Abstract
Dental caries results from prolonged plaque acidification that leads to the establishment of a cariogenic microflora and demineralization of the tooth. Urease enzymes of oral bacteria hydrolyze urea to ammonia, which can neutralize plaque acids. To begin to examine the relationship between plaque ureolytic activity and the incidence of dental caries, recombinant, ureolytic strains of Streptococcus mutans were constructed. Specifically, the ureABCEFGD operon from Streptococcus salivarius 57.I was integrated into the S. mutans chromosome in such a way that the operon was transcribed from a weak, cognate promoter in S. mutans ACUS4 or a stronger promoter in S. mutans ACUS6. Both strains expressed NiCl2-dependent urease activity, but the maximal urease levels in ACUS6 were threefold higher than those in ACUS4. In vitro pH drop experiments demonstrated that the ability of the recombinant S. mutans strains to moderate a decrease in pH during the simultaneous metabolism of glucose and urea increased proportionately with the level of urease activity expressed. Specific-pathogen-free rats that were infected with ACUS6 and fed a cariogenic diet with drinking water containing 25 mM urea and 50 μM NiCl2 had relatively high levels of oral urease activity, as well as dramatic decreases in the prevalence of smooth-surface caries and the severity of sulcal caries, relative to controls. Urease activity appears to influence plaque biochemistry and metabolism in a manner that reduces cariogenicity, suggesting that recombinant, ureolytic bacteria may be useful to promote dental health.
Despite significant gains in the control and treatment of dental caries, this disease remains widespread and costly to treat. Caries formation results from the acidification of dental plaque driven by microbial metabolism of dietary carbohydrate. The pH of dental plaque can fall to values as low as 4.0 (35), causing dissolution of the tooth enamel (37). In addition, frequent consumption of fermentable carbohydrate leads to extended periods of plaque acidification, which encourages the emergence of an acid-tolerant, cariogenic plaque microflora enriched in organisms such as mutans streptococci and lactobacilli (6). The enrichment of dental plaque with cariogenic microorganisms is generally accompanied by the loss of less acid-tolerant, less cariogenic organisms, which are abundant in so-called healthy dental plaque. These organisms include the sanguis group streptococci and Actinomyces naeslundii (4), which, interestingly, are also capable of neutralizing the acids produced by glycolysis through the generation of ammonia from salivary substrates.
Caries development is usually a prolonged process involving cycles of demineralization and remineralization. Periods of plaque acidification and tooth demineralization are normally followed by phases of alkalinization with a return to more neutral plaque pH values (35), which promotes remineralization at the tooth surface (22, 23). It is when the phases of demineralization dominate that a carious lesion develops. Whereas the processes by which dental plaque becomes acidified have been intensively studied, the alkalinization phase and pH homeostasis during fasting periods are rather poorly understood. A number of mechanisms are thought to contribute to the alkalinization of dental plaque, including clearance of acids and sugars by saliva, buffering by salivary and bacterial components, and production of alkali by plaque bacteria, which occurs primarily through the metabolism of urea to ammonia by microbial urease activity.
Urea is secreted continuously in the range of 3 to 10 mM in saliva and crevicular fluids of healthy individuals (15) and is rapidly hydrolyzed by the urease enzymes of oral microflora. Existing data indirectly support a major role for ureolysis in plaque pH homeostasis. Elevated salivary urea and ammonia concentrations are correlated with marked reductions in the extent and duration of plaque acidification following a carbohydrate challenge (20). Urea hydrolysis can neutralize plaque acids (33) and may positively influence plaque ecology by preventing the pH from falling to levels that select for the outgrowth of aciduric, cariogenic microorganisms (6, 8, 9). In addition, ammonia released by ureolysis can promote remineralization of the tooth enamel (22, 26, 27). Clinical studies indicate that caries-resistant patients have elevated resting plaque pH values and that these values are not lowered to the same extent as those in caries-susceptible individuals following a carbohydrate intake (1). Margolis et al. (22) have confirmed that caries-resistant subjects have more alkaline resting plaque pH values than do caries-susceptible individuals and have correlated this, in part, with increased ammonium concentrations in plaque. Studies of patients with chronic renal failure have also shown that these patients, who have salivary urea concentrations often greater than 50 mM, have alkaline plaque pH levels and a very low incidence of dental caries, despite ingestion of a diet that is dominated by carbohydrates (29; E. P. Syrrakou, M.S. thesis, University of Rochester, Rochester, N.Y.).
Recently, attention has focused on the concept that the development of cariogenic plaque may result not only from the extensive acidification of dental plaque but also from a diminution in the alkali-generating capacity of oral biofilms colonizing a carious lesion (9). Loss of ammonia-producing bacteria from the complex populations on the tooth surface would reduce the capacity of plaque to neutralize acids and slow the return of plaque pH to more neutral values. This concept is consistent with two observations: (i) the resting plaque pH in healthy individuals is higher than the pH of the saliva which bathes the plaque, and (ii) the depth and duration of plaque acidification is greater in caries-prone subjects. Also reinforcing this concept is the demonstration that individuals with low salivary ureolytic capacity have a markedly diminished capacity to blunt glycolytic acidification (34). More recently, recombinant Streptococcus mutans strains carrying plasmid-borne urease genes were used in vitro to demonstrate directly that the levels of urease commonly found in healthy plaque are sufficient to offset a pH drop by using physiologically relevant levels of urea, even in the presence of a 10-fold molar excess of glucose (12). Importantly, the ureolytic capacity of dental plaque within a carious lesion and the prevalence of alkali-generating bacteria following sustained acidification of dental plaque remain unexplored.
Unfortunately, testing of hypotheses related to the base-producing capacity of biofilms and oral health in well-controlled clinical studies has yet to be undertaken. A variety of accepted animal caries models are available for testing the effects of various carbohydrates and therapeutics on caries formation, with the rat model appearing to be the most appropriate and widely accepted. However, these caries models are not readily adaptable to the study of alkali generation because of a lack of a suitable test organism and differences in the endogenous flora of rats and humans with regard to urease activity (see below). In clinical studies, essentially all of the data relating ureolysis to plaque pH homeostasis and oral health are restricted to total plaque from healthy individuals (5, 19, 30, 33). To address some of these deficiencies, recombinant ureolytic strains of the cariogenic plaque bacterium S. mutans which could be implanted in the plaque of experimental animals were constructed to test the hypothesis that enhancing the alkali-generating capacity of plaque could reduce the incidence of caries formation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study and their relevant characteristics are described in Table 1. Escherichia coli was grown and maintained in L broth (31), and S. mutans strains were grown and maintained in brain heart infusion (BHI) medium in the presence of the appropriate antibiotics when necessary. S. mutans UA159 StR was a streptomycin-resistant spontaneous mutant selected by plating S. mutans UA159 (UAB159) on BHI agar plates (1.5% Bacto agar) supplemented with 500 μg of streptomycin per ml. All growth media were obtained from Difco (Detroit, Mich.) and all chemicals were obtained from Sigma (St. Louis, Mo.) unless otherwise noted.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| S. mutans | ||
| UA159 | Wild type; Ure− Lac+ | Laboratory stock |
| UA159 StR | Spontaneous Str mutant of UA159 | This study |
| ACUS4 | UA159 with ure cluster and sp integrated at lac; Ure+ Spr Lac− | This study |
| ACUS5 | UA159 with sp integrated at lac; Ure− Spr Lac− | This study |
| ACUS6 | UA159 containing ure, sp and tet integrated at lac; Ure+ Spr Tcr Lac− | This study |
| ACUS8 | UA159 containing sp and tet integrated at lac; Ure− Spr Tcr Lac− | This study |
| E. coli | ||
| DH10B | General cloning host | Promega |
| Plasmids | ||
| pVA2216 | S. mutans integration vector; Apr Tcr | 17 |
| pACUS1 | pSU20 harboring sp cassette and ure from S. salivarius 57.I; Cmr Spr | This study |
| pACUS2 | pVA2216 with tet replaced by sp and ure from pACUS1; Apr Tcs Spr | This study |
| pAC21 | pSU20 harboring tet between sp and 0.74 kb of ure sequence 5′ to ureA on the ACUS4 chromosome; Cmr Spr Tcr | This study |
ure, ureABCEFGD and a partial ureI open reading frame; Spr, spectinomycin resistance; Tcr, tetracycline resistance; Apr, ampicillin resistance; Str, streptomycin resistance; Cmr, chloramphenicol resistance; Lac, lactose metabolism.
