Abstract
We describe a woman with a history of relapsing acute optic neuritis and perineuritis. Testing failed to confirm a specific diagnosis; hence, she was diagnosed with seronegative neuromyelitis optica spectrum disorder and treated with the immunotherapy rituximab, later in conjunction with mycophenolate mofetil. She achieved a durable remission for 9 years until she presented with paresthesia affecting her left fifth digit, right proximal thigh, and left foot, while also reporting a 25-pound weight loss over the prior 3 months. New imaging demonstrated a longitudinally extensive and enhancing optic nerve, in conjunction with multifocal enhancing lesions within the spinal cord, in a skip-like distribution. The differential diagnosis is discussed.
Case Presentation
In September 2020, a 67-year-old woman presented with a 2-day history of left eye burning and difficulty reading fine print (Figure 1).1 Her clinical history dates to January 2009 when she presented with a 10-day history of progressive, painful central vision loss in her right eye. The ophthalmologic examination at that time was remarkable for a visual acuity (VA) of 20/50 OD and 20/25 OS. There was decreased color vision in the right eye, a right relevant afferent pupillary defect, and right optic disc swelling with areas of hemorrhage. The aquaporin-4 antibody (anti-AQP4; assessed by cell-based assay [CBA]) was negative, and she was diagnosed with right anterior optic neuritis (ON).
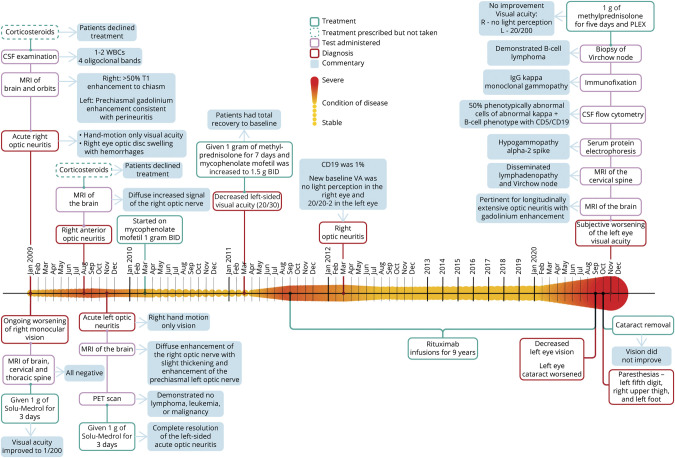
Figure 1. Chronologic Heat Map.
In this figure, we detail the condition of the patient over time. The longitudinal axis (left to right) depicts the condition of disease, where the smaller amplitude and lighter color indicates greater stability of disease. Alternately, the expanded amplitude of the colored heat map (above and below the horizontal linear axis over time) designates increased disease activity (whether on a clinical or paraclinical basis) or complications of the treatment of disease. Other fields of information are added either above or below the heat map and include information about treatments, diagnoses, commentaries adding contextual perspectives, and results from specific test assessments from each most relevant period of clinical decision-making. Each field is consistently color-coded throughout as defined in the figure legend.1
MRI of the brain and orbits (with fat suppression) with and without contrast (Figure 2) showed greater than 50% T1 enhancement of the right optic nerve extending to the level of the optic chiasm and with prechiasmatic involvement of the contralateral optic nerve, most consistent with an inflammatory process localized to the orbital segments of the optic nerves and sheath (i.e., perineuritis). MRI of the cervical and thoracic spinal cord was unremarkable. CT of the chest was normal.
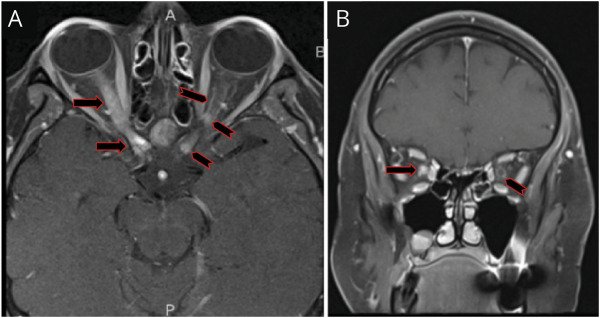
Figure 2. T1, Fat-Suppressed, Postcontrast MRI of the Orbits.
