At a Glance Commentary
Scientific Knowledge on the Subject
Despite many decades of research on the pathogenesis and treatment of chronic obstructive pulmonary disease (COPD), the medical community has failed to decrease its morbidity and mortality to the same degree that has been achieved in other major noncommunicable diseases, such as coronary artery disease, stroke, and certain malignancies. An important factor contributing to this slow progress may be that the current COPD definition and taxonomy fail to identify the disorder at its early stages, before airflow limitation becomes evident. It also defines the entity as a single “disease” with most efforts devoted to the study of the pathogenetic mechanisms of only one major cause of COPD (cigarette smoking), failing to expand the horizon about the heterogeneity of processes contributing to its final clinical presentation.
What This Study Adds to the Field
Evidence gathered over the last 3 decades has prompted us to propose an updated definition and taxonomy of COPD, based on a pragmatic approach that can accommodate new changes over time. This proposal states that “COPD is a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, expectoration) due to persistent abnormalities of the airways (bronchitis, bronchiolitis), alveoli (emphysema), and/or pulmonary vessels, confirmed by spirometrically determined airflow limitation and/or objective evidence of structural or physiological pulmonary dysfunction.” We hope that stakeholders may find this definition to be more inclusive, practical, and useful, so that specific studies can be designed and conducted for different types of COPD.
Attempts to define concepts and things date back to the time of Aristotle, around 2,400 years ago, when he proposed that a definition should state the essential nature or being of something (1). However, this abstract concept (2–4) may not translate well to medical practice, where precise definitions are needed to communicate to colleagues and patients and to conduct epidemiological, clinical, translational, and discovery research in the presence of new evidence brought about by scientific progress. Since Aristotle’s initial discussions, the last 24 centuries have witnessed the appearance and evolution of definitions of many things that surround us, including diseases (5). In medicine, accurate and precise definitions (nosology) and classification of diseases (taxonomy) allow thinking, speaking, and writing about observable phenomena in an understandable and unified way.
Definition of Disease and Diagnostic Criteria
The definition and the diagnostic criteria (terms often mixed or confused) of a disease represent different constructs. A definition must describe its essential attributes while avoiding circularity; it must also not consist of terms that are synonymous with it. It should not be too wide or too narrow (not miss or include anything to which the term should not be applied). It should also be clear, understandable, and positive, attempting to avoid concepts derived by exclusions. Following these basic rules, Scadding provided a definition of disease (6) that captures these attributes: “a disease is the sum of the abnormal characteristics displayed by a group of living organisms by which they differ from the norm of their species and that places them at a biological disadvantage.” In his writings, Scadding proposed that some diseases could be defined by collecting symptoms (e.g., migraine) and/or signs (e.g., scoliosis), by the presence of abnormal function (e.g., arterial hypertension), by characteristic structural abnormalities (e.g., cancers), and/or by cause (e.g., tuberculosis or, more recently, coronavirus disease [COVID-19]). Complex diseases may be defined by combining two or more characteristics, but independent of the characteristics used, once a definition is agreed on, it is practical to adopt a nomenclature (taxonomy) that uses names and terms to classify subgroups within the disease that share similar characteristics. It follows that, as knowledge increases, the definition and classification of diseases can and should change over time. Such was the case of tuberculosis (7), first labeled as “phthisis” during Hippocratic times and then as “consumption” or “white plague” in Europe until the 19th century. Believed to be different from scrofula and Pott’s disease for decades, it was not until the common tuberculosis bacillus was found to be the cause of these different diseases that a unified definition was developed. This has happened to many diseases and will continue to occur as our knowledge continues to expand.
On the other hand, diagnostic criteria are used to operationalize the proposed definition and may be included or not in the definition. As highlighted by Snider (8), diagnostic criteria are “features of the disease that are found, by empiric research, to best distinguish the disease from other diseases that resemble it.” Some diseases, such as arterial hypertension, include diagnostic criteria in their definition (9), whereas others, such as diabetes, do not but then provide different operative diagnostic criteria based on specific stages or types of tests (10).
Reasons to Update the Definition and Taxonomy of Chronic Obstructive Pulmonary Disease
As is true for most disease, the definition of chronic obstructive pulmonary disease (COPD) has evolved over time (11, 12), having last been refined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) in 2022 as follows: “COPD is a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases and influenced by host factors including abnormal lung development. Significant comorbidities may have an impact on morbidity and mortality” (13). Despite many decades of research on the pathogenesis and treatment of COPD and the development and implementation of several beneficial pharmacological and therapeutic interventions, the medical community has failed to decrease its morbidity and mortality to the same degree that has been achieved in other major noncommunicable diseases, such as coronary artery disease, stroke, and certain malignancies (14, 15). Many factors may account for this, including its complex nature with heterogeneous effects on airways, pulmonary vasculature, and lung parenchyma and the dynamic impact of acute exacerbations. An important limitation, however, may be that the current COPD definition and taxonomy fail to identify the disorder at its early stages, before airflow limitation becomes evident. It also defines the entity as a single disease, limiting the role of different causes of COPD leading to the same functional or physiologic abnormality. Indeed, the labeling of COPD as a disease has funneled our efforts to study the pathogenetic mechanisms of only one major cause of COPD (cigarette smoking), failing to expand the horizon about the heterogeneity of processes contributing to its final clinical presentation.
