Abstract
N,N‐dimethyltryptamine (DMT) is a psychedelic compound that is believed to have potential as a therapeutic option in several psychiatric disorders. The number of clinical investigations with DMT is increasing. However, very little is known about the pharmacokinetic properties of DMT as well as any relationship between its exposure and effects. This study aimed to characterize population pharmacokinetics of DMT as well as the relationship between DMT plasma concentrations and its psychedelic effects as measured through subjective intensity ratings. Data were obtained from 13 healthy subjects after intravenous administration of DMT. The data were analyzed using nonlinear mixed‐effects modeling in NONMEM. DMT plasma concentrations were described by a two‐compartment model with first‐order elimination leading to formation of the major metabolite indole 3‐acetic acid. The relationship between plasma concentrations and psychedelic intensity was described by an effect site compartment model with a sigmoid maximum effect (E max) response. DMT clearance was estimated at 26 L/min, a high value indicating elimination of DMT to be independent of blood flow. Higher concentrations of DMT were associated with a more intense experience with the concentration of DMT at the effect site required to produce half of the maximum response estimated at 95 nM. The maximum achievable intensity rating was 10 and the simulated median maximum rating was zero, 2, 4, 8, and 9 after doses of 1, 4, 7, 14, and 20 mg, respectively. The model can be useful in predicting suitable doses for clinical investigations of DMT based on the desired intensity of the subjective experience.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
N,N‐dimethyltryptamine (DMT) is a psychedelic compound being investigated as a therapeutic option in psychiatric disorders. Very little is known about its pharmacokinetic/pharmacodynamic properties.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study aimed to investigate the pharmacokinetics of DMT and to quantify the relationship between DMT concentrations and intensity of the psychedelic experience after intravenous administration of DMT to 13 healthy subjects.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
DMT pharmacokinetics were characterized, showing a large DMT clearance of 26 L/min. The relationship between DMT concentrations and psychedelic intensity was also described. The applicability of the model was demonstrated through simulations of obtained intensity at different dose levels, illustrating what doses may be required for suboptimal and maximal responses, respectively.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This is the first time the pharmacokinetics/pharmacodynamics of DMT has been modeled. We believe this model can be useful in guiding DMT dosing in future clinical research. Further, the work demonstrates the benefits of using a quantitative pharmacokinetic/pharmacodynamic approach in future clinical development of psychedelics in general.
INTRODUCTION
Globally, ~ 300 million people are estimated to suffer from depressive disorders, making this one of the single largest contributors to non‐fatal health losses. 1 Around the same number of people suffer from a range of anxiety disorders. Still, a substantial part of the patient population does not respond to standard treatment. 2 The need for new therapeutic options in psychiatric disorders has contributed to renewed interest in the classic serotonergic psychedelics. 3 , 4 , 5 , 6 Early studies have shown these compounds to have remarkably long‐lasting effects after only a single or few dose occasions. 3 , 4 , 5 , 6
N,N‐dimethyltryptamine (DMT; [molecular weight 188.27 g/mol]) is a naturally occurring compound, belonging to the classical serotonergic psychedelics. It is a weak base with a pKa of 8.68 and a logP of 2.573. 7 DMT is the main psychoactive component in ayahuasca, a plant‐based tea traditionally used by indigenous peoples in South America. 8 Ayahuasca has shown potential as a treatment alternative in depression. 9 , 10 However, it is not clear whether these effects can be attributed to DMT alone. Nevertheless, DMT has been shown to elicit antidepressive effects in preclinical models. 11 DMT is believed to exert its effect primarily through agonism at the 5‐HT2A receptor, although several other targets have been identified, including various 5‐HT receptors, the sigma‐1 receptor, as well as trace amine receptors. 12 , 13 , 14 Furthermore, DMT is believed to be produced endogenously and is being used as a tool in research investigating the neurobiology of the human consciousness. 15
DMT is not orally available when administered alone. 16 This is believed to be mainly due to extensive metabolism by monoamine oxidase (MAO)‐A with the formation of the inactive metabolite indole 3‐acetic acid (IAA). Most research on DMT has thus been performed through administration of ayahuasca because the brew contains harmala alkaloids which acts as inhibitors of MAO‐A, making DMT available to the system. After ayahuasca administration, several other metabolites have been identified. With the exception of IAA, DMT N‐oxide has been identified as the most prominent. 17 However, no DMT N‐oxide was observed after intravenous administration of DMT, 18 indicating that the metabolic pattern may be influenced by the route of administration as well as the presence or absence of MAO inhibitors. Further, research shows that the fraction excreted unchanged in urine is less than 1% after oral intake of DMT/ayahuasca, whereas this fraction was observed to increase to ~ 10% after smoking of DMT. 16 , 17 After intravenous administration, DMT is rapidly eliminated leading to a very short half‐life. 19 However, reports thoroughly examining DMT disposition in humans are scarce. In one study, aimed at exploring target‐controlled intravenous infusion to extend effect duration, pharmacokinetic (PK) parameters were suggested. 20 However, these estimates have to be regarded with caution with some values being physiologically implausible. Thus, no reliable estimates of the PK parameters describing DMT disposition in humans have been published to date.
