Abstract
Objective:
To better characterize infection stillbirth in terms of pathogenesis and microbiology.
Methods:
We conducted a secondary analysis of 512 stillbirths in a prospective, multisite, geographically, racially and ethnically diverse, population-based study of stillbirth in the United States. Cases underwent evaluation that included maternal interview, chart abstraction, biospecimen collection, fetal autopsy, and placental pathology. Recommended evaluations included syphilis and parvovirus serology. Each case was assigned probable and possible causes of death using the INCODE Stillbirth Classification System. Cases where infection was assigned as a probable or possible cause of death were reviewed. For these cases, clinical scenario, autopsy, maternal serology, culture results and placental pathology were evaluated.
Results:
For 66 (12.9%) cases of stillbirth, infection was identified as a probable or possible cause of death. Of these, 36% (95% CI 35% - 38%) were categorized as a probable and 64% (95% CI 62% - 65%) as a possible cause of death. Infection-related stillbirth occurred earlier than non-infection related stillbirth (median gestational age 22 weeks vs 28 weeks, p= 0.001). Fetal bacterial culture results were available in 47 cases (71%), of which 35 (53%) grew identifiable organisms. The predominant species were Escherichia coli (19; 29%), group B streptococcus (8; 12%), and enterococcus (8; 12%). Placental pathology revealed chorioamnionitis in 50 (76%), funisitis in 27 (41%), villitis in 11 (17%), deciduitis in 35 (53%), necrosis in 27 (41%), and viral staining in 7 (11%) cases. Placental pathology found inflammation or evidence of infection in 65 (99%) cases and fetal autopsy in 26 (39%) cases. In infection stillbirth cases, the likely causative non-bacterial organisms identified were parvovirus in 2 (3%) cases, syphilis in 1 (2%) case, cytomegalovirus in 5 (8%) cases and herpes in 1 (2%) case.
Conclusions:
Of infection-related stillbirth cases in a large U.S. cohort, E. coli, group B streptococcus and enterococcus were the most common bacterial pathogens and cytomegalovirus the most common viral pathogen.
Précis:
Most cases of infection-related stillbirth are due to bacterial pathogens, of which Escherichia coli, group B streptococcus, and enterococcus are the most common.
INTRODUCTION
In the United States, stillbirth (defined as fetal death at ≥ 20 weeks of gestation) affects about one in 168 pregnancies (6 per 1,000 pregnancies), or 23,595 per year based on 2015 data.1 Causes of stillbirth include genetic abnormalities, obstetric complications, maternal medical diseases and abnormalities of the placenta and umbilical cord. Maternal or fetal infection (or both) is an important cause of fetal death for which prospective, well-characterized studies are lacking. Approximately 10 to 20% of stillbirths have been reported to be caused by infection in high-income countries.2,3 The percentage is likely higher in low-income countries.2
The proportion of stillbirths due to infection is uncertain because clinical symptoms or signs of infection may not be apparent and because systematic evaluation for infection is not always conducted when assessing stillbirth. In addition, although many organisms have been reported to cause sporadic stillbirths, large studies have not reported the specific responsible organisms. Thus, our primary objective was to evaluate and characterize stillbirth related to infection using clinical, histologic and microbiologic data. We also sought to characterize the organisms associated with stillbirth, as well as the utility of various tests for the identification of infection-related stillbirth, in a well-characterized, large and diverse U.S. cohort.
METHODS
The Stillbirth Collaborative Research Network (SCRN) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development conducted a multi-center, ethnically, racially and geographically diverse case-control study including stillbirths and a representative sample of live births enrolled in five catchment areas defined by county boundaries between March 2006 and September 2008. Detailed methods have previously been published.4 Stillbirth was defined as death at the time of delivery at ≥ 20 weeks of gestation with Apgar scores of 0 and 0 at 1 and 5 minutes. Gestational age was determined by multiple sources and an algorithm as previously described.4 Participants gave written informed consent and Institutional Review Board approval was obtained from each clinical site and the data-coordinating center.
The protocol included medical record abstraction, maternal interview, biospecimen collection and placental pathologic examination for all participants, with postmortem examination of stillbirths, which included freezing sections of placenta and membranes in stillbirths and live births and liver in stillbirths.5,6 Sections of frozen placenta and liver were stored at – 80°C. In cases of stillbirth, clinicians were advised to obtain a standard set of “clinically indicated” laboratory studies.7 These included serologic tests for syphilis and parvovirus as well as tests to assess non-infectious causes of stillbirth.4 However, all of these tests were not obtained in every case of stillbirth, either due to patient or health care provider preference or due to lack of stored serum sample.3,8 Parvovirus serology also was assessed using maternal sera collected at the time of enrollment in cases that did not have previous testing. ELISA was used to determine if parvovirus IgM and IgG antibodies were present. (ARUP, Salt Lake City, Utah).