Axial (A) image demonstrates longitudinally extensive enhancement of the right optic nerve extending into the prechiasmatic area (black and red arrow) along with faint contrast enhancement of the left optic nerve (black and red chevron) in a pattern of subtle and very thin ‘tram tracking’, signifying optic nerve sheath enhancement (i.e., perineuritis). Furthermore, there are nodular enhancements involving the left optic nerve sheath at the orbital apex, but also the intracanalicular, prechiasmatic portion of the optic nerve, with very subtle ‘tram tracking’ (black and red chevrons). Coronal image (B) shows diffuse right optic nerve enhancement (black and red arrow) and perineural enhancement of the left optic nerve (black and red chevron), in keeping with the ‘donut sign’ of perineuritis.
CSF examination showed 1–2 white blood cells (WBCs) and 738 and 8 red blood cells (RBCs) in tubes 1 and 4, respectively. Glucose was 111 mg/dL (serum glucose 150–160 mg/dL), and protein was 32 mg/dL. There were 4 oligoclonal bands unique to the CSF. Flow cytometry was unrevealing.
Additional assessments included thyroid-stimulating hormone (which was low at 0.18 IU/mL [ref: 0.50–4.50 IU/mL]) with a normal free T4. Other negative or unremarkable tests included serum angiotensin-converting enzyme (ACE), anti–double stranded DNA antibodies, anti-neutrophilic cytoplasmic antibodies, anti-nuclear antibodies, anti-SSA (anti–Sjögren syndrome–related antigen A autoantibodies), and anti-SSB (anti–Sjögren syndrome–related antigen B autoantibodies), complement levels of C3 and C4, cardiolipin antibody panel, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, RBC folate, vitamin B6, vitamin B1, methylmalonic acid, homocysteine, and beta-2-glycoprotein antibodies.
Subsequently, her right eye vision worsened to hand motion only and was associated with disc edema and multifocal hemorrhages, prompting treatment with IV methylprednisolone for 3 days, followed by an 11-day oral prednisone taper. Vision on the right remained at hand motion only.
In August 2009, she again was diagnosed with right anterior ON. A brain MRI revealed increased thickening and diffuse abnormal enhancement of the right optic nerve. Repeated and now borderline positivity for anti-SSA and an elevated serum ACE (81 U/L; normal 9–67 U/L) prompted lip biopsy and a total body PET scan, both of which were unremarkable.
In November 2009, the patient presented to the emergency department (ED) with painful, new left eye vision loss. VA was 20/20-2 in the left eye, in conjunction with decreased VA in the right eye to hand motion only. She was diagnosed with left-sided acute ON. Brain MRI showed diffuse enhancement of the right optic nerve with slight thickening and enhancement of the prechiasmal left optic nerve. She received both IV and oral corticosteroids, which led to complete resolution of her left-sided ON. The steroid-sparing agent mycophenolate mofetil (MMF) was initiated in March of 2010 at 1 g oral twice daily, based on a working diagnosis of seronegative neuromyelitis optica spectrum disorder (NMOSD).
Approximately 1 year later, the patient was noted to have slightly decreased left eye VA (20/30) and decreased color vision. She again received IV and oral corticosteroids, and the dose of MMF was increased to 1.5 g twice daily with subsequent recovery to her baseline VA. Despite dose escalation of MMF, 4 months later, the patient experienced recurrent left ON (Figure 2). She was then started on rituximab. She experienced yet another episode of right-sided ON in March of 2012 (Figure 1), at which time her CD19 level was at 1%, a level that can be associated with rituximab failure (typically when the CD19% is between 1% and 2.5%).2 Monthly monitoring of CD19 counts was initiated so that rituximab redosing was instituted when CD19 levels exceeded 0%.
The patient remained stable on rituximab for approximately 9 years, until September 2020, when she noted worsening of her vision in the left eye. She then developed paresthesia in her left 5th digit, right upper thigh, and left foot. Referred to the ED, she was found to have a minimally reactive left pupil but no pain with extraocular eye movements. Serum anti-AQP4 and myelin oligodendrocyte glycoprotein (MOG) antibodies, assessed by CBA, were negative. CSF analysis and infectious workup were unrevealing. The patient reported a 25-pound weight loss over the prior 3 months, and her peripheral WBC count had been consistently elevated >13,000 WBCs/μL.
