Abstract
Anti‐angiogenic antibodies are widely used in the treatment of neovascular macular degeneration. Human antibody targeting C‐type lectin domain family 14 member A (CLEC14a) is potential therapeutic agents owing to its antiangiogenic activity. In the present study, we aimed to predict the human intraocular pharmacokinetic (PK) properties of an anti‐CLEC14a antibody. I‐125 labeled aflibercept and anti‐CLEC14a antibody were intravitreally injected into mice, rats, and rabbits. Single photon emission computed tomography/computed tomography imaging was performed, and the intraocular radioactivity concentration (%ID/ml) was obtained. The PK parameters in those three animal species were obtained by compartmental analysis. The PK parameters in humans were estimated by allometric scaling of the animal PK parameters with consideration of the hydrodynamic radius of the antibody. The mean half‐life values of intraocular I‐125‐labeled aflibercept in mice, rats, and rabbits were 1.13 days, 1.25 days, and 4.91 days, respectively, by analysis with a one‐compartment model. The predicted human half‐life of intraocular aflibercept was 5.75 days based on vitreal volume by allometric scaling. The half‐life values of intraocular I‐125‐labeled anti‐CLEC14a in mice, rats and rabbits were 1.05 days, 1.84 days, and 6.37 days, respectively, by analysis with a one‐compartment model. The predicted human half‐life of intraocular anti‐CLEC14a was 10.29 days based on vitreal volume. According to the hydrodynamic volume of the anti‐CLEC14a, the predicted human half‐life of intraocular anti‐CLEC14a was 9.81 days. The PK characteristics of the intraocular anti‐CLEC14a antibody were evaluated noninvasively in animals using I‐125 labeling, and the intraocular PK characteristics in humans were predicted using these animal data. This methodology can be applied for the development of new antiangiogenic antibodies to treat macular degeneration.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Allometric scaling based on multiple species of animals could be used in first‐in‐human studies.
WHAT QUESTION DID THIS STUDY ADDRESS?
Could molecular imaging using radiolabeled antibodies be used in the pharmacokinetic (PK) analysis of intravitreally injected antibodies and precise prediction of human PK parameters?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Molecular imaging using radiolabeled antibodies can be used in PK analysis.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Methodologies using molecular imaging can be applied for the development of new antibodies to treat macular degeneration by intravitreal injection.
INTRODUCTION
Intravitreal injection of anti‐angiogenic antibodies is currently the primary treatment option for neovascular macular degenerative disease, owing to its excellent efficacy in decreasing visual impairment. 1 , 2 , 3 Intravitreal anti‐angiogenic antibodies such as ranibizumab (Genetech Inc.), pegaptanib (OSI Pharmaceuticals), and aflibercept (Regeneron) are approved by the US Food and Drug Administration for the treatment of exudative macular degeneration and are widely used worldwide. 4 , 5 , 6 Among them, the aflibercept intravitreal injection has a higher affinity for VEGF than bevacizumab or ranibizumab and is more frequently used in intravitreal injection. 7 , 8 , 9 , 10 The greater VEGF binding affinity leads to a substantially longer duration of action in the eye, which is crucial in the management of neovascular macular diseases because it can reduce the incidence of rare serious adverse events that can result from the intravitreal injection procedure. 7
C‐type lectin domain family 14 member A (CLEC14a) is expressed exclusively in endothelial cells and plays an essential role in endothelial cell–cell contacts in angiogenesis. 11 CLEC14a is expressed at a very low level in healthy human and primate tissue but expressed in the vasculature of a range of solid human tumors. 12 A monoclonal antibody targeting CLEC14a has been reported to suppress tumor angiogenesis and tumor growth in mice. 13 , 14 Based on this anti‐angiogenic activity, the intravitreal injection of an anti‐CLEC14a antibody could be another potential application for the management of neovascular macular disease.