Each stillbirth was assigned a cause of death using an algorithm developed by the SCRN investigators, termed INCODE (initial causes of fetal death).9 The algorithm was designed to account for the facts that causes of stillbirth are often uncertain and that multiple factors may contribute. Conditions were considered to either be “probable” or “possible” causes of stillbirth, or present but unlikely to be contributors to the stillbirth.9 Probable causes were conditions with a high likelihood of directly causing the stillbirth based upon the best available evidence at the time INCODE was developed. An example of this would be a case in which fetal hydrops is present and parvovirus is identified on maternal serology or placental and fetal pathology. Possible causes were those that were possibly involved in a pathophysiologic sequence that led to the death, without being a direct cause of the stillbirth, such as a case in which there is histologic evidence of infection in vital organs without culture or PCR proven infection. If a condition associated with stillbirth was ascertained but did not meet criteria for probable or possible cause, it was considered “present.” For example, a case in which a culture or PCR result was positive for infection, but no histologic or clinical infectious sequelae were noted would be classified as present. In the initial analysis of causes of death among 512 stillbirths, infection was determined to be a probable or possible cause in 66 (12.9%) cases.8 Of these, 65% were antepartum and 35% were intrapartum stillbirths. Infection accounted for a higher proportion of stillbirths relatively early in gestation, especially prior to 23 weeks of gestation, and were associated with a higher percentage of stillbirths in non-Hispanic blacks compared to non-Hispanic whites or Hispanics.8 Our primary analysis includes specific details of these 66 cases with probable or possible infectious causes of stillbirth. Following our review, one case was excluded from our analysis after a second review of the INCODE cause of death was not consistent with a possible or probable infection cause of death. Another case was included as a possible infection cause of death as it was twin B of a pregnancy in which twin A had a possible infection cause of death.
We also categorized the pathophysiologic pathway to infection-related stillbirth based on the clinical scenario and results of serology and histologic examinations. These pathways include direct fetal infection; direct fetal infection causing fetal anomalies or pathologic condition; placental infection leading to placental insufficiency; severe maternal illness; infection involved in a previable or periviable preterm birth; and cases of isolated placental inflammation.2,9 Direct fetal infection was defined by evidence of fetal infection on blood or lung cultures as well as inflammation on histologic fetal examination. Cases with infections which can cause a fetal anomaly or malformation (e.g. cytomegalovirus or parvovirus) were designated by findings of these organisms on culture or histology or by pathognomonic findings in the fetus or placenta. Cases with culture or PCR proven infection in combination with placentitis or villitis or pathognomonic findings of an organism known to cause placental inflammation leading to placental insufficiency (e.g. syphilis) were categorized into this group. Severe maternal illness was defined by documented maternal infection process leading to systemic shock (i.e. hypotension, end organ injury) or need for intensive care unit admission. Cases in which infection was involved in a preterm labor process (preterm prelabor rupture of membranes, cervical insufficiency, preterm contractions) at a previable or periviable gestational age were included in the preterm labor group. Cases for which isolated placental inflammation was noted without fitting into one of the above groups were categorized as placental inflammation only. Records were individually reviewed to verify the clinical history and test results for all cases included in this analysis.
Characteristics were compared between those with and without infection among stillbirths as well as between stillbirths with infection and live birth controls. The sampling design of the study was taken into account with SAS survey procedures and weighting adjustment for the staggered start for enrollment at the 59 hospitals and differential participation rates in the study design to ensure the controls (live births) were representative of the delivery population of the catchment area as previously described.4 The Rao-Scott chi-squared test or Fisher’s exact test were used for all categorical univariate comparisons and the f-test for numeric univariate comparisons. The utility of a diagnostic test to determine a probable or possible cause of death was determined in this cohort of cases. A test result was considered a “pertinent positive” if it detected a cause of death or confirmed a cause of death that was suspected based on clinical presentation. A “pertinent negative” test was defined as a result that ruled out a potential cause of death based on the clinical scenario.10
RESULTS
A total of 663 women with 676 stillbirths were enrolled; of these, 500 women with 512 stillbirths that had complete fetal postmortem examination were included in this analysis (Figure 1). Characteristics of this cohort have previously been described.8 Among the 500 women, 495 (99.0%) had serologic testing for syphilis, 451 (90.2%) for parvovirus, 38 (7.6%) for cytomegalovirus (CMV), and 56 (11.2%) for toxoplasmosis. Results of vaginal culture for group B streptococcus (GBS) were available for 105 (20.5%).
Figure 1.
