Abstract
Oms66 is a Borrelia burgdorferi outer membrane porin protein whose role in Lyme disease pathogenesis and immunity has not been well established. Oms66 was solubilized from whole-cell lysates of strain B313 (which is derived from B31 but lacks OspA, -B, -C, and -D) and purified to homogeneity by fast-protein liquid chromatography. Purified native Oms66 (nOms66), which retained the ability to form large channels in a planar lipid bilayer model membrane system, and denatured Oms66 (hOms66) were used to immunize New Zealand White rabbits. The resulting Oms66 antisera were tested in a complement-dependent borreliacidal assay in parallel with basal serum and with serum from rabbits immune to reinfection with B. burgdorferi (IRS). IRS showed high-titer complement-dependent killing of both strains B31 and B313. Sera from animals immunized with nOms66 showed high-titer complement-dependent killing activity against strain B313 but exhibited no killing of B31. By comparison, serum generated from immunizations with hOms66 showed no killing activity against either strain. Following adsorption of antiserum to nOms66 with recombinant Oms66 (rOms66), the serum antibodies no longer bound to rOms66 or to nOms66 that had been denatured with 8 M urea. However, the antibodies still bound to nOms66 and killing activity against B313 was retained, thus suggesting that native, conformational epitopes are targets of this bactericidal activity. Six C3H HeJ mice were immunized with nOms66 and were challenged using “host-adapted” B. burgdorferi B31 by skin implantation of infected mouse ear tissue. Four of the six mice were protected against both localized and disseminated infection. These findings indicate that native Oms66 can elicit potent bactericidal activity and significant protective immunity against host-adapted organisms.
The abundant Borrelia burgdorferi lipoprotein, OspA, is currently being used in a recombinant form as a human and animal vaccine against Lyme disease (40, 43, 45). However, OspA is now recognized to be downregulated when organisms are within a mammalian host (14, 27, 31), thereby potentially limiting vaccine effectiveness if there is transmission of any viable spirochetes from the tick. In fact, the OspA vaccine reduces the risk of acquiring Lyme disease by only 49 to 68% after two injections and by 76 to 92% after three injections (40, 43). In addition, protection against heterologous strains may be limited (24, 29). Because of these concerns about the OspA vaccine, there has been a search for additional protective immunogens.
There is evidence that such protective immunogens exist. Immunization with decorin binding proteins and with OspB and OspC from B. burgdorferi shows some degree of protection in mice (16, 19, 30). Protective immunity has also been conferred in hamsters by a whole-cell vaccine that does not include OspA (33). Infection of the rabbit with B. burgdorferi elicits immunity that is fully protective against challenge by both in vitro-cultivated organisms and host-adapted organisms acquired from infected tissue. These host-adapted Borrelia spp. have been shown, by reverse transcription-PCR in rabbits, to no longer express OspA (E. S. Shang, C. I. Champion, X. Wu, J. T. Skare, D. R. Blanco, J. N. Miller, and M. A. Lovett, submitted for publication), as has been established for mice (3), suggesting that immunogens other than OspA have induced a protective immune response.
A surface-exposed (6, 7, 35) outer membrane protein of B. burgdorferi designated p66 is expressed during human infection, as judged by the presence of specific antibodies in patients with Lyme disease (7). This protein is conserved within Borrelia species, although there is some sequence variability between B. burgdorferi sensu lato strains (5). Recently, this protein has also been shown to bind integrins, suggesting a possible role as an adhesin (11). In parallel studies designed to identify B. burgdorferi porins, a 66-kDa protein, which formed a large channel in lipid bilayer studies, was identified and designated Oms66. Determination of partial amino acid sequences of Oms66 revealed identity with p66 (41). The host immune response to Oms66, along with immunity induced by vaccination with this protein, has not been fully elucidated to date. Recent studies by Bunikis et al. (4) have shown that, in vitro, the accessibility of Oms66 to specific antibodies is blocked by OspA. It has not been reported whether Oms66 is accessible to antibody binding in vivo in the absence of OspA. Since humoral immunity has been shown to be important in resolving B. burgdorferi infection (1, 16, 21), these studies were designed to determine if antibodies to Oms66 could kill in vitro-cultivated B. burgdorferi not expressing OspA and if immunization with Oms66 conferred protection against infection with host-adapted B. burgdorferi no longer expressing OspA.
Evidence from crystal structures has shown that the native conformation of typical bacterial porins is that of a β-barrel (12, 46) and that the surface-exposed regions of porins are generally limited to loops that are formed between β-strands (20, 44). Assuming that Oms66 has a porin-like structure, based on its channel-forming properties, its native conformation could be a critical determinant of some of its surface-exposed epitopes. Furthermore, since it is well established that protection elicited by an immunogen can be dependent on its native conformation (17, 18, 32, 34), the significance of Oms66 native conformation has been investigated in this study.
MATERIALS AND METHODS
Strains and plasmids.
B. burgdorferi strains used included B31, a tick isolate from New York, and B313, which was kindly provided by Alan Barbour. Strain B313 is of the B31 lineage, but it lacks the lp38 and lp54 plasmids and therefore the ability to express OspA, -B, and -D (38). These organisms were grown in BSKII medium containing 10% rabbit serum (Sigma, St. Louis, Mo.) and no gelatin and were incubated at 34°C. Escherichia coli DH5α was used for all cloning experiments. The oms66 gene was cloned into pCR-TOPO for DNA sequencing (Invitrogen, Carlsbad, Calif.), and into pGEX 4T-1 (Pharmacia, Alameda, Calif.) for recombinant expression.
Genetic constructs.
The PCR was used to amplify the oms66 gene from B. burgdorferi B313, using B313 chromosomal DNA as a template. The primers used, which included a BamHI restriction site at the 5′ end of the gene, and an XhoI site at the 3′ end of the gene, were as follows: CCGGGATCCGCAGACGCATTAAAGGAAAAAGAT and CGGCTCGAGTTAGCTTCCGCTGTAGGCTAT. Amplification was carried out using 0.25 μg of genomic DNA template at 94°C for 30 s, 52°C for 30 s, and 72°C for 2 min for 28 cycles in a Perkin-Elmer DNA Thermal Cycler (Perkin-Elmer Corp., Norwalk, Conn.). Cloning of the resulting product into the BamHI and XhoI sites in pGEX 4T-1 generated a plasmid designated as pOms66. This construct was engineered such that expression of a GST-Oms66 fusion protein, followed by thrombin cleavage, would release a full length Oms66 protein with two additional amino acids at the N terminus.
