inst), Desmoid Tumor Research Foundation, Merck (Inst), Philogen (Inst), Gradalis (Inst), Deciphera (Inst), Takeda (Inst), Incyte (Inst), SpringWorks Therapeutics (Inst), Adaptimmune (Inst), Advenchen Laboratories (Inst), Bavarian Nordic (Inst), BTG (Inst), PTC Therapeutics (Inst), GlaxoSmithKline (Inst), FORMA Therapeutics (Inst), TRACON Pharma (Inst), Ayala Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Boehringer Ingelheim (Inst), Salarius Pharmaceuticals (Inst), Theseus Pharmaceuticals (Inst), Monopar Therapeutics (Inst), C4 Therapeutics (Inst), InhibRx (Inst), Noxopharm (Inst), Rain Therapeutics (Inst)
Rodrigo Ruiz-Soto
Employment: Deciphera
Stock and Other Ownership Interests: Deciphera, Immunogen
Patents, Royalties, Other Intellectual Property: I am an inventor in three patents with Immunogen, I transferred the rights to Immunogen. I have not received (and I will not receive) any royalties, I am an inventor in pending patents at Deciphera. I have transferred the rights to Deciphera, I have not received (and will not receive) any royalties
John R. Zalcberg
Leadership: ICON Group
Stock and Other Ownership Interests: Biomarin, Opthea, Amarin Corporation, Concert Pharmaceuticals, Frequency Therapeutics, Gilead Sciences, Madrigal Pharmaceuticals, UniQure, Zogenix, Orphazyme, Moderna Therapeutics, Twist Bioscience, Novavax
Honoraria: Specialised Therapeutics, Merck Serono, Targovax, Halozyme, Gilead Sciences, Deciphera
Consulting or Advisory Role: Merck Serono, Targovax, Merck Sharp & Dohme, Halozyme, Lipotek, Specialised Therapeutics, Center for Emerging & Neglected Diseases (CEND), Deciphera, REVOLUTION MEDICINE, FivePHusion, Genor BioPharma, 1Globe Health Institute, Novotech
Research Funding: Merck Serono (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), IQvia (Inst), Mylan (Inst), Ipsen (Inst), Eisai (Inst), Medtronic (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Merck Serono, AstraZeneca, Merck Sharp & Dohme, Deciphera, Sanofi
Julie Meade
Employment: Deciphera, Mirati Therapeutics
Stock and Other Ownership Interests: Deciphera, Mirati Therapeutics
Travel, Accommodations, Expenses: Deciphera, Mirati Therapeutics
Jean-Yves Blay
Leadership: Innate Pharma
Honoraria: Roche, AstraZeneca, PharmaMar, MSD, BMS, Bayer, Ignyta, Deciphera
Consulting or Advisory Role: Roche, Pharmamar, Blueprint Medicines, Bayer, Deciphera, Karyopharm Therapeutics
Research Funding: GlaxoSmithKline (Inst), Pharmamar (Inst), Novartis (Inst), Bayer (Inst), Roche (Inst), BMS (Inst), MSD (Inst), DEciphera (Inst), AstraZeneca (Inst), OSE Pharma (Inst)
Travel, Accommodations, Expenses: Roche
Jonathan Trent
Consulting or Advisory Role: Blueprint Medicines, Deciphera, Daiichi Sankyo, Epizyme, Agios, C4 Therapeutics, Bayer, AADI Bioscience, LLC, Foghorn Therapeutics, Boehringer Ingelheim, Cogent Medicine, SERVIER
Matthew L. Sherman
Employment: Deciphera, Pieris Pharmaceuticals
Leadership: Deciphera, Pieris Pharmaceuticals
Stock and Other Ownership Interests: Deciphera, Pieris Pharmaceuticals
No other potential conflicts of interest were reported.
See accompanying editorial on page 3903
PRIOR PRESENTATION
Presented at the 2022 ASCO Plenary Series, virtual, January 25, 2022.