DNA manipulations.
DNA isolation and recombinant DNA manipulations were carried out using established procedures (31). Genetically competent S. mutans (28) was prepared by growing S. mutans UA159 in BHI broth supplemented with 10% heat-inactivated horse serum (Life Technologies, Inc., Gaithersburg, Md.) at 37°C in a 5% CO2 aerobic atmosphere. Competent S. mutans was transformed with plasmid DNA isolated from E. coli. Transformants were selected and maintained on BHI agar plates containing spectinomycin (250 μg/ml) or tetracycline (5 μg/ml) as needed. Integration of the antibiotic resistance and urease genes into the S. mutans chromosome was confirmed by Southern blotting (31).
Determination of enzyme activity.
Urease activity was assayed in intact cells as previously described (10). To examine the nickel dependence of urease biogenesis, recombinant S. mutans strains were grown to an optical density at 600 nm of ca. 0.6 in BHI supplemented with NiCl2 at concentrations of 0, 25, 50, 75 or 100 μM. The cells were concentrated by centrifugation, washed, resuspended in 10 mM sodium phosphate (pH 7.0), and assayed for urease activity. Activity was expressed in units (U), defined as the amount of enzyme required to hydrolyze 1 μmol of urea per min, and was normalized to cell dry weight. To examine urease activity expressed in the plaques of experimental animals, the mandibles and the maxillary palate of each animal were individually placed in sterile sodium phosphate buffer on ice and sonicated for 30 s at 10-s intervals. The suspensions from the left and right mandibles from all animals in each group were pooled, as were the suspensions from the maxillary palate from all animals in each group. The samples were centrifuged at 2,900 × g for 20 min at 4°C, resuspended in 4 ml of sodium phosphate buffer, and stored on ice. The amount of ammonia released was measured after a 6.5-h incubation in the presence of urea, with a no-urea-added control to detect any ammonia liberated from other sources. Activity was normalized to protein content as determined by the method of Bradford (7).
In vitro pH drop experiments.
Recombinant S. mutans was grown in BHI broth supplemented with 0 or 25 μM NiCl2 to an optical density at 600 nm of ca. 0.6. The cells were concentrated by centrifugation, washed, and resuspended in 1/10 the initial volume in ice-cold pH drop buffer (10 mM KCl, 1 mM MgCl2). The glycolytic capacities of the cells were determined as described by Belli and Marquis (3). The ureolytic capacities of the strains and the influence of urea metabolism on environmental acidification as a result of glycolysis were determined by initiating the pH drop and simultaneously adding 56 mM glucose and 5 or 25 mM urea (12). The pH of the cell suspensions was monitored continuously for 1 h using a KCl glass electrode connected to either a Corning 320 or a Beckman φ10 meter.
Animal studies.
Eighteen litters of female Sprague-Dawley rat pups with their dams were obtained from Charles River Breeding Laboratories (Wilmington, Mass.). Prior to infection, the dams were screened for mutans streptococci by plating from oral swabs onto mitis salivarius (MS) agar on MS agar supplemented with bacitracin (1 μg/ml) (MSB agar). No animals were found that harbored detectable levels of mutans streptococci. Animals were also screened for and found to be free of sialoacroadenitis virus by immunologic screening, since this virus can affect salivary gland function, which in turn could affect the development of caries. At 19 days of age, the pups were weaned, grouped into nine groups of 16 animals, and caged in plastic cages. The rats were then infected on two consecutive days by oral swabbing with cultures of the various strains of S. mutans (Table 2) that had been grown to mid-exponential phase in low-molecular-weight medium (39). One group of animals was infected with S. mutans UA159 StR, four groups (groups 2 to 5) were infected with the ureolytic strain S. mutans ACUS6, and four groups (groups 6 to 9) were infected with the nonureolytic control strain, S. mutans ACUS8. Two days after inoculation, successful infection was confirmed by plating oral swabs from each animal on MSB agar and on MSB agar containing appropriate antibiotics.
TABLE 2.
Infecting strains, phenotypes, and diet fed to experimental animalsa
| Group | Strain | Characteristics | Diet |
|---|---|---|---|
| 1 | UA159 StR | Lac+ Ure− Str | Diet 2000, 5% sucrose water |
| 2 | ACUS6 | Lac− Ure+ Spr Tcr | Diet 2000, 5% sucrose water |
| 3 | ACUS6 | Lac− Ure+ Spr Tcr | Diet 2000, 5% sucrose water + 50 μM NiCl2 |
| 4 | ACUS6 | Lac− Ure+ Spr Tcr | Diet 2000, 5% sucrose water + 50 mM Urea |
| 5 | ACUS6 | Lac− Ure+ Spr Tcr | Diet 2000, 5% sucrose water + 50 μM NiCl2 + 50 mM urea |
| 6 | ACUS8 | Lac− Ure+ Spr Tcr | Diet 2000, 5% sucrose water |
| 7 | ACUS8 | Lac− Ure+ Spr Tcr | Diet 2000, 5% sucrose water + 50 μM NiCl2 |
| 8 | ACUS8 | Lac− Ure+ Spr Tcr | Diet 2000, 5% sucrose water + 50 mM urea |
| 9 | ACUS8 | Lac− Ure+ Spr Tcr | Diet 2000, 5% sucrose water + 50 μM NiCl2 + 50 mM urea |
Nine groups of 16 rats were infected with the indicated strains. Details of the infection, monitoring, and delivery of diet can be found in Materials and Methods.
