Abstract
Background and Objectives
Some disease-modifying treatments impair response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines in multiple sclerosis (MS), potentially increasing the risk of breakthrough infections. We aimed to investigate longitudinal SARS-CoV-2 antibody dynamics and memory B cells after 2 and 3 messenger RNA (mRNA) vaccine doses and their association with the risk of COVID-19 in patients with MS on different treatments over 1 year.
Methods
Prospective observational cohort study in patients with MS undergoing SARS-CoV-2 mRNA vaccinations. Antispike (anti-S) immunoglobulin G (IgG) titers were measured by chemiluminescence microparticle immunoassay. Frequencies of spike-specific memory B cells were measured on polyclonal stimulation of peripheral blood mononuclear cells and screening of secreted antibodies by ELISA.
Results
We recruited 120 patients with MS (58 on anti-CD20 antibodies, 9 on sphingosine 1-phosphate (S1P) receptor modulators, 15 on cladribine, 24 on teriflunomide (TFL), and 14 untreated) and collected 392 samples up to 10.8 months after 2 vaccine doses. When compared with untreated patients, anti-CD20 antibodies (β = −2.07, p < 0.001) and S1P modulators (β = −2.02, p < 0.001) were associated with lower anti-S IgG, while TFL and cladribine were not. Anti-S IgG decreased with months since vaccine (β = −0.14, p < 0.001), independently of treatments. Within anti-CD20 patients, anti-S IgG remained higher in those with greater baseline B-cell counts and were not influenced by postvaccine anti-CD20 infusions. Anti-S IgG increase after a 3rd vaccine was mild on anti-CD20 and S1P modulators. Spike-specific memory B-cell responses were weaker on S1P modulators and anti-CD20 than on TFL and influenced by postvaccine anti-CD20 infusions. The frequency of breakthrough infections was comparable between DMTs, but the risk of COVID-19 was predicted by the last measured anti-S IgG titer before infection (OR = 0.56, 95% CI = 0.37–0.86, p = 0.008).
Discussion
Postvaccine anti-S IgG titers decrease over time regardless of MS treatment and are associated with breakthrough COVID-19. Both humoral and specific memory B-cell responses are diminished on S1P modulators. Within anti-CD20–treated patients, B-cell count at first vaccine determines anti-S IgG production, whereas postvaccine anti-CD20 infusions negatively affect spike-specific memory B cells.
Postvaccine antibody titers against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) decrease over time in the general population, reflecting a progressive reduction in vaccine efficacy and the need of vaccine boosters.1,2 In addition to circulating antibodies, vaccine-induced memory responses (both T and B cells) are also relevant for developing a rapid immune reaction in case of virus encounter.3-5
Patients with multiple sclerosis (MS) represent a fragile population with higher susceptibility to severe COVID-19, especially those with greater age, with neurologic disability, with comorbidities, or treated with some disease-modifying treatments (DMTs).6 Several studies have shown that anti-CD20 monoclonal antibodies and sphingosine 1-phosphate receptor modulators (S1P-mod) impair both antibody production and memory adaptive immune responses to SARS-CoV-2 vaccines,7-10 with recent data suggesting an increased frequency of breakthrough infections on such DMTs.11-13 It is, however, unclear to what extent the higher susceptibility to breakthrough infections is mediated by impaired humoral or cellular adaptive responses, rather than by other potential DMT-related mechanisms.
Several questions remain to be answered to optimize vaccine response in MS, and this is relevant for future expected COVID-19 waves. We therefore aimed to investigate the effect of various DMTs on longitudinal antibody dynamics, efficacy of boosters, specific memory B-cell responses, and risk of COVID-19 within a prospective cohort of patients with MS on different DMTs and followed-up for over 1 year.
Methods
Study Population and Blood Samples
This is a prospective single-center observational cohort study initiated in February 2021 at the Neurocenter of Southern Switzerland.8 Inclusion criteria were as follows: a diagnosis of MS14; age older than 18 years; and being scheduled for SARS-CoV-2 messenger RNA (mRNA) vaccine (mRNA-1273 or BNT162b2).15,16 Exclusion criteria were as follows: medical treatments influencing response to vaccines other than MS DMTs; having experienced a symptomatic laboratory-confirmed SARS-CoV-2 infection.
Serum samples were collected at t0 (within 2 weeks before the first vaccine dose), t1 (21–35 days after the second dose), and at each following clinical visit (as required by treating neurologist). Serum samples were also collected at different time points after a 3rd vaccine dose (if administered). Ethylene diamine tetra-acetic acid blood samples were additionally collected in a subgroup of patients between 3 and 5 months after the 2nd vaccine dose for the assessment of SARS-COV-2–specific memory B-cell response.
All patients with MS included in the study were regularly seen at our center (usually every 3 months, as required by treating neurologist). Breakthrough COVID-19 was defined as a positive molecular PCR test for SARS-CoV-2, and patients were asked to report such events as quickly as possible to the treating neurologist by telephone. Patients were also directly asked for the occurrence of COVID-19 during each clinical visit. Samples collected after breakthrough infections were excluded from all analyses.
SARS-CoV-2 Serological Analysis and Baseline Cell Sorting
Quantification of serum immunoglobulin G (IgG) antibodies against SARS-CoV-2 spike (S) protein (including the receptor-binding domain) was performed using a commercial chemiluminescence microparticle immunoassay by Abbot (quantification limits 21–40,000 AU/mL, cutoff for positivity = 50 AU/mL).17 Total CD19+ B cells and CD19+CD27+ memory B cells were also measured at t0 using fluorescence-activated cell sorting.
