Abstract
Objectives:
The National Patient-Centered Clinical Research Network (PCORnet) supports observational and clinical research using healthcare data. The PCORnet Antibiotics and Childhood Growth Study is one of PCORnet’s inaugural observational studies. The objectives of this manuscript are to describe (1) the processes used to integrate and analyze data from children across 36 participating institutions and (2) the cohort characteristics and prevalence of antibiotic use.
Methods:
We included children in the cohort if they had at least one same-day height and weight measured in each of three age periods: 1) before 12 months, 2) 12 to 30 months, and 3) after 24 months. We distributed statistical queries that each institution ran on its local version of the PCORnet Common Data Model, with aggregate data returned for analysis. We defined overweight or obesity as age-sex-specific body mass index ≥ 85th, obesity ≥95th percentile, and severe obesity ≥120% of the 95th percentile.
Results:
681,739 children met the cohort inclusion criteria and were racially/ethnically diverse (24.9% black, 17.5% Hispanic). Before 24 months, 55.2% of children received at least one antibiotic prescription; 21.3% received a single antibiotic prescription, 14.3% received four or more, and 33.3% received a broad spectrum antibiotic. Overweight and obesity prevalence was 27.6% at 4 to <6 years of age (n=362,044) and 36.2% at 9 to <11 years (n=58,344).
Conclusion:
The PCORnet Antibiotics study is a large, national longitudinal observational study in a diverse population that will examine the relationship between early antibiotic use and subsequent growth patterns in children.
Introduction
The widespread availability of healthcare data through electronic health records (EHRs) and other data sources provide unique opportunities to conduct pragmatic clinical trials and observational studies on a large scale. The National Patient-Centered Clinical Research Network (PCORnet) is a distributed research network that uses healthcare data to facilitate multi-site clinical trials and observational research studies.1–3 PCORnet has 13 Clinical Data Research Networks (CDRNs) that contribute health information for over 128 million patients. Within the CDRNs, data are organized in a Common Data Model (CDM) that allows for standardization across institutions and the development of efficient and reusable tools to capture and analyze data.4
This type of data infrastructure is essential for patient-centered research that requires large samples sizes, such as studies of rare diseases or studies that require assessments of heterogeneity of treatment effects, with various types of exposures among specific subgroups. To help develop infrastructure for observational research in PCORnet, the Patient-Centered Outcome Research Institute (PCORI) funded two initial observational studies to explore diverse research questions and launch PCORnet into a research-ready data system.3,5 One of these studies, the PCORnet Antibiotics and Childhood Growth Study, was the first effort in PCORnet to establish a large pediatric cohort across the network and the first study in PCORnet to actively characterize prescribing data. Having access to a large pediatric cohort also will enable assessments of different types, timing, and doses of antibiotics on weight outcomes, which has been difficult to do with smaller studies.
Our objective for this study was to evaluate the utility of this cohort for conducting comparative effectiveness research on medications and growth in young children. In this manuscript, we describe: (1) the processes used to integrate, synchronize, and analyze data from children across 36 health care institutions organized in 10 CDRNs; and (2) the cohort characteristics, including antibiotic use before 24 months of age and prevalence of overweight and obesity from early to mid-childhood.
Methods
Participating Institutions and the PCORnet Common Data Model
Created by the Affordable Care Act of 2010, PCORI is a funding agency that supports patient-centered comparative effectiveness research (CER) within five priority areas: “evaluating prevention, diagnosis, and treatment options; improving health systems; enhancing communication and dissemination of evidence; addressing disparities in health and health care; and improving CER methods and data infrastructure.”6 PCORI created PCORnet to expand the data infrastructure available for comparative effectiveness research, in a manner that incorporates the input of stakeholders.2 In addition to the 13 participating CDRNs including data from nearly 100 healthcare systems, PCORnet has 20 People-Powered Research Networks (PPRNs) that are focused on specific diseases or populations (contributing to both stakeholder engagement efforts and data) and 2 Health Plan Research Networks that are working to link health insurance claims data to PCORnet EHR data.
In PCORnet, data are organized by Network Partners. These Network Partners include data from either one contributing healthcare institution, or in the case of centralized Network Partners, from multiple institutions. In this study, 28 Network Partners are participating, and these partners hold data for 36 institutions across 10 CDRNs, including integrated delivery systems, free-standing children’s hospitals, and federally qualified health centers (Supplemental Table 1). To participate in the study, Network Partners had to meet data quality standards that were set forth by the PCORnet Coordinating Center. These included assessments of data model conformance, missingness in required tables and variables, and data plausibility in date and vital measure fields.7 Required tables included enrollment, encounters, demographics, vitals, diagnoses, and procedures. The study team additionally required that Network Partners had the capacity to create a pediatric cohort that met the study’s inclusion criteria, and could identify antibiotic prescriptions. Of the 44 institutions initially planned for inclusion, we removed 8 from the study for the following reasons: did not meet Coordinating Center data quality standards (1) or did not meet them by February 1, 2017 (1); did not have access to outpatient prescription medications in their CDM (2); were unable to map their prescribing data to RxNorm codes needed for the study by February 1, 2017 (1); were unwilling to share individual-level data (2); or chose not to participate because the site had a small pediatric population available in their CDM (1). A team of stakeholders from participating CDRNs and 4 of the PPRNs, including parents, providers, health system representatives, and patient advocates, closely informed the study conception and design and has provided ongoing feedback throughout the study.