Once infection had been established, animals were paired and placed in suspended cages. The experimental and control groups are outlined in Table 2. Briefly, all animals were given an ad libitum diet consisting of Diet 2000 (56% sucrose) and drinking water sweetened with 5% sucrose. NiCl2 (50 μM) was also added to the drinking water of groups 3 and 7; urea (50 mM) was added to the drinking water of groups 4 and 8; and the drinking water of groups 5 and 9 was supplemented with both 50 mM urea and 50 μM NiCl2. The water was supplemented with nickel to activate the urease apoenzyme in vivo. The rats consumed food and water ad libitum for 5 weeks and then were sacrificed by CO2 asphyxiation and decapitated. The jaws were removed and sonicated in 5 ml (right mandible) or 3 ml (left mandible and maxillary palate) of sterile sodium phosphate buffer (pH 7.0) on ice for 30 s at 10-s intervals. Microbiological assessment was carried out using the sonicate from the left mandible. Undiluted and diluted (1:100 in sodium phosphate buffer) samples of the sonicate were plated, using an Autoplate 3000 (Spiral Biotech), on sheep blood (SB) agar for determination of total cultivable flora, on MSB to determine total mutans streptococcal counts, and on MSB containing the appropriate antibiotics to quantify recombinant populations. All the plates were incubated at 37°C for 2 days in a 5% CO2 aerobic atmosphere, and the sheep blood agar plates were incubated for an additional day aerobically at 37°C. Colonies from the MS selective agar plates were examined for the stable maintenance of the urease genes by gridding to urea agar (Difco) which was modified by the addition of 15 g of Todd-Hewitt broth per liter and supplemented with 50 μM NiCl2. The urease activity present in the rat plaque flora was determined as described above. All jaws were scored for caries by the method of Keyes (17). Statistical comparisons of the data from the rat study were made using the Tukey-Kramer honestly significant difference (HSD) test.
RESULTS
Construction of recombinant, ureolytic S. mutans.
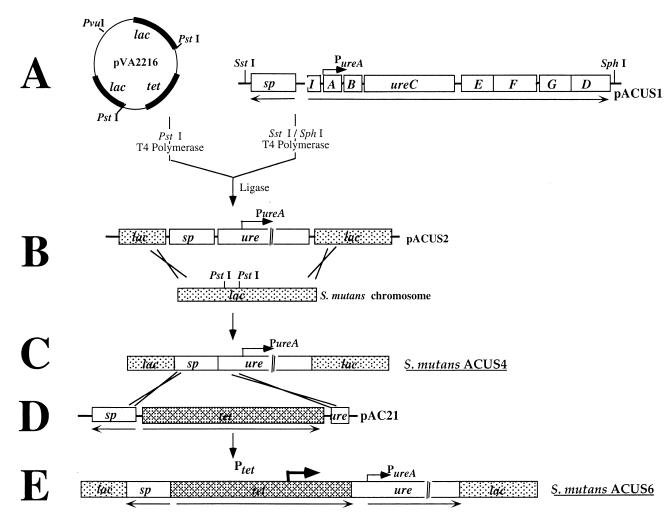
The strategy used for constructing recombinant, ureolytic S. mutans is depicted in Fig. 1. Briefly, a spectinomycin resistance gene (38) was cloned 5′, and in the opposite orientation, to the urease (ure) gene cluster on pMC12. Plasmid pMC12 (10) harbors the intact ureABC genes, encoding the enzyme subunits, and the ureEFGD genes, encoding the accessory proteins required for activation of the apoenzyme from S. salivarius 57.I (Fig. 1A). Recombinant bacteria harboring pMC12 express urease, but the amount of active enzyme produced is dependent on the concentration of NiCl2 in the growth medium (10). The urease and spectinomycin genes were cloned as a cassette into the S. mutans lac sequences on the integration vector pVA2216 (18), with concomitant replacement of the tetracycline resistance gene. The resulting plasmid, pACUS2 (Fig. 1B), was linearized by restriction digestion at a unique PvuI site within the vector and used to transform competent S. mutans UA159. Allelic exchange between homologous plasmid and chromosomal sequences resulted in the establishment of the spectinomycin/urease cassette in the UA159 lac locus (Fig. 1C). Spectinomycin-resistant transformants were identified by selection on BHI agar supplemented with spectinomycin and were transferred to modified urea agar plates, which contained a pH indicator dye, to screen for the expression of urease activity. Colonies which produced a pink color, indicating the production of alkali from urea, were isolated and found to express urease activity when grown in the presence of 50 μM NiCl2. One of the positive clones was selected and named ACUS4.
FIG. 1.
Construction of recombinant, ureolytic S. mutans. The ureABCEFGD genes and a partial ureI open reading frame from S. salivarius were cloned, adjacent to a Spr gene, within S. mutans lac sequences on the integration vector, pVA2216 (18). Introduction of the recombinant vector, pACUS2, into S. mutans allowed integration of the genes within the chromosomal lac locus by allelic exchange. The ure genes in the resulting strain, S. mutans ACUS4, were transcribed from a putative weak cognate promoter, PureA. To construct a strain expressing higher levels of urease activity, a second integration vector, pAC21, was constructed that harbored tet flanked by Spr and ure sequences. The introduction of pAC21 into ACUS4 allowed homologous recombination such that tet was integrated 5′ to ure in ACUS4. As a result, the ure operon in this strain, S. mutans ACUS6, was transcribed from a strong promoter within tet, Ptet, at a constitutive level. Two control strains were also constructed using a similar strategy (data not shown). S. mutans ACUS5 and ACUS8 were inactivated at the lac locus by integration of the Spr gene alone or together with tet, respectively. Arrows beneath the gene clusters indicate transcriptional direction. Rows A to E are as described in the text.
In the ureolytic strain S. mutans ACUS4, ure transcription was apparently dependent on the activity of a putative weak promoter 5′ to ureA that is thought to drive constitutive transcription at a relatively low level (11). ACUS4 was weakly ureolytic (0.025 U mg of cell dry weight−1) relative to the source of the urease genes, S. salivarius 57.I, which under similar conditions produces in excess of 1 U mg of cell dry weight−1. To examine how the amount of urease activity could differentially influence dental health in vivo, a strain of S. mutans which would produce higher levels of urease than ACUS4 was constructed. A tetracycline (tet) cassette (14), which was found to have a comparatively strong, outward-reading promoter (K. A. Clancy and R. A. Burne, unpublished data), was integrated 5′ to the ure cluster on the ACUS4 chromosome. Briefly, S. mutans ACUS4 was transformed with an integration vector, pAC21, harboring tet flanked by the spectinomycin resistance (Spr) gene at the 5′ end and a 0.74-kbp DNA fragment at the 3′ end, which was identical to the 5′ region of the ure cluster on the ACUS4 chromosome (Fig. 1D). A double-crossover recombination event resulted in the integration of tet 5′ to, and in the same orientation as, the ure cluster on the ACUS4 chromosome (Fig. 1E). Tcr and Spr transformants were isolated by selection on BHI agar supplemented with spectinomycin and tetracycline, and the expression of ure genes by the transformants was determined by using urea indicator agar plates and by assaying for urease activity after growth in the presence of 50 μM NiCl2.
To be able to control for any alteration in the cariogenicity of recombinant S. mutans that may have been caused by inactivation of lac or by the presence or expression of antibiotic resistance genes, two additional S. mutans strains were constructed. S. mutans ACUS5 was constructed by transforming UA159 with a pVA2216-based plasmid in which the tet gene was replaced by the Spr gene. Similarly, the transformation of UA159 with a pVA2216-based replicon containing Spr cloned adjacent to tet resulted in S. mutans ACUS8, a UA159 derivative with tet and Spr genes inserted at the lac locus.
