Abstract
Rationale
The long-term effects of using a high-flow nasal cannula for chronic hypercapnic respiratory failure caused by chronic obstructive pulmonary disease remain unclear.
Objectives
To assess whether long-term high-flow nasal cannula use reduces the number of exacerbations and improves other physiological parameters in patients with chronic hypercapnic respiratory failure caused by chronic obstructive pulmonary disease.
Methods
We enrolled 104 participants (aged ⩾40 yr) with daytime hypercapnia (Global Initiative for Chronic Obstructive Lung Disease stages 2–4) receiving long-term oxygen therapy (⩾16 h/d for ⩾1 mo) and randomly assigned them to high-flow nasal cannula/long-term oxygen therapy and long-term oxygen therapy groups. The primary endpoint was the moderate or severe exacerbation rate. We compared changes from baseline in arterial blood gas values, peripheral oxygen saturation, pulmonary function, health-related quality-of-life scores, and the 6-minute-walk test.
Measurements and Main Results
High-flow nasal cannula use significantly reduced the rate of moderate/severe exacerbations (unadjusted mean count 1.0 vs. 2.5, a ratio of the adjusted mean count between groups [95% confidence interval] of 2.85 [1.48–5.47]) and prolonged the duration without moderate or severe exacerbations. The median time to first moderate or severe exacerbation in the long-term oxygen therapy group was 25 (14.1–47.4) weeks; this was not reached in the high-flow nasal cannula/long-term oxygen therapy group. High-flow nasal cannula use significantly improved health-related quality of life scores, peripheral oxygen saturation, and specific pulmonary function parameters. No safety concerns were identified.
Conclusions
A high-flow nasal cannula is a reasonable therapeutic option for patients with stable hypercapnic chronic obstructive pulmonary disease and a history of exacerbations.
Clinical trial registered with www.umin/ac.jp (UMIN000028581) and www.clinicaltrials.gov (NCT03282019).
Keywords: chronic obstructive pulmonary disease, hypercapnia, oxygen inhalation therapy, pulmonary disease, respiratory insufficiency
At a Glance Commentary
Scientific Knowledge on the Subject
Advanced-stage chronic obstructive pulmonary disease leads to chronic respiratory insufficiency, which is associated with high mortality and exacerbation recurrence. Several clinical trials have shown the potential efficacy of high-flow nasal cannula oxygen therapy for chronic hypercapnic respiratory failure caused by chronic obstructive pulmonary disease; however, the long-term effects remain unclear.
What This Study Adds to the Field
This is the first randomized clinical trial to evaluate the number of severe or moderate exacerbations in patients with stable hypercapnic chronic obstructive pulmonary disease who were randomly administered either long-term oxygen therapy alone or long-term oxygen therapy plus high-flow nasal cannula oxygen therapy for 52 weeks. Our study revealed that treatment with domiciliary high-flow nasal cannula oxygen therapy reduced the number of exacerbations and prolonged the period of chronic obstructive pulmonary disease without moderate or severe exacerbations. Our findings suggest that the use of domiciliary high-flow nasal cannula oxygen therapy may be an appropriate therapeutic option for this patient population.
Advanced-stage chronic obstructive pulmonary disease (COPD) causes severe airflow limitations, severely limited performance, and systemic complications (1), progressively leading to chronic respiratory insufficiency, which is often characterized by hypercapnia or hypoxia. Once patients develop chronic hypercapnic respiratory failure (CHRF), their prognosis and condition worsen. Moreover, they tend to develop exacerbations, which are associated with high mortality and exacerbation recurrence (2).
High-flow nasal cannula (HFNC) oxygen therapy is a gas delivery system that provides heated, humidified air via a nasal cannula, with supplemental oxygen, as required. HFNC creates a low level of positive airway pressure, has a washout effect on the pharyngeal dead space, decreases inspiratory resistance, and can improve mucus clearance (3–5). Through their physiological effect, short-term use of an HFNC improves the breathing pattern and reduces inspiratory effort and hypercapnia in patients with stable hypercapnic COPD (6, 7). HFNC use has been widely studied in adult ICUs, among patients with acute hypoxemic respiratory failure (8) or acute hypercapnic respiratory failure (9), and after extubation (10).
A recent meta-analysis of several randomized trials (11) showed that HFNC reduced the PaCO2 and number of exacerbations in patients with stable COPD and improved health-related quality of life (HRQOL). However, most current studies have focused on the short-term effects of HFNC, and data on its long-term effects are sparse. In a randomized controlled trial that assessed the effect of 12-month HFNC use in an at-home setting in Denmark (12), consistent HFNC use reduced the number of COPD exacerbations and hospitalizations. This Danish study recruited patients with COPD with chronic hypoxemic respiratory failure, with only half of them being hypercapnic at inclusion. In another randomized trial (13), HFNC significantly reduced the number of days with exacerbation and increased the time to the first exacerbation in a mixed population of patients with COPD and bronchiectasis for 12 months. However, it remains unclear whether long-term HFNC use can be effective and safe for patients with the most severe COPD with CHRF.
Therefore, we hypothesized that long-term HFNC use in patients with COPD with CHRF would reduce the number of COPD exacerbations and improve mortality rates, HRQOL, and physiological parameters. Some of the results of these studies have been reported previously in the form of a preprint (14).
Methods
This randomized controlled trial included patients from 42 Japanese hospitals and grouped them equally to either long-term oxygen therapy (LTOT) alone or LTOT with domiciliary HFNC (HFNC/LTOT). Table E1 in the online supplement summarizes the inclusion and exclusion criteria. Briefly, individuals aged 40 years or older with daytime hypercapnia (PaCO2 ⩾45 mm Hg and pH ⩾7.35) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) (15) stages 2–4 disease receiving LTOT for at least 16 hours per day for at least 1 month before providing informed consent were recruited. Patients were required to have had an exacerbation (moderate or severe as judged by the investigators) within the past 1 year. Patients were in a stable condition and free from a COPD exacerbation (of any severity) within the 4 weeks before enrollment. We excluded patients who had used nocturnal noninvasive ventilation (NIV) within the past 4 weeks or an HFNC within the past 1 year and those with a history of obstructive sleep apnea syndrome or highly suspected to have obstructive sleep apnea syndrome on the basis of clinical findings. All patients were receiving optimal medical therapy according to GOLD guidelines (15) in addition to LTOT at the time of enrollment. Changes in medication, the commencement of rehabilitation, or use of NIV were not permitted throughout the study period, except temporarily (<14 d) in patients with COPD exacerbations. The study was approved by the Kobe University Clinical Research Ethical Committee (C180079). Written informed consent was obtained from all patients before participation. This trial was registered with the University Hospital Medical Information Network Clinical Trials Registry (www.umin/ac.jp) (UMIN000028581) before enrollment of the first study participant and with ClinicalTrials.gov (NCT03282019).
