Randomized controlled trials of monoclonal antibodies (mAbs) for the treatment of COVID-19 excluded pregnant persons. A retrospective, propensity score–matched, cohort study was conducted in a large health care system to examine the effect of several mAbs on hospitalization, obstetric-associated safety outcomes, and death during pregnancy.
Visual Abstract. Monoclonal Antibody Treatment in Pregnancy.
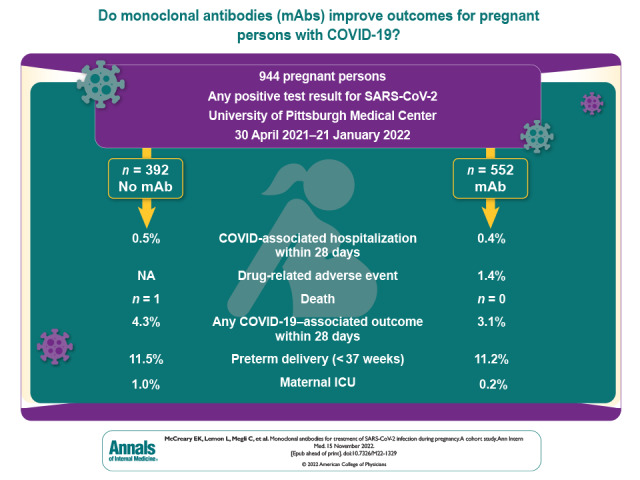
Randomized controlled trials of monoclonal antibodies (mAbs) for the treatment of COVID-19 excluded pregnant persons. A retrospective, propensity score–matched, cohort study was conducted in a large health care system to examine the effect of several mAbs on hospitalization, obstetric-associated safety outcomes, and death during pregnancy.
Abstract
Background:
Monoclonal antibody (mAb) treatment decreases hospitalization and death in high-risk outpatients with mild to moderate COVID-19. However, no studies have evaluated adverse events and effectiveness of mAbs in pregnant persons compared with no mAb treatment.
Objective:
To determine the frequency of drug-related adverse events and obstetric-associated safety outcomes after treatment with mAb compared with no mAb treatment of pregnant persons, and the association between mAb treatment and a composite of 28-day COVID-19–related hospital admission or emergency department (ED) visit, COVID-19–associated delivery, or mortality.
Design:
Retrospective, propensity score–matched, cohort study.
Setting:
UPMC Health System from 30 April 2021 to 21 January 2022.
Participants:
Persons aged 12 years or older with a pregnancy episode and any documented positive SARS-CoV-2 test (polymerase chain reaction or antigen test).
Intervention:
Bamlanivimab and etesevimab, casirivimab and imdevimab, or sotrovimab treatment compared with no mAb treatment.
Measurements:
Drug-related adverse events, obstetric-associated safety outcomes among persons who delivered, and a risk-adjusted composite of 28-day COVID-19–related hospital admission or ED visit, COVID-19–associated delivery, or mortality.
Results:
Among 944 pregnant persons (median age [interquartile range (IQR)], 30 years [26 to 33 years]; White (79.5%; n = 750); median Charlson Comorbidity Index score [IQR], 0 [0 to 0]), 552 received mAb treatment (58%). Median gestational age at COVID-19 diagnosis or treatment was 179 days (IQR, 123 to 227), and most persons received sotrovimab (69%; n = 382). Of those with known vaccination status, 392 (62%) were fully vaccinated. Drug-related adverse events were uncommon (n = 8; 1.4%), and there were no differences in any obstetric-associated outcome among 778 persons who delivered. In the total population, the risk ratio for mAb treatment of the composite 28-day COVID-19–associated outcome was 0.71 (95% CI, 0.37 to 1.4). The propensity score–matched risk ratio was 0.61 (95% CI, 0.34 to 1.1). There were no deaths among mAb-treated patients compared with 1 death in the nontreated control patients. There were more non-COVID-19–related hospital admissions in the mAb-treated persons in the unmatched cohort (14 [2.5%] vs. 2 [0.5%]; risk ratio, 5.0; 95% CI, 1.1 to 21.7); however, there was no difference in the propensity score–matched rates, which were 2.5% mAb-treated vs. 2% untreated (risk ratio, 1.3; 95% CI, 0.58% to 2.8%).
Limitations:
Drug-related adverse events were patient and provider reported and potentially underrepresented. Symptom severity at the time of SARS-CoV-2 testing was not available for nontreated patients.