The recombinant S. mutans strains were unable to grow with lactose as the sole carbohydrate source, consistent with the insertional inactivation of the lac operon. Southern hybridization to chromosomal DNA from recombinant strains confirmed that integration of the ure genes and selective markers occurred in the lac locus. No appreciable differences were found between the growth rates in batch cultures of S. mutans ACUS4, ACUS5, ACUS6, and ACUS8 compared to the wild-type strain, indicating that inactivation of the lac locus, as well as harboring the foreign genes, did not adversely affect the growth of the recombinants. All S. mutans strains stably maintained and expressed the recombinant genes following routine passage in the absence of antibiotic selection for >12 months.
Characterization of urease expression by recombinant, ureolytic S. mutans.
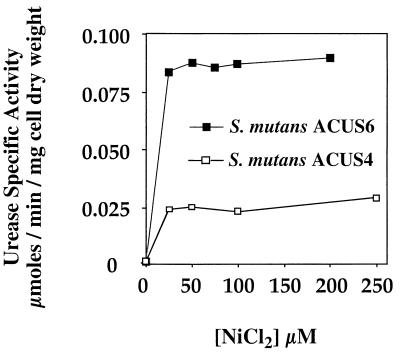
S. mutans ACUS4 and S. mutans ACUS6 expressed urease activity, whose level was dependent on the concentration of exogenous NiCl2 present in the growth medium (Fig. 2). As is commonly observed when urease genes are expressed in nonureolytic hosts (24), addition of NiCl2 to the growth medium was needed to observe significant urease activity, because nonureolytic strains lack the high-affinity transport proteins that are needed to scavenge sufficient Ni2+ from the environment. When no additional NiCl2 was added to the growth medium, ACUS4 and ACUS6 were poorly ureolytic, expressing only 2 × 10−4 U and 5 × 10−4 U mg of cell dry weight−1, respectively. However, when grown in the presence of 25 μM NiCl2, ACUS4 expressed approximately 0.025 U mg of cell dry weight−1. When grown in 25 μM NiCl2, ACUS6 expressed greater than threefold more activity than ACUS4 did (approximately 0.08 U mg of cell dry weight−1). Increasing the concentration of nickel to 50 μM slightly increased the level of urease activity expressed by ACUS6. S. mutans ACUS5 and ACUS8 were nonureolytic regardless of the concentration of exogenous NiCl2 added to the growth medium (data not shown).
FIG. 2.
Nickel-dependent urease activity in recombinant, ureolytic S. mutans. S. mutans ACUS4 and S. mutans ACUS6 were grown to an optical density of 0.6 in BHI supplemented with nickel chloride and assayed for urease activity. Activity was expressed in units (micromoles of urea hydrolyzed per minute per milligram of cell dry weight. The graph is representative of results obtained with at least four separately grown bacterial cultures of each strain. In control experiments, no urease activity was detected for the parent strain, S. mutans UA159, or the recombinant strains, S. mutans ACUS5 and ACUS8, regardless of the concentration of nickel chloride (data not shown).
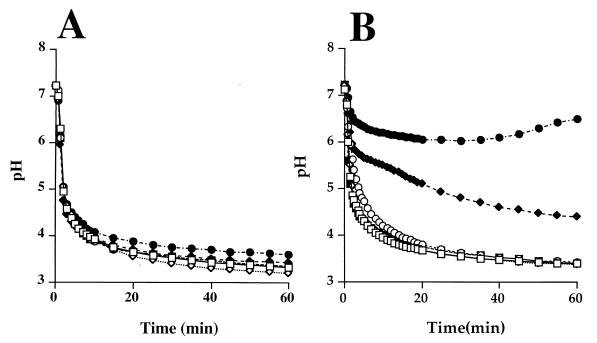
Relationship of urease activity to environmental pH-modulating capacity in vitro.
The ability to modulate the level of activity of ureolytic S. mutans isolates by nickel supplementation and promoter strength allowed for exploration of the relationship between the level of urease activity and the capacity of the strains to retard glycolytic pH reduction. The organisms were grown in the absence of NiCl2 and allowed to metabolize a solution of excess glucose. All strains reduced the suspension pH to a final value of approximately 3.5 at a comparable rate (Fig. 3; some data not shown). Growth in the presence of 25 μM NiCl2 did not reduce the capacity of these strains to metabolize glucose and acidify the environment (Fig. 3). When grown in the presence of NiCl2 and allowed to metabolize glucose and 25 mM urea, S. mutans ACUS4 reduced the extent of the pH fall by approximately 0.5 pH unit (Fig. 3A). The moderation of environmental acidification, as a result of urea metabolism, was more pronounced in the more strongly ureolytic strain, S. mutans ACUS6 (Fig. 3B). Specifically, when grown in the same concentration of NiCl2 (25 μM) and expressing threefold more urease activity than ACUS4 (Fig. 2), cell suspensions of ACUS6 metabolizing glucose and as little as 5 mM urea had a final pH value almost 1.5 pH units higher than did those metabolizing glucose alone. Increasing the concentration of urea to 25 mM resulted in a final cell suspension pH of 6.5 at the end of the experiment (1 h), which was 3.0 pH units higher than that achieved following the metabolism of glucose alone. While it was apparent that the concentration of urea influenced the pH-moderating capacity of the ureolytic strains, the absolute level of urease present was an essential determinant in the depth and duration of the acidification. For example, when ACUS6 was grown in the absence of NiCl2 and allowed to metabolize glucose and 25 mM urea, the final suspension pH was just 0.2 pH unit higher than when it metabolized glucose alone (Fig. 3B). Also, the nonureolytic control strains, S. mutans ACUS5 and ACUS8, had an identical pH drop profile to the wild-type strain, S. mutans UA159 (data not shown). No moderation of pH in the presence of urea was observed using the nonureolytic strains.
FIG. 3.
Moderation of glycolytic acidification as a function of urease activity and urea availability. ACUS6 and ACUS8 were grown in BHI in the absence (open symbols) or presence (solid symbols) of 25 μM nickel chloride to an optical density of ca. 0.6. Glucose was added to the cell suspension to initiate glycolysis together with 0 mM (□, ■), 5 mM (✧, ⧫), or 25 mM (○, ●) urea. Data points were collected every 30 s over a 1-h period. Data points for every second for 15 min, every minute for 5 min, and every 5 min for 40 min are presented. The urease activity expressed by these strains was 0.27 × 10−3 and 23.0 × 10−3 U mg of cell dry weight−1 for ACUS4 and 0.5 × 10−3 and 82.5 × 10−3 U mg of cell dry weight−1 for ACUS6 in the absence and presence of nickel chloride, respectively.
Animal studies.