The time course of psychedelic effects has been observed to follow DMT plasma concentrations somewhat closely with a duration of less than 30 min after intravenous bolus administration. 15 , 21 However, the relationship has never been quantified.
Consequently, despite the increasing number of clinical investigations with DMT, much work remains in terms of understanding the basic clinical pharmacology of this compound. Having an increased understanding of the underlying PK characteristics as well as how variability in PK might affect the observed response is essential in going forward with DMT as a potential future treatment option. This knowledge could aid in developing new dose regimens, such as infusion protocols to enable the study of DMT over extended periods of time, as well as enabling dose adjustments according to patient characteristics. It would also be beneficial in terms of guiding dose level decisions in future studies with DMT based on a PK/pharmacodynamic (PK/PD) target.
In this work, a population‐based PK and PK/PD analysis was performed on data from a clinical study where DMT was administered intravenously to 13 healthy subjects. 15 The present analysis aimed to characterize population PK of DMT as well as the population PK/PD relationship between DMT plasma concentration and psychedelic experience as measured through real‐time subjective intensity ratings. The psychedelic experience is an important factor to consider in clinical research with DMT and is believed to correlate to the therapeutic outcome in the treatment of depression. 22 Hence, the results of this study may be useful in guiding future clinical development of DMT.
METHODS
Clinical trial
A placebo controlled clinical trial was performed at the National Institute of Health Research (NIHR) Imperial Clinical Research Facility in 13 healthy subjects (7 men, median age 33 years [range 22–48 years]). The study was conducted according to the principles laid down in the revised Declaration of Helsinki (2000), the International Committee on Harmonization Good Clinical Practices guidelines, and the UK National Health Service Research Governance Framework, and was approved by the National Research Ethics (NRES) Committee London – Brent and the Health Research Authority. All subjects provided written informed consent to participate in the study. The study has been described in more detail elsewhere. 15
In brief, the study used a fixed order design where subjects received a placebo administration at their first visit and DMT during their second visit, which took place a week later. DMT was administered as an intravenous bolus dose and each subject received one of four doses of DMT fumarate: 7 mg (n = 3), 14 mg (n = 4), 18 mg (n = 1), or 20 mg (n = 5). Nine blood samples per subject were drawn at staggered timepoints into chilled EDTA tubes from an indwelling forearm vein catheter up to 60 min after drug administration. Plasma was harvested and stored at −80°C before being shipped to Gothenburg on dry ice for bioanalysis. Subjective effects were obtained by asking for ratings of subjective intensity of the psychedelic experience on a scale from zero to 10, where zero is no effect and 10 is the most intense experience imaginable, every minute during the first 20 min.
Bioanalysis
DMT and metabolite levels in plasma were quantified using a previously described liquid chromatography tandem mass spectrometry method. 18 The method was validated for DMT, DMT N‐oxide, and IAA over ranges of 0.25–200, 15–200, and 500–5000 nM, respectively. Lower limits of quantification (LLOQ) were set at 0.25, 15, and 500 nM for DMT, DMT N‐oxide, and IAA, respectively. IAA concentrations were measured as a change from baseline due to high endogenous levels of IAA. The method was shown to be accurate and precise over these ranges. Samples above the upper limit of quantification were diluted before re‐analysis.
Modeling approach
Data were analyzed using nonlinear mixed‐effects modeling in NONMEM version 7.4.3. (ICON Development Solutions). 23 Pirana (version 3.0.0) and Perl‐speaks‐NONMEM (version 5.2.6) 24 was used for model automation and diagnostics. R (version 4.1.1) was used for model diagnostics and visualization. Models were fitted using the first‐order conditional estimation with interaction method. Samples below the LLOQ were excluded from analysis (n = 3 and n = 9 for DMT and IAA, respectively). In addition, one sample was excluded due to measured DMT concentration that was high above the expected range, most likely explained by contamination of the sample.
The analysis was performed using a sequential approach where a population PK model was first established to describe plasma concentrations of DMT. The model was then extended to include the metabolite IAA. Finally, the PK/PD model was developed using a “Population PK Parameters and Data” approach where population PK parameters are fixed but individual PK parameters are estimated simultaneously with PD parameters. 25 All DMT fumarate doses were converted and expressed as nanomoles of DMT base. Between‐subject variability (BSV) was described by exponential random effects following a log‐normal distribution with mean zero and variance ω 2.
Pharmacokinetic model development
One‐ and two‐compartment models with first‐order elimination from the central compartment were fitted to the DMT observations and assessed. BSV was evaluated on clearance (CL), central volume of distribution, intercompartmental clearance (Q), and peripheral volume of distribution. Once a base model describing the plasma concentrations of DMT had been established, the metabolite IAA was added to the model. IAA was assumed to be a primary metabolite of DMT with a fixed metabolic fraction of 1 where the IAA formation rate equals the elimination rate of DMT. IAA disposition was described using a one‐compartment model with first‐order elimination. BSV was evaluated on apparent IAA clearance (CL[m]) and apparent IAA volume of distribution (V[m]). Residual variability was evaluated separately for DMT and IAA using additive, proportional, or combined error models.
