To the Editor: Bronchial asthma is a respiratory disease with pathological features that mainly comprise changes in airway inflammation, airway remodeling, and airway hyperresponsiveness. During an asthma attack, a large number of inflammatory genes are activated, and the expression of inflammatory mediators increases, eventually leading to airway hyperresponsiveness and remodeling. Nuclear factor-κB (NF-κB) is composed of transcriptionally active dimers of the Rel family, which is closely related to the expression of inflammatory genes during an asthma attack.
Histone deacetylases (HDACs) are the key enzymes that regulate histone and non-histone deacetylation. Our previous studies revealed that an HDAC6 inhibitor (Tubastatin A HCl), an HDAC 8 inhibitor (PCI-34051), and a broad-spectrum HDAC inhibitor (Givinostat) could effectively alleviate the airway inflammation, remodeling, and hyperresponsiveness of asthma.[1] Although the anti-inflammatory effect of the HDAC8 inhibitor was slightly better than that of the HDAC6 inhibitor, the HDAC6 inhibitor was more efficacious in relieving asthmatic airway remodeling and hyperresponsiveness, possibly because of differences in the expression characteristics of their respective targets in the lung tissue of asthmatic mice. This study aimed to clarify the target of HDAC8 in the pathogenesis of asthma and its mechanism for regulating airway inflammation.
Female BALB/C mice (specific-pathogen-free grade, 6–8 weeks, 18–22 g) were purchased from Liaoning Chang-sheng Biotech Co., Ltd. (animal license number: SCXK [Liao 2010–0001]). All procedures were approved by the Institutional Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Forty-eight mice were randomly divided into four groups with 12 mice in each group: normal (control) group, asthma group, dexamethasone group, and PCI-34051 group. All mice except for those in the normal group were sensitized with ovalbumin (OVA, 20 μg; Sigma, St. Louis, MO, USA) and aluminum hydroxide gel (Al[OH]3, 2 mg; Sigma) via intraperitoneal administration on days 1, 8, and 15 [Figure 1A]. Seven days after the last sensitization, OVA (20 mg/mL) atomization was performed with an ultrasonic atomizing device at 3 mL/min for 30 min, 3 times/week for 8 weeks. Dexamethasone (2.0 mg/kg, Zhuo Feng, Zhengzhou, China) and PCI-34051 (0.5 mg/kg, Selleckchem, Houston, TX, USA) were administered via intraperitoneal injection 30 min before stimulation. Normal saline was used instead of OVA in the normal control group.
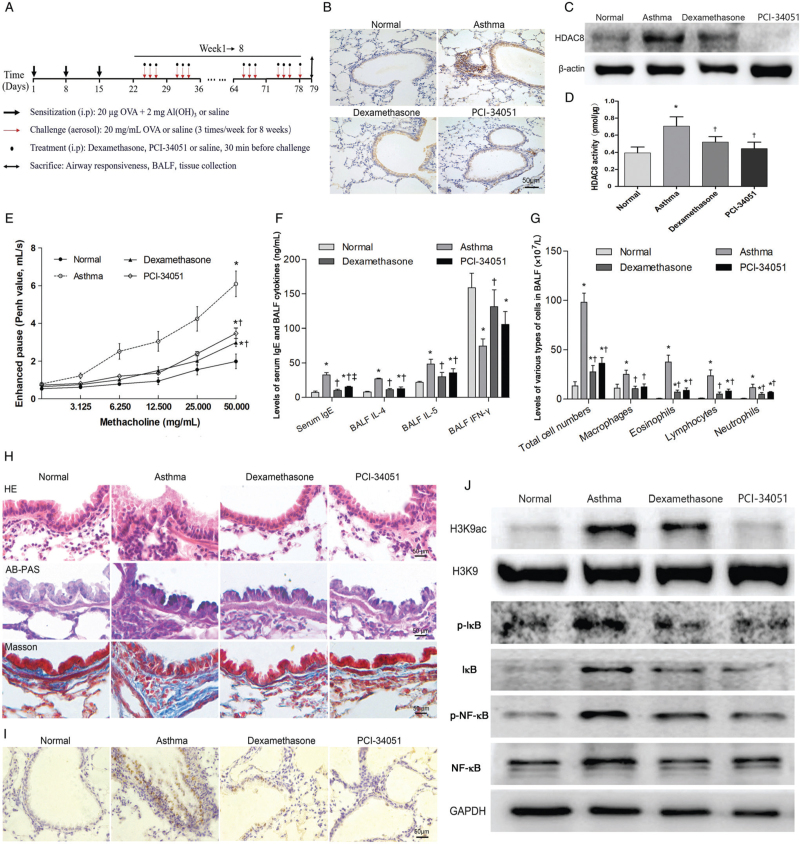
Figure 1.