The prediction of human pharmacokinetic (PK) parameters based on preclinical animal data is critical, especially before first‐in‐human studies. The procedure for the intravitreal injection of anti‐angiogenic drugs is related to rare but serious adverse effects. 7 In the case of anti‐CLEC14a antibody, PK parameter prediction using interspecies allometric scaling considering the hydrodynamic radius of the antibody could be used in preparation for first‐in‐human intravitreal injection to avoid invasive intravitreal injection, which could lead to cardiovascular effects and less frequent cardiovascular accidents owing to the relation of the antibody to VEGF‐dependent angiogenesis. 14 , 15 , 16 , 17 Thus, human PK parameter prediction would be beneficial before a first‐in‐human study and could reduce the incidence of serious adverse effects. 18 Allometric scaling is based on the body size of animal species, and PK parameters are calculated from constants and body weight or body surface area; thus, scaling based on multiple species is more precise than that based on single species. 19 The human intravitreal antibody half‐life was predicted, and reasonable agreement with the experimental data in case studies of aflibercept, brolucizumab, and PEGylated Fabs was achieved. 16
Molecular imaging using radiolabeled drugs is used to determine the PKs and biodistribution of drugs in vivo. 20 , 21 , 22 , 23 With the administration of radiolabeled drugs, the systemic distribution and PKs can be measured by single‐photon emission computed tomography (SPECT) or positron emission tomography (PET), both of which are noninvasive molecular imaging methods that would be an excellent method in the exploration of new drugs. This approach maximizes noninvasiveness by replacing blood sampling and reducing the euthanization of animals at each timepoint. Molecular imaging using radiolabeled drugs in vivo could be used to evaluate the whole body temporally and spatially with visualization, 24 which is especially beneficial if the target organ is difficult to assess. In the present study, we aimed to predict the human PK parameters of a novel anti‐angiogenic antibody using allometric scaling, especially using noninvasive molecular imaging.
MATERIALS AND METHODS
Animals
Review and approval for the animal study was obtained from the Seoul National University Bundang Hospital Institutional Animal Care and Use Committee, and all procedures followed the guidelines of the Association for Research in Vision and Ophthalmology for research in animals (BA1609‐209/604–01). For allometric scaling, nine wild‐type 6‐week‐old male C57BL/6 mice (22–23 g), seven male Sprague–Dawley rats (200–230 g), and five male Dutch belted rabbits (1.5–2 kg) each were used to investigate the intraocular PK parameters of anti‐CLEC14a. Six wild‐type 6‐week‐old male C57BL/6 mice (22–23 g) with choroidal neovascularization (CNV), three male Sprague–Dawley rats (200–230 g) with CNV, and three male Dutch belted rabbits (1.5–2 kg) each were used to investigate the intraocular PK parameters of aflibercept. CNV was induced in the mice and rats as described previously and in the supplementary information. 25
Materials and radiolabeling
An antibody targeting CLEC14a was generated as described in a previous study. 26 Aflibercept and anti‐CLEC14a were radiolabeled using I‐125 (PerkinElmer) iodination tubes in phosphate buffer for 15 min (500 μCi of I‐125 for 100 μg of protein). Radiolabeled antibodies were purified, and excess iodide was removed by gel infiltration. The radiochemical purity of the radiolabeled antibodies was determined by silica‐gel‐based radio–thin‐layer chromatography (Bio Scan AC‐3000) using a solvent of 0.9% NaCl, followed by purification to 96% purity (Figure S1).
Intravitreal injection procedure and SPECT/CT imaging
Intravitreal injection of radiolabeled aflibercept and anti‐CLEC14a was performed in mice and rats after anesthesia was induced by the inhalation of 2% isoflurane in oxygen and intramuscular injection of Zoletil (Virbac Laboratories) and xylazine hydrochloride (Bayer AG). Topical anesthesia was then established by administering proparacaine hydrochloride eye drops to the eye after mydriatic eye drops (Mydrin‐P; Santen Pharmaceutical Co.) and povidone iodine (5%, w/v). A Hamilton syringe (Hamilton Company) with a 34‐gauge needle was used for injections in mice and rats, and a 30‐gauge needle was used for intravitreal injections in rabbits. The doses of I‐125‐labeled aflibercept and anti‐CLEC14a radioactivity per volume administered to mice, rats and rabbits were 1.41 MBq in 2 μl (2.0 mg/0.05 ml), 2.67 MBq in 4 μl (2.0 mg/0.05 ml) and 3.7 MBq in 20 μl (2.0 mg/0.05 ml), respectively, per animal. After intravitreal injection, in vivo SPECT/CT imaging of the whole body was performed using one animal SPECT/CT scanner (Nano SPECT/CT; Bioscan, Inc.) for mice and rats and another SPECT/CT scanner (NM/CT670, GE Healthcare) for rabbits under anesthesia. Images were obtained over the course of 2 weeks (5 h and 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, and 14 days). Animal SPECT images were acquired in 24 projections (60 s/projection) with helical scanning, an energy window peak of 28 keV, and a width of 10%. Computed tomography (CT) images were then acquired with a frame tube voltage of 45 kVp, a current of 0.22 mA, and a circular gantry rotation of 360 degrees.
Image and PK data analysis
Image analysis was performed using the PMOD fusion software tool version 4.1 (PMOD Technologies). The radioactivity of I‐125‐labeled aflibercept and anti‐CLEC14a antibody in the injected eye was determined by placing fitting a volume of interest to the ocular cavity. The result was decay‐corrected and calculated as a percentage of the injected dose (%ID). PK analysis was performed by compartmental analysis using PKanalix version 2021R1 (Lixoft).
