PURPOSE
To compare taxane maintenance chemotherapy, paclitaxel (P) and paclitaxel poliglumex (PP), with surveillance (S) in women with ovarian, peritoneal, or fallopian tube (O/PC/FT) cancer who attained clinical complete response after first-line platinum-taxane therapy.
METHODS
Women diagnosed with O/PC/FT cancer who attained clinical complete response after first-line platinum-taxane–based chemotherapy were randomly allocated 1:1:1 to S or maintenance, P 135 mg/m2 once every 28 days for 12 cycles, or PP at the same dose and schedule. Overall survival (OS) was the primary efficacy end point.
RESULTS
Between March 2005 and January 2014, 1,157 individuals were enrolled. Grade 2 or worse GI adverse events were more frequent among those treated with taxane (PP: 20%, P: 27% v S: 11%). Grade 2 or worse neurologic adverse events occurred more often with taxane treatment (PP: 46%, P: 36% v S: 14%). At the fourth scheduled interim analysis, both taxane regimens passed the OS futility boundary and the Data Monitoring Committee approved an early release of results. With a median follow-up of 8.1 years, 653 deaths were reported; none were attributed to the study treatment. Median survival durations were 58.3, 56.8, and 60.0 months for S, P, and PP, respectively. Relative to S, the hazard of death for P was 1.091 (95% CI, 0.911 to 1.31; P = .343) and for PP, it was 1.033 (95% CI, 0.862 to 1.24; P = .725). The median times to first progression or death (PFS) were 13.4, 18.9, and 16.3 months for S, P, and PP, respectively. Hazard ratio = 0.801; 95% CI, 0.684 to 0.938; P = .006 for P and hazard ratio = 0.854; 95% CI, 0.729 to 1.00; P = .055 for PP.
CONCLUSION
Maintenance therapy with P and PP did not improve OS among patients with newly diagnosed O/tubal/peritoneal cancer, but may modestly increase PFS. GI and neurologic toxicities were more frequent in the taxane treatment arms.
INTRODUCTION
Ovarian cancer presents a substantial burden to our public health. In 2021, it is estimated that there will be more than 21,410 new cases of ovarian cancer and almost 13,770 deaths from ovarian cancer in the United States.1 Advanced-stage ovarian, peritoneal, and fallopian tube (FT) carcinomas present a significant therapeutic challenge. These cancers tend to be a chronic process, characterized by repetitive recurrences, often with multiple surgical interventions and several chemotherapeutic regimens. The majority of patients experience a complete clinical response to primary therapy, a combination of surgery and a platinum/taxane regimen.2,3 However, most patients with complete clinical responses recur within 2-3 years and survival can vary from a few months to many years.4
CONTEXT
Key Objective
Does maintenance therapy, with a single-agent taxane, in patients demonstrating a complete clinical response to primary therapy for advanced ovarian, peritoneal, or fallopian tube cancer improve overall survival or progression-free survival, and if so, what were the associated toxicities?
Knowledge Generated
Although maintenance taxane therapy did not improve overall survival in this population, the progression-free survival was modestly increased. However, the maintenance taxane therapy was associated with significantly greater GI and neurologic adverse events.
Relevance
Given the modest survival gains associated with maintenance taxane therapy, and the associated toxicities, in the context of other current maintenance therapies, such as poly (ADP-ribose) polymerase inhibitors, it is unlikely that maintenance therapy with single-agent taxanes will play a significant role in the management of patients with advanced ovarian, peritoneal, or fallopian tube cancer.
This observation has led to an interest in evaluating maintenance therapy to improve either progression-free survival (PFS) or overall survival (OS). One of the first studies to explore this strategy was SWOG-9701/GOG-178. The use of maintenance single-agent paclitaxel (P), a cycle-specific agent, was considered attractive because of its antiangiogenic activity and relative safety (no secondary malignancies or organ toxicity). In addition, retrospective data suggested clinical activity of single-agent P in the recurrent setting.5 This SWOG/GOG trial was closed at a planned interim analysis when it was noted that 12 cycles (v three cycles) of maintenance of monthly single-agent P extended the PFS (median PFS 28 months v 21 months, P = .0023; hazard ratio [HR], 0.43).6 Six years later, the OS for this trial was reported and showed no statistical difference between the two arms. These results were potentially influenced by crossover (allowed at interim analysis), in addition to the impact of subsequent therapies.7 The accumulative neurotoxicity on the 12-cycle arm, 23% grade 2 and 10% grade 3 and 4, was of concern.