HDAC8 regulates NF-κB-related Inflammation In asthmatic mice through H3K9 acetylatlon. (A) Protocol for establishing the mouse asthma model by treatment with dexamethasone or PCI-34051. Immunohistochemistry (B) and western blotting (C) of HDAC8 in lung tissues. (D) Quantification of HDAC8 enzymatic activity in lung tissues. (E) Penh values following stimulation with increasing concentrations of acetyl-β-methacholine chloride. (F) Quantification of the levels of IgE in serum and cytokines in BALF. (G) Quantification of different cell types in BALF. (H) Histopathological staining of lung sections with H&E, AB-PAS, and Masson's trichrome. (I) Immunohistochemistry of H3K9ac in lung tissues. (J) Phosphorylation and acetylation of NF-κB pathway components on western blotting. Scale bar represents 50 μm. ∗P < 0.05 vs. normal control group, †P < 0.05 vs. asthma group, ‡P < 0.05 vs. dexamethasone group. AB-PAS: Alcian blue-periodic acid Schiff; Al(OH)3: Aluminum hydroxide gel; BALF: Bronchoalveolar lavage fluid; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; H&E: Hematoxylin and eosin; H3K9: Histone H3 lysine 9; H3K9ac: Acetylation of histone H3 lysine 9; HDAC8: Histone deacetylase 8; IκB/p-IκB: Inhibitor of κB/phosphorylated IκB; i.p: Intraperitoneal injection; IFN-γ: Interferon-γ; IgE: Immunoglobulin E; IL: Interleukin; NF-κB: Nuclear factor-κB; OVA: Ovalbumin; PCI-34051: Histone deacetylase 8 inhibitor; Penh: Enhanced pause; p-NF-κB: Phosphorylated nuclear factor-κB.
Airway responsiveness was measured using non-invasive whole-body plethysmography (Emka Technologies, Paris, France). Enhanced pause (Penh) was recorded to detect changes in airway responsiveness following stimulation with acetyl-β-methacholine chloride (Sigma) at concentrations of 0 mg/mL, 3.125 mg/mL, 6.250 mg/mL, 12.500 mg/mL, 25.000 mg/mL, and 50.000 mg/mL. Bronchoal-veolar lavage fluid (BALF) was collected and the total number of cells was counted. A 50-μL aliquot of the resuspended cells was smeared for Wright-Giemsa staining for a random, blind calculation of the numbers of cells of different types. Levels of interleukin-4 (IL-4), interleukin-5 (IL-5), and interferon-γ (IFN-γ) in the BALF supernatant, and immunoglobulin E (IgE) in the serum, were measured using enzyme-linked immunosorbent assay kits (Cloud-Clone, Houston, TX, USA) according to the manufacturer's instructions.
Lung tissues from each group were fixed with paraformaldehyde, embedded in paraffin, and stained with hematoxylin and eosin (H&E), Alcian blue-periodic acid Schiff (AB-PAS) and Masson's trichrome to evaluate the inflammatory infiltration, goblet cell metaplasia, and collagen deposition. Pathological changes were observed under a light microscope and measured using Image-Pro Plus software (version 6.0, Media Cybernetics, Rockville, MD, USA). Inflammatory infiltration was graded on the following scale: 0 points, no inflammatory cells around the bronchus; 1 point, a few inflammatory cells around the bronchus; 2 points, uneven distribution of inflammatory cells around the bronchus, but not clustered; 3 points, a large number of inflammatory cells around the bronchus with even distribution and rare agglomeration; and 4 points, a large number of inflammatory cells clustered around the bronchus.
Nuclear HDAC8 activity in lung lysates (right lower lobe) was examined using a fluorometric assay kit (BioVision, San Francisco, CA, USA) according to the manufacturer's protocol. The expression level of HDAC8 and histone H3 lysine 9 (H3K9) acetylation pattern in lung tissue were assessed by western blotting and immunohistochemistry, respectively. The activation level of NF-κB signaling was detected using western blotting. The values are expressed as the mean ± standard deviation. Statistical analysis was performed using the SPSS 23.0 software (IBM, Armonk, NY, USA). Comparisons of groups were performed by t test. P values < 0.05 were considered statistically significant.
Our study showed that HDAC8 was highly expressed in mouse epithelial cells, alveolar interstitial cells, and inflammatory cells around the airways during asthma pathogenesis [Figure 1B]. Quantitative analysis revealed that the expression level and enzymatic activity of HDAC8 decreased significantly under inhibition by dexamethasone or PCI-34051 in asthmatic lung tissue [Figure 1C and 1D]. Additionally, PCI-34051 treatment reduced airway hyper-responsiveness and inflammation, which were characterized by decreased Penh values (Figure 1E; methacholine concentration 50 mg/mL, P < 0.001), decreased levels of serum IgE (Figure 1F; P < 0.001), IL-4 (Figure 1F; P < 0.001), and IL-5 (Figure 1F; P = 0.007), and reduced numbers of total inflammatory cells (Figure 1G; P < 0.001) and eosinophils (Figure 1G; P < 0.001) in the BALF. Histopathological staining with HE, AB-PAS, and Masson trichrome revealed that treatment with PCI-34051 significantly decreased inflammatory cell infiltration, mucus accumulation, and subepithelial collagen deposition in asthmatic lung tissue [Figure 1H].