Interventions
Patients who met the eligibility criteria were randomized to receive either LTOT or domiciliary HFNC/LTOT; all continued their prescribed LTOT during the daytime. The HFNC/LTOT group used HFNC for ⩾4 h/night during sleep at flow rates of 30–40 L/min combined with LTOT and the myAIRVO 2 device during the daytime, if they preferred. The HFNC was administered using this device, which provides humidification and high-flow medical gas via an Optiflow nasal cannula interface (Fisher and Paykel Healthcare). The investigator could adjust the nocturnal oxygen flow rate to maintain an oxygen saturation as measured by pulse oximetry (SpO2) ⩾88%. If patients reported discomfort with HFNC use, the investigator could downtitrate the flow rate to a minimum of 20 L/min. The set temperature was initiated at 37°C. If the participants complain of discomfort due to high temperature, the temperature may be used at 34°C or 31°C. Before initiating HFNC, doctors and nurses trained and habituated participants to the HFNC.
Follow-Up and Measurements
All participants were seen in follow-up throughout the 52-week study period, and study visits were scheduled every 4 weeks. Participants completed a daily diary to record any worsening in upper respiratory tract symptoms (e.g., nasal discharge and sore throat), worsening in lower respiratory tract symptoms (e.g., dyspnea, sputum, cough, and wheezing), fever, or use of systemic corticosteroids or antibiotics (16). A COPD exacerbation was diagnosed if one of the following symptom patterns was experienced for at least 2 consecutive days: at least two of three major symptoms (worsening of dyspnea, sputum purulence, or sputum volume) or any one major symptom in addition to one minor symptom (worsening of nasal discharge, wheezing, sore throat, cough, or fever) (17). In case of an interval between a consecutive and next consecutive COPD exacerbation, and if the interval was ⩽7 days, two consecutive COPD exacerbations were counted as a COPD exacerbation. A mild COPD exacerbation was defined as being resolved without use of systemic corticosteroids or antibiotics. A moderate COPD exacerbation was defined as necessitating treatment with systemic corticosteroids and/or antibiotics. Systemic corticosteroids and antibiotics were administered if deemed necessary by the principal investigator, subinvestigator, or treating physician for up to 14 days. Administration of treatment within 7 days of completion of previous therapy was considered to have been for the same exacerbation. A severe COPD exacerbation was defined as requiring hospitalization, including an emergency admission. The central judgment committee assessed the presence and severity of COPD exacerbations based on a daily diary.
At each visit, doctors verified the COPD treatment regimen and adherence to HFNC use by checking the total time spent on the myAIRVO 2 device from the device data (for the HFNC/LTOT group alone) and evaluated any adverse events (AEs), hospital admissions, and SpO2. In addition, participants underwent HRQOL (18, 19), sleep quality, and dyspnea (20–23) assessments; pulmonary function testing (24, 25); and a 6-minute-walk test (American Thoracic Society guidelines) (26) at 12, 24, and 52 weeks at the admission or outpatient clinic. A deviation of up to 1 week was permitted. Detailed follow-up and measurements are provided in the online supplement.
Study Outcomes
The primary endpoint was the rate of moderate/severe COPD exacerbations over the 52-week period. The secondary endpoints were the time to the first COPD exacerbation (all severity degrees, moderate/severe, and severe only), changes in physiological parameters, and others (Table E2). In terms of initiation of NIV, there were no criteria specific to this study, and the principal investigator, subinvestigator, or treating physician made their decisions on the basis of Japanese guidelines (27). Among these definitions, changes represented the differences in values between baseline and at each scheduled measurement.
Statistical Methods
To calculate ratios of the adjusted means of exacerbation counts between the groups, we used a multivariate generalized linear regression model with a negative binomial distribution controlling sex, GOLD stage, and age as covariates. We also used Kaplan-Meier analysis, mixed models repeated measurements (MMRMs), Fisher’s exact test, t test, and analysis of covariance. Descriptive statistics summarized the means and SDs. We also compared the group differences in the measurements between the baseline and each visit with the least squared mean (LSM) by MMRM.
All analyses were performed using SAS 9.4 (SAS Institute) and Ri386 3.4.3 (www.r-project.org/). The significance level was 0.05, and we did not adjust it for repeated tests, considering this study’s exploratory nature. We defined the safety and full analysis sets as data sets intended to analyze “patient backgrounds and AEs” and “primary and secondary endpoints,” respectively. The online supplement provides the details of the statistical analysis.
Sample Size Determination
We aimed to recruit 120 patients, considering approximately 10% of potential dropouts and patient recruitment feasibility. The minimum evaluable size/treatment was 53 patients. We determined this sample size because it could detect an effect size of one COPD exacerbation (moderate or severe) per year, considering previous articles (12, 13). Thus, this sample size can detect the difference in the mean counts between the groups with a power of 90% and a two-tailed type I error rate of 5%. We also assumed that a population SD was 1.58, corresponding to a mean exacerbation count of 2.5 per year (12, 13, 16), considering that the count can follow a Poisson-type distribution with which the mean is equal to the variance.
