SUMMARY
In aging, skeletal muscle strength and regenerative capacity declines due, in part, to functional impairment of muscle stem cells (MuSCs), yet the underlying mechanisms remain elusive. Here we capitalize on mass-cytometry to identify high CD47 expression as a hallmark of dysfunctional MuSCs (CD47hi) with impaired regenerative capacity that predominate with aging. The prevalent CD47hi MuSC subset suppresses the residual functional CD47lo MuSC subset through a paracrine signaling loop, leading to impaired proliferation. We uncover that elevated CD47 levels on aged MuSCs result from increased U1 snRNA expression, which disrupts alternative polyadenylation. The deficit in aged MuSC function in regeneration can be overcome either by morpholino-mediated blockade of CD47 alternative polyadenylation or antibody blockade of CD47 signaling, leading to improved regeneration in aged mice, with therapeutic implications. Our findings highlight a previously unrecognized age-dependent alteration in CD47 levels and function in MuSCs, which underlies reduced muscle repair in aging.
Keywords: Aging, muscle stem cells, regeneration, CyTOF, paracrine signaling, thrombospondin-1, CD47 signaling, in vivo antibody-blockade, alternative polyadenylation, U1 snRNA, antisense morpholino oligonucleotide
eTOC
Porpiglia and colleagues identify a dysfunctional CD47hi muscle stem cell (MuSC) subset in aged mice, which arises from increased U1snRNA-driven CD47 alternative polyadenylation. CD47hi MuSCs trigger deleterious thrombospondin-1/CD47 signaling. A thrombospondin-1 antibody or a morpholino to the U1 site on CD47 restores aged MuSC function and muscle regeneration in vivo.
Graphical Abstract

INTRODUCTION
Muscle stem cells, also known as satellite cells, reside within skeletal muscle tissue in niches juxtaposed to myofibers and are required for skeletal muscle maintenance and regeneration throughout life1–8. There is abundant evidence that age-related loss of muscle mass and strength, also known as sarcopenia, is paralleled by loss of MuSC function5. However, the mechanisms underlying this loss remain poorly understood, limiting the development of therapies. Changes in cell extrinsic regulators such as fibronectin, wnt, fibroblast growth factor-2 (FGF-2), and apelin in the muscle microenvironment diminish MuSC function with aging9–13. In addition, MuSCs isolated from aged mice exhibit intrinsic defects due to aberrant p38 MAPK, JAK/STAT and TGF-β signaling, which lead to a decrease in the proportion of functional MuSCs, hindering muscle regeneration14–18. The absence of markers for distinguishing and prospectively isolating dysfunctional MuSCs has limited mechanistic insights and the development of therapeutic strategies to enhance aged MuSC function in regeneration.
Here we capitalize on multiparametric single-cell mass cytometry19–21, which allows the discovery of previously unrecognized cell subsets within rare stem cell populations, to determine whether alterations in the relative abundance of MuSC subsets or in the regulation of their signaling networks is responsible for the decline of MuSC function in the course of aging. High dimensional single-cell analysis of MuSCs enabled us to identify two functionally and molecularly distinct subsets, defined by differential cell surface expression of CD47. CD47lo MuSCs exhibit high regenerative capacity, whereas CD47hi MuSCs are defective in self-renewal. With aging, a shift in polyadenylation site choice leads to a marked increase in the relative abundance of CD47hi MuSCs with impaired regenerative capacity.
CD47, known as the “don’t eat me signal” on cancer cells22,23, has only recently been implicated in myogenesis. A recent study revealed that CD47 signaling promotes proliferation of young MuSCs in the context of hypertrophy modeled by mechanical stress, leading to myonuclear accretion24. Here we uncover a role for CD47 in MuSC function in regeneration and aging. We show that in contrast to young MuSCs, CD47 is markedly increased on the cell surface of aged MuSCs due to alternative polyadenylation resulting from increased U1 snRNA expression. Further, our study uncovers an unexpected age-dependent role for CD47 signaling in a paracrine loop that inhibits proliferation of the residual functional CD47lo aged MuSC subset and has a pleiotropic negative effect on regenerative function in aged muscle. To overcome this age-related dysfunction, we identify two means to surmount aberrant CD47 signaling, via antisense morpholino oligonucleotides (AMOs) or immune blockade, which lead to enhanced regeneration. These findings have broad implications for sarcopenia and provide fresh insights into aged stem cell dysfunction of broad relevance to regenerative medicine.
RESULTS
CD47 expression levels distinguish functionally and molecularly distinct aged muscle stem cell subsets.
We sought to identify cell surface markers that could distinguish MuSC subsets whose relative proportion is altered in the course of aging. We interrogated our previously described cell surface marker screen of single Pax7-ZsGreen muscle cells20 and found heterogeneous CD47 expression in Pax7-ZsGreen+ MuSCs (Fig. 1A). To determine whether CD47, in combination with myogenic transcription factors, could resolve distinct MuSC subsets, we performed high dimensional CyTOF analysis. We added a CD47 antibody to our previously established CyTOF panel, which contained antibodies against surface markers CD9 and CD104, to distinguish MuSCs from progenitors P1 and P2, CD44 and CD98, to identify activated MuSCs, and myogenic transcription factors20. Analysis of the high-dimensional CyTOF data, using X-shift clustering paired with single-cell force directed layout visualization, revealed three distinct clusters within the α7integrin+/CD34+ MuSC population, which were distinguished by differential expression of CD47 and the transcription factor Pax7 (Fig. S1A, Fig.1B). The most prominent cluster was Pax7hi/CD47lo (Fig. 1B, left), and the remaining two clusters were Pax7lo/CD47int-hi (Fig. 1B, right). Further analysis of these subsets by CyTOF revealed that the CD47hi subset expressed higher levels of the transcription factor Myf5, which marks activated stem cells, compared to the CD47lo subset. Moreover, the expression of the myogenic transcription factors MyoD and Myogenin, characteristic of committed and differentiated cells, was low in both MuSC subsets, as expected (Fig. S1B).
Figure 1. CD47 expression levels distinguish functionally and molecularly distinct aged muscle stem cell subsets.
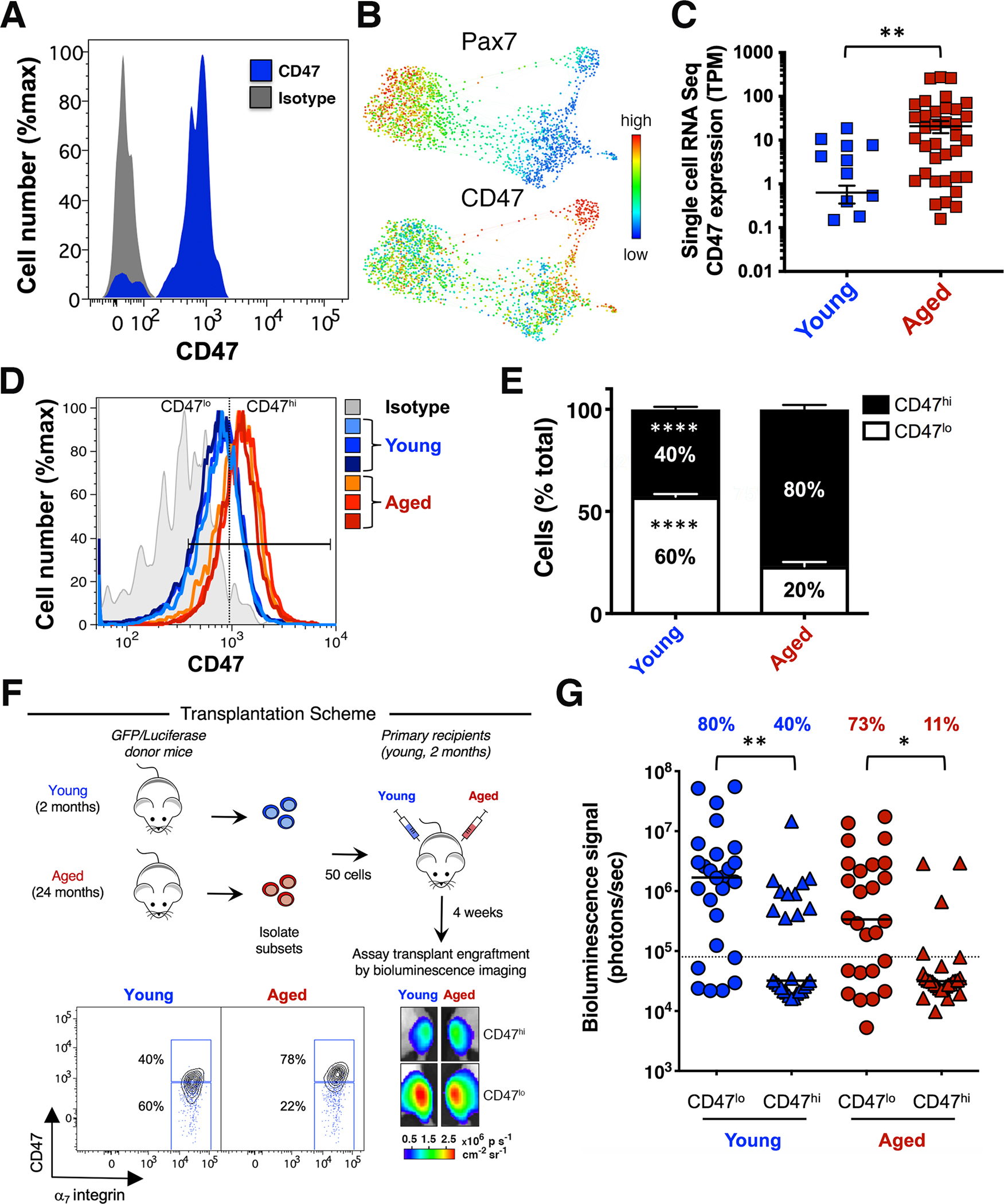
(A) Cell surface marker analysis of MuSCs from Pax7-ZsGreen reporter mice20. Histogram overlay shows CD47 expression in ZsGreen+ cells (blue) and the CD47 isotype control (gray). (B) CyTOF analysis of the MuSC population in TA and GA muscle isolated from young mice. Gated Live/Lineage−/α7integrin+/CD34+ MuSCs were analyzed with the X-shift algorithm (K=30) yielding 3 clusters that were visualized using single-cell force-directed layout20. Expression levels of Pax7 and CD47 distinguish unique MuSC subsets (representative experiment, n= 3 mice; 4 independent experiments). (C) Scatter plot shows CD47 expression in MuSCs from young and aged mice, measured by single-cell RNA-seq analysis (mean ± SEM). Two-tailed unpaired t-test analysis with Welch’s correction. (D) Histogram overlay shows CD47 expression in MuSCs from young (blue) and aged (red) mice and the FMO + CD47 isotype control (gray) (representative experiment, n= 3 young and 3 aged mice). (E) Stacked columns indicate the relative proportion of CD47lo and CD47hi MuSC subsets in young and aged mice (mean ± SEM from n=9 mice, 3 independent experiments). Two-way ANOVA analysis with Sidak correction for multiple comparisons. (F) Scheme depicting the in vivo assay of regenerative capacity. Overlaid blue dots in the biaxial plot indicate the FMO + CD47 isotype control. Representative BLI images at 4 weeks post-transplant are shown (lower right panel). (G) Scatter plot shows the percentage of transplants from each condition that engrafted above threshold (dashed line) into recipient tissue and the BLI signal intensity (y axis). Line represents the median BLI signal (n= 26 mice, 3 independent experiments). Kruskal Wallis test with significance determined by Dunn’s multiple comparisons test.
Compared to young, aged MuSCs showed a marked increase in CD47 mRNA and protein expression, measured by single-cell RNA-seq and flow cytometry, respectively (Fig. 1C, D). Both the proportion of CD47hi MuSCs and the CD47 signal intensity increased with aging (Fig. 1D, E; Fig. S1C, D). These data demonstrate the presence of two previously unrecognized subsets within the MuSC population, CD47lo and CD47hi, whose relative abundance shifts during aging, leading to accumulation of the CD47hi subset.
To assess their regenerative potential, CD47lo and CD47hi MuSC subsets were isolated from young and aged GFP/Luciferase mice, transplanted into the irradiated Tibialis Anterior (TA) muscles of immunodeficient NOD/SCID mice and assessed four weeks post-transplantation by bioluminescence imaging (BLI)25. Strikingly, the CD47lo subset isolated from young and aged donor mice exhibited the highest regenerative potential (Fig. 1F, G) and gave rise to a larger GFP+ fraction of the total MuSC population in primary recipients than did CD47hi MuSCs (S1E). These findings suggest that elevated CD47 may play a role in the reduced engraftment seen in the unfractionated aged MuSC population18.
Alternative polyadenylation regulates CD47 expression at the onset of myogenic differentiation and is altered in aged muscle stem cells.
To understand the mechanism underlying the elevated CD47 expression in aged MuSCs, we noted that in human cell lines CD47 undergoes alternative polyadenylation, leading to differential subcellular protein localization26,27. CD47 mRNA isoforms with a short 3’ UTR generate CD47 proteins that traffic to the endoplasmic reticulum, whereas CD47 mRNA isoforms with a long 3’ UTR bind a complex containing the RNA binding protein HuR to generate CD47 proteins that localize to the cell surface26. Since HuR has been reported to increase at the onset of myogenic differentiation28–30, we hypothesized that the long CD47 mRNA isoform, with consequent CD47 surface expression, was preferentially expressed during myogenic differentiation and also aberrantly expressed in aged MuSCs.
To test this hypothesis, we measured surface and intracellular CD47 protein levels by flow cytometry. We found that young MuSCs had a greater proportion of cells expressing intracellular CD47 than progenitors from either young or aged mice, where CD47 expression was predominantly on the cell surface (Fig. 2A; S2A, B). This was confirmed by confocal microscopy (Fig. S2C, D). However, aged MuSCs had a significantly greater proportion of cells expressing surface CD47 and at higher levels compared to young MuSCs (Fig. 2A–C). These results show that CD47 localization on the cell surface, which increases with myogenic progression, increases prematurely in aged MuSCs.
Figure 2. Alternative polyadenylation regulates CD47 expression and is altered in aged muscle stem cells.