Human PK parameter and mean elimination half‐life prediction
First, allometric scaling was performed, based on linear regression with log–log transformation, known as the general allometric equation (GAE), and the allometric power function was obtained to predict human PK parameters. Exponents and coefficients were obtained based on eyeball volume (PK parameter = α × vitreal volumeb). Human PK parameters were predicted by using exponents and coefficients obtained using 70 kg as the human body weight and 4 ml as the human eyeball volume. The GAE was applied using R software version 4.0.2 (The R Foundation for Statistical Computing, Austria).
Second, using consensus values from model‐based meta‐analysis, the predicted mean elimination terminal half‐life (t 1/2) was obtained using the hydrodynamic radius of the antibody. 16 We leveraged the existing t 1/2 information for a given Fab and extrapolate the t 1/2 of anti‐CLEC14a (IgG) in the rabbits. In case of anti‐CLEC14a, equations can be written as
Where = 3.0 nm, and = 6.51 days, with knowledge of typical F ab t 1/2 value in humans. 16
The hydrodynamic radius of anti‐CLEC14a was roughly estimated using the hydrodynamic radius converter website based on molecular weight, which was 4.54 nm. (https://www.fluidic.com/toolkit/hydrodynamic‐radius‐converter/).
RESULTS
Ocular PK properties of aflibercept
The log‐scale radioactivity of aflibercept in the intraocular space (expressed as %ID/ml) showed a rapid decline in mice and rats and a relatively gradual decrease in rabbits (Figure 1). A one‐compartment model best described the radioactivity of intraocular aflibercept, and the diagnostic plot of the one‐compartment model suggested that the model showed adequate predictive performance (Figure S2). As shown in the radioactivity concentration‐time profiles, the t 1/2 of intraocular aflibercept was longer in rabbits (152.95 h) than mice or rats (25.17 and 44.26 h, respectively; Table 1).
FIGURE 1.

Mean intraocular I‐125 aflibercept radioactivity concentration‐time profiles in mice, rats and rabbits after a single intravitreal injection (semi‐log scale). The radioactivity at each timepoint is expressed as a percentage of the injected dose
TABLE 1.
Ocular PK parameters of radiolabeled aflibercept in mice, rats, and rabbits
| Parameters | Mouse | Rat | Rabbit |
|---|---|---|---|
| t 1/2, h | 27.15 ± 9.58 | 29.95 ± 15.97 | 117.73 ± 25.97 |
| AUC0‐∞, %ID∙ml/h | 91961.88 ± 43165.64 | 44766.08 ± 5603.21 | 11937.7 ± 3210.98 |
| V d, ml | 0.046 ± 0.01 | 0.10 ± 0.058 | 1.44 ± 0.11 |
| CL, %ID/h | 0.0014 ± 0.00072 | 0.0023 ± 0.00026 | 0.0089 ± 0.0027 |
Note: Data are presented as mean ± SD.
Abbreviations: AUC0‐∞, area under the plasma concentration‐time curve from time zero to infinity; CL, clearance; PK, pharmacokinetic; t 1/2, terminal half‐life; V d, volume of distribution.
Ocular PK properties of anti‐CLEC14a
The log‐scale radioactivity of anti‐CLEC14a in the intraocular space (expressed as %ID/ml) showed a rapid decline in mice and rats and a gradual decrease in rabbits, analogous to that of aflibercept (Figure 2). A diagnostic plot of the predicted value using a one‐compartment model against the observed data for each individual animal is shown in the supplementary information, and the diagnostic plot of the one‐compartment model suggested that the model showed adequate predictive performance (Figure S3). Fused SPECT/CT images of mice, rats and rabbits over time after receiving an intravitreal injection of anti‐CLEC14a are shown in Figures S4–S6. The t 1/2 of intraocular anti‐CLEC14a in mice, rats, and rabbits was 1.05 days, 1.84 days, and 6.37 days, respectively, longer than that of aflibercept in rats and rabbits. The PK parameters based on the one‐compartment model in the three species are shown in Table 2.
FIGURE 2.

Mean intraocular I‐125 anti‐CLEC14a radioactivity concentration‐time profiles in mice, rats, and rabbits after a single intravitreal injection (semi‐log scale). The radioactivity at each timepoint is expressed as a percentage of the injected dose
TABLE 2.
