Abstract
STUDY QUESTION
Can human umbilical cord platelet-rich plasma (hUC-PRP) efficiently treat endometrial damage and restore fertility in a preclinical murine model?
SUMMARY ANSWER
Local application of hUC-PRP promotes tissue regeneration and fertility restoration in a murine model of Asherman syndrome and endometrial atrophy (AS/EA).
WHAT IS KNOWN ALREADY
AS/EA are well-described endometrial pathologies that cause infertility; however, there are currently no gold-standard treatments available. Recent reports have described the successful use of human platelet-rich plasma in reproductive medicine, and its regenerative potential is further enhanced using hUC-PRP, due to the ample growth factors and reduced pro-inflammatory cytokines in the latter.
STUDY DESIGN, SIZE, DURATION
hUC-PRP (n = 3) was processed, characterized and delivered locally to endometrial damage in a murine model (n = 50). The hUC-PRP was either used alone or loaded into a decellularized porcine endometrium-derived extracellular matrix (EndoECM) hydrogel; endometrial regeneration, fertility outcomes and immunocompatibility were evaluated 2 weeks following treatment administration.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Umbilical cord blood was obtained from women in childbirth. Endometrial damage (mimicking AS/EA) was induced using ethanol in 8-week-old C57BL/6 mice, and treated with the most concentrated hUC-PRP sample 4 days later. Characterization of hUC-PRP and immunotolerance was carried out with multiplex technology, while uterine samples were analyzed by immunohistochemistry and quantitative PCR. The number of embryos and their morphology was determined visually.
MAIN RESULTS AND THE ROLE OF CHANCE
Platelet density was enhanced 3-fold in hUC-PRP compared to that in hUC blood (P < 0.05). hUC-PRP was enriched with growth factors related to tissue regeneration (i.e. hepatocyte growth factor, platelet-derived growth factor-BB and epidermal growth factor), which were released constantly (in vitro) when hUC-PRP was loaded into EndoECM. Both treatments (hUC-PRP alone and hUC-PRP with EndoECM) were immunotolerated and caused significantly regeneration of the damaged endometrium, evidenced by increased endometrial area, neoangiogenesis, cell proliferation and gland density and lower collagen deposition with respect to non-treated uterine horns (P < 0.05). Additionally, we detected augmented gene expression of Akt1, VEGF and Ang, which are involved in regenerative and proliferation pathways. Finally, hUC-PRP treatment restored pregnancy rates in the mouse model.
LARGE SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
This proof-of-concept pilot study was based on a murine model of endometrial damage and the use of EndoECM requires further validation prior to clinical implementation for women affected by AS/EA.
WIDER IMPLICATIONS OF THE FINDINGS
The local administration of hUC-PRP has high impact and is immunotolerated in a murine model of AS/EA, as has been reported in other tissues, making it a promising candidate for heterologous treatment of these endometrial pathologies.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the Ministerio de Ciencia, Innovación y Universidades; Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital, Generalitat Valenciana; and Instituto de Salud Carlos III. The authors do not have any conflicts of interest to declare.
Keywords: endometrium, human umbilical cord, platelet-rich plasma, extracellular matrix hydrogel, regenerative medicine
WHAT DOES THIS MEAN FOR PATIENTS?
This study evaluated whether the platelet-rich plasma derived from the human umbilical cord blood (hUC-PRP) can be used to treat two disorders of the uterus (Asherman’s syndrome and endometrial atrophy) that cause infertility. Mouse models of these disorders confirmed the beneficial effects of hUC-PRP treatment, which produces enhanced regenerative factors. Specifically, we observed no apparent harm in treated animals and an improvement in their endometrial health and their achievement of pregnancy. Finally, hUC-PRP can be easily preserved, is independent of the patient’s comorbidities, and is microbiologically safe. Overall, we have confirmed that hUC-PRP can heal the uterus and propose this treatment as a potential alternative for infertile patients affected by these endometrial disorders.
Introduction
The uterus, the largest organ within the female reproductive system, is composed of an external serous membrane (termed the perimetrium), a thick middle muscular (termed the myometrium) and an inner mucous layer (termed the endometrium) (Simón et al., 2009). The human endometrium is a highly dynamic tissue, which repeatedly undergoes a cycle of proliferation, differentiation and renewal, approximately every 28 days, in preparation for a potential blastocyst implantation and subsequent pregnancy. Key components of these processes include the endogenous epithelial and stromal stem cells, endometrial microvessels and other exogenous factors (including hormones, bone marrow cells and paracrine factors) (de Miguel-Gómez et al., 2021b). While endometrial renewal is usually efficient, pathologies or disorders, such as Asherman’s syndrome or endometrial atrophy (EA), may result in recurrent pregnancy loss or infertility (Galliano et al., 2015). Asherman syndrome (AS) is a rare disease characterized by the presence of intrauterine adhesions and fibrosis inside the uterine cavity and/or endocervix (Yu et al., 2008) caused by iatrogenic trauma to the endometrium, Müllerian duct malformation, uterine artery embolization or even the insertion of intrauterine devices (Conforti et al., 2013). EA, also known as thin/refractory endometrium, is characterized by an inadequate endometrial growth and poor vascularity (Mahajan and Sharma, 2016) resulting from iatrogenic causes (i.e. repeated or vigorous curettages or myomectomies, or indiscriminate use of drugs such as clomiphene citrate) or intrinsic factors (e.g. inflammation elicited by acute or chronic infections).
There currently exists a discord regarding the gold-standard treatment of these endometrial pathologies. Cell therapies based on human bone marrow-derived stem cells (Singh et al., 2014; Santamaria et al., 2016), umbilical cord-derived mesenchymal stem cells (Cao et al., 2018) or menstrual-derived mesenchymal stem cells (Tan et al., 2016; Hu et al., 2019; Ma et al., 2020) have been proposed as promising treatments for AS/EA management. However, the current clinical strategies involving these biological products may be invasive or have a low engraftment (Terrovitis et al., 2010; Von Bahr et al., 2012; Gharibeh et al., 2022) and have thus encouraged the search for alternative non-invasive treatments.
Based on the premise that the biomolecules secreted by stem cells are sufficient to activate tissue regeneration, especially in the endometrium (de Miguel-Gómez et al., 2020), human platelet-rich plasma (hPRP) has gained substantial importance in regenerative medicine within the fields of in dentistry (Sachdeva et al., 2015; Fan et al., 2020; Xu et al., 2020), dermatology (Giordano et al., 2017; Elghblawi, 2018; Moneib et al., 2018), orthopedics (Memeo et al., 2014; Le et al., 2019; Berney et al., 2020), neurology (Malahias et al., 2018) and gynecology, which includes the management of vaginal (Kim et al., 2017), ovarian (Cakiroglu et al., 2022) and uterine disorders (Chrysanthopoulou et al., 2017; Turan et al., 2018; Sfakianoudis et al., 2019; Kim et al., 2019a). Applications in the endometrium (Chang et al., 2015; Zadehmodarres et al., 2017; Kim et al., 2019a) have mostly been positive; however, recent controversial results have been reported in terms of fertility restoration (Javaheri et al., 2020; Lin et al., 2021).
Notably, hPRP is a fraction of blood, easily obtained via gradient density centrifugation, and distinguished for clinically providing supra-physiologic platelet concentrations. Although precise concentrations have not yet been established, some authors consider PRP to simply have more platelets than normal (Saiz et al., 2020), while others claim a minimum of 800 000 (Mazzotta et al., 2022) or 1 000 000 platelets/ml (Rebulla et al., 2016) are sufficient to provide a regenerative effect, and another group argued that excessive platelet concentrations may decrease the effectiveness of the treatment (Gentile and Garcovich, 2020). Nevertheless, there is consensus that hPRP can be considered a safe and effective treatment that promotes tissue repair. Once activated, the integrity of the plasma membrane of platelets is compromised, releasing the various growth factors (e.g. platelet-derived growth factor-BB (PDGF-BB), epidermal growth factor (EGF), hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF)) contained within their α-granules into the surroundings (Cecerska-Heryć et al., 2022). Although the main advantage of autologous hPRP therapy is the immunotolerant nature of its components, its quality can vary due to patient age and/or comorbidities, and different processing protocols (Le et al., 2019). Further, the large volumes of blood required to prepare sufficient hPRP may be detrimental to a patient’s hemodynamic stability (Tadini et al., 2015a). On the other hand, since human umbilical cord platelet-rich plasma (hUC-PRP) is derived from women of reproductive age, it becomes a reliable, microbiologically safe and consistent therapeutic option, avoiding extra health burdens to patients (Caiaffa et al., 2021). In this regard, the optimal impact of hPRP therapy is achievable when the plasma is obtained from younger women, and blood sample processing is commercially standardized (Castellano et al., 2017). Indeed, human umbilical cord (hUC) plasma releases more growth factors and less pro-inflammatory cytokines, compared to adult plasma in vitro (Parazzi et al., 2010; Ehrhart et al., 2018), and can restore ovarian function in mice (Buigues et al., 2021). Moreover, our group previously reported the efficacy of commercial hUC plasma in a murine model of endometrial damage (de Miguel-Gómez et al., 2021a). Although hUC-PRP was proposed as a supplement for cell culture (Subiran et al., 2021) and has recently been applied in clinical trials for diabetic foot ulcer (Volpe et al., 2017), dystrophic recessive epidermolysis bullosa (Tadini et al., 2015b), corneal lesions (Samarkanova et al., 2021) and knee osteoarthritis (Caiaffa et al., 2021), certain parameters remain to be investigated for its use in endometrial regeneration, and ultimately, fertility restoration.