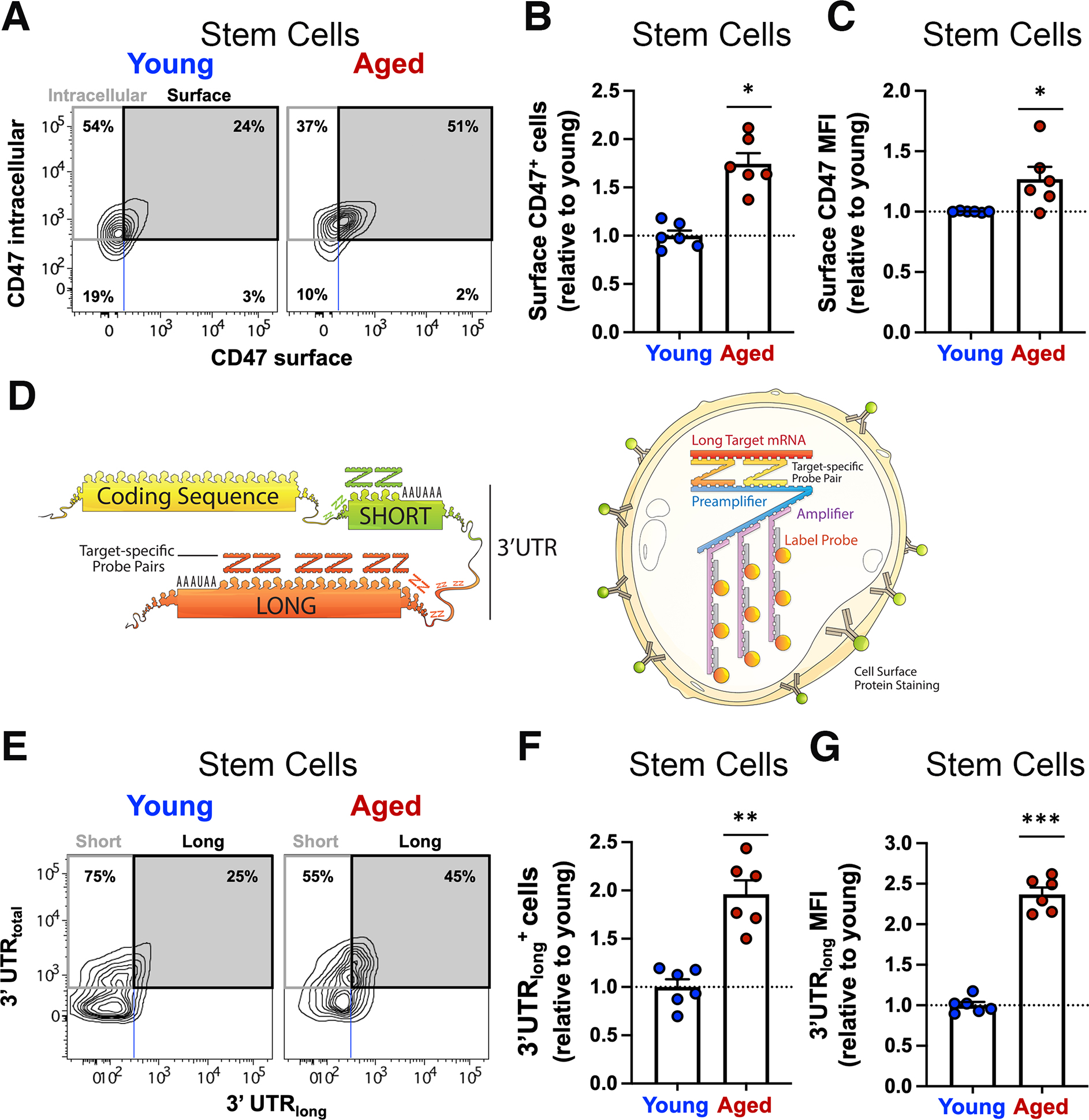
(A, B) Surface and intracellular CD47 protein expression was measured by flow cytometry in young and aged muscle cells. (A) Contour plot of intracellular CD47-PECy7 (y axis) by surface CD47-BV605 (x axis) in young and aged MuSCs (representative sample, n=3 mice). (B) Bar graph indicates the abundance of aged stem cells expressing surface CD47 relative to young (mean ± SEM, n=6 mice, 2 independent experiments). Two-tailed paired t-test analysis. (C) Bar graph shows the expression level of CD47 protein on the surface of aged MuSCs, quantified relative to young MuSCs (mean ± SEM from n=6 mice, 2 independent experiments). Two-tailed paired t-test analysis. (D) Scheme depicting the CD47 coding sequence followed by the 3’ untranslated region (UTR) (left) and the PrimeFlow RNA assay (right). (E, F) CD47 mRNA isoforms distribution was measured by PrimeFlow RNA assay in young and aged muscle. (E) Contour plot of CD47 mRNA-3’UTRtotal (y axis) by CD47 mRNA-3’UTRlong (x axis) shows the distribution of cells expressing the short (upper left quadrant) or the long CD47 mRNA isoform (upper right quadrant) as a fraction of total CD47 mRNA (upper left and right quadrants) in young (left panels) and aged (right panels) MuSCs (representative sample). (F) Bar graph indicates the abundance of aged MuSCs expressing the long CD47 mRNA isoform, quantified relative to young MuSCs (mean ± SEM, n=6 mice, 2 independent experiments). Two-tailed paired t-test analysis. (G) Bar graph shows the expression level of the long CD47 mRNA isoform in aged MuSCs, quantified relative to young MuSCs (mean ± SEM, n=6 mice, 2 independent experiments).
To map and determine the abundance of the different CD47 mRNA isoforms, we performed transcript alignment from different species, in combination with analysis of published datasets obtained by 3’ region extraction and deep sequencing of murine cell lines31, and identified three previously unrecognized murine CD47 mRNA isoforms with 3’UTRs of different lengths that showed, from shortest to longest, progressively lower usage (Fig. S2E, F). We focused our analysis on the most prevalent isoforms containing the polyadenylation sites, PAS1 or PAS2, herein referred to as short and long, respectively (Fig. S2E, F; Fig. 2D, left panel).
To measure the distribution of short and long CD47 mRNA isoforms simultaneously in stem and progenitor cells, we used branched DNA technology to label individual CD47 mRNA isoforms in single-cells with target-specific probes32,33 (Fig. 2D, right panel). We stained single-cell suspensions of muscle cells from either young or aged mice, first with antibodies against the surface markers α7 integrin and CD9, to distinguish both stem (α7 integrin+/CD9int) and progenitor (α7 integrin+/CD9hi) cells (Fig. S2G), and then intracellularly with probes specific to the different CD47 mRNA isoforms (Fig. 2D).
In aged mice, the proportion of cells and levels of expression of the long CD47 mRNA isoform were comparable in both stem and progenitor cells (Fig. 2E; Fig. S2H, I). This contrasted with young mice where more progenitor cells than MuSCs expressed the long CD47 mRNA isoform (Fig. 2E, and Fig. S2H, I). Furthermore, the relative abundance of MuSCs expressing the long CD47 mRNA isoform and the expression level of the long CD47 mRNA isoform were higher in aged compared to young MuSCs (Fig. 2F, G). We validated these results using isoform specific q-RT-PCR (Fig. S2J). These data indicate alternative polyadenylation as the mechanism underlying differential surface CD47 expression.
U1 snRNA drives alternative polyadenylation and skews the balance toward long CD47 mRNA isoforms in aged muscle stem cells.
To gain mechanistic insight into CD47 mRNA alternative polyadenylation in aged MuSCs, we aligned transcripts from different species, with a focus on the region upstream of the proximal polyadenylation site (PAS1). This analysis identified a conserved binding motif for U1 small nuclear RNA (U1 snRNA), an established component of the RNA splicing machinery34,35 (Fig. 3A). Critically, U1 snRNA also plays a role in alternative polyadenylation facilitating proximal polyadenylation site readthrough6,36,37. We hypothesized that differential expression of U1 snRNA could underlie the increased abundance of the long CD47 mRNA isoform in aged MuSCs. Consistently, U1 snRNA expression was significantly increased in aged compared to young MuSCs (Fig. 3B), whereas other known alternative polyadenylation factors were unchanged38 (Fig. S3A).
Figure 3. U1 snRNA drives alternative polyadenylation and skews the balance toward long CD47 mRNA isoforms in aged muscle stem cells.

(A) CD47 mRNA sequences upstream of polyadenylation site 1 (PAS1) from indicated species were aligned. A highly conserved U1 snRNA binding site (blue box) was identified upstream of PAS1 (red box). (B) Scatter plot shows the expression levels of U1 snRNA in sorted aged MuSCs relative to young MuSCs, measured by q-RT-PCR (mean ± SEM, n= 4 young and 4 aged mice, 2 independent experiments). Two-tailed paired t test. (C) Bar graph shows the relative abundance of CD47 mRNA with long 3’ UTR, as a fraction of the total CD47 mRNA, in young and aged MuSCs treated in vitro with AMOs to PAS1, U1 snRNA binding site on CD47 transcript (CD47 U1), and control (ctr) (mean ± SEM, 4 independent experiments). Two-way ANOVA analysis. (D, E) Surface CD47 protein expression was measured by flow cytometry in young and aged MuSCs treated in culture with AMOs to PAS1, CD47 U1 and control AMO. (D) Bar graph shows the proportion of young and aged MuSCs expressing surface CD47 upon treatment with PAS1 (red) or CD47 U1 (blue) AMO, relative to ctr AMO treated MuSCs (mean ± SEM, 3 independent experiments). Two-way ANOVA. (E) Bar graph shows the expression level of surface CD47 protein on young (left) and aged (right) MuSCs treated as described above, measured as MFI and quantified relative to control AMO treated MuSCs (mean ± SEM, 3 independent experiments). Two-way ANOVA. (F) MuSCs sorted from young and aged GFP/Luciferase mice were treated overnight with AMOs to CD47 U1 or control (ctr) AMO and transplanted (100 cells/injection) into the TA muscle of hindlimb-irradiated NOD/SCID mice. To monitor MuSC engraftment, mice were imaged weekly by BLI. Scatter plot shows the BLI signal intensity of the engrafted transplants, 3 weeks after transplantation. Line represents the median BLI signal with interquartile range (n= 14 mice, 2 independent experiments). Wilcoxon test. (G) Model for post-transcriptional regulation of CD47 in aged MuSCs. U1 snRNA, which is upregulated in aged MuSCs, binds to its site on CD47 3’UTR upstream of PAS1, blocks usage of CD47 PAS1 site, and shifts the balance toward the long isoform, leading to increased surface CD47 expression. Treatment of aged MuSCs with the CD47U1 AMO (intervention), which competes with U1 snRNA for its binding site, prevents alternative polyadenylation, leads to increased production of the short CD47 mRNA isoform and decreased surface CD47 expression, and improves MuSC engraftment to levels similar to those of young MuSCs. The speed dial shows that increasing levels of CD47U1 AMO shift the balance towards the short CD47 isoform and a more youthful molecular phenotype.
To test the role of U1 snRNA in CD47 mRNA alternative polyadenylation, we designed an AMO complementary to the U1 snRNA binding site (CD47 U1 AMO), to block the binding of U1 snRNA to the CD47 mRNA 3’ UTR. In addition, we designed an AMO complementary to a conserved sequence in the PAS1 (PAS1 AMO), to compete with the polyadenylation complex and force the selection of a more distal PAS. Treatment with the PAS1 AMO led to a significant increase in the relative abundance of the long CD47 mRNA isoform and surface CD47 protein levels in young but not aged MuSCs (Fig. 3C–E), where the relative abundance of the long CD47 mRNA isoform and surface CD47 protein levels were already elevated prior to treatment (Fig. 2A, E). By contrast, treatment with the CD47 U1 AMO led to a significant decrease in the relative abundance of the long CD47 mRNA isoform, with a consequent decrease in surface CD47 protein levels in aged MuSCs (Fig. 3C–E). These findings suggest that U1 snRNA controls CD47 alternative polyadenylation and that increased U1 snRNA expression shifts the balance toward long CD47 mRNA isoform in aged MuSCs. Consistent with alternative polyadenylation as a key regulatory mechanism for surface CD47 expression, we found that aged MuSCs exhibited increased expression of the complex that controls CD47 localization (Elav1, Set, and Rac1)26(Fig. S3B–D).
To determine the biological significance of reversing the relative abundance of the short and long CD47 mRNA isoforms in aged MuSCs, we performed transplantation assays with MuSCs treated with the CD47 U1 or control AMO. Strikingly, aged MuSCs treated with CD47 U1 AMO had significantly higher regenerative capacity compared to controls (Fig. 3F). Altogether, these data demonstrate that blocking U1 snRNA binding to CD47 mRNA in aged MuSCs rejuvenates their function in regeneration, by shifting the balance of CD47 mRNA isoforms toward the short isoform, and decreasing surface CD47 expression (Fig. 3G).
Aberrant thrombospondin-1 signaling via CD47 inhibits the proliferative capacity of aged muscle stem cells.
CD47, a transmembrane protein that belongs to the immunoglobulin superfamily, has dual functions as ligand and receptor39. Also known as the “don’t eat me signal” on neoplastic cells, CD47 is a ligand for SIRP-α, a receptor present on the surface of immune cells, through which CD47 inhibits phagocytosis22,23. In addition, CD47 is known as Integrin Associated Protein because it interacts with integrins40. Finally, CD47 also serves as a receptor for thrombospondin-1, a secreted cellular matrix protein that inhibits the proliferation of endothelial cells39,41–43. During aging, thrombospondin-1 accumulates in several tissues, including skeletal and cardiac muscle and its expression in skeletal muscle decreases in response to exercise44–47. We found that thrombospondin-1 transcripts are increased in aged compared to young MuSCs (Fig. 4A). To establish whether increased thrombospondin-1 expression could account for the reduced proliferative potential of aged MuSCs, we investigated the thrombospondin-1/CD47 signaling axis.
Figure 4. Aberrant thrombospondin-1 signaling via CD47 inhibits the proliferative capacity of aged muscle stem cells.