The Gynecologic Oncology Group (GOG) believed that the P maintenance question was an important one to answer and thus led to the development of this trial, GOG-212. However, there was concern regarding the additional neurotoxicity and persistent alopecia. Accordingly, incorporated into the trial design was a novel taxane, CT-2103 (paclitaxel poliglumex [PP]; Opaxio, also previously known as Xyotax) reported in phase I trials to have less neurotoxicity and less alopecia.8 Also, since the drug was dissolved in an aqueous solution, the administration time was a 10-minute infusion and hypersensitivity reactions were less common than with P. In a phase II trial with heavily pretreated patients with ovarian, tubal, and peritoneal carcinoma, grade 2 and 3 neurotoxicity totaled 30%, higher than that noted in phase I trials.9,10 Other potential advantages of the conjugated taxane were a prolonged distribution phase, more rapid administration, and uptake by pinocytosis bypassing multidrug resistance.
METHODS
Patients
This study was conducted in patients with newly diagnosed advanced-stage (stage III or IV), International Federation of Gynecology and Obstetrics epithelial ovarian, FT, or primary peritoneal cancer. All epithelial ovarian cell types were eligible, except for tumors of low malignant potential and any mixed tumors with a sarcomatous component. Patients could have primary surgery or received neoadjuvant chemotherapy, provided that they underwent interval surgical debulking after at least one cycle, but not more than six cycles of platinum/taxane chemotherapy. All patients must have received at least two cycles of chemotherapy after the interval surgery. After their primary surgical procedure, patients could have had either optimal (≤ 1 cm residual) or suboptimal residual disease.
Patients must have completed at least five cycles and not more than eight cycles of platinum (intravenous or intraperitoneal) and P- or docetaxel-based chemotherapy. They must have completed the chemotherapy within 12 weeks of starting the clinical trial. On the basis of a normal examination, the absence of symptoms, normal computed tomography imaging, and a normal cancer antigen-125 (CA-125), they must have been considered to be in a complete clinical response.
Patients must have had a GOG performance status of 0, 1, or 2 and adequate bone marrow, renal, hepatic, and neurologic function. Patients with prior treatment with bevacizumab were excluded. All patients provided written informed consent. Eligibility and ineligibility criteria are presented in the Data Supplement (online only).
The complete Protocol (online only) is available online. The National Cancer Institute's Cancer Therapy and Evaluation Program approved the study, as did the Institutional Review Boards for the participating centers. The GOG Pathology Committee confirmed the correct histologic diagnosis with review by three committee pathologists. The authors wrote the manuscript and are responsible for the accuracy and quality of the reported data and the integrity of the protocol.
Study Design
GOG-212 was a three-arm, randomized, open-label phase III trial comparing two taxane maintenance regimens with surveillance (S) in women diagnosed with primary advanced-stage (stage III or IV) ovarian or FT carcinoma, or primary peritoneal carcinoma. The randomly allocated study regimens consisted of S, P 135 mg/m2 once every 28 days × 12, or PP 135 mg/m2 once every 28 days × 12. The random assignment ratio was 1:1:1. Dose reductions to 100 mg/m2 (P, once every 28 days) and 80 mg/m2 (PP, once every 28 days) were indicated on the basis of hematologic and no hematologic toxicities (see the Treatment Modifications in the Data Supplement). After demonstrating disease progression, further treatment recommendations were at the discretion of the investigators.
The enrollment of patients was web-based. Enrollment for each patient occurred after completion of first-line treatment, and consent for maintenance therapy had been stochastically determined. A minimization procedure11 was used to dynamically allocate one of the three study treatments to each enrollee after electronically checking eligibility. The random assignment procedure tended to allocate study treatments equally within each of the following stratification factor: stage of disease at diagnosis (stage III v stage IV), presence of macroscopic disease after debulking surgery (yes or no), prior taxane treatment (docetaxel v P), and route of prior platinum treatment (intraperitoneal v intravenous). The patient's assigned study treatment was not disclosed until the patient had been successfully enrolled. This report includes an accounting of all enrolled patients.
End Points and Assessments
The primary end point of the study was OS, and PFS was a secondary efficacy end point. For each patient, the duration of OS and PFS was measured from the date of enrollment. Death because of any cause was considered an uncensored event. For those patients alive at last contact, survival duration was censored on the date of last contact. The duration of PFS was measured on the date of radiographic, pathologic, and clinical evidence of new disease; rising CA-125; or death, whichever occurred first. For progression on the basis of rising CA-125, a patient needed two CA-125 measurements on two separate dates that were at least twice her nadir value or the laboratory normal value. In those cases, when an elevated CA-125 prompted a radiographic assessment and showed new disease, the radiographic assessment was used to determine the PFS duration.
Safety end points included frequency and severity of adverse events (AEs), which were assessed according to Common Toxicity Criteria version 3.0. Patient-reported outcomes (PROs) were monitored with the FACT-O,12,13 and patient-reported neuropathy was assessed with the GOG-NTX4.14 PROs were to be assessed before courses 3, 5, and 7 and on completion of treatment.