A small pilot study demonstrated that all the strains would implant and that the level of urease could be modulated by provision of nickel in the drinking water (data not shown). Then, using ACUS6 and the otherwise isogenic, nonureolytic strain ACUS8, a full-scale experiment was undertaken to determine if increases in the levels of urease enzyme in vivo could moderate caries formation. As outlined in Table 2, rats were infected with either a streptomycin-resistant derivative of the wild-type strain, S. mutans UA159 StR, the ureolytic recombinant S. mutans ACUS6, or the nonureolytic recombinant strain S. mutans ACUS8. All animals were provided with Diet 2000 containing 56% sucrose and one of the following: (i) sweetened (5% sucrose) drinking water, (ii) sweetened water supplemented with 50 μM NiCl2, (iii) sweetened water supplemented with 50 mM urea, or (iv) sweetened water supplemented with 50 μM NiCl2 and 50 mM urea. No differences were seen in weight gains of the various groups of animals during the course of the study.
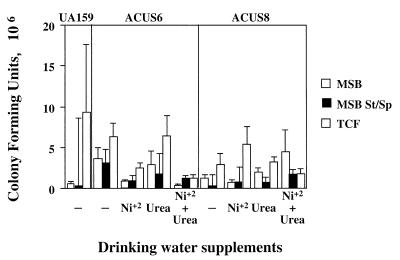
S. mutans ACUS6 and S. mutans ACUS8 implanted and colonized rat dentition as efficiently as did the wild-type strain, S. mutans UA159 StR. Microbiological assessment of the rat jaw microflora determined that the bacterial counts for mutans streptococci and total cultivable flora were not statistically different among the groups of rats (Fig. 4). In addition, the transfer of at least 150 colonies from each group from MSB-spectinomycin agar plates to modified urea agar plates determined that the ureolytic recombinant strains had stably maintained the correct phenotype following passage through the animal host.
FIG. 4.
Microbiological assessment of the microflora on the rat dentition. The right mandible from each animal was sonicated in 5 ml of sterile sodium phosphate buffer (pH 7.0). Diluted (1:100) and undiluted aliquots were plated on MSB to determine total mutans counts, on MSB containing streptomycin and spectinomycin (St/Sp) to determine total recombinant populations, and on SB agar to determine total cultivable flora (TCF). The graph represents the mean bacterial counts for all animals in each group. Error bars represent the standard error of the mean. Statistical analysis was carried out using the Tukey-Kramer HSD test.
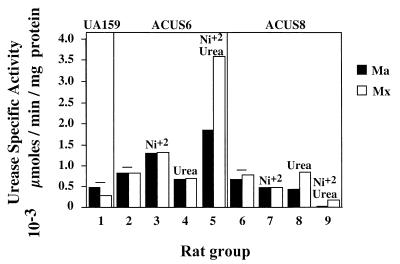
The level of urease activity expressed in the plaques of the experimental animals is presented in Fig. 5. The sonicate from the mandibles and maxillary palate from animals fed the cariogenic diet alone or with just urea provided in the drinking water expressed very low levels of urease activity. In animals infected with the nonureolytic strain, ACUS8, the addition of NiCl2 alone or together with urea to the drinking water did not increase the level of plaque urease specific activity. In contrast, plaques obtained from animals infected with the ureolytic strain, ACUS6, had increased levels of urease activity when NiCl2 was included in the drinking water. Compared to the urease activity in animals fed the cariogenic diet alone, the urease activity increased almost twofold when the animals were provided with NiCl2 and increased fourfold when NiCl2 was given in combination with urea. Notably, the level of urease activity was 20-fold (maxillary palate) and 70-fold (mandibles) higher when ACUS6-infected animals were provided with NiCl2 and urea than when ACUS8-infected animals were given the same dietary supplements.
FIG. 5.
Urease specific activity expressed by S. mutans ACUS6 and ACUS8 in vivo. The left and right mandibles (Ma) from all rats in each group were sonicated in sterile sodium phosphate (pH 7.0). The sonicate was pooled and assayed for urease activity as described above. Similarly, the pooled sonicate from the maxillae (Mx) of all rats in each group was assayed for urease activity. Activity is expressed as units per milligram of protein. Group 1 was infected with the wild-type strain. Groups 2 to 5 were infected with the ureolytic recombinant S. mutans ACUS6, and groups 6 to 9 were infected with the nonureolytic control strain S. mutans ACUS8. The supplements to the drinking water are indicated above the bars in the graph. Groups, 1, 2, and 6 received 5% sucrose-sweetened water (−), the same water supplemented with 50 μM NiCl2 was provided to groups 3 and 7, the same water supplemented with 50 mM urea was provided to groups 4 and 8, and the same water supplemented with both 50 μM NiCl2 and 50 mM urea was provided to groups 5 and 9.
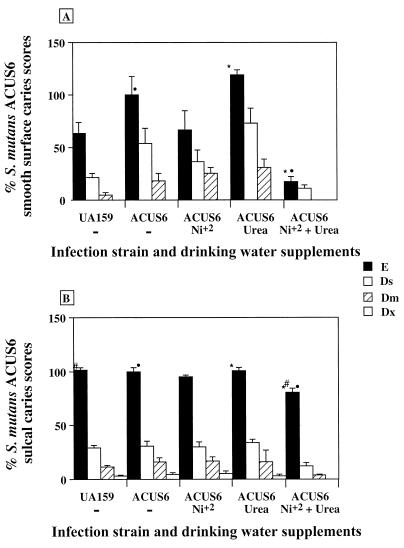
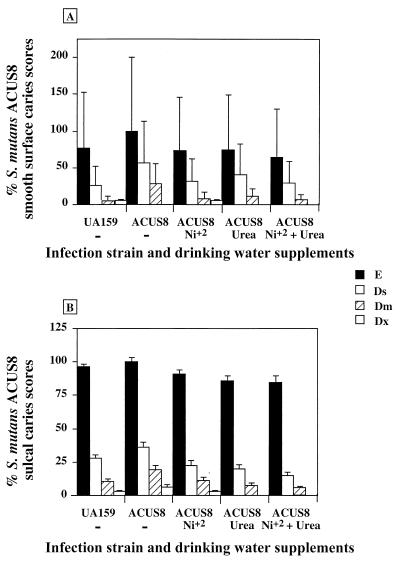
The jaws from all animals were scored for caries by the Keyes method (17). The inactivation of lac and the presence of the antibiotic resistance or urease genes in the recombinant strains did not reduce the cariogenic capacity of S. mutans. S. mutans UA159 StR, ACUS6, and ACUS8 elicited smooth-surface scores of approximately, 9, 14, and 12 (mean scores), respectively, and total sulcal scores of approximately 36, 35, and 37 (mean scores), respectively (Fig. 6 and 7). The addition of only urea to the drinking water had no effect on caries scores for the nonureolytic strain, S. mutans ACUS8. Notably, there was an increase of about 1.2-fold in the smooth-surface caries scores in animals infected with S. mutans ACUS6 in the group receiving only the urea supplement, although the difference was not statistically significant. In contrast, there was an apparent reduction of almost 50% in the total smooth-surface caries scores for animals infected with ACUS6 when the drinking water was supplemented with nickel alone. Similarly, under the same conditions, there was an apparent reduction in smooth-surface caries scores for animals infected with ACUS8, with the greatest decrease in Ds (slight dentinal involvement) caries scores. However, when subjected to the comparatively stringent Tukey-Kramer analysis, the caries scores were not statistically different from those in animals that did not have their drinking water supplemented with NiCl2. In all cases, the addition of NiCl2 to the drinking water had no effect on sulcal caries scores.