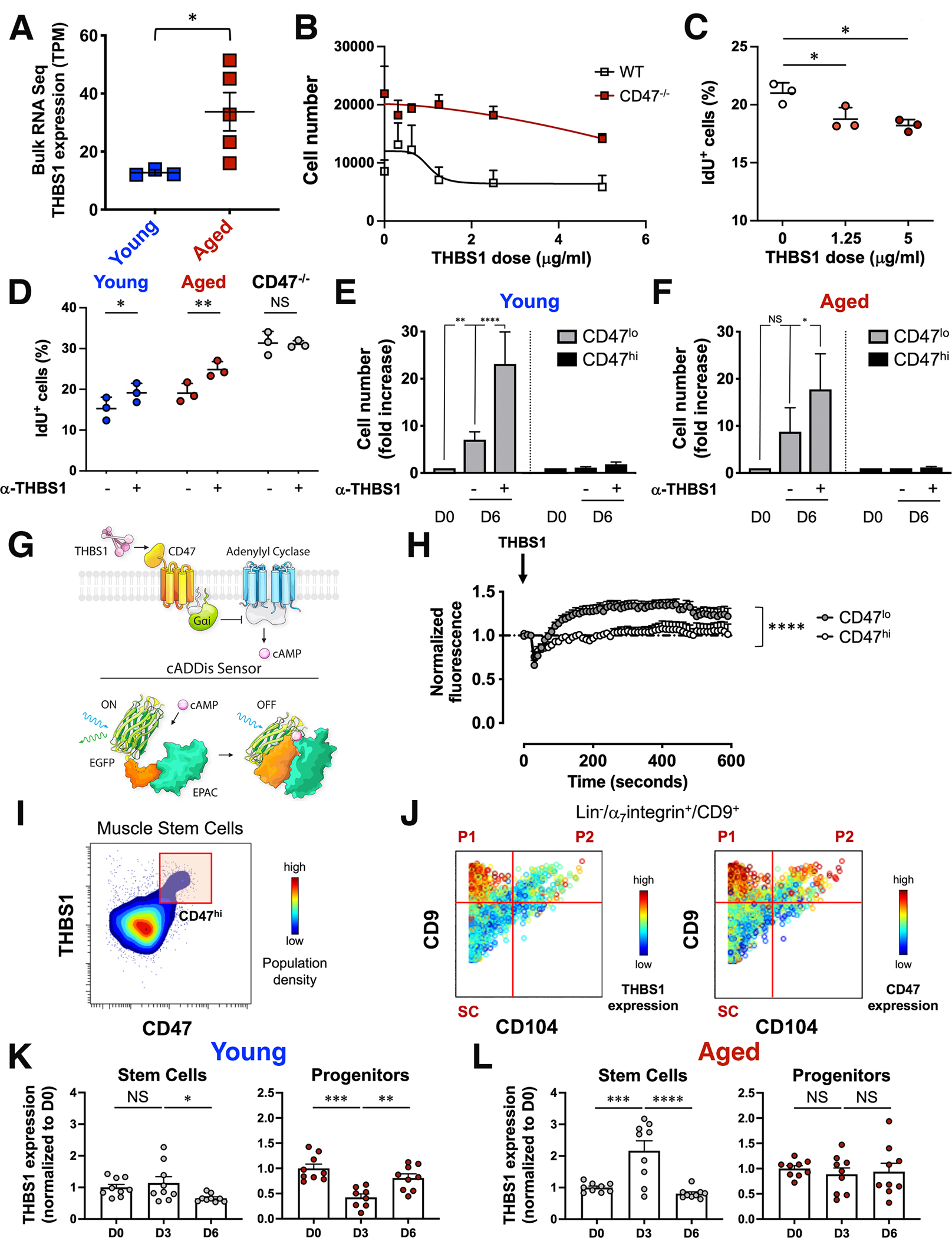
(A) Scatter plot shows thrombospondin-1 (THBS1) mRNA levels in bulk RNAseq data from young and aged MuSCs (mean ± SEM, n=3 young and 5 aged mice). Two-tailed unpaired t-test analysis with Welch’s correction. (B) Sorted MuSCs from young wild type and CD47−/− mice were treated in vitro with increasing doses of recombinant THBS1 and proliferation was measured by cell count (mean ± SEM, n=14 wild type and 4 CD47−/− replicates, 4 independent experiments). (C) Scatter plot shows the fraction of young IdU+ MuSCs in response to a 6-day in vitro treatment with increasing doses of recombinant THBS1 (mean ± SD, n= 3 young mice). One-way ANOVA analysis with Tukey’s correction for multiple comparisons. (D) Scatter plot shows the fraction of IdU+ MuSCs in response to a 6-day treatment of sorted wild type (young, aged) and CD47−/− aged MuSCs with a blocking antibody to THBS1 (α-THBS1) (mean ± SD, n= 3 mice per genotype). Two-way ANOVA with Sidak’s correction for multiple comparisons. (E, F) CD47lo and CD47hi MuSCs subsets, sorted from young and aged mice, were treated in vitro on biomimetic hydrogels in the presence (+) or absence (−) of a blocking antibody to THBS1 (α-THBS1), and cell number was quantified by cell count at day 0 (D0) or at day 6 (D6) after treatment. Bar graph shows cell number normalized to D0 for each subset (mean ± SEM from n= 7 young and 3 aged replicates). Two-way ANOVA analysis with Tukey’s correction for multiple comparisons. (G) (Top scheme) THBS1–CD47–cAMP signaling axis. THBS1–CD47 signals through Guanine nucleotide-binding protein Gi’s alpha subunit which inhibits adenylyl cyclases to reduce cAMP levels. (Bottom scheme) The cADDis downward sensor detects changes in cAMP concentration in living cells. cAMP binding to the cADDis downward sensor reduces GFP fluorescence. (H) Sorted CD47lo and CD47hi young MuSCs, transduced with a baculovirus encoding the cAMP sensor, were treated with THBS1 and individually imaged with a confocal microscope for 5 minutes at 10 sec intervals. The graph shows the average normalized GFP fluorescence of CD47lo and CD47hi MuSCs over time (mean ± SEM, 4 mice, 2 independent experiments). Two-tailed paired t-test analysis. (I, J) CyTOF analysis of young hindlimb muscle. (I) Representative contour plot of THBS1 (y axis) by CD47 (x axis) colored by population density shows a CD47hi MuSC subset that expresses high levels of THBS1. (J) Representative biaxial dot plots of CD9 (y axis) by CD104 (x axis) colored by channel show the expression of THBS1 (left) and CD47 (right) in stem (SC) and progenitor cells (P1, P2). (K, L) Intracellular THBS1 protein measurement by CyTOF during an injury time course. Bar graphs show THBS1 levels at day 3 (D3) and day 6 (D6) post injury, normalized to day 0 (D0), in SC (left) and P1 (right) population from young (K) and aged (L) mice (mean ± SEM, n=9 mice from 2 independent experiments). One-way ANOVA analysis with Tukey’s correction for multiple comparisons.
First, to determine whether exposure to thrombospondin-1 suppresses MuSC proliferation in vitro, MuSCs from young wild type and CD47−/− mice were cultured in the presence of increasing concentrations of recombinant thrombospondin-1. At increasing doses of thrombospondin-1, the number of total, Iododeoxyuridine (IdU)+, and ki67+ MuSCs significantly decreased, while the apoptosis marker cleaved PARP remained low, demonstrating that thrombospondin-1 suppresses MuSC proliferation (Fig. 4B, C; Fig. S4A, B). Consistently, transcripts for the cell cycle inhibitors p21 (CDKN1a), p27(CDKN1b) and p57 (CDKN1c), as well as p57 protein, increased significantly after thrombospondin-1 treatment (Fig. S4C, D). Notably, CD47−/− MuSCs exhibited constitutively high proliferation rates and did not respond to thrombospondin-1 treatment, confirming that thrombospondin-1 mediated suppression of proliferation depends on CD47 (Fig. 4B).
Thrombospondin-1 blockade restores the proliferative capacity of aged muscle stem cells in vitro.
To assess whether thrombospondin-1 blockade could mitigate the proliferative defect of aged MuSCs in vitro, we treated them with a thrombospondin-1 antibody that blocked its interaction with CD4748. Proliferation of aged MuSCs was restored to levels comparable to untreated young MuSCs, whereas control CD47−/− MuSCs were refractory to this treatment, underscoring the specificity of the blocking antibody (Fig. S4E, F). Finally, an in vitro IdU incorporation assay followed by CyTOF analysis confirmed that the significant increase in cell number upon thrombospondin-1 blockade was due to a significant increase in cell proliferation (Fig. 4D). To establish the cellular target of thrombospondin-1, we sorted CD47lo and CD47hi MuSC subsets from young and aged mice and treated them with the blocking antibody to thrombospondin-1 for one week. Strikingly, proliferation significantly increased in the treated CD47lo MuSC subset isolated from young and aged mice, but not in the CD47hi subset (Fig. 4E, F). These findings demonstrate that thrombospondin-1 preferentially suppresses the proliferation of CD47lo MuSCs and suggest that MuSC subsets that express different levels of CD47 exhibit different sensitivities to thrombospondin-1, with consequences for recruitment of intracellular signaling pathways, cell cycle phase and cell fate determination.
To determine whether the CD47lo and CD47hi MuSC subsets differed in their cell cycle status, we performed CyTOF analysis of MuSCs harvested during an in vivo time course of recovery from acute injury. Upon injury CD47lo/Pax7hi MuSCs incorporated high levels of IdU demonstrating increased proliferation, which was reflected in the increased relative abundance (Fig. S4G, H). By contrast, the CD47hi/Pax7lo MuSCs incorporated little IdU and their relative abundance decreased during the course of injury (Fig. S4G, H). These findings provide additional evidence that CD47lo MuSCs represent self-renewing stem cells.
To investigate differential signaling responses to thrombospondin-1 stimulation, we focused on cAMP signaling, a key regulator of cell proliferation49,50, as thrombospondin-1 decreases cAMP levels through activation of Gi proteins51,52. Basal cAMP levels were significantly higher in sorted CD47lo MuSCs compared to CD47hi MuSCs (Fig. S4I). To determine whether CD47lo and CD47hi MuSC subsets differed in their ability to modulate cAMP signaling in response to thrombospondin-1 stimulation, we employed live single-cell confocal imaging of sorted MuSC subsets expressing a fluorescent cAMP downward sensor53 (Fig. 4G). Upon thrombospondin-1 treatment, the GFP signal significantly increased in the CD47lo but not in the CD47hi MuSC subset (Fig. 4H), indicating a decrease in cAMP levels, and demonstrating that thrombospondin-1 signals through cAMP in the CD47lo but not in the CD47hi MuSC subset.
To identify the cellular source of thrombospondin-1, we simultaneously stained young and aged muscle cells with antibodies against cell surface markers that distinguish myogenic stem and progenitor cells and an antibody against thrombospondin-1, which was detected intracellularly. Data analysis showed that the CD47hi MuSC subset expressed greater thrombospondin-1 levels than the CD47lo MuSC subset (Fig. 4I).
To investigate whether thrombospondin-1 had a physiological role during regeneration, we extended our analysis of thrombospondin-1 expression to cells within the Lin−/α7integrin+/CD9+ myogenic compartment, which includes, in addition to the stem cells, progenitor populations P1 and P2, distinguished by co-expression of CD9 and CD104, as we described previously20. Strikingly, P1 progenitor cells expressed significantly greater levels of both thrombospondin-1 and CD47 compared to MuSCs (Fig. 4J).
We hypothesized that during regeneration, after MuSCs expand, their P1 progeny secrete thrombospondin-1 creating a negative feedback loop that prevents MuSC exhaustion and promotes MuSC return to quiescence. To test this, we investigated the dynamics of thrombospondin-1 expression in vivo, in the context of recovery from acute injury. We measured thrombospondin-1 expression intracellularly in muscle stem and progenitor cells from young and aged mice by CyTOF. Thrombospondin-1 expression was low in young MuSCs compared to young P1 progenitor cells and further decreased at day 6 post injury (Fig. 4K, left panel). In young P1 progenitor cells thrombospondin-1 expression changed dynamically, decreasing significantly at day 3 post injury, a time during which MuSCs expand dramatically20, and increasing significantly at day 6 post injury (Fig. 4K, right panel). By contrast, thrombospondin-1 expression in the aged myogenic compartment exhibited a marked dysregulation. In aged MuSCs, thrombospondin-1 expression levels significantly increased at day 3 post injury, and decreased by day 6 (Fig. 4L, left panel). In aged P1 progenitor cells, thrombospondin-1 expression levels were low and did not change (Fig. 4L, right panel). The dynamic change in thrombospondin-1 expression in young progenitor cells suggests a mechanism by which progenitor cell population density influences MuSC expansion. We propose that MuSCs sense progenitor cell population density through the thrombospondin-1 secreted by progenitor cells after MuSC expansion, which acts in a paracrine fashion to suppress proliferation of the neighboring MuSCs and promote their return to quiescence.
Thrombospondin-1 blockade in vivo activates aged muscle stem cells and increases strength in the absence of injury.
To test this model in vivo, we treated Pax7Cre-ERT2;Rosa26LSL-Luc mice with a thrombospondin-1 blocking antibody or IgG control regimen, and monitored endogenous MuSC numbers by BLI (Fig. 5A). Remarkably, in vivo treatment with the thrombospondin-1 blocking antibody was sufficient to significantly expand MuSCs in the absence of injury compared to IgG control (Fig. 5A), suggesting that thrombospondin-1 prevents MuSC activation during homeostasis. This led to a significant increase in Pax7+ cells, and increased myofiber size and grip strength, compared to IgG control (Fig. S5 A–F).
Figure 5. Thrombospondin-1 blockade in vivo activates aged muscle stem cells and increases muscle strength in absence of injury.

(A) Experimental scheme (upper panel). Endogenous MuSC expansion was assayed by BLI in Pax7Cre-ERT2;Rosa26LSL-Luc mice upon intramuscular injection in the TA and GA muscles (three times, at two-days interval) with a blocking antibody to THBS1 (α-THBS1) or a control IgG (contralateral leg). Graph shows BLI signal intensity (y axis) over time for each treatment group (mean ± SEM, n = 7 mice per condition, 2 independent experiments). Multiple t-test with Holm-Sidak correction for multiple comparisons. (B) Experimental scheme (upper panel). Young and aged wild type mice were treated with α-THBS1 as in (A) and muscle tissue was collected for CyTOF analysis at the end of treatment. (Left) Representative biaxial dot plots of CD98 (y axis) by CD44 (x axis) colored by channel show IdU incorporation in activated MuSCs (upper right quadrant). (Upper right) Scatter plot indicates the proportion of CD98+/CD44+ activated MuSCs (mean ± SEM, n = 6 mice, 2 independent experiments). Two-tailed paired t-test. (Lower right) Scatter plot shows the proportion of IdU+ cells in the MuSC population (mean ± SEM, n = 6 mice, 2 independent experiments). Two-tailed paired t-test. (C) Experimental scheme. Wild type young and aged mice were treated with α-THBS1 as in (A) and hindlimb muscle tissue was collected for CyTOF analysis, 6 days after the last antibody injection. (D) Gated Live/Lineage−/α7integrin+/CD9+ cells were clustered using the X-shift algorithm (K=50) yielding 61 clusters that were visualized using single-cell force-directed layout. The expression levels of the myogenic transcription factor Pax7 and the surface marker CD47 were overlaid and are shown in the panel composite (n= 3 per condition). (E) Bar graph shows the change in the proportion of each MuSC subset in aged mice treated with α-THBS1, relative to IgG control (n= 3 mice per condition). One-tailed paired t-test. (F) Bar graph shows the fraction of Pax7+ cells in the Live/Lineage−/α7integrin+/CD9+ cell population for each condition (n= 3 mice per condition). One-tailed unpaired t-test (young and aged IgG ctr); one-tailed paired t-test (aged IgG ctr and α-THBS1). (G, H) Young and aged mice were treated with α-THBS1 as in (A) and hindlimb muscle tissue was collected for histology 10 days after the end of treatment. (G) Scatter plot shows the fraction of Pax7+ cells in IgG treated and α-THBS1 treated TA muscles 10 days after the end of treatment (n=3 per condition). Paired t-test. (H) Myofiber cross sectional area (CSA) was quantified in IgG treated and α-THBS1 treated TA muscles and curve fitting was performed (n=3 per condition). Chi-square test. (I, J) Wild type young and aged mice, and young CD47−/− mice were treated with α-THBS1 as in (A) and grip strength (I) and tetanic force (J) were measured 10 days after the end of treatment. (I) Scatter plot shows grip strength measurements in α-THBS1 treated muscles, normalized to IgG treated contralateral muscles (mean ± SEM, n=9 young wild type; n=8 aged wild type and young CD47−/−, 3 independent experiments). Two-tailed paired t-test. (J) Scatter plot shows tetanic force measurements in α-THBS1 treated muscles, normalized to IgG treated contralateral muscles (mean ± SEM, n=9 young wild type; n=7 aged wild type and young CD47−/−, 3 independent experiments). One-tailed paired t-test.
