To the Editor:
A protease–antiprotease imbalance is a feature of many chronic lung diseases, with concentrations and activity of several MMPs (matrix metalloproteinases) correlating with disease pathology. MMP-12 plays a prominent role in lung tissue remodeling owing to its capacity to degrade elastin and other extracellular matrix constituents but is also increasingly recognized for immunomodulatory functions central to the regulation of innate immunity. MMP-12 is elevated in patients with asthma and, in some instances, associated with more severe forms of disease (1, 2). Mouse models of allergic airway disease (AAD) have defined a central role for MMP-12 in driving airway remodeling and recruitment of innate immune cells (3, 4). However, the role of MMP-12 in regulating adaptive immunity in chronic lung diseases has been neglected.
In a mouse model of house dust mite (HDM)-induced AAD (Figure 1A) (5), C57BL/6 wild-type (WT) mice showed a strong induction of airway MMP-12, which was absent in Mmp12−/− (KO) mice (Figure 1B). Although numbers of CD3+ T cells were comparable between WT and KO animals (Figure 1C), lung B cells were significantly reduced in HDM-treated KO animals (Figure 1D). Numbers of B cells in the lung draining mediastinal lymph nodes (Figure 1E) and peripheral blood (Figure 1F) were not significantly different in allergen-exposed WT and KO mice, indicating the reduced B cell response in KO animals was lung specific. Chronic HDM exposure induces the formation of tertiary lymphoid structures containing ectopic germinal centers in the lungs of mice. Significantly fewer germinal center B cells were present in the lungs of HDM-exposed KO mice relative to WT control mice (Figure 1G), but T follicular helper cell numbers were comparable (Figure 1H).
Figure 1.
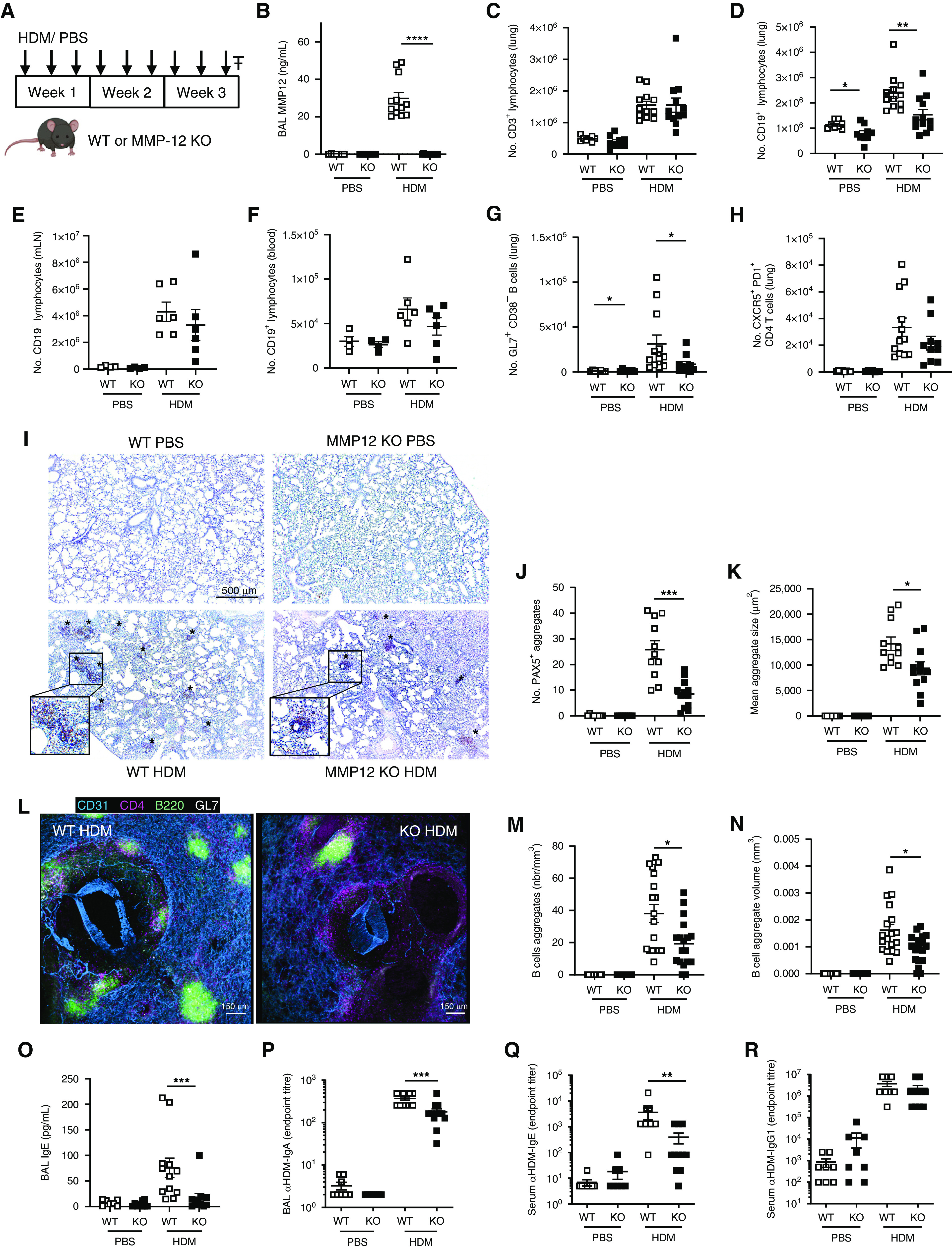
MMP-12 (matrix metalloproteinase-12) regulates formation of pulmonary B cell aggregates in a mouse model of allergic airway disease. (A) Male, 6- to 8-week-old C57BL/6 wild type (WT) and Mmp12(−/−) (KO) mice (purchased from JAX and subsequently bred in-house) were administered 25 μg house dust mite (HDM) extract (Citeq Biologics) or 50 µl sterile phosphate-buffered saline (PBS) intranasally three times per week for 3 weeks. Mice were culled (₸) 24 hours after the final dose of HDM or PBS (5). All animal procedures and care conformed