PURPOSE
ATHENA (ClinicalTrials.gov identifier: NCT03522246) was designed to evaluate rucaparib first-line maintenance treatment in a broad patient population, including those without BRCA1 or BRCA2 (BRCA) mutations or other evidence of homologous recombination deficiency (HRD), or high-risk clinical characteristics such as residual disease. We report the results from the ATHENA–MONO comparison of rucaparib versus placebo.
METHODS
Patients with stage III-IV high-grade ovarian cancer undergoing surgical cytoreduction (R0/complete resection permitted) and responding to first-line platinum-doublet chemotherapy were randomly assigned 4:1 to oral rucaparib 600 mg twice a day or placebo. Stratification factors were HRD test status, residual disease after chemotherapy, and timing of surgery. The primary end point of investigator-assessed progression-free survival was assessed in a step-down procedure, first in the HRD population (BRCA-mutant or BRCA wild-type/loss of heterozygosity high tumor), and then in the intent-to-treat population.
RESULTS
As of March 23, 2022 (data cutoff), 427 and 111 patients were randomly assigned to rucaparib or placebo, respectively (HRD population: 185 v 49). Median progression-free survival (95% CI) was 28.7 months (23.0 to not reached) with rucaparib versus 11.3 months (9.1 to 22.1) with placebo in the HRD population (log-rank P = .0004; hazard ratio [HR], 0.47; 95% CI, 0.31 to 0.72); 20.2 months (15.2 to 24.7) versus 9.2 months (8.3 to 12.2) in the intent-to-treat population (log-rank P < .0001; HR, 0.52; 95% CI, 0.40 to 0.68); and 12.1 months (11.1 to 17.7) versus 9.1 months (4.0 to 12.2) in the HRD-negative population (HR, 0.65; 95% CI, 0.45 to 0.95). The most common grade ≥ 3 treatment-emergent adverse events were anemia (rucaparib, 28.7% v placebo, 0%) and neutropenia (14.6% v 0.9%).
CONCLUSION
Rucaparib monotherapy is effective as first-line maintenance, conferring significant benefit versus placebo in patients with advanced ovarian cancer with and without HRD.
INTRODUCTION
Maintenance treatment may delay disease recurrence or progression for patients with ovarian cancer who have achieved a complete response (CR) or partial response (PR) to first-line chemotherapy.1-4 Efficacy with the poly(ADP-ribose) polymerase (PARP) inhibitors olaparib and niraparib as maintenance treatment varies on the basis of molecular characteristics,2-7 with the greatest progression-free survival (PFS) benefit observed in patients with ovarian cancer harboring BRCA mutations (eg, BRCA1 or BRCA2), followed by patients with other homologous recombination deficiency (HRD). Similar findings were observed in the ARIEL3 (ClinicalTrials.gov identifier: NCT01968213) study of maintenance treatment with the PARP inhibitor rucaparib in recurrent ovarian cancer; yet, the overall primary analysis demonstrated significantly improved PFS with rucaparib versus placebo regardless of HRD test status (BRCA mutations and genomic loss heterozygosity [LOH], a molecular feature of HRD).8 Given this broad efficacy of rucaparib in the recurrent setting, we hypothesized that rucaparib may be effective as first-line maintenance therapy across a diverse patient population with newly diagnosed ovarian cancer.
CONTEXT
Key Objective
Poly(ADP-ribose) polymerase inhibitors have shown efficacy as first-line maintenance treatment for patients with ovarian cancer. However, questions remain about which patients may benefit from their use. Given the broad efficacy of rucaparib in the recurrent setting, we evaluated the efficacy of rucaparib as maintenance in a diverse patient population with newly diagnosed ovarian cancer.
Knowledge Generated
In the first-line setting, rucaparib monotherapy maintenance treatment significantly improved progression-free survival compared with placebo in the intent-to-treat population and population of patients harboring tumors with evidence of homologous recombination deficiency, as well as the non-nested subgroup of patients with tumors without evidence of homologous recombination deficiency (HRD-negative).
Relevance
ATHENA–MONO demonstrates that rucaparib monotherapy is an effective first-line maintenance option that provides clinical benefit to a broad population of patients with newly diagnosed ovarian cancer.
ATHENA is an international, multicenter, randomized, double-blind, phase III trial consisting of four treatment arms (rucaparib, nivolumab, rucaparib + nivolumab, and placebo).9 The study has two separate and fully independently powered comparisons evaluating rucaparib monotherapy (ATHENA–MONO) and rucaparib + nivolumab (ATHENA–COMBO) as maintenance treatment for patients with newly diagnosed advanced ovarian cancer. Here, we report the efficacy and safety results from the ATHENA–MONO comparison of rucaparib maintenance treatment versus placebo. The results for ATHENA–COMBO are not yet mature and will be reported separately.
METHODS
Study Design
ATHENA (GOG-3020/ENGOT-ov45; ClinicalTrials.gov identifier: NCT03522246) is led by the GOG Foundation and conducted in partnership with the European Network of Gynecological Oncological Trial Groups (under ENGOT model C10) and NRG Oncology–Japan. Patients were enrolled at 200 centers in 24 countries in Asia, Australia/New Zealand, Europe, and North America. The study was approved by national or local institutional review boards and conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice Guidelines. Patients provided informed consent before participation.
Patients
Eligible patients were ≥ 18 years and had newly diagnosed, histologically confirmed, advanced (International Federation of Gynecology and Obstetrics stage III-IV), high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer. Patients had completed cytoreductive surgery (R0/complete resection was permitted) before chemotherapy or following neoadjuvant chemotherapy; had completed four to eight cycles of first-line platinum-doublet treatment, including a minimum of four cycles of a platinum/taxane combination (bevacizumab was only allowed during the chemotherapy phase), and achieved an investigator-assessed response; had sufficient formalin-fixed paraffin-embedded tumor tissue available for planned analyses and a known BRCA mutation result (either positive or negative) via central testing; had an Eastern Cooperative Oncology Group performance status of 0-1; and had adequate organ function. Full eligibility criteria are provided in Appendix Table A1 (online only).
Random Assignment
Within 8 weeks of day 1 of their last cycle of chemotherapy, patients were randomly assigned 4:1 to oral rucaparib + intravenous (IV) placebo or oral placebo + IV placebo.
Random assignment was computer-generated (block size of 10). Patients were stratified by HRD classification (BRCA mutation, BRCA wild-type/LOH high [LOH ≥ 16%], BRCA wild-type/LOH low [LOH < 16%], and BRCA wild-type/LOH indeterminate), disease status after chemotherapy (no residual disease v residual disease), and timing of surgery (primary surgery v interval debulking). The study was conducted in a double-blinded manner: patients, investigators, site staff, and the study sponsor were blinded to assignments, and study treatments were manufactured to be identical in appearance.
Procedures
Tumor HRD test status (BRCA mutations and genomic LOH) was determined centrally using the FoundationOne CDx next-generation sequencing assay (Foundation Medicine Inc, Cambridge, MA; full details in the Data Supplement [online only]).
Patients received rucaparib 600 mg or placebo orally twice a day starting on cycle 1 day 1 and placebo IV every 4 weeks starting on cycle 2 day 1 in 28-day cycles. Rucaparib treatment could continue until 24 months after initiation of placebo IV administration, disease progression, death, or unacceptable toxicity. Additional details of dose modification criteria are available in the Data Supplement.
Disease assessments per RECIST v1.1 were conducted at screening, every 12 weeks relative to cycle 2 day 1 for the first 3 years, and every 24 weeks thereafter until radiologic progressive disease. Safety was assessed from first administration of study drug until 28 days after the last dose of oral drug. After 28 days, only adverse events of special interest (myelodysplastic syndrome [MDS] and acute myeloid leukemia [AML]) and serious adverse events considered as potentially study drug–related were to be reported. Patients who discontinued treatment were followed for subsequent treatments, secondary malignancy, and survival every 12 weeks after the 28-day safety follow-up visit until death, loss to follow-up, consent withdrawal, or study closure.
Outcomes
The primary end point for ATHENA–MONO was investigator-assessed PFS per RECIST. Secondary end points included overall survival (OS); investigator-assessed objective response rate (ORR) in patients with measurable disease at baseline; duration of response (DOR) for patients with investigator-assessed confirmed radiographic CR or PR; and blinded independent central review (BICR)–assessed PFS per RECIST. Key exploratory end points included analysis of PFS in subgroups on the basis of patient characteristics and assessment of patient-reported outcomes using the Functional Assessment of Cancer Therapy—Ovarian questionnaire (see study Protocol, online only).
To assess safety, treatment-emergent adverse events (TEAEs) were classified using the Medical Dictionary for Drug Regulatory Activities v24.0 and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v5.0. We also assessed safety via physical examinations, laboratory assessments, electrocardiogram, and vital signs.
Statistical Analysis
The significance level for ATHENA–MONO was set at a two-sided P = .025 because of the overall family-wise type I error rate being split equally between ATHENA–MONO and ATHENA–COMBO. Assuming approximately 40% of patients enrolled had BRCA-mutant or BRCA wild-type/LOH high carcinoma (HRD population), a sample size of at least 500 patients was required for ATHENA–MONO to yield ≥ 90% power at this significance level to show a statistically significant difference in PFS with a hazard ratio (HR) of 0.45 in the HRD population and 0.60 in the intent-to-treat (ITT) population (all randomly assigned patients).
An ordered step-down multiple comparison procedure was used,11 testing the primary efficacy end point of investigator-assessed PFS first in the HRD population and then, if statistically significant at the two-sided .025 significance level, testing in the ITT population. Analysis of the key secondary end points of final OS and ORR were to follow in a similar ordered step-down procedure. Once significance was not achieved for one test, significance was not declared for all subsequent analyses. BICR-assessed PFS and DOR were evaluated as standalone, secondary end points.
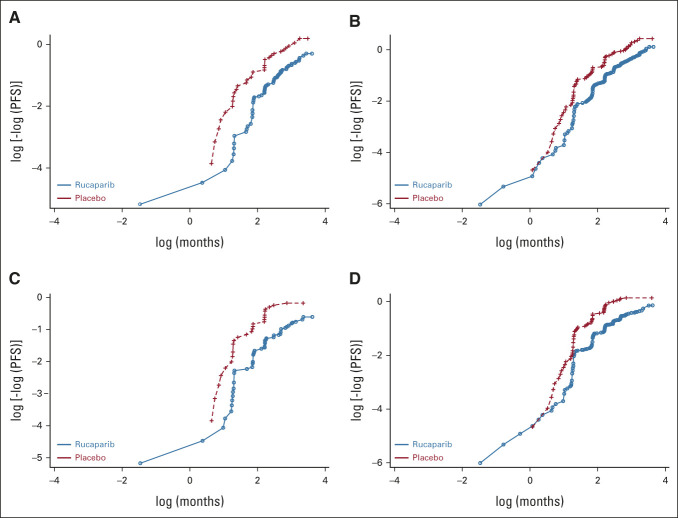
Investigator- and BICR-assessed PFS were analyzed using a stratified log-rank test between randomized treatment groups; we also used a stratified Cox proportional hazards model to estimate the HR with 95% CI between the groups. The proportional hazards assumption (ie, constant relative hazard) was verified graphically using log-log plots (Appendix Fig A1, online only). Investigator-assessed confirmed ORR was evaluated in the subgroup of patients with RECIST measurable disease at baseline and compared between treatment groups using a chi-square test.
Investigator-assessed DOR was analyzed in the subgroup of patients with a confirmed CR or PR. DOR was analyzed using a Cox proportional hazards model and a log-rank test between randomized treatment groups. OS will be considered mature when 70% of death events have been collected. Safety data were summarized descriptively for all patients who received at least one dose of oral study treatment. TEAEs leading to treatment interruption, dose reduction, or discontinuation of oral study drug are reported.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The independent data monitoring committee monitored enrollment and reviewed the safety and efficacy of the trial approximately every 6 months, including maturity of PFS events. Additional details of the statistical analyses can be found in the Data Supplement.