SUPPORT
The INTRIGUE study was funded by Deciphera Pharmaceuticals, LLC.
CLINICAL TRIAL INFORMATION
NCT03673501 (INTRIGUE)
AUTHOR CONTRIBUTIONS
Conception and design: Sebastian Bauer, Robin L. Jones, Jean-Yves Blay, Hans Gelderblom, Suzanne George, Patrick Schöffski, Margaret von Mehren, John R. Zalcberg, Jonathan Trent, Steven Attia, Ying Su, Julie Meade, Tao Wang, Matthew L. Sherman, Rodrigo Ruiz-Soto, Michael C. Heinrich
Provision of study materials or patients: Sebastian Bauer, Robin L. Jones, Jean-Yves Blay, Hans Gelderblom, Suzanne George, Patrick Schöffski, Margaret von Mehren, John R. Zalcberg, Yoon-Koo Kang, Albiruni Abdul Razak, Jonathan Trent, Steven Attia, Axel Le Cesne, Michael C. Heinrich
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ripretinib Versus Sunitinib in Patients With Advanced Gastrointestinal Stromal Tumor After Treatment With Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sebastian Bauer
Honoraria: Novartis, Pfizer, Bayer, Pharmamar, GlaxoSmithKline, Deciphera
Consulting or Advisory Role: Blueprint Medicines, Bayer, Lilly, Deciphera, Nanobiotix, Daiichi Sankyo, Exelixis, Janssen-Cilag, ADC Therapeutics, Mundipharma, GlaxoSmithKline, Adcendo, Boehringer Ingelheim
Research Funding: Blueprint Medicines, Novartis, Incyte (Inst)
Travel, Accommodations, Expenses: Pharmamar
Yoon-Koo Kang
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol Myers Squibb, Zymeworks, ALX Oncology, Amgen, Novartis, Macrogenics, Surface Oncology, Blueprint Medicines
Tao Wang
Employment: Deciphera
Stock and Other Ownership Interests: Deciphera, Pfizer
Robin L. Jones
Consulting or Advisory Role: Lilly, Immune Design, Merck Serono, Adaptimmune, Daiichi Sankyo, Eisai, Morphotek, TRACON Pharma, Immodulon Therapeutics, Deciphera, PharmaMar, Blueprint Medicines, Clinigen Group, Epizyme, Boehringer Ingelheim, Bayer, Karma Oncology, UpToDate, Athenex, Immunicum, Mundipharma, SpringWorks Therapeutics, SynOX
Research Funding: GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: PharmaMar
Albiruni Abdul Razak
Consulting or Advisory Role: Adaptimmune, Bayer, GlaxoSmithKline, Medison, Inhibrx
Research Funding: Deciphera, Karyopharm Therapeutics, Pfizer, Roche/Genentech, Bristol Myers Squibb, MedImmune, Amgen, GlaxoSmithKline, Blueprint Medicines, Merck, AbbVie, Adaptimmune, Iterion Therapeutics, Neoleukin Therapeutics, Daiichi Sankyo, Symphogen, Rain Therapeutics
Expert Testimony: Medison
Suzanne George
Stock and Other Ownership Interests: Abbott Laboratories
Honoraria: CStone Pharmaceuticals
Consulting or Advisory Role: Blueprint Medicines, Deciphera, Bayer, Lilly, UpToDate, Research to Practice, MORE Health, Daiichi, Kayothera, Immunicum
Research Funding: Blueprint Medicines (Inst), Deciphera (Inst), Daiichi Sankyo RD Novare (Inst), Merck (Inst), Eisai (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst), Theseus Pharmaceuticals (Inst), BioAtla
Patents, Royalties, Other Intellectual Property: UptoDate
Expert Testimony: Bayer
Other Relationship: Research to Practice, WCG
Axel Le Cesne
Honoraria: Bayer, PharmaMar, Deciphera
Michael C. Heinrich
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Blueprint Medicines, Deciphera, Theseus Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Patent on treatment of GIST-licensed to Novartis (Inst)
Patrick Schöffski
Honoraria: Blueprint Medicines
Consulting or Advisory Role: Deciphera, Ellipses Pharma, Blueprint Medicines, Transgene, Exelixis, Boehringer Ingelheim, Studiecentrum voor Kernenergie (SCK CEN), SQZ Biotechnology, Adcendo, PharmaMar, Merck, Medpace
Research Funding: CoBioRes NV (Inst), Eisai (Inst), G1 Therapeutics (Inst), PharmaMar (Inst), Genmab (Inst), Merck (Inst), Sartar Therapeutics (Inst), ONA Therapeutics (Inst)
Ying Su
Employment: Codiak Biosciences, Deciphera
Stock and Other Ownership Interests: Deciphera, Codiak Biosciences