Axial T1 MRI sequences with gadolinium revealed evidence of a longitudinally extensive segment of left optic nerve enhancement (Figure 3). Furthermore, T1 postcontrast MRIs of the spinal cord demonstrated several areas of contrast enhancement, in conjunction with the incidental identification of extensively disseminated mediastinal and retroperitoneal lymphadenopathy.
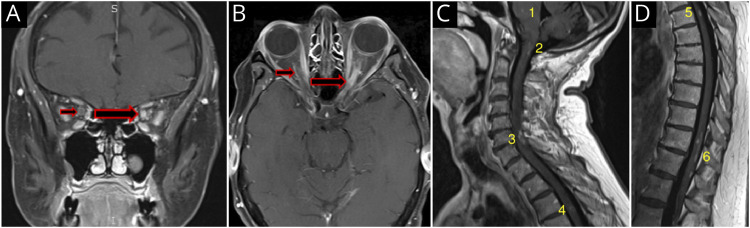
Figure 3. Coronal (A) and Axial (B) Fat-Suppressed, T1 Postcontrast MRI of the Orbits Demonstrating Right Optic Nerve Sheath Enhancement on the Coronal View, Consistent With the ‘Donut Sign’ of Perineuritis (Small Red Arrow).
There is also homogeneous enhancement of the left optic nerve (large black and red arrow). The axial image in B shows subtle thin linear ‘tram tracking’ of the right optic nerve (small black and red arrow), as well as a longitudinally extensive enhancing left optic nerve, also with features of ‘tram tracking’ (large black and red arrow). Sagittal T1 imaging (C) reveals punctate enhancement in the pons (1), an open “u”-shaped enhancing lesion, with a small vertical linear enhancement extending caudally from the “u” lesion in the dorsal cervicomedullary region (2), a ring-like configuration of enhancement at C6-7 (3), and a subtle small ring of enhancement at T3-4 (4). Sagittal T1 imaging (D) demonstrates a punctate area of enhancement in the upper (5) and a complex multifocal pattern of punctate and linear enhancements in the lower thoracic spinal cord (6).
Differential Diagnosis
Our patient initially presented with several episodes of longitudinally extensive anterior ON, some with chiasmatic and perineural involvement signifying perineuritis, in conjunction with evidence of optic disc hemorrhages, all red flags for typical demyelinating ON. The triggers for these recurrent attacks were varied and most notably included the failure of MMF treatment in conjunction with CD19 hyper-repopulation while receiving treatment with rituximab (Figure 1). The later development of leptomeningeal enhancement seen on MRI led us to consider other disorders and widen the differential diagnosis.
Inflammatory and Infectious Considerations
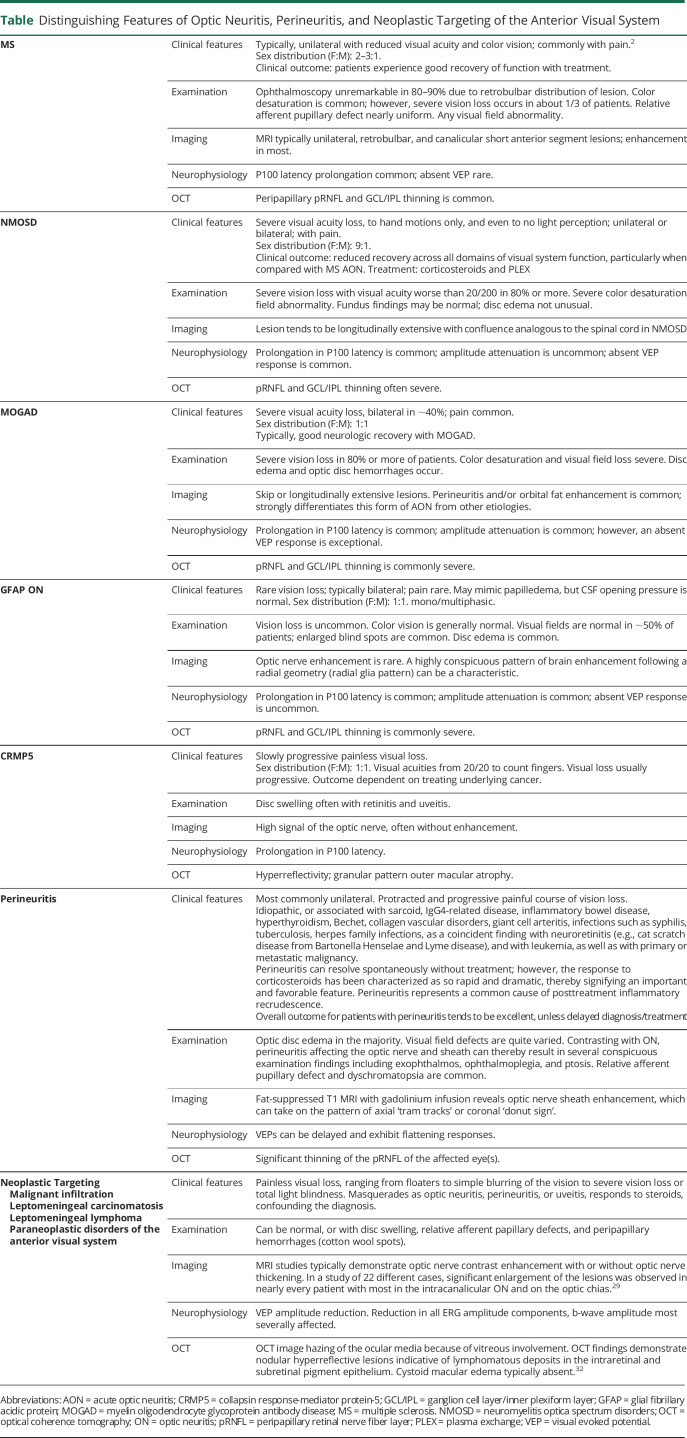