Statistical Considerations
Initially, the targeted enrollment for this study was 1,550 patients. However, during the conduct of this study, bevacizumab became increasingly prescribed as maintenance therapy in this population of patients, which decreased the expected accrual rate. Hence, the targeted enrollment was reduced to 1,100 patients in 2008 before any interim analyses of the primary end point. The minimum clinically relevant treatment effect size and the overall type I and II errors remained the same. The redesign included interim analyses scheduled when approximately 35%, 50%, 65%, and 80% of the number of deaths required for the final analysis had occurred. The final analysis of OS was to occur when at least 301 deaths were reported among those randomly assigned to S. With the type I error set to 0.025 (one tail, accounting for interim analyses) for each pair-wise comparison of an experimental treatment with S, this sample size provides 90% power for an experimental treatment, which reduces the hazard of death by 25% (HR, 0.75). The planned interim analyses included guidelines for stopping the study for efficacy and futility. The asymmetric decision boundaries were based on O'Brien-Fleming–like spending functions for type I and II errors.15 The Kaplan-Meier procedure was used to estimate survivorship functions. Since there were no established strong prognostic factors in this patient population, an unstratified log rank was used to test the primary hypothesis concerning OS and the secondary hypothesis concerning PFS. These tests included all patients enrolled on the trial regardless of whether they were determined to be ineligible. A proportional hazards model was used to estimate treatment HRs and their corresponding CIs. Exploratory assessments of homogeneity of the treatment effect across patient subgroups were performed with a proportional hazard model that included main effect terms for treatment and subgroup effects and interaction terms for treatment-by-subgroup effects. The P values from these exploratory analyses were not adjusted for multiplicity. These analyses do not arise from prespecified hypothesis but are presented to provide supportive documentation for the robustness of the study's primary conclusion.
RESULTS
Patients
Between March 2005 and January 2014, 1,157 individuals were enrolled. The first three interim analyses were conducted as planned in November 2012, November 2014, and May 2016. In each instance, the independent Data Monitoring Committee voted to continue the study as planned. However, after reviewing the results of the fourth interim analysis, conducted in May 2016, the independent Data Monitoring Committee voted to stop the study for futility and approved an early release of the results. Therefore, for this report, the study data were frozen and locked on February 26, 2019.
There were seven (1.8%), 13 (3.4%), and 14 (3.6%) deemed ineligible in the PP, P, and S groups, respectively. Eight (2.0%) patients who were assigned to PP and 10 (2.6%) assigned to P did not initiate their study treatment, whereas 12 (3.1%) patients assigned to S initiated an anticancer therapy before demonstrating protocol-defined clinical disease progression. Overall, there have been 43 (4%) patients who withdrew consent for follow-up or reported lost to follow-up. A CONSORT diagram is presented in Figure 1.
FIG 1.
CONSORT diagram—all enrolled patients. AE, adverse event; OS, overall survival; P, paclitaxel; PFS, progression-free survival; PP, paclitaxel poliglumex; PRO, patient-reported outcome; S, surveillance.
Patient and disease characteristics are presented in Table 1. Both patient and disease characteristics among the three treatment groups were similar. The majority of patients had stage III, high-grade serous carcinoma of the ovary.
TABLE 1.
Patient and Disease Characteristics
AEs
Table 2 summarizes the maximum grade of selected AEs by randomly assigned treatment for the 1,127 patients who initiated their assigned study treatment. The 18 individuals who did not begin their assigned study treatment are not included in these tables. A complete tabulation of AEs is included in the Data Supplement.
TABLE 2.
Maximum Common Toxicity Criteria Version 3.0 Grade for Selected Adverse Events
AEs occurred more frequently among those treated with a taxane, which are grade 3 or worse neutropenia (PP: 21.6%, P: 16.6%; S: 0.5%; P < .01), grade 3 or worse sensory neuropathy (PP: 10.0%, P: 5.4%, S: 0.8%; P < .001), grade 3 or worse hypokalemia (PP: 2.4%, P: 0.5%, S: 0.0%; P = .001), grade 3 or worse joint, bone, muscle pain (PP: 8.2%, P: 4.8%, S: 2.9%, P = .004), grade 2 alopecia (PP: 19.3%, P: 41.2%, S: 10.1%; P < .001), and grade 2 or worse fatigue (PP: 17.1%, P: 25.1%, S: 5.7%; P < .001). None of the deaths that have been reported have been attributed to the study treatment. There was only one case reported of grade 3 or worse febrile neutropenia, and this occurred on P. There were 44 (44 of 379, 11.6%) patients on PP who stopped treatment because of neurotoxicity, and 31 (31 of 374, 8.3%) who stopped P because of neurotoxicity.
OS and PFS
The Data Monitoring Committee recommended public release of the results after the fourth scheduled interim analysis, which determined that the relative death hazards passed the futility boundaries for both taxane regimens. These results were presented at the Society of Gynecologic Oncology in 2017. The current report includes results of additional patient follow-up.
