PURPOSE
Evidence-based guidelines recommend cascade genetic counseling and testing for hereditary cancer syndromes, providing relatives the opportunity for early detection and prevention of cancer. The current standard is for patients to contact and encourage relatives (patient-mediated contact) to undergo counseling and testing. Direct relative contact by the medical team or testing laboratory has shown promise but is complicated by privacy laws and lack of infrastructure. We sought to compare outcomes associated with patient-mediated and direct relative contact for hereditary cancer cascade genetic counseling and testing in the first meta-analysis on this topic.
MATERIALS AND METHODS
We conducted a systematic review and meta-analysis in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PROSPERO No.: CRD42020134276). We searched key electronic databases to identify studies evaluating hereditary cancer cascade testing. Eligible trials were subjected to meta-analysis.
RESULTS
Eighty-seven studies met inclusion criteria. Among relatives included in the meta-analysis, 48% (95% CI, 38 to 58) underwent cascade genetic counseling and 41% (95% CI, 34 to 48) cascade genetic testing. Compared with the patient-mediated approach, direct relative contact resulted in significantly higher uptake of genetic counseling for all relatives (63% [95% CI, 49 to 75] v 35% [95% CI, 24 to 48]) and genetic testing for first-degree relatives (62% [95% CI, 49 to 73] v 40% [95% CI, 32 to 48]). Methods of direct contact included telephone calls, letters, and e-mails; respective rates of genetic testing completion were 61% (95% CI, 51 to 70), 48% (95% CI, 37 to 59), and 48% (95% CI, 45 to 50).
CONCLUSION
Most relatives at risk for hereditary cancer do not undergo cascade genetic counseling and testing, forgoing potentially life-saving medical interventions. Compared with patient-mediated contact, direct relative contact increased rates of cascade genetic counseling and testing, arguing for a shift in the care delivery paradigm, to be confirmed by randomized controlled trials.
INTRODUCTION
Cascade genetic testing is the process of extending genetic testing to the at-risk relatives of an individual found to carry a germline pathogenic variant. In families with a hereditary cancer syndrome, identifying asymptomatic carriers offers the opportunity to reduce cancer incidence, morbidity, and mortality and is cost-effective.1-10 The Centers for Disease Control and Prevention Office of Public Health Genomics have designated cascade genetic testing as a tier one genomic application for hereditary breast and ovarian cancers and Lynch syndrome.11 Furthermore, mathematical modeling suggests that the combination of genetic testing at the time of cancer diagnosis and cascade testing by 70% of at-risk relatives could identify all four million individuals with a cancer-associated pathogenic variant in the United States in less than a decade.12 However, fewer than 20% of individuals with a hereditary cancer syndrome are aware of their underlying pathogenic variant.2,3,13,14 Furthermore, when pathogenic variants are identified, families face many barriers to completion of cascade testing, likely contributing to underutilization of this critical service.15-20 Finally, racial and ethnic minorities experience even more pronounced under-recognition of hereditary cancer syndromes,21-25 leading the American Association for Cancer Research, the American Cancer Society, ASCO, and the National Cancer Institute all to cite a critical need to improve genetic cancer risk assessment and testing for minority populations.26
CONTEXT
Key Objective
Does direct relative contact for hereditary cancer syndrome cascade testing improve rates of genetic counseling and testing as compared with the current standard of care, patient-mediated family contact?
Knowledge Generated
Currently, the majority of people with a hereditary cancer syndrome are not aware and, therefore, cannot use potentially life-saving medical interventions. Our study found that, in families with hereditary cancer syndromes, direct relative contact by the medical team or testing laboratory resulted in higher uptake of genetic counseling and genetic testing among at-risk relatives compared with the patient-mediated approach.
Relevance
Currently, patients shoulder the responsibility of disseminating information on hereditary cancer syndromes among relatives, resulting in genetic counseling and testing by only about one third of at-risk relatives. Our findings demonstrate the power of direct relative contact, arguing for a shift in the care delivery paradigm, to be confirmed by randomized controlled trials.
A growing body of literature suggests that health system–led direct contact of relatives is acceptable to clinicians and patients and more successful than patient-mediated contact.27-30 However, current Health Insurance Portability and Accountability Act privacy laws prohibit health care providers from disclosing genetic information to relatives. As a result, the affected patients must shoulder the burden for coordinating cascade testing for their families.31 This is often complicated by the difficulty in communicating complex health information, strained family relationships, and competing demands, as patients may also be coping with a new cancer diagnosis that prompted their genetic testing.32 Further complicating matters, health care systems, and health care policies have been largely oriented toward treatment of disease and not prevention. The primary objective of the current study was to conduct a systematic review and meta-analysis of the literature on the success of cascade genetic counseling and testing among all relatives for hereditary cancer syndromes via patient-mediated and direct relative contact. Secondary aims were to explore rates of relative disclosure and the influence of sex, degree of relation, race, ethnicity, specific cancer syndrome, and insurance status on completion of cascade genetic counseling and testing. Although similar systematic reviews have been completed,28-30 to our knowledge, this study is the first meta-analysis.
MATERIALS AND METHODS
Overview
The current study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and was preregistered with PROSPERO (registration No.: CRD42020134276).33 A comprehensive literature search was conducted on July 23, 2021, using the following bibliographic databases from inception: Ovid MEDLINE (In-Process and Other Non-Indexed Citations and Ovid MEDLINE 1946 to present), Ovid EMBASE (1974 to present), and Cochrane Library (Wiley). No article type, date, or language restrictions were included in the search. Search concepts included cascade screening, genetic counseling, and cancer. The full Ovid MEDLINE search strategy is available in Appendix Table A1 (online only).
Inclusion and Exclusion Criteria
Eligible manuscripts included all primary English language research studies with the objective of evaluating cascade genetic counseling and testing for hereditary cancer syndromes, including a focus on disclosure of results to relatives, completion of genetic counseling, and completion of genetic testing. Commentaries, systematic reviews, meta-analyses, and case reports were not included. Studies were evaluated to determine if cascade testing was patient-mediated or via direct relative contact. See the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for a comprehensive review of reasons for publication exclusion (Fig 1).
FIG 1.
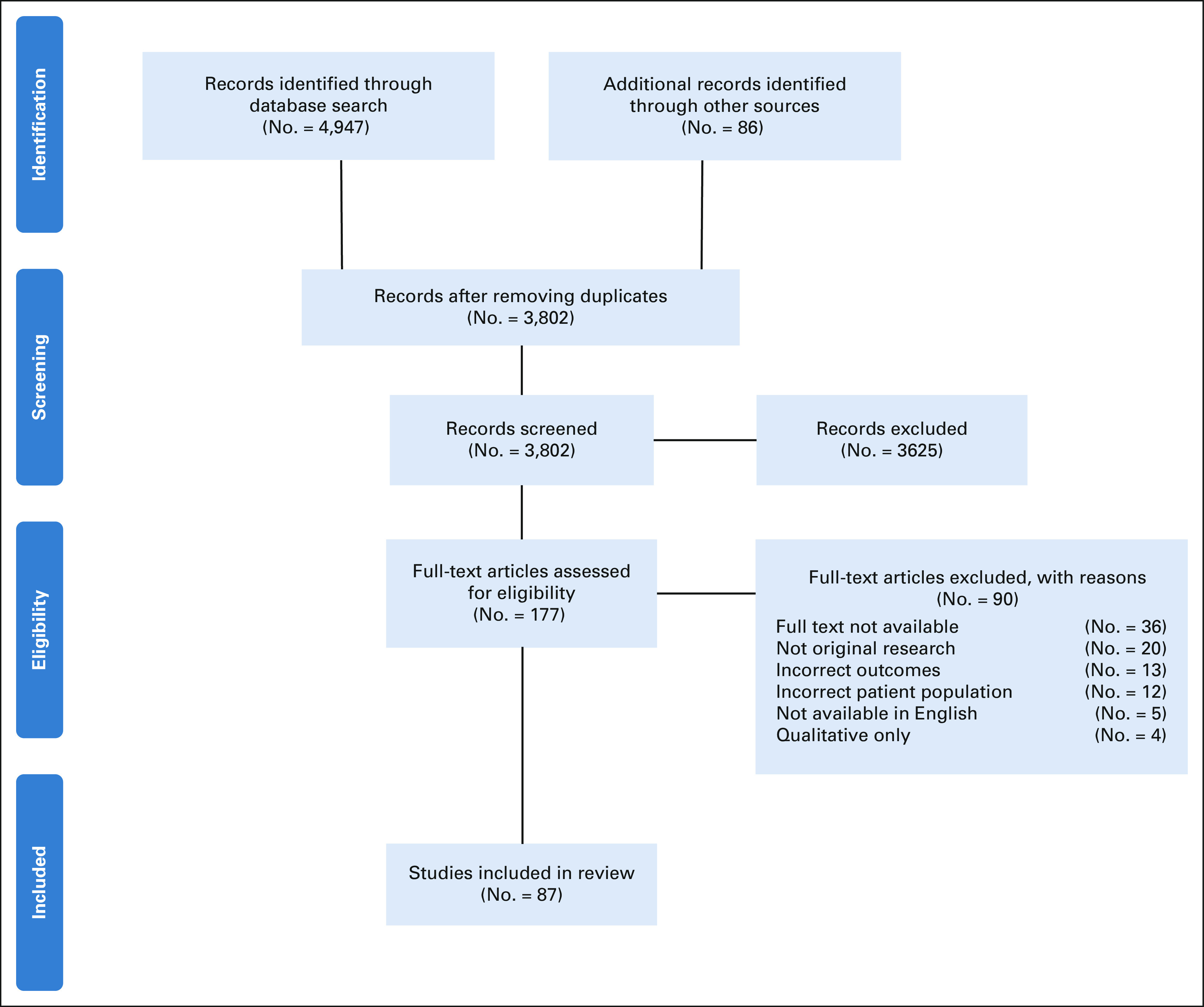
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data Extraction
Manuscripts were independently evaluated by two reviewers, and disagreements were discussed with a third reviewer. Data were extracted by one reviewer and checked by two additional reviewers. Studies were coded according to a priori–specified characteristics, including study type, intervention, participant characteristics, and risk of bias.