In this study, we analyzed hUC-PRP to elucidate its composition (in comparison with adult hPRP) and kinetics of growth factors release, as well as its immunocompatibility in vivo, impact on endometrial tissue regeneration, and ultimately, fertility restoration in a mouse model. Further, since we previously described the local application of an immunotolerated decellularized porcine endometrium-derived extracellular matrix (EndoECM) hydrogel loaded with growth factors, to enhance endometrial regeneration (López-Martínez et al., 2021b), we additionally studied the application of hUC-PRP alone, or loaded into EndoECM, in an established preclinical murine model of AS/EA to analyze the combined effect.
Materials and methods
Study design
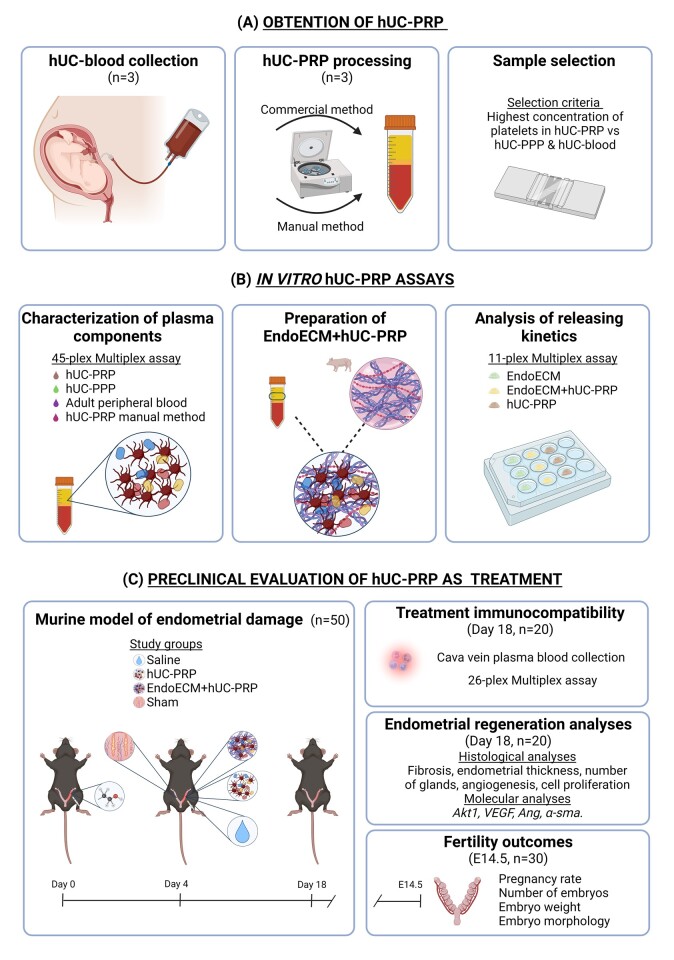
All patients signed an institutionally accepted general research waiver to express their written consent prior to donating their biological samples. All of the animal procedures described in this study were performed in accordance with Directive 2010/63/EU and the Ethics Committee for Animal Welfare of University of Valencia (A-20210203145327). An overview of the study is depicted in Fig. 1.
Figure 1.
Study design. (A) The hUC-PRP was generated by collecting hUC blood from women in childbirth, isolating the PRP fraction via commercial (closed) and manual (open) systems, and selecting the most concentrated sample. (B) In vitro hUC-PRP assays included the characterization of plasma components, the loading of the hUC-PRP into the EndoECM, and a comparison of the releasing kinetics of hUC-PRP with or without EndoECM. (C) To analyze the efficacy of hUC-PRP as a preclinical treatment, we studied the immunocompatibility of EndoECM and hUC-PRP, endometrial regeneration (n = 20) and fertility outcomes of a murine model (following natural mating; n = 30) of endometrial damage. α-sma, actin alpha 2, smooth muscle; Akt1, AKT serine/threonine kinase 1; Ang, angiogenin; EndoECM, decellularized porcine endometrium-derived extracellular-matrix hydrogel; hUC, human umbilical cord; hUC-PRP, platelet-rich plasma from the hUC; hUC-PPP, platelet-poor plasma from the hUC; VEGF, vascular endothelial growth factor.
Collection of hUC blood, processing and selection of hUC-PRP
hUC blood was donated from women (aged 20–32) in childbirth, who were healthy and delivered a healthy newborn, following Hospital Politécnico y Universitario La Fe standard operating procedures for hUC blood donation. Briefly, hUC blood was collected after delayed hUC clamping using a blood collection bag containing anticoagulant (731712, Grifols, Barcelona, Spain). Then, hUC blood was immediately stored at 4°C until its processing.
The hUC-PRP was first prepared using a commercialized ‘closed’ system (HyTissue® PRP20, P7-4020, Fidia, Madrid, Spain), where each 20 ml hUC blood sample was transferred into specialized tubes, and centrifuged as described by the manufacturer, then used to isolate and extract ∼6 ml of platelet-poor plasma (hUC-PPP, upper fraction) and 4 ml of hUC-PRP (lower fraction). To evaluate if the quality of resulting platelet concentration was maintained with respect to traditional PRP methodology, all samples were dually processed using the traditional ‘open’ system involving double centrifugation, following protocols previously established by our group (de Miguel-Gómez et al., 2021a). In this case, the hUC blood samples were centrifuged at 280×g for 8 min to separate plasma, followed by centrifugation at 400×g for 15 min to collect the concentrated hUC-PRP from the lower third fraction.
The number of platelets in the whole hUC blood, hUC-PRP and hUC-PPP samples was quantified using a platelet counting fluid (1700090, SPINREACT, Girona, Spain) on a Neubauer chamber. The most enriched hUC-PRP sample was selected for subsequent analyses, and aliquots were stored at −80°C until further use. After thawing samples, plasma was systematically activated with 5% CaCl2 (at 0.1 mol/l of hUC-PRP).
Multiplex analyses for hUC-PRP and hUC-PPP characterization
A panel of 45 protein biomarkers was analyzed in the hUC-PRP (extracted using both methods) and hUC-PPP, using the Cytokine/Chemokine/Growth Factor 45-Plex 387 Human ProcartaPlex™ Panel 1 (EPX450-12171-901, Thermo Fisher Scientific, Vienna, Austria) with Luminex xMAP® Technology. The results were compared to our previously published analyses of PRP obtained from adult peripheral blood (de Miguel-Gómez et al., 2021a).
Preparation of EndoECM supplemented with hUC-PRP
Uteri from healthy sows were processed as we previously described (López-Martínez et al., 2021a), to produce the EndoECM, in accordance with ISO 9001 quality management, in relation to the safe and legal procurement of animal organs from the slaughterhouse. Then, as established in other studies using extracellular matrix hydrogels (Zhang et al., 2020a), 15% (v/v) of thawed hUC-PRP was supplemented in 6 mg/ml of EndoECM to prepare the EndoECM + hUC-PRP treatment.
In vitro kinetics of growth factors release
The releasing kinetics of the hUC-PRP with/without EndoECM was analyzed in vitro, according to a protocol adapted from Yang et al. (2011). Briefly, 150 µl drops of the EndoECM, EndoECM + hUC-PRP or hUC-PRP were introduced into individual wells (n = 2 wells per group) of a 24-well plate and incubated at 37°C (with continuous gentle agitation) for 30 min to promote hydrogel gelation. Then, 500 µl of Dulbecco’s phosphate-buffered saline (dPBS, P5493, Sigma-Aldrich, MI, USA) was added to the well. The dPBS was entirely recovered and replaced, without disturbing the gelled drop, six hours later, and then every second day, over a 14-day period. The cumulative releasing kinetics were analyzed using the Growth Factor 11-Plex Human ProcartaPlex™ Panel (EPX110-12170-901, Thermo Fisher Scientific) with Luminex xMAP® Technology, as the cumulative growth factor concentration released at each point with respect to the total concentration released at the end of the assay.
The murine model for uterine damage
Eight-week-old immunocompetent inbred mice (n = 50, C57BL/6NCrl, Charles River Laboratories, Saint-Germain-Nuelles, France) were housed in the animal facilities of the Central Research Unit of the Medicine Faculty at University of Valencia. Uterine damage was induced using ethanol as we recently described (López-Martínez et al., 2021b). Mice were designated for endometrial regeneration (n = 5 per group) or fertility-related (n = 10 per group) analyses, and the following groups were randomly assigned using an online tool (https://www.random.org; Haahr, 2021): (i) saline (treated with dPBS), (ii) hUC-PRP or (iii) EndoECM + hUC-PRP. For the regeneration experiments, a fourth group with no uterine damage, (iv) sham group, was included as a positive control. Supplementary Table SI includes details of all interventions and husbandry.
Multiplex analyses for hUC-PRP immunocompatibility
A terminal blood sample was collected from the mice designated to analyze endometrial regeneration, placed in tubes with EDTA (1.8mg/ml) to prevent coagulation and kept on ice until centrifuged at 1600×g for 10 min at 4°C to isolate the plasma fraction, and finally stored at −80°C until further analysis. To assess the immunotolerance of the mice to the hUC-PRP with/without EndoECM, the plasma was evaluated using the Cytokine & Chemokine 26plex-Mouse ProcartaPlex™ Panel 1 (EPX260-26088-901, Thermo Fisher Scientific) with Luminex xMAP® Technology.