Antigen Specific Memory B Cell Repertoire Analysis (AMBRA)
To determine the frequency of antigen-specific memory B cells, peripheral blood mononuclear cells were isolated by Ficoll-Paque Plus (Cytiva) gradient and seeded in replicative cultures of 96-well U-bottom plates at 3 × 104 cells/well.18 Cells were stimulated with 2.5 μg/mL of R848 (Invivogen) in Roswell Park Memorial Institute 1640 complete medium supplemented with 2 mM glutamine, 1% (v/v) nonessential amino acids, 1% (v/v) sodium pyruvate, 50 U/mL of penicillin, 50 μg/mL of streptomycin, 50 U/mL of Kanamycin, 0.1% Beta-Mercaptethanol (all from Gibco), 10% fetal bovine serum (Hyclone), 0.5% Transferrin (30 μg/mL) (LubioScience), and 1,000 U/mL of interleukin-2 (produced in house from transfected J558L cells). Cells were cultured at 37°C, 5% CO2 for 12 days. Specific IgG antibodies were measured in the culture supernatants using in-house–developed ELISA.
ELISA
To measure antibodies in the memory B-cell culture supernatants, 96-well plates were coated overnigt at 4°C with 2.5 μg/mL of the recombinant SARS-CoV-2 (2019-nCoV) S protein (S1 + S2 extracellular domain) in phosphate-buffered saline (PBS) (Sino Biological Inc). Uncoated plates were used as control (no Ag). Plates were washed and blocked with Blocker Casein in PBS supplemented with 0.05% Tween 20 (Sigma Aldrich) for 1 hour at room temperature. Plates were subsequently incubated with 25 μL of undiluted supernatant for 1 hour at room temperature. After washing with PBS containing 0.1% Tween-20 (PBS-T), alkaline phosphatase–conjugated goat anti-human IgG (dilution 1:500; from Southern Biotech) was added and incubated for 45 minutes at room temperature. Plates were washed 3 times with PBS-T, and 4-nitrophenyl phosphate (Sigma-Aldrich) substrate was added, and the absorbance of 405 nm was measured by a microplate reader (BioTek). The frequency of S-specific B cells was calculated according to the Poisson distribution and expressed per million cells. Cultures containing IgG antibodies reacting to uncoated plates were excluded from the analysis.
Statistical Analysis
Variables were expressed as median with interquartile range or counts with percentages. Multivariate mixed-effect linear models with individual patients as random effect were used to test several variables (fixed effects) for association with longitudinal log-transformed postvaccine antispike (anti-S) IgG, adjusted by age and sex. Mann-Whitney and Wilcoxon matched-pair tests were also used to test for differences in anti-S IgG between groups as appropriate. Logistic regression was used to test variables for association with the occurrence of COVID-19 during follow-up. For this analysis, the last measured anti-S IgG titer before infection was used for patients who developed COVID-19, and the mean anti-S IgG titer across all time points was used for those who did not develop COVID-19.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consents were obtained from all patients included in the study. The study was approved by Canton Ticino ethics committee (CETI3863).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Longitudinal Changes in SARS-CoV-2 Anti-S IgG Titers After 2 Vaccine Doses
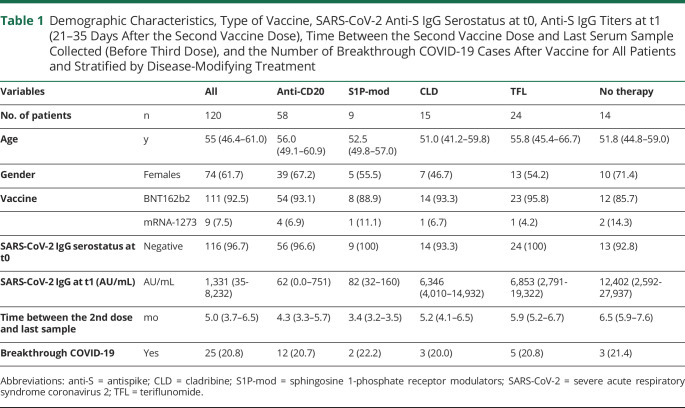
A total of 120 patients were recruited between February 25, 2021, and April 16, 2021. Of them, 58 were treated with anti-CD20 antibodies (ocrelizumab = 32, rituximab = 25, and ofatumumab = 1), 9 treated with S1P-mod (fingolimod = 7, ozanimod = 2), 15 treated with cladribine (CLD), 24 treated with teriflunomide (TFL), and 14 were untreated. All patients received 2 mRNA vaccine doses (BNT162b2 = 111, mRNA-1273 = 9, median time between doses = 28 [28–30] days) and had serum collected at t0 and t1. Six patients had no further samples, 114 had ≥1, 37 had ≥2, and 1 had 3 additional samples (total number of samples = 392, up to 10.8 months after the 2nd dose). Table 1 summarizes baseline demographic characteristics, anti-S IgG titers measured at t1 and time interval between the 2nd vaccine dose and last serum sample collected (before potential 3rd vaccine dose).
Table 1.