RESULTS
Patients
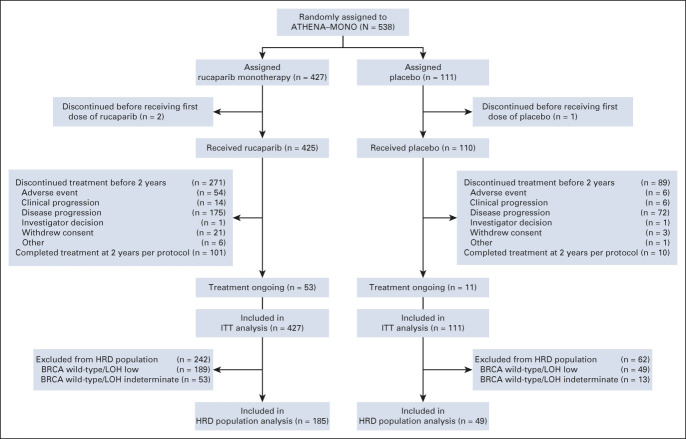
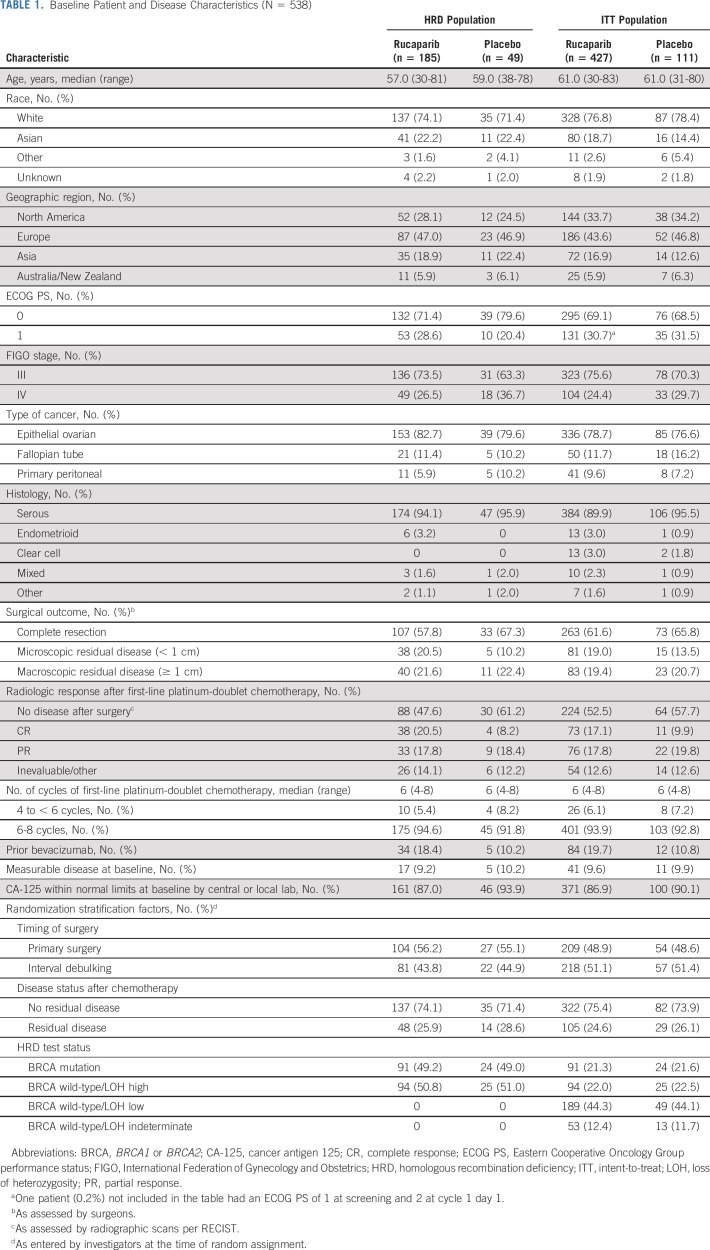
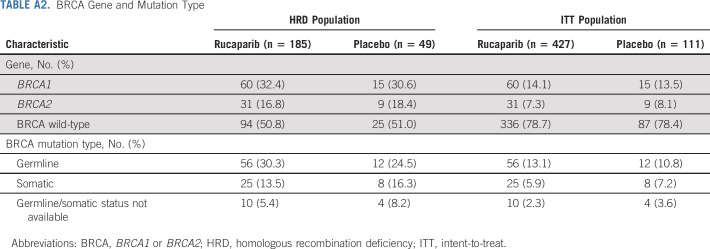
Between October 1, 2018, and September 30, 2020, 427 patients were randomly allocated to the rucaparib monotherapy group and 111 to the placebo group (Fig 1). Baseline patient, disease, and genomic characteristics are provided in Table 1 and Appendix Table A2 (online only). Most patients did not have a BRCA mutation (rucaparib, 336 [78.7%]; and placebo, 87 [78.4%]).
FIG 1.
CONSORT diagram of patients. BRCA, BRCA1 or BRCA2; HRD, homologous recombination deficiency; ITT, intent-to-treat; LOH, loss of heterozygosity.
TABLE 1.
Baseline Patient and Disease Characteristics (N = 538)
The study is ongoing, with 53 patients (12.4%) in the rucaparib group and 11 patients (9.9%) in the placebo group still receiving treatment. Median duration of follow-up was 26.1 months (95% CI, 25.8 to 26.9) for rucaparib versus 26.2 months (95% CI, 24.0 to 27.7) for placebo.
Efficacy
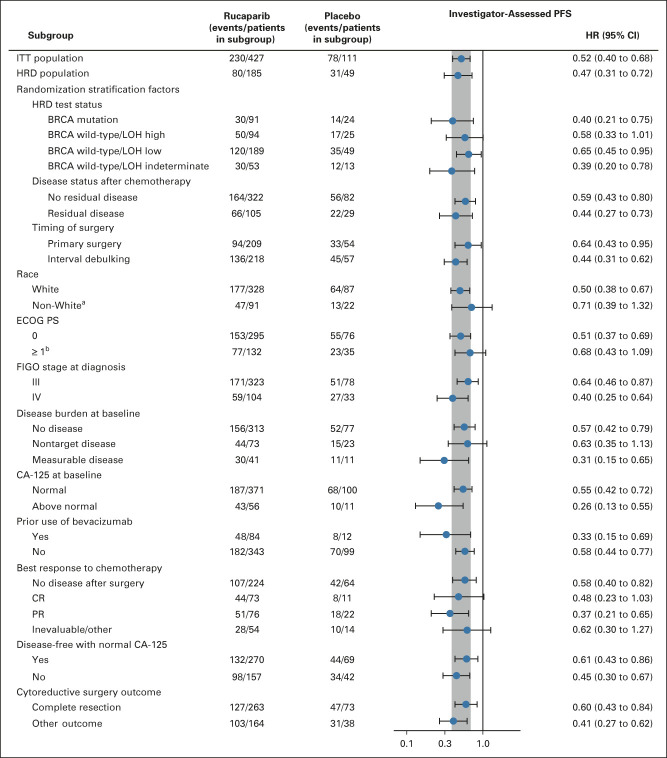
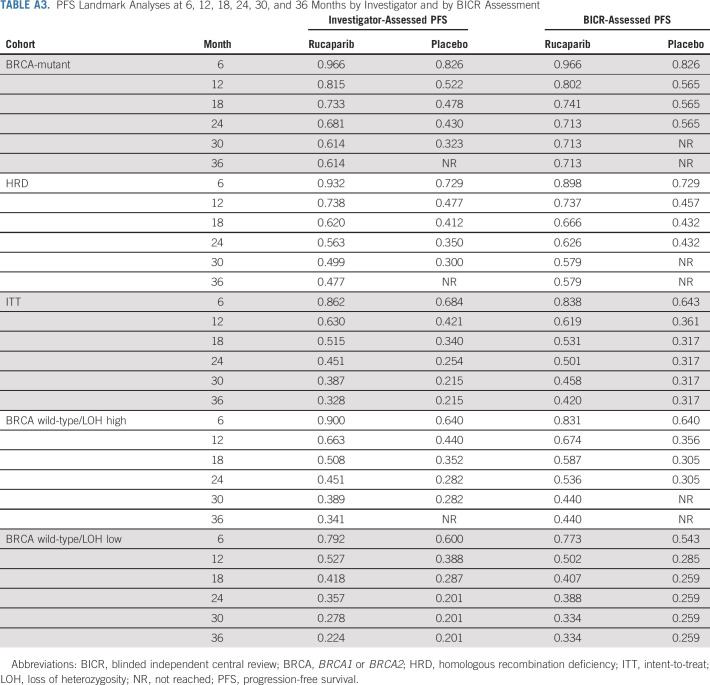
Per the step-down multiple comparison procedure, investigator-assessed PFS was first analyzed in the HRD population (185 [43.3%] patients in the rucaparib group and 49 [44.1%] patients in the placebo group). Median PFS was 28.7 months (95% CI, 23.0 to not reached) in the rucaparib group versus 11.3 months (95% CI, 9.1 to 22.1) in the placebo group (log-rank P = .0004; HR, 0.47; 95% CI, 0.31 to 0.72; Fig 2A). In the ITT population, median PFS was 20.2 months (95% CI, 15.2 to 24.7) in the rucaparib group versus 9.2 months (95% CI, 8.3 to 12.2) in the placebo group (log-rank P < .0001; HR, 0.52; 95% CI, 0.40 to 0.68; Fig 2B). At 24 months, 45.1% of rucaparib-treated patients in the ITT population were progression-free versus 25.4% with placebo (Appendix Table A3, online only). Exploratory subgroup analyses of investigator-assessed PFS in the ITT population showed that there was greater clinical benefit with rucaparib versus placebo for all subgroups (Fig 3), including by tumor HRD classification: BRCA-mutant (Fig 4A), BRCA wild-type/LOH high (Fig 4B), and BRCA wild-type/LOH low (HRD-negative; Fig 4C).
FIG 2.
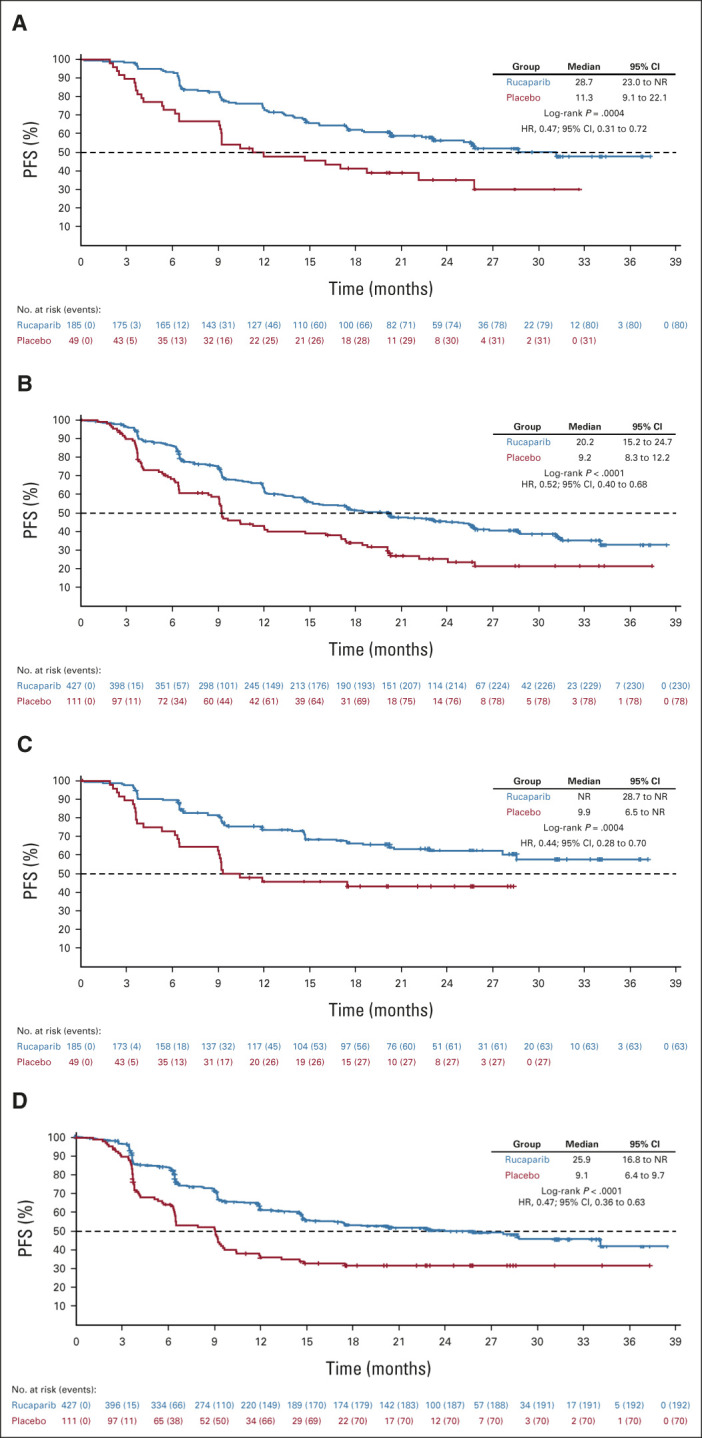
PFS by investigator in the (A) homologous recombination deficiency population and (B) intent-to-treat population and PFS by BICR for the same populations (C and D, respectively). For BICR analyses, nominal P values, not adjusted for multiplicity, are shown. BICR, blinded independent central review; HR, hazard ratio; NR, not reached; PFS, progression-free survival.
FIG 3.
Investigator-assessed PFS in subgroups in the ITT population. The vertical gray band corresponds to the 95% CI of the ITT population. aExcludes patients with unknown race. bOne rucaparib-treated patient had an ECOG PS of 1 at screening and 2 at cycle 1 day 1. BRCA, BRCA1 or BRCA2; CA-125, cancer antigen 125; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; HRD, homologous recombination deficiency; ITT, intent-to-treat; LOH, loss of heterozygosity; PFS, progression-free survival; PR, partial response.
FIG 4.