Histological analysis of endometrial tissue regeneration
Left uterine horns were fixed with 4% paraformaldehyde overnight, dehydrated, embedded in paraffin and serially sectioned in a vertical position (4 µm). Alternatively, the right horns were collected, and dried-stored at −80°C until further processing.
Following Masson’s Trichrome staining (MT, HT-15, Sigma-Aldrich), we assessed whole endometrial area/thickness in sections at 25× magnification in QuPath (Bankhead et al., 2017), excluding myometrium and uterine lumen visually. Gland density was established as the number of glands per mm2, within four fields of views (at 200× magnification) from two MT-stained cross-sections. Collagen deposition was quantified using Image J software (selecting the MT option from the vector drop-down menu of the Color Deconvolution plugin) to assess the proportion of fibrotic tissue (i.e. collagen area, stained in blue) within the endometrium.
Endometrial cell proliferation was assessed by immunohistochemistry with Ki67 (1:300 dilution, ab15580, Abcam, MA, USA). The proliferation index was calculated as percentage of endometrial Ki67+ by total number of endometrial cells, using QuPath software in 200× fields. Angiogenesis was evaluated by double immunofluorescence with Image-Pro Plus (Media Cybernetics, CA, USA), using fluorescein-labeled Griffonia (Bandeiraea) Simplicifolia Lectin I (GSL I, 1:200 dilution, FL-1101, Vector Laboratories, CA, USA) and alpha-smooth muscle actin (α-SMA, 1:300 dilution, C6198, Sigma-Aldrich). Notably, new blood vessels were exclusively stained by GSL I, while mature vessels were detected by the co-expression of both antibodies. Neoangiogenesis was quantified as the lectin-positive area minus the α-SMA-positive area, divided by the total analyzed area (López-Martínez et al., 2021b).
Molecular analysis of endometrial tissue regeneration
The endometrial tissue was isolated from the myometrium by applying gentle pressure on the uterine horns, with the back of curved forceps, to expel the endometrial tissue (Cheng et al., 2011; Ferrero et al., 2017). Total RNA from the endometria (n = 20) and myometria (from the sham group; n = 5) was extracted using the RNeasy® Mini Kit (74014, Qiagen, Hilden, Germany), and reverse transcribed using the PrimeScript™ RT Reagent Kit (RR037A, Takara Bio, Japan). Both RNA and DNA concentrations were quantified with a NanoDrop™ 2000c Spectrophotometer (Thermo Fisher Scientific). Gene expression of α-Sma (only in the sham group, to verify the exactitude of the endometrial-myometrium dissection), and VEGF, Akt1 and Ang1 (in all endometria) was evaluated by real-time quantitative PCR (RT-qPCR) using Power-Up SYBR Green (A25742, Applied Biosystems, USA) and the StepOnePlus™ Real-Time PCR System (Applied Biosystems). Specific primer sequences (IDT, Leuven, Belgium) and details about the RT-qPCR protocol are presented in Supplementary Table SII. Expression data were normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) housekeeping gene expression, and the ΔΔCt method (Livak and Schmittgen, 2001) was used to calculate the relative gene expression level or fold change (FC) with respect to the sham group.
Fertility outcomes
Natural mating was considered successful by the presence of a vaginal plug (which was monitored daily), and the achievement of pregnancy was evaluated 14.5 days later (E14.5). Mice were sacrificed to collect uteri, and to record the number of embryos present, in addition to their weight and morphology.
Statistical analysis
GraphPad Prism software v 8.3 (www.graphpad.com; La Jolla, CA, USA) was used for statistical analysis and graphical representation. Normally distributed data were analyzed by one-way ANOVA, while non-normally distributed data were analyzed by the Kruskal–Wallis test. Both were followed by a t-test or Mann–Whitney U tests for 2-by-2 comparisons, respectively. In all cases, P < 0.05 was considered statistically significant.
Results
hUC-PRP: quantity and quality analysis
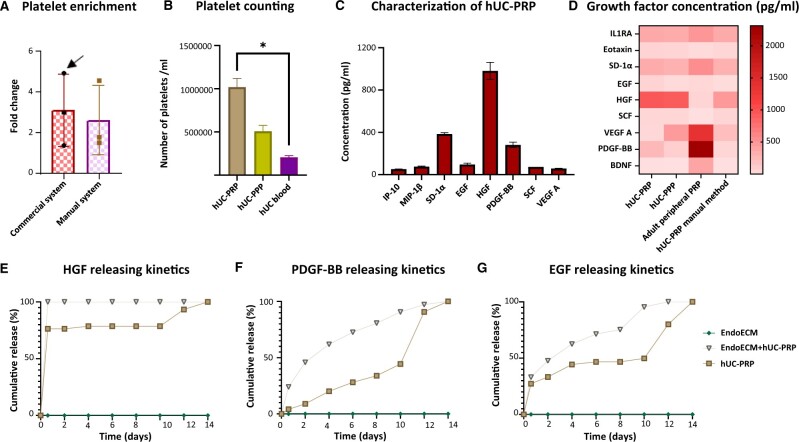
The commercialized system for hUC-PRP obtention proved to have similar efficacy to the traditional manual methodology. We found no differences in platelet enrichment between both strategies, which respectively had FCs of 3.10 ± 1.77 and 2.63 ± 1.67 compared to the whole hUC platelets number (Fig. 2A). Specifically, the most enriched hUC-PRP sample contained 2-fold more platelets per milliliter than hUC-PPP and a significant 5-fold enrichment with respect to whole hUC blood (1 018 000 ± 101 482 platelets/ml in hUC-PRP vs 508 500 ± 67 060 platelets/ml in hUC-PPP vs 207 000 ± 18 938 platelets/ml in whole hUC blood, P < 0.05, Fig. 2B) and it was selected for further characterization.
Figure 2.
hUC-PRP characterization, composition and in vitro releasing kinetics. (A) Comparison of platelet enrichment using commercial and manual methods for PRP extraction. Platelet enrichment was defined as the number of platelets in the hUC-PRP, divided by the number of platelets in whole hUC blood. The most enriched sample (indicated with an arrow) was selected for subsequent analyses. (B) Comparison of platelet density in hUC-PRP, hUC-PPP and whole hUC blood. (C) Predominant results from the multiplex protein assay for cytokines, chemokines and growth factors in hUC-PRP. (D) Comparative heat-map of protein differences found among hUC-PRP and hUC-PPP extracted with a commercialized system, PRP from adult peripheral blood and hUC-PRP extracted with double centrifugation. (E–G) Releasing kinetics of (E) HGF, (F) PDGF-BB and (G) EGF in EndoECM + hUC-PRP, EndoECM and hUC-PRP conditions. The cumulative release was defined as the cumulative concentration released at each point, with respect to the total concentration released on day 14. Data in A–D are presented as a mean of three replicates ± SD. *P < 0.05. EGF, epidermal growth factor; EndoECM, decellularized porcine endometrium-derived extracellular-matrix hydrogel; HGF, hepatic growth factor; hUC, human umbilical cord; hUC-PRP, platelet-rich plasma from hUC; hUC-PPP, platelet-poor plasma from hUC; IP10, C-X-C motif chemokine 10; MIP-1β, C-C motif chemokine 4; PDGF-BB, platelet-derived growth factor-BB; SCF, stem cell factor; SDF1α, stromal cell-derived factor 1 A; VEGF A, vascular endothelial growth factor.
Of the 45 biomarkers analyzed in hUC-PRP, eight growth factors were distinguished for having a concentration >50 pg/ml, including interferon gamma-induced protein 10 (IP-10), macrophage inflammatory protein 1 beta (MIP-1β), stromal-derived factor 1 alpha (SDF-1α), EGF, HGF, PDGF-BB, stem cell factor (SCF) and VEGF alpha (VEGF-A) (Fig. 2C). Although hUC-PRP was enriched with PDGF-BB and EGF, it had less VEGF-A than hUC-PPP (Fig. 2D). Further, considering both PRP and PPP fractions together, the hUC plasma was ample in HGF, Eotaxin and SCF, but more scarce in brain-derived neurotrophic factor (BDNF), SD-1α, interleukin-1 receptor antagonist (IL1RA), and especially PDGF-BB, compared to the adult peripheral blood PRP. Finally, hUC-PRP obtained by the commercialized system concentrated HGF, PDGF-BB and EGF more efficiently than that obtained by the manual system. The complete panel characterization is included in Supplementary Table SIII.
In vitro kinetics of growth factor release
The drops of EndoECM hydrogel with/and without hUC-PRP gelled adequately, enabling the addition of dPBS to the wells without disrupting the drops, and maintained their shape for up to two weeks. The kinetics of the principal growth factors (i.e. HGF, PDGF-BB and EGF) released by hUC-PRP was analyzed in vitro. Alone, hUC-PRP released 50% of its HGF during an initial burst within the first 6 h of culture (Fig. 2E). Meanwhile, the initial release from EndoECM + hUC-PRP was increased and followed by a more linear pattern until Day 14. Further, hUC-PRP initially released PDGF-BB slowly, only amplifying production during the last 4 days, whereas hUC-PRP with the hydrogels provided constant release (Fig. 2F). As for EGF, different treatments behaved more similarly exposing a constant liberation of the growth factor within the first 2 days, but EndoECM + hUC-PRP again produced again a more constant release in the following days (Fig. 2G). Supplementary Table SIV describes the kinetics of the eleven growth factors over 14 days.