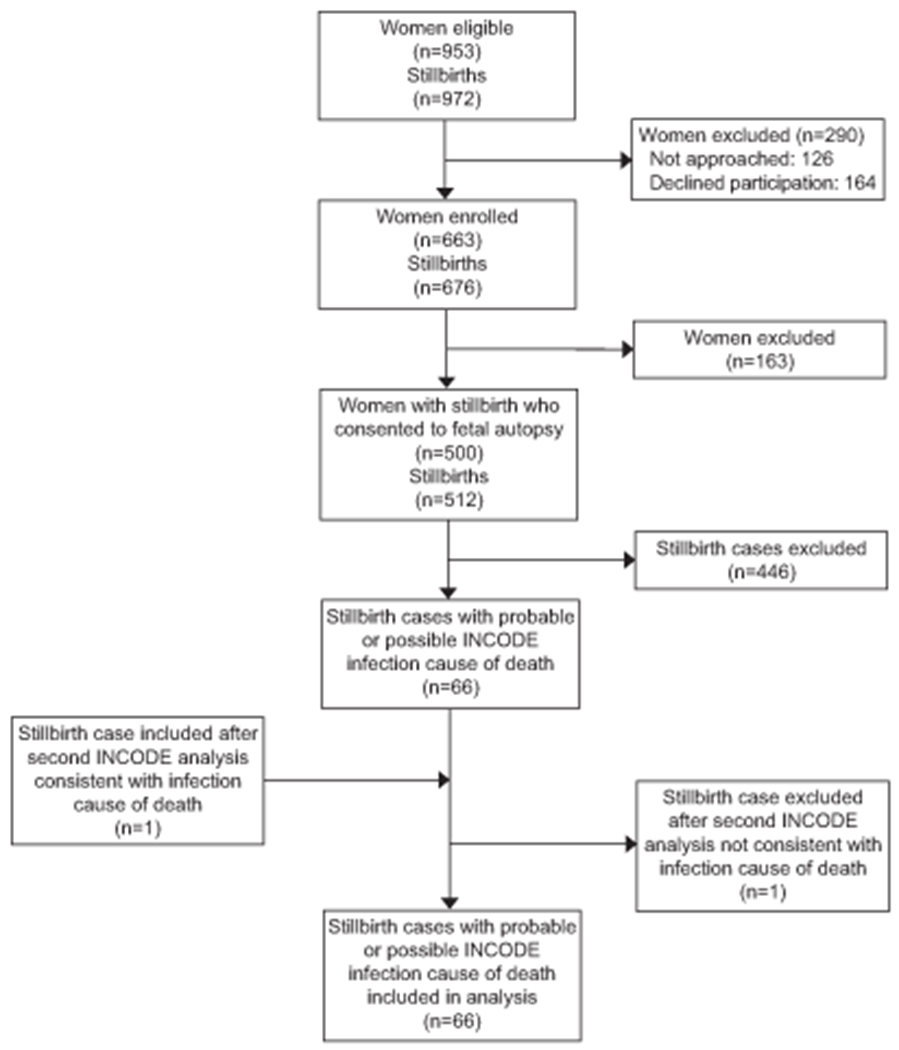
Enrollment of stillbirths. INCODE, initial causes of fetal death. Livebirth enrollment: 3,083 women identified; 394 (13%) not approached; 759 (25%) refused to participate; 1,930 (63%) consented for the study. Reference 4 details the complete study design and recruitment experience of the parent study.
In stillbirths at 37 weeks of gestation or greater, 75.0% of cases had group B streptococcus results (63 of 84 cases). Of the cases with a probable or possible infection cause of death, fetal bacterial culture results (obtained at the discretion of the pathologist from fetal blood and lung) were available in 47 cases, of which 35 had growth and identification of various organisms. The remaining cases did not have culture data available. Of the cases of stillbirth thought to be due to infection, five (8%) had placental culture results with polymicrobial growth that in most cases did not correlate with the clinical scenario or likely pathogenic organism. In this study, placental cultures were not systematically obtained. Accordingly, these findings should be interpreted with caution.
In the 66 infection stillbirths, 36% (95% CI 35% - 38%) were categorized as a probable infection stillbirth and 64% (95% CI 62%-65%) as a possible infection stillbirth. Of these, 21 (32%) had infection as the only cause of death suspected. There were 45 (68%) stillbirths with more than one possible or probable cause of death including infection. In the multifactorial cases, spontaneous preterm delivery at non-viable gestation ages occurred in 31 (69%) cases, followed by placental (11; 24%), genetic (5; 11%), maternal medical conditions (5; 11%), other causes (3; 7%), hypertensive disorders (2; 4%) and umbilical cord causes (2; 4%). Infection stillbirths occurred at lower gestational ages (p = 0.001), were more often intrapartum (p <0.001) and were more frequent in non-Hispanic black race-ethnicity (p <0.001) as compared to non-infection stillbirths (Table 1). As compared to live births by gestational age, infection stillbirth was more common among non-Hispanic black women (p <0.001) and was less often associated with hypertensive disorders of pregnancy (p = 0.002; Table 2).
Table 1.