Margaret von Mehren
Consulting or Advisory Role: Deciphera, GlaxoSmithKline
Research Funding: Novartis (Inst), Deciphera (Inst), Gradalis (Inst), Genmab (Inst), ASCO (Inst), Solaris Health (Inst)
Patents, Royalties, Other Intellectual Property: Cell line
Other Relationship: NCCN
Hans Gelderblom
Research Funding: Novartis (Inst), Ipsen (Inst), Deciphera (Inst), Daiichi (Inst)
Steven Attia
Research Funding: AB Science (Inst), TRACON Pharma (Inst), Bayer (Inst), Novartis (Inst), Lilly (Inst), Immune Design (Inst), Karyopharm Therapeutics (Inst), Epizyme (Inst), Blueprint Medicines (Inst), Genmab (Inst), CBA Pharma (Inst), Desmoid Tumor Research Foundation, Merck (Inst), Philogen (Inst), Gradalis (Inst), Deciphera (Inst), Takeda (Inst), Incyte (Inst), SpringWorks Therapeutics (Inst), Adaptimmune (Inst), Advenchen Laboratories (Inst), Bavarian Nordic (Inst), BTG (Inst), PTC Therapeutics (Inst), GlaxoSmithKline (Inst), FORMA Therapeutics (Inst), TRACON Pharma (Inst), Ayala Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Boehringer Ingelheim (Inst), Salarius Pharmaceuticals (Inst), Theseus Pharmaceuticals (Inst), Monopar Therapeutics (Inst), C4 Therapeutics (Inst), InhibRx (Inst), Noxopharm (Inst), Rain Therapeutics (Inst)
Rodrigo Ruiz-Soto
Employment: Deciphera
Stock and Other Ownership Interests: Deciphera, Immunogen
Patents, Royalties, Other Intellectual Property: I am an inventor in three patents with Immunogen, I transferred the rights to Immunogen. I have not received (and I will not receive) any royalties, I am an inventor in pending patents at Deciphera. I have transferred the rights to Deciphera, I have not received (and will not receive) any royalties
John R. Zalcberg
Leadership: ICON Group
Stock and Other Ownership Interests: Biomarin, Opthea, Amarin Corporation, Concert Pharmaceuticals, Frequency Therapeutics, Gilead Sciences, Madrigal Pharmaceuticals, UniQure, Zogenix, Orphazyme, Moderna Therapeutics, Twist Bioscience, Novavax
Honoraria: Specialised Therapeutics, Merck Serono, Targovax, Halozyme, Gilead Sciences, Deciphera
Consulting or Advisory Role: Merck Serono, Targovax, Merck Sharp & Dohme, Halozyme, Lipotek, Specialised Therapeutics, Center for Emerging & Neglected Diseases (CEND), Deciphera, REVOLUTION MEDICINE, FivePHusion, Genor BioPharma, 1Globe Health Institute, Novotech
Research Funding: Merck Serono (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), IQvia (Inst), Mylan (Inst), Ipsen (Inst), Eisai (Inst), Medtronic (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Merck Serono, AstraZeneca, Merck Sharp & Dohme, Deciphera, Sanofi
Julie Meade
Employment: Deciphera, Mirati Therapeutics
Stock and Other Ownership Interests: Deciphera, Mirati Therapeutics
Travel, Accommodations, Expenses: Deciphera, Mirati Therapeutics
Jean-Yves Blay
Leadership: Innate Pharma
Honoraria: Roche, AstraZeneca, PharmaMar, MSD, BMS, Bayer, Ignyta, Deciphera
Consulting or Advisory Role: Roche, Pharmamar, Blueprint Medicines, Bayer, Deciphera, Karyopharm Therapeutics
Research Funding: GlaxoSmithKline (Inst), Pharmamar (Inst), Novartis (Inst), Bayer (Inst), Roche (Inst), BMS (Inst), MSD (Inst), DEciphera (Inst), AstraZeneca (Inst), OSE Pharma (Inst)
Travel, Accommodations, Expenses: Roche
Jonathan Trent
Consulting or Advisory Role: Blueprint Medicines, Deciphera, Daiichi Sankyo, Epizyme, Agios, C4 Therapeutics, Bayer, AADI Bioscience, LLC, Foghorn Therapeutics, Boehringer Ingelheim, Cogent Medicine, SERVIER
Matthew L. Sherman
Employment: Deciphera, Pieris Pharmaceuticals
Leadership: Deciphera, Pieris Pharmaceuticals
