ABSTRACT
Objective:
There is still limited information on the clinical characteristics and outcomes of cystic fibrosis (CF) patients with COVID-19 in Brazil. The objective of this study was to describe the cumulative incidence of COVID-19 in CF patients, as well as their clinical characteristics and outcomes.
Methods:
This was a prospective cohort study involving unvaccinated adult CF patients and conducted during the first year of the SARS-CoV-2 pandemic in the city of Porto Alegre, in southern Brazil. The clinical course of the disease was rated on the WHO Ordinal Scale for Clinical Improvement. The primary outcome was the number of incident cases of COVID-19.
Results:
Between April 30, 2020 and April 29, 2021, 98 CF patients were included in the study. Seventeen patients were diagnosed with COVID-19. For the CF patients, the annual cumulative incidence of COVID-19 was 17.3%, similar to that for the general population, adjusted for age (18.5%). The most common symptoms at diagnosis of COVID-19 were cough (in 59%), dyspnea (in 53%), fatigue (in 53%), and fever (in 47%). Only 6 (35%) of the patients required hospitalization, and 3 (17.6%) required oxygen support. Only 1 patient required mechanical ventilation, having subsequently died.
Conclusions:
During the first year of the SARS-CoV-2 pandemic in southern Brazil, the cumulative incidence rate of COVID-19 was similar between CF patients and the general population. More than 50% of the CF patients with SARS-CoV-2 infection had a mild clinical presentation, without the need for hospital admission, and almost the entire sample recovered completely from the infection, the exception being 1 patient who had advanced lung disease and who died.
Keywords: Cystic fibrosis, COVID-19, SARS-CoV-2
RESUMO
Objetivo:
Ainda não há informações suficientes sobre as características clínicas e desfechos de pacientes com fibrose cística (FC) e COVID-19 no Brasil. O objetivo deste estudo foi descrever a incidência cumulativa de COVID-19 em pacientes com FC, bem como suas características clínicas e desfechos.
Métodos:
Estudo prospectivo de coorte com adultos com FC não vacinados, realizado na cidade de Porto Alegre, no sul do Brasil, durante o primeiro ano da pandemia de SARS-CoV-2. A evolução clínica da COVID-19 foi avaliada por meio da WHO Ordinal Scale for Clinical Improvement (escala ordinal de evolução clínica, elaborada pela OMS). O desfecho primário foi o número de casos incidentes de COVID-19.
Resultados:
Entre 30 de abril de 2020 e 29 de abril de 2021, 98 pacientes com FC foram incluídos no estudo. Dezessete pacientes receberam diagnóstico de COVID-19. Nos pacientes com FC, a incidência cumulativa anual de COVID-19 foi de 17,3%, semelhante à observada na população geral, ajustada pela idade (18,5%). Os sintomas mais comuns no momento do diagnóstico de COVID-19 foram tosse (em 59%), dispneia (em 53%), fadiga (em 53%) e febre (em 47%). Apenas 6 (35%) dos pacientes necessitaram de hospitalização, e 3 (17,6%) necessitaram de suporte de oxigênio. Apenas 1 paciente necessitou de ventilação mecânica e, posteriormente, morreu.
Conclusões:
Durante o primeiro ano da pandemia de SARS-CoV-2 no sul do Brasil, a taxa de incidência cumulativa de COVID-19 foi semelhante nos pacientes com FC e na população geral. Mais de 50% dos pacientes com FC e infecção por SARS-CoV-2 apresentaram manifestações clínicas leves, sem necessidade de internação hospitalar, e quase toda a amostra se recuperou completamente da infecção, à exceção de 1 paciente, que apresentava doença pulmonar avançada e morreu.
