Abstract
Adenoid cystic carcinoma (ACC) usually arises in the salivary glands, and is a rare tumor, accounting for 1% of all head and neck cancer cases. According to estimates, there are 3-4.5 cases of ACC for every one million individuals. Numerous studies have reported the association between ACC and microRNAs (miRNAs/miRs). miRNAs are endogenous, non-coding small RNAs, 19-25 nt in length, that can regulate target gene expression at the post-transcriptional level. The aberrant expression of miRNAs may be associated with the prognosis and treatment of patients, as well as with tumorigenesis and tumor development. miRNAs are becoming reliable biomarkers for disease detection due to their varied characteristics, and miRNA target-based therapies are increasingly being used in clinical practice. The present review provides a brief introduction to ACC and the biogenesis of miRNAs. A summary of the miRNAs that have been validated by in vitro or in vivo studies is then presented, describing their role in ACC.
Keywords: adenoid cystic carcinoma, miRNA, biomarker, therapy, pathogenesis
1. Introduction
Adenoid cystic carcinoma (ACC) is an aggressive malignancy that usually arises in the salivary glands; it represents 10% of salivary gland tumors and 1% of head and neck cancer cases. According to estimates, there are 3-4.5 cases of ACC for every one million individuals. Although ACC affects individuals of all ages, its peak occurrence is between the ages of 40 and 60 years, and there is a slight female preponderance (60% female and 40% male) (1). ACC is the most common malignant tumor of the minor salivary glands, and is also not uncommon in the sublingual, submandibular and parotid salivary glands. The lungs, cervix and skin, as well as the glandular tissue of the breast, lacrimal glands, paranasal sinuses and nasopharynx, are examples of uncommon locations of this type of tumor (1). A number of factors are considered to be potential causes of ACC, particularly exposure to ionizing radiation (2). Notably, an increased incidence of subsequent salivary gland cancer has been observed in women diagnosed with breast cancer. However, the associations between smoking, alcohol consumption and ACC remain unclear (2).
2. Clinical features
Pain, facial nerve dysfunction and nerve ending invasion are features found in the majority of patients with ACC (3). In a previous study, 30% of patients with ACC were shown to have ulcerations, 48% exhibited pain and 98% reported a mass; the duration of symptoms ranged from 1 month to 4 years (4). The symptoms of ACC may differ, depending on the location of the tumor. The tumor represents a mass in the major salivary glands, and facial nerve palsy may occur when the tumor is located in the parotid gland. Tumors are usually common in the palate, and thus ulcerations and fistula can also be observed. When the tumor occurs in the larynx, the first presenting symptom may be dyspnea; however, in tumors occurring in the nose and paranasal sinuses, epistaxis and eye symptoms, as well as deep facial pain and nasal obstruction, may be the forefront symptoms (4). With an occurrence rate of 16.1-72.7%, the common malignant feature of ACC is distant metastasis (DM). The lungs are the most common metastatic site, accounting for 74.5-94.4% of cases of metastasis (5).
3. Histopathology and diagnostic imaging
With regard to ACC, according to the histological appearance, 'cylindromas' are the initial histopathological term. A minimal cytoplasm and angulated hyperchromatic nuclei can be noted in ACC cells, and the affected tissue is usually eosinophilic or clear. Although myoepithelial differentiation predominates, ACC exhibits biphasic differentiation with both myoepithelial and secretory glandular elements (6). ACC exhibits different ratios of the three distinct growth patterns known as solid, tubular and cribriform. Of these, cribriform is the most frequent subtype (7).
The preferred test for identifying bone invasion patterns in ACC is computed tomography. Heterogeneous bone remodeling and enhancement are often observed in low-grade ACC, while in high-grade ACC, 'worm'-like and osteolytic lesions, adjacent bone compression resorption-like changes and bone destruction are more commonly observed (8).
Magnetic resonance imaging is the primary method for demonstrating the involvement of the skull base. A 'dural tail sign' is displayed, while tumor cells spread along nerves to the anterior cranial fossa, and then consequently affect the dura mater. Soft meningeal enhancement, nodular enhancement or dural thickening >5 mm is usually suggestive of dural infiltration (5,8,9).
4. Treatment
The therapeutic strategy for ACC depends on the tumor stage and grade. Regardless of the primary tumor site, surgery is the standard of care for non-metastatic ACC. The primary goal is a complete surgical excision (10). Irrespective of prior treatment, in the setting of distant metastatic disease, and resectable, recurrent locoregional disease, the appropriate treatment is specified by the American Society of Clinical Oncology guidelines (5). Under these circumstances, if the metastatic disease does not exhibit rapid progression or is considered imminently lethal, treatment should include appropriate surgical reconstruction and rehabilitation, and palliative care. In the case that complete surgical resection is feasible and if following primary tumor treatment, the time to pulmonary relapse is >36 months, the surgical treatment of oligometastatic disease should also be considered. According to the literature, patients treated with post-operative radiotherapy exhibit better local control (9,11).
Currently, microRNAs (miRNAs/miRs) are attractive molecular biomarker candidates, as they can be repeatedly extracted from a variety of biological samples, and are often stable and tolerant in a wide range of storage conditions. In addition, miRNAs can be easily detected and accurately quantified by a variety of widely used standard techniques, such as small RNA sequencing, microarrays and reverse transcription-quantitative PCR (12). Furthermore, miRNAs could be used as alternative therapeutic targets. The combination of miRNA therapeutics and chemotherapy, radiotherapy and immunotherapy has exhibited promising results (13). Thus, the development of new forms of miRNAs may provide significant clinical benefits for cancer patients; however, further research is needed in this field (13).
5. Biogenesis of miRNAs
As vital post-transcriptional regulators of gene expression, miRNAs are part of a large family of RNAs, 21 nt in length, and have significantly improved our understanding of the post-transcriptional regulation of gene expression. miRNAs control the activity of 50% of protein-coding genes in mammals, according to research (14).
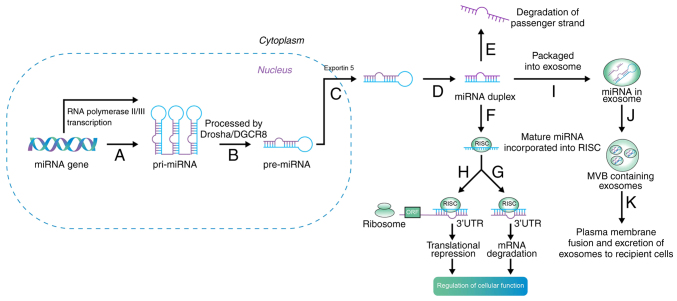
The biogenesis and maturation of miRNAs first occurs in the nucleus, and subsequently, with the aid of proteins and enzymes, biogenesis and maturation occur in the cytoplasm (15). Briefly, as long primary transcripts (pri-miRNAs), miRNAs are initially produced by RNA polymerase II in the nucleus. Drosha and Dicer, two enzymes from the RNase III family, are bound by pri-miRNAs, which fold into hairpin structures. In the nucleus, the microprocessor complex is formed by Drosha with DGCR8 microprocessor complex subunit, and the 70 nt precursor miRNA (pre-miRNA) hairpin is then liberated by the primary transcript. The mature miRNA/miRNA duplex is produced by Dicer when exportin-5 exports pre-miRNA to the cytoplasm (Fig. 1) (16,17).
Figure 1.
Biogenesis of miRNA. Step A: miRNAs are initially produced by RNA polymerase II or III in the nucleus, and transcribed into pri-miRNA. Step B: Drosha and Dicer are bound by the pri-miRNAs; this microprocessor complex is formed by drosha with DGCR8, and then pre-miRNA is liberated. Step C: Exportin-5 exports pre-miRNA to the cytoplasm. Step D: The mature miRNA/miRNA duplex are produced by dicer. Step E: Inactive strands are degraded. Step F: The incorporation of the mature strand into RISC. The gene expression is suppressed by RISC with Step G: mRNA degradation or Step H: translational repression, then regulates cellular function. Step I: In addition, exosomes can package miRNAs. Step J: Exosomes are then compartmentalized into an MVB. Step K: The plasma membrane is fused by the MVB, and then miRNA-containing exosomes are transferred to recipient cells and influence gene regulation. miRNA, microRNA; pri-, primary; pre-, precursor; RISC, RNA-induced silencing complex; mRNA, messenger RNA; MVB, multivesicular body; 3′UTR, 3′-untranslated region; ORF, open reading frame; DGCR8, DGCR8 microprocessor complex subunit.
To construct the RNA-induced silencing complex (RISC), transactivation-responsive RNA-binding protein and Argonaute 2 are bound by mature miRNAs. Although related to RISC, inactive strands are degraded, and other active strands remain within the RISC (18). Through partial complementary sequences, miRNAs can recognize target mRNAs. The miRNA/mRNA complexes cannot complete protein synthesis when miRNAs undergo partial base pairing with target mRNAs (19). miRNAs can not only promote translational repression, by deadenylating the target mRNA poly-A tail, but can also promote target mRNA degradation. miRNA-based translational repression is often overwhelmed by the process of mRNA destabilization in a rapid manner. Dysregulated miRNAs can affect various intracellular signaling pathways via these two mechanisms of miRNAs, and thus influence the development of diseases, including cancer (20).
Since mature miRNAs regulate the expression of multiple target genes, the dysregulation of miRNAs may cause abnormal gene expression profiles in cells, which may then lead to organ injury or even to cancer (20). Furthermore, miRNAs can be well preserved in a variety of specimens, such as formalin-fixed tissue blocks, urine and blood plasma or serum. Compared with proteins, miRNAs are more measurable due to their increased sensitivity (12). There is currently increasing interest in creating miRNAs as biomarkers for various molecular diagnostic applications, such as autoimmune diseases, cardiovascular diseases and cancer. miRNA profiling has therefore gained interest from researchers in a variety of biological and medical research fields (21,22).
Increasingly, studies have indicated that miRNAs and the biogenesis machinery have a critical influence on the development of cancer (23,24). For instance, the dysregulation of miRNA biogenesis enzymes, tumor-suppressor miRNAs and miRNAs with oncogenic functions are related to the development of cancer (25). Therefore, miRNAs are crucial for cancer research. As aforementioned, miRNAs are becoming reliable biomarkers for disease detection due to their varied characteristics (they can be repeatedly extracted from a variety of biological samples, and are often stable and tolerant in a wide range of storage conditions), and miRNA target-based therapies are increasingly being used in clinical practice; however, further research is warranted in this area.