Risk of Bias and Analytic Strategy
The Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) was applied to assess the risk of bias for studies reporting on direct relative contact for cascade testing.34 The Joanna Briggs Institute Critical Appraisal Checklist for cohort studies and the Joanna Briggs Institute Critical Appraisal Checklist for analytical cross-sectional studies, as appropriate, were applied to studies reporting on proband-mediated contact of relatives to determine the extent to which studies addressed risk of bias in their design, conduct, and analysis.35 All risk of bias and ratings assessments were independently assessed by two reviewers, and disagreements were discussed with a third reviewer.
Statistical Analysis
Meta-analyses for the proportion of at-risk relatives that completed genetic counseling and genetic testing were conducted using R software (Version 3.6.1[07/05/19], R Foundation for Statistical Computing, Vienna, Austria). Statistical heterogeneity was tested through the chi-square test (ie, Cochrane Q test), and a P value ≤ .20 was used to indicate the presence of heterogeneity. Statistical heterogeneity was also assessed by the inconsistency statistic (I2). A random effects analysis was used to calculate pooled proportions and means. The random effects analysis is more conservative and allows for more variability in the individual study proportion estimates when generating the pooled proportion. The pooled proportion was calculated using the Freeman-Tukey Double arcsine transformation, and the 95% CI was calculated using the Clopper-Pearson interval. The DerSimonian-Laird estimator was used to estimate the between-study variance. For the outcome proportions of interest, the results of each study were expressed as binary proportions with exact 95% CIs. For each meta-analysis, a funnel plot was constructed and reviewed, displaying the study proportion against study precision, estimated by the standard error, to assess for publication bias. Sensitivity analyses were performed for the outcomes of interest (rates of cascade genetic counseling and genetic testing) to investigate the impact of date of publication, study country of origin, study design, method of data collection, and study quality on our aggregated results.
RESULTS
Study Characteristics
Eighty-seven publications of original research were included in our systematic review. Seventy-one observational studies provided data that allowed for inclusion in the meta-analysis (29 prospective studies, 27 cross-sectional studies, and 15 retrospective studies). Seventeen studies report on rates of cascade genetic counseling including six studies on direct relative contact, eight studies on proband-mediated relative contact, and three on both modes of contact. Fifty studies report on rates of cascade genetic testing including 12 studies on direct relative contact, 34 studies on proband-mediated relative contact, and four on both modes of contact. Thirty studies report on rates of disclosure of genetic information by the probands to their at-risk relatives (Appendix Tables A2 and A3, online only).
Cumulative Patient Characteristics
A total of 14,736 probands and 33,223 at-risk relatives were evaluated for completion of cascade genetic counseling and testing. Study publication dates spanned from 1996 to 2021 and included 21 countries: United States (37), the Netherlands (eight), United Kingdom (five), France (five), Australia (four), Finland (four), Norway (three), Belgium (three), Singapore (two), Canada (four), Israel (two), Trinidad and Tobago, the Bahamas, Germany, Spain, Ireland, Italy, Korea, Malaysia, South Africa, Sweden, and both United States and Canada (one each). Across all studies, the median reported proband age was 51.5 years (range, 18-93 years) and the relative age was 47.4 years (18-85 years). Fifty-nine studies included information on the proband's sex. Among the 13,266 probands in these studies, 10,854 (81.8%) were female and 2,412 (18.2%) were male. Forty-eight studies included information on relatives' sex. Among the 19,590 relatives in these studies, 10,777 (55.0%) were female and 8,813 (45.0%) were male.
Thirty-five studies included information on proband race and ethnicity. Among the 9,686 probands in these studies, 6,777 (70.0%) identified as White, 1,735 (17.9%) as Hispanic/Latino, 604 (6.2%) as Asian, 221 (2.3%) as Black, and 7 (0.1%) as Native American. Among this group, 1,816 (18.7%) probands identified as Ashkenazi Jewish. Ten studies included information on relatives' race and ethnicity.15,36-44 Among the 2,543 relatives included in these studies, 1,876 (73.8%) identified as White, 394 (15.5%) as Asian, 195 (7.7%) as Hispanic/Latino, 58 (2.3%) as Black, and 20 (0.8%) as Native American. Among this group, 137 (5.4%) relatives identified as Ashkenazi Jewish. Further proband and relative characteristics for each study included in the systematic review are reported in Appendix Table A2.
Cumulative Rates of Cascade Genetic Counseling and Testing
Among the cohort of all patients included in the meta-analysis, 48% (95% CI, 38 to 58) of relatives underwent cascade genetic counseling. Female relatives had higher rates of completion of genetic counseling compared with male relatives (60% [95% CI, 43 to 75] v 31% [95% CI, 20 to 44]).
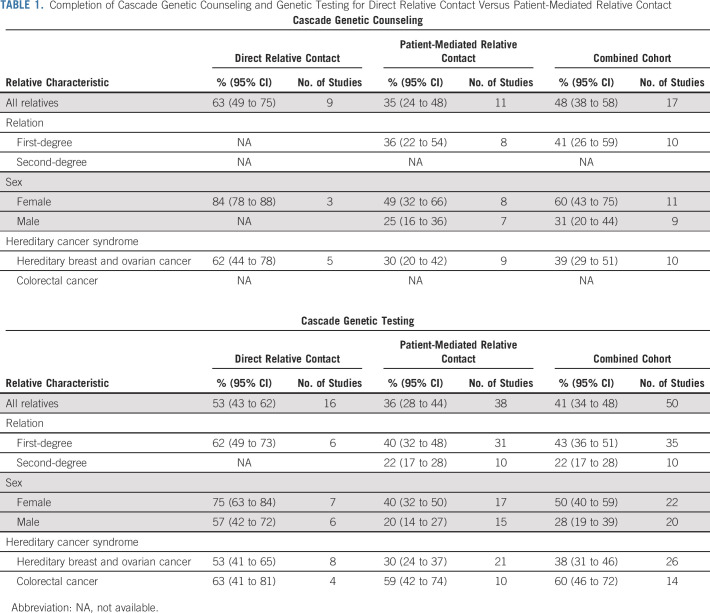
Among the cohort of all patients included in the meta-analysis, 41% (95% CI, 34 to 48) of relatives underwent cascade genetic testing. The method of measuring completion of cascade genetic testing differed between studies. Thirty-nine studies included review of genetic testing as a part of the study design, eight studies relied on self-report by the proband, one study relied on self-report by the relatives, one study used both reviews of results as part of study design and proband self-report, and one study did not describe the method of outcome measurement (Appendix Table A3). First-degree relatives were significantly more likely to complete genetic testing compared with second-degree relatives (43% [95% CI, 36 to 51] v 22% [95% CI, 17 to 28]). Female relatives were significantly more likely to complete genetic testing than male relatives (50% [95% CI, 40 to 59] v 28% [95% CI, 19 to 39]). Relatives in families with a colorectal cancer syndrome had higher rates of genetic testing compared with families with hereditary breast and ovarian cancers (60% [95% CI, 46 to 72] v 38% [95% CI, 31 to 46]; Table 1).
TABLE 1.
Completion of Cascade Genetic Counseling and Genetic Testing for Direct Relative Contact Versus Patient-Mediated Relative Contact
Eighteen studies included information on proband disclosure of the pathogenic variant to relatives. Among 3,779 probands with data available on disclosure, 94% (95% CI, 88 to 97) reported disclosing their genetic test results to at least one at-risk relative. Nineteen studies included information on relatives to whom probands disclosed information about the pathogenic variant identified. Among 12,751 at-risk relatives (determined either via interview with at-risk relatives or review of a proband's pedigree), 72% (95% CI, 64 to 79) were informed of their genetic risk by the proband.