Six hundred fifty-three deaths from cancer, 21 deaths from other causes, and 33 from unknown causes had been reported, and 450 patients (38.9% of the total patients enrolled) were alive at last contact. The median duration of follow-up was 8.1 years. No deaths were attributed to the study treatment.
The median OS was 58.3, 56.8, and 60.0 months for S, P, and PP treatments, respectively (Fig 2). Compared with S, the relative hazard of death for P was 1.091 (95% CI, 0.991 to 1.307; P = .343) and for PP, it was 1.033 (95% CI, 0.862 to 1.239; P = .725). The differences in survivorship can be reasonably ascribed to random variation.
FIG 2.

Overall survival by treatment group (randomized treatment). P, paclitaxel; PP, paclitaxel poliglumex; S, surveillance.
PFS was a secondary end point. The median PFS was 13.4, 18.9, and 16.3 months for S, P, and PP treatments, respectively. The PFS plots for each treatment are provided in Figure 3. Relative to S, for the P maintenance, the hazard of the first progression or death was 19.9% lower (HR, 0.801; 97.5% CI, 0.684 to 0.938; P = .006). For PP, the hazard of the first progression or death was 14.6% lower (HR, 0.854; 97.5% CI, 0.729 to 1.00; P = .055).
FIG 3.
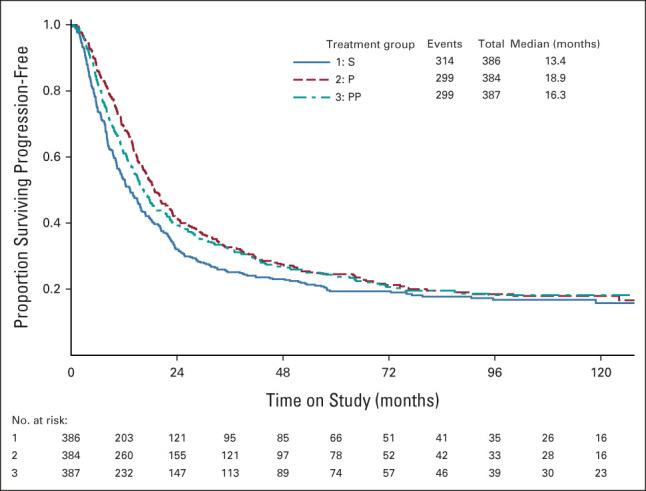
PFS by treatment group (randomized treatment). P, paclitaxel; PFS, progression-free survival; PP, paclitaxel poliglumex; S, surveillance.
PROs
The FACT/GOG-NTX was used to monitor the change in patient-reported neurologic symptoms before treatment and after 6 months on study. Decreasing scores indicate worsening neurologic symptoms, and differences of 1.2 points or larger are considered clinically meaningful (Calhoun et al14). Relative to the S group, those on P had on average a 1.1-unit decline (worsening) in NTX scores (P < .001) and those on PP a 2.4-unit decrease in scores (P < .001). Moreover, compared with P, the decline on PP was greater (P < .001).
Exploratory Data Analysis
It is recognized that patients with no gross residual disease after the primary cytoreduction have superior survival outcomes compared with patients with gross residual tumor. Since a lower tumor burden might have been better controlled with maintenance therapy, we looked at the outcomes on the basis of residual tumor (Data Supplement). In addition, serous and nonserous histology might have different sensitivity to chemotherapy and outcomes of these patients are also illustrated in Figure 3 in the Final Report. Neither of these characteristics appeared to identify a category of patient who would benefit from the maintenance strategy. We also assessed the survival of patients on the basis of their baseline CA-125. Patients with a CA-125 ≤ 10 IU demonstrated a better survival, but this cut point does not appear to be predictive for a taxane effect on OS.
DISCUSSION
This clinical trial was challenging to conduct, requiring almost 9 years to complete accrual, an extended interval for any cancer trial. Not surprisingly, an observation versus treatment trial in patients with cancer might have accrual challenges. Accrual was initially slow, as patients struggled with the concept of enrolling for an additional 12 months of therapy versus observation. This was especially true when the trial was initially presented at the end of their primary therapy, a time most patients focus upon the hallmark of completing treatment and not embarking on an additional year of treatment, especially in a randomized fashion. Over time, our investigators were coached to introduce the trial as early as possible when a complete clinical response seemed likely. This strategy resulted in enhanced accrual.