In the present review, through a preliminary literature search, a large amount of literature reporting on miRNAs, as well as ACC, was identified; however, no studies were identified that summarize the mechanisms of miRNAs in ACC. The present review thus aimed to summarize the miRNAs that have been experimentally validated and have pathogenic mechanisms in ACC. It is hoped that the present review may provide a basis for future research on miRNAs in ACC.
6. miRNAs in ACC
In ACC, the molecular mechanisms underlying the molecular alterations responsible for oncogenic activity remain elusive. There are a number of studies reporting the association between miRNAs and ACC. For example, Kiss et al (26) found that miRNA profiles in breast and salivary ACC (SACC) differed from those in corresponding normal tissues. miR-9-5p is considered to be a potential biomarker and therapeutic target (27). miR-6835-3p, miR-4676, miR-1180 and certain other miRNAs are related to the overall survival and recurrence-free survival of patients with ACC (28). miR-20a and miR-17 have been shown to be associated with the poor outcomes of patients (29). miR-375, miR-150 and miR-455-3p have been identified as aberrantly expressed miRNAs in SACC (30). Zhao et al (31) suggested that miR-29a-3p may bind with AKT serine/threonine kinase 2, and has an influence on lacrimal gland ACC (LACC). The present review discusses the miRNAs that have been validated by in vitro or in vivo studies, and describes their role in ACC (Table I).
Table I.
miRNAs in ACC.
| miRNA | Upstream | Downstream | Expression | Function | Prognosis | (Refs.) |
|---|---|---|---|---|---|---|
| miR-130a | MYB | NDRG2 | + | MYB activates the expression of miR-130a, and then leads to NDRG2 downregulation | Poor | (32) |
| miR-21 | - | PDCD4 Bcl-2 | + | Through modulation of PDCD4 and Bcl-2 expression, miR-21 can suppress cell apoptosis and then increase cell proliferation and metastasis | Poor | (39) |
| miR-93-5p | - | BRMS1L | + | May promote EMT, and by targeting BRMS1L, regulate Wnt signaling | Poor | (48) |
| miR-103-3p | - | TPD52 | + | Metastatic properties of SACC are maintained by the feedback regulation between TPD52 and miR-103a-3p | Poor | (50) |
| miR-222 | - | PUMA | + | Promotes the ability of proliferation and migration of ACC cells | Poor | (53) |
| miR-155 | - | EGFR/NF-κB | + | Facilitates cell cycle progression and promotes invasion in ACC and that the EGFR/NF-κB pathway might participate in mediating the effects of miRNA155 | Poor | (62) |
| miR-320a | - | ITGB3 | − | Inhibits SACC metastasis by silencing ITGB3 | Favorable | (73) |
| miR-140-5P | - | Survivin | − | Suppresses SACC cell proliferation and invasion, and induces cell apoptosis by regulating survivin expression | Favorable | (81) |
| miR-187 | CXCR5 | - | − | CXCR5 facilitates PNI through downregulating miR-187 | Favorable | (88) |
| miR-101-3p | - | Pim-1 | − | Suppresses cell proliferation, invasion and enhances chemotherapeutic sensitivity in SACC by targeting Pim-1 | Favorable | (97) |
| miR-98 | - | N-RAS | − | Possibly acts as a tumor suppressor in SACC by negatively regulating the oncogene N-RAS | Favorable | (100) |
| miR-125a-5p | - | P38/MAPK | − | Downregulation of miR-125a-5p promotes SACC progression through p38 signal pathway | Favorable | (109) |
| miR-582-5p | - | FOXC1 | − | miR-582-5p can inhibit invasion and migration in SACC cell lines | Favorable | (113) |
ACC, adenoid cystic carcinoma; SACC, salivary ACC; MYB, V-myb myeloblastosis viral oncogene homolog; NDRG2, N-myc downstream-regulated gene 2; PDCD4, programmed cell death protein 4; Bcl-2, B-cell lymphoma-2; BRMS1L, breast cancer metastasis suppressor 1 like; TPD52, tumor protein D52; PUMA, p53 upregulated modulator of apoptosis; EGFR, epidermal growth factor receptor; ITGB3, integrin β3; CXCR5, C-X-C chemokine receptor type 5; pim-1, proto-oncogene serine/threonine-protein kinase; N-RAS, Ras proteins; MAPK, mitogen-activated protein kinases; FOXC1, forkhead box C1; miRNA/miR, microRNA; EMT, epithelial-mesenchymal transition; PNI, perineural invasion.
miR-130a
miR-130a is located on chromosome 11 (32). As previously demonstrated, miR-130a is aberrantly expressed in a number of types of cancer; for instance, it is overexpressed in esophageal cancer tissue (33), osteosarcoma (34), non-small cell lung cancer (35), basal cell carcinoma (36), adult T-cell leukemia (37) and gastric cancer (38), although it is underexpressed in chronic lymphocytic leukemia (39), prostate carcinoma (40), glioblastoma (41), hepatocellular carcinoma cells (42), ovarian cancer (43), breast cancer (44) and cervical cancer (45).
As a member of a transcription factor family, MYB proto-oncogene, transcription factor (MYB) is associated with human malignancies, such as melanoma, pancreatic and esophageal cancer (46). N-myc downstream-regulated gene 2 (NDRG2), a tumor suppressor gene, can inhibit metastasis, attenuate tumor progression and increase tumor sensitivity to anticancer drugs. In various aggressive tumors, NDRG2 is suppressed and its expression is related to patient prognosis (47). It has been found that NDRG2 expression is downregulated in SACC tissue samples, and in mouse models, it may promote the metastasis and growth of xenograft tumors. According to the literature research, by targeting NDRG2, miR-130a can suppress NDRG2 expression (48). In SACC samples, miR-130a expression has been inversely linked to NDRG2 in vitro or in vivo; the overexpression of miR-130a can increase SACC proliferation and invasion. In addition, miR-130a-modulated cell invasion, colony formation and proliferation are reversed by the restoration of NDRG2 expression. Furthermore, through binding to the miR-130a promoter, MYB activates the expression of miR-130a, and then leads to NDRG2 downregulation (48). On the whole, the MYB/miR-130a/NDRG2 axis may present an effective strategy for the treatment of SACC.
miR-21
miR-21 is located on chromosome 17q23.2 (49). miR-21 can affect cell proliferation through a variety of targets, including phosphatase and tensin homolog (PTEN), sprouty RTK signaling antagonist 2 and programmed cell death protein 4 (PDCD4) (50). miR-21 is overexpressed in various human tumors, such as oral, prostate, breast and colorectal cancer (51-54). These findings indicate that miR-21 may play a vital role in tumorigenesis.
The role of miR-21 in ACC has also been investigated. In 2015, Jiang et al (55) found that through the miR-21/PDCD4/STAT3 pathway, miR-21 may regulate SACC progression. In SACC cell lines and tissue samples, miR-21 was shown to be overexpressed and to enhance the cellular capacity for migration and invasion. As a tumor suppressor gene, PDCD4 is the direct target of miR-21, and miR-21 can suppress PDCD4. It was also found that, in SACC samples, the low expression of PDCD4 and the high expression of phosphorylated (p-)STAT3 were linked to the high expression of miR-21. In a study by Wang et al (56), miR-21 inhibitor reduced lung metastatic SACC cell resistance to simvastatin, which was found be effective against the growth of various types of cancer, including breast (57), anaplastic thyroid (58), and lung (59) cancer. Yan et al (60) also indicated that PDCD4, PTEN and B-cell lymphoma-2 (Bcl-2) may be the potential targets of miR-21, and through modulation of PDCD4 and Bcl-2 expression, miR-21 can suppress cell apoptosis, and increase cell proliferation and metastasis. miR-21 thus has potential for use as a therapeutic target in SACC.
miR-93-5p
miR-93 is derived from the paralogue of the miR-17-92 cluster. SMAD family member 7, vascular endothelial growth factor A, STAT3, SRY-box transcription factor 4, AKT serine/threonine kinase 3, erb-b2 receptor tyrosine kinase 2, cyclin B1 and p21 are identified targets of miR-93, suggesting that through diverse mechanisms, miR-93 may function as a tumor suppressor (61). miR-93-5p is associated with a variety of cancer types, including epithelial ovarian carcinoma (61), colorectal cancer (62), gastric cancer (63) and hepatocellular carcinoma (64).
As part of the Sin3A-histone deacetylase co-repressor complex, breast cancer metastasis suppressor 1 like (BRMS1L) may suppress target gene transcription (65). As a mediator downstream of the p53 pathway, BRMS1L can also inhibit the invasion and migration of cancer cells, which are critical processes in cancer metastasis (66). In LACC, miR-93-5p enhances cell tumorigenesis by targeting BRMS1L (67). In LACC tissues, it has been found that miR-93-5p expression is increased, and miR-93-5p can prevent the apoptosis of LACC cells; with the overexpression of miR-93-5p, an evident enhancement of the invasion and migration of LACC cells has been observed (67). miR-93-5p targets BRMS1L, and miR-93-5p can then inhibit the protein expression of BRMS1L. When BRMS1L expression is increased, the invasive and migratory potential of LACC cells is significantly inhibited (67). Mutated Wnt pathway components can also affect cancer and multiple growth-related pathologies. It has also been indicated that through BRMS1L, miR-93-5p can regulate Wnt signaling; the elucidation of the exact mechanisms involved may result in the development of effective treatment strategies that may markedly decrease the morbidity and mortality of patients with LACC (67).
miR-103-3p
According to the literature, miR-103a-3p functions as an oncogene; in gastric cancer, endometrial carcinoma and hepatocellular carcinoma, miR-103a-3p expression is upregulated (68). In SACC, by targeting tumor protein D52 (TPD52), miR-103a-3p promotes metastasis (69). Unlike in healthy tissues, miR-103a-3p expression is high in SACC tissues. By assessing the clinicopathological features of 52 patients with SACC, the high expression of miR-103a-3p was found to be associated with lung metastasis and local regional recurrence (69). When miR-103a-3p expression was knocked down, cell migration was suppressed, and cell functions may be affected via the epithelial-mesenchymal transition process. TPD52, a member of the TPD52-like protein family, is mapped to chromosome 8q21. TPD52 exerts differential effects on various tumor types. In breast and prostate cancer, the expression of TPD52 is high (70,71), while in lung cancer and liposarcoma, low expression is observed (72,73). TPD52 may promote cell invasion, migration, proliferation and survival; however, research also suggests that TPD52 may act as a suppressor in the progression of tumors. Compared with that in SACC tissues, TPD52 expression is markedly higher in healthy tissues, and in SACC, TPD52 may be the direct target of miR-103a-3p. As previously demonstrated, when TPD52 is overexpressed, the migration of SACC-LM (highly metastatic) cells is significantly suppressed, suggesting that the migration of SACC cells is inhibited by TPD52; miR-103a-3p overexpression decreases TPD52 expression in SACC cells when the cellular expression of TPD52 is excessive (69).