Conclusion:
In pregnant persons with mild to moderate COVID-19, adverse events after mAb treatment were mild and rare. There was no difference in obstetric-associated safety outcomes between mAb treatment and no treatment among persons who delivered. There was no difference in 28-day COVID-19–associated outcomes and non-COVID-19–related hospital admissions for mAb treatment compared with no mAb treatment in a propensity score–matched cohort.
Primary Funding Source:
No funding was received for this study.
Monoclonal antibody (mAb) treatment is associated with decreased hospitalization and death in outpatients with mild to moderate COVID-19 (1–3). The Emergency Use Authorizations (EUAs) for these compounds were updated in May 2021 to list pregnancy as a risk factor for progression to severe disease, and several guidelines recommend use for pregnant persons with COVID-19 (3–6). Yet, no studies evaluate the effectiveness of mAbs targeting SARS-CoV-2 among pregnant persons, data are limited on the safety of mAbs in pregnancy, and immunoglobulin-based therapy remains controversial (7–13).
The purpose of this study is to estimate the rate of drug-related adverse events from mAb treatment among a cohort of pregnant persons with SARS-CoV-2 infection, the rate of obstetric-associated safety outcomes among all persons who delivered, and the risk-adjusted association between mAb treatment and composite 28-day COVID-19–related hospital admission or emergency department (ED) visit, COVID-19–associated delivery, or mortality compared with no mAb treatment.
Methods
This study was approved by the UPMC Quality Improvement Review Committee and the University of Pittsburgh Institutional Review Board. Methods and results are reported in accordance with the RECORD (Reporting of Studies Conducted Using Observational Routinely Collected Health Data) statement (14). The study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guideline (see the Supplement STROBE and Extended RECORD Checklist) (15).
This was a retrospective cohort study of mAb EUA-eligible persons (aged 12 years or older) with a pregnancy episode and any documented positive SARS-CoV-2 test (polymerase chain reaction or antigen test) in the UPMC Health System from 30 April 2021 to 21 January 2022. Patients treated with mAbs were compared with matched nontreated control patients. For the entire study period, any pregnant person was eligible for mAb per health system guidelines and could receive treatment as long as the person had a referral order placed by a prescriber and was still within the appropriate symptom onset time window for treatment (that is, 7 days or fewer).
Before 23 December 2021, all mAb-treated patients received a mAb product (for example, bamlanivimab–etesevimab, casirivimab–imdevimab, or sotrovimab) via a central management and inventory allocation system, which has been previously described (16–19). A small minority of patients received subcutaneous casirivimab and imdevimab to accommodate surging patient referrals and staffing shortages (20). From 23 December 2021 through 21 January 2022, all patients received intravenous sotrovimab due to the emergence of the Omicron variant. Starting 28 September 2021, pregnant patients and patients with immunocompromised conditions were given priority for mAb treatment due to drug scarcity. Patients reviewed the U.S. Food and Drug Administration (FDA) EUA Fact Sheet(s) and verbally consented to treatment with any available mAb before mAb administration.
Patients were considered mAb-treated if they received any mAb in an outpatient infusion center, urgent care facility, or obstetric triage area, and nontreated if they did not receive mAb. Patients were excluded if they had no previous care at UPMC in the preceding 12 months, received mAb for postexposure prophylaxis, or received mAb while at inpatient status or in a nonobstetric ED. The obstetric ED was used as an outpatient referral center during this time period and therefore the persons treated there were included in this analysis as their clinical status was deemed “outpatient” rather than “emergency department.” To account for immortal time bias between SARS-CoV-2 testing and mAb treatment, nontreated persons with a hospital admission within 1 day of their positive SARS-CoV-2 test result were excluded (more detail on the framework of analytic decisions is available in Supplement Table 1) (21). Both groups required 28-day follow-up. For nontreated control patients, the 28-day outcome ascertainment period started on the day after the SARS-CoV-2 test positive result. For treated patients, the 28-day outcome ascertainment period started on the day of mAb treatment.
Outcomes
The primary descriptive safety outcomes were the rates of drug-related adverse events reported by patients or providers at each treatment site among persons who received mAb, and obstetric-associated outcomes (that is, gestational age at delivery, birthweight, stillbirth, neonatal intensive care unit (NICU) admission, diagnosis of hypertension at time of delivery, severe maternal morbidity [defined in accordance with Centers for Disease Control and Prevention guidelines], and maternal ICU visit) for all persons who delivered in the study timeframe within the UPMC Health System (22). To determine mAb effectiveness, the primary outcome was the risk-adjusted association between mAb treatment and a composite of 28-day COVID-19–related hospital admission or ED visit, COVID-19–associated delivery, or mortality. A COVID-19–related hospital admission was defined as an antepartum admission for supportive oxygen or additional respiratory support. A COVID-19–associated delivery was identified as a delivery indicated secondary to COVID-19–related complications including maternal respiratory failure or fetal distress with evidence of pathognomonic SARS-CoV-2 placentitis (23–25). Secondary effectiveness outcomes included 28-day non-COVID-19–related admissions and rates of individual components of the composite outcome.