Stock and Other Ownership Interests: Deciphera, Pieris Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rubin BP, Heinrich MC, Corless CL: Gastrointestinal stromal tumour. Lancet 369:1731-1741, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Søreide K, Sandvik OM, Søreide JA, et al. : Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 40:39-46, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, et al. : Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577-580, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Heinrich MC, Corless CL, Duensing A, et al. : PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299:708-710, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Szucs Z, Thway K, Fisher C, et al. : Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol 13:93-107, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Vallilas C, Sarantis P, Kyriazoglou A, et al. : Gastrointestinal stromal tumors (GISTs): Novel therapeutic strategies with immunotherapy and small molecules. Int J Mol Sci 22:493, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Druker BJ, Talpaz M, Resta DJ, et al. : Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344:1031-1037, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Blanke CD, Rankin C, Demetri GD, et al. : Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26:626-632, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Novartis Pharmaceuticals Corporation : Gleevec (Imatinib Mesylate) Tablets, for Oral Use. Prescribing Information, 2020. https://www.novartis.us/sites/www.novartis.us/files/gleevec_tabs.pdf [Google Scholar]
- 10.National Comprehensive Cancer Network . NCCN Guidelines Gastrointestinal Stromal Tumors (GISTs) Version 1.2022, 2022. https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf [Google Scholar]
- 11.Hao Z, Sadek I: Sunitinib: The antiangiogenic effects and beyond. OncoTargets Ther 9:5495-5505, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfizer Labs . Sutent (Sunitinib Malate) Capsules, for Oral Use. Prescribing Information, 2021. https://labeling.pfizer.com/showlabeling.aspx?id=607 [Google Scholar]
- 13.Demetri GD, van Oosterom AT, Garrett CR, et al. : Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 368:1329-1338, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Heinrich MC, Maki RG, Corless CL, et al. : Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 26:5352-5359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemunaitis J, Bauer S, Blay JY, et al. : Intrigue: Phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Future Oncol 16:4251-4264, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Smith BD, Kaufman MD, Lu WP, et al. : Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell 35:738-751.e9, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Blay JY, Serrano C, Heinrich MC, et al. : Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 21:923-934, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deciphera Pharmaceuticals, LLC : QINLOCKTM (Ripretinib) Tablets: US Prescribing Information, 2021. https://www.qinlockhcp.com/Content/files/qinlock-prescribing-information.pdf [Google Scholar]
- 19.Janku F, Abdul Razak AR, Chi P, et al. : Switch control inhibition of KIT and PDGFRA in patients with advanced gastrointestinal stromal tumor: A phase I study of ripretinib. J Clin Oncol 38:3294-3303, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu C, Dignam JJ: Biomarker-driven oncology clinical trials: Key design elements, types, features, and practical considerations. JCO Precis Oncol 10.1200/PO.19.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polley MC, Korn EL, Freidlin B: Phase III precision medicine clinical trial designs that integrate treatment and biomarker evaluation. JCO Precis Oncol 10.1200/PO.18.00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demetri GD, Reichardt P, Kang YK, et al. : Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:295-302, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finlay AY, Khan GK: Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin Exp Dermatol 19:210-216, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Fayers P Bottomley A; EORTC Quality of Life Group, et al. : Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer 38:S125-S133, 2002. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 25.Dmitrienko A, Tamhane AC, Bretz F: Multiple Testing Problems in Pharmaceutical Statistics (ed 1). New York, NY, Chapman and Hall/CRC, 2009 [Google Scholar]