These findings indicate that the metastatic properties of SACC are maintained by the feedback regulation between TPD52 and miR-103a-3p. In summary, the miR-103a-3p/TPD52 axis may be critical in SACC pathogenesis, and may provide further insight into potential therapeutic targets or novel biomarkers.
miR-222
Encoding on chromosome X (Xp11.3), miR-221 and miR-222 are highly homologous, functioning as a cluster (miR-221/222). Acting as an oncogene, this cluster may overcome the status of cell quiescence and may promote cell proliferation, survival and metastasis (74). miR-222 is involved in colorectal, gastric, prostate, pancreatic, liver and breast cancer, and thus has potential for use as a diagnostic and prognostic biomarker (75).
In oral squamous cell carcinoma, miR-222 expression is positive (76). As previously demonstrated, in ACC, when miR-222 was knocked down, the proliferation and migration of ACC cells was inhibited, and apoptosis was significantly induced (77). Moreover, the expression of p53 upregulated modulator of apoptosis (PUMA) was increased (77). Belonging to the Bcl-2 protein family, PUMA is a BH3-only protein; PUMA gene mutation or absence may result in reduced rates of apoptosis, suggesting that PUMA plays a crucial role in the process of apoptosis (78). miR-222 and PUMA expression are negatively regulated; thus, the elaboration of their association is essential for the study of ACC (77).
miR-155
As one of the most multifunctional miRNAs, miR-155 is related to inflammation, immunological modulation and tumor development (79). miR-155 is involved in several types of cancer, including gastric (80), lung (81), kidney (82) and breast (83) cancer, and head and neck squamous cell carcinoma (84). The expression of miR-155 in malignant pathologies suggests its possible use as a diagnostic biomarker (85).
As demonstrated in the study by Liu et al (86), miR-155 plays a critical role in the invasion and growth of SACC. Compared with that in normal tissues, miR-155 expression in ACC is markedly increased. By knocking down miR-155, cell proliferation was inhibited, indicating that, in SACC, miR-155 may promote cell proliferation and facilitate cell cycle progression, which suggests the promoting effects of miR-155 on SACC carcinogenesis. Indeed, the knockdown of miR-155 markedly inhibited the invasive ability of SACC cells, and in nude mice, the silencing of miR-155 inhibited the pulmonary metastasis of SACC cells. In addition, there was a correlation between miR-155 and the EGFR⁄NF-κB pathway. The EGFR⁄NF-κB pathway plays a role in the growth and metastasis of several malignant tumors. EGFR overexpression can induce metastasis, invasion, angiogenesis and tumorigenesis (87-89). The EGFR⁄NF-κB pathway may be related to the effects of miR-155 on ACC carcinogenesis. The mechanisms underlying the interactions between EGFR⁄NF-κB and miR-155 warrant further investigations. In addition, through the ubiquitin-like modifier activating enzyme 2 pathway, miR-155 affects SACC metastasis (90).
miR-320a
As a member of the miR-320 family, miR-320a is located on chromosome 8p21.3 (91,92). It has been found that miR-320a expression is decreased in various tumors, and by downregulating target gene expression, miR-320a may function as a tumor suppressor (93). For instance, in hepatocellular carcinoma, by targeting high mobility group box 1, miR-320a could function as a suppressor of tumor and play a critical role in the invasion-metastasis cascade (94). In breast cancer, by modulating the expression of Rab protein Rab11a, miR-320a could also function as a tumor suppressor and biomarker (95). By targeting ras association domain family 8, miR-320a enhanced the proliferation and invasion of epithelial ovarian cancer cells (96). In summary, miR-320a plays a critical role in cancer.
In the study by Sun et al (97), it was found that in highly metastatic ACC cells in the lungs, miR-320a was the most markedly downregulated miRNA. The cells were then transfected with miRNA mimics to increase miR-320a expression, and this markedly inhibited the adhesion, invasion and migration of SACC cells. This indicated that, in SACC cells, reduced miR-320a expression can result in enhanced invasiveness. Based on a series of measurements, integrin β3 (ITGB3) was considered to be the target of miR-320a. ITGB3 has been found in variety of cancer types, such as breast cancer (98), nasopharyngeal carcinoma (99) and human non-small cell lung cancer (100). In SACC cells, by targeting ITGB3, miR-320a could regulate cell invasiveness (97). In addition, through in vivo experiments, the overexpression of miR-320a in ACC cells was found to suppress metastasis to the liver or lungs of tumor-bearing mice, which suggested that the metastasis of ACC xenografts was suppressed by miR-320a overexpression (97). Furthermore, miR-320a overexpression reduced IGTB3 expression, and by silencing ITGB3, miR-320a inhibited SACC metastasis (97). In summary, in metastatic SACC cells, miR-320a is downregulated, which leads to the overexpression of ITGB3, and the upregulation of ITGB3 enhances cell invasion and metastasis. miR-320a may thus be a promising therapeutic target and prognostic biomarker for SACC.
miR-140-5p
miR-140-5p has been reported to function as a tumor suppressor in hypopharyngeal, biliary tract and colorectal cancer (101). Rothman et al (102) demonstrated that the downregulation of miR-140-5p regulated cellular proliferation and migration in hepatocellular carcinoma, and lung and breast cancer. These findings indicate that miR-140-5p plays a vital role in cancer.
Survivin (BIRC5), a 142-amino acid, 16.5-kDa protein, is the smallest member of the family of inhibitors of apoptosis proteins (103). Compared with the levels in adult differentiated tissues, overexpression is noted in tumors; in addition, in several human neoplasms, survivin is related to a poor prognosis. This apoptotic inhibitor has a notable influence on both the inhibition of cell death and the promotion of cancer cell survival (104). In SACC, miR-140-5p inhibits metastasis and progression by targeting survivin (105). miRNA array screening identified that miR-140-5p expression was decreased in SACC, while the overexpression of miR-140-5p suppressed cell proliferation and invasion, and induced apoptosis, inhibiting tumor growth (105). In SACC tissues, the expression of survivin was found to be high and the overexpression of survivin was related to a poor prognosis of patients with SACC (105). miR-140-5p could target the 3′-untranslated region of survivin directly, and inversely regulate survivin. SACC cell proliferation and invasion were suppressed by the inhibition of survivin, and the inhibition of survivin could also induce cell apoptosis (105). By contrast, the enforced expression of survivin could counteract the tumor suppressive effects of miR-140-5p. It was also illustrated in SACC that miR-140-5p functions as a tumor suppressor. By regulating survivin expression, miR-140-5p has the potential to suppress the proliferation and invasion of SACC cells, inducing cell apoptosis (105). On the whole, miR-140-5p has the potential to be a promising target for the treatment of SACC.
miR-187
The human miR-187 gene is located at 18q12.2. Among the cancer-related miRNAs, the study of miR-187 has attracted increasing attention in recent years. It has been found that the expression of miR-187 varies markedly in various tumor types (106). For instance, in breast cancer, as an independent prognostic factor, miR-187 may confer an increased invasive potential in vitro (107). By targeting disabled homolog-2, miR-187 can regulate ovarian cancer progression (108), and by targeting FGF9, miR-187 can suppress lung cancer cell proliferation (109). In summary, miR-187 plays a vital role in cancer.
As a member of the G-protein coupled receptor superfamily, C-X-C chemokine receptor type 5 (CXCR5) can evoke inflammatory responses and promote lymphocyte migration (110). It has been suggested that in a number of human cancer types, CXCR5 is highly expressed and may be associated with tumor occurrence, invasion and metastasis (111). In SACC, CXCR5 induces perineural invasion (PNI) by inhibiting miR-187 (112). In SACC samples, CXCR5 expression is increased, which may result in SACC-LM cell PNI, invasion and migration, while the silencing of CXCR5 attenuates migration, invasion and PNI (112). As the main cells of peripheral nerves, Schwann cells may be beneficial for the maintenance of axons and may be fundamental to the development and survival of nerves. The inhibition of the expression of CXCR5 leads to a downregulation of Schwann cell hallmarks (112). As previously demonstrated, miR-187 is the downstream miRNA of CXCR5. At the nerve invasion frontier, miR-187 expression exhibits a downward tendency, and by inhibiting miR-187, CXCR5 can promote Schwann cell marker expression. By suppressing miR-187, CXCR5 induces the differentiation of tumor cells into Schwann-like cells, facilitating the PNI of SACC (112). Thus, miR-187 is essential for the study of SACC.
miR-101-3p
In various malignancies, miR-101 is one of the downregulated miRNAs, and the genomic loss of miRNA-101 may confer a proliferative advantage on cancer (113). miR-101-3p expression has been found in various types of cancer, including ovarian cancer (114), cholangiocarcinoma (115), and gastric (116), breast (117) and bladder cancer (118).
PIM kinase is a part of the family of serine/threonine kinases (119). Pim-1 is a proto-oncogene, and the dysregulation of Pim-1 may result in tumorigenesis and in malignant progression (120). In SACC, miR-101-3p can enhance chemotherapeutic sensitivity, and can suppress the proliferation and invasion of cells by targeting Pim-1 (121). Compared with that in normal parotid glands, miR-101-3p expression in ACC tissues is markedly reduced. The overexpression of miR-101-3p can inhibit ACC cell proliferation and invasion, while the silencing of miR-101-3p reverses the phenomenon, indicating that miR-101-3p may play a crucial role in the ACC inhibition of progression (121). Moreover, Pim-1 expression is inversely associated with the expression of miR-101-3p; by directly downregulating Pim-1, miR-101-3p can inhibit ACC cell invasion and proliferation (121). In addition, in cells treated with cisplatin, miR-101-3p expression was markedly downregulated, and the expression of Pim-1 was notably increased, indicating that in SACC cells, miR-101-3p may enhance sensitivity to cisplatin (121). In summary, miR-101-3p may prove to be a promising therapeutic target for patients with ACC.
miR-98
As part of the mature let-7 family, miR-98 was initially found to be downregulated in leukemia cell lines (122). In nasopharyngeal carcinoma, it was also found that miR-98 expression was markedly decreased. In addition, miR-98 was abnormally expressed in various types of cancer, including lung, breast, colorectal cancer and glioma. Consequently, miR-98 is widely considered as a tumor suppressor gene (122,123).