Data Sources and Definitions
Demographic, obstetric, and clinical data were abstracted from the UPMC Clinical Data Warehouse (CDW). The CDW stores all discrete data entered into each electronic medical record across the health system. Race was patient-identified and classified as Black, White, or Other (included Alaska Native, American Indian, Asian, Filipino, Indian, Native Hawaiian, or Pacific Islander). Dates entered in pregnancy episodes were used to calculate the estimated gestational age at the time of positive SARS-CoV-2 test or mAb treatment. Sociodemographic data, medical history, and administrative claims data for all outpatient and in-hospital encounters were collected with diagnoses and procedures coded based on the International Classification of Diseases, Ninth and Tenth revisions (ICD-9 and ICD-10, respectively) (26, 27).
Adverse events were defined as any reaction that occurred during the observation period after mAb injection or infusion (for example, rash, shortness of breath, and so forth) and were recorded by practitioners at each infusion center in a secure electronic file-sharing application. Nursing and physician staff also used an internal, nonpunitive, patient safety reporting system (Risk Master, UPMC and Computer Sciences Corporation) for adverse reactions and medication errors. Delivery outcomes were recorded in delivery forms in the medical record.
The primary effectiveness outcome was identified using hospitalizations and ED visits from the CDW. Hospital discharge disposition of “ceased to breathe” corresponded to in-hospital mortality, and deaths after discharge were identified with the Death Master File from the Social Security Administration (28). Detailed chart review was performed by a subspecialist in maternal–fetal medicine (C.M.) to stratify admission and delivery indication for the primary and secondary outcomes. The study team (C.M.) reviewed cases with estimated gestational age under 98 days at the time of event to identify early miscarriages. Vaccination status was confirmed by a clinical pharmacist (A.O.) using the electronic medical record, medical referral orders, and the Pennsylvania Statewide Immunization Information System (PA-SIIS).
Post hoc Exploratory Analyses
To confirm our findings, we conducted multivariable logistic regression conditionally in an inverse probability weighted sample based on the propensity score, adjusting for the propensity score. In the population with common support and inverse probability weights, logit models accounted for weighting to model the primary outcome and margins were used to determine the adjusted predicted risk and risk difference. Finally, COVID-associated visit was modeled using logit models adding the propensity score as an adjustment variable and calculating the associated risk (for details, see the Supplement).
To understand how treatment may be different by vaccination status, we stratified the population by unvaccinated versus fully vaccinated at time of event. Patients were classified as fully vaccinated when there was evidence in the electronic health record of at least 2 doses of an approved COVID-19 messenger RNA technology vaccine (for example, Pfizer, Moderna) or a single dose of an approved virus-based technology vaccine (for example, Johnson & Johnson). In these subpopulations, we first conducted a crude logistic regressions modeling likelihood of 28-day COVID-associated admission, then conditionally adjusted for the propensity scores. This was repeated exploring the secondary outcome of non-COVID-19 admission within 28 days of event. Results are again presented as predicted risk per 100 pregnant people, the risk difference between the treated and nontreated, and as adjusted risk ratios with 95% CIs. All analyses were performed in Stata IC (version 16 software package; StataCorp), and statistical significance corresponded to a P < 0.050.
Statistical Analyses
Baseline characteristics and adverse events were compared by treatment status, visualizing the standardized mean differences as appropriate. To understand the risk-adjusted association between treatment and outcome, we evaluated the association conditionally in a propensity score–matched sample. Results are presented as the risk difference between the treated and nontreated and as adjusted risk ratios with 95% CIs.