FIG. 6.
Mean caries scores from animals infected with the ureolytic recombinant S. mutans ACUS6. All four jaw quadrants from each animal were scored for smooth-surface (A) and sulcal (B) caries by the method of Keyes (17). The graph represents the mean caries score and standard error of the mean from all animals in each group as a percentage of total enamel (E) scores for ACUS6 infected animals fed a cariogenic diet, where 100% = 14 in panel A and 100% = 35 in panel B. All animals were fed a cariogenic diet with sweetened drinking water. Nickel chloride and urea were added to the drinking water as indicated underneath the bar chart. Statistical comparisons were made using the Tukey-Kramer HSD test (P = 0.05). Symbols above the bars indicate groups that are statistically different from control groups in caries scores. Only differences in E scores are indicated. E, total caries; Ds, slight dentinal involvement; Dm, moderate dentinal involvement; and Dx, extensive dentinal involvement.
FIG. 7.
Mean caries scores from animals infected with the nonureolytic recombinant S. mutans ACUS8. All four jaw quadrants from each animal were scored for smooth-surface (A) and sulcal (B) caries. The graph represents the mean caries score and standard error of the mean from all animals in each group as a percentage of total (E) scores for ACUS8-infected animals fed a cariogenic diet, where 100% = 12 in panel A and 100% = 37 in panel B. All animals were fed a cariogenic diet with sweetened drinking water. Nickel chloride and urea were added to the drinking water as indicated underneath the bar chart. Statistical comparisons were made using the Tukey-Kramer HSD test (P ≥ 0.05). The symbols used are identical to those in Fig. 6.
Supplementation of the drinking water with both NiCl2 (to increase urease activity) and urea (as a substrate for base production) resulted in very low caries scores in animals infected with ACUS6. In this case, there was at least an 80% reduction in smooth-surface caries scores relative to those in animals fed the cariogenic diet and sweetened drinking water alone or supplemented with urea. Additionally, there was a significant reduction in total sulcal caries in these animals relative to animals infected with either UA159 and fed the cariogenic diet (21% reduction) and relative to animals infected with ACUS6 and fed the cariogenic diet and sweetened drinking water alone (20% reduction) or drinking water supplemented with only urea (20% reduction). There was no additional reduction in caries in animals harboring the control strain, ACUS8, following the provision of both NiCl2 and urea in the drinking water beyond that seen for the group receiving nickel alone.
DISCUSSION
Previously, a recombinant strain harboring the S. salivarius 57.I ure cluster, S. mutans ACO4, had been constructed and used in vitro to test hypotheses relating pH-moderating potential to ureolytic capacity (12). Those studies established the feasibility of expressing recombinant ure genes in cariogenic plaque bacteria and demonstrated in principle that some minimum level of alkali-generating capacity was necessary to significantly blunt the pH fall due to glycolysis. In ACO4, the ure cluster was plasmid borne and the segregational stability was dependent on maintenance of antibiotic pressure. Once the urease genes were integrated into the chromosome in ACUS4 and ACUS6, they were stably maintained and expressed in the absence of antibiotic selection, without detectable deleterious effects on cell growth or glycolytic capacity. Consequently, as demonstrated in this communication, strains with the ACUS designation were suitable for exploration of the role of ammonia generation in vivo.
The requirement of the ureolytic S. mutans strains for nickel to produce an active urease, as well as the differential level of ure transcription in ACUS4 and ACUS6, turned out to be a major advantage in this study. Simply by modulating the concentration of available NiCl2 in the growth medium, populations of cells were generated that expressed low, intermediate, or high levels of urease activity. In vitro analysis confirmed that increasing the ureolytic capacity of plaque bacteria increased their capacity to moderate acidification by glycolysis and reinforced that some critical level of urease activity was necessary to prevent the fall of the pH to values at which caries occurs. Clearly, ACUS6 produced a sufficient quantity of urease to have a considerable impact on the environmental pH whereas AUCS4 did not. Recombinant strains that could produce a functional urease in the absence of exogenous nickel would be useful in future in vitro and in vivo studies, and efforts to isolate the genes for the S. salivarius nickel uptake system are under way.
The decrease in caries in animals receiving drinking water supplemented with NiCl2 is not entirely surprising. Nickel is a trace element in the biosphere which may be essential for animal nutrition. However, high concentrations of nickel can be highly toxic and carcinogenic, presumably through inhibition of enzyme activities and through DNA damage induced by lipid peroxidation, respectively (32). Notably, the levels of nickel in the drinking water were below that which we have determined to be toxic for S. mutans or to have significant impact on the growth of oral streptococci. Almost assuredly, then, the effects of nickel on caries seen in this study were not exerted through enhancement of urease in naturally ureolytic organisms, since the addition of nickel alone to drinking water did not enhance urease activity in controls. Instead, the decreases in caries formation were probably elicited through the toxicity of nickel to the bacteria and the inhibition of enzymes.
The decision to carry out the rat caries experiments with ACUS6 as the test strain was based on its pH-moderating capacity in the pH drop experiments. Specifically, the level of urease activity achieved by ACUS6 was 0.2 μmol of NH4 produced min−1 mg of protein−1. In this case, the rate of ammonia production from urea was close to the glycolytic rate reported for S. mutans (0.08 to 0.25 μmol of lactate produced mg of cell dry weight−1) (3), so that with sufficient concentrations of urea, ACUS6 would be predicted to produced enough ammonia to neutralize the organic acids produced by glycolysis. Also, the maximum ureolytic rate demonstrated by ACUS6 grown in the presence of nickel was comparable to values predicted by Dibdin and Dawes (13) to be sufficient to moderate plaque acidification, preventing enamel demineralization.
Based on the in vitro studies, ACUS6 should have been only weakly ureolytic in animals which did not receive supplemental NiCl2. Consistent with this prediction was the finding that urease levels in ACUS6-infected rats were comparable to those measured in animals infected with wild-type or the nonureolytic control strain ACUS8 when nickel was absent. In contrast, the provision of nickel and urea to animals harboring ACUS6 increased urease activity significantly and resulted in statistically meaningful reductions in both smooth-surface and sulcal caries. The additional decrease in caries was not observed in ACUS8-infected animals. Thus, the presence of ACUS6 and the production of urease by this strain was the key factor in the inhibition of caries. The most probable mechanism by which caries inhibition occurred was the direct effect of ammonia production on plaque pH. However, another possible effect of the presence of ammonia-producing streptococci may have been to influence the overall ecology of plaque, such that changes in plaque structure or composition also contributed to the decline in caries. Regardless, it is clear that the presence of recombinant, ammonia-producing organisms can moderate the initiation and progression of dental caries in experimental animals.