As demonstrated in the study by Liu et al (124), miR-98 expression was downregulated in SACC tissues compared with that in adjacent normal tissues; in highly metastatic ACC-M cell lines, miR-98 expression was found to be lower, whereas N-Ras expression was higher. As part of a family of oncoproteins, N-Ras is commonly mutated in cancer, and mutations in N-Ras can lead to the activation of downstream serine/threonine kinases, which may enhance cell survival and cellular transformation, and promote cell cycle progression (125,126). In SACC, miR-98 is related to N-Ras, and miR-98 may negatively regulate N-Ras expression, suggesting that miR-98 may target N-Ras directly (124). miR-98 overexpression can decrease cell clonogenicity and viability, while N-Ras is evidently related to tumor size and clinical phase, suggesting that by targeting N-RAS translation, miR-98 can function as a tumor suppressor (124). In addition, the expression levels of p-AKT and p-ERK are decreased by the overexpression of miR-98, indicating the inactivation of the RAS/MAPK/ERK and PI3K/AKT pathways (124). Thus, the role of miR-98 in SACC may be crucial.
miR-125a-5p
miR-125a-5p is part of an evolutionarily conserved cluster of three miRNA genes within 727 bp of one another on chr19q13.41 (127). In several human cancer types, including medulloblastoma, and lung, ovarian and breast cancer, the expression of miR125a-5p is downregulated (128). In oral squamous cell carcinoma, miR-125a-5p expression is also markedly downregulated (129). These findings indicate that miR-125a-5p may function as a tumor suppressor.
The p38/MAPK signaling pathway is considered to regulate various physiological processes, including cell proliferation, differentiation and apoptosis (130). Recently, increasing evidence has suggested that p38 signaling is activated during the tumorigenesis of several human malignancies (131,132). In SACC, by targeting the p38/JNK/ERK signaling pathway, miR-125a-5p is associated with SACC progression (133). Firstly, it was found that miR-125a-5p was downregulated in primary SACC tissues, and lower miR-125a-5p expression levels were positively linked to a metastatic phenotype (133). In SACC cells, miR-125a-5p downregulation markedly promoted migration and invasion, while miR-125a-5p overexpression markedly inhibited migration and invasion. It was also shown that the inhibition of miR-125a-5p upregulated the expression of p-p38/JNK/ERK, while the overexpression of miR-125a-5p may decrease the activation of p-p38, JNK and ERK; based on this inference, miR-125a-5p regulates SACC progression through the p38/JNK/ERK signaling pathway (133). This suggests that miR-125a-5p has potential for use as a prognostic biomarker and therapeutic target for SACC.
miR-582-5p
In prostate cancer, gastric cancer, colorectal carcinoma, hepatocellular carcinoma and bladder cancer, miR-582-5p functions as a tumor suppressor. However, miR-582-5p does not only always act as a tumor inhibitor. miR-582-5p can enhance the survival of glioblastoma stem cells, and the overexpression of miR-582-5p can promote the growth of prostate cancer (134).
Forkhead box C1 (FOXC1) plays a critical role in the development of normal embryonic tissues and can regulate the development of several organs (135). FOXC1 also exerts a notable effect on tumor development and metastasis (136). In SACC, by targeting FOXC1, miR-582-5p can inhibit invasion and migration (137). In SACC cell lines and tissues, miR-582-5p has been found to be significantly downregulated. Following the overexpression of miR-582-5p by transfection, SACC cell invasion and metastasis were inhibited, and proliferation was promoted in vitro (137). miR-582-5p can target FOXC1, and the expression of FOXC1 is inversely related to the expression of miR-582-5p. FOXC1 expression is reduced when miR-582-5p expression is increased, and SACC cell invasion and migration are markedly inhibited (137). In addition, in a xenograft tumor model, tumorigenesis and lung metastasis were inhibited by enhanced miR-582-5p expression (137). These findings suggest the potential of miR-582-5p as a prognostic biomarker and therapeutic target for patients with SACC.
In addition to the aforementioned miRNAs, there are also some experimentally validated miRNAs. For instance, by the regulation of the miR-146b-5p/ATP-citrate lyase axis, cancer susceptibility candidate 9 can facilitate the malignant phenotypes of SACC cells (138). By targeting mTOR, miR-144-3p inhibits the proliferation and induces the apoptosis of SACC cells (139). By targeting PTEN, miR-23b-3p may exert a critical effect by enhancing angiogenesis and local vascular microleakage (140). In SACC, miR-5191 expression has been found to be downregulated; an increase in the expression of miR-5191 led to the inhibition of tumorigenesis and pulmonary metastasis, indicating that miR-5191 was associated with an improved prognosis (141). The expression of miR-338-5p/3p was also lower in SACC cell lines, and may impair motility and invasion by targeting the γ2 chain gene (142). Through sponging with miR-143-3p, long non-coding RNA ADAMTS9 antisense RNA 2 can promote SACC cell migration and invasion (143). From the aforementioned summarized findings, it can be seen that miRNAs have a critical influence on the development of ACC, and can be used as biomarkers and therapeutic targets; some miRNAs are associated with a good prognosis, while others have opposite functions. Thus, further research on miRNAs in ACC is necessary. Among the miRNAs aforementioned, miR-6835-3p has hardly been reported in other cancer types, and perhaps it may be a unique biomarker, as well as a therapeutic target for ACC; however, further research into this miRNA is required in order to fully understand its functions. In addition, miR-5191 has rarely been reported in other cancer types, and thus the study of this miRNA in ACC may also prove useful.
7. Conclusions and future perspectives
There is strong evidence in support of the role of miRNAs in a number of cancer types, including ACC. Along with the alterations of miRNA biogenesis mechanisms, alterations in the levels of miRNA several upstream targets, including mutated protein controls, transcription factors and epigenetic controls, can also lead to alterations in miRNA levels. Thus, the dysregulation of miRNAs may lead to oncogenic or tumor-suppressive effects, consequently influencing the onset, progression and diffusion of ACC (144).
The incidence of PNI in ACC is ~43.2%, and PNI may be an independent factor associated with a poor prognosis. DM is common, with an incidence of 40-50%. The natural history of ACC is defined by a disease-free window followed by treatment failure, locoregional recurrence and distant metastasis, with a guarded prognosis and propensity for indolent progression. Thus, the early diagnosis of ACC is essential (5).
As there are still some limitations to the currently available diagnostic methods, and a better prognosis is often achieved from an early and accurate diagnosis, new diagnostic and prognostic biomarkers are being developed using miRNAs for patients with ACC (145). As also aforementioned in the summary of relevant miRNAs, each miRNA exhibits alterations in expression, and the majority of these affect tumor cell invasion, metastasis and proliferation; some can also influence tumor development by affecting their targets, and some can affect the therapeutic effects of certain drugs. Thus, miRNAs can be used as biomarkers for the diagnosis of ACC and to predict the prognosis of patients with ACC.
In addition, several studies have reported that other miRNAs also play a role in the diagnosis, progression and prognosis of ACC (146-148). In bodily fluids, these miRNAs are circulating and their stability can be easily maintained. Thus, miRNAs can be non-invasive, promising, affordable, easily accessible and novel testing tools for patients with ACC with a personalized management plan. In addition, as miRNAs control multiple target genes, the combination of several miRNAs may enhance sensitivity; compared with individual miRNA assays, circulating and multiple miRNA-based profiles may present a considerable and effective diagnostic and prognostic tool. This may reveal how each miRNA affects tumor development and elucidate the biological effects of miRNA regulation on the multistep process leading to ACC (144,145,149).
Under conditions of DM or symptomatic locoregional recurrence, surgery and radiotherapy are not available treatment options for patients with ACC, but chemotherapy is then suitable for the disease; thus, future studies are required to focus on the development and delivery of miRNA-based drugs (150). Further attention needs to be paid to the control of the off-target effects of miRNA therapeutics, improvements in miRNA delivery and the optimization of miRNA-based drug stability. Moreover, in order to improve non-miRNA treatment, more efforts need be focused on the use of such non-miRNA treatments (radiotherapy and chemotherapy) linked to miRNAs, which play a critical role in the regulation of the treatment response (151).
In conclusion, the present review provides a brief introduction into ACC and the biogenesis of miRNAs. miRNAs that have been validated by in vitro or in vivo studies are presented, and their role in ACC is described. Various miRNAs exhibit differential expression and regulation in ACC, and can function as either tumor suppressor genes or oncogenes. The present review may provide the basis for future research, which is required to examine the role of miRNAs in ACC.
Acknowledgments
Not applicable.
Funding Statement
This study was supported by Anhui Medical University School of Stomatology Discipline Construction Follow-up Project (grant no. 2020kqsy02).
Availability of data and materials
Not applicable.