Propensity scores were generated using multivariate logistic regression modeling the likelihood of receiving mAb treatment. Variables included in the model were decided a priori and based on clinical judgment and were captured at the time of test or mAb administration to mimic baseline characteristics in a clinical trial. These covariates include age, race, estimated gestational age, and vaccination status at time of event, month and year of event, insurance type (commercial, public, self-pay), area deprivation index score, parity, gravidity, diagnosis of preexisting hypertension, chronic hypertension, pregestational diabetes, gestational diabetes, asthma, rheumatoid arthritis, infertility, anxiety, depression, chronic heart failure, irritable bowel syndrome or disorder, hyperlipidemia, endometriosis, history of deep venous thrombosis or venous thromboembolism, use of corticosteroids, use of 17-alpha-hydroxyprogesterone caproate, history of chemotherapy, tobacco use, alcohol use, illicit drug use, Charlson Comorbidity Index, body mass index, and blood pressures at the closest office visit within 1 year prior, and indicators for missingness of any data. The timing of the positive SARS-CoV-2 result or treatment date was included in the propensity score model to account for unmeasured differences in variant waves. Missing data were replaced with the respective group's median value, and indicators of missingness were included in the propensity score.
Propensity score distributions were assessed to ensure balance across treatment groups. Optimal propensity score matching allowing replacement was used to estimate the average treatment effect. Because an untreated patient could match to several treated patients, appropriate frequency weights were generated. Covariates were compared before and after matching to ensure that the model balanced the covariates and that all standardized differences were not greater than 10% postmatching. In the propensity score–matched cohort, logit models and adjusted predictive margins were used to display the adjusted risk per 100 pregnant persons.
Role of the Funding Source
Some of the sotrovimab used in this study was donated by GlaxoSmithKline/Vir Biotechnology. The study received no other funding.
Results
Study Population
Of 1140 pregnant persons with a positive SARS-CoV-2 test, 944 (83%) were included in the cohort, among whom 552 (58%) were treated with mAb. Patients were primarily excluded if they received mAb treatment in a nonobstetric ED (n = 86; 7.5%), had an inpatient admission (n = 44; 3.8%), or were hospitalized within 24 hours of positive test (n = 56; 4.9%). Most patients were young (median age, 30 years [interquartile range (IQR), 26 to 33 years]), were White (79.5%; n = 750), and had few comorbidities (median Charlson Comorbidity Index, 0 [IQR, 0 to 0]). Of those with known vaccination status, 392 (62%) were fully vaccinated. The mAb-treated patients were older (median age, 30.0 vs. 28.9 years; standardized mean difference [SMD], −0.23), were more likely to have commercial insurance (68.3% vs. 55.1%; SMD, 0.27), and had a lower median area of deprivation index score (64 [IQR, 44 to 82] vs. 71 [IQR, 54 to 88]; SMD, 0.30). The mAb-treated patients were more likely to have a history of infertility (12.3% vs. 6.6%; SMD, 0.20) and to be fully vaccinated (48.0% vs. 32.4%; SMD, 0.56). The median gestational age at time of COVID-19 diagnosis or treatment was 179 days (IQR, 122.5 to 226.5 days) (Supplement Figure 1). Among treated persons, most (58%; n = 320) received mAb within 4 days of symptom onset. The most common mAb was sotrovimab (69%; n = 382) compared with casirivimab and imdevimab (20%; n = 110) and bamlanivimab and etesevimab (11%; n = 60). The mean time from SARS-CoV-2 test result to mAb treatment (SD) was 1 day (SD, 1.5) (Table 1). Five patients received federally donated sotrovimab; the rest of the population received company-donated sotrovimab.
Table 1.
Comparison of Characteristics of Pregnant mAb-Treated Versus Nontreated Persons

Adverse Events From mAb Treatment and Obstetric-Associated Safety Outcomes
Drug-related adverse events were mild and occurred in 8 patients treated with mAb (1.4%). No patients experienced a severe infusion-related reaction. Among the 778 persons who delivered in the follow-up period, there were no differences between groups for gestational age at delivery (273 days [IQR, 263 to 277 days] vs. 273 days [IQR, 266 to 277 days]; treated vs. untreated), birthweight (3290 g [IQR, 2930 to 3660 g] vs. 3240 g [IQR, 2924 to 3620 g]; treated vs. untreated), NICU admission, stillbirths, severe maternal morbidity, hypertension at delivery, or maternal ICU admission (Table 2).
Table 2.