ON in multiple sclerosis is typically unilateral with mild to moderate vision loss with diffuse or central visual field loss (Table).3 Ophthalmoscopy is typically unremarkable acutely (given the retrobulbar localization of the initial lesion in most), and MRI might demonstrate unilateral, retrobulbar, and/or canalicular short anterior segment lesions with optic nerve enhancement acutely in most (Table).4
Table.
Distinguishing Features of Optic Neuritis, Perineuritis, and Neoplastic Targeting of the Anterior Visual System
NMOSD and MOG antibody disease (MOGAD) ON commonly results in severe vision loss and is frequently bilateral, with distinguishing features on MRI and on fundus examination (Table).5
Both NMOSD and MOGAD may present with longitudinally extensive optic nerve and spinal cord (≥3 vertebral segments) lesions on MRI, with more frequent involvement of the lower cord and conus associated with MOGAD.5 Alternately, NMOSD is more likely to affect the intracranial portion of the optic nerve, optic tracts, and optic chiasm and can also feature periependymal, fornix, and hypothalamic lesions.5,6
MOGAD ON is more likely to be associated with optic disc edema, with or without hemorrhage, often in conjunction with inflammation of the optic nerve sheath, which can extend into the intraorbital portion of the nerve.7 Indeed, the AQP4 and MOG antibodies were negative in our patient. However, given that NMOSD can be associated with either B- or T-cell lymphomas, we hypothesize that the reduced efficacy derived from B-cell therapy in AQP4 antibody–negative patients could signify that the underlying pathobiology can be contingent on cellular rather than humoral immune mechanisms.8-10
Neurosarcoidosis can present with either a relapsing subacute optic neuropathy that resembles a demyelinating ON or with a slowly progressive optic neuropathy. About a third of cases show concurrent intraocular inflammation (pan uveitis), and MRI shows optic nerve involvement in 75% of cases. Response to treatment with corticosteroids is adequate, although relapses are common.11
Sarcoidosis and other granulomatous disorders (such as granulomatosis with polyangiitis), infectious etiologies (such as tuberculosis, herpetic infections, Bartonella, and Lyme disease), hyperthyroidism, collagen vascular disorders among other entities can also result in optic perineuritis in addition to distinctive clinical manifestations compared with ON in isolation such as exophthalmos, ophthalmoplegia, ptosis, in the correct clinical context (similar to that seen with MOGAD) as well as with meningeal enhancement and thickening.