The PCORnet CDM consists of 15 tables and over 100 variables available for research.4 An in-depth assessment of data usability and consistency was necessary before conducting statistical analyses, a process called study-specific data characterization. For the PCORnet Antibiotics and Childhood Growth Study, this process included capturing site-level aggregate data on study-specific variables (e.g., demographics, diagnoses, medications, vital signs). The study team analyzed this data to determine which sites met data quality eligibility requirements, while providing initial information on the cohort of interest.
Distributed Statistical Queries of the Network
Sites extract data from their local EHR systems and other healthcare data repositories, such as insurance claims, and transform those data to meet CDM standards. The PCORnet distributed research network model addresses governance and privacy concerns by allowing institutions to maintain data locally, rather than create a network-wide centralized database. Queries written to conform to the CDM standards are distributed to Network Partners for local execution, resulting in the return of standardized output that can be aggregated with other partners. To produce statistical query packages for distribution, either for data characterization or study analyses, PCORnet follows a standard workflow, informed by the setup of the Food and Drug Administration’s (FDA) Sentinel program.8 The FDA Sentinel program utilizes claims data from health insurers to examine drug safety across the United States. The workflow begins with the development of scientific specifications that describe the purpose of the query and the intended analyses, serving as a blueprint for the programming team. The programmers then develop a SAS statistical query to capture relevant data from Network Partners or to conduct analyses. Study teams are also responsible for generating and reviewing codes for relevant variables used for the query. This study used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and SNOMED-CT codes for diagnoses, and RxNorm and National Drug Code (NDC) codes for medications.
After query program development has been completed and tested with simulated data at the PCORnet Coordinating Center and at sites for beta testing, the program is distributed to the network via the PopMedNet Query Tool (QT).9 Once results have been returned from all responding sites, responses are collapsed into a single summary report for review and analyses by the study team (Supplemental Figure 1) in the case of aggregate data, and into analytic files for other types of data, such as patient-level data.
Study Cohort
The PCORnet Antibiotics and Childhood Growth study cohort included children from birth-<11 years of age. Inclusion criteria were one or more encounters with same-day length/height and weight measured in each of the following intervals: 0-<12 months; 12-<30 months; and 24+ months of age. The latter two age periods overlapped to allow for the possibility that children had their two-year-old well-child visit soon after their second birthday. Thus, children with only two measures were eligible for inclusion if the second was between 24 and 30 months of age; less than 1% of the children met the cohort criteria with only two measures. Children were excluded from the cohort if they did not have a male or female designation. Most Network Partners had data available from 2009 or 2010 until mid to late 2016, with a few exceptions. Only one had data that began prior to 2000; another Network Partner’s data availability ended in 2015.
Variables and Data Analysis
All data presented in this manuscript were from descriptive analyses of all participating institutions. We presented some descriptive data anonymously at the Network Partner level for those 23 Network Partners that had at least 5,000 children in the cohort. Demographics were defined according to the PCORnet CDM standards. Drug codes for antibiotics were identified using a two-pronged approach. First, we captured NDC codes for antibiotics using the functional classification system from First Databank (FDB).10 Crosswalks available from the National Library of Medicine were used to convert the NDC code list into RxNorm codes,11 the prescription classification system used for this study. Second, to identify additional systemic antibiotic codes in RxNorm, we separately captured RxNorm semantic clinical drug form (SCDF) terms using the Anatomic Therapeutic Classification system.12 Under the RxNorm hierarchy, we also collected all less specific codes that were related to the SCDF terms, to maximize capture of antibiotics; these included drug component, ingredient, brand name, multi-ingredient, and precise ingredient codes. We further captured more specific codes related to the SCDF terms, including semantic clinical drug or pack and semantic branded drug or pack codes. During manual review of these lists, we excluded anti-protozoal medications, antibiotics not available in the United States, veterinary medicines, and most intravenous medications. This led to a final antibiotic list of oral medications and intravenous or intramuscular ceftriaxone and penicillin, medications likely to be prescribed in the outpatient or emergency department setting. This restriction ensured consistency across the network – several did not have inpatient medications – and allowed this study to focus on antibiotics whose use we believed could be potentially more modifiable than most intravenous medications.