Results
Patients
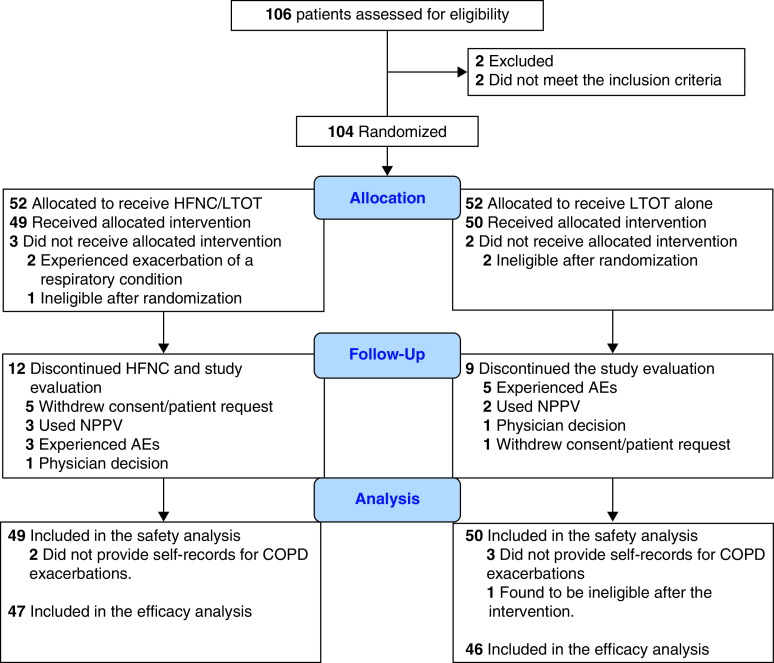
We initially enrolled 104 patients. Five were excluded because of a lack of study treatments. The remaining 99 patients were included in the safety set. Because 6 patients did not provide self-records for COPD exacerbations, we excluded them from the safety set; therefore, there were 93 patients in the full analysis set (HFNC/LTOT, 47 patients; LTOT, 46 patients; Figure 1). All patients in the safety and full analysis sets provided safety and efficacy data for our intention-to-treat basis analysis. Twelve patients in the HFNC/LTOT group and nine patients in the LTOT group terminated the evaluation of the interventions. Table 1 shows the patients’ demographic data, smoking histories, GOLD stages, and currently prescribed drugs; there were no statistically significant differences between the groups.
Figure 1.
Flow diagram of participant inclusion and exclusion. AE = adverse event; COPD = chronic obstructive pulmonary disease; HFNC = high-flow nasal cannula oxygen therapy; LTOT = long-term oxygen therapy; NPPV = noninvasive positive pressure ventilation.
Table 1.
Patient Demographic Data, Smoking Status, Global Initiative for Chronic Obstructive Lung Disease Stage, and Prescribed Drugs
| Characteristic | HFNC/LTOT, n (%) | LTOT, n (%) |
|---|---|---|
| Sex | ||
| Male | 44 (89.8) | 44 (88.0) |
| Female | 5 (10.2) | 6 (12.0) |
| Age, yr | ||
| Mean | 72.9 | 75.16 |
| SD | 7.43 | 6.67 |
| BMI, kg/m2 | ||
| Mean | 20.21 | 20.38 |
| SD | 3.57 | 3.64 |
| Smoking history | ||
| Current | 0 (0.0) | 2 (4.0) |
| Past | 48 (98.0) | 48 (96.0) |
| Never | 1 (2.0) | 0 (0.0) |
| Cigarettes per day, n | ||
| Mean | 31.67 | 30 |
| SD | 14.7 | 13.85 |
| Yr | ||
| Mean | 39.63 | 39.84 |
| SD | 11.17 | 9.35 |
| GOLD stage | ||
| 2 | 1 (2.0) | 4 (8.0) |
| 3 | 10 (20.4) | 11 (22.0) |
| 4 | 38 (77.6) | 35 (70.0) |
| LAMA* | ||
| N | 1 (2.0) | 7 (14.0) |
| Y | 48 (98.0) | 43 (86.0) |
| LABA* | ||
| N | 2 (4.1) | 4 (8.0) |
| Y | 47 (95.9) | 46 (92.0) |
| Inhaled steroids* | ||
| N | 22 (44.9) | 19 (38.0) |
| Y | 27 (55.1) | 31 (62.0) |
| Arterial blood gas | ||
| pH | ||
| Mean | 7.38 | 7.39 |
| SD | 0.02 | 0.02 |
| PaCO2 | ||
| Mean | 51.38 | 50.50 |
| SD | 4.96 | 5.03 |
| PaO2 | ||
| Mean | 80.37 | 84.10 |
| SD | 21.75 | 21.85 |
| Pulmonary function | ||
| FEV1 | ||
| Mean | 0.64 | 0.66 |
| SD | 0.22 | 0.19 |
| Percent predicted FEV1 | ||
| Mean | 25.59 | 27.08 |
| SD | 8.40 | 8.94 |
| FEV1/FVC | ||
| Mean | 32.65 | 32.53 |
| SD | 8.86 | 10.14 |
Definition of abbreviations: BMI = body mass index; GOLD = Global Initiative for Chronic Obstructive Lung Disease; HFNC = high-flow nasal cannula oxygen therapy; LABA = long-acting β-agonist; LAMA = long-acting muscarinic agent; LTOT = long-term oxygen therapy.
Duplicate counts.
Treatments
The mean ± SD duration of HFNC/LTOT use was 7.3 ± 3.0 h/d. During the study, including baseline data, the mean ± SD oxygen flow rates in the HFNC/LTOT and LTOT groups at rest were 1.53 ± 0.95 and 1.64 ± 1.00 L/min, respectively (P = 0.577). The mean ± SD total flow rate in the HFNC/LTOT group was 28.5 ± 4.57 L/min.
COPD Exacerbation Rate
The unadjusted sample means (HFNC/LTOT and LTOT) of the rates of 1) all-severity, 2) moderate/severe, and 3) severe-only COPD exacerbations were 3.8 and 5.3, 1.0 and 2.5, and 0.3 and 0.5, respectively (Figure E1). The rate data followed negative binomial distributions.
The multivariate generalized linear regression model showed that the adjusted ratios (95% confidence intervals) of the mean exacerbation count in the LTOT group compared with that in the HFNC/LTOT group were 1.40 (0.91–2.16), 2.85 (1.48–5.47), and 1.54 (0.74–3.22) for 1), 2), and 3), respectively, in the preceding paragraph. Regarding the primary endpoint, the adjusted mean rate of moderate/severe exacerbations in HFNC/LTOT was better than that in LTOT (P = 0.002), and the estimated statistical power was 0.72. The P values for treatment effects for 1) and 3) were statistically insignificant, and these outcomes belonged to secondary endpoints.