Descritores: Fibrose cística, COVID-19, SARS-CoV-2
INTRODUCTION
COVID-19 emerged in the city of Wuhan, in Hubei province, China, as a pneumonia of unknown origin. In February of 2020, the WHO declared the outbreak of the novel coronavirus an international public health emergency. 1 Health outcomes of individuals infected with SARS-CoV-2 range from the lack of any symptoms to severe illness and death. 2 In addition to advanced age, suspected risk factors for developing severe illness caused by SARS-CoV-2 infection include the presence of comorbidities. 3 - 5
Cystic fibrosis (CF) is a recessive genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, mostly affecting Caucasians and predominantly involving the lungs by impairing mucociliary airway clearance. 6 , 7 In patients with CF, the airways become susceptible to dramatic inflammation and chronic infection. 8 Persistent lower airway infection with inflammation is the major cause of morbidity and mortality and contributes to a decline in lung function. 7
Pulmonary exacerbations have an impact on survival in patients with CF, reducing health-related quality of life, adversely affecting sleep and neurobehavioral performance, and increasing health care costs. 9 Pulmonary exacerbations are usually caused by bacteria that are typically associated with the disease, such as Staphylococcus aureus, Pseudomonas aeruginosa, and Burkholderia cepacia complex. 10 Viruses are commonly found during respiratory exacerbations, particularly the influenza A virus, the influenza B virus, and rhinovirus. 11 In comparison with nonviral exacerbations, viral exacerbations are associated with worse severity and quality-of-life scores. 9 During the 2009 influenza A (H1N1) pandemic, infection with influenza A (H1N1) virus was associated with transient but significant morbidity in most patients with CF. In a small number of CF patients with severe lung disease, influenza was associated with respiratory deterioration, need for mechanical ventilation, and even death. 12
Patients with CF should be considered to have an increased risk of developing severe manifestations in case of SARS-CoV-2 infection. Surprisingly, the results of studies conducted in 2020 and examining SARS-CoV-2 infection in patients with CF showed that the infection rate was lower in CF patients than in the general population. 8
The first case of COVID-19 in Brazil was diagnosed on February 26, 2020, 13 , 14 and the first case of COVID-19 in the state of Rio Grande do Sul, in southern Brazil, was diagnosed on March 10, 2020. 15 There is still a lack of information on the clinical characteristics and outcomes of CF patients diagnosed with COVID-19 in Brazil.
The objective of this prospective cohort study was to describe the cumulative incidence, clinical characteristics, and outcomes of incident cases of COVID-19 in unvaccinated adult CF patients during the first year of the COVID-19 pandemic.
METHODS
Study design and population
This was a prospective study conducted between April 30, 2020 and April 29, 2021 and describing the clinical characteristics and outcomes of incident cases of COVID-19 in a cohort of 98 CF patients monitored under the auspices of the Hospital de Clínicas de Porto Alegre (HCPA) adult CF program. The HCPA is a tertiary care teaching hospital located in the city of Porto Alegre, which is the capital of the state of Rio Grande do Sul, in southern Brazil.
In the state of Rio Grande do Sul, the social isolation measures to limit the spread of SARS-CoV-2 were implemented on March 17, 2020. Following the implementation of the measures, all health care facilities sought ways to restrict the circulation of individuals, avoiding elective medical appointments, elective procedures, and ancillary tests. Patients being followed at the HCPA CF outpatient clinic began to be monitored by telephone consultation. A WhatsApp group including patients and health care professionals was created in order to provide health care recommendations during the COVID-19 pandemic. A health questionnaire was sent to all CF patients every two weeks in order to gather information on respiratory symptoms, complaints consistent with or diagnosis of COVID-19, use of oral antibiotics, need for emergency care, and need for hospital admission. This information was collected prospectively and monitored by the multidisciplinary health care team.
The inclusion criteria were as follows: having been diagnosed with CF on the basis of clinical features and a positive sweat chloride test (> 60 mmol/L) or, in the case of patients with a borderline sweat test, the presence of a known disease-causing mutation in each copy of the CFTR gene 16 ; and being ≥ 17 years of age before April 30, 2020. All participating patients were monitored under the auspices of the HCPA adult CF program during the COVID-19 pandemic. The primary outcome of the study was the number of incident cases of COVID-19 over a one-year period.
Study measures and procedures
One member of our research team reviewed all patient electronic medical records at the HCPA. Data on the following variables were recorded at study entry: age; sex; ethnicity; age at CF diagnosis; presence of (homozygous or heterozygous) F508del mutation; BMI; pancreatic status; CF-related diabetes; a history of massive hemoptysis requiring bronchial artery embolization; pneumothorax; a previous diagnosis of allergic bronchopulmonary aspergillosis; CF-related liver disease; liver and/or lung transplantation; chronic infection with P. aeruginosa, B. cepacia, methicillin-susceptible S. aureus, methicillin-resistant S. aureus, and/or nontuberculous mycobacteria; and use of inhaled dornase alpha, inhaled colistimethate sodium, inhaled tobramycin, and/or oral azithromycin. In addition, the latest spirometry and six-minute walk test results were reviewed in order to record FVC, FEV1, FEV1/FVC, the six-minute walk distance (6MWD), and oxygen saturation. FVC and FEV1 were expressed in liters (L) and in percentage of the predicted values for age, height, and sex. 17
In the present study, pancreatic insufficiency was defined as the use of enzymes, whereas pancreatic sufficiency was defined as no use of enzymes. Chronic infection was defined as having three or more positive isolates during the previous 12 months. CF-related diabetes was defined as the use of insulin.