Other Studies
Other studies explored covariates, but the data were not presented in a manner where they could be quantitatively meta-analyzed. Two studies evaluated the impact of race and ethnicity on cascade testing and found that relatives in White families were more likely to complete cascade genetic testing compared with relatives from Black, Asian, Native American, and Hispanic/Latino families.15,37 One study evaluated insurance status and cascade testing and found that being insured versus uninsured was associated with higher uptake of cascade genetic testing (odds ratio, 3.74 [95% CI, 2.06 to 6.80]).39
Thirteen studies reported on the impact of relative age and uptake of cascade genetic testing. Among these studies, ten demonstrated that older relative age was associated with increased likelihood of completing cascade testing19,45-52,119 and three suggested the opposite, that younger relatives were more likely to complete cascade testing.36,41,53 Ten studies reported on the impact of parenthood and cascade testing, with seven studies reporting that probands with children were more likely to complete cascade testing19,49-51,53-55 and three studies demonstrating no association between parenthood and cascade testing uptake.46,56,57 Three studies evaluated the role of familial support and found that relatives who reported greater family support and those belonging to more cohesive families were more likely to undergo cascade genetic testing15,36,58 (Table 2).
TABLE 2.
Studies of Proband and Relative Characteristics That Influence Cascade Genetic Testing for Hereditary Cancer Syndromes
Patient-Mediated Cascade Genetic Counseling and Testing
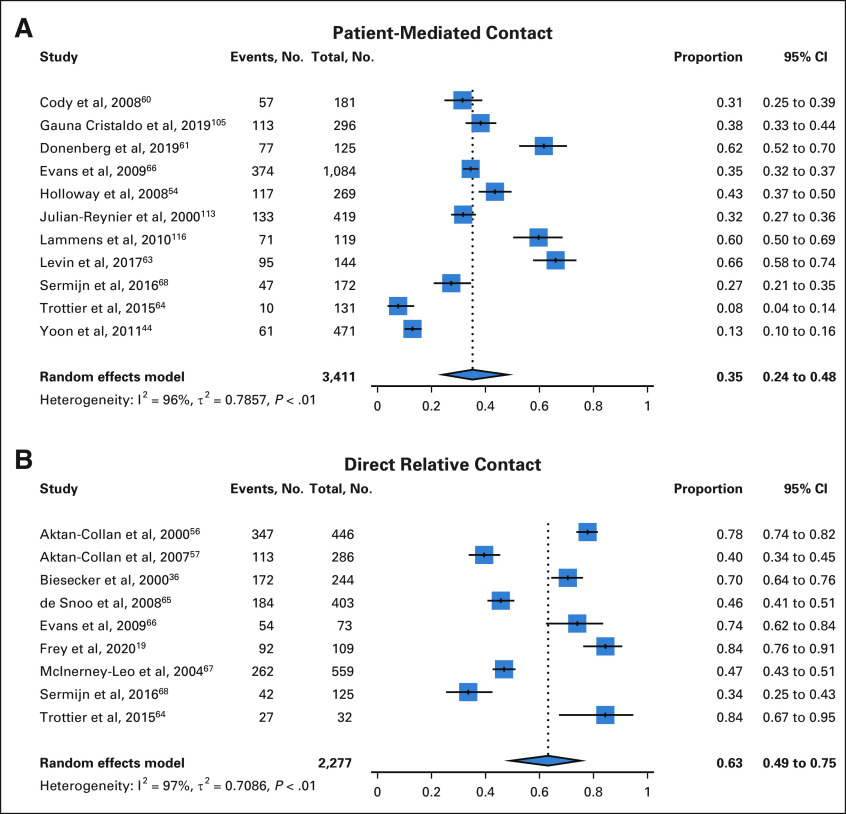
Thirty-eight studies evaluated patient-mediated cascade genetic counseling and testing for hereditary cancer syndromes, whereby the responsibility for communicating with relatives is placed on the affected proband/patient. Among 3,411 relatives with genetic counseling data available, 35% (95% CI, 24 to 48) completed genetic counseling with the patient-mediated approach (Fig 2). Rates of genetic counseling were higher for female versus male relatives (49% [95% CI, 32 to 66] v 25% [95% CI, 16 to 36]).
FIG 2.
Cascade genetic counseling: pooled proportions with patient-mediated contact (A) and direct relative contact (B).
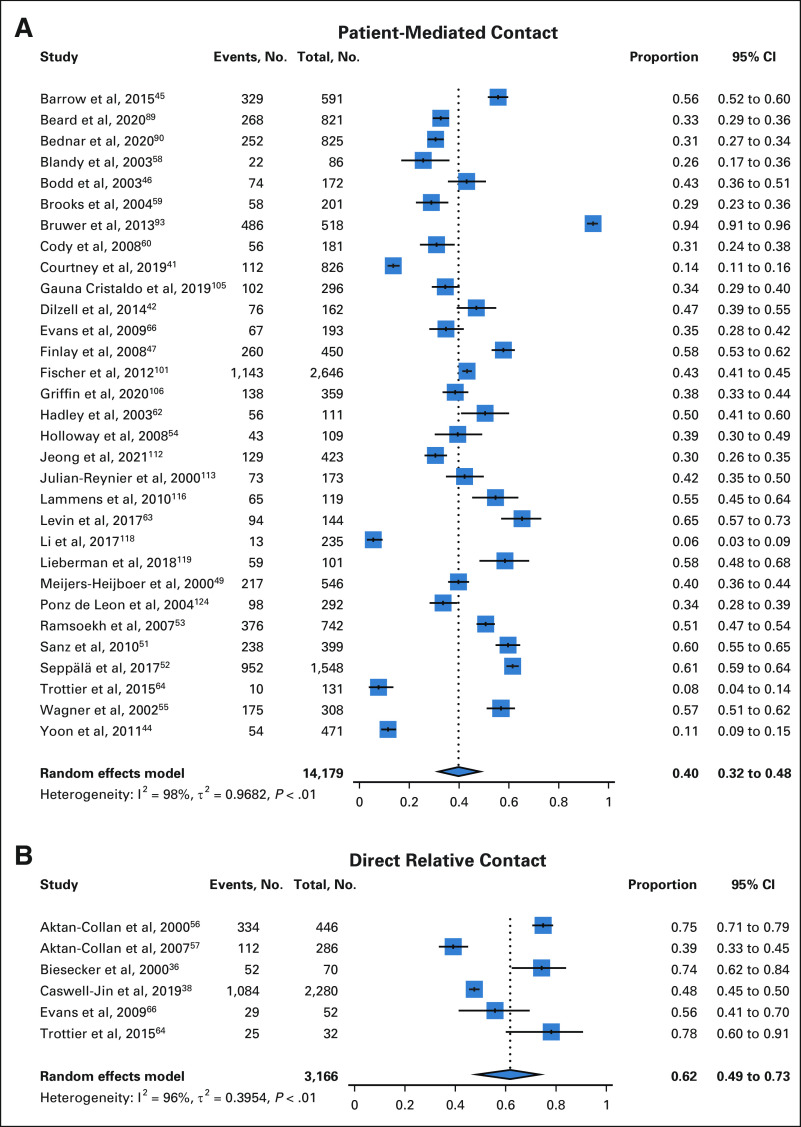
Among 21,519 relatives with data available on completion of patient-mediated cascade genetic testing, 36% (95% CI, 28 to 44) completed genetic testing (Fig 3). Rates of genetic testing were significantly higher for first-degree versus second-degree relatives (40% [95% CI, 32 to 48] v 22% [95% CI, 17 to 28]) and female versus male relatives (40% [95% CI, 32 to 50] v 20% [95% CI, 14 to 27]).
FIG 3.