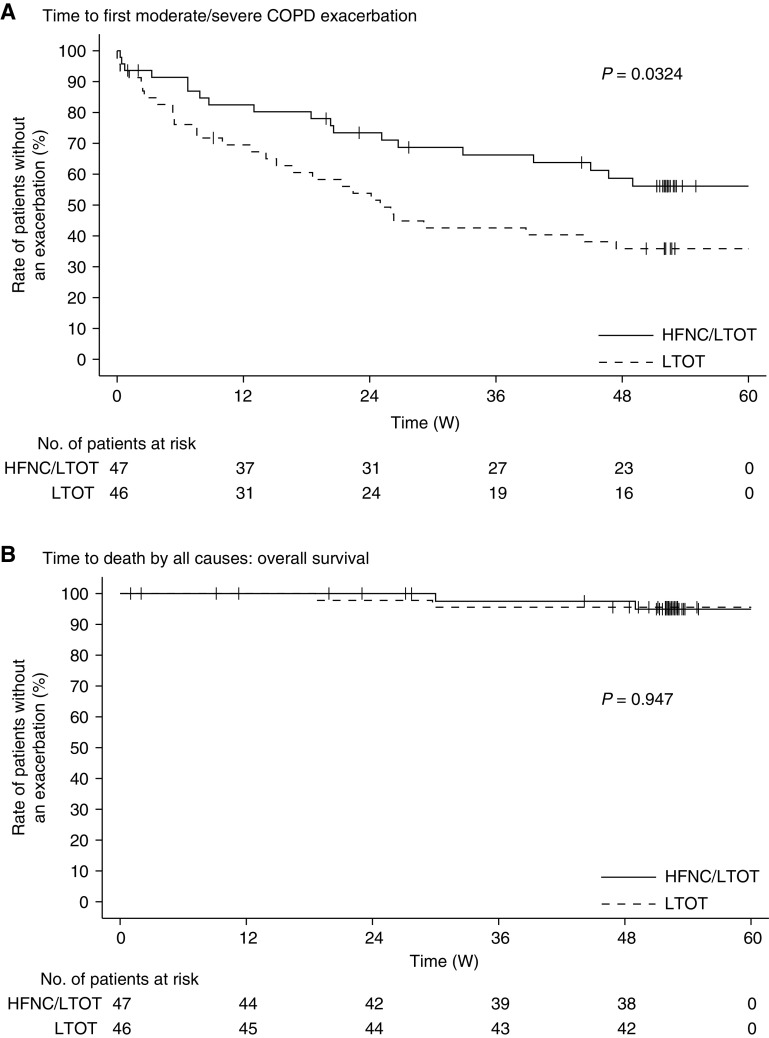
Time to the First Moderate/Severe COPD Exacerbation and Overall Survival
On the basis of findings of the log-rank test for the time to the first moderate or severe COPD exacerbation, we rejected the null hypothesis that the two survival curves were identical (P = 0.032) (Figure 2). The median time to first moderate/severe COPD exacerbation (95% confidence interval) in the LTOT group was 25 (14.1–47.4) weeks; however, the median time to first moderate/severe COPD exacerbation was not reached in the HFNC/LTOT group. The rates (i.e., percentages) of patients without an exacerbation at the 52nd week in the LTOT and HFNC/LTOT groups were 35.9% and 56.1%, respectively. Conversely, we could not reject the null hypothesis for overall survival (P = 0.947). Two patients in each treatment group died during the study. The overall survival rates (percentages) at the 52nd week in the LTOT and HFNC/LTOT groups were 95.6% and 94.9%, respectively. The median time to death was not reached in either of the treatment groups.
Figure 2.
Time to the first moderate or severe chronic obstructive pulmonary disease (COPD) exacerbation and overall survival (OS). The upper (A) and lower (B) graphs show the Kaplan-Meier curves for the time to the first moderate or severe COPD exacerbation and OS, respectively. The solid and broken lines indicate the high-flow nasal cannula oxygen therapy/long-term oxygen therapy (HFNC/LTOT) and LTOT-alone groups, respectively. W = weeks.
St. George’s Respiratory Questionnaire for COPD: Total, Symptom, Activity, and Impact Scores and other HRQOL Scores
Only the total score at 24 weeks (P = 0.044) and the impact score at 12 weeks (P = 0.028) showed significant differences in the LSMs between the groups (Figure 3). The LSMs (95% confidence intervals) of the total impact scores for the HFNC/LTOT and LTOT groups at this point were −3.57 (−8.19, 1.04) and 3.81 (−0.75, 8.36), respectively. There were no significant differences in quality-adjusted life-years between the treatment groups (P = 0.270). At 12, 24, and 52 weeks (Figure 3), we did not observe any statistically significant differences in the LSMs of changes in severe respiratory insufficiency scores or Pittsburgh Sleep Quality Index–Japanese version scores between the groups (all P > 0.05). There were no significant differences in the modified Medical Research Council scores between the groups at baseline or at 12, 24, or 52 weeks (all P > 0.05).
Figure 3.