The amplified oms66 gene was cloned into the T-tailed pCR-TOPO vector as described in the Invitrogen cloning manual. This construct was used for DNA sequence analysis, which was performed at the University of Montana Molecular Biology Sequencing Facility using the dideoxy chain termination method (39).
Purification of native Oms66.
B. burgdorferi 313 were inoculated into 2.5 liters of media and were incubated at 34°C for 5 days. Cells (approximately 2.5 × 1011) were then pelleted by centrifugation at 10,000 × g for 20 min and washed three times in phosphate-buffered saline (PBS). Final cell pellets were then subjected to a sequential detergent solubilization. This was done by first resuspending the pellets in 40 ml of 1% sodium lauryl sarcosinate. The suspension was then frozen at −20°C, thawed, and sonicated for 15 s. After sonication, insoluble material, which included most of the Oms66, was pelleted at 100,000 × g for 1 h at 4°C. The supernatant was removed, and the pellet was then resuspended in 20 ml of 0.5% octyl-polyoxyethylene (OPOE), sonicated, and centrifuged as described above. The supernatant was removed, and the pellet, which again included most of the Oms66, was resuspended in 20 ml of 1% hydrogenated Triton X-100 (hTX-100) and again sonicated and centrifuged. The supernatant from the hTX-100 solubilization was subjected to anion-exchange and chromatofocusing chromatography using a fast-protein liquid chromatography (FPLC) system (Pharmacia, Alameda, Calif.). For anion exchange, the buffer used was 50 mM Tris (pH 8.0), containing either 0.02% hTX-100 or 0.75% octyl glucoside, and elution was performed with a salt gradient of 0 to 1 M NaCl. For chromatofocusing, the starting buffer was 25 mM bis-Tris (pH 6.7), containing either 0.1% hTX-100 or 0.75% octyl glucoside, and the eluent was 10% Polybuffer 74 (Pharmacia), pH 5.0, containing either 0.1% hTX-100 or 0.75% octyl glucoside.
Lipid bilayer assays.
The method used was essentially as described previously (42). Briefly, Oms66 protein (25 ng), diluted in 0.1% Triton X-100, was added to a 1.0 M KCl–10 mM Tris (pH 8.0) solution bathing a lipid bilayer composed of azolectin (Avanti Polar Lipids, Alabaster, Ala.). Agar-agar silver chloride-silver electrodes were placed on each side of the membrane. One electrode was connected to a voltage source, and the other was connected to a current amplifier and chart recorder (with the output being monitored on a storage oscilloscope). A voltage of 50 mV was applied across the membrane, and conductance increases were recorded.
Production of recombinant Oms66.
E. coli DH5α, harboring the pOms66 plasmid, was grown in 6 liters of Luria broth containing 100 μg of ampicillin per ml. After the cells were grown to mid-log phase, expression of the Oms66-GST fusion protein was induced by the addition of 1.0 mM (final concentration) isopropyl-β-d-thiogalactopyranoside (IPTG; Pharmacia). Expression was allowed to proceed for 3 h, at which time the cells were pelleted and resuspended in 100 ml of PBS. Cells were then lysed by sonication, followed by the addition of Triton X-100 to a final concentration of 1%. The suspension was incubated for 1 h at room temperature, and insoluble material was pelleted by centrifugation at 10,000 × g for 15 min. The insoluble pellet was resuspended in 50 ml a solution containing 50 mM Tris (pH 8.0) and 8.0 M urea. Insoluble material was pelleted at 10,000 × g for 15 min. The supernatant was then dialyzed three times in 1 liter of 50 mM Tris (pH 8.0). After dialysis, 100 U of thrombin (Pharmacia) was added to the sample, followed by incubation for 16 h at 22°C. Five milliliters of the sample was solubilized in 5× solubilization mix (2% sodium dodecyl sulfate [SDS], 7.5% glycerol, 5% 2-mercaptoethanol, and 0.125 M Tris [pH 6.8] for a 1× solution) and was subjected to SDS-polyacrylamide gel electrophoresis (PAGE) on a preparative scale. After electrophoresis, gels were electroblotted onto polyvinylidine difluoride (PVDF) membranes (Millipore, Bedford, Mass.), and the membranes were stained with amido black. The 66-kDa band corresponding to the cleaved Oms66 moiety of the fusion protein was excised from the membrane. The protein was eluted from the membrane by incubating membrane strips for 30 min at 22°C in 50 mM Tris (pH 8.0) with 2% SDS and 1% Triton X-100. After the incubation, the strips were centrifuged at 10,000 × g for 15 min, and the supernatant was saved.
Western blots and dot blot analysis.
Following SDS-PAGE and electroblotting onto PVDF membranes, Western blot analyses were carried out using the Amersham ECL System (Amersham, Piscataway, N.J.). For dot blot analysis, preparations of the Oms66 protein were spotted onto nitrocellulose membranes with a pore size of 0.45 μm (Schleicher & Schuell, Keene, N.H.). The preparations used included native protein in 50 mM Tris (pH 8.0), native protein that had been heated for 5 min at 95°C, native protein that had been dried down and resuspended in an 8 M urea solution, and recombinant Oms66 in 50 mM Tris (pH 8.0). In each case, 2 μl of protein solution, containing 0.5 μg of protein, was spotted onto the membrane, dried, and then subjected to enhanced chemiluminescence detection. A 1:1,000 dilution of anti-Oms66 serum, prepared as described below, was used for antigen detection.
Immunization and challenge of animals.
Oms66 protein was used in either its native conformation or in a denatured form. Native Oms66 (nOms66) was in a solution containing 50 mM Tris (pH 8.0), 150 mM NaCl, and 0.02% Triton X-100. The denatured form (hOms66) was prepared by boiling native Oms66 in a 2% SDS solution, followed by acetone precipitation and then resuspension in the original Tris–NaCl–Triton X-100 buffer. Each protein preparation (without the addition of an adjuvant) was used to immunize a single adult New Zealand White rabbit (Irish Farms, Norco, Calif.). A primary injection of 5 μg of protein was administered subcutaneously in the back of the neck. Four weeks following the primary injections, rabbits were anesthetized with 45 mg of xylazine and 8.8 mg of ketamine per kg and injected with 1 μg of protein via the popliteal lymph node as follows. The popliteal region was shaved and swabbed with ethanol. The lymph node was then located and palpitated until it was visible beneath the skin, and the antigen was injected through the skin, directly into the lymph node. Boost immunizations were repeated at 2 and 3 months by popliteal lymph node injection. Serum was obtained prior to immunization (basal serum) and also prior to and 2 weeks after each boost. Infection-derived immune rabbit serum was produced by infecting rabbits intradermally with 6 × 107 virulent B. burgdorferi B31. Serum was obtained 2 weeks after infection at which time the animals were shown to have cleared the infection and were resistant to challenge reinfection. Immune serum was stored at −76°C until ready for use.