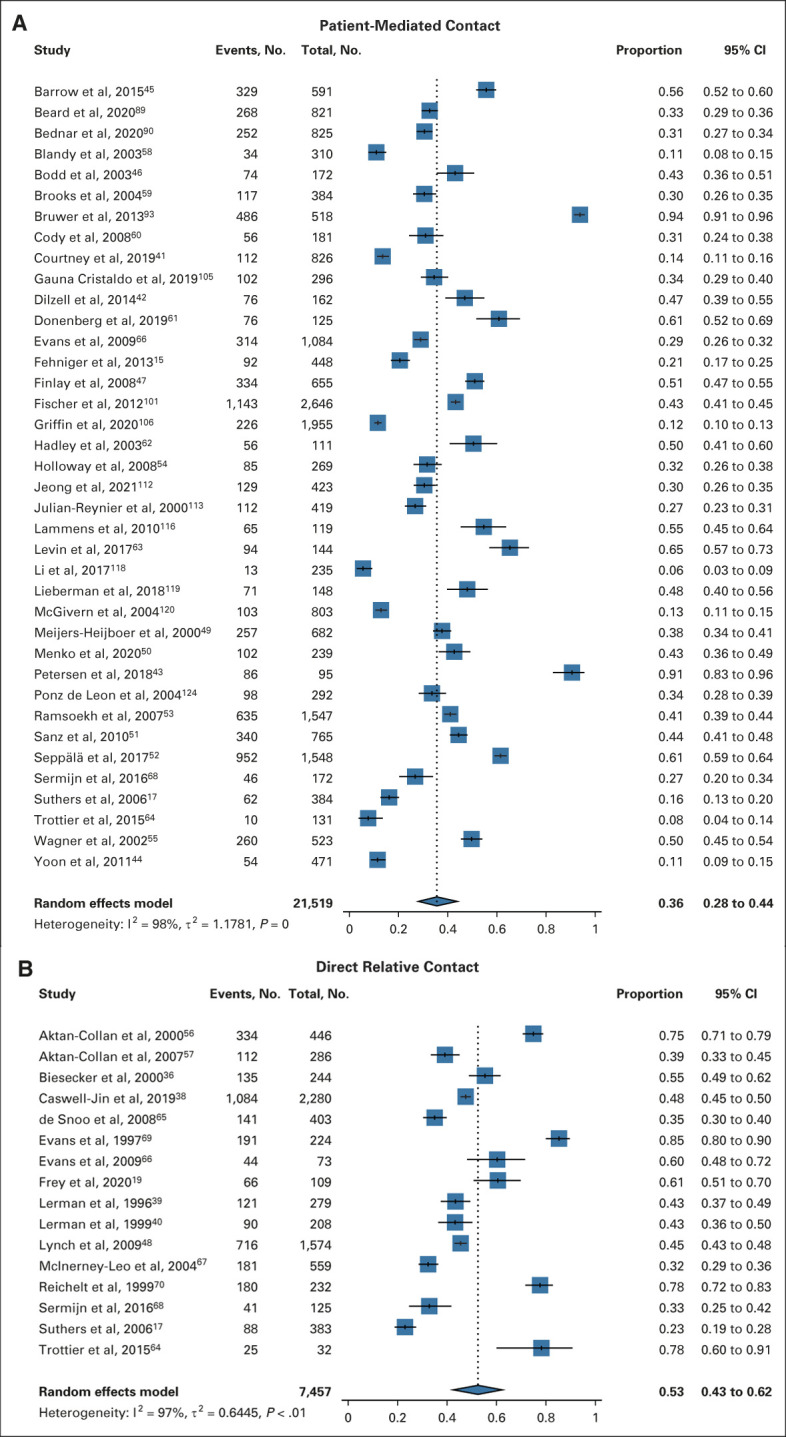
Cascade genetic testing: pooled proportions with patient-mediated contact (A) and direct relative contact (B).
Eleven studies evaluated patient-mediated cascade testing whereby the patient was provided by the medical team with an informational letter to share with at-risk relatives.42,47,49,53,55,59-64 Provision of a letter resulted in genetic counseling for 37% (95% CI, 15 to 67) of relatives and genetic testing for 41% (95% CI, 31 to 52) of relatives. When a letter was not provided, 34% (95% CI, 24 to 45) of relatives underwent genetic counseling and 33% (95% CI, 24 to 44) underwent genetic testing.
Twenty-one studies included information on cascade testing via patient-mediated relative contact for hereditary breast and ovarian cancer families. In this population, 30% (95% CI, 24 to 37) of relatives completed genetic testing, including 35% (95% CI, 27 to 44) of first-degree relatives, 23% (95% CI, 17 to 31) of second-degree relatives, 37% (95% CI, 28 to 48) of female relatives, and 16% (95% CI, 12 to 22) of male relatives. Ten studies included information on cascade testing via patient-mediated relative contact for colorectal cancer syndrome families. In this population, 59% (95% CI, 42 to 74) of relatives completed genetic testing.
Direct Relative Contact Cascade Genetic Counseling and Testing
Sixteen studies evaluated direct relative contact cascade genetic counseling and/or testing for hereditary cancer syndromes, whereby relatives are contacted by the medical team or testing laboratory. Fifteen studies investigated direct relative contact through outreach by the medical team; one study evaluated an online initiative for direct contact organized by the genetic testing laboratory.38
Among 2,277 relatives with genetic counseling data available, 63% (95% CI, 49 to 75) completed genetic counseling with direct relative contact (Fig 2). Among 7,457 relatives with genetic testing data available, 53% (95% CI, 43 to 62) completed cascade genetic testing with direct relative contact (Fig 3). Rates of genetic testing with direct relative contact were higher for female versus male relatives (75% [95% CI, 63 to 84] v 57% [95% CI, 42 to 72]).
Three methods of direct relative contact were described: (1) letter, (2) e-mail, and (3) telephone call. Direct contact via a letter resulted in genetic counseling for 55% (95% CI, 42 to 68) of relatives and genetic testing for 48% (95% CI, 37 to 59) of relatives.17,36,39,40,48,56,57,65-69 Direct contact via an e-mail resulted in genetic testing for 48% (95% CI, 45 to 50) of relatives.38 Direct contact via a telephone call resulted in genetic counseling for 84% (95% CI, 76 to 91) of relatives and genetic testing for 61% (95% CI, 51 to 70) of relatives.19
Eight studies included information on cascade testing via direct relative contact for hereditary breast and ovarian cancer families.36,39,48,64,66-68,70 Among this group, 53% (95% CI, 41 to 65) of relatives completed genetic testing including 69% (95% CI, 57 to 79) of first-degree relatives, 71% (95% CI, 60 to 81) of female relatives, and 51% (95% CI, 28 to 73) of male relatives. Four studies included information on cascade testing via direct relative contact for colorectal cancer syndrome families.40,56,57,69 Among this group, 63% (95% CI, 41 to 81) of relatives completed genetic testing.
Direct Relative Contact Versus Patient-Mediated Relative Contact
Direct relative contact resulted in genetic counseling for 63% (95% CI, 49 to 75) of relatives versus 35% (95% CI, 24 to 48) with patient-mediated relative contact (Table 1 and Fig 2). None of the included studies evaluating direct relative contact provided information on genetic counseling rates for first-degree versus second-degree relatives. Direct relative contact resulted in genetic testing for 53% (95% CI, 43 to 62) of all relatives versus 36% (95% CI, 28 to 44) with patient-mediated relative contact (Table 1 and Fig 3). For first-degree relatives, direct relative contact resulted in genetic testing of 62% (95% CI, 49 to 73) of relatives versus 40% (95% CI, 32 to 48) with patient-mediated relative contact (Fig 4). Four nonrandomized studies included both direct relative contact and patient-mediated relative contact.17,64,66,68 Among these four studies, direct relative contact resulted in genetic testing for 48% (95% CI, 26 to 70) of relatives versus 20% (95% CI, 10 to 30) with patient-mediated relative contact.
FIG 4.
Cascade genetic testing of first-degree relatives: pooled proportions with patient-mediated contact (A) and direct relative contact (B).
Sensitivity Analyses
Sensitivity analyses were performed for the rates of patient-mediated and direct relative contact cascade genetic counseling and genetic testing for all relatives. Grouping studies by date of publication, study country of origin, study design, method of data collection, and study quality did not change the trends of our aggregate results (Appendix Table A3).
Quality of Evidence/Risk of Bias/Publication Bias
Study quality was assessed using as appropriate ROBINS-I,34 the Joanna Briggs Institute Critical Appraisal Checklist for cohort studies, or the Joanna Briggs Institute Critical Appraisal Checklist for analytical cross-sectional studies. The majority of studies assessed via ROBINS-I were found to be at moderate risk of bias. Studies assessed using the Joanna Briggs instruments were deemed appropriate to include in this review. The funnel plots suggest reduced representation of smaller studies with both low and high genetic counseling and genetic testing proportions (Appendix Table A4 and Fig A1, online only).
DISCUSSION
We have reviewed systematically the available literature on cascade genetic counseling and testing for cancer syndromes. This topic is of critical importance as, for hereditary cancer syndromes, the clinical benefit, sustainability, and cost-effectiveness of genetic counseling and testing are dependent on successful cascade testing for at-risk relatives.9,10 Our review, to our knowledge, the first meta-analysis addressing this topic, confirms that the majority of at-risk relatives do not undergo genetic counseling nor testing and that direct relative contact significantly increases completion of cascade genetic counseling for all relatives and genetic testing for first-degree relatives as compared with patient-mediated relative contact.
This review identified factors that may affect a relative's likelihood of completing cascade genetic counseling and genetic testing. Studies included in this analysis suggest that uptake of cascade genetic counseling is higher in female versus male relatives, first-degree versus more distant relatives, and families with a colorectal cancer syndrome versus hereditary breast and ovarian cancer syndrome. Limited data suggest that White race and being insured were associated with higher rates of cascade testing completion. We have also identified other factors that may contribute to success of cascade testing including relatives' age, parenthood status, and familial support although the sample size was extremely limited. A growing literature suggests that genetic testing likely presents a unique set of challenges among medically underserved and vulnerable populations. Many factors can influence a person's decision about genetic testing including race, ethnicity, sex, education level, affordability, insurance, and concerns about discrimination.71-78 Idos et al20 reported on multiplex cancer gene panel testing in a racially, ethnically, and socioeconomically diverse cohort (41% Hispanic, 26% Spanish-speaking only, and 30% achieved a highest level of education of high school or less) and found that 38% of relatives underwent cascade genetic testing. However, among studies included in our review that provided information on relative race, 74% of the population identified as White, emphasizing the critical need for trials that explore genetic medicine in diverse patient populations. Elucidating barriers to cascade genetic testing is essential so that cascade testing strategies can be designed to target those relatives least likely to use potentially life-saving medical interventions. Furthermore, this aligns with the call put forth by several organizations to improve genetic cancer risk assessment and testing for minority populations.26 We identified three strategies for direct relative contact, letter (by mail), e-mail, and telephone call, with telephone calls demonstrating the highest rates of completion of genetic counseling and genetic testing.