The enhancement and thickening can be associated with highly characteristic imaging signatures such as ‘tram tracks’ (on axial cuts) and the ‘donut sign’ (on coronal cuts) (Table).4,11 Autoimmune etiologies of ON (e.g., Sjögren syndrome and systemic lupus erythematosus) can feature retrobulbar optic nerve enhancement.5 IgG4-related ophthalmic disease manifestations include ON with inflammation of the optic nerve sheath, compressive optic neuropathy, myositis, and infraorbital redundant fat inflammation.12 Individuals with IgG4-related disease with neurologic involvement typically have systemic disease (e.g., recurrent pancreatitis). Seronegative autoimmune optic neuropathy, such as chronic relapsing inflammatory optic neuropathy or relapsing isolated ON, may present with severe bilateral (simultaneous or sequential) ON with MRI features including retrobulbar optic nerve enhancement with occasional nerve swelling (Table).4,12
Malignant Infiltration, Leptomeningeal Disease, and Paraneoplastic Disorders
Our patient was stable on treatment with rituximab for several years. If one considers seronegative NMOSD to be the cause of her recurrent bouts of ON, it is safe to assume that rituximab helped keep her neuroinflammatory disorder under control for several years before the most recent symptoms associated with the discovery of extensive systemic lymphadenopathy (Table).
The CNS infiltration of malignant lymphocytoplasmic cells is recognized as the Bing-Neel syndrome (BNS), a constellation of neurologic manifestations secondary to Waldenstrom macroglobulinemia (WM). Although BNS is typically identified in those with established WM, in 15%–36% of cases, it represents the initial clinical presentation of the disorder.13 The early recognition of the BNS carries considerable implications, given that the administration of the Bruton tyrosine kinase, ibrutinib, has been demonstrated to produce response rates of greater than 90%.14 A particular case of the BNS that involved enhancement of the optic nerves and the left optic sheath exhibited a rapid, dramatic, and durable response to ibrutinib lasting more than 36 months.15
A paraneoplastic syndrome resulting from her hematologic malignancy could explain her episodes of ON preceding the diagnosis of smoldering lymphoma. Although, this is unlikely due to the rare occurrence of paraneoplastic disorders with hematologic malignancies, and our patient's protracted time course of visual stability.16 Moreover, the underlying malignancy in paraneoplastic disorders is typically identified within the first few years after neurologic symptom onset.
ON, because of paraneoplastic etiologies such as with the collapsin response mediator protein 5, may present with optic disc edema and may coexist with retinitis and uveitis.17 Although the optic nerve may show high signal abnormality, the nerve rarely enhances in this condition, and the optic neuropathy is progressive in nature, which was different than our patient.
Histologic Diagnosis
Serum protein electrophoresis showed hypogammaglobulinemia and a spike in the alpha-2 region. CSF flow cytometry demonstrated 50% phenotypically abnormal Ig kappa light chain–positive and CD5+CD19+ B cells. Immunofixation results showed a low concentration of IgG kappa monoclonal gammopathy with suppression of normal IgG levels. Biopsy of the left supraclavicular lymph node confirmed a B-cell lymphoma, likely chronic lymphocytic lymphoma (CLL) vs small lymphocytic lymphoma.
Final Diagnostic Considerations and Treatment Plan
Recurrent bilateral ON and perineuritis in our patient were thought to be secondary to seronegative NMOSD, and her chronic immunosuppression most likely unmasked the hematologic malignancy. Although lymphoma can cause recurrent, asynchronous bilateral ON, it was felt that the spinal cord leptomeningeal infiltration observed later in her disease course was secondary to CNS infiltration of her systemic lymphoma.14 With the lymphoma diagnosis confirmed, treatment with the Bruton tyrosine kinase (BTK) inhibitor acalabrutinib was initiated, and rituximab was continued. Rituximab is often part of the armamentarium for the treatment of CLL in younger individuals, whereas BTK inhibitors are superior to standard chemotherapy for treatment of CLL in older adults.18
Combination therapy with the addition of the anti-CD20 monoclonal antibody rituximab to a BTK inhibitor for the treatment of CLL has been evaluated in several studies, without superior outcomes compared with monotherapy with a BTK inhibitor.18 However, such may be related to the ability of BTK inhibitors to downregulate the cell surface expression of CD20, with the potential consequence of rendering subsequent anti-CD20 therapy of little to no utility. With this principle in mind, combination therapy in our patient with recurrent ON and lymphoma might be an effective and reasonably safe strategy, albeit while specifically using a stepwise sequence, administering anti-CD20 therapy first, followed by the application of the BTK inhibitor.