S. mutans UA159 StR was included in this study as a positive control for the elicitation of dental caries since this strain implants and causes smooth-surface and sulcal caries in rodents (39). Although the values were not statistically different, S. mutans UA159 StR appeared to be modestly less cariogenic on smooth surfaces than the ACUS strains when animals received the control diet only. As indicated, UA159 StR is a spontaneously arising streptomycin-resistant strain of S. mutans. Decreases in the virulence of streptomycin-resistant strains of S. mutans have been reported previously (2). Thus, the most appropriate control for comparison of the cariogenicity of ACUS6 is ACUS8, since these strains harbor the same antibiotic resistance genes, lack lactose catabolism capacity, and differ only in their possession of urease genes.
It appears as though the limiting factor in ammonia generation from urea in the healthy human may be urea, not a lack of enzymatic activity. Specifically, urea is not found in any appreciable quantity in dental plaque (5), probably because it is rapidly hydrolyzed by the relatively high levels of urease present in total samples of plaque and saliva (around 1 U mg [wet weight]−1 in healthy individuals) (34). However, it has also been shown (34) that individuals who produce low levels of urease have a poor capacity to offset glycolytic acidification when provided with urea. It is also important to consider that caries is a site-specific disease and that loss of alkali-generating potential at a carious site could have devastating consequences without having any detectable impact on the total oral ureolytic potential. In the rat caries model, ambient levels of urea provided in saliva were insufficient to elicit dramatic changes in caries, even in the presence of organisms producing an active urease. However, addition to the drinking water, which is ingested only periodically, of as little as 50 mM urea was sufficient to inhibit smooth-surface and sulcal caries, provided that animals were infected with recombinant streptococci producing an active urease. It is also essential to note that inhibition of caries by the urease-producing streptococci occurred in the face of an overwhelming cariogenic challenge. Therefore, it can be concluded that relatively small differences in urea concentrations and in the amount of urease enzyme may dramatically affect caries initiation and progression.
The finding that increasing base production in dental plaque reduces not only smooth-surface caries but also sulcal scores is important. Sulcal surfaces are areas where the diet and acidic breakdown products are likely to be retained, more so than on the smooth surfaces of the teeth; therefore, the sulci are generally subjected to a more severe acid attack and are more prone to caries development. Clinically, sulcal and interproximal caries are far more common than smooth surface caries and fluoride is far less effective against these types of caries. It therefore appears that in contrast to some other caries prevention strategies, enhancement of ammonia generation in plaque may be an effective way to combat the more common types of carious lesions.
Bacterial strains with diminished virulence have been used in replacement strategies to control dental caries. Early studies with variants of S. salivarius showed that less cariogenic bacteria could reduce caries by preempting colonization by more virulent S. mutans strains (36). Implantable strains of S. mutans that are deficient in lactate dehydrogenase (16) and express a heterologous alcohol dehydrogenase gene also appear to be potentially effective for controlling caries by replacement therapy. Other approaches have explored expressing glucanase enzymes in commensal oral streptococci to degrade the adhesive plaque glucans produced by mutans streptococci (21). Use of alkali-generating bacteria should now also be considered potentially useful for replacement therapy. A strain of S. mutans was selected for the present study because of the compatibility with the rat caries model. Future studies could be done with noncariogenic or less cariogenic resident plaque streptococci, since most oral streptococci can be genetically manipulated. In fact, we have constructed ureolytic strains of S. gordonii, showing that the use of other bacterial hosts that colonize the teeth is certainly feasible. One clear advantage that ammonia-producing plaque bacteria have over other approaches is that they may favorably modify the supragingival plaque ecology by fostering an environment that inhibits the emergence of a cariogenic flora, rather than targeting a specific pathogenic agent or perturbing the normal metabolic or physiologic pathways, which could compromise competitive fitness.
A key consideration in the utility of replacement strains is their ability to compete with endogenous strains and the fact that ablation of endogenous activities or introduction of foreign genes can compromise the fitness of a bacterium. Arguably, the introduction of genes which produce ammonia from urea into oral streptococci may instead create strains which are better able to compete than the parent. First, urea can diffuse through the membrane, so that no energy is required to transport this compound. Once cleaved, the ammonia can neutralize the cytoplasm, raising the intracellular pH and creating a diminished requirement for the organisms to spend ATP to extrude protons. Recently, we have also found that the ureolytic oral bacteria S. salivarius (Y. M. Chen, C. A. Weaver, and R. A. Burne, submitted for publication) and A. naeslundii (25) can efficiently use the ammonia from urea as a source of nitrogen. Thus, recombinant ureolytic bacteria may gain a competitive advantage from a bioenergetic standpoint because they can access a source of nitrogen unavailable to other oral bacteria.
The economic importance of dental caries has resulted in a large research effort focusing on preventing caries formation. Fluoridation of water and dental hygiene products are effective but not completely so. Other approaches to control caries include immunization and use of antimicrobial agents. This communication provides compelling evidence to support the idea that increasing the alkali-generating capacity of plaque, perhaps by using recombinant ureolytic bacteria, may help to protect against caries formation. Modulation of the alkali-generating potential of dental plaque may also have great potential because it does not target particular etiologic agents and instead may work by fostering an ecologically healthy oral environment, which naturally controls the emergence of pathogenic microorganisms.
ACKNOWLEDGMENTS
This study was supported by grant DE10362 from the National Institute of Dental and Craniofacial Research.
We thank Margaret Chen for critical reading of the manuscript.