Our results should be viewed in light of several limitations. The majority of studies evaluated for risk of bias using ROBINS-I were found to be at moderate risk of bias. The funnel plots may indicate decreased publication of smaller studies with both low and high genetic counseling and genetic testing proportions. However, this is unlikely to skew the summary estimates in favor of uptake of genetic counseling and genetic testing because only the absence of smaller studies with low testing proportions would be indicative of publication bias. Several studies measured completion of cascade genetic testing on the basis of proband or relative self-report. Ideally, future studies will include a review of the genetic testing results to confirm completion of the recommended appropriate genetic testing. Finally, the primary outcomes for this study were completion of cascade genetic counseling and genetic testing for all relatives via patient-mediated and direct relative contact. We found that direct relative contact significantly improved rates of completion of genetic counseling. The rate of genetic testing was higher for direct relative contact compared with patient-mediated contact, and this result was close to statistical significance; completion was significantly higher on the subgroup analysis of first-degree relatives. These limitations highlight the need for well-designed prospective randomized controlled trials addressing this topic.
Our findings offer a meta-analysis to confirm prior systematic reviews that direct relative contact results in greater uptake of cascade genetic counseling and genetic testing for familial cancer syndromes as compared with patient-mediated relative contact. These findings, combined with the growing body of evidence that direct contact is acceptable to patients, their at-risk relatives, and providers, suggest a change in the paradigm of cascade testing.17,19,27,57,79,80
Favoring direct relative contact are focus groups, suggesting that direct contact programs are viewed as an acceptable complement to existing patient-mediated cascade screening efforts and ethical arguments increasingly supporting the notion that, in the context of shared genetic information, the clinician has responsibility not just to the patient but also to at-risk relatives.27,81-84 Furthermore, Health Insurance Portability and Accountability Act privacy rules allow for several avenues of direct clinician contact of at-risk relatives including with the patient's consent.85 Other possible avenues include direct relative contact facilitated by the testing laboratory as described by Caswell-Jin et al,38 provider-to-provider contact, and contact permitted via the public health exception. However, the public health exemption has largely been used in the context of communicable diseases with the potential for imminent harm. Although other countries have explored public health approaches to cascade testing, this is yet to be explored in the United States.85,86 Of note, providers and patients have voiced concern about privacy protection and control over information flow in the setting of direct contact cascade testing programs.27 Well-designed studies are needed to clarify the acceptability of direct contact programs for both probands and relatives and to explore the legal, financial, and resource implications of direct contact cascade testing programs in the United States and abroad.
In conclusion, although cascade genetic testing for cancer syndromes is a tier-one application of genomic medicine per the Centers for Disease Control and Prevention,11 we have found that the majority of at-risk relatives do not undergo this potentially life-saving intervention. Our meta-analysis confirms prior literature that direct relative contact significantly increases completion of cascade genetic counseling for all relatives and genetic testing for first-degree relatives as compared with patient-mediated relative contact. Future randomized comparative effectiveness studies must explore strategies of direct relative contact and facilitate pursuit of genetic counseling and testing in under-represented patient subgroups to promote equitable identification of the millions of Americans unknowingly harboring cancer-associated pathogenic variants, moving toward the promise of precision medicine.12
ACKNOWLEDGMENT
We extend our sincere gratitude to Ms Meher Jamy for her assistance in preparing this manuscript.
APPENDIX
FIG A1.
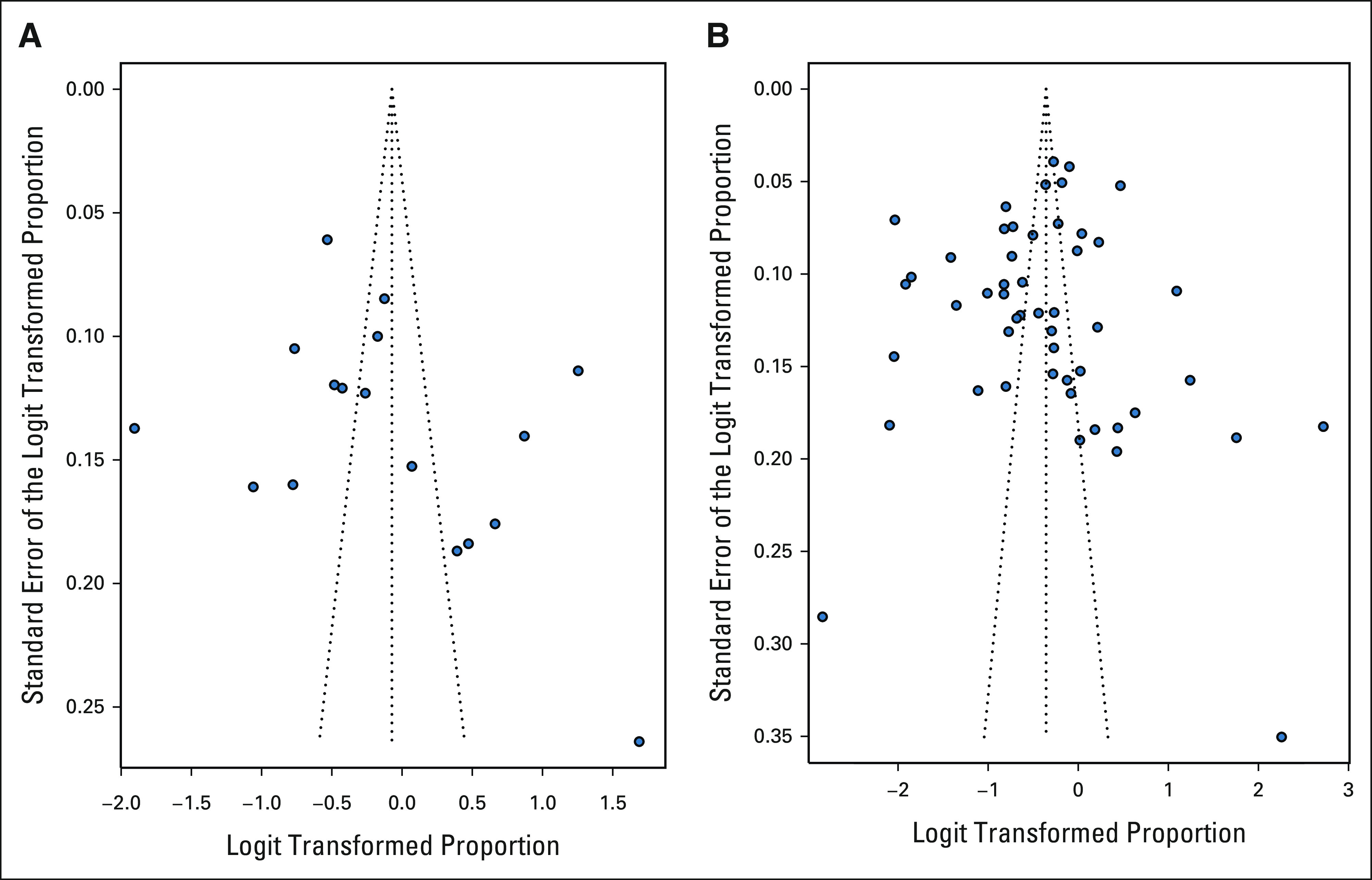
Funnel plots for genetic counseling and genetic testing outcomes. (A) Cascade genetic counseling, and (B) cascade genetic testing.
TABLE A1.
Ovid MEDLINE Search Strategy
TABLE A2.
Demographics of Probands and Relatives for Included Studies (No. = 87)
TABLE A3.
Sensitivity Analyses for All Relatives Completing Cascade Genetic Counseling and Genetic Testing
TABLE A4.
Risk of Bias in Nonrandomized Studies of Interventions (n = 16)
Jose Alejandro Rauh-Hain
Consulting or Advisory Role: Schlesinger Associates, Guidepoint Inc
Charlene Thomas
Travel, Accommodations, Expenses: Inovio Pharmaceuticals, Nektar, Pfizer
Kevin Holcomb
Research Funding: Fujirebio Diagnostics
Expert Testimony: Johnson and Johnson, Inc
Allison W. Kurian
Research Funding: Myriad Genetics (Inst)
Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, Invitae, Genentech
Kenneth Offit
Patents, Royalties, Other Intellectual Property: Patent pending on therapeutic applications of targeting ERCC3 mutations in cancer. Diagnosis & treatment of ercc3-mutant cancer US20210137850A1
Other Relationship: AnaNeo Therapeutics, Inc
No other potential conflicts of interest were reported.
SUPPORT
M.K.F. was supported by the following grant: NIH/NCATS Grant No. KL2-TR-002385. R.N.S. was supported by the following grants: National Cancer Institute Grant No. K07CA216326 and R01CA211723 and Patient-Centered Outcomes Research Institute Grant No. IHS-2017C3-9211. P.J.C. and C.T. were supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01). S.L. was supported by the following grant: National Cancer Institute Grant No. U01CA233056. J.G.H. and K.O. were supported by the following grant: National Cancer Institute Grant No. P30 CA008748.