Herein we propose to update the current definition and taxonomy of COPD by incorporating the most important recent advances in the understanding of this complex disease. The first concept is that COPD results not only from the consumption of cigarettes but also from other causes, such as biomass exposure (16–19), poverty (20), infections such as tuberculosis (21–24), or even asthma (25–31). COPD from different causes evolves in different patterns compared with that of the classic COPD related to cigarette smoking. Furthermore, in vast regions of the world, and particularly in women, these factors, and not cigarette smoking, are the most important causes of COPD (32–34). Second, the advent of novel tools, such as computed tomography (CT) of the chest, has provided evidence that structural lung abnormalities can be detected in the absence of airflow limitation (35), and the term pre-COPD has been proposed to describe these individuals (36, 37). As an example, controversy exists about whether a person with CT-diagnosed emphysema with normal spirometry has COPD and, importantly, how that individual should be treated. The same can be said of individuals with preserved ratio impaired spirometry (PRISM) who have symptoms and increased risk of poor outcomes (38). Individuals with PRISM with low FVC to TLC ratio are at a high risk of developing COPD (39). Third, general population studies have shown that symptoms (cough and sputum) can identify middle-aged subjects with high risk of developing persistent airflow limitation (40). Finally, events occurring during pregnancy and throughout childhood and adolescence can profoundly impact lung development and result in airflow limitation without an obligatory rapid decline in lung function over time (a feature previously believed to be cardinal to COPD) (41–43). Despite these considerations, the prevalent thought today maintains that COPD is solely one disease, caused by cigarette smoking. Pharmacological trials and most other interventional studies have centered on a narrow segment of older patients (60–70 yr of age) with COPD who have a significant history of smoking (44–47). An updated COPD definition and taxonomy should promote development of transformative therapies that can prevent and alter disease trajectory throughout the entire lifespan (48).
Causes of COPD
In some regions of the world, many COPD cases do not have a history of cigarette smoking (20, 49, 50). Therefore, an updated taxonomy should recognize and identify the causes of COPD to better study its natural course and enable development of specific therapies to improve outcomes. Again, taking lessons from other fields, the American Diabetic Association classifies the diabetes into four types based on etiology that range from autoimmunity to specific causes, such as cystic fibrosis or pancreatitis (10). Recognizing the different causes of COPD that have been identified so far, a potential subclassification may be considered as follows.
Genetic COPD (COPD-G)
The causal relationship between one single gene defect serpin family A member 1 (SERPINA1) and the development of emphysema was established in 1965 (51). This particular type of COPD (alpha-1 antitrypsin deficiency) characteristically affects younger individuals, particularly those who smoked cigarettes, and primarily presents with lower lobe emphysema. Clinically, it may be associated with bronchiectasis, asthma, or cirrhosis. Importantly, it is underdiagnosed and can be treated with specific augmentation therapy, thereby justifying its own subgrouping (52). Other genetic abnormalities also increase the risk of developing chronic airflow limitation and associated multimorbidity (53–57). Yet, it is the combined effect of many common variants, each of them with a small effect size rather than a rare variant with a large effect size (e.g., SERPINA1), that contributes to the clinical presentation of the disease. In fact, tobacco smoking only causes COPD in a subgroup of susceptible smokers (58). The presence of many genetic loci variants (59) suggests the possible importance of these genes in COPD development. A better understanding of the genetic basis of COPD could help classify and eventually develop novel targeted therapies (60).
COPD Due to Abnormal Lung Development (COPD-D)
Lungs are not fully developed at birth. They grow and mature through infancy and adolescence, reaching peak lung function between 16–18 years of age in females and 20–25 years in males (61). Many environmental exposures (e.g., passive smoking or exposure to indoor pollution from biomass), repeated infections, poor nutrition, and genetic factors can alter lung growth in utero, during infancy, and during adolescence and contribute to COPD later in life (41). In fact, only about half of adult patients fulfilling the current COPD diagnostic criteria have followed a lung function trajectory characterized by an enhanced rate of decline with age starting from normal peak lung function in early adulthood, whereas the remainder start with a lower peak lung function in early adulthood and are diagnosed with COPD in late adulthood without enhanced lung function decline (62). It is possible that cases attributed to asthma during childhood (see below, COPD-A) may relate to the fact that a child with lung developmental problems is likely to experience some form of respiratory symptoms (e.g., dyspnea on exercise, cough, wheezing) and be diagnosed with (and treated for) asthma if taken to a pediatrician (63, 64).
Environmental COPD
Cigarette smoking COPD (COPD-C)
This is the best studied type of COPD (13, 65, 66). A significant volume of research associates COPD with an abnormal inflammatory response to the inhaled products of cigarette combustion (26). However, most smokers respond to the inhalation of cigarettes with inflammation, but not all develop airflow limitation, suggesting that other mechanisms may be responsible. These mechanisms (67, 68) include: 1) an imbalance between proteases and antiproteases; 2) an abnormal immunological reaction that results in some degree of autoimmunity and lung destruction (69); and 3) uncontrolled autophagy (70), enhanced apoptosis (71), and/or a process of accelerated lung aging (72, 73).
Biomass and pollution exposure COPD (COPD-P)
Biomass exposure is an important cause of COPD in certain regions of the world (32, 34, 50), particularly for women (74). Detailed studies suggest that this represents a different and specific type of COPD, showing that these patients have less emphysema and more airways involvement, as well as slower lung function decline, than patients with cigarette-induced COPD (33, 75). Data from some, but not all, studies that have implemented substitution of open-flame biomass burning with ventilated cooking furnaces have shown a reduction of COPD prevalence and symptoms (76), and air cleaners seem to improve symptoms (77). However, not a single randomized trial of any specific therapy has been conducted in this population.
COPD Due to Infections (COPD-I)
Clinicians around the world see nonsmoking subjects with persistent airflow limitation due to previous lung infections. The infections may occur during infancy, caused by respiratory syncytial virus and other viruses (78, 79) or a history of treated tuberculosis (18, 21, 24, 50). The interaction between infectious processes and limitation to airflow is poorly defined, but it may be due to abnormal repair processes that lead to fibrosis and airway and parenchymal distortion. Few studies have addressed the therapeutic implications of this type of COPD. Recent research (80, 81), however, has shown that the presence of bronchiectasis (a potential result of these postinfectious complications) in patients with COPD conveys a poor prognosis (82), supporting the need to specifically address this type of COPD. In Western countries, as patients infected with HIV are living into their 50s and beyond with the advent of highly active antiretroviral therapy, COPD has become highly prevalent in this population, especially among smokers (83).