To investigate the magnitude of the MuSC response to thrombospondin-1 blockade in vivo, in young and aged mice, we measured IdU incorporation in activated MuSCs20 (CD44+/CD98+, Fig. 5B). Strikingly, aged mice that received thrombospondin-1 blockade exhibited significant increases in the proportion of activated and proliferating MuSCs (Fig. 5B), suggesting that in vivo thrombospondin-1 blockade boosts the proliferative capacity of aged MuSCs in resting muscle.
To determine whether activated aged MuSCs returned to quiescence following in vivo thrombospondin-1 blockade, hindlimb muscles from aged mice treated with the anti-thrombospondin-1 regimen were collected 6 days post treatment (Fig. 5C). High-resolution CyTOF analysis of the entire myogenic compartment, using X-shift clustering paired with single-cell force directed layout visualization, revealed that thrombospondin-1 blockade in aged mice was sufficient to increase the number of Pax7hi stem cells to a level similar to that seen in IgG control treated young mice (Fig. 5D, F). Moreover, these Pax7hi stem cells expressed low levels of CD47, suggesting that thrombospondin-1 blockade facilitated the expansion of CD47lo MuSCs in aged mice (Fig. 5D, E).
Thrombospondin-1 blockade in aged mice resulted in a significant increase in Pax7+ cells and muscle cross-sectional area (Fig. 5 G, H), as well as increased grip strength and tetanic force (Fig. 5I, J), compared to IgG control. Notably, a similar treatment did not elicit these effects in CD47−/− mice, confirming the specificity of the blocking antibody (Fig. 5 G–J). These results show that thrombospondin-1 blockade leads to a remarkable MuSC activation, increased myofiber size, and increased muscle strength in aged mice in absence of injury.
Thrombospondin-1 blockade in vivo enhances the regenerative response of aged muscle leading to increased strength.
To investigate whether thrombospondin-1 blockade could enhance the regenerative response of aged MuSCs in vivo, we treated young and aged mice with the anti-thrombospondin-1 regimen described above and then acutely injured them by notexin injection. Hindlimb muscles were harvested 3- and 6-days post injury and analyzed by CyTOF (Fig. 6A). We found that at day 3 post injury, when MuSC expansion is normally at its peak1,20, in vivo thrombospondin-1 blockade led to a significant increase in the proportion of aged MuSCs (Lin−/α7integrin+/CD9int), compared to IgG control (Fig. 6B), as well as an increase in the proportion of aged activated (CD98+/CD44+) and proliferating (IdU+) MuSCs (Fig. 6C), suggesting improved regenerative capacity. Moreover, thrombospondin-1 blockade was sufficient to significantly increase the number of Pax7hi cells in the aged myogenic compartment at day 6 post injury (Fig. S6A), suggesting that the expanded aged MuSC population was able to self-renew.
Figure 6. Thrombospondin-1 blockade in vivo enhances the regenerative response of aged muscle leading to increased strength.

(A) Experimental scheme. Wild type young and aged mice were injected intramuscularly in the TA and GA muscles with a blocking antibody to THBS1 or IgG control prior to injury and muscle tissue was collected for CyTOF analysis 3- or 6-days post injury. (B) Scatter plot shows the proportion of MuSCs within the myogenic compartment at day 3 post injury for each condition (mean ± SEM, n=6 per condition, 2 independent experiments). Two-way ANOVA with Sidak’s correction for multiple comparisons. (C) (Left) Representative biaxial dot plots of CD98 by CD44 colored by channel, show IdU incorporation in activated MuSCs (upper right quadrant). (Upper right) Scatter plot shows the proportion of CD98+/CD44+ activated MuSCs (mean ± SEM, n=6 per condition, 2 independent experiments). One-tailed paired t-test. (Lower right) Scatter plot shows the proportion of IdU+ cells in the MuSC population (mean ± SEM, n=6 per condition; 2 independent experiments). One-tailed paired t-test. (D, E) Wild type young and aged mice, and young CD47−/− mice were treated with α-THBS1 as in (A) and grip strength (D) and tetanic force (E) were measured 10 days post injury. (D) Scatter plot shows grip strength measurements in α-THBS1 treated muscles, normalized to IgG treated contralateral muscles (mean ± SEM, n=8 for each group; 3 independent experiments). Two-tailed paired t-test. (E) Scatter plot shows tetanic force measurements in α-THBS1 treated muscles, normalized to IgG treated contralateral muscles (mean ± SEM, n=9 young wild type and CD47−/−, n=6 aged wild type; 3 independent experiments). One-tailed paired t-test. (F) Model. Skeletal muscle injury leads to MuSC activation. In young muscle during regeneration, progenitor cells participate in a negative feedback loop whereby they produce THBS1 to limit MuSC proliferation, and promote MuSC return to quiescence, therefore preventing MuSC exhaustion. In aged muscle accumulation of a dysfunctional CD47hi MuSC subset, which precociously secretes THBS1, creates a dysregulated microenvironment that inhibits the proliferation and function of the CD47lo MuSC subset, impairing muscle regeneration.
To determine whether thrombospondin-1 blockade in vivo could accelerate recovery from injury, we performed both histological analysis of skeletal muscle and functional assessments of strength. Consistent with the increased MuSC activation, we found increased eMHC+ fibers at day 6 post injury (Fig. S6B, E) and increased myofiber size at day 10 post injury in mice treated with the thrombospondin-1 blocking antibody compared to IgG treated controls (Fig. S6C–E). Moreover, treated mice exhibited a significant increase in grip strength and tetanic force at day 10 post injury (Fig. 6D, E). No significant increase in muscle strength was found in CD47−/− mice, confirming the specificity of the blocking antibody. Taken together our data indicate that transient modulation of thrombospondin-1 signaling in vitro and in vivo by a thrombospondin-1 blocking antibody treatment represents a promising therapy to restore the regenerative potential of aged MuSCs.
DISCUSSION
The loss of muscle mass and strength with aging is a major predictor of poor health outcome, with a cost of billions of healthcare dollars54–57. Sarcopenia, now recognized as a disease by the World Health Organization58, is due in part to the accumulation of dysfunctional stem cells that have lost the ability to proliferate and regenerate the tissue14,16–18. Previous studies from our group and others have identified cell intrinsic and extrinsic signaling pathways that are dysregulated in aged MuSCs, leading to defects in quiescence, self-renewal and proliferation9–18. However, a major barrier to elucidating the molecular mechanisms responsible for the age-associated decline in MuSC regenerative capacity has been the heterogeneity of the MuSC population, underscoring the need for single-cell studies. To resolve the molecular and functional heterogeneity of the MuSC population and distinguish dysfunctional subsets that can be purified and characterized during aging, we capitalized on mass cytometry19,21. Here we uncover a role for the surface marker CD47 and its differential expression levels in the regulation of skeletal muscle stem cell fate and function in homeostasis and aging.
While CD47 is expressed on all cells, its expression levels are transiently altered in different contexts, modulating its function22,59–62. Previous studies have shown that CD47 expression is increased on the surface of hematopoietic stem cells (HSCs) upon damage-induced mobilization and that this increase protects HSCs from being cleared by phagocytes during immune surveillance, a process that is hijacked by cancer cells22,23. Elevated expression of CD47 on CD4 T cells has been shown to define functional long-lived memory T cell precursors62. Finally, decreased CD47 levels mediate age-related clearance of red blood cells, providing evidence for changes in CD47 expression with age59. Here we show that in contrast to aged red blood cells with low CD47 levels, aged MuSCs have elevated CD47 levels, which may prevent their clearance. This dysfunctional CD47hi MuSC subset, characterized by poor regenerative capacity, accumulates and becomes predominant with age.
We sought to establish the mechanism by which the dysfunctional CD47hi MuSC subset arises with aging. We identified an increased abundance of CD47 mRNA isoforms with long 3’UTR in aged MuSCs and showed that this isoform switch results from alternative polyadenylation, which generates CD47 mRNA isoforms that dictate different protein localization and function. Importantly, we identify U1 snRNA as a key factor responsible for the premature switch to the long CD47 mRNA isoform in aged MuSCs. Further, we show that blocking U1 snRNA binding to CD47 by AMOs can restore CD47 expression and normal regenerative function to aged MuSCs by shifting the balance of CD47 mRNA isoforms toward the short isoform, as seen in young MuSCs. Previously, alternative polyadenylation was shown to play a role in modulating MuSC function in different muscles, by generating isoforms of Pax3 with different susceptibility to miRNA binding, leading to translational repression6. Our findings underscore the importance of another mechanism, protein localization, by which post-transcriptional control regulates muscle stem cell fate.
To understand the role of CD47 in the regulation of MuSC fate and function we investigated its interaction with thrombospondin-1, a negative regulator of cell proliferation, as this pathway has previously been implicated in self-renewal and reprogramming43,63. Moreover, thrombospondin-1 upregulation during the course of aging has been previously reported in several tissues, including kidney, heart and skeletal muscle44–47. We found that in aged muscle in the context of injury, CD47hi MuSCs secrete high levels of thrombospondin-1, which inhibits the expansion of the residual functional CD47lo MuSC subset, in a paracrine loop leading to global impairment of aged MuSC proliferation and regeneration (Fig. 6F). Further, we showed that thrombospondin-1-mediated suppression of cell proliferation occurs through inhibition of cAMP production. Importantly, we demonstrate that transient thrombospondin-1 blockade in two different in vivo contexts, in acute muscle injury as well as in resting muscle in the absence of injury, is sufficient to promote the activation and self-renewal of the aged CD47lo MuSC subset and markedly enhance aged muscle regenerative response, leading to a significant increase in strength. Intriguingly, in young muscles undergoing hypertrophy in response to mechanical stress, thrombospondin-1/CD47 signaling induced by an exogenous thrombospondin-1 peptide was found to promote MuSC proliferation via a mechanism that requires calcitonin receptor downregulation and is independent of cAMP24. Our findings underscore the complexity of CD47 signaling and reveal an age-dependent alteration in the role of CD47 in MuSC function.
Our work suggests that cancer immunotherapy may impact cancer cachexia. CD47 upregulation has been identified as a means by which cancer cells escape immune clearance, by engaging SIRPα on immune cells22,23. This interaction is currently being targeted systemically for cancer immunotherapy23,64. Further investigation is warranted to determine whether immunotherapies that block CD47/SIRPα signaling can interfere with thrombospondin-1/CD47 signaling in skeletal muscle. This could lead to MuSC activation and increased MuSC proliferation and counteract the muscle wasting that accompanies cancer.
In summary, here we identify a hallmark of dysfunctional aged MuSCs, elevated CD47, which enables their isolation and characterization. We describe the mechanism by which CD47 is increased on the cell surface, alternative polyadenylation. The purification of defective aged MuSCs, using differential CD47expression, enabled unprecedented mechanistic insights and allowed us to design studies to overcome aged MuSC dysfunction in vivo and ameliorate muscle regeneration in aging by two complementary approaches: (i) decrease of CD47 expression by AMO-mediated blockade of alternative polyadenylation or (ii) transient antibody blockade of thrombospondin-1/CD47 signaling. These findings have therapeutic potential and are of broad significance to the fields of aging and regenerative medicine.