M.K.F. and M.D.A. are cofirst authors of this work.
AUTHOR CONTRIBUTIONS
Conception and design: Melissa K. Frey, Xuan Li, Jose Alejandro Rauh-Hain, Haley Moss, Becky Baltich Nelson, Kenneth Offit, Ravi N. Sharaf
Administrative support: Hannah Bergeron, Kenneth Offit
Provision of study materials or patients: Haley Moss, Steven Lipkin
Collection and assembly of data: Melissa K. Frey, Muhammad Danyal Ahsan, Hannah Bergeron, Rana K. Fowlkes, Priyanka Narayan, Evelyn Cantillo, Ravi N. Sharaf
Data analysis and interpretation: Melissa K. Frey, Muhammad Danyal Ahsan, Jenny Lin, Rana K. Fowlkes, Roni Nitecki, Jose Alejandro Rauh-Hain, Charlene Thomas, Paul J. Christos, Jada G. Hamilton, Eloise Chapman-Davis, Kevin Holcomb, Allison W. Kurian, Steven Lipkin, Ravi N. Sharaf
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cascade Testing for Hereditary Cancer Syndromes: Should We Move toward Direct Relative Contact? A Systematic Review and Meta-Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jose Alejandro Rauh-Hain
Consulting or Advisory Role: Schlesinger Associates, Guidepoint Inc
Charlene Thomas
Travel, Accommodations, Expenses: Inovio Pharmaceuticals, Nektar, Pfizer
Kevin Holcomb
Research Funding: Fujirebio Diagnostics
Expert Testimony: Johnson and Johnson, Inc
Allison W. Kurian
Research Funding: Myriad Genetics (Inst)
Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, Invitae, Genentech
Kenneth Offit
Patents, Royalties, Other Intellectual Property: Patent pending on therapeutic applications of targeting ERCC3 mutations in cancer. Diagnosis & treatment of ercc3-mutant cancer US20210137850A1
Other Relationship: AnaNeo Therapeutics, Inc
No other potential conflicts of interest were reported.
REFERENCES
- 1.Tuffaha HW, Mitchell A, Ward RL, et al. : Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet Med 20:985-994, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Committee on Gynecologic Practice : ACOG Committee Opinion No. 727: Cascade testing: Testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol 131:e31-e34, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Randall LM, Pothuri B, Swisher EM, et al. : Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol 146:217-224, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Offit K: The future of clinical cancer genomics. Semin Oncol 43:615-622, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finch AP, Lubinski J, Møller P, et al. : Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol 32:1547-1553, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, You R, Wang X, et al. : Effectiveness of prophylactic surgeries in BRCA1 or BRCA2 mutation carriers: A meta-analysis and systematic review. Clin Cancer Res 22:3971-3981, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Engel C, Rahner N, Schulmann K, et al. : Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 8:174-182, 2010 [DOI] [PubMed] [Google Scholar]
- 8.de Jong AE, Hendriks YM, Kleibeuker JH, et al. : Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 130:665-671, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Ladabaum U, Wang G, Terdiman J, et al. : Strategies to identify the lynch syndrome among patients with colorectal cancer: A cost-effectiveness analysis. Ann Intern Med 155:69-79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makhnoon S, Tran G, Levin B, et al. : Uptake of cancer risk management strategies among women who undergo cascade genetic testing for breast cancer susceptibility genes. Cancer 127:3605-3613, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center for Disease Control and Prevention : Tier 1 Genomic Applications Toolkit for Public Health Departments
- 12.Offit K, Tkachuk KA, Stadler ZK, et al. : Cascading after peridiagnostic cancer genetic testing: An alternative to population-based screening. J Clin Oncol 38:1398-1408, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manchanda R, Burnell M, Gaba F, et al. : Randomised trial of population-based BRCA testing in Ashkenazi Jews: Long-term outcomes. BJOG 127:364-375, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Drohan B, Roche CA, Cusack JC, et al. : Hereditary breast and ovarian cancer and other hereditary syndromes: Using technology to identify carriers. Ann Surg Oncol 19:1732-1737, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Fehniger J, Lin F, Beattie MS, et al. : Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns 22:603-612, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Katapodi MC, Viassolo V, Caiata-Zufferey M, et al. : Cancer predisposition cascade screening for hereditary breast/ovarian cancer and lynch syndromes in Switzerland: Study protocol. JMIR Res Protoc 6:e184, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suthers GK, Armstrong J, McCormack J, et al. : Letting the family know: Balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet 43:665-670, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharaf RN, Myer P, Stave CD, et al. : Uptake of genetic testing by relatives of lynch syndrome probands: A systematic review. Clin Gastroenterol Hepatol 11:1093-1100, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Frey MK, Kahn RM, Chapman-Davis E, et al. : Prospective feasibility trial of a novel strategy of facilitated cascade genetic testing using telephone counseling. J Clin Oncol 38:1389-1397, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Idos GE, Kurian AW, Ricker C, et al. : Multicenter prospective cohort study of the diagnostic yield and patient experience of multiplex gene panel testing for hereditary cancer risk. JCO Precis Oncol 3:1-12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy DE, Byfield SD, Comstock CB, et al. : Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genet Med 13:349-355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Childers KK, Maggard-Gibbons M, Macinko J, et al. : National distribution of cancer genetic testing in the United States: Evidence for a gender disparity in hereditary breast and ovarian cancer. JAMA Oncol 4:876-879, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolaidis C, Duquette D, Mendelsohn-Victor KE, et al. : Disparities in genetic services utilization in a random sample of young breast cancer survivors. Genet Med 21:1363-1370, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Cragun D, Weidner A, Lewis C, et al. : Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 123:2497-2505, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuinness JE, Trivedi MS, Silverman T, et al. : Uptake of genetic testing for germline BRCA1/2 pathogenic variants in a predominantly Hispanic population. Cancer Genet 235-236:72-76, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polite BN, Adams-Campbell LL, Brawley OW, et al. : Charting the future of cancer health disparities research: A position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. J Clin Oncol 35:3075-3082, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Henrikson NB, Blasi P, Figueroa Gray M, et al. : Patient and family preferences on health system-led direct contact for cascade screening. J Pers Med 11:538, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menko FH, Ter Stege JA, van der Kolk LE, et al. : The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and lynch syndrome: A systematic review of the literature and implications for clinical practice. Fam Cancer 18:127-135, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Roberts MC, Dotson WD, DeVore CS, et al. : Delivery of cascade screening for hereditary conditions: A scoping review of the literature. Health Aff (Millwood) 37:801-808, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baroutsou V, Underhill-Blazey ML, Appenzeller-Herzog C, et al. : Interventions facilitating family communication of genetic testing results and cascade screening in hereditary breast/ovarian cancer or lynch syndrome: A systematic review and meta-analysis. Cancers (Basel) 13:925, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Health Insurance Portability and Accountability Act of 1996, H.R. 3103, 104th Congress.