Pharmacokinetic/pharmacodynamic model development
Based on graphical exploration of the data, effect compartment models were evaluated to describe the relationship between DMT concentrations and subjective psychedelic intensity ratings. The effect compartment model assumes that the response is mediated through DMT levels in a compartment corresponding to a theoretical biophase (i.e., the brain in this case). The change in concentration in the effect compartment is described according to:
where k e0 is the effect compartment equilibrium rate constant, C p is the plasma concentration of DMT, and C e represents the theoretical concentration in the effect compartment.
The drug effect was assessed using maximum effect (E max) or sigmoid E max models as follows:
where E 0 is the baseline response, E max is the maximum response, EC50,e is the concentration of DMT at the effect site required to produce half of the maximum response, and the Hill coefficient γ describes the steepness of the relationship.
BSV was evaluated on k e0, EC50,e, and γ. The subjective ratings were handled as continuous data but with the addition of a logit transformation to restrict the predicted values between zero and 10. This was expressed for every observation j of each individual i as:
| (1) |
where λ is the individual prediction and ε ij is the residual error, additive on the logit scale and following a normal distribution.
Model evaluation
Model discrimination between nested models was based on objective function value (OFV) where a change in OFV of −3.84 was considered a significant model improvement at p = 0.05 under the assumption that ΔOFV is approximately chi‐squared distributed. The final model was evaluated by assessing plausibility of parameter estimates, goodness‐of‐fit (GOF) plots, and visual predictive checks (VPCs). Sampling importance resampling (samples/resamples = 5000/1000) was performed to determine precision of the parameter estimates and to calculate 95% confidence intervals. 26 The covariance output was used as the proposal distribution without an inflation factor. Parameter precision was considered acceptable if %RSE was less than or equal to 30% for fixed effects and less than or equal to 50% for BSV parameters.
Simulations
The final PK/PD model was used to evaluate the predicted effect at different dose levels. For five different intravenous bolus doses (1, 4, 7, 14, and 20 mg), the achieved DMT exposure and effect were simulated in 100 subjects, respectively. Dose levels were set to demonstrate a range of doses that would likely cause non‐existent (1 mg) to significant (20 mg) psychedelic experiences. Simulations were performed in R using the package mrgsolve.
RESULTS
A total of 93 (19, 29, 6, and 39 for the 7, 14, 18, and 20 mg doses, respectively) and 87 (18, 27, 6, and 36 for the 7, 14, 18, and 20 mg doses, respectively) plasma concentration observations of DMT and IAA, respectively, as well as 273 (84, 63, 21, and 105 for the 7, 14, 18, and 20 mg doses, respectively) subjective intensity ratings, were included in the analysis. The individual concentration‐time curves of DMT and IAA have been previously published elsewhere. 18 No subjective psychedelic effects or measurable concentrations of DMT were observed after placebo administration. Consequently, observations after placebo administration were not modeled.
Population pharmacokinetic model
No indications of PK nonlinearity were observed in the data. DMT plasma concentrations were well‐described by a two‐compartment PK model with first‐order elimination as a single elimination pathway leading to the formation of IAA. A two‐compartment model led to a significant improvement in model fit compared to a one‐compartment model (ΔOFV = −41.64). In addition, GOF plots indicated a better fit when using two compartments as compared to one. BSV was incorporated on CL. The data did not allow for estimation of more than one BSV parameter. IAA observations were described by a one‐compartment PK model with first‐order elimination. BSV was included on V(m). Proportional residual error models were used for both DMT and IAA. Precisions were below the prespecified criteria of 30 and 50 %RSE for all parameters except Q, for which it was 37%. The VPC illustrates that the predictive performance of the final model is adequate (Figure 1). Estimated parameters of the final PK model are summarized in Table 1. GOF plots of the final PK model are demonstrated in Figure S1.
FIGURE 1.