For mouse immunizations, soluble, nOms66 (in 50 mM Tris, 0.02% hTX100) was used to immunize six 8-week-old C3H HeJ mice. The initial injection and the first three boost immunizations were administered subcutaneously with a dose of 1 μg for the primary injection and a dose of 500 ng for each boost. The fourth boost immunization (1 μg) was given intrasplenically as follows. Mice were anesthetized using 200 mg of ketamine and 10 mg of xylazine per kg. The skin over the abdominal area covering the spleen was cut aseptically, exposing the spleen beneath the visceral peritoneum, and 50 μl of soluble protein (1 μg) was injected directly into the spleen, followed by suturing of the overlying skin.
Two weeks after the final boost immunization, mice were challenged with infected tissue from a donor mouse as previously described (2, 13). The donor mouse (adult C3H HeJ) was prepared by infection with 104 virulent B. burgdorferi B31. After 10 days, the mouse was anesthetized, and an ear was excised and cut into 2-by-2-mm tissue slices. These tissue slices were immediately implanted into the mice that were to be challenged. The implant procedure involved anesthetizing the mice, followed by aseptically making a small (4-mm) incision on the back of the mouse. The donor tissue was then placed beneath the skin, and the wound was closed. Three weeks after challenge, mice were sacrificed, and tissue biopsies were cultured in BSKII media containing 50 μg of rifampin and 100 μg of phosphomycin per ml. The cultures were examined every 3 days for growth of B. burgdorferi, and cultures were classified as negative only after 5 weeks of incubation.
Serum adsorptions.
rOms66 was conjugated to Sepharose 4B beads (Pharmacia) as follows: 500 μg of rOms66, in 1 ml of 0.1 M NaHCO3 (pH 8.0)–0.5 M NaCl, was added to 500 μl of CNBr-activated Sepharose 4B that had been washed in 1 mM HCl. The solution was mixed for 2 h at 22°C and then washed with the NaHCO3-NaCl buffer. The remaining active groups on the beads were blocked by incubating for 2 h in a solution of 0.1 M Tris (pH 8.0). The final product was then washed three times in alternating cycles of low pH buffer (0.1 M NaCH3COO, pH 4.0) and high pH buffer (0.1 M Tris, pH 8.0; 0.5 M NaCl). After the coupling process, 500 μl of anti-nOms66 rabbit serum was added directly to 150 μl the rOms66-conjugated beads and to Sepharose 4B beads that were not coupled to a ligand. The suspensions were incubated for 2 h at 22°C, and the beads were then pelleted at 500 × g and removed. The adsorption was repeated three times.
Bactericidal assays.
For borreliacidal assays, sera were tested for complement-dependent killing activity as follows. In a microtiter plate, serial dilutions of test sera were made in normal rabbit serum (NRS) which was heat inactivated (56°C for 30 min). Then, 10 μl of each test serum dilution was combined with 55 μl of PBS, 25 μl of guinea pig serum (GPS) (Sigma) as a source of active complement, and 10 μl of a B. burgdorferi suspension. The organisms used were harvested during the logarithmic phase of growth by centrifugation at 2,000 × g for 10 min, followed by a washing in a 1:1 mixture of PBS and NRS. The concentration of organisms was adjusted to 2 × 108 organisms/ml, such that 2 × 106 organisms were added to each well. Assays were performed using either B. burgdorferi B313, or virulent, low-passage B. burgdorferi B31. B31 was obtained from culture of skin biopsies of infected rabbits and was passaged only once prior to use. In order to determine whether complement-mediated killing was responsible for bactericidal activity, assays were also performed using heat-inactivated guinea pig serum. To ensure that complement alone was not killing the organisms, a control was included using 25 μl of GPS, 55 μl of PBS, 10 μl of NRS, and 10 μl of organisms without the addition of immune serum. All test mixtures were made in duplicate and were incubated at 34°C for 6 h, at which time 5 μl of each sample was transferred to 200 μl of BSKII medium. The mixture was incubated an additional 12 h, and another 5 μl was then transferred to 200 μl of medium. In order to test for residual complement activity, 50 μl of 5% sheep blood cells, sensitized with a 1:1,000 dilution of hemolysin (anti-sheep red blood cell antibody), was then added to each of the test wells, which were incubated at 37°C for 1 h. Lysis of blood cells after 1 h indicated the presence of residual complement. Sample mixtures transferred into fresh medium were incubated at 34°C for up to 8 days. Microscopic analysis was performed by dark-field microscopy every 2 days to look for the presence of viable B. burgdorferi. Cultures were judged to be negative if there was no growth evident by 8 days. The dilution at which no viable organisms were observed was considered the 100% killing endpoint.
Immunoelectron microscopy (IEM).
A total of 108 motile B. burgdorferi B31 and B313 cells were pelleted at 2,000 × g for 10 min. The pellets were resuspended in 20 μl of BSKII medium, followed by the addition of test sera, diluted in BSKII medium. Final dilutions of 1:40 were used for anti-nOms66 serum, anti-hOms66 serum, infection immune serum, and basal serum. The adsorbed serum was diluted 1:10, thus giving a final effective concentration of 1:40 (since the adsorbed serum was diluted 1:4 in the adsorption procedure). Organisms were incubated with the sera for 4 h, after which time 1.5 ml of a solution containing 0.15 M NaCl, 10 mM CaCl2, and 10 mM MgCl2 (SCM) was added. Organisms were pelleted at 2,000 × g for 10 min and were washed again in 1.5 ml of SCM. The organisms were then resuspended in 20 μl of SCM and incubated on a Parlodion 300 mesh copper grid for 10 min at 22°C. Grids were then washed four times in SCM and blocked for 30 min using 50% normal goat serum (NGS) and 50% SCM at room temperature in a humidified chamber. Grids were then washed eight times in SCM, followed by incubation for 1 h at 22°C in a humidified chamber with a 1:20 dilution (in 10% NGS–90% SCM) of goat anti-rabbit immunoglobulin which was conjugated to 10-nm colloidal gold particles (Sigma). Grids were washed eight times in SCM and eight times in distilled water and were then stained for 30 s using 2% uranyl acetate in water. Grids were then washed six times in distilled water and examined in a JEOL electron microscope using an accelerating voltage of 80 kV. For the enumeration of gold particle binding, particles were counted on the surface of 10 organisms.
RESULTS
Expression and purification of recombinant Oms66.