Safety of mAb Treatment in Pregnant Persons: Deliveries Through 7 July 2022

Association of mAb Treatment With Outcome
In the propensity score–matched cohort, clinical characteristics were similar between groups (Supplement Figure 2). Using replacement and appropriate weighting, 223 untreated patients were matched to all 552 treated pregnant persons. Before matching, more patients were treated in the later months of the study period. However, testing and treatment by month were similar after matching (Supplement Figure 3). In the primary analysis in the propensity-matched cohort, the composite 28-day risk-adjusted frequency of a COVID-19–associated outcome was 3.1 per 100 persons (95% CI, 1.6 to 4.5) in mAb-treated compared with 5.1 per 100 persons (95% CI, 3.2 to 6.9) in nontreated control patients (risk difference, −2.0 per 100 persons [95% CI, −4.3 to 0.33]; risk ratio, 0.61 [95% CI, 0.34 to 1.1]). The adjusted risk ratio was near 1.0 in both the propensity score–inverse probability weighted (risk ratio, 0.97 [95% CI, 0.49 to 1.9]) and propensity score–adjusted models (risk ratio, 0.96 [95% CI, 0.48 to 1.9]) comparing mAb treated with untreated persons (Supplement Table 2). In the total cohort, 3 patients had propensity scores not in common support.
The individual components of the composite outcome were similar between groups (Table 3). There were no deaths among mAb-treated patients compared with 1 death in the nontreated control patients. There were more non-COVID-19–related hospital admissions in the mAb-treated patients in the unmatched total population (14 [2.5%] vs. 2 [0.5%]; risk ratio, 5.0 [95% CI, 1.1 to 21.7]). The risk ratio in the propensity-matched cohort was 1.3 (95% CI, 0.58 to 2.8), and the risk difference in the propensity-adjusted cohort was 2.2 per 100 people (95% CI, 0.59 to 3.8) (Table 3 and Supplement Table 2). Common indications for non-COVID-19 admissions were pyelonephritis (n = 3), preterm contractions (n = 2), and intrahepatic cholestasis of pregnancy (n = 2).
Table 3.
Detailed Outcomes Within 28 Days of Test or Treatment in Pregnant mAb-Treated Versus Nontreated Patients

In post hoc exploratory analyses among the subset of vaccinated patients (n = 392), the composite 28-day propensity-adjusted, risk-adjusted frequency of a COVID-19–associated outcome was 2.6 per 100 persons (95% CI, 0.53 to 4.7) in mAb-treated compared with 0.63 per 100 persons (95% CI, −0.61 to 1.9) in nontreated control patients (risk difference, 2 per 100 persons [95% CI, −0.47 to 4.4]; Supplement Table 3, Supplement Figures 4 and 5). For unvaccinated patients (n = 178), the composite 28-day propensity-adjusted, risk-adjusted frequency of a COVID-19–associated outcome was 4.2 per 100 persons (95% CI, 0.61 to 7.8) in mAb-treated compared with 10.2 per 100 persons (95% CI, 2.5 to 18) in nontreated control patients (risk difference, −6 per 100 persons [95% CI, −14.5 to 2.5]). Propensity scores for treatment, stratified by mAb, are available in Supplement Figure 6. The ICD coding documented during delivery admission is available in Supplement Table 4.
Discussion
In a cohort study of 944 pregnant persons, drug-related adverse events after mAb treatment were rare and there was no difference in obstetric-associated outcomes among persons who delivered. The risk-adjusted frequency of 28-day COVID-19–associated outcomes for mAb treatment compared with no mAb treatment was similar and there was no difference in non-COVID-19–related hospital admissions in the propensity-matched cohort.
Prior work reported on the use of mAbs in pregnant persons with COVID-19 in small case series (29, 30). These data demonstrate no serious treatment-related adverse events. We expand on this work with the largest safety and effectiveness evaluation of mAb treatment compared with nontreatment in pregnant persons to our knowledge, including both a description of clinically adjudicated adverse events and COVID-19–related outcomes. Importantly, mAb treatment seems safe in pregnancy with respect to drug-related adverse events and obstetric-associated outcomes (that is, gestational age at delivery, birthweight, stillbirth, NICU admission, hypertension, severe maternal morbidity, and maternal ICU visit).
Although COVID-19–associated outcomes were similar between groups, the event frequency for hospitalization and death were lower than previously reported frequencies for the general population for both mAb-treated and nontreated groups (31–33). The low event frequency may reflect the younger population with few comorbidities and inclusion of fully vaccinated patients in the cohort. The neutral finding from effectiveness models may be due to the sample size of the cohort or the absence of a true treatment effect. Our study cannot evaluate whether mAbs are associated with a difference in risk-adjusted COVID-19–related outcomes for pregnant persons at higher risk for COVID-19–related complications, such as those with several comorbidities, other immunocompromising conditions, and/or unvaccinated status.