- 32.Chivers Seymour K, Addington-Hall J, Lucassen AM, et al. : What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research. J Genet Couns 19:330-342, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 339:b2700, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne JA, Hernán MA, Reeves BC, et al. : ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munn Z, Moola S, Lisy K, et al. : Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 13:147-153, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Biesecker BB, Ishibe N, Hadley DW, et al. : Psychosocial factors predicting BRCA1/BRCA2 testing decisions in members of hereditary breast and ovarian cancer families. Am J Med Genet 93:257-263, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Braley EF, Bedard AC, Nuk J, et al. : Patient ethnicity and cascade genetic testing: A descriptive study of a publicly funded hereditary cancer program. Fam Cancer 21:369-374, 2021 [DOI] [PubMed] [Google Scholar]
- 38.Caswell-Jin JL, Zimmer AD, Stedden W, et al. : Cascade genetic testing of relatives for hereditary cancer risk: Results of an online initiative. J Natl Cancer Inst 111:95-98, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerman C, Narod S, Schulman K, et al. : BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. JAMA 275:1885-1892, 1996 [PubMed] [Google Scholar]
- 40.Lerman C, Hughes C, Trock BJ, et al. : Genetic testing in families with hereditary nonpolyposis colon cancer. JAMA 281:1618-1622, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Courtney E, Chok AK, Ting Ang ZL, et al. : Impact of free cancer predisposition cascade genetic testing on uptake in Singapore. NPJ Genom Med 4:22, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dilzell K, Kingham K, Ormond K, et al. : Evaluating the utilization of educational materials in communicating about Lynch syndrome to at-risk relatives. Fam Cancer 13:381-389, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Petersen J, Koptiuch C, Wu YP, et al. : Patterns of family communication and preferred resources for sharing information among families with a Lynch syndrome diagnosis. Patient Educ Couns 101:2011-2017, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon SY, Thong MK, Taib NA, et al. : Genetic counseling for patients and families with hereditary breast and ovarian cancer in a developing Asian country: An observational descriptive study. Fam Cancer 10:199-205, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Barrow P, Green K, Clancy T, et al. : Improving the uptake of predictive testing and colorectal screening in lynch syndrome: A regional primary care survey. Clin Genet 87:517-524, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Bodd TL, Reichelt J, Heimdal K, et al. : Uptake of BRCA1 genetic testing in adult sisters and daughters of known mutation carriers in Norway. J Genet Couns 12:405-417, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Finlay E, Stopfer JE, Burlingame E, et al. : Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test 12:81-91, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch HT, Snyder CL, Lynch JF, et al. : Family information service participation increases the rates of mutation testing among members of families with BRCA1/2 mutations. Breast J 15:S20-S24, 2009. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meijers-Heijboer EJ, Verhoog LC, Brekelmans CT, et al. : Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet 355:2015-2020, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Menko FH, Jeanson KN, Bleiker EMA, et al. : The uptake of predictive DNA testing in 40 families with a pathogenic BRCA1/BRCA2 variant. An evaluation of the proband-mediated procedure. Eur J Hum Genet 28:1020-1027, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanz J, RamónCajal yT, Torres A, et al. : Uptake of predictive testing among relatives of BRCA1 and BRCA2 families: A multicenter study in northeastern Spain. Fam Cancer 9:297-304, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Seppälä TT, Pylvänäinen K, Mecklin JP: Uptake of genetic testing by the children of Lynch syndrome variant carriers across three generations. Eur J Hum Genet 25:1237-1245, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramsoekh D, van Leerdam ME, Tops CM, et al. : The use of genetic testing in hereditary colorectal cancer syndromes: Genetic testing in HNPCC, (A)FAP and MAP. Clin Genet 72:562-567, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Holloway SM, Bernhard B, Campbell H, et al. : Uptake of testing for BRCA1/2 mutations in South East Scotland. Eur J Hum Genet 16:906-912, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Wagner A, Tops C, Wijnen JT, et al. : Genetic testing in hereditary non-polyposis colorectal cancer families with a MSH2, MLH1, or MSH6 mutation. J Med Genet 39:833-837, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aktan-Collan K, Mecklin JP, Järvinen H, et al. : Predictive genetic testing for hereditary non-polyposis colorectal cancer: Uptake and long-term satisfaction. Int J Cancer 89:44-50, 2000 [PubMed] [Google Scholar]
- 57.Aktan-Collan K, Haukkala A, Pylvänäinen K, et al. : Direct contact in inviting high-risk members of hereditary colon cancer families to genetic counselling and DNA testing. J Med Genet 44:732-738, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blandy C, Chabal F, Stoppa-Lyonnet D, et al. : Testing participation in BRCA1/2-positive families: Initiator role of index cases. Genet Test 7:225-233, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Brooks L, Lennard F, Shenton A, et al. : BRCA1/2 predictive testing: A study of uptake in two centres. Eur J Hum Genet 12:654-662, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Cody N, Green A, McDevitt T, et al. : Cascade screening in BRCA1/2 mutation carriers. Ir Med J 101:140-142, 2008 [PubMed] [Google Scholar]
- 61.Donenberg T, George S, Ali J, et al. : A clinically structured and partnered approach to genetic testing in Trinidadian women with breast cancer and their families. Breast Cancer Res Treat 174:469-477, 2019 [DOI] [PubMed] [Google Scholar]
- 62.Hadley DW, Jenkins J, Dimond E, et al. : Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med 163:573-582, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Levin T, Mæhle L: Uptake of genetic counseling, genetic testing and surveillance in hereditary malignant melanoma (CDKN2A) in Norway. Fam Cancer 16:257-265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trottier M, Lunn J, Butler R, et al. : Strategies for recruitment of relatives of BRCA mutation carriers to a genetic testing program in the Bahamas. Clin Genet 88:182-186, 2015 [DOI] [PubMed] [Google Scholar]
- 65.de Snoo FA, Riedijk SR, van Mil AM, et al. : Genetic testing in familial melanoma: Uptake and implications. Psychooncology 17:790-796, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Evans DG, Binchy A, Shenton A, et al. : Comparison of proactive and usual approaches to offering predictive testing for BRCA1/2 mutations in unaffected relatives. Clin Genet 75:124-132, 2009 [DOI] [PubMed] [Google Scholar]
- 67.McInerney-Leo A, Biesecker BB, Hadley DW, et al. : BRCA1/2 testing in hereditary breast and ovarian cancer families: Effectiveness of problem-solving training as a counseling intervention. Am J Med Genet A 130A:221-227, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Sermijn E, Delesie L, Deschepper E, et al. : The impact of an interventional counselling procedure in families with a BRCA1/2 gene mutation: Efficacy and safety. Fam Cancer 15:155-162, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans DG, Maher ER, Macleod R, et al. : Uptake of genetic testing for cancer predisposition. J Med Genet 34:746-748, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reichelt JG, Dahl AA, Heimdal K, et al. : Uptake of genetic testing and pre-test levels of mental distress in Norwegian families with known BRCA1 mutations. Dis Markers 15:139-143, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayden S, Mange S, Duquette D, et al. : Large, prospective analysis of the reasons patients do not pursue BRCA genetic testing following genetic counseling. J Genet Couns 26:859-865, 2017 [DOI] [PubMed] [Google Scholar]
- 72.Armstrong K, Calzone K, Stopfer J, et al. : Factors associated with decisions about clinical BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev 9:1251-1254, 2000 [PubMed] [Google Scholar]
- 73.Ropka ME, Wenzel J, Phillips EK, et al. : Uptake rates for breast cancer genetic testing: A systematic review. Cancer Epidemiol Biomarkers Prev 15:840-855, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Kieran S, Loescher LJ, Lim KH: The role of financial factors in acceptance of clinical BRCA genetic testing. Genet Test 11:101-110, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Godard B, Pratte A, Dumont M, et al. : Factors associated with an individual's decision to withdraw from genetic testing for breast and ovarian cancer susceptibility: Implications for counseling. Genet Test 11:45-54, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Thompson HS, Valdimarsdottir HB, Duteau-Buck C, et al. : Psychosocial predictors of BRCA counseling and testing decisions among urban African-American women. Cancer Epidemiol Biomarkers Prev 11:1579-1585, 2002 [PubMed] [Google Scholar]
- 77.Olaya W, Esquivel P, Wong JH, et al. : Disparities in BRCA testing: When insurance coverage is not a barrier. Am J Surg:562-565, 1982 [DOI] [PubMed] [Google Scholar]
- 78.Hallowell N, Ardern-Jones A, Eeles R, et al. : Men's decision-making about predictive BRCA1/2 testing: The role of family. J Genet Couns 14:207-217, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Hadfield SG, Horara S, Starr BJ, et al. : Family tracing to identify patients with familial hypercholesterolaemia: The second audit of the Department of Health Familial Hypercholesterolaemia Cascade Testing Project. Ann Clin Biochem 46:24-32, 2009 [DOI] [PubMed] [Google Scholar]
- 80.van den Heuvel LM, Smets EMA, van Tintelen JP, et al. : How to inform relatives at risk of hereditary diseases? A mixed-methods systematic review on patient attitudes. J Genet Couns 28:1042-1058, 2019 [DOI] [PubMed] [Google Scholar]
- 81.Weaver M: The double helix: Applying an ethic of care to the duty to warn genetic relatives of genetic information. Bioethics 30:181-187, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Doukas DJ, Berg JW: The family covenant and genetic testing. Am J Bioeth 1:3-10, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Parker M, Lucassen AM: Genetic information: A joint account? BMJ 329:165-167, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grill K, Rosén A: Healthcare professionals' responsibility for informing relatives at risk of hereditary disease. J Med Ethics 47:e12, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henrikson NB, Wagner JK, Hampel H, et al. : What guidance does HIPAA offer to providers considering familial risk notification and cascade genetic testing? J Law Biosci 7:lsaa071, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van El CG, Baccolini V, Piko P, et al. : Stakeholder views on active cascade screening for familial hypercholesterolemia. Healthcare (Basel) 6:108, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aktan-Collan KI, Kaariainen HA, Kolttola EM, et al. : Sharing genetic risk with next generation: Mutation-positive parents' communication with their offspring in Lynch Syndrome. Fam Cancer 10:43-50, 2011 [DOI] [PubMed] [Google Scholar]