The oms66 gene from B. burgdorferi B313 was amplified by PCR and was then sequenced to confirm that the gene was identical to that of strain B31 (data not shown). The amplified gene was then cloned in frame into pGEX 4T-1, resulting in expression of a glutathione S-transferase (GST)-Oms66 fusion protein. Fusion protein expression resulted in an insoluble product that was subsequently solubilized with 8 M urea. After removal of the urea by dialysis, thrombin was used to cleave the Oms66 protein from its GST fusion partner. This resulted in a full-length, recombinant Oms66 protein (rOms66) that contained two extra amino acids at the N terminus (Gly and Ser). The GST moiety could not be used for affinity purification of the fusion protein because denaturation by urea abrogated binding to glutathione-conjugated Sepharose beads. Therefore, the cleaved Oms66 protein was purified to homogeneity by gel purification (data not shown).
Purification of native Oms66.
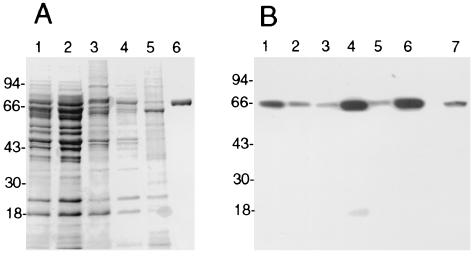
nOms66 was isolated from B. burgdorferi B313. This strain is deficient in the expression of a number of abundant lipoproteins (38), which facilitated the purification of nOms66. A sequential detergent solubilization procedure on insoluble extracts of the B313 strain was used in order to enrich for the Oms66 protein and to eliminate contaminating proteins. The first two detergents used in the extraction procedure (sodium lauryl sarcosinate and octyl-polyoxyethylene, respectively) were shown to release many contaminating proteins without releasing Oms66 from the insoluble extract (Fig. 1B, lanes 2 and 3). Subsequently, the supernatant from the final hTX-100 detergent solubilization was markedly enriched with the Oms66 protein (Fig. 1B, lane 4). After ion-exchange chromatography and chromatofocusing, the Oms66 protein was shown, by gel analysis, to be pure and free from other B. burgdorferi proteins (Fig. 1A, lane 6). In addition, Western immunoblot analysis, using serum obtained from B. burgdorferi infection-immune rabbits, showed that no B. burgdorferi proteins other than Oms66 were detectable (Fig. 1B, lane 7). During sequential steps of purification, fractions containing the Oms66 protein were analyzed using a planar lipid bilayer model membrane system. This provided a method to monitor for the presence of Oms66 and also enabled the determination of whether or not the purified protein maintained pore-forming ability. Upon the addition of 20 ng of protein to a lipid bilayer apparatus, a total of 176 channel insertion events were recorded, and the average single-channel conductance was found to be 9.8 nS. This was in accordance with previous findings indicating that Oms66 formed a large channel with a conductance of 9.62 nS (41). Given that the Oms66 protein retained pore-forming activity following the purification procedure, it was presumed that native conformation was also maintained.
FIG. 1.
Purification of Oms66 from B. burgdorferi B313. (A) Coomassie blue-stained SDS-PAGE gel showing the purification of Oms66. Lane 1 shows whole organisms solubilized in SDS-PAGE sample buffer. Lanes 2, 3, and 4 show the soluble material after the sequential extraction of whole organisms with 0.5% sodium lauryl sarcosinate, 0.5% octyl polyoxyethylene, and 1.0% hydrogenated Triton X-100, respectively. Lane 5 is the insoluble pellet, and lane 6 is FPLC-purified Oms66. (B) Western blot of the samples described for panel A, with an additional lane (lane 7) containing FPLC-purified Oms66. Lanes 1 to 6 were probed with anti-Oms66 antisera, and lane 7 was probed with anti-B. burgdorferi immune serum from infection-immune rabbits.
Immunization with nOms66 elicits conformationally determined bactericidal antibodies.
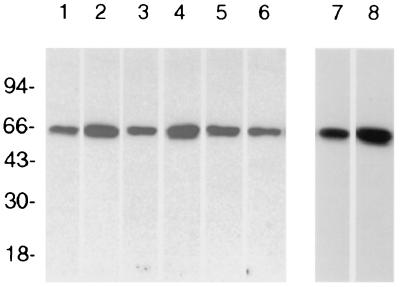
Because of the limited amount of attainable, purified nOms66, rabbits and mice were immunized with small amounts of protein (as low as 1 μg and 500 ng, respectively) via the popliteal lymph node and spleen, respectively. As shown in Fig. 2, a single rabbit and six mice immunized with purified nOms66 developed serum antibodies that reacted specifically with Oms66 on Western blots of whole organisms. These sera were then tested for complement-dependent bactericidal activity against both B. burgdorferi B31 and B313. As shown in Table 1, both the rabbit serum and the mouse sera possessed equally high titers (1:320 dilution) of complement-dependent bactericidal activity against strain B313. This activity was eight times greater than that observed using serum from infection-derived immune rabbits (1:40 dilution). No bactericidal activity was observed in the presence of heat-inactivated guinea pig serum as a complement source, indicating the complement-dependent nature of this activity. In comparison, these same sera showed no bactericidal activity against strain B31, even though this strain expresses Oms66. Recently, Bunikis and Barbour (4) have shown that Oms66 (p66) from strain B31 is closely associated with the major surface lipoprotein OspA and that OspA may hinder the access of antibodies to Oms66. Thus, one explanation for our findings is that anti-Oms66 antibody does not bind to Oms66 on the surface of strain B31 due to steric hindrance by OspA.
FIG. 2.
Western blot reactivities of serum from immunized animals. Each lane contains 5 × 106 B. burgdorferi B31. Lanes 1 to 6 were probed with antisera from mice (mouse 1 to mouse 6, respectively) immunized with nOms66. Lane 7 was probed with anti-nOms66 generated in a rabbit, and lane 8 was probed with anti-hOms66 generated in a rabbit.
TABLE 1.
Borreliacidal assay results: killing titers of immune sera against B. burgdorferi B31 and B313
| Immune serum | 100% killing titer (reciprocal)a
|
||
|---|---|---|---|
| B313 (basal) | B313 (4th boost) | B31 (4th boost) | |
| Mouseb | |||
| Oms66-1 | <20 | 320 | <20 |
| Oms66-2 | <20 | 320 | <20 |
| Oms66-3 | <20 | 320 | <20 |
| Oms66-4 | <20 | 320 | <20 |
| Oms66-5 | <20 | 320 | <20 |
| Oms66-6 | <20 | 160 | <20 |
| Rabbit | |||
| nOms66 | <20 | 320 | <20 |
| Adsorbed nOms66 | <20 | 160c | <20 |
| hOms66 | <20 | <20 | <20 |
| Infection immune | <20 | 40 | 160 |
100% killing indicates that after incubating organisms with immune serum and complement for 6 h, no viable organisms remained.