Our research team recorded patient clinical information and patient answers to the health questionnaire between April 30, 2020 and April 29, 2021, identifying the incident cases of COVID-19. The diagnostic criteria for COVID-19 were a positive RT-PCR from nasal and pharyngeal swabs, chest CT findings consistent with COVID-19, a firm clinical diagnosis of COVID-19 made in a hospital setting, or any combination of the three. By the end of the study, on April 29, 2021, none of the participating patients had been vaccinated against COVID-19. The clinical course of COVID-19 was classified in accordance with the WHO Ordinal Scale for Clinical Improvement. 18 Death was defined as a patient who died for any reason.
Ethics
The study was approved by the Research Ethics Committee of the HCPA (Protocol no. 2020-0225) and by Plataforma Brasil (Protocol no. 33225520400005327). Written informed consent was obtained at recruitment. The study was in accordance with international and national standards for clinical studies in humans (Declaration of Helsinki and Brazilian governmental regulation-Plataforma Brasil).
Sample size calculation
No sample size calculation was performed a priori. The sample size was equal to the number of incident cases of COVID-19 during the study period.
Statistical analysis
Statistical analysis was performed with the IBM SPSS Statistics software package, version 22.0 (IBM Corporation, Armonk, NY, USA). We performed a descriptive analysis of the study variables. The normality of the data distribution was examined with quantile-quantile plots and the Shapiro-Wilk test. Qualitative data were expressed as number of cases and proportion. Quantitative data were expressed as mean ± standard deviation or median and interquartile range. Categorical comparisons were performed with the chi-square test with Yates’ correction (when appropriate) or Fisher’s exact test. Continuous variables were compared by means of a t-test or the Wilcoxon-Mann-Whitney test. Cumulative incidence was calculated as the number of new cases of COVID-19 divided by the total number of individuals in the population at risk for the study period (1 year), being also calculated at 6-month intervals. The annual cumulative incidence rate of COVID-19 in the state of Rio Grande do Sul was also calculated, being adjusted for age. 19 The chi-square test of independence was used in order to compare the annual cumulative incidence of COVID-19 between CF patients and the general population.
RESULTS
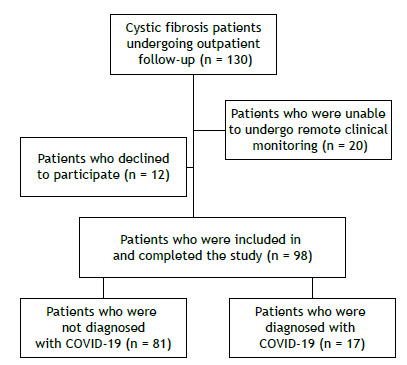
Of a total of 130 patients being followed under the auspices of the HCPA adult CF program, 98 were included in the study. Twelve patients declined to participate, and 20 were unable to undergo remote clinical monitoring (Figure 1). Between April 30, 2020 and April 29, 2021, 17 CF patients were diagnosed with COVID-19. Of those, 14 had positive SARS-CoV-2 RT-PCR test results and 3 had clinical symptoms consistent with COVID-19 and positive SARS-CoV-2 serology. Of those 3 patients, 2 had typical chest CT findings of COVID-19. By the end of the study, on April 29, 2021, none of the participating patients had been vaccinated against COVID-19.
Figure 1. Flow chart of the patient selection process.
The characteristics of the study participants at study entry are presented in Table 1. Most (67%) of the participating patients were female. The mean age of the patients was 29.2 ± 8.8 years, and the median age at diagnosis was 3 years. The mean BMI was 21.7 ± 2.7 kg/m2. Twenty-one percent of the patients were homozygous for the F508del mutation, and 33% were heterozygous for the F508del mutation. Seventy-nine percent had exocrine pancreatic insufficiency, 25% had CF-related diabetes, 30% had CF-related liver disease, and 70% were chronically infected with P. aeruginosa. The mean percent predicted FEV1 was 59.3 ± 25.2%, and the mean percent predicted 6MWD was 69.8 ± 13.5%. Four patients had previously undergone lung transplantation, and 1 had previously undergone liver transplantation.