Immunotolerance of hUC-PRP in mice
Following centrifugation of terminal blood samples from recipient mice, <100 µl of plasma was recovered. Multiplex analyses revealed immunocompatibility and immunotolerance to both hUC-PRP and the EndoECM hydrogel, as no statistical differences were found in the expression of 26 immune biomarkers between these treatments and the saline treatment or sham groups. Expressions of interleukin-5, interleukin-6, tumor necrosis factor alpha and interferon gamma were the most prominent (Fig. 3). The complete immune characterization is presented in Supplementary Table SV.
Figure 3.
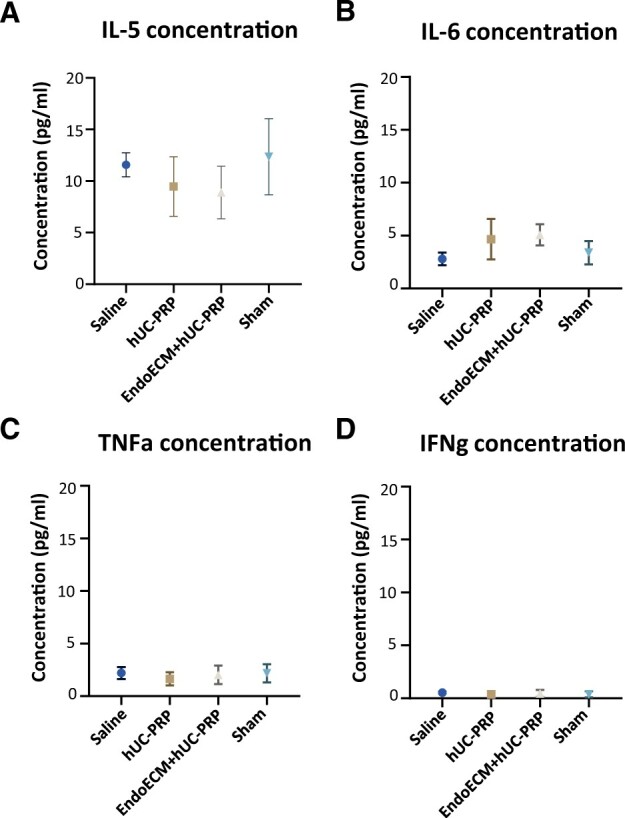
hUC-PRP immunocompatibility. Concentration of (A) IL-5, (B) IL-6, (C) TNFa and (D) IFNg in murine plasma collected 14 days following administration of saline, hUC-PRP or EndoECM + hUC-PRP, or a sham intervention, n = 5 per group. Data are presented as a mean of three replicates ± SD. P < 0.05. EndoECM, decellularized porcine endometrium-derived extracellular-matrix hydrogel; hUC-PRP, platelet-rich plasma from hUC; IFNg, interferon gamma; IL-5, interleukin 5; IL-6, interleukin 6; TNFa, tumor necrosis factor alpha.
In vivo endometrial regeneration of uterine horns following hUC-PRP treatment
All mice presented synchronized estrous cycles, as verified by vaginal cytology (Supplementary Fig. S1A and B).
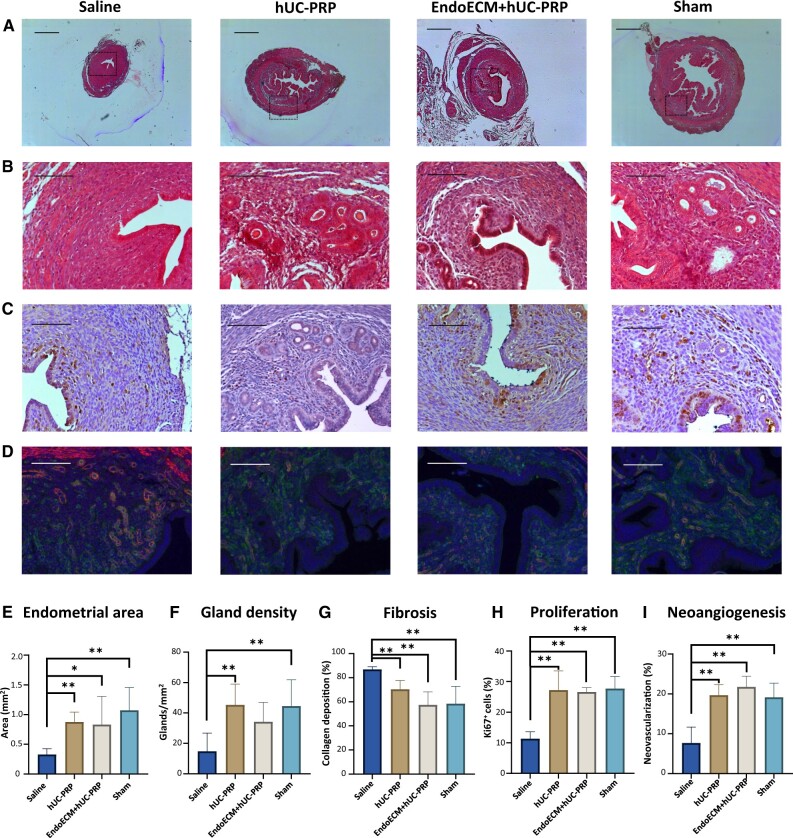
Although ethanol-induced damage statistically reduced the endometrial surface area, treating horns with hUC-PRP with/without EndoECM hydrogel did not show any visible reaction of discomfort or pain in the treated mice, and restored the endometrium such that its area was comparable to the untreated horns of mice that underwent sham surgery causing no damage (0.33 ± 0.1 mm2, 0.88 ± 0.17 mm2, 0.83 ± 0.47 mm2 and 1.08 ± 0.383 mm2 in uterine horns treated with uteri treated with saline, hUC-PRP, EndoECM + hUC-PRP or sham, respectively, P < 0.05, Fig. 4A and E). In mice that underwent sham surgery, the stromal layer of the endometrial tissue presented an organized structure, including epithelium and secretory glands. Comparably, both treatments involving hUC-PRP increased gland density, with respect to saline. This difference was statistically significant for the hUC-PRP-treated and non-damaged sham samples with respect to the saline-treated group; however, only a trend was found for mice treated with EndoECM + hUC-PRP (14.78 ± 11.97, 45.39 ± 13.58, 34.26 ± 12.74 and 44.59 ± 17.21 glands/mm2 in uteri treated with saline, hUC-PRP, EndoECM + hUC-PRP or sham, respectively, P < 0.05, Fig. 4B and F).
Figure 4.
Histological analysis of the damaged murine endometrium 14 days post-treatment. Representative cross-sections of murine uterine horns that were either damaged with ethanol and treated with saline, hUC-PRP or EndoECM + hUC-PRP, or manipulated without causing any damage (sham). Tissue regeneration was evaluated using Masson’s Trichrome staining for (A) endometrial area, (B) gland density and collagen deposition, (C) Ki-67 immunostaining and (D) GSL I (green) and α-sma (red) double immunostaining of new (green) and mature (red and green) blood vessels in the endometrium. Scale bars are set to 500 µm (A) or 100 µm (B to D). Histological quantification of (E) endometrial area, (F) gland density, (G) fibrosis, (H) proliferation and (I) neoangiogenesis. *, P < 0.05; **, P < 0.01; EndoECM, decellularized porcine endometrium-derived extracellular-matrix hydrogel; hUC-PRP, platelet-rich plasma from human umbilical cord blood.
Finally, collagen deposition was evaluated to study fibrosis. Compared to saline-treated uterine horns, the hUC-PRP-treated horns had statistically smaller collagen deposits. This difference was more pronounced for EndoECM + hUC-PRP, which in turn, was comparable with undamaged uteri (86.88 ± 2.27%, 70.34 ± 7.39%, 57.45 ± 10.80% and 58.34 ± 14.52% in the uteri treated with saline, hUC-PRP, EndoECM + hUC-PRP or sham, respectively, P < 0.05, Fig. 4B and G).
Restoring the endometrial function with cell proliferation and neovascularization
Endometrial function was significantly recovered with both treatments, as evidenced by the significantly augmented proliferation index with respect to the saline-treated group (11.34 ± 2.25%, 27.23 ± 6.30%, 26.61 ± 1.42% and 27.71% ± 4.00% in uteri treated with saline, hUC-PRP, EndoECM + hUC-PRP or sham, respectively; P < 0.05; Fig. 4C and H) and a well-distributed neovascularization. Angiogenesis promoted by hUC-PRP was similar to that in the sham group, and further amplified following treatment with EndoECM + hUC-PRP. In both cases, angiogenesis was statistically improved with respect to saline-treated uteri (7.70 ± 3.99%, 19.69 ± 2.64%, 21.77 ± 2.67% and 19.14 ± 3.55% in uteri treated with saline, hUC-PRP, EndoECM + hUC-PRP or sham, respectively, P < 0.05, Fig. 4D and I).