Characteristics of non-infection stillbirths and infection stillbirths
| Stillbirth, No Infection * N = 446 | Infection-related Stillbirth * N = 66 | P-value | ||
|---|---|---|---|---|
| Maternal age (years) | 0.555 | |||
| <20 | N | 54 | 6 | |
| % | 12.1 | 9 | ||
| 20-34 | N | 321 | 53 | |
| % | 72.0 | 80 | ||
| 35-39 | N | 53 | 5 | |
| % | 11.9 | 8 | ||
| 40+ | N | 18 | 2 | |
| % | 4.0 | 3 | ||
| Gestational age (weeks) | Median | 28 | 22 | 0.001 |
| IQR | (23, 35) | (21, 32) | ||
| Intrapartum stillbirth | <0.001 | |||
| Intrapartum | N | 63 | 24 | |
| % | 14.1 | 36 | ||
| Antepartum | N | 383 | 42 | |
| % | 85.9 | 64 | ||
| Maternal race/ethnicity | <0.001 | |||
| White, non-Hispanic | N | 171 | 12 | |
| % | 38.3 | 18 | ||
| Black, non-Hispanic | N | 86 | 29 | |
| % | 19.3 | 45 | ||
| Hispanic | N | 160 | 16 | |
| % | 35.9 | 25 | ||
| Other | N | 29 | 8 | |
| % | 6.5 | 12 | ||
| Missing | N | 0 | 1 | |
| Body mass index (kg/m2) | 0.488† | |||
| <30 | N | 307 | 44 | |
| % | 71.1 | 69 | ||
| 30-39 | N | 96 | 17 | |
| % | 22.2 | 27 | ||
| 40-49 | N | 26 | 2 | |
| % | 6.0 | 3 | ||
| 50+ | N | 3 | 1 | |
| % | 0.7 | 1 | ||
| Missing | N | 14 | 2 | |
| Chronic hypertension | 0.485 | |||
| Yes | N | 46 | 5 | |
| % | 10.3 | 8 | ||
| No | N | 399 | 61 | |
| % | 89.7 | 92 | ||
| Missing | N | 1 | 0 | |
| Preeclampsia/gestational hypertension | 0.292 | |||
| Yes | N | 48 | 4 | |
| % | 11.1 | 7 | ||
| No | N | 383 | 56 | |
| % | 88.9 | 93 | ||
| Missing | N | 15 | 6 | |
| Pregestational diabetes | 0.558† | |||
| Yes | N | 24 | 2 | |
| % | 5.4 | 3 | ||
| No | N | 421 | 64 | |
| % | 94.6 | 97 | ||
| Missing | N | 1 | 0 | |
| Gestational diabetes | 0.758† | |||
| Yes | N | 23 | 2 | |
| % | 5.2 | 3 | ||
| No | N | 423 | 64 | |
| % | 94.8 | 97 | ||
| Insurance/method of payment | 0.367 | |||
| No Insurance | N | 28 | 3 | |
| % | 6.3 | 5 | ||
| Any public/private assistance | N | 225 | 39 | |
| % | 50.7 | 60 | ||
| Veterans Affairs/commercial health insurance/health maintenance organization (HMO) | N | 191 | 23 | |
| % | 43.0 | 35 | ||
| Missing | N | 2 | 1 | |
| Maternal education | 0.577 | |||
| 0-11 (none, primary, some secondary) | N | 87 | 16 | |
| % | 20.7 | 26 | ||
| 12 (completed secondary) | N | 117 | 18 | |
| % | 27.9 | 29 | ||
| 13+ (college) | N | 216 | 28 | |
| % | 51.4 | 45 | ||
| Missing | N | 26 | 4 | |
| Tobacco use | 0.268 | |||
| None | N | 343 | 51 | |
| % | 81.0 | 82 | ||
| <10 cigarettes/day | N | 37 | 8 | |
| % | 8.8 | 13 | ||
| 10+ cigarettes /day | N | 43 | 3 | |
| % | 10.2 | 5 | ||
| Missing | N | 23 | 4 | |
| Lifetime drug use | 0.309 | |||
| Never used | N | 294 | 39 | |
| % | 69.8 | 64 | ||
| Ever used, without addiction | N | 110 | 17 | |
| % | 26.1 | 28 | ||
| Ever used, with addiction | N | 17 | 5 | |
| % | 4.1 | 8 | ||
| Missing | N | 25 | 5 | |
Column percentages are displayed;
Fisher’s Exact Test
Table 2.
Characteristics of infection stillbirths and live births by gestational age
| Variable | < 37 Weeks Gestation | ≥ 37 Weeks Gestation | ||||
|---|---|---|---|---|---|---|
| Infection-related Stillbirths * Nw = 55 N = 54 (%) | Livebirths * Nw = 155 N = 485 (%) | P-value | Infection-related Stillbirths * Nw = 10 N = 10 (%) | Livebirths * Nw = 1285 N = 1447 (%) | P-value | |
| Maternal age (years) | 0.052 | 0.132† | ||||
| <20 | 3 (6) | 16 (10.1) | 2 (20) | 133 (10.3) | ||
| 20-34 | 47 (85) | 120 (77.5) | 6 (60) | 969 (75.4) | ||
| 35-39 | 4 (7) | 15 (9.6) | 1 (10) | 157 (12.2) | ||
| 40+ | 1 (2) | 4 (2.8) | 1 (10) | 26 (2.1) | ||
| Maternal race/ethnicity | <.001 | 0.040† | ||||
| White, non-Hispanic | 8 (14) | 57 (36.6) | 4 (40) | 603 (46.9) | ||
| Black, non-Hispanic | 28 (52) | 25 (16.5) | 4 (40) | 143 (11.2) | ||
| Hispanic | 13 (25) | 64 (41.3) | 1 (10) | 438 (34.1) | ||
| Other | 5 (9) | 9 (5.6) | 1 (10) | 101 (7.8) | ||
| Missing | 1 | 0 | 0 | 0 | ||
| Body mass index (kg/m2) | 0.593† | 0.375† | ||||
| <30 | 34 (64) | 100 (70.9) | 10 (100) | 964 (77.2) | ||
| 30-39 | 16 (31) | 31 (22.2) | 0 (0) | 220 (17.6) | ||
| 40-49 | 2 (4) | 8 (5.3) | 0 (0) | 56 (4.4) | ||