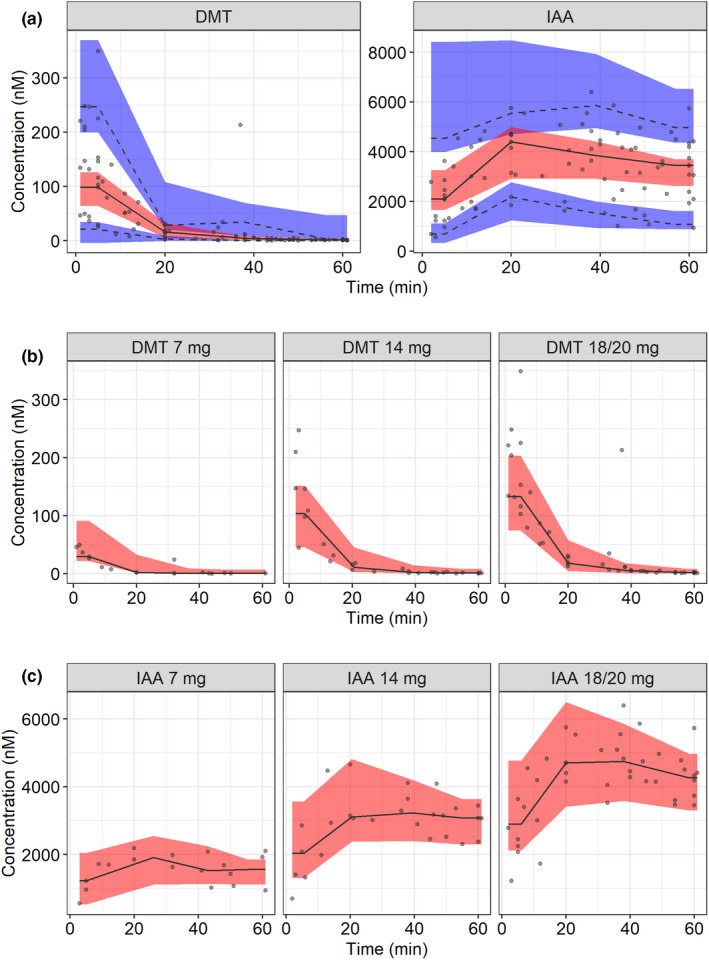
Visual predictive check (VPC; n = 1000) of (a) the final pharmacokinetic (PK) model of N,N‐dimethyltryptamine (DMT) and the metabolite indole 3‐acetic acid (IAA) across all dose levels; (b) the final PK model of DMT stratified by dose level, and (c) the final PK model of IAA stratified by dose level in 13 healthy subjects after intravenous bolus dose. Circles are observations, solid lines are medians of the observations, dashed lines are 5th and 95th percentiles of the observations, the red areas are the 95% confidence intervals of the median of the simulated data, and the blue areas are the 95% confidence intervals of the 5th and 95th percentiles of the simulated data. The dose stratified VPCs are presented with median predictions only, due to the low sample size of each panel. Doses are expressed as DMT fumarate.
TABLE 1.
Final pharmacokinetic and pharmacodynamic parameter estimates
| Parameter | Estimate (95% CI) | %RSE |
|---|---|---|
| CL, L/min | 26.0 (20.6–33.6) | 15.1 |
| Q, L/min | 2.99 (1.87–5.44) | 36.6 |
| Vc, L | 221 (181–273) | 12.1 |
| Vp, L | 59.0 (48.0–82.7) | 18.5 |
| CL(m), L/min | 0.093 (0.076–0.11) | 10.6 |
| V(m), L | 9.55 (8.07–11.1) | 9.8 |
| BSV CL, CV% | 47.3 (37.5–65.3) | 41.0 |
| BSV V(m), CV% | 29.0 (21.7–41.5) | 45.0 |
| Residual error DMT, CV% | 50.0 (43.9–58.9) | 19.2 |
| Residual error IAA, CV% | 19.0 (16.8–21.8) | 16.3 |
| E max | 10 FIX | |
| EC50,e, nM | 94.7 (75.4–123) | 14.9 |
| k e0, min−1 | 1.38 (1.16–1.94) | 17.5 |
| γ | 2.87 (2.63–3.02) | 4.2 |
| BSV EC50,e, CV% | 38.6 (29.1–50.4) | 34.1 |
| BSV γ, CV% | 77.3 (58.1–103) | 36.6 |
| Residual error intensity ratings, SD | 0.82 (0.55–0.90) | 7.8 |
Abbreviations: BSV, between subject variability; CI, confidence interval; CL, clearance for N,N‐dimethyltryptamine; CL(m), clearance for indole 3‐acetic acid; CV%, coefficient of variation percentage; DMT, N,N‐dimethyltryptamine; EC50,e, the concentration in the effect compartment needed to achieve 50% of the maximum response; E max, maximum achieved psychedelic intensity; IAA, indole 3‐acetic acid; k e0, the effect compartment equilibrium rate constant; Q, intercompartmental clearance for N,N‐dimethyltryptamine; Vc, central volume of distribution for N,N‐dimethyltryptamine; V(m), volume of distribution for indole 3‐acetic acid; Vp, peripheral volume of distribution for N,N‐dimethyltryptamine; γ, Hill coefficient describing the steepness of the relationship.
Population pharmacokinetic/pharmacodynamic model
A short delay was observed in response as compared to DMT concentrations. The relationship between DMT plasma concentrations and subjective intensity ratings was described by an effect compartment model with a sigmoid E max response, where the rate by which equilibrium between plasma and effect compartment concentration is established is described by k e0, here estimated to 1.38 min−1. E max was fixed to a value of 10 because this was the highest allowed rating and because it was considered achievable for all individuals due to the subjective nature of the rating. All subjects had a baseline value of zero.
BSV was incorporated on EC50,e and the Hill factor γ. A correlation between the two BSV terms was observed and estimated to 93%. However, this was not included in the final model because it led to ill conditioning of the model (condition number increased from 394 to 2790). The final PK/PD parameters are summarized in Table 1. A VPC is shown in Figure 2 and illustrates the predictive performance of the final model. A schematic of the final PK/PD model is shown in Figure 3. GOF plots of the final PK/PD model are demonstrated in Figure S1.
FIGURE 2.