Demographic Characteristics, Type of Vaccine, SARS-CoV-2 Anti-S IgG Serostatus at t0, Anti-S IgG Titers at t1 (21–35 Days After the Second Vaccine Dose), Time Between the Second Vaccine Dose and Last Serum Sample Collected (Before Third Dose), and the Number of Breakthrough COVID-19 Cases After Vaccine for All Patients and Stratified by Disease-Modifying Treatment
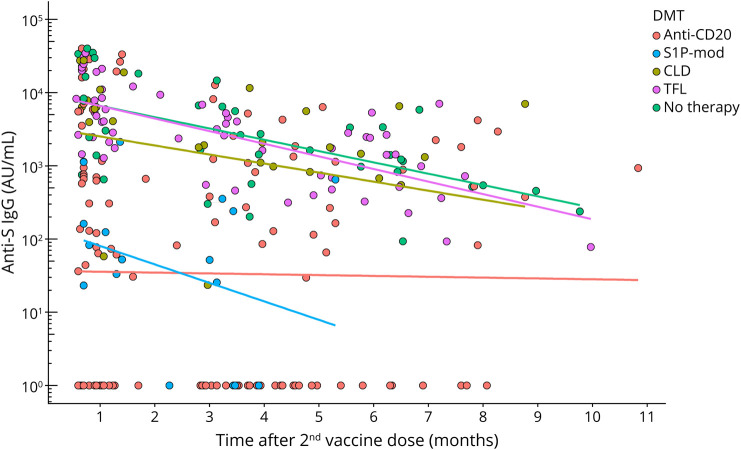
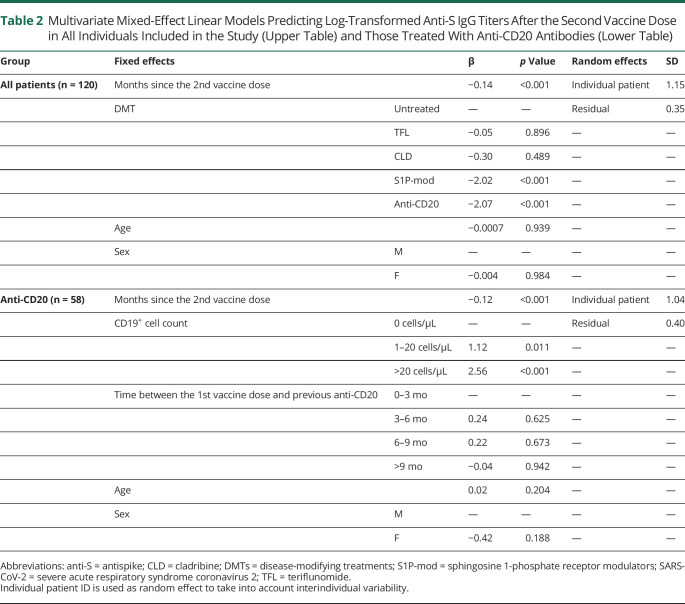
A mixed-effect model was created with DMTs, months between the 2nd vaccine dose and serum sample as fixed effects predicting anti-S IgG, and individual patients as random effect. Compared with untreated patients, anti-CD20 and S1P-mod were associated with lower anti-S IgG titers over follow-up, while TFL and CLD were not (Figure 1 and Table 2). Anti-S IgG also progressively decreased with months since 2nd vaccine dose (Figure 1 and Table 2), and this was independent of DMTs. Age and sex were not associated with anti-S IgG titers.
Figure 1. Longitudinal Dynamics of SARS-Cov-2 Anti-S IgG Titers in Patients With MS Under Treatment With Anti-CD20 Antibodies, S1P Modulators (S1P-Mod), Cladribine (CLD), Teriflunomide (TFL), and No Therapy.
Anti-S IgG titers are shown on a logarithmic scale. Anti-S = antispike; MS = multiple sclerosis.
Table 2.
Multivariate Mixed-Effect Linear Models Predicting Log-Transformed Anti-S IgG Titers After the Second Vaccine Dose in All Individuals Included in the Study (Upper Table) and Those Treated With Anti-CD20 Antibodies (Lower Table)
Determinants of SARS-CoV-2 Anti-S IgG After 2 Vaccine Doses in Anti-CD20–Treated Patients
We have previously shown that baseline CD19+ B cells and time since previous anti-CD20 infusion were associated with anti-S IgG production at 1 month after vaccination.8 We extended this analysis using a mixed-effect model with CD19+ B-cell count at t0, months between the 1st vaccine dose and previous anti-CD20 infusion, and months between the 2nd vaccine dose and serum sample as fixed effects. Anti-S IgG titers decreased with longer time since the 2nd vaccine dose, but remained constantly higher in patients with greater CD19+ B-cell counts at t0, especially those with >20 CD19+ B cells/μL (β = 2.56, p < 0.001, Figure 2A and Table 2). Time (months) since the last prevaccine anti-CD20 infusion was associated with anti-S IgG in univariate (3–6 vs 0–3: β = 0.38, p = 0.537; 6–9 vs 0–3: β = 1.15, p = 0.055; >9 vs 0–3: β = 1.78, p = 0.005), but not in the multivariate model (Table 2). Notably, the number of CD19+CD27+ memory B cells at t0 was low, with all but 1 patient having ≤2 CD19+CD27+ memory B cells/μL (Figure 2B).
Figure 2. Determinants of Anti-S IgG Titers in Patients With MS Treated With Anti-CD20 Antibodies.