Authors' contributions
YL and FG collected the related literature and drafted the manuscript. YH, JX and XH revised and edited the manuscript. YW and RC performed revision of the manuscript, and conducted project administration and funding acquisition. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nightingale J, Lum B, Ladwa R, Simpson F, Panizza B. Adenoid cystic carcinoma: A review of clinical features, treatment targets and advances in improving the immune response to monoclonal antibody therapy. Biochim Biophys Acta Rev Cancer. 2021;1875:188523. doi: 10.1016/j.bbcan.2021.188523. [DOI] [PubMed] [Google Scholar]
- 2.Cantù G. Adenoid cystic carcinoma. An indolent but aggressive tumour. Part A: From aetiopathogenesis to diagnosis. Acta Otorhinolaryngol Ital. 2021;41:206–214. doi: 10.14639/0392-100X-N1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z, Pan J, Chen J, Wu S, Wu T, Ye H, Zhang H, Nie X, Huang C. Multicentre clinicopathological study of adenoid cystic carcinoma: A report of 296 cases. Cancer Med. 2021;10:1120–1127. doi: 10.1002/cam4.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A, Haigentz M, Jr, Takes RP, et al. Adenoid cystic carcinoma of the head and neck-an update. Oral Oncol. 2015;51:652–661. doi: 10.1016/j.oraloncology.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Fang Y, Peng Z, Wang Y, Gao K, Liu Y, Fan R, Zhang H, Xie Z, Jiang W. Current opinions on diagnosis and treatment of adenoid cystic carcinoma. Oral Oncol. 2022;130:105945. doi: 10.1016/j.oraloncology.2022.105945. [DOI] [PubMed] [Google Scholar]
- 6.Jaso J, Malhotra R. Adenoid cystic carcinoma. Arch Pathol Lab Med. 2011;135:511–515. doi: 10.5858/2009-0527-RS.1. [DOI] [PubMed] [Google Scholar]
- 7.Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid cystic carcinoma: A review of recent advances, molecular targets, and clinical trials. Head Neck. 2016;38:620–627. doi: 10.1002/hed.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seshadri M, Rich LJ. Ultrasound guided generation of PDOX models of adenoid cystic carcinoma. EBioMedicine. 2019;42:38. doi: 10.1016/j.ebiom.2019.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha H, Keam B, Ock CY, Kim TM, Kim JH, Chung EJ, Kwon SK, Ahn SH, Wu HG, Sung MW, Heo DS. Role of concurrent chemoradiation on locally advanced unresectable adenoid cystic carcinoma. Korean J Intern Med. 2021;36:175–181. doi: 10.3904/kjim.2019.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guazzo E, Bowman J, Porceddu S, Webb L, Panizza B. Advanced adenoid cystic carcinoma of the skull base-the role of surgery. Oral Oncol. 2019;99:104466. doi: 10.1016/j.oraloncology.2019.104466. [DOI] [PubMed] [Google Scholar]
- 11.Atallah S, Marc M, Schernberg A, Huguet F, Wagner I, Mäkitie A, Baujat B. Beyond surgical treatment in adenoid cystic carcinoma of the head and neck: A literature review. Cancer Manag Res. 2022;14:1879–1890. doi: 10.2147/CMAR.S355663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabris L, Ceder Y, Chinnaiyan AM, Jenster GW, Sorensen KD, Tomlins S, Visakorpi T, Calin GA. The potential of MicroRNAs as prostate cancer biomarkers. Eur Urol. 2016;70:312–322. doi: 10.1016/j.eururo.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi S, Xie H, Peng X, Yin W, Tao Y, Wang X. miRNA-based biomarkers, therapies, and resistance in cancer. Int J Biol Sci. 2020;16:2628–2647. doi: 10.7150/ijbs.47203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 15.Chen JQ, Papp G, Szodoray P, Zeher M. The role of microRNAs in the pathogenesis of autoimmune diseases. Autoimmun Rev. 2016;15:1171–1180. doi: 10.1016/j.autrev.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Brief Bioinform. 2014;15:1–19. doi: 10.1093/bib/bbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 18.Achkar NP, Cambiagno DA, Manavella PA. miRNA biogenesis: A dynamic pathway. Trends Plant Sci. 2016;21:1034–1044. doi: 10.1016/j.tplants.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Hata A, Lieberman J. Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci Signal. 2015;8:re3. doi: 10.1126/scisignal.2005825. [DOI] [PubMed] [Google Scholar]
- 20.Lee TJ, Yuan X, Kerr K, Yoo JY, Kim DH, Kaur B, Eltzschig HK. Strategies to modulate MicroRNA functions for the treatment of cancer or organ injury. Pharmacol Rev. 2020;72:639–667. doi: 10.1124/pr.119.019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: Approaches and considerations. Nat Rev Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dragomir MP, Knutsen E, Calin GA. Classical and noncanonical functions of miRNAs in cancers. Trends Genet. 2022;38:379–394. doi: 10.1016/j.tig.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Hussen BM, Hidayat HJ, Salihi A, Sabir DK, Taheri M, Ghafouri-Fard S. MicroRNA: A signature for cancer progression. Biomed Pharmacother. 2021;138:111528. doi: 10.1016/j.biopha.2021.111528. [DOI] [PubMed] [Google Scholar]
- 25.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 26.Kiss O, Tőkés AM, Vranic S, Gatalica Z, Vass L, Udvarhelyi N, Szász AM, Kulka J. Expression of miRNAs in adenoid cystic carcinomas of the breast and salivary glands. Virchows Arch. 2015;467:551–562. doi: 10.1007/s00428-015-1827-3. [DOI] [PubMed] [Google Scholar]
- 27.Auxzilia Preethi K, Chandralekha Selvakumar S, Sekar D. MicroRNAs and it's targets in the treatment of salivary adenoid cystic carcinoma. Oral Oncol. 2022;133:106053. doi: 10.1016/j.oraloncology.2022.106053. [DOI] [PubMed] [Google Scholar]
- 28.Andreasen S, Tan Q, Agander TK, Hansen TVO, Steiner P, Bjørndal K, Høgdall E, Larsen SR, Erentaite D, Olsen CH, et al. MicroRNA dysregulation in adenoid cystic carcinoma of the salivary gland in relation to prognosis and gene fusion status: A cohort study. Virchows Arch. 2018;473:329–340. doi: 10.1007/s00428-018-2423-0. [DOI] [PubMed] [Google Scholar]
- 29.Mitani Y, Roberts DB, Fatani H, Weber RS, Kies MS, Lippman SM, El-Naggar AK. MicroRNA profiling of salivary adenoid cystic carcinoma: Association of miR-17-92 upregulation with poor outcome. PLoS One. 2013;8:e66778. doi: 10.1371/journal.pone.0066778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AL, Al-Samadi A, Sperandio M, Soares AB, Teixeira LN, Martinez EF, Demasi APD, Araújo VC, Leivo I, Salo T, Passador-Santos F. MiR-455-3p miR-150 and miR-375 are aberrantly expressed in salivary gland adenoid cystic carcinoma and polymorphous adenocarcinoma. J Oral Pathol Med. 2019;48:840–845. doi: 10.1111/jop.12894. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J, Liu X, Lin J, Jiang M, Xu F, Zhang C, Tang Q, Zhu L, Dong L, Lin T. AKT2 identified as a potential target of mir-29a-3p via microRNA profiling of patients with high proliferation lacrimal gland adenoid cystic carcinoma. Exp Eye Res. 2022;219:109067. doi: 10.1016/j.exer.2022.109067. [DOI] [PubMed] [Google Scholar]
- 32.Zhang HD, Jiang LH, Sun DW, Li J, Ji ZL. The role of miR-130a in cancer. Breast Cancer. 2017;24:521–527. doi: 10.1007/s12282-017-0776-x. [DOI] [PubMed] [Google Scholar]
- 33.Liu SG, Qin XG, Zhao BS, Qi B, Yao WJ, Wang TY, Li HC, Wu XN. Differential expression of miRNAs in esophageal cancer tissue. Oncol Lett. 2013;5:1639–1642. doi: 10.3892/ol.2013.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Yan D, Wu W, Zhu J, Ye W, Shu Q. MicroRNA-130a promotes the metastasis and epithelial-mesenchymal transition of osteosarcoma by targeting PTEN. Oncol Rep. 2016;35:3285–3292. doi: 10.3892/or.2016.4719. [DOI] [PubMed] [Google Scholar]
- 35.Wang XC, Tian LL, Wu HL, Jiang XY, Du LQ, Zhang H, Wang YY, Wu HY, Li DG, She Y, et al. Expression of miRNA-130a in nonsmall cell lung cancer. Am J Med Sci. 2010;340:385–388. doi: 10.1097/MAJ.0b013e3181e892a0. [DOI] [PubMed] [Google Scholar]
- 36.Sand M, Skrygan M, Sand D, Georgas D, Hahn SA, Gambichler T, Altmeyer P, Bechara FG. Expression of microRNAs in basal cell carcinoma. Br J Dermatol. 2012;167:847–855. doi: 10.1111/j.1365-2133.2012.11022.x. [DOI] [PubMed] [Google Scholar]
- 37.Ishihara K, Sasaki D, Tsuruda K, Inokuchi N, Nagai K, Hasegawa H, Yanagihara K, Kamihira S. Impact of miR-155 and miR-126 as novel biomarkers on the assessment of disease progression and prognosis in adult T-cell leukemia. Cancer Epidemiol. 2012;36:560–565. doi: 10.1016/j.canep.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Jiang H, Yu WW, Wang LL, Peng Y. miR-130a acts as a potential diagnostic biomarker and promotes gastric cancer migration, invasion and proliferation by targeting RUNX3. Oncol Rep. 2015;34:1153–1161. doi: 10.3892/or.2015.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovaleva V, Mora R, Park YJ, Plass C, Chiramel AI, Bartenschlager R, Döhner H, Stilgenbauer S, Pscherer A, Lichter P, Seiffert M. miRNA-130a targets ATG2B and DICER1 to inhibit autophagy and trigger killing of chronic lymphocytic leukemia cells. Cancer Res. 2012;72:1763–1772. doi: 10.1158/0008-5472.CAN-11-3671. [DOI] [PubMed] [Google Scholar]
- 40.Boll K, Reiche K, Kasack K, Mörbt N, Kretzschmar AK, Tomm JM, Verhaegh G, Schalken J, von Bergen M, Horn F, Hackermüller J. MiR-130a, miR-203 and miR-205 jointly repress key oncogenic pathways and are downregulated in prostate carcinoma. Oncogene. 2013;32:277–285. doi: 10.1038/onc.2012.55. [DOI] [PubMed] [Google Scholar]