Network Partners did not routinely have days supplied available for prescriptions. Thus, we defined exposure to antibiotics by the number of episodes of antibiotics prescribed. The time window for an antibiotic episode was seven days, such that any antibiotic prescriptions within a seven-day period of another prescription were joined together into a single episode. Narrow spectrum antibiotics included amoxicillin, penicillin, and dicloxacillin; broad spectrum included all other antibiotics, including penicillin combinations, such as amoxicillin/clavulanic acid. To define whether the episode was for a broad or narrow spectrum antibiotic, we used the highest spectrum antibiotic prescribed within the episode.
Diagnostic codes were identified for potential confounders or effect modifiers, such as asthma and prematurity, as well as diagnoses for chronic conditions. For complex chronic conditions, we used the list of ICD-9-CM code clusters developed by Feudtner, et al.13 Using these diagnoses, we added to the list by searching an Optum database to ensure that we captured all relevant codes for these diagnoses. For those sites that used only SNOMED-CT codes for diagnoses, we translated the final ICD-9-CM code list to SNOMED-CT using an established crosswalk.14
Computation of BMI z-scores utilized the World Health Organization (WHO) growth standards for children <24 months of age: underweight if age-and sex-specific BMI was <2.3rd percentile, normal weight if 2.3rd-<97.7th percentile, and overweight/obesity if ≥97.7th percentile.15 We used the Centers for Disease Control and Prevention (CDC) NHANES 2000 growth charts to classify weight status of children ≥24 months of age: underweight if <5th percentile, normal weight 5th-<85th, overweight 85th-<95th percentile, obesity ≥95th percentile, and severe obesity ≥120% of the 95th percentile.16 We removed implausible values of BMI z-scores less than −5 and greater than 8, accounting for the recommended bounds for both the WHO (−5, +5) and CDC (−4, +8). We utilized a SAS-based summary program to aggregate data across responding Network Partners and format into a readable excel report for analyses and characterization.
Results
The final study cohort included 681,739 children, which was 38% of all children who had at least one same-day height/weight measurement <12 months of age and 71% of children who also had at least one additional same-day height and weight measurement12-<30 months of age (Supplemental Figure 2). Slightly more than half were male (52.3%), and the cohort was racially diverse, with 53.4% White, 24.9% Black or African American, 5.9% other race, and 4.2% Asian (Table 1). Although there was substantial missing data on Hispanic ethnicity, 17.5% of the cohort was identified as Hispanic. All 28 Network Partners contributed to the cohort; the largest CDRN contributors were PEDSnet (46% of the cohort), PORTAL (22%), ADVANCE (9%), and Mid-South (8%).
Table 1.
Descriptive Statistics for Study Cohort
| Sex, N (%) | |
| Male | 356,875 (52.3%) |
| Female | 324,864 (47.7%) |
| Race, N (%) | |
| White | 363,759 (53.4%) |
| Black, African American | 170,007 (24.9%) |
| Asian | 28,356 (4.2%) |
| Multiple race | 14,184 (2.1%) |
| Native Hawaiian, Other Pacific Islander | 2,930 (0.4%) |
| American Indian, Alaska Native | 2,862 (0.4%) |
| Other | 39,885 (5.9%) |
| Refuse/Unknown/No info | 59,684 (8.8%) |
| Hispanic, N (%) | |
| Yes | 119,059 (17.5%) |
| No | 445,878 (65.4%) |
| Other | 2,125 (0.3%) |
| Refuse/Unknown/No Info | 114,660 (16.8%) |
| Birth-related Diagnoses, N (%) | |
| Prematurity | 52,752 (7.7%) |
| Heavy for gestational age newborn | 9,907 (1.5%) |
| Low birth weight | 5,828 (0.9%) |
| Macrosomia | 425 (0.06%) |
| Chronic Conditions/Categories, N (%) | |
| Asthma | 94,399 (13.8%) |
| Cardiovascular | 46,862 (6.9%) |
| Other congenital defect | 42,094 (6.2%) |
| Respiratory | 21,455 (3.1%) |
| Metabolic | 17,005 (2.5%) |
| Neuromuscular | 16,906 (2.5%) |
| Renal | 16,397 (2.4%) |
| Hematological/Immunological | 14,547 (2.1%) |
| Endocrine | 6,602 (1.0%) |
| Malignant Neoplasms | 6,593 (1.0%) |
| Gastrointestinal | 6,015 (0.9%) |
Birth-related diagnoses were rarely documented in the electronic health records, except for prematurity, with 7.7% of children having a code for prematurity documented (Table 1). By comparison, the prevalence of US preterm births in 2014 was 9.5%.17 Because several of the participating health systems were referral centers, the rate of chronic conditions was high, including 13.8% who had a least one diagnostic code documented for asthma, and 6.9%, 6.2%, and 3.1% for cardiovascular, other defects (mostly congenital defects), and respiratory diagnoses, respectively, <5 years of age.