Oms66-1 to -6 represent results for the six different mice that were immunized with nOms66 protein.
The titer for the adsorbed serum was calculated with the assumption that the starting serum was diluted 1:4 compared to the unadsorbed serum.
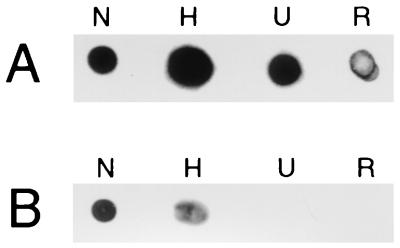
In an effort to determine whether native conformational epitopes of Oms66 were important to this demonstrated bactericidal activity, rabbit anti-nOms66 antiserum was adsorbed with denatured rOms66 to remove antibodies directed at nonconformational epitopes. As shown by SDS-PAGE immunoblot analysis using rOms66, this adsorption resulted in the removal of all detectable antibodies to rOms66 (Fig. 3, lane 3). Adsorption with control beads showed, by densitometric analysis, that reactivity was reduced by only fourfold compared to nonadsorbed serum (Fig. 3, lanes 1 and 2). This same finding of a complete loss of reactivity was also observed by dot blot analysis when the adsorbed serum was reacted against rOms66 (Fig. 4, column R) and nOms66 denatured in 8 M urea (Fig. 4, column U). Similarly, when nOms66 was heated at 95°C for 5 min prior to dot blotting, a significant loss of reactivity was also observed with the adsorbed serum (Fig. 4, column H). However, the adsorbed serum showed a minimal loss of reactivity when reacted against nOms66 (Fig. 4, column N). Interestingly, when tested for bactericidal activity against strain B313 (Table 1), the adsorbed serum maintained a high level of complement-dependent killing activity. In contrast, a rabbit immunized with the heated and denatured preparation of nOms66 (hOms66) elicited a significant anti-Oms66 antibody response (Fig. 2, lane 8), but serum from this animal showed no bactericidal activity against strain B313 (Table 1). Taken together, these findings suggest that native conformational epitopes on Oms66 are the primary targets of complement-dependent bactericidal antibody.
FIG. 3.
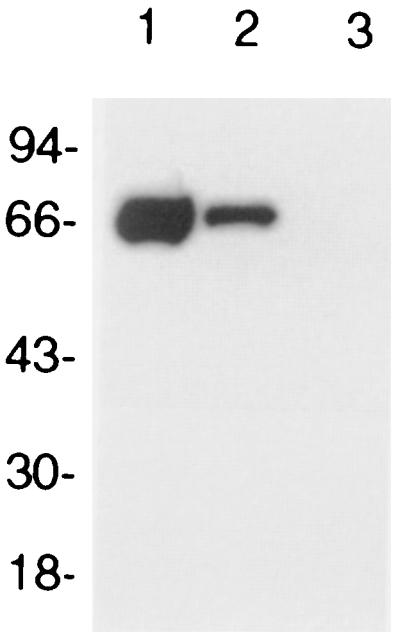
Western blot of adsorbed serum. A total of 1 μg of recombinant Oms66 was electrophoresed in each lane. Lane 1 was probed with anti-nOms66, lane 2 was probed with anti-nOms66 adsorbed with Sepharose 4B beads, and lane 3 was probed with anti-nOms66 adsorbed with Sepharose 4B beads conjugated to recombinant Oms66. Sera were used at a 1:1,000 dilution.
FIG. 4.
Dot blot of Oms66 probed with anti-Oms66 sera. (A) Dot blot probed with unadsorbed anti-nOms66 serum. (B) Dot blot probed with anti-nOms66 serum that had been adsorbed with recombinant Oms66 protein. N, native Oms66 protein; H, native Oms66 that had been heated to 95°C for 5 min; U, Oms66 denatured by the addition of 8 M urea; R, recombinant Oms66. Each spot contains 0.5 μg of the corresponding Oms66 protein.
Demonstration of conformationally determined epitopes of Oms66 on the surface of B. burgdorferi.
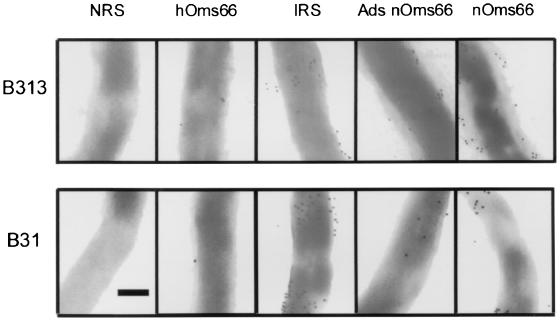
IEM was performed to compare the abilities of different rabbit antisera to bind whole B. burgdorferi. The sera tested were antiserum against nOms66 (anti-nOms66), anti-nOms66 that was adsorbed with recombinant Oms66 (Ads-anti-nOms66), antiserum against heated and denatured nOms66 (anti-hOms66), serum from rabbits infected with B. burgdorferi and shown to be immune to challenge reinfection (IRS), and NRS. All organisms used in the enumeration of particle binding were structurally intact, as judged by the absence of extracellular flagella and by the maintenance of cell structure. As is seen in Fig. 5 and Table 2, anti-nOms66 antibody showed significant binding to the surface of strain B313 (17.1 gold particles/μm2), whereas only 5.1 gold particles/μm2 bound to strain B31. This result correlated with the findings of bactericidal activity described above and confirmed that the accessibility of antibody binding to Oms66 on strain B31 is limited as Bunikis and Barbour have reported (4). The surface binding of anti-nOms66 on strain B313 was 2.28 times greater than that of IRS binding on these same organisms, a finding which is also consistent with the greater level of bactericidal activity of anti-nOms66 compared to IRS. The Ads-anti-nOms66 also bound to the surface of strain B313, although the amount of binding was reduced almost twofold (9.8 particles/μm2) relative to the nonadsorbed serum. Interestingly, this level of binding by Ads-anti-nOms66 was comparable to that of IRS (7.5 particles/μm2), yet Ads-anti-nOms66 showed fourfold-greater killing activity against strain B313 than IRS. These findings suggest that there is not a simple correlation between the amount of surface antibody binding and the bactericidal activity observed. In comparison, NRS and anti-hOms66 showed little or no binding to the surface of either strain B31 or B313, a result again consistent with their observed lack of bactericidal activity.
FIG. 5.