| 50+ | 1 (1) | 2 (1.6) | 0 (0) | 10 (0.8) | ||
| Missing | 2 | 14 | 0 | 35 | ||
| Chronic hypertension | 0.853 | 0.999† | ||||
| Yes | 5 (9) | 18 (11.7) | 0 (0) | 69 (5.4) | ||
| No | 50 (91) | 136 (88.3) | 10 (100) | 1206 (94.6) | ||
| Missing | 0 | 1 | 0 | 10 | ||
| Preeclampsia/gestational hypertension | 0.002 | 0.999† | ||||
| Yes | 4 (8) | 31 (21.3) | 0 (0) | 91 (7.3) | ||
| No | 47 (92) | 116 (78.7) | 10 (100) | 1152 (92.7) | ||
| Missing | 4 | 8 | 0 | 42 | ||
| Pregestational diabetes | 0.684† | 0.118† | ||||
| Yes | 1 (2) | 7 (4.7) | 1 (10) | 15 (1.2) | ||
| No | 54 (98) | 147 (95.3) | 9 (90) | 1258 (98.8) | ||
| Missing | 0 | 1 | 0 | 12 | ||
| Gestational diabetes | 0.450† | 0.557† | ||||
| Yes | 1 (2) | 8 (5.4) | 1 (10) | 98 (7.8) | ||
| No | 54 (98) | 145 (94.6) | 9 (90) | 1162 (92.2) | ||
| Missing | 0 | 2 | 0 | 25 | ||
| Insurance/method of payment | 0.101 | 0.676† | ||||
| No Insurance | 3 (6) | 6 (4.1) | 0 (0) | 45 (3.5) | ||
| Any Public/private assistance | 32 (59) | 99 (64.1) | 6 (60) | 600 (46.8) | ||
| Veterans Affairs/commercial health insurance/health maintenance organization (HMO) | 19 (35) | 49 (31.8) | 4 (40) | 638 (49.7) | ||
| Missing | 1 | 1 | 0 | 2 | ||
| Maternal education | 0.545 | 0.470† | ||||
| 0-11 (none, primary, some secondary) | 12 (23) | 31 (21.9) | 3 (40) | 219 (17.9) | ||
| 12 (completed secondary) | 17 (33) | 53 (37.3) | 1 (10) | 299 (24.5) | ||
| 13+ (college) | 23 (44) | 58 (40.8) | 5 (50) | 702 (57.6) | ||
| Missing | 3 | 13 | 1 | 65 | ||
| Tobacco use | 0.176† | 0.481† | ||||
| None | 43 (82) | 125 (87.9) | 7 (90) | 1060 (86.6) | ||
| <10 cigarettes/day | 8 (15) | 9 (6.3) | 1 (10) | 79 (6.5) | ||
| 10+ cigarettes /day | 2 (3) | 8 (5.8) | 0 (0) | 84 (6.9) | ||
| Missing | 2 | 13 | 2 | 62 | ||
| Lifetime drug use | 0.494 | 0.530† | ||||
| Never used | 31 (60) | 94 (70.1) | 7 (90) | 840 (69.2) | ||
| Ever used, without addiction | 17 (32) | 36 (26.7) | 1 (10) | 350 (28.8) | ||
| Ever used, with addiction | 4 (8) | 4 (3.2) | 0 (0) | 24 (2) | ||
| Missing | 3 | 21 | 2 | 71 | ||
Nw represents weighted N, column percentages are displayed,
Fisher’s Exact Test
The predominant organisms responsible for probable and possible causes of infection-related death, stratified by timing of stillbirth were E. coli (19; 29%), group B streptococcus (8; 12%), and enterococcus (8; 12%). Most cases occurred prior to 24 weeks’ gestation (59%), with 11% at 24-28 weeks’, 5% at 29-31 weeks’, 12% at 32-36 weeks’, and 14% at 37-42 weeks’ (Table 3).
Table 3.
Organisms identified in infection-related stillbirths from fetal culture stratified by gestational age
| Variable | GA <24 | GA 24 0/7-28 6/7 | GA 29 0/7-31 6/7 | GA 32 0/7-36 6/7 | GA 37+ | Total |
|---|---|---|---|---|---|---|
| N (%) | 39 (59) | 7 (11) | 3 (5) | 8 (12) | 9 (14) | 66 (100) |
| Fetal culture results, N (%) | ||||||
| Positive culture | 22 (33) | 2 (3) | 0 | 5 (8) | 6 (9) | 35 (53) |
| No growth | 6 (9) | 1 (2) | 2 (3) | 2 (3) | 1 (1) | 12 (18) |
| No data | 11 (17) | 4 (6) | 1 (2) | 1 (1) | 2 (3) | 19 (29) |
| Organisms found, N (%)* | ||||||
| E. Coli | 13 (20) | 0 | 0 | 2 (3) | 4 (6) | 19 (29) |
| Group B Streptococcus | 5 (8) | 0 | 0 | 2 (3) | 1 (1) | 8 (12) |
| Enterococcus | 4 (6) | 2 (3) | 0 | 1 (1) | 1 (2) | 8 (12) |
| Bacillus | 2 (3) | 0 | 0 | 0 | 1 (2) | 3 (5) |
| Enterobacter | 1 (2) | 1 (1) | 0 | 0 | 0 | 2 (3) |
| Other | 12 (18) | 3 (5) | 0 | 2 (3) | 1 (1) | 18 (27) |
Organisms are not mutually exclusive, 16 cases (%) had multiple organisms present GA: gestational age (weeks)
The most useful tests were placental pathology and fetal autopsy with a pertinent positive result in 89% and 55% of cases respectively (Table 4). In the 66 cases, placental pathology revealed chorioamnionitis in 50 (76%), funisitis in 27 (41%), villitis in 11 (17%), deciduitis in 35 (53%), necrosis in 27 (41%), and viral staining or inclusion bodies in 7 (11%) cases. Overall, placental pathology found inflammation or evidence of infection in 65 (99%) cases. Fetal autopsy detected inflammation or evidence of infection in 26 (39%) cases.