- 41.Qiu S, Lin S, Hu D, Feng Y, Tan Y, Peng Y. Interactions of miR-323/miR-326/miR-329 and miR-130a/miR-155/miR-210 as prognostic indicators for clinical outcome of glioblastoma patients. J Transl Med. 2013;11:10. doi: 10.1186/1479-5876-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, Huang P, Qiu J, Liao Y, Hong J, Yuan Y. MicroRNA-130a is down-regulated in hepatocellular carcinoma and associates with poor prognosis. Med Oncol. 2014;31:230. doi: 10.1007/s12032-014-0230-2. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Huang L, Zhao Y, Tan W. Downregulation of miR-130a contributes to cisplatin resistance in ovarian cancer cells by targeting X-linked inhibitor of apoptosis (XIAP) directly. Acta Biochim Biophys Sin (Shanghai) 2013;45:995–1001. doi: 10.1093/abbs/gmt113. [DOI] [PubMed] [Google Scholar]
- 44.Pan Y, Wang R, Zhang F, Chen Y, Lv Q, Long G, Yang K. MicroRNA-130a inhibits cell proliferation, invasion and migration in human breast cancer by targeting the RAB5A. Int J Clin Exp Pathol. 2015;8:384–393. [PMC free article] [PubMed] [Google Scholar]
- 45.He L, Wang HY, Zhang L, Huang L, Li JD, Xiong Y, Zhang MY, Jia WH, Yun JP, Luo RZ, Zheng M. Prognostic significance of low DICER expression regulated by miR-130a in cervical cancer. Cell Death Dis. 2014;5:e1205. doi: 10.1038/cddis.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 47.Kim G, Lim S, Kim KD. N-myc downstream-regulated gene 2 (NDRG2) function as a positive regulator of apoptosis: A new insight into NDRG2 as a tumor suppressor. Cells. 2021;10:2649. doi: 10.3390/cells10102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Zhang CY, Xia RH, Han J, Sun B, Sun SY, Li J. The MYB/miR-130a/NDRG2 axis modulates tumor proliferation and metastatic potential in salivary adenoid cystic carcinoma. Cell Death Dis. 2018;9:917. doi: 10.1038/s41419-018-0966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li YF, Jing Y, Hao J, Frankfort NC, Zhou X, Shen B, Liu X, Wang L, Li R. MicroRNA-21 in the pathogenesis of acute kidney injury. Protein Cell. 2013;4:813–819. doi: 10.1007/s13238-013-3085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bautista-Sánchez D, Arriaga-Canon C, Pedroza-Torres A, De La Rosa-Velázquez IA, González-Barrios R, Contreras-Espinosa L, Montiel-Manríquez R, Castro-Hernández C, Fragoso-Ontiveros V, Álvarez-Gómez RM, Herrera LA. The promising role of miR-21 as a cancer biomarker and its importance in RNA-based therapeutics. Mol Ther Nucleic Acids. 2020;20:409–420. doi: 10.1016/j.omtn.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das PK, Islam F, Lam AK. The roles of cancer stem cells and therapy resistance in colorectal carcinoma. Cells. 2020;9:1392. doi: 10.3390/cells9061392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dioguardi M, Caloro GA, Laino L, Alovisi M, Sovereto D, Crincoli V, Aiuto R, Coccia E, Troiano G, Lo Muzio L. Circulating miR-21 as a potential biomarker for the diagnosis of oral cancer: A systematic review with meta-analysis. Cancers (Basel) 2020;12:936. doi: 10.3390/cancers12040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribas J, Lupold SE. The transcriptional regulation of miR-21, its multiple transcripts, and their implication in prostate cancer. Cell Cycle. 2010;9:923–929. doi: 10.4161/cc.9.5.10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S, Yang X, Yang J, Zhen J, Zhang D. Serum microRNA-21 as a potential diagnostic biomarker for breast cancer: A systematic review and meta-analysis. Clin Exp Med. 2016;16:29–35. doi: 10.1007/s10238-014-0332-3. [DOI] [PubMed] [Google Scholar]
- 55.Jiang LH, Ge MH, Hou XX, Cao J, Hu SS, Lu XX, Han J, Wu YC, Liu X, Zhu X, et al. miR-21 regulates tumor progression through the miR-21-PDCD4-Stat3 pathway in human salivary adenoid cystic carcinoma. Lab Invest. 2015;95:1398–1408. doi: 10.1038/labinvest.2015.105. [DOI] [PubMed] [Google Scholar]
- 56.Wang C, Li T, Yan F, Cai W, Zheng J, Jiang X, Sun J. Effect of simvastatin and microRNA-21 inhibitor on metastasis and progression of human salivary adenoid cystic carcinoma. Biomed Pharmacother. 2018;105:1054–1061. doi: 10.1016/j.biopha.2018.05.157. [DOI] [PubMed] [Google Scholar]
- 57.Yao X, Xie R, Cao Y, Tang J, Men Y, Peng H, Yang W. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J Nanobiotechnology. 2021;19:311. doi: 10.1186/s12951-021-01058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen MC, Tsai YC, Tseng JH, Liou JJ, Horng S, Wen HC, Fan YC, Zhong WB, Hsu SP. Simvastatin inhibits cell proliferation and migration in human anaplastic thyroid cancer. Int J Mol Sci. 2017;18:2690. doi: 10.3390/ijms18122690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu X, Pan Y, Ma H, Li W. Simvastatin inhibits proliferation and induces apoptosis in human lung cancer cells. Oncol Res. 2013;20:351–357. doi: 10.3727/096504013X13657689382897. [DOI] [PubMed] [Google Scholar]
- 60.Yan F, Wang C, Li T, Cai W, Sun J. Role of miR-21 in the growth and metastasis of human salivary adenoid cystic carcinoma. Mol Med Rep. 2018;17:4237–4244. doi: 10.3892/mmr.2018.8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X, Chen S, Xiu YL, Sun KX, Zong ZH, Zhao Y. RhoC is a major target of microRNA-93-5P in epithelial ovarian carcinoma tumorigenesis and progression. Mol Cancer. 2015;14:31. doi: 10.1186/s12943-015-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X, Liu J, Zhang Q, Liu B, Cheng Y, Zhang Y, Sun Y, Ge H, Liu Y. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J Exp Clin Cancer Res. 2020;39:65. doi: 10.1186/s13046-019-1507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma DH, Li BS, Liu JJ, Xiao YF, Yong X, Wang SM, Wu YY, Zhu HB, Wang DX, Yang SM. miR-93-5p/IFNAR1 axis promotes gastric cancer metastasis through activating the STAT3 signaling pathway. Cancer Lett. 2017;408:23–32. doi: 10.1016/j.canlet.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Shi X, Liu TT, Yu XN, Balakrishnan A, Zhu HR, Guo HY, Zhang GC, Bilegsaikhan E, Sun JL, Song GQ, et al. microRNA-93-5p promotes hepatocellular carcinoma progression via a microRNA-93-5p/MAP3K2/c-Jun positive feedback circuit. Oncogene. 2020;39:5768–5781. doi: 10.1038/s41388-020-01401-0. [DOI] [PubMed] [Google Scholar]
- 65.Gong C, Qu S, Lv XB, Liu B, Tan W, Nie Y, Su F, Liu Q, Yao H, Song E. BRMS1L suppresses breast cancer metastasis by inducing epigenetic silence of FZD10. Nat Commun. 2014;5:5406. doi: 10.1038/ncomms6406. [DOI] [PubMed] [Google Scholar]
- 66.Koyama R, Tamura M, Nakagaki T, Ohashi T, Idogawa M, Suzuki H, Tokino T, Sasaki Y. Identification and characterization of a metastatic suppressor BRMS1L as a target gene of p53. Cancer Sci. 2017;108:2413–2421. doi: 10.1111/cas.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao J, Jin X, Shi Y, Zhang H. miR-93-5p enhance lacrimal gland adenoid cystic carcinoma cell tumorigenesis by targeting BRMS1L. Cancer Cell Int. 2018;18:72. doi: 10.1186/s12935-018-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Z, Zhang Q, Yuan W, Li X, Chen C, Guo Y, Shao B, Dang Q, Zhou Q, Wang Q, et al. MiR-103a-3p promotes tumour glycolysis in colorectal cancer via hippo/YAP1/HIF1A axis. J Exp Clin Cancer Res. 2020;39:250. doi: 10.1186/s13046-020-01705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu M, Chen CW, Yang LQ, Yang WW, Du ZH, Li YR, Li SL, Ge XY. MicroRNA-103a-3p promotes metastasis by targeting TPD52 in salivary adenoid cystic carcinoma. Int J Oncol. 2020;57:574–586. doi: 10.3892/ijo.2020.5069. [DOI] [PubMed] [Google Scholar]
- 70.Shehata M, Bièche I, Boutros R, Weidenhofer J, Fanayan S, Spalding L, Zeps N, Byth K, Bright RK, Lidereau R, Byrne JA. Nonredundant functions for tumor protein D52-like proteins support specific targeting of TPD52. Clin Cancer Res. 2008;14:5050–5060. doi: 10.1158/1078-0432.CCR-07-4994. [DOI] [PubMed] [Google Scholar]
- 71.Rubin MA, Varambally S, Beroukhim R, Tomlins SA, Rhodes DR, Paris PL, Hofer MD, Storz-Schweizer M, Kuefer R, Fletcher JA, et al. Overexpression, amplification, and androgen regulation of TPD52 in prostate cancer. Cancer Res. 2004;64:3814–3822. doi: 10.1158/0008-5472.CAN-03-3881. [DOI] [PubMed] [Google Scholar]
- 72.Wang SJ, Li YJ, Gao B, Li XL, Li YT, He HY. Long non-coding RNA 00152 slicing represses the growth and aggressiveness of hemangioma cell by modulating miR-139-5p. Biomed Pharmacother. 2019;120:109385. doi: 10.1016/j.biopha.2019.109385. [DOI] [PubMed] [Google Scholar]
- 73.Tennstedt P, Bölch C, Strobel G, Minner S, Burkhardt L, Grob T, Masser S, Sauter G, Schlomm T, Simon R. Patterns of TPD52 overexpression in multiple human solid tumor types analyzed by quantitative PCR. Int J Oncol. 2014;44:609–615. doi: 10.3892/ijo.2013.2200. [DOI] [PubMed] [Google Scholar]
- 74.Ravegnini G, Cargnin S, Sammarini G, Zanotti F, Bermejo JL, Hrelia P, Terrazzino S, Angelini S. Prognostic role of miR-221 and miR-222 expression in cancer patients: A systematic review and meta-analysis. Cancers (Basel) 2019;11:970. doi: 10.3390/cancers11070970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song J, Ouyang Y, Che J, Li X, Zhao Y, Yang K, Zhao X, Chen Y, Fan C, Yuan W. Potential value of miR-221/222 as diagnostic, prognostic, and therapeutic biomarkers for diseases. Front Immunol. 2017;8:56. doi: 10.3389/fimmu.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang F, Zhao W, Zhou L, Zhang L, Liu Z, Yu D. miR-222 regulates the cell biological behavior of oral squamous cell carcinoma by targeting PUMA. Oncol Rep. 2014;31:1255–1262. doi: 10.3892/or.2014.2985. [DOI] [PubMed] [Google Scholar]