Table 1. Characteristics of the study participants at study entry and comparison between patients with and without COVID-19.a .
| Characteristic | Total | Group | p* | |
|---|---|---|---|---|
| COVID-19 | No COVID-19 | |||
| (N = 98) | (n = 17) | (n = 81) | ||
| Age, years | 29.2 ± 8.8 | 28.2 ± 9.3 | 29.4 ± 8.72 | 0.598 |
| Sex | 0.500 | |||
| Male | 32 (33) | 2 (6) | 30 (94) | |
| Ethnicity | 1.000 | |||
| White | 98 (100) | 17 (17.5) | 81 (82.6) | |
| Age at CF diagnosis | 3 (0-17) | 8 (0-23) | 3 (0-17) | 0.652 |
| BMI, kg/m2 | 21.7 ± 2.7 | 19.8 ± 2.5 | 22.1 ± 2.6 | 0.001 |
| F508del mutation | 0.028 | |||
| Homozygous | 21 (21) | 5 (24) | 16 (76) | |
| Heterozygous | 33 (34) | 1 (3)† | 32 (97)† | |
| Other | 44 (45) | 11 (25) | 33 (75) | |
| Pancreatic insufficiency | 77 (79) | 12 (16) | 65 (84) | 0.515 |
| CFRD | 24 (25) | 2 (8) | 22 (92) | 0.228 |
| Pneumothorax | 1 (1) | 0 | 1 (100) | 1.000 |
| Massive hemoptysis (> 100 mL) | 19 (19) | 4 (21) | 15 (79) | 0.736 |
| Bronchial artery embolization | 8 (8) | 2 (25) | 6 (75) | 0.624 |
| ABPA | 17 (17.3) | 3 (17.6) | 14 (82.4) | 1.000 |
| CF-related liver disease | 28 (30) | 5 (18) | 23 (82) | 1.000 |
| Liver transplantation | 1 (1) | 0 | 1 (100) | 1.000 |
| Lung transplantation | 4 (4) | 0 | 4 (100) | 1.000 |
| Pseudomonas aeruginosa | 67 (70) | 12 (18) | 55(82) | 0.770 |
| MSSA | 60 (62) | 11 (18) | 49 (82) | 0.777 |
| MRSA | 9 (9.4) | 1 (11) | 8 (89) | 1.000 |
| Burkholderia cepacia | 22 (23) | 4 (18) | 18 (82) | 0.757 |
| MNT | 6 (6.3) | 0 | 6 (100) | 0.585 |
| Dornase alpha | 84 (86) | 13 (15.5) | 71 (84.5) | 0.257 |
| Inhaled colistimethate sodium | 51(52) | 7 (13.7) | 44 (86) | 0.472 |
| Inhaled tobramycin | 33 (34) | 5 (15) | 28 (85) | 0.899 |
| Azithromycin | 75 (76.5) | 13 (17) | 62 (83) | 1.000 |
| FVC, % predicted | 75.5 ± 21.8 | 73.9 ± 20.0 | 75.9 ± 22.3 | 0.740 |
| FEV1, % predicted | 59.3 ± 25.2 | 60.9 ± 28.1 | 59.0 ± 24.7 | 0.779 |
| FEV1/FVC, % | 76.9 ± 16.1 | 77.5 ± 21.1 | 76.8 ± 15.0 | 0.877 |
| SpO2, % | 94 (2.5) | 95 (2.8) | 94 (2.5) | 0.239 |
| 6MWD, % predicted | 69.81 ± 13.47 | 64.36 ± 12.89 | 70.94 ± 13.43 | 0.142 |
CF: cystic fibrosis; CFRD: cystic fibrosis-related diabetes; ABPA: allergic bronchopulmonary aspergillosis; MSSA: methicillin-susceptible Staphylococcus aureus; MRSA: methicillin-resistant Staphylococcus aureus; NTM: nontuberculous mycobacteria; and 6MWD: six-minute walk distance. aData are presented as n (%), median ± SD, or median (IQR). *Chi-square test for categorical variables. Student’s t-test or Mann-Whitney U test for continuous variables. †Adjusted standard residual > 1.96 or < −1.96 (implies significantly different proportions).