Discussion
CNS invasion by systemic hematologic malignancies has been well described and can involve the brain, the spine, or the leptomeninges and can present with cranial nerve palsies, headache, radiculopathy, or spinal cord signs.19 Non-Hodgkin lymphoma and systemic low-grade lymphomas invade the CNS in approximately 8% and 3% of cases, respectively.20 This can be contrasted with the common involvement of leptomeninges and brain parenchyma observed in up to 70% of cases of primary CNS lymphoma. The latter also presents with ocular involvement in about 25% of newly diagnosed cases.21 The rate of CNS invasion by other systemic malignancies such as CLL appears to be less common and remains understudied.22 A series of 7 patients with indolent B-cell lymphoma and CNS involvement demonstrated the following common characteristics: bone marrow involvement at the time of lymphoma diagnosis (6/7 patients) and systemic or CNS high-grade histologic transformation (4/7 patients), which were not present in our patient.23
Autopsy studies suggest that malignant cell infiltration of the optic nerve occurs in only approximately 18% of acute and 16% of chronic systemic leukemia cases.24,25 However, the extrapolation of an incident rate of such an important disease process from autopsy investigations represents a potentially serious confounding error of ascertainment bias. Alternately, our broader search leads us to conclude that the potential for both neoplastic infiltration and the predilection to cause leptomeningeal carcinomatosis/lymphoma of the anterior visual segments is considerably more common.26-30 Systematic ascertainment has demonstrated a rate as high as 90%,31 with a concordance rate of systemic involvement in greater than half of those with histopathologic confirmation of lymphomatous infiltration of the optic nerve.29
A study involving patients with confirmed leptomeningeal carcinomatosis revealed that vision loss was the predominant ocular symptom in over 70%, while half reported that their visual disturbance was their initial and the corresponding symptom which prompted them to seek out medical attention.31 Along with mimicking the symptoms and semiology of ON and perineuritis, optic nerve malignant infiltration and leptomeningeal carcinomatosis can initially exhibit corticosteroid treatment response characteristics reminiscent of a broad spectrum of neuroimmunologic and neuroinflammatory disorders of the optic nerve and optic nerve sheath.
The potential for the erroneous underestimation of the true incidence rates for the involvement of the anterior visual system in cancer should mandate that the methodology for future staging investigations includes both a prospective and systematic search for the pathobiology of visual system syndromes that occur in these patients (Table).27 To this point, a potential diagnostic confounder in the case of our patient was that optic nerve biopsy was not performed, principally due to the invasive nature of the procedure. However, it is crucial for us to underscore here, that given the complexity surrounding the diagnosis of malignant infiltration and leptomeningeal carcinomatosis localized to the anterior visual system, when less invasive diagnostic methods fail to elucidate the pathobiology of such processes, then directed biopsy must be considered imperative, and even of justifiable urgency.27
Acknowledgment
The authors thank the National Multiple Sclerosis Society and Mr. Jason Ooi and Dr. Matthew Parsons for their development of Figure 1 which details the complex chronological semiology of this case.
Glossary
- ACE
angiotensin-converting enzyme
- anti-AQP4
aquaporin-4 antibody
- BNS
Bing-Neel syndrome
- BTK
Bruton tyrosine kinase
- CBA
cell-based assay
- CLL
chronic lymphocytic lymphoma
- ED
emergency department
- MMF
mycophenolate mofetil
- MOG
myelin oligodendrocyte glycoprotein
- MOGAD
MOG antibody disease
- NMOSD
neuromyelitis optica spectrum disorder
- ON
optic neuritis
- RBC
red blood cell
- VA
visual acuity
- WBC
white blood cell
- WM
Waldenstrom macroglobulinemia
Appendix. Authors
Study Funding
This work was funded by the National Multiple Sclerosis Society. The authors thank Mr. Jason Ooi and Dr. Matthew Parsons for the development of Figure 1, which was funded by the Frohman Foundation: Innovating Precision Care Through Discovery in Molecular Medicine.