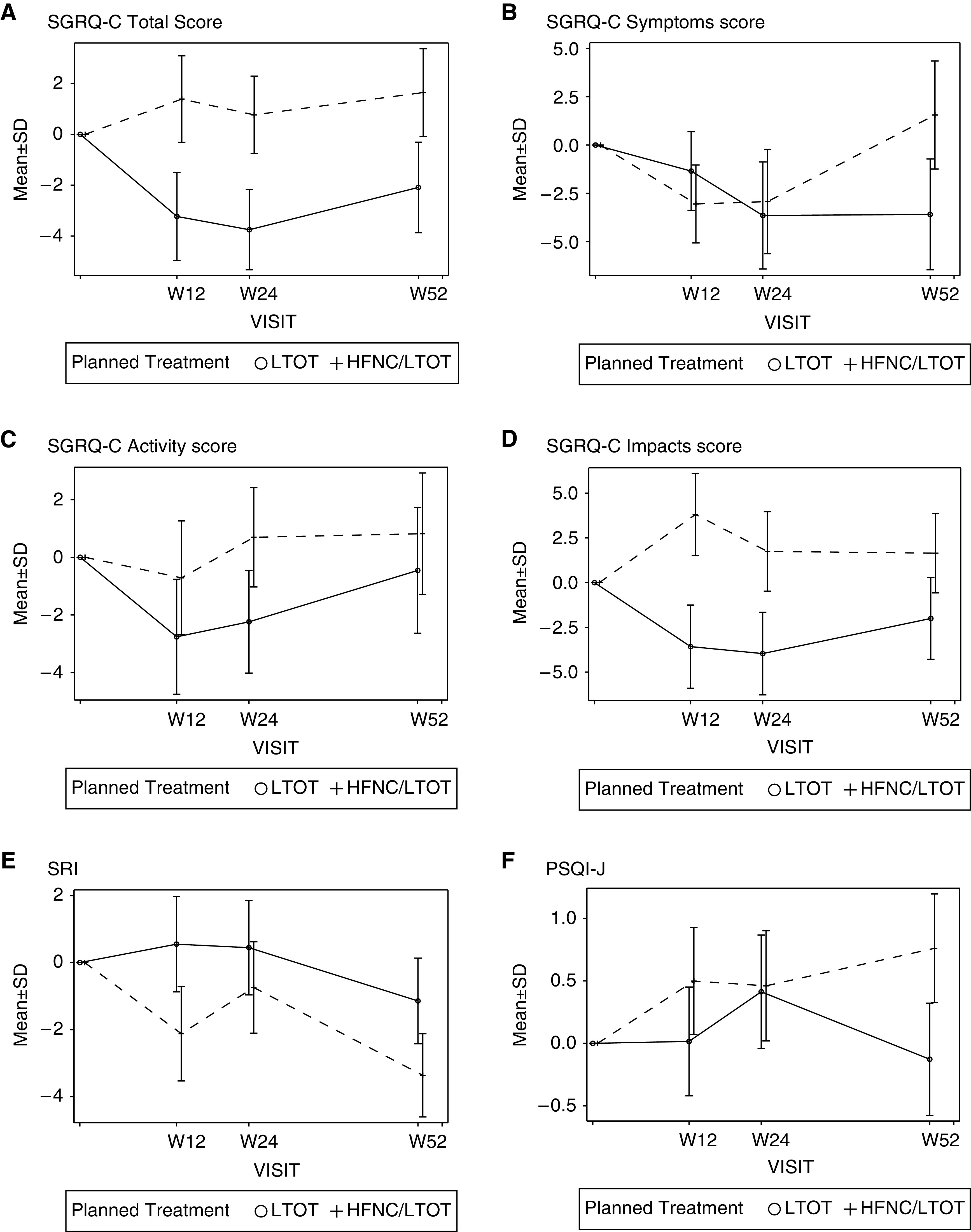
Least squared means (LSMs) with SEs for health-related quality of life (HRQOL) scores. (A) St. George’s Respiratory Questionnaire for Chronic Obstructive Pulmonary Disease (SGRQ-C) total score. (B) SGRQ-C symptom score. (C) SGRQ-C activity score. (D) SGRQ-C impact score. (E) Severe Respiratory Insufficiency (SRI) Questionnaire score. (F) Pittsburgh Sleep Quality Index–Japanese version (PSQI-J) score. Solid and broken lines indicate the high-flow nasal cannula oxygen therapy/long-term oxygen therapy (HFNC/LTOT) and LTOT-alone groups, respectively. W = weeks.
Arterial Blood Gas Analyses and SpO2
Table 2 and Table E3 in the online supplement show box plots and descriptive statistics for arterial blood gas measurements, respectively. There were no significant differences in the mean values, except for the PaCO2 at 12 weeks (P = 0.039); however, we could not confirm this significant difference using the LSM with MMRM (P = 0.058). The unadjusted mean ± SD of the differences in the PaCO2 between baseline and 12, 24, and 52 weeks in the HFNC/LTOT group were −1.65 ± 4.9, −0.86 ± 5.28, and 0.54 ± 7.23, respectively, and those in the LTOT group were 0.69 ± 5.28, 0.72 ± 5.33, and 1.54 ± 6.13, respectively (between treatments, P = 0.039, 0.181, and 0.520, respectively). At baseline, the sample mean ± SE of SpO2 values for the HFNC/LTOT and LTOT groups were 94.8 ± 3.15 and 95.3 ± 2.51, respectively, with no significant difference (P = 0.331). Figure E2 in the online supplement shows the LSM changes in SpO2 at observation points (4–52 wk). We observed statistically significant differences between the treatment groups only at 52 weeks (HFNC/LTOT vs. LTOT LSM ± SE, 1.01 ± 0.33% vs. −0.20 ± 0.32%, respectively).
Table 2.