Ocular PK parameters of radiolabeled anti‐CLEC14a in mice, rats, and rabbits
| Parameters | Mouse | Rat | Rabbit |
|---|---|---|---|
| t 1/2, h | 25.17 ± 28.02 | 44.26 ± 10.39 | 152.95 ± 13.25 |
| AUC0‐∞, %ID∙ml/h | 79140.4 ± 106,619 | 13099.27 ± 8631.72 | 4292.94 ± 541.55 |
| V d, ml | 0.053 ± 0.022 | 0.65 ± 0.32 | 5.16 ± 0.25 |
| CL, %ID/h | 0.0032 ± 0.022 | 0.011 ± 0.0062 | 0.024 ± 0.0031 |
Note: Data are presented as mean ± SD.
Abbreviations: AUC0‐∞, area under the plasma concentration‐time curve from time zero to infinity; CL, clearance; PK, pharmacokinetic; t 1/2, terminal half‐life; V d, volume of distribution.
Human ocular PK parameter prediction for aflibercept and anti‐CLEC14a using allometric scaling
Allometric scaling of ocular PK parameters was also performed based on both the body weight and the vitreal volume of the three species. The volume of distribution (V d) and the clearance (CL) of aflibercept and anti‐CLEC14a increased as the animal's vitreal volume increased (Figures 3 and 4, aflibercept and anti‐CLEC14a, respectively). Predicted human PK parameters are shown in Table 3. The predicted t 1/2 of human intraocular aflibercept was 138 h based on the vitreal volume. The predicted t 1/2 of anti‐CLEC14a was 247 h using the vitreal volume for allometric scaling, longer than that of aflibercept.
FIGURE 3.
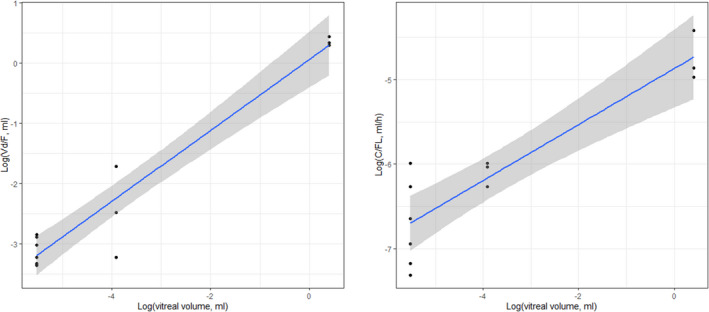
Allometric scaling of aflibercept parameters in three species of animals. Log–log scale of V d (left panel) and CL (right panel) based on vitreal volume. CL, clearance; V d, volume of distribution
FIGURE 4.

Allometric scaling of anti‐CLEC14a parameters in three species of animals. Log–log scale of V d (left panel) and CL (right panel) based on vitreal volume. CL, clearance; V d, volume of distribution
TABLE 3.
Prediction of human CL, V d, and half‐life of aflibercept and anti‐CLEC14a using allometric scaling based on vitreal volume
| Aflibercept | Anti‐CLEC14a | |
|---|---|---|
| CL, %ID/h | 0.012 (0.0042–0.035) | 0.0414 (0.00549–0.312) |
| V d, ml | 2.41 (0.827–7.01) | 14.8 (2.64–82.6) |
| t 1/2, h | 138 | 247 |
Note: Data are presented as mean predictive value (95% confidence interval).
Abbreviations: CL, clearance; t 1/2, terminal half‐life; V d, volume of distribution.
Second, the hydrodynamic radius was estimated based on the molecular weight of the antibody. Anti‐CLEC14a antibody used in the present study had molecular weight of approximately 150 kDa and a hydrodynamic radius of 4.54 nm. Based on the equation introduced by Caruso et al., 16 the predicted half‐life was 9.85 days.
DISCUSSION
In the present study, we predicted the intraocular PK parameters of anti‐angiogenic antibodies in humans by allometric scaling of the parameters in mice, rats, and rabbits based on the hydrodynamic radius. First, we analyzed the PK parameters of intraocular aflibercept in those three species and predicted the human intraocular PK parameters by allometric scaling. The predicted t 1/2 of aflibercept in human vitreous was shorter than the previously reported intraocular half‐life of aflibercept in humans (~9–11 days). 16 , 27 After predicting human intraocular PK parameters using allometric scaling, we analyzed the PK parameters of intraocular anti‐CLEC14a in the same three animal species and performed allometric scaling. Second, we predicted the t 1/2 of human intravitreal anti‐CLEC14a based on the hydrodynamic radius.