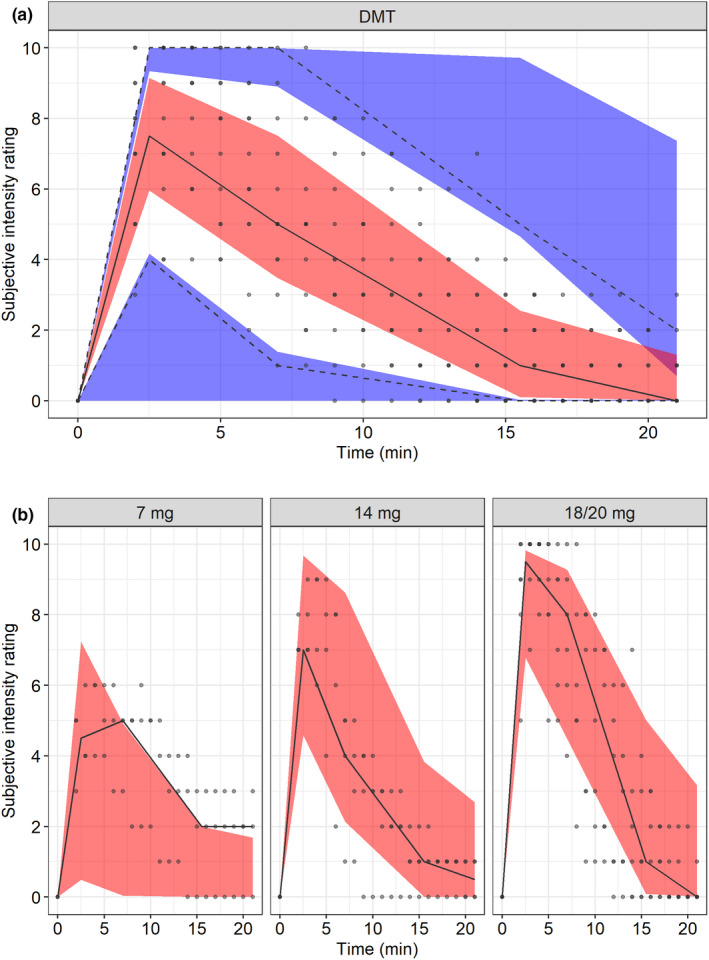
Visual predictive check (VPC; n = 1000) of (a) the final pharmacokinetic/pharmacodynamic (PK/PD) model of the psychedelic intensity produced by N,N‐dimethyltryptamine (DMT) versus time across all dose levels and (b) the final PK/PD model of the psychedelic intensity produced by DMT versus time stratified by dose after intravenous administration in 13 healthy subjects. Circles are observations, the solid line is the median of the observations, dashed lines are 5th and 95th percentiles of the observations, the red areas are the 95% confidence intervals of the median of the simulated data, and the blue areas are the 95% confidence intervals of the 5th and 95th percentiles of the simulated data. The dose stratified VPCs are presented with median predictions only, due to the low sample size of each panel. Doses are expressed as DMT fumarate.
FIGURE 3.
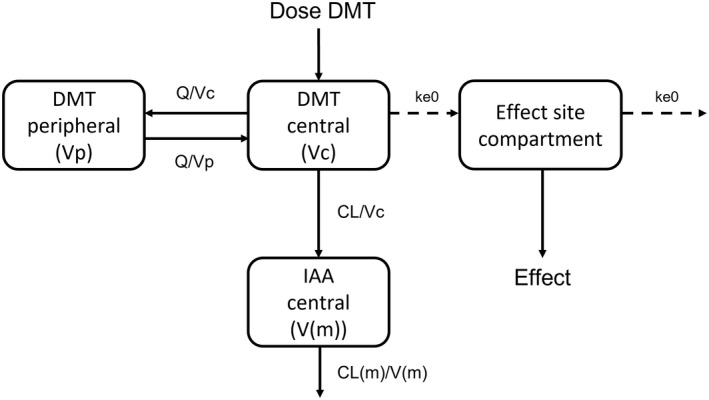
Schematic presentation of the final pharmacokinetic/pharmacodynamic model of N,N‐dimethyltryptamine (DMT), indole 3‐acetic acid (IAA), and the psychedelic intensity produced by DMT. CL, clearance for N,N‐dimethyltryptamine; Vc, central volume of distribution for N,N‐dimethyltryptamine; Q, intercompartmental clearance for N,N‐dimethyltryptamine; Vp, peripheral volume of distribution for N,N‐dimethyltryptamine; CL(m), apparent clearance for indole 3‐acetic acid; V(m), apparent volume of distribution for indole 3‐acetic acid; k e0, the effect compartment equilibrium rate constant.
Simulations
The simulated distribution of the maximum achieved effect at each respective dose level is shown in Figure 4. A median maximum effect of 0, 2, 4, 8, and 9 were achieved at the doses 1, 4, 7, 14, and 20 mg, respectively. The proportion of the population achieving a maximum rating above five increased from zero at the lowest dose to 4, 42, 92, and 100% at each respective dose level.
FIGURE 4.