Accrual to maintenance trials has been challenging for others also. Pecorelli et al16 investigated the potential benefit of an additional six cycles of P and recruited only 200 patients over 7 years. Because of the low accrual, they performed an unplanned futility analysis, concluding that an additional six cycles of P did not improve either PFS or OS in a similar population of patients that we studied. The Italian results conflicted with the SWOG-9701/GOG-178 study reported in 2003. But the study populations differed. In the Italian study, half of the patients underwent a pathologic confirmation of the complete response. The PFS in the observation arm was 30 months, compared with 14 months in the SWOG-9701/GOG-178, and none of these patients underwent a second-look procedure. Both studies were challenged with patient noncompliance. In the Italian study, maintenance therapy was given to 15% of patients on the observation arm, 6% of patients on the treatment arm received no P, and 3% withdrew from the study prematurely. In comparison, in our study, maintenance therapy was given to 3.1% of patients on the observation arm and 2.3% of patients on the treatment arms received no P.
The decision to use OS rather than PFS as the primary outcome was controversial, and in retrospect, considering the evolution of drug approval/registration to include PFS, a primary end point of PFS could have been considered. In this study, maintenance therapy failed to demonstrate an OS benefit; however, PFS was superior for patients who received the P maintenance. In addition, the treatment with both the taxanes resulted in significant adverse side effects, with the progression of sensory neuropathy being the most significant concern.
Other recent clinical trials evaluating the potential benefit of maintenance therapy, with bevacizumab, pazopanib, or a poly (ADP-ribose) polymerase inhibitor, have reported improved PFS, but no benefit in OS for the general population. Also, numerous trials over the past 3 years have reported evaluating the use of poly (ADP-ribose) polymerase inhibitors in the primary maintenance setting. Each of these trials demonstrated improved PFS. However, the only study to date reporting OS is the PRIMA trial although it was an interim, not final analysis, with HR 0.70 with 95% CI, 0.44 to 1.11 (Table 3). To date, it is important to note that most maintenance trials have demonstrated improved PFS, but not OS.
TABLE 3.
Trials Evaluating Maintenance Therapies for the Treatment of Women Diagnosed With Advanced Ovarian Cancer
Information regarding the germline or somatic status of the patients in this trial was not available or analyzed.
In conclusion, maintenance treatment with P or PP was associated with GI and neurologic AEs, but neither study regimen prolonged OS.
ACKNOWLEDGMENT
The following NRG Oncology/Gynecologic Oncology Group member institutions participated in this study: Ohio State University Comprehensive Cancer Center, University of Oklahoma Health Sciences Center, University of California Medical Center at Irvine-Orange Campus, University of Iowa Hospitals and Clinics, Wayne State University/Karmanos Cancer Institute, Walter Reed National Military Medical Center, University of Texas Southwestern Medical Center, Rush University Medical Center, Women's Cancer Center of Nevada, Roswell Park Comprehensive Cancer Center, University of Colorado Cancer Center-Anschutz Cancer Pavilion, University of Kentucky, Mayo Clinic, Duke University Medical Center, Moffitt Cancer Center and Research Institute, Gynecologic Oncology of West Michigan PLLC, Abington Memorial Hospital, University of Minnesota Medical Center-Fairview, University of Pittsburgh Cancer Institute, Washington University School of Medicine, University of North Carolina at Chapel Hill, MD Anderson Cancer Center, Case Western Reserve University, University of Wisconsin Hospital and Clinics, Women and Infants Hospital, University of New Mexico, Cancer Research for the Ozarks NCORP, Gynecologic Oncology Network/Brody School of Medicine, Northern Indiana Cancer Research Consortium, University of Alabama at Birmingham, Cleveland Clinic Foundation, Abramson Cancer Center of the University of Pennsylvania, Penn State Milton S Hershey Medical Center, Michigan Cancer Research Consortium Community Clinical Oncology Program, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Tacoma General Hospital, Colorado Cancer Research Program NCORP, Fred Hutchinson Cancer Research Center, State University of New York Downstate Medical Center, Fox Chase Cancer Center, The Hospital of Central Connecticut, Cancer Research Consortium of West Michigan NCORP, University of Mississippi Medical Center, University of California at Los Angeles Health System, University of Virginia, University of Texas—Galveston, Froedtert and the Medical College of Wisconsin, Mount Sinai School of Medicine, University of Massachusetts Memorial Health Care, Saint Vincent Hospital, Cooper Hospital University Medical Center, University of Cincinnati, Wake Forest University Health Sciences, Tufts-New England Medical Center, Stony Brook University Medical Center, Delaware/Christina Care CCOP, Northwestern University, University of Chicago, Yale University, Central Illinois CCOP, Yale University, Central Illinois CCOP, Upstate Carolina CCOP, William Beaumont Hospital, Wichita CCOP, Aurora Women's Pavilion of Aurora West Allis Medical Center, Memorial Sloan Kettering Cancer Center, Thomas Jefferson University Hospital, Ellis Fischel Cancer Center, Georgia Center for Oncology Research and Education (CORE), Baystate Medical Center, Kansas City CCOP, Geisinger Medical Center, Saint Louis-Cape Girardeau CCOP, UCSF-Mount Zion, University of Illinois, Carle Cancer Center, Metro-Minnesota CCOP, Virginia Commonwealth University, and Virginia Mason CCOP.