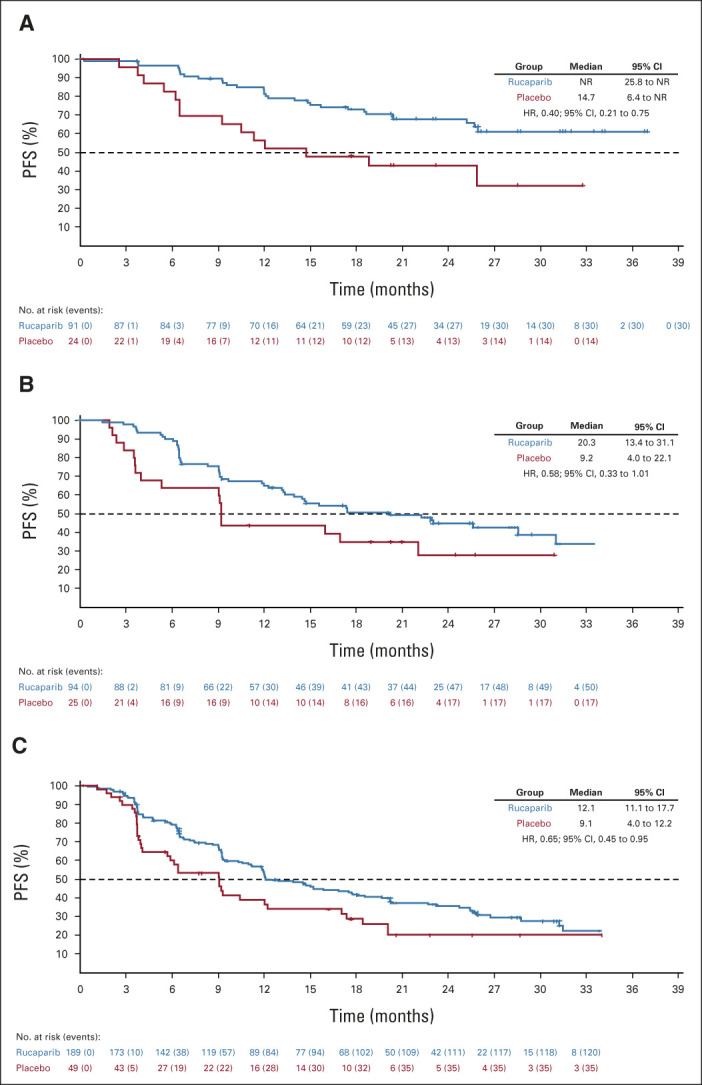
PFS by investigator in (A) patients with BRCA-mutant tumors, (B) patients with BRCA wild-type/LOH high tumors, and (C) patients in the homologous recombination deficiency-negative subgroup (BRCA wild-type/LOH low tumors). BRCA, BRCA1 or BRCA2; HR, hazard ratio; LOH, loss of heterozygosity; NR, not reached; PFS, progression-free survival.
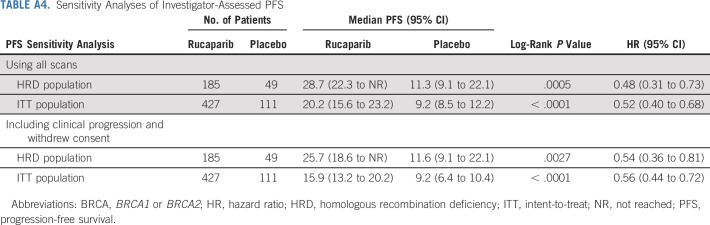
For the standalone secondary end point of BICR-assessed PFS, the results were consistent with those for investigator-assessed PFS in the HRD population (Fig 2C), ITT population (Fig 2D), and HRD subgroups (Appendix Fig A2, online only). Similarly, sensitivity analyses of investigator-assessed PFS support the statistically significant results of the primary end point, indicating that the time to disease progression was not affected by censoring of patients (Appendix Table A4, online only).
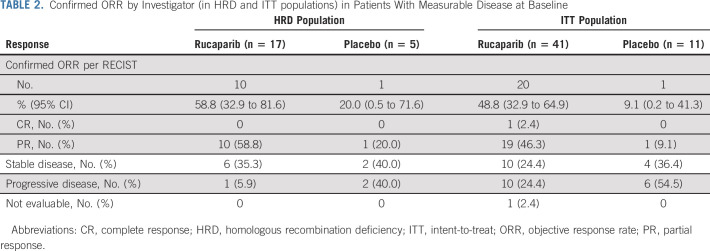
As of the data cutoff, OS results were immature; in the ITT population, 24.7% of death events had occurred. As significance could not be established for this end point, in accordance with the prespecified step-down procedure to adjust for multiplicity, significance could not be claimed for the subsequent ORR analyses at this time.
Confirmed objective responses were observed among rucaparib-treated patients with RECIST measurable disease at baseline (Table 2), including in 10/17 patients (ORR, 58.8% [95% CI, 32.9 to 81.6]) in the HRD population and 20/41 patients (ORR, 48.8% [95% CI, 32.9 to 64.9]) in the ITT population. An objective response was observed in 1/5 placebo-treated patients in the HRD population (ORR, 20.0% [95% CI, 0.5 to 71.6]) and 1/11 patients in the ITT population (ORR, 9.1% [95% CI, 0.2 to 41.3]). Median DOR in the HRD and ITT populations for rucaparib-treated responders versus the one placebo-treated responder, respectively, was 16.7 months (95% CI, 5.7 to not reached) versus 5.5 months (95% CI, not evaluable), and 22.1 months (95% CI, 8.4 to not reached) versus 5.5 months (95% CI, not evaluable; Appendix Fig A3, online only).
TABLE 2.
Confirmed ORR by Investigator (in HRD and ITT populations) in Patients With Measurable Disease at Baseline
Safety
The safety population for the ATHENA–MONO comparison included 425 patients and 110 patients who received at least one dose of oral rucaparib or oral placebo, respectively. Median treatment duration was 14.7 (range, 0.1-32.7) months in the rucaparib group and 9.9 (range, 0.9-25.9) months in the placebo group. Median dose intensity was 0.88 (interquartile range, 0.680-0.995) in the rucaparib group and 1.00 (interquartile range, 0.970-1.000) in the placebo group.
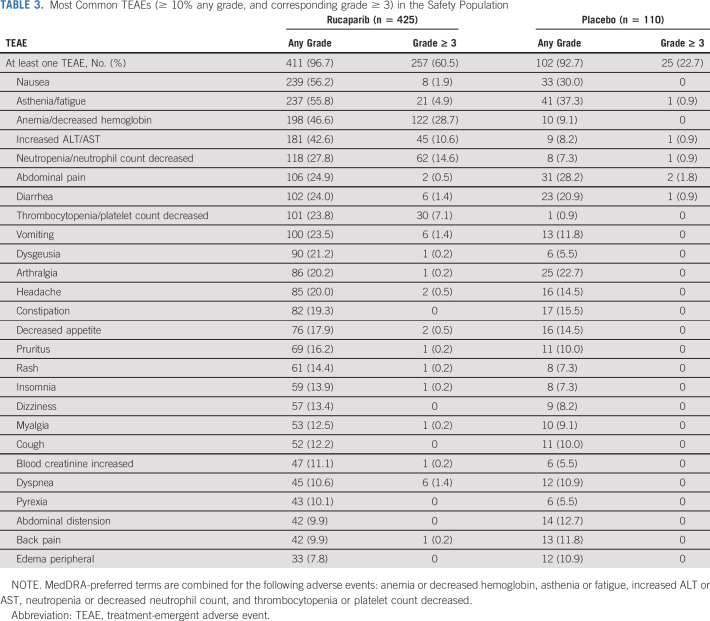
A TEAE of any grade occurred in 411 (96.7%) patients in the rucaparib group and 102 (92.7%) in the placebo group (Table 3). The most common TEAEs (reported in ≥ 40% of patients in either group) were nausea, asthenia/fatigue, anemia/decreased hemoglobin, and increased ALT/AST. TEAEs of grade ≥ 3 were reported in 257 (60.5%) patients in the rucaparib group and 25 (22.7%) in the placebo group, with the most common in the rucaparib group being anemia/decreased hemoglobin and neutropenia/neutrophil count decreased. The most common grade ≥ 3 TEAE reported in the placebo group was hypertension, reported in four (3.6%) patients with placebo and seven (1.6%) patients with rucaparib.
TABLE 3.
Most Common TEAEs (≥ 10% any grade, and corresponding grade ≥ 3) in the Safety Population
The majority of the increased ALT/AST events were grade 1 or 2; ALT and AST levels generally normalized over the course of treatment without other signs of liver injury (Appendix Fig A4, online only). None of the cases of ALT/AST elevation met Hy's law criteria for drug-induced liver injury.
MDS and AML were reported in two patients in the rucaparib group (one MDS during treatment [0.2%] and one AML during long-term follow-up [0.2%]; additional details in the Data Supplement) and no patients in the placebo group.
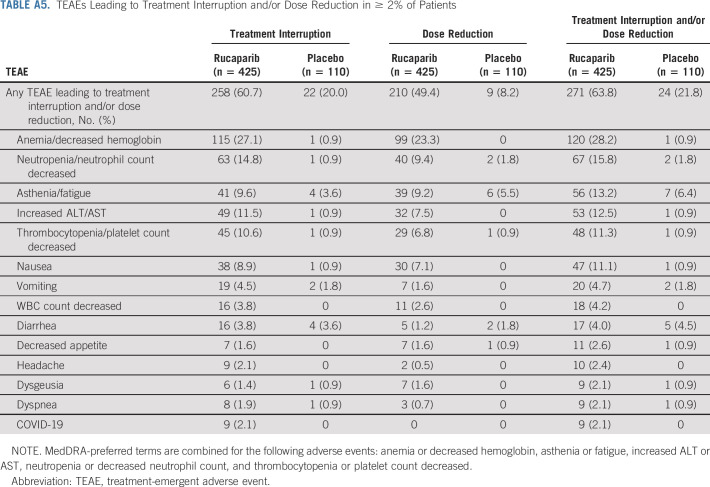
Treatment interruption of oral study drug because of a TEAE occurred in 258 (60.7%) patients in the rucaparib group and 22 (20.0%) in the placebo group (Appendix Table A5, online only). Dose reduction because of a TEAE occurred in 210 (49.4%) patients in the rucaparib group and nine (8.2%) in the placebo group (Table A5). TEAEs led to discontinuation for 50 (11.8%) and six (5.5%) patients in the rucaparib and placebo groups, respectively (Appendix Table A6, online only); the most common TEAE leading to discontinuation of rucaparib was anemia/decreased hemoglobin.
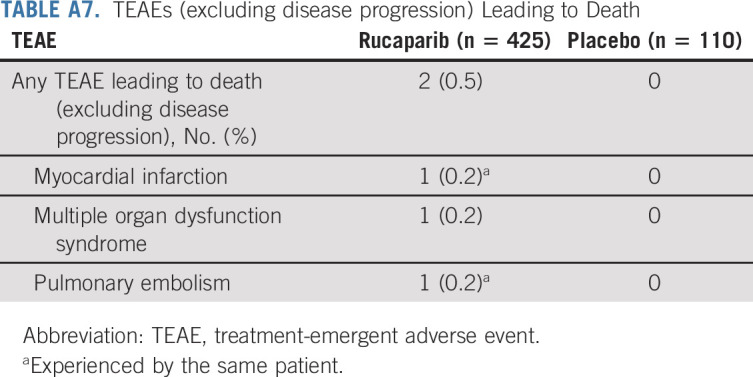
As of the cutoff date, death due to a TEAE (excluding disease progression) occurred in two (0.5%) patients in the rucaparib group (one because of myocardial infarction and pulmonary embolism and one because of multiple organ dysfunction syndrome; Appendix Table A7, online only); neither was considered related to rucaparib. No patients in the placebo group died because of a TEAE.
Patient-Reported Outcomes
Changes from baseline in Functional Assessment of Cancer Therapy—Ovarian Trial Outcome Index scores were similar between rucaparib and placebo in the ITT population (Appendix Fig A5, online only).
DISCUSSION
In ATHENA–MONO, rucaparib maintenance treatment significantly improved PFS versus placebo for patients with newly diagnosed advanced ovarian cancer regardless of BRCA or HRD status. Analysis of patient populations on the basis of HRD status indicate that the improvement in PFS observed with rucaparib in the ITT population was not driven solely by BRCA or HRD subgroups, with substantial PFS benefit also observed among patients with HRD-negative (ie, BRCA wild-type/LOH low) tumors, commonly considered to be homologous recombination proficient. Together, these data suggest that rucaparib maintenance treatment can provide benefit for a broad set of patients who have responded to first-line chemotherapy. The BICR assessment demonstrated that PFS benefit across subgroups is consistent with that seen by investigator assessment, as evaluated by HRs. The medians for PFS were generally higher with BICR versus investigator assessment, which is a trend previously described in other studies and could reflect bias associated with informative censoring.12 Approximately 10% of patients in the current analysis had measurable disease at baseline, and approximately half of these patients who received rucaparib maintenance had a deepening of response with confirmed reductions in tumor burden, including in patients with HRD-negative tumors.