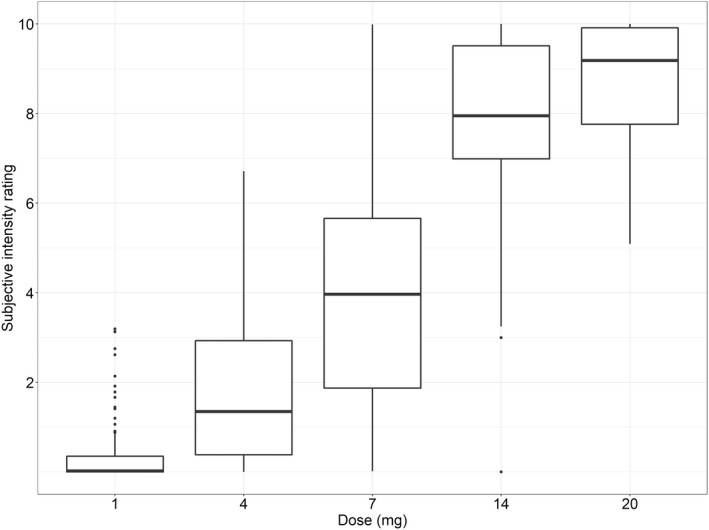
Distribution of simulated maximum achieved effect at five different intravenously administrated N,N‐dimethyltryptamine (DMT) doses in 100 individuals, respectively. The horizontal line in the middle of the boxes is the median, the boxes indicate the 25th and the 75th percentiles, and whiskers extend between the 5th and the 95th percentiles. Doses are expressed as DMT fumarate.
DISCUSSION
DMT is one of several serotonergic psychedelics that have recently gained an increased amount of attention as potential therapeutic tools in treatment of a number of psychiatric disorders. Despite the increasing number of clinical investigations with DMT, very little is known about the PK and PK/PD properties of DMT. In this work the population PK of DMT and its metabolite IAA as well as the PK/PD relationship between DMT concentrations and subjective psychedelic intensity was characterized. To the best of our knowledge, this is the first time the relationship between DMT plasma concentrations and its effects have been characterized using a modeling approach.
DMT plasma concentrations were best described using a two‐compartment PK model. To be able to estimate any PK parameters of the metabolite, the assumption was made that DMT was completely metabolized into IAA via a first‐order elimination pathway. The estimated PK parameters of DMT and IAA are summarized in Table 1. Noteworthy is the extremely high clearance obtained for DMT. The obtained plasma clearance of 26 L/min is clearly above the cardiac output of an average healthy individual (~5 L/min). Although blood clearance of DMT might be lower, it seems unlikely that the blood:plasma ratio should be sufficiently high to fully account for this large clearance value. This is also supported by a separate experiment where DMT was added in known amounts to whole blood. Plasma was harvested and the measured concentrations of DMT in plasma corresponded well with the nominal concentrations in whole blood (data not shown). Hence, the results indicate a degradation of DMT that is independent of organ blood flow. Because DMT is primarily metabolized by MAO‐A, this could potentially be explained by the presence of MAO‐A in tissues throughout the body, including the blood vessels. 27 , 28 However, further research is needed to confirm this and to obtain a better understanding of the elimination of DMT from the human body. In addition, a relatively high BSV in clearance was observed. No full covariate analysis was performed in this work due to the low number of individuals. However, no trends were observed with regards to sex or age (data not shown). Nevertheless, there are several potential factors that could cause variability in clearance between different individuals, including for example body size. In addition, polymorphisms in MAO‐A affecting drug metabolism have been previously reported 29 and we believe this should be further investigated as a potential source of variability in DMT PK in future studies.
The assumption was made that DMT is completely metabolized into IAA via a single elimination pathway. The metabolic pattern of DMT has not yet been fully elucidated. Previous studies have identified several other metabolites of DMT after intake of ayahuasca, however, the inclusion of MAO inhibitors might shift the metabolic pathways and it is unclear which metabolites are formed after intravenous injection of DMT. For example, we have previously shown that DMT N‐oxide, a metabolite that has been observed after oral intake of ayahuasca, was not present in plasma samples from the individuals in the present study. 18 Consequently, setting the metabolic fraction to one was deemed to be the most appropriate option with the data available. Although IAA is not believed to be an active metabolite, it was incorporated in the model for descriptive purposes. A complete understanding of DMT metabolism and any potential active metabolites might, however, improve the understanding of both PK and PD aspects in the future.