Immunoelectron microscopy. B. burgdorferi B31 and B313 were incubated with various sera. Sera used included NRS; hOms66, which is antiserum to denatured Oms66; IRS; Ads nOms66, which is antiserum to native Oms66 that had been adsorbed with recombinant Oms66; and nOms66, which is antiserum to native Oms66. After incubation, the cells were fixed to grids and were incubated with anti-rabbit immunoglobulin G conjugated to 10-nm colloidal gold. Each micrograph was taken at the same magnification. The bar represents 0.25 μm.
TABLE 2.
Enumeration of antibody binding to the surface of B. burgdorferi
| Organism | Antiserum | No. of gold particles bound/μm |
|---|---|---|
| B313 | nOms66 | 17.1 |
| B31 | nOms66 | 5.1 |
| B313 | Adsorbed nOms66 | 9.8 |
| B31 | Adsorbed nOms66 | 3.4 |
| B313 | Infection immune | 7.5 |
| B31 | Infection immune | 13.7 |
| B313 | hOms66 | 1.0 |
| B31 | hOms66 | 0.5 |
| B313 | Basal | 0.4 |
| B31 | Basal | 0.7 |
Protection conveyed by immunization with nOms66.
In order to determine if nOms66 immunization elicited protective immunity, six immunized mice were challenged by skin implantation using tissue from mice previously infected with B. burgdorferi B31 (strain B313 is avirulent and thus cannot be utilized for challenge studies). Barthold et al. (3) have shown that OspA is not expressed by host-adapted B. burgdorferi during mouse infection. As shown in Table 3, six naive mice challenged with host-adapted organisms all developed local and disseminated infection as shown by positive culture results of their skin (six of six), ear (six of six), and joint tissues (six of six). In addition, four of six spinal cord cultures and five of six bladder cultures were positive for this group. In comparison, four of the six Oms66 immunized mice were completely protected following implant challenge using host-adapted organisms. All four of these animals showed no infection, as judged by negative cultures of their skin, ear, joint, spinal cord, and bladder tissues. Two of the six immunized mice, however, did show both local skin and disseminated infection. Thus, these results demonstrate significant protection against host-adapted B. burgdorferi B31 following immunization with native Oms66.
TABLE 3.
Mouse protection data: results from culture of extracted tissues at 3 weeks after challenge
| Mouse | Presence (+) or absence (−)a of B. burgdorferi in:
|
||||
|---|---|---|---|---|---|
| Skin | Ear | Joint | Spinal cord | Bladder | |
| Oms66-1 | − | − | − | − | − |
| Oms66-2 | − | − | − | − | − |
| Oms66-3 | + | + | + | − | + |
| Oms66-4 | − | − | − | − | − |
| Oms66-5 | + | + | + | + | − |
| Oms66-6 | − | − | − | − | − |
| Naive-1 | + | + | + | + | + |
| Naive-2 | + | + | + | + | + |
| Naive-3 | + | + | + | − | + |
| Naive-4 | + | + | + | + | + |
| Naive-5 | + | + | + | + | + |
| Naive-6 | + | + | + | − | − |
+, Tissue was culture positive for B. burgdorferi; −, tissue remained culture negative after 5 weeks of incubation.
DISCUSSION
There has been considerable research directed towards the study of the Oms66 (p66) outer membrane protein of B. burgdorferi. It has been shown to function as a porin and an adhesin (11, 41), and both of these functions suggest a role for this protein in pathogenesis. This is the first report of its potential role as a protective immunogen. Our initial studies were carried out in order to determine whether antiserum produced against a native form of the Oms66 protein could elicit bactericidal activity. Subsequent studies were designed to determine the protective immunogenicity of the protein. In vitro killing activity has been correlated with protective immunity for a variety of bacterial pathogens (15, 28, 37, 47), as well as with B. burgdorferi (36). In addition to assays that originally used dark-field microscopy to monitor the killing of spirochetes through loss of motility, bactericidal assays for B. burgdorferi have been developed using dyes and fluorimetry (8, 9, 23, 26, 36) and by plating for viable counts (10). These borreliacidal assays have been an important parameter for assessing the activity of immune serum against B. burgdorferi (10, 22, 25). However, since we considered a 100% killing end point as a more meaningful correlate of protection, we used the presence or absence of growth, as observed microscopically, instead of colorimetric assays to ascertain the killing titer. Our assay utilized 25% guinea pig serum as the source of complement, which permitted growth of both B31 and B313 in the absence of immune serum. However, experiments undertaken in the original characterization of B. burgdorferi B313 indicated that growth of this strain was inhibited by complement (38). In our studies, complete abrogation of growth occurred only with the addition of immune serum to the complement source. The lack of complement-dependent growth inhibition can be partially explained by the fact that the complement source was diluted approximately 1:40 when organisms were transferred from the assay mixture into the BSKII growth medium. It is still possible that there was some degree of antibody-independent complement killing or complement-dependent growth inhibition, but since we were judging 100% killing titers, these differences were not observed. Moreover, since the amount of complement is consistent in each test well, yet killing activity increases with the amount of specific antibody added, it was evident that the killing activity against B313 was dependent upon specific antibody and was not simply due to complement sensitivity of B313.
The observation that B313, in contrast to B31, was killed by a high dilution of the nOms66 antiserum was particularly interesting. Previous studies have indicated that strain B313 has increased surface immunogold labeling of Oms66 (p66) compared to that of B31 (6). It has been suggested that this difference is the result of abundant surface lipoproteins that may be blocking the accessibility of antibodies to the Oms66 protein in B31. Recently, the direct observation that Osp lipoproteins limit the access of antibody and trypsin to Oms66 has further supported this concept (4). This same phenomenon of blocking access to Oms66 may explain why B31, which contains an abundance of surface exposed lipoproteins, was not killed by anti-Oms66 immune serum, whereas B313, which lacks OspA, -B, -C, and -D, was killed. The converse could also explain the decreased sensitivity of B313 to IRS. The Osp lipoproteins are extremely immunogenic, and antibodies to these proteins dominate in an immune response. The absence of these lipoproteins on the surface of an organism would thus render it less susceptible to the anti-Osp killing activity of infection immune serum.
The maintenance of bactericidal activity of the adsorbed anti-nOms66 serum and the lack of killing activity of the anti-hOms66 serum raise important issues regarding the nature of the Oms66 epitopes that are the targets for killing antibody. Removal of antibodies that bind epitopes on denatured rOms66 appears to have no effect on bactericidal activity. This suggests that conformational epitopes on the native protein, compared to the linear epitopes, are the key targets with respect to bactericidal activity. The failure of anti-hOms66 serum to kill B313 further supports this notion.