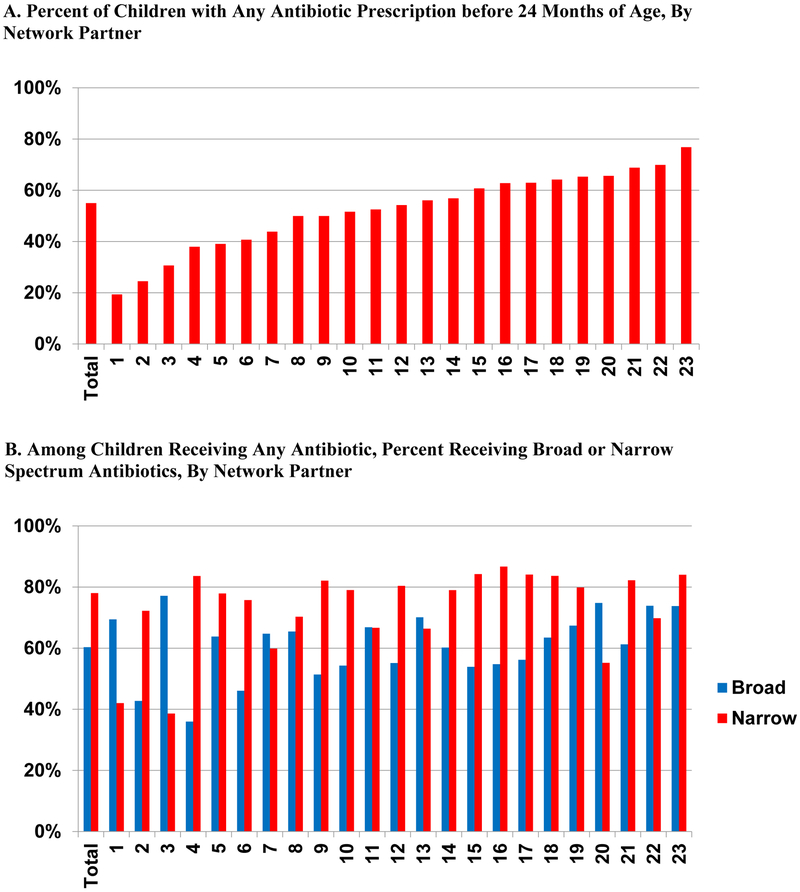
Among all children in the cohort, 55.2% received at least one prescription for an antibiotic <24 months of age (Table 2). Among the 23 Network Partners with at least 5,000 children in the cohort, the fraction of children prescribed at least one antibiotic ranged from 19% to 77%; 16 of the 23 Network Partners, including most of the larger ones, had prescribing rates above 50% (Figure 1a). For all children in the cohort, 21.3% had one prescription episode and 14.3% had 4+ prescription episodes <24 months (Table 2).
Table 2.
Exposure to Antibiotics and Other Medications Before Age 2 Years
| No Antibiotics, N (%) | 305,489 (44.8%) |
| Any Antibiotic, N (%) | 376,250 (55.2%) |
| All Antibiotics, N (%) | |
| 1 | 144,949 (21.3%) |
| 2 | 83,157 (12.2%) |
| 3 | 50,870 (7.5%) |
| 4+ | 97,267 (14.3%) |
| Narrow Spectrum, N (%) | |
| 1 | 159,295 (23.4%) |
| 2 | 76,486 (11.2%) |
| 3 | 34,326 (5.0%) |
| 4+ | 23,512 (3.4%) |
| Broad Spectrum, N (%) | |
| 1 | 114,830 (16.8%) |
| 2 | 47,440 (7.0%) |
| 3 | 24,848 (3.6%) |
| 4+ | 39,926 (5.9%) |
| Amoxicillin, N (%) | |
| 1 | 159,083 (23.3%) |
| 2 | 76,096 (11.2%) |
| 3 | 34,068 (5.0%) |
| 4+ | 22,793 (3.3%) |
| Amoxicillin/Clavulanic Acid, N (%) | |
| 1 | 67,435 (9.9%) |
| 2 | 19,121 (2.8%) |
| 3 | 6,797 (1.0%) |
| 4+ | 4,280 (0.6%) |
| Azithromycin, N (%) | |
| 1 | 46,191 (6.8%) |
| 2 | 10,836 (1.6%) |
| 3 | 3,719 (0.5%) |
| 4+ | 2,371 (0.3%) |
Figure 1.
PCORnet Antibiotics Study, Prescribing Rate and Breakdown of Antibiotic Spectrum by Network Partner
This figure presents the percent of children who receive an antibiotic prescription before 24 months of age overall for the cohort and by Network Partner for those contributing at least 5,000 children to the cohort (A) and the percent of children receiving any narrow and broad spectrum antibiotic before 24 months of age overall and by Network Partner (B). The denominator for A includes all children in the cohort; for B, it includes all children who received at least one antibiotic prescription before 24 months of age. Among the 23 Network Partners presented, 16 had antibiotic prescribing rates of greater than 50% and had higher narrow spectrum antibiotic prescribing rates than broad.