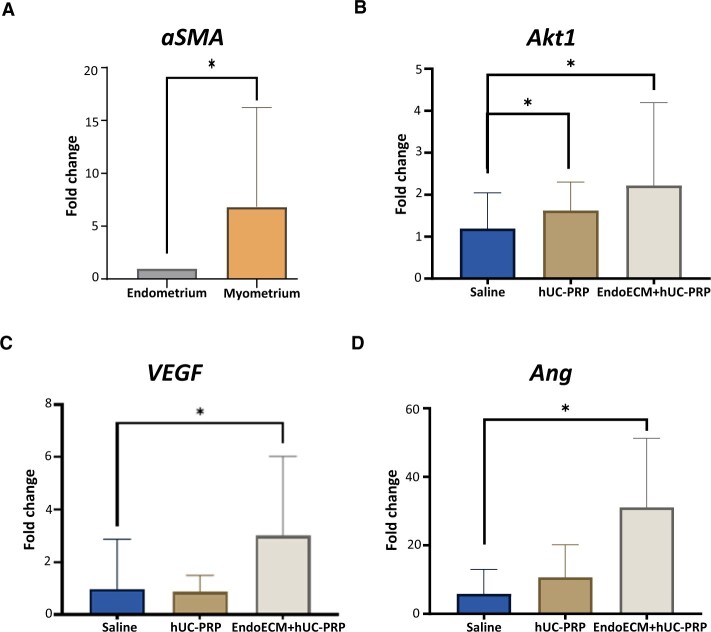
Key molecular mechanisms involved in the endometrial recovery
Murine endometrial tissue was isolated from the rest of the uterine horn to carry out molecular analyses. A significant 6.83 ± 9.47 FC in aSMA gene expression was found between endometrial and myometrial samples, confirming adequate endometrial isolation (P < 0.05; Fig. 5A). Then, when endometrial samples from both treatments involving hUC-PRP were analyzed, we found Akt1 was significantly overexpressed compared to saline treatment (FCs of 1.19 ± 0.85, 1.62 ± 0.68 and 2.22 ± 1.98 for treatments with saline, hUC-PRP and EndoECM + hUC-PRP, respectively; P < 0.05; Fig. 5B). However, in comparison to the saline-treated uteri, only EndoECM + hUC-PRP induced significant overexpression of VEGF (FCs of 0.98 ± 1.89, 0.87 ± 0.63 and 3.00 ± 3.03 respectively, P < 0.05; Fig. 5C) and Ang (FCs of 5.83 ± 7.18, 10.68 ± 9.53 and 31.06 ± 20.22, respectively; P < 0.05; Fig. 5D).
Figure 5.
Molecular analysis of the damaged murine endometrium 14 days post-treatment. Murine uterine horns were damaged with ethanol and treated with saline, hUC-PRP or EndoECM + hUC-PRP, and mRNA was extracted. Relative gene expression of aSMA (A), Akt1 (B), VEGF (C) and Ang (D). Data were normalized with respect to the gene expression from the sham group and presented as a mean ± SD. For all the experiments, n = 5 per group. P < 0.05 was considered statistically significant. *, P < 0.05. Akt1, thymoma viral proto-oncogene 1; Ang, angiogenin; aSMA, actin alpha 2, smooth muscle, aorta; EndoECM, decellularized porcine endometrium-derived extracellular-matrix hydrogel; hUC-PRP, platelet-rich plasma from human umbilical cord blood; VEGF, vascular endothelial growth factor.
hUC-PRP treatment restores fertility in mice
Profound unilateral uterine damage was induced in our model using ethanol, as demonstrated by a significantly reduced pregnancy rate of 33.33% in the saline-treated horns with respect to the contralateral non-damaged horns. The basic hUC-PRP treatment restored fertility, by producing pregnancy rates of 66.67% in the damaged horns, which were not statistically different from the pregnancy rates in the sham group. However, the EndoECM + hUC-PRP produced similar rates to the saline-treated horns, indicating that when administered in this vehicle, it was not enough to reverse the damage (Fig. 6A). We also noted a similar trend across the treatments for the number of embryos per horn (Fig. 6B). Notably, embryo weight at E14.5 was comparable between the damaged horns in the hUC-PRP-based treatment groups and the sham horns (Fig. 6C), and they presented normal morphology and size (Fig. 6D).
Figure 6.
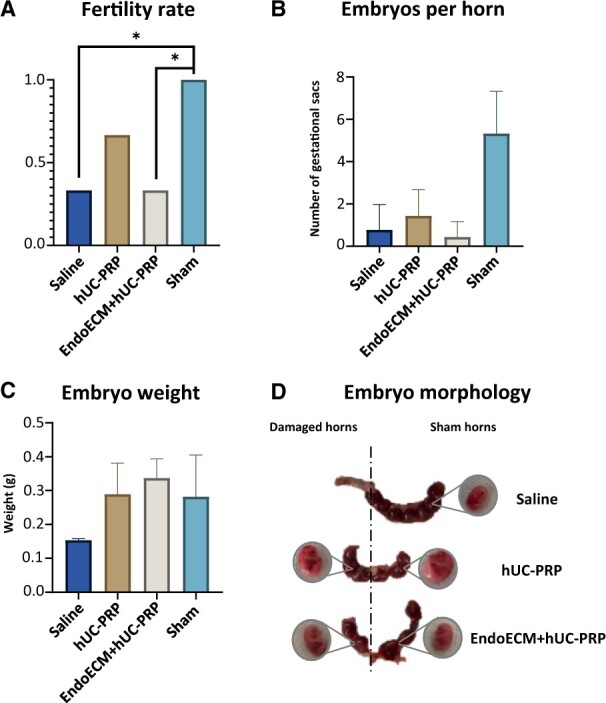
Achievement of pregnancy 14 days following unilateral treatment to the uterine horns of mice with ethanol-damaged endometrium. Fertility restoration was assessed by (A) pregnancy rate (number of embryos per horn by total number of embryos), (B) number of embryos per horn, (C) embryo weight and (D) representative images of gestational uterine horns and embryo morphology, 14 days after confirmed mating by vaginal plug (stage E14.5 of embryo development). Right uterine horns were treated with saline, hUC-PRP or EndoECM + hUC-PRP, while contralateral left horns only underwent sham surgery. (A–C) Data are presented as mean ± SD, n = 10 per group. A statistical significance of P < 0.05 is indicated by the asterisk. hUC-PRP, platelet-rich plasma from human umbilical cord; EndoECM, decellularized porcine endometrium-derived extracellular-matrix hydrogel.
Discussion
The adequacy of instilling human adult peripheral blood PRP to clinically treat endometrial pathologies is widely accepted, due to the ample growth factors and cytokines it releases (Chang et al., 2015; Zadehmodarres et al., 2017; Kim et al., 2019a). Since hUC-PRP contains even more growth factors and less pro-inflammatory cytokines (Parazzi et al., 2010), it has recently emerged as an effective therapeutic alternative in diverse medical fields (Tadini et al., 2015b; Volpe et al., 2017; Caiaffa et al., 2021; Samarkanova et al., 2021), but its use has not yet been reported for endometrial pathologies. Interestingly, two independent studies recently compared the efficacy of hUC-PRP versus adult PRP for bone regeneration and hip osteoarthritis and showed similar results. Although their findings were influenced by illness severity, both groups still agreed that there were more biological advantages when using hUC-PRP, as it comes from younger sources, can easily be cryopreserved, is independent of the patient’s comorbidities and is microbiologically safe (Mazzotta et al., 2022; Rani et al., 2022).
The state-of-the-art treatments used for patients with AS/EA employ bioengineering-based techniques, organoids and PRP that act in the target tissue by enhancing cytokine induction, growth factor production or regulation of the Th1/Th2 response, producing an improvement, thickening and regeneration of the endometrium, by activating tissue remodeling processes, and cell proliferation (de Miguel-Gómez et al., 2021a; Gharibeh et al., 2022). In this study, we obtained and characterized hUC-PRP, in addition to demonstrating the regenerative effect it has (either alone or loaded into an EndoECM hydrogel) on a damaged murine endometrium, and how it can restore fertility.
The two protocols we used for hUC-PRP processing seem adequate for clinical use, as both the traditional ‘open’ and commercialized ‘closed’ systems similarly concentrated platelets. However, for the clinical setting, the elevated cost of closed system kits for isolating hUC-PRP may be justified by their added safety and sterility, which cannot be guaranteed with open systems, because they are not manufactured to protect from external contaminants (WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy, 2010; Karakas et al., 2020). Prior to administering heterologous PRP (processed in the laboratory setting) into the recipient organism, it is crucial to ensure the product’s sterility. In this regard, our methodology provides a reproducible, safe, practical yet affordable PRP bio-product that will facilitate applications in regenerative medicine and alternative therapeutic approaches. Although costlier, it seems more appropriate to use closed-system kits in clinics to ensure patients’ safety. Further, using the commercial system, we demonstrated that hUC-PRP is enriched with several factors that play important roles in the endometrium. Identified factors included: HGF, which has been related with proliferation (Yoshida et al., 2004) and decidualization (Zhang, 2010); PDGF-BB, which is largely involved in tissue contraction and migration of stromal cells (Gargett and Masuda, 2010); EGF, which promotes endometrial growth (Ejskjaer et al., 2005) and early pregnancy (Large et al., 2014); and SDF-1α, which enhances endometrial receptivity (Koo et al., 2021). Altogether, these factors show substantial potential for endometrial-specific regeneration and support the translation of hUC-PRP for clinical treatment of endometrial disorders, such as AS and EA. Additionally, this study highlighted the xenogeneic and immunotolerant nature of the hUC-PRP, 2 weeks after treatment, with the analysis of more than 25 cytokines/chemokines in the mice’s blood, setting the preclinical foundation for its heterologous use in patients. The use of hUC-PRP has also been widely supported by its application in diabetic foot ulcers (Volpe et al., 2017), epidermolysis bullosa (Tadini et al., 2015b), corneal lesions (Samarkanova et al., 2021) or osteoarthritis (Caiaffa et al., 2021), which altogether demonstrate there are no complications or severe adverse events occurring with hUC-PRP treatment.