The safety profile for rucaparib in ATHENA–MONO is consistent with that of rucaparib in other settings13-15 and other PARP inhibitors in the first-line maintenance setting.2,3,5 The most common nonhematologic TEAEs observed with rucaparib were generally low grade, and the majority of grade ≥ 3 events were hematologic TEAEs previously associated with PARP inhibitors. The incidence of MDS/AML in ATHENA–MONO was consistent with other PARP inhibitor studies in the first-line setting.2,3,5 No clinically meaningful differences in patient-reported outcomes were detected between study groups, suggesting that rucaparib maintenance treatment did not negatively affect patients' health-related quality of life versus placebo.
The SOLO-1 study of olaparib was the first to demonstrate benefit of first-line maintenance treatment of a PARP inhibitor, but the patient population was restricted to women who harbored BRCA mutations.2 The PRIMA study of niraparib then expanded the assessment of first-line maintenance PARP inhibitor treatment to all molecular subgroups.3 However, the PRIMA study excluded patients with International Federation of Gynecology and Obstetrics stage III disease who had no visible residual disease after primary debulking surgery, skewing the study population toward those with advanced disease. Notably, PRIMA also implemented an individualized dosing algorithm on the basis of weight and platelet levels late in the study because of high rates of grade ≥ 3 thrombocytopenia reported with niraparib.16,17 Most patients who enrolled after individualized dosing was adopted received the lower starting dose of niraparib 200 mg once a day, which, when compared with efficacy results with niraparib 300 mg once a day, was found to have less robust PFS benefit versus placebo, particularly in the HRD-negative (homologous recombination proficient) population.17 ATHENA–MONO enrolled a broad population of women with newly diagnosed ovarian cancer who had responded to first-line treatment, with no restrictions on HRD status or surgical outcome (including R0/complete resection). The results presented here demonstrate clear benefit for rucaparib maintenance across HRD subgroups and in the ITT population, including those with stage III cancer without residual disease, expanding our knowledge of the populations that may benefit from PARP inhibitor maintenance treatment. Additionally, the safety profile of rucaparib supports a single starting dose of 600 mg twice a day, and the availability of four dose reduction steps in the study offers flexibility for managing side effects.
Strengths of the study include that ATHENA–MONO had the highest proportion of patients with BRCA wild-type (78.6%) and HRD-negative tumors (44.2%) among phase III clinical studies evaluating first-line maintenance with a PARP inhibitor,1-4 giving further weight to the observed effectiveness of rucaparib maintenance treatment in patients typically thought to be less sensitive to a PARP inhibitor. ATHENA–MONO also enrolled a population that included patients with a complete resection or nonmeasurable disease after surgery but cancer antigen 125 response, a population that could be considered more real-world compared with other studies in its inclusion of patients without certain prognostically high-risk clinical characteristics. A limitation of the ATHENA–MONO analysis was the relatively small number of placebo group patients; although the 4:1 random assignment to rucaparib and placebo was considered advantageous for encouraging participation, the placebo group sample size limits the interpretation of some subgroup analyses. Regardless, despite the smaller number of placebo group patients, analyses of PFS demonstrated a clear trend toward rucaparib benefit versus placebo across subgroups.
In summary, ATHENA–MONO demonstrates that rucaparib monotherapy is effective in the first-line maintenance setting with benefit observed in a broad patient population with advanced ovarian cancer, including those with and without HRD tumors.
ACKNOWLEDGMENT
The authors thank the patients who participated in ATHENA–MONO, as well as their families and caregivers. A list of ATHENA collaborators can be found in Appendix 1 (online only). The authors also thank the investigators for their contributions to the administration and execution of the studies, the Clovis Oncology study teams for clinical development and operational support, and Vivian Chen of Clovis Oncology for assistance in manuscript preparation. Medical writing and editorial support funded by Clovis Oncology were provided by Nathan Yardley and Stephen Bublitz of Ashfield MedComms, an Ashfield Health company.
APPENDIX 1. ATHENA Collaborators
ENGOT
Belgium (BGOG)—Toon Van Gorp (UZ Leuven); Ignace Vergote (UZ Leuven); Czech Republic (CEEGOG)—Marketa Pospiskova (Krajska nemocnice T. Bati Zlin); Lukas Rob \(Fakultni nemocnice Kralovske Vinohrady Praha); Maria Zvarikova (Masarykuv onkologicky ustav Brno); Denmark (NSGO)—Charlotte A Haslund (Aalborg University Hospital); Bente Lund (Aalborg University Hospital); Gitte-Bettina Nyvang (Odense University Hospital); Finland (NSGO)—Maarit Anttila (Kuopio University Hospital); Hanna Sallinen (Kuopio University Hospital); Germany (AGO)—Tanja Fehm (Universitaetsklinikum Duesseldorf); Jens Gerber (Städtisches Klinikum Dessau); Frederik Marme (University Hospital Mannheim); Andreas Schneeweiss (NCT Heidelberg); Florian Zettl (Klinikum Traunstein-Hamatologie/Onkologie); Greece (HeCOG)—Athina Christopoulou (General Hospital of Patras); George Fountzilas (Euromedica General Clinic, B' Oncology Clinic); Anna Koumarianou (Attikon General University Hospital); Amanda Psyrri (University Hospital Attikon); Flora Zagouri (Alexandra Hospital); Ireland (CTI)—Paula Calvert (University Hospital Waterford); Dearbhaile Collins (Cork University Hospital); Greg Korpanty (University Hospital Limerick); Conleth Murphy (Bon Secours Hospital); Israel (ISGO)—Mario Beiner (Meir Medical Center); Inbar Ben-Shachar (Ziv Medical Center); Ilan Bruchim (Hillel Yaffe MC); Jacob Korach (Sheba Medical Center); Ora Rosengarten (Shaare Zedek Medical Center); Tamar Safra (Tel Aviv Sourasky Medical Center); Ayelet Shai (Galilee Medical Center); Italy (MITO)—Roberto Bordonaro (Arnas Garibaldi); Enrico Breda (Ospedale S. Giovanni Calibita Fatebenefratelli); Domenico Cristiano Corsi (Ospedale S. Giovanni Calibita Fatebenefratelli); Rocco De Vivo (Ospedale san Bortolo); Elena Geuna (Candiolo Cancer Institute—IRCCS); Domenica Lorusso (Policlinico Agostino Gemelli); Clara Natoli (University G. D'Annunuzio-Chieti); Innocenza Palaia (Azienda Ospedaliera Universitaria Policlinico Umberto I); Pierluigi Benedetti Panici (Azienda Ospedaliera Universitaria Policlinico Umberto I); Carmela Pisano (Instituto Nazionale Tumori—IRCCS); Daniela Sambataro (Arnas Garibaldi); Roberto Sorio (Centro di Riferimento Oncologico CRO—IRCCS); Michele De Tursi (University G. D'Annunuzio-Chieti); Giorgio Valabrega (Candiolo Cancer Institute—IRCCS); Poland (PGOG)—Wieslawa Bednarek (Medical University of Lublin); Anita Chudecka-Glaz (Samodzielny Publiczny Szpital Kliniczny Nr 2 Pomorskiego Uniwersytetu Medycznego w Szczecinie); Pawel Knapp (Uniwersytecki Szpital Kliniczny); Beata Mackowiak-Matejczyk (Bialostockie Centrum Onkologii Oddzial Onkologii Ginekologicznej); Joanna Pikiel (Szpitale Pomorskie Sp. zoo); Andrzej Roszak (Wielkopolskie Centrum Onkologii); Włodzimierz Sawicki (Szpital Magodent); Spain (GEICO)—Constanza Maximano Alonso (Hospital Universitario Puerta de Hierro - Majadahonda); Jeronimo Martinez-Garcia (Hospital Virgen de la Arrixaca); Maria Iglesias Gonzalez (Hospital Son Llatzer); Cristina Martin Lorente (Hospital De La Santa Creu I Sant Pau); Nuria Ruiz-Miravet (Consorcio Hospitalario Provincial Castellon); Ana Oaknin (Vall d'Hebron Institute of Oncology); Jose Fuentes Pradera (Hospital Nuestra Senora De Valme); Purificacion Martinez Del Prado (Hospital Universitario Basurto); Maria Del Mar Gordon Santiago (Hospital de Jerez); Isabel Palacio Vazquez (Hospital Universitario Central de Asturias); Sweden (NSGO)—Josefin Fernebro (Karolinska University Hospital); Gabriel Lindahl (Onkologiska Kliniken, Linkoping); Susanne Malander (Skåne University Hospital); Turkey (TRSGO)—Ozturk Ates (Dr Abdurrahman Yurtaslan Ankara Oncology Education and Research Hospital, Clinic of Medical Oncology); Fuat Demirkiran (Istanbul University, Cerrahpasa School of Medicine); Tevfik Guvenal (Celal Bayar University Faculty of Medicine); Fatih Köse (Baskent University Hospital, Adana Dr Turgut Noyan Application and Research Center, Kisla Campus); Cagatay Taskiran (Koc University School of Medicine, Koc University Hospital); United Kingdom (NCRI)—Roshan Agarwal (Northampton General Hospital); Susana Banerjee (The Royal Marsden NHS Foundation Trust - Royal Marsden Hospital); Clare Barlow (Musgrove Park Hospital); Emma Cattell (Musgrove Park Hospital); Maxine Flubacher (Poole Hospital NHS Foundation Trust); Rebecca Herbertson (University Hospitals Sussex NHS Foundation, Royal Sussex County Hospital, Clinical Trials Pharmacy, Clinical Trials Pharmacy, Clinical Research Facility); Jane Hook (St James University Hospital); David Jackson (St James University Hospital); Rachel Jones (South West Wales Cancer centre—Singleton Hospital); Rebecca Kristeleit (Guy's and St Thomas' NHS Foundation Trust); Louise Li (The James Cook University Hospital Medical Oncology); Iain McNeish (Imperial College London); Rowan Miller (Barts and the London NHS Trust; University College London Hospital—NHS Foundation Trust); Ana Montes (Guy's and St Thomas' NHS Foundation Trust [Guy's Cancer Centre]); Sarah Moon (University Hospitals of Morecambe Bay NHS Foundation Trust); Fiona Nussey (Western General Hospital); Christine Parkinson (Addenbrookes Hospital); Rachel Plant (Poole Hospital NHS Foundation Trust); Axel Walther (Bristol Cancer Institute Medical Oncology); Justin Waters (East Kent Hospitals University NHS Foundation trust Medical Oncology).
GOG
United States—Charles Anderson (Willamette Valley Cancer Institute and Research Center); Joyce Barlin (Womens Cancer Care Associates); Kian Behbakht (UC Health Cancer Center Investigational Pharmacy); David Bender (University of Iowa Hospitals and Clinics Investigational Pharmacy Service); Caroline Billingsley (University of Cincinnati Medical Center); Albert Bonebrake (Ferrell-Duncan Clinic); Leslie Bradford (Maine Medical Center); William Bradley (Froedtert Hospital/Inpatient Pharmacy); Joseph Buscema (Arizona Oncology Associates, PC—HOPE); Paul Celano (Greater Baltimore Medical Center); Setsuko Chambers (The University of Arizona Cancer Center—North Campus); Cynthia Chua (Oncology Hematology Care Inc); Noelle Cloven (Texas Oncology—Fort Worth Cancer Center); Christopher Darus (Maine Medical Center); Robert Coleman (US Oncology Research); John Diaz (Baptist Health Medical Group Oncology LLC); Babak Edraki (John Muir Medical Center—Concord Campus, Pharmacy Department); Ramez Eskander (University of California San Diego); Marina Frimer (Northwell Health); Stephanie Gaillard (SKCCC at Johns Hopkins, Oncology Investigational Drug Service); Joseph de la Garza (Texas Oncology—San Antonio Medical Center); Sharad Ghamande (Georgia Cancer Center at Augusta University); Darlene Gibbon (Summit Medical Group); Michael Gold (Oklahoma Cancer Specialists and Research Institute); Mary Gordinier (Norton Cancer Institute); Ronald Harris (Broome Oncology LLC); Thomas Herzog (University of Cincinnati College of Medicine); Robert Holloway (Florida Hospital); Camille Jackson (Stephenson Cancer Center); Amanda Jackson (University of Cincinnati Medical Center); Andrea Jewell (University of Kansas Cancer Center); Edward Grendys Jr (Florida Gynecologic Oncology); Christine Lee (Texas Oncology-The Woodlands, Gynecologic Oncology); Linda Van Le (University of North Carolina Chapel Hill); Ramey Littell (Kaiser Permanente—San Francisco); Joseph Lucci (Memorial Hermann—TMC Investigational Drugs Services Pharmacy); Ling Ma (Rocky Mountain Cancer Centers); Michael McCollum (Virginia Oncology Associates); Colleen McCormick (LMG Gynecologic Oncology); Kristi McIntyre (Texas Oncology—Dallas Presbyterian Hospital); Sanaz Memarzadeh (UCLA Women's Health Clinical Research Unit); Mark Messing (Texas Oncology-Bedford); Bradley Monk (HonorHealth Research Institute); Timothy Moore (Columbus NCORP); Kathleen Moore (Stephenson Cancer Center); Mark Morgan (University of Pennsylvania); Robert Morris (Karmanos Cancer Institute); Cassandra Niemi (Northwest Cancer Specialists, P.C.); Alexander Olawaiye (UPMC Magee-Womens Hospital); David O'Malley (The Ohio State University); Brian Orr (UPMC Magee-Womens Hospital); Steven Papish (Summit Medical Group); Shiroo Parshad (Community Health Network); Bhavana Pothuri (New York University Medical Center); Emily Prendergast (Metro Minnesota [MMCORC]); Scott Richard (Thomas Jefferson University); Debra Richardson (Stephenson Cancer Center); Peter Rose (Cleveland Clinic); Erin Salinas (Northwest Cancer Specialists, P.C.); Alessandro Santin (Yale University); Mark Shahin (Abington Memorial Hospital); Sudarshan Sharma (Dr Sudarshan K. Sharma Ltd—Gynecologic Oncology); Nicola Spirtos (Women's Cancer Center of Nevada); David Spriggs (Massachusetts General Hospital); David Starks (Avera Cancer Institute); Michael Teneriello (Texas Oncology-Austin Central); Meaghan Tenney (Northside Hospital); Premal Thaker (Washington University School of Medicine); Fred Ueland (University of Kentucky); Lydia Usha (Rush University Medical Center); Katrina Wade (Ochsner Medical Center); David Warshal (MD Anderson Cancer Center at Cooper); Theresa Werner (Huntsman Cancer Institute); Gina Westhoff (LMG Gynecologic Oncology); Shannon Westin (The University of Texas MD Anderson Cancer Center); Jenny Whitworth (Baptist MD Anderson Cancer Center); Lyndsay Willmott (Arizona Oncology Associates, PC—HAL); Oladapo Yeku (Dana Farber Cancer Institute; Massachusetts General Hospital).