Narrow spectrum antibiotics, nearly all amoxicillin, were more commonly prescribed with 43.0% of all children in the cohort (over three-quarters of children who received any antibiotics) receiving at least one prescription for a narrow spectrum antibiotic <24 months of age and 33.3% receiving at least one broad spectrum antibiotic (Table 2). Across the 23 Network Partners with at least 5,000 children, 16 had higher rates of prescribing for narrow spectrum antibiotics (Figure 1b).
Nearly 1/4 of children (23.4%) received one narrow spectrum antibiotic episode, and 3.4% received 4+ compared to 16.8% and 5.9% for broad spectrum (Table 2). Amoxicillin/clavulanic acid and azithromycin were prescribed at least once for 14.3% and 9.2% of children, respectively.
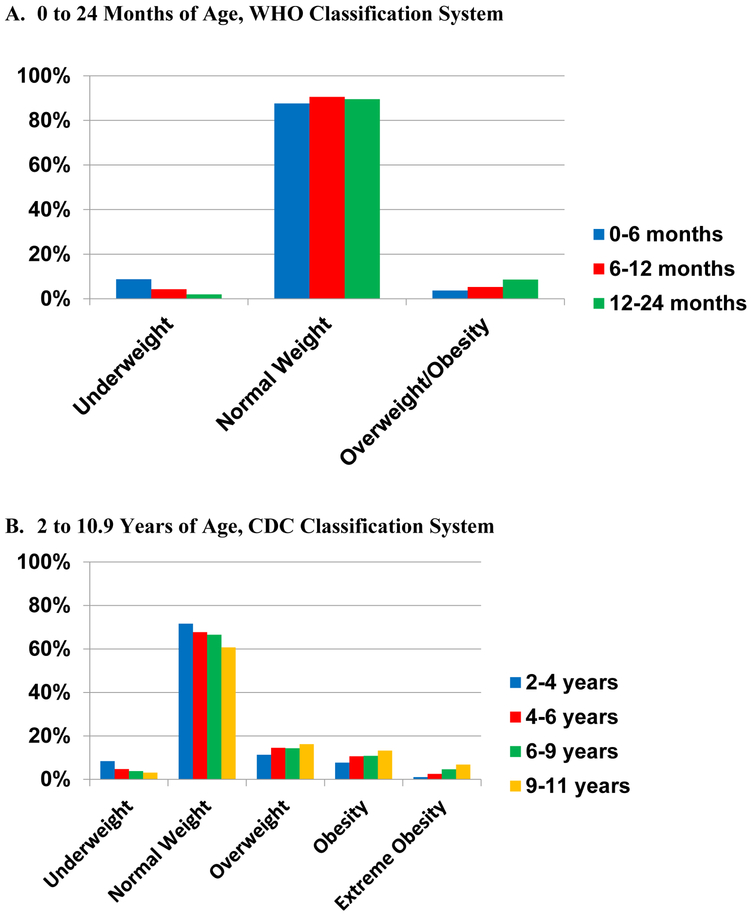
Mean BMI z-scores were −0.30 (WHO, SD 1.31) at 0-<6 months of age, rising to 0.59 (NHANES, SD 1.18) at 9-<11 years of age (Supplemental Table 2). The prevalence of overweight and obesity across age groups was 3.7% among 0-<6-month-old children, 27.6% among 4-<6 year olds, and 36.2% among 9-<11 year olds (Figure 2, Supplemental Tables 3 and 4). The prevalence of severe obesity was 2.5% and 6.8% respectively for 4-<6 and 9-<11 year olds. There was a range in prevalence of overweight and obesity across the 23 Network Partners with at least 5,000 children in the cohort (Supplemental Figure 3). For example, prevalence of obesity among 4-<6-year-old children had a range of 5% to 22%.
Figure 2.
PCORnet Antibiotics Study, Cohort Weight Class
The study calculated age- and sex-specific BMI (BMI z-score) for children at each age period. World Health Organization (WHO) growth standards for children were utilized before 24 months of age: underweight if BMI z-score was <2.3rd percentile, normal weight if 2.3rd to <97.7th percentile, and overweight/obesity if ≥97.7th percentile (A). The Centers for Disease Control and Prevention (CDC) NHANES 2000 growth charts for children were utilized for children from 2 to 10.9 years: underweight if BMI z-score <5th percentile, normal weight 5th to <85th, overweight 85th to <95th percentile, obesity ≥95th percentile, and severe obesity ≥120% of the 95th percentile (B). Rates of overweight, obesity, and severe obesity were higher for old children.
Discussion
Using the PCORnet data network infrastructure, we assembled a study cohort of 681,739 children with multiple measures of height and weight captured in EHRs across 36 health care institutions. More than half of these children had height and weight data at ages 4- <6 years, and 9% at ages 9-<11 years. The cohort has broad diversity in geography, demographics, and care settings and provides a rich data source for the longitudinal study of childhood growth.