Hydrogels are by far the most prominently used bioengineering strategy for female reproductive medicine (Francés-Herrero et al., 2022a) and are composed of hydrophilic polymeric networks, which can deliver controlled drug-release into target wounds (Narayanaswamy and Torchilin, 2019). Since ECM-based hydrogels are proven to mimic the physicochemical properties of the tissue of origin, we hypothesized they could provide the perfect environment for tissue regeneration (Francés-Herrero et al., 2022b). Due to their spatiotemporal control over the mobilization of therapeutic agents into the tissue of interest, hydrogels loaded with peripheral blood PRP have previously been demonstrated to be highly effective therapeutic strategies, promoting tissue regeneration, wound healing efficiency, vascularization and suitable biocompatibility (Xu et al., 2017; Zhang et al., 2020a,b, 2021). In corroboration, our in vitro analysis of the kinetics of the EndoECM and/or hUC-PRP demonstrated a sustained release of factors present in hUC-PRP (including HGF, PDGF-BB and EGF) over a 14-day period. With EndoECM + hUC-PRP, we observed relatively linear cumulative deliveries of HGF and PDGF-BB, while hUC-PRP on its own displayed a more irregular release of these growth factors. Since the latter are mitogenic and play critical roles in wound-healing and immunomodulation (Evrova and Buschmann, 2017), we postulated that a sustained release of these factors could be highly advantageous and could be enhanced by using the EndoECM as carrier for hUC-PRP. Finally, we demonstrated how the biological activity of these factors promoted endometrial regeneration in a mouse model with uterine damage.
A few studies have described the beneficial effect of adult PRP (Kim et al., 2020, 2022; Zhou et al., 2020) and commercialized hUC plasma (de Miguel-Gómez et al., 2021a) in preclinical murine models of AS/EA, and have they reported findings similar to the ones presented herein, in terms of endometrial regeneration, augmented angiogenesis, cell proliferation and ability to achieve pregnancy. Interestingly, aging has a detrimental effect on plasma composition (Castellano et al., 2017), and our group previously observed that uterine horns treated with hUC plasma had endometrial function partly mediated by an increase in HOXA10 overexpression, with respect to adult PRP (de Miguel-Gómez et al., 2021a). Regardless, the use of donated hUC-PRP is an innovative approach, since hUC-PRP contains a higher concentration of growth factors and anti-inflammatory molecules than adult peripheral PRP (Mazzotta et al., 2022), and more platelets than commercial hUC plasma (Everts et al., 2020). One of the distinguishing features of AS and EA is the presence of a functional fibrotic and thin endometrial tissue, which can have negative repercussions on the reproductive outcomes of affected women. The uteri of our preclinical model were damaged with ethanol, to mimic the severe endometrial injury in these conditions (i.e. loss of luminal epithelium and stroma integrity, few proliferative cells, decreased angiogenesis and acute fibrosis), as previously described (Kim et al., 2019b; de Miguel-Gómez et al., 2021b). In this study, endometrial tissue regeneration was dually achieved by the treatment with hUC-PRP or EndoECM + hUC-PRP. Specifically, hUC-PRP reduced endometrial fibrosis, increased endometrial area and gland density, and restored the functionality of the tissue by enhancing neovascularization, cell proliferation and activating regenerative molecular pathways (i.e. PI3K/Akt), which are involved in cell survival and decidualization (Fabi et al., 2017) or re-epithelialization (by increasing VEGF gene expression) (Abraham et al., 2021). As such, fertility outcomes in mice treated with hUC-PRP were comparable to the undamaged mice (i.e. those who underwent a sham surgery), suggesting that they were able to completely overcome the endometrial damage. Alternatively, when the hUC-PRP was loaded into the EndoECM hydrogel, molecular alterations led to improved tissue regeneration, but the injured endometrium was not completely restored, preventing the mice from achieving similar pregnancy rates to the sham group. We suspect these findings are related to the low concentration of hUC-PRP mixed with the EndoECM hydrogel (15% v/v; which had previously been described as sufficient in the pancreas (Zhang et al., 2020a) and other tissues (Francés-Herrero et al., 2022b), but might not be adequate in the endometrium) and suggest testing higher concentrations of hUC-PRP in future investigations. Interestingly, we found embryo weight at 2 weeks of gestation (E14.5) was similar with respect to control embryos, indicating the treatments did not alter early embryo development.
Despite the innovation this study offers regarding the application of hUC-PRP in preclinical models of endometrial damage, it presents some limitations. First, the kinetics of the main growth factors released from the hUC-PRP were analyzed under controlled conditions, and these may differ slightly in vivo. Second, we only superficially evaluated murine embryo development at E14.5, and thus a more in-depth study of the pups’ weight and morphology at birth, along with their genetics, and epigenetics, can be investigated in the future, to fully understand the impact of hUC-PRP treatment long-term. Lastly, we used a single hUC-PRP sample to treat all the damaged endometria of our preclinical model, to standardize treatment between recipient mice, similar other groups reported in clinical trials (Tadini et al., 2015b; Volpe et al., 2017; Everts et al., 2020; Caiaffa et al., 2021; Samarkanova et al., 2021). Our hUC-PRP showed standard platelet content, cytokines concentration and composition with respect to adult hPRP, and therefore the slight variability among samples should not significantly affect the treatment’s efficacy (Murphy et al., 2012; Buzzi et al., 2018).
Finally, our results, along with previous experimental and clinical findings of hUC-PRP treatment in different tissues, support the hypothesis that hUC-PRP has the ability to restore the injured endometrium, and is thus a suitable candidate for therapeutic management of patients with endometrial pathologies. While the use of ECM hydrogels with higher concentrations of hUC-PRP is currently underway, the potential of this delivery system offers promising results.
Conclusions
This study aimed to prospectively characterize the hUC-PRP of healthy women, obtained using a commercially available system and demonstrate the promising potential of this easily obtainable blood derivative for xenogenic and heterologous applications. There are many advantages to using hUC-PRP rather than other PRP sources, including the standardization of isolation protocols, a youthful composition and the independence from patient’s comorbidities and viral infection, which altogether enhance the regenerative properties of the treatment. The hUC-PRP and EndoECM-hUC-PRP are immunotolerated by mice and were shown to improve endometrial regeneration in an AS/EA murine model. In addition, the hUC-PRP restored fertility in this model, as evidenced by normal gestations. Once translated to clinical practice, these novel therapies may provide alternatives for the clinical management of endometrial pathologies.
Supplementary Material
Acknowledgements
The authors are thankful to the hUC blood donors, the medical team who collected the samples, the staff of the animal facilities of the Central Research Unit of the Faculty of Medicine of the University of Valencia and Mercavalencia for donating the sow uteri necessary to produce the EndoECM hydrogel. Finally, the authors thank Rosalba Lopez for her editing services in preparing this manuscript for publication.
Authors’ roles
A.R.-E.: experimental studies and procedures, manuscript drafting and analysis. L.d.M.-G., E.F.-H. and M.G.-Á., A.F., I.M.-T. and M.G.-C.: experimental studies and procedures. A.D.: animal surgery and care. A.P.: study design and critical discussion. I.C.: study design, analysis, manuscript drafting and critical discussion. All authors contributed to the interpretation of the results and the editing of the article.
Funding
This research was funded by the Ministerio de Ciencia, Innovación y Universidades (FPU19/04850 (A.R.-E.), FPU18/06327 (E.F.-H.), FPU20/00251 (M.G.-Á.)); Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital, Generalitat Valenciana (APOTIP/2021/042 (L.d.M.-G.)); and Instituto de Salud Carlos III co-funded by the European Union (ISCIII, Fondo Social Europeo El FSE invierte en tu futuro, CP19/00149, PI17/01039 and PI21/00305 (I.C.)).
Conflict of interest
None declared.
Contributor Information
Adolfo Rodríguez-Eguren, IVI Foundation, Health Research Institute La Fe, Valencia, Spain.
Lucía de Miguel-Gómez, IVI Foundation, Health Research Institute La Fe, Valencia, Spain.
Emilio Francés-Herrero, IVI Foundation, Health Research Institute La Fe, Valencia, Spain; University of Valencia, Valencia, Spain.
María Gómez-Álvarez, IVI Foundation, Health Research Institute La Fe, Valencia, Spain.
Amparo Faus, IVI Foundation, Health Research Institute La Fe, Valencia, Spain.
Macarena Gómez-Cerdá, IVI Foundation, Health Research Institute La Fe, Valencia, Spain.
Inés Moret-Tatay, Inflammatory Bowel Disease Research Group/Multiplex Analysis Unit, Health Research Institute La Fe, Valencia, Spain.
Ana Díaz, University of Valencia, Valencia, Spain.
Antonio Pellicer, University of Valencia, Valencia, Spain; IVI-RMA Rome, Rome, Italy.
Irene Cervelló, IVI Foundation, Health Research Institute La Fe, Valencia, Spain.
Data availability
The data underlying this article are available in the article and in its Supplementary Material.