Larry J. Copeland
Consulting or Advisory Role: Tarveda Therapeutics, Myriad Genetics, GlaxoSmithKline, Elevar Therapeutics, Toray Industries, Rubius Therapeutics, Sorrento Therapeutics, Celsion, Corcept Therapeutics
Mark F. Brady
Consulting or Advisory Role: Cel-Sci
Robert A. Burger
Employment: Genentech/Roche, Mersana
Stock and Other Ownership Interests: Genentech/Roche, Mersana
Consulting or Advisory Role: Myriad Genetics
David Cella
Stock and Other Ownership Interests: FACIT.org
Consulting or Advisory Role: AbbVie, GlaxoSmithKline, Pfizer, Astellas Pharma, Novartis, Bristol Myers Squibb, Ipsen, Celcuity, Immunogen, Fulcrum Therapeutics
Research Funding: Novartis (Inst), Ipsen (Inst), Pfizer (Inst), PledPharma (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Regeneron (Inst), Clovis Oncology (Inst)
David M. O'Malley
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, Tesaro, Novocure, Genentech/Roche, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics, Novartis, Seattle Genetics, BBI Healthcare, Arquer Diagnostics, Toray Industries, Takeda, InxMed, Celsion, Arcus Biosciences, Sutro Biopharma, Atossa Therapeutics, Laekna Therapeutics, Onconova Therapeutics, VBL Therapeutics, Vincerx Pharma, Adaptimmune, Roche
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seattle Genetics (Inst), Iovance Biotherapeutics (Inst), Leap Therapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology, Inc (Inst), Acerta Pharma (Inst), Advaxis (Inst), Arcus Biosciences (Inst), Deciphera (Inst), Exelixis (Inst), Roche (Inst), Incyte (Inst), Karyopharm Therapeutics (Inst), Ludwig Institute for Cancer Research (Inst), Novartis (Inst), Novocure (Inst), OncoQuest (Inst), BeiGene (Inst), Pfizer (Inst), Precision Therapeutics (Inst), Sanofi (Inst), Sutro Biopharma (Inst), GlaxoSmithKline (Inst), Verastem (Inst)
Daron G. Street
Consulting or Advisory Role: AmerisourceBergen
Speakers' Bureau: Coherus Biosciences
Travel, Accommodations, Expenses: AmerisourceBergen
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology, Merck, Eisai, AstraZeneca, Genmab
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology, Eisai, Genmab
Research Funding: AbbVie (Inst), Genentech/Roche (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Robert T. Morris
Honoraria: GlaxoSmithKline, Clovis Oncology, AstraZeneca, Merck
Consulting or Advisory Role: GlaxoSmithKline, AstraZeneca, Clovis Oncology
Speakers' Bureau: AstraZeneca, Clovis Oncology, GlaxoSmithKline, Merck
William J. Lowery
Employment: Anthem
Consulting or Advisory Role: Seattle Genetics
Speakers' Bureau: AstraZeneca
David S. Miller
Consulting or Advisory Role: Eisai, AstraZeneca, Karyopharm Therapeutics, Incyte, Merck Sharp & Dohme, Asymmetric Therapeutics, Boston Biomedical Research Institute, Tarveda Therapeutics, Myriad Genetic Laboratories, GlaxoSmithKline, AbbVie, Incyte, EMD Serono, Seattle Genetics, Clinical Education Alliance, Eisai, ITeos Therapeutics, Novocure, Novartis, Immunogen, Agenus
Speakers' Bureau: Clovis Oncology, Genentech
Research Funding: US Biotest (Inst), Advenchen Laboratories (Inst), Tesaro (Inst), Xenetic Biosciences (Inst), Advaxis (Inst), Janssen (Inst), Aeterna Zentaris (Inst), TRACON Pharma (Inst), Pfizer (Inst), Immunogen (Inst), Mateon Therapeutics (Inst), Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Millennium Pharmaceuticals (Inst), Aprea AB (Inst), Regeneron (Inst), NVision (Inst), Leap Therapeutics (Inst), Novartis (Inst), Syros Pharmaceuticals (Inst), Karyopharm Therapeutics (Inst), Agenus (Inst), Akesobio (Inst), EMD Serono, Incyte (Inst)
Saketh Guntupalli
Consulting or Advisory Role: Clovis Oncology, Tesaro
Research Funding: Bristol Myers Squibb
Robert S. Mannel
Stock and Other Ownership Interests: Edwards Lifesciences, Stryker, Danaher
No other potential conflicts of interest were reported.
DISCLAIMER
No honoraria were paid to any of the authors. The analysis, interpretation, and content of this manuscript are the responsibility of the authors.