The intraocular PK characteristics of aflibercept observed in this study are similar to previously reported results in nonclinical and clinical studies. The radioactivity concentration decreased more rapidly in mice and rats than in rabbits, but this elimination pattern could be possible considering the rapid elimination of the antibody from the rabbit eye due to the small size of the ocular cavity. Similar trends of radioactivity over time have been reported after the intravitreal injection of 89Zr‐labeled aflibercept and 89Zr‐labeled deferoxamine in rats and 99mTc‐labeled compounds in mice was shown. 28 , 29 Other studies have reported a more gradual decrease in the concentration of intravitreally injected FITC‐labeled dextran. 30 Because the radioisotope used to label the antibody is very small compared to the protein of the antibody itself, a rapid decline in the intravitreal concentration of the radiolabeled antibody is not likely to be due to the radiolabeling itself. We understand that the elimination of macromolecules through the anterior chamber is determined by their size, 31 and the data from mice and rats indicated a rapid decline in the radiolabeled antibody concentration.
In rabbits, the half‐life of intravitreally injected aflibercept ranges from 3.92 days to 4.79 days according to the manufacturer, conventional immunoassays, and PET/CT. 8 , 9 , 20 In our study, the half‐life of intravitreally injected aflibercept in rabbits was 4.91 days, comparable to the value reported by the manufacturer. The difference in half‐life could be due to differences in methodologies (PET/CT imaging, immunoassay, and SPECT/CT imaging) and rabbit breeds. In the case of rats, the half‐life was 4.69 days in the Zr‐89‐labeled aflibercept PET/CT study, longer than the half‐life in rats with CNV (1.25 days) in the present study. 28 A low number of rats and possible injection errors could contribute to underestimation of the half‐life. In the case of mice, the half‐life was 1.13 days; to our knowledge, our study is the first to evaluate the PK parameters of aflibercept in mice. Based on the PK parameters in these three species, the human intraocular V d and CL of aflibercept were predicted. The predicted half‐life of human intraocular aflibercept was 5.75 days based on vitreal volume. The half‐life of intraocular aflibercept in patients with age‐related macular degeneration has been reported to be ~9–11 days, longer than the predicted half‐life based on body weight in our study. 16 , 27
To predict the PK parameters of intravitreally injected anti‐CLEC14a in humans, we performed allometric scaling based on data from three species. Allometric scaling is widely used for the prediction of human PK parameters, including premature ocular PK parameters. 32 , 33 However, due to numerous observed failures, various methods are adopted for the better prediction of PK parameters. 34 , 35 The rule of exponent technique is generally accepted, and the exponent obtained is based on body weight. 36 In our study, the body weight of rabbits was ~1.5 kg, and the human body weight was assumed to be 70 kg. However, the prediction would be incorrect considering the rabbit and human eyeball volume. The human eye has a larger vitreous cavity but has a larger serum compartment than the rabbit eye. 37 Therefore, we performed allometric scaling based on both body weight and vitreal volume. The predicted half‐life of intraocular aflibercept was comparable to previously reported values when allometric scaling was based on body weight.
Drugs are eliminated from the vitreous cavity via biotransformation or physical elimination to the blood circulation, 38 and anterior and posterior routes are available for intravitreally injected drugs. The proportion of macromolecule CL mediated by the retinal pigment epithelium has been reported to be relatively small, accounting for approximately only 3–20% of the injected dose. 39 Therefore, intravitreally injected anti‐VEGF could be eliminated mainly via the anterior route. Based on anterior‐route drug elimination, another robust prediction of human intravitreal PK parameters was suggested, using rabbits as a reference animal. 38 A reliable rabbit‐to‐human comparison was performed and a good correlation of PK parameters between the human and rabbit eye was found, with comparable absolute values. However, the intravitreal half‐life of aflibercept in humans predicted by that equation (human CLivt [ml/h] = 1.41 × rabbit CLivt +0.04) using our data (52.8 h, using a fixed V d of 4 ml) is not compatible with the known intravitreal half‐life of aflibercept in humans (9–11 days, ~250 h). We used radioactivity for the determination of intravitreal concentrations, and the difference in assay methodology could be one of the reasons for the discrepancy.