REFERENCES
- 1.Abelson D C, Mandel I D. The effect of saliva on plaque pH in vivo. J Dent Res. 1981;60:1634–1638. doi: 10.1177/00220345810600090101. [DOI] [PubMed] [Google Scholar]
- 2.Bammann L L, Clark W B, Gibbons R J. Impaired colonization of gnotobiotic rats by streptomycin-resistant strains of Streptococcus mutans. Infect Immun. 1978;22:721–726. doi: 10.1128/iai.22.3.721-726.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belli W A, Marquis R E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991;57:1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender G R, Marquis R E. Membrane ATPases and acid tolerance of Actinomyces viscosus and Lactobacillus casei. Appl Environ Microbiol. 1987;53:2124–2128. doi: 10.1128/aem.53.9.2124-2128.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas S D, Kleinberg I. Effect of urea concentration on its utilization, on the pH and the formation of ammonia and carbon dioxide in a human salivary sediment system. Arch Oral Biol. 1971;16:759–780. doi: 10.1016/0003-9969(71)90121-x. [DOI] [PubMed] [Google Scholar]
- 6.Bowden G H, Hamilton I R. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and “S. mitior” growing in continuous culture. Can J Microbiol. 1987;33:824–827. doi: 10.1139/m87-143. [DOI] [PubMed] [Google Scholar]
- 7.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw D J, McKee A S, Marsh P D. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities. J Dent Res. 1989;68:1298–1302. doi: 10.1177/00220345890680090101. [DOI] [PubMed] [Google Scholar]
- 9.Burne R A. Oral streptococci... products of their environment. J Dent Res. 1998;77:445–452. doi: 10.1177/00220345980770030301. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Clancy K A, Burne R A. Streptococcus salivarius urease: genetic and biochemical characterization and expression in a dental plaque streptococcus. Infect Immun. 1996;64:585–592. doi: 10.1128/iai.64.2.585-592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y-Y M, Weaver C A, Mendelsohn D R, Burne R A. Transcriptional regulation of the Streptococcus salivarius 57.I urease operon. J Bacteriol. 1998;180:5769–5775. doi: 10.1128/jb.180.21.5769-5775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clancy A, Burne R A. Construction and characterization of a recombinant ureolytic Streptococcus mutans and its use to demonstrate the relationship of urease activity to pH modulating capacity. FEMS Microbiol Lett. 1997;151:205–211. doi: 10.1111/j.1574-6968.1997.tb12571.x. [DOI] [PubMed] [Google Scholar]
- 13.Dibdin G H, Dawes C. A mathematical model of the influence of salivary urea on the pH of fasted dental plaque and on the changes occurring during a cariogenic challenge. Caries Res. 1998;32:70–74. doi: 10.1159/000016432. [DOI] [PubMed] [Google Scholar]
- 14.Flannagan S E, Zitzow L A, Su Y A, Clewell D B. Nucleotide sequence of the 18-kb conjugative transposon Tn916 from Enterococcus faecalis. Plasmid. 1994;32:350–354. doi: 10.1006/plas.1994.1077. [DOI] [PubMed] [Google Scholar]
- 15.Golub L M, Borden S M, Kleinberg I. Urea content of gingival crevicular fluid and its relationship to periodontal disease in humans. J Periodontal Res. 1971;6:243–251. doi: 10.1111/j.1600-0765.1971.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 16.Hillman J D, Chen A, Snoep J L. Genetic and physiological analysis of the lethal effect of l-(+)-lactate dehydrogenase deficiency in Streptococcus mutans: complementation by alcohol dehydrogenase from Zymomonas mobilis. Infect Immun. 1996;64:4319–4323. doi: 10.1128/iai.64.10.4319-4323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keyes P H. Dental caries in the molar teeth of rats. I. Distribution of lesions induced by high carbohydrate, low-fat diets. J Dent Res. 1958;37:1077–1087. doi: 10.1177/00220345580370060801. [DOI] [PubMed] [Google Scholar]
- 18.Kiska D L, Macrina F L. Genetic regulation of fructosyltransferase in Streptococcus mutans. Infect Immun. 1994;62:1241–1251. doi: 10.1128/iai.62.4.1241-1251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleinberg I. Effect of urea concentrations on human plaque pH in situ. Arch Oral Biol. 1967;12:1475–1484. doi: 10.1016/0003-9969(67)90183-5. [DOI] [PubMed] [Google Scholar]
- 20.Kleinberg I, Kanapka J A, Craw D. Effect of saliva and salivary factors on the metabolism of the mixed oral flora. In: Stiles H M, Loesche W J, O'Brien T C, editors. Microbial aspects of dental caries. Washington, D.C.: Information Retrieval Inc.; 1976. pp. 433–464. [Google Scholar]
- 21.Kubo S, Kubota H, Ohnishi Y, Morita T, Matsuya T, Matsushiro A. Expression and secretion of an Arthrobacter dextranase in the oral bacterium Streptococcus gordonii. Infect Immun. 1993;61:4375–4381. doi: 10.1128/iai.61.10.4375-4381.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margolis H C, Duckworth J H, Moreno E C. Composition of pooled resting plaque fluid from caries-free and caries susceptible individuals. J Dent Res. 1988;67:1468–1475. doi: 10.1177/00220345880670120601. [DOI] [PubMed] [Google Scholar]
- 23.Marquis R E, Burne R A, Parsons D T, Casiano-Colon A C. Arginine deiminase and alkali generation in plaque. In: Bowen W H, Tabak L A, editors. Cariology for the nineties. Rochester, N.Y: University of Rochester Press; 1993. pp. 309–318. [Google Scholar]
- 24.Mobley H L T, Island M D, Hausinger R P. Molecular biology of ureases. Microbiol Rev. 1995;59:451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morou-Bermudez E, Burne R A. Genetic and physiologic characterization of urease of Actinomyces naeslundii. Infect Immun. 1999;67:504–512. doi: 10.1128/iai.67.2.504-512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce E I, Schamschula R G, Cooper M H. Increases in fluoride, calcium, and phosphate in dental plaque resulting from the use of a mineralizing mouthrinse containing urea and monofluorophosphate. J Dent Res. 1983;62:818–820. doi: 10.1177/00220345830620071101. [DOI] [PubMed] [Google Scholar]
- 27.Pearce E I, Wakefield J S, Sissons C H. Therapeutic mineral enrichment of dental plaque visualized by transmission electron microscopy. J Dent Res. 1991;70:90–94. doi: 10.1177/00220345910700021701. [DOI] [PubMed] [Google Scholar]
- 28.Perry D, Kuramitsu H K. Genetic linkage among cloned genes of Streptococcus mutans. Infect Immun. 1989;57:805–809. doi: 10.1128/iai.57.3.805-809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson S, Woodhead J, Crall J. Caries resistance in children with chronic renal failure: plaque pH, salivary pH, and salivary composition. Pediatr Res. 1985;19:796–799. doi: 10.1203/00006450-198508000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Salako N O, Kleinberg I. Incidence of selected ureolytic bacteria in human dental plaque from sites with different salivary access. Arch Oral Biol. 1989;34:787–791. doi: 10.1016/0003-9969(89)90029-0. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch J E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Savolainen H. Biochemical and clinical aspects of nickel toxicity. Rev Environ Health. 1996;11:167–173. doi: 10.1515/reveh.1996.11.4.167. [DOI] [PubMed] [Google Scholar]
- 33.Singer D L, Chaterjee R, Denepitiya L, Kleinberg I. A comparison of the acid-base metabolisms of pooled human dental plaque and salivary sediment. Arch Oral Biol. 1983;28:29–35. doi: 10.1016/0003-9969(83)90023-7. [DOI] [PubMed] [Google Scholar]
- 34.Sissons C H, Cutress T W, Pearce E I. Kinetics and product stoichiometry of ureolysis by human salivary bacteria and artificial mouth plaques. Arch Oral Biol. 1985;30:781–790. doi: 10.1016/0003-9969(85)90132-3. [DOI] [PubMed] [Google Scholar]
- 35.Stephan R M. Changes in hydrogen-ion concentration on tooth surfaces and in carious lesions. J Am Dent Assoc. 1940;27:718–723. [Google Scholar]
- 36.Tanzer J M, Kurasz A B, Clive J. Inhibition of ecological emergence of mutans streptococci naturally transmitted between rats and consequent caries inhibition by Streptococcus salivarius TOVE-R infection. Infect Immun. 1985;49:76–83. doi: 10.1128/iai.49.1.76-83.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theuns H M, van Dijk J W, Driessens F C, Groeneveld A. Effect of the pH of buffer solutions on artificial carious lesion formation in human tooth enamel. Caries Res. 1984;18:7–11. doi: 10.1159/000260740. [DOI] [PubMed] [Google Scholar]
- 38.Trieu-Cuot P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5"-aminoglycoside phosphotransferase type III. Gene. 1983;23:331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- 39.Wexler D L, Penders J E, Bowen W H, Burne R A. Characteristics and cariogenicity of a fructanase-defective Streptococcus mutans strain. Infect Immun. 1992;60:3673–3681. doi: 10.1128/iai.60.9.3673-3681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]