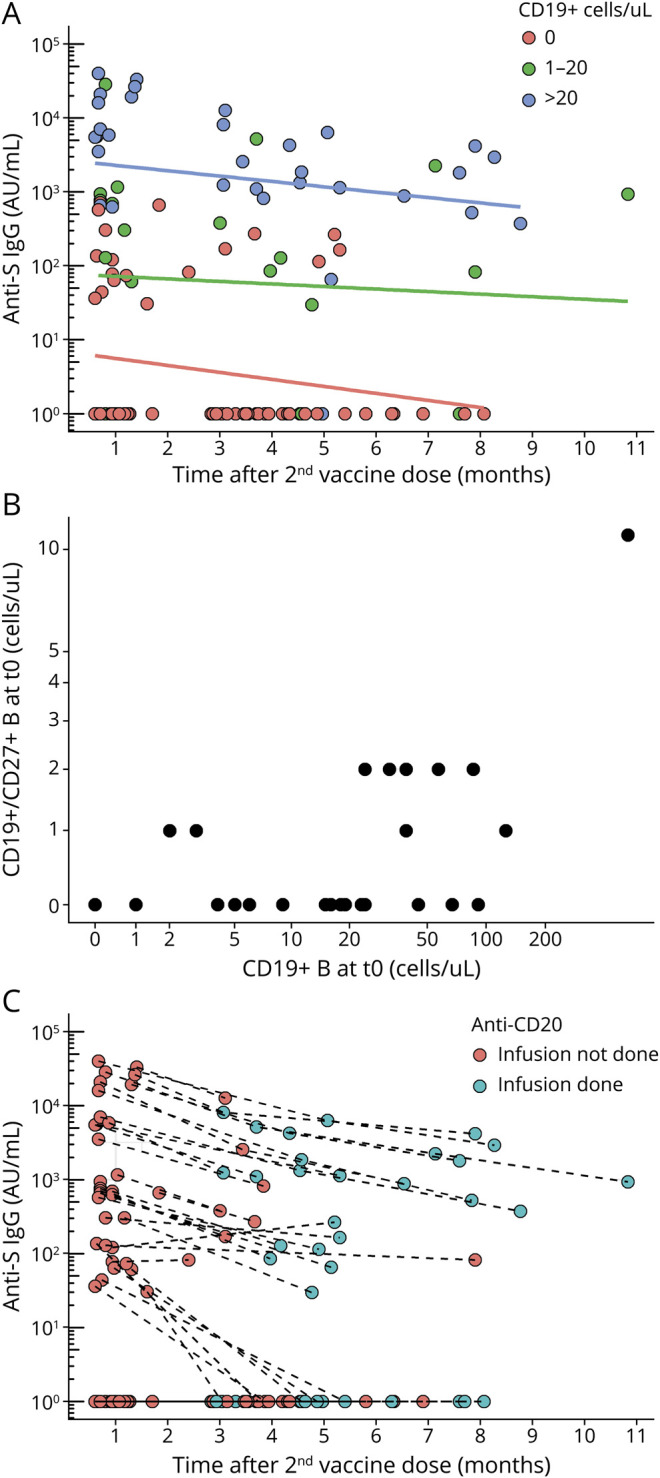
(A) Relation between baseline CD19+ B-cell count at t0 and temporal trends in anti-S IgG titers in anti-CD20–treated patients. (B) Relation between total CD19+ B-cell count at t0 and CD19+CD27+ memory B-cell count at t0 in anti-CD20–treated patients; (C) Effect of administration of anti-CD20 infusions after the 2nd vaccine dose on anti-S IgG titers; a blue circle indicates that the patient has in the meanwhile received an anti-CD20 infusion. Anti-S IgG titers and cell counts are shown on a logarithmic scale. Anti-S = antispike; IgG = immunoglobulin G.
We investigated whether anti-CD20 infusions performed after the 2nd vaccine dose also influenced following anti-S IgG titers. Twenty-six patients were seropositive at t1, and 18 of them received anti-CD20 infusions after t1. Anti-S IgG titers decreased over time regardless of whether anti-CD20 infusions were performed after t1 (Figure 2C). Patients who received compared with those who did not receive anti-CD20 after t1 had similar anti-S IgG at the following sample (629.7 [69.5–1,727.8] vs 324.3 [146.9–1,252.9], respectively; Mann-Whitney p = 0.911).
Humoral Response to the Third Vaccine Dose
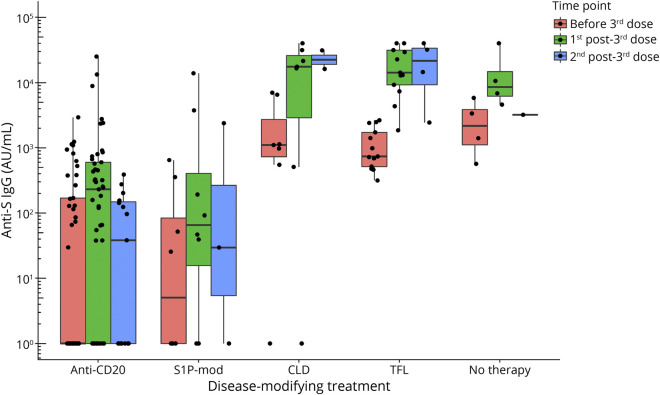
Based on experts' recommendation, patients with postvaccine anti-S IgG lower than 2,000 AU/mL were given priority access to receive a 3rd vaccine dose. Seventy-seven patients received a 3rd dose at 6.9 (5.8–8.0) months after the 2nd dose (anti-CD20 n = 45, S1P-mod n = 8, TFL n = 13, CLD n = 7, untreated n = 4) and had at least 1 sample collected afterward (median time to sample = 35 [29–47] days). A second following sample was also available from 25 patients at a median of 124 (101–152) days after the 3rd vaccine dose.
The increase in anti-S IgG titers at the 1st sample after the 3rd vaccine dose was mild on anti-CD20 (229 [0–598] AU/mL) and S1P-mod (68.2 [28.7–1,083] AU/mL), but larger on TFL (14,237 [9,268–31,463] AU/mL) and CLD (17,462 [8,516–26,466] AU/mL, Figure 3). Compared with untreated patients, post-3rd dose anti-S IgG titers were lower in those on anti-CD20 (β = −2.23, p < 0.001) and S1P-mod (β = −2.25, p = 0.003) and inversely associated with time elapsed between the 3rd dose and serum sample (β = −0.14, p < 0.001, Figure 3).
Figure 3. Changes in SARS-CoV-2 Anti-S IgG Titers After a Third Vaccine Dose in Patients Stratified by Disease-Modifying Treatment.