References
- Abraham S, Sanjay G, Majiyd NA, Chinnaiah A.. Encapsulated VEGF121-PLA microparticles promote angiogenesis in human endometrium stromal cells. J Genet Eng Biotechnol 2021;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG. et al. QuPath: open source software for digital pathology image analysis. Sci Rep 2017;7:16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney M, McCarroll P, Glynn L, Lenehan B.. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci 2020;190:1021–1025. [DOI] [PubMed] [Google Scholar]
- Buigues A, Marchante M, de Miguel-Gómez L, Martinez J, Cervelló I, Pellicer A, Herraiz S.. Stem cell-secreted factor therapy regenerates the ovarian niche and rescues follicles. Am J Obstet Gynecol 2021;225:65.e1–65.e14. [DOI] [PubMed] [Google Scholar]
- Buzzi M, Versura P, Grigolo B, Cavallo C, Terzi A, Pellegrini M, Giannaccare G, Randi V, Campos EC.. Comparison of growth factor and interleukin content of adult peripheral blood and cord blood serum eye drops for cornea and ocular surface diseases. Transfus Apher Sci 2018;57:549–555. [DOI] [PubMed] [Google Scholar]
- Caiaffa V, Ippolito F, Abate A, Nappi V, Santodirocco M, Visceglie D.. Allogenic platelet concentrates from umbilical cord blood for knee osteoarthritis: preliminary results. Med Glas (Zenica) 2021;18:1–7. [DOI] [PubMed] [Google Scholar]
- Cakiroglu Y, Yuceturk A, Karaosmanoglu O, Kopuk SY, Korun ZEU, Herlihy N, Scott RT, Tiras B, Seli E.. Ovarian reserve parameters and IVF outcomes in 510 women with poor ovarian response (POR) treated with intraovarian injection of autologous platelet rich plasma (PRP). Aging 2022;14:2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, Wang J, Bai D, Wang J, Wang L. et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther 2018;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Mosher KI, Abbey RJ, McBride AA, James ML, Berdnik D, Shen JC, Zou B, Xie XS, Tingle M. et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 2017;544:488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecerska-Heryć E, Goszka M, Serwin N, Roszak M, Grygorcewicz B, Heryć R, Dołęgowska B.. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev 2022;64:84–94. [DOI] [PubMed] [Google Scholar]
- Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, Liang X.. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med 2015;8:1286–1290. [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Licence D, Cook E, Luo F, Arends MJ, Smith SK, Print CG, Charnock-Jones DS.. Activation of mutated K-ras in donor endometrial epithelium and stroma promotes lesion growth in an intact immunocompetent murine model of endometriosis. J Pathol 2011;224:261–269. [DOI] [PubMed] [Google Scholar]
- Chrysanthopoulou EL, Pergialiotis V, Perrea D, Κourkoulis S, Verikokos C, Doumouchtsis SK.. Platelet rich plasma as a minimally invasive approach to uterine prolapse. Med Hypotheses 2017;104:97–100. [DOI] [PubMed] [Google Scholar]
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A.. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol 2013;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel-Gómez L, Ferrero H, López-Martínez S, Campo H, López-Pérez N, Faus A, Hervás D, Santamaría X, Pellicer A, Cervelló I.. Stem cell paracrine actions in tissue regeneration and potential therapeutic effect in human endometrium: a retrospective study. BJOG 2020;127:551–560. [DOI] [PubMed] [Google Scholar]
- de Miguel-Gómez L, López-Martínez S, Campo H, Francés-Herrero E, Faus A, Díaz A, Pellicer A, Domínguez F, Cervelló I.. Comparison of different sources of platelet-rich plasma as treatment option for infertility-causing endometrial pathologies. Fertil Steril 2021a;115:490–500. [DOI] [PubMed] [Google Scholar]
- de Miguel-Gómez L, López-Martínez S, Francés-Herrero E, Rodríguez-Eguren A, Pellicer A, Cervelló I.. Stem cells and the endometrium: from the discovery of adult stem cells to pre-clinical models. Cells 2021b;10:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart J, Sanberg PR, Garbuzova-Davis S.. Plasma derived from human umbilical cord blood: Potential cell-additive or cell-substitute therapeutic for neurodegenerative diseases. J Cell Mol Med 2018;22:6157–6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejskjaer K, Sørensen BS, Poulsen SS, Mogensen O, Forman A, Nexø E.. Expression of the epidermal growth factor system in human endometrium during the menstrual cycle. Mol Hum Reprod 2005;11:543–551. [DOI] [PubMed] [Google Scholar]
- Elghblawi E. Platelet-rich plasma, the ultimate secret for youthful skin elixir and hair growth triggering. J Cosmet Dermatol 2018;17:423–430. [DOI] [PubMed] [Google Scholar]
- Everts P, Onishi K, Jayaram P, Lana JF, Mautner K.. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci 2020;21:7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrova O, Buschmann J.. In vitro and in vivo effects of PDGF-BB delivery strategies on tendon healing: a review. Eur Cell Mater 2017;34:15–39. [DOI] [PubMed] [Google Scholar]
- Fabi F, Grenier K, Parent S, Adam P, Tardif L, Leblanc V, Asselin E.. Regulation of the PI3K/Akt pathway during decidualization of endometrial stromal cells. PLoS One 2017;12:e0177387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Perez K, Dym H.. Clinical uses of platelet-rich fibrin in oral and maxillofacial surgery. Dent Clin North Am 2020;64:291–303. [DOI] [PubMed] [Google Scholar]
- Ferrero H, Buigues A, Martínez J, Simón C, Pellicer A, Gómez R.. A novel homologous model for noninvasive monitoring of endometriosis progression. Biol Reprod 2017;96:302–312. [DOI] [PubMed] [Google Scholar]
- Francés-Herrero E, Lopez R, Hellström M, de Miguel-Gómez L, Herraiz S, Brännström M, Pellicer A, Cervelló I.. Bioengineering trends in female reproduction: a systematic review. Hum Reprod Update 2022a;28:798–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francés-Herrero E, Rodríguez-Eguren A, Gómez-Álvarez M, de Miguel-Gómez L, Ferrero H, Cervelló I.. Future challenges and opportunities of extracellular matrix hydrogels in female reproductive medicine. IJMS 2022b;23:3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano D, Bellver J, Díaz-García C, Simón C, Pellicer A.. Art and uterine pathology: how relevant is the maternal side for implantation? Hum Reprod Update 2015;21:13–38. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Masuda H.. Adult stem cells in the endometrium. Mol Hum Reprod 2010;16:818–834. [DOI] [PubMed] [Google Scholar]
- Gentile P, Garcovich S.. Systematic review—the potential implications of different platelet-rich plasma (PRP) concentrations in regenerative medicine for tissue repair. Int J Mol Sci 2020;21:5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibeh N, Aghebati-Maleki L, Madani J, Pourakbari R, Yousefi M, Ahmadian Heris J.. Cell-based therapy in thin endometrium and Asherman syndrome. Stem Cell Res Ther 2022;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano S, Romeo M, Lankinen P.. Platelet-rich plasma for androgenetic alopecia: Does it work? Evidence from meta analysis. J Cosmet Dermatol 2017;16:374–381. [DOI] [PubMed] [Google Scholar]
- Haahr M. RANDOM.ORG: True Random Number Service. 2021. Available at: https://www.random.org (1 October 2021, date last accessed).