PCORnet’s architecture strikes a balance between facilitating large-scale collaborative research and managing institutional risks. The PCORnet Antibiotics and Childhood Growth Study, engaging 28 of 82 (34%) Network Partners, provides an early test of the network, including valuable information about network governance and operations, patient engagement, use of prescribing and anthropometric data, and characteristics of the population. Most contributing healthcare institutions in the network are anchored by large hospitals in urban or suburban areas, which may explain the high rates of complex chronic conditions. Overall, however, the clinical data collected align well with expectations for pediatric populations.
Results from this descriptive study demonstrate that PCORnet is well-suited for pediatrics observational epidemiologic research, with capacity to create large cohorts for the study of healthcare exposures and outcomes. PCORnet can also provide meaningful national surveillance data on healthcare utilization and outcomes. In this cohort, over half of included children received at least one prescription for antibiotics by their second birthday. While recommended first-line antibiotics were most common, one-third of children received at least one broad-spectrum antibiotic, and one in seven received 4+ courses. This results in a significant exposed group for both antibiotics overall and for subset analyses by type and extent of exposure. The rate of antibiotic exposure is comparable to prior studies.18–20
Anthropometric measures for this cohort are similar to national survey estimates. For most age categories, the median BMI z-score is slightly positive. Prevalence of obesity in our cohort was 13.1% from ages 4-<6 years and 20.0% from ages 9-<11 years, compared to 9% among 2-<6 year olds and 18% among 6-<12 year olds in NHANES data spanning 2011 to 2014.21 Prevalence of severe obesity was 2.5% for 4-<6 year olds and 6.8% for 9-<11 year olds, compared to 1.7% for 2-<6 years olds and 4.3% for 6-<12 year olds in NHANES 2011–2014. The slightly higher prevalence in our cohort may reflect differences in age ranges, given the higher prevalence of childhood obesity as age increases or differences in patient mix between those children continuously enrolled in PCORnet health systems versus the US population.21,22 It may also reflect characteristics of the cohort, which includes a larger proportion of African-American children and children living in urban settings than the US population.21
The objective of the PCORnet Antibiotics and Childhood Growth Study is to better characterize the relationship between antibiotics and childhood obesity in the United States. This study is powered to examine multiple potential associations of antibiotics and weight, including the effects of types, timing, and frequency of antibiotic use in the first 2 years of life on BMI, obesity, and growth. Quantifying the precise effect size of this association will provide pertinent information to patients and clinicians regarding potential obesogenic risks of antibiotic prescriptions. Compared to prior studies that have examined the relationship between antibiotic use and growth in children, this cohort provides the largest and most diverse population for study. Prior reports have included single health system or single region studies in the US, including children in Northern California (260,556 children) or central Pennsylvania (142,824 children), as well as smaller European national studies such as in England (11,532 children) or Denmark (28,354 children).23–26 Representation of African-American children in this cohort is higher than the entire US population (25% vs 12%); the proportion of patients identifying as Hispanic is similar to the US population. This cohort demonstrates a modest overrepresentation of several chronic conditions, which may be due to the inclusion of several large health systems providing tertiary care. Also, the prevalence of chronic disease could have been higher solely because the cohort includes children who have received healthcare services.
In addition to the overrepresentation of urban environments and chronic conditions, it is important to note several other limitations. Most significantly, available data did not include other variables that influence the risk of childhood obesity, including maternal health or gestational factors, as well as environmental, social, behavioral, and dietary factors. The PCORnet study will address the absence of gestational data by linking maternal and child health records for a subset of the cohort, allowing inclusion of maternal BMI, child birth weight, and maternal weight gain. Another limitation was that antibiotic exposure was measured using prescribing records, which may overestimate exposure in cases where children do not complete prescribed courses of antibiotics. Prescribing records also may underestimate exposure because some prescribing may occur in healthcare settings not covered by a contributing institution and would therefore be missed. Similarly, our identification of chronic conditions relies on one or more occurrences of diagnosis codes recorded during clinical encounters and may overestimate prevalence due to use of these codes for “rule-out” diagnostic evaluations. When we capture and analyze individual-level data on participants, rather than aggregate data only as for this manuscript, we will be able to compensate for this by requiring repeated presence of codes, consistent with the chronic nature of the conditions.
More generally, the use of routine clinical data for the study may result in missingness (e.g., patients with race or Hispanic ethnicity not recorded), misestimation (e.g., higher use of diagnostic codes when ruling out conditions), or loss of detail (e.g., use of ICD-9-CM in billing data, losing specificity of diagnoses primarily recorded in the EHR). These limitations are common to studies using EHR and administrative data.
Conclusion
The PCORnet Antibiotics and Childhood Growth cohort demonstrates the capacity of PCORnet to facilitate capture of data on large pediatric populations for research, including early childhood growth, chronic conditions, infectious diagnoses, and antibiotic usage. This study provides valuable surveillance information on antibiotic utilization and weight and will be the largest to date to examine the relationship between antibiotic use in early life on weight outcomes in childhood. The large sample size and detailed clinical data will allow us to examine relationships among the type, timing, and level of antibiotic exposure to determine whether these factors are related to weight outcomes. PCORnet can provide significant opportunities to explore precise research inquiries in pediatrics.