Limitations of the study
This study describes the short-term effects of thrombospondin-1 blockade. Clinically, we envision thrombospondin-1 blockade as a transient intervention, for example prior to or after surgery, or to treat patients suffering from disuse-induced atrophy due to illness or recovery from hip or knee replacement. We anticipate that such a treatment would activate the functional stem cells in the muscle of aged patients and accelerate recovery. Given the role of thrombospondin-1 as a tumor suppressor, we cannot rule out the possibility that long-term treatment with the blocking antibody would lead to stem cell exhaustion.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Helen Blau (hblau@stanford.edu)
Materials availability
This study did not generate new unique reagents
Data and Code Availability
RNAseq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
CyTOF data have been deposited at Flowrepository.org and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-mouse Thrombospondin-1 (clone A6.1) | eBioscience | Cat# 14-9756-82 |
| anti-mouse α7 integrin (clone 3C12) | MBL international | Cat# K0046-3 |
| anti-mouse CD29 (clone 9EG7) | BD Biosciences | Cat# 553715 |
| anti-mouse CXCR4 (clone 2B11) | BD Biosciences | Cat# 551852 |
| anti-mouse CD31 (clone MEC 13.3) | BD Biosciences | Cat# 550274 |
| anti-mouse CD45 (clone 30-F11) | BD Biosciences | Cat# 553076 |
| anti-mouse CD11b (clone M1/70) | BD Pharmigen | Cat# 557394 |
| anti-mouse Sca-1 (clone E13-161.7) | BD Biosciences | Cat# 553333 |
| anti-mouse CD106 (clone MVCAM.A) | Biolegend | Cat# 105701 |
| anti-mouse CD9 (clone KMC8) | eBiosciences | Cat# 14-0091-82 |
| anti-mouse CD44 (clone IM-7) | BD Biosciences | Cat# 553131 |
| anti-mouse CD47 (clone MIAP301) | Biolegend | Cat# 127502 |
| Rat IgG2a, k | Biolegend | Cat# 400502 |
| anti-Rat IgG2a PerCP-eFluor 710 | eBioscience | Cat# 46-4817-82 |
| anti-mouse CD90.2 (clone 30-H12) | BD Biosciences | Cat# 553009 |
| anti-mouse CD98 (clone 4F2) | Biolegend | Cat# 128202 |
| anti-mouse CD104 (clone 346-11A) | BD Biosciences | Cat# 553745 |
| anti-mouse Pax7 (clone PAX7) | Santa Cruz Biotechnology | Cat# sc-81648 |
| anti-mouse Myf5 (clone C-20) | Santa Cruz Biotechnology | Cat# sc-302 |
| anti-mouse MyoD (clone 5.8A) | BD Pharmigen | Cat# 554130 |
| anti-mouse Myogenin (clone F5D) | BD Pharmigen | Cat# 556358 |
| anti-pRb-166Er | Fluidigm | Cat# 3166011A |
| anti-Mouse CD45 (30-F11)-89Y | Fluidigm | Cat# 3089005B |
| anti-Mouse CD11b (M1/70) -148Nd | Fluidigm | Cat# 3148003B |
| anti-human/mouse CD44-Yb171 | Fluidigm | Cat# 3171003B |
| anti-Laminin | Abcam | Cat# ab11575 |
| anti-eMHC (clone F1.562) | DSHB | N/A |
| Biotin anti-mouse CD11b (Clone M1/70) | BD Biosciences | Cat# 553309 |
| Biotin anti-mouse CD45 (Clone 30F11) | BD Biosciences | Cat# 553078 |
| Biotin anti-mouse Sca1 (Clone E13-161.7) | BD Biosciences | Cat# 553334 |
| Biotin anti-mouse CD31 (Clone 390) | eBioscience | Cat# 13-0311-82 |
| anti α7integrin-PE (clone R2F2) | AbLab | Cat# 10ST215 |
| anti-CD34-eFluor660 (clone RAM34) | eBioscience | Cat# 50-0341-82 |
| Streptavidin APC-Cy7 | BD Biosciences | Cat# 554063 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 16% Paraformaldehyde aqueous solution (PFA) | Electron Microscopy Sciences | Cat# 15711 |
| 4’,6 diamidino-2-phenylindole (DAPI) | Life Technologies | Cat# D1306 |
| D-Luciferin | Caliper Life Sciences | Cat# 122796 |
| Collagenase type 2 | Worthington | Cat# LS004176 |
| Dispase II | Thermo Fisher Scientific | Cat# 17105041 |
| Tamoxifen | VWR | Cat# AAJ63509-03 |
| Dimethyl Sulfoxide (DMSO) | Sigma | Cat# D8418 |
| Notexin | Latoxan | Cat# L8104 |
| Cisplatin | Sigma-Aldrich | Cat# P4394 |
| Cell-ID intercalator-Ir | Fluidigm | Cat# 201192B |
| 5-Iodo-2′-deoxyuridine | Sigma Aldrich | Cat# I7125 |
| Perm/Wash Buffer I | BD Biosciences | Cat# 557885 |
| Endoporter-PEG | Gene Tools | Cat# OT-EP-PEG-1 |
| DMEM, low glucose, pyruvate | Thermo Fisher Scientific | Cat# 11885-092 |
| Ham’s F-10 Nutrient Mix | Invitrogen | Cat# 11550-043 |
| Horse Serum | Fisher-Scientific | Cat# 26-050-088 |
| Penicillin/Streptomycin | Gibco | Cat# 15140122 |
| Recombinant Human FGF-basic | Peprotech | Cat# 100-18B |
| Critical Commercial Assays | ||
| PrimeFlow RNA Assay Kit | Thermo Fisher Scientific | Cat# 88-18005-204 |
| Custom Probe-CD47long 3’UTR | Thermo Fisher Scientific | Assay ID: VB6-6001206 |
| Custom Probe-CD47total | Thermo Fisher Scientific | Assay ID: VB1-3030381 |
| RNeasy Micro Kit | Qiagen | Cat# 74004 |
| High-Capacity cDNA reverse transcription kit | Applied Biosystems | Cat# 4368814 |
| TaqMan gene expression Master Mix | Applied Biosystems | Cat# 4370048 |
| SYBR Green PCR Master Mix | Life Technologies | Cat# 4309155 |
| Green Down cADDis cAMP Assay Kit | Montana Molecular | Cat# D0200G |
| Cyclic AMP XP Assay Kit | Cell Signaling Technology | Cat# 4339 |
| Ovation RNA-Seq System V2 kit | NuGen | Cat# 7102-08 |
| TruSEQ RNA Library Preparation Kit v2 | Illumina | Cat# RS-122-2101 |
| Nextera DNA Library Prep Kit | Illumina | Cat# FC-121-1030 |
| Deposited Data | ||
| RNAseq data | This study | GSE198249 |
| CyTOF Data | This study | FR-FCM-Z5RA, FR-FCM-Z5RB, FR-FCM-Z5RC, FR-FCM-Z5RD, FR-FCM-Z5RE, FR-FCM-Z5RG, FR-FCM-Z5RF |
| Experimental Models: Cell Lines | ||
| Primary Myoblasts | In house | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | Jackson Laboratories | Strain# 000664 |
| Mouse: Pax7tm1(cre/ERT2)Gaka (Pax7Cre-ERT2) | Jackson Laboratories | Strain# 017763 |
| Mouse: Gt(ROSA)26Sortm1(Luc)Kael (Rosa26LSL-Luc) | Jackson Laboratories | Strain# 005125 |
| Mouse: B6.129S7-Cd47tm1Fpl/J | Jackson Laboratories | Strain# 003173 |
| Mouse: NOD.Cg-Prkdcscid/J | Jackson Laboratories | Strain# 001303 |
| Oligonucleotides | ||
| q-RT-PCR Primer Sequences | Integrated DNA Technologies | Table S1 |
| Antisense Morpholino Oligonucleotides Sequences | Gene Tools | Table S2 |
| TaqMan gene expression assay (cdkn1a-FAM) | Applied Biosystems | Mm00432448_m1 |
| TaqMan gene expression assay (cdkn1b-FAM) | Applied Biosystems | Mm00438168_m1 |
| TaqMan gene expression assay (cdkn1c-FAM) | Applied Biosystems | Mm00438170_m1 |
| TaqMan gene expression assay (GADPH-VIC) | Applied Biosystems | 4352339E |
| Software and Algorithms | ||
| Flowjo | FlowJo, LLC | www.flowjo.com |
| Cytobank | 65 | www.cytobank.org |
| Matlab or R – Normalizer | 70 |
https://github.com/nolanlab/bead-normalization/wiki/Normalizing-FCS-Files
https://github-com.laneproxy.stanford.edu/ParkerICI/premessa—R |
| Matlab or R – Single Cell Debarcoder | 66 |
https://github.com/nolanlab/single-cell-debarcoder
https://github-com.laneproxy.stanford.edu/ParkerICI/premessa—R |
| Vortex | 67 | https://github.com/nolanlab/vortex/ |
| STAR | 68 | https://github.com/alexdobin/STAR/releases |
| RSEM | 69 | https://github.com/bli25ucb/RSEM_tutorial |
| DESeq2 | 70 | http://www.bioconductor.org/packages/DESeq2/ |
| Living Image v.4.5 | Perkin Elmer | Perkinelmer.com |
| Aurora Scientific Dynamic Muscle Data Acquisition and Analysis Software | Aurora Scientific | https://aurorascientific.com/products/muscle-physiology/muscle-daq-software/615a-dynamic-muscle-control/ |
| Keyence Analysis Software (BZ-X700 Microscope) | Keyence | www.keyence.com |
| CRISP | 71 | github.com/will-yx/ |
| FiberNet | 71 | github.com/will-yx/ |
| Graphpad Prism 9 | GraphPad Software, Inc | www.graphpad.com |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All animal protocols were approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC) (protocol #10509 and #29398) and experiments were performed in compliance with the institutional guidelines of Stanford University. C57BL/6 young adult mice (2–4 months, Strain# 000664) were purchased from Jackson Laboratories (Bar Harbor, ME). C57BL/6 aged mice (22–24 months) were purchased from the US National Institute on Aging (NIA). Mice ubiquitously expressing a green fluorescent protein (GFP) transgene and mice ubiquitously expressing a firefly luciferase (Fluc) transgene driven by the ACTB promoter (L2G85 strain) were obtained and genotyped as described previously25,72. Double transgenic GFP/Luciferase mice were generated by breeding the above strains and were confirmed by appropriate PCR-based strategies to validate the genotype. Immunodeficient NOD/SCID (NOD.Cg-Prkdcscid/J, Strain# 001303) young adult mice (2 months) were purchased from Jackson Laboratories (Bar Harbor, ME). CD47−/− mice72 (B6.129S7-Cd47tm1Fpl/J, Strain# 003173) were obtained from Jackson Laboratory (Bar Harbor, ME)., bred at Stanford University and genotyped according to the recommended PCR-based protocol. Double-transgenic Pax7Cre-ERT2;Rosa26LSL-Luc were generated by crossing Pax7Cre-ERT2 mice obtained from Jackson Laboratory (Pax7tm1(cre/ERT2)Gaka, Strain# 017763) and Rosa26LSL-Luc obtained from Jackson Laboratory (Gt(ROSA)26Sortm1(Luc)Kael, Strain# 005125). We validated these genotypes by appropriate PCR-based strategies. All mice used in these studies were females except for experiments in Fig. S5 were both males and females were analyzed and no significant difference between males and females was reported in the experimental outcome. The number of animals for each data set and all relevant details regarding the sample size are reported in the figure legend.
Primary cell cultures
MuSCs isolated from young and aged C57BL/6 female mice by Fluorescence Activated Cell Sorting (FACS), were used for AMO treatment, followed by RNA and protein expression measurements. CD47lo and CD47hi MuSCs, sorted from young female mice, were used for live cAMP assay. MuSCs sorted from young and aged C57BL/6 and CD47−/− female mice were used for THBS1 treatment and blockade, followed by cell proliferation, RNA and protein expression measurements. MuSCs sorted from young and aged GFP/Luciferase female mice were used for AMO treatment, followed by transplantation experiments. Primary myoblasts prepared from young C57BL/6 female mice were used for measurements of protein subcellular localization. Myogenic medium containing DMEM/F10 (50:50), 20% FBS, 2.5 ng·ml−1 fibroblast growth factor-2 (FGF-2, also known as βFGF) and 1% penicillin–streptomycin was used to culture MuSCs and primary myoblasts at 37°C in the tissue culture incubator, unless otherwise indicated below.
METHOD DETAILS
Muscle tissue dissociation
Tibialis Anterior (TA) and Gastrocnemius (GA) muscles were dissected and subjected to mechanical dissociation using the gentleMACS™ dissociator (Miltenyi Biotech), followed by collagenase (0.25%; Worthington) and dispase II (0.04 U/ml; Thermofisher Scientific) enzymatic digestion at 37°C for 90 minutes. The resulting cell suspension was passed through a standard syringe needle and subsequently through a 70-μm nylon filter (BD Biosciences, San Jose, CA).
Isolation of muscle stem cells and their subsets
Muscle tissue was isolated and digested to a single-cell suspension as described above. Cells were first incubated with a purified antibody reactive to murine CD47 (Biolegend, San Diego, CA) for 30 minutes at 4°C, then washed and incubated with an anti-Rat IgG2a secondary antibody conjugated to Percp-eFluor710 (eBioscience, San Diego, CA). Cells were then incubated with biotinylated antibodies reactive to murine CD45, CD11b, CD31, and Sca1 (BD Biosciences, San Jose, CA) for 30 minutes at 4°C and washed. Cells were then incubated with streptavidin magnetic beads (Miltenyi Biotech, Auburn, CA), streptavidin-APC-Cy7 (Invitrogen, Carlsbad, CA), α7 integrin-PE antibody (AbLab, Vancouver, Canada), CD34-eFluor660 antibody (eBioscience, San Diego, CA). After magnetic depletion of the biotin-positive cells, the lineage-negative cells were stained with DAPI and sorted on a FACSAria cell sorter or a Sony SH800 cell sorter in purity mode, using FACS Diva software (BD Biosciences, San Jose, CA) or SH800 software (Sony). To isolate MuSCs, we first gated on viable cells (DAPI negative), then on cells negative for the lineage markers (CD45, CD31, CD11b, and Sca1), finally on cells positive for both CD34 and α7 integrin, which represent the muscle stem cell fraction. To simultaneously isolate CD47lo and CD47hi subsets we first gated on viable cells (DAPI negative), then on cells negative for the lineage markers (CD45, CD31, CD11b, and Sca1) and positive for α7 integrin and CD34. Finally, we sorted CD47lo and CD47hi subsets based on their differential CD47 expression. Flow cytometry scatter plots were generated using FlowJo (Treestar, Ashland, OR).
Transplantation of muscle stem cells and their subsets
Sorted cell populations were transplanted immediately following FACS isolation into the TA muscle of recipient mice as previously described25, or 16 hours post treatment with antisense morpholino oligonucleotides, as described below. Cells from GFP/Luciferase mice were transplanted into the TA muscle of gender-matched, hindlimb-irradiated NOD/SCID mice (Jackson Laboratories, Bar Harbor, ME). NOD/SCID mice (2–4 months of age, median 2 months) were anesthetized with ketamine (2.4 mg per mouse) and xylazine (240 μg per mouse) by intraperitoneal injection and irradiated by a single dose of 18 Gy administered to the hindlimbs, with the rest of the body shielded in a lead jig. Transplantation was performed within three days post-irradiation. Freshly isolated cells were counted by hemocytometer and resuspended at desired cell concentrations in PBS with 2.5% goat serum and 1 mM EDTA. Cells were transplanted by intramuscular injection into the TA muscle in a 10 μl volume. To evaluate stem cell repopulation, we collected and digested primary recipient muscles four weeks after transplantation and stained them with antibodies to lineage markers (CD45, CD11b, CD31 and Sca1), surface markers α7 integrin and CD34, as well as with DAPI, to determine viability. To identify donor cells, we gated for cells negative for DAPI and the lineage markers and positive for α7 integrin and CD34 and the donor marker GFP.
Biomimetic Hydrogel Fabrication
We fabricated polyethylene glycol (PEG) hydrogels from PEG precursors, synthesized as described previously73. We produced hydrogels by using the published formulation to achieve 12-kPa (Young’s modulus) stiffness hydrogels in 1-mm thickness, which is the optimal condition for culturing MuSCs and maintaining stem-cell fate in culture73.
Bulk RNA-Seq
α7-integrin+/CD34+ MuSCs were isolated as described above. RNA was isolated using Qiagen RNeasy Micro kit from 5,000 –10,000 cells and cDNA generated and amplified using NuGEN Ovation RNA-Seq System v2 kit. Libraries were constructed from cDNA with the TruSEQ RNA Library Preparation Kit v2 (Illumina) and sequenced to 30–40 × 106 1 × 75-bp reads per sample on a HiSEQ 2500 from the Stanford Functional Genomics Facility, purchased using NIH S10 Shared Instrument Grant S10OD018220. For analysis, RNA sequences were aligned against the Mus musculus mm10 reference genome using the Spliced Transcripts Alignment to a Reference (STAR) software. RNA-Seq by Expectation-Maximization (RSEM) software package was used for annotating reads to transcripts. RNA abundance was quantified into transcripts per million (TPM) values as well as total read counts. A counts matrix containing the number of read counts for each gene and each sample was obtained. This matrix was analyzed by DESeq to calculate statistical analysis of significance of genes between samples.
Single-cell RNA-seq
MuSCs sorted from young and aged mice were counted and diluted to 500 cells per microliter and subsequently loaded, captured and stained with viability dyes (LIVE/DEAD cell viability assay; Molecular Probes, Life Technologies) on a small-sized microfluidc RNA-seq chip (Fluidigm) using the C1 system. Captured cells were imaged using phase-contrast and fluorescence microscopy to determine live/dead status. Lysis, reverse transcription and cDNA preamplification was performed using the SMARTer Ultra Low RNA Kit for the Fluidigm C1 System (Clontech) according to Fluidigm’s manual for mRNA sequencing on the C1 system. Resulting cDNA was harvested and analyzed on the Fragment analyzer™ by Advanced Analytical. Cells with a concentration higher than 0.05 ng/ul were selected for library preparation. Library preparation was performed using Nextera XT DNA Sample Preparation Kit (Illumina), as described in the Fluidigm manual. Following library preparation, cells were pooled and sequenced on an Illumina NextSeq instrument using 2×75 paired end reads on a NextSeq high output kit (Illumina). Reads were mapped to the Mus musculus mm10 reference genome using STAR. RSEM was used for determining transcript abundance (TPM). A counts matrix containing the number of counts for each gene and each sample was obtained. This matrix was analyzed by DESeq to calculate statistical analysis of significance of genes between samples.