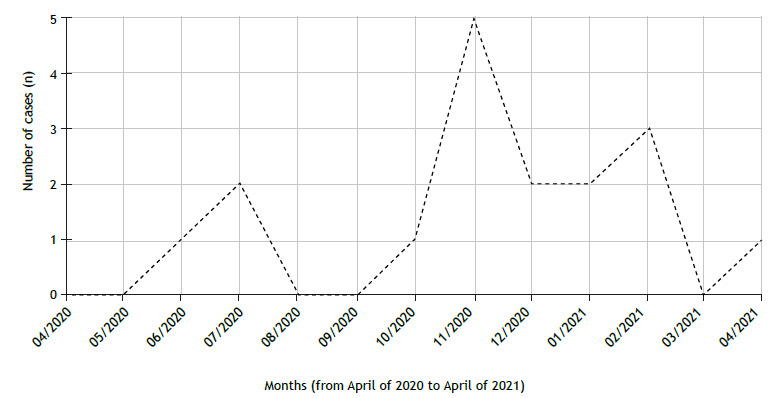
In the state of Rio Grande do Sul on April 29, 2021, there were records of 1,091,191 confirmed cases of SARS-CoV-2 infection, with an age-adjusted annual cumulative incidence rate of 18.5%. 16 For the CF patients, the annual cumulative incidence was 17.3%, and the cumulative incidence for the first and second 6-month periods was 4.1% and 13.3%, respectively. We found that the annual cumulative incidence of COVID-19 was not significantly different between the patients with CF and the general population (p = 0.738). The risk of SARS-CoV-2 infection did not differ between the patients with CF and the general population (OR, 0.94; 95% CI, 0.64-1.37 vs. OR, 1.06; 95% CI, 0.75-1.50). The distribution of COVID-19 cases in the study period is presented in Figure 2.
Figure 2. Distribution of COVID-19 cases during the study period.
A comparison between patients with and without COVID-19 is presented in Table 1. The BMI was lower in patients with SARS-CoV-2 infection (19.8 ± 2.5 kg/m2) than in those without SARS-CoV-2 infection (22.1 ± 2.6 kg/m2; p = 0.001). There was a significant difference between the proportion of F508del mutations (homozygous, heterozygous, and other mutations) between the two groups (p = 0.028), with a lower proportion of heterozygosis in patients with COVID-19. There was no difference between the groups regarding percent predicted FVC (73.9 ± 20.0% vs. 75.9 ± 22.3%; p = 0.740), percent predicted FEV1 (60.9 ± 28.1% vs. 59.0 ± 24.7%; p = 0.779), or percent predicted 6MWD (64.4 ± 12.9% vs. 70.9 ± 13.4%; p = 0.142). There were no differences between the groups regarding the other variables. None of the patients who had undergone lung transplantation had SARS-CoV-2 infection.
Table 2 shows the main symptoms at diagnosis of COVID-19, patient management, and respiratory support. The most common symptoms at diagnosis of COVID-19 were cough (in 59%), dyspnea (in 53%), fatigue (in 53%), fever (in 47%), and increased sputum volume (in 41%). Only 6 patients (35%) required hospitalization. Three patients (17.6%) required oxygen support during their hospital stay. Only 1 patient required ICU admission and noninvasive ventilation, followed by endotracheal intubation and mechanical ventilation. This patient died.
Table 2. Main symptoms at diagnosis, patient management, and respiratory support in cystic fibrosis patients with COVID-19.
| Variable | n (%) |
|---|---|
| Symptoms at diagnosis | |
| Fever | 8 (47) |
| Dyspnea | 9 (53) |
| Cough | 10 (59) |
| Increased sputum volume | 7 (41) |
| Fatigue and/or asthenia | 9 (53) |
| Headache | 4 (23.5) |
| Myalgia and/or arthralgia | 4 (23.5) |
| Ageusia and/or anosmia | 4 (23.5) |
| Hemoptysis | 1 (5.8) |
| Nausea, vomiting, and/or diarrhea | 4 (23.5) |
| Patient management | |
| Outpatient care | 11 (64.7) |
| Hospitalization | 6 (35) |
| Medical ward | 5 (83) |
| ICU | 1 (16) |
| Respiratory support | |
| Additional oxygen therapy | 3 (17.6) |
| Noninvasive ventilation | 1 (5.8) |
| High-flow nasal cannula oxygen therapy | 0 |
| Invasive ventilation | 1 (5.8) |
| ECMO | 0 |
ECMO: extracorporeal membrane oxygenation.