NRG Oncology—Japan
Japan—Tsukasa Baba (Iwate Medical University); Keiichi Fujiwara (Saitama Medical University International Medical Center); Kenichi Harano (National Cancer Center Hospital East); Kosei Hasegawa (Saitama Medical University International Medical Center); Takashi Hirakawa (Gunma University Hospital); Koji Horie (Saitama Cancer Center); Shoji Kamiura (Osaka International Cancer Institute); Hisamori Kato (Kanagawa Cancer Center); Noriyuki Katsumata (Nippon Medical School Musashikosugi Hospital); Akira Kikuchi (Niigata Cancer Center Hospital); Junichi Kodama (Hiroshima City Hiroshima Citizens Hospital); Etsuko Miyagi (Yokohama City University Hospital); Shoji Nagao (Hyogo Cancer Center); Hidekatsu Nakai (Kindai University Hospital); Kazuto Nakamura (Gunma Prefectural Cancer Center); Toyomi Satoh (University of Tsukuba Hospital); Masashi Takano (National Defense Medical College Hospital); Kenji Tamura (National Cancer Center Hospital); Kimio Ushijima (Kurume University Hospital); Satoshi Yamaguchi (Hyogo Cancer Center); Mayu Yunokawa (The Cancer Institute Hospital of JFCR).
Australia
Sally Baron-Hay (Northern Cancer Institute); Tony Bonaventura (Newcastle Private Hospital); Andrew Dean (St John of God Subiaco Hospital); Michael Friedlander (Prince of Wales Hospital); Bo Gao (Westmead Hospital); Jeffrey Goh (Royal Brisbane and Women's Hospital); Anne Hamilton (Peter MacCallum Cancer Center); Martin Oehler (Burnside War Memorial Hospital).
Canada
James Bentley (NSHA-QEII Health Science Centre); Paul Bessette (Centre Integre Universitaire de sante et de service sociaux de l'Estri-Centre hospitalier univeritaire de Sherbrooke [CIUSSS—CHUS]); Theresa Chan (BC Cancer Surrey Pharmacy Department); Susan Ellard (BC Cancer - Kelowna); Jenny Ko (BC Cancer—Abbotsford); Clare Reade (Juravinski Cancer Centre); Xing Zeng (McGill University Health Centre—Glen Site); Xiaofu Zhu (Cross Cancer Institute); Bohdariannna Zorniak (Cross Cancer Institute).
Princess Margaret Consortium—Alon Altman (CancerCare Manitoba); Laurie Elit (Juravinski Cancer Centre); Prafull Ghatage (Alberta Health Services—Tom Baker Cancer Centre); Amit Oza (Princess Margaret Cancer Centre); Diane Provencher (Centre Hospitalier de l'Université de Montreal); Johanne Weberpals (The Ottawa Hospital Cancer Centre); Stephen Welch (London Health Sciences Centre).
New Zealand
Jennifer Fernando (Palmerston North Hospital); Joanna Jones (Tauranga Hospital); Archana Srivastava (Waikato Hospital); Michelle Vaughan (Christchurch Hospital); Michelle Wilson (Auckland City Hospital).
Romania
Adriana Mariana Balint (Spitalul Clinic Municipal Dr Gavril Curteanu); Tudor Eliade Ciuleanu (Institutul Oncologic Prof Dr Ion Chiricuta); Dana Clement (Institutul Regional de Oncologie Iasi); Filip Dumitru (S.C. Oncopremium Team S.R.L); Doina Elena Ganea (Spitalul Judetean de Urgenta Sfantul Ioan cel Nou Suceava); Dan Lungulescu (S.C. Oncolab S.R.L.); Simona Mihutiu (Spitalul Clinic Municipal Dr Gavril Curteanu); Serban-Mircea Negru (ONCOMED Timisoara); Iuliana Pantelimon (Quantum Medical Center S.R.L.); Michael Schenker (Centrul de Oncologie Sf.Nectarie); Alina Cristina Turcu (Spitalul Municipal Ploiesti); Anghel Adrian Udrea (S.C. Medisprof S.R.L.).
Russia
Mikhail Dvorkin (BHI of Omsk Region Clinical Oncology Dispensary); Alexander Fedenko (P. Hertsen Moscow Oncology Research Institute); Dmitry Kirtbaya (SBHI Oncological Dispensary No. 2 of the Ministry of Health of Krasnodar Territory); Alla Lisyanskaya (Saint-Petersburg State Budget Inst of Healthcare City Clin. Oncology Dispensary); Olga Mikheeva (Limited Liability Company MedPomosch); Vladimir Moiseenko (SBHI Saint Petersburg Research Center specialized types of medical care [Oncology]); Sergey Orlov (FSBEI HE I.P. Pavlov SPbSMU MoH Russia); Vadim Shirinkin (SBHI Orenburg Regional Clinical Oncology Dispensary); Pavel Skopin (Federal State Budgetary Educational Institution of Higher Education); Ekaterina Solovyeva (Arkhangelsk Clinical Oncological Dispensary); Dmitry Udovitsa (SBHI Oncological Dispensary No. 2 of the Ministry of Health of Krasnodar Territory); Vladimir Vladimirov (GBUZ SK Pyatigorsk Interdistrict Oncology Dispensary).
Singapore
David Tan (National University Hospital); Yee Chay Wen (National Cancer Centre Singapore).
South Korea
Suk-Joon Chang (Ajou University Hospital); Min Chul Choi (CHA Bundang Medical Center); Seob Jeon (Soonchunhyan University Hospital Cheonan); Yong-Man Kim (Asan Medical Center); Byoung-Gie Kim (Samsung Medical Center); Jae-Weon Kim (Seoul National University Hospital); Yong Beom Kim (Seoul National University Bundang Hospital); Jung-Yun Lee (Severance Hospital, Yonsei University Health System); Kwang-Beom Lee (Gachon University Gil Medical Center); Jae Kwan Lee (Korea University Guro Hospital); Myong Cheol Lim (National Cancer Center); Sang-Young Ryu (Korea Cancer Center Hospital); Seung-Hyuk Shim (Konkuk University Medical Center); Kyeong So (Konkuk University Medical Center).
Taiwan
Chih-Long Chang (Mackay Memorial Hospital); Wen-Fang Cheng (National Taiwan University Hospital); Hung-Hsueh Chou (Chang Gung Medical Foundation- Linkou Branch); Tang-Yuan Chu (Hualien Tzu Chi Hospital); Sheng-Mou Hsiao (Far Eastern Memorial Hospital); Wen-Tseng Huang (Chi Mei Hospital, Liouying); Chieh-Yi Kang (Chi Mei Medical Center); Wen-Shiung Liou (Kaohsiung Veterans General Hospital); Wei-Min Liu (Taipei Medical University Hospital); Chien-Hsing Lu (Taichung Veterans General Hospital); Peng-Hui Wang (Taipei Veterans General Hospital); Lian-Shung Yeh (China Medical University Hospital); Mu-Hsien Yu (Tri-Service General Hospital).
FIG A1.
Plots of the log of the cumulative hazard for PFS by investigator in (A) the homologous recombination-deficiency population and (B) the intent-to-treat population and PFS by blinded independent central review for the same populations (C and D, respectively). PFS, progression-free survival.
FIG A2.
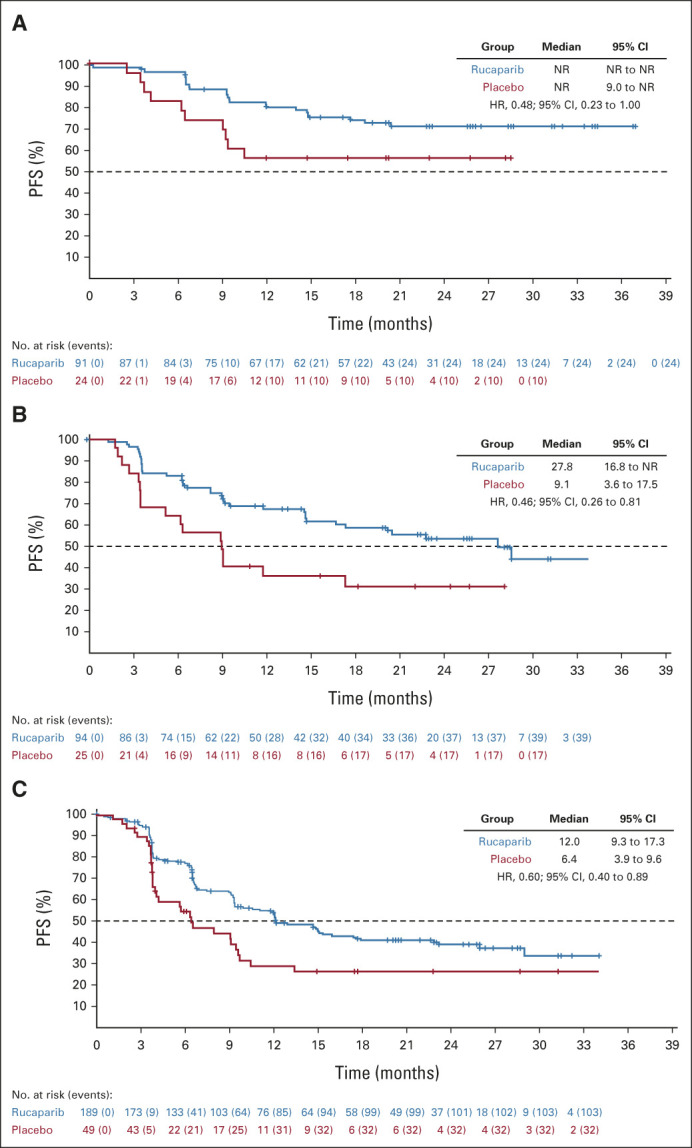
PFS by blinded independent central review in (A) patients with BRCA-mutant tumors, (B) patients with BRCA wild-type/LOH high tumors, and (C) patients in the homologous recombination deficiency-negative subgroup (BRCA wild-type/LOH low tumors). BRCA, BRCA1 or BRCA2; HR, hazard ratio; LOH, loss of heterozygosity; NR, not reached; PFS, progression-free survival.
FIG A3.
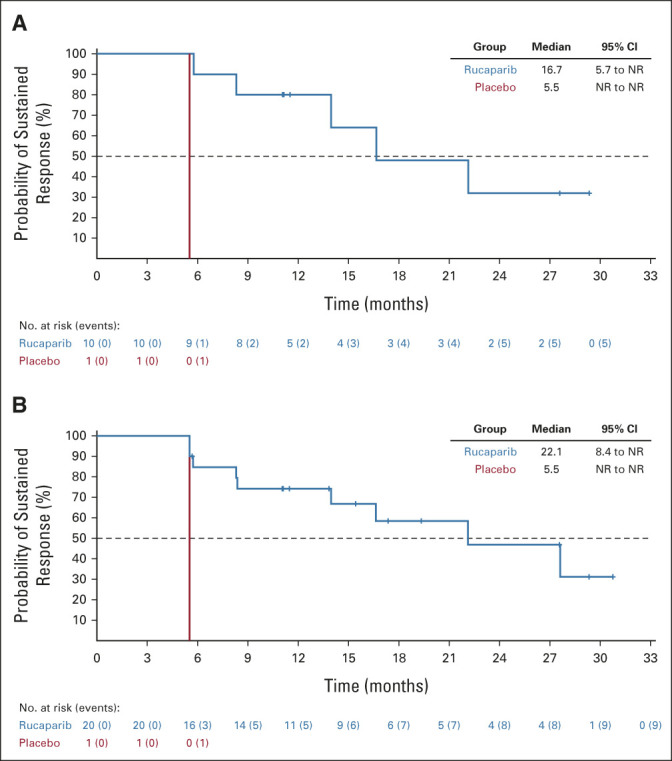
Duration of RECIST response in the (A) homologous recombination deficiency population and (B) intent-to-treat population. NR, not reached.