In the present analysis, we first established a PK model, whereafter typical parameters were fixated when modeling subjective intensity ratings. The study subjects had been asked to rate their experience on a scale from zero to 10 right before and during the first 20 min after DMT administration. For the purpose of modeling, the observed data was treated as continuous, even though it was actually integer scale. However, to avoid producing predictions outside the boundaries of the scale, a logit transformation was used to restrict the values between zero and 10. The applied transformation only allows predictions to approach the boundaries of the scale asymptotically. Hence, exact values of zero or 10 cannot be predicted. However, for obtaining a basic understanding of the achieved effect at different concentrations, values of 9.99 or 0.001 can be assumed to be equivalent to 10 and zero, respectively. A slight delay in response as compared to DMT concentrations were observed. Because changes in perception are usually coupled to changes in brain signaling, something that occurs rapidly as a response to a stimulus, it was concluded that the most likely explanation would be a delay in distribution to the effect site rather than a delay in developing the response. Consequently, an effect compartment model with a sigmoid E max response was chosen as the final model because it appropriately represents the hypothesized mechanism behind the observed delay. The effect compartment model assumes that drug needs to be distributed from plasma to the effect compartment, and that only the drug in the effect compartment contributes to the observed effects. The value of ke0 provides information of the effect delay. The estimated value of 1.38 min−1 can be seen to indicate that it takes ~2 min for DMT concentrations in the brain to equilibrate with blood. The addition of a Hill coefficient, estimated to be approximately three, significantly improved the model fit. The Hill factor describes the sigmoidicity of the relationship between effect and concentration with a higher value indicating a steeper slope. Hence, a Hill factor of three indicates that within 20–80% of maximal response, a small change in concentration results in a large change in response. A BSV of 39 and 77% were estimated for EC50,e and gamma, respectively. Because the intensity rating is a subjective effect measure and thus error‐prone, it is not surprising that some variability in EC50,e would be observed. Most likely, there will be some inconsistency in the reporting of intensity even within the same individual. In general, there are difficulties in working with subjective response measures in terms of robustness. However, in studies with DMT as well as with other psychedelics, the experience must be taken into consideration as some evidence suggests a correlation between subjective measures assessing the quality of the psychedelic experience and therapeutic outcomes related to improvements in mood and addictive disorders. 22 Consequently, understanding how plasma concentrations of DMT relate to the intensity of this experience is a key factor in setting appropriate dose levels in future clinical studies.
The EC50,e of the psychedelic intensity was estimated at 95 nM in this study. Interestingly, this is similar to in vitro EC50 values associated with activation of the 5‐HT2A receptor (201–269 nM). 30 This potentially supports the idea that the psychedelic effects of DMT are mainly mediated through agonism at the 5‐HT2A receptor.
Although this model may not allow for accurate predictions of how the response varies over time in a diverse population, due to the limited data, we believe it can be useful in understanding what effect and associated variability can be expected at different concentrations and doses. To illustrate this, simulations were performed to predict the maximum achieved psychedelic intensity rating at different dose levels. The median maximum achieved effect rating increased from four to eight between the 7 mg and 14 mg dose, respectively, whereas the corresponding proportion of the population achieving a maximum response above five increased from 42 to 92%. At the highest dose level (20 mg) the median maximum effect was nine and the predicted proportion achieving a maximum effect above five was 100%. For the purpose of illustrating the applicability of the obtained results, an intensity rating of five was set as an arbitrary limit of what would constitute a fully psychedelic experience. Hence, if the psychedelic experience is used as the target outcome, these results indicate that increasing the dose above 14 mg might not provide any substantial increase in benefit as 92% of the population is predicted to already achieve an intensity above five. This is consistent with previous research suggesting the intravenous bolus dose for a psychedelic threshold to be close to 14 mg of DMT fumarate. 21 Further, some research suggests that DMT might be effective in protection and recovery from ischemic injuries 31 , 32 and, in contrast to when treating psychiatric disorders, this might call for doses where the aim is to keep the response at a “sub‐psychedelic” level, here assumed to correspond to a rating below five. In this case, the results indicate that a dose at or below 7 mg would be needed. However, it is clear from the spread in the maximum achieved effect across the population at the 4 and 7 mg doses that if a “medium” intensity is desirable, individually tailored doses will most likely be necessary.
Overall, the final model adequately described the PK and psychedelic intensity data. Even though this was a relatively small study, it is a first step toward gaining an increased understanding of the PK/PD characteristics of DMT. We believe that this model can be useful in predicting suitable doses for clinical investigations of DMT based on the desired intensity of the subjective experience (and its potential relationship to desired therapeutic effects). This model may also provide the basis for determining dose parameters suitable for extended administration of DMT, which would be a significant step forward in the clinical development of DMT. However, the large variability observed in the data highlights the need for larger studies with the ability to investigate potential covariates and causes for these observations.
AUTHOR CONTRIBUTIONS
E.E., C.T., R.C.‐H., D.R., and M.A. wrote the manuscript. E.E., C.T., R.C.‐H., and M.A. designed the research. E.E., C.T., R.C.‐H., and M.A. performed the research. E.E., C.T., R.C.‐H., D.R., and M.A. analyzed the data.
FUNDING INFORMATION
The clinical study was funded by the Centre for Psychedelic Research, Imperial College London. E.E. is partly supported by the Sahlgrenska Academy at the University of Gothenburg.
CONFLICT OF INTEREST
C.T. provides consultation work for Entheon Biomedical. R.C‐H. is an advisor for Entheon Biomedical and has previously been an advisor for Small Pharma. All other authors declared no competing interests for this work.
Supporting information
Figure S1
Eckernäs E, Timmermann C, Carhart‐Harris R, Röshammar D, Ashton M. Population pharmacokinetic/pharmacodynamic modeling of the psychedelic experience induced by N,N‐dimethyltryptamine – Implications for dose considerations. Clin Transl Sci. 2022;15:2928‐2937. doi: 10.1111/cts.13410
REFERENCES
- 1. Institute of Health Metrics and Evaluation . Global Health Data Exchange (GHDx). February 3.