There were several previous studies which showed that intravitreal half‐life of antibodies correlates well with the hydrodynamic molecular radius. 16 , 40 The molecular size of anti‐CLEC14a (150 kDa) is bigger than aflibercept (115 kDa) and the predicted intravitreal half‐life considering hydrodynamic radius of anti‐CLEC14a and aflibercept in humans were 9.85 days and 9.8 days, respectively. In a previous study presented by Del Amo et al., there was no apparent differences of CL and V d around 100 kDa. 39 The relatively large V d of intravitreal anti‐CLEC14a than aflibercept would likely contribute to a longer half‐life despite the large CL, which was in the range of the CL and V d reported by Del Amo et al. CLEC14a is a type I transmembrane protein with an extracellular C‐type lectin domain related to cell–cell contact in angiogenesis, 41 and CLEC14a expression has been confirmed in the retina. 42 , 43 Target‐mediated elimination kinetics would apply, and drug binding to the extracellular target would influence the overall drug CL. 38
Owing to its inhibition of angiogenesis, the use of anti‐CLEC14a antibody has been applied in the treatment of neovascular age‐related macular degeneration. 44 Anti‐VEGF antibodies are widely used in the management of neovascular degenerative macular diseases, but the duration and efficacy of these treatments still need to be improved. 45 , 46 In the present study, we found that the intraocular half‐life of the anti‐CLEC14a antibody is longer than that of aflibercept in rabbits, and longer half‐life was also predicted in humans. Based on this finding, we could expect that anti‐CLEC14a will have a longer injection interval than conventional anti‐VEGF antibodies.
There are several limitations to the present study. First, the animal models among the species used for allometric scaling were heterogeneous. CNV was induced in the mice and rats but not the rabbits. The role of a diseased blood‐retinal barrier (BRB), such as that in CNV, in ocular PKs still needs to be elucidated, but there is no strong evidence that damage to the BRB would influence major changes in ocular PKs. 38 Therefore, we performed allometric scaling based on vitreal volume and other prediction methods based on previous studies that did not include both mouse and rat data for precise prediction. 16 , 39 The heterogeneous conditions of the three species of animals could impact the predicted human PK parameters. To minimize heterogeneous conditions, PK evaluation with the intraocular anti‐CLEC14a antibody was performed in the intact eyes of all three animal species. Another limitation of the study is that we analyzed whole ocular cavities, which could not be discriminated as three spaces, as in previous studies. 9 , 47 Nonetheless, we were able to perform PK evaluation without euthanizing animals for invasive sampling. The formation of antidrug antibodies is another limitation of preclinical animal studies, which necessitates nonhuman primate studies. 48 De Zafra et al. investigated several sponsors with intravitreal administration and antidrug antibody formation in rabbits and found that interpretation of the PKs would be influenced by ocular inflammation. In the present study, we did not investigate the presence of antidrug antibodies. We performed a single intravitreal injection followed by SPECT/CT imaging, which truly follows the 3R principle (replacement, reduction, and refinement). A single intravitreal injection is the least immunogenic compared to multiple intravitreal injections. Using a single intravitreal injection followed by SPECT/CT imaging over possibly less than 14 days of evaluation and caution thereafter would be optimal in consideration of antidrug antibody formation. However, an antidrug antibody investigation should be included in future preclinical studies.
In conclusion, the PK characteristics of intravitreal aflibercept and an anti‐CLEC14a antibody were evaluated noninvasively in animals using I‐125 labeling, and the intravitreal PK characteristics in humans were predicted by allometric scaling of those animal data. The molecular weight of the antibody was directly related to the half‐life, and prediction of the human CL with a fixed V d could not be applied according to the antibody. This methodology can be applied for the development of new antiangiogenic antibodies to treat macular degeneration.
AUTHOR CONTRIBUTIONS
S.P. and J.O. wrote the manuscript. S.P. and H.Y.L. designed the research. Y.W.L. and S.J.K. performed the research. S.P., S.L., and J.O. analyzed the data. S.L. contributed new reagents/analytical tools.
FUNDING INFORMATION
This research was supported by a grant (NRF‐2013M3A9D5072560) from Korea Mouse Phenotyping Projects of the National Research Foundation (NRF) funded by the Ministry of Science and ICT, Republic of Korea.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