Disclosure
D.A. Pimentel Maldonado has no disclosures. R. Lisak, over the past 2 years, has been funded for research support by the NIH, National Multiple Sclerosis Society (USA), Mallinckrodt Pharmaceuticals, Genentech, Teva Pharmaceuticals, Novartis, MedImmune, and Chugai. He has served as a consultant to GLG, Syntimmune, Alexion, AlphaSites, Insights Consulting, Informa Pharma Consulting, and Slingshot Consulting. He has served on the speaker's bureau for Teva Pharmaceuticals (nonbranded talks only). S.L. Galetta has received consultant fees from Genentech. L.J. Balcer is editor-in-chief of the Journal of Neuro-Ophthalmology. T. Varkey has no disclosures. A. Goodman has served as a consultant and received honoraria from Janssen and Novartis. He has received research support from the following: Biogen, Genzyme Sanofi, and Atara. J. Graves unrelated to the current work, over the past year has grant/contract research support from the National MS Society, Biogen, and Octave Biosciences. She serves on a steering committee for a trial supported by Novartis. She has received honoraria for a nonpromotional, educational activity for Sanofi-Genzyme. She has received speaker fees from Alexion and BMS and served on an advisory board for Genentech. S.D. Newsome has received consultant fees for scientific advisory boards from Biogen, Genentech, Celgene, EMD Serono, Novartis, and Greenwich Biosciences; is an advisor for Autobahn Therapeutics and BioIncept; is a clinical adjudication committee member for a MedDay Pharmaceuticals clinical trial; and has received research funding (paid directly to institution) from Biogen, Novartis, Genentech, National MS Society, Department of Defense, and Patient-Centered Outcomes Institute. M.K. Racke is an employee of and owns stock in Quest Diagnostics. S.S. Zamvil is Deputy Editor of Neurology, Neuroimmunology and Neuroinflammation and is a member of the advisory board for the International Society of Neuroimmunology. He has served as a consultant and received honoraria from Biogen Idec, EMD Serono, Genzyme, Novartis, Roche/Genentech, and Teva Pharmaceuticals, Inc., and has served or serves on Data Safety Monitoring Boards for Lilly, BioMS, Teva, and Opexa Therapeutics. Currently, S.S. Zamvil receives research grant support from the NIH, the NMSS, The Maisin Foundation, Biogen, and Celgene. E.M. Frohman has received speaker honoraria from Genzyme, Novartis, Janssen, and Alexion. T.C. Frohman has received speaker fees from Alexion. Go to Neurology.org/NN for full disclosure.
: References
- 1.Anadani N, Hyland M, Cruz RA, et al. Treating MS after surviving PML: discrete strategies for rescue, remission, and recovery patient 1: from the national multiple sclerosis society case conference proceedings. Neurol Neuroimmunol Neuroinflamm. 2021;8(1):e929. doi: 10.1212/NXI.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg BM, Graves D, Remington G, et al. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler. 2012;18(7):1022-1026. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima H, Hosokawa T, Sugino M, et al. Visual field defects of optic neuritis in neuromyelitis optica compared with multiple sclerosis. BMC Neurol. 2010;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett JL. Optic neuritis. Continuum (Minneap Minn). 2019;25(5):1236-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramanathan S, Prelog K, Barnes EH, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22:470-482. [DOI] [PubMed] [Google Scholar]
- 6.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63(7):964-968. [DOI] [PubMed] [Google Scholar]
- 7.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. Myelin oligodendrocyte glycoprotein antibody–positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. 2018;195:8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackburn K, Wang C, Greenberg B. Two cases of aquaporin-4 positive neuromyelitis optica associated with T-cell lymphoma. J Neuroimmunol. 2020;338:577092. [DOI] [PubMed] [Google Scholar]
- 9.Nasralla S, Abboud H. Is neuromyelitis optica without AQP4-IgG a T-cell mediated disease? Insights from checkpoint inhibitor immune-related adverse events. Mult Scler Relat Disord. 2020;46:102451. [DOI] [PubMed] [Google Scholar]
- 10.Gibril M, Walters R. Neuromyelitis optica spectrum disorder as a paraneoplastic syndrome: a rare and challenging diagnosis. BMJ Case Rep. 2021;14(5):e239389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidd DP, Burton BJ, Graham EM, Plant GT. Optic neuropathy associated with systemic sarcoidosis. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petzold A, Plant GT. Chronic relapsing inflammatory optic neuropathy: a systematic review of 122 cases reported. J Neurol. 2014;261(1):17-26. [DOI] [PubMed] [Google Scholar]
- 13.Minnema MC, Kimby E, D'Sa S, et al. Guideline for the diagnosis, treatment and response criteria for Bing-Neel syndrome. Haematologica. 2017;102(1):43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. New Engl J Med. 2013;369(1):32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Neil DS, Francescone MA, Khan K, et al. A case of bing-neel syndrome successfully treated with ibrutinib. Case Rep Hematol. 2018;2018:8573105. Erratum in: Case Rep Hematol. 2018;2018:7610201. Bachir A [corrected to Alobeid B]. doi: 10.1155/2018/8573105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan Y, Khan W, Thalambedu N, Ashfaq AA, Ullah W. Optic neuritis: a rare paraneoplastic phenomenon of Hodgkin's lymphoma. Cureus. 2019:11(7):e5181. Accessed April 25, 2022. cureus.com/articles/21433-optic-neuritis-a-rare-paraneoplastic-phenomenon-of-hodgkins-lymphoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross SA, Salomao DR, Parisi JE, et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann Neurol. 2003;54(1):38-50. [DOI] [PubMed] [Google Scholar]
- 18.Rogers A, Woyach JA. BTK inhibitors and anti-CD20 monoclonal antibodies for treatment-naïve elderly patients with CLL. Ther Adv Hematol. 2020;11:2040620720912990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bierman P, Giglio P. Diagnosis and treatment of central nervous system involvement in non-Hodgkin's lymphoma. Hematology/Oncology Clin. 2005;19(4):597-609. [DOI] [PubMed] [Google Scholar]
- 20.Tomita N, Kodama F, Sakai R, et al. Predictive factors for central nervous system involvement in non-Hodgkin's lymphoma: significance of very high serum LDH concentrations. Leuk Lymphoma. 2000;38(3-4):335-343. [DOI] [PubMed] [Google Scholar]
- 21.Grommes C, Rubenstein JL, DeAngelis LM, Ferreri AJM, Batchelor TT. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019;21(3):296-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moazzam AA, Drappatz J, Kim RY, Kesari S. Chronic lymphocytic leukemia with central nervous system involvement: report of two cases with a comprehensive literature review. J Neurooncol. 2012;106(1):185-200. [DOI] [PubMed] [Google Scholar]
- 23.Spectre G, Gural A, Amir G, Lossos A, Siegal T, Paltiel O. Central nervous system involvement in indolent lymphomas. Ann Oncol. 2005;16(3):450-454. [DOI] [PubMed] [Google Scholar]
- 24.Kincaid MC, Green WR. Ocular and orbital involvement in leukemia. Surv Ophthalmol. 1983;27(4):211-232. [DOI] [PubMed] [Google Scholar]
- 25.Kattah JC, Suski ET, Killen JY, Smith FP, Limaye SR. Optic neuritis and systemic lymphoma. Am J Ophthalmol. 1980;89(3):431-436. [DOI] [PubMed] [Google Scholar]
- 26.Millar MJ, Tumuluri K, Murali R, Ng T, Beaumont P, Maloof A. Bilateral primary optic nerve lymphoma. Ophthalmic Plast Reconstr Surg. 2008;24(1):71-73. [DOI] [PubMed] [Google Scholar]
- 27.Ahle G, Touitou V, Cassoux N, et al. Optic nerve infiltration in primary central nervous system lymphoma. JAMA Neurol. 2017;74(11):1368-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong D, Danesh-Meyer H, Pon JA. Infiltrative lymphomatous optic neuropathy in non-Hodgkin lymphoma. J Clin Neurosci. 2015;22(9):1513-1515. [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Zhao J, Song H, Wei S, Zhou H, Xu Q. Orbital magnetic resonance imaging may contribute to the diagnosis of optic nerve lymphoma. Front Neurol. 2020;11:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young B, Eggenberger E, Kaufman D. Current electrophysiology in ophthalmology: a review. Curr Opin Ophthalmol. 2012;23(6):497-505. doi: 10.1097/ICU.0b013e328359045e. [DOI] [PubMed] [Google Scholar]
- 31.Lanfranconi S, Basilico P, Trezzi I, et al. Optic neuritis as isolated manifestation of leptomeningeal carcinomatosis: a case report and systematic review of ocular manifestations of neoplastic meningitis. Neurol Res Int. 2013;2013:892523. 10.1155/2013/892523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu TY, Ibrahim M, Bittencourt M, Sepah YJ, Do DV, Nguyen QD. Retinal optical coherence tomography manifestations of intraocular lymphoma. J Ophthalmic Inflamm Infect. 2012. Dec;2(4):215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]