Table 4.
Test utility in infection stillbirth cases
| Test Utility in Stillbirths with a Possible or Probable Infection Cause of Death | ||||
|---|---|---|---|---|
| Test | Total Tested N (% of total infection cases) | Positive Result N (% of cases tested) | Pertinent Positive Result* N (% of cases tested) | Normal Placental Pathology and Autopsy with Positive Screening Test N (% of cases tested) |
| Placental Pathology | 66 (100) | 63 (96) | 59 (89) | NA |
| Fetal Autopsy | 66 (100) | 36 (55) | 36 (55) | NA |
| Parvovirus Serology | 55 (83) | 5 (9) | 2 (4) | 3 (6) |
| Syphilis Testing | 65 (99) | 1 (2) | 1 (2) | 0 |
| Cytomegalovirus Serology | 11 (17) | 2 (18) | 2 (18) | 0 |
| Herpes Simplex Virus Serology | 16 (24) | 2 (13) | 1 (6) | 0 |
| Fetal Cultures | 47 (71) | 35 (75) | 25 (53) | 0 |
| Placental Cultures | 5 (8) | 5 (100) | 1 (20) | 0 |
A pertinent positive result is a test result which confirms a clinically suspected cause of death or one which identifies a potential cause of death. Reference 10 describes the methodology behind our assessment of test utility in stillbirth evaluation.
Serologic tests used as a “screen” for infection did not increase the diagnostic yield in any cases (Table 4). In infection-related stillbirths (n=66), five cases had a positive serologic result (positive IgM) for parvovirus. However, in three of these cases there was no evidence of parvovirus infection clinically or on placental pathology and fetal autopsy. Thus, parvovirus infection was not considered to be a probable or possible cause of death and the test was not “useful” in the diagnostic evaluation.
Fetal cultures (blood, lung) provided a pertinent positive result in 53% of cases tested with yield limited by contamination or lack of growth in many cases. Data regarding placental cultures were available for only a small subset of cases and may have been confounded by contamination. Accordingly, these results are not included in our analysis.
Of the overall SCRN cohort of 512 stillbirth cases, there were few cases due to “TORCH” infections, a historic acronym for toxoplasmosis, syphilis (other), rubella, CMV, and herpes. These infections were assessed in the context of results from serologic screening (Table 4), histopathology and clinical correlates of infection. CMV was noted on placental pathology or fetal autopsy in 6 cases, and in 5 of these cases infection was a probable or possible cause of death. Acute parvovirus was the probable or possible cause of death in 2 cases in which non-immune hydrops was present. Syphilis serology was found to be a probable cause of death in 1 case with spirochetes present on histologic examination.. Herpes was the probable cause of death in one case with positive immunohistochemical staining on placental pathology. No cases of pathogenic toxoplasmosis, varicella-zoster or rubella were found, although these were not systematically evaluated. Of the nine cases in which TORCH infection was a probable or possible cause of death, all had positive findings either on placental pathology (8 of 9 cases) or fetal autopsy (7 of 9 cases). Cases with positive serology in the absence of findings on autopsy or placental histology were not identified as probable or possible infection-related causes of death.
In our analysis of pathophysiologic pathways leading to infection-related stillbirth, we found 10 cases (15%) of direct fetal infection and 6 cases (9%) of direct fetal infection involving an organism associated with fetal malformations. Of these latter 6 cases, 4 were due to cytomegalovirus and 2 to parvovirus with fetal autopsy findings consistent with these infections. We identified 5 cases (8%) of placental infection leading to placental insufficiency including one case involving syphilis. There were no cases of severe maternal illness following extensive medical record abstraction. We found 31 cases (47%) that fit primarily into the preterm birth pathophysiologic pathway. All cases with the exception of one occurred at a previable gestational age. The remaining 14 cases (21%) had only placental inflammation without characteristics fitting the above groups.
DISCUSSION
Of the 12.9% of cases of stillbirth probably or possibly due to infection, a majority (53%) were associated with bacterial pathogens. The most common organisms were E. coli, group B streptococcus and enterococcus. There were eight viral infections (12%) including 5 with CMV, 2 with parvovirus B19, and one due to herpes virus as well as one case of syphilis.