The nature of antibody binding to Oms66 was further addressed in protein blotting and immunoelectron microscopy studies. It is clear from the dot blots that the denaturation process abrogates binding of antibodies directed against conformational epitopes. The adsorbed sera retained some reactivity with heated nOms66, but upon more rigorous denaturation (heating in urea and precipitation using acetone) reactivity was completely eliminated. IEM results confirm the results of the bactericidal assays. The anti-nOms66 sera and the corresponding adsorbed sera both bind significantly to B313, whereas the anti-hOms66 serum shows negligible binding. It is noteworthy that the anti-nOms66 serum did bind to B31. This finding is in accord with previous studies (6), but it is nonetheless somewhat surprising, given that there was no in vitro bactericidal activity against B31 despite the apparent binding. However, the adsorbed sera shows insignificant binding to B31, and there are approximately twice as many particles bound to B313 with the unadsorbed serum compared to the adsorbed serum. This may indicate that there are a number of linear epitopes, recognized by the unadsorbed sera and accessible in B31, that do not play an important role in protective immunity. On the other hand, this could also relate to the relative abundance of antibodies in each serum, as judged by bactericidal assays; the adsorbed serum had a killing titer which was 0.5 times that of the unadsorbed serum, so lower numbers of bound gold particles could be expected.
As shown in this study, retaining the native conformation of Oms66 was essential for eliciting bactericidal antibodies. It has also been shown recently that a protective epitope of the lipoprotein OspC is conformational (18). Thus, all immunizations for protection studies were carried out using Oms66 protein in its native state. The protein was assayed for pore-forming ability after purification, and maintenance of this activity indicated that the protein was in its native conformation. Another important aspect of this study is the fact that the mice were challenged using skin implants from infected mice. It has been shown that host-adapted organisms downregulate OspA expression, thus presumably making Oms66 accessible to killing antibodies. In support of this concept is our previous finding that mice immunized with nOms66 are not protected following challenge with low-passage in vitro-cultivated organisms (data not shown).
While immunizations resulted in bactericidal activity as well as complete protection for four of six mice, it is possible that the immune response to Oms66 was not maximized. Relatively low amounts of protein were injected (1 μg) in a detergent-soluble form without any adjuvant; it is conceivable that further modifications in the immunization methodology may result in a significantly greater degree of protection. Another issue that must be addressed in future studies is whether or not there is heterologous protection, considering that the Oms66 protein shows variability in what is proposed to be a surface-exposed region (5).
The numbers of host-adapted B. burgdorferi used for the challenge of each immunized mouse were not determined in this study. It should be noted that these immunized mice were challenged with portions of the ears of B. burgdorferi-infected mice. Barthold has reported numbers of B. burgdorferi in the mouse ear ranging from zero to 3,200 (3). It is conceivable that the numbers of B. burgdorferi vary considerably from mouse to mouse or that different portions of the ear, e.g., the pinna, contain more spirochetes than do the more cartilaginous portions of the ear. The susceptibility of two of the six mice to infection could reflect challenge with substantially greater numbers of host-adapted Borrelia organisms. It is unlikely that differences in mouse antibody titers were involved with the variability in protection since the killing antibody titers were similar in all cases (Table 1), as were the Western blot reactivities of all of the mouse sera (Fig. 2).
In summary, our study has shown that immunization with Oms66 produces bactericidal antibodies and confers protection against challenge with host-adapted B. burgdorferi B31. The recognition of conformational epitopes is a critical property of the antibodies essential for in vitro bactericidal activity. Studies are currently in progress to define the Oms66 epitopes essential for bactericidal activity and to evaluate the optimize Oms66 immunization protocols in this regard.
ACKNOWLEDGMENTS
We thank Sven Bergstrom for providing antiserum to recombinant Oms66 and Bruce Kagan for the use of instruments for lipid bilayer analysis. We thank Joan Strange at the University of Montana Molecular Biology Sequencing Facility for her technical assistance with DNA sequencing. We are grateful to Ellen Shang for her helpful discussion.
This work was supported by National Institutes of Health grants AI-29733 to M. A. Lovett and AI-37312 to J. N. Miller.
REFERENCES
- 1.Barthold S W. Antigenic stability of Borrelia burgdorferi during chronic infections of immunocompetent mice. Infect Immun. 1993;61:4955–4961. doi: 10.1128/iai.61.12.4955-4961.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthold S W. Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect Immun. 1999;67:36–42. doi: 10.1128/iai.67.1.36-42.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein a immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunikis J, Barbour A G. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunikis J, Luke C J, Bunikiene E, Bergstrom S, Barbour A G. A surface-exposed region of a novel outer membrane protein (P66) of Borrelia spp. is variable in size and sequence. J Bacteriol. 1998;180:1618–1623. doi: 10.1128/jb.180.7.1618-1623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunikis J, Noppa L, Bergstrom S. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease Borrelia. FEMS Microbiol Lett. 1995;131:139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x. [DOI] [PubMed] [Google Scholar]
- 7.Bunikis J, Noppa L, Ostberg Y, Barbour A G, Bergstrom S. Surface exposure and species specificity of an immunoreactive domain of a 66-kilodalton outer membrane protein (P66) of the Borrelia spp. that cause Lyme disease. Infect Immun. 1996;64:5111–5116. doi: 10.1128/iai.64.12.5111-5116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callister S M, Jobe D A, Schell R F, Pavia C S, Lovrich S D. Sensitivity and specificity of the borreliacidal-antibody test during early Lyme disease: a “gold standard”? Clin Diagn Lab Immunol. 1996;3:399–402. doi: 10.1128/cdli.3.4.399-402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callister S M, Schell R F, Lim L C L, Jobe D A, Case K L, Bryant G L, Molling P E. Detection of borreliacidal antibodies by flow cytometry. An accurate, highly specific serodiagnostic test for Lyme disease. Arch Intern Med. 1994;154:1625–1632. [PubMed] [Google Scholar]
- 10.Cassatt D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coburn J, Chege W, Magoun L, Bodary S C, Leong J M. Characterization of a candidate Borrelia burgdorferi beta(3)-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–940. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- 12.Cowan S W, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit R A, Jansonius J N, Rosenbusch J P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 13.de Silva A M, Fikrig E, Hodzic E, Kantor F S, Telford III S R, Barthold S W. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J Infect Dis. 1998;177:395–400. doi: 10.1086/514200. [DOI] [PubMed] [Google Scholar]