PRIOR PRESENTATION
Presented in part at the Scientific Plenary Session of the 48th Annual SGO meeting, National Harbor, MD, March 12, 2017.
SUPPORT
Supported by the National Cancer Institute: U10CA180822 (NRG Oncology SDMC) and U10CA180868 (NRG Oncology Operations and UG1CA189867 [NCORP] as well as Cell Therapeutics Inc; see the Acknowledgment section).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Larry J. Copeland, Mark F. Brady, Robert A. Burger, William H. Rodgers, David Cella, Krishnansu S. Tewari, Nick M. Spirtos, Robert S. Mannel, Philip J. DiSaia
Administrative support: David S. Miller
Provision of study materials or patients: Helen Q. Huang, Robert T. Morris, William J. Lowery, David S. Miller, Nick M. Spirtos, Robert S. Mannel, Philip J. DiSaia
Collection and assembly of data: Larry J. Copeland, Mark F. Brady, Daron G. Street, Krishnansu S. Tewari, William J. Lowery, David S. Miller, Summer B. Dewdney, Nick M. Spirtos, Frederick R. Ueland, Gretchen E. Glaser, Robert S. Mannel
Data analysis and interpretation: Larry J. Copeland, Mark F. Brady, Robert A. Burger, Helen Q. Huang, David Cella, Krishnansu S. Tewari, David P. Bender, Robert T. Morris, David S. Miller, Saketh Guntupalli, Gretchen E. Glaser, Philip J. DiSaia
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III Randomized Trial of Maintenance Taxanes Versus Surveillance in Women With Advanced Ovarian/Tubal/Peritoneal Cancer: A Gynecologic Oncology Group 0212:NRG Oncology Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Larry J. Copeland
Consulting or Advisory Role: Tarveda Therapeutics, Myriad Genetics, GlaxoSmithKline, Elevar Therapeutics, Toray Industries, Rubius Therapeutics, Sorrento Therapeutics, Celsion, Corcept Therapeutics
Mark F. Brady
Consulting or Advisory Role: Cel-Sci
Robert A. Burger
Employment: Genentech/Roche, Mersana
Stock and Other Ownership Interests: Genentech/Roche, Mersana
Consulting or Advisory Role: Myriad Genetics
David Cella
Stock and Other Ownership Interests: FACIT.org
Consulting or Advisory Role: AbbVie, GlaxoSmithKline, Pfizer, Astellas Pharma, Novartis, Bristol Myers Squibb, Ipsen, Celcuity, Immunogen, Fulcrum Therapeutics
Research Funding: Novartis (Inst), Ipsen (Inst), Pfizer (Inst), PledPharma (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Regeneron (Inst), Clovis Oncology (Inst)
David M. O'Malley
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, Tesaro, Novocure, Genentech/Roche, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics, Novartis, Seattle Genetics, BBI Healthcare, Arquer Diagnostics, Toray Industries, Takeda, InxMed, Celsion, Arcus Biosciences, Sutro Biopharma, Atossa Therapeutics, Laekna Therapeutics, Onconova Therapeutics, VBL Therapeutics, Vincerx Pharma, Adaptimmune, Roche
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seattle Genetics (Inst), Iovance Biotherapeutics (Inst), Leap Therapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology, Inc (Inst), Acerta Pharma (Inst), Advaxis (Inst), Arcus Biosciences (Inst), Deciphera (Inst), Exelixis (Inst), Roche (Inst), Incyte (Inst), Karyopharm Therapeutics (Inst), Ludwig Institute for Cancer Research (Inst), Novartis (Inst), Novocure (Inst), OncoQuest (Inst), BeiGene (Inst), Pfizer (Inst), Precision Therapeutics (Inst), Sanofi (Inst), Sutro Biopharma (Inst), GlaxoSmithKline (Inst), Verastem (Inst)
Daron G. Street
Consulting or Advisory Role: AmerisourceBergen
Speakers' Bureau: Coherus Biosciences
Travel, Accommodations, Expenses: AmerisourceBergen
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology, Merck, Eisai, AstraZeneca, Genmab
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology, Eisai, Genmab
Research Funding: AbbVie (Inst), Genentech/Roche (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Robert T. Morris
Honoraria: GlaxoSmithKline, Clovis Oncology, AstraZeneca, Merck
Consulting or Advisory Role: GlaxoSmithKline, AstraZeneca, Clovis Oncology
Speakers' Bureau: AstraZeneca, Clovis Oncology, GlaxoSmithKline, Merck
William J. Lowery
Employment: Anthem
Consulting or Advisory Role: Seattle Genetics
Speakers' Bureau: AstraZeneca
David S. Miller
Consulting or Advisory Role: Eisai, AstraZeneca, Karyopharm Therapeutics, Incyte, Merck Sharp & Dohme, Asymmetric Therapeutics, Boston Biomedical Research Institute, Tarveda Therapeutics, Myriad Genetic Laboratories, GlaxoSmithKline, AbbVie, Incyte, EMD Serono, Seattle Genetics, Clinical Education Alliance, Eisai, ITeos Therapeutics, Novocure, Novartis, Immunogen, Agenus
Speakers' Bureau: Clovis Oncology, Genentech
Research Funding: US Biotest (Inst), Advenchen Laboratories (Inst), Tesaro (Inst), Xenetic Biosciences (Inst), Advaxis (Inst), Janssen (Inst), Aeterna Zentaris (Inst), TRACON Pharma (Inst), Pfizer (Inst), Immunogen (Inst), Mateon Therapeutics (Inst), Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Millennium Pharmaceuticals (Inst), Aprea AB (Inst), Regeneron (Inst), NVision (Inst), Leap Therapeutics (Inst), Novartis (Inst), Syros Pharmaceuticals (Inst), Karyopharm Therapeutics (Inst), Agenus (Inst), Akesobio (Inst), EMD Serono, Incyte (Inst)