- Hu J, Song K, Zhang J, Zhang Y, Tan BZ.. Effects of menstrual blood-derived stem cells on endometrial injury repair. Mol Med Rep 2019;19:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri A, Kianfar K, Pourmasumi S, Eftekhar M.. Platelet-rich plasma in the management of Asherman’s syndrome: an RCT. Int J Reprod Biomed 2020;18:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas L, Ercan D, Karabay E, Gumuslu S, Karabacak R.. How can clinically-safe and effective platelet rich plasma (PRP) be obtained in a laboratory? Ann Med Res 2020;27:2933–2942. [Google Scholar]
- Kim H, Shin JE, Koo HS, Kwon H, Choi DH, Kim JH.. Effect of autologous platelet-rich plasma treatment on refractory thin endometrium during the frozen embryo transfer cycle: a pilot study. Front Endocrinol (Lausanne) 2019a;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park M, Paek JY, Lee WS, Song H, Lyu SW.. Intrauterine infusion of human platelet-rich plasma improves endometrial regeneration and pregnancy outcomes in a murine model of Asherman’s syndrome. Front Physiol 2020;11:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MK, Yoon JA, Yoon SY, Park M, Lee WS, Lyu SW, Song H.. Human platelet-rich plasma facilitates angiogenesis to restore impaired uterine environments with Asherman’s syndrome for embryo implantation and following pregnancy in mice. Cells 2022;11:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Park ES, Kim TH.. Rejuvenation using platelet-rich plasma and lipofilling for vaginal atrophy and lichen sclerosus. J Menopausal Med 2017;23:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Choi B, Bin Lim JW, Kim YJ, Kim SY, Ku SY.. Efficient production of murine uterine damage model. Tissue Eng Regen Med 2019b;16:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo HS, Yoon M-J, Hong S-H, Ahn J, Cha H, Lee D, Ko J-E, Kwon H, Choi DH, Lee K-A. et al. CXCL12 enhances pregnancy outcome via improvement of endometrial receptivity in mice. Sci Rep 2021;11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large MJ, Wetendorf M, Lanz RB, Hartig SM, Creighton CJ, Mancini MA, Kovanci E, Lee K-F, Threadgill DW, Lydon JP. et al. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet 2014;10:e1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le ADK, Enweze L, DeBaun MR, Dragoo JL.. Platelet-rich plasma. Clin Sports Med 2019;38:17–44. [DOI] [PubMed] [Google Scholar]
- Lin Y, Qi J, Sun Y.. Platelet-rich plasma as a potential new strategy in the endometrium treatment in assisted reproductive technology. Front Endocrinol (Lausanne) 2021;12:1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- López-Martínez S, Campo H, de Miguel-Gómez L, Faus A, Navarro AT, Díaz A, Pellicer A, Ferrero H, Cervelló I.. A natural xenogeneic endometrial extracellular matrix hydrogel toward improving current human in vitro models and future in vivo applications. Front Bioeng Biotechnol 2021a;9:639688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Martínez S, Rodríguez-Eguren A, de Miguel-Gómez L, Francés-Herrero E, Faus A, Díaz A, Pellicer A, Ferrero H, Cervelló I.. Bioengineered endometrial hydrogels with growth factors promote tissue regeneration and restore fertility in murine models. Acta Biomater 2021b;135:113–125. [DOI] [PubMed] [Google Scholar]
- Ma H, Liu M, Li Y, Wang W, Yang K, Lu L, He M, Deng T, Li M, Wu D.. Intrauterine transplantation of autologous menstrual blood stem cells increases endometrial thickness and pregnancy potential in patients with refractory intrauterine adhesion. J Obstet Gynaecol Res 2020;46:2347–2355. [DOI] [PubMed] [Google Scholar]
- Mahajan N, Sharma S.. The endometrium in assisted reproductive technology: How thin is thin? J Hum Reprod Sci 2016;9:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malahias MA, Nikolaou VS, Johnson EO, Kaseta MK, Kazas ST, Babis GC.. Platelet-rich plasma ultrasound-guided injection in the treatment of carpal tunnel syndrome: A placebo-controlled clinical study. J Tissue Eng Regen Med 2018;12:e1480–e1488. [DOI] [PubMed] [Google Scholar]
- Mazzotta A, Pennello E, Stagni C, del Piccolo N, Boffa A, Cenacchi A, Buzzi M, Filardo G, Dallari D.. Umbilical cord PRP vs. autologous PRP for the treatment of hip osteoarthritis. JCM 2022;11:4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memeo A, Verdoni F, De Bartolomeo O, Albisetti W, Pedretti L.. A new way to treat forearm post-traumatic non-union in young patients with intramedullary nailing and platelet-rich plasma. Injury 2014;45:418–423. [DOI] [PubMed] [Google Scholar]
- Miguel‐Gómez L, Romeu M, Pellicer A, Cervelló I.. Strategies for managing Asherman’s syndrome and endometrial atrophy: since the classical experimental models to the new bioengineering approach. Mol Reprod Dev 2021b;88:527–543. [DOI] [PubMed] [Google Scholar]
- Moneib HA, Youssef SS, Aly DG, Rizk MA, Abdelhakeem YI.. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: a comparative study. J Cosmet Dermatol 2018;17:495–501. [DOI] [PubMed] [Google Scholar]
- Murphy MB, Blashki D, Buchanan RM, Yazdi IK, Ferrari M, Simmons PJ, Tasciotti E.. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials 2012;33:5308–5316. [DOI] [PubMed] [Google Scholar]
- Narayanaswamy R, Torchilin VP.. Hydrogels and their applications in targeted drug delivery. Molecules 2019;24:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzi V, Lazzari L, Rebulla P.. Platelet gel from cord blood: a novel tool for tissue engineering. Platelets 2010;21:549–554. [DOI] [PubMed] [Google Scholar]
- Rani N, Perut F, Granchi D, Sante G, Di Pennello E, Mazzotta A, Dallari D, Baldini N.. Ultrasound-guided injection of platelet-rich plasma or cord blood platelet-rich plasma in nonunion: a randomized controlled trial. Regen Med 2022;17:271–281. [DOI] [PubMed] [Google Scholar]
- Rebulla P, Pupella S, Santodirocco M, Greppi N, Villanova I, Buzzi M, Fazio N, De Grazzini G, Argiolas M, Bergamaschi P. et al. ; Italian Cord Blood Platelet Gel Study Group (see Appendix 1). Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus 2016;14:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva GS, Sachdeva LT, Goel M, Bala S.. Regenerative endodontic treatment of an immature tooth with a necrotic pulp and apical periodontitis using platelet-rich plasma (PRP) and mineral trioxide aggregate (MTA): a case report. Int Endod J 2015;48:902–910. [DOI] [PubMed] [Google Scholar]
- Saiz LC, Leache L, Erviti J, Ramírez N. Informe Plasma rico en plaquetas (PRP) Mejora de la adecuación de la práctica asistencial y clínica. MAPAC. 2020. https://www.navarra.es/NR/rdonlyres/B392D594-8010-4121-8F7B-7BA4BE674260/466687/INF_PRP_def.pdf (26 May 2022, date last accessed).
- Samarkanova D, Martin S, Bisbe L, Puig J, Calatayud-Pinuaga M, Rodriguez L, Azqueta C, Coll R, Casaroli-Marano R, Madrigal A. et al. Clinical evaluation of allogeneic eye drops from cord blood platelet lysate. Blood Transfus 2021;19:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, Palmero J, Remohí J, Pellicer A, Simón C.. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod 2016;31:1087–1096. [DOI] [PubMed] [Google Scholar]
- Sfakianoudis K, Simopoulou M, Nitsos N, Lazaros L, Rapani A, Pantou A, Koutsilieris M, Nikas Y, Pantos K.. Successful implantation and live birth following autologous platelet-rich plasma treatment for a patient with recurrent implantation failure and chronic endometritis. In Vivo 2019;33:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón C., Horcajadas, J.A., García-Velasco, J., Pellicer, A. In: Editorial Médica Panamericana (1st ed.). El Endometrio Humano: Desde la investigación a la clínica. Buenos Aires, 2009;2–42. [Google Scholar]
- Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S.. Autologous stem cell transplantation in refractory Asherman’s syndrome: a novel cell based therapy. J Hum Reprod Sci 2014;7:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subiran C, Kristensen SG, Andersen CY.. Umbilical cord blood–derived platelet-rich plasma: a clinically acceptable substitute for fetal bovine serum? Fertil Steril 2021;115:336–337. [DOI] [PubMed] [Google Scholar]
- Tadini G, Guez S, Pezzani L, Marconi M, Greppi N, Manzoni F, Rebulla P, Esposito S.. Preliminary evaluation of cord blood platelet gel for the treatment of skin lesions in children with dystrophic epidermolysis bullosa.Blood Transfusion. 2015a;13:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadini G, Pezzani L, Ghirardello S, Rebulla P, Esposito S, Mosca F.. Cord blood platelet gel treatment of dystrophic recessive epidermolysis bullosa. BMJ Case Rep 2015b;2015:bcr2014207364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, Xu X, Kong L.. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod 2016;31:2723–2729. Hum Reprod. [DOI] [PubMed] [Google Scholar]
- Terrovitis JV, Smith RR, Marbán E.. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res 2010;106:479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan G, Bahat PY, Aydın A, Özgör BY.. Evaluation of platelet-rich plasma injection activity in the treatment of abnormal uterine bleeding. Turk J Obstet Gynecol 2018;15:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P, Marcuccio D, Stilo G, Alberti A, Foti G, Volpe A, Princi D, Surace R, Pucci G, Massara M.. Efficacy of cord blood platelet gel application for enhancing diabetic foot ulcer healing after lower limb revascularization. Semin Vasc Surg 2017;30:106–112. [DOI] [PubMed] [Google Scholar]
- Von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K.. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012;30:1575–1578. [DOI] [PubMed] [Google Scholar]
- WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. Geneva: World Health Organization; 2010. 3, Blood-sampling systems. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138666/ (25 May 2022, date last accessed). [PubMed]
- Xu J, Gou L, Zhang P, Li H, Qiu S.. Platelet-rich plasma and regenerative dentistry. Aust Dent J 2020;65:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Al-Ani MK, Sun Y, Xu W, Pan L, Song Y, Xu ZL, Pan X, Yang L.. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J Tissue Eng Regen Med 2017;11:1173–1184. [DOI] [PubMed] [Google Scholar]
- Yang HS, Shin J, Bhang SH, Shin JY, Park J, Im G, Kim CS, Kim BS.. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp Mol Med 2011;43:622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Matsumoto K, Tomioka D, Bessho K, Itami S, Yoshikawa K, Nakamura T.. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors 2004;22:111–119. [DOI] [PubMed] [Google Scholar]
- Yu D, Wong YM, Cheong Y, Xia E, Li TC.. Asherman syndrome–one century later. Fertil Steril 2008;89:759–779. [DOI] [PubMed] [Google Scholar]
- Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L.. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod 2017;21:54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Miao H, Wang D, Qiu H, Zhu Y, Yao X, Guo Y, Wang Z.. Pancreatic extracellular matrix and platelet-rich plasma constructing injectable hydrogel for pancreas tissue engineering. Artif Organs 2020a;44:e532–e551. [DOI] [PubMed] [Google Scholar]
- Zhang L, Qiu H, Wang D, Miao H, Zhu Y, Guo Q, Guo Y, Wang Z.. Enhanced vascularization and biocompatibility of rat pancreatic decellularized scaffolds loaded with platelet-rich plasma. J Biomater Appl 2020b;35:313–330. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yao D, Zhao W, Zhang R, Yu B, Ma G, Li Y, Hao D, Xu FJ.. Engineering platelet-rich plasma based dual-network hydrogel as a bioactive wound dressing with potential clinical translational value. Adv Funct Mater 2021;31:2009258. [Google Scholar]
- Zhang X. Hepatocyte growth factor system in the mouse uterus: variation across the estrous cycle and regulation by 17-beta-estradiol and progesterone. Biol Reprod 2010;82:1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shen H, Wu Y, Zhao X, Pei J, Mou Z, Dong J, Hua X.. Platelet-rich plasma therapy enhances the beneficial effect of bone marrow stem cell transplant on endometrial regeneration. Front Cell Dev Biol 2020;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its Supplementary Material.