- 2. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905‐1917. [DOI] [PubMed] [Google Scholar]
- 3. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life‐threatening cancer: a randomized double‐blind trial. J Psychopharmacol. 2016;30:1181‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life‐threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30:1165‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis AK, Barrett FS, May DG, et al. Effects of psilocybin‐assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiat. 2021;78:481‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carhart‐Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment‐resistant depression: six‐month follow‐up. Psychopharmacology. 2018;235:399‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brito‐da‐Costa AM, Dias‐da‐Silva D, Gomes NGM, Dinis‐Oliveira RJ, Madureira‐Carvalho Á. Toxicokinetics and Toxicodynamics of ayahuasca alkaloids N,N‐dimethyltryptamine (DMT), Harmine, harmaline and tetrahydroharmine: clinical and forensic impact. Pharmaceuticals. 2020;13:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dobkin de Rios M. Ayahuasca – the healing vine. Int J Soc Psychiatry. 1971;17:256‐269. [DOI] [PubMed] [Google Scholar]
- 9. Sanches RF, de Lima Osório F, dos Santos RG, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol. 2016;36:77‐81. [DOI] [PubMed] [Google Scholar]
- 10. Palhano‐Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment‐resistant depression: a randomized placebo‐controlled trial. Psychol Med. 2019;49:655‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cameron LP, Benson CJ, Dunlap LE, Olson DE. Effects of N, N‐dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chem Neurosci. 2018;9:1582‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N‐dimethyltryptamine (DMT) is an endogenous sigma‐1 receptor regulator. Science. 2009;323:934‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68:264‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006;79:223‐246. [DOI] [PubMed] [Google Scholar]
- 15. Timmermann C, Roseman L, Schartner M, et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep. 2019;9:16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riba J, McIlhenny EH, Bouso JC, Barker SA. Metabolism and urinary disposition of N,N‐dimethyltryptamine after oral and smoked administration: a comparative study. Drug Test Anal. 2015;7:401‐406. [DOI] [PubMed] [Google Scholar]
- 17. Riba J, McIlhenny EH, Valle M, Bouso JC, Barker SA. Metabolism and disposition of N,N‐dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test Anal. 2012;4:610‐616. [DOI] [PubMed] [Google Scholar]
- 18. Eckernäs E, Bendrioua A, Cancellerini C, et al. Development and application of a highly sensitive LC‐MS/MS method for simultaneous quantification of N,N‐dimethyltryptamine and two of its metabolites in human plasma. J Pharm Biomed Anal. 2022;212:114642. [DOI] [PubMed] [Google Scholar]
- 19. Strassman RJ, Qualls CR. Dose‐response study of N,N‐dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51:85‐97. [DOI] [PubMed] [Google Scholar]
- 20. Gallimore AR, Strassman RJ. A model for the application of target‐controlled intravenous infusion for a prolonged immersive DMT psychedelic experience. Front Pharmacol. 2016;7:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose‐response study of N,N‐dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98‐108. [DOI] [PubMed] [Google Scholar]
- 22. Yaden DB, Griffiths RR. The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2021;4:568‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beal S, Boeckmann L, Sheiner LB NONMEM users guide; 1989.
- 24. Lindbom L, Pihlgren P, Jonsson EN. PsN‐toolkit – a collection of computer intensive statistical methods for non‐linear mixed effect modeling using NONMEM. Comput Methods Prog Biomed. 2005;79:241‐257. [DOI] [PubMed] [Google Scholar]
- 25. Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best‐case performance. J Pharmacokinet Pharmacodyn. 2003;30:387‐404. [DOI] [PubMed] [Google Scholar]
- 26. Dosne AG, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43:583‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramonet D, Rodríguez M, Saura J, et al. Localization of monoamine oxidase a and B and semicarbazide‐sensitive amine oxidase in human peripheral tissues. Inflammopharmacology. 2003;11:111‐117. [DOI] [PubMed] [Google Scholar]
- 28. Rodríguez MJ, Saura J, Billett EE, Finch CC, Mahy N. Cellular localization of monoamine oxidase a and B in human tissues outside of the central nervous system. Cell Tissue Res. 2001;304:215‐220. [DOI] [PubMed] [Google Scholar]
- 29. Ariffin NM, Islahudin F, Kumolosasi E, Makmor‐Bakry M. Effects of MAO‐A and CYP450 on primaquine metabolism in healthy volunteers. Parasitol Res. 2019;118:1011‐1018. [DOI] [PubMed] [Google Scholar]
- 30. Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB. Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology. 2014;231:875‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nardai S, László M, Szabó A, et al. N,N‐dimethyltryptamine reduces infarct size and improves functional recovery following transient focal brain ischemia in rats. Exp Neurol. 2020;327:113245. [DOI] [PubMed] [Google Scholar]
- 32. Nemes B, Pető K, Németh N, et al. N,N‐dimethyltryptamine prevents renal ischemia‐reperfusion injury in a rat model. Transplant Proc. 2019;51:1268‐1275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