COPD and Asthma (COPD-A)
The extent to which asthma contributes to the genesis of COPD has been much debated (84, 85), but it is recognized that patients with severe, uncontrolled asthma can develop chronic airflow limitation without other environmental risk factors (31). In some patients with asthma, detailed physiological and pathological studies demonstrate varying degrees of loss of lung elastic recoil and emphysema (28, 86). The overlap of asthma and COPD has been the subject of recent interest and debate (87–89). Finally, as discussed above, children with abnormal lung development (now a recognized cause of COPD in adults) can be mislabeled as having asthma. Future studies with biologics that are effective in selected cases of severe asthma are being conducted in populations with COPD-A to investigate their potential therapeutic efficacy (90).
COPD of Unknown Cause (COPD-U)
Well-conducted epidemiologic studies have observed that no (currently known) risk factors can be identified in a significant proportion of adults with COPD who thus qualify (for now) as idiopathic (91). A large observational study in Austria has shown that there are many risks factors associated with low lung function that differ by age but interact and accumulate over the lifespan (92) (Figure 1). Hence, COPD-U may be the result of the interaction of several of these factors (sex, occupational exposure, education, dysanaptic lung growth, epigenetic phenomena, and poverty [20, 93] capable of influencing the normal lung function trajectory over a lifetime) (94). COPD-U remains poorly studied but may have a better prognosis than those cited above (95).
Figure 1.
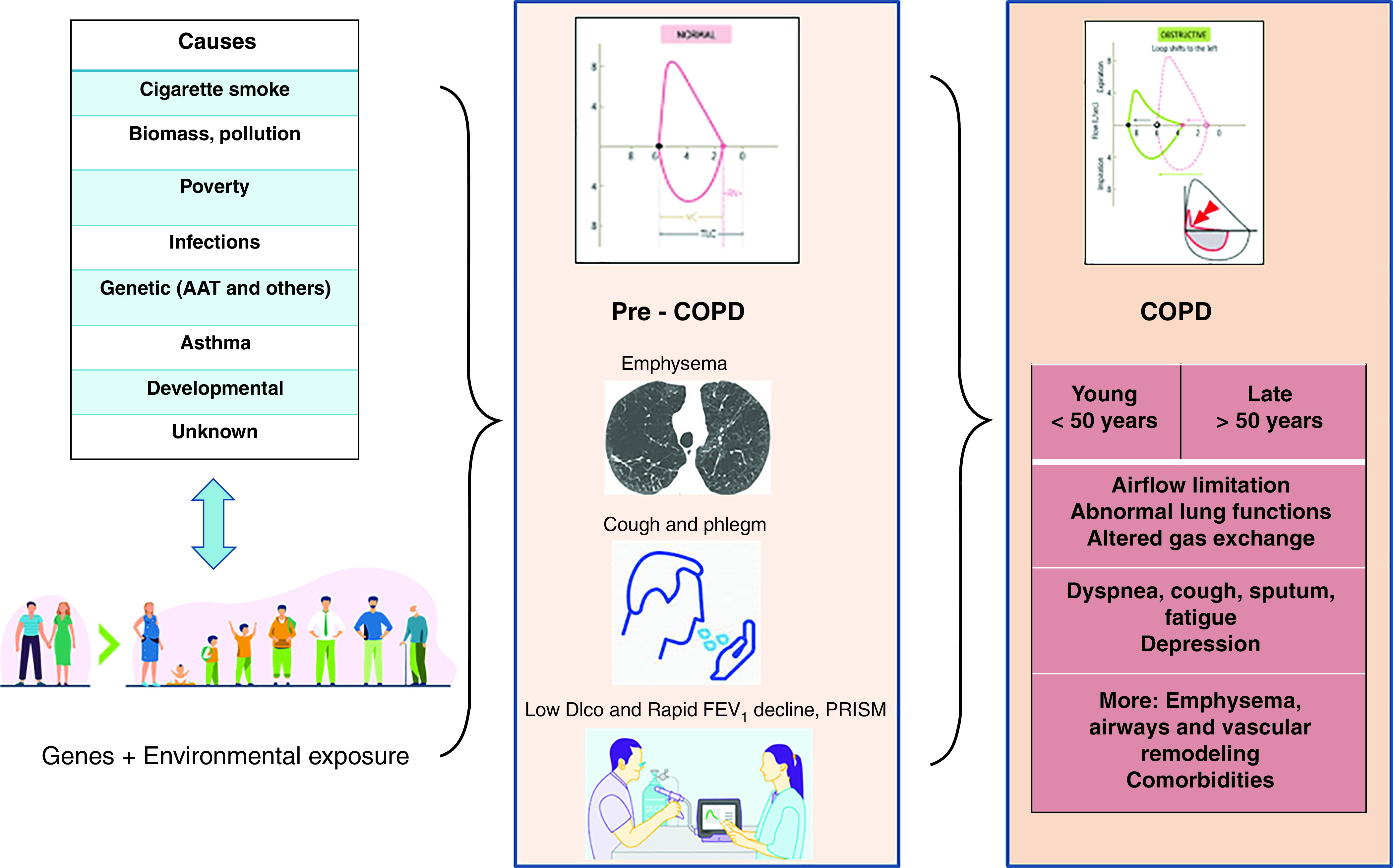
Chronic obstructive pulmonary disease (COPD) results from an interaction between genetic predisposition of some individuals with different causative agents over time. It goes through a period (pre-COPD) where the operational threshold that is used to define airflow limitation has not been reached, but symptoms, structural abnormalities detectable with imaging, or functional dysfunction detectable with lung function testing may identify individuals at higher risk of COPD development. Finally, a full-blown spirometrically detectable phase is reached either at young (<50 yr) or older (⩾50 yr) age. AAT = alpha-1-antitrypsin deficiency; PRISM = preserved ratio impaired spirometry.
COPD of Mixed Causes (COPD-M)
Finally, some patients may have several of the causal factors noted above. These cases will benefit from detailed studies of their clinical expression, predominant pathobiological mechanisms, and natural course to better evaluate potential therapies.