strictly to the UK Home Office Guidelines under the Animals (Scientific Procedures) Act 1986, and protocols were approved by the Home Office of Great Britain. (B) MMP-12 concentrations in BAL fluid were determined by ELISA (Abcam). (C and D) CD45+ (30-F11, BioLegend), CD3+ (145-2C11, eBioScience) T cells (C) and CD45+, CD3−, CD19+ (1D3, BioLegend) B cells (D) within lungs of mice were enumerated from single-cell suspensions by flow cytometry (5). (E and F) B cell numbers were enumerated in mediastinal lymph nodes (mLN; E) and peripheral blood (F) by flow cytometry. (G and H) Lung GL7+ (GL7, BioLegend), CD38− (90, BioLegend) germinal center B cells (G) and CXCR5+ (L138D7, BioLegend), PD1+ (RMP1-30, BioLegend) T follicular helper cells (H) were enumerated by flow cytometry. (I–K) Representative images of formalin-fixed, wax-embedded lung sections (4 μm) (5) stained for PAX5 (30 ng/ml anti-PAX5 Antibody EPR3730[2]; Abcam). B cell aggregates (brown; *) were scored from four fields of view per section using a Leica DFC300FX microscope, and individual aggregate size was enumerated using Fiji software. (L) Representative images of agarose-inflated, paraformaldehyde-fixed precision-cut lung slices (PCLSs; 200 mm transverse sections) stained with B220 (RA3-6B2; Biolegend), CD4 (RM4-5; Biolegend), CD31 (polyclonal goat IgG; R&D Systems), and GL7 (GL7, Biolegend). PCLSs were imaged on an inverted laser scanning confocal SP5 (Leica Microsystems) using a 20× objective at a resolution of 512 × 512 pixels. (M and N) PCLS image analysis and rendering was performed using IMARIS software 8.1 version (Bitplane, Oxford Instruments) on six fields of view per PCLS. (O) Concentration of total IgE was determined in BAL fluid of mice by ELISA (eBioscience). (P) Concentration of HDM-specific IgA in BAL fluid was determined using an in-house ELISA, whereby serially diluted BAL fluid was incubated with plates precoated with HDM (50 μg/ml; Citeq), and bound IgA was subsequently detected using Clonotyping System-HRP (Southern Biotech). Data are expressed as the lowest dilution at which antibody is still detectable. (Q) Concentrations of HDM-specific IgE and (R) HDM-specific IgG1 were determined in the serum by ELISA using the same method outlined in P. Results are depicted as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 using Mann-Whitney statistical test.