- 14.de Silva A M, Telford S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeMaria T F, Murwin D M, Leake E R. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun. 1996;64:5187–5192. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fikrig E, Barthold S W, Marcantonio N, Deponte K, Kantor F S, Flaveil R A. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect Immun. 1992;60:657–661. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore R D, Jr, Kappel K J, Dolan M C, Burkot T R, Johnson B J. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilmore R D, Mbow M L. Conformational nature of the Borrelia burgdorferi B31 outer surface protein C epitope. Infect Immun. 1999;67:5463–5469. doi: 10.1128/iai.67.10.5463-5469.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M P, Dorward D W, Höök M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–53. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoenger A, Pagès J M, Fourel D, Engel A. The orientation of porin OmpF in the outer membrane of Escherichia coli. J Mol Biol. 1993;233:400–413. doi: 10.1006/jmbi.1993.1520. [DOI] [PubMed] [Google Scholar]
- 21.Hu L T, Klempner M S. Host-pathogen interactions in the immunopathogenesis of Lyme disease. J Clin Immunol. 1997;17:354–365. doi: 10.1023/a:1027308122565. [DOI] [PubMed] [Google Scholar]
- 22.Kochi S K, Johnson R C, Dalmasso A P. Complement-mediated killing of the Lyme disease spirochete Borrelia burgdorferi: role of antibody in formation of an effective membrane attack complex. J Immunol. 1991;146:3964–3970. [PubMed] [Google Scholar]
- 23.Lim L C, Liu Y F, Schell K, Lovrich S D, Callister S M, Schell R F. Detection of borreliacidal antibody by using acridine orange and flow cytometry. Clin Diagn Lab Immunol. 1994;1:44–50. doi: 10.1128/cdli.1.1.44-50.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovrich S D, Callister S M, DuChateau B K, Lim L C, Winfrey J, Day S P, Schell R F. Abilities of OspA proteins from different seroprotective groups of Borrelia burgdorferi to protect hamsters from infection. Infect Immun. 1995;63:2113–9. doi: 10.1128/iai.63.6.2113-2119.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovrich S D, Callister S M, Schmitz J L, Alder J D, Schell R F. Borreliacidal activity of sera from hamsters infected with the Lyme disease spirochete. Infect Immun. 1991;59:2522–2528. doi: 10.1128/iai.59.8.2522-2528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J, Coughlin R T. A simple colorimetric micro assay for borreliacidal activity of antisera. J Microb Methods. 1993;17:145–153. [Google Scholar]
- 27.Margolis N, Rosa P A. Regulation of expression of major outer surface proteins in Borrelia burgdorferi. Infect Immun. 1993;61:2207–2210. doi: 10.1128/iai.61.5.2207-2210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin D, Cadieux N, Hamel J, Brodeur B R. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J Exp Med. 1997;185:1173–1183. doi: 10.1084/jem.185.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuzawa T, Kurita T, Yanagihara Y. Negative finding in cross-protective activity of Japanese Borrelia isolates against infection with three species of Lyme disease Borrelia in outbred mice. Microbiol Immunol. 1997;41:733–736. doi: 10.1111/j.1348-0421.1997.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 30.Mbow M L, Gilmore R D, Titus R G. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect Immun. 1999;67:5470–5472. doi: 10.1128/iai.67.10.5470-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery R R, Malawista S E, Feen K J M, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munodzana D, McElwain T F, Knowles D P, Palmer G H. Conformational dependence of Anaplasma marginale major surface protein 5 surface-exposed B-cell epitopes. Infect Immun. 1998;66:2619–2624. doi: 10.1128/iai.66.6.2619-2624.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norton Hughes C A, Engstrom S M, Coleman L A, Kodner C B, Johnson R C. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect Immun. 1993;61:5115–5122. doi: 10.1128/iai.61.12.5115-5122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal S, Theodor I, Peterson E M, de la Maza L M. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15:575–82. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 35.Probert W S, Allsup K M, Lefebvre R B. Identification and characterization of a surface-exposed, 66-kilodalton protein from Borrelia burgdorferi. Infect Immun. 1995;63:1933–1939. doi: 10.1128/iai.63.5.1933-1939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousselle J C, Callister S M, Schell R F, Lovrich S D, Jobe D A, Marks J A, Wieneke C A. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J Infect Dis. 1998;178:733–741. doi: 10.1086/515382. [DOI] [PubMed] [Google Scholar]
- 37.Sacchi C T, Goria M C, de Lemos A P, Bradileone M C. Considerations on the use of Neisseria meningitidis class 5 proteins as meningococcal BC vaccine components. Vaccine. 1995;13:112–118. doi: 10.1016/0264-410x(95)80021-5. [DOI] [PubMed] [Google Scholar]
- 38.Sadziene A, Thomas D D, Barbour A G. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect Immun. 1995;63:1573–1580. doi: 10.1128/iai.63.4.1573-1580.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigal L H, Zahradnik T, Weinstein A, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, AdlerKlein D, Doherty T, Evans J, Malawista S E, Molloy P, Seidner A, Sabetta J, Simon H J, Klempner M S, Mays J, Marks D. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339:1638–1639. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 41.Skare J T, Mirzabekov T A, Shang E S, Blanco D R, ErdjumentBromage H, Bunikis J, Bergstrom S, Tempst P, Kagan B L, Miller J N, Lovett M A. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun. 1997;65:3654–3661. doi: 10.1128/iai.65.9.3654-3661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skare J T, Shang E S, Foley D M, Blanco D R, Champion C I, Mirzabekov T, Sokolov Y, Kagan B L, Miller J N, Lovett M A. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J Clin Investig. 1995;96:2380–2392. doi: 10.1172/JCI118295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 44.Struyvé M, Visser J, Adriaanse H, Benz R, Tommassen J. Topology of PhoE porin: the ‘eyelet’ region. Mol Microbiol. 1993;7:131–140. doi: 10.1111/j.1365-2958.1993.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Hoecke C, Comberbach M, De Grave D, Desmons P, Fu D, Hauser P, Lebacq E, Lobet Y, Voet P. Evaluation of the safety, reactogenicity and immunogenicity of three recombinant outer surface protein (OspA) lyme vaccines in healthy adults. Vaccine. 1996;14:1620–1626. doi: 10.1016/s0264-410x(96)00146-6. [DOI] [PubMed] [Google Scholar]
- 46.Weiss M S, Abele U, Weckesser J, Welte W, Schiltz E, Schulz G E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991;254:1627–30. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- 47.Wijewardana T G, Sutherland A D. Bactericidal activity in the sera of mice vaccinated with Pasteurella multocida type A. Vet Microbiol. 1990;24:55–62. doi: 10.1016/0378-1135(90)90050-6. [DOI] [PubMed] [Google Scholar]