Putting It All Together
Each person is the product of the genome (G) inherited from their parents and its interaction with the environment (E) over time (T), or, as proposed very recently, “GETomics” (96). The different causes of COPD ought to be noted in the evaluation of patients, whether in the clinic or for research purposes, particularly because some causes may coexist (e.g., prematurity, infection, and smoking) and influence the course of patients and their response to therapies. There is a need to conduct trials designed for each type of COPD (48). Whichever the cause(s), the disease will start at some point and will likely go through a clinically silent period, followed by a symptomatic period (particularly with cough and phlegm or exertional dyspnea) (40, 97). In some persons, there are structural abnormalities detected on chest CT (35, 98) and/or functional changes, such as rapid lung function decline (99) or low diffusing capacity of lungs for carbon monoxide (100, 101) including PRISM (38, 39) but, in all cases, without spirometric evidence for airflow limitation. Because these subjects are no longer healthy, and a variable proportion of them (between 10% and 25%) may eventually develop airflow limitation, this pre-COPD phase represents a targetable group in whom to attempt to delay or, better yet, reverse disease progression (48). In some individuals, spirometric evidence for airflow limitation is already present at initial contact, in which case the diagnosis of COPD is evident whether at younger (<50 yr) or older (⩾51 yr) ages (42, 102).
Arriving at a Revised COPD Definition
If, as the evidence above indicates, COPD has different causes and pathobiological mechanisms, sometimes coexisting and interacting in the same individual but all eventually resulting in lung abnormalities with airflow limitation, as the GOLD definition states (13), then the definition should not be limited to one disease caused by one single agent, as is the prevalent thought in most healthcare workers and certainly in the literature. This raises the question of whether COPD should be considered a syndrome or a disease (103). In this regard, one can speak of a syndrome when a consistently recognizable pattern of symptoms and signs is associated with specific clinical entity (i.e., fibromyalgia) (3, 4). However, whether a nosologic entity defined only in clinical-descriptive terms is called a syndrome or a disease does not actually matter, provided that verbal usages are made explicit and applied consistently (6). Because COPD does have identifiable causes, operational structural and functional diagnostic criteria, and a well-recognized accepted terminology that already includes the nominalistic recognized label of a disease, we propose to continue labeling it as such, acknowledging that it can be caused by one or more mechanisms and present clinically in a number of different ways. It may be argued that the term COPD ought to be changed too. However, it is important to be practical and recognize the advantages and benefits of the current acronym. COPD is currently recognized by the major health organizations in the world (104) and accepted in the medical literature, including the International Coding of Diseases (105). To agree on a new acronym would be to start again on a road that has taken us a long time and effort to pursue. Keeping the term COPD but adjusting its meaning to include the expanding knowledge of the disease should make specific goals and better management of patients more achievable.
An updated definition may be useful (and needed) because it expands the horizon to include individuals with abnormal structural or functional lung abnormalities, who at the moment of contact may not have reached the spirometric threshold diagnostic for COPD but are likely to have increased risk of developing chronic airflow limitation (36, 37, 106), thus enabling preventive and earlier interventions in these subjects. In fact, a similar broader view of disease is already used in other complex chronic noncommunicable diseases like diabetes, whose current definition states “Diabetes is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of different organs, especially the eyes, kidneys, nerves, heart, and blood vessels” (10). Notice that this definition includes multiple causes and pathobiological mechanistic pathways all resulting in hyperglycemia (operational definition). The same can be said of COPD, which has different causes with varied clinical expressions. Also note that the definition of diabetes includes no operational diagnostic criteria. Instead, practical thresholds of surrogate markers (glycemia or hemoglobin A1C) are used to define different disease stages. Following this reasoning and accepting the rules that guide the construction of a disease definition, we propose that COPD should be defined as follows:
COPD is a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, expectoration) due to persistent abnormalities of the airways (bronchitis, bronchiolitis), and/or alveoli (emphysema), that often results in progressive airflow limitation.
Diagnostic Criteria
Currently, the diagnosis of COPD requires the presence of not–fully reversible airflow limitation during an FVC maneuver (13), with an FEV1/FVC < 0.7, as values below this threshold are associated with poor outcomes (107, 108). However, because several measurable symptoms, structural changes, and pulmonary functional abnormalities may precede the arbitrary threshold of airflow limitation (40, 99, 100), it is possible to outline diagnostic criteria for pre-COPD stages enhancing our capacity to incorporate other important stages in the definition of the COPD disease complex (Table 1).
Table 1.
Operational Descriptors and Threshold Values of the Diagnostic Criteria to Identify Persons (pre–Chronic Obstructive Pulmonary Disease [COPD]) at Higher Risk of Developing COPD and Those with Confirmed Disease (COPD)
| Diagnostic Criteria |
||
|---|---|---|
| Pre-COPD | COPD | |
| Spirometry post-bronchodilator | FEV1/FVC ⩾ 0.7 | FEV1/FVC < 0.7 |
| and | and | |
| Symptoms | Chronic bronchitis (cough and phlegm daily for 3 mo over 3 yr) | Chronic bronchitis, dyspnea, fatigue, comorbidities |
| and/or | and/or | |
| Function | DlCO < 80% predicted or FEV1 decline >40 ml/yr or PRISM | Low DlCO, PAH, lung hyperinflation, hypoxia, hypercapnia |
| and/or | and/or | |
| Structure | Emphysema (>5% in CT-measured algorithms or diagnosed visually), vascular remodeling | Emphysema, airways thickening, vascular remodeling |
Definition of abbreviations: CT = computed tomography; DlCO = diffusing capacity for carbon monoxide; PAH = pulmonary arterial hypertension; PRISM = preserved ratio impaired spirometry.
Terminology of COPD
Recently, GOLD has agreed on a series of terms that can help achieve a uniform language of expression (109) and hopefully avoid terminological confusion as follows:
-
•
Early COPD. The word “early” means “near the beginning of a process.” Because COPD can start early in life and take a long time to manifest clinically, identifying early COPD in the clinic is very difficult. Furthermore, biological early, which relates to initial mechanisms that eventually lead to COPD, should be differentiated from clinical early, which reflects the initial perception of symptoms, functional limitation, and/or structural abnormalities. Based on this, GOLD proposes to use the term early COPD only to discuss biological early, when appropriate.