Supplementary Material
What’s New.
PCORnet provides an unprecedented opportunity to conduct research using healthcare data. In 36 healthcare institutions, we assembled a large cohort to examine antibiotics and childhood growth. More than 1/2 of children received an antibiotic prescription before 2 years of age.
Acknowledgements
This work was supported through the Patient-Centered Outcomes Research Institute (PCORI) Program Award (OBS-1505–30699). All statements in this manuscript are solely those of the authors and do not necessarily represent the views of PCORI, its Board of Governor, or Methodology Committee. The PCORnet Childhood Antibiotic Study Team includes a diverse group of investigators, research staff, clinicians, community members, and parent caregivers. All members of the team including the study’s Executive Antibiotic Stakeholder Advisory Group (EASAG) contributed to the study design, data acquisition, and interpretation of results. The Study Team would like to thank the leaders of the participating PCORnet Clinical Data Research Networks (CDRNs), Patient Powered Research Networks (PPRNs), and PCORnet Coordinating Center as well as members of the PCORI team for their support and commitment to this project.
Funding: This work was supported through the Patient-Centered Outcomes Research Institute (PCORI) Program Award (OBS-1505–30699). The authors have no conflicts of interest to report.
Abbreviations:
- PCORnet
National Patient-Centered Clinical Research Network
- BMI
Body mass index
- CDRNs
Clinical data research networks
- CDM
Common Data Model
- EHR
Electronic health record
Appendix 1. Members of the PCORnet Antibiotics and Childhood Growth Study Group
William Adams, MD, Department of Pediatrics, Boston University School of Medicine, Boston, Massachusetts
Brad Appelhans, PhD, Department of Preventive Medicine, Rush Medical College, Chicago, Illinois
Andrew Brickman, PhD, Strategic Clinical Initiatives, Health Choice Network, Terr Doral, Florida
Jiang Bian, MS, PhD, College of Medicine, University of Florida, Gainesville, Florida
Matthew F. Daley, MD, Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado
Arthur Davidson, MD, MPH, Denver Public Health, Denver, Colorado
Amanda Dempsey, MD, PhD, MPH, Department of Pediatrics, University of Colorado School of Medicine, Denver, Colorado
Lara R. Dugas, PhD, MPH, Department of Public Health Sciences, Loyola University, Chicago, Illinois
Ihuoma Eneli, MD, MS, Nationwide Children’s Hospital, Columbus, Ohio
Stephanie L. Fitzpatrick, Center for Health Research, Kaiser Permanente North West, Portland, Oregon
William Heerman, MD, MPH, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee
Michael Horberg, MD, MAS, Kaiser Permanente Mid-Atlantic Permanente Research Institute, Rockville, Maryland
Daniel S. Hsia, MD, Pennington Biomedical Research Center, Baton Rouge, Louisiana
Jenny Ingber, PhD, American Museum of Natural History, New York, New York
Carmen R. Isasi, MD, PhD, Department of Epidemiology and Population Health, Montefiore, Bronx, New York
David M. Janicke, PhD, ABPP, Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, Florida
Doug Kane, MS, Kaiser Permanente Washington Health Research Institute, Seattle, Washington
Elyse Kharbanda, MD, MPH, Health Partners Institute
David Meltzer, MD, PhD, Center for Health and Social Sciences,University of Chicago Medicine, Chicago, Illinois
Mary Jo Messito, MD, Department of Pediatrics, New York University School of Medicine, New York, New York
Prakash Nadkarni, MD, Department of Internal Medicine, Carver College of Medicine, University of Iowa, Iowa City, Iowa
Kevin O’Bryan, MD, Department of Pediatrics,St Louis Children’s Hospital, St. Louis, Missouri
Holly Peay, MS, PhD, RTI International, Research Triangle Park, North Carolina
Jon Puro, MPA/HA, OCHIN Inc, Portland, Oregon
Daksha Ranade, MPH, MBA, Research Informatics Department,Seattle Children’s Hospital, Seattle, Washington
Goutham Rao, MD, Case Western Reserve University and University Hospitals of Cleveland, Cleveland, Ohio
Alfredo Tirado-Ramos, PhD, University of Texas Health Science Center at San Antonio, San Antonio, Texas
Maria Rayas, MD, University of Texas Health Science Center at San Antonio, San Antonio, Texas
Hanieh Razzaghi, MPH, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania
Iben M. Ricket, Louisiana Public Health Institute, New Orleans, Louisiana
Marc Rosenman, MD, Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University Feinberg School of Medicine, Chicago, Illinois
Robert M. Siegel, MD, FAAP, Heart Institute, Cincinnati Children’s Hospital, Cincinnati, Ohio