FIG A4.
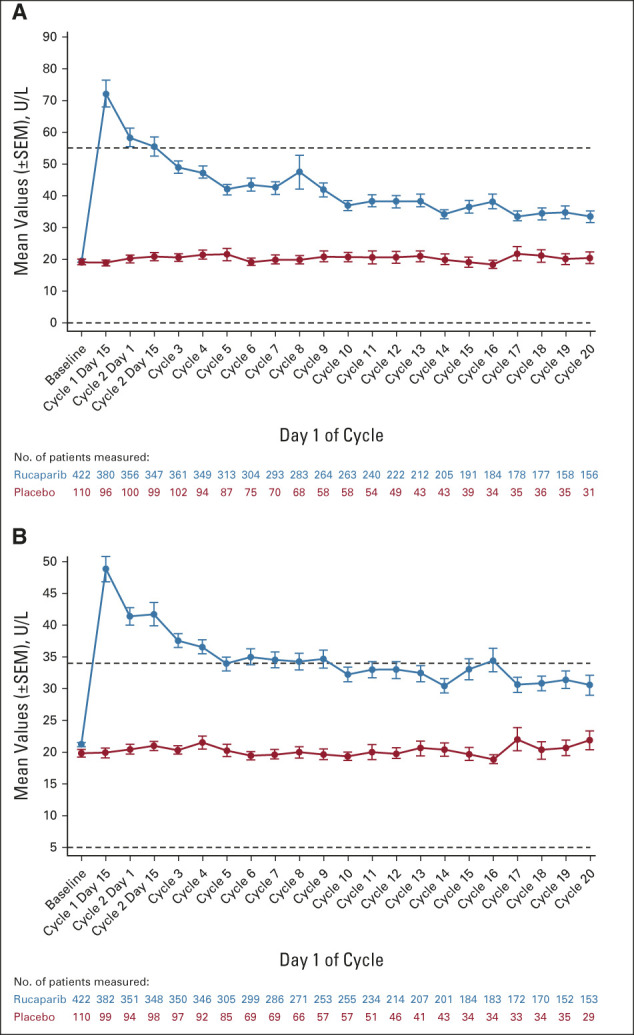
Changes from baseline in (A) ALT and (B) AST. Horizontal dotted lines in graphs represent the upper and lower limits of normal for each laboratory parameter.
FIG A5.
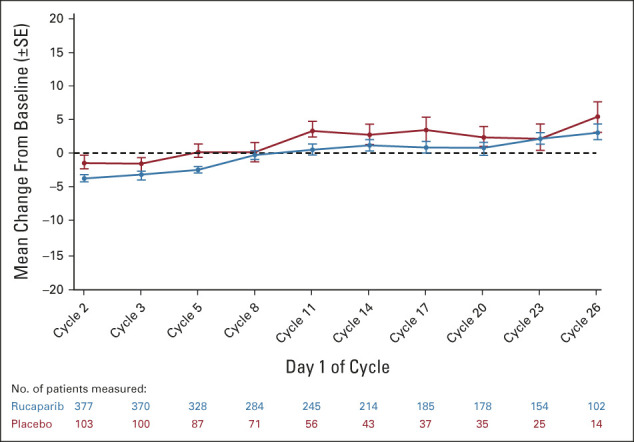
Change from baseline in Functional Assessment of Cancer Therapy—Ovarian Trial Outcome Index score in the intent-to-treat population.
TABLE A1.
Inclusion and Exclusion Criteria
TABLE A2.
BRCA Gene and Mutation Type
TABLE A3.
PFS Landmark Analyses at 6, 12, 18, 24, 30, and 36 Months by Investigator and by BICR Assessment
TABLE A4.
Sensitivity Analyses of Investigator-Assessed PFS
TABLE A5.
TEAEs Leading to Treatment Interruption and/or Dose Reduction in ≥ 2% of Patients
TABLE A6.
TEAEs (excluding disease progression) Leading to Treatment Discontinuation
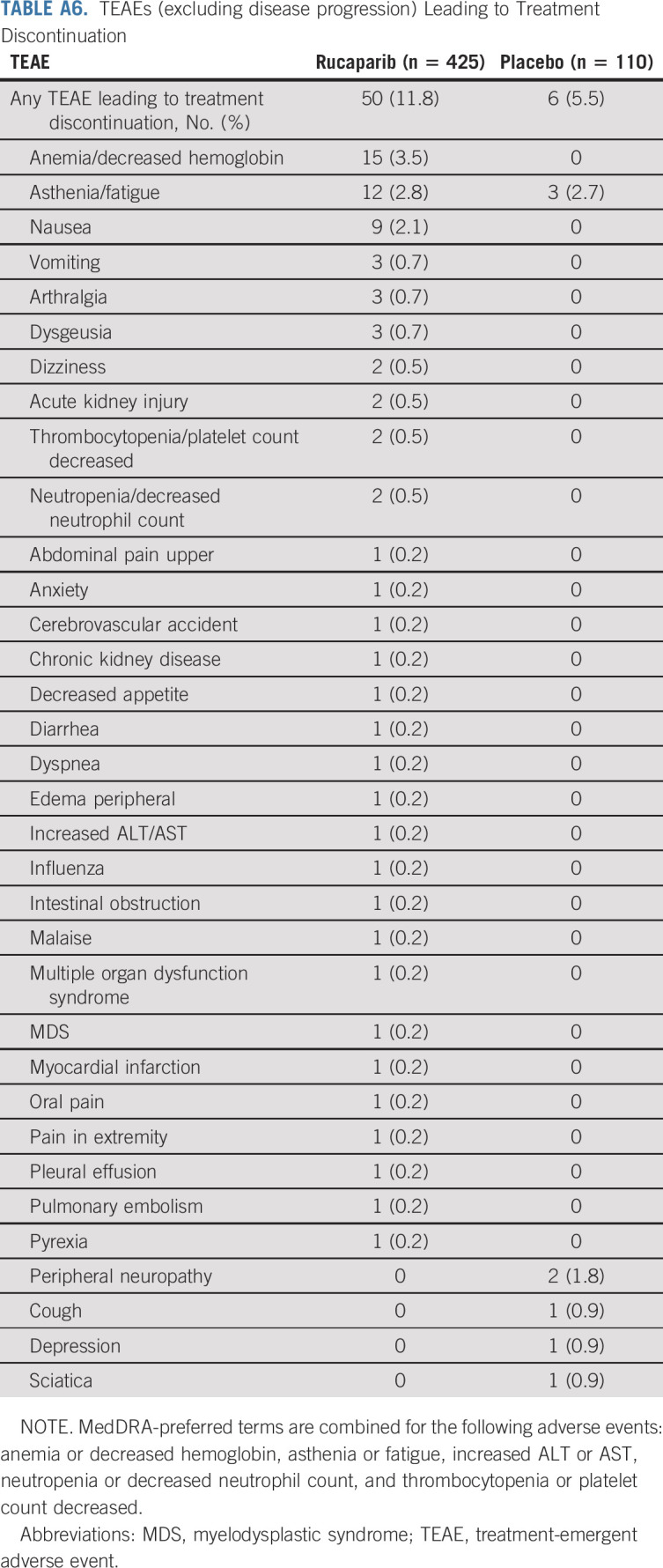
TABLE A7.
TEAEs (excluding disease progression) Leading to Death
Bradley J. Monk
Leadership: US Oncology
Honoraria: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, GOG Foundation, Elevar Therapeutics, Novocure, Gradalis, Karyopharm Therapeutics, Bayer, EMD Serono/Merck, Macrogenics, Sorrento Therapeutics, US Oncology, Myriad Pharmaceuticals, Novartis, OncoC4, Pieris Pharmaceuticals
Consulting or Advisory Role: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Myriad Pharmaceuticals, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure, Bayer, Elevar Therapeutics, EMD Serono/Merck, Gradalis, US Oncology, Novartis, Pieris Pharmaceuticals, OncoC4
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, TESARO/GSK, Merck
Research Funding: Novartis (Inst), Amgen (Inst), Genentech (Inst), Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), Immunogen (Inst), Regeneron (Inst), Nucana (Inst)
Myong Cheol Lim
Consulting or Advisory Role: GI Innovation, Boryung, AstraZeneca, Takeda, CKD Pharm, Genexine, Hospicare
Research Funding: AbbVie (Inst), AstraZeneca (Inst), Amgen (Inst), Astellas Pharma, BeiGene (Inst), Cellid (Inst), CKD Pharm (Inst), Clovis Oncology (Inst), Genexine (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Merck (Inst), MSD (Inst), OncoQuest (Inst), Pfizer (Inst), Roche (Inst), Eisai (Inst)
David M. O'Malley
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, Tesaro, Novocure, Genentech/Roche, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics, Novartis, Seattle Genetics, BBI Healthcare, Arquer Diagnostics, Toray Industries, Takeda, InxMed, Celsion, Arcus Biosciences, Sutro Biopharma, Novocure, Atossa Therapeutics, Laekna Therapeutics, Onconova Therapeutics, VBL Therapeutics, Vincerx Pharma, Adaptimmune, Roche
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Immunogen (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seattle Genetics (Inst), Iovance Biotherapeutics (Inst), Leap Therapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology Inc (Inst), Acerta Pharma (Inst), Advaxis (Inst), Ajinomoto (Inst), Arcus Biosciences (Inst), Deciphera (Inst), Exelixis (Inst), Roche (Inst), Incyte (Inst), Karyopharm Therapeutics (Inst), Ludwig Institute for Cancer Research (Inst), Novartis (Inst), NovoCure (Inst), OncoQuest (Inst), BeiGene (Inst), Pfizer (Inst), Precision Therapeutics (Inst), Sanofi (Inst), Seattle Genetics (Inst), Sutro Biopharma (Inst), GlaxoSmithKline (Inst), Verastem (Inst)
Ana Oaknin
Consulting or Advisory Role: Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab, Mersana, GSK, Deciphera, Agenus, Corcept Therapeutics, Eisai, EMD Serono, F. Hoffmann-La Roche, Medison, Merck Sharp & Dohme, Novocure, prIME Oncology, Shattuck Labs, Sutro Biopharma, ITeos Therapeutics, Amgen
Research Funding: AbbVie (Inst), Abililty Pharmaceuticals (Inst), Advaxis (Inst), Aeterna Zentaris (Inst), Aprea Therapeutics (Inst), Clovis Oncology Inc (Inst), Eisai (Inst), Roche (Inst), Regeneron (Inst), Agenus (Inst), AstraZeneca (Inst), BeiGene (Inst), Belgian Gynaecological Oncology Group (BGOG) (Inst), Bristol Myers Squibb International Corporation (BMS) (Inst), Corcept Therapeutics (Inst), Immunogen (Inst), Iovance Biotherapeutics (Inst), Iovance Lilly (Inst), Medimmune (Inst), Merck (Inst), Merck Sharp & Dohme (Inst), Mundipharma Research (Inst), Novartis Farmacéutica (Inst), Seagen (Inst), Seattle Genetics (Inst), Sutro Biopharma (Inst), Tesaro (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Michelle K. Wilson
Research Funding: Foundation Medicine (Inst)
Robert L. Coleman
Employment: US Oncology
Leadership: Onxeo
Stock and Other Ownership Interests: McKesson
Consulting or Advisory Role: Clovis Oncology, Genentech/Roche, AstraZeneca/MedImmune, Genmab, Tesaro, OncoMed, Sotio, Oncolytics, AbbVie/Stemcentrx, Immunogen, AbbVie, Agenus, Novocure, Merck, OncXerna Therapeutics, Alkermes, Gradalis, Regeneron
Research Funding: AstraZeneca/MedImmune, Esperance Pharmaceuticals, Array BioPharma, Clovis Oncology, Johnson & Johnson, Merck, Roche/Genentech, Abbott/AbbVie, Immunogen (Inst), Mirati Therapeutics (Inst), Amgen (Inst), Pfizer (Inst), Lilly (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Merck, AstraZeneca/MedImmune, Array BioPharma, Clovis Oncology, Roche/Genentech, Research to Practice, GOG Foundation, Clovis Oncology, Sotio, Vaniam Group
Domenica Lorusso
Consulting or Advisory Role: PharmaMar, AstraZeneca, Clovis Oncology, GlaxoSmithKline, MSD, Genmab, Amgen, Seattle Genetics, Immunogen, Merck Serono, Oncoinvest, Corcept Therapeutics, Sutro Biopharma
Speakers' Bureau: AstraZeneca, Clovis Oncology, GlaxoSmithKline, MSD, PharmaMar
Research Funding: PharmaMar (Inst), Clovis Oncology (Inst), GlaxoSmithKline (Inst), MSD (Inst), AstraZeneca (Inst), Genmab (Inst), Seattle Genetics (Inst), Immunogen (Inst), Incyte (Inst), Novartis (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, PharmaMar, AstraZeneca, Clovis Oncology, GlaxoSmithKline
Uncompensated Relationships: Gynecologic Cancer InterGroup
Paul Bessette
Consulting or Advisory Role: Merck, Eisai
Research Funding: Tesaro, Merck, AstraZeneca, Clovis Oncology
Sharad Ghamande
Consulting or Advisory Role: Seattle Genetics
Speakers' Bureau: Tesaro/GSK, Eisai
Research Funding: Jounce Therapeutics (Inst), Astellas Pharma (Inst), Akeso Biopharma (Inst), Merck Serono (Inst), Incyte (Inst), Ellipses Pharma (Inst), Aravive (Inst), GlaxoSmithKline (Inst), Merck (Inst), Roche (Inst), Genentech (Inst), Takeda (Inst), Seattle Genetics (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), AbbVie (Inst), Tesaro (Inst)
Diane Provencher
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Research Funding: AstraZeneca, AbbVie
Emily Prendergast
Consulting or Advisory Role: GOG Foundation, HERON
Oladapo Yeku
Honoraria: Medscape
Consulting or Advisory Role: Celldex, GIMV NV, TigaTx Inc, hC Bioscience
Michael Schenker
Research Funding: Bristol Myers Squibb, Roche, Amgen, MSD, Pfizer/EMD Serono, Lilly, Astellas Pharma, AstraZeneca, GlaxoSmithKline, Regeneron, Novartis, AbbVie, Gilead Sciences, Sanofi/Regeneron, Mylan, BIOVEN, Clovis Oncology, Tesaro, BeiGene, Five Prime Therapeutics
Travel, Accommodations, Expenses: Bristol Myers Squibb
Joyce N. Barlin
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, OncoC4
Speakers' Bureau: AstraZeneca, Clovis Oncology, Merck
Toon Van Gorp
Consulting or Advisory Role: Eisai Europe (Inst), OncXerna Therapeutics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), MSD/Merck (Inst)
Research Funding: Amgen (Inst), Roche (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: MSD/Merck (Inst), Immunogen (Inst), PharmaMar (Inst), AstraZeneca (Inst)
Gabriel Lindahl
Honoraria: GlaxoSmithKline
Travel, Accommodations, Expenses: GlaxoSmithKline
Dearbhaile C. Collins
Honoraria: Pfizer, Genmab, Takeda, Amgen, Seattle Genetics
Consulting or Advisory Role: Seattle Genetics, MSD
Research Funding: Pfizer (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Kathleen Moore
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: GOG Partners, NRG Oncology (Inst)
Honoraria: Research To Practice, Prime Oncology, Physicans' Education Resource, Great Debates and Updates
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, Tesaro (Inst), VBL Therapeutics, Merck, Aravive, Eisai, Vavotar Life Sciences, Mersana (Inst), Myriad Genetics, Alkermes (Inst), Blueprint Pharmaceuticals (Inst), GlaxoSmithKline/Tesaro (Inst), I-Mab (Inst), InxMed (Inst), Mereo BioPharma (Inst), OncXerna Therapeutics, Onconova Therapeutics, Novartis
Research Funding: PTC Therapeutics (Inst), Lilly (Inst), Merck (Inst), Tesaro (Inst), Genentech (Inst), Clovis Oncology (Inst), Lilly Foundation (Inst), Regeneron (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Verastem (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Agenus (Inst), Takeda (Inst), Forty Seven (Inst), Stem CentRx (Inst), Immunogen (Inst), Bayer (Inst), Novogen (Inst), AbbVie/Stemcentrx (Inst), Artios (Inst), Bolt Biotherapeutics (Inst), Amgen (Inst), Daiichi Sankyo/Lilly (Inst), Cyteir (Inst), Immunocore (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: GOG Partners (Inst)
Frederik Marme
Honoraria: Roche/Genentech, Novartis, Pfizer, AstraZeneca, Tesaro, Clovis Oncology, Eisai, Celgene, Genomic Health, PharmaMar, Amgen, MSD Oncology, Immunomedics (Inst), Seattle Genetics, Myriad Genetics, Pierre Fabre, GlaxoSmithKline, Agendia, Lilly, Gilead Sciences
Consulting or Advisory Role: AstraZeneca (Inst), Tesaro, Pfizer, Roche (Inst), Genomic Health, CureVac, Amgen, Vaccibody (Inst), Immunomedics (Inst), Eisai, GlaxoSmithKline, Gilead Sciences
Research Funding: Roche/Genentech (Inst), Novartis (Inst), AstraZeneca (Inst), Tesaro (Inst), Clovis Oncology (Inst), MSD Oncology (Inst), Vaccibody (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, AstraZeneca
Shannon N. Westin