Summary of Results for Primary and Selected Secondary Endpoints at 52 Weeks
| Endpoints | Items | Statistics | HFNC/LTOT (n = 47) |
n | LTOT (n = 46) |
n | P Values |
|---|---|---|---|---|---|---|---|
| Primary | COPD exacerbation rate (moderate/severe) | Ratio of the mean count (95% CI) | Reference level | 2.85 (1.48–5.47) | 0.002 | ||
| Unadjusted mean count (SD) | 1.0 (1.8) | 2.5 (3.8) | |||||
| Secondary | COPD exacerbation rate (severe) | Ratio of the mean count (95% CI) | Reference level | 1.54 (0.74–3.22) | 0.250 | ||
| Unadjusted mean count (SD) | 0.3 (0.5) | 0.5 (0.9) | |||||
| COPD exacerbation rate (all) | Ratio of the mean count (95% CI) | Reference level | 1.40 (0.91–2.16) | 0.126 | |||
| Unadjusted mean count (SD) | 3.8 (4.0) | 5.3 (4.4) | |||||
| Modified MRC scale score at 52 wk | Count | Grade 0 | 0 | Grade 0 | 0 | 0.922 | |
| Grade 1 | 2 | Grade 1 | 3 | ||||
| Grade 2 | 10 | Grade 2 | 9 | ||||
| Grade 3 | 17 | Grade 3 | 21 | ||||
| Grade 4 | 7 | Grade 4 | 7 | ||||
| SpO2, %* | LSM (SE) | 1.01 ± 0.33% | 37 | −0.20 ± 0.32% | 40 | 0.010 | |
| pH† | Sample mean (SD) | 7.38 (0.03) | 37 | 7.38 (0.04) | 38 | 0.118 | |
| Difference‡ | 0.00 (0.03) | 37 | −0.01 (0.03) | 38 | |||
| PaCO2† | Sample mean (SD) | 50.87 (8.28) | 37 | 51.65 (8.57) | 38 | 0.520 | |
| Difference‡ | 0.54 (7.23) | 37 | 1.54 (6.13) | 38 | |||
| PaO2† | Sample mean (SD) | 84.82 (23.36) | 37 | 77.37 (14.53) | 38 | 0.063 | |
| Difference‡ | 4.21 (26.51) | 37 | −7.04 (24.94) | 38 | |||
| VC† | Sample mean (SD) | 2.24 (0.50) | 36 | 2.27 (0.61) | 39 | 0.351 | |
| Difference‡ | −0.04 (0.27) | 35 | 0.03 (0.33) | 36 | |||
| FVC† | Sample mean (SD) | 2.05 (0.56) | 36 | 2.07 (0.63) | 39 | 0.888 | |
| Difference‡ | −0.02 (0.31) | 35 | −0.03 (0.33) | 38 | |||
| FEV1† | Sample mean (SD) | 0.66 (0.25) | 36 | 0.65 (0.21) | 39 | 0.265 | |
| Difference‡ | 0.00 (0.07) | 35 | −0.02 (0.08) | 39 | |||
| DlCO† | Samplex mean (SD) | 6.90 (2.21) | 30 | 6.18 (3.32) | 28 | 0.850 | |
| Difference‡ | −0.35 (1.68) | 21 | −0.46 (2.04) | 15 | |||
| 6-min-walk distance† | Sample mean (SD) | 238.5 (88.62) | 32 | 222.25 (109.96) | 36 | 0.177 | |
| Difference‡ | 8.80 (72.17) | 31 | −12.85 (55.34) | 34 | |||
| Time to NIV† | Sample mean (SD) | 188.0 ± 8.9 | 3 | 234.7 ± 178.3 | 3 | NA |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; HFNC = high-flow nasal cannula oxygen therapy; LSM = least squares mean; LTOT = long-term oxygen therapy; MRC = Medical Research Council; NA = not applicable; NIV = noninvasive ventilation; SpO2 = oxygen saturation as measured by pulse oximetry; VC = vital capacity.
LSM at 52 weeks.
Changes in the sample means at 52 weeks.
Difference between the baseline and 52-week data.
Pulmonary Function
Between the HFNC/LTOT and LTOT groups, we observed statistically significant differences (mean ± SE) only in FVC (2.14 ± 0.54 vs. 2.07 ± 0.62 L, respectively; P = 0.017) and percent predicted FVC (66.74 ± 15.74% vs. 65.41 ± 17.79%, respectively; P = 0.015) at 24 weeks and in FEV1 (0.68 ± 0.23 vs. 0.65 ± 0.21 L, respectively; P = 0.045) and percent predicted FEV1 (26.89 ± 9.23% vs. 26.86 ± 9.32%, respectively; P = 0.026) at 12 weeks (Table E4). There were no other statistically significant differences among the treatment groups at any observation point.
Six-Minute-Walk Test
At 12, 24, and 52 weeks, no significant differences were observed between the treatment groups in the mean values of changes in walking distance; between pre- and posttest SpO2; in walking distance between baseline and 12, 24, and 52 weeks; and in the modified Borg scores (all P > 0.05).
Time to NIV
Only three patients in each treatment group required long-term NIV. The mean ± SE values of NIV duration for the HFNC/LTOT and LTOT groups were 188.0 ± 8.9 and 234.7 ± 178.3 days, respectively. Univariate analysis could not be performed, considering that the number of patients associated with this event was limited.
AEs
Most AEs of at least a moderate degree occurred in several patients in the HFNC/LTOT and LTOT groups: infections and infestations and respiratory, thoracic, and mediastinal disorders 26.5% versus 32.0% and 38.8% versus 42.0%, respectively (Table E5).
Discussion
We evaluated the efficacy and safety of HFNC combined with LTOT with LTOT alone in patients with hypercapnic COPD over 52 weeks and found that HFNC/LTOT could reduce the number of moderate or severe COPD exacerbations and prolong the duration without moderate or severe COPD exacerbations. The St. George’s Respiratory Questionnaire for COPD (SGRQ-C) total (Week 24) and impact (Week 12) scores, SpO2 (Week 52), FVC/percent predicted FVC (Week 24), and FEV1/percent predicted FEV1 (Week 12) were variables that showed statistically significant differences between the treatment groups. AEs were infrequent in both groups, suggesting that HFNC/LTOT is a safe treatment. To the best of our knowledge, this is the first study demonstrating that HFNC may be effective and safe for most patients with severe COPD and CHRF.
Previous studies conducted in similar settings support our finding that domiciliary HFNC can reduce the number of moderate or severe COPD exacerbations. In a randomized trial, Storgaard and colleagues (12) reported that 12 months of HFNC in a home setting could reduce the numbers of COPD exacerbations and hospitalizations in patients with chronic hypoxic COPD; however, only half of these patients had hypercapnia. In another randomized trial, Rea and colleagues (13) demonstrated that HFNC significantly reduced the number of exacerbation days and increased the time to the first exacerbation in a mixed population of patients with COPD and bronchiectasis.
This study, however, differs in several important ways from the previous studies. Our study involved the novel rationale that the most severely sick subgroup of patients with COPD would be the most likely to benefit from domiciliary HFNC. Therefore, this study enrolled only patients with severe COPD with chronic hypercapnia and hypoxia, using LTOT at baseline, which is an important difference from the previous trials. In addition, adherence to HFNC use was relatively good in this trial (mean ± SD, 7.3 ± 3.0 h/d) compared with that in previous trials. An HFNC was used only 1–2 h/d in the study by Rea and colleagues (13), and its use was continued during the observation period in only half of the patients in the study by Storgaard and colleagues (12). Last, the number of institutions from which participants were enrolled was higher in this study; thus, the results can be generalized to a larger population.