In this study, parvovirus and syphilis were the only viruses and spirochetes that were systematically evaluated. Accordingly, we cannot make definitive conclusions regarding the prevalence of viral and spirochete pathogens in stillbirth cases overall. However, a positive viral serologic result in the absence of corresponding pathologic findings on fetal autopsy or placental histology is unlikely to represent a possible or probable cause of death. Therefore, our findings are likely accurate in regard to the relative paucity of known viral pathogens in comparison to bacterial organisms in infection-related stillbirth in the United States. Our data do not support the routine screening for “TORCH” infections with maternal serology in the absence of other evidence for an infectious cause of stillbirth.
All cases of stillbirth linked to bacterial infection had abnormal findings suggestive of infection on placental histology, autopsy or both. Our results suggest that fetal culture alone, in the absence of other findings suggestive of infection (clinical features, placental pathology, and fetal autopsy) are insufficient to support infection as a probable or possible cause of death. A suggested algorithm for the evaluation of infectious causes of stillbirth is shown in figure 2.
Figure 2.
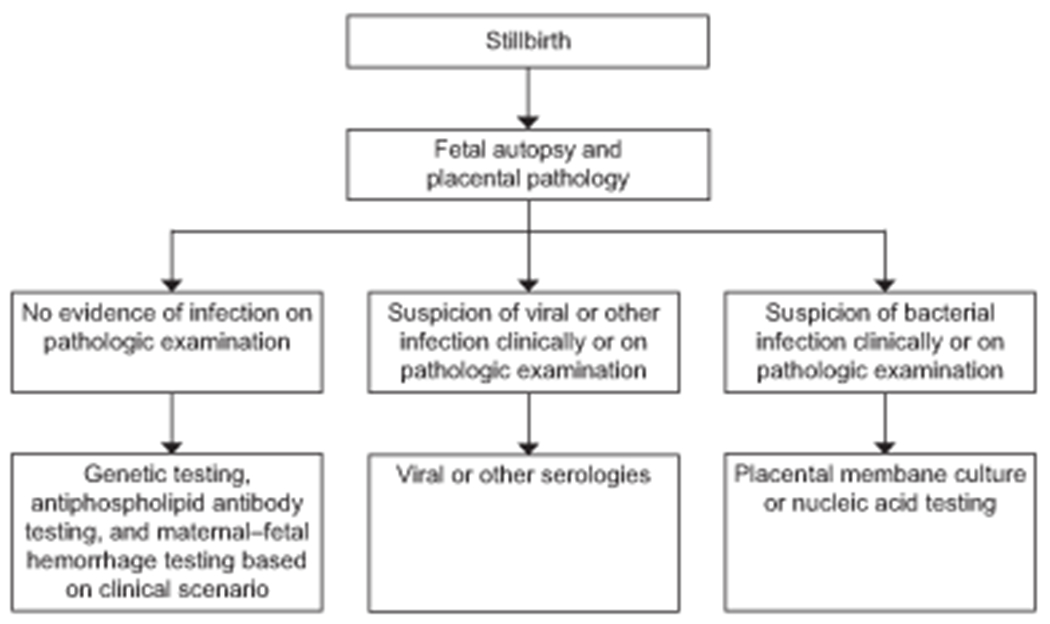
Algorithm for the evaluation of infection-related stillbirth.
Non-Hispanic black race was associated with infection-related stillbirth. In addition, stillbirth due to infection was more likely to occur at previable or periviable gestational ages and to be associated with spontaneous preterm birth. There is considerable overlap in the pathophysiology of spontaneous preterm birth and many stillbirths associated with infection. Typically, this occurs when spontaneous preterm labor occurs at a previable or periviable gestation. This preterm labor process often leads to an intrapartum stillbirth, which often would have been avoided with interventions such as cesarean delivery at a later gestational age. Thirty-one (47%) cases in this cohort were due to this pathophysiologic sequence.
We performed a literature search using the search terms “stillbirth” and “infection”. Existing data on infection stillbirth are comprised primarily of case series examining specific bacterial or viral pathogens with only a small number evaluating infection stillbirth in a prospective, comprehensive fashion. A study from Sweden noted positive cultures for E. coli, Enterococcus feacalis, and group B streptococcus (as well as a likely contaminant, Coagulase negative staphylococcus) in stillbirths.11 There also were a number of organisms with very small numbers of positive cultures. It is not clear that infection was the unequivocal cause of death in all of these cases.11 Several papers focused on group B streptococcus, since it causes serious neonatal morbidity and mortality in addition to stillbirth. A systematic review of 17 studies (most prior to 2000) noted a group B streptococcus (GBS) stillbirth rate of 0.04 – 0.9 per 1,000 births.12 The proportion of stillbirths associated with GBS was 0 – 12.1%.12 Another systematic review and meta-analysis of 14 studies including 5 after 2000 noted that 1% (95% CI, 0 – 2%) of stillbirths are associated with GBS in high income countries and 4% (95% CI, 2 – 6%) in Africa.13 Both studies acknowledge the low quality of available data.12,13
A lack of systematic cultures for infectious pathogens (including assessment of a panel of viral pathogens and identification of mycoplasmas and ureaplasmas) is a weakness of our study. It is possible that a more comprehensive approach could identify additional infectious causes of stillbirth. Also, molecular techniques such as PCR may identify additional causes of stillbirth. Strengths of the study include its prospective nature, unbiased enrollment, use of controls, racial, ethnic, and geographic diversity, extensive and systematic evaluation including autopsy and placental histology in all cases, and use of a rigorous tool to assign causes of death.