Quantitative RT-PCR
We isolated RNA from muscle stem or progenitor cells using the RNeasy Micro Kit (Qiagen). We reverse-transcribed cDNA from total mRNA from each sample using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). We subjected cDNA to RT-PCR using TaqMan Assays (Applied Biosystems) or SYBR Green PCR master mix (Applied Biosystems) in an ABI 7900HT Real-Time PCR System (Applied Biosystems). We cycled samples at 95 °C for 10 min and then 40 cycles at 95 °C for 15 s and 60 °C for 1 min. To quantify relative transcript levels, we used 2−ΔΔCt to compare treated and untreated samples and expressed the results relative to Rnu2 or Gapdh (Fig. 3B, Fig. S4D). Transcript levels for the long isoform of CD47 were expressed relative to Cd47 total levels (Fig. S2H, Fig. 3C). For SYBR Green qRT-PCR, we used the following primer sequences:
Gapdh_Fw: aggtcggtgtgaacggatttg; Gapdh_Rev: tgtagaccatgtagttgaggtca
CD47total_Fw: cctgttctggggaaagtttgg; CD47total_Rev: aggatggctccaacaaccac
CD47long_Fw: gatcggaggcatgacaaagc; CD47long_Rev: gcaacaaagagacaagaggcg
Rnu1_Fw: cttacctggcaggggagata; Rnu1_Rev: atccggagtgcaatggataa
Rnu2_Fw ctcggccttttggctaagat; Rnu2_Rev tgtcctcggatagaggacgta
TaqMan Assays (Applied Biosystems) were used to quantify cdkn1a, cdkn1b, cdkn1c in samples according to the manufacturer instructions with the TaqMan Universal PCR Master Mix reagent kit (Applied Biosystems). Transcript levels were expressed relative to Gapdh levels. Multiplex qPCR enabled target signals (FAM) to be normalized individually by their internal Gapdh signals (VIC).
Flow cytometry staining for surface and intracellular CD47
Muscle tissue was isolated and digested to a single-cell suspension as described above. Cells were incubated with biotinylated antibodies reactive to CD45, CD11b, CD31, and Sca1 (BD Biosciences, San Jose, CA) for 30 minutes at 4°C and washed. Cells were then incubated with streptavidin-APC-Cy7 (Invitrogen, Carlsbad, CA), α7 integrin-PE antibody (AbLab, Vancouver, Canada), CD9-APC antibody (eBioscience, San Diego, CA) and CD47-BV605 antibody (BD Biosciences, San Jose, CA) for 30 minutes on ice and washed. Cells were fixed in 1.6% paraformaldehyde, permeabilized with BD Perm/Wash buffer I (cat# 557885, BD Biosciences, San Jose, CA), washed and stained intracellularly with the CD47-PECy7 antibody (Biolegend, San Diego, CA) in BD Perm/Wash buffer I. Finally, cells were washed twice with staining buffer and analyzed on a BD-LSRII flow cytometer using FACS Diva Software (BD Biosciences, San Jose, CA).
Immunofluorescence (IF) staining for surface and intracellular CD47 protein
Sorted MuSCs (Lin−/α7integrin+/CD34+) were seeded on laminin coated chamber slides and stained either prior to or after permeabilization, as detailed below to detect surface and intracellular CD47 expression, respectively. Chamber slides were imaged by confocal microscopy. For surface staining of CD47, cells were fixed for 15 min at room temperature in 4% PFA, washed with PBS, blocked for 15 min at 4°C in 5% Normal Goat Serum in PBS and then incubated with rat anti-mouse CD47 (BD Biosciences, Cat# 555297) primary antibody for 1h at 4°C in PBS. After washing in PBS, cells were incubated with anti-rat IgG2a, k conjugated to Alexa Fluor 647 secondary antibody for 1 h at 4°C in PBS. Cells were washed and stained with DAPI. Images were acquired on a confocal microscope.
For intracellular staining of CD47, cells were fixed for 15 min at room temperature in 4% PFA, washed with PBS, blocked for 45 min at 4°C in PBS containing 5% Normal Goat Serum and 0.2% saponin. Cells were then incubated with rat anti-mouse CD47 (BD Biosciences, Cat# 555297) primary antibody overnight at 4°C in PBS containing 1% BSA and 0.2% saponin. After washing in PBS, cells were incubated with anti-rat IgG2a,k conjugated to Alexa Fluor 647 secondary antibody for 1 h at 4C in PBS containing 1%BSA and 0.2% saponin. Cells were washed and stained with DAPI. Images were acquired on a Marianas spinning disk confocal (SDC) microscope (Intelligent Imaging Innovations).
PrimeFlow RNA assay
Target-specific RNA in situ hybridization probes, spanning 600–800 bases upstream of CD47 PAS1 and PAS2, were custom-designed by Thermo Fisher Scientific to differentiate between the short and long 3’UTR isoforms of CD47 mRNA. TA and GA muscles were isolated from young (2–4 months) and aged (22–24 months) mice and digested to single cell suspensions that were stained using antibodies against lineage markers (CD45, CD11b, CD31, Sca1)-APC-Cy7, α7 integrin-PE, CD9-APC and CD47-BV605. Samples were then processed for the PrimeFlow RNA Assay (cat # 88-18005-204, Thermo Fisher Scientific), according to manufacturer’s instructions. Cells were fixed, permeabilized, and stained for intracellular CD47 using CD47-PE-Cy7 antibody. Finally, RNA in situ hybridization was performed using custom-designed probes targeting the total (Assay ID: VB1-3030381) or long (Assay ID: VB6-6001206) 3’UTR isoform of CD47 mRNA. The total and long 3’UTR isoforms of CD47 mRNA were labeled with AF647 and AF750, respectively.
Antisense Morpholino Oligonucleotides treatment
MuSCs sorted from young and aged mice were plated in Ham’s F10, 10% Horse Serum and 1% Penicillin/Streptomycin at 37°C in 24 well plates and transfected with antisense morpholino oligonucleotides (AMO) at a concentration of 15μM with Endoporter (Gene Tools, LLC), according to the manufacturer’s instructions. For RNA preparation, media was changed 16h-post transfection and samples were collected at 24h-post transfection. For flow cytometric analysis of CD47 expression, media was changed 16h-post transfection and samples were collected at 32h-post transfection. For transplantation assays cells were collected 16h-post transfection and transplanted as described above. We used the following AMO sequences:
Control AMO: ggttacaatctaagatcaaacgacg; CD47_PAS1 AMO: cacagcacatcatattttttttatt; CD47_U1 AMO: agtacagctgctttgaatataaact
MuSC treatment with THBS1 or THBS1-blocking antibody in vitro
Following isolation, we resuspended MuSCs in myogenic cell culture medium containing DMEM/F10 (50:50), 20% FBS, 2.5 ng·ml−1 fibroblast growth factor-2 (FGF-2, also known as βFGF) and 1% penicillin–streptomycin. We seeded MuSC suspensions at a density of 500 cells per cm2 surface area. We maintained cell cultures at 37°C in 5% CO2 and changed medium every other day. For thrombospondin-1 treatment studies (dose response curve), we added recombinant thrombospondin-1 (R&D Systems) at the indicated concentrations 24h-post isolation and cultured the cells for a total of 6 days, changing the media every two days. Cell number was determined by manual cell counting. For thrombospondin-1 blockade studies, we added the thrombospondin-1 blocking antibody (10μg/ml) (A6.1, Thermo Fisher Scientific) to MuSCs cultured on biomimetic hydrogels 24h-post isolation and cultured the cells for a total of 6 days, changing the media every two days. Cell number was determined by manual cell counting.
In vitro IdU incorporation
MuSCs cultured on biomimetic hydrogel and treated with thrombospondin-1 or with the thrombospondin-1 blocking antibody (A6.1, Thermo Fisher Scientific) for 6 days were pulsed with IdU at a concentration of 10μM for 1 hour and then harvested and processed for CyTOF staining as described above.
Live Cell cAMP assay
CD47lo and CD47hi MuSC subsets were sorted from young mice, seeded at 3000 cells/well in a 96-well cell imaging plate (Eppendorf, 0030741013) and transduced the following day with the cAMP Difference Detector in situ (cADDis) BacMam sensor (Montana Molecular, D0200G) according to manufacturer’s instructions53. Briefly, cells were infected with 10μl of BacMam sensor stock in a total of 150μl of media containing 2mM Sodium Butyrate (Montana Molecular) for 30 min at room temperature, followed by 6h in the 37°C tissue culture incubator. BacMam was removed and replaced with media containing 1mM Sodium Butyrate for 16–24h. Prior to imaging, cells were incubated in PBS for 20 min at room temperature. Images were acquired on a Marianas spinning disk confocal (SDC) microscope (Intelligent Imaging Innovations) (40x, epi-fluorescence) every 10 seconds for 5 min, with thrombospondin-1 (5μg/ml) added after 30 seconds. Red fluorescence was used to determine a mask and background subtracted green and red fluorescent intensity over time was determined using Slidebook (Intelligent Imaging Innovations).
cAMP competitive ELISA
Sorted MuSCs were cultured overnight in 96 well plates and cAMP measurements were performed on cell lysates using the Cyclic AMP XP® Assay Kit (cat # 4339, Cell Signaling Technology) according to the manufacturer’s instructions.
Bioluminescence imaging
Bioluminescence imaging (BLI) was performed using a Xenogen-100 system, as previously described25, at 3–4 weeks post-transplant, or before and after in vivo anti-thrombospondin-1 treatment for Pax7Cre-ERT2;Rosa26LSL-Luc. The system is comprised of a light-tight imaging chamber, a charge-coupled device (CCD) camera with a cryogenic refrigeration unit and the appropriate computer system (Living-Image Software, Caliper LifeSciences). Briefly, the animals were anesthetized under isofluorane and injected intraperitoneally with a 100μl volume of luciferin diluted in PBS (0.1mmol/Kg body weight, Caliper LifeSciences). For transplantation, immediately after injection, images were acquired each minute for a total of 15 min and data were stored for subsequent analysis, using Living Image software (Caliper Life Sciences). Bioluminescence images acquired at 12 min post-luciferin injection were used for analysis. Bioluminescence signal was calculated by drawing a consistent region-of-interest (ROI) over each hindlimb and quantifying the resulting signal. A bioluminescence signal value of 80,000 photons/s represented our detection threshold, as this level was previously determined to represent the presence of one or more GFP-positive muscle fiber in transplanted tissue. For measuring endogenous muscle stem cell expansion, immediately after injection, images were acquired at 1, 5, 30, 60, 120 seconds and data were stored for subsequent analysis, using Living Image software (Caliper Life Sciences). Bioluminescence images acquired at 30 seconds post-luciferin injection were used for analysis as described above.
In vivo thrombospondin-1 blockade
A blocking antibody to thrombospondin-1 was administered by intramuscular injection into the Tibialis Anterior (TA) (15μl of a 250μg/ml solution diluted in sterile PBS) and Gastrocnemius (GA) (30μl of a 250μg/ml solution diluted in sterile PBS) muscles (experimental leg) of young and aged C57BL6 mice, as well as Pax7Cre-ERT2; Rosa26LSL-Luc mice. IgG control at the same concentration was injected in the contralateral leg, to control for damage induced by needle injury. The antibody regimen included three injections, at two-day intervals.
In vivo IdU labeling
Mice were weighed, anesthetized with isofluorane and injected intraperitoneally with IdU eight hours prior to sacrificing the animal (150 mg/kg body weight per injection).
Skeletal muscle injury
Mice (8–10 weeks) were acutely injured by a single 10 μl intramuscular injection of notexin (10 μg/ml; Latoxan, France) into the TA muscle and two injections in the GA muscle at the indicated time points.
Endogenous muscle stem cell lineage tracing
Pax7CreERT2; Rosa26LSL-Luc mice were treated with five consecutive daily intraperitoneal injections at a tamoxifen dose of 75mg/kg body weight to activate luciferase expression following Pax7-dependent Cre-mediated recombination. Ten days after the last tamoxifen injection, mice were subjected to repeated anti-thrombospondin-1 injections (3 injections, at two days intervals) and imaged by BLI.
Grip strength measurement
Mice were put on a BioSeb grip tester and their grip strength was measured for one hindlimb at the time74. Mice were measured three-six times and the average was used to calculate grip strength. Values were normalized to the IgG control leg.
In vivo muscle force measurement
The peak isometric tetanic force of the ankle plantarflexors was assessed as previously described75,76. Briefly, the foot of anesthetized mice was placed on a footplate attached to a servomotor (model 300C-LR; Aurora Scientific). Two Pt-Ir electrode needles (Aurora Scientific) were inserted percutaneously over the tibial nerve, just posterior/posterior-medial to the knee. The ankle joint was secured at a 90° angle. The peak isometric tetanic force was achieved by varying the current delivered to the tibial nerve at a frequency of 150 Hz and a 0.1-ms square wave pulse. We performed three tetanic measurements on each muscle, with 1 min recovery between each measurement. Force measurement acquisition was blinded. The researchers performing the force measurements were unaware of treatment conditions. Data were collected with the Aurora Scientific Dynamic Muscle Data Acquisition and Analysis Software.
Histology and Immunohistochemistry (IHC) in muscle cryosections
TA muscle were cryopreserved and frozen in an isopentane/liquid nitrogen double bath and stored at −80C until analysis. Frozen TA muscle tissue was prepared for histology as previously described25. For Laminin and PAX7 staining, we fixed transverse sections from muscles using 4% PFA, blocked and permeabilized them using PBS/1% BSA/0.3% Triton X-100, and incubated them overnight with primary anti-Laminin (Abcam, cat # ab11575, 1:500) and anti-PAX7 (Santa Cruz Biotechnology, cat# sc-81648, 1:50) antibodies. We washed the slides and then incubated them with secondary antibodies (goat-anti mouse-IgGAF555 and goat-anti Rabbit-IgGAF647, Jackson ImmunoResearch Laboratories, 1:200). We counterstained nuclei with DAPI (Invitrogen). For Laminin and embryonic Myosin Heavy Chain (eMHC) staining, we permeabilized muscle sections using PBS/1% BSA/0.3% Triton X-100 and incubated them overnight with primary anti-Laminin (Abcam, cat # ab11575, 1:500), anti-Laminin (Abcam, cat # ab11575, 1:500) and anti-PAX7 (Santa Cruz Biotechnology, cat# sc-81648, 1:50) antibodies. We washed the slides and then incubated them with secondary antibodies (goat-anti mouse-IgGAF555 and goat-anti Rabbit-IgGAF647, Jackson ImmunoResearch Laboratories, 1:200). We counterstained nuclei with DAPI (Invitrogen). For Laminin and embryonic Myosin Heavy Chain (eMHC) staining, we permeabilized muscle sections using PBS/1% BSA/0.3% Triton X-100 and incubated them overnight with primary anti-Laminin (Abcam, cat # ab11575, 1:500), anti-eMHC (DSHB, clone# F1.652, 1:10) antibodies. We washed the samples and then incubated them with secondary antibodies (goat-anti mouse-IgGAF555 and goat-anti Rabbit-IgGAF647, Jackson ImmunoResearch Laboratories, 1:200). We counterstained nuclei with DAPI (Invitrogen). Images were acquired using KEYENCE BZ-X700 all in one fluorescence microscope with 20x/0.75 N.A. objective for eMHC and Laminin staining or 40X objective for PAX7 and Laminin staining. Individual fields were stitched and analyzed using the CRISP image processing algorithm77. Image processing of the skeletal muscle immunofluorescent histological sections, for semi-automated fiber detection and analysis of eMHC+ fibers, fiber cross-sectional area and minimum feret’s diameter, was performed using two independent methods, the SMASH plugin for MATLAB78 and the Fibernet algorithm developed in our laboratory71, yielding comparable results. Pax7+ cells were quantified manually using ImageJ by 3 independent researchers yielding comparable results. Data analyses were blinded.