Supporting information
Appendix S1
Park S, Lee YW, Oh J, Kim SJ, Lee S, Lee H‐Y. Pharmacokinetic evaluation of radiolabeled intraocular anti‐CLEC14a antibody in preclinical animal species and application in humans. Clin Transl Sci. 2022;15:2938‐2946. doi: 10.1111/cts.13412
REFERENCES
- 1. Jager RD, Mieler WF, Miller JW. Age‐related macular degeneration. N Engl J Med. 2008;358(24):2606‐2617. [DOI] [PubMed] [Google Scholar]
- 2. Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single‐blinded, randomised, controlled, phase 2b, non‐inferiority trial. Lancet (London, England). 2017;389(10085):2193‐2203. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell P, Liew G, Gopinath B, Wong TY. Age‐related macular degeneration. Lancet (London, England). 2018;392(10153):1147‐1159. [DOI] [PubMed] [Google Scholar]
- 4. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age‐related macular degeneration. N Engl J Med. 2006;355(14):1432‐1444. [DOI] [PubMed] [Google Scholar]
- 5. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age‐related macular degeneration. N Engl J Med. 2006;355(14):1419‐1431. [DOI] [PubMed] [Google Scholar]
- 6. Chapman JA, Beckey C. Pegaptanib: a novel approach to ocular neovascularization. Ann Pharmacother. 2006;40(7–8):1322‐1326. [DOI] [PubMed] [Google Scholar]
- 7. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap‐eye) in wet age‐related macular degeneration. Ophthalmology. 2012;119(12):2537‐2548. [DOI] [PubMed] [Google Scholar]
- 8. Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92(5):667‐668. [DOI] [PubMed] [Google Scholar]
- 9. Park SJ, Choi Y, Na YM, et al. Intraocular pharmacokinetics of intravitreal aflibercept (Eylea) in a rabbit model. Invest Ophthalmol Vis Sci. 2016;57(6):2612‐2617. [DOI] [PubMed] [Google Scholar]
- 10. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two‐year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kong DH, Kim MR, Jang JH, Na HJ, Lee S. A review of anti‐angiogenic targets for monoclonal antibody cancer therapy. Int J Molec Sci. 2017;18(8):1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robinson J, Whitworth K, Jinks E, Nagy Z, Bicknell R, Lee SP. An evaluation of the tumour endothelial marker CLEC14A as a therapeutic target in solid tumours. J Pathol Clin Res. 2020;6(4):308‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mura M, Swain RK, Zhuang X, et al. Identification and angiogenic role of the novel tumor endothelial marker CLEC14A. Oncogene. 2012;31(3):293‐305. [DOI] [PubMed] [Google Scholar]
- 14. Kim TK, Park CS, Jang J, et al. Inhibition of VEGF‐dependent angiogenesis and tumor angiogenesis by an optimized antibody targeting CLEC14a. Mol Oncol. 2018;12(3):356‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Semeraro F, Morescalchi F, Parmeggiani F, Arcidiacono B, Costagliola C. Systemic adverse drug reactions secondary to anti‐VEGF intravitreal injection in patients with neovascular age‐related macular degeneration. Curr Vasc Pharmacol. 2011;9(5):629‐646. [DOI] [PubMed] [Google Scholar]
- 16. Caruso A, Füth M, Alvarez‐Sánchez R, et al. Ocular half‐life of intravitreal biologics in humans and other species: Meta‐analysis and model‐based prediction. Mol Pharm. 2020;17(2):695‐709. [DOI] [PubMed] [Google Scholar]
- 17. Agrahari V, Mandal A, Agrahari V, et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016;6(6):735‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li C, Zhang C, Deng R, et al. Prediction of human pharmacokinetics of antibody–drug conjugates from nonclinical data. Clin Transl Sci. 2019;12(5):534‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fogli S, Del Re M, Rofi E, Posarelli C, Figus M, Danesi R. Clinical pharmacology of intravitreal anti‐VEGF drugs. Eye (Lond). 2018;32(6):1010‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christoforidis JB, Williams MM, Kothandaraman S, Kumar K, Epitropoulos FJ, Knopp MV. Pharmacokinetic properties of intravitreal I‐124‐aflibercept in a rabbit model using PET/CT. Curr Eye Res. 2012;37(12):1171‐1174. [DOI] [PubMed] [Google Scholar]
- 21. Boss DS, Olmos RV, Sinaasappel M, Beijnen JH, Schellens JHM. Application of PET/CT in the development of novel anticancer drugs. Oncologist. 2008;13(1):25‐38. [DOI] [PubMed] [Google Scholar]
- 22. Park HS, Kim E, Moon BS, Lim NH, Lee BC, Kim SE. In vivo tissue pharmacokinetics of carbon‐11‐labeled clozapine in healthy volunteers: a positron emission tomography study. CPT Pharmacometrics Syst Pharmacol. 2015;4(5):305‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy PS, Patel N, McCarthy TJ. Has molecular imaging delivered to drug development? Philos Trans A Math Phys Eng Sci. 2017;375(2107):20170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson CJ, Lewis JS. Current status and future challenges for molecular imaging. Philos Transact A Math Phys Eng Sci. 2017;375(2107):20170023. [DOI] [PubMed] [Google Scholar]