-
•
Mild COPD. The term “mild” has been used interchangeably with that of early disease. This assumption is incorrect; as noted above, early refers to time, whereas mild refers to disease severity, as GOLD recommends. In COPD it is expressed as the value of FEV1 as a function of predicted normal values.
-
•
COPD in young patients. The term “young” directly relates to the chronological age of the subject as agreed on by the World Health Organization. Given that lung function peaks at around 20–25 years and that young patients may develop COPD similar to older patients (102, 110), GOLD considers young those patients with COPD from 20–50 years of age (42).
-
•
Pre-COPD. This term (36, 37) identifies individuals of any age who have respiratory symptoms and structural and/or functional abnormalities in the absence of airflow limitation and who may (or not) develop persistent airflow limitation (i.e., COPD).
Conclusions
The knowledge related to COPD has changed significantly from a quasi-orphan, unpreventable, and untreatable disease when first described as emphysema by Rene Laennec in 1821 (111) to one where research has opened windows to its pathogenesis, causal agents, clinical expressions, and therapy. Supported by the knowledge acquired over decades, here we propose to keep the accepted acronym of COPD while enlarging the meaning of the term to include several causal agents and stages that can benefit from different preventive and therapeutic strategies. This proposal should help plan studies aimed at a better understanding of the pathobiological mechanisms involved in the genesis of these different types of COPD. Most of what we know today is derived from studies centered almost exclusively on COPD due to cigarette smoking, yet these results have been extrapolated directly to other causes of COPD in the many guidelines developed around the world. Some of these COPDs may be amenable to different effective primary and secondary prevention and treatments that have not been adequately explored. Finally, the content of this proposal should not be viewed as the final word. Rather, we expect that it will improve with the advances brought by increased knowledge of the cause, nature, and evolution of COPD.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202204-0671PP on August 1, 2022
Author disclosures are available with the text of this article at www.atsjournals.org
References
- 1.Posterior analytics II https://plato.stanford.edu/entries/aristotle-metaphysics/#SubsDefi.
- 2.Locke JE. 1997. Penguin Books. [Google Scholar]
- 3. Scadding JG. Essentialism and nominalism in medicine: logic of diagnosis in disease terminology. Lancet . 1996;348:594–596. doi: 10.1016/s0140-6736(96)02049-1. [DOI] [PubMed] [Google Scholar]
- 4. Pearce JM. Disease, diagnosis or syndrome? Pract Neurol . 2011;11:91–97. doi: 10.1136/jnnp.2011.241802. [DOI] [PubMed] [Google Scholar]
- 5. Livingstone-Banks J. The case for a meta-nosological investigation of pragmatic disease definition and classification. J Eval Clin Pract . 2018;24:1013–1018. doi: 10.1111/jep.13012. [DOI] [PubMed] [Google Scholar]
- 6. Scadding JG. Health and disease: what can medicine do for philosophy? J Med Ethics . 1988;14:118–124. doi: 10.1136/jme.14.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armocida E, Martini M. Tuberculosis: a timeless challenge for medicine. J Prev Med Hyg . 2020;61:E143–E147. doi: 10.15167/2421-4248/jpmh2020.61.2.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snider GL. Nosology for our day: its application to chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2003;167:678–683. doi: 10.1164/rccm.200203-204PP. [DOI] [PubMed] [Google Scholar]
- 9. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA . 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care . 2014;37:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 11. Snider GL. Chronic obstructive pulmonary disease: a definition and implications of structural determinants of airflow obstruction for epidemiology. Am Rev Respir Dis . 1989;140:S3–S8. doi: 10.1164/ajrccm/140.3_Pt_2.S3. [DOI] [PubMed] [Google Scholar]
- 12. Lowe KE, Regan EA, Anzueto A, Austin E, Austin JHM, Beaty TH, et al. COPDGene 2019: redefining the diagnosis of chronic obstructive pulmonary disease Chronic Obstr Pulm Dis (Miami) 2019. 6 384 399 31710793 [Google Scholar]
- 13. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med . 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 14. Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. U.S. Burden of Disease Collaborators The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA . 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet . 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruce N, Perez-Padilla R, Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ . 2000;78:1078–1092. [PMC free article] [PubMed] [Google Scholar]
- 17. Ramírez-Venegas A, Sansores RH, Pérez-Padilla R, Regalado J, Velázquez A, Sánchez C, et al. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med . 2006;173:393–397. doi: 10.1164/rccm.200504-568OC. [DOI] [PubMed] [Google Scholar]
- 18. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet . 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 19. Viegi G, Simoni M, Scognamiglio A, Baldacci S, Pistelli F, Carrozzi L, et al. Indoor air pollution and airway disease. Int J Tuberc Lung Dis . 2004;8:1401–1415. [PubMed] [Google Scholar]
- 20. Townend J, Minelli C, Mortimer K, Obaseki DO, Al Ghobain M, Cherkaski H, et al. The association between chronic airflow obstruction and poverty in 12 sites of the multinational BOLD study. Eur Respir J . 2017;49:1601880. doi: 10.1183/13993003.01880-2016. [DOI] [PubMed] [Google Scholar]
- 21. Bateman ED, Jithoo A. Lung diseases in South Africa: an overview. Novartis Found Symp . 2006;279:4–11. [PubMed] [Google Scholar]
- 22. Amaral AF, Coton S, Kato B, Tan WC, Studnicka M, Janson C, et al. BOLD Collaborative Research Group Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J . 2015;46:1104–1112. doi: 10.1183/13993003.02325-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caballero A, Torres-Duque CA, Jaramillo C, Bolívar F, Sanabria F, Osorio P, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study) Chest . 2008;133:343–349. doi: 10.1378/chest.07-1361. [DOI] [PubMed] [Google Scholar]