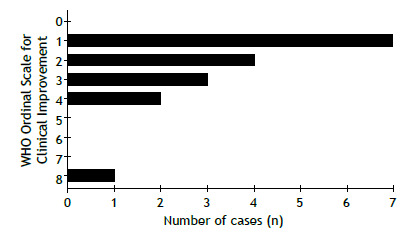
The WHO Ordinal Scale for Clinical Improvement for COVID-19 is shown in Figure 3. Seven patients (41%) were rated 1 (no limitation of activities), 4 (23.5%) were rated 2 (limitation of activities), 3 (17.6%) were rated 3 (hospitalized, no oxygen therapy), 2 (11.8%) were rated 4 (oxygen by mask or nasal prongs), and 1 (5.9%) was rated 8 (death). The patient who died had advanced lung disease (a percent predicted FEV1 of 29%) and was on the waiting list for lung transplantation.
Figure 3. The World Health Organization Ordinal Scale for Clinical Improvement (for COVID-19). 0: no clinical or virological evidence of infection; 1: no limitation of activities; 2: limitation of activities; 3: hospitalized, no oxygen therapy; 4: oxygen by mask or nasal prongs; 5: noninvasive ventilation or high-flow oxygen; 6: intubation and mechanical ventilation; 7: ventilation + additional organ support (vasopressors, renal replacement therapy, or ECMO); and 8: death.
DISCUSSION
In this prospective cohort study performed in southern Brazil between April of 2020 and April of 2021, we described the clinical presentation and outcomes of incident cases of COVID-19 in unvaccinated adult CF patients during the first year of the SARS-CoV-2 pandemic. The cumulative incidence rate in our cohort of patients with CF was 17.3% during the study period, similar to that observed for the general population of the state of Rio Grande do Sul, which was 18.5% (adjusted for age). 19 When comparing clinical characteristics between the study participants, we found that those with COVID-19 had a lower BMI and a lower proportion of heterozygous F508del mutations than did those without COVID-19. More than 50% of the CF patients infected with SARS-CoV-2 had a mild clinical presentation, without the need for hospital admission. Almost the entire sample recovered completely from the infection, the exception being 1 patient who had advanced lung disease and who died.
Other studies have shown a lower incidence of SARS-CoV-2 infection in CF patients during the first wave than in the general population, 20 - 26 the incidence of SARS-CoV-2 infection being lower in the aforementioned studies than in the current study. One of the first reports on the low incidence rate of SARS-CoV-2 infection in people with CF was published by Colombo et al. on April of 2020. 20 In a multinational retrospective study, Cosgriff et al. 21 estimated that the incidence of COVID-19 in CF patients in the 15- to 57-year age bracket was 0.07%, compared with 0.15% in the general population. Between March 1 and June 30, 2020, Corvol et al. 23 conducted a large prospective study in France involving CF patients in the 9- to 60-year age bracket and found a 0.41% incidence of COVID-19, an incidence that was 93% lower than that in the general population. In a prospective multicenter cohort study conducted between February and July of 2020 and in which 50% of the patients were ≥ 18 years of age, Colombo et al. 25 reported that the cumulative incidence of SARS-CoV-2 infection was 2.4/1,000 population. However, in a retrospective study investigating the 38-country European Cystic Fibrosis Society Patient Registry, Naehrlich et al. 26 reported that the incidence of confirmed SARS-CoV-2 infection in people with CF was 2.70 cases per 1,000 population, an incidence that was not significantly different from that of SARS-CoV-2 infection in the general population. When incidence was analyzed by age group, the incidence of SARS-CoV-2 infection was significantly higher in people with CF than in the general population for those under 15 years of age, those in the 15- to 24-year age bracket, and those in the 25- to 49-year age bracket. 26
There are several possible explanations for the high cumulative incidence rate of COVID-19 in people with CF in the current study. First, testing in early 2020 was very restricted to symptomatic cases with more severe respiratory symptoms. Therefore, many cases of asymptomatic or mild infection in the general population probably went undetected. Second, people with CF may have been tested more frequently than was the general population because of increased surveillance and established care routines. Third, the current study included only adult CF patients, who usually have more severe lung disease than do younger CF patients. Consequently, adult CF patients usually take early action to treat respiratory symptoms and to be tested for SARS-CoV-2 infection. Fourth, this was a prospective study, and all patients were closely monitored with regard to their health status.
There are three CF centers in the state of Rio Grande do Sul, all of which are located in Porto Alegre (the capital of the state), and our center is the largest. In addition, the HCPA Adult CF Center is one of the largest adult CF centers in Brazil. Because almost all of the patients followed at the HCPA Adult CF Center were living in the state of Rio Grande do Sul at the time, we considered that this sample of adult CF patients was representative of the adult CF population of the state of Rio Grande do Sul.