The mAb-treated patients experienced more non-COVID-19 admissions compared with patients who did not receive mAb treatment in the unmatched cohort. We cannot determine whether these admissions were related to mAb treatment or not. A time-to-event analysis revealed only 2 admissions within the first week of receiving mAb: 1 seizure in a patient with known epilepsy and 1 episode of hyperglycemia (Supplement Figure 7). The absolute difference in non-COVID hospitalizations (14 events vs. 2 events) suggests that unmeasured differences may be present between groups unrelated to mAb treatment; indeed, there was no significant difference in events in the propensity-matched cohort. The mAb-treated patients had higher rates of vaccination, receipt of fertility care, and more commercial insurance, and therefore may be more likely to access health care for a non-COVID-19 reason compared with patients who did not receive mAb. Monitoring bias, when providers followed patients who received mAb more closely than those who did not receive mAb, may also have contributed to the finding. Importantly, this study did not show a difference in hypertension-related preterm deliveries or severe maternal morbidity.
Recent clinical practice guidelines from the National Institutes of Health, Centers for Disease Control and Prevention, American College of Obstetricians and Gynecologists, and Society for Maternal-Fetal Medicine recognize pregnant persons as higher risk for severe disease from SARS-CoV-2 infection (34–36). Severe disease is most common in pregnant people aged 35 to 44 years compared with younger patients, and COVID-19 can increase risk for preterm birth or stillbirth amongst all pregnant people (37, 38). However, these data derive from the Alpha and Delta variant eras. More recent data suggest that those with mild to moderate disease do not have increased rates of adverse neonatal outcomes, and that Omicron-infected persons experience less severe disease than persons infected with previous variants (39, 40). In the meantime, current guidelines recommend against withholding treatment of COVID-19, including mAb, from pregnant or lactating persons because of theoretical safety concerns (39). In the context of this study, pregnant persons with minimal comorbidities and low risk for severe disease in the Omicron variant era may not benefit from treatment with the routine use of mAbs. However, it is unknown whether mAbs would benefit (or harm) pregnant persons with additional risk factors for severe disease, and whether different mAbs are variably effective against different SARS-CoV-2 variants in pregnant persons.
The study has several limitations. First, drug-related adverse events were patient and provider reported as a part of routine care and potentially underrepresented. Second, symptom severity at the time of testing and treatment (whether symptomatic or asymptomatic) was not available for nontreated patients. However, the average time from SARS-CoV-2 test result to treatment was 1 day, suggesting that immortal time bias between mAb-treated and nontreated is very unlikely. Third, vaccine status data are incomplete due to patients receiving vaccines outside of the health system or state, and the inability to document whether a patient is truly unvaccinated or if data are simply missing. Fourth, fully vaccinated was defined as having at least 2 messenger RNA vaccines or a single dose of an approved virus-based technology vaccine, which is inconsistent with updated vaccine schedules. Fifth, as with any observational study, these findings do not provide causal inference, as many unmeasured confounders may be present. We used several modeling approaches and found consistent results. Sixth, most patients in the cohort received sotrovimab when the Omicron variant was dominant in our geographic region, due to a large increase in SARS-CoV-2–positive cases during that time and pregnancy being added to the EUA as a risk factor, improving patient and provider acceptance of mAb therapy. These data may not be generalizable to other variants, regions, or time periods.
In conclusion, in pregnant persons with mild to moderate COVID-19, adverse events after mAb treatment were mild and rare. There was no difference in obstetric-associated safety outcomes between mAb treatment and no treatment among persons who delivered. There was no significant difference in 28-day COVID-19–associated outcomes and non-COVID-19–related hospital admissions for mAb treatment compared with no mAb treatment in a propensity score–matched cohort.
Supplementary Material
Appendix: UPMC Magee Monoclonal Antibody Treatment Group Members
All authors in this group have made contributions to the conception, design, or analysis of the work; have revised the work critically for important intellectual content; have given final approval of the version to be published; and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This group consists of the following individuals who contributed to this work but did not author it: Rich Beigi, Maribeth McLaughlin, Hyagriv Simhan, Harold Wiesenfeld, Scarlet Lau, Michael Haley, Sandy Trizzino, Ashley Steiner, Lauren Wiser, Michelle Adam, Tina Borneman, David T. Huang, Richard J. Wadas, Russell Meyers, J. Ryan Bariola, Mark Schmidhofer, Graham Snyder, Donald M. Yealy, Derek C. Angus, Tami Minnier, Judith A. Shovel, Debbie Albin, Oscar C. Marroquin, Kevin Collins, Adam King, Kevin E. Kip, Mary Kay Wisniewski, Colleen Sullivan, Meredith Axe, William Garrard, Stephanie Montgomery, Ghady Haidar, Paula L. Kip, Rachel L. Zapf, Sharen Ziska, Jessica Shirley, and Rebecca Medva.