- 24. Menezes AM, Hallal PC, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, et al. Latin American Project for the Investigation of Obstructive Lung Disease (PLATINO) Team Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J . 2007;30:1180–1185. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- 25. Roflumilast: APTA 2217, B9302-107, BY 217, BYK 20869. Drugs R D . 2004;5:176–181. doi: 10.2165/00126839-200405030-00009. [DOI] [PubMed] [Google Scholar]
- 26. Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers . 2015;1:15076. doi: 10.1038/nrdp.2015.76. [DOI] [PubMed] [Google Scholar]
- 27. Castaldi PJ, Benet M, Petersen H, Rafaels N, Finigan J, Paoletti M, et al. Do COPD subtypes really exist? COPD heterogeneity and clustering in 10 independent cohorts. Thorax . 2017;72:998–1006. doi: 10.1136/thoraxjnl-2016-209846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gelb AF, Yamamoto A, Verbeken EK, Nadel JA. Unraveling the pathophysiology of the asthma-COPD overlap syndrome: unsuspected mild centrilobular emphysema is responsible for loss of lung elastic recoil in never smokers with asthma with persistent expiratory airflow limitation. Chest . 2015;148:313–320. doi: 10.1378/chest.14-2483. [DOI] [PubMed] [Google Scholar]
- 29. Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. BOLD Collaborative Research Group COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest . 2011;139:752–763. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mannino DM, Gan WO, Wurst K, Davis KJ. Asthma and chronic obstructive pulmonary disease overlap: the effect of definitions on measures of burden. Chronic Obstr Pulm Dis (Miami) . 2017;4:87–96. doi: 10.15326/jcopdf.4.2.2016.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med . 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Díaz E, Bruce N, Pope D, Lie RT, Díaz A, Arana B, et al. Lung function and symptoms among indigenous Mayan women exposed to high levels of indoor air pollution. Int J Tuberc Lung Dis . 2007;11:1372–1379. [PubMed] [Google Scholar]
- 33. Ramírez-Venegas A, Sansores RH, Quintana-Carrillo RH, Velázquez-Uncal M, Hernandez-Zenteno RJ, Sánchez-Romero C, et al. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am J Respir Crit Care Med . 2014;190:996–1002. doi: 10.1164/rccm.201404-0720OC. [DOI] [PubMed] [Google Scholar]
- 34. Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest . 2010;138:3–6. doi: 10.1378/chest.10-0645. [DOI] [PubMed] [Google Scholar]
- 35. Oelsner EC, Smith BM, Hoffman EA, Folsom AR, Kawut SM, Kaufman JD, et al. Associations between emphysema-like lung on CT and incident airflow limitation: a general population-based cohort study. Thorax . 2018;73:486–488. doi: 10.1136/thoraxjnl-2017-210842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Celli BR, Agustí A. COPD: time to improve its taxonomy? ERJ Open Res . 2018;4:00132-2017. doi: 10.1183/23120541.00132-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han MK, Agusti A, Celli BR, Criner GJ, Halpin DMG, Roche N, et al. From Gold 0 to pre-COPD. Am J Respir Crit Care Med . 2021 doi: 10.1164/rccm.202008-3328PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wan ES, Fortis S, Regan EA, Hokanson J, Han MK, Casaburi R, et al. COPDGene Investigators Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am J Respir Crit Care Med . 2018;198:1397–1405. doi: 10.1164/rccm.201804-0663OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fortis S, Comellas A, Kim V, Casaburi R, Hokanson JE, Crapo JD, et al. Low FVC/TLC in Preserved Ratio Impaired Spirometry (PRISm) is associated with features of and progression to obstructive lung disease. Sci Rep . 2020;10:5169. doi: 10.1038/s41598-020-61932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med . 2016;193:662–672. doi: 10.1164/rccm.201511-2210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med . 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 42. Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Calverley PMA, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2018;197:1540–1551. doi: 10.1164/rccm.201710-2028PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med . 2019;7:358–364. doi: 10.1016/S2213-2600(18)30529-0. [DOI] [PubMed] [Google Scholar]
- 44. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med . 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 45. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med . 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 46. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. IMPACT Investigators Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med . 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 47. Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. ETHOS Investigators Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med . 2020;383:35–48. doi: 10.1056/NEJMoa1916046. [DOI] [PubMed] [Google Scholar]
- 48. Martinez FJ, Agusti A, Celli BR, Han MK, Allinson JP, Bhatt SP, et al. Treatment trials in young patients with chronic obstructive pulmonary disease and pre-chronic obstructive pulmonary disease patients: time to move forward. Am J Respir Crit Care Med . 2022;205:275–287. doi: 10.1164/rccm.202107-1663SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Halpin DMG, Celli BR, Criner GJ, Frith P, López Varela MV, Salvi S, et al. It is time for the world to take COPD seriously: a statement from the GOLD board of directors. Eur Respir J . 2019;54:1900914. doi: 10.1183/13993003.00914-2019. [DOI] [PubMed] [Google Scholar]
- 50. Obaseki DO, Erhabor GE, Gnatiuc L, Adewole OO, Buist SA, Burney PG. Chronic airflow obstruction in a Black African population: results of BOLD study, Ile-Ife, Nigeria. COPD . 2016;13:42–49. doi: 10.3109/15412555.2015.1041102. [DOI] [PubMed] [Google Scholar]
- 51. Silverman EK, Sandhaus RA. Clinical practice: alpha1-antitrypsin deficiency. N Engl J Med . 2009;360:2749–2757. doi: 10.1056/NEJMcp0900449. [DOI] [PubMed] [Google Scholar]