When we compared clinical characteristics between patients, we found that those with COVID-19 had a lower BMI than did those without COVID-19. This finding is suggestive of more severe disease in patients with SARS-CoV-2 infection. Again, this could indicate that people with more severe disease usually take early action to be tested for SARS-CoV-2 infection. 26 The study by Colombo et al. 25 compared patients who tested positive by molecular testing (cases) and those who tested negative (controls). In contrast to our study, controls were older than cases, whereas the two groups were comparable in terms of sex, CFTR genotype, comorbidities, CF maintenance therapy, and respiratory function prior to SARS-CoV-2 infection.
In the current study, the main symptoms at diagnosis were cough, dyspnea, fatigue/asthenia, fever, and increased sputum volume. These are consistent with the symptoms commonly experienced by the general population and with the findings reported in other cohorts of CF patients with COVID-19. 23 , 24 , 27
The course of COVID-19 was mild in more than 50% of our patients, with only 1 patient having developed severe illness requiring ventilatory support and ICU care, and later progressing to death. Of the other patients who were hospitalized, 3 did not require any respiratory support. This is surprising given that viral infections tend to have worse outcomes in CF patients. However, it should be noted that in our study there were only 4 patients who had undergone lung transplantation and only 1 who had undergone liver transplantation. McClenaghan et al. 28 reported that 11 of the 181 individuals analyzed in their study were admitted to the ICU. Of those, 7 had undergone transplantation. A total of 7 patients died, 3 of which had undergone transplantation. Corvol et al. 23 reported that 19 of 31 patients were hospitalized, and 11 had undergone transplantation. In our cohort, none of the patients who had undergone lung or liver transplantation acquired COVID-19, and this might explain why morbidity and mortality were low in our study.
Our study has several limitations. First, the investigation was done in a single center. Second, of a total of 130 patients with CF followed at our center, only 98 were included in the study. Third, the restricted testing strategy adopted during the first phase of the COVID-19 pandemic, with only symptomatic patients being tested by PCR and limited use of serological tests, might have resulted in an underestimation of the infection rate, especially in the general population. Fourth, our study represents the situation in the first year of the COVID-19 pandemic, before COVID-19 vaccination.
In conclusion, during the first year of the SARS-CoV-2 pandemic in southern Brazil, the cumulative incidence rate of COVID-19 in CF patients was 17.3%, similar to that observed for the general population (adjusted for age), which was 18.5%. More than 50% of the CF patients with SARS-CoV-2 infection had a mild clinical presentation, without the need for hospital admission. Almost the entire sample recovered completely from the infection, the exception being 1 patient who had advanced lung disease and who died.
Footnotes
Study carried out at the Hospital de Clínicas de Porto Alegre, Porto Alegre (RS) Brasil.
Financial support: This study received financial support from the Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA, Research Incentive Fund of the Porto Alegre Hospital de Clínicas; Grant no. 2020-0225). LBJ is the recipient of a fellowship grant from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development) Programa Institucional de Bolsas de Iniciação Científica (PIBIC, Institutional Program for Young Investigator Grants) - Universidade Federal do Rio Grande do Sul (UFRGS, Federal University of Rio Grande do Sul; Grant no. 36257).