Anti-S IgG titers are shown on a logarithmic scale. Anti-S = antispike; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Frequency of Antigen-Specific Memory B Cells
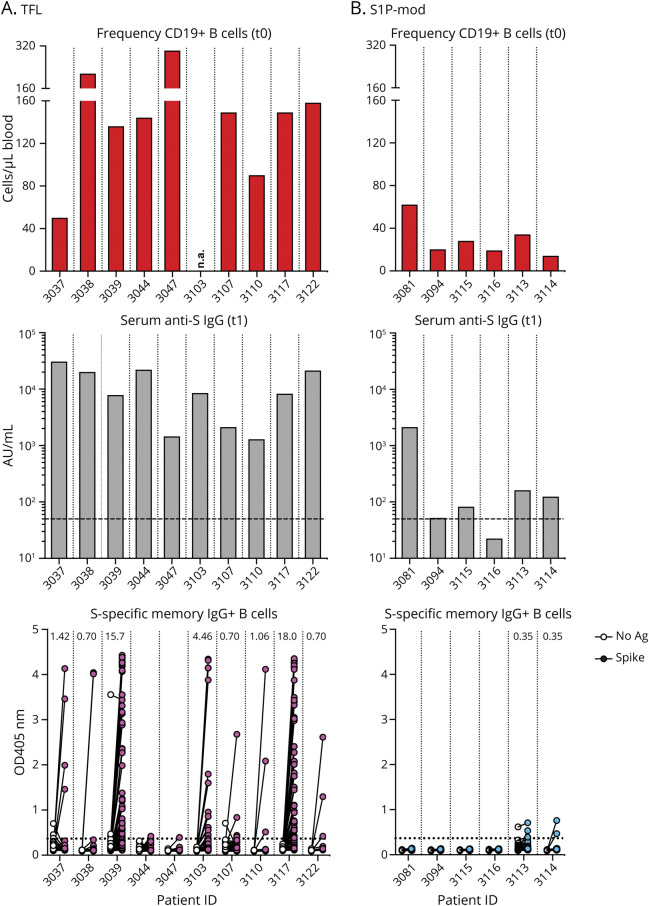
We were interested in investigating whether those DMTs negatively influencing the humoral response to the vaccine (i.e., anti-CD20 and S1P-mod) also impaired the development of virus-specific memory B cells, when compared with DMTs with no influence on vaccine-induced anti-S IgG titers (e.g., TFL). SARS-CoV-2–specific circulating memory B cells were therefore measured from samples collected at 4.4 (3.5–5.0) months after the 2nd vaccine dose from a total of 40 patients (anti-CD20 n = 24, S1P-mod n = 6, TFL n = 10).
Figure 4 shows the results obtained from the analysis of patients on TFL and S1P-mod. The figure also reports for each patient the frequency of CD19+ B cells at t0 (before the 1st vaccine dose) and serum anti-S IgG antibody titers measured at t1 (21–35 days after the 2nd vaccine dose). SARS-CoV-2–specific memory B cells were found, albeit at variable frequencies, in 8 of 10 patients on TFL, with no clear association with either CD19+ B-cell frequency at t0 or anti-S IgG titers at t1 (Figure 4A). By contrast, SARS-CoV-2–specific memory B cells were undetectable or very low in all patients on S1P-mod (Figure 4B). Some specific memory B cells against SARS-CoV-2 were detected in the 2 patients treated with ozanimod (3113 and 3114), whereas the remaining 4 patients on fingolimod had no response at all (Figure 4B).
Figure 4. SARS-CoV-2–Specific IgG+ Memory B-Cell Response Measured in Patients With MS on Treatment With Teriflunomide (A) and S1P Modulators (B), With Relative CD19+ B-Cell Counts at t0 (Before First Vaccine Dose) and Anti-S IgG Measured at t1 (21–35 Days After the Second Dose).
Numbers in the bottom panels indicate frequency of S-specific memory B cells in 10e6 peripheral blood mononuclear cells (PBMCs). Anti-S = antispike; MS = multiple sclerosis; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
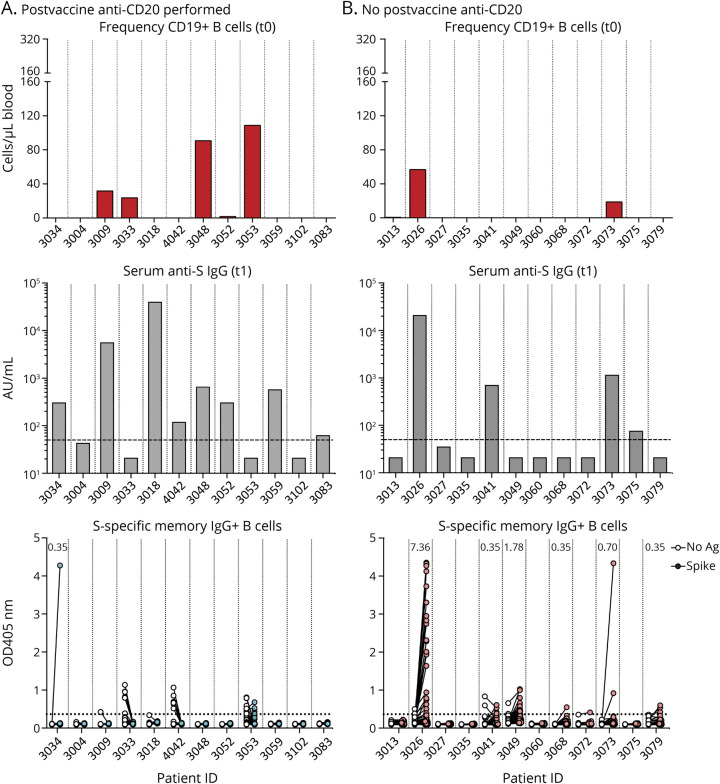
The same analysis was performed in patients on anti-CD20 treatment, and these were stratified according to whether patients received (n = 12, Figure 5A) or did not receive (n = 12, Figure 5B) anti-CD20 infusions between the 2nd vaccine dose and SARS-CoV-2–specific memory B-cell measurement. With the exception of patient 3034 (in which a mild response was observed), specific memory B cells were undetectable in all patients who received anti-CD20 after the 2nd vaccine dose, even in those individuals with greater CD19+ B-cell counts at t0 and anti-S IgG titers at t1 (Figure 5A). Specific memory B cells were also absent in most of the patients who did not receive anti-CD20 after the 2nd vaccine dose, but clearly measurable responses were present in the only 2 patients who had detectable CD19+ B cells at t0 and anti-S IgG at t1 (3026 and 3073, Figure 5B).