- 77.Zhou Z, Zhou L, Jiang F, Zeng B, Wei C, Zhao W, Yu D. Downregulation of miR-222 induces apoptosis and cellular migration in adenoid cystic carcinoma cells. Oncol Res. 2017;25:207–214. doi: 10.3727/096504016X14732772150460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, He Y, Xiang J, Feng L, Wang Y, Chen R. The functional mechanism of MicroRNA in oral lichen planus. J Inflamm Res. 2022;15:4261–4274. doi: 10.2147/JIR.S369304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y, Tian Z, Tan Y, Lian G, Chen S, Chen S, Li J, Li X, Huang K, Chen Y. Bmi-1-induced miR-27a and miR-155 promote tumor metastasis and chemoresistance by targeting RKIP in gastric cancer. Mol Cancer. 2020;19:109. doi: 10.1186/s12943-020-01229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Roosbroeck K, Fanini F, Setoyama T, Ivan C, Rodriguez-Aguayo C, Fuentes-Mattei E, Xiao L, Vannini I, Redis RS, D'Abundo L, et al. Combining anti-Mir-155 with chemotherapy for the treatment of lung cancers. Clin Cancer Res. 2017;23:2891–2904. doi: 10.1158/1078-0432.CCR-16-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kulkarni P, Dasgupta P, Hashimoto Y, Shiina M, Shahryari V, Tabatabai ZL, Yamamura S, Tanaka Y, Saini S, Dahiya R, Majid S. A lncRNA TCL6-miR-155 interaction regulates the Src-Akt-EMT network to mediate kidney cancer progression and metastasis. Cancer Res. 2021;81:1500–1512. doi: 10.1158/0008-5472.CAN-20-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bacci M, Giannoni E, Fearns A, Ribas R, Gao Q, Taddei ML, Pintus G, Dowsett M, Isacke CM, Martin LA, et al. miR-155 drives metabolic reprogramming of ER+ breast cancer cells following long-term estrogen deprivation and predicts clinical response to aromatase inhibitors. Cancer Res. 2016;76:1615–1626. doi: 10.1158/0008-5472.CAN-15-2038. [DOI] [PubMed] [Google Scholar]
- 84.Hess AK, Müer A, Mairinger FD, Weichert W, Stenzinger A, Hummel M, Budach V, Tinhofer I. MiR-200b and miR-155 as predictive biomarkers for the efficacy of chemoradiation in locally advanced head and neck squamous cell carcinoma. Eur J Cancer. 2017;77:3–12. doi: 10.1016/j.ejca.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 85.Gulei D, Raduly L, Broseghini E, Ferracin M, Berindan-Neagoe I. The extensive role of miR-155 in malignant and non-malignant diseases. Mol Aspects Med. 2019;70:33–56. doi: 10.1016/j.mam.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Liu L, Hu Y, Fu J, Yang X, Zhang Z. MicroRNA155 in the growth and invasion of salivary adenoid cystic carcinoma. J Oral Pathol Med. 2013;42:140–147. doi: 10.1111/j.1600-0714.2012.01189.x. [DOI] [PubMed] [Google Scholar]
- 87.Biswas DK, Iglehart JD. Linkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancer. J Cell Physiol. 2006;209:645–652. doi: 10.1002/jcp.20785. [DOI] [PubMed] [Google Scholar]
- 88.Cascinu S, Verdecchia L, Valeri N, Berardi R, Scartozzi M. New target therapies in advanced pancreatic cancer. Ann Oncol. 2006;17(Suppl 5):v148–v152. doi: 10.1093/annonc/mdj971. [DOI] [PubMed] [Google Scholar]
- 89.Bianco R, Gelardi T, Damiano V, Ciardiello F, Tortora G. Rational bases for the development of EGFR inhibitors for cancer treatment. Int J Biochem Cell Biol. 2007;39:1416–1431. doi: 10.1016/j.biocel.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 90.Feng X, Matsuo K, Zhang T, Hu Y, Mays AC, Browne JD, Zhou X, Sullivan CA. MicroRNA profiling and target genes related to metastasis of salivary adenoid cystic carcinoma. Anticancer Res. 2017;37:3473–3481. doi: 10.21873/anticanres.11715. [DOI] [PubMed] [Google Scholar]
- 91.Jin Y, Chen X, Gao ZY, Liu K, Hou Y, Zheng J. The role of miR-320a and IL-1β in human chondrocyte degradation. Bone Joint Res. 2017;6:196–203. doi: 10.1302/2046-3758.64.BJR-2016-0224.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shang C, Zhang H, Guo Y, Hong Y, Liu Y, Xue Y. MiR-320a down-regulation mediates bladder carcinoma invasion by targeting ITGB3. Mol Biol Rep. 2014;41:2521–2527. doi: 10.1007/s11033-014-3110-0. [DOI] [PubMed] [Google Scholar]
- 93.Xie N, Jia Z, Li L. miR-320a upregulation contributes to the development of preeclampsia by inhibiting the growth and invasion of trophoblast cells by targeting interleukin 4. Mol Med Rep. 2019;20:3256–3264. doi: 10.3892/mmr.2019.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lv G, Wu M, Wang M, Jiang X, Du J, Zhang K, Li D, Ma N, Peng Y, Wang L, et al. miR-320a regulates high mobility group box 1 expression and inhibits invasion and metastasis in hepatocellular carcinoma. Liver Int. 2017;37:1354–1364. doi: 10.1111/liv.13424. [DOI] [PubMed] [Google Scholar]
- 95.Wang B, Yang Z, Wang H, Cao Z, Zhao Y, Gong C, Ma L, Wang X, Hu X, Chen S. MicroRNA-320a inhibits proliferation and invasion of breast cancer cells by targeting RAB11A. Am J Cancer Res. 2015;5:2719–2729. doi: 10.1158/1538-7445.AM2015-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L, Chen H, He F, Zhang S, Li A, Zhang A, Zhang A. MicroRNA-320a promotes epithelial ovarian cancer cell proliferation and invasion by targeting RASSF8. Front Oncol. 2021;11:581932. doi: 10.3389/fonc.2021.581932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun L, Liu B, Lin Z, Yao Y, Chen Y, Li Y, Chen J, Yu D, Tang Z, Wang B, et al. MiR-320a acts as a prognostic factor and inhibits metastasis of salivary adenoid cystic carcinoma by targeting ITGB3. Mol Cancer. 2015;14:96. doi: 10.1186/s12943-015-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fuentes P, Sesé M, Guijarro PJ, Emperador M, Sánchez-Redondo S, Peinado H, Hümmer S, Ramón Y, Cajal S. ITGB3-mediated uptake of small extracellular vesicles facilitates intercellular communication in breast cancer cells. Nat Commun. 2020;11:4261. doi: 10.1038/s41467-020-18081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL, Lv JW, Huang XD, Liu RQ, Chen F, He XJ, et al. Long noncoding RNA FAM225A promotes nasopharyngeal carcinoma tumorigenesis and metastasis by acting as ceRNA to sponge miR-590-3p/miR-1275 and upregulate ITGB3. Cancer Res. 2019;79:4612–4626. doi: 10.1158/0008-5472.CAN-19-0799. [DOI] [PubMed] [Google Scholar]
- 100.Zhao B, Han H, Chen J, Zhang Z, Li S, Fang F, Zheng Q, Ma Y, Zhang J, Wu N, Yang Y. MicroRNA let-7c inhibits migration and invasion of human non-small cell lung cancer by targeting ITGB3 and MAP4K3. Cancer Lett. 2014;342:43–51. doi: 10.1016/j.canlet.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 101.Fang Z, Yin S, Sun R, Zhang S, Fu M, Wu Y, Zhang T, Khaliq J, Li Y. miR-140-5p suppresses the proliferation, migration and invasion of gastric cancer by regulating YES1. Mol Cancer. 2017;16:139. doi: 10.1186/s12943-017-0708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M, Morrell NW, Thomas M, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126:2495–2508. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelly RJ, Lopez-Chavez A, Citrin D, Janik JE, Morris JC. Impacting tumor cell-fate by targeting the inhibitor of apoptosis protein survivin. Mol Cancer. 2011;10:35. doi: 10.1186/1476-4598-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martínez-García D, Manero-Rupérez N, Quesada R, Korrodi-Gregório L, Soto-Cerrato V. Therapeutic strategies involving survivin inhibition in cancer. Med Res Rev. 2019;39:887–909. doi: 10.1002/med.21547. [DOI] [PubMed] [Google Scholar]
- 105.Qiao Z, Zou Y, Zhao H. MicroRNA-140-5p inhibits salivary adenoid cystic carcinoma progression and metastasis via targeting survivin. Cancer Cell Int. 2019;19:301. doi: 10.1186/s12935-019-1018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng W, Sha H, Sun X, Zou R, Zhu Y, Zhou G, Feng J. Role and mechanism of miR-187 in human cancer. Am J Transl Res. 2020;12:4873–4884. [PMC free article] [PubMed] [Google Scholar]
- 107.Mulrane L, Madden SF, Brennan DJ, Gremel G, McGee SF, McNally S, Martin F, Crown JP, Jirström K, Higgins DG, et al. miR-187 is an independent prognostic factor in breast cancer and confers increased invasive potential in vitro. Clin Cancer Res. 2012;18:6702–6713. doi: 10.1158/1078-0432.CCR-12-1420. [DOI] [PubMed] [Google Scholar]
- 108.Chao A, Lin CY, Lee YS, Tsai CL, Wei PC, Hsueh S, Wu TI, Tsai CN, Wang CJ, Chao AS, et al. Regulation of ovarian cancer progression by microRNA-187 through targeting disabled homolog-2. Oncogene. 2012;31:764–775. doi: 10.1038/onc.2011.269. [DOI] [PubMed] [Google Scholar]
- 109.Liang Z, Xu J, Ma Z, Li G, Zhu W. MiR-187 suppresses non-small-cell lung cancer cell proliferation by targeting FGF9. Bioengineered. 2020;11:70–80. doi: 10.1080/21655979.2019.1706287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bagaeva LV, Rao P, Powers JM, Segal BM. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J Immunol. 2006;176:7676–7685. doi: 10.4049/jimmunol.176.12.7676. [DOI] [PubMed] [Google Scholar]