Footnotes
This article was published at Annals.org on 15 November 2022.
* For members of the UPMC Magee Monoclonal Antibody Treatment Group, see the Appendix.
References
- 1. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med. 2021;385:e81. [PMID: ] doi: 10.1056/NEJMoa2108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bariola JR, McCreary EK, Wadas RJ, et al. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis. 2021;8:ofab254. [PMID: ] doi: 10.1093/ofid/ofab254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dougan M, Nirula A, Azizad M, et al; BLAZE-1 Investigators. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med. 2021;385:1382-92. [PMID: ] doi: 10.1056/NEJMoa2102685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817-26. [PMID: ] doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Regeneron Pharmaceuticals. Fact Sheet For Health Care Providers Emergency Use Authorization (Eua) Of Regen-Cov®. Accessed at www.regeneron.com/downloads/treatment-covid19-eua-fact-sheet-for-hcp.pdf?msclkid=d381de7caaa411eca33873cac3ce1dec on 18 August 2022.
- 6. Eli Lilly. Fact Sheet For Health Care Providers. Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. Accessed at https://pi.lilly.com/eua/bam-and-ete-eua-factsheet-hcp.pdf?msclkid=dd47bf39aaa411ecaf236f035e0670b2 on 18 August 2022.
- 7. Chang MH, Cowman K, Guo Y, et al. A real-world assessment of tolerability and treatment outcomes of COVID-19 monoclonal antibodies administered in pregnancy [Letter]. Am J Obstet Gynecol. 2022;226:743-5. [PMID: ] doi: 10.1016/j.ajog.2022.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayer C, VanHise K, Caskey R, et al. Monoclonal antibodies casirivimab and imdevimab in pregnancy for coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021;138:937-9. [PMID: ] doi: 10.1097/AOG.0000000000004603 [DOI] [PubMed] [Google Scholar]
- 9. Hirshberg JS, Cooke E, Oakes MC, et al. Monoclonal antibody treatment of symptomatic COVID-19 in pregnancy: initial report [Letter]. Am J Obstet Gynecol. 2021;225:688-9. [PMID: ] doi: 10.1016/j.ajog.2021.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thilagar BP, Ghosh AK, Nguyen J, et al. Anti-spike monoclonal antibody therapy in pregnant women with mild-to-moderate coronavirus disease 2019 (COVID-19) [Letter]. Obstet Gynecol. 2022;139:616-8. [PMID: ] doi: 10.1097/AOG.0000000000004700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manciulli T, Modi G, Campolmi I, et al. Treatment with anti-SARS-CoV-2 monoclonal antibodies in pregnant and postpartum women: first experiences in Florence, Italy. Infection. 2022;50:1139-1145. [PMID: ] doi: 10.1007/s15010-022-01777-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hyrich KL, Verstappen SM. Biologic therapies and pregnancy: the story so far. Rheumatology (Oxford). 2014;53:1377-85. [PMID: ] doi: 10.1093/rheumatology/ket409 [DOI] [PubMed] [Google Scholar]
- 13. Palmeira P, Quinello C, Silveira-Lessa AL, et al. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. [PMID: ] doi: 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benchimol EI, Smeeth L, Guttmann A, et al; RECORD Working Committee. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12:e1001885. [PMID: ] doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vandenbroucke JP, von Elm E, Altman DG, et al; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163-94. [PMID: ] doi: 10.7326/0003-4819-147-8-200710160-00010-w1 [DOI] [PubMed] [Google Scholar]
- 16. McCreary EK, Bariola JR, Minnier T, et al. Launching a comparative effectiveness adaptive platform trial of monoclonal antibodies for COVID-19 in 21 days. Contemp Clin Trials. 2022;113:106652. [PMID: ] doi: 10.1016/j.cct.2021.106652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woltemate TJ, Wadas RJ, McCreary EK, et al. Emergency department implementation of monoclonal antibody infusion for the treatment of coronavirus disease 2019: a template for rapid deployment. J Am Coll Emerg Physicians Open. 2021;2:e12550. [PMID: ] doi: 10.1002/emp2.12550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bariola JR, McCreary EK, Khadem T, et al. Establishing a distribution network for COVID-19 monoclonal antibody therapy across a large health system during a global pandemic. Open Forum Infect Dis. 2021;8:ofab151. [PMID: ] doi: 10.1093/ofid/ofab151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang DT, McCreary EK, Bariola JR, et al. The UPMC OPTIMISE-C19 (OPtimizing Treatment and Impact of Monoclonal antIbodieS through Evaluation for COVID-19) trial: a structured summary of a study protocol for an open-label, pragmatic, comparative effectiveness platform trial with response-adaptive randomization [Letter]. Trials. 2021;22:363. [PMID: ] doi: 10.1186/s13063-021-05316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCreary EK, Bariola JR, Wadas RJ, et al. Association of subcutaneous or intravenous administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in adults with COVID-19. JAMA Netw Open. 2022;5:e226920. [PMID: ] doi: 10.1001/jamanetworkopen.2022.6920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492-9. [PMID: ] doi: 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. How Does CDC Identify Severe Maternal Morbidity? Accessed at www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm on 18 August 2022.