- 52. Chapman KR, Chorostowska-Wynimko J, Koczulla AR, Ferrarotti I, McElvaney NG. Alpha 1 antitrypsin to treat lung disease in alpha 1 antitrypsin deficiency: recent developments and clinical implications. Int J Chron Obstruct Pulmon Dis . 2018;13:419–432. doi: 10.2147/COPD.S149429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL, et al. ICGN Investigators; ECLIPSE Investigators; COPDGene Investigators A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet . 2012;21:947–957. doi: 10.1093/hmg/ddr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hobbs BD, de Jong K, Lamontagne M, Bossé Y, Shrine N, Artigas MS, et al. COPDGene Investigators; ECLIPSE Investigators; LifeLines Investigators; SPIROMICS Research Group; International COPD Genetics Network Investigators; UK BiLEVE Investigators; International COPD Genetics Consortium Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat Genet . 2017;49:426–432. doi: 10.1038/ng.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beane J, Vick J, Schembri F, Anderlind C, Gower A, Campbell J, et al. Characterizing the impact of smoking and lung cancer on the airway transcriptome using RNA-Seq. Cancer Prev Res (Phila) . 2011;4:803–817. doi: 10.1158/1940-6207.CAPR-11-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang L, Lee S, Gim J, Qiao D, Cho M, Elston RC, et al. Family-based rare variant association analysis: a fast and efficient method of multivariate phenotype association analysis. Genet Epidemiol . 2016;40:502–511. doi: 10.1002/gepi.21985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qiao D, Lange C, Beaty TH, Crapo JD, Barnes KC, Bamshad M, et al. Lung GO; NHLBI Exome Sequencing Project; COPDGene Investigators Exome sequencing analysis in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2016;193:1353–1363. doi: 10.1164/rccm.201506-1223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ . 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ragland MF, Benway CJ, Lutz SM, Bowler RP, Hecker J, Hokanson JE, et al. genetic advances in chronic obstructive pulmonary disease: insights from COPDGene. Am J Respir Crit Care Med . 2019;200:677–690. doi: 10.1164/rccm.201808-1455SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. DeMeo DL, Hersh CP, Hoffman EA, Litonjua AA, Lazarus R, Sparrow D, et al. Genetic determinants of emphysema distribution in the national emphysema treatment trial. Am J Respir Crit Care Med . 2007;176:42–48. doi: 10.1164/rccm.200612-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med . 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 62. Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med . 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 63. Agustí A, Celli B. Natural history of COPD: gaps and opportunities. ERJ Open Res . 2017;3 doi: 10.1183/23120541.00117-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet . 2018;391:350–400. doi: 10.1016/S0140-6736(17)30879-6. [DOI] [PubMed] [Google Scholar]
- 65. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol . 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 66. Agustí A, Hogg JC. Update on the pathogenesis of COPD: reply. N Engl J Med . 2019;381:2484. doi: 10.1056/NEJMc1914437. [DOI] [PubMed] [Google Scholar]
- 67. Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annu Rev Med . 2003;54:113–129. doi: 10.1146/annurev.med.54.101601.152209. [DOI] [PubMed] [Google Scholar]
- 68. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet . 2017;389:1931–1940. doi: 10.1016/S0140-6736(17)31222-9. [DOI] [PubMed] [Google Scholar]
- 69. Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med . 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 70. Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med . 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 71. Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest . 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest . 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 73. Divo MJ, Celli BR, Poblador-Plou B, Calderón-Larrañaga A, de-Torres JP, Gimeno-Feliu LA, et al. EpiChron—BODE Collaborative Group Chronic obstructive pulmonary disease (COPD) as a disease of early aging: evidence from the EpiChron cohort. PLoS One . 2018;13:e0193143. doi: 10.1371/journal.pone.0193143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Perez TA, Castillo EG, Ancochea J, Pastor Sanz MT, Almagro P, Martínez-Camblor P, et al. Sex differences between women and men with COPD: a new analysis of the 3CIA study. Respir Med . 2020;171:106105. doi: 10.1016/j.rmed.2020.106105. [DOI] [PubMed] [Google Scholar]
- 75. Liu S, Zhou Y, Wang X, Wang D, Lu J, Zheng J, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax . 2007;62:889–897. doi: 10.1136/thx.2006.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou Y, Zou Y, Li X, Chen S, Zhao Z, He F, et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med . 2014;11:e1001621. doi: 10.1371/journal.pmed.1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hansel NN, Putcha N, Woo H, Peng R, Diette GB, Fawzy A, et al. Randomized clinical trial of air cleaners to improve indoor air quality and chronic obstructive pulmonary disease health: results of the CLEAN AIR study. Am J Respir Crit Care Med . 2022;205:421–430. doi: 10.1164/rccm.202103-0604OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Baraldi E, Bonadies L, Manzoni P. Evidence on the link between respiratory syncytial virus infection in early life and chronic obstructive lung diseases. Am J Perinatol . 2020;37:S26–S30. doi: 10.1055/s-0040-1714345. [DOI] [PubMed] [Google Scholar]
- 79. Satia I, Cusack R, Greene JM, O’Byrne PM, Killian KJ, Johnston N. Prevalence and contribution of respiratory viruses in the community to rates of emergency department visits and hospitalizations with respiratory tract infections, chronic obstructive pulmonary disease and asthma. PLoS One . 2020;15:e0228544. doi: 10.1371/journal.pone.0228544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gao YH, Guan WJ, Xu G, Tang Y, Gao Y, Lin ZY, et al. Macrolide therapy in adults and children with non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis. PLoS One . 2014;9:e90047. doi: 10.1371/journal.pone.0090047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hnin K, Nguyen C, Carson KV, Evans DJ, Greenstone M, Smith BJ. Prolonged antibiotics for non-cystic fibrosis bronchiectasis in children and adults. Cochrane Database Syst Rev . 2015:CD001392. doi: 10.1002/14651858.CD001392.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, Donat-Sanz Y, Serra PC, Lerma MA, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2013;187:823–831. doi: 10.1164/rccm.201208-1518OC. [DOI] [PubMed] [Google Scholar]