Figure 5. SARS-CoV-2–Specific IgG+ Memory B-Cell Response Measured in Patients With MS on Treatment With Anti-CD20 Who Received Anti-CD20 Infusions After the Second Vaccine Dose (A) and Did Not Receive Anti-CD20 Infusions After the Second Vaccine Dose (B), With Relative CD19+ B-Cell Counts at t0 (Before First Vaccine Dose) and Anti-S IgG Measured at t1 (21–35 Days After the Second Dose).
Numbers in the bottom panels indicate frequency of S-specific memory B cells in 10e6 peripheral blood mononuclear cells (PBMCs). Anti-S = antispike; MS = multiple sclerosis; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Association Between Anti-S IgG and SARS-CoV-2 Memory B-Cell Response With Risk of COVID-19
Twenty-five patients developed COVID-19 during follow-up (age = 53.9 [46.0–57.9], 17 [68%] females, 21 [84%] after 3 vaccine doses and 4 [16%] after 2 vaccine doses). COVID-19 cases were equally distributed across treatments with 12/58 (20.7%) patients on anti-CD20, 3/15 (20%) on CLD, 2/9 (22.2%) on S1P-mod, and 5/24 (20.8%) on TFL and 3/14 (21.4%) untreated. No COVID-19 case was life-threatening or required mechanical ventilation; 2 patients were hospitalized (both on anti-CD20). Seven patients (6 on anti-CD20 and 1 on S1P-mod) received early treatment (≤5 days from the beginning of symptoms) with monoclonal antibodies against S-protein of SARS-CoV-2 (sotrovimab).
COVID-19 patients had a last serum sample collected at 72 (50–102) days before infection. The median anti-S IgG at these time points was 324.6 (0.0–1,457.5) AU/mL, when compared with 676.0 (29.5–4,605.3) AU/mL considering all remaining samples. Last measured anti-S IgG titer predicted risk of infection (OR = 0.56, 95% CI = 0.37–0.86, p = 0.008), and this was independent of age, sex, number of vaccine doses received, and DMTs (all not significant). Regarding, memory B-cell response, the proportion of patients who developed COVID-19 was 2/14 (14.3%) among those who tested positive for SARS-CoV-2–specific memory B cells and 6/26 (23.1%) among those who tested negative for SARS-CoV-2–specific memory B cells (OR = 0.55, 95% CI = 0.10–3.21, p = 0.511).
Discussion
The development of vaccines against SARS-CoV-2 has played a major role in the prevention of COVID-19 and, most importantly, of severe forms of disease over the last year. However, antibody response to SARS-CoV-2 vaccines can be weakened by specific MS DMTs. We made use of a large cohort of patients with MS with repeated serial samples and detailed clinical follow-up over 1 year to describe longitudinal anti-S IgG dynamics after 2 and 3 vaccine doses, the development of SARS-CoV-2 specific memory B cell responses, and their potential relation with breakthrough COVID-19.
We confirm that anti-CD20 and S1P-mod negatively influence anti-S IgG production at 1 month after 2 vaccine doses, whereas this response is not impaired by other DMTs such as TFL and CLD. Afterward, anti-S IgG titers progressively declined over time independently of DMTs, as observed in the general population.19 Similarly, anti-CD20 and S1P-mod negatively influence the humoral response to a 3rd vaccine dose, whereas patients on TFL and CLD had a rapid increase in anti-S IgG titers, comparable with that of untreated patients. These findings are consistent with recent reports20-22 and suggest that one should expect only a mild increase in anti-S IgG with additional boosters in anti-CD20–treated and S1P-mod–treated MS patients.
Among patients treated with anti-CD20 antibodies, we observed a highly variable postvaccine humoral production ranging from absent to very high anti-S IgG titers over follow-up, with the main determinant of such diverse response being the CD19+ B-cell count during vaccine administration. We found a mild anti-S IgG production already in patients with ≥1 CD19+ B cells/μL during the 1st vaccine dose, as in the study conducted by Kornek et al.,23 but a threshold of 20 CD19+ B cells/μL was associated with sustained high anti-S IgG titers over time, similar to those observed in untreated patients. This was achieved even in the presence of very low CD19+CD27+ memory B-cell counts, which is relevant because of the evidence supporting their pathogenic role in MS and the associated risk of disease reactivation.24,25
Several reports have suggested the time interval between the last anti-CD20 infusion and vaccination determines the extent of anti-S IgG production.7,26-28 We replicated this in our cohort, but the association disappeared when baseline CD19+ B-cell counts were included in the same regression model. Timing of CD19+ B-cell repopulation can largely vary after anti-CD20 infusions between patients and within the same individual.29,30 Our results suggest CD19+ B-cell count (rather than time since last anti-CD20 infusion) provides a more reliable prediction of the humoral response to SARS-CoV-2 vaccines. It is of interest that we did not find any evidence that anti-CD20 infusions administered after vaccination could negatively influence anti-S IgG titers and patients who received anti-CD20 after the 2nd vaccine dose did not have a more rapid drop in anti-S IgG. This implies that vaccine-induced antibody production predominantly takes place within a rather limited period of time and that following B-cell depletion does not directly influence circulating antibodies.