Histological analysis revealed a reduction in the number and size of pulmonary B cell aggregates (Figures 1I–1K), and MMP-12 concentrations correlated with total PAX5+ area (r = 0.5098; P = 0.0013). Interrogation of precision-cut lung slices by confocal microscopy demonstrated the colocalization of germinal center B cells and CD4+ T cells (Figure 1L) and validated that the number of B cell aggregates (Figure 1M) and the individual aggregate volume (Figure 1N) were reduced in HDM-exposed KO mice.
In keeping with the diminished pulmonary B cell aggregates in HDM-KO mice, concentrations of airway total IgE (HDM-specific IgE below limits of detection) and HDM-specific IgA were also significantly reduced (Figures 1O and 1P). Systemic HDM-specific IgE was also reduced in HDM-treated KO mice (Figure 1Q), whereas IgG1 was comparable to WTs (Figure 1R). Airway MMP-12 positively correlated with IgE (r = 0.612; P < 0.0001) and IgA (r = 0.482; P = 0.002). Furthermore, mast cell protease-1, a surrogate for IgE-induced mast cell degranulation, was reduced in HDM-treated KO mice relative to WT control mice and showed a significant correlation with IgE (r = 0.681; P < 0.0001). These data show that MMP-12 is important for functional B cell aggregate formation and local antibody responses during AAD.
CXCL13 (chemokine (C-X-C motif) ligand 13) is a B cell chemokine demonstrated to drive aggregate formation in tertiary lymphoid structures in mouse models of AAD (6), and CXCL12 has been demonstrated to be important in distinct lung inflammatory models (7). MMP-12 can modulate the activity of CXC chemokines by proteolytic processing (8). Although lung CXCL13 (Figure 2A) and CXCL12 (Figure 2B) concentrations were comparable between HDM-treated WT and KO mice, MMP-12 proteolytically cleaves CXCL13, but not CXCL12, in vitro (Figure 2C). Mass spectrometry analysis demonstrated that recombinant CXCL13 (9,812 Da) yields a product of 8,239 Da upon exposure to MMP-12. Deconvolution of these data suggested that MMP-12 cleaves CXCL13 between Ser-94 (P1) and Leu-95 (P1′) at the chemokine’s C-terminal domain (Figure 2D). MMP-12 cleavage of CXCL13 was subsequently assessed in vivo in allergen-exposed mice by Western blot. Antibodies to CXCL13 frequently target its C-terminus and were thus found to bind MMP-12–cleaved CXCL13 with reduced affinity resulting in lower-intensity bands (Figures 2E and 2F). CXCL13 concentrations present in BAL fluid (BALF) and lungs of HDM-treated mice were below the limit of detection by Western blot (Figures 2E and 2F). When BALF of WT HDM-exposed mice was incubated ex vivo with CXCL13, it cleaved the chemokine in an analogous manner to MMP-12; however, no cleavage was observed with KO BALF (Figure 2E). CXCL13 immunoprecipitations of lung homogenate from HDM-treated WT and KO mice were performed to enrich the chemokine. Full-length CXCL13 was detectable in immunoprecipitations of MMP-12 KO mice, whereas the truncated form was detectable in WT immunoprecipitations (Figure 2F). Therefore, MMP-12 cleavage of CXCL13 occurs in vivo in HDM-exposed mice.