Antibody conjugation with metal isotopes
Purified antibodies were conjugated to the indicated metals for mass cytometry analysis using the MaxPAR antibody conjugation kit (Fluidigm, South San Francisco, CA) according to the manufacturer’s instruction. Following labeling, antibodies were diluted in Candor PBS Antibody Stabilization solution (Candor Bioscience GmbH, Wangen, Germany) to 0.2 mg/ml and stored long-term at 4°C. Each antibody clone and lot was titrated to optimal staining concentrations using murine myoblasts, as well as murine muscle and spleen cell suspensions.
Mass cytometry staining
Dead cells were stained using cisplatin (Sigma Aldrich, Cat# P4394) as previously described79. In particular, cells were resuspended in 1ml serum-free DMEM at 2×106 cells/ml and cisplatin was added at a final concentration of 25 μM for 1 minute at room temperature. The reaction was quenched with 3ml of DMEM/10% FBS. Samples were centrifuged at 500g for 10 minutes and cell pellets were resuspended in cell staining media (CSM: PBS with 0.5% bovine serum albumin (BSA) and 0.02% sodium azide) and fixed with 1.6% paraformaldehyde (PFA) for 10 minutes on ice. Cells were washed with CSM and stained with antibodies against surface markers included in the mass cytometry panel for 1 hour at room temperature. Cells were washed twice with CSM and permeabilized with methanol for 10 minutes on ice. Cells were washed twice with CSM and stained with antibodies against intracellular markers included in the mass cytometry panel for 1 hour at room temperature. Cells were washed twice with CSM and stained with 1 ml of 191/193Ir DNA intercalator (Fluidigm, South San Francisco, CA) diluted (1:5000) in PBS with 1.6% PFA for 20 minutes at room temperature.
Mass cytometry measurement
Cells were acquired on the CyTOF 2 mass cytometer (Fluidigm, South San Francisco, CA) at an event rate of approximately 500 cells per second as previously described. The instrument was run in high-resolution mode (Mass resolution ~700) with internally calibrated dual-count detection. Samples were normalized using beta beads.
X-Shift analysis and graphic display of single-cell mass cytometry data
Events were clustered based on a combination of surface markers and myogenic transcription factors using the X-Shift algorithm and clusters were validated. In order to visualize the spatial relationships between the cell types within these X-shift clusters, ≤2,000 (Fig. 1B) or ≤10,000 (Fig. 5D) randomly sampled cells from each cluster were subjected to a force-directed layout. All conditions from a single experiment were processed simultaneously so that the resulting map would capture all populations present in the entire dataset.
QUANTIFICATION AND STATISTICAL ANALYSIS
All animal experiments were randomized. No statistical method was used to predetermine sample size. The investigators were not blinded to allocation during experiments and outcome assessment, except for force measurement assays and histological analyses. Transplantation experiments were performed with at least n=12 mice, two independent experiments. The line indicated in the scatter plots or bar graphs represents the median (Fig. 1G; Fig. 3F), mean ± SEM (Fig. 1C, E; Fig. S1B, D; Fig. 2B, C, F, G; Fig. S2B, I, J; Fig. 3B–E; Fig. S3A–D; Fig. 4A, B, E, F, H, K, L; Fig. S4D–F, H, I; Fig. 5A–B, I–J; Fig. S5B, D–F; Fig. 6B–E; Fig. S6A) or mean ± SD (Fig. S1E; Fig. S2D; Fig. 4C, D; Fig. S4A–C; Fig. 5F,G; Fig. S6B, D). The sample size of each experimental group and the statistical details of each experiment are described in the corresponding figure legend. ANOVA or multiple t-test analysis were performed for experiments with 3 or more groups, as indicated in the legend. For comparisons between two groups, unpaired or paired Student’s t-test was used. Analyses were conducted using GraphPad Prism 9 software (GraphPad Software). Statistical significance was determined by p≤0.05. *, **, *** and **** represent statistical significance at p≤0.05, p≤0.01, p≤0.001 and p≤0.0001, respectively.
Supplementary Material
Table S2 (related to Figure 3). Antisense Morpholino Oligonucleotide (AMO) Sequences
Highlights.
Elevated CD47 levels define a dysfunctional aged muscle stem cell (MuSC) subset
U1 snRNA upregulation drives CD47 alternative polyadenylation in aged MuSCs
Thrombospondin-1/CD47 paracrine signaling suppresses aged MuSC proliferation
In vivo thrombospondin-1 blockade restores regeneration and strength in aged muscle
ACKNOWLEDGEMENTS
We thank Kassie Koleckar for technical assistance, Drs. David Burns, Glenn Markov, Megan Mayerle and Gaspard Pardon for valuable discussions, the Stanford Shared FACS Facility for technical support, Drs. Anthony Jose and Nori Ueno from Thermo Fisher Scientific for support with the PrimeFlow RNA assay, and Dr. Miro Koulnis and Max Koulnis for help with illustrations. We thank Dr. Garry Nolan for his critical insight into single-cell analysis and for sharing resources. This study was supported by BD Biosciences Stem Cell grant (E.P.); NIH grants NS089533 and AG020961, CIRM grant RB5-07469 and the Baxter Foundation (H.M.B.).
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
Footnotes
DECLARATION OF INTERESTS
H.M.B. is cofounder of Rejuvenation Technologies Inc. and Epirium Bio. H.M.B. and E.P. are named inventors on patent application No. PCT/US2021/038549, held by Stanford University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Blau HM, Cosgrove BD, and Ho ATV (2015). The central role of muscle stem cells in regenerative failure with aging. Nat Med 21, 854–862. 10.1038/nm.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feige P, Brun CE, Ritso M, and Rudnicki MA (2018). Orienting Muscle Stem Cells for Regeneration in Homeostasis, Aging, and Disease. Cell Stem Cell 23, 653–664. 10.1016/j.stem.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MAURO A (1961). Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9, 493–495. 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt M, Schüler SC, Hüttner SS, von Eyss B, and von Maltzahn J (2019). Adult stem cells at work: regenerating skeletal muscle. Cellular and Molecular Life Sciences 76, 2559–2570. 10.1007/s00018-019-03093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sousa-Victor P, and Muñoz-Cánoves P (2016). Regenerative decline of stem cells in sarcopenia. Mol Aspects Med 50, 109–117. 10.1016/j.mam.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 6.de Morree A, Klein JDD, Gan Q, Farup J, Urtasun A, Kanugovi A, Bilen B, van Velthoven CTJ, Quarta M, and Rando TA (2019). Alternative polyadenylation of Pax3 controls muscle stem cell fate and muscle function. Science (1979) 366, 734–738. 10.1126/science.aax1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlikowski B, Pulliam C, Betta ND, Kardon G, and Olwin BB (2015). Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet Muscle 5, 42. 10.1186/s13395-015-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M, and Kardon G (2015). Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun 6, 7087. 10.1038/ncomms8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakkalakal J. v., Jones KM, Basson MA, and Brack AS (2012). The aged niche disrupts muscle stem cell quiescence. Nature 490, 355–360. 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukjanenko L, Karaz S, Stuelsatz P, Gurriaran-Rodriguez U, Michaud J, Dammone G, Sizzano F, Mashinchian O, Ancel S, Migliavacca E, et al. (2019). Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell 24, 433–446.e7. 10.1016/j.stem.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukjanenko L, Jung MJ, Hegde N, Perruisseau-Carrier C, Migliavacca E, Rozo M, Karaz S, Jacot G, Schmidt M, Li L, et al. (2016). Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med 22, 897–905. 10.1038/nm.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinel C, Lukjanenko L, Batut A, Deleruyelle S, Pradère JP, le Gonidec S, Dortignac A, Geoffre N, Pereira O, Karaz S, et al. (2018). The exerkine apelin reverses age-associated sarcopenia. Nat Med 24, 1360–1371. 10.1038/s41591-018-0131-6. [DOI] [PubMed] [Google Scholar]
- 13.Rozo M, Li L, and Fan C-M (2016). Targeting β1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat Med 22, 889–896. 10.1038/nm.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, and Olwin BB (2014). P38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med 20, 265–271. 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brett JO, Arjona M, Ikeda M, Quarta M, de Morrée A, Egner IM, Perandini LA, Ishak HD, Goshayeshi A, Benjamin DI, et al. (2020). Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat Metab 2, 307–317. 10.1038/s42255-020-0190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, and Rudnicki MA (2014). Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med 20, 1174–1181. 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, Latella L, and Sacco A (2014). STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med 20, 1182–1186. 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, and Blau HM (2014). Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med 20, 255–264. 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornson ZB, Nolan GP, and Fantl WJ (2013). Single-cell mass cytometry for analysis of immune system functional states. Curr Opin Immunol 25, 484–494. 10.1016/j.coi.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porpiglia E, Samusik N, van Ho AT, Cosgrove BD, Mai T, Davis KL, Jager A, Nolan GP, Bendall SC, Fantl WJ, et al. (2017). High-resolution myogenic lineage mapping by single-cell mass cytometry. Nat Cell Biol 19, 558–567. 10.1038/ncb3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitzer MH, and Nolan GP (2016). Mass Cytometry: Single Cells, Many Features. Cell 165, 780–791. 10.1016/j.cell.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, and Weissman IL (2009). CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 138, 271–285. 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, van Rooijen N, and Weissman IL (2009). CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 138, 286–299. 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneshige A, Kaji T, Zhang L, Saito H, Nakamura A, Kurosawa T, Ikemoto-Uezumi M, Tsujikawa K, Seno S, Hori M, et al. (2022). Relayed signaling between mesenchymal progenitors and muscle stem cells ensures adaptive stem cell response to increased mechanical load. Cell Stem Cell 29, 265–280.e6. 10.1016/j.stem.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Sacco A, Doyonnas R, Kraft P, Vitorovic S, and Blau HM (2008). Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506. 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkovits BD, and Mayr C (2015). Alternative 3′ UTRs act as scaffolds to regulate membrane protein localization. Nature 522, 363–367. 10.1038/nature14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma W, and Mayr C (2018). A Membraneless Organelle Associated with the Endoplasmic Reticulum Enables 3′UTR-Mediated Protein-Protein Interactions. Cell 175, 1492–1506.e19. 10.1016/j.cell.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apponi LH, Corbett AH, and Pavlath GK (2011). RNA-binding proteins and gene regulation in myogenesis. Trends Pharmacol Sci 32, 652–658. 10.1016/j.tips.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Figueroa A, Cuadrado A, Fan J, Atasoy U, Muscat GE, Munoz-Canoves P, Gorospe M, Munoz A, Mun A, Figueroa A, et al. (2003). Role of HuR in Skeletal Myogenesis through Coordinate Regulation of Muscle Differentiation Genes. Mol Cell Biol 23, 4991–5004. 10.1128/MCB.23.14.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Giessen K, and Gallouzi I-E (2007). Involvement of Transportin 2-mediated HuR Import in Muscle Cell Differentiation. Mol Biol Cell 18, 3250–3263. 10.1091/mbc.e07-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoque M, Ji Z, Zheng D, Luo W, Li W, You B, Park JY, Yehia G, and Tian B (2013). Analysis of alternative cleavage and polyadenylation by 3′ region extraction and deep sequencing. Nat Methods 10, 133–139. 10.1038/nmeth.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Buuren N, Tellinghuisen TL, Richardson CD, and Kirkegaard K (2018). Transmission genetics of drug-resistant hepatitis C virus. Elife 7, 1–21. 10.7554/eLife.32579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hensel JA, Heineman BD, Kimble AL, Jellison ER, Reese B, and Murphy PA (2021). Identification of splice regulators of fibronectin-EIIIA and EIIIB by direct measurement of exon usage in a flow-cytometry based CRISPR screen. Sci Rep 11, 19835. 10.1038/s41598-021-99079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilsen TW (2003). The spliceosome: The most complex macromolecular machine in the cell? BioEssays 25, 1147–1149. 10.1002/bies.10394. [DOI] [PubMed] [Google Scholar]
- 35.Wahl MC, Will CL, and Lührmann R (2009). The Spliceosome: Design Principles of a Dynamic RNP Machine. Cell 136, 701–718. 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Berg MG, Singh LN, Younis I, Liu Q, Pinto AM, Kaida D, Zhang Z, Cho S, Sherrill-Mix S, Wan L, et al. (2012). U1 snRNP determines mRNA length and regulates isoform expression. Cell 150, 53–64. 10.1016/j.cell.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaida D, Berg MG, Younis I, Kasim M, Singh LN, Wan L, and Dreyfuss G (2010). U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature 468, 664–668. 10.1038/nature09479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian B, and Manley JL (2016). Alternative polyadenylation of mRNA precursors. Nat Rev Mol Cell Biol 18, 18–30. 10.1038/nrm.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oldenborg P-A (2013). CD47: A Cell Surface Glycoprotein Which Regulates Multiple Functions of Hematopoietic Cells in Health and Disease. ISRN Hematol 2013, 1–19. 10.1155/2013/614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown EJ, and Frazier WA (2001). Integrin Associated protein (CD47) and its ligands. Trends Cell Biol 11, 130–135. [DOI] [PubMed] [Google Scholar]
- 41.Feng N, Wang Z, Zhang Z, He X, Wang C, and Zhang L (2015). MiR-487b promotes human umbilical vein endothelial cell proliferation, migration, invasion and tube formation through regulating THBS1. Neurosci Lett 591, 1–7. 10.1016/j.neulet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Gao Q, Chen K, Gao L, Zheng Y, and Yang YG (2016). Thrombospondin-1 signaling through CD47 inhibits cell cycle progression and induces senescence in endothelial cells. Cell Death Dis 7, e2368. 10.1038/cddis.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur S, Soto-Pantoja DR, Stein E. v., Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, et al. (2013). Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep 3, 1–12. 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoier B, Passos M, Bangsbo J, and Hellsten Y (2013). Intense intermittent exercise provides weak stimulus for vascular endothelial growth factor secretion and capillary growth in skeletal muscle. Exp Physiol 98, 585–597. 10.1113/expphysiol.2012.067967. [DOI] [PubMed] [Google Scholar]