In addition to humoral immunity, cellular mechanisms are also induced by vaccines, and many studies have shown potent specific CD4+ and CD8+ T-cell responses against SARS-CoV-2 after mRNA vaccination, even in patients treated with anti-CD20 antibodies.9,31-33 The memory B-cell response has been instead less investigated, with studies showing how these are also negatively influenced by anti-CD20 and S1P-mod.9,10 We observed that a large proportion of patients treated with TFL have a good frequency of circulating memory B cells specific for SARS-CoV-2 S antigen, even several months after vaccination. By contrast, this memory B-cell response was impaired in patients treated with S1P-mod. There were some hints for a possible difference between ozanimod (mild response) and fingolimod (no response), which is interesting but needs to be interpreted with caution given the small sample size.
Diminished/absent specific memory B-cell responses were also found in patients treated with anti-CD20, with the remarkable exception of those few individuals who had detectable CD19+ B cells during the 1st vaccine dose and did not receive further anti-CD20 infusions between vaccine and memory B measurements. Taken together with the results of anti-S IgG measurements, these data suggest that prevaccine anti-CD20 infusions and related CD19+ B-cell count during vaccination influence both immediate anti-S IgG production and the development of SARS-CoV-2–specific memory B cells, whereas postvaccine anti-CD20 infusions do not affect circulating anti-S IgG titers but remove SARS-CoV-2–specific memory B cells.
Despite not being powered to provide insights into the severity of COVID-19, we highlight how all breakthrough infections in our cohort were mild to moderate in severity, similar to what has been recently reported by other studies.11-13 One relevant question that needed to be addressed was whether vaccine-induced circulating SARS-CoV-2 antibody titers and cellular responses actually reflect the risk of experiencing breakthrough COVID-19. Studies support this hypothesis in the general population, with an inverse association between neutralizing antibody titers and risk of breakthrough COVID-19.34,35 Some recent studies have also suggested patients with MS treated with anti-CD20 and S1P-mod are at a higher risk of breakthrough COVID-19, but whether this effect is related to lower circulating SARS-CoV-2 antibodies is less clear.11-13 In our cohort, the last anti-S IgG titer measured in the 25 patients who developed breakthrough symptomatic COVID-19 was lower than all remaining samples and was a better predictor of infection than the DMT being used. As to memory B-cell responses, individuals with evidence of SARS-CoV-2–specific memory B cells had numerically lower cases of symptomatic COVID-19 during follow-up when compared with those with no SARS-CoV-2–specific memory B cells. The difference was not statistically significant, but the study was not powered to investigate this. Taken together, these results suggest that lower anti-S IgG titers likely influence the risk of breakthrough COVID-19 in MS (probably more than the specific DMTs themselves). The presence of specific memory B cells may also be relevant to build a rapid immune response to the virus and decrease the chance of developing symptoms, but this needs to be confirmed by other studies.
Strengths of the study include the prospective design of data and sample collection, which minimizes the risk of recall bias (especially for COVID-19) and loss of follow-up. Second, the collection of longitudinal samples allowed us to study anti-S IgG dynamics over time overall and stratified by various DMTs and biological parameters, in contrast with most of the studies performed to date in which postvaccine humoral response has been measured at single time points. Third, the availability of regularly collected clinical data and the exclusion of samples after confirmed COVID-19 made it possible to disentangle vaccine-induced anti-S IgG production from the potential contribution of infectious events. Moreover, this is, to our knowledge, the first study able to integrate clinical data, prevaccine biological parameters, humoral and cellular markers of response to vaccines at multiple time points, and risk of breakthrough COVID-19 from the same large cohort of patients with MS.
Limitations of the study include the small number of patients on treatments other than anti-CD20 and the lack of data regarding additional MS DMTs (e.g., dimethyl fumarate and natalizumab), but previous studies have not shown concerns for therapies other than anti-CD20 and S1P-mod for SARS-CoV-2 vaccine response. Anti-S IgG measurements were performed in all patients at t0 and t1, but the timing of following samples was variably distributed across patients. However, potential interindividual differences and the effect of time were statistically taken into account. We cannot exclude that some patients had asymptomatic COVID-19 after vaccination or mild symptoms that did not prompt individuals to perform a molecular PCR test, but all patients included in the study have been carefully followed up at our center and were suggested to be tested for COVID-19 even in case of very mild symptoms. An association between lower anti-S IgG titers and breakthrough COVID-19 was found, but the study was not powered to detect differences in severity of disease. Similarly, the study was not powered to detect a statistically significant association between specific memory B response and the risk of breakthrough COVID-19. Finally, we did not include data on memory T-cell responses in this work. Other studies have already investigated this showing a robust specific T-cell response after SARS-CoV-2 vaccine, which could at least partly explain the similar frequency of breakthrough infections across DMTs with diverse effect on vaccine-induced humoral response.
To conclude, we demonstrate how vaccine-induced SARS-CoV-2 anti-S antibody titers progressively decrease over time in patients with MS independently of DMTs and are associated with risk of breakthrough COVID-19. Both short-term production of anti-S IgG and specific memory B-cell responses are diminished in individuals treated with S1P-mod and anti-CD20 antibodies. Within patients on anti-CD20, prevaccine and postvaccine infusions differentially influence humoral and memory B-cell responses to the vaccine. Given that postponing anti-CD20 infusions by a few months can be considered relatively safe,30 and anti-CD20 treatment is associated with a higher risk of severe COVID-19,6,13 we suggest that a personalized dosing time regimen could represent a strategy to optimize vaccine response, particularly in selected patients with additional risk factors for worse COVID-19 outcomes (e.g., older age, high disability, and comorbidities). Larger studies are needed to further investigate the potential influence of MS DMTs on the outcome and severity of breakthrough COVID-19, with potential clinical implications for administering further vaccine doses and providing early treatment.
Acknowledgment
The authors thank the participants to the study, the nurses Mara Gola and Nadia Grassi for blood collection, and Sandra Jovic for technical assistance.