- 26.Li J, Gao J, Hong J, Shen L: Efficacy and safety of sunitinib in Chinese patients with imatinib-resistant or -intolerant gastrointestinal stromal tumors. Future Oncol 8:617-624, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Yoon DH, Ryu MH, Ryoo BY, et al. : Sunitinib as a second-line therapy for advanced GISTs after failure of imatinib: Relationship between efficacy and tumor genotype in Korean patients. Invest New Drugs 30:819-827, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Reichardt P, Demetri GD, Gelderblom H, et al. : Correlation of KIT and PDGFRA mutational status with clinical benefit in patients with gastrointestinal stromal tumor treated with sunitinib in a worldwide treatment-use trial. BMC Cancer 16:22, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer S, Heinrich MC, George S, et al. : Clinical activity of ripretinib in patients with advanced gastrointestinal stromal tumor harboring heterogeneous KIT/PDGFRA mutations in the phase III INVICTUS study. Clin Cancer Res 27:6333-6342, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debiec-Rychter M, Sciot R, Le Cesne A, et al. : KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42:1093-1103, 2006 [DOI] [PubMed] [Google Scholar]
- 31.George S, Chi P, Heinrich MC, et al. : Ripretinib intrapatient dose escalation after disease progression provides clinically meaningful outcomes in advanced gastrointestinal stromal tumour. Eur J Cancer 155:236-244, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalcberg JR, Heinrich MC, George S, et al. : Clinical benefit of ripretinib dose escalation after disease progression in advanced gastrointestinal stromal tumor: An analysis of the INVICTUS study. Oncologist 26:e2053-e2060, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute : Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf [Google Scholar]
- 34.Benedict C, DuHamel K, Nelson CJ: Reduction in social activities mediates the relationship between diarrhea and distress in rectal/anal cancer survivors. Psychooncology 27:691-694, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lui M, Gallo-Hershberg D, DeAngelis C: Development and validation of a patient-reported questionnaire assessing systemic therapy induced diarrhea in oncology patients. Health Qual Life Outcomes 15:249, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manfi Al-Rudayni AH, Gopinath D, Kannan Maharajan M, et al. : Impact of oral mucositis on quality of life in patients undergoing oncological treatment: A systematic review. Transl Cancer Res 9:3126-3134, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fauske L, Hompland I, Lorem G, et al. : Perspectives on treatment side effects in patients with metastatic gastrointestinal stromal tumour: A qualitative study. Clin Sarcoma Res 9:6, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen F, Yildiz I, Basaran M, et al. : Impaired coronary flow reserve in metastatic cancer patients treated with sunitinib. J BUON 18:775-781, 2013 [PubMed] [Google Scholar]
- 39.George S, Blay JY, Casali PG, et al. : Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 45:1959-1968, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Reichardt P, Kang YK, Rutkowski P, et al. : Clinical outcomes of patients with advanced gastrointestinal stromal tumors: Safety and efficacy in a worldwide treatment-use trial of sunitinib. Cancer 121:1405-1413, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]