Tony Solomonides, DPhil, MSc, NorthShore University HealthSystem, Evanston, Illinois
Elsie M. Taveras, MD, MPH, MassGeneral Hospital for Children and Harvard Medical School, Boston, Massachusetts
Bradley Taylor, BS, Medical College of Wisconsin, Milwaukee, Wisconsin
Veeral Tolia, MD, Baylor Scott & White Health, Fort Worth, Texas
Zachary Willis, MD, MPH, Department of Pediatrics,University of North Carolina at Chapel Hill, Chapel Hill, South Carolina
Jeffrey VanWormer, PhD, Center for Clinical Epidemiology and Population Health, Marshfield Clinic Research Institute, Marshfield, Wisconsin
Tim Wysocki, PhD, ABPP, Nemours Children’s Health System, Jacksonville, Florida
Xiaobo Zhou, PhD, University of Texas, Health Science Center of Houston, Houston, Texas
References
- 1.Collins FS, Hudson KL, Briggs JP, Lauer MS. PCORnet: turning a dream into reality. J Am Med Inform Assoc. 2014;21(4):576–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient-centered clinical research network. Journal of the American Medical Informatics Association : JAMIA. 2014;21(4):578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toh S, Rasmussen-Torvik LJ, Harmata EE, et al. The National Patient-Centered Clinical Research Network (PCORnet) Bariatric Study Cohort: Rationale, Methods, and Baseline Characteristics. JMIR Res Protoc. 2017;6(12):e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PCORnet: Common Data Model (CDM) specification, version 3.0. 2017; http://www.pcornet.org/wp-content/uploads/2014/07/2015-07-29-PCORnet-Common-Data-Model-v3dot0-RELEASE.pdf. Accessed July 12, 2017.
- 5.PCORnet. Demonstration studies. 2017; http://pcornet.org/demonstration-studies/. Accessed December 13, 2017.
- 6.Selby JV, Lipstein SH. PCORI at 3 years--progress, lessons, and plans. N Engl J Med. 2014;370(7):592–595. [DOI] [PubMed] [Google Scholar]
- 7.Qualls LG PT, Hammill BG, Topping J, Louzao DM, Brown JS, Curtis LH, Marsolo K. Evaluating foundational data quality in the National Patient-Centered Clinical Research Network (PCORnet®). EGEMS. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platt R. FDA’s Mini-Sentinel program to evaluate the safety of marketed medical products. https://www.brookings.edu/wp-content/uploads/2013/01/Richard-Platt-Presentation.pdf: Harvard Pilgrim Health CareInstitute; 2013. [Google Scholar]
- 9.PopMedNet. http://www.popmednet.org/?page_id=41. Accessed July 12, 2017.
- 10.First Databank. [Website]. http://www.fdbhealth.com/, 2017.
- 11.U.S. National Library of Medicine. RxNorm 2004; https://www.nlm.nih.gov/research/umls/rxnorm/, 2017.
- 12.U.S. National Library of Medicine. Unified Medical Language System® (UMLS®) - Metathesaurus 2009; https://www.nlm.nih.gov/research/umls/knowledge_sources/metathesaurus/index.html. Accessed July 7, 2017.
- 13.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. [DOI] [PubMed] [Google Scholar]
- 14.Observational Health Data Sciences and Informatics (OHDSI). [Website]. 2017; http://www.ohdsi.org/web/wiki/doku.php?id=documentation:vocabulary.
- 15.WHO Multicentre Growth Reference Study Group. Geneva: World Health Organization. World Health Organization; 2006. [Google Scholar]
- 16.Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. Defining Childhood Obesity [Webpage]. 2016; https://www.cdc.gov/obesity/childhood/defining.html#modalIdString_CDCTable_0. Accessed August 16, 2017.
- 17.Ferre C, Callaghan W, Olson C, Sharma A, Barfield W. Effects of Maternal Age and Age-Specific Preterm Birth Rates on Overall Preterm Birth Rates - United States, 2007 and 2014. MMWR Morb Mortal Wkly Rep. 2016;65(43):1181–1184. [DOI] [PubMed] [Google Scholar]
- 18.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. [DOI] [PubMed] [Google Scholar]
- 19.McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287(23):3096–3102. [DOI] [PubMed] [Google Scholar]
- 20.Youngster I, Avorn J, Belleudi V, et al. Antibiotic Use in Children - A Cross-National Analysis of 6 Countries. J Pediatr. 2017;182:239–244 e231. [DOI] [PubMed] [Google Scholar]
- 21.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168(11):1063–1069. [DOI] [PubMed] [Google Scholar]
- 23.Li DK, Chen H, Ferber J, Odouli R. Infection and antibiotic use in infancy and risk of childhood obesity: a longitudinal birth cohort study. Lancet Diabetes Endocrinol. 2017;5(1):18–25. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz BS, Pollak J, Bailey-Davis L, et al. Antibiotic use and childhood body mass index trajectory. Int J Obes (Lond). 2016;40(4):615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond). 2013;37(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond). 2011;35(4):522–529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.