In conclusion, most cases of infection-related stillbirth based on traditional clinical and pathological criteria in a large and diverse U.S. cohort were associated with bacterial infections. The most common bacterial pathogens were E. coli, GBS and Enterococcus. We suggest that prevention efforts should target these organisms. To this end, the World Health Organization has research underway to develop a GBS vaccine which would have a major impact on stillbirths and neonatal complications due to GBS worldwide.14
Stillbirth due to infection was more common in non-Hispanic black women and shared pathophysiology with spontaneous preterm birth. Thus, efforts to reduce those infections may also reduce spontaneous preterm births as well as stillbirths. There were also several cases of CMV as well as a small number due to parvovirus and syphilis. Given these data, we do not recommend routine TORCH serology or cultures in the absence of clinical, placental or autopsy evidence of infection as part of the routine evaluation for stillbirth.
Supplementary Material
Acknowledgments
This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764).
This work, including the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review and approval of the manuscript, was supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U10-HD045953 Brown University, Rhode Island; U10-HD045925 Emory University, Georgia; U10-HD045952 University of Texas Medical Branch at Galveston, Texas; U10-HDO45955 University of Texas Health Sciences Center at San Antonio, Texas; U10-HD045944 University of Utah Health Sciences Center, Utah; and U01-HD045954 RTI International, RTP.
Financial Disclosure
Donald J Dudley received funds for serving on the British Journal of Obstetrics and Gynecology Editorial Board. Radek Bukowski is an advisor and holds stock in Savran Technologies Inc., a company that developed technology to isolate ultra-rare cells from blood for non-invasive diagnostics. The other authors did not report any potential conflicts of interest.
Footnotes
Presented in part at the SMFM 37th Annual Conference, January 29 – February 2, 2018, Dallas, TX.
Comments and views of the author(s) do not necessarily represent the views of the NICHD.
References
- 1.MacDorman MF, Gregory EC. Fetal and perinatal mortality: United States, 2013. Natl Vital Stat Rep 2015;64:1–24. [PubMed] [Google Scholar]
- 2.Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet 2010; 375: 1482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Stillbirth Collaborative Research Network Writing group. Causes of death among stillbirths. JAMA 2011;306:2459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker CB, Hogue CJR, Koch MA, Willinger M, Reddy U, Thorsten VR, et al. , for the Stillbirth Collaborative Research Network. Stillbirth Collaborative Research Network: Design, methods and recruitment experience. Paediatric and Perinatal Epidemiology 2011;25:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Shehata B, Thorsten VR, et al. The Stillbirth Collaborative Research Network (SCRN) Placental and Umbilical Cord Examination. Am J Perinatol 2011;28:781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Abramowsky CR, Thorsten V, et al. The stillbirth collaborative research network postmortem examination protocol. Am J Perinatol 2012; 29:187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Management of Stillbirth. ACOG Practice Bulletin No. 102. American College of Obstetricians and Gynecologists. Obstet Gynecol 2009;113:113:748–61. [DOI] [PubMed] [Google Scholar]
- 8.Stillbirth Collaborative Research Network Writing Group. Association between stillbirth and risk factors known at pregnancy confirmation. JAMA 2011;306:2469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley DJ, Goldenberg R, Conway D, Silver RM, Saade GR, Varner MW, et al. A new system for determining the causes of stillbirth. Obstet Gynecol 2010;116:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page JM, Christiansen-Lindquist L, Thorsten V, Parker CB, Reddy UM, Dudley DJ, et al. Diagnostic Tests for Evaluation of Stillbirth: Results From the Stillbirth Collaborative Research Network. Obstet Gynecol 2017. Apr;129(4):699–706. [DOI] [PubMed] [Google Scholar]
- 11.Tolockiene E, Morsing E, Holst E, Herbst A, Ljungh A, Svenningsen N, et al. Intrauterine infection may be a major cause of stillbirth in Sweden. Acta Obstet Gynecol Scand 2001;80:511–18. [PubMed] [Google Scholar]
- 12.Nan C, Dangor Z, Cutland CL, Edwards MS, Madhi SA, Cunnington MC. Maternal group B streptococcus-related stillbirth: a systematic review. BJOG 2015;122:1437–45. [DOI] [PubMed] [Google Scholar]
- 13.Seale AC, Blencowe H, Bianchi-Jassir F, Embleton N, Bassat Q, Ordi J, et al. Stillbirth with group B streptococcus disease worldwide: systematic review and meta-analysis. Clin Inf Dis 2017;65(S2):S125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group B Streptococcus Vaccine Development Technology Roadmap. Priority activities for development, testing, licensure and global availability of Group B streptococcus vaccines. Geneva: World Health Organization; 2017. License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