- 45.Hoier B, Walker M, Passos M, Walker PJ, Green A, Bangsbo J, Askew CD, and Hellsten Y (2013). Angiogenic response to passive movement and active exercise in individuals with peripheral arterial disease. J Appl Physiol 115, 1777–1787. 10.1152/japplphysiol.00979.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isenberg JS, and Roberts DD (2020). Thrombospondin-1 in maladaptive aging responses: A concept whose time has come. Am J Physiol Cell Physiol 318, C45–C63. 10.1152/ajpcell.00089.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kivelä R, Silvennoinen M, Lehti M, Jalava S, Vihko V, and Kainulainen H (2008). Exercise-induced expression of angiogenic growth factors in skeletal muscle and in capillaries of healthy and diabetic mice. Cardiovasc Diabetol 7, 13. 10.1186/1475-2840-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Annis DS, Murphy-Ullrich JE, and Mosher DF (2006). Function-blocking antithrombospondin-1 monoclonal antibodies. Journal of Thrombosis and Haemostasis 4, 459–468. 10.1111/j.1538-7836.2006.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumaz N, and Marais R (2005). Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. FEBS Journal 272, 3491–3504. 10.1111/j.1742-4658.2005.04763.x. [DOI] [PubMed] [Google Scholar]
- 50.Stork PJS, and Schmitt JM (2002). Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12, 258–266. 10.1016/S0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Lindberg FP, and Frazier WA (1999). Integrin-associated protein stimulates α2β1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases. Journal of Cell Biology 147, 389–399. 10.1083/jcb.147.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao M, Roberts DD, and Isenberg JS (2011). Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res 63, 13–22. 10.1016/j.phrs.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tewson PH, Martinka S, Shaner NC, Hughes TE, and Quinn AM (2016). New DAG and cAMP Sensors Optimized for Live-Cell Assays in Automated Laboratories. J Biomol Screen 21, 298–305. 10.1177/1087057115618608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rockwood K, and Mitnitski A (2007). Frailty in relation to the accumulation of deficits. Journals of Gerontology - Series A Biological Sciences and Medical Sciences 62, 722–727. 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 55.Rolland Y, Czerwinski S, van Kan GA, Morley JE, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y, et al. (2008). Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. Journal of Nutrition, Health and Aging, 433–450. 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, and Onder G (2012). Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clinical Nutrition 31, 652–658. 10.1016/j.clnu.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, and Pereira SL (2019). Economic Impact of Hospitalizations in US Adults with Sarcopenia. J Frailty Aging 8, 93–99. 10.14283/jfa.2019.10. [DOI] [PubMed] [Google Scholar]
- 58.Cao L, and Morley JE (2016). Sarcopenia Is Recognized as an Independent Condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J Am Med Dir Assoc 17, 675–677. 10.1016/j.jamda.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Khandelwal S, van Rooijen N, and Saxena RK (2007). Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion (Paris) 47, 1725–1732. 10.1111/j.1537-2995.2007.01348.x. [DOI] [PubMed] [Google Scholar]
- 60.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, and Lindberg FP (2000). Role of CD47 as a marker of self on red blood cells. Science (1979) 288, 2051–2054. 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 61.Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, and Brown EJ (1995). In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J Cell Sci 108, 3419–3425. 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- 62.Van VQ, Raymond M, Baba N, Rubio M, Wakahara K, Susin SA, and Sarfati M (2012). CD47 high Expression on CD4 Effectors Identifies Functional Long-Lived Memory T Cell Progenitors. The Journal of Immunology 188, 4249–4255. 10.4049/jimmunol.1102702. [DOI] [PubMed] [Google Scholar]
- 63.Soto-Pantoja DR, Kaur S, and Roberts DD (2015). CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol 50, 212–230. 10.3109/10409238.2015.1014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, and Weissman IL (2019). Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer 19, 568–586. 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kotecha N, Krutzik PO, and Irish JM (2010). Web-based analysis and publication of flow cytometry experiments. Curr Protoc Cytom. 10.1002/0471142956.cy1017s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zunder ER, Lujan E, Goltsev Y, Wernig M, and Nolan GP (2015). A continuous molecular roadmap to iPSC reprogramming through progression analysis of single-cell mass cytometry. Cell Stem Cell 16, 323–337. 10.1016/j.stem.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samusik N, Good Z, Spitzer MH, Davis KL, and Nolan GP (2016). Automated mapping of phenotype space with single-cell data. 13, 493–496. 10.1038/nmeth.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li B, and Dewey CN (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323. 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang YX, Holbrook CA, Hamilton JN, Garoussian J, Afshar M, Su S, Schürch CM, Lee MY, Goltsev Y, Kundaje A, et al. (2022). A single cell spatial temporal atlas of skeletal muscle reveals cellular neighborhoods that orchestrate regeneration and become disrupted in aging. bioRxiv. 10.1101/2022.06.10.494732. [DOI] [Google Scholar]
- 72.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, and Brown EJ (1996). Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science (1979) 274, 795–798. 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 73.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, and Blau HM (2010). Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science (1979) 329, 1078–1081. 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serrano AL, and Muñoz-Cánoves P (2021). Mouse models of muscle fibrosis. In Methods in Molecular Biology (Humana Press Inc.), pp. 357–370. 10.1007/978-1-0716-1382-5_24. [DOI] [PubMed] [Google Scholar]
- 75.Mintz EL, Passipieri JA, Lovell DY, and Christ GJ (2016). Applications of in vivo functional testing of the rat tibialis anterior for evaluating tissue engineered skeletal muscle repair. Journal of Visualized Experiments 2016. 10.3791/54487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheth KA, Iyer CC, Wier CG, Crum AE, Bratasz A, Kolb SJ, Clark BC, Burghes AHM, and Arnold WD (2018). Muscle strength and size are associated with motor unit connectivity in aged mice. Neurobiol Aging 67, 128–136. 10.1016/j.neurobiolaging.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang A. v., Kraft P, Holbrook CA, Schürch CM, Ho ATV, and Blau HM (2021). Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science (1979) 371. 10.1126/science.abc8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith LR, and Barton ER (2014). SMASH - semi-automatic muscle analysis using segmentation of histology: A MATLAB application. Skelet Muscle 4. 10.1186/2044-5040-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fienberg HG, Simonds EF, Fantl WJ, Nolan GP, and Bodenmiller B (2012). A platinum-based covalent viability reagent for single-cell mass cytometry. Cytometry Part A 81 A, 467–475. 10.1002/cyto.a.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2 (related to Figure 3). Antisense Morpholino Oligonucleotide (AMO) Sequences
Data Availability Statement
RNAseq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
CyTOF data have been deposited at Flowrepository.org and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-mouse Thrombospondin-1 (clone A6.1) | eBioscience | Cat# 14-9756-82 |
| anti-mouse α7 integrin (clone 3C12) | MBL international | Cat# K0046-3 |
| anti-mouse CD29 (clone 9EG7) | BD Biosciences | Cat# 553715 |
| anti-mouse CXCR4 (clone 2B11) | BD Biosciences | Cat# 551852 |
| anti-mouse CD31 (clone MEC 13.3) | BD Biosciences | Cat# 550274 |
| anti-mouse CD45 (clone 30-F11) | BD Biosciences | Cat# 553076 |
| anti-mouse CD11b (clone M1/70) | BD Pharmigen | Cat# 557394 |
| anti-mouse Sca-1 (clone E13-161.7) | BD Biosciences | Cat# 553333 |
| anti-mouse CD106 (clone MVCAM.A) | Biolegend | Cat# 105701 |
| anti-mouse CD9 (clone KMC8) | eBiosciences | Cat# 14-0091-82 |
| anti-mouse CD44 (clone IM-7) | BD Biosciences | Cat# 553131 |
| anti-mouse CD47 (clone MIAP301) | Biolegend | Cat# 127502 |
| Rat IgG2a, k | Biolegend | Cat# 400502 |
| anti-Rat IgG2a PerCP-eFluor 710 | eBioscience | Cat# 46-4817-82 |
| anti-mouse CD90.2 (clone 30-H12) | BD Biosciences | Cat# 553009 |
| anti-mouse CD98 (clone 4F2) | Biolegend | Cat# 128202 |
| anti-mouse CD104 (clone 346-11A) | BD Biosciences | Cat# 553745 |
| anti-mouse Pax7 (clone PAX7) | Santa Cruz Biotechnology | Cat# sc-81648 |
| anti-mouse Myf5 (clone C-20) | Santa Cruz Biotechnology | Cat# sc-302 |
| anti-mouse MyoD (clone 5.8A) | BD Pharmigen | Cat# 554130 |
| anti-mouse Myogenin (clone F5D) | BD Pharmigen | Cat# 556358 |
| anti-pRb-166Er | Fluidigm | Cat# 3166011A |
| anti-Mouse CD45 (30-F11)-89Y | Fluidigm | Cat# 3089005B |
| anti-Mouse CD11b (M1/70) -148Nd | Fluidigm | Cat# 3148003B |
| anti-human/mouse CD44-Yb171 | Fluidigm | Cat# 3171003B |
| anti-Laminin | Abcam | Cat# ab11575 |
| anti-eMHC (clone F1.562) | DSHB | N/A |
| Biotin anti-mouse CD11b (Clone M1/70) | BD Biosciences | Cat# 553309 |
| Biotin anti-mouse CD45 (Clone 30F11) | BD Biosciences | Cat# 553078 |
| Biotin anti-mouse Sca1 (Clone E13-161.7) | BD Biosciences | Cat# 553334 |
| Biotin anti-mouse CD31 (Clone 390) | eBioscience | Cat# 13-0311-82 |
| anti α7integrin-PE (clone R2F2) | AbLab | Cat# 10ST215 |
| anti-CD34-eFluor660 (clone RAM34) | eBioscience | Cat# 50-0341-82 |
| Streptavidin APC-Cy7 | BD Biosciences | Cat# 554063 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 16% Paraformaldehyde aqueous solution (PFA) | Electron Microscopy Sciences | Cat# 15711 |
| 4’,6 diamidino-2-phenylindole (DAPI) | Life Technologies | Cat# D1306 |
| D-Luciferin | Caliper Life Sciences | Cat# 122796 |
| Collagenase type 2 | Worthington | Cat# LS004176 |
| Dispase II | Thermo Fisher Scientific | Cat# 17105041 |
| Tamoxifen | VWR | Cat# AAJ63509-03 |
| Dimethyl Sulfoxide (DMSO) | Sigma | Cat# D8418 |
| Notexin | Latoxan | Cat# L8104 |
| Cisplatin | Sigma-Aldrich | Cat# P4394 |
| Cell-ID intercalator-Ir | Fluidigm | Cat# 201192B |
| 5-Iodo-2′-deoxyuridine | Sigma Aldrich | Cat# I7125 |
| Perm/Wash Buffer I | BD Biosciences | Cat# 557885 |
| Endoporter-PEG | Gene Tools | Cat# OT-EP-PEG-1 |
| DMEM, low glucose, pyruvate | Thermo Fisher Scientific | Cat# 11885-092 |
| Ham’s F-10 Nutrient Mix | Invitrogen | Cat# 11550-043 |
| Horse Serum | Fisher-Scientific | Cat# 26-050-088 |
| Penicillin/Streptomycin | Gibco | Cat# 15140122 |
| Recombinant Human FGF-basic | Peprotech | Cat# 100-18B |
| Critical Commercial Assays | ||
| PrimeFlow RNA Assay Kit | Thermo Fisher Scientific | Cat# 88-18005-204 |
| Custom Probe-CD47long 3’UTR | Thermo Fisher Scientific | Assay ID: VB6-6001206 |
| Custom Probe-CD47total | Thermo Fisher Scientific | Assay ID: VB1-3030381 |
| RNeasy Micro Kit | Qiagen | Cat# 74004 |
| High-Capacity cDNA reverse transcription kit | Applied Biosystems | Cat# 4368814 |
| TaqMan gene expression Master Mix | Applied Biosystems | Cat# 4370048 |
| SYBR Green PCR Master Mix | Life Technologies | Cat# 4309155 |
| Green Down cADDis cAMP Assay Kit | Montana Molecular | Cat# D0200G |
| Cyclic AMP XP Assay Kit | Cell Signaling Technology | Cat# 4339 |
| Ovation RNA-Seq System V2 kit | NuGen | Cat# 7102-08 |
| TruSEQ RNA Library Preparation Kit v2 | Illumina | Cat# RS-122-2101 |
| Nextera DNA Library Prep Kit | Illumina | Cat# FC-121-1030 |
| Deposited Data | ||
| RNAseq data | This study | GSE198249 |
| CyTOF Data | This study | FR-FCM-Z5RA, FR-FCM-Z5RB, FR-FCM-Z5RC, FR-FCM-Z5RD, FR-FCM-Z5RE, FR-FCM-Z5RG, FR-FCM-Z5RF |
| Experimental Models: Cell Lines | ||
| Primary Myoblasts | In house | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | Jackson Laboratories | Strain# 000664 |
| Mouse: Pax7tm1(cre/ERT2)Gaka (Pax7Cre-ERT2) | Jackson Laboratories | Strain# 017763 |
| Mouse: Gt(ROSA)26Sortm1(Luc)Kael (Rosa26LSL-Luc) | Jackson Laboratories | Strain# 005125 |
| Mouse: B6.129S7-Cd47tm1Fpl/J | Jackson Laboratories | Strain# 003173 |
| Mouse: NOD.Cg-Prkdcscid/J | Jackson Laboratories | Strain# 001303 |
| Oligonucleotides | ||
| q-RT-PCR Primer Sequences | Integrated DNA Technologies | Table S1 |
| Antisense Morpholino Oligonucleotides Sequences | Gene Tools | Table S2 |
| TaqMan gene expression assay (cdkn1a-FAM) | Applied Biosystems | Mm00432448_m1 |
| TaqMan gene expression assay (cdkn1b-FAM) | Applied Biosystems | Mm00438168_m1 |
| TaqMan gene expression assay (cdkn1c-FAM) | Applied Biosystems | Mm00438170_m1 |
| TaqMan gene expression assay (GADPH-VIC) | Applied Biosystems | 4352339E |
| Software and Algorithms | ||
| Flowjo | FlowJo, LLC | www.flowjo.com |
| Cytobank | 65 | www.cytobank.org |
| Matlab or R – Normalizer | 70 |
https://github.com/nolanlab/bead-normalization/wiki/Normalizing-FCS-Files
https://github-com.laneproxy.stanford.edu/ParkerICI/premessa—R |
| Matlab or R – Single Cell Debarcoder | 66 |
https://github.com/nolanlab/single-cell-debarcoder
https://github-com.laneproxy.stanford.edu/ParkerICI/premessa—R |
| Vortex | 67 | https://github.com/nolanlab/vortex/ |
| STAR | 68 | https://github.com/alexdobin/STAR/releases |
| RSEM | 69 | https://github.com/bli25ucb/RSEM_tutorial |
| DESeq2 | 70 | http://www.bioconductor.org/packages/DESeq2/ |
| Living Image v.4.5 | Perkin Elmer | Perkinelmer.com |
| Aurora Scientific Dynamic Muscle Data Acquisition and Analysis Software | Aurora Scientific | https://aurorascientific.com/products/muscle-physiology/muscle-daq-software/615a-dynamic-muscle-control/ |
| Keyence Analysis Software (BZ-X700 Microscope) | Keyence | www.keyence.com |
| CRISP | 71 | github.com/will-yx/ |
| FiberNet | 71 | github.com/will-yx/ |
| Graphpad Prism 9 | GraphPad Software, Inc | www.graphpad.com |