- 23. Huynh A, Sehn JK, Goldfarb IT, et al. SARS-CoV-2 placentitis and intraparenchymal thrombohematomas among COVID-19 infections in pregnancy. JAMA Netw Open. 2022;5:e225345. [PMID: ] doi: 10.1001/jamanetworkopen.2022.5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwartz DA, Avvad-Portari E, Babál P, et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury. Arch Pathol Lab Med. 2022;146:660-76. [PMID: ] doi: 10.5858/arpa.2022-0029-SA [DOI] [PubMed] [Google Scholar]
- 25. Stenton S, McPartland J, Shukla R, et al. SARS-COV2 placentitis and pregnancy outcome: a multicentre experience during the Alpha and early Delta waves of coronavirus pandemic in England. EClinicalMedicine. 2022;47:101389. [PMID: ] doi: 10.1016/j.eclinm.2022.101389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). 2011.
- 27. Centers for Disease Control and Prevention. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). 2021.
- 28. Social Security Administration. Social Security master file of Social Security number holders and applications: death information. 13 October 2021. Accessed at www.ssa.gov/dataexchange/request_dmf.html on 18 August 2022.
- 29. Richley M, Rao RR, Afshar Y, et al. Neutralizing monoclonal antibodies for coronavirus disease 2019 (COVID-19) in pregnancy: a case series. Obstet Gynecol. 2022;139:368-72. [PMID: ] doi: 10.1097/AOG.0000000000004689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levey NH, Forrest AD, Spielman DW, et al. Outcomes of pregnant patients treated with REGEN-COV during the COVID-19 pandemic. Am J Obstet Gynecol MFM. 2022;4:100673. [PMID: ] doi: 10.1016/j.ajogmf.2022.100673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang DT, McCreary EK, Bariola JR, et al. Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 delta variant surge: a cohort study and randomized comparative effectiveness trial. JAMA Netw Open. 2022;5:e2220957. [PMID: ] doi: 10.1001/jamanetworkopen.2022.20957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCreary EK, Bariola JR, Minnier TE, et al. The comparative effectiveness of COVID-19 monoclonal antibodies: a learning health system randomized clinical trial. Contemp Clin Trials. 2022;119:106822. [PMID: ] doi: 10.1016/j.cct.2022.106822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCreary EK, Bariola JR, Wadas RJ, et al. Association of subcutaneous or intravenous administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in adults with COVID-19. JAMA Netw Open. 2022;5:e226920. [PMID: ] doi: 10.1001/jamanetworkopen.2022.6920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. The American College of Obstetricians and Gynecologists. Supporting You and Your Patients During COVID-19. Accessed at www.acog.org/covid-19 on 18 August 2022.
- 35. National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Accessed at www.covid19treatmentguidelines.nih.gov on 18 August 2022. [PubMed]
- 36. Centers for Disease Control and Prevention. Pregnant and Recently Pregnant People, At Increased Risk for Severe Illness from COVID-19. Accessed at www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnant-people.html on 18 August 2022.
- 37. Metz TD, Clifton RG, Hughes BL, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748-59. [PMID: ] doi: 10.1001/jama.2022.1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1640-5. [PMID: ] doi: 10.15585/mmwr.mm7047e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. National Institutes of Health. COVID-19 Treatment Guidelines. Special Considerations in Pregnancy. Accessed at www.covid19treatmentguidelines.nih.gov/special-populations/pregnancy on 18 August 2022.
- 40. Metz TD, Clifton RG, Hughes BL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021;137:571-80. [PMID: ] doi: 10.1097/AOG.0000000000004339 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