Figure 2.
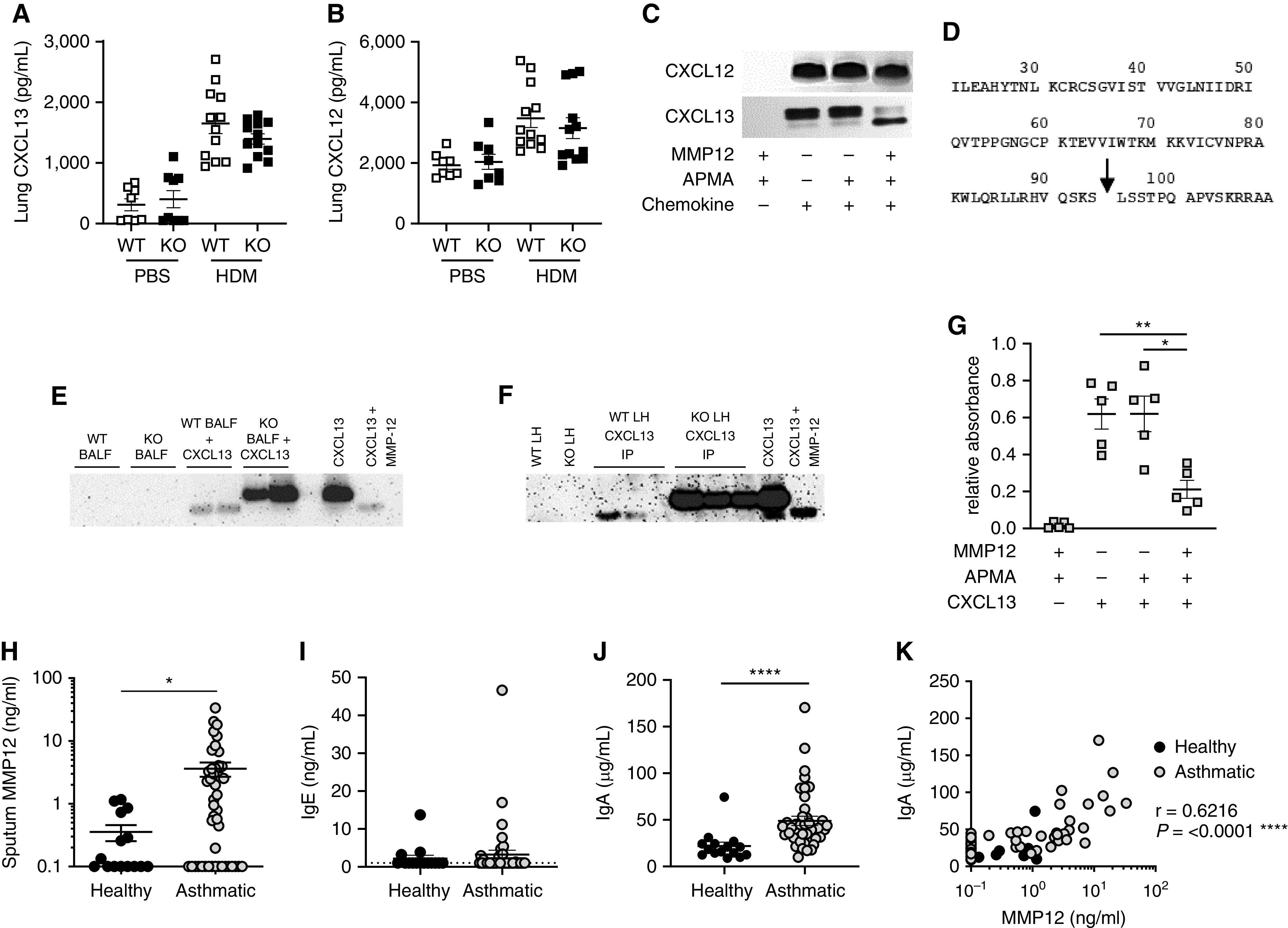
MMP-12 (matrix metalloproteinase-12) proteolytically processes CXCL13 to modulate glycosaminoglycan binding and correlates with airway antibody responses in patients with asthma. (A and B) The concentrations of CXCL13 (A) and CXCL12 (B) within lung homogenate (5) were measured by ELISA (R&D Systems). (C) Recombinant mouse CXCL13 and CXCL12 (both 100 μg/ml; Peprotech) were incubated with APMA (4-Aminophenylmercuric acetate)-activated (Sigma Aldrich) recombinant mouse MMP-12 (10 μg/ml; Abcam) for 8 hours at 37°C. The reaction products were separated by gel electrophoresis using NuPAGE 4–12% Bis-Tris Protein Gels (Thermo Fisher) and detected using Pierce Silver Stain kit. (D) Identification of murine MMP-12 cleavage products by an Applied Biosystems 4800 MALDI-TOF mass spectrometer in linear mode; MMP-12 cleavage site within murine CXCL13 denoted by arrow. (E) BAL fluid (BALF) from HDM-exposed wild-type (WT) or Mmp12−/− (KO) mice was incubated with CXCL13 (50 ng; Peprotech) for 16 hours at 37°C. The reaction products were separated by gel electrophoresis using NuPAGE 4–12% Bis-Tris Protein Gels (Thermo Fisher). Comparable amounts of WT and MMP-12 KO BALF alone or recombinant CXCL13 alone (with or without MMP-12 cleavage as described above) were run as controls. Proteins were transferred to PVDF (polyvinylidene fluoride) membrane and probed with biotinylated anti-CXCL13 antibody (0.2 μg/ml; R&D Systems), followed by Streptavidin-HRP conjugate (R&D Systems). (F) Immunoprecipitations (IPs) were performed on pooled lung homogenate (LH) from HDM-exposed WT or MMP-12 KO mice using biotinylated anti-CXCL13 antibody (10 μg; R&D Systems) and Streptavidin Dynabeads (Invitrogen). Samples were interrogated by Western blot as detailed above. WT and MMP-12 KO LH or recombinant CXCL13 (with or without MMP-12 cleavage as defined above) were run as controls. (G) CXCL13–MMP-12 reaction products (10 pmol CXCL13/well) were assessed for their capacity to bind biotinylated heparan sulfate (Sigma). (H–K) Analysis of sputum samples from a cohort (5) of 15 healthy volunteers and 42 patients with moderate to severe asthma (defined by British Thoracic Society guideline criteria [disease steps 3–5]; Research Ethics Committee Reference 10/H1010/7). (H) Sputum MMP-12 measured by ELISA (Abcam). (I and J) Sputum IgE (I) and IgA (J) measured by ELISA (Invitrogen). (K) Correlation between sputum MMP-12 and IgA. Solid symbols represent healthy control subjects; open symbols represent patients with asthma. Results depicted as mean ± SEM. (A, B, G–J) *P < 0.05, **P < 0.01, and ****P < 0.0001 using Mann-Whitney statistical test. Correlation analysis (K) was performed using Spearman rank test.
The excised fragment at the C-terminus of CXCL13 contains a binding site for the glycosaminoglycan heparan sulfate of the extracellular matrix (ECM) (9). Accordingly, MMP-12–cleaved CXCL13 demonstrated a reduced capacity to bind to biotinylated heparan sulfate (Figure 2G). Previous work demonstrated that cathepsin B operates within lymph nodes to cleave CXCL13 at a comparable site to MMP-12 and that ensuing cleavage-dependent liberation of the chemokine from the ECM was critical for formation of B cell follicles (10). We suggest that MMP-12 cleavage of CXCL13 performs an analogous role within the lung, thus rationalizing the lung-specific deficiency observed in B cell follicle formation in HDM-treated KO mice.