Saketh Guntupalli
Consulting or Advisory Role: Clovis Oncology, Tesaro
Research Funding: Bristol Myers Squibb
Robert S. Mannel
Stock and Other Ownership Interests: Edwards Lifesciences, Stryker, Danaher
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021 [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP, Hoskins WJ, Brady MF, et al. : Cyclophosphamide and cisplatin versus paclitaxel and cisplatin: A phase III randomized trial in patients with suboptimal stage III/IV ovarian cancer (from the Gynecologic Oncology Group). Semin Oncol 23:40-47, 1996 [PubMed] [Google Scholar]
- 3.Armstrong DK, Bundy B, Wenzel L, et al. : Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34-43, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Rose PG, Java JJ, Salani R, et al. : Nomogram for predicting individual survival after recurrence of advanced-stage, high-grade ovarian carcinoma. Obstet Gynecol 133:245-254, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markman M, Hakes T, Barakat R, et al. : Follow-up of Memorial Sloan-Kettering Cancer Center patients treated on National Cancer Institute Treatment Referral Center protocol 9103: Paclitaxel in refractory ovarian cancer. J Clin Oncol 14:796-799, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Markman M, Liu PY, Wilczynski S, et al. : Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol 21:2460-2465, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Markman M, Liu PY, Moon J, et al. : Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: Follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gynecol Oncol 114:195-198, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemunaitis J, Cunningham C, Senzer N, et al. : Phase I study of CT-2103, a polymer-conjugated paclitaxel, and carboplatin in patients with advanced solid tumors. Cancer Invest 23:671-676, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Boddy AV, Plummer ER, Todd R, et al. : A phase I and pharmacokinetic study of paclitaxel poliglumex (XYTOAX), investigating both 3-weekly and 2-weekly schedules. Clin Cancer Res 11:7834-7840, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Sabbatini P, Aghajanian C, Dizon D, et al. : Phase II study of CT-2103 in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. J Clin Oncol 22:4523-4531, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Pocock SJ, Simon R: Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 31:103-115, 1975 [PubMed] [Google Scholar]
- 12.Cella DF, Tulsky DS, Gray G, et al. : The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol 11:570-579, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. : Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol 19:1809-1817, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Calhoun EA, Welshman EE, Chang C-H, et al. : Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 13:741-748, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Pampallona S, Tsiatis AA, Tsiatis AA: Group sequential designs for one-sided and two-sided hypothesis testing with provision for early stopping in favor of the null hypothesis. J Stat Plan Inference 42:19-35, 1994 [Google Scholar]
- 16.Pecorelli S, Favalli G, Gadducci A, et al. : Phase III trial of observation versus six courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after six courses of paclitaxel/platinum-based chemotherapy: Final results of the after-6 protocol 1. J Clin Oncol 27:4642-4648, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Burger RA, Brady MF, Bookman MA, et al. : Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Perren TJ, Swart AM, Pfisterer J, et al. : A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484-2496, 2011 [DOI] [PubMed] [Google Scholar]
- 19.du Bois A, Floquet A, Kim J-W, et al. : Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 32:3374-3382, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Vergote I, du Bois A, Floquet A, et al. : Overall survival results of AGO-OVAR16: A phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol Oncol 155:186-191, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Kim J-W, Mahner S, Wu L-Y, et al. : Pazopanib maintenance therapy in East Asian women with advanced epithelial ovarian cancer: Results from AGO-OVAR16 and an East Asian study. Int J Gynecol Cancer 28:2-10, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Ray-Coquard IR, Pautier P, Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 [DOI] [PubMed] [Google Scholar]
- 24.González-Martín A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Coleman RL, Fleming GF, Brady MF, et al. : Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 381:2403-2415, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]