- 88.Alegre N, Perre PV, Bignon YJ, et al. : Psychosocial and clinical factors of probands impacting intrafamilial disclosure and uptake of genetic testing among families with BRCA1/2 or MMR gene mutations. Psychooncology 28:1679-1686, 2019 [DOI] [PubMed] [Google Scholar]
- 89.Beard VK, Bedard AC, Nuk J, et al. : Genetic testing in families with hereditary colorectal cancer in British Columbia and Yukon: A retrospective cross-sectional analysis. CMAJ Open 8:E637-E642, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bednar EM, Sun CC, McCurdy S, et al. : Assessing relatives' readiness for hereditary cancer cascade genetic testing. Genet Med 22:719-726, 2020 [DOI] [PubMed] [Google Scholar]
- 91.Bradbury AR, Dignam JJ, Ibe CN, et al. : How often do BRCA mutation carriers tell their young children of the family's risk for cancer? A study of parental disclosure of BRCA mutations to minors and young adults. J Clin Oncol 25:3705-3711, 2007 [DOI] [PubMed] [Google Scholar]
- 92.Bradbury AR, Patrick-Miller L, Egleston BL, et al. : When parents disclose BRCA1/2 test results: Their communication and perceptions of offspring response. Cancer 118:3417-3425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bruwer Z, Futter M, Ramesar R: Communicating cancer risk within an African context: Experiences, disclosure patterns and uptake rates following genetic testing for Lynch syndrome. Patient Educ Couns 92:53-60, 2013 [DOI] [PubMed] [Google Scholar]
- 94.Cheung EL, Olson AD, Yu TM, et al. : Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiol Biomarkers Prev 19:2211-2219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Claes E, Evers-Kiebooms G, Boogaerts A, et al. : Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet A 116A:11-19, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Conley CC, Ketcher D, Reblin M, et al. : The big reveal: Family disclosure patterns of BRCA genetic test results among young Black women with invasive breast cancer. J Genet Couns 29:410-422, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cragun D, Weidner A, Tezak A, et al. : Family communication of genetic test results among women with inherited breast cancer genes. J Genet Couns 30:701-709, 2021 [DOI] [PubMed] [Google Scholar]
- 98.Eijzenga W, de Geus E, Aalfs CM, et al. : How to support cancer genetics counselees in informing at-risk relatives? Lessons from a randomized controlled trial. Patient Educ Couns 101:1611-1619, 2018 [DOI] [PubMed] [Google Scholar]
- 99.Elrick A, Ashida S, Ivanovich J, et al. : Psychosocial and clinical factors associated with family communication of cancer genetic test results among women diagnosed with breast cancer at a young age. J Genet Couns 26:173-181, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ersig AL, Hadley DW, Koehly LM: Colon cancer screening practices and disclosure after receipt of positive or inconclusive genetic test results for hereditary nonpolyposis colorectal cancer. Cancer 115:4071-4079, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fischer C, Engel C, Sutter C, et al. : BRCA1/2 testing: Uptake, phenocopies, and strategies to improve detection rates in initially negative families. Clin Genet 82:478-483, 2012 [DOI] [PubMed] [Google Scholar]
- 102.Forrest LE, Burke J, Bacic S, et al. : Increased genetic counseling support improves communication of genetic information in families. Genet Med 10:167-172, 2008 [DOI] [PubMed] [Google Scholar]
- 103.Gaff CL, Collins V, Symes T, et al. : Facilitating family communication about predictive genetic testing: Probands' perceptions. J Genet Couns 14:133-140, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Garcia C, Sullivan MW, Lothamer H, et al. : Mechanisms to increase cascade testing in hereditary breast and ovarian cancer: Impact of introducing standardized communication aids into genetic counseling. J Obstet Gynaecol Res 46:1835-1841, 2020 [DOI] [PubMed] [Google Scholar]
- 105.Gauna Cristaldo FB, Touzani R, Apostolidis T, et al. : Uptake of genetic counseling among adult children of BRCA1/2 mutation carriers in France. Psychooncology 28:1894-1900, 2019 [DOI] [PubMed] [Google Scholar]
- 106.Griffin NE, Buchanan TR, Smith SH, et al. : Low rates of cascade genetic testing among families with hereditary gynecologic cancer: An opportunity to improve cancer prevention. Gynecol Oncol 156:140-146, 2020 [DOI] [PubMed] [Google Scholar]
- 107.Hagoel L, Dishon S, Almog R, et al. : Proband family uptake of familial-genetic counselling. Psychooncology 9:522-527, 2000 [DOI] [PubMed] [Google Scholar]
- 108.Hall ET, Parikh D, Caswell-Jin JL, et al. : Pathogenic variants in less familiar cancer susceptibility genes: What happens after genetic testing? JCO Precis Oncol 2:1-10, 2018 [DOI] [PubMed] [Google Scholar]
- 109.Hayat Roshanai A, Lampic C, Rosenquist R, et al. : Disclosing cancer genetic information within families: Perspectives of counselees and their at-risk relatives. Fam Cancer 9:669-679, 2010 [DOI] [PubMed] [Google Scholar]
- 110.Healey E, Taylor N, Greening S, et al. : Quantifying family dissemination and identifying barriers to communication of risk information in Australian BRCA families. Genet Med 19:1323-1331, 2017 [DOI] [PubMed] [Google Scholar]
- 111.Hughes C, Lerman C, Schwartz M, et al. : All in the family: Evaluation of the process and content of sisters' communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet 107:143-150, 2002 [DOI] [PubMed] [Google Scholar]
- 112.Jeong GW, Shin W, Lee DO, et al. : Uptake of family-specific mutation genetic testing among relatives of patients with ovarian cancer with BRCA1 or BRCA2 mutation. Cancer Res Treat 53:207-211, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Julian-Reynier C, Sobol H, Sevilla C, et al. : Uptake of hereditary breast/ovarian cancer genetic testing in a French national sample of BRCA1 families. The French Cancer Genetic Network. Psychooncology 9:504-510, 2000 [DOI] [PubMed] [Google Scholar]
- 114.Kardashian A, Fehniger J, Creasman J, et al. : A pilot study of the Sharing Risk Information Tool (ShaRIT) for families with hereditary breast and ovarian cancer syndrome. Hered Cancer Clin Pract 10:4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kegelaers D, Merckx W, Odeurs P, et al. : Disclosure pattern and follow-up after the molecular diagnosis of BRCA/CHEK2 mutations. J Genet Couns 23:254-261, 2014 [DOI] [PubMed] [Google Scholar]
- 116.Lammens CR, Aaronson NK, Wagner A, et al. : Genetic testing in Li-Fraumeni syndrome: Uptake and psychosocial consequences. J Clin Oncol 28:3008-3014, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Landsbergen K, Verhaak C, Kraaimaat F, et al. : Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer 4:115-119, 2005 [DOI] [PubMed] [Google Scholar]
- 118.Li ST, Yuen J, Zhou K, et al. : Impact of subsidies on cancer genetic testing uptake in Singapore. J Med Genet 54:254-259, 2017 [DOI] [PubMed] [Google Scholar]
- 119.Lieberman S, Lahad A, Tomer A, et al. : Familial communication and cascade testing among relatives of BRCA population screening participants. Genet Med 20:1446-1454, 2018 [DOI] [PubMed] [Google Scholar]
- 120.McGivern B, Everett J, Yager GG, et al. : Family communication about positive BRCA1 and BRCA2 genetic test results. Genet Med 6:503-509, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Montgomery SV, Barsevick AM, Egleston BL, et al. : Preparing individuals to communicate genetic test results to their relatives: Report of a randomized control trial. Fam Cancer 12:537-546, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patenaude AF, Dorval M, DiGianni LS, et al. : Sharing BRCA1/2 test results with first-degree relatives: Factors predicting who women tell. J Clin Oncol 24:700-706, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Peters MLB, Stobie L, Dudley B, et al. : Family communication and patient distress after germline genetic testing in individuals with pancreatic ductal adenocarcinoma. Cancer 125:2488-2496, 2019 [DOI] [PubMed] [Google Scholar]
- 124.Ponz de Leon M, Benatti P, Di Gregorio C, et al. : Genetic testing among high-risk individuals in families with hereditary nonpolyposis colorectal cancer. Br J Cancer 90:882-887, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ricker CN, Koff RB, Qu C, et al. : Patient communication of cancer genetic test results in a diverse population. Transl Behav Med 8:85-94, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Segal J, Esplen MJ, Toner B, et al. : An investigation of the disclosure process and support needs of BRCA1 and BRCA2 carriers. Am J Med Genet A 125A:267-272, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Smith KR, Zick CD, Mayer RN, et al. : Voluntary disclosure of BRCA1 mutation test results. Genet Test 6:89-92, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Stoffel EM, Ford B, Mercado RC, et al. : Sharing genetic test results in Lynch syndrome: Communication with close and distant relatives. Clin Gastroenterol Hepatol 6:333-338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taber JM, Chang CQ, Lam TK, et al. : Prevalence and correlates of receiving and sharing high-penetrance cancer genetic test results: Findings from the Health Information National Trends Survey. Public Health Genomics 18:67-77, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tercyak KP, Peshkin BN, DeMarco TA, et al. : Parent-child factors and their effect on communicating BRCA1/2 test results to children. Patient Educ Couns 47:145-153, 2002 [DOI] [PubMed] [Google Scholar]
- 131.Troian J, Apostolidis T, Touzani R, et al. : Parental disclosure of positive BRCA1/2 mutation status to children 10 years after genetic testing. Psychol Health Med 25:756-766, 2020 [DOI] [PubMed] [Google Scholar]
- 132.Vadaparampil ST, Malo T, de la Cruz C, et al. : Do breast cancer patients tested in the oncology care setting share BRCA mutation results with family members and health care providers? J Cancer Epidemiol 2012:498062, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wagner Costalas J, Itzen M, Malick J, et al. : Communication of BRCA1 and BRCA2 results to at-risk relatives: A cancer risk assessment program's experience. Am J Med Genet C Semin Med Genet 119C:11-18, 2003 [DOI] [PubMed] [Google Scholar]
- 134.Hughes C, Lynch H, Durham C, et al. : Communication of BRCA1/2 test results in hereditary breast cancer families. Cancer Res Ther Control 8:51-59, 1999 [Google Scholar]