The clinical significance of our findings was interrogated in a previously described (5) patient cohort of healthy volunteers and patients with moderate to severe asthma. Sputum MMP-12 was significantly elevated in patients with asthma relative to healthy control subjects (Figure 2H). Sputum IgE concentrations were generally very low and often undetectable (potentially attributable to sequestration by FcεR1-expressing cells) (Figure 2I). However, IgA was elevated in the airways of patients with asthma (Figure 2J) and significantly correlated with MMP-12 concentrations (Figure 2K), supportive of a role for this protease in local antibody responses in patients with asthma.
Although a protease imbalance is a hallmark of many chronic lung diseases, and MMP-12 has been associated with structural changes and modulation of innate immunity in this context, our studies also now suggest a prominent role for MMP-12 in defining B cell and local antibody responses in the lung in the context of asthma. We also highlight the capacity of MMP-12 to proteolytically modify the critical B cell chemokine CXCL13, reducing its capacity to bind to ECM heparan sulfate. Additional studies are needed to unequivocally demonstrate the significance of this MMP-12 processing of CXCL13 in defining pulmonary B cell and local airway antibody responses in mouse models of AAD and in patients with asthma.
Lung B cell aggregates have been previously reported in patients with asthma at a greater size and number than in healthy volunteers and correlating with eosinophilic infiltration, airway wall thickening, and disease severity (11), suggestive of a role in potentiating pulmonary pathology. Furthermore, allergen-specific IgE+ B cells have been described within these aggregates in the context of AAD in patients (12), again associating with pathological features of disease. Mucosal production of IgE and ensuing degranulation of mast cells is a major pathway of the inflammatory response underlying asthma. Secretory IgA responses to allergens in patients with asthma have been suggested to play a pathogenic role through eosinophil activation, with IgA shown to induce eosinophil degranulation in vitro and airway IgA concentrations correlating with eosinophil cationic protein during late asthmatic responses (13). Moreover, it is plausible that our findings could also be of broader significance in the context of chronic inflammation or host immunity to infectious challenge.
Acknowledgments
Acknowledgment
The authors thank Lorraine Lawrence for histological sectioning. Mass spectrometry was performed at the Centre for Integrative Systems Biology and Bioinformatics (CISBIO) mass spectrometry core facility, managed by Dr. Paul Hitchen, at Imperial College London. This report includes independent research supported by National Institute for Health Research South Manchester Respiratory and Allergy Clinical Research Facility at Manchester University National Health Service (NHS) Foundation Trust (Wythenshawe) and by the National Institute for Health and Care Research (NIHR) Manchester Biomedical Research Centre.
Footnotes
Supported by Rosetrees Trust grant M612 (R.J.S. and C.M.L.), British Heart Foundation Centre of Research Excellence (CRE) Research Fellowship from Imperial College RE/18/4/34215 (R.J.), Wellcome Trust grants 107059/Z/15/Z (C.M.L.) and 209458/Z/17/Z (R.J.S.), the 7th European Community Framework Programme FP7 Ideas: European Research Council Marie Curie Intra-European Fellowship FP7-PEOPLE-2013-IEF No627374 (T.P.), and the Manchester Biomedical Research Centre (A.S.). Parts of this research were supported by a precompetitive, open innovation award to the Manchester Collaborative Centre for Inflammation Research by AstraZeneca and GlaxoSmithKline. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
Author Contributions: C.J.P., D.F.P., and R.J.S. designed and interpreted the experiments, performed statistical analysis, and prepared the manuscript. C.J.P., with the assistance of D.F.P., T.P., R.J., and R.J.S., performed the majority of the experiments. A.M.G., T.H., G.T., and A.S. were instrumental in the acquisition and processing of samples from the primary patient cohort. J.P., J.A.H., and C.M.L. provided key reagents and contributed discussions throughout the work.