- 111.Hussain M, Adah D, Tariq M, Lu Y, Zhang J, Liu J. CXCL13/CXCR5 signaling axis in cancer. Life Sci. 2019;227:175–186. doi: 10.1016/j.lfs.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 112.Zhang M, Wu JS, Xian HC, Chen BJ, Wang HF, Yu XH, Pang X, Dai L, Jiang J, Liang XH, Tang YL. CXCR5 induces perineural invasion of salivary adenoid cystic carcinoma by inhibiting microRNA-187. Aging (Albany NY) 2021;13:15384–15399. doi: 10.18632/aging.203097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gui T, Shen K. miRNA-101: A potential target for tumor therapy. Cancer Epidemiol. 2012;36:537–540. doi: 10.1016/j.canep.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 114.Liang H, Yu T, Han Y, Jiang H, Wang C, You T, Zhao X, Shan H, Yang R, Yang L, et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol Cancer. 2018;17:119. doi: 10.1186/s12943-018-0870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu Y, Yao Y, Jiang X, Zhong X, Wang Z, Li C, Kang P, Leng K, Ji D, Li Z, et al. SP1-induced upregulation of lncRNA SPRY4-IT1 exerts oncogenic properties by scaffolding EZH2/LSD1/DNMT1 and sponging miR-101-3p in cholangiocarcinoma. J Exp Clin Cancer Res. 2018;37:81. doi: 10.1186/s13046-018-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cao S, Lin L, Xia X, Wu H. lncRNA SPRY4-IT1 regulates cell proliferation and migration by sponging miR-101-3p and regulating AMPK expression in gastric cancer. Mol Ther Nucleic Acids. 2019;17:455–464. doi: 10.1016/j.omtn.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Zhang X, Gao D, Fang K, Guo Z, Li L. Med19 is targeted by miR-101-3p/miR-422a and promotes breast cancer progression by regulating the EGFR/MEK/ERK signaling pathway. Cancer Lett. 2019;444:105–115. doi: 10.1016/j.canlet.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 118.Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X, Wang M, Huang C, Wang L, Zeng F, Jiang G. LncRNA SPRY4-IT1 sponges miR-101-3p to promote proliferation and metastasis of bladder cancer cells through up-regulating EZH2. Cancer Lett. 2017;388:281–291. doi: 10.1016/j.canlet.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 119.Xu J, Xiong G, Cao Z, Huang H, Wang T, You L, Zhou L, Zheng L, Hu Y, Zhang T, Zhao Y. PIM-1 contributes to the malignancy of pancreatic cancer and displays diagnostic and prognostic value. J Exp Clin Cancer Res. 2016;35:133. doi: 10.1186/s13046-016-0406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pang W, Tian X, Bai F, Han R, Wang J, Shen H, Zhang X, Liu Y, Yan X, Jiang F, Xing L. Pim-1 kinase is a target of miR-486-5p and eukaryotic translation initiation factor 4E, and plays a critical role in lung cancer. Mol Cancer. 2014;13:240. doi: 10.1186/1476-4598-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu XY, Liu ZJ, He H, Zhang C, Wang YL. MicroRNA-101-3p suppresses cell proliferation, invasion and enhances chemotherapeutic sensitivity in salivary gland adenoid cystic carcinoma by targeting Pim-1. Am J Cancer Res. 2015;5:3015–3029. [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou H, Huang Z, Chen X, Chen S. miR-98 inhibits expression of TWIST to prevent progression of non-small cell lung cancers. Biomed Pharmacother. 2017;89:1453–1461. doi: 10.1016/j.biopha.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 123.Huang SD, Yuan Y, Zhuang CW, Li BL, Gong DJ, Wang SG, Zeng ZY, Cheng HZ. MicroRNA-98 and microRNA-214 post-transcriptionally regulate enhancer of zeste homolog 2 and inhibit migration and invasion in human esophageal squamous cell carcinoma. Mol Cancer. 2012;11:51. doi: 10.1186/1476-4598-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, Zhang W, Guo H, Yue J, Zhuo S. miR-98 functions as a tumor suppressor in salivary adenoid cystic carcinomas. Onco Targets Ther. 2016;9:1777–1786. doi: 10.2147/OTT.S98534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thumar J, Shahbazian D, Aziz SA, Jilaveanu LB, Kluger HM. MEK targeting in N-RAS mutated metastatic melanoma. Mol Cancer. 2014;13:45. doi: 10.1186/1476-4598-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y, Velho S, Vakiani E, Peng S, Bass AJ, Chu GC, Gierut J, Bugni JM, Der CJ, Philips M, et al. Mutant N-RAS protects colorectal cancer cells from stress-induced apoptosis and contributes to cancer development and progression. Cancer Discov. 2013;3:294–307. doi: 10.1158/2159-8290.CD-12-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bhatlekar S, Manne BK, Basak I, Edelstein LC, Tugolukova E, Stoller ML, Cody MJ, Morley SC, Nagalla S, Weyrich AS, et al. miR-125a-5p regulates megakaryocyte proplatelet formation via the actin-bundling protein L-plastin. Blood. 2020;136:1760–1772. doi: 10.1182/blood.2020005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nishida N, Mimori K, Fabbri M, Yokobori T, Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y, Mori M. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17:2725–2733. doi: 10.1158/1078-0432.CCR-10-2132. [DOI] [PubMed] [Google Scholar]
- 129.Liu X, Zhao T, Yuan Z, Ge S. MIR600HG sponges miR-125a-5p to regulate glycometabolism and cisplatin resistance of oral squamous cell carcinoma cells via mediating RNF44. Cell Death Discov. 2022;8:216. doi: 10.1038/s41420-022-01000-w. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 130.Falcicchia C, Tozzi F, Arancio O, Watterson DM, Origlia N. Involvement of p38 MAPK in synaptic function and dysfunction. Int J Mol Sci. 2020;21:5624. doi: 10.3390/ijms21165624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 132.Han J, Sun P. The pathways to tumor suppression via route p38. Trends Biochem Sci. 2007;32:364–371. doi: 10.1016/j.tibs.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 133.Liang Y, Ye J, Jiao J, Zhang J, Lu Y, Zhang L, Wan D, Duan L, Wu Y, Zhang B. Down-regulation of miR-125a-5p is associated with salivary adenoid cystic carcinoma progression via targeting p38/JNK/ERK signal pathway. Am J Transl Res. 2017;9:1101–1113. [PMC free article] [PubMed] [Google Scholar]
- 134.Zeng X, Ma X, Guo H, Wei L, Zhang Y, Sun C, Han N, Sun S, Zhang N. MicroRNA-582-5p promotes triple-negative breast cancer invasion and metastasis by antagonizing CMTM8. Bioengineered. 2021;12:10126–10135. doi: 10.1080/21655979.2021.2000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han B, Bhowmick N, Qu Y, Chung S, Giuliano AE, Cui X. FOXC1: An emerging marker and therapeutic target for cancer. Oncogene. 2017;36:3957–3963. doi: 10.1038/onc.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gilding LN, Somervaille TCP. The diverse consequences of FOXC1 deregulation in cancer. Cancers (Basel) 2019;11:184. doi: 10.3390/cancers11020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang WW, Chen B, Lei CB, Liu GX, Wang YG, Yi C, Wang YY, Zhang SY. miR-582-5p inhibits invasion and migration of salivary adenoid cystic carcinoma cells by targeting FOXC1. Jpn J Clin Oncol. 2017;47:690–698. doi: 10.1093/jjco/hyx073. [DOI] [PubMed] [Google Scholar]
- 138.Xie H, Tang J, Lu L, Li B, Wang M. CASC9 plays a role in salivary adenoid cystic carcinoma in vitro by upregulation of ACLY. Oral Dis. 2022;28:352–363. doi: 10.1111/odi.13759. [DOI] [PubMed] [Google Scholar]
- 139.Huo F, Zhang C, He H, Wang Y. MicroRNA-144-3p inhibits proliferation and induces apoptosis of human salivary adenoid carcinoma cells via targeting of mTOR. Biotechnol Lett. 2016;38:409–416. doi: 10.1007/s10529-015-2007-x. [DOI] [PubMed] [Google Scholar]
- 140.Hou CX, Sun NN, Han W, Meng Y, Wang CX, Zhu QH, Tang YT, Ye JH. Exosomal microRNA-23b-3p promotes tumor angiogenesis and metastasis by targeting PTEN in salivary adenoid cystic carcinoma. Carcinogenesis. 2022;43:682–692. doi: 10.1093/carcin/bgac033. [DOI] [PubMed] [Google Scholar]
- 141.Li Z, Zhang Q, Su H, Li HY, Cao G, Xu JK, Wang JL, Niu CZ, Zhang F, Yang J, Chen W. miR-5191 acts as a tumor suppressor in salivary adenoid cystic carcinoma by targeting Notch-2. Oral Dis. 2022;28:1871–1881. doi: 10.1111/odi.13841. [DOI] [PubMed] [Google Scholar]
- 142.Wang S, Zhang L, Shi P, Zhang Y, Zhou H, Cao X. Genome-wide profiles of metastasis-associated mRNAs and microRNAs in salivary adenoid cystic carcinoma. Biochem Biophys Res Commun. 2018;500:632–638. doi: 10.1016/j.bbrc.2018.04.122. [DOI] [PubMed] [Google Scholar]
- 143.Xie S, Yu X, Li Y, Ma H, Fan S, Chen W, Pan G, Wang W, Zhang H, Li J, Lin Z. Upregulation of lncRNA ADAMTS9-AS2 promotes salivary adenoid cystic carcinoma metastasis via PI3K/Akt and MEK/Erk signaling. Mol Ther. 2018;26:2766–2778. doi: 10.1016/j.ymthe.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bertoli G, Cava C, Castiglioni I. MicroRNAs: New biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5:1122–1143. doi: 10.7150/thno.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 146.Ju R, Huang Y, Guo Z, Han L, Ji S, Zhao L, Long J. The circular RNAs differential expression profiles in the metastasis of salivary adenoid cystic carcinoma cells. Mol Cell Biochem. 2021;476:1269–1282. doi: 10.1007/s11010-020-03989-z. [DOI] [PubMed] [Google Scholar]
- 147.Xu Q, Liu X, Chen W, Zhang Z. Inhibiting adenoid cystic carcinoma cells growth and metastasis by blocking the expression of ADAM 10 using RNA interference. J Transl Med. 2010;8:136. doi: 10.1186/1479-5876-8-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Andreasen S. Molecular features of adenoid cystic carcinoma with an emphasis on microRNA expression. Apmis. 2018;126(Suppl 140):S7–S57. doi: 10.1111/apm.12828. [DOI] [PubMed] [Google Scholar]
- 149.Liang H, Gong F, Zhang S, Zhang CY, Zen K, Chen X. The origin, function, and diagnostic potential of extracellular microRNAs in human body fluids. Wiley Interdiscip Rev RNA. 2014;5:285–300. doi: 10.1002/wrna.1208. [DOI] [PubMed] [Google Scholar]
- 150.Papaspyrou G, Hoch S, Rinaldo A, Rodrigo JP, Takes RP, van Herpen C, Werner JA, Ferlito A. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: A review. Head Neck. 2011;33:905–911. doi: 10.1002/hed.21458. [DOI] [PubMed] [Google Scholar]
- 151.Le Tourneau C, Razak AR, Levy C, Calugaru V, Galatoire O, Dendale R, Desjardins L, Gan HK. Role of chemotherapy and molecularly targeted agents in the treatment of adenoid cystic carcinoma of the lacrimal gland. Br J Ophthalmol. 2011;95:1483–1489. doi: 10.1136/bjo.2010.192351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.