- 83. Bigna JJ, Kenne AM, Asangbeh SL, Sibetcheu AT. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob Health . 2018;6:e193–e202. doi: 10.1016/S2214-109X(17)30451-5. [DOI] [PubMed] [Google Scholar]
- 84. Sluiter HJ, Koëter GH, de Monchy JG, Postma DS, de Vries K, Orie NG. The Dutch hypothesis (chronic non-specific lung disease) revisited. Eur Respir J . 1991;4:479–489. [PubMed] [Google Scholar]
- 85. Barnes PJ. Against the Dutch hypothesis: asthma and chronic obstructive pulmonary disease are distinct diseases. Am J Respir Crit Care Med . 2006;174:240–243. [Discussion, pp. 243–244.]. doi: 10.1164/rccm.2604008. [DOI] [PubMed] [Google Scholar]
- 86. Senhorini A, Ferreira DS, Shiang C, Silva LF, Dolhnikoff M, Gelb AF, et al. Airway dimensions in fatal asthma and fatal COPD: overlap in older patients. COPD . 2013;10:348–356. doi: 10.3109/15412555.2012.752806. [DOI] [PubMed] [Google Scholar]
- 87. Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis. PLoS One . 2015;10:e0136065. doi: 10.1371/journal.pone.0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2015;191:758–766. doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sin DD, Miravitlles M, Mannino DM, Soriano JB, Price D, Celli BR, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J . 2016;48:664–673. doi: 10.1183/13993003.00436-2016. [DOI] [PubMed] [Google Scholar]
- 90. Busse WW. Biologicals for asthma in patients with asthma-COPD overlap. Lancet Respir Med . 2017;5:175–177. doi: 10.1016/S2213-2600(17)30055-3. [DOI] [PubMed] [Google Scholar]
- 91. Burney P, Patel J, Minelli C, Gnatiuc L, Amaral AFS, Kocabas A, et al. BOLD Collaborative Research Group Prevalence and population-attributable risk for chronic airflow obstruction in a large multinational study. Am J Respir Crit Care Med . 2021;203:1353–1365. doi: 10.1164/rccm.202005-1990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Breyer-Kohansal R, Faner R, Breyer MK, Ofenheimer A, Schrott A, Studnicka M, et al. Factors associated with low lung function in different age bins in the general population. Am J Respir Crit Care Med . 2020;202:292–296. doi: 10.1164/rccm.202001-0172LE. [DOI] [PubMed] [Google Scholar]
- 93. Morrow JD, Cho MH, Hersh CP, Pinto-Plata V, Celli B, Marchetti N, et al. DNA methylation profiling in human lung tissue identifies genes associated with COPD. Epigenetics . 2016;11:730–739. doi: 10.1080/15592294.2016.1226451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bolton CE, Bush A, Hurst JR, Kotecha S, McGarvey L, Stocks J, et al. Are early life factors considered when managing respiratory disease? A British Thoracic Society survey of current practice. Thorax . 2012;67:1110. doi: 10.1136/thoraxjnl-2012-202637. [DOI] [PubMed] [Google Scholar]
- 95. Labonté LE, Tan WC, Li PZ, Mancino P, Aaron SD, Benedetti A, et al. Canadian Respiratory Research Network; CanCOLD Collaborative Research Group Undiagnosed chronic obstructive pulmonary disease contributes to the burden of health care use: data from the CanCOLD study. Am J Respir Crit Care Med . 2016;194:285–298. doi: 10.1164/rccm.201509-1795OC. [DOI] [PubMed] [Google Scholar]
- 96. Agustí A, Melén E, DeMeo DL, Breyer-Kohansal R, Faner R. Pathogenesis of chronic obstructive pulmonary disease: understanding the contributions of gene-environment interactions across the lifespan. Lancet Respir Med . 2022;10:512–524. doi: 10.1016/S2213-2600(21)00555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med . 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. McAllister DA, Ahmed FS, Austin JH, Henschke CI, Keller BM, Lemeshow A, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One . 2014;9:e93221. doi: 10.1371/journal.pone.0093221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Petersen H, Sood A, Polverino F, Owen CA, Pinto-Plata V, Celli BR, et al. The course of lung function in middle-aged heavy smokers: incidence and time to early onset of chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2018;198:1449–1451. doi: 10.1164/rccm.201805-0861LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Harvey BG, Strulovici-Barel Y, Kaner RJ, Sanders A, Vincent TL, Mezey JG, et al. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur Respir J . 2015;46:1589–1597. doi: 10.1183/13993003.02377-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Alcaide AB, Sanchez-Salcedo P, Bastarrika G, Campo A, Berto J, Ocon MD, et al. Clinical features of smokers with radiological emphysema but without airway limitation. Chest . 2017;151:358–365. doi: 10.1016/j.chest.2016.10.044. [DOI] [PubMed] [Google Scholar]
- 102. Sanchez-Salcedo P, Divo M, Casanova C, Pinto-Plata V, de-Torres JP, Cote C, et al. Disease progression in young patients with COPD: rethinking the Fletcher and Peto model. Eur Respir J . 2014;44:324–331. doi: 10.1183/09031936.00208613. [DOI] [PubMed] [Google Scholar]
- 103. Beasley R, Weatherall M, Travers J, Shirtcliffe P. Time to define the disorders of the syndrome of COPD. Lancet . 2009;374:670–672. doi: 10.1016/S0140-6736(09)61541-5. [DOI] [PubMed] [Google Scholar]
- 104.Institute for Health Metrics and Evaluation https://www.healthdata.org/gbd/2019.
- 105.ICD10 Data. https://www.icd10data.com/ICD10CM/Codes/J00-J99/J40-J47
- 106. Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med . 2019;381:1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 107. Almagro P, Martinez-Camblor P, Soriano JB, Marin JM, Alfageme I, Casanova C, et al. Finding the best thresholds of FEV1 and dyspnea to predict 5-year survival in COPD patients: the COCOMICS study. PLoS One . 2014;9:e89866. doi: 10.1371/journal.pone.0089866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xiong H, Huang Q, Shuai T, Zhu L, Zhang C, Zhang M, et al. Assessment of comorbidities and prognosis in patients with COPD diagnosed with the fixed ratio and the lower limit of normal: a systematic review and meta-analysis. Respir Res . 2020;21:189. doi: 10.1186/s12931-020-01450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Global Initiative for Chronic Obstructive Lung Disease. https://goldcopd.org/
- 110. Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease: the Copenhagen General Population Study. Am J Respir Crit Care Med . 2020;201:671–680. doi: 10.1164/rccm.201908-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.R L. A treatise on the diseases of the chest. London: T. Underwood, and C. Underwood; 1821. [Google Scholar]