Originally Published in Press as DOI: 10.1164/rccm.202109-2082LE on August 9, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Chaudhuri R, McSharry C, Brady J, Donnelly I, Grierson C, McGuinness S, et al. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease and asthma: relationship to disease severity. J Allergy Clin Immunol . 2012;129:655–663.e8. doi: 10.1016/j.jaci.2011.12.996. [DOI] [PubMed] [Google Scholar]
- 2. Araujo BB, Dolhnikoff M, Silva LF, Elliot J, Lindeman JH, Ferreira DS, et al. Extracellular matrix components and regulators in the airway smooth muscle in asthma. Eur Respir J . 2008;32:61–69. doi: 10.1183/09031936.00147807. [DOI] [PubMed] [Google Scholar]
- 3. Lanone S, Zheng T, Zhu Z, Liu W, Lee CG, Ma B, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J Clin Invest . 2002;110:463–474. doi: 10.1172/JCI14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pouladi MA, Robbins CS, Swirski FK, Cundall M, McKenzie AN, Jordana M, et al. Interleukin-13-dependent expression of matrix metalloproteinase-12 is required for the development of airway eosinophilia in mice. Am J Respir Cell Mol Biol . 2004;30:84–90. doi: 10.1165/rcmb.2003-0051OC. [DOI] [PubMed] [Google Scholar]
- 5. Patel DF, Peiró T, Shoemark A, Akthar S, Walker SA, Grabiec AM, et al. An extracellular matrix fragment drives epithelial remodeling and airway hyperresponsiveness. Sci Transl Med . 2018;10:eaaq0693. doi: 10.1126/scitranslmed.aaq0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baay-Guzman GJ, Huerta-Yepez S, Vega MI, Aguilar-Leon D, Campillos M, Blake J, et al. Role of CXCL13 in asthma: novel therapeutic target. Chest . 2012;141:886–894. doi: 10.1378/chest.11-0633. [DOI] [PubMed] [Google Scholar]
- 7. Fleige H, Ravens S, Moschovakis GL, Bölter J, Willenzon S, Sutter G, et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med . 2014;211:643–651. doi: 10.1084/jem.20131737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood . 2008;112:3455–3464. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- 9. Monneau YR, Luo L, Sankaranarayanan NV, Nagarajan B, Vivès RR, Baleux F, et al. Solution structure of CXCL13 and heparan sulfate binding show that GAG binding site and cellular signalling rely on distinct domains. Open Biol . 2017;7:170133. doi: 10.1098/rsob.170133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cosgrove J, Novkovic M, Albrecht S, Pikor NB, Zhou Z, Onder L, et al. B cell zone reticular cell microenvironments shape CXCL13 gradient formation. Nat Commun . 2020;11:3677. doi: 10.1038/s41467-020-17135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elliot JG, Jensen CM, Mutavdzic S, Lamb JP, Carroll NG, James AL. Aggregations of lymphoid cells in the airways of nonsmokers, smokers, and subjects with asthma. Am J Respir Crit Care Med . 2004;169:712–718. doi: 10.1164/rccm.200308-1167OC. [DOI] [PubMed] [Google Scholar]
- 12. Slavin RG, Gleich GJ, Hutcheson PS, Kephart GM, Knutsen AP, Tsai CC. Localization of IgE to lung germinal lymphoid follicles in a patient with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol . 1992;90:1006–1008. doi: 10.1016/0091-6749(92)90479-l. [DOI] [PubMed] [Google Scholar]
- 13. Pilette C, Durham SR, Vaerman JP, Sibille Y. Mucosal immunity in asthma and chronic obstructive pulmonary disease: a role for immunoglobulin A? Proc Am Thorac Soc . 2004;1:125–135. doi: 10.1513/pats.2306032. [DOI] [PubMed] [Google Scholar]