REFERENCES
- 1.World Health Organization (WHO) COVID-19 Public Health Emergency of International Concern (PHEIC). Global research and innovation forum. Geneva: WHO; c2021. https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum [Google Scholar]
- 2.Mitchell A, Chiwele I. BMJ Best Practice. BMC Publishing Group; 2021. Coronavirus Disease 2019 (COVID-19) [Google Scholar]
- 3.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM. Comorbidity and its impact on 1590 patients with COVID-19 in China a nationwide analysis. Eur Respir J. 2020;55(5):2000547–2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy [published correction appears in. JAMA. 2021;325(20):2120–2120. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China a retrospective cohort study [published correction appears in. Lancet. 2020;395(10229):1038–1038. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z. Identification of the cystic fibrosis gene cloning and characterization of complementary DNA [published correction appears in. Science. 1989;245(4925):1437–1437. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 7.Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 8.Fainardi V, Longo F, Chetta A, Esposito S, Pisi G. Sars-CoV-2 infection in patients with cystic fibrosis An overview. Acta Biomed. 2020;91(3):e2020035. doi: 10.23750/abm.v91i3.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt JM. Treatment of pulmonary exacerbations in cystic fibrosis. Eur Respir Rev. 2013;22(129):205–216. doi: 10.1183/09059180.00006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ. Cystic fibrosis pulmonary guidelines treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 11.Wat D, Gelder C, Hibbitts S, Cafferty F, Bowler I, Pierrepoint M. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7(4):320–328. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viviani L, Assael BM, Kerem E. ECFS H1N1 study group Impact of the A (H1N1) pandemic influenza (season 2009-2010) on patients with cystic fibrosis. J Cyst Fibros. 2011;10(5):370–376. doi: 10.1016/j.jcf.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária (ANVISA) Portos, aeroportos e fronteiras. Coronavírus. Linha do tempo. Brasília: ANVISA; https://www.gov.br/anvisa/pt-br/assuntos/paf/coronavirus/linha-do-tempo [Google Scholar]
- 14.Biernath A. Um ano de coronavírus no Brasil: os bastidores da descoberta do primeiro caso oficial. BBC News Brasil; 2021. https://www.bbc.com/portuguese/brasil-56189539 [Google Scholar]
- 15.Brasil. Estado do Rio Grande do Sul. Secretaria de Vigilância em Saúde . Confirmado o primeiro caso de novo coronavírus no Rio Grande do Sul. Porto Alegre: Secretaria de Vigilância em Saúde; c2020. https://saude.rs.gov.br/confirmado-o-primeiro-caso-de-novo-coronavirus-no-rio-grande-do-sul [Google Scholar]
- 16.Farrell PM, White TB, Ren CL, Hempstead SE, Accurso F, Derichs N. Diagnosis of Cystic Fibrosis Consensus Guidelines from the Cystic Fibrosis Foundation [published correction appears in. J Pediatr. 2017;184:243–243. doi: 10.1016/j.jpeds.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 17.Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33(4):397–397. doi: 10.1590/S1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) COVID-19 Therapeutic Trial Synopsis. Geneva: WHO; c2020. https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis [Google Scholar]
- 19.Brasil. Estado do Rio Grande do Sul. Secretaria de Vigilância em Saúde . Boletim Epidemiológico Covid-19. Porto Alegre: Secretaria de Vigilância em Saúde; c2021. https://coronavirus.rs.gov.br/informe-epidemiologico [Google Scholar]
- 20.Colombo C, Burgel PR, Gartner S, van Koningsbruggen-Rietschel S, Naehrlich L, Sermet-Gaudelus I. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir Med. 2020;8(5):e35–e36. doi: 10.1016/S2213-2600(20)30177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosgriff R, Ahern S, Bell SC, Brownlee K, Burgel PR, Byrnes C. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros. 2020;19(3):355–358. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bezzerri V, Lucca F, Volpi S, Cipolli M. Does cystic fibrosis constitute an advantage in COVID-19 infection . Ital. J Pediatr. 2020;46(1):143–143. doi: 10.1186/s13052-020-00909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corvol H, de Miranda S, Lemonnier L, Kemgang A, Reynaud Gaubert M, Chiron R. First Wave of COVID-19 in French Patients with Cystic Fibrosis. J Clin Med. 2020;9(11):3624–3624. doi: 10.3390/jcm9113624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mondejar-Lopez P, Quintana-Gallego E, Giron-Moreno RM, Cortell-Aznar I. Ruiz de Valbuena-Maiz M.Diab-Caceres L Impact of SARS-CoV-2 infection in patients with cystic fibrosis in Spain Incidence and results of the national CF-COVID19-Spain survey. Respir Med. 2020;170:106062–106062. doi: 10.1016/j.rmed.2020.106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo C, Alicandro G, Daccó V, Gagliano V, Morlacchi LC, Casciaro R. SARS-CoV-2 infection in cystic fibrosis A multicentre prospective study with a control group, Italy, February-July 2020. PLoS One. 2021;16(5):e0251527. doi: 10.1371/journal.pone.0251527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naehrlich L, Orenti A, Dunlevy F, Kasmi I, Harutyunyan S, Pfleger A. Incidence of SARS-CoV-2 in people with cystic fibrosis in Europe between February and June 2020. J Cyst Fibros. 2021;20(4):566–577. doi: 10.1016/j.jcf.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathew HR, Choi MY, Parkins MD, Fritzler MJ. Systematic review cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic. BMC Pulm Med. 2021;21(1):173–173. doi: 10.1186/s12890-021-01528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClenaghan E, Cosgriff R, Brownlee K, Ahern S, Burgel PR, Byrnes CA. The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J Cyst Fibros. 2020;19(6):868–871. doi: 10.1016/j.jcf.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