This author is a Social Media Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Roche, AstraZeneca, Genentech, Medscape, Clovis Oncology, Gerson Lehrman Group, Vaniam Group, Merck, BioAscent, Curio Science, OncLive, Targeted Oncology, Curio Science, GlaxoSmithKline, Eisai, Zentalis, Agenus, EQRX, Lilly, Vincerx Pharma, Mereo BioPharma, Immunogen, Mersana
Research Funding: AstraZeneca (Inst), Novartis (Inst), Bayer (Inst), Cotinga Pharmaceuticals (Inst), Clovis Oncology (Inst), Roche/Genentech (Inst), GOG Foundation (Inst), Mereo BioPharma (Inst), Bio-Path Holdings, Inc (Inst), GlaxoSmithKline (Inst), OncXerna Therapeutics (Inst), Zentalis (Inst)
Iain A. McNeish
Honoraria: Clovis Oncology, AstraZeneca
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, Carrick Therapeutics, Roche, BeiGene, Scancell Ltd, GlaxoSmithKline, Epsila Bio
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Danny Shih
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Travel, Accommodations, Expenses: Clovis Oncology
Kevin K. Lin
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Travel, Accommodations, Expenses: Clovis Oncology
Sandra Goble
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Stephanie Hume
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Travel, Accommodations, Expenses: Clovis Oncology
Keiichi Fujiwara
Honoraria: Kyowa Hakko Kirin, Zeria Pharmaceutical, Nippon Kayaku, Chugai Pharma, Eisai, Taiho Pharmaceutical, Daiichi Sankyo, Ono Pharmaceutical, Takeda
Consulting or Advisory Role: MSD, Taiho Pharmaceutical, Eisai, Takeda, Genmab, NanoCarrier
Research Funding: Eisai (Inst), Kaken Pharmaceutical (Inst), Chugai Pharma (Inst), Immunogen (Inst), Oncotherapeutics (Inst), AstraZeneca (Inst), Zeria Pharmaceutical (Inst), Ono Pharmaceutical (Inst), MSD (Inst), Regeneron (Inst), Merck KGaA (Inst), Genmab (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: MSD
Rebecca S. Kristeleit
Honoraria: Clovis Oncology, Roche/Genentech, AstraZeneca, Tesaro, Merck Sharp & Dohme, Basilea Pharmaceutical, Incyte, Shattuck Labs, GlaxoSmithKline
Consulting or Advisory Role: Clovis Oncology, Roche/Genentech, Sotio, Cerulean Pharma, Basilea
Travel, Accommodations, Expenses: Clovis Oncology, Basilea, Valirx
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting, June 3-7, 2022, Chicago, IL.
SUPPORT
Supported by Clovis Oncology Inc; supported in part by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20,014; C.P.).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Requests for deidentified data sets for the results reported in this publication will be made available to qualified researchers following submission of a methodologically sound proposal to medinfo@clovisoncology.com. Data will be made available for such requests following online publication of this article and for 1 year thereafter in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Data will be provided by Clovis Oncology. Clovis Oncology does not share identified participant data or a data dictionary.
AUTHOR CONTRIBUTIONS
Conception and design: Bradley J. Monk, Robert L. Coleman, Tamar Safra, Frederik Marme, Shannon N. Westin, Iain A. McNeish, Kevin K. Lin, Sandra Goble, Stephanie Hume, Keiichi Fujiwara, Rebecca S. Kristeleit
Provision of study materials or patients: Bradley J. Monk, Christine Parkinson, Robert L. Coleman, Paul Bessette, Athina Christopoulou, Diane Provencher, Emily Prendergast, Oladapo Yeku, Michael Schenker, Hung-Hsueh Chou, Toon Van Gorp, Gabriel Lindahl, Dearbhaile C. Collins, Kathleen Moore, Frederik Marme, Shannon N. Westin, Iain A. McNeish, Rebecca S. Kristeleit
Collection and assembly of data: Bradley J. Monk, Christine Parkinson, Myong Cheol Lim, David M. O'Malley, Ana Oaknin, Robert L. Coleman, Domenica Lorusso, Paul Bessette, Sharad Ghamande, Athina Christopoulou, Diane Provencher, Emily Prendergast, Fuat Demirkiran, Olga Mikheeva, Oladapo Yeku, Anita Chudecka-Glaz, Michael Schenker, Ramey D. Littell, Tamar Safra, Hung-Hsueh Chou, Vít Drochýtek, Joyce N. Barlin, Toon Van Gorp, Fred Ueland, Charles Anderson, Kathleen Moore, Frederik Marme, Shannon N. Westin, Danny Shih, Sandra Goble, Stephanie Hume, Keiichi Fujiwara, Rebecca S. Kristeleit
Data analysis and interpretation: Bradley J. Monk, Myong Cheol Lim, David M. O'Malley, Ana Oaknin, Michelle K. Wilson, Robert L. Coleman, Sharad Ghamande, Oladapo Yeku, Michael Schenker, Tamar Safra, Mark A. Morgan, Toon Van Gorp, Gabriel Lindahl, Dearbhaile C. Collins, Kathleen Moore, Frederik Marme, Shannon N. Westin, Iain A. McNeish, Kevin K. Lin, Sandra Goble, Stephanie Hume, Keiichi Fujiwara, Rebecca S. Kristeleit
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA–MONO/GOG-3020/ENGOT-ov45)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Bradley J. Monk
Leadership: US Oncology
Honoraria: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, GOG Foundation, Elevar Therapeutics, Novocure, Gradalis, Karyopharm Therapeutics, Bayer, EMD Serono/Merck, Macrogenics, Sorrento Therapeutics, US Oncology, Myriad Pharmaceuticals, Novartis, OncoC4, Pieris Pharmaceuticals
Consulting or Advisory Role: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Myriad Pharmaceuticals, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure, Bayer, Elevar Therapeutics, EMD Serono/Merck, Gradalis, US Oncology, Novartis, Pieris Pharmaceuticals, OncoC4
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, TESARO/GSK, Merck
Research Funding: Novartis (Inst), Amgen (Inst), Genentech (Inst), Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), Immunogen (Inst), Regeneron (Inst), Nucana (Inst)
Myong Cheol Lim
Consulting or Advisory Role: GI Innovation, Boryung, AstraZeneca, Takeda, CKD Pharm, Genexine, Hospicare
Research Funding: AbbVie (Inst), AstraZeneca (Inst), Amgen (Inst), Astellas Pharma, BeiGene (Inst), Cellid (Inst), CKD Pharm (Inst), Clovis Oncology (Inst), Genexine (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Merck (Inst), MSD (Inst), OncoQuest (Inst), Pfizer (Inst), Roche (Inst), Eisai (Inst)
David M. O'Malley
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, Tesaro, Novocure, Genentech/Roche, Immunogen, GOG Foundation, Translational Genomics Research Institute, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron, Sorrento Therapeutics, Rubius Therapeutics, Elevar Therapeutics, Novartis, Seattle Genetics, BBI Healthcare, Arquer Diagnostics, Toray Industries, Takeda, InxMed, Celsion, Arcus Biosciences, Sutro Biopharma, Novocure, Atossa Therapeutics, Laekna Therapeutics, Onconova Therapeutics, VBL Therapeutics, Vincerx Pharma, Adaptimmune, Roche
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Immunogen (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seattle Genetics (Inst), Iovance Biotherapeutics (Inst), Leap Therapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology Inc (Inst), Acerta Pharma (Inst), Advaxis (Inst), Ajinomoto (Inst), Arcus Biosciences (Inst), Deciphera (Inst), Exelixis (Inst), Roche (Inst), Incyte (Inst), Karyopharm Therapeutics (Inst), Ludwig Institute for Cancer Research (Inst), Novartis (Inst), NovoCure (Inst), OncoQuest (Inst), BeiGene (Inst), Pfizer (Inst), Precision Therapeutics (Inst), Sanofi (Inst), Seattle Genetics (Inst), Sutro Biopharma (Inst), GlaxoSmithKline (Inst), Verastem (Inst)
Ana Oaknin
Consulting or Advisory Role: Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab, Mersana, GSK, Deciphera, Agenus, Corcept Therapeutics, Eisai, EMD Serono, F. Hoffmann-La Roche, Medison, Merck Sharp & Dohme, Novocure, prIME Oncology, Shattuck Labs, Sutro Biopharma, ITeos Therapeutics, Amgen
Research Funding: AbbVie (Inst), Abililty Pharmaceuticals (Inst), Advaxis (Inst), Aeterna Zentaris (Inst), Aprea Therapeutics (Inst), Clovis Oncology Inc (Inst), Eisai (Inst), Roche (Inst), Regeneron (Inst), Agenus (Inst), AstraZeneca (Inst), BeiGene (Inst), Belgian Gynaecological Oncology Group (BGOG) (Inst), Bristol Myers Squibb International Corporation (BMS) (Inst), Corcept Therapeutics (Inst), Immunogen (Inst), Iovance Biotherapeutics (Inst), Iovance Lilly (Inst), Medimmune (Inst), Merck (Inst), Merck Sharp & Dohme (Inst), Mundipharma Research (Inst), Novartis Farmacéutica (Inst), Seagen (Inst), Seattle Genetics (Inst), Sutro Biopharma (Inst), Tesaro (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Michelle K. Wilson
Research Funding: Foundation Medicine (Inst)
Robert L. Coleman
Employment: US Oncology
Leadership: Onxeo
Stock and Other Ownership Interests: McKesson
Consulting or Advisory Role: Clovis Oncology, Genentech/Roche, AstraZeneca/MedImmune, Genmab, Tesaro, OncoMed, Sotio, Oncolytics, AbbVie/Stemcentrx, Immunogen, AbbVie, Agenus, Novocure, Merck, OncXerna Therapeutics, Alkermes, Gradalis, Regeneron
Research Funding: AstraZeneca/MedImmune, Esperance Pharmaceuticals, Array BioPharma, Clovis Oncology, Johnson & Johnson, Merck, Roche/Genentech, Abbott/AbbVie, Immunogen (Inst), Mirati Therapeutics (Inst), Amgen (Inst), Pfizer (Inst), Lilly (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Merck, AstraZeneca/MedImmune, Array BioPharma, Clovis Oncology, Roche/Genentech, Research to Practice, GOG Foundation, Clovis Oncology, Sotio, Vaniam Group
Domenica Lorusso
Consulting or Advisory Role: PharmaMar, AstraZeneca, Clovis Oncology, GlaxoSmithKline, MSD, Genmab, Amgen, Seattle Genetics, Immunogen, Merck Serono, Oncoinvest, Corcept Therapeutics, Sutro Biopharma
Speakers' Bureau: AstraZeneca, Clovis Oncology, GlaxoSmithKline, MSD, PharmaMar
Research Funding: PharmaMar (Inst), Clovis Oncology (Inst), GlaxoSmithKline (Inst), MSD (Inst), AstraZeneca (Inst), Genmab (Inst), Seattle Genetics (Inst), Immunogen (Inst), Incyte (Inst), Novartis (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, PharmaMar, AstraZeneca, Clovis Oncology, GlaxoSmithKline