The rate of moderate or severe COPD exacerbations decreased in this study; however, the effects on physiological parameters and HRQOL were modest. Moreover, the favorable changes in the SGRQ-C total and impact scores, SpO2, and some pulmonary function parameters were only transitory. Therefore, these changes cannot clearly explain the observed improvement in COPD exacerbations. SpO2 significantly improved at 52 weeks; however, a longer observation period is necessary to confirm whether this improvement persists. Nevertheless, the reduction in COPD exacerbations may have resulted from the combined effects of those favorable physiological changes.
Worsened airflow (lower FEV1) is associated with an increased risk of COPD exacerbations; however, the role of other potential contributors, including a prior history of exacerbations, poor quality of life, chronic hypercapnia, impaired sleep quality, and chronic mucus hypersecretion, has been reported (28, 29). Considering these predisposing factors, it is reasonable to associate a combination of multiple factors (although each factor’s contribution is limited) with reduced COPD exacerbations. Furthermore, the method of maintaining a daily diary for assessing COPD exacerbations may be more sensitive than other parameters for detecting changes in disease. Moreover, the improvement in pulmonary function parameters observed in the HFNC group may have resulted from reduced COPD exacerbations, which could accelerate lung function decline (30). Conversely, several parameters, such as HRQOL and the 6-minute-walk test, may take a longer time to improve because they have been established over a long period.
Despite the significant improvements in the SGRQ-C and PaCO2 in our pilot trial (31), in this trial, there were nonsignificant improvements of only 1–2 mm Hg in the PaCO2 and 4–8 points (these changes were larger than the minimal clinically important difference of 4 units [32]) in the SGRQ-C total score. These improvements are smaller than those reported in previous studies: PaCO2 3–8 mm Hg (31, 33, 34) and SGRQ-C total score 7.8 points (31). Furthermore, the HFNC did not prevent all-severity or severe exacerbations, even though it reduced moderate or severe exacerbations. The lack of a significant and continuous improvement in these parameters and moderate effect on exacerbations may have occurred because we evaluated participants over a longer period. In addition, we included patients with the most severe COPD only; therefore, the results were influenced by the treatments and progression of COPD. We also evaluated blood gas measurements in the daytime, several hours after HFNC cessation, and did not evaluate carbon dioxide at night. These factors may have led to an underestimation of improvements in physiological parameters.
The mechanisms by which HFNC use leads to a long-term improvement remain unclear; however, HFNC use has two major advantages over conventional oxygen delivery systems, which result in the reduction of moderate or severe exacerbations and improved physiological effects. One is better secretion clearance due to humidification, and the other is improved ventilation efficiency and subsequent improvement in muscle strength. Patients with COPD are likely to have exacerbations caused by decreased mucociliary function. HFNC use can enhance pulmonary mucociliary function and improve clearance of secretions (12). Improved secretory clearance can lead to fewer exacerbations requiring treatment and improved quality of life. In addition, patients with CHRF experience muscle fatigue caused by decreased ventilatory efficiency (34), and the physiologic effects of HFNC use may improve muscle fatigue by improving ventilatory efficiency. HFNC use may also improve muscle weakness caused by deterioration of muscle oxidative metabolism (35). In this study, to maximize these physiological effects, the HFNC was used mainly at night, when hypoventilation is more likely to occur, and minimally during the day to avoid sedentary behavior.
Limitations
This study has some limitations. First, both patients and clinicians could have identified a sham device. The use of a sham device was impossible, considering the difficulty in blinding patients to flow, heat, and humidity. To partially overcome this, a central review panel that was blinded to treatment allocation diagnosed the presence of a COPD exacerbation, the primary outcome, on the basis of the diaries of participants. Second, the significance level for repeated measurements was not adjusted, despite the study’s exploratory purpose. However, according to the Bonferroni correction for multiple testing, the significance levels should be 0.0125, 0.004, and 0.004 for the SGRQ-C impact score and for pulmonary function testing and SpO2, respectively, which are less than the P values observed for repeated measurements. We did not achieve the target sample size because of a lack of availability of patients, although we estimated that the statistical power was sufficient because we rejected the null hypothesis for the primary endpoint. Finally, when a patient dropped out in 6 months and had two counts of exacerbation, we estimated that the patient experienced four counts in 1 year. The extrapolation indeed had bias, especially in patients who dropped out very early. However, the time to the first exacerbation also showed a significant difference between the HFNC/LTOT and LTOT groups; hence, we considered that the primary endpoint analysis did not have a severe bias to the conclusion.
In conclusion, in the patients with stable hypercapnia with a recent history of COPD exacerbations, HFNC reduced the number of moderate or severe COPD exacerbations and prolonged the duration without moderate or severe COPD exacerbations over the 52-week study period. Although our data could not clearly explain the mechanisms underlying this improvement, HFNC use may be a reasonable therapeutic option for such patients.
Acknowledgments
Acknowledgment
The authors thank Editage (www.editage.jp) for their writing support.
Footnotes
Sponsored by the Foundation for Biomedical Research and Innovation at Kobe with funding from Teijin Pharma under the study contract.
Author Contributions: K.N., T.H., N.C., T.J., R.T., A.S., F.T., T. Kadowaki, A.W., M.F., T. Kitajima, S.S., and K. Tomii contributed to the conception and design of the trial. K.N. and K. Tomii interpreted the data and wrote the manuscript in collaboration with all coauthors. Y.N. and T. Kikuchi performed the statistical analysis. All authors read and approved the final manuscript. The corresponding author had final responsibility for submitting the manuscript for publication.