- 25. Ahn SJ, Lee HY, Hong HK, et al. Preclinical SPECT imaging of choroidal neovascularization in mice using integrin‐binding [(99m)Tc]IDA‐D‐[c(RGDfK)](2). Mol Imaging Biol. 2019;21(4):644‐653. [DOI] [PubMed] [Google Scholar]
- 26. Ki MK, Jeoung MH, Choi JR, et al. Human antibodies targeting the C‐type lectin‐like domain of the tumor endothelial cell marker clec14a regulate angiogenic properties in vitro. Oncogene. 2013;32(48):5449‐5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Do DV, Rhoades W, Nguyen QD. Pharmacokinetic study of intravitreal aflibercept in humans with neovascular age‐related macular degeneration. Retina (Philadelphia, PA). 2020;40(4):643‐647. [DOI] [PubMed] [Google Scholar]
- 28. Luaces‐Rodríguez A, Del Amo EM, Mondelo‐García C, et al. PET study of ocular and blood pharmacokinetics of intravitreal bevacizumab and aflibercept in rats. Eur J Pharm Biopharm. 2020;154:330‐337. [DOI] [PubMed] [Google Scholar]
- 29. Schmitt M, Hippeläinen E, Raviña M, et al. Intravitreal pharmacokinetics in mice: SPECT/CT imaging and scaling to rabbits and humans. Mol Pharm. 2019;16(10):4399‐4404. [DOI] [PubMed] [Google Scholar]
- 30. Sadeghi A, Puranen J, Ruponen M, et al. Pharmacokinetics of intravitreal macromolecules: scaling between rats and rabbits. Eur J Pharm Sci. 2021;159:105720. [DOI] [PubMed] [Google Scholar]
- 31. Keshet Y, Gal‐Or O, Schaap Fogler M, et al. Aflibercept clearance through the drainage system in a rat model. International journal of retina and vitreous. 2021;7(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eissing T. Allometric considerations on proteins administered intravitreally to children. CPT Pharmacometrics Syst Pharmacol. 2018;7(11):703‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spandau U. What is the optimal dosage for intravitreal bevacizumab for retinopathy of prematurity? Acta Ophthalmol. 2013;91(2):e154. [DOI] [PubMed] [Google Scholar]
- 34. Tang H, Hussain A, Leal M, Mayersohn M, Fluhler E. Interspecies prediction of human drug clearance based on scaling data from one or two animal species. Drug Metab Dispos. 2007;35(10):1886‐1893. [DOI] [PubMed] [Google Scholar]
- 35. Boxenbaum H. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J Pharmacokinet Biopharm. 1982;10(2):201‐227. [DOI] [PubMed] [Google Scholar]
- 36. Mahmood I, Balian JD. Interspecies scaling: predicting clearance of drugs in humans. Three different approaches. Xenobiotica. 1996;26(9):887‐895. [DOI] [PubMed] [Google Scholar]
- 37. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114(12):2179‐2182. [DOI] [PubMed] [Google Scholar]
- 38. Del Amo EM, Rimpelä AK, Heikkinen E, et al. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:134‐185. [DOI] [PubMed] [Google Scholar]
- 39. Del Amo EM, Urtti A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp Eye Res. 2015;137:111‐124. [DOI] [PubMed] [Google Scholar]
- 40. Shatz W, Hass PE, Mathieu M, et al. Contribution of antibody hydrodynamic size to vitreal clearance revealed through rabbit studies using a species‐matched fab. Mol Pharm. 2016;13(9):2996‐3003. [DOI] [PubMed] [Google Scholar]
- 41. Rho SS, Choi HJ, Min JK, et al. Clec14a is specifically expressed in endothelial cells and mediates cell to cell adhesion. Biochem Biophys Res Commun. 2011;404(1):103‐108. [DOI] [PubMed] [Google Scholar]
- 42. Lee S, Rho S‐S, Park H, et al. Carbohydrate‐binding protein CLEC14A regulates VEGFR‐2– and VEGFR‐3–dependent signals during angiogenesis and lymphangiogenesis. J Clin Invest. 2017;127(2):457‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khan KA, McMurray JL, Mohammed F, Bicknell R. C‐type lectin domain group 14 proteins in vascular biology, cancer and inflammation. FEBS J. 2019;286(17):3299‐3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park SW, Park UC, Hong IH, et al. Human antibody targeting C‐type lectin‐like domain of CLEC14a as a potential therapy for neovascular age‐related macular degeneration. Invest Ophthalmol Vis Sci. 2019;60(9):2988. [Google Scholar]
- 45. Smith SD, Kapoor K, Wagner A. High‐dose, High‐frequency intravitreal aflibercept for Wet AMD. Invest Ophthalmol Vis Sci. 2020;61(7):4234. [Google Scholar]
- 46. Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc. 2012;87(1):77‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park SJ, Oh J, Kim YK, et al. Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor‐Trap in a rabbit model. Eye (Lond). 2015;29(4):561‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Zafra CLZ, Sasseville VG, Matsumoto S, et al. Inflammation and immunogenicity limit the utility of the rabbit as a nonclinical species for ocular biologic therapeutics. Regulatory toxicology and pharmacology: RTP. 2017;86:221‐230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