Previous studies have shown that the NF-κB signaling pathway is activated during the inflammatory response in asthma, and that NF-κB-related inflammatory gene expression is closely related to the acetylation level of the H3K9 site.[2,3] In this research, the H3K9 acetylation level was significantly higher in mouse asthmatic lung tissue compared with that in normal tissue and was predominantly distributed in airway epithelial cells and inflammatory cells [Figure 1I]. The activation level of the NF-κB signaling pathway was also higher in asthmatic lung tissue, with upregulated expression of NF-κB, phosphorylated NF-κB, inhibitor of κB(IκB), and phosphorylated-IκB [Figure 1J]. Treatment with dexamethasone or PCI-34051 effectively inhibited H3K9 acetylation and NF-κB pathway activation [Figure 1J]. As an important family member of Class I HDACs, HDAC8 plays significant regulatory roles in apoptosis, proliferation, and differentiation of T cells, differentiation and contraction of smooth muscle cells, and other pathophysiological processes. Using the high performance liquid chromatography method, Aramsangtienchai et al[4] found that HDAC8 could regulate the acetylation levels of histones at H3K9, H2BK12, and H3K18 and affect downstream gene transcription. Ha et al[5] also found that HDAC8 plays an important role during apoptosis, regulating the acetylation level of H3K27, and affecting the transcription of downstream genes such as BNIP3 and MLN64 in RAW264.7 macrophages induced by anthrax toxin.
During asthma, the activation of the NF-κB signaling pathway in lung tissue is directly involved in regulating the expression of a large number of inflammatory genes. A previous study has confirmed that acetylation modification of histone-related sites can change the spatial conformation between histones and chromatin, loosening the structure to expose the promoter regions of target genes to NF-κB binding.[6] Among such histone modification sites (H3K9, H3K14, H3K18, H3K27, H3K36, and others), the acetylation and methylation modifications of H3K9 directly participate in and regulate the transcription of NF-κB-dependent inflammatory genes. Therefore, it is speculated that the anti-inflammatory function of HDAC8 inhibitors may be exerted via the regulation of H3K9 acetylation and modulation of the level of stimulation by the NF-κB pathway. Our results further support the speculation that the role of HDAC8 in the pathogenesis of asthma is to regulate the level of acetylation of H3K9 in lung tissue, thereby affecting activation of the NF-κB pathway and related inflammation. However, the exact epigenetic mechanism by which HDAC8 affects the expression of NF-κB-related inflammatory genes remains unclear and needs further discussion.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81770021) and the postdoctoral program (No. 2018M641749).
Conflicts of interest
None.
Footnotes
How to cite this article: Ren Y, Li M, Bai S, Su X. Histone deacetylase 8 regulates NF-κB-related inflammation in asthmatic mice through H3K9 acetylation. Chin Med J 2022;135:2110–2112. doi: 10.1097/CM9.0000000000001963
References
- 1.Ren Y, Su X, Kong L, Li M, Zhao X, Yu N, et al. Therapeutic effects of histone deacetylase inhibitors in a murine asthma model. Inflamm Res 2016; 65:995–1008. doi: 10.1007/s00011-016-0984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Guan XJ, Peng XH, Cui ZL, Luan CY, Guo XJ. Acetylation of lysine 9 on histone H3 is associated with increased pro-inflammatory cytokine release in a cigarette smoke-induced rat model through HDAC1 depression. Inflamm Res 2015; 64:513–526. doi: 10.1007/s00011-015-0832-y. [DOI] [PubMed] [Google Scholar]
- 3.Bagul PK, Deepthi N, Sultana R, Banerjee SK. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J Nutr Biochem 2015; 26:1298–1307. doi: 10.1016/j. jnutbio.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Aramsangtienchai P, Spiegelman NA, He B, Miller SP, Dai L, Zhao Y, et al. HDAC8 catalyzes the hydrolysis of long chain fatty acyl lysine. ACS Chem Biol 2016; 11:2685–2692. doi: 10.1021/acschem-bio.6b00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha SD, Han CY, Reid C, Kim SO. HDAC8-mediated epigenetic reprogramming plays a key role in resistance to anthrax lethal toxin-induced pyroptosis in macrophages. J Immunol 2014; 193:1333–1343. doi: 10.4049/jimmunol.1400420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghizzoni M, Haisma HJ, Maarsingh H, Dekker FJ. Histone acetyltransferases are crucial regulators in NF-κB mediated inflammation. Drug Discov Today 2011; 16:504–511. doi: 10.1016/j.dru-dis.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]