Uncompensated Relationships: Gynecologic Cancer InterGroup
Paul Bessette
Consulting or Advisory Role: Merck, Eisai
Research Funding: Tesaro, Merck, AstraZeneca, Clovis Oncology
Sharad Ghamande
Consulting or Advisory Role: Seattle Genetics
Speakers' Bureau: Tesaro/GSK, Eisai
Research Funding: Jounce Therapeutics (Inst), Astellas Pharma (Inst), Akeso Biopharma (Inst), Merck Serono (Inst), Incyte (Inst), Ellipses Pharma (Inst), Aravive (Inst), GlaxoSmithKline (Inst), Merck (Inst), Roche (Inst), Genentech (Inst), Takeda (Inst), Seattle Genetics (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), AbbVie (Inst), Tesaro (Inst)
Diane Provencher
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Research Funding: AstraZeneca, AbbVie
Emily Prendergast
Consulting or Advisory Role: GOG Foundation, HERON
Oladapo Yeku
Honoraria: Medscape
Consulting or Advisory Role: Celldex, GIMV NV, TigaTx Inc, hC Bioscience
Michael Schenker
Research Funding: Bristol Myers Squibb, Roche, Amgen, MSD, Pfizer/EMD Serono, Lilly, Astellas Pharma, AstraZeneca, GlaxoSmithKline, Regeneron, Novartis, AbbVie, Gilead Sciences, Sanofi/Regeneron, Mylan, BIOVEN, Clovis Oncology, Tesaro, BeiGene, Five Prime Therapeutics
Travel, Accommodations, Expenses: Bristol Myers Squibb
Joyce N. Barlin
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, OncoC4
Speakers' Bureau: AstraZeneca, Clovis Oncology, Merck
Toon Van Gorp
Consulting or Advisory Role: Eisai Europe (Inst), OncXerna Therapeutics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), MSD/Merck (Inst)
Research Funding: Amgen (Inst), Roche (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: MSD/Merck (Inst), Immunogen (Inst), PharmaMar (Inst), AstraZeneca (Inst)
Gabriel Lindahl
Honoraria: GlaxoSmithKline
Travel, Accommodations, Expenses: GlaxoSmithKline
Dearbhaile C. Collins
Honoraria: Pfizer, Genmab, Takeda, Amgen, Seattle Genetics
Consulting or Advisory Role: Seattle Genetics, MSD
Research Funding: Pfizer (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Kathleen Moore
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: GOG Partners, NRG Oncology (Inst)
Honoraria: Research To Practice, Prime Oncology, Physicans' Education Resource, Great Debates and Updates
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, Tesaro (Inst), VBL Therapeutics, Merck, Aravive, Eisai, Vavotar Life Sciences, Mersana (Inst), Myriad Genetics, Alkermes (Inst), Blueprint Pharmaceuticals (Inst), GlaxoSmithKline/Tesaro (Inst), I-Mab (Inst), InxMed (Inst), Mereo BioPharma (Inst), OncXerna Therapeutics, Onconova Therapeutics, Novartis
Research Funding: PTC Therapeutics (Inst), Lilly (Inst), Merck (Inst), Tesaro (Inst), Genentech (Inst), Clovis Oncology (Inst), Lilly Foundation (Inst), Regeneron (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Verastem (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Agenus (Inst), Takeda (Inst), Forty Seven (Inst), Stem CentRx (Inst), Immunogen (Inst), Bayer (Inst), Novogen (Inst), AbbVie/Stemcentrx (Inst), Artios (Inst), Bolt Biotherapeutics (Inst), Amgen (Inst), Daiichi Sankyo/Lilly (Inst), Cyteir (Inst), Immunocore (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: GOG Partners (Inst)
Frederik Marme
Honoraria: Roche/Genentech, Novartis, Pfizer, AstraZeneca, Tesaro, Clovis Oncology, Eisai, Celgene, Genomic Health, PharmaMar, Amgen, MSD Oncology, Immunomedics (Inst), Seattle Genetics, Myriad Genetics, Pierre Fabre, GlaxoSmithKline, Agendia, Lilly, Gilead Sciences
Consulting or Advisory Role: AstraZeneca (Inst), Tesaro, Pfizer, Roche (Inst), Genomic Health, CureVac, Amgen, Vaccibody (Inst), Immunomedics (Inst), Eisai, GlaxoSmithKline, Gilead Sciences
Research Funding: Roche/Genentech (Inst), Novartis (Inst), AstraZeneca (Inst), Tesaro (Inst), Clovis Oncology (Inst), MSD Oncology (Inst), Vaccibody (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, AstraZeneca
Shannon N. Westin
This author is a Social Media Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Roche, AstraZeneca, Genentech, Medscape, Clovis Oncology, Gerson Lehrman Group, Vaniam Group, Merck, BioAscent, Curio Science, OncLive, Targeted Oncology, Curio Science, GlaxoSmithKline, Eisai, Zentalis, Agenus, EQRX, Lilly, Vincerx Pharma, Mereo BioPharma, Immunogen, Mersana
Research Funding: AstraZeneca (Inst), Novartis (Inst), Bayer (Inst), Cotinga Pharmaceuticals (Inst), Clovis Oncology (Inst), Roche/Genentech (Inst), GOG Foundation (Inst), Mereo BioPharma (Inst), Bio-Path Holdings, Inc (Inst), GlaxoSmithKline (Inst), OncXerna Therapeutics (Inst), Zentalis (Inst)
Iain A. McNeish
Honoraria: Clovis Oncology, AstraZeneca
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, Carrick Therapeutics, Roche, BeiGene, Scancell Ltd, GlaxoSmithKline, Epsila Bio
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Danny Shih
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Travel, Accommodations, Expenses: Clovis Oncology
Kevin K. Lin
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Travel, Accommodations, Expenses: Clovis Oncology
Sandra Goble
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Stephanie Hume
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Travel, Accommodations, Expenses: Clovis Oncology
Keiichi Fujiwara
Honoraria: Kyowa Hakko Kirin, Zeria Pharmaceutical, Nippon Kayaku, Chugai Pharma, Eisai, Taiho Pharmaceutical, Daiichi Sankyo, Ono Pharmaceutical, Takeda
Consulting or Advisory Role: MSD, Taiho Pharmaceutical, Eisai, Takeda, Genmab, NanoCarrier
Research Funding: Eisai (Inst), Kaken Pharmaceutical (Inst), Chugai Pharma (Inst), Immunogen (Inst), Oncotherapeutics (Inst), AstraZeneca (Inst), Zeria Pharmaceutical (Inst), Ono Pharmaceutical (Inst), MSD (Inst), Regeneron (Inst), Merck KGaA (Inst), Genmab (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: MSD
Rebecca S. Kristeleit
Honoraria: Clovis Oncology, Roche/Genentech, AstraZeneca, Tesaro, Merck Sharp & Dohme, Basilea Pharmaceutical, Incyte, Shattuck Labs, GlaxoSmithKline
Consulting or Advisory Role: Clovis Oncology, Roche/Genentech, Sotio, Cerulean Pharma, Basilea
Travel, Accommodations, Expenses: Clovis Oncology, Basilea, Valirx
No other potential conflicts of interest were reported.
REFERENCES
- 1.Coleman RL, Fleming GF, Brady MF, et al. : Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 381:2403-2415, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Martin A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Ray-Coquard I, Pautier P, Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Moore KN, Colombo N, et al. : Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 22:1721-1731, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Poveda A, Floquet A, Ledermann JA, et al. : Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 22:620-631, 2021 [DOI] [PubMed] [Google Scholar]
- 7.del Campo JM, Matulonis UA, Malander S, et al. : Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. J Clin Oncol 37:2968-2973, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman RL, Oza AM, Lorusso D, et al. : Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:1949-1961, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monk BJ, Coleman RL, Fujiwara K, et al. : ATHENA (GOG-3020/ENGOT-ov45): A randomized, phase III trial to evaluate rucaparib as monotherapy (ATHENA–MONO) and rucaparib in combination with nivolumab (ATHENA–COMBO) as maintenance treatment following frontline platinum-based chemotherapy in ovarian cancer. Int J Gynecol Cancer 31:1589-1594, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergote I, Coleman RL, Pignata S, et al. : Joint ENGOT and GOG Foundation requirements for trials with industry partners. Gynecol Oncol 154:255-258, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Qiu Z, Guo W, Lynch G: On generalized fixed sequence procedures for controlling the FWER. Stat Med 34:3968-3983, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Stone A, Gebski V, Davidson R, et al. : Exaggeration of PFS by blinded, independent, central review (BICR). Ann Oncol 30:332-338, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Kristeleit R, Lisyanskaya A, Fedenko A, et al. : Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): An international, open-label, randomised, phase 3 trial. Lancet Oncol 23:465-478, 2022 [DOI] [PubMed] [Google Scholar]
- 14.Ledermann JA, Oza AM, Lorusso D, et al. : Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): Postprogression outcomes and updated safety from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol 21:710-722, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swisher EM, Lin KK, Oza AM, et al. : Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol 18:75-87, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Mirza MR, Gonzalez Martin A, Graybill W, et al. : Evaluation of an individualized starting-dose of niraparib in the PRIMA/ENGOT-OV26/GOG-3012 study. J Clin Oncol 38, 2020. (suppl 15; abstr 6050) [Google Scholar]
- 17.European Medicines Agency : EPAR—Assessment Report—Variation (Zejula-H-C-003943-II-0019—EMA/531223/2020). 2022. https://www.ema.europa.eu/documents/variation-report/zejula-h-c-003943-ii-0019-epar-assessment-report-variation_en.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for deidentified data sets for the results reported in this publication will be made available to qualified researchers following submission of a methodologically sound proposal to medinfo@clovisoncology.com. Data will be made available for such requests following online publication of this article and for 1 year thereafter in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Data will be provided by Clovis Oncology. Clovis Oncology does not share identified participant data or a data dictionary.