Data sharing statement: All requests for raw and analyzed data and materials will be reviewed by the corresponding author to verify whether the request is subject to confidentiality obligations.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202201-0199OC on June 30, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J . 2007;30:993–1013. doi: 10.1183/09031936.00082507. [DOI] [PubMed] [Google Scholar]
- 2. Foucher P, Baudouin N, Merati M, Pitard A, Bonniaud P, Reybet-Degat O, et al. Relative survival analysis of 252 patients with COPD receiving long-term oxygen therapy. Chest . 1998;113:1580–1587. doi: 10.1378/chest.113.6.1580. [DOI] [PubMed] [Google Scholar]
- 3. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med . 2009;103:1400–1405. doi: 10.1016/j.rmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 4. Hasani A, Chapman TH, McCool D, Smith RE, Dilworth JP, Agnew JE. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis . 2008;5:81–86. doi: 10.1177/1479972307087190. [DOI] [PubMed] [Google Scholar]
- 5. Möller W, Feng S, Domanski U, Franke KJ, Celik G, Bartenstein P, et al. Nasal high flow reduces dead space. J Appl Physiol (1985) . 2017;122:191–197. doi: 10.1152/japplphysiol.00584.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pisani L, Fasano L, Corcione N, Comellini V, Musti MA, Brandao M, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax . 2017;72:373–375. doi: 10.1136/thoraxjnl-2016-209673. [DOI] [PubMed] [Google Scholar]
- 7. Bräunlich J, Mauersberger F, Wirtz H. Effectiveness of nasal highflow in hypercapnic COPD patients is flow and leakage dependent. BMC Pulm Med . 2018;18:14. doi: 10.1186/s12890-018-0576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. FLORALI Study Group REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med . 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 9. Rittayamai N, Phuangchoei P, Tscheikuna J, Praphruetkit N, Brochard L. Effects of high-flow nasal cannula and non-invasive ventilation on inspiratory effort in hypercapnic patients with chronic obstructive pulmonary disease: a preliminary study. Ann Intensive Care . 2019;9:122. doi: 10.1186/s13613-019-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernández G, Vaquero C, González P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA . 2016;315:1354–1361. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 11. Bonnevie T, Elkins M, Paumier C, Medrinal C, Combret Y, Patout M, et al. Nasal high flow for stable patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD . 2019;16:368–377. doi: 10.1080/15412555.2019.1672637. [DOI] [PubMed] [Google Scholar]
- 12. Storgaard LH, Hockey HU, Laursen BS, Weinreich UM. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis . 2018;13:1195–1205. doi: 10.2147/COPD.S159666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rea H, McAuley S, Jayaram L, Garrett J, Hockey H, Storey L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med . 2010;104:525–533. doi: 10.1016/j.rmed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Nagata K, Horie T, Chohnabayashi N, Jinta T, Tsugitomi R, Shiraki A.FLOCOP Study Investigators. Domiciliary high-flow nasal cannula oxygen therapy for stable hypercapnic COPD patients: a prospective, multicenter, open-label, randomized controlled trial [preprint] 2021. [DOI]
- 15. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med . 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 16. Terada K, Muro S, Sato S, Ohara T, Haruna A, Marumo S, et al. Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax . 2008;63:951–955. doi: 10.1136/thx.2007.092858. [DOI] [PubMed] [Google Scholar]
- 17. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med . 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 18. Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George Respiratory Questionnaire. Chest . 2007;132:456–463. doi: 10.1378/chest.06-0702. [DOI] [PubMed] [Google Scholar]
- 19. Windisch W, Freidel K, Schucher B, Baumann H, Wiebel M, Matthys H, et al. The Severe Respiratory Insufficiency (SRI) Questionnaire: a specific measure of health-related quality of life in patients receiving home mechanical ventilation. J Clin Epidemiol . 2003;56:752–759. doi: 10.1016/s0895-4356(03)00088-x. [DOI] [PubMed] [Google Scholar]
- 20. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res . 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doi Y, Minowa M, Uchiyama M, Okawa M, Kim K, Shibui K, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res . 2000;97:165–172. doi: 10.1016/s0165-1781(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 22. Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res . 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax . 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25. Sasaki H. Nakamura M. Kida A. Kambe M. Takahashi K. Fujimura M. Reference values of spirogram and arterial blood gas levels in Japanese. J Jpn Respir Soc . 2001;39:S1–S17. [Google Scholar]
- 26. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med . 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 27. Akashiba T, Ishikawa Y, Ishihara H, Imanaka H, Ohi M, Ochiai R, et al. The Japanese Respiratory Society noninvasive positive pressure ventilation (NPPV) guidelines (second revised edition) Respir Investig . 2017;55:83–92. doi: 10.1016/j.resinv.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 28. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2018;198:464–471. doi: 10.1164/rccm.201710-2029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shorofsky M, Bourbeau J, Kimoff J, Jen R, Malhotra A, Ayas N, et al. Canadian Respiratory Research Network CanCOLD Collaborative Research group. Impaired sleep quality in COPD is associated with exacerbations: the CanCOLD cohort study. Chest . 2019;156:852–863. doi: 10.1016/j.chest.2019.04.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halpin DMG, Decramer M, Celli BR, Mueller A, Metzdorf N, Tashkin DP. Effect of a single exacerbation on decline in lung function in COPD. Respir Med . 2017;128:85–91. doi: 10.1016/j.rmed.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 31. Nagata K, Kikuchi T, Horie T, Shiraki A, Kitajima T, Kadowaki T, et al. Domiciliary high-flow nasal cannula oxygen therapy for patients with stable hypercapnic chronic obstructive pulmonary disease. A multicenter randomized crossover trial. Ann Am Thorac Soc . 2018;15:432–439. doi: 10.1513/AnnalsATS.201706-425OC. [DOI] [PubMed] [Google Scholar]
- 32. Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med . 2014;189:250–255. doi: 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- 33. Fraser JF, Spooner AJ, Dunster KR, Anstey CM, Corley A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax . 2016;71:759–761. doi: 10.1136/thoraxjnl-2015-207962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bräunlich J, Seyfarth HJ, Wirtz H. Nasal high-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med . 2015;10:27. doi: 10.1186/s40248-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen YH, Huang CC, Lin HL, Cheng SL, Wu HP. Effects of high flow nasal cannula on exercise endurance in patients with chronic obstructive pulmonary disease. J Formos Med Assoc . 2022;121:381–387. doi: 10.1016/j.jfma.2021.05.018. [DOI] [PubMed] [Google Scholar]