Glossary
- anti-S
antispike
- CLD
cladribine
- DMTs
disease-modifying treatments
- IgG
immunoglobulin G
- mRNA
messenger RNA
- MS
multiple sclerosis
- PBS
phosphate-buffered saline
- S1P-mod
sphingosine 1-phosphate receptor modulators
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- TFL
teriflunomide
Appendix. Authors
Footnotes
COVID-19 Resources: NPub.org/COVID19
Study Funding
This work was supported by the Swiss Multiple Sclerosis Society (Research Grant No. 2021-03) and by senior (to C. Zecca) and junior (to G. Disanto) grants from Area Formazione accademica, Ricerca e Innovazione (AFRI). FS and the Institute for Research in Biomedicine are supported by the Helmut Horten Foundation.
Disclosure
G. Disanto, A. Galante, M. Cantù, R Sacco, F. Mele, J.J. Jessica Eisler, F. Keller, E. Bernasconi, F. Sallusto, C. Zecca, and C. Gobbi report no disclosures deemed relevant to this manuscript. Go to Neurology.org/NN for full disclosure.
References
- 1.Munro APS, Janani L, Cornelius V, et al. , COV-BOOST study group. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Frenck RW, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385(17):1627-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mateus J, Dan JM, Zhang Z, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science. 2021;374(6566):eabj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrera G, Picozza M, D'Orso S, et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci Immunol. 2021;6(66):eabl5344. [DOI] [PubMed] [Google Scholar]
- 5.Ciabattini A, Pastore G, Fiorino F, et al. Evidence of SARS-CoV-2-specific memory B cells six months after vaccination with the BNT162b2 mRNA vaccine. Front Immunol. 2021;12:740708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sormani MP, Schiavetti I, Carmisciano L, et al. COVID-19 severity in multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. 2022;7(5):e787. [Google Scholar]
- 7.Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2022;72:103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Disanto G, Sacco R, Bernasconi E, et al. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol .2021;78(12):1529-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: up to 6 months cross-sectional study. J Neuroimmunol. 2021;361:577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garjani A, Patel S, Bharkhada D, et al. Impact of mass vaccination on SARS-CoV-2 infections among multiple sclerosis patients taking immunomodulatory disease-modifying therapies in England. Mult Scler Relat Disord. 2022;57:103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose DR, Mahadeen AZ, Carlson AK, et al. Clinical features and outcomes of COVID-19 despite SARS-CoV-2 vaccination in people with multiple sclerosis. Mult Scler J Exp Transl Clin. 2021;7(4):20552173211057110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiavetti I, Cordioli C, Stromillo ML, et al. Breakthrough SARS-CoV-2 infections in MS patients on disease modifying therapies. Mult Scler. 2022 Nov;28(13):2106-2111. [DOI] [PubMed] [Google Scholar]
- 14.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. [DOI] [PubMed] [Google Scholar]
- 15.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polack FP, Thomas SJ, Kitchin N, et al. , C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maine GN, Lao KM, Krishnan SM, et al. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J Clin Virol. 2020;133:104663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinna D, Corti D, Jarrossay D, Sallusto F, Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol. 2009;39(5):1260-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collier ARY, Yu J, McMahan K, et al. Differential kinetics of immune responses elicited by covid-19 vaccines. N Engl J Med. 2021;385(21):2010-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreyer-Alster S, Menascu S, Mandel M, et al. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J Neurol Sci. 2022;434:120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.König M, Torgauten HM, Tran TT, et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol. 2022;79(3):307-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maglione A, Morra M, Meroni R, Matta M, Clerico M, Rolla S. Humoral response after the booster dose of anti-SARS-CoV-2 vaccine in multiple sclerosis patients treated with high-efficacy therapies. Mult Scler Relat Disord. 2022;61:103776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornek B, Leutmezer F, Rommer PS, et al. B cell depletion and SARS-CoV-2 vaccine responses in neuroimmunologic patients. Ann Neurol. 2022;91(3):342-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiSano KD, Gilli F, Pachner AR. Memory B cells in multiple sclerosis: emerging players in disease pathogenesis. Front Immunol. 2021;12:676686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine. 2017;16:41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyang TV, Evans JP, Miller JS, et al. Neutralizing antibody responses against SARS-CoV-2 in vaccinated people with multiple sclerosis. Mult Scler J. 2022;8(1):20552173221087357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitzalis M, Idda ML, Lodde V, et al. Effect of different disease-modifying therapies on humoral response to BNT162b2 vaccine in Sardinian multiple sclerosis patients. Front Immunol. 2021;12:781843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Räuber S, Korsen M, Huntemann N, et al. Immune response to SARS-CoV-2 vaccination in relation to peripheral immune cell profiles among patients with multiple sclerosis receiving ocrelizumab. J Neurol Neurosurg Psychiatry. 2022;93(9):978-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellwardt E, Ellwardt L, Bittner S, Zipp F. Monitoring B-cell repopulation after depletion therapy in neurologic patients. Neurol Neuroimmunol Neuroinflamm. 2018;5(4):e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker D, MacDougall A, Kang AS, Schmierer K, Giovannoni G, Dobson R. CD19 B cell repopulation after ocrelizumab, alemtuzumab and cladribine: implications for SARS-CoV-2 vaccinations in multiple sclerosis. Mult Scler Relat Disord. 2022;57:103448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brill L, Rechtman A, Zveik O, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78(12):1510-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell–specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98:e541-e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Marco L, D'Orso S, Pirronello M, et al. Assessment of T-cell reactivity to the SARS-CoV-2 omicron variant by immunized individuals. JAMA Netw Open. 2022;5(4):e2210871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chau NVV, Ngoc NM, Nguyet LA, et al. An observational study of breakthrough SARS-CoV-2 Delta variant infections among vaccinated healthcare workers in Vietnam. eClinicalMedicine. 2021;41:101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. New Engl J Med. 2